over the last few decades, the renin-angiotensin system (RAS) has seen the discovery of several new members, both in the periphery and in the central nervous system (CNS), including substrates, enzymes, and receptors, and one may wonder why textbooks are still teaching an oversimplified and outdated version of this system. Far from being the straightforward cascade containing one substrate [angiotensinogen], two peptides, [angiotensin (ANG) I, ANG II], two enzymes [renin, angiotensin-converting enzyme (ACE)] and one receptor (AT1), the RAS currently includes nearly a dozen of ANG fragments, some active and some inactive, more than two dozen peptidases, and at least six different receptors. Moreover, the RAS now consists of several axes upstream and downstream of the classical cascade. While sometimes, these newcomers are considered to be “missing links,” more often they are viewed with skepticism. In addition, it is now well accepted that local RASs are present in most organs and tissues, including but not limited to the heart, kidney, vasculature, adipose tissue, pancreas, and brain, and they are involved in the local regulation of these tissues.
Since its first description by Ganten et al. (3), in the early 1970s, the brain RAS has been the subject of controversy and debate. Is ANG II generated in the brain or does it travel from the periphery? Is ANG III the real ligand for the AT1 receptor in the CNS? Is Mas the receptor for ANG (1-7)? Is there a non-AT1, non-AT2 receptor? What is the binding site for ANG IV? Although some of these questions have been answered, for example, it is now well accepted that ANG II in the CNS can be generated locally, and it also can enter the brain via the circumventricular organs, other claims still spark debates.
Probably the oldest controversy for the brain RAS concerns the presence of renin in the CNS. Because renin levels in the brain are usually low and likely not homogenous throughout the various nuclei, they have been difficult to assess. As a consequence, over the years, different theories have emerged, generally involving alternate pathways for the synthesis of ANG II. Accordingly, evidence has shown that the octapeptide could be produced via tonin, chymase, cathepsins, and other peptidases (see Ref. 11 for a full list). One of the latest possibilities for a renin-independent synthesis of ANG II involves the recently discovered ANG (1-12) peptide (12), which could eventually be transformed successively into ANG (1-10), then ANG II, through the action of ACE. However, as recently reviewed by Grobe et al. (4), genetic studies argue against renin-independent pathways, essentially because of the lack of phenotype in transgenic animals overexpressing angiotensinogen in the brain and support the presence of this enzyme in the CNS (1, 7). In addition, evidence has shown the existence of a nonsecreted intracellular form of the enzyme, renin-b, which is functional in the brain of rodents and humans (5).
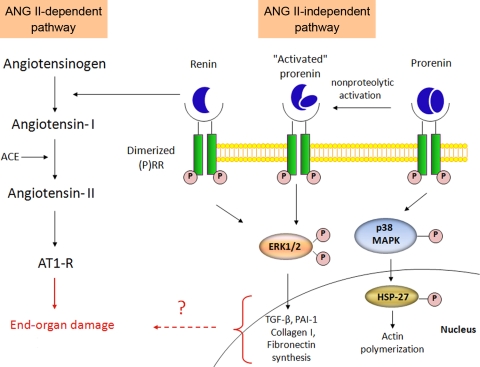
A new piece of the puzzle is presented in this issue (2) by Dr. Genevieve Nguyen's group. Here, the authors show evidence of the (pro)renin receptor [(P)RR] in the CNS. The (P)RR was discovered (8) and cloned from the X chromosome (9) by the Nguyen group. It is known to bind both renin and the “inactive” prorenin, resulting in increased activity of both enzymes, thus leading to enhanced formation of ANG I (Fig. 1). Interestingly, binding of the receptor also leads to the activation of ANG II-independent intracellular signaling pathways, ultimately leading to the expression of profibrotic genes, such as TGF-β, PAI-1, and others.
Fig. 1.
ANG II-dependent and independent pathways for renin signaling. Both renin and prorenin can bind the dimerized (pro)renin receptor [(P)RR], leading to the activation of different signaling pathways. Binding of prorenin to the receptor results in either direct activation of downstream MAP kinases signaling, or in the nonproteolytic activation of prorenin, facilitating the activation of ERK signaling cascades that can also be triggered by renin binding. Activation of these signaling cascades results in actin polymerization and the expression for profibrotic genes, leading to TGF-β, PAI-1, collagen-I, and fibronection synthesis. Binding of renin to the (P)RR also activates an ANG II-dependent pathway leading to the formation of ANG II and the activation of the AT1 receptor. Activation of both pathways ultimately leads to end-organ damage. Abbreviations: TGF-β, transforming growth factor β; PAI-1, plasminogen-activator inhibitor-1.
Although the presence of the (P)RR was previously identified by this group in the brain (8) and by others in primary neurons (10), this is the first time, almost 15 years after its discovery, that the localization of the receptor is being mapped throughout the brain. Importantly, in this paper, Contrepas et al. (2), using in situ hybridization, show the presence of the (P)RR mRNA in various brain regions, some of them, like the subfornical organ (SFO), paraventricular nucleus, the supraoptic nucleus, the nucleus of the tractus solitarius (NTS), or the rostral ventrolateral medulla, being famous for their involvement in the central regulation of cardiovascular function and volume homeostasis. Of particular interest are the data showing that the (P)RR mRNA was highly expressed in the SFO and the NTS. Indeed, these two regions are very much involved in the fine-tuning of blood pressure (BP) regulation by being an “open window” to the periphery, and thus, sensitive to blood-borne RAS components, for the SFO, and by receiving information on the BP status, via the baroreceptor reflex afferents, for the NTS. Therefore, the presence of the (P)RR in these important nuclei suggests that it may play a role in the central regulation of BP, which opens new avenues in this field.
The (P)RR in the brain, like other components of the RAS, is involved in additional functions, beyond the regulation of BP. Indeed, while ACE is required to maintain fertility and ACE2 serves as a receptor for the SARS coronavirus (6), mutation of the (P)RR is associated with X-linked mental retardation and epilepsy, and thus, (P)RR seems to be important for brain development and cognition. Therefore, another major finding by Contrepas et al. (2) is that the mutated version of the (P)RR, responsible for the mental retardation, while still capable of binding renin and forming dimers with the native receptor, affects the trafficking of this receptor to the neurite tips in cultured neurons. Moreover, for these mutated homodimers, the ANG II-independent signaling pathway was impaired, supporting the idea that the mutated receptor may act as a dominant negative and suggesting that ERK signaling plays an important role in neuronal differentiation. However, with one controversy almost resolved, another is coming, and several questions already need to be answered. For example, is the trafficking of the (P)RR to the neurite tips, part of a mechanism that would allow ANG II formation near the synaptic cleft? We previously mentioned that renin levels are assumed to be low in the brain, on the other hand, prorenin levels have been shown, in the plasma, to be 10 to 100 times higher than renin. Is the same true in the brain? Could prorenin, or another form of renin (4), be more relevant as enzymes in the brain or could its interaction with the (P)RR be the key to increased renin activity in the CNS? Also, Raizada's group (10) previously showed that the (P)RR, via a non-AT1-mediated pathway involving MAP kinases, was able to inhibit neuronal activity in vitro. This is rather unexpected since activation of the RAS is usually associated with neuronal excitation, and it has potentially interesting consequences. Indeed, upon confirmation in vivo, and assuming that this particular pathway could be selectively activated, this would confer on (P)RR some therapeutic properties, for example, during neurogenic hypertension when the overactivity of the RAS needs to be subdued. Obviously, these questions are only the tip of the iceberg, and many more are likely to emerge.
In conclusion, the present study by Contrepas et al. (2) provides a major advancement of our knowledge of the function of the (P)RR in the brain in mental retardation and potentially the regulation of cardiovascular function. Although these data do not necessarily show that renin is generated in the CNS, they definitely support the fact that renin is playing major roles in the brain.
Acknowledgments
This work was supported by a grant from the National Institutes of Health Heart Lung and Blood Institute (HL-093178) to E. Lazartigues.
REFERENCES
- 1.Allen AM, O'Callaghan EL, Hazelwood L, Germain S, Castrop H, Schnermann J, Bassi JK. Distribution of cells expressing human renin-promoter activity in the brain of a transgenic mouse. Brain Res 1243: 78–85, 2008. [DOI] [PubMed] [Google Scholar]
- 2.Contrepas A, Walker J, Koulakoff A, Franek KJ, Qadri F, Giaume C, Corvol P, Schwartz CE, Nguyen G. A role of the (pro)renin receptor in neuronal cell differentiation. Am J Physiol Regul Integr Comp Physiol (May 27, 2009). doi: 10.1152/ajpregu.90832.2008. [DOI] [PMC free article] [PubMed]
- 3.Ganten D, Minnich JL, Grenger P, Hayduk K, Brecht HM, Barbeau A, Boucher R, Genest J. Angiotensin-forming enzyme in brain tissue. Science 173: 64–65, 1971. [DOI] [PubMed] [Google Scholar]
- 4.Grobe JL, Xu D, Sigmund CD. An intracellular renin-angiotensin system in neurons: fact, hypothesis, or fantasy. Physiology 23: 187–193, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lavoie JL, Liu X, Bianco RA, Beltz TG, Johnson AK, Sigmund CD. Evidence supporting a functional role for intracellular renin in the brain. Hypertension 47: 461–466, 2006. [DOI] [PubMed] [Google Scholar]
- 6.Lazartigues E, Feng Y, Lavoie JL. The two fACEs of the tissue renin-angiotensin systems: implication in cardiovascular diseases. Curr Pharm Des 13: 1231–1245, 2007. [DOI] [PubMed] [Google Scholar]
- 7.Morimoto S, Cassell MD, Sigmund CD. The brain renin-angiotensin system in transgenic mice carrying a highly regulated human renin transgene. Circ Res 90: 80–86, 2002. [DOI] [PubMed] [Google Scholar]
- 8.Nguyen G, Delarue F, Berrou G, Rondeau E, Sraer JD. Specific receptor binding of renin on human mesangial cells in culture increases plasminogen activator inhibitor-1 antigen. Kidney Int 50: 1897–1903, 1996. [DOI] [PubMed] [Google Scholar]
- 9.Nguyen G, Delarue F, Burckle C, Bouzhir L, Giller T, Sraer JD. Pivotal role of the renin/prorenin receptor in angiotensin II production and cellular responses to renin. J Clin Invest 109: 1417–1427, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shan Z, Cuadra AE, Sumners C, Raizada MK. Characterization of a functional (pro)renin receptor in rat brain neurons. Exp Physiol 93: 701–708, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Speth R, Karamyan V. The significance of brain aminopeptidases in the regulation of the actions of angiotensin peptides in the brain. Heart Fail Rev 13: 299–309, 2008. [DOI] [PubMed] [Google Scholar]
- 12.Trask AJ, Jessup JA, Chappell MC, Ferrario CM. Angiotensin-(1–12) is an alternate substrate for angiotensin peptide production in the heart. Am J Physiol Heart Circ Physiol 294: H2242–H2247, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]