Abstract
Nesfatin-1 is a recently discovered hypothalamic peptide that was shown to suppress food intake through a melanocortin-3/4 receptor-dependent mechanism. Since nesfatin-1 mRNA is detected in the paraventricular nucleus of the hypothalamus, and because many peptides that alter food intake also influence cardiovascular function, we tested the ability of centrally administered nesfatin-1 to affect mean arterial pressure (MAP) in conscious, freely moving rats. Significant increases in MAP were observed following intracerebroventricular administration of nesfatin-1. Pretreatment with either the melanocortin-3/4 receptor antagonist, SHU9119 (intracerebroventricular), or the α-adrenergic antagonist, phentolamine (intra-arterial), abrogated the rise in MAP induced by nesfatin-1, indicating that nesfatin-1 may interact with the central melanocortin system to increase sympathetic nerve activity and lead to an increase in MAP. Thus we have identified a novel action of nesfatin-1, in addition to its anorexigenic effects, to stimulate autonomic nervous system activity.
Keywords: autonomics, hypothalamus, appetite
nesfatin-1 was first described by Oh-I and colleagues (15) as a hypothalamic peptide capable of markedly reducing food intake and body weight when chronically administered into the third cerebroventricle of rats. This effect was shown to occur through a leptin-independent, melanocortin-3/4 receptor (MC3/4R)-dependent mechanism (15). Immunohistochemical and in situ hybridization studies revealed that nesfatin-1 is produced in the hypothalamus, particularly in the paraventricular, arcuate, and supraoptic nuclei and the lateral hypothalamic area (1, 8, 9, 15). Electrophysiological analyses demonstrated that nesfatin-1 hyperpolarizes neuropeptide Y neurons in the arcuate nucleus (16), suggesting that the anorexigenic action of the peptide was due to an inhibition of the activity of neurons producing the orexigen, neuropeptide Y. Additionally, bath-applied nesfatin-1 directly affects the membrane potential of paraventricular neurons, although no correlations could be made between the response of neurons to nesfatin-1 and their electrophysiological identity, molecular phenotype, or baseline membrane potential (17). It has been shown recently that nesfatin-1 immunoreactivity can be detected throughout the hypothalamus in neurons that produce oxytocin, vasopressin, melanin-concentrating hormone, corticotropin-releasing hormone, proopiomelanocortin (POMC), and somatostatin (8), and in both magnocellular and parvocellular neurons of the paraventricular nucleus of the hypothalamus (PVN). These findings suggest that nesfatin-1 may affect other hypothalamically mediated functions, in addition to appetite control. Because peptides that act in hypothalamus to influence feeding often affect cardiovascular function as well [e.g., orexin (7), adrenomedullin (26), α-melanocyte-stimulating hormone (α-MSH) (3)], we sought to determine whether centrally administered nesfatin-1 could induce changes in mean arterial pressure (MAP). We found that, indeed, intracerebroventricular (icv) injection of nesfatin-1, in doses that inhibit feeding, led to an increase in MAP in conscious, unrestrained rats, and that this effect, like that on feeding, appeared to be mediated by a MC3/4R-dependent mechanism.
MATERIALS AND METHODS
Animals.
All procedures have been approved by the Saint Louis University Animal Care and Use Committee. Adult male rats (Harlan Sprague-Dawley; 250–300 g) were individually housed with free access to food and water under controlled conditions (lights on 0600–1800, 23–25°C). Rats were anesthetized with a mixture of ketamine (60 mg/ml; Ketaset, Fort Dodge Animal Health, Fort Dodge, IA) and xylazine (8 mg/ml; TranquiVed, VedCo, Saint Joseph, MO) at 0.1 ml/100 g body wt, as previously described (22). A stainless steel cannula (23 gauge, 17 mm) was implanted into the right lateral cerebroventricle with the aid of a stereotaxic device. Animals were allowed to recuperate for at least 5 days and were observed daily to ensure health and recovery to presurgery body weight. Cannula patency and placement were confirmed by the dipsogenic effect of centrally administered angiotensin II (50 pmol). For cardiovascular studies, a second surgery was performed to implant a carotid cannula under ketamine-xylazine anesthesia, as described previously (25). The carotid cannula was exteriorized between the shoulders and filled with heparinized saline (200 U/ml, 0.9% NaCl). MAP was monitored the following day after animals were habituated to a quiet room for at least 1 h. The carotid cannula was flushed with heparinized saline (200 U/ml, 0.9% NaCl) and connected to a pressure transducer (DigiMed Blood Pressure Analyzer, Micro-Med, Louisville, KY). Baseline MAP and heart rate (HR) recordings were collected at 1-min intervals for a minimum of 30 min. For all experiments, preinjection baseline was calculated as the average MAP or HR for 5 min before icv injection of nesfatin-1 or saline. Animals were administered saline vehicle or vehicle containing 10, 20, 60, 180, or 540 pmol nesfatin-1, and MAP was recorded at 1-min intervals for 1 h. Significant differences in change in MAP or HR from baseline were determined by nonparametric statistics (see below), comparing change at each time point between saline-injected controls and each dosing group. Doses of nesfatin-1 used for all experiments were taken from Oh-I et al. and adjusted so that the feeding response matched the response seen previously (15). This was necessitated by the fact that our cannulas were implanted in the lateral cerebroventricle, whereas those of Oh-I et al. (15) were in the third ventricle. To test whether the effect of nesfatin-1 on MAP could be blocked with the melanocortin-3/4 antagonist, SHU9119, rats were pretreated with 300 pmol SHU9119 (Phoenix Pharmaceuticals, Burlingame, CA), administered icv 15 min before administration of saline, or 180 pmol nesfatin-1, and MAP was recorded for 1 h. To determine the effect of α-adrenergic blockade on nesfatin-1-induced MAP increase, rats were pretreated intra-arterially (ia) with phentolamine-HCl (10 mg/kg body wt, Sigma-Aldrich, St. Louis, MO) 15 min before icv injection of saline or 180 pmol nesfatin-1 when stable and, as expected, reduced, baseline MAP was established. As an internal control to ensure vascular reactivity to pressor hormones during α-adrenergic blockade, additional rats were also pretreated with phentolamine before ia administration of angiotensin II (1.0 μg/kg body wt) (27).
To examine the effect of nesfatin-1 on food intake, rats (250–300 g) were habituated to metabolic cages (Nalgene, Harvard Apparatus, Holliston, MA) for at least 3 days, with free access to lab chow (catalog no. 1811156, Test Diet, Richmond, IN) and tap water. Food and water intakes and body weight were monitored daily. Three protocols were conducted. In the first protocol, food and water bottles were removed from the cages of ad libitum-fed and -watered animals at 1650, and icv injections of saline vehicle or vehicle containing 10, 20, or 60 pmol nesfatin-1 (Phoenix Pharmaceuticals) were conducted. Food and water were replaced 10 min later, and intakes were recorded at 30-min intervals until 2100 and at 1200 and 1700 the following day. Body weights were recorded at 1700 on day 2. In the second protocol, animals were pretreated with the MC3/4R antagonist, SHU9119 (300 pmol in 2-μl saline, Phoenix Pharmaceuticals) icv, before administration of 60 pmol nesfatin-1 or vehicle at 1650. In the third protocol, animals were denied food (water present) for 18 h before icv injection of saline vehicle or vehicle containing 60 or 180 pmol nesfatin-1 at 1000. Food and water were returned 10 min later. Intakes were recorded every 30 min for 5 h.
To determine whether centrally administered nesfatin-1 caused any changes in locomotor behavior, rats were studied using the Opto-Max Activity Monitor (Columbus Instruments, Columbus, OH). Animals were allowed to rest in their home cages in the recording room for a minimum of 1 h. Individual rats were then placed in the recording chamber for a 1-h habituation period, during which time baseline locomotor behaviors were monitored. Rats received either saline vehicle, nesfatin-1 (60 or 180 pmol), SHU9119 (300 pmol), or SHU9119 and 180-pmol nesfatin-1 icv, and behavior was recorded at 1-min intervals for 2 h.
Data were analyzed using either a Mann-Whitney U-test (for cardiovascular) or ANOVA with Scheffé's multiple comparison (for metabolic and behavioral experiments). A nonparametric statistical analysis (Mann-Whitney U) was used to evaluate blood pressure data, since the data were transformed to be expressed as change from preinjection baseline, due to the natural variation of resting blood pressure between rats (28).
RESULTS
In conscious, freely moving rats, two of the doses (60 and 180 pmol) of nesfatin-1 tested caused significant increases in MAP (Fig. 1, A and B). The rise in MAP began within 2 min following icv injection, peaked at ∼5 min, and was sustained for 16 min (180 pmol nesfatin-1) and 17 min (60 pmol nesfatin-1). The lowest doses of nesfatin-1 (10 and 20 pmol) elicited no significant increases in MAP. The highest dose tested (540 pmol) appeared to increase MAP, but this effect failed to attain significance (see Supplemental Fig. 1A in the online version of this article). The greatest peak change from baseline, of ∼9 mmHg (average preinjection baselines: saline = 115.0 ± 2.3 mmHg; 10-pmol nesfatin-1 = 116.3 ± 3.9 mmHg; 20-pmol nesfatin-1 = 122.4 ± 3.5 mmHg; 60-pmol nesfatin-1 = 123.2 ± 3.0 mmHg; 180-pmol nesfatin-1 = 116.2 ± 2.3 mmHg; 540-pmol nesfatin-1 = 128.8 ± 3.3 mmHg), occurred in rats administered 180-pmol nesfatin-1. Analysis of variance indicated potential differences in MAP among the groups at baseline (F5 = 2.83, P = 0.021). However, post hoc testing using Scheffé's multiple comparisons failed to reveal any significant, individual, between-group differences (e.g., saline vs. 10-pmol nesfatin-1, P = 1.0; saline vs. 20-pmol nesfatin-1, P = 0.71; saline vs. 60-pmol nesfatin-1, P = 0.57; saline vs. 180-pmol nesfatin-1, P = 1.0; saline vs. 540-pmol nesfatin-1, P = 0.1). When analyzed as area under the curve (AUC) for the 20-min interval following icv injection, a bell-shaped response curve was observed (Fig. 1B, i.e., the effect resolved at a higher dose with no significant difference noted between the groups treated with 180- and 540-pmol nesfatin-1). Although nesfatin-1 increased MAP, HR was not significantly increased by nesfatin-1 administration (average of HR for 5 min before injection: saline = 415 ± 36 beats/min; 10-pmol nesfatin-1 = 350 ± 8 beats/min; 20-pmol nesfatin-1 = 365 ± 11 beats/min; 60-pmol nesfatin-1 = 387 ± 8 beats/min; 180-pmol nesfatin-1 = 363 ± 21 beats/min; 540-pmol nesfatin-1 = 370 ± 13 beats/min) (Supplementary Fig. 1B). Since many centrally acting peptides influence MAP by increasing or decreasing sympathetic nerve activity, we tested the ability of the mixed α1- and α2-adrenergic antagonist phentolamine to block the nesfatin-1-induced rise in MAP. The ia administration of phentolamine HCl (10 mg/kg), given 15 min before icv injection of nesfatin-1, lowered resting MAP, as expected (average preinjection baselines: saline/saline = 119.4 ± 4.3 mmHg; saline/nesfatin-1 = 126.1 ± 3.2 mmHg; phentolamine/saline = 89.5 ± 2.7 mmHg; phentolamine/nesfatin-1 = 84.8 ± 4.8 mmHg) and abrogated the rise in MAP induced by nesfatin-1 (Fig. 1C). The dose of phentolamine used in these studies, however, did not inhibit the ability of the animals to vasoconstrict, as rats that were pretreated with phentolamine responded to a pressor dose (27) of ia-administered angiotensin II (Supplementary Fig. 2).
Fig. 1.
Nesfatin-1 leads to an increase in mean arterial pressure that is blocked by phentolamine pretreatment. Rats bearing intracerebroventricular (icv) and carotid cannulas were connected to a pressure transducer and injected icv at time 0 with saline vehicle, or vehicle containing 10, 20, 60, 180, or 540 pmol nesfatin-1. A: central administration of 60 or 180 pmol nesfatin-1 led to significant alterations of mean arterial pressure (MAP), which resolved within 20 min of injection. B: significant increases in MAP are apparent when viewed as area under curve (AUC) for the entire 20-min observation period. C: pretreatment with the α-adrenergic antagonist phentolamine blocked the rise in MAP induced by nesfatin-1. Rats were pretreated with 10 mg/kg phentolamine or 0.1 ml/kg saline vehicle intra-arterially (IA) 15 min before icv administration of 180 pmol nesfatin-1 or saline vehicle at time 0. Data were evaluated using a Mann-Whitney U-test. *P < 0.05 and **P < 0.01 vs. saline-injected controls.
Since the action of nesfatin-1 to inhibit food intake is abrogated by pretreatment with SHU9119, we evaluated whether SHU9119 could also block the ability of nesfatin-1 to elevate MAP. In the experiments reported by Oh-I et al. (15), injections were made into the third ventricle. Since our injections were made into the lateral cerebroventricle, we initially had to determine a dose of SHU9119 that was capable of abrogating the anorexigenic action of nesfatin-1 in our animals, given the differing route of administration.
We confirmed the finding of Oh-I et al. (15) that icv administration of nesfatin-1 significantly reduces food intake in ad libitum-fed rats (Fig. 2A). Rats administered the highest dose tested (60 pmol nesfatin-1) consumed less food throughout the first 4 h of testing. This decrease in food intake reached significance at 1930 and continued until 2100. While, compared with vehicle-treated controls, less food was consumed by rats administered 10 or 20 pmol nesfatin-1 at all times, this difference attained significance only at 2100 in the 20 pmol group. Administration of 60 pmol nesfatin-1 also significantly decreased water consumption compared with vehicle-injected controls, and this difference had already attained significance by 1800 (Fig. 2B). The effect of nesfatin-1 on food and water intakes had resolved by noon the following day, so that, although still decreased, no significant differences were observed among nesfatin-1-treated rats or vehicle-injected control animals at noon or 1700 (24-h food intakes: saline = 8.13 ± 0.20 g/100 g body wt, 10 pmol nesfatin-1 = 6.97 ± 0.49 g/100 g body wt, 20 pmol nesfatin-1 = 6.67 ± 0.64 g/100 g body wt, 60 pmol nesfatin-1 = 6.88 ± 0.34 g/100 g body wt; 24-h water intakes: saline = 14.4 ± 0.85 ml/100 g body wt, 10 pmol nesfatin-1 = 11.63 ± 1.21 ml/100 g body wt, 20 pmol nesfatin-1 = 11.2 ± 1.26 ml/100 g body wt, 60 pmol nesfatin-1 = 11.73 ± 1.27 ml/100 g body wt). Our data also confirm that nesfatin-1-induced anorexia can be attenuated by pretreatment with the MC3/4R anatagonist SHU9119 (300 pmol in 2 μl saline, icv) (Fig. 3A). Pretreatment with SHU9119 similarly reversed the inhibitory effect of nesfatin-1 on water drinking (Fig. 3B), an effect not reported by Oh-I and colleagues (15). At 24 h, the anorexigenic and antidipsogenic effects of nesfatin-1 alone had resolved (not significantly different from vehicle-injected controls), although rats treated with SHU9119 and 60 pmol nesfatin-1 had eaten significantly more at 24 h than rats treated with nesfatin-1 alone (24-h food intakes: saline/saline = 8.13 ± 0.2 g/100 g body wt, SHU9119/saline = 8.14 ± 0.57 g/100 g body wt, saline/nesfatin-1 = 6.67 ± 0.64 g/100 g body wt; SHU9119/nesfatin-1 = 8.62 ± 0.38 g/100 g body wt; 24-h water intakes: saline/saline = 14.4 ± 0.85 ml/100 g body wt, SHU9119/saline = 15.83 ± 2.64 ml/100 g body wt, saline/nesfatin-1 = 11.21 ± 1.26 ml/100 g body wt, SHU9119/nesfatin-1 = 14.29 ± 0.56 ml/100 g body wt). In addition, pretreatment with SHU9119 not only reversed the body weight loss induced by nesfatin-1, but also led to increased body weight gain over vehicle-injected control rats (body weight change: saline/saline = 4.47 ± 0.95 g/100 g body wt, SHU9119/saline = 10.34 ± 1.84 g/100 g body wt, saline/nesfatin-1 = 3.11 ± 2.54 g/100 g body wt, SHU9119/nesfatin-1 = 16.53 ± 2.09 g/100 g body wt). In our hands, a higher dose of SHU9119 (300 pmol) was required to reverse the inhibitory effect of nesfatin-1 than that (20 pmol) reported by Oh-I and colleagues (15). In addition to reducing food intake and water drinking in ad libitum-fed rats, nesfatin-1 also decreased food and water intake in overnight-fasted rats (Fig. 4), a finding that has not been previously reported in the literature.
Fig. 2.
Nesfatin-1 reduces food intake. Centrally administered nesfatin-1 led to a dose-related, significant decrease in food intake (A) and water drinking (B) when injected at 1650 into ad libitum-fed and watered rats. Data were analyzed with ANOVA and Scheffé's multiple comparison. *P < 0.05, **P < 0.01, and ***P < 0.001, vs. saline vehicle-injected controls.
Fig. 3.
The anorexigenic and antidipsogenic actions of nesfatin-1 are blocked by pretreatment with SHU9119. Pretreatment with the melanocortin-3/4 receptor antagonist, SHU9119 (300 pmol, icv) abrogated the effect of nesfatin-1 on both food intake (A) and water drinking (B) in ad libitum-fed and watered rats. Data were analyzed with ANOVA and Scheffé's multiple comparison. **P < 0.01; ***P < 0.001.
Fig. 4.
Nesfatin-1 reduces food intake in overnight-fasted rats. Rats were fasted (no food, water present) for 18 h before icv injection of either saline vehicle, or vehicle containing 20, 60, or 180 pmol nesfatin-1. There were no significant differences between control or nesfatin-1-treated animals for the 20 or 60 pmol dose. However, significance was attained using the 180-pmol dose of nesfatin-1 compared with saline vehicle injected controls for both food (A) and water (B) intake. Significance was determined with ANOVA and Scheffé's multiple comparison. **P < 0.01; ***P < 0.001.
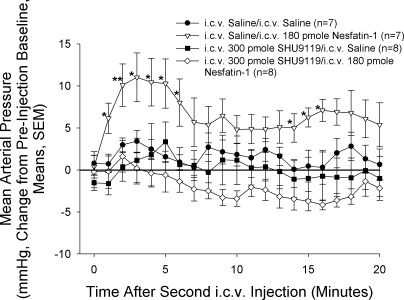
Having established an effective dose of SHU9119, we then pretreated animals 15 min before icv injection of nesfatin-1 (180 pmol) or saline vehicle with 300 pmol SHU9119 or saline icv. SHU9119 completely blocked the rise in MAP induced by nesfatin-1 (Fig. 5), but did not alter basal MAP (average preinjection baselines: saline/saline = 123.6 ± 5.2 mmHg, saline/nesfatin-1 = 115.5 ± 4.4 mmHg, SHU9119/saline = 126.0 ± 3.6 mmHg, SHU9119/nesfatin-1 = 129.6 ± 5.1 mmHg).
Fig. 5.
Pretreatment with SHU9119 blocks nesfatin-1-induced rise in MAP. Rats were pretreated with an icv injection of either 300 pmol SHU9119 or 2-μl saline vehicle 15 min before icv injection, with either saline vehicle or 180 pmol nesfatin-1 at time 0. While nesfatin-1 led to a significant rise in MAP, this effect was blocked by pretreatment with SHU9119. Significance was determined using a Mann-Whitney U-test. *P < 0.05, **P < 0.01 vs. saline-injected controls.
While recording MAP, we noted that icv injection of nesfatin-1 altered the spontaneous activity of the rats. We, therefore, placed rats in an Opto-Max behavior-monitoring device, and, after 1 h of habituation, administered nesfatin-1 icv. We evaluated time spent in the margin, which can be interpreted as an indicator of anxiety. Rats that received either 60 or 180 pmol nesfatin-1 spent significantly (P < 0.05) more time (as determined by ANOVA/Scheffé's multiple comparison) in the margin than rats that received saline vehicle (AUC for 60-min evaluation period: saline = 94 ± 35 s; 60 pmol nesfatin-1 = 266 ± 58 s; 180 pmol nesfatin-1 = 550 ± 182 s). Nesfatin-1-treated animals also traveled farther in the margin, although this effect did not attain statistical significance (AUC for 60-min evaluation period: saline = 572 ± 170 in.; 60 pmol nesfatin-1 = 1,071 ± 172 in.; 180 pmol nesfatin-1 = 997 ± 230 in.). The effect of nesfatin-1 on time spent in the margin was abrogated by pretreatment with SHU9119 (Supplemental Fig. 3).
DISCUSSION
Nesfatin-1 is produced in, and influences the membrane potential of, neurons in the PVN (17), an area of the brain that communicates extensively with preautonomic centers in brain stem and spinal cord. Additionally, nesfatin-1-like immunoreactivity has been detected in neurons producing oxytocin, vasopressin, corticotropin-releasing hormone, POMC, melanin-concentrating hormone, cocaine- and ampthetamine-regulated transcript, and somatostatin (8). These findings indicate that nesfatin-1 may be involved in functions other than feeding behavior, such as cardiovascular regulation, neuroendocrine control of stress hormone secretion, or behavioral arousal. In these studies, we sought to evaluate the effect of centrally administered nesfatin-1 on MAP as an indicator of cardiovascular function. We found that central administration of nesfatin-1 resulted in significant increases in MAP. Although the nesfatin-1-induced elevation in MAP appeared modest, the magnitude of the rise in MAP is similar to that we have observed for other neuropeptides that also can either inhibit (prolactin-releasing peptide; Ref. 23) or stimulate (orexin; Ref. 21) feeding. Central injection of nesfatin-1 did not lead to significant increases in HR (see Supplemental Fig. 1B). This apparent lack of effect could be because of high variability in HR caused by increased locomotor activity. The nesfatin-1-induced increase in MAP could be blocked by pretreatment with SHU9119, a MC3/4R antagonist previously shown to block nesfatin-1-induced anorexia (15). While it has been reported that central administration of SHU9119 leads to an increase in food intake (6, 20), this effect was not observed in our experiments or in the experiments of Oh-I et al. (15). This may be due to differences in dose of peptide employed, as we and Oh-I et al. (15) administered less antagonist than reported previously by others. The initial report of the effect of nesfatin-1 on food intake did not include studies in fasted animals. In our studies, nesfatin-1 also altered food intake in overnight-fasted rats, although a higher dose of peptide was required to observe this effect. This may be due to the fact that the hypoglycemic challenge elicited by fasting is a stronger stimulus than light cycle-entrained feeding behavior.
The effect of nesfatin-1 on MAP also could be blocked by peripheral administration of the nonselective adrenergic antagonist phentolamine. Thus nesfatin-1 may act through MC3/4R-containing neurons to activate preautonomic centers, such as hypothalamic nuclei or hindbrain regions, including the nucleus tractus solitarius (NTS), (where nesfatin-1 and POMC mRNA have been localized), resulting in increased sympathetic nervous system activity and an elevation in MAP. While the central melanocortin system may be best known for its involvement in appetite regulation (3), our findings implicating the melanocortin system in cardiovascular control are not unprecedented. Injection of α-MSH into the lateral cerebroventricle of conscious rabbits led to an increase in MAP and sympathetic nerve activity, an effect that was abrogated by exogenous administration of the endogenously produced MC3/4R antagonist, agouti-related peptide (12). In rats, MSH agonists and NH2-terminal fragments have been reported to acutely increase MAP (18) and alter baseline pressures when injected chronically (10). The central melanocortin system also was shown to augment leptin-induced elevations in MAP and sympathetic nerve activity (5) and may play a role in the elevated blood pressure of spontaneously hypertensive rats (4). A major site of action for these effects may be MC4R-containing nuclei of the caudal brain stem (i.e., rostroventrolateral medulla, medullary raphe, NTS, and parabrachial nucleus), as Skibicka and Grill (24) have recently shown increased energetic and autonomic effects in decerebrate animals injected with the melanocortin agonist melanotan II into the fourth ventricle.
There are several lines of evidence that suggest the central melanocortin system is involved in anxiety-like behaviors, in addition to its regulatory roles in feeding behavior and cardiovascular control. Central administration of melanocortin agonists reduced the number of licks made by rats in the Vogel test (2) and reduced the time spent in the open arms of an elevated plus maze (19). Additionally, treatment with SHU9119 reduced anxiety-related behaviors induced by stress (11). A recent report has shown that central administration of nesfatin-1 decreased time spent in the open arms of an elevated plus maze and increased the startle response in rats (13). These results agree with our data showing that rats treated with nesfatin-1 icv exhibit increased margin time compared with rats that received saline vehicle, indicating that nesfatin-1 may be involved in stress or anxiety pathways in the brain. It appears that this effect of nesfatin-1 is also mediated by the central melanocortin system, since the increase in time spent in the margin can be blocked by pretreatment with SHU9119. It is possible that the elevation of MAP caused by nesfatin-1 is secondary to the apparent effect of the peptide on anxiety. However, this is not likely, as similar doses of nesfatin-1 did not significantly increase HR.
In summary, we have confirmed the anorexigenic and apparent anxiogenic actions of nesfatin-1 and have demonstrated that the peptide is involved in cardiovascular regulation, as measured by changes in MAP. The effect of nesfatin-1 on MAP appears to be mediated through a MC3/4R-dependent mechanism, as has already been shown for its effect on food intake (15). These data, in accordance with recent reports showing colocalization of nesfatin-1-producing cells with POMC neurons (8), suggest that nesfatin-1 may be intimately involved in the central melanocortin system, possibly playing a regulatory role. Since nesfatin-1 has yet to be visualized in axon terminals, it is conceivable that the peptide is a nonconventional neurotransmitter/neuromodulator that acts in an autocrine or paracrine fashion to affect its own or neighboring neurons, as opposed to the more classical axon terminal/synapse mechanism, as has been suggested by Broberger and colleagues (8).
Perspectives and Significance
Probably the most remarkable aspect of nesfatin-1 is its anorexigenic potency. Nesfatin-1 dramatically reduces food intake (15) when injected at picomole doses. How these doses compare to endogenous levels of nesfatin-1 is difficult to ascertain, since neither the stability of nesfatin-1 nor the levels present in the synaptic cleft of brain nuclei that are important in appetite regulation are currently known. It is known, however, that a 24-h fast reduces mRNA and protein levels of nesfatin-1 in the PVN compared with ad libitum-fed controls (15). Importantly, chronic administration of an antisense oligonucleotide directed against nesfatin-1 mRNA led to decreased hypothalamic nesfatin-1 content and exaggerated food intake and body weight gain over controls, indicating that nesfatin-1 may be a physiological regulator of food intake (15). To determine whether nesfatin-1 plays an important role in the physiological regulation of cardiovascular function, future experiments utilizing antisense oligonucleotides could be employed. Although nesfatin-1 has been shown to colocalize with many neuropeptides in the hypothalamus, the fact that the peptide is found in parvocellular neurons (8) and has direct membrane effects in parvocellular neurons in both PVN (16) and the arcuate (17) is of particular interest. Parvocellular neurons in the PVN have been shown to project to areas of the brain stem important in cardiovascular control. Since we injected the peptide into the lateral cerebroventricle, nesfatin-1 could be acting in extra-hypothalamic sites as well, such as the NTS. Foo et al. (8) have shown that nesfatin-1 is produced in the NTS, and the NTS is one of the few sites of extra-hypothalamic POMC expression in the brain. Our data indicate that nesfatin-1 acts through central melanocortin receptors to increase sympathetic activity, establishing POMC neurons in the NTS as good candidates for the site of action of nesfatin-1.
GRANTS
G. L. C. Yosten is supported by a predoctoral fellowship [National Institutes of Health (NIH) Grant 5T32GM008306]. W. K. Samson is supported by NIH Grant HL66023.
Supplementary Material
REFERENCES
- 1.Brailoiu GC, Dun SL, Brailoiu E, Inan S, Yang J, Chang JK, Dun NJ. Nesfatin-1: distribution and interaction with a G protein-coupled receptor in the rat brain. Endocrinology 148: 5088–5094, 2007. [DOI] [PubMed] [Google Scholar]
- 2.Chaki S, Ogawa S, Toda Y, Funakoshi T, Okuyama S. Involvement of the melanocortin MC4 receptor in stress-related behavior in rodents. Eur J Pharmacol 474: 95–101, 2003. [DOI] [PubMed] [Google Scholar]
- 3.Cone R Anatomy and regulation of the central melanocortin system. Nature Neuroscience 8: 571–578, 2005. [DOI] [PubMed] [Google Scholar]
- 4.da Silva AA, do Carmo JM, Kanyicska B, Dubinion J, Brandon E, Hall JE. Endogenous melanocortin system activity contributes to the elevated arterial pressure in spontaneously hypertensive rats. Hypertension 51: 884–890, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dunbar JC, Lu H. Leptin-induced increase in sympathetic nervous and cardiovascular tone is mediated by proopiomelanocortin (POMC) products. Brain Res Bull 50: 215–221, 1999. [DOI] [PubMed] [Google Scholar]
- 6.Fan W, Boston BA, Kesterson RA, Hruby VJ, Cone RD. Role of melanocortinergic neurons in feeding and the agouti obesity syndrome. Nature 385: 165–168, 1997. [DOI] [PubMed] [Google Scholar]
- 7.Ferguson AV, Samson WK. The orexin/hypocretin system: a critical regulator of neuronendocrine and autonomic function. Front Neuroendocrinol 24: 141–150, 2003. [DOI] [PubMed] [Google Scholar]
- 8.Foo K, Brismar H, Broberger C. Distribution and neuropeptide coexistence of nucleobindin-2 mRNA/nesfatin-like immunoreactivity in the rat CNS. Neuroscience 156: 563–579, 2008. [DOI] [PubMed] [Google Scholar]
- 9.Kohno D, Nakata M, Maejima Y, Shimizu H, Sedbazar U, Yoshida N, Dezaki K, Onaka T, Mori M, Yada T. Nesfatin-1 neurons in paraventricular and supraoptic nuclei of the rat hypothalamus coexpress oxytocin and vasopressin and are activated by refeeding. Endocrinology 149: 1295–1301, 2008. [DOI] [PubMed] [Google Scholar]
- 10.Kuo JJ, da Silva AA, Hall JE. Hypothalamic melanocortin receptors and chronic regulation of arterial pressure and renal function. Hypertension 41: 768–774, 2003. [DOI] [PubMed] [Google Scholar]
- 11.Liu J, Garza JC, Truong HV, Henschel J, Zhang W, Lu XY. The melanocortinergic pathway is rapidly recruited by emotional stress and contributes to stress-induced anorexia and anxiety-like behavior. Endocrinology 148: 5531–5540, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Matsumura K, Tsuchihashi T, Abe I, Iida M. Central alpha-melanocyte-stimulating hormone acts at melanocortin-4 receptor to activate sympathetic nervous system in conscious rabbits. Brain Res 948: 145–148, 2002. [DOI] [PubMed] [Google Scholar]
- 13.Merali Z, Cayer C, Kent P, Anisman H. Nesfatin-1 increases anxiety- and fear-related behaviors in the rat. Psychopharmacology 201: 115–123, 2008. [DOI] [PubMed] [Google Scholar]
- 15.Oh-I S, Shimizu H, Satoh T, Okada S, Adachi S, Inoue K, Eguchi H, Yamamoto M, Imaki T, Hashimoto K, Tsuchiya T, Monden T, Horiguchi K, Yamada M, Mori M. Identification of nesfatin-1 as a satiety molecule in the hypothalamus. Nature 443: 709–712, 2006. [DOI] [PubMed] [Google Scholar]
- 16.Price CJ, Hoyda TD, Samson WK, Ferguson AV. Nesfatin-1 influences the excitability of paraventricular nucleus neurones. J Neuroendocrinol 20: 245–250, 2008. [DOI] [PubMed] [Google Scholar]
- 17.Price CJ, Samson WK, Ferguson AV. Nesfatin-1 inhibits NPY neurons in the arcuate nucleus. Brain Res 1230: 99–106, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ramaekers D, Beckers F, Demeulemeester H, Bert C, Denef C, Aubert AE. Effects of melanocortins on cardiovascular regulation in rats. Clin Exp Pharmacol Physiol 29: 549–558, 2002. [DOI] [PubMed] [Google Scholar]
- 19.Rao TL, Kokare DM, Sarkar S, Khisti RT, Chopde CT, Subhedar N. GABAergic agents prevent alpha-melanocyte stimulating hormone induced anxiety and anorexia in rats. Pharmacol Biochem Behav 76: 417–423, 2003. [DOI] [PubMed] [Google Scholar]
- 20.Rossi M, Kim MS, Morgan GA, Small CJ, Edwards CMB, Sunter D, Abusnana S, Goldstone AP, Russell SH, Stanley SA, Smith DM, Yagaloff K, Ghatei MA, Bloom SR. A C-terminal fragment of Agouti-related protein increases feeding and antagonizes the effect of alpha-melanocyte stimulating hormone in vivo. Endocrinology 139: 4428–4431, 1998. [DOI] [PubMed] [Google Scholar]
- 21.Samson WK, Bagley SL, Ferguson AV, White MM. Hypocretin/orexin type 1 receptor in brain: role in cardiovascular control and the neuroendocrine response to immobilization stress. Am J Physiol Regul Integr Comp Physiol 292: R382–R387, 2007. [DOI] [PubMed] [Google Scholar]
- 22.Samson WK, Murphy TC, Resch ZT. Central mechanisms for the hypertensive effects of preproadrenomedullin-derived peptides in conscious rats. Am J Physiol Regul Integr Comp Physiol 274: R1505–R1509, 1998. [DOI] [PubMed] [Google Scholar]
- 23.Samson WK, Resch ZT, Murphy TC. A novel action of the newly described prolactin-releasing peptides: cardiovascular regulation. Brain Res 858: 19–25, 2000. [DOI] [PubMed] [Google Scholar]
- 24.Skibicka KP, Grill HJ. Energetic responses are triggered by caudal brainstem melanocortin receptor stimulation and mediated by local sympathetic effector circuits. Endocrinology 149: 3605–3616, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Taylor MM, Bagley SL, Samson WK. Intermedin/adrenomedullin-2 acts within central nervous system to elevate blood pressure and inhibit food and water intake. Am J Physiol Regul Integr Comp Physiol 288: R919–R927, 2005. [DOI] [PubMed] [Google Scholar]
- 26.Taylor MM, Keown CA, Samson WK. Involvement of the central adrenomedullin peptides in the baroreflex. Regul Pept 112: 87–93, 2003. [DOI] [PubMed] [Google Scholar]
- 27.Yang R, Smolders I, De Bundel D, Fouyn R, Halberg M, Demaegdt H, Vanderheyden P, Dupont AG. Brain and peripheral angiotensin II type 1 receptors mediate renal vasoconstrictor and blood pressure responses to angiotensin IV in the rat. J Hypertens 26: 998–1007, 2008. [DOI] [PubMed] [Google Scholar]
- 28.Zar JH Biostatistical Analysis (2nd Ed.). Englewood Cliffs, NJ: Prentice-Hall, 1984.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.