Abstract
The nucleus tractus solitarius (NTS), a major hindbrain area involved in cardiovascular regulation, receives primary afferent fibers from peripheral baroreceptors and chemoreceptors. Hydrogen peroxide (H2O2) is a relatively stable and diffusible reactive oxygen species (ROS), which acting centrally, may affect neural mechanisms. In the present study, we investigated effects of H2O2 alone or combined with the glutamatergic antagonist kynurenate into the NTS on mean arterial pressure (MAP) and heart rate (HR). Conscious or anesthetized (urethane and α-chloralose) male Holtzman rats (280–320 g) were used. Injections of H2O2 (125 to 1500 pmol/40 nl) into the intermediate NTS of anesthetized rats evoked dose-dependent and transient hypotension (−18 ± 3 to −55 ± 11 mmHg) and bradycardia (−16 ± 5 to −116 ± 40 bpm). Injection of the catalase inhibitor 3-amino-1,2,4-triazole (100 nmol/40 nl) into the NTS also produced hypotension and bradycardia. Previous injection of the ionotropic l-glutamate receptor antagonist kynurenate (7 nmol/40 nl) attenuated by 48% the bradycardic response, without changing the hypotension evoked by H2O2 (500 pmol/40 nl) in anesthetized rats. The antioxidant l-ascorbate (600 pmol/80 nl) injected into the NTS attenuated the bradycardic (42%) and hypotensive (67%) responses to H2O2 (500 pmol/40 nl) into the NTS. In conscious rats, injection of H2O2 (50 nmol/100 nl) into the NTS also evoked intense bradycardia (−207 ± 8 bpm) and hypotension (−54 ± 6 mmHg) that were abolished by prior injection of kynurenate (7 nmol/100 nl). The results show that H2O2 into the NTS induces hypotension and bradycardia probably due to activation of glutamatergic mechanisms.
Keywords: reactive oxygen species, catalase inhibition, kynurenic acid, blood pressure, heart rate
the nucleus tractus solitarius (NTS), located in the dorsomedial medulla oblongata (11), is the site at which primary cardiovascular sensory afferents, such as those arising from arterial baroreceptors, peripheral chemoreceptors, and cardiopulmonary receptors terminate. Consequently, the NTS plays a pivotal role in relaying signals from these afferents to other areas in the brain, especially within the medulla (11). The importance of NTS in controlling cardiovascular functions was demonstrated when electrolytic lesion, centered on the intermediate one-third of the nucleus, produced severe hypertension within minutes, which was maintained even after rats had recovered from the anesthesia (14). The excitatory amino acid transmitter l-glutamate plays an important role in the transmission of cardiovascular reflexes within the NTS [reviewed in (27, 30, 32)]. For this reason, identifying mechanisms that affect l-glutamate release or its signaling pathways in the NTS is a key to understanding how cardiovascular reflex signals are processed centrally.
In the past two decades, subtoxic, endogenously generated reactive oxygen species (ROS) have been demonstrated to act as neuromodulators in the central nervous system (CNS) (3, 6, 10, 34, 37, 39). Among a variety of ROS, hydrogen peroxide (H2O2) is of particular interest because it is a relatively stable oxidant with small molecular weight and highly diffusible across biological membranes (4, 8). The local production of H2O2 in the brain can vary according to metabolic demands and factors like the level of NADPH oxidase activity (35, 36) and even the degree of activation of l-glutamate receptors (9).
Previous studies have demonstrated that H2O2 can affect glutamatergic transmission through a number of mechanisms, including inhibition of calcium-dependent l-glutamate release (39), inhibition of sodium-dependent l-glutamate uptake by astrocytes (34), and direct inhibition of ionotropic l-glutamate receptors by acting on their redox modulatory sites (1–3). Inhibition of l-glutamate release and inhibition of l-glutamate receptor activity by increased levels of H2O2 may produce a net inhibitory effect on local glutamatergic neurotransmission in the NTS, thus impairing the efficiency of reflex adjustments of blood pressure. On the other hand, inhibition of l-glutamate uptake by ROS might enhance glutamatergic transmission, thereby potentially facilitating reflex control of blood pressure. Therefore, considering that ROS can modulate glutamatergic transmission by different mechanisms, the net cardiovascular effect of ROS in the NTS is difficult to predict.
Evidence available in the literature indicates that redox state of NTS may potentially affect the development and/or maintenance of cardiovascular dysfunction. For example, increased ROS scavenging by viral mediated overexpression of superoxide dismutase (SOD) and/or catalase in the NTS or in the rostral ventrolateral medulla reduces blood pressure in spontaneously hypertensive rats (17, 23). Viral mediated overexpression of SOD in forebrain areas also reduces the pressor response to central ANG II (38). Furthermore, a previous study from our laboratory also demonstrated that H2O2 delivered into the fourth brain ventricle of conscious rats elicits a dose-dependent increase in blood pressure along with a decrease in heart rate (21). Changes in redox state directly in the NTS by H2O2 production or scavenging may affect glutamatergic transmission in this area and consequently cardiovascular regulation; however, this possibility has not been investigated yet. In this study, we investigated whether increasing H2O2 levels in the NTS acutely would affect arterial blood pressure and heart rate and whether these effects were linked to activation of ionotropic glutamate receptors in the same area.
MATERIALS AND METHODS
Animals
Male Holtzman rats (280 to 320 g, n = 27) from the main breeding stock of the Dentistry School-São Paulo State University animal facility were used. Animals were housed in individual cages in a room with controlled temperature (22 ± 3°C) and humidity (40 to 60%) and received rat chow (Guabi Rat Chow, Paulinia, SP, Brazil) and water ad libitum. Lights were on from 7 AM to 7 PM. All experiments were done in accordance with the Brazilian Society for Neuroscience and Behavior Guidelines for Animal Experimentation and had the approval of the institutional animal care and use committee of the Federal University of São Paulo/Escola Paulista de Medicina.
Drugs
Hydrogen peroxide (H2O2) was prepared in saline 0.9% (vehicle) from a stock solution (30% wt/wt; Sigma, St. Louis, MO) to final concentrations ranging from 3.1 to 500 mmol/l. Ascorbic acid and kynurenic acid (Sigma) were neutralized with sodium bicarbonate (1 mol/l) and diluted in vehicle to the desired final concentrations.
Animal Instrumentation
For experiments conducted in anesthetized rats, femoral artery and vein polyethylene cannulas (PE-10 connected to PE-50; Clay Adams, Parsippany, NJ) were implanted in animals anesthetized with ketamine (80 mg/kg) and xylazine (7 mg/kg) delivered by intraperitoneal injection. The free end of each catheter was tunneled subcutaneously and exteriorized at the back of the neck.
After a recovery period of 48 h, the arterial cannula was connected to a Statham-Gould pressure transducer. The transducer signal was amplified and directed to an analog-to-digital converter for data acquisition (PowerLab 16Sp; ADInstruments, Sydney, Australia). Data were digitized at 1,000 Hz. Heart rate (HR) and mean arterial pressure (MAP) were derived online from the pulsatile arterial pressure signal with Chart 5 for Windows software (ADInstruments).
For experiments conducted in conscious rats, stainless-steel guide cannulas directed to the NTS were implanted in rats anesthetized with ketamine (80 mg/kg ip) combined with xylazine (7 mg/kg ip) (Cristalia Produtos Químicos e Farmacêuticos, Itapira, SP, Brazil). Rats were placed in a Stoelting stereotaxic instrument, and an incision was made through the skin on the skull to expose bregma and lambda that were positioned at the same horizontal plane. A stainless-steel cannula (14.0 mm × 0.6 mm OD) was implanted so that its tip was located 14.5 mm caudal and 7.5 mm ventral to bregma and 0.5 mm lateral to the midline. Two jeweler screws were implanted in the skull, and the cannula was anchored to the screws with acrylic cement. At the end of the surgery, rats received an intramuscular injection with 30,000 IU of penicillin (Fort Dodge Saúde Animal, Campinas, SP, Brazil) and were housed in individual cages with chow and water ad libitum.
Three days after stereotaxic surgery, also under ketamine/xylazine anesthesia, PE cannulas (PE-10 connected to PE-50, Clay Adams) filled with heparinized saline (125 IU/ml) were inserted into the aorta through the right femoral artery for measurement of pulsatile arterial pressure, MAP, and HR, according to procedures indicated above.
Experimental Protocols in Anesthetized Rats
After an initial recording of MAP and HR performed in freely moving conscious rats, anesthesia was induced and maintained with a mixture of urethane (830 mg/kg) plus α-chloralose (55 mg/kg iv) through the venous line. The level of anesthesia was adjusted for each animal to completely inhibit corneal and withdraw reflexes. Rats were tracheotomized to facilitate spontaneous respiration. The rats were then placed in a stereotaxic instrument (David Kopf), and a longitudinal incision was performed on the dorsal part of the neck between the occipital and the second vertebrae. The muscle was retracted so that the occipital bone and the atlanto-occipital membrane were easily visualized. Part of the atlanto-occipital membrane was removed, and a partial occipital craniotomy was performed. After removing the pia-mater, the dorsal surface of the medulla was completely exposed, allowing clear visualization of the area postrema and easy access to the injection site in the NTS. Injections were performed 0.50 mm rostral and 0.40 to 0.50 mm lateral to callamus scriptorius, and 0.40 to 0.50 mm ventral to the medulla surface using a glass micropipette coupled to a nitrogen pressure injection system (Picospritzer).
Dose-response curve for H2O2.
In a group of seven rats, after arterial pressure and HR stabilized, vehicle and l-glutamate (500 pmol) were injected (40 nl) unilaterally into the NTS. Thereafter, different doses of H2O2 (125, 250, 500, 1000, and 1,500 pmol/40 nl/rat) were randomly injected into the same site. The interval separating each injection was 10 min.
Catalase inhibition in the NTS.
To test whether endogenously generated H2O2 could produce cardiovascular responses similar to those produced by exogenous H2O2, the catalase inhibitor 3-amino-1,2,4-triazole (ATZ) was injected (100 nmol/40 nl) unilaterally into the NTS of another group of rats (n = 4). The dose of ATZ was selected based on published data, indicating the tissue concentration of ATZ needed to inhibit up to 95% of brain catalase activity (5). Injections of vehicle were performed prior to injections of ATZ.
Effects of H2O2 after blockade of ionotropic glutamate receptors in the NTS.
To determine whether blood pressure and HR effects of H2O2 in the NTS were linked to changes in glutamatergic transmission, the effect of H2O2 (500 pmol/40 nl) was tested again, after injection of the ionotropic glutamate receptor antagonist kynurenate (7 nmol/40 nl) in another group of rats (n = 7). Injections of H2O2 were performed 15 min before and again 10 and 60 min after injection of kynurenate into the same site.
Effects of H2O2 after local injection of l-ascorbate.
To confirm that effects of H2O2 in the NTS were linked to its oxidant action, H2O2 (500 pmol/40 nl) was injected 15 min before and 20 min after injection of the endogenous antioxidant l-ascorbate (600 pmol/80 nl) in another group of rats (n = 6).
Experimental Protocols in Conscious Rats
Experiments were performed in unanesthetized freely moving rats, ∼48 h after cannulation surgery. To minimize any impact of stress on experimental outcomes, rats were allowed to stabilize for a period of at least 1 h before experiments started.
Unilateral injections into the NTS were made with a 5-μl Hamilton syringe connected by PE-10 tube to an injector cannula made of 32-gauge stainless-steel tubing. The injector, when fully inserted, protruded 2 mm beyond the tip of the guide cannula. Injections of 100 nl each were performed over a period of 5 to 10 s.
Effects of H2O2 before and after local blockade of glutamate receptors in the NTS of conscious rats.
Effects of H2O2 (50 nmol/100 nl) in the NTS of conscious freely moving rats was tested after the injection site was identified as a site from which injection of l-glutamate produced a robust decrease in mean arterial pressure and HR (20). After site identification, H2O2 (50 nmol/100 nl) was injected into the NTS. Cardiovascular responses to H2O2 were then tested again 5 and 60 min after the injection of kynurenate (7 nmol/100 nl) into the same site. To confirm the efficacy of kynurenate, l-glutamate was injected into the same site 10 min later (i.e., 5 min after injection of H2O2).
Histology
At the end of each experiment, injection sites were marked with 2% Evan's Blue solution (40 to 100 nl, depending on the protocol). Immediately after the injection, animals were deeply anesthetized with sodium thiopental (70 mg/kg of body wt iv) and perfused through the heart with 0.9% NaCl (30 ml) followed by 10% buffered formalin (300 ml). Brains were removed and kept in 10% buffered formalin solution for at least 24 h. Coronal slices of 50 μm were cut on a freezing microtome, mounted on glass slides, stained with Neutral red, and viewed under light microscopy to evaluate the location and distribution of dye at the injection site. If the site encompassed the NTS, the histology was considered positive, and the results from those animals were used for data analysis.
Data Analysis
Data analysis typically consisted of measuring the maximal change in MAP and HR, taking as baseline 20 s of recording just before each NTS microinjection. Results are reported as means ± SE. ANOVA for repeated measures followed by post hoc pairwise multiple comparisons Student-Newman-Keuls test, or paired t-tests were used. Dose-response curves were determined by a nonlinear fit using a sigmoidal dose-response curve with a variable slope. All data were statistically analyzed using Prism software version 5.00 (GraphPad Software, San Diego CA). Differences were considered significant when the probability of a Type I error was less than 5% (P < 0.05).
RESULTS
Cardiovascular Responses to H2O2 Injected Into the NTS in Anesthetized Rats
Urethane combined with α-chroralose anesthesia did not affect baseline MAP (126 ± 2 mmHg) vs. preanesthesia (122 ± 2 mmHg, n = 24) but produced a small bradycardia (361 ± 7 bpm) vs. preanesthesia (387 ± 10 bpm, paired t-test, P < 0.05).
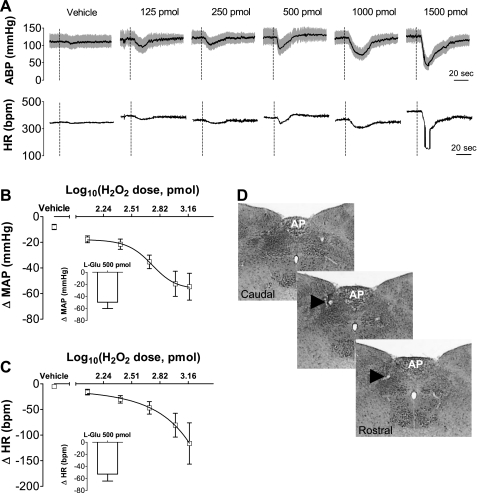
The plot of the changes in MAP × log dose H2O2 showed a sigmoid dose-dependent pattern with a maximum hypotensive response of −56 ± 13 mmHg and a slope of −3.2 mmHg/pmol. The ED50 was 525 pmol, and the Emax was −18 ± 10 mmHg pmol. The plot of the changes in HR × log dose H2O2 showed a dose-dependent transient bradycardia without a plateau (Fig. 1).
Fig. 1.
Changes in arterial blood pressure (ABP) and heart rate (HR) produced by H2O2 injected into the intermediate nucleus tractus solitarius (NTS) of anesthetized rats. A: ABP and HR traces from an animal randomly injected with vehicle or H2O2 (125, 250, 500, 1,000, and 1,500 pmol/40 nl) into the intermediate NTS. B: nonlinear regression curve showing changes in mean arterial pressure (ΔMAP). C: changes in heart rate (ΔHR) induced by H2O2 injected into the intermediate NTS. Insets show MAP and HR responses evoked by injections of l-glutamate (500 pmol) at the same site. D: photomicrographs showing the location of H2O2 injections sites into the intermediate NTS (arrows). AP, area postrema. Dashed vertical lines in A show the moment of injection into the NTS. Results in B and C are expressed as means ± SE. Vehicle, n = 6 and H2O2, n = 4–7.
Injections of H2O2 (500, 1,000, and 1,500 pmol/40 nl) into the intermediate NTS produced depressor responses (−35 ± 5, −52 ± 10, and −55 ± 11 mmHg, respectively, vs. vehicle: −8 ± 2 mmHg) and H2O2 (1,000 and 1,500 pmol/40 nl) into the NTS also produced bradycardia (−81 ± 23 and −116 ± 40 bpm, respectively, vs. vehicle: −5 ± 2 bpm) (Fig. 1). Hypotensive and bradycardic responses were transient, reaching a peak within 20 s, with full recovery of baseline MAP and HR levels within 30 to 90 s.
The changes in MAP produced by H2O2 at the doses of 125 and 250 pmol/40 nl into the intermediate NTS (−18 ± 3 and −22 ± 4 mmHg, respectively), and the changes in HR produced by H2O2 at the doses of 125, 250, and 500 pmol/40 nl (−16 ± 5, −30 ± 7, and −47 ± 12 bpm, respectively) did not reach statistical significance compared with vehicle (Fig. 1).
To confirm that injections reached sites into intermediate NTS that produce cardiovascular effects similar to those previously reported (25), before the injections of H2O2, rats received injections of l-glutamate into the intermediate NTS. Consistent with results from previous studies, injection of l-glutamate (500 pmol/40 nl) produced hypotension (−41 ± 7 mmHg) and bradycardia (−42 ± 5 bpm).
The photomicrographs in Fig. 1D show a typical injection site into the intermediate NTS in a rat representative of the groups studied.
Cardiovascular Responses Produced by ATZ Injection in the NTS of Anesthetized Rats
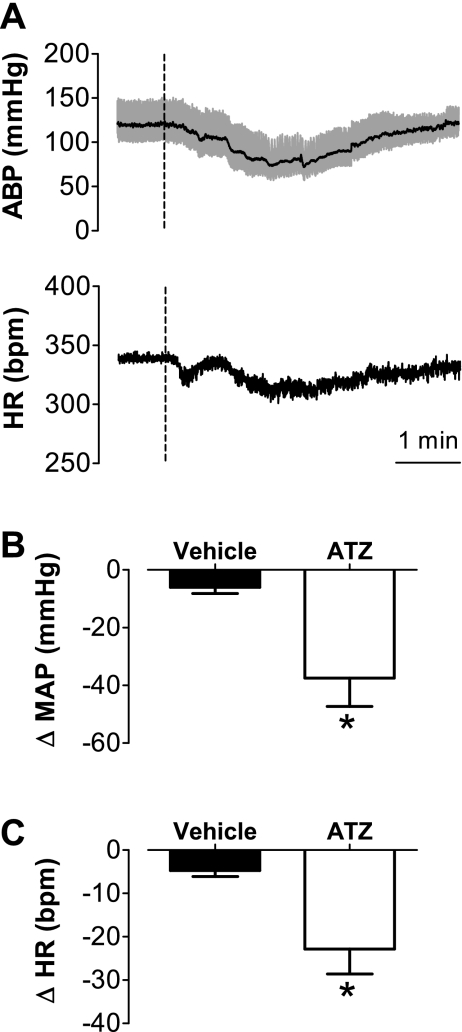
The catalase inhibitor ATZ was injected into the NTS to evaluate changes in MAP and HR evoked by accumulation of H2O2 produced endogenously.
The injection of ATZ (100 nmol/40 nl) also produced hypotensive (−37 ± 10 mmHg vs. vehicle: −6 ± 2 mmHg, t-test, P < 0.05, n = 4) and bradycardic (−23 ± 6 bpm vs. vehicle: −5 ± 1 bpm, t-test, P < 0.05, n = 4) responses (Fig. 2). These responses had a slower onset compared with those resulting from H2O2 injection, reaching their peaks within 2 to 3 min after ATZ injection and lasting for 5 to 6 min before fully returning to baseline (Fig. 2).
Fig. 2.
Changes in arterial blood pressure and heart rate produced by 3-amino-1,2,4-triazole (ATZ) injected into the intermediate NTS of anesthetized rats. A: ABP and HR traces from an animal treated with ATZ (100 nmol/40 nl) into the intermediate NTS. Changes in mean arterial pressure (ΔMAP) (B) changes in mean arterial pressure (ΔMAP) (C) induced by ATZ or vehicle injected into the intermediate NTS. Dashed vertical lines in A show the moment of injection into the NTS. Results in B and C are expressed as means ± SE. *Significantly different from vehicle (P < 0.05; unpaired t-test); n = 4.
Effects of Ionotropic Glutamate Receptors Blockade in the NTS on H2O2-Evoked Cardiovascular Responses in Anesthetized Rats
To assess the role of ionotropic glutamate receptors in mediating cardiovascular effects of H2O2 in the NTS, the effects of H2O2 into the intermediate NTS was tested 10 min before and 10 and 60 min after injection of the ionotropic glutamate receptor antagonist kynurenate into the same site.
Prior injection of kynurenate (7 nmol/40 nl) into the NTS reduced the bradycardic response to H2O2 (500 pmol) by about 48% (−31 ± 5 bpm before vs. −16 ± 2 bpm 10 min after; n = 7, paired t-test, P < 0.05) but had no effect on the hypotension (−39 ± 5 mmHg before vs. −36 ± 4 mmHg 10 min after; paired t-test, P > 0.5) (Fig. 3). A normal bradycardic response was produced by H2O2 into the NTS 60 min after kynurenate injection (−52 ± 14 bpm), an indication that the attenuation of the H2O2-evoked reduction in HR was not due to local tissue damage by the injected volume. H2O2-evoked changes in MAP remained unaltered 60 min after kynurenate (−35 ± 7 mmHg).
Fig. 3.
Changes in arterial blood pressure and heart rate produced by H2O2 alone or combined with kynurenate injected into the intermediate NTS. ABP and HR traces of an anesthetized rat (A) and a conscious rat (B) that received H2O2 into the intermediate NTS before and after injection of kynurenate (KYN) into the same area. Changes in MAP (C) and changes in heart rate (HR) (D) to H2O2 into the intermediate NTS before, 10, and 60 min after kynurenate injection into the same area in anesthetized rats (n = 7). Changes in mean arterial pressure (E) and changes in heart rate (F) to H2O2 into the intermediate NTS before, 5 to 10 and 60 min after kynurenate injection into the same area in conscious rats (n = 5). Doses of H2O2 were 50 nmol and 500 pmol, respectively, in conscious and anesthetized rats. The dose of kynurenate was 7 nmol in both groups. Dashed vertical lines in A and B show the moment of injection into the NTS. Results in C–F are expressed as means ± SE. *Significantly different from before KYN (P < 0.05, ANOVA followed by Student-Newman-Keuls test).
Cardiovascular Effects of H2O2 Alone or Combined with the Blockade of Glutamate Receptors in the NTS of Conscious Rats
Baseline MAP and HR of freely moving conscious rats were 106 ± 2 mmHg and 362 ± 13 bpm, respectively. Injection of H2O2 (50 nmol/100 nl) into the intermediate NTS of conscious rats evoked an intense bradycardia (−208 ± 19 bpm, n = 5) and an initial hypotension (−55 ± 7 mmHg, n = 5) that reached the peak within 5 s and returned to baseline within 30 s (Fig. 3).
Prior (5 min) injection of kynurenate (7 nmol/100 nl) into the NTS completely blocked HR and MAP responses evoked by H2O2 in the same site (−13 ± 4 bpm and −2 ± 2 mmHg, respectively) (Fig. 3).
The pressor and bradycardic responses to H2O2 injected into the NTS 60 min after kynurenate (−60 ± 4 mmHg and −169 ± 14 bpm, respectively) were not different from the responses before kynurenate (−55 ± 7 mmHg and −208 ± 19 bpm, respectively) (Fig. 3). Injections of l-glutamate (1 nmol/100 nl) into the intermediate NTS of conscious rats also produced hypotension (−52 ± 5 mmHg) and a bradycardic response (−206 ± 27 bpm), as did the H2O2 injection.
Effects of l-Ascorbate in the NTS on Cardiovascular Responses to H2O2 in Anesthetized Rats
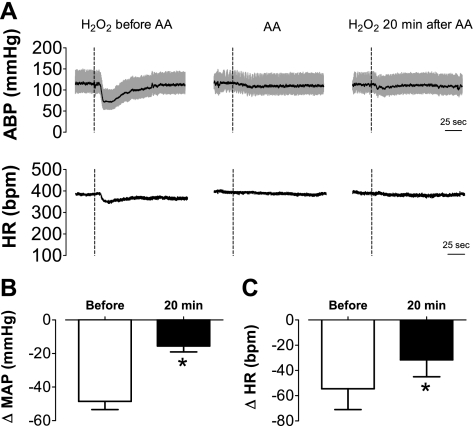
To test whether effects of H2O2 into the NTS on MAP and HR could be prevented by antioxidant treatment, responses to injection of H2O2 (500 pmol/40 nl) were recorded 15 min before and 20 min after injection of pH neutral l-ascorbate (600 pmol/80 nl) into the same site.
The treatment with l-ascorbate reduced depressor (−48 ± 5 mmHg before vs. −16 ± 3 mmHg 20 min after; n = 6, paired t-test, P < 0.05) and bradycardic (−55 ± 16 bpm before vs. −32 ± 13 bpm 20 min after, paired t-test, P < 0.05) responses to H2O2 injection into the NTS (Fig. 4). Injection of l-ascorbate produced a transient fall in MAP (−24 ± 6 mmHg vs. vehicle: −6 ± 3 mmHg; n = 6, t-test, P < 0.05) but did not significantly change HR (−15 ± 6 bpm vs. vehicle: −8 ± 3 bpm, t-test, P > 0.05).
Fig. 4.
Changes in arterial blood pressure and heart rate produced by H2O2 alone or combined with l-ascorbate injected into the intermediate NTS. A: ABP and HR traces showing the effects of H2O2 (500 pmol) into the intermediate NTS before and 20 min after the injection of l-ascorbate (600 pmol) into the same site of an anesthetized rat. Changes in MAP (B) and changes in heart rate (HR) (C) induced by H2O2 into the intermediate NTS before and 20 min after ascorbate (AA) injection into the same area (n = 6). Dashed vertical lines in A show the moment of injection into the NTS. Results in B and C are expressed as means ± SE. *Significantly different from before l-ascorbate (P < 0.05, paired t-test).
DISCUSSION
The present results show that changes in the redox state of the intermediate NTS produced by H2O2 delivery or catalase inhibition by ATZ evoked transient hypotensive and bradycardic responses in conscious or anesthetized rats, likely as a result of activation of baroreflex circuitry within the NTS, which may change efferent autonomic activity to the cardiovascular system. Except for the hypotension in anesthetized rats, these responses were attenuated by blocking local ionotropic glutamate receptors with kynurenate. The hypotension and bradycardia produced by H2O2 in the NTS were also reduced by local pretreatment with the antioxidant l-ascorbate, which suggests that cardiovascular effects depend on oxidative modifications produced by H2O2 within the NTS.
Although cellular mechanisms were not addressed in the present study, the results suggest that increased levels of H2O2 in the NTS have a net effect on the autonomic nervous system that is strong enough to change MAP and HR. The fact that injections of H2O2 evoked transient responses with blood pressure and heart rate returning to preinjection levels in a relative short time (∼1 to 2 min) suggests that whatever the underlying cellular mechanisms might be, they do not appear to cause either permanent oxidative modifications or cell damage. Moreover, responses to low doses of H2O2 were also observed, even after a first injection of high dose in the same animal, suggesting minimum detrimental effects within the range of H2O2 concentrations used in this study. One caveat that might be considered is that the concentrations of H2O2 injected in this study (3 to 500 mmol/l) are higher than the average extracellular concentration of H2O2 measured under conditions, such as reperfusion after brief ischemia (3 to 100 μmol/l) (16, 18). However, the actual concentration of H2O2 in the NTS was considerably less than the concentration of the solution injected, because of the dilution in the extracellular fluid during diffusion from the injection site, peroxidase activity, or low-molecular-weight antioxidants present in the CNS tissue (13, 28).
To test whether the balance between tonic production vs. tonic scavenging of H2O2 in the NTS determines the extent to which H2O2 is capable of interfering in the control of MAP and HR, an experiment treating NTS with catalase inhibitor ATZ was performed. Blocking catalase activity in the NTS with ATZ, we expected the concentration of H2O2 to rise and, consequently, reductions of MAP and HR similar to those produced by injections of H2O2 should be produced. Indeed, results demonstrated that the inhibition of catalase in the NTS evoked hypotension and bradycardia with a slow developing profile compared with the response produced by direct injection of H2O2. Differences in the time course of responses to H2O2 and ATZ may be explained by the time required for increasing the concentration of H2O2 following inhibition of catalase. These results suggest that the balance between production of H2O2 and its scavenging by catalase might be an important factor limiting endogenous actions of H2O2 in the NTS and, therefore, its influence on cardiovascular control.
Both bradycardic and hypotensive responses to H2O2 were attenuated by previous injection of l-ascorbate into the NTS. This indicates that effects of H2O2 are likely dependent on oxidative processes rather than nonspecific effects, such as an increase in O2 levels secondary to H2O2 degradation. l-ascorbate is normally present in the cerebrospinal fluid at concentrations ranging from 0.2 to 0.4 mM (7, 22, 26) and can scavenge stable (H2O2), as well as more labile (O2•− and HO•) ROS (26). Attenuation of H2O2-evoked responses following l-ascorbate injection may involve greater H2O2 scavenging or conversion of molecular targets oxidized by H2O2 to a reduced form, or both. However, l-ascorbate alone into the NTS also reduced MAP, suggesting that molecular mechanisms involved in the effects of l-ascorbate need further investigation.
Injection of H2O2 into the NTS produced a greater fall in HR in conscious freely moving rats than in anesthetized rats, whereas hypotensive responses were similar. Injection of l-glutamate at the same site also produced robust bradycardic and depressor responses. The pattern of blood pressure change in response to l-glutamate in conscious rats is not consistent with results from previous studies, which showed pressor responses to l-glutamate injections in the NTS of conscious rats (12, 20). Perhaps the reason for these differences is the more rostral position of injection sites in the present study that may activate mainly baroreflex-related neurons that produces hypotension and bradycardia.
Bradycardia and hypotension evoked by H2O2 into the NTS resemble cardiovascular responses to baroreflex activation or those induced by l-glutamate injected into the intermediate NTS in anesthetized rats (12, 19, 20, 33). l-glutamate is the main excitatory amino acid in the central nervous system and is the major excitatory transmitter released by visceral afferent input to NTS (12, 19, 20, 30, 32, 33). The bradycardic and hypotensive responses to H2O2 into the NTS in conscious rats and the bradycardic response to H2O2 into the NTS in anesthetized rats were abolished by pretreatment with kynurenate into the NTS, indicating that the effects of H2O2 in the NTS might depend on ionotropic glutamatergic receptor activation. The recovery of the H2O2-evoked responses after the usual time required for clearance of kynurenate from the tissue refutes the possibility that effects of kynurenate could be an indirect effect of tissue damage. However, H2O2-evoked hypotension in anesthetized rats was not affected by kynurenate injection into the NTS, which suggests that hypotension to H2O2 into the NTS in this situation is not dependent on the activation of ionotropic l-glutamate receptors. Clearly, this is a controversial result but is similar to reports in the literature showing that kynurenate was not effective at inhibiting the bradycardic or hypotensive response to l-glutamate in the NTS of anesthetized rats (19, 31). This suggests that kynurenate-sensitive l-glutamate receptors might not be the only l-glutamate receptors that participate in control of the blood pressure in the NTS, at least during anesthesia. Activation of metabotropic l-glutamate receptors in the NTS also induce hypotension in anesthetized animals (15, 19, 24). Therefore, the hypotensive responses to H2O2 in anesthetized rats may depend on activation of this class of receptors or another mechanism not investigated in the present study. Because kynurenate abolished bradycardia in response to H2O2 in the NTS, the remaining hypotension is probably the result of withdrawal of sympathetic tone to the vasculature. Further studies are necessary to determine the mechanism through which H2O2 in the NTS might evoke sympathoinhibition and hypotension in anesthetized rats.
Different mechanisms with opposite effects have been proposed for the modulation of glutamatergic neurotransmission by H2O2. Studies in vitro have demonstrated that H2O2 can block sodium-dependent glutamate uptake by glial cells, which may lead to a gradual accumulation of extracellular glutamate. The latter has the potential to result in neuronal hyperexcitability or even excitotoxicity (29, 34). If such processes take place, when H2O2 levels rise in the NTS, the concentration of glutamate also rises over time increasing neuronal activity and/or the number of active neurons to cause MAP and HR to fall. On the other hand, other studies have shown that ROS may also have an inhibitory action on glutamatergic neurotransmission, especially by direct inhibition of glutamate receptors (1–3). In addition, it has been shown that H2O2 produces a long-lasting inhibition of calcium-dependent glutamate release from cerebrocortical synaptosomes (39), which would be inconsistent with H2O2 having facilitatory action on glutamatergic transmission in the NTS. Although different mechanisms with opposite effects might be activated by H2O2 in the NTS, the inhibition of glutamate uptake is the one that can explain hypotension and bradycardia. Further investigations are necessary to clarify the cellular mechanisms modified by H2O2 in the NTS and to confirm the involvement of glutamatergic mechanisms on the cardiovascular responses to H2O2 in the NTS.
Perspectives and Significance
The present results show that H2O2 into the NTS affects cardiovascular regulation possibly through modifications in glutamatergic neurotransmission. These findings invite further investigations on the role that H2O2 could play in modulating autonomic functions under physiological and pathological conditions. Besides central mechanisms involved in cardiovascular regulation, future studies might also investigate how H2O2 level and its influence on central glutamatergic transmission may affect central mechanisms related to the control of other physiological functions like hormone secretion. Furthermore, the modulation of glutamatergic neurotransmission in the NTS by H2O2 levels must also be confirmed and clarified in future studies by combining PEG-catalase or ATZ with glutamate into the NTS.
GRANTS
This research was supported by public funding from Fundação de Amparo à Pesquisa do Estado de São Paulo, Conselho Nacional de Pesquisa/Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (L. M. Cardoso, J. V. Menani, and E. Colombari) and, in part, by the National Institutes of Health Grant HL-071645 (G. M. Toney).
Acknowledgments
The authors thank Reginaldo C. Queiroz, Silas P. Barbosa, Silvia Fóglia, and Ana L. V. de Oliveira for animal care and expert technical assistance and Silvana A. D. Malavolta for secretarial assistance.
REFERENCES
- 1.Abele R, Lampinen M, Keinanen K, Madden DR. Disulfide bonding and cysteine accessibility in the alpha-amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptor subunit GluRD. Implications for redox modulation of glutamate receptors. J Biol Chem 273: 25,132–25,138, 1998. [DOI] [PubMed] [Google Scholar]
- 2.Aizenman E, Hartnett KA, Reynolds IJ. Oxygen free radicals regulate NMDA receptor function via a redox modulatory site. Neuron 5: 841–846, 1990. [DOI] [PubMed] [Google Scholar]
- 3.Aizenman E, Lipton SA, Loring RH. Selective modulation of NMDA responses by reduction and oxidation. Neuron 2: 1257–1263, 1989. [DOI] [PubMed] [Google Scholar]
- 4.Antunes F, Cadenas E. Estimation of H2O2 gradients across biomembranes. FEBS Lett 475: 121–126, 2000. [DOI] [PubMed] [Google Scholar]
- 5.Aragon CM, Rogan F, Amit Z. Dose- and time-dependent effect of an acute 3-amino-1,2,4-triazole injection on rat brain catalase activity. Biochem Pharmacol 42: 699–702, 1991. [DOI] [PubMed] [Google Scholar]
- 6.Avshalumov MV, Chen BT, Koos T, Tepper JM, Rice ME. Endogenous hydrogen peroxide regulates the excitability of midbrain dopamine neurons via ATP-sensitive potassium channels. J Neurosci 25: 4222–4231, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Avshalumov MV, Chen BT, Rice ME. Mechanisms underlying H2O2-mediated inhibition of synaptic transmission in rat hippocampal slices. Brain Res 882: 86–94, 2000. [DOI] [PubMed] [Google Scholar]
- 8.Bienert GP, Schjoerring JK, Jahn TP. Membrane transport of hydrogen peroxide. Biochim Biophys Acta 1758: 994–1003, 2006. [DOI] [PubMed] [Google Scholar]
- 9.Bondy SC, Lee DK. Oxidative stress induced by glutamate receptor agonists. Brain Res 610: 229–233, 1993. [DOI] [PubMed] [Google Scholar]
- 10.Chen BT, Avshalumov MV, Rice ME. H2O2 is a novel, endogenous modulator of synaptic dopamine release. J Neurophysiol 85: 2468–2476, 2001. [DOI] [PubMed] [Google Scholar]
- 11.Ciriello J, Hochstencach SL, Roder S. Central projections of baroreceptor and chemoreceptor afferent fibers in the rat. In: Nucleus of the Solitaty Tract, edited by Robin A. Barraco. Boca Raton, FL: CRC Press, 1994.
- 12.Colombari E, Bonagamba LG, Machado BH. Mechanisms of pressor and bradycardic responses to l-glutamate microinjected into the NTS of conscious rats. Am J Physiol Regul Integr Comp Physiol 266: R730–R738, 1994. [DOI] [PubMed] [Google Scholar]
- 13.Desagher S, Glowinski J, Premont J. Astrocytes protect neurons from hydrogen peroxide toxicity. J Neurosci 16: 2553–2562, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Doba N, Reis DJ. Acute fulminating neurogenic hypertension produced by brainstem lesions in the rat. Circ Res 32: 584–593, 1973. [DOI] [PubMed] [Google Scholar]
- 15.Foley CM, Vogl HW, Mueller PJ, Hay M, Hasser EM. Cardiovascular response to group I metabotropic glutamate receptor activation in NTS. Am J Physiol Regul Integr Comp Physiol 276: R1469–R1478, 1999. [DOI] [PubMed] [Google Scholar]
- 16.Hyslop PA, Zhang Z, Pearson DV, Phebus LA. Measurement of striatal H2O2 by microdialysis following global forebrain ischemia and reperfusion in the rat: correlation with the cytotoxic potential of H2O2 in vitro. Brain Res 671: 181–186, 1995. [DOI] [PubMed] [Google Scholar]
- 17.Kishi T, Hirooka Y, Kimura Y, Ito K, Shimokawa H, Takeshita A. Increased reactive oxygen species in rostral ventrolateral medulla contribute to neural mechanisms of hypertension in stroke-prone spontaneously hypertensive rats. Circulation 109: 2357–2362, 2004. [DOI] [PubMed] [Google Scholar]
- 18.Lei B, Adachi N, Arai T. The effect of hypothermia on H2O2 production during ischemia and reperfusion: a microdialysis study in the gerbil hippocampus. Neurosci Lett 222: 91–94, 1997. [DOI] [PubMed] [Google Scholar]
- 19.Leone C, Gordon FJ. Is l-glutamate a neurotransmitter of baroreceptor information in the nucleus of the tractus solitarius? J Pharmacol Exp Ther 250: 953–962, 1989. [PubMed] [Google Scholar]
- 20.Machado BH, Bonagamba LG. Microinjection of l-glutamate into the nucleus tractus solitarii increases arterial pressure in conscious rats. Brain Res 576: 131–138, 1992. [DOI] [PubMed] [Google Scholar]
- 21.Maximo CL, de Almeida Colombari DS, Vanderlei MJ, Alves CD Jr, Colombari E. Cardiovascular responses produced by central injection of hydrogen peroxide in conscious rats. Brain Res Bull 71: 37–44, 2006. [DOI] [PubMed] [Google Scholar]
- 22.Miele M, Fillenz M. In vivo determination of extracellular brain ascorbate. J Neurosci Methods 70: 15–19, 1996. [DOI] [PubMed] [Google Scholar]
- 23.Nozoe M, Hirooka Y, Koga Y, Sagara Y, Kishi T, Engelhardt JF, Sunagawa K. Inhibition of Rac1-derived reactive oxygen species in nucleus tractus solitarius decreases blood pressure and heart rate in stroke-prone spontaneously hypertensive rats. Hypertension 50: 62–68, 2007. [DOI] [PubMed] [Google Scholar]
- 24.Pawloski-Dahm C, Gordon FJ. Evidence for a kynurenate-insensitive glutamate receptor in nucleus tractus solitarii. Am J Physiol Heart Circ Physiol 262: H1611–H1615, 1992. [DOI] [PubMed] [Google Scholar]
- 25.Reis DJ, Granata AR, Perrone MH, Talman WT. Evidence that glutamic acid is the neurotransmitter of baroreceptor afferent terminating in the nucleus tractus solitarius (NTS). J Auton Nerv Syst 3: 321–334, 1981. [DOI] [PubMed] [Google Scholar]
- 26.Rice ME Ascorbate regulation and its neuroprotective role in the brain. Trends Neurosci 23: 209–216, 2000. [DOI] [PubMed] [Google Scholar]
- 27.Sapru Carotid chemoreflex HN. Neural pathways and transmitters. Adv Exp Med Biol 410: 357–364, 1996. [PubMed] [Google Scholar]
- 28.Sokolova T, Gutterer JM, Hirrlinger J, Hamprecht B, Dringen R. Catalase in astroglia-rich primary cultures from rat brain: immunocytochemical localization and inactivation during the disposal of hydrogen peroxide. Neurosci Lett 297: 129–132, 2001. [DOI] [PubMed] [Google Scholar]
- 29.Sorg O, Horn TF, Yu N, Gruol DL, Bloom FE. Inhibition of astrocyte glutamate uptake by reactive oxygen species: role of antioxidant enzymes. Mol Med 3: 431–440, 1997. [PMC free article] [PubMed] [Google Scholar]
- 30.Sved AF, Gordon FJ. Amino acids as central neurotransmitters in the barorecepor reflex pathway. News Physiol Sci 9: 243–246, 1994. [Google Scholar]
- 31.Talman WT Kynurenic acid microinjected into the nucleus tractus solitarius of rat blocks the arterial baroreflex but not responses to glutamate. Neurosci Lett 102: 247–252, 1989. [DOI] [PubMed] [Google Scholar]
- 32.Talman WT Glutamatergic transmission in the nucleus tractus solitarii: from server to peripherals in the cardiovascular information superhighway. Braz J Med Biol Res 30: 1–7, 1997. [DOI] [PubMed] [Google Scholar]
- 33.Talman WT, Granata AR, Reis DJ. Glutamatergic mechanisms in the nucleus tractus solitarius in blood pressure control. Fed Proc 43: 39–44, 1984. [PubMed] [Google Scholar]
- 34.Volterra A, Trotti D, Tromba C, Floridi S, Racagni G. Glutamate uptake inhibition by oxygen free radicals in rat cortical astrocytes. J Neurosci 14: 2924–2932, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang G, Anrather J, Glass MJ, Tarsitano MJ, Zhou P, Frys KA, Pickel VM, Iadecola C. Nox2, Ca2+, and protein kinase C play a role in angiotensin II-induced free radical production in nucleus tractus solitarius. Hypertension 48: 482–489, 2006. [DOI] [PubMed] [Google Scholar]
- 36.Wang G, Anrather J, Huang J, Speth RC, Pickel VM, Iadecola C. NADPH oxidase contributes to angiotensin II signaling in the nucleus tractus solitarius. J Neurosci 24: 5516–5524, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zimmerman MC, Davisson RL. Redox signaling in central neural regulation of cardiovascular function. Prog Biophys Mol Biol 84: 125–149, 2004. [DOI] [PubMed] [Google Scholar]
- 38.Zimmerman MC, Lazartigues E, Lang JA, Sinnayah P, Ahmad IM, Spitz DR, Davisson RL. Superoxide mediates the actions of angiotensin II in the central nervous system. Circ Res 91: 1038–1045, 2002. [DOI] [PubMed] [Google Scholar]
- 39.Zoccarato F, Valente M, Alexandre A. Hydrogen peroxide induces a long-lasting inhibition of the Ca(2+)-dependent glutamate release in cerebrocortical synaptosomes without interfering with cytosolic Ca2+. J Neurochem 64: 2552–2558, 1995. [DOI] [PubMed] [Google Scholar]