Abstract
The (pro)renin receptor [(P)RR] plays a pivotal role in the renin-angiotensin system. Experimental models emphasize the role of (P)RR in organ damage associated with hypertension and diabetes. However, a mutation of the (P)RR gene, resulting in frame deletion of exon 4 [Δ4-(P)RR] is associated with X-linked mental retardation (XLMR) and epilepsy pointing to a novel role of (P)RR in brain development and cognitive function. We have studied (P)RR expression in mouse brain, as well as the effect of transfection of Δ4-(P)RR on neuronal differentiation of rat neuroendocrine PC-12 cells induced by nerve growth factor (NGF). In situ hybridization showed a wide distribution of (P)RR, including in key regions involved in the regulation of blood pressure and body fluid homeostasis. In mouse neurons, the receptor is on the plasma membrane and in synaptic vesicles, and stimulation by renin provokes ERK1/2 phosphorylation. In PC-12 cells, (P)RR localized mainly in the Golgi and in endoplasmic reticulum and redistributed to neurite projections during NGF-induced differentiation. In contrast, Δ4-(P)RR remained cytosolic and inhibited NGF-induced neuronal differentiation and ERK1/2 activation. Cotransfection of PC-12 cells with (P)RR and Δ4-(P)RR cDNA resulted in altered localization of (P)RR and inhibited (P)RR redistribution to neurite projections upon NGF stimulation. Furthermore, (P)RR dimerized with itself and with Δ4-(P)RR, suggesting that the XLMR and epilepsy phenotype resulted from a dominant-negative effect of Δ4-(P)RR, which coexists with normal transcript in affected males. In conclusion, our results show that (P)RR is expressed in mouse brain and suggest that the XLMR and epilepsy phenotype might result from a dominant-negative effect of the Δ4-(P)RR protein.
Keywords: brain (P)RR expression, functional (P)RR, X-linked mental retardation
the classical renin-angiotensin system (RAS) is an intravascular enzymatic cascade that generates ANG II, considered the biologically active peptide, and plays a major role in the control of blood pressure, as well as fluid and salt balance. A receptor for renin and its proenzyme form, prorenin, called (pro)renin receptor [(P)RR] was recently cloned (20). The binding of renin and of prorenin results in increased angiotensin generation at the cell surface. Activation of (P)RR results in the activation of the MAP kinases ERK1/2 pathway and in upregulation of profibrotic genes, independently of ANG II generation (9, 10, 13, 21). These biochemical characteristics have generated a special interest in potential nonhemodynamic functions of renin ad prorenin. The receptor is abundantly expressed in adult human (20) and rat (14) organs, including the brain. Although overexpression of (P)RR is associated with a renal (15) and cardiovascular phenotype (3), it is surprising that alterations in the (P)RR gene, ATP6ap2 point to an essential role of (P)RR in cell survival and development of the central nervous system. In zebra fish, (P)RR/ATP6AP2 is expressed at a very early stage of development (www.zfin.org; ZFIN ID: ZDB-FIG-070117–571), and a mutation in the (P)RR gene resulted in the death of fish before the end of embryogenesis, leading to the conclusion that (P)RR is an embryonic-essential gene. Additionally, the mutant was characterized by severe malformations of the central nervous system and of the eye (2).
Recently, we reported the only known mutation in (P)RR in humans that resulted in the presence of a mRNA with an in-frame deletion of exon 4 [Δ4-(P)RR], along with normal (P)RR mRNA. The patients suffered from X-linked mental retardation and epilepsy without detectable cardiovascular or renal abnormalities (23), supporting a predominant role of (P)RR during the development of the central nervous system. Studies on lymphocytic cells immortalized from these patients showed that the Δ4-(P)RR protein could still bind renin, but it had lost the ability to phosphorylate the MAP kinases ERK1/2 upon renin stimulation, suggesting a dominant negative effect on (P)RR-mediated ERK1/2 activation.
The purpose of this study was to determine the site of expression of (P)RR in brain and the presence of a functional (P)RR in primary neurons in culture. Furthermore, we studied the effect of Δ4-(P)RR expression on neuronal differentiation of PC-12 neuroendocrine cells, and we investigated the possibility of oligomerization of (P)RR as a potential mechanism of the dominant-negative effect of Δ4-(P)RR.
MATERIALS AND METHODS
Plasmid construction.
RNA from a patient having the Δ4-(P)RR mutation and from a normal control were prepared using GeneElute Mammalian RNA Miniprep Kit (Sigma, St. Louis, MO). cDNA was generated from the mRNAs template using SuperScript first-strand synthesis kit (Invitrogen, Carlsbad, CA) and cloned into pcDNA3.1 (directional TOPO expression kit and for CT-GFP fusion TOPO TA expression kit, Invitrogen). Constructs were confirmed by sequencing.
In situ hybridization.
Adult mouse brains were embedded in paraffin and cut in 4-μm-thick sections. Coronal brain sections were studied by in situ hybridization using the full-length cDNA mouse (P)RR reversed transcribed and labeled with 35S-dUTP as a riboprobe as described (12). Sense probe was used as a negative control.
Cell culture and transfection.
Primary neuronal cultures were prepared from the cortices of 16-day-old OF1 mouse embryos. Briefly, cells were mechanically dissociated with a flame-polished Pasteur pipette in PBS supplemented with glucose (33 mM). Cells were plated on polyornithine-coated 12-well plates (5 × 105 cells per well) or glass coverslips in 24-well plates (5 × 104 cells per coverslip) in neurobasal medium containing B27 supplement (1:50) glutamax (2 mM) and penicillin/streptomycin. They were used after 1 wk of culture in vitro.
Rat neuroendocrine PC-12 cells were obtained from American Type Culture Collection and cultured in DMEM, 10% horse serum, 5% FBS, l-glutamine, and penicillin/streptomycin. PC-12 cells were plated on poly-l-lysine-coated plates 1 day prior to transfection in 1% FBS/DMEM. Cells were transfected with Lipofectamine Plus reagent or Lipofectamine 2000 at a ratio of reagent to DNA of 1.5 (μl/μg) following the manufacturers' protocol. Tissue culture reagents were from Sigma and Invitrogen.
Immunofluorescence study.
Mouse neurons cultured on glass coverslips were fixed in 4% paraformaldehyde for 20 min and permeabilized or not with Triton-X100 0.5% for 15 min at 4°C. Nonspecific binding was blocked by incubation with PBS containing 3% normal goat serum. The cells were then incubated with purified rabbit IgG against human (P)RR (Ab 1623) and antisynaptophysin (Sigma S5768, 1/5,000). After extensive washes, the cells were incubated with secondary Alexa 488 coupled anti-rabbit IgG and Alexa 555 coupled anti-mouse IgG (Molecular Probes). After 3 washes, the cells were mounted in polyvinyl alcohol mounting medium with DABCO (Fluka, Buchs, Switzerland) and examined with a confocal microscope (Leica, Wetzlar, Germany).
PC-12 cells were transfected with the indicated plasmids. Twenty-four hours after transfection, the cells were washed with PBS and maintained in 1% FBS/DMEM or 1% FBS/DMEM with 100 ng/ml of nerve growth factor (NGF) for 36–40 h on poly-l-lysine- and laminin-coated coverslips. The coverslips were washed twice with PBS, fixed in 4% paraformaldehyde, permeablized with Triton X-100, and blocked in 2% horse serum, 0.4% BSA, PBS for 30 min with RNaseA (Qiagen). The coverslips were incubated with anti-V5 primary antibody (Invitrogen; 1:400) for 1 h, washed 3 times, and then incubated with Alexa Fluor chicken anti-mouse 488 (1:800) or Alexa Fluor goat anti-mouse 594 for 1 h. Nuclei were stained by incubating coverslips in 0.5 μM Sytox-orange, or with anti-laminin B followed by Alexa Fluor chicken anti-goat 647. Coverslips were mounted onto microscope slides in SlowFade Gold antifade mounting medium. The morphology of the cells was appreciated by staining actin fibers with Alexa Fluor Phalloidin 633. Confocal images were obtained using a Zeiss LSM510 microscope. All reagents were from Molecular Probes (Carlsbad, CA) unless otherwise indicated.
ERK1/2 phosphorylation induced by renin and by NGF.
Mouse neurons, in 12-well plates, were serum deprived for 18 h prior to stimulation by human renin (Sigma). Renin (10 nM) was diluted in neurobasal medium and added to the cells incubated at 37°C in the presence of the AT1 receptor blocker losartan (Los 10 μM) and AT2 receptor blocker PD123319 (10 μM). The reaction was stopped at 10, 20, and 30 min by withdrawal of the incubation medium, and the cells were lysed by the addition of lysis buffer, centrifuged for 15 min at 12,000 g at 4°C to remove the cellular debris. The supernatant was analyzed by SDS-10% PAGE and by Western blot analysis for phosphorylated and total ERK1/2 (Cell Signaling, Beverly, MA), as described previously (20).
To measure endogenous response of PC-12 cells to renin stimulation, cells were plated onto poly-l lysine coated 6-well dishes in 1% FBS/DMEM/PenStrepGlut media and incubated overnight at 37°C. The PC-12 cells were then washed twice with PBS and kept in DMEM overnight. Cells were stimulated with 10 nM renin or normal growth medium for 10 min, and ERK1/2 phosphorylation was analyzed by Western blot. To measure the response of PC-12 cells transfected with (P)RR-encoding plasmids, PC-12 cells were plated in 1% FBS/DMEM in 6-well plastic tissue culture plates. The PC-12 cells were then transfected with 4 μg of the appropriate plasmid using Lipofectamine Plus reagent. Twenty-four hours after transfection, the cells were washed with PBS and incubated overnight in 1% FBS/DMEM prior to stimulation. The cells were stimulated for 5 min with 10% FBS/DMEM or 100 ng/ml of NGF in DMEM. Cells were immediately washed twice with cold PBS and lysed; then ERK1/2 phosphorylation was analyzed by Western blot.
Dimerization.
PC-12 cells were transfected with a total of 4 μg of the indicated plasmids in 60-mm dishes. Twenty-four hours after transfection they were washed with PBS and placed in 1% FBS/DMEM without or with 100 ng/ml of NGF for 36–40 h. Forty-eight hours later, the cells were washed twice with PBS and lysed. The supernatant was cleared by centrifugation, and 300–400 μg of protein was incubated for 4 h with 1 μg rabbit anti-V5 (Sigma) on a spinning rotator at 4°C. Magnetic beads conjugated with anti-rabbit antibody were added to the lysate-antibody mixture and incubated on the rotator at 4°C for 2 h. The beads were washed 6 times boiled in Laemmli sample buffer, separated by SDS-PAGE, and transferred to a nitrocellulose membrane. Coimmunoprecipitated green fluorescent protein (GFP)-tagged protein was detected by Western blot analysis using an anti-GFP antibody (1:2,000; Roche Diagnostics, Mannheim, Germany), an anti-mouse horseradish peroxidase-conjugated antibody (1:5,000; Pierce, Rockford, IL) and West Pico Chemiluminescense (Pierce).
Statistical analysis.
The statistical significance was determined by use of Student's t-test.
RESULTS
Expression of the (pro)renin receptor in adult mouse brain.
We studied the sites of expression of (P)RR in adult mouse brain by in situ hybridization with a 35S-labeled riboprobe (Fig. 1, 2). The mRNA encoding (P)RR is expressed in most cerebral areas but more importantly in the key regions involved in the regulation of blood pressure and body fluid homeostasis such as the subfornical organ (Fig. 1A), the supraoptic nucleus (Fig. 1B), the rostral ventrolateral medulla (Fig. 1C), the paraventricular nuclei (Fig. 1D), and the nucleus tractus solitarius (Fig. 1E). The labeling is predominant in neuronal cells identified by their large and pale nuclei and in cells of the choroid plexus (Fig. 2M). In contrast, glial cells in the white matter, as well as in the gray matter, identified as having smaller and compact nuclei, showed no significant labeling above background (Fig. 2, F and N). In the cortex, the strongest expression was observed in neurons of layers 4 and 6b (Fig. 2, D and F), as well as in layer 2 of the piriform cortex (Fig. 2E), whereas almost no labeling was visible in layer 1 (Fig. 2C). The pyramidal cells of the hippocampus showed a very strong labeling in the CA3 field (Fig. 2G) that was more intense than in CA2 or CA1 (Fig. 2H), while the granule cells of the dentate gyrus (Fig. 2, I and J) exhibited very low labeling. The thalamus was strongly labeled with some heterogeneity among the different nuclei (Fig. 2, K and L). A summary of the relative abundance of (P)RR expression in the different regions of the adult mouse brain is presented in Table 1.
Fig. 1.
Expression of (P)RR in key brain regions involved in the regulation of blood pressure and body fluid homeostasis by in situ hybridization with radiolabeled (P)RR antisense riboprobes and counterstained with toluidine blue. (P)RR is highly expressed in neurons of the subfornical organ (SFO; A); the supraoptic nucleus (SO; B); the rostral ventrolateral medulla (RVLM; C); the paraventricular nuclei (PaV; D) and the nucleus tractus solitarius (NTS; E). Mve, medial vestibular nucleus; SpVe, spinal vestibular nucleus; vsc, ventral spinocerebellar tract; 3V, third ventricle.
Fig. 2.
Expression of (P)RR in adult mouse forebrain by in situ hybridization with radiolabeled riboprobes (A and B) Low-magnification bright field images of mouse brain sections counterstained with toluidine blue after ISH with (P)RR antisense probe (A) or sense probe (B). A strong expression of (P)RR mRNA was detected in the cortex, the thalamus, and the pyramidal cell layer of CA3. A sense probe did not display any specific signal. C–N: higher magnification images showing the hybridization signals obtained with the antisense probe in the cerebral cortex (C–F; 1, 2, 4, 6b refer to the cortical layers and WM referes to the white matter), the hippocampal formation (F–K; PL refers to pyramidal cell layer in CA1, CA2, and CA3 fields; GL refers to granule cell layer in the dentate gyrus); the thalamus (K, L; DLG and VPL refer to dorsal lateral geniculate nucleus and ventral posteromedial thalamic nucleus, respectively); and the choroid plexus (M; LV indicates the lateral ventricle). Background levels obtained with the sense probe hybridized to a serial section are shown in for the dentate gyrus (J) and for the cortex (N), white matter, and CA1 field. Scale bar shown in N corresponds to 1,000 μm (A and B) and 60 μm (C–N).
Table 1.
Distribution of (P)RR mRNA by in situ hybridization in normal adult mouse brain
| Brain Area | Distribution |
|---|---|
| Cortex | +++ |
| Caudate putamen | + |
| Magnocellular paraventricular hypothalamus nucleus | ++ |
| Supraoptic nucleus | + |
| Hippocampus | +++ |
| Superior olivary nucleus | ++ |
| Nucleus tractus solitary | +++ |
| Rostroventrolateral medulla | + |
| Nucleus ambigus | + |
| Subfornical organ | +++ |
| Area postrema | + |
| Dentate gyrus | + |
| Dorsal lateral geniculate nucleus | + |
| Ventral posteromedial thalamic nucleus | ++ |
| Hypothalamic paraventricumar nucleus | ++ |
The observation was made by two investigators, and the brain areas were identified according to a mouse brain atlas (1). The number of plus signs (+) indicates the level of intensity of (P)RR expression studied by in situ hybridization with 35S-DUTP riboprobe.
Mouse neurons in culture express a functional (P)RR.
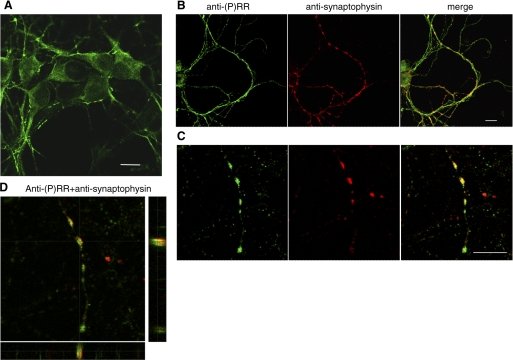
In primary neurons in culture, the immunofluorescence staining with anti-(P)RR antibody showed a punctuate pattern of staining in the cells with a reinforcement of the staining along the plasma membranes and along the neurites (Fig. 3A). A short form of (P)RR was shown to coprecipitate with the vacuolar proton-ATPase (V-ATPase) (18), an essential component of synaptic vesicles and necessary for the concentration and the maturation of neurotransmitters (22). We, thus, performed double staining with anti-(P)RR and with anti-synaptophysin, a glycoprotein found in every synaptic vesicles of all neurons in the brain. The confocal analysis showed that (P)RR and synaptophysin colocalized (Fig. 3, B–D), indicating that (P)RR was present in synaptic vesicles, but not every synaptophysin-positive vesicle was stained with (P)RR.
Fig. 3.
Staining of (P)RR in primary neurons. A–C: neurons fixed in paraformaldehyde and permeabilized by Triton-X100 were stained with purified IgG to (P)RR (ab1623 diluted 1/1,000). A: (P)RR staining shows a punctated pattern inside the neuronal body with reinforcement at the plasma membrane and along the neurites. B, C: localization of (P)RR in synaptic vesicle. B: Anti-(P)RR staining showed a punctuated labeling in neurons bodies and in neurites in primary neurons in culture. The double staining with monoclonal anti-synaptophysin that stains synaptic vesicles showed that (P)RR colocalized with synaptophysin. Higher magnification of vesicles in neurites (C) showed that not every synaptophysin-positive vesicle (green) was positive for (P)RR (red). Scales bars are 10 μm. D: higher magnification the colocalization of (P)RR with synatophysin in a synaptic vesicle.
Next, we wanted to know whether (P)RR localized in the membranes of neurons could be stimulated by renin. Preliminary experiments indicated that human renin could bind to mouse mesangial cells and activate mouse (P)RR (9). It was also recently confirmed that (P)RR in mouse vascular smooth muscle cells could be activated by human renin (9). Therefore, we used human recombinant renin to stimulate (P)RR in mouse neurons, in the presence of ANG II receptor type 1 and type 2 blockers to prevent any ERK1/2 phosphorylation potentially due to ANG II generation. Our results showed that renin induced a clear phosphorylation of ERK1/2 that reached 400 ± 8% after 10 min and 560 ± 10% after 30 min. The extent of ERK phosphorylation and the time course were similar to that observed in other cell types and indicate that the prorenin receptor expressed on the cell surface of neurons in culture is functional (Fig. 4A). Altogether, our results show that (P)RR is expressed in adult mouse brain and that its expression is mainly associated with neurons where it functions via the MAP kinases ERK1/2 signaling.
Fig. 4.
Renin-induced phosphorylation of ERK1/2 on neurons and PC-12 cells. A: primary mouse neurons were cultured for 7 days and serum deprived overnight prior to stimulation. Renin (10 nM) induced ERK phosphorylation detectable at 10 min and lasted for at least 30 min. The graph summarizes the time course of ERK1/2 phosphorylation induced by renin in neurons; the errors bars represent the SE of 3 independent experiments performed in triplicate. B: PC-12 cells were serum starved and then stimulated with 10 nM renin or 10% serum. C: NGF-induced ERK1/2 activation is inhibited in PC-12 cells expressing Δ4-(P)RR. Cells were transfected with plasmids encoding (P)RR or Δ4-(P)RR and stimulated with serum or NGF. Cell lysates were analyzed by Western blot for ERK1/2 and phospho-ERK1/2. Data in this figure represent mean ± SD of 4 experiments and ***P < 0.001 by Student's t-test.
Effect of transfection of Δ4-(P)RR on NGF-induced ERK1/2 phosphorylation and neuronal differentiation of PC12 cells.
Next, we studied the effect of Δ4-(P)RR transfection on ERK1/2 signaling, the cellular distribution of the mutant Δ4-(P)RR, and its effect on neuronal differentiation. To this end, we used rat neuroendocrine PC-12 cells that can be induced to differentiate into neurons by incubation with NGF. Stimulation of PC-12 cells by renin induced a 200% increase of ERK1/2 phosphorylation. However, the cells were capable of a greater response, as indicated by a 700% increased in ERK1/2 phosphorylation when they were stimulated with serum (Fig. 4B). PC-12 cells were then transfected with wild-type (wt)-(P)RR, Δ4-(P)RR, or both, or with a control plasmid and stimulated with serum or NGF. Following NGF stimulation the extent of ERK1/2 activation in cells transfected with wt-(P)RR showed a moderate 133% increase compared with control cells, whereas ERK1/2 activation was significantly decreased by 28% in Δ4-(P)RR-expressing cells (P < 0.001 compared with control stimulated with NGF) and by 38% in cells double transfected with wt-(P)RR and Δ4-(P)RR (P < 0.001 compared with control stimulated with NGF) (Fig. 4C).
Effect of the coexpression of (P)RR and of Δ4-(P)RR on (P)RR distribution, evidence for a dimerization of (P)RR.
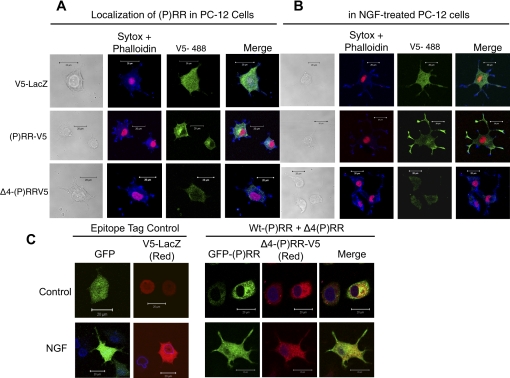
We examined the possibility that the decrease of ERK phosphorylation observed with Δ4-(P)RR transfection could be due to altered localization of (P)RR on the plasma membrane when it was cotransfected with Δ4-(P)RR. In the absence of anti-(P)RR antibodies able to specifically detect Δ4-(P)RR, we used a V5-tagged Δ4-(P)RR construct transfected into PC-12 and analyzed the cells with anti-V5 antibody. PC-12 cells were also transfected with V5-tagged (P)RR as a control. Immunofluorescence studies of PC-12 cells transfected with V5-tagged (P)RR showed that it was located in vesicles throughout the cell with an increased concentration in a perinuclear region likely corresponding to the Golgi apparatus (Fig. 5 A). The addition of NGF caused a shift in the localization of the (P)RR protein to the tips of neurite extensions (Fig. 5B). In contrast, transfection of cells with V5-tagged Δ4-(P)RR resulted in a diffuse, lower expression level and lack of localization to the perinuclear region. Additionally, Δ4-(P)RR protein did not localize to neurite tips after NGF stimulation. As a control, LacZ-V5-transfected PC-12 cells stimulated with NGF exhibited strong expression of LacZ-V5. However, LacZ-V5 was dispersed throughout the cell and did not redistribute to neurite tips, even though the PC-12 cells were responsive to NGF and formed neurite extensions, as visualized by actin staining with Alexa Fluor Phalloidin 633 (Fig. 5, A and B).
Fig. 5.
Cellular distribution of (P)RR and Δ4-(P)RR. Following transfection, the cells were treated without NGF (A) or with NGF (B) and subjected to immunofluorescence staining. V5-tagged proteins are stained with AlexaFluor-488 (green) and nuclei stained with Sytox-orange (red). Actin is stained with Phalloidin-633 (blue). Scale bar represents 10 μm. C: effects of cotransfection of (P)RR and Δ4(P)RR on cellular distribution of (P)RR. PC-12 cells were cotransfected with plasmids encoding (P)RR-GFP (green) and Δ4(P)RR-V5, cultured in the presence or absence of NGF, and subjected to immunofluorescence staining. Δ4(P)RR-V5 is stained with AlexaFluor-594 (red) and nuclei stained anti-lamin B and detected with Alexa Fluor-647 (blue). Scale bar represents 20 μm.
To study the effect of Δ4-(P)RR on (P)RR distribution and on neuronal differentiation we coexpressed (P)RR and Δ4-(P)RR, recapitulating the heterozygous condition observed in patients with XLMR and epilepsy related to the mutation in the (P)RR/ATP6AP2 gene (23). We cotransfected (P)RR and Δ4-(P)RR differentially tagged with either GFP or V5 into PC-12 cells. Coexpression of (P)RR and Δ4-(P)RR resulted in an altered localization of (P)RR, which exhibited less of a perinuclear localization and did not move to occupy neurite tips when stimulated with NGF (Fig. 5C).
It has been suggested that (P)RR may form dimers (20, 24), and the decreased response to renin in PC12 cells coexpressing (P)RR and Δ4-(P)RR was suggestive of a dominant-negative effect of Δ4-(P)RR. We, therefore, performed coimmunoprecipitation experiments to study whether (P)RR and Δ4-(P)RR could form dimers. Coimmunoprecipitation experiments using lysates from double-transfected PC-12 cells indeed indicated that both (P)RR and Δ4-(P)RR were able to form both homodimers and heterodimers [(P)RR/(P)RR, (P)RR/Δ4-(P)RR, and Δ4-(P)RR/Δ4-(P)RR dimers] (Fig. 6).
Fig. 6.
Dimerization of (P)RR and Δ4(P)RR. PC12 cells were transfected with plasmids encoding GFP or V5 tagged (P)RR or Δ4-(P)RR and cultured for 48 h. Coimmunoprecipitation of cell lysates was performed using anti-V5 antibody and Western blot analysis using anti-GFP antibody. Ly, cell lysate; IP, immunoprecipitates.
DISCUSSION
The central RAS is known to control cardiovascular function and fluid homeostasis (19, 25). All components of the RAS are localized in the brain, and renin has been previously reported in the hypothalamus and cerebellar cortex in the mouse and rat brain and in neurons in most areas of the human brain (1, 6, 7, 11, 16, 27). Our results now show that (P)RR is also expressed widely in the adult mouse brain and at high levels in nuclei important for the regulation of blood pressure and body fluid homeostasis, such as the subfornical organ, the paraventricular nucleus, the area postrema, the nucleus tractus solitarii, the rostroventrolateral medulla, and the nucleus ambiguus. But to our surprise, we also found very high (P)RR expression in regions that are not involved in cardiovascular and fluid homeostasis, further supporting the role of the RAS in cardiovascular unrelated functions, such as learning, memory, and cognition (8). The strongest expression observed in thalamic relay nuclei. Furthermore, the in situ hybridization results indicated that (P)RR was abundantly expressed specifically in neurons, and stimulation of neurons in culture with renin induced the phosphorylation of ERK1/2. This confirms the synthesis of a membrane-associated (P)RR and previous data obtained with hypothalamic neurons (26).
The cellular distribution of (P)RR was an unexpected result, as we also found (P)RR in synaptic vesicles in addition to the membrane-associated (P)RR. This result is supported by the demonstration of the presence of truncated (P)RR in purified synaptic vesicles demonstrated by mass spectrometry (29). This localization questions the function of (P)RR in synaptic vesicles, which is out of reach of extracellular renin and prorenin. It should be stressed that before the cloning of the (pro)renin receptor, as such, a truncated form of (P)RR composed of the transmembrane and cytoplasmic domain was reported to coprecipitate with the membrane sector part of the V-ATPase (18), a multimeric protein essential for the control of pH in intracellular compartments and for the concentration and the maturation of neurotransmitters in synaptic vesicles (22). The gene coding for (P)RR was, therefore, called ATP6ap2 for ATP6-associated protein 2. The existence of this truncated form of (P)RR indicates that a processing of (P)RR has occurred. Indeed, we recently demonstrated that (P)RR is cleaved intracellularly by furin to generate a soluble form, which is secreted, and a small truncated form composed of the transmembrane and cytoplasmic domain and corresponding to the fragment of (P)RR coprecipitating with the V-ATPase (5). The facts that the (pro)renin receptor is extremely conserved among species and that the greater homology resides in the portion encompassing the transmembrane and intracellular region of the molecule corresponding to the truncated (P)RR associated with the V-ATPase, suggest for this portion an essential highly conserved function (4, 17). Altogether, these data suggest a close physical proximity of a truncated fragment of (P)RR/ATP6ap2 with the V-ATPase. However, to date, there is no argument for a functional link between truncated (P)RR and the V-ATPase, except the fact that a gene trap in the ATP6ap2 gene ortholog of zebra fish gives a phenotype similar to the knockout of V-ATPase subunits (2). However, our study by immunofluorescence failed to show the presence of (P)RR in every synaptic vesicle, indicating that at least in mouse neurons, (P)RR is not essential for the V-ATPase activity and/or structure. This issue will be only solved with the generation of (P)RR tissue specific knockout mice.
Recently, we described a large family with mental retardation, which linkage analysis localized the X-linked mental retardation (XLMR) and epilepsy syndrome (XMRE, OMIM #300423) to Xp11.3-Xp21.1 between markers DXS1003 and DXS1237. In the course of identifying the candidate gene for XLMR, we completed a gene catalog in the 25 genes and found that the only mutation was a A silent C>T transition (c. 321C>T) in K8355 in the (P)RR/ATP6AP2 gene.
The involvement of the 321C>T transition in the exon-skipping event and in the patient's phenotype was confirmed by comparison of the genomic DNA sequence from the patient and a healthy control and by screening of 600 healthy control males and 300 healthy females. In addition, to exclude the other genes located in the interval as causative for the disease, the 25 genes at either the genomic or the RNA level were sequenced without finding additional mutations in the family. This mutation resulted in the synthesis of Δ4-(P)RR and affects the ectodomain of (P)RR involved in the binding of renin; therefore, one would expect impaired renin binding. In fact, renin binding was normal, as well as (P)RR-bound renin enzymatic activity, but the signaling via ERK1/2 was affected (23). As it was described that (P)RR could form dimers (20, 24) one possibility would be that (P)RR dimerization would be responsible for a the dominant-negative effect. The results of cotransfection experiments of PC-12 cells with both (P)RR and Δ4-(P)RR constructs, indeed, showed that Δ4-(P)RR coimmunoprecipitated with (P)RR and that it resulted in inhibition of normal (P)RR cellular localization. The reason why cotransfection of (P)RR and Δ4-(P)RR also affects of ERK activation response to NGF is still unclear, but these results stress the importance of ERK signaling for neuronal differentiation (28, 30).
In conclusion, our study showed that the truncated Δ4 form of the (pro)renin receptor acts like a negative dominant, impairing the redistribution of full-length receptor in the neurite tips and inhibiting PC-12 cells differentiation into neurons upon stimulation by nerve growth factor. This dominant-negative effect could be responsible for impaired ERK1/2 activation induced by renin and subsequently for altered neuronal differentiation.
Perspectives and Significance
While support for locally produced brain angiotensin peptides is strong, the biochemical pathways responsible for this production remains unclear. It is accepted that renin is produced in the brain, and recent study by Allen et al. (1) has even suggested that renin was more extensively produced than other components of the RAS. The main issue remains whether this form of renin which is likely produced as prorenin (the inactive proenzyme form of renin) in the brain, can display any enzymatic activity locally, because only the cells of the juxtaglomerular apparatus and of the adrenal gland can process prorenin into a mature, catalytically active renin by cleaving the prosegment of prorenin. However, in one circumstance, prorenin can display enzymatic activity without cleavage of the prosegment—when prorenin is bound to (P)RR and is “nonproteolytically” activated (20, 21). Our demonstration of high levels of expression of the (pro)renin receptor in the brain now provides a mechanism for angiotensin peptides generation in the brain by prorenin.
From the study of the mutated (P)RR responsible for mental retardation and epilepsy phenotype, we also suggest that the expression and the activation of the (pro)renin receptor is necessary for appropriate neuronal cell differentiation.
GRANTS
This work was supported, in part, by a grant from National Institute of Child Health and Human Development (HD26202) to C. E. Schwartz and a grant from the South Carolina Department of Disabilities and Special Needs.
Acknowledgments
This article is dedicated to the memory of Ethan Francis Schwartz, 1996-1998.
REFERENCES
- 1.Allen AM, O'Callaghan EM, Hazelwood L, Germain S, Castrop H, Schnermann J, Bassi JK. Distribution of cells expressing human renin-promoter activity in the brain of a transgenic mouse. Brain Res 1243: 78–85, 2008. [DOI] [PubMed] [Google Scholar]
- 2.Amsterdam A, Nissen RM, Sun Z, Swindell EC, Farrington S, Hopkins N. Identification of 315 genes essential for early zebrafish development. Proc Natl Acad Sci USA 101: 12,792–12,797, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burckl CA, Danser AHJ, Müller DN, Garrelds IM, Gasc JM, Popova E, Plehm R, Peters J, Bader M, Nguyen G. Elevated blood pressure and heart rate in human renin receptor transgenic rats. Hypertension 47: 552–556, 2006. [DOI] [PubMed] [Google Scholar]
- 4.Burckl C, Bader M. Prorenin and its ancient receptor. Hypertension 48: 549–551, 2006. [DOI] [PubMed] [Google Scholar]
- 5.Cousin C, Bracquart D, Contrepas A, Corvol P, Muller L, Nguyen G. Soluble form of the (Pro)renin receptor generated by intracellular cleavage by furin is secreted in plasma. Hypertension 53: 1077–1082, 2009. [DOI] [PubMed] [Google Scholar]
- 6.Doobay MF, Talman LS, Obr T, Tian X, Davisson RL, Lazartigues E. Differential expression of neuronal ACE2 in transgenic mice with overexpression of the brain renin-angiotensin system. Am J Physiol Regul Integr Comp Physiol 292: R373–R381, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dzau VJ, Ingelfinger J, Pratt RE, Ellison KE. Identification of renin and angiotensinogen messenger RNA sequences in mouse and rat brains. Hypertension 8: 544–548, 1986. [DOI] [PubMed] [Google Scholar]
- 8.Dzau VJ, Bernstein K, Celermajer D, Cohen J, Dahlöf B, Deanfield J, Diez J, Drexler H, Ferrari R, van Gilst W, Hansson L, Hornig B, Husain A, Johnston C, Lazar H, Lonn E, Lüscher T, Mancini J, Mimran A, Pepine C, Rabelink T, Remme W, Ruilope L, Ruzicka M, Schunkert H, Swedberg K, Unger T, Vaughan D, Weber M. Working Group on Tissue Angiotensin-Converting Enzyme, International Society of Cardiovascular Pharmacotherapy. The relevance of tissue angiotensin-converting enzyme: manifestations in mechanistic and endpoint data. Am J Cardiol 88: 1L–20L, 2001. [DOI] [PubMed] [Google Scholar]
- 9.Feldt S, Maschke U, Dechend R, Luft FC, Müller DN. The putative (pro)renin receptor blocker HRP fails to prevent (pro)renin signaling. J Am Soc Nephrol 19: 743–748, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Feldt S, Batenburg WW, Mazak I, Maschke U, Wellner M, Kvakan H, Dechend R, Fiebeler A, Burckle C, Contrepas A, Danser AHJ, Bader M, Nguyen G, Luft FC, Müller DN. Prorenin and renin-induced extracellular signal-regulated kinase 1/2 activation in monocytes is not blocked by aliskiren or the handle-region peptide. Hypertension 51: 682–688, 2008. [DOI] [PubMed] [Google Scholar]
- 11.Fuxe K, Ganten D, Hökfelt T, Locatelli V, Poulsen K, Stock G, Rix E, Taugner R. Renin-like immunocytochemical activity in the rat and mouse brain. Neurosci Lett 18: 245–250, 1980. [DOI] [PubMed] [Google Scholar]
- 12.Gasc JM, Shanmugam S, Sibony M, Corvol P. Tissue-specific expression of type 1 angiotensin II receptor subtypes. An in situ hybridization study. Hypertension 24: 531–537, 1994. [DOI] [PubMed] [Google Scholar]
- 13.Huang Y, Noble NA, Zhang J, Xu C, Border WA. Renin-stimulated TGF-β1 expression is regulated by a mitogen-activated protein kinase in mesangial cells. Kidney Int 72: 45–52, 2007. [DOI] [PubMed] [Google Scholar]
- 14.Huang Y, Border WA, Noble NA. Functional renin receptors in renal mesangial cells. Curr Hypertens Rep 9: 133–139, 2007. [DOI] [PubMed] [Google Scholar]
- 15.Kaneshiro Y, Ichihara A, Sakoda M, Takemitsu T, Nabi AH, Uddin MN, Nakagawa T, Nishiyama A, Suzuki F, Inagami T, Itoh H. Slowly progressive, angiotensin II-independent glomerulosclerosis in human (pro)renin receptor-transgenic rats. J Am Soc Nephrol 18: 1789–1795, 2007. [DOI] [PubMed] [Google Scholar]
- 16.Lavoie JL, Cassell MD, Gross KW, Sigmund CD. Adjacent expression of renin and angiotensinogen in the rostral ventrolateral medulla using a dual-reporter transgenic model. Hypertension 43: 1116–1119, 2004. [DOI] [PubMed] [Google Scholar]
- 17.L'Huillier N, Sharp MGF, Dunbar DR, Mullins JJ. On the relationship between the renin receptor and the vacuolar proton ATPase membrane sector associated protein (M8–9). In: The Local Cardiac Renin Angiotensin-Aldosterone System, edited by Frolich ED and Re, RN. New York: Springer, 2006, chap. 3, p. 17–34.
- 18.Ludwig J, Kerscher S, Brandt U, Pfeiffer K, Getlawi F, Apps DK, Schägger H. Identification and characterization of a novel 9.2-kDa membrane sector-associated protein of vacuolar proton-ATPase from chromaffin granules. J Biol Chem 273: 10939–10947, 1998. [DOI] [PubMed] [Google Scholar]
- 19.McKinley MJ, Albiston AL, Allen AM, Mathai ML, May CN, McAllen RM, Oldfield BJ, Mendelsohn FA, Chai SY. The brain renin-angiotensin system: location and physiological roles. Int J Biochem Cell Biol 35: 901–918, 2003. [DOI] [PubMed] [Google Scholar]
- 20.Nguyen G, Delarue F, Burcklé C, Bouzhir L, Giller T, Sraer JD. Pivotal role of the renin/prorenin receptor in angiotensin II production and cellular responses to renin. J Clin Invest 109: 1417–1427, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nguyen G, Danser AH. Prorenin and (pro)renin receptor: a review of available data from in vitro studies and experimental models in rodents. Exp Physiol 93: 557–563, 2008. [DOI] [PubMed] [Google Scholar]
- 22.Nishi T, Forgac M. The vacuolar (H+)-ATPases—nature's most versatile proton pumps. Nat Rev Mol Cell Biol 3: 94–103, 2002. [DOI] [PubMed] [Google Scholar]
- 23.Ramser J, Abidi FE, Burckl C, Lenski C, Toriello H, Wen G, Lubs HA, Engert S, Stevenson RE, Meindl A, Schwartz CE, Nguyen G. A unique exonic splice enhancer mutation in a family with X-linked mental retardation and epilepsy points to a novel role of the renin receptor. Hum Mol Genet 14: 1019–1027, 2005. [DOI] [PubMed] [Google Scholar]
- 24.Schefe JH, Menk M, Reinemund J, Effertz K, Hobbs RM, Pandolfi PP, Ruiz P, Unger T, Funke-Kaiser H. A novel signal transduction cascade involving direct physical interaction of the renin/prorenin receptor with the transcription factor promyelocytic zinc finger protein. Circ Res 99: 1355–1366, 2006. [DOI] [PubMed] [Google Scholar]
- 25.Schinke M, Baltatu O, Böhm M, Peters J, Rascher W, Bricca G, Lippoldt A, Ganten D, Bader M. Blood pressure reduction and diabetes insipidus in transgenic rats deficient in brain angiotensinogen. Proc Natl Acad Sci USA 96: 3975–3980, 1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shan Z, Cuadra AE, Sumners C, Raizada MK. Characterization of a functional (pro)renin receptor in rat brain neurons. Exp Physiol 93: 701–708, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Slater EE, Defendini R, Zimmerman EA. Wide distribution of immunoreactive renin in nerve cells of human brain. Proc Natl Acad Sci USA 77: 5458–5460, 1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sweat JD Mitogen-activated protein kinases in synaptic plasticity and memory. Curr Opin Neurobiol 14: 311–317, 2004. [DOI] [PubMed] [Google Scholar]
- 29.Takamori S, Holt M, Stenius K, Lemke EA, Grønborg M, Riedel D, Urlaub H, Schenck S, Brügger B, Ringler P, Müller SA, Rammner B, Gräter F, Hub JS, De Groot BL, Mieskes G, Moriyama Y, Klingauf J, Grubmüller H, Heuser J, Wieland F, Jahn R. Molecular anatomy of a trafficking organelle. Cell 127: 831–846, 2006. [DOI] [PubMed] [Google Scholar]
- 30.Weeber EJ, Sweatt JD. Molecular neurobiology of human cognition. Neuron 14: 845–848, 2002. [DOI] [PubMed] [Google Scholar]