Abstract
Populations of intrathoracic extracardiac neurons transduce myocardial ischemia, thereby contributing to sympathetic control of regional cardiac indices during such pathology. Our objective was to determine whether electrical neuromodulation using spinal cord stimulation (SCS) modulates such local reflex control. In 10 anesthetized canines, middle cervical ganglion neurons were identified that transduce the ventricular milieu. Their capacity to transduce a global (rapid ventricular pacing) vs. regional (transient regional ischemia) ventricular stress was tested before and during SCS (50 Hz, 0.2 ms duration at 90% MT) applied to the dorsal aspect of the T1 to T4 spinal cord. Rapid ventricular pacing and transient myocardial ischemia both activated cardiac-related middle cervical ganglion neurons. SCS obtunded their capacity to reflexly respond to the regional ventricular ischemia, but not rapid ventricular pacing. In conclusion, spinal cord inputs to the intrathoracic extracardiac nervous system obtund the latter's capacity to transduce regional ventricular ischemia, but not global cardiac stress. Given the substantial body of literature indicating the adverse consequences of excessive adrenergic neuronal excitation on cardiac function, these data delineate the intrathoracic extracardiac nervous system as a potential target for neuromodulation therapy in minimizing such effects.
Keywords: cardiac pacing, ventricular ischemia, middle cervical ganglion neuron, cardiac nervous system, neurocardiology
delivering high-frequency electrical stimuli to the dorsal aspect of the T1–T4 thoracic spinal cord [spinal cord stimulation (SCS)] can alleviate the symptoms associated with chronic refractory angina pectoris (19, 23, 29, 32). SCS also stabilizes ventricular electrical alterations that attend regional ventricular ischemia (14), reduces the size of infarcts induced by transient myocardial ischemia (37), and protects against ischemia-induced ventricular tachycardia/ventricular fibrillation in animal models with chronic myocardial infarction and heart failure (27).
Electrical neuromodulation impacts on the dynamic interactions among autonomic afferent and efferent neurons mediated via, as yet poorly defined, spinal cord and peripheral neural circuits (18, 20, 35, 43). Centrally, SCS reduces the release of neuropeptides from cardiac nociceptive afferent neurons (17), reduces intrasegmental and supraspinal transmission of cardiac nociceptive information (20, 35), and increases the activity of a subpopulation of thoracic sympathetic preganglionic efferent neurons (17, 18). Peripherally, SCS reduces the activity generated by intrinsic cardiac neurons by ∼70% at baseline as well as when they are activated by transient ventricular ischemia (13, 21). Transection of the ansae subclavia eliminates the suppressor effects of SCS on intrinsic cardiac neural activity, indicating that responses are due primarily to the influence of spinal cord neurons acting via the sympathetic nervous system (21). The ability SCS to reduce infarct size secondary to transient myocardial ischemia is blocked by prior adrenergic blockade (37), likewise indicating a primary role for SCS-mediated control of sympathetic efferent neurons regulating the stressed heart.
Intrathoracic extracardiac neurons are in constant communication with intrinsic cardiac ones in the overall coordination of regional cardiac indices (3, 11). Neurons in intrathoracic extracardiac ganglia receive major inputs from the spinal cord preganglionic neurons that are under the control of the intermediolateral cell column that, in turn, receives inputs from spinal and supraspinal components of the cardiac neuronal hierarchy (2, 20). Furthermore, inputs arising from spinal cord neurons are known to exert influences on middle cervical ganglion neurons involved in short-loop intrathoracic cardio-cardiac reflexes (5, 6). It remains to be established whether central neuronal inputs to the intrathoracic extracardiac nervous system influence the latter's capacity to transduce the cardiac milieu. Thus, the present experiments were designed 1) to determine whether spinal cord inputs influence the capacity of middle cervical ganglion neurons to transduce the ventricular milieu and 2) to determine whether SCS influences how these neurons respond to regional vs. global ventricular stress, thereby impacting upon intrathoracic extracardiac sympathetic reflex function.
MATERIALS AND METHODS
Animal preparation.
These experiments were performed in accordance with the guidelines for animal experimentation described in the “Guiding Principles for Research Involving Animals and Human Beings” (1), in accordance with the Guide to the Care and Use of Experimental Animals set up by the Canadian Council on Animal Care, and with the approval of the IACUC of the University of Montreal.
Adult mongrel dogs (n = 10; 20–25 kg) of either sex were anesthetized with sodium thiopental (25 mg/kg iv, supplemented as required), intubated, and maintained under positive-pressure ventilation. After surgery, anesthesia was maintained with α-chloralose (75 mg/kg iv bolus, with repeat doses of 12.5 mg/kg iv, as required). Noxious stimuli were applied to a paw periodically to ascertain the adequacy of anesthesia. Body temperature was maintained between 37.5 and 38.5°C by water-jacketed Micro-Temp heating units (Zimmer, Dover, OH). Fluid replacement (physiological saline) and α-chloralose were administered via a femoral vein catheter.
SCS.
After placing the animal in the prone position, the epidural space was entered with a Touhy needle via a small skin incision in the caudal dorsal thorax. A four-pole lead (Octrode; Advanced Neuromodulation Systems, Plano, TX) was advanced rostrally in the epidural space to the T1–T4 spinal cord level, utilizing anterior-posterior fluoroscopy. The tip of the lead was positioned slightly to the left of midline, according to current clinical practice (33). The most cranial pole of the lead was positioned at the T1 level with the caudal pole at the T3-T4 level. Electrical current was delivered via its rostral and caudal poles to verify their functional position. Stimuli were delivered via a stimulus isolation unit and constant current generator (model SIU 5B; Grass Instruments, Quincy, MA) connected to a stimulator (model S88 stimulator; Grass Instruments). Increasing stimulus intensity (with the rostral pole as cathode) to motor threshold (MT) intensity induced muscle contractions in the proximal forepaw and shoulder. Stimulation with the caudal pole as cathode at MT activated thoracic paravertebral muscles, resulting in a twisting movement of the trunk. Animals were rotated to the decubitus position and MT was reestablished. In each experimental protocol, SCS was delivered for 9 min at 50 Hz, 200-μs duration and at a current intensity of 90% MT (range 0.19 and 0.78 mA; mean 0.43 mA). The rostral and caudal poles were chosen as cathode and anode, respectively, according to current clinical practice (32, 33). MT was checked periodically during the experiment and did not vary significantly from initial levels.
Cardiovascular instrumentation.
Heart rate was monitored via a lead II ECG. Left ventricular chamber pressure was recorded via a Millar catheter introduced into the left ventricular cavity via the right femoral artery. A transthoracic incision exposed the heart and the left middle cervical ganglia associated with the left thoracic vagosympathetic trunk. After opening the pericardial sac, two miniature solid-state pressure transducers (model P19; Konigsberg Instruments, Pasadena CA) were inserted into the left ventricle midwall in the perfusion beds of the ventral descending and circumflex coronary arteries. Aortic pressure was monitored via a Bentley Trantec model 800 transducer connected to a Cordis no. 6 catheter that was inserted into the ascending aorta via a femoral artery. These cardiac indices were monitored throughout the experiments via an eight-channel rectilinear recorder (Nihon Kohden, Tokyo, Japan), digitized (power 1401; Cambridge Electronics Design), and analyzed (Spike 2, Cambridge Electronics Design) to determine mean hemodynamic values at baseline and in response to each stressor.
Coronary artery occlusion.
A 3-0 silk thread was placed around the ventral descending coronary artery about 2 cm from its origin, distal to the site of origin of the first major branch of that artery. Another silk thread was placed around the circumflex coronary artery about 3 cm from its origin, distal to the origin of its first major branch. These threads were led through short polyethylene tubing segments for subsequent snaring of each artery for 30 s. These occlusion sites were chosen to obviate inducing too large a ventricular ischemic zone to minimize subsequent ventricular arrhythmia induction.
Cardiac pacing protocol.
Rapid ventricular pacing was performed by delivering trains of super-threshold electrical stimuli at a rate of 180 pulses/min to the right ventricle for 5-min periods of time. This was accomplished via bipolar electrodes that had been sutured to the epicardium of the right ventricular outflow tract.
Middle cervical ganglion neuronal activity.
The left middle cervical ganglion was identified and left in place, attached to surrounding tissues to maintain its stability. The activity generated by neurons in that ganglion was identified by using methods that have been described previously (6, 11). The recording microelectrode utilized had a 250-μm shank diameter, an exposed tip of 10 μm, and an impedance of 9–11 MΩ at 1,000 Hz (model ME 25-10-2; Frederick Haer). The indifferent electrode was attached to the mediastinum adjacent to that middle cervical ganglion.
This ganglion was explored with the electrode tip placed from its ventral surface to its underlying regions, such that the activity generated by neurons in various loci within that ganglion could be identified. Action potentials generated by the somata and/or dendrites of individual neurons can be recorded from a locus within a middle cervical ganglion for hours using this methodology (6). Action potentials so identified were differentially amplified by means of a Princeton Applied Research model 113 amplifier that had band pass filters set at 300 Hz to 10 kHz and an amplification range of ×100–500. The output of this device was further amplified (×50–200) and filtered (band width 100 Hz to 2 kHz) by means of an optically isolated amplifier (Applied Microelectronics Institute, Halifax, NS, Canada). The amplified neuronal signals, together with the cardiovascular signals, were digitized (Cambridge Electronics Design, power 1401 data acquisition system) and analyzed using the Spike 2 software package (Cambridge Electronics Design). Ganglionic loci were identified from which action potentials with signal-to-noise ratios > 3:1 could be recorded. The activity generated by individual neuronal somata was identified by the amplitude and configuration (waveform recognition) of the recorded action potentials via the Spike 2 program. Using these techniques and criteria, action potentials generated by individual cell bodies and/or dendrites rather than axons of passage can be recorded for extended period of time (6, 11). As such, once an active locus was identified the activity generated by its spontaneously active neurons was studied throughout the duration of the various protocols outlined below (cardiac stressors).
To identify middle cervical ganglion neurons that transduced the left ventricular milieu, the activity generated by neurons within various loci of the left middle cervical ganglion was recorded as gentle mechanical stimuli (delivered by a saline-soaked cotton applicator) were applied for 2–3 s to various loci on the ventral and ventrolateral surface of the left ventricle. It is known that most middle cervical ganglion neurons that transduce the ventricular mechanical milieu also transduce its local chemical milieu (12). Once a neuron was identified that responded to repeat (×3) local mechanical deformation of epicardial regions adjacent to either the left anterior descending or circumflex coronary artery, its spontaneous activity was recorded during 5–10 min control states to establish baseline values for that index.
Cardiac stressors.
The ventricles were then paced at 180 beats/min for 5 min. After at least 5 min, the artery that perfused the identified sensory field was occluded 3–4 times. That is, the ventral descending or circumflex coronary artery was occluded individually for 30 s, depending on which vessels perfused its identified sensory field. At least 5 min occurred between these interventions for preparation stabilization. The order that these vessels were occluded varied among animals. The order of applying these two protocols (ventricular pacing vs. coronary artery occlusion) also varied among animals.
SCS protocol.
To test the effects of activating the dorsal aspect of the thoracic spinal cord (SCS) on the activity generated by cardiac-related middle cervical ganglion neurons, in four preliminary animals 2-, 5-, 10-, and 15-min periods of SCS were studied in the absence of stressors. SCS yielded similar neuronal activity changes at up to 15 min of SCS (27 ± 23 impulses/min baseline to 38 ± 27 impulses/min at SCS termination, P = 0.36). To minimize the potential for long-term effects on neuronal activity post-SCS (13, 21), SCS was instituted in each experimental protocol for only 9 min thereafter. For the global stress of rapid cardiac pacing, SCS was started 2 min before and maintained for 2 min after the allocated 5-min periods of cardiac pacing (total 9 min). These data were compared with those obtained during 5-min of pacing alone. With respect to coronary artery occlusion (duration 30 s), occlusions were performed throughout the final 30 s of SCS (i.e., the occlusion and SCS terminated simultaneously) and compared with the response to 30 s coronary artery occlusion by itself. One hour elapsed between each protocol in which no interventions were instituted.
Data analysis.
Spontaneous cardiodynamic fluctuations were minimal during control periods, heart rate varying < 5 beats/min, and systolic pressure fluctuating < 5 mmHg. Thus, thresholds for classifying induced cardiovascular changes were chosen to be greater than these ranges. Action potentials arising from a locus within each middle cervical ganglion studied were characterized by means of their differing configurations (waveform recognition) by employing the Spike 2 program. Neuronal activity and cardiovascular indices recorded simultaneously before each intervention were compared with those indices derived during each intervention, average activity representing the same population recorded throughout all interventions. The grouped data so derived are expressed as means ± SD. SigmaStat 3.1 (Systat Software) with one-way ANOVA with post hoc comparisons (Holm-Sidak test) was used to test for differences within and between groups. A significance of P < 0.05 was used.
RESULTS
Neuronal activity.
Spontaneously active neurons identified in one locus of each middle cervical ganglion were analyzed by means of their unique action potential waveform configurations (employing Spike-2 program). Only those that were activated by gently mechanical stimuli applied to circumscribed epicardial loci on either the left ventricular ventral or lateral epicardial wall entered this study. As a consequence we were able to identify in control states 3–5 neurons in an active ganglionic locus. Collectively, neurons so identified generated about 40 impulses/min (Table 1). Although most neurons generated activity throughout the cardiac cycle (Fig. 1), limited numbers generated cardiac-related activity usually during ventricular isovolumetric contraction (Fig. 2). Application of focal mechanical stimuli to these select ventricular epicardial loci activated these neurons two- to threefold, doing so similarly upon repeat local mechanical stimulus application. Neurons so identified became the subject of subsequent investigation.
Table 1.
Middle cervical ganglion neuronal activity
| Ventricular Pacing | Coronary Artery Occlusion | |
|---|---|---|
| Control Stressors | ||
| Control | 36.7±28.1 | 41.5±35.6 |
| Intervention | 79.8±47.5* | 111.0±61.4* |
| Stressor with SCS | ||
| Control | 30.8±24.4 | 19.4±16.2 |
| SCS | 26.8±19.7 | 19.0±14.0 |
| SCS and intervention | 72.5±36.9*+ | 29.5±20.2# |
Data are impulses/min; mean ± SD; n = 10 dogs. Activity is the average of 3–5 identified ventricular middle cervical ganglion neurons generated per animal. Neuronal activity increased during the rapid ventricular pacing, as well as brief periods of regional ventricular ischemia. Spinal cord stimulation (SCS) did not change neuronal activity overall; it suppressed neuronal responses to regional ventricular ischemia but not those initiated by ventricular pacing.
P ≤ 0.02 control vs. intervention;
P ≤ 0.02 SCS vs. SCS and intervention;
P ≤ 0.01 intervention vs. SCS and intervention.
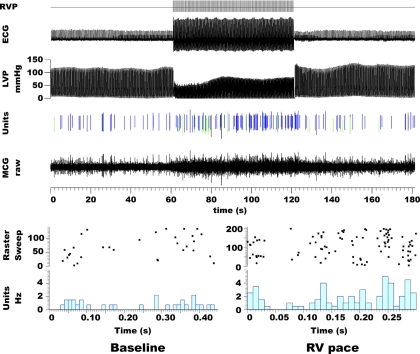
Fig. 1.
Ventricular pacing modified cardiac-related middle cervical ganglion (MCG) neuronal activity. Top: rapid right ventricular pacing (RVP; initiated throughout the RVP signal on upper trace) induced a fall in left ventricular pressure (LVP) while activating 3 neurons identified from raw MCG neuronal activity (line below) by their unique action potential configurations (green, blue, and black vertical lines). Bottom: raster sweep plots of the action potentials generated by these 3 neurons during consecutive cardiac cycles for 60 s during control states (baseline) and right ventricular pacing (RV pace). Time 0 is defined by QRS onset. Activity increased during pacing, rapidly returning to control values once pacing ceased (cf, unit activity). Timing bars are located below each panel.
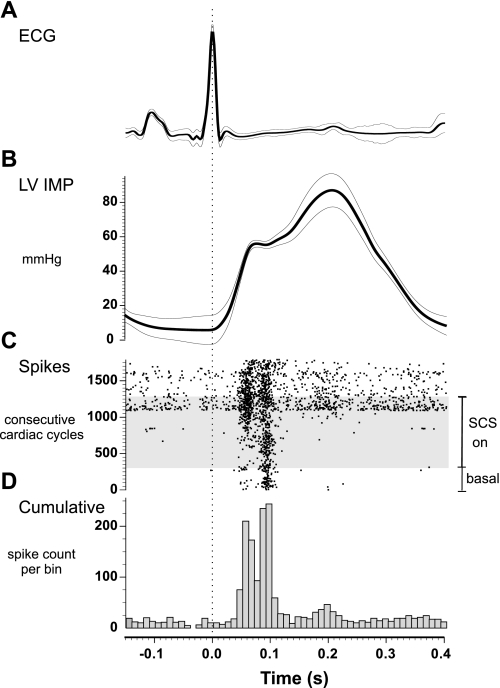
Fig. 2.
Spinal cord stimulation (SCS) modified the activity generated by MCG neurons associated with left ventricular mechanosensory neurites. Lead II electrocardiogram (ECG) (A) and left ventricular intramyocardial pressure (LV IMP) (B) show QRS averaged values (dark line) recorded during each of 1,792 consecutive cardiac cycles (± 1 SD thin lines). C: dots in the raster sweep plot represent action potentials generated by 4 neurons recorded during the 1,792 cardiac cycles. This included activity recorded 2 min before (basal) and during 9 min of continuous SCS (SCS on = gray shaded area), as well as 4 min following SCS (top of raster plot). The horizontal time 0 was set to the R wave of the ECG. Activity increased as SCS persisted (gray zone in raster plot), including after SCS termination (top of this plot C); increased activity demonstrated less phase locking to the cardiac cycle. D: cumulative index of all action potentials recorded during these 1,792 consecutive cardiac cycles demonstrating that activity was maximal during maximum local isovolumetric dynamics of their receptor fields.
Rapid ventricular pacing.
Cardiac pacing increased the activity generated by identified neurons, whether pacing was tested for 1-min periods (Fig. 1) or for the 5-min periods used to subsequently test the effects of SCS (Fig. 3). Pacing the ventricles at 180 beats/min for 5 min enhanced neuronal activity by ∼117% (range of neuronal activity from 1.4 to 68.4 impulses/min at baseline to 4.8 to 128.1 impulses/min during pace) (Table 1). All identified neurons responded with increased activity that occurred in excess of that related to increasing the heart rate (50% increase rate; 120 beats/min→180 beats/min). Furthermore, in some instances pacing also initiated cyclic bursts of activity that persisted after terminating pacing (Fig. 3A). Left ventricular chamber and aortic systolic pressures fell with pace onset. These indices gradually returned to baseline values as pacing continued such that at the end of pacing (when neuronal activity was quantified) aortic pressure (128 ± 7/100 ± 6 mmHg) was comparable to that of control states (130 ± 7/100 ± 6 mmHg).
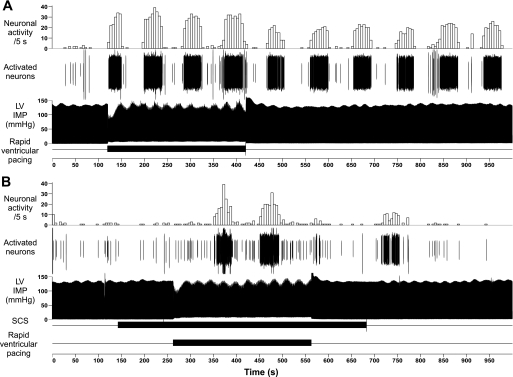
Fig. 3.
A: rapid ventricular pacing (horizontal bar below) increased neuronal activity. A maximum of 5 identified middle cervical ganglion neurons were recorded that generated increased (cyclic bursts) activity during pacing, something that persisted for some time after discontinuing pacing. B: 9 min of SCS (started 2 min prior to pacing; horizontal bar below) induced a lesser response to pacing (lowest horizontal bar) and one that extinguished soon after discontinuing SCS. In each panel, from above downward, are: neuronal activity averaged over 5-s sequential intervals, discriminated neuronal activity (activated neurons), and intramyocardial pressure (LV IMP) recorded adjacent to the identified sensory neurite field. The horizontal bar below represents SCS application time (applied 2 min before and continuing for 2 min after pacing).
Coronary artery occlusion.
The activity generated by identified neurons increased during transient occlusion of either the left anterior descending or circumflex coronary artery, depending on whether the location of the mechanosensory field associated with identified neurons in individual animals was perfused by one or the other artery (Fig. 4A; Table 1). Repeated artery occlusion induced similar neuronal responses. Intramyocardial systolic pressure in the nonoccluded ventricular zone remained unchanged overall (106 ± 11 to 98 ± 8 mmHg; not significant); corresponding intramyocardial pressures in the risk zone fell ∼18% during the transient coronary occlusion. Transient coronary artery occlusion did not alter heart rate (121 ± 8 to 120 ± 10 beats/min) or aortic pressure (130 ± 10/98 ± 7 to 130 ± 11/95 ± 8 mmHg) overall.
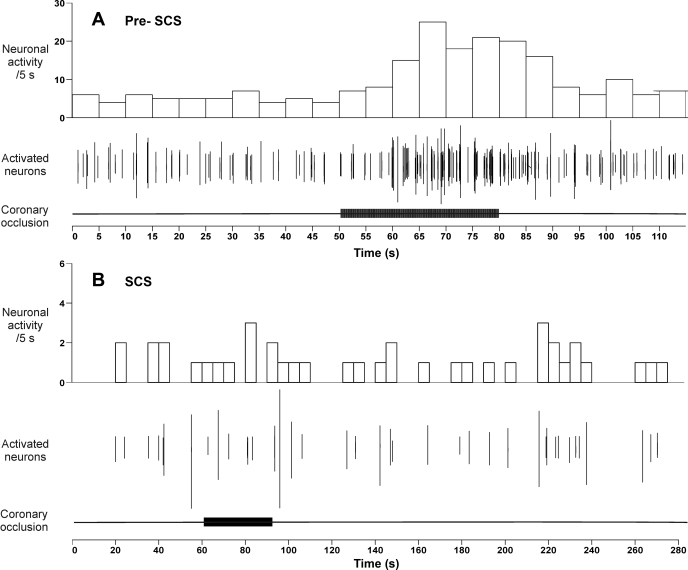
Fig. 4.
Effects of left anterior descending coronary artery occlusion (30-s duration; horizontal bars below each panel) on middle cervical ganglion neuronal activity before (Pre-SCS) (A) and during (B) SCS. The time scales of these 2 records differ in order to emphasize the lack of long-term effects of ischemia after SCS. Neuronal activity and activated neuron traces are as defined in Fig 1.
SCS.
Nine minutes of SCS did not alter heart rate, left ventricular chamber pressure, or aortic pressure overall (data not shown). SCS did not change neuronal activity overall (Table 1), even though in some instances activity of a few neurons was changed (Fig. 2).
Stressors in the presence of SCS.
When ventricular pacing was instituted in the presence of SCS, the enhancement of neuronal activity was similar to that which had occurred during that intervention in the absence of SCS (Table 1). However, in two of the animals, SCS reduced the period of time that pacing modified neuronal activity (Fig. 3). In contrast, regional ventricular ischemia no longer enhanced the activity generated by identified middle cervical ganglion neurons when instituted in the presence of SCS (Fig. 4; Table 1).
DISCUSSION
In basal states, SCS is known to suppress the activity generated by populations of intrinsic cardiac neurons (13, 21). It also reduces the responsiveness of such neurons to regional ventricular ischemia (13, 21). Data obtained from the current study indicate that SCS modified populations of neuronal somata located in intrathoracic extracardiac ganglia. Furthermore, it targets neurons in classical peripheral sympathetic ganglia that in turn target cardiac indices. Although SCS did not suppress overall basal activity generated by the subpopulations of intrathoracic sympathetic extracardiac neurons involved in transducing the ventricular milieu identified in this study, it did obtund their capacity to transduce regional ventricular ischemia.
Reflex control of heart function is dependent upon complex interactions within both peripheral and central neuronal elements of the cardiac neuronal hierarchy (2, 3, 20). Neuromodulation, targeted at various levels of the cardiac neuronal hierarchy, has the capacity to modify reflex function within the hierarchy (17, 22) and the cardiomyocytes themselves (37). Against the stress of acute myocardial ischemia, SCS reduces electrical instability of the ventricle (27) and infarct size (37), the later at least being dependent on modulation of adrenergic nerve function (37). In animal models of reduced coronary reserve, SCS mitigates the ST segment deviations associated with chemical activation of intrathoracic adrenergic neurons (14). SCS by itself (18), or in combination with coronary artery occlusion (17), increases cFos expression within subpopulations of high thoracic sympathetic preganglionic neurons. In the current study, while the average response of middle cervical ganglia neurons to SCS did not change over the stimulation times evaluated (up to 15 min), a subset of these neurons were augmented, an effect that outlived the stimulation (Fig. 2). Considering the heterogeneous neuroanatomical organization of the extracardiac intrathoracic autonomic ganglia (24, 25), including afferent, efferent, and local circuit neurons, it is not unexpected that the integrated reflex response to stressors is partially reflective of the characteristics of their imposed afferent and efferent neuronal inputs. As the vast majority (∼70%) of neurons in the middle cervical ganglia that respond to local epicardial mechanical deformation or chemical stimuli are local circuit in nature (5, 6), presumably this population of polymodal neurons represented the principal neuronal population evaluated in this study. Presumably that is, in part, why most identified neurons were not directly activated by SCS. Moreover, SCS exerts its cardioprotective effects (14, 27, 37) without interfering with the direct flow-through pre- to postganglionic efferent neuronal projections (34). We have proposed instead that SCS exerts it cardioprotective effects, in part, by modulating reflex processing within intrathoracic ganglia (13, 14).
Significant subpopulations of neurons within intrathoracic extracardiac and intrinsic cardiac ganglia perform a major processing function: interconnecting neurons located within individual or separate intrathoracic ganglia. Local circuit neurons perform this function (3, 8, 10, 41). This neuron population forms the substrate for the processing of cardiovascular afferent information within peripheral autonomic ganglia, even when such ganglia are disconnected from the influence of central neurons (4, 7). Local circuit neurons transduce cardiovascular afferent information reflexly to modulate the sympathetic and parasympathetic efferent outflows to cardiac effector sites (11). This population also receives significant descending inputs from central neurons (9, 28, 41). These neurons are crucial for overall information processing within the intrathoracic nervous system and, as such, represent a principal modulator of the final efferent neuronal outflows to different cardiac regions (3, 10). We hypothesize that the population of local circuit neurons represents the preferential target for SCS-derived neuromodulation and that such inhibition limits excessive reflex responses of the cardiac nervous system responding to the stress of acute myocardial ischemia. It is known that the inputs so activated in the spinal cord project to intrathoracic extracardiac neurons via the ansae (8, 9). Whether this occurs via the efferent or afferent axonal projections from the spinal cord remains to be explored, although the former is the most likely occurrence. In conjunction with SCS-induced effects to increase myocyte resistance to ischemic stress (14, 37), we propose that within the risk zone the supply/demand imbalance is thereby reduced, as is sensory activation within that zone and this is fundamental to the clinical experience of angina relief associated with SCS neuromodulation therapy (32).
Middle cervical ganglion neurons involved in transducing the cardiac mechanical and/or chemical milieu (11) become excited by global cardiac stresses such as that attending rapid ventricular pacing. Such an intervention alters the mechanical and chemical milieu of both ventricles, thereby enhancing sensory inputs derived from multiple cardiac afferent neurons that influence populations of middle cervical cardiac neurons (6) such that they become activated (Table 1). In preliminary experiments in which relatively short-term ventricular pacing was tested, activity increased soon after initiating pacing (Fig. 1). These data indicate that cardiac pacing influences the intrathoracic cardiac nervous system, an influence that extinguishes with time once pacing is terminated (Figs. 1 and 3).
The present study indicated that SCS is ineffective at overcoming excessive sensory inputs arising from a global cardiac stress such as that initiated by rapid ventricular pacing. In accord with that observation, such spinal cord inputs are likewise ineffectual in reducing pacing-induced ST segment deviations in animal models with reduced coronary reserve (14). This may reflect an overriding direct local metabolic effect of cardiac pacing on the interstitial milieu and resultant global increase in cardiac afferent neuronal transduction. Against such pacing-induced afferent input barrage, the capacity for higher centers to modulate intrathoracic reflex processing may be limited. However, while maximal reflex responses within the middle cervical ganglia neurons to an imposed pacing stress is maintained during SCS (Table 1), the duration of sympathoexcitation can in some instances be truncated (Fig. 3).
Regional ventricular ischemia creates boundary conditions whereby more limited ventricular sensory neurite fields are affected relative to the surrounding interstitium. Such a cardiac substrate would initiate differential sensory inputs to middle cervical ganglion cardiac neurons to enhance the activity of intrathoracic extracardiac local circuit neurons (3, 6). Differential transduction of regional boundary conditions likely represents a substantially different neuronal substrate on which descending neuronal projections might act, especially when considering the processing elements within the intrathoracic extracardiac ganglia, the local circuit neurons (12). Presumably that is why the enhancement of neuronal activity subsequent to ischemia transduction rendered this population more sensitive to SCS. Taken together, these data indicate that spinal cord inputs to the intrathoracic extracardiac nervous system are capable of altering its capacity to transduce the ischemic myocardium by primarily targeting neuronal processing elements within its ganglia, the local circuit neurons.
Perspectives and Significance
The reflex response of the cardiac neuronal hierarchy to imposed cardiac stress is a primary determinant of resultant cardiac responses. During acute myocardial ischemia, individuals that exhibit excessive and imbalanced sympathoexcitation are at increased risk for sudden cardiac death and/or progression into congestive heart failure (16, 30, 40). While β-adrenergic blockade has documented efficacy to reduce adverse consequences of such cardiopathology (16, 38), there are multiple untoward effects associated with its use (42). The cardiac neuronal hierarchy has emerged as a novel therapeutic target for managing cardiac disease via pharmacological (36, 39), physical (26, 31), and/or electrical (15, 37, 43) means to target specific nexus points within the associated neural networks. That SCS blunts/stabilizes intrathoracic extracardiac neuronal transduction of regional ventricular ischemia supports the concept that SCS imparts its cardioprotective effects on cardiac electrical stability (27) and myocyte viability (37) via multiple intrathoracic neuronal populations.
GRANTS
This study was supported by the Canada Institutes of Health Research (to R. Cardinal and J. A. Armour), the Quebec Heart and Stroke Foundation (to J. A. Armour and R. Cardinal), and the National Heart, Lung, and Blood Institute Grant HL-71830 (to J. L. Ardell).
Acknowledgments
The authors gratefully acknowledge the technical assistance of Caroline Bouchard.
REFERENCES
- 1.American Physiological Society. Guiding principles for research involving animals and human beings. Am J Physiol Regul Integr Comp Physiol 283: R281–R283, 2002. [DOI] [PubMed] [Google Scholar]
- 2.Andresen MC, Kunze DL, Mendelowitz D. Central nervous system regulation of the heart. In: Basic and Clinical Neurocardiology, edited by Armour JA and Ardell JL. New York: Oxford University Press, 2004, p. 187–219.
- 3.Ardell JL Intrathoracic neuronal regulation of cardiac function. In: Basic and Clinical Neurocardiology, edited by Armour JA and Ardell JL. New York: Oxford University Press, 2004, p. 118–152.
- 4.Ardell JL, Butler CK, Smith FM, Hopkins DA, Armour JA. Activity of in vivo atrial and ventricular neurons in chronic decentralized canine hearts. Am J Physiol Heart Circ Physiol 260: H713–H721, 1991. [DOI] [PubMed] [Google Scholar]
- 5.Armour JA Synaptic transmission in chronically decentralized middle cervical and stellate ganglia of the dog. Can J Physiol Pharmacol 61: 1149–1155, 1983. [DOI] [PubMed] [Google Scholar]
- 6.Armour JA Activity of in situ middle cervical ganglion neurons in dogs, using extracellular recording techniques. Can J Physiol Pharmacol 63: 704–716, 1985. [DOI] [PubMed] [Google Scholar]
- 7.Armour JA Neuronal activity recorded extracellularly in chronic decentralized in situ canine middle cervical ganglia. Can J Physiol Pharmacol 64: 1038–1046, 1986. [DOI] [PubMed] [Google Scholar]
- 8.Armour JA Anatomy and function of the intrathoracic neurons regulating the mammalian heart. In: Reflex Control of the Circulation, edited by Zucker IH and Gilmore JP. Boca Raton, Fl: CRC, 1991, p. 1–37.
- 9.Armour JA Cardiac neuronal hierarchy in health and disease. Am J Physiol Regul Integr Comp Physiol 287: R262–R271, 2004. [DOI] [PubMed] [Google Scholar]
- 10.Armour JA Potential clincial relevance of the ‘little brain’ on the mammalian heart. Exp Physiol 93: 165–176, 2008. [DOI] [PubMed] [Google Scholar]
- 11.Armour JA, Collier K, Kember G, Ardell JL. Differential selectivity of cardiac neurons in separate intrathoracic autonomic ganglia. Am J Physiol Regul Integr Comp Physiol 274: R939–R949, 1998. [DOI] [PubMed] [Google Scholar]
- 12.Armour JA, Kember G. Cardiac sensory neurons. In: Basic and Clinical Neurocardiology, edited by Armour JA and Ardell JL. New York: Oxford University Press, 2004, p. 79–117.
- 13.Armour JA, Linderoth B, Arora RC, DeJongste MJL, Ardell JL, Kingma JG, Hill M, Foreman RD. Long-term modulation of the intrinsic cardiac nervous system by spinal cord neurons in normal and ischemic hearts. Auton Neurosci 95: 71–79, 2002. [DOI] [PubMed] [Google Scholar]
- 14.Cardinal R, Ardell JL, Linderoth B, Vermeulen M, Foreman RD, Armour JA. Spinal cord activation differentially modulates ischemic electrical responses to different stressors in canine ventricles. Auton Neurosci 111: 37–47, 2004. [DOI] [PubMed] [Google Scholar]
- 15.Cardinal R, Pagé PL, Vermeulen M, Bouchard C, Ardell JL, Foreman RD, Armour JA. Spinal cord stimulation suppresses bradycardias and atrial tachyarrhythmias induced by mediastinal nerve stimulation in dogs. Am J Physiol Regul Integr Comp Physiol 291: R1369–R1375, 2006. [DOI] [PubMed] [Google Scholar]
- 16.Dell'Italia LJ, Ardell JL. Sympathetic nervous system in the evolution of heart failure. In: Basic and Clinical Neurocardiology, edited by Armour JA and Ardell JL. New York: Oxford University Press, 2004, p. 340–367.
- 17.Ding XH, Ardell JL, Hua F, McAuley RJ, Sutherly K, Daniel JJ, Williams CA. Modulation of cardiac ischemia-sensitive afferent neuron signaling by preemptive C2 spinal cord stimulation: effect on substance P release. Am J Physiol Regul Integr Comp Physiol 294: R93–R101, 2008. [DOI] [PubMed] [Google Scholar]
- 18.Ding XH, Hau F, Sutherly K, Ardell JL, Williams CA. C2 spinal cord stimulation induces dynorphin release from the rat T4 spinal cord: potential modulation of ischemia-sensitive neurons. Am J Physiol Regul Integr Comp Physiol 295: R1519–R1528, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eddicks S, Maier-Hauff K, Schenk M, Müller A, Baumann G, Theres H. Thoracic spinal cord stimulation improves functional status and relieves symptoms in patients with refractory angina pectoris: the first placebo-controlled randomized study. Heart 93: 585–590, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Foreman RD, DeJongste MJL, Linderoth B. Integrative control of cardiac function by cervical and thoracic spinal neurons. In: Basic and Clinical Neurocardiology, edited by Armour JA and Ardell JL. New York: Oxford University Press, 2004, p. 153–186.
- 21.Foreman RD, Linderoth B, Ardell JL, Barron KW, Chandler MJ, Hull SS, TerHorst GJ, DeJongste MJL, Armour JA. Modulation of intrinsic cardiac neurons by spinal cord stimulation: implications for therapeutic use in angina pectoris. Cardiovasc Res 47: 367–375, 2000. [DOI] [PubMed] [Google Scholar]
- 22.Foreman RD, Linderoth B, DeJongste MJL, Ardell JL, Armour JA. Central and peripheral mechanisms evoked by spinal cord stimulation (SCS) for angina pectoris. In: Management of Acute and Chronic Pain, edited by Krames E and Reig E. Bologna, Italy: Monduzzi Editore, 2000, p. 597–604.
- 23.Hautvast RW, DeJongste MJL, Staal MJ, Van Gilst VH, Lie KI. Spinal cord stimulation in chronic intractable angina pectoris: a randomized, controlled efficacy study. Am Heart J 136: 114–120, 1998. [DOI] [PubMed] [Google Scholar]
- 24.Hopkins DA, Armour JA. Localization of sympathetic postganglionic and parasympathetic preganglionic neurons which innervate different regions of the dog heart. J Comp Neurol 229: 186–198, 1984. [DOI] [PubMed] [Google Scholar]
- 25.Hopkins DA, Armour JA. Ganglionic distribution of afferent neurons innervating the canine heart and cardiopulmonary nerves. J Auton Nerv Syst 26: 213–222, 1989. [DOI] [PubMed] [Google Scholar]
- 26.Hou Y, Scherlag BJ, Lin J, Zhang Y, Lu Z, Truong K, Patterson E, Lazzara R, Jackman WM, Po SS. Ganglionated plexi modulate extrinsic cardiac neuronal input. J Am Coll Cardiol 50: 61–68, 2007. [DOI] [PubMed] [Google Scholar]
- 27.Issa ZF, Zhou X, Ujhelyi MR, Rosenberger J, Bhakta D, Groh WJ, Miller JM, Zipes DP. Thoracic spinal cord stimulation reduces the risk of ischemic ventricular arrhythmias in a post-infarction heart failure canine model. Circulation 111: 3217–3220, 2005. [DOI] [PubMed] [Google Scholar]
- 28.Kember G, Fenton GA, Armour JA, Kalyaniwalla N. Competition model for aperiodic stochastic resonance in a Fitzhugh-Nagumo model of cardiac sensory neurons. Phys Rev E Stat Nonlin Soft Matter Phys 63: 1–6, 2001. [DOI] [PubMed] [Google Scholar]
- 29.Kim MC, Kini A, Sharma SK. Refractory angina pectoris: mechanism and therapeutic options. J Am Coll Cardiol 39: 923–934, 2002. [DOI] [PubMed] [Google Scholar]
- 30.La Rovere MT, Bersano C, Gnemmi M, Specchia G, Schwartz PJ. Exercise-induced increase in baroreflex sensitivity predicts improved prognosis after myocardial infaction. Circulation 106: 945–949, 2002. [DOI] [PubMed] [Google Scholar]
- 31.Lemola K, Chartier D, Yeh YH, Dubuc M, Cartier R, Armour JA, Ting M, Sakabe M, Shiroshita-Takeshita A, Comtois P, Nattel S. Pulmonary vein region ablation in experimental vagal atrial fibrillation: role of pulmonary veins versus autonomic ganglia. Circulation 117: 470–477, 2008. [DOI] [PubMed] [Google Scholar]
- 32.Mannheimer C, Camici P, Chester MR, Collins A, DeJongste MJL, Eliasson T, Follath F, Hellemans I, Herlitz J, Luscher T, Pasic M, Thelle D. The problem of chronic refractory angina: report from the ESC Joint Study Group on Treatment of Refractory Angina. Eur Heart J 23: 355–370, 2002. [DOI] [PubMed] [Google Scholar]
- 33.Mannheimer C, Eliasson T, Andersson B, Bergh CH, Augustinsson LE, Emanuelsson H, Waagstein F. Effects of spinal cord stimulation in angina pectoris induced by pacing and possible mechanisms of action. Br Med J 307: 477–480, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Olgin JE, Takahashi T, Wilson E, Vereckei A, Steinberg H, Zipes DP. Effects of thoracic spinal cord stimulation on cardiac autonomic regulation of the sinus and atrioventricular nodes. J Cardiov Electrophysiol 13: 475–481, 2002. [DOI] [PubMed] [Google Scholar]
- 35.Qin C, Farber JP, Linderoth B, Shahid A, Foreman RD. Neuromodulation of thoracic intraspinal visceroreceptive transmission by electrical stimulation of spinal cord dorsal column and somatic afferents in rats. J Pain 9: 71–78, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Richer LP, Vinet A, Kus T, Cardinal R, Ardell JL, Armour JA. α-Adrenoceptor blockade modifies neurally induced atrial arrhythmias. Am J Physiol Regul Integr Comp Physiol 295: R1175–R1180, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Southerland EM, Milhorn D, Foreman RD, Linderoth B, DeJongste MJL, Armour JA, Subramanian V, Singh M, Singh K, Ardell JL. Preemptive, but not reactive, spinal cord stimulation mitigates transient ischemia-induced infarction via cardiac adrenergic neurons. Am J Physiol Heart Circ Physiol 292: H311–H317, 2007. [DOI] [PubMed] [Google Scholar]
- 38.Squire I Neurohumoral intervention to reduce sudden cardiac death in heart failure: what is the optimal pharmacological strategy? Heart Fail Rev 9: 337–345, 2004. [DOI] [PubMed] [Google Scholar]
- 39.Tallaj J, Wei CC, Hankes GH, Holland M, Rynders P, Dillon AR, Ardell JL, Armour JA, Lucchesi PA, Dell'Italia LJ. β1-adrenergic receptor blockade attenuates angiotensin II-mediated catecholamine release into the cardiac interstitium in mitral regurgitation. Circulation 108: 225–230, 2003. [DOI] [PubMed] [Google Scholar]
- 40.Vanoli E, Adamson PB, Foreman RD, Schwartz PJ. Prediction of unexpected sudden death among healthy dogs by a novel marker of autonomic neural activity. Heart Rhythm 5: 300–305, 2008. [DOI] [PubMed] [Google Scholar]
- 41.Waldmann M, Thompson GW, Kember G, Ardell JL, Armour JA. Stochastic behavior of atrial and ventricular intrinsic cardiac neurons. J Appl Physiol 101: 1–7, 2006. [DOI] [PubMed] [Google Scholar]
- 42.Westfall TC, Westfall DP. Adrenergic agonists and antagonists. In: Goodman & Gilman's: The Pharmacolgoical Basis of Therapeutics, edited by Brunton LL, Lazo JS, and Parker KL. New York: McGraw-Hill, 2006, p. 237–295.
- 43.Wu M, Linderoth B, Foreman RD. Putative mechanisms behind effects of spinal cord stimulation on vascular diseases: a review of experimental studies. Auton Neurosci 138: 9–23, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]