Abstract
Smoking increases cardiovascular risk in young women and eliminates the protective effect of the premenopausal state. Increased sympathetic nervous system (SNS) activity is associated with increased cardiovascular risk. One potential mechanism by which smoking increases risk is through chronic SNS activation. It has been reported that premenopausal women have high SNS activity during the midluteal (ML) phase, which falls to low levels during the early follicular (EF) phase. We tested the hypothesis that smoking disrupts this fall in SNS activity during the EF phase. We measured blood pressure and muscle sympathetic nerve activity (MSNA) using microneurography in 11 premenopausal female smokers and 11 age-matched nonsmoking controls at two separate times during the ovarian cycle: ML phase (8–10 days after the luteinizing hormone surge) and EF phase (days 1–4 of the menstrual cycle). The change in MSNA from the EF phase to the ML phase was significantly different between the smoking and nonsmoking groups (P = 0.036). Whereas there was a significant decrease in MSNA from the ML phase to the EF phase among nonsmoking controls (22.7 ± 3.3 vs. 17.9 ± 2.8 bursts/min, P = 0.012), MSNA remained elevated during the EF phase in smokers (22.5 ± 3.8 vs. 26.8 ± 4.0 bursts/min, P = 0.28). Surprisingly, mean arterial pressure (MAP) was significantly lower during the ML phase than in the EF phase in both nonsmokers (P = 0.0080) and smokers (P = 0.0094). We conclude that smoking disrupts the ovarian pattern of SNS activity by preventing the normal fall in MSNA during the EF phase of the ovarian cycle. Such chronic alterations may contribute to the pathogenesis of cardiovascular risk in young smoking females.
Keywords: cigarette smoking, muscle sympathetic nerve activity, menstrual cycle, premenopausal women
smoking remains the most important modifiable risk factor for heart disease in both men and women. Premenopausal women are relatively protected from heart disease and lag behind men by 10 years in the development of coronary heart disease; however, the protective effect of the premenopausal state is entirely erased by cigarette smoking. Smoking has a substantially greater adverse effect on cardiovascular risk in women than in men and is especially hazardous in young women. Women who smoke 20 cigarettes per day have a 6-fold increased incidence of myocardial infarction (MI), as opposed to a 3-fold increased incidence in smoking men, and female smokers ages 35–44 have a relative risk for MI of 7.1, a much larger effect than that seen in young men (23).
The mechanisms underlying the greater adverse effect of smoking in young women are unclear. One potential mechanism may be through chronic activation of the sympathetic nervous system (SNS), since cigarette smoking is known to acutely increase central SNS outflow, and SNS overactivity is an independent risk factor for cardiovascular disease. In premenopausal women, SNS activity may fluctuate with the ovarian cycle. Some, but not all, investigators have reported that SNS activity is highest during the midluteal (ML) phase during which estrogen and progesterone levels are high, and SNS activity falls during the early follicular (EF) phase at which time estrogen and progesterone levels are low (4, 5, 11, 13, 17, 19). The purpose of this study was to 1) evaluate the chronic effects of smoking on SNS activity in premenopausal female smokers and 2) examine the ovarian pattern of SNS activity in premenopausal smokers. We hypothesized that premenopausal smokers might have greater SNS activity during both the EF and ML phases of the ovarian cycle (i.e., the ovarian pattern is intact but shifted upward) or an altered ovarian pattern of SNS activity characterized by a failure to dip SNS activity during the EF phase, leading to a greater overall burden of SNS activity and thereby contributing to increased cardiovascular risk.
MATERIALS AND METHODS
Subjects.
A total of 22 premenopausal women participated in this study: 11 regular smokers and 11 age-matched nonsmoking controls. A smoker was defined as currently smoking at least three or more cigarettes daily for at least 1 yr. Nonsmoking controls had no previous history of cigarette smoking or significant passive smoke exposure. All participants were healthy, premenopausal women between the ages of 18 and 45 yr, with no medical problems and taking no regular medications. No subjects were in a training program or did regular endurance exercise. All participants had regular menstrual cycles of ∼26–30 days in duration and had not taken oral contraceptives within the last year. The experimental protocol was approved by the Institutional Review Board of the University of California, Los Angeles, and written, informed consent was obtained from each participant.
Experimental protocol.
All participants were studied a total of two times: once during the EF phase (1–4 days after the onset of menstruation) and once during the ML phase (8–10 days after the luteinizing hormone surge was detected by the home ovulation prediction kit). The order of the two visits was randomized, and each participant was studied at the same time of day during both visits. Participants were instructed to maintain their usual smoking habits between the two study visits. The onset of the EF phase was identified by the start of menstruation (first day of full menstrual flow), and the ML phase was identified using a home ovulation prediction kit (OvuQuick; Quidel) All participants were instructed to abstain from food, exercise, caffeine, and smoking for at least 12 h before each study visit. Each participant underwent a urine pregnancy test before each study to rule out pregnancy. The study room was quiet, semidark, and temperate (∼21°C). Participants were placed in a supine position and fitted with a blood pressure cuff on the upper arm for intermittent automatic blood pressure monitoring and electrocardiogram (ECG) patch electrodes for continuous heart rate (HR) recordings. The leg was positioned for microneurography, and the tungsten microelectrode was inserted and manipulated to obtain a satisfactory muscle sympathetic nerve activity (MSNA) recording. After 10 min of rest, baseline blood pressure and HR were measured, and baseline MSNA was recorded for 10 min. Blood pressure was measured every third minute throughout the study.
Measurements.
Arterial blood pressure was measured with an automated sphygmomanometer (Dynamap). Each data point of blood pressure was the mean of at least three consecutive readings. The mean arterial pressure (MAP) was calculated as SBP (systolic blood pressure) + DBP (diastolic blood pressure). HR was monitored with continuous electrocardiography. Multiunit postganglionic sympathetic nerve activity directed to muscle (MSNA) was recorded directly from the peroneal nerve by microneurography, using standard technique (28, 29). A tungsten microelectrode (tip diameter 5–15 μm; Bioengineering Services, University of Iowa) was inserted into the nerve, and a reference microelectrode was inserted subcutaneously 1–2 cm from the recording electrode. The microelectrodes were connected to a preamplifier (gain 1,000) and an amplifier (gain 50–100). Nerve signals were filtered (700–2,000 Hz; Nerve Traffic analyzer, model 662C-3; Bioengineering Services, University of Iowa), rectified, and integrated (time constant 0.1 s) to obtain a mean voltage display of sympathetic nerve activity that was recorded on a paper recorder. Lead II of the ECG was recorded simultaneously with the neurogram. MSNA was distinguished by previously described standards (8–10, 16, 18), and a satisfactory MSNA neurogram exhibited a signal-to-noise ratio of >3:1. Sympathetic bursts were identified by visual inspection of nerve bursts by a single investigator without knowledge of the participant's status as smoker vs. control or the phase of the menstrual cycle. MSNA was expressed as burst frequency (bursts/min). To control for variations in resting HR, MSNA was also expressed as bursts per 100 heart beats (bursts/100 HB).
Respiratory rate and perceived stress.
To assess the possibility that respiratory rate or perceived stress was different during the different menstrual phases in smokers or nonsmokers and thus may have contributed to differences in MSNA levels, we performed additional measurements of respiratory rate and perceived stress in five smokers and five nonsmokers who met the above inclusion/exclusion criteria. Spontaneous, resting respiratory rate was measured over 3 min and averaged per minute. Perceived stress was measured on a five-point scale: 0, not stressful; 1, somewhat stressful; 2, stressful; 3, very stressful; and 4, very, very stressful (1).
Data analysis.
Statistical analysis was performed using the SAS program (SAS Institutes). Baseline characteristics were compared using independent two-tailed t-tests. Two-way ANOVA with repeated measures was performed using PROC GLM to determine differences between groups (smokers vs. controls) and within groups with respect to changes in MSNA, MAP, SBP, DBP, HR, respiratory rate, and perceived stress from the ML phase to the EF phase. Results are means ± SE. A P value <0.05 was considered statistically significant.
RESULTS
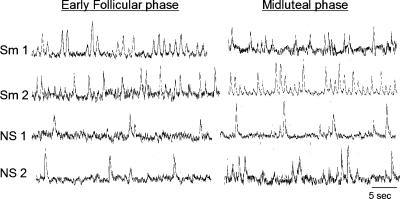
Smokers and nonsmoking controls were well-matched in age and body mass index (Table 1). Blood pressure and HR were similar between the two groups during both the EF and ML phases. Smokers were currently smoking an average of 8.0 cigarettes per day, for a mean duration of 8.3 yr. Figure 1 depicts examples of sympathetic neurograms from two smokers and two nonsmokers during the EF and ML phases of the ovarian cycle. When MSNA was quantified as bursts per minute, there was a trend (P = 0.08) toward greater baseline MSNA in smokers (26.8 ± 4.0 bursts/min) compared with controls (17.9 ± 2.8 bursts/min) during the EF phase. This difference became significant (P = 0.039) when MSNA was quantified as bursts per 100 heart beats, and baseline MSNA was significantly greater in smokers (38.8 ± 5.0 bursts/100 HB) compared with controls (25.0 ± 3.6 bursts/100 HB) during the EF phase. During the ML phase, MSNA was virtually identical between the two groups (smokers vs. controls: 22.5 ± 3.8 vs. 22.7 ± 3.3 bursts/min, respectively; P = 0.96), and results were similar when MSNA was quantified as bursts per 100 heart beats.
Table 1.
Baseline characteristics and hemodynamics during the EF and ML phases
| Nonsmokers | Smokers | P Value | |
|---|---|---|---|
| n | 11 | 11 | |
| Age, yr | 30.1±2.1 | 31.6±2.4 | 0.63 |
| Body mass index, kg/m2 | 23.8±1.3 | 25.8±1.8 | 0.36 |
| Early follicular phase | |||
| MAP, mmHg | 83.9±4.0 | 84.0±3.4 | 0.99 |
| SBP, mmHg | 120.1±6.1 | 116.8±4.5 | 0.67 |
| DBP, mmHg | 65.8±3.1 | 67.5±3.2 | 0.70 |
| HR, beats/min | 72.0±3.8 | 69.3±4.5 | 0.65 |
| MSNA, bursts/min | 17.9±2.8 | 26.8±4.0 | 0.082 |
| MSNA, bursts/100 HB | 25.0±3.6 | 38.8±5.0 | 0.039* |
| Midluteal phase | |||
| MAP, mmHg | 76.3±3.3 | 76.7±2.8 | 0.97 |
| SBP, mmHg | 110.2±5.1 | 109.8±4.4 | 0.96 |
| DBP, mmHg | 59.2±2.5 | 60.1±2.3 | 0.79 |
| HR, beats/min | 68.9±2.9 | 76.3±3.9 | 0.15 |
| MSNA, bursts/min | 22.7±3.3 | 22.5±3.8 | 0.96 |
| MSNA, bursts/100 HB | 32.6±4.0 | 30.3±5.6 | 0.73 |
| Smoking history | |||
| Cigarettes smoked per day (mean) | 8.0±1.3 | ||
| Years of smoking | 8.3±2.5 |
Values are means ± SE of measurements during the early follicular (EF) and midluteal (ML) phases of the ovulatory cycle; n = no. of subjects. MAP, mean arterial pressure; SBP, systolic blood pressure; DBP, diastolic blood pressure; HR, heart rate. Muscle sympathetic nerve activity (MSNA) is shown as both bursts per minute and bursts per 100 heart beats (HB).
P < 0.05.
Fig. 1.
Sympathetic neurograms. Original nerve recordings from 2 representative smokers (Sm) and 2 nonsmokers (NS) during both the early follicular (EF) and midluteal (ML) phases of the ovarian cycle.
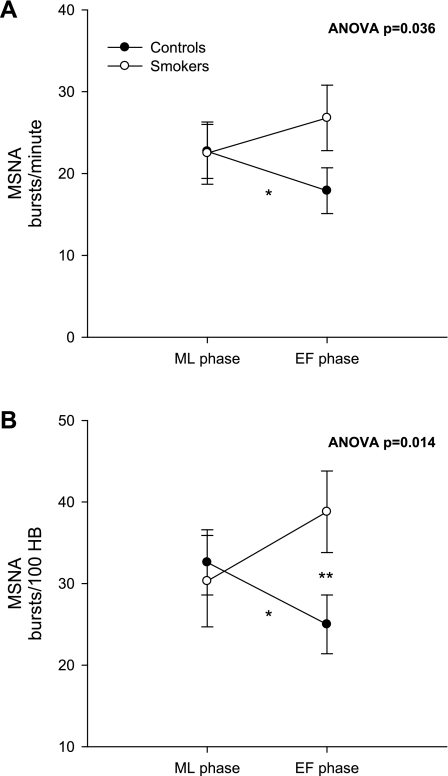
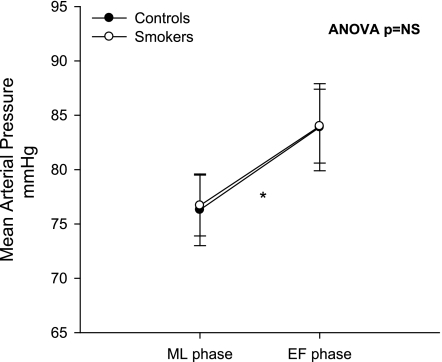
The overall ANOVA F-test revealed that the change in MSNA from the EF phase to the ML phase was significantly different between the smoking and nonsmoking groups (Fig. 2) when MSNA was quantified as both bursts per minute (A; P = 0.036) and bursts per 100 heart beats (B; P = 0.014). Within-subjects analysis showed that there was a significant decrease in MSNA from the ML phase to the EF phase in nonsmoking controls (P = 0.012); however, there was no decrease in MSNA during the EF phase of the ovarian cycle in smokers (P = 0.28). MAP was significantly lower during the ML phase than in the EF phase in both nonsmokers (P = 0.0080) and smokers (P = 0.0094) (Fig. 3). These effects were not likely due to order effect, since the order of study visits was randomized. MAP was 76.3 ± 3.3 mmHg during the ML phase and increased to 83.9 ± 4.0 mmHg during the EF phase in nonsmokers, and MAP increased from 76.7 ± 2.8 mmHg during the ML phase to 84.0 ± 3.4 mmHg during the EF phase in smokers. There was no significant difference in the change in MAP during the two phases of the ovarian cycle between the nonsmoking and smoking groups (P = 0.92).
Fig. 2.
Muscle sympathetic nerve activity (MSNA) during the ML and EF phases of the ovarian cycle. In A, MSNA is quantified as bursts per minute during the ML and EF phases in controls and smokers. In B, MSNA is quantified as bursts per 100 heart beats (bursts/100 HB) during the ML and EF phases. A single asterisk indicates a significant within-group difference in MSNA during the ML vs. EF phase in controls; a double asterisk indicates a significant between-group difference in MSNA during the EF phase in controls vs. smokers. The overall ANOVA F-test indicated a significant difference in ovarian pattern of MSNA between the 2 groups when quantified as bursts/min (A; P = 0.036) and bursts/100 HB (B; P = 0.014).
Fig. 3.
Mean arterial pressure (MAP) during the ML and EF phases of the ovarian cycle in controls and smokers. A single asterisk indicates a significant within-group difference in controls and smokers. Overall ANOVA F-test was not significant (NS) for a between-group difference.
Respiratory rate and perceived stress.
To exclude the possibility that differences in respiratory rate or perceived stress during the different phases of the menstrual cycle could account for lack of fall in MSNA during the EF phase in smokers but not in nonsmokers, additional measurements of respiratory rate and perceived stress were performed in five smokers and five nonsmokers who met previously specified inclusion/exclusion criteria (demographics similar to subjects in Table 1). Data are shown in Table 2. The spontaneous respiratory rate was not different during the EF vs. ML phases of the menstrual cycle in smokers (14.2 ± 0.4 vs. 13.8 ± 0.4 breaths/min, P = 0.18) or nonsmokers (17.0 ± 0.4 vs. 17.4 ± 0.4 breaths/min, P = 0.37). Perceived stress was not different during EF vs. ML phases of the menstrual cycle in smokers (0.4 ± 0.1 vs. 0, P = 0.18) or nonsmokers (0 vs. 0.1 ± 0.04, P = 0.37).
Table 2.
Respiratory rate and perceived stress during the EF and ML phases
|
Respiratory rate, breaths/min |
Perceived Stress (Scale 0–4)
|
|||
|---|---|---|---|---|
| EF | ML | EF | ML | |
| Smokers | ||||
| SD | 14 | 14 | 0 | 0 |
| CG | 12 | 11 | 0 | 0 |
| DR | 14 | 14 | 1 | 0 |
| GA | 17 | 16 | 1 | 0 |
| MG | 14 | 14 | 0 | 0 |
| Nonsmokers | ||||
| LC | 16 | 16 | 0 | 0 |
| TH | 16 | 17 | 0 | 0 |
| GA | 19 | 20 | 0 | 0.5 |
| GA | 15 | 15 | 0 | 0 |
| TH | 19 | 19 | 0 | 0 |
Perceived stress was measured on a 5-point scale: 0, not stressful; 1, somewhat stressful; 2, stressful; 3, very stressful; and 4, very, very stressful (1). Respiratory rates and perceived stress were not different during the different phases of the menstrual cycle in either smokers or nonsmokers.
DISCUSSION
The major novel finding of the present study is that the ovarian pattern of SNS activity is altered in premenopausal female smokers. Whereas MSNA falls significantly during the EF phase compared with the ML phase in nonsmoking controls, smokers fail to have a decline in MSNA during the EF phase. Thus female smokers have a greater total burden of SNS activity that could contribute to the increased risk of cardiovascular disease and elimination of the “female coronary advantage” in this population.
Studies have shown that smoking acutely activates neural SNS outflow (21). When the pressor effect of smoking was attenuated by administration of sodium nitroprusside, smoking significantly increased MSNA up to threefold. Skin sympathetic nerve activity (SSNA), which is not affected by the baroreflex, was also found to be elevated during smoking (21). In addition, smoking acutely and transiently increases blood pressure and HR (15, 21, 22) and increases plasma catecholamine levels (15). Although these prior studies have shown that smoking increases SNS activity acutely, it is unknown whether these effects are long-lasting and extend beyond the acute increases observed while the subject is actively smoking. We have demonstrated the first evidence that smoking has chronic effects on SNS activity that are evident even when the subject is not acutely smoking. When MSNA was quantified as bursts per 100 heart beats, resting MSNA was significantly greater in smokers (who had abstained from smoking for at least 12 h) than in controls during the EF phase. We have shown that the normal pattern of MSNA fluctuation with the ovarian cycle is disrupted in female smokers, and rather than declining during the EF phase, MSNA remains significantly elevated.
Whether or not SNS activity fluctuates with the ovarian cycle in premenopausal women has been a subject of debate. Minson et al. (19) found that resting MSNA was significantly higher during the ML phase (when both estrogen and progesterone are markedly elevated) than in the EF phase (when the ovary is least hormonally active). Accordingly, plasma norepinephrine levels have been shown to be significantly higher during the ML phase than the EF phase (14). However, other studies, including two recent studies by Carter et al. and Fu et al., have demonstrated no significant difference between the ML and EF phases in MSNA at rest (4, 5, 11, 13, 17). Interestingly, Fu et al. (13) did report a greater sympathetic nerve response to upright tilt during the ML phase compared with the EF phase. Our finding that resting MSNA fluctuates with the ovarian cycle in normal controls is the first study that confirms the findings of Minson et al. (19), and we have shown that in healthy, nonsmoking females, MSNA is significantly higher during the ML phase and lower during the EF phase. In contrast, smoking eliminates this normal decline in resting MSNA during the EF phase.
The mechanisms by which smoking might disrupt the normal pattern of MSNA fluctuation with the ovarian cycle are unclear. One possibility is that the spontaneous, resting respiratory rates differ between smokers and nonsmokers. Narkiewicz et al. (20) reported that in healthy men, subjects with the respiratory rates in the highest tertile had significantly higher MSNA levels compared with those in the lowest tertile. In our additional studies, we found no difference in respiratory rates according to phase of the menstrual cycle in either smokers or nonsmokers. In fact, smokers tended to have lower spontaneous resting respiratory rates compared with nonsmokers, eliminating respiratory rate as an explanation for the sustained elevation in sympathetic nerve activity in smokers throughout the menstrual cycle. Another potential explanation is that perceived stress may differ in smokers compared with nonsmokers according to the phase of the menstrual cycle, and this may be heightened by abstinence from cigarettes for 12 h as specified in the study protocol. However, the self-reported perception of stress was not different during the different phases of the menstrual cycle in either smokers or nonsmokers, eliminating this potential mechanism as well.
Another potential mechanism for our findings includes smoking-induced impairment of sympathetic baroreceptor responsivity. In support of this possibility is the observation that, in controls, there was a significant increase in MAP during the EF phase compared with the ML phase, with a concomitant decrease in MSNA from the EF to ML phase, possibly mediated by the baroreflexes. However, in the smoking group, although there was a significant increase in MAP from the ML phase to the EF phase, there was no significant reduction in MSNA as was observed in the control group.
Finally, another potential mechanism for altered SNS pattern among smokers includes decreased NO availability. Both active and passive smoking decreases NO availability (2, 6), and NO is known to decrease central SNS outflow. Smoking could decrease the sympathoinhibitory effect of NO on the central nervous system in premenopausal women (24, 26).
Limitations.
We recognize several limitations to this study. First, although we were precise in determining the phase of the ovarian cycle by using ovulation predictor kits and menstrual cycle documentation, we did not measure estrogen and progesterone levels directly in our subjects. Second, the participants of this study were instructed to maintain usual smoking habits during the study period; however, we are unaware whether the amount of cigarette smoking might have changed between study visits possibly due to participation in a smoking-related study or to hormonal changes throughout the ovarian cycle. Prior studies have shown that the frequency of smoking increases significantly during the luteal phase compared with the follicular phase, and cigarette cravings, withdrawal symptoms, and relapses after smoking cessation occur more often during the luteal phase (3, 7, 12, 25, 27). Third, the impact of 12 h of smoking withdrawal on MSNA activity may vary during the different phases of the menstrual cycle. If this were the case, one might expect perceived stress to increase as symptoms of smoking withdrawal increased. However, in our study, despite a 12-h abstinence from smoking, perceived stress was quite low and did not vary with the different phases of the menstrual cycle. Finally, sympathetic baroreflexes were not tested directly in these experiments; thus the mechanistic role of impaired baroreflexes on the altered ovarian pattern of SNS activity in smokers remains untested at this time. However, although we did not pharmacologically manipulate blood pressure to measure the baroreflex curve, we did observe a difference in the relationship between blood pressure and MSNA between the two groups, when MSNA was measured in the same individuals at two significantly different blood pressures that were influenced by the ovarian cycle. The MSNA response to pharmacological manipulation of blood pressure during the two phases of the ovarian cycle must be tested in future studies to determine whether abnormal sympathetic baroreflexes account for the altered ovarian pattern of SNS activity in smokers.
In conclusion, this study demonstrates that smoking alters the normal pattern of SNS activity with the ovarian cycle. Unlike nonsmoking controls, premenopausal women who were regular smokers did not have a decline in MSNA during the EF phase. In smokers, MSNA levels remained high during the EF phase (and similar to levels during the ML phase) despite a greater blood pressure during the EF phase. Resting MSNA quantitated as bursts per 100 heart beats was significantly greater in smokers than in controls during the EF phase, even after abstinence from smoking for at least 12 h. These chronic alterations in SNS activity with the ovarian cycle might contribute to the pathogenesis of increased cardiovascular risk in young female smokers. Future studies examining when and if smoking cessation restores the normal ovarian pattern of SNS activity are warranted.
GRANTS
This work was supported by National Institutes of Health Grants 9F32 DK080995, 1R01 HL084525, and M01 RR00865.
REFERENCES
- 1.Callister R, Suwarno NO, Seals DR. Sympathetic activity is influenced by task difficulty and stress perception during mental challenge in humans. J Physiol 454: 373–387, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Campisi R, Czernin J, Schoder H, Sayre JW, Marengo FD, Phelps ME, Schelbert HR. Effects of long-term smoking on myocardial blood flow, coronary vasomotion, and vasodilator capacity. Circulation 98: 119–125, 1998. [DOI] [PubMed] [Google Scholar]
- 3.Carpenter MJ, Upadhyaya HP, LaRowe SD, Saladin ME, Brady KT. Menstrual cycle phase effects on nicotine withdrawal and cigarette craving: a review. Nicotine Tob Res 8: 627–638, 2006. [DOI] [PubMed] [Google Scholar]
- 4.Carter JR, Lawrence JE. Effects of the menstrual cycle on sympathetic neural responses to mental stress in humans. J Physiol 585: 635–641, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carter JR, Lawrence JE, Klein JC. Menstrual cycle alters sympathetic neural responses to orthostatic stress in young, eumenorrheic women. Am J Physiol Endocrinol Metab 297: E85–E91, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Celermajer DS, Adams MR, Clarkson P, Robinson J, McCredie R, Donald A, Deanfield JE. Passive smoking and impaired endothelium-dependent arterial dilatation in healthy young adults. N Engl J Med 334: 150–155, 1996. [DOI] [PubMed] [Google Scholar]
- 7.DeBon M, Klesges RC, Klesges LM. Symptomatology across the menstrual cycle in smoking and nonsmoking women. Addict Behav 20: 335–343, 1995. [DOI] [PubMed] [Google Scholar]
- 8.Delius W, Hagbarth KE, Hongell A, Wallin BG. General characteristics of sympathetic activity in human muscle nerves. Acta Physiol Scand 84: 65–81, 1972. [DOI] [PubMed] [Google Scholar]
- 9.Delius W, Hagbarth KE, Hongell A, Wallin BG. Manoeuvres affecting sympathetic outflow in human skin nerves. Acta Physiol Scand 84: 177–186, 1972. [DOI] [PubMed] [Google Scholar]
- 10.Delius W, Hongell A, Hongell A, Wallin BG. Manoeuvres affecting sympathetic outflow in human muscle nerves. Acta Physiol Scand 84: 82–94, 1972. [DOI] [PubMed] [Google Scholar]
- 11.Ettinger SM, Silber DH, Gray KS, Smith MB, Yang QX, Kunselman AR, Sinoway LI. Effects of the ovarian cycle on sympathetic neural outflow during static exercise. J Appl Physiol 85: 2075–2081, 1998. [DOI] [PubMed] [Google Scholar]
- 12.Franklin TR, Ehrman R, Lynch KG, Harper D, Sciortino N, O'Brien CP, Childress AR. Menstrual cycle phase at quit date predicts smoking status in an NRT treatment trial: a retrospective analysis. J Womens Health (Larchmt) 17: 287–292, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fu Q, Okazaki K, Shibata S, Shook RP, VanGunday TB, Galbreath MM, Reelick MF, Levine BD. Menstrual cycle effects on sympathetic neural responses to upright tilt. J Physiol 587: 2019–2031, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goldstein DS, Levinson P, Keiser HR. Plasma and urinary catecholamines during the human ovulatory cycle. Am J Obstet Gynecol 146: 824–829, 1983. [DOI] [PubMed] [Google Scholar]
- 15.Grassi G, Seravalle G, Calhoun DA, Bolla GB, Giannattasio C, Marabini M, Del Bo A, Mancia G. Mechanisms responsible for sympathetic activation by cigarette smoking in humans. Circulation 90: 248–253, 1994. [DOI] [PubMed] [Google Scholar]
- 16.Hagbarth KE, Hallin RG, Hongell A, Torebjork HE, Wallin BG. General characteristics of sympathetic activity in human skin nerves. Acta Physiol Scand 84: 164–176, 1972. [DOI] [PubMed] [Google Scholar]
- 17.Lawrence JE, Ray CA, Carter JR. Vestibulosympathetic reflex during the early follicular and midluteal phases of the menstrual cycle. Am J Physiol Endocrinol Metab 294: E1046–E1050, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mano T, Iwase S, Toma S. Microneurography as a tool in clinical neurophysiology to investigate peripheral neural traffic in humans. Clin Neurophysiol 117: 2357–2384, 2006. [DOI] [PubMed] [Google Scholar]
- 19.Minson CT, Halliwill JR, Young TM, Joyner MJ. Influence of the menstrual cycle on sympathetic activity, baroreflex sensitivity, and vascular transduction in young women. Circulation 101: 862–868, 2000. [DOI] [PubMed] [Google Scholar]
- 20.Narkiewicz K, van de Borne P, Montano N, Hering D, Kara T, Somers VK. Sympathetic neural outflow and chemoreflex sensitivity are related to spontaneous breathing rate in normal men. Hypertension 47: 51–55, 2006. [DOI] [PubMed] [Google Scholar]
- 21.Narkiewicz K, van de Borne PJ, Hausberg M, Cooley RL, Winniford MD, Davison DE, Somers VK. Cigarette smoking increases sympathetic outflow in humans. Circulation 98: 528–534, 1998. [DOI] [PubMed] [Google Scholar]
- 22.Niedermaier ON, Smith ML, Beightol LA, Zukowska-Grojec Z, Goldstein DS, Eckberg DL. Influence of cigarette smoking on human autonomic function. Circulation 88: 562–571, 1993. [DOI] [PubMed] [Google Scholar]
- 23.Njolstad I, Arnesen E, Lund-Larsen PG. Smoking, serum lipids, blood pressure, and sex differences in myocardial infarction A 12-year follow-up of the Finnmark Study. Circulation 93: 450–456, 1996. [DOI] [PubMed] [Google Scholar]
- 24.Owlya R, Vollenweider L, Trueb L, Sartori C, Lepori M, Nicod P, Scherrer U. Cardiovascular and sympathetic effects of nitric oxide inhibition at rest and during static exercise in humans. Circulation 96: 3897–3903, 1997. [DOI] [PubMed] [Google Scholar]
- 25.Perkins KA, Levine M, Marcus M, Shiffman S, D'Amico D, Miller A, Keins A, Ashcom J, Broge M. Tobacco withdrawal in women and menstrual cycle phase. J Consult Clin Psychol 68: 176–180, 2000. [DOI] [PubMed] [Google Scholar]
- 26.Sartori C, Lepori M, Scherrer U. Interaction between nitric oxide and the cholinergic and sympathetic nervous system in cardiovascular control in humans. Pharmacol Ther 106: 209–220, 2005. [DOI] [PubMed] [Google Scholar]
- 27.Snively TA, Ahijevych KL, Bernhard LA, Wewers ME. Smoking behavior, dysphoric states and the menstrual cycle: results from single smoking sessions and the natural environment. Psychoneuroendocrinology 25: 677–691, 2000. [DOI] [PubMed] [Google Scholar]
- 28.Vallbo AB, Hagbarth KE, Wallin BG. Microneurography: how the technique developed and its role in the investigation of the sympathetic nervous system. J Appl Physiol 96: 1262–1269, 2004. [DOI] [PubMed] [Google Scholar]
- 29.Wallin BG, Fagius J. Peripheral sympathetic neural activity in conscious humans. Annu Rev Physiol 50: 565–576, 1988. [DOI] [PubMed] [Google Scholar]