Abstract
We examined food intake in chronically maintained decerebrate rats in response to two antimetabolic drugs known to stimulate food intake, 2-mercaptoacetate (MA) and 2-deoxy-d-glucose (2DG). MA reduces fatty acid oxidation, and 2DG reduces glucose utilization. Because previous work has shown that insulin-induced hypoglycemia increases food intake in decerebrate rats, we predicted that 2DG would have this same effect. MA-induced feeding requires vagal sensory neurons that terminate in the hindbrain. Cholecystokinin-induced suppression of feeding, which likewise requires vagal sensory neurons, has been shown to suppress food intake in decerebrate rats. Therefore, we predicted that MA's effects on feeding would also persist in decerebrate rats. In our experiments, the test diet (40% milk, diluted with water) was infused intraorally through a chronic cheek fistula. We found that sham controls consumed 258% and 230% of their baseline milk intake in response to 2DG and MA, respectively. Decerebrates consumed 239% of their baseline milk intake in response to 2DG, but did not increase their intake in response to MA. Because decerebration separates the hindbrain from the forebrain, these results indicate that 2DG-induced glucoprivation is capable of acting within the hindbrain to activate fundamental reflex circuitry for consummatory feeding responses, as shown previously for hypoglycemia. In contrast, MA affects food consumption only after forebrain processing of MA-induced vagal afferent signals and in the presence of intact ascending and descending neural pathways.
Keywords: glucoprivic feeding, lipoprivic feeding, consummatory feeding responses, vagus nerve, hindbrain
decerebration is a procedure in which all neural connections between the hindbrain and forebrain are surgically transected. Decerebrate rats can be maintained chronically, and studies of such rats have generated important insights into the fundamental functional organization of the nervous system, including the organization of ingestive behavior and certain metabolic controls (7–9, 12, 13, 16, 17, 22, 39). Decerebrate rats cannot engage in appetitive behaviors, but they retain consummatory ingestive reflexes that allow them to accept or reject liquid food infused directly into their mouths. Previous work has shown that these reflexes can be modulated in decerebrate rats by certain signals known to control food intake in intact rats, including CCK and glucagon-like peptide-1 (GLP-1), which inhibit food intake (15, 17), and insulin-induced hypoglycemia (8), which stimulates food intake. Because the decerebration procedure restricts the central neural integration of feeding stimuli and responses to the caudal brain stem, the fact that GLP-1 and CCK reduce food intake in decerebrate rats indicates that neural circuits responsive to these peptides are capable of directly controlling hindbrain consummatory feeding reflexes without forebrain input.
In the present experiment, we examine the effect of decerebration on consummatory feeding responses induced by systemic administration of 2-mercaptoacetate (MA), which reduces fatty acid oxidation (2), and 2-deoxy-d-glucose (2DG), which reduces glucose utilization (4). The stimuli arising from these antimetabolic drugs activate the only two known controls of food intake that appear to be derived from ongoing substrate metabolism. Thus, it is of fundamental significance to understand the neural organization of these two controls.
The lipoprivic control is dependent on vagal sensory neurons. Surgical transection of the subdiaphragmatic vagus nerve (23, 34), chemical lesion of vagal afferents by systemic capsaicin administration (33), lesion of sensory terminals in the nucleus of the solitary tract (6), subdiaphragmatic vagal deafferentation (3), and lesions in the visceral afferent projection to the lateral parabrachial nucleus and central nucleus of the amygdala (6, 31) all abolish MA-induced feeding. Mercaptoacetate-induced Fos expression in the nucleus of the solitary tract (NTS) and other brain sites is also eliminated by subdiaphragmatic vagotomy (29). Because vagal afferent neurons terminate in the hindbrain, the hindbrain is likely to be involved in the processing of signals involved in MA-induced feeding. Moreover, the suppression of feeding in response to CCK, which also requires vagal sensory neurons (42–44), is not abolished by decerebration (15). Therefore, we hypothesized that MA would stimulate feeding even in chronic decerebrate rats.
The glucoprivic control can be activated by central glucoprivation (25) and does not require the vagus nerve. However, glucoprivic feeding is heavily dependent on the hindbrain. Glucoreceptor cells capable of eliciting a feeding response to localized glucose deficit are located in the hindbrain (1, 8, 27, 30), and elicitation of a number of key glucoregulatory responses, including glucoprivic feeding, require hindbrain catecholamine neurons (19, 20, 24, 28, 35). Furthermore, it has previously been shown that hypoglycemic doses of insulin, which also produce glucoprivation, increase feeding in decerebrate rats (8). Therefore, we predicted that 2DG would increase consummatory feeding responses in decerebrate rats. However, insulin is a hormone with multiple actions on peripheral substrate utilization and complex actions throughout the neuroaxis (36, 45). In previous work reporting hypoglycemia-induced feeding in decerebrate rats, no controls were included in which glucose was clamped at normal levels to evaluate the effects of hyperinsulinema itself on food intake. Although the central effects of insulin are usually associated with decreased feeding in the intact rat (45), the effects of insulin on consummatory feeding responses have not been studied in the normoglycemic decerebrate rat. If the present study reveals that 2DG, like insulin-induced hypoglycemia, increases consummatory feeding responses, a crucial role for insulin per se in producing these effects could be ruled out.
MATERIALS AND METHODS
Animals.
Adult male Sprague-Dawley rats were obtained from Simonsen Laboratories (Gilroy, CA). Rats were housed individually in suspended wire-mesh cages, under standard American Association for Accreditation of Laboratory Animal Care-approved conditions, in a temperature-controlled room (21 ± 1°C), and with a 12:12-h light-dark cycle (0700 to 1900) until decerebration was completed. Rats had ad libitium access to pellet rat chow (Harlan Teklad F6 Rodent Diet W, Madison, WI) and tap water prior to complete decerebration. All protocols were approved by Washington State University Institutional Animal Care and Use Committee.
Preparation of animals.
Rats were prepared for experimentation by stereotaxic decerebration. The decerebration was conducted in two stages, using a procedure previously described in detail (14). A midline incision was made in the skin to expose the skull, and a fine cut was made in the coronal plane across the dorsal surface of the skull 60% of the distance from bregma to lambda. A hemisection of the neuroaxis was made at the mesodiencephalic juncture using a hand-held blunted L-shaped spatula. The skin incision was closed. Each rat was allowed to recover for 2 wk before the procedure was repeated on the other side to produce a complete transection of the neuroaxis. The control rats were treated in the same manner (sham-operated), but the spatula was not inserted into the brain. Cheek fistulas were implanted at the time of the second surgery in both chronic decerebrate (CD) and control animals. Cheek fistulas were implanted through the skin rostral to the first premolar. The design of the fistula and implantation procedure have been described previously (19).
Care of decerebrate animals.
Following decerebration, rats were housed as pairs in Plexiglas cages. Cages were placed on top of a wire mesh tray over a heating pad, connected to a timer set for a 30 min on/60 min off heating cycle. Cage bedding was changed daily. Control and CD rats were fed by gavage 3 times each day (9 ml/feeding), with a nutritionally complete liquid diet (2.5 kcal/ml), used in previous studies to maintain chronic decerebrate rats (16, 26). The powdered diet was suspended in water and freshly made before each feeding. Feedings occurred at 0800, 1500, and 2200. This procedure was adequate to maintain body weights in both controls and decerebrates. During the first week after the complete decerebration, body temperatures were monitored closely, every 1–2 h during the light phase of the light-dark cycle, using a rectal thermometer. If an animal's body temperature fell below 34 C, the animal was placed near a heating unit and monitored until body temperature recovered to normal. After the first week, body temperatures were measured only once a day, just prior to the morning gavage feeding, unless closer observation was needed. Feeding tests were initiated ∼1 wk after surgery. Both groups were habituated to the test cage, and the intraoral infusion protocol prior to the feeding tests.
Feeding tests.
Decerebrate and control rats were tested for feeding in response to MA (thioglycolic acid, 68 mg/kg, 46 mg/kg or 91 mg/kg ip; Sigma-Aldrich, St. Louis, MO), 2DG (Sigma-Aldrich, 200 mg/kg sc), and 0.9% sterile saline (1 ml/kg). One saline test was conducted before the 2DG test and one was conducted before the MA test. Feeding tests were initiated 2 h after the morning gavage feeding. Before each feeding test, fistulas were cleared with a blunted 26-gauge needle and an infusion tip, connected to a syringe pump by Silastic tubing, was screwed onto the fistula. The tubing was supported above the rat's head so that the rat could move freely in the test cage. For feeding tests, liquid food (lactose-free whole milk diluted to a 40% solution with tap water) was infused intraorally through the cheek fistula (0.8 ml/min) beginning 60 min after drug or saline injection. The milk was infused in successive 5-min intervals with a 1-min break between. Infusions continued until rats refused the milk solution three consecutive times by allowing the milk to drop out of its mouth (15-s refusal, followed by a 30-s break). Spillage was monitored. The amount of milk consumed minus the spill was calculated. After the testing was complete, the calibrated flow rate and remaining volume were checked to ensure that the calibrated and actual flow rates did not differ. Previously, we showed that both MA and 2DG stimulate consummatory feeding in intact rats using the same milk test diet and the same protocol for intraoral milk delivery and the same drug doses (19). Tests were conducted in the following order: saline, 2DG, saline, MA, with a rest day following the 2DG test.
Evaluation of decerebration.
The brain tissue was embedded into a 12% gelatin block, cryoprotected by soaking in a 20% sucrose solution, sectioned sagittally (40 μm) using a cryostat and stained with cresyl violet. The completeness of the decerebration was then assessed by microscopic examination.
Data analysis.
Test results from CD and sham groups were analyzed separately using one-way repeated-measures ANOVA followed by the Holm-Sidak post hoc test. The differences were considered significant if P < 0.05. Results from the two saline tests were pooled for each group for statistical analysis.
RESULTS
All animals included in the decerebrate group were found to have complete decerebrations. Decerebrate rats weighed 270 g ± 7.0 at the time of surgery and 278 g ± 6.7 at the end of the test period. Sham controls weighed 276 g ± 12.9 and 313 g ± 12.8 at the time of surgery and at the end of testing, respectively.
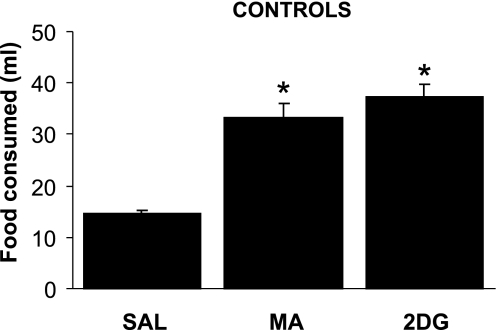
Figure 1 shows that shams (n = 4) increased milk consumption significantly above their saline intake in response to both 2DG and MA [F (2, 3) = 46.2, P = 0.001]. Shams consumed 14.5 ± 0.65 ml of milk after saline injection (13.5 ± 1.9 and 15.5 ± 0.35 ml, respectively, in the first and second saline tests), 37.4 ± 2.4 ml after 2DG and 33.3 ± 2.8 ml after MA.
Fig. 1.
Consumption of intraorally delivered milk by sham control rats after saline (SAL; 0.9%, 1 ml/kg), 2-mercaptoacetate (MA; 68 mg/kg) or 2-deoxy-d-glucose (2DG; 200 mg/kg) administration. Sham controls significantly increased their milk consumption in response to both MA and 2DG. Lines above bars indicate means ± SE. *P ≤ .001 vs. saline.
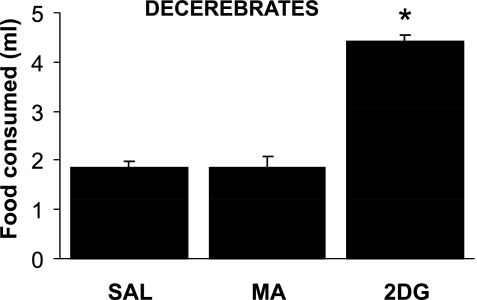
Figure 2 shows that decerebrates (n = 9) significantly increased their milk consumption in response to 2DG, but not to MA [F (2, 8) = 24.1, P = 0.001]. Decerebrates consumed 1.84 ± 0.14 ml in response to saline (1.81 ± 0.16 and 1.88 ± 0.19 ml, in the two tests), 4.41 ± 0.47 ml in response to 2DG, and 1.86 ± 0.22 ml in response to MA. Because MA failed to increase food intake in decerebrates at 68 mg/kg, which in our hands is a highly reliable suprathreshold dose for stimulation of food intake, we conducted a more limited experiment using a lower and a higher dose of MA (46 mg/kg and 91 mg/kg, respectively). Sham controls (n = 4) consumed 9.1 ± 2.0 ml of milk in response to saline and 13.8 ± 1.2, 13.5 ± 1.9, and 15.6 ± 1.7 ml in response to 46, 68, and 91 mg/kg MA, respectively. However, none of these doses increased intake above baseline levels (2.6 ml of milk) in the decerebrate rat (n = 1), so the remaining decerebrate rats were not retested at these doses.
Fig. 2.
Consumption of intraorally delivered milk by sham control rats after saline (0.9%, 1 ml/kg), MA (68 mg/kg), or 2DG (200 mg/kg) administration in chronically maintained decerebrate rats. Decerebrates significantly increased their milk consumption in response to 2DG, but not in response to MA. This indicates that glucoprivation increases food intake, in part, by acting directly on hindbrain consummatory feeding reflex circuitry, while effects of MA on consummatory feeding require forebrain processing of afferent metabolic signals and intact ascending and descending neural pathways. Lines above bars indicate means ± SE. *P ≤ 0.001 vs. saline.
DISCUSSION
Decerebrate rats increased their milk consumption in response to 2DG-induced glucoprivation. This finding strengthens and extends previous results showing that insulin-induced hypoglycemia increases feeding in decerebrate rats (8) and is consistent with the assumption that feeding evoked by both 2DG and insulin-induced hypoglycemia is elicited by decreased intracellular glucose metabolism (41). Although both basal and 2DG-stimulated milk consumption were much reduced in decerebrates compared with the intake of sham controls, both groups increased their intake by approximately the same percentage, compared with their own baseline responses (240% and 258%, respectively). In contrast to 2DG, MA did not increase consummatory feeding responses in decerebrate rats. Consummatory responses stimulated by MA thus appear to require projections descending from brain sites rostral to the level of decerebration, whereas 2DG is capable of stimulating consummatory reflex circuitry by actions within the hindbrain.
Decerebrate rats increased their intake in response to 2DG by the same percentage above their own baseline as in the controls. However, as noted, the absolute magnitude of both the baseline intake and the response in the decerebrates was much reduced compared with the control intakes. This same phenomenon has been reported previously in decerebrate rats (8) and is undoubtedly related to the loss of voluntary control of motor function in these rats, including loss of descending facilitatory control of ingestive reflexes from forebrain sites involved in motivation and appetitive responses and loss of their capacity to engage in or benefit from higher-order processing of sensory input from the hindbrain to forebrain sites (for a review, see Ref. 12). Which specific pathways control responsiveness of consummatory feeding reflexes is not known.
The present results, indicating differences in the underlying neural organization of MA- and 2DG-induced feeding, are consistent with other data. MA-, but not 2DG-induced feeding, is eliminated by vagotomy (34). Rather, receptors that detect glucoprivation and elicit feeding and other key glucoregulatory responses are present in the hindbrain (1, 8, 27, 30). In addition, hindbrain catecholamine neurons are required for glucoprivic feeding but not for the feeding response to MA (19, 28). Lesions of the lateral parabrachial nucleus (6) or central nucleus of the amygdala (31) abolish MA-induced, but not 2DG-induced, feeding. Furthermore, GABA receptor antagonists in the accumbens shell and ventral tegmental area that reduce the feeding response to MA do not reduce 2DG-induced feeding (21). Finally, MA and 2DG activate different hypothalamic peptidergic neurons (10, 11). MA has been shown to increase expression of hypothalamic melanin-concentrating hormone mRNA, but not neuropeptide Y (NPY) or agouti gene-related protein mRNA, which are increased during glucoprivation (11, 40). Our demonstration that decerebrate rats increase consummatory feeding in response to 2DG, but not MA, further sharpens the distinctiveness of these two metabolic controls.
Results of the present experiment indicate that the neural organization of MA-induced feeding differs fundamentally from the organization of the pathways mediating satiety effects of peripheral CCK. Effects of both MA (3, 33, 34, 38) and CCK (42–44) on food intake require vagal sensory neurons. However, vagal afferents mediating responses to CCK make connections in the hindbrain that are sufficient to modulate consummatory responses (9, 17), whereas afferents mediating responses to MA apparently do not.
Several brain sites rostral to the NTS that are activated by MA (29) and required for MA-induced appetitive feeding responses (6, 31) may be required for elicitation of consummatory responses to MA. Important brain regions involved with MA feeding include the NTS, lateral parabrachial nucleus, and central nucleus of the amygdala. Our decerebration protocol disconnects the amygdala from the hindbrain and may also cause collateral damage to the lateral parabrachial nucleus, which is proximal to the level of transection. Since the lateral parabrachial nucleus and the central nucleus of the amygdala are reciprocally interconnected with each other and with the NTS (37), their activation by vagal sensory input may control consummatory response circuitry via recurrent descending pathways to the hindbrain. The ventral tegmental area and the nucleus accumbens shell, structures of general importance for feeding and other motivated behaviors, have also been implicated in MA-induced appetitive feeding responses (21) but may facilitate consummatory responses as well. The hypothalamus is also likely to be involved in MA-induced feeding. Increased Fos expression in the paraventricular nucleus of the hypothalamus has been reported in response to reduced fatty acid oxidation by methylpalmoxirate (18). However, MA-induced feeding is not impaired by electrolytic lesion of the paraventricular nucleus (6) or by NPY-saporin lesions in the basomedial hypothalamus (5). Clearly, additional investigation will be required to clarify and elaborate the contributions of the hypothalamus and other brain sites to MA-induced consummatory and appetitive feeding responses.
Perspectives and Significance
The physiological role of the lipoprivic control of feeding in overall energy homeostasis is unclear. However, it may be that this control adjusts macronutrient selection to assure appropriate caloric intake. Previous work with purified macronutrient diets showed that rats injected with MA increased their intake of protein and carbohydrate, but did not increase their intake of fat, even when it was the only caloric source available (32). This response differed from the response to 2DG, which increased intake of all three macronutrients. This pattern of macronutrient selection suggests that the response to MA may require higher-order processing of taste and metabolic signals. A control that monitors ongoing metabolism and integrates taste and metabolic signals to appropriately adjust food choice may serve to enhance the animal's ability to fulfill its caloric and macronutrient requirements from diverse food sources. Additional work will be required to determine whether the lipoprivic control contributes to such a process. However, the dependence of this control on sites conveying both visceral sensory and taste information, including the nucleus of the solitary tract, the parabrachial complex, and the central nucleus of the amygdala, is compatible with this function (6, 29, 31, 34). The pattern of reciprocal interconnectivity described for these critical sites (37) suggests that sensory integration is an important aspect of their function. If so, this would be consistent with the failure of the lipoprivic control to enhance consummatory feeding responses in decerebrate rats in which this type of processing cannot occur.
GRANTS
This work was supported by National Institutes of Health Grant DK040498 and DK00345072 to S. Ritter.
Acknowledgments
The authors thank Dr. F. W. Flynn of the University of Wyoming for assistance with the decerebration technique and postsurgical husbandry of the decerebrate rats.
REFERENCES
- 1.Andrew SF, Dinh TT, Ritter S. Localized glucoprivation of hindbrain sites elicits corticosterone and glucagon secretion. Am J Physiol Regul Integr Comp Physiol 292: R1792–R1798, 2007. [DOI] [PubMed] [Google Scholar]
- 2.Bauche F, Sabourault D, Giudicelli Y, Nordmann J, Nordmann R. Inhibition in vitro of acyl-CoA dehydrogenases by 2-mercaptoacetate in rat liver mitochondria. Biochem J 215: 457–464, 1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brandt K, Arnold M, Geary N, Langhans W, Leonhardt M. Vagal afferents mediate the feeding response to mercaptoacetate but not to the beta (3) adrenergic receptor agonist CL 316,243. Neurosci Lett 411: 104–107, 2007. [DOI] [PubMed] [Google Scholar]
- 4.Brown J Effects of 2-deoxy-d-glucose on carbohydrate metabolism: review of the literature and studies in the rat. Metabolism 11: 1098–1112, 1962. [PubMed] [Google Scholar]
- 5.Bugarith K, Dinh TT, Li AJ, Speth RC, Ritter S. Basomedial hypothalamic injections of neuropeptide Y conjugated to saporin selectively disrupt hypothalamic controls of food intake. Endocrinology 146: 1179–1191, 2005. [DOI] [PubMed] [Google Scholar]
- 6.Calingasan NY, Ritter S. Lateral parabrachial subnucleus lesions abolish feeding induced by mercaptoacetate but not by 2-deoxy-d-glucose. Am J Physiol Regul Integr Comp Physiol 265: R1168–R1178, 1993. [DOI] [PubMed] [Google Scholar]
- 7.DiRocco RJ, Grill HJ. The forebrain is not essential for sympathoadrenal hyperglycemic response to glucoprivation. Science 204: 1112–1114, 1979. [DOI] [PubMed] [Google Scholar]
- 8.Flynn FW, Grill HJ. Insulin elicits ingestion in decerebrate rats. Science 221: 188–190, 1983. [DOI] [PubMed] [Google Scholar]
- 9.Flynn FW, Grill HJ. Intraoral intake and taste reactivity responses elicited by sucrose and sodium chloride in chronic decerebrate rats. Behav Neurosci 102: 934–941, 1988. [DOI] [PubMed] [Google Scholar]
- 10.Fraley GS, Dinh TT, Ritter S. Immunotoxic catecholamine lesions attenuate 2DG-induced increase of AGRP mRNA. Peptides 23: 1093–1099, 2002. [DOI] [PubMed] [Google Scholar]
- 11.Fraley GS, Ritter S. Immunolesion of norepinephrine and epinephrine afferents to medial hypothalamus alters basal and 2-deoxy-d-glucose-induced neuropeptide Y and agouti gene-related protein messenger ribonucleic acid expression in the arcuate nucleus. Endocrinology 144: 75–83, 2003. [DOI] [PubMed] [Google Scholar]
- 12.Grill HJ, Kaplan JM. The neuroanatomical axis for control of energy balance. Front Neuroendocrinol 23: 2–40, 2002. [DOI] [PubMed] [Google Scholar]
- 13.Grill HJ, Kaplan JM. Sham feeding in intact and chronic decerebrate rats. Am J Physiol Regul Integr Comp Physiol 262: R1070–R1074, 1992. [DOI] [PubMed] [Google Scholar]
- 14.Grill HJ, Norgren R. Neurological tests and behavioral deficits in chronic thalamic and chronic decerebrate rats. Brain Res 143: 299–312, 1978. [DOI] [PubMed] [Google Scholar]
- 15.Grill HJ, Smith GP. Cholecystokinin decreases sucrose intake in chronic decerebrate rats. Am J Physiol Regul Integr Comp Physiol 254: R853–R856, 1988. [DOI] [PubMed] [Google Scholar]
- 16.Harris RB, Kelso EW, Flatt WP, Bartness TJ, Grill HJ. Energy expenditure and body composition of chronically maintained decerebrate rats in the fed and fasted condition. Endocrinology 147: 1365–1376, 2006. [DOI] [PubMed] [Google Scholar]
- 17.Hayes MR, Skibicka KP, Grill HJ. Caudal brainstem processing is sufficient for behavioral, sympathetic, and parasympathetic responses driven by peripheral and hindbrain glucagon-like-peptide-1 receptor stimulation. Endocrinology 149: 4059–4068, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Horn CC, Friedman MI. Methyl palmoxirate increases eating behavior and brain Fos-like immunoreactivity in rats. Brain Res 781: 8–14, 1998. [DOI] [PubMed] [Google Scholar]
- 19.Hudson B, Ritter S. Hindbrain catecholamine neurons mediate consummatory responses to glucoprivation. Physiol Behav 82: 241–250, 2004. [DOI] [PubMed] [Google Scholar]
- 20.I'Anson H, Sundling LA, Roland SM, Ritter S. Immunotoxic destruction of distinct catecholaminergic neuron populations disrupts the reproductive response to glucoprivation in female rats. Endocrinology 144: 4325–4331, 2003. [DOI] [PubMed] [Google Scholar]
- 21.Kandov Y, Israel Y, Kest A, Dostova I, Verasammy J, Bernal SY, Kasselman L, Bodnar RJ. GABA receptor subtype antagonists in the nucleus accumbens shell and ventral tegmental area differentially alter feeding responses induced by deprivation, glucoprivation and lipoprivation in rats. Brain Res 1082: 86–97, 2006. [DOI] [PubMed] [Google Scholar]
- 22.Kaplan JM, Seeley RJ, Grill HJ. Daily caloric intake in intact and chronic decerebrate rats. Behav Neurosci 107: 876–881, 1993. [PubMed] [Google Scholar]
- 23.Langhans W, Scharrer E. Evidence for a vagally mediated satiety signal derived from hepatic fatty acid oxidation. J Auton Nerv Syst 18: 13–18, 1987. [DOI] [PubMed] [Google Scholar]
- 24.Li AJ, Wang Q, Dinh TT, Ritter S. Simultaneous silencing of Npy and Dbh expression in hindbrain A1/C1 catecholamine cells suppresses glucoprivic feeding. J Neurosci 29: 280–287, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miselis RR, Epstein AN. Feeding induced by intracerebroventricular 2-deoxy-d-glucose in the rat. Am J Physiol 229: 1438–1447, 1975. [DOI] [PubMed] [Google Scholar]
- 26.Nautiyal KM, Dailey ME, Brito, N, Brito MN, Harris RB, Bartness TJ, Grill HJ. Energetic responses to cold temperatures in rats lacking forebrain-caudal brainstem connections. Am J Physiol Regul Integr Comp Physiol 295: R789–R798, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ritter RC, Slusser PG, Stone S. Glucoreceptors controlling feeding and blood glucose: location in the hindbrain. Science 213: 451–452, 1981. [DOI] [PubMed] [Google Scholar]
- 28.Ritter S, Bugarith K, Dinh TT. Immunotoxic destruction of distinct catecholamine subgroups produces selective impairment of glucoregulatory responses and neuronal activation. J Comp Neurol 432: 197–216, 2001. [DOI] [PubMed] [Google Scholar]
- 29.Ritter S, Dinh TT. 2-Mercaptoacetate and 2-deoxy-d-glucose induce Fos-like immunoreactivity in rat brain. Brain Res 641: 111–120, 1994. [DOI] [PubMed] [Google Scholar]
- 30.Ritter S, Dinh TT, Zhang Y. Localization of hindbrain glucoreceptive sites controlling food intake and blood glucose. Brain Res 856: 37–47, 2000. [DOI] [PubMed] [Google Scholar]
- 31.Ritter S, Hutton B. Mercaptoacetate-induced feeding is impaired by central nucleus of the amygdala lesions. Physiol Behav 58: 1215–1220, 1995. [DOI] [PubMed] [Google Scholar]
- 32.Ritter S, Ritter JB, Cromer L. 2-Deoxy-d-glucose and mercaptoacetate induce different patterns of macronutrient ingestion. Physiol Behav 66: 709–715, 1999. [DOI] [PubMed] [Google Scholar]
- 33.Ritter S, Taylor JS. Capsaicin abolishes lipoprivic but not glucoprivic feeding in rats. Am J Physiol Regul Integr Comp Physiol 256: R1232–R1239, 1989. [DOI] [PubMed] [Google Scholar]
- 34.Ritter S, Taylor JS. Vagal sensory neurons are required for lipoprivic but not glucoprivic feeding in rats. Am J Physiol Regul Integr Comp Physiol 258: R1395–R1401, 1990. [DOI] [PubMed] [Google Scholar]
- 35.Ritter S, Watts AG, Dinh TT, Sanchez-Watts G, Pedrow C. Immunotoxin lesion of hypothalamically projecting norepinephrine and epinephrine neurons differentially affects circadian and stressor-stimulated corticosterone secretion. Endocrinology 144: 1357–1367, 2003. [DOI] [PubMed] [Google Scholar]
- 36.Saltiel AR, Pessin JE. Insulin signaling pathways in time and space. Trends Cell Biol 12: 65–71, 2002. [DOI] [PubMed] [Google Scholar]
- 37.Saper CB Central autonomic system. In: The Rat Nervous System, edited by Paxinos, G. San Diego: Academic, 1995, p. 107–135.
- 38.Scharrer E, Langhans W. Control of food intake by fatty acid oxidation. Am J Physiol Regul Integr Comp Physiol 250: R1003–R1006, 1986. [DOI] [PubMed] [Google Scholar]
- 39.Seeley RJ, Grill HJ, Kaplan JM. Neurological dissociation of gastrointestinal and metabolic contributions to meal size control. Behav Neurosci 108: 347–352, 1994. [PubMed] [Google Scholar]
- 40.Sergeyev V, Broberger C, Gorbatyuk O, Hokfelt T. Effect of 2-mercaptoacetate and 2-deoxy-d-glucose administration on the expression of NPY, AGRP, POMC, MCH and hypocretin/orexin in the rat hypothalamus. Neuroreport 11: 117–121, 2000. [DOI] [PubMed] [Google Scholar]
- 41.Smith GP, Epstein AN. Increased feeding in response to decreased glucose utilization in the rat and monkey. Am J Physiol 217: 1083–1087, 1969. [DOI] [PubMed] [Google Scholar]
- 42.Smith GP, Jerome C, Cushin BJ, Eterno R, Simansky KJ. Abdominal vagotomy blocks the satiety effect of cholecystokinin in the rat. Science 213: 1036–1037, 1981. [DOI] [PubMed] [Google Scholar]
- 43.Smith GP, Jerome C, Norgren R. Afferent axons in abdominal vagus mediate satiety effect of cholecystokinin in rats. Am J Physiol Regul Integr Comp Physiol 249: R638–R641, 1985. [DOI] [PubMed] [Google Scholar]
- 44.South EH, Ritter RC. Capsaicin application to central or peripheral vagal fibers attenuates CCK satiety. Peptides 9: 601–612, 1988. [DOI] [PubMed] [Google Scholar]
- 45.Woods SC, Porte D Jr. The role of insulin as a satiety factor in the central nervous system. Adv Metab Disord 10: 457–468, 1983. [DOI] [PubMed] [Google Scholar]