Abstract
Systemic inflammation is associated with either fever or hypothermia. Fever, a response to mild systemic inflammation, is mediated by cyclooxygenase (COX)-2 and not by COX-1. However, it is still disputed whether COX-2, COX-1, neither, or both mediate(s) responses to severe systemic inflammation, and, in particular, the hypothermic response. We compared the effects of SC-236 (COX-2 inhibitor) and SC-560 (COX-1 inhibitor) on the deep body temperature (Tb) of rats injected with a lower (10 μg/kg iv) or higher (1,000 μg/kg iv) dose of LPS at different ambient temperatures (Tas). At a neutral Ta (30°C), the rats responded to LPS with a polyphasic fever (lower dose) or a brief hypothermia followed by fever (higher dose). SC-236 (2.5 mg/kg iv) blocked the fever induced by either LPS dose, whereas SC-560 (5 mg/kg iv) altered neither the febrile response to the lower LPS dose nor the fever component of the response to the higher dose. However, SC-560 blocked the initial hypothermia caused by the higher LPS dose. At a subneutral Ta (22°C), the rats responded to LPS with early (70–90 min, nadir) dose-dependent hypothermia. The hypothermic response to either dose was enhanced by SC-236 but blocked by SC-560. The hypothermic response to the higher LPS dose was associated with a fall in arterial blood pressure. This hypotensive response was attenuated by either SC-236 or SC-560. At the onset of LPS-induced hypothermia and hypotension, the functional activity of the COX-1 pathway (COX-1-mediated PGE2 synthesis ex vivo) increased in the spleen but not liver, lung, kidney, or brain. The expression of splenic COX-1 was unaffected by LPS. We conclude that COX-1, but not COX-2, mediates LPS hypothermia, and that both COX isoforms are required for LPS hypotension.
Keywords: body temperature, thermoregulation, fever, inflammation
so strongly is systemic inflammation associated with changes in deep body temperature (Tb) that every clinical definition of the systemic inflammatory response syndrome includes a change in Tb (11, 48). Whereas the majority (∼90%) of patients with systemic inflammation have an increased Tb, a number of them (∼10%) have a lowered Tb (6, 17). Thermoregulatory manifestations are also present in animal models of systemic inflammation. In a rat model of systemic inflammation induced by bacterial LPS, the pattern of Tb change depends on the ambient temperature (Ta) and the LPS dose. At a neutral or supraneutral Ta (warm environment), fever is the prevailing response; the fever is monophasic when the dose of LPS is low (just suprathreshold), but it turns polyphasic as the dose increases (66, 68, 69, 71, 79); a mild hypothermia may precede the polyphasic fever when the dose of LPS is high (68). At a subneutral Ta (cool environment), hypothermia followed by fever is the predominant response; the magnitude of the hypothermia increases along with the LPS dose (9, 52, 70). Our studies show that LPS-induced fever and hypothermia are both physiological responses brought about by brain-driven changes in thermoeffector activity (3, 4, 70). Whereas the biological value of fever is thought to be related to its immunostimulant and antibacterial effects (43), the biological value of hypothermia may be related to energy conservation when inflammation is severe enough to compromise tissue perfusion or threaten energy reserves (72, 82).
There is no doubt that cyclooxygenase (COX) plays a critical role in the genesis of fever by catalyzing the conversion of arachidonic acid to prostaglandin (PG) H2, the immediate precursor of febrigenic PGE2 (10, 35, 55, 66). Clinical fevers (5) and all phases of experimental, LPS-induced fever (13, 49, 81, 83, 90) are thought to be mediated by COX-2, the inducible isoform, and not by COX-1, the predominantly constitutive isoform. An involvement of COX in LPS-induced hypothermia has also been suggested (7, 19, 86), and it is believed to be associated with the formation of potentially cryogenic PGD2 from COX-derived PGH2 (86). However, studies of the COX isoforms involved in LPS hypothermia have yielded contradictory results. Dogan et al. (20) and Akarsu and Mamuk (2) reported a suppression of LPS hypothermia by a preferential (valeryl-salicylate) or selective (SC-560) COX-1 inhibitor in rats, thus suggesting that this response is mediated by COX-1. Zhang et al. (90) reported the opposite, hypothermia-enhancing, effect of SC-560 in the rat, thus suggesting that products of COX-1 inhibit LPS hypothermia in the same species. Further complicating the picture, Dogan et al. (20, 21) and Zhang et al. (90) found that a preferential (nimesulide) or selective (SC-236) inhibitor of COX-2 attenuated LPS hypothermia in rats, thus suggesting mediation by COX-2.
The present study was conducted to clarify which COX isoform, if any, mediates hypothermia in systemic inflammation. We compared the effects of SC-560 and SC-236 on the Tb responses of rats to different doses of LPS at different Tas. Because Tb responses to high doses of LPS are associated with hypotension (46, 62, 70), arterial blood pressure was monitored in a subset of experiments. As a follow up to our finding that the COX-1 inhibitor, but not the COX-2 inhibitor, blocked LPS-induced hypothermia, we measured the expression of COX-1 at the mRNA and protein levels and the functional activity of the COX-1 pathway at the onset of the LPS-induced responses.
METHODS
Animals
The study was conducted in male Wistar rats (Harlan, Indianapolis, IN) that weighed 290–380 g at the time of experiments. Initially, the rats were housed three per standard cage; after surgery, they were housed individually. The cages were kept in a rack equipped with a Smart Bio-Pack ventilation system (model SB4100) and Thermo-Pak temperature control system (model TP2000; Allentown Caging Equipment, Allentown, NJ); the temperature of the incoming air was maintained at 28°C. Standard rat chow and tap water were available ad libitum. The room was on a 12:12-h light-dark cycle (lights on at 7:00 AM). The cage space was enriched with artificial “rat holes” (cylindrical confiners made of stainless-steel wire). In addition to spending time in the confiners voluntarily, the rats were systematically habituated to being located in the confiners (7 daily training sessions, 4 h each). The same confiners were used later in the experiments. Rodents are readily adaptable to confinement to an extent that habituated rodents respond to it with neither stress fever (71) nor other signs of stress (1, 32, 56, 78). Each rat was used in an experiment once and euthanized with sodium pentobarbital (100 mg/kg iv) immediately thereafter. All procedures were conducted under protocols approved by the St. Joseph's Hospital and Medical Center's Animal Care and Use Committee.
Surgical Preparation
Four days before an experiment, every rat was implanted with an intravenous catheter; some rats were also implanted with intra-arterial catheters. The procedures were performed under ketamine-xylazine-acepromazine (55.6, 5.5, and 1.1 mg/kg ip, respectively) anesthesia and antibiotic (enrofloxacin, 1.1 mg/kg sc) protection. During surgery, a rat was maintained on a board warmed to 37°C by a Deltaphase isothermal pad (Braintree Scientific, Braintree, MA).
For venous catheterization, a small longitudinal incision was made on the left ventral surface of the neck. The left jugular vein was exposed, freed from its surrounding connective tissue, and ligated. A silicone catheter (ID 0.5 mm, OD 0.9 mm) filled with heparinized (10 U/ml) saline was passed into the superior vena cava through the jugular vein and secured in place with ligatures. The free end of the catheter was knotted, tunneled under the skin to the nape, and exteriorized. The skin was sutured.
For arterial catheterization, the right ventral surface of the neck was incised, and the right carotid artery was isolated and clamped by a microclip. The tip of a PE-50 catheter (ID 0.6 mm, OD 1.0 mm) filled with heparinized saline was placed into the artery, the clip was removed, and the catheter was moved toward the aorta. The catheter was secured in place with ligatures. The free end of the catheter was heat-sealed and exteriorized at the nape. The skin was sutured.
To prevent postsurgical hypothermia, the animals were allowed to recover from anesthesia in an environmental chamber (model 3940; Forma Scientific, Marietta, OH) set to 28.0°C. The intravenous catheters were flushed with heparinized saline every other day; the intra-arterial catheters were flushed daily.
Experimental Setup
On the day of the experiment, each rat was placed in a confiner. For measurement of colonic temperature (an index of Tb), a copper-constantan thermocouple was inserted in the colon, 10 cm beyond the anal sphincter. The thermocouple was fixed to the base of the tail with adhesive tape and plugged into a data logger (Cole-Parmer, Vernon Hills, IL), which conveyed the data to a personal computer. The rat was transferred to an environmental chamber (Forma Scientific) set to either a neutral (30.0°C) or subneutral (22.0°C) Ta (67). The venous catheter was extended with a length of PE-50 tubing filled with saline, and the extension was passed through a wall port and connected to a syringe filled with the drug of interest. This setup permits intravenous drug administration without disturbing a rat and without causing a marked stress response that often presents a major limitation in thermoregulation experiments (69, 73).
If present, the arterial catheter was used for recording arterial pressure. A PE-50 tubing extension of the arterial catheter was passed through a wall port and connected to a differential pressure transducer (Columbus Instruments, Columbus, OH). The analog output of the transducer was converted by the Datamax logger interface (Columbus Instruments) into a digital signal, which was fed into a personal computer. The pulsatile arterial pressure data were collected and processed using the Datamax software (Columbus Instruments). Mean arterial pressure was calculated from a time integral of the pulsatile pressure.
Drug Administration
COX inhibitors.
Selective COX-1 and COX-2 inhibitors (SC-560 and SC-236, respectively) were gifts from Pfizer (Groton, CT). In vitro studies demonstrate that SC-560 is ∼700 times more potent to inhibit COX-1 than COX-2 (76), whereas SC-236 is ∼1,800 times more potent to inhibit COX-2 than COX-1 (65). SC-560 and SC-236 were dissolved in ethanol to a final concentration of 16 and 8 mg/ml, respectively. These solutions were aliquoted and stored at −80°C until the day of the experiment. On the day of the experiment, an aliquot was warmed to room temperature, and infused intravenously at a rate of 31 μl/kg/min for 10 min. Control rats were infused with the vehicle at the same low rate. This infusion protocol produced neither hemolysis (determined based the color of the plasma) nor other signs of ethanol toxicity. The doses of SC-560 and SC-236 delivered over the 10-min infusion were 5 and 2.5 mg/kg, respectively. At these in-vivo doses, SC-560 maximally inhibits COX-1 without affecting COX-2, whereas SC-236 maximally inhibits COX-2 without affecting COX-1 (31, 53, 54).
LPS.
E. coli 0111:B4 LPS was purchased from Sigma-Aldrich (St. Louis, MO). A stock suspension of LPS (5 mg/ml) in pyrogen-free saline was stored at −20°C. On the day of the experiment, the stock was diluted to a final concentration of either 10 or 1,000 μg/ml. The diluted LPS suspension or saline was bolus injected (1 ml/kg) through the extension of the venous catheter 20 min after completion of the 10-min-long infusion of SC-560, SC-236, or their vehicle. The resultant doses of LPS (10 or 1,000 μg/kg iv) have been repeatedly shown to cause a mild polyphasic fever (the lower dose) or a brief hypothermia followed by fever (the higher dose) at a neutral Ta, whereas they cause a dose-dependent hypothermia at a subneutral Ta (68–71, 79).
Functional Activity of the COX-1 Pathway and COX-1 Expression
COX-1 pathway activity was assessed on the basis of the ex vivo production of PGE2 that is blocked by SC-560. We selected the COX-1-mediated synthesis of PGE2 as a measure of the functional activity of the COX-1 pathway because the immediate product of the reaction catalyzed by COX-1, PGH2, is unstable. Among the multiple products synthesized in the next step (by several PGE, D, F, and I synthases and by thromboxane synthases), PGE2 is reasonably stable and the most robustly produced during inflammation in a wide spectrum of organs and tissues throughout the body (35). Furthermore, at least in some situations, the critical, rate-limiting step of inflammation-associated PGE2 synthesis seems to be the one catalyzed by COX and not the one catalyzed by terminal synthases (8). If one accepts that LPS-induced hypothermia is mediated by PGD2 (which may not be the case; see Refs. 27, 44), an alternative approach would be to use the COX-1-mediated PGD2 synthesis as a measure of COX-1 pathway activity. However, PGD2 is much less stable than PGE2, whereas some more stable products of PGD2, such as 15-deoxy-Δ12,14-PGJ2, increase (rather than decrease) deep Tb in rats (A. A. Steiner, A. S. Dragic, J. Pan, A. A. Romanovsky; unpublished observation). Hence, the stability of PGE2 and the robustness of its synthesis under inflammatory conditions provide a solid justification for the use of COX-1-mediated PGE2 synthesis as a measure of COX-1 pathway activity. It should be understood, however, that this measure reflects the enzymatic activity not only of COX-1, but also that of several PGE terminal synthases, and depends both on how COX-1 is coupled with each synthase and on which enzyme in each COX-1-synthase pair catalyzes the critical step.
Tissues for the functional activity assay were harvested from rats 50 min after injection of LPS (1,000 μg/kg) or saline at a Ta of 22.0 °C. This time point corresponds to the maximum rate of fall in Tb during LPS hypothermia. At the time of tissue harvesting, rats were anesthetized intravenously with ketamine-xylazine-acepromazine (5.6, 0.6, and 0.1 mg/kg, respectively). Following transcardiac perfusion with 30 ml of saline (10 ml/min), the entire brain, right kidney, spleen, right lung, and the central lobe of the liver were collected. Each tissue was rinsed with PBS (0.01 M, pH 7.4) and transferred to a polypropylene conical tube. PBS was added to each tube to achieve a PBS:tissue ratio of 5:1 (wt:wt), and the tissue was then homogenized on ice using an ultrasonic cell disruptor. Aliquots of the homogenate were preincubated (25°C, 15 min) with or without SC-560 (0.6 μM). At this concentration, SC-560 inhibits COX-1 activity by >95% (76). Preincubation was followed by incubation (37°C, 10 min) of the homogenate with arachidonic acid (30 μM). Enzymatic reactions were stopped by heating the homogenate to 65°C for 5 min. The homogenate was then centrifuged (13,000 g, 10 min, 4°C), and the supernatant and pellet were stored separately at −80°C. The supernatant was assayed for PGE2 by radioimmunoassay using a rabbit antibody raised against a PGE2-albumin complex (Sigma-Aldrich) and for total protein by the Bradford method (Bio-Rad, Hercules, CA); the assays were conducted according to the manufacturers' instructions. The concentrations of PGE2 and total protein were used to calculate the COX-1 pathway activity (PGE2 concentration/protein concentration/duration of arachidonic acid incubation). The COX-1 pathway activity was calculated by subtracting the ex vivo activity of a sample incubated in the absence of SC-560 from the activity of the same sample incubated in the presence of SC-560.
In view of the fact that LPS increased the functional activity of the COX-1 pathway in spleen but not in other tissues (see results), the expression of COX-1 was measured in spleen samples only. The pellets from the samples processed for functional activity were subjected to Western blot analysis for determination of COX-1 protein content, as follows. The pellet was reconstituted by sonication in PBS containing the Complete EDTA-free Protease Inhibitor Cocktail (Roche Applied Science, Indianapolis, IN). As a positive control for detection of COX-1, rat platelets were obtained from platelet-rich plasma and sonicated like the pellets. The reconstituted pellet (35 μg total protein) or platelet lysate (35 μg total protein) was resolved by SDS-polyacrylamide gel electrophoresis, transferred to a polyvinylidene fluoride membrane, and probed with a rabbit anti-COX-1 polyclonal antibody (1:1,000; Cayman, Ann Arbor, MI) for 12 h at 4°C. Blots were then incubated with a horseradish peroxidase-conjugated secondary antibody (anti-rabbit IgG, 1:20,000; Sigma-Aldrich) for 1 h at room temperature. The blot was developed using the Western Lightning Chemiluminescence Reagent (Perkin Elmer, Boston, MA) and X-ray film (Kodak, Rochester, NY). The Western blots were analyzed by densitometry using Adobe Photoshop CS3 extended edition: after digital (TIF) images of the films were color inverted, the mean gray value of a box drawn around a band of interest was subtracted from the value of a same-size box drawn over the background; the resulting value (arbitrary units) represented the intensity of the band.
For quantification of COX-1 mRNA levels by quantitative real-time RT-PCR, spleen samples were collected (as described above) from a separate set of rats. In addition to samples from LPS- and saline-injected rats, samples from untreated rats were collected and used as reference samples for calculating relative expression values (see below). Total RNA was isolated from tissue samples using TRIzol (Invitrogen, Carlsbad, CA) and treated with Turbo DNA-free (Ambion, Austin, TX), as described in detail elsewhere (34). RNA integrity was determined by a 20100 Bioanalyzer (Agilent, Santa Clara, CA). Total RNA was reverse transcribed to cDNA by random hexamer priming using SuperScript III First-Strand Synthesis System (Invitrogen). For quantitative real-time PCR, a LightCycler (Roche Applied Science, Indianapolis, IN) was used. The concentration of double-stranded DNA amplicon was monitored using Light Cycler FastStart DNA Master Plus SYBR Green I (Roche Applied Science). Primers for COX-1 (gene of interest) were 5′-ACTGGAAACCCAGCACATTC (forward) and 5′-ACTCCTCCCTCCAGAAGAGC (reverse); annealing temperature was 62°C. Primers for β-actin (housekeeping gene) were 5′-CGAGTCCGCGTCCACCCGCGA (forward) and 5′-GACGACGAGCGCAGCGATATC (reverse); annealing temperature was 62°C. The relative expression R of the gene of interest was calculated according to the formula: Ri,t = 2(Nh,t –Nh,c) –(Ni,t –Ni,c), where N is the threshold cycle number, i.e., the number of the amplification cycle in which fluorescence of a given sample becomes significantly different from the baseline signal (36). The indexes i and h refer to the gene of interest and housekeeping gene, respectively; the index t refers to individual samples from rats treated with either LPS or saline; and the index c refers to control samples (namely, samples pooled from untreated rats). This equation is based on the inverse proportionality between N and log2C, where C is the initial template concentration in the PCR sample. The physical meaning of Ri,t is the concentration of mRNA of interest (COX-1) in a sample from a treated (with LPS or saline) animal divided by the concentration of the same message in the simultaneously run untreated controls, in which each concentration is normalized for the concentration of a housekeeping mRNA (β-actin) in the same sample. Gene amplification was verified by running agarose gel electrophoresis of each amplicon obtained during the exponential phase of PCR amplification.
Statistical Analyses
The Tb and blood pressure responses were compared across treatments and time points by a two-way ANOVA. The data on COX-1 pathway activity were compared across treatments and organs by a two-way ANOVA. The COX-1 protein and mRNA levels in the spleen were compared across treatments by Student's t-test. All analyses were performed using Statistica Advanced 8.0 (StatSoft, Tulsa, OK). The data are reported as means ± SE.
RESULTS
LPS-induced hypothermia is blocked by SC-560 but enhanced by SC-236.
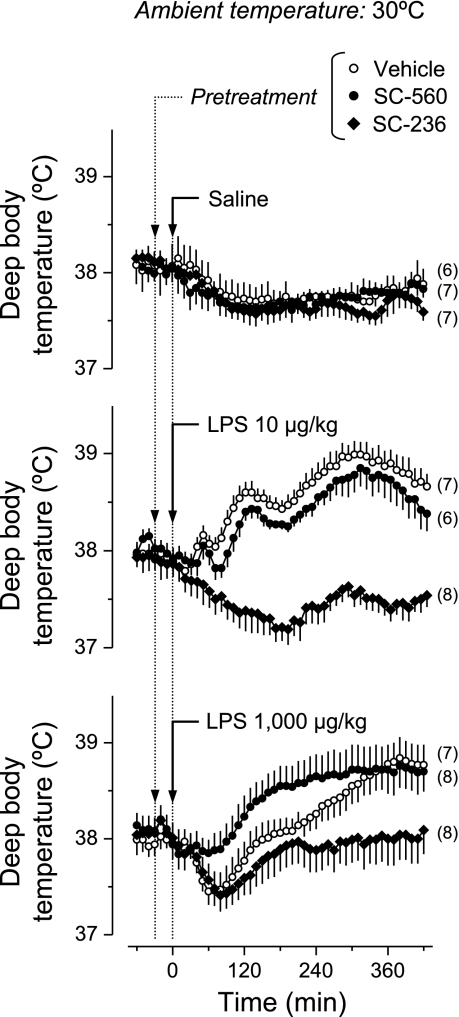
We studied the effects of a COX-1 inhibitor (SC-560), a COX-2 inhibitor (SC-236), or their vehicle on the thermoregulatory responses of rats injected with a lower dose of LPS (10 μg/kg), a higher dose of LPS (1,000 μg/kg), or saline at a neutral (30°C) or subneutral (22°C) Ta. Baseline Tb ranged from 37.5 to 38.5°C; values near the upper end of the range were recorded at the neutral Ta, whereas values near the lower end of the range were recorded at the subneutral Ta. Regardless of Ta, no thermoregulatory response was observed in the saline-treated rats pretreated with SC-560, SC-236, or their vehicle (Figs. 1 and 2). At the neutral Ta (Fig. 1), the vehicle-pretreated rats responded to the lower dose of LPS with a typical polyphasic fever, which consisted of three consecutive Tb rises peaking at ∼50, 120, and 300 min. All phases of the febrile response were abrogated by SC-236 (P < 1.0 × 10−5, 40–420 min), but none was affected by SC-560. At the same Ta, the vehicle-pretreated rats responded to the higher dose of LPS with a small drop in Tb followed by a long-lasting fever. SC-560 blocked the initial hypothermia (P < 2.2 × 10−5, 40–120 min), whereas SC-236 blocked the subsequent febrile response (P < 2.2 × 10−5, 160–420 min).
Fig. 1.
Effects of COX-1 and COX-2 inhibitors on the thermal responses to LPS at a neutral ambient temperature (Ta). Rats kept at a Ta of 30°C were pretreated with a COX-1 inhibitor (SC-560; 5 mg/kg iv), a COX-2 inhibitor (SC-236; 2.5 mg/kg iv), or their vehicle before the intravenous administration of LPS (doses indicated) or saline. Arrowheads indicate the time at which pretreatment (10-min infusion) started; arrows indicate the time of LPS injection. The number of animals in each group (n) is indicated. Compared with the vehicle pretreatment, SC-236 blocked the febrile response to LPS (P < 1.0 × 10−5, 40–420 min for the lower LPS dose; P < 2.2 × 10−5, 160–420 min for the higher LPS dose), whereas SC-560 blocked the initial hypothermic response to the higher LPS dose (P < 2.2 × 10−5, 40–120 min).
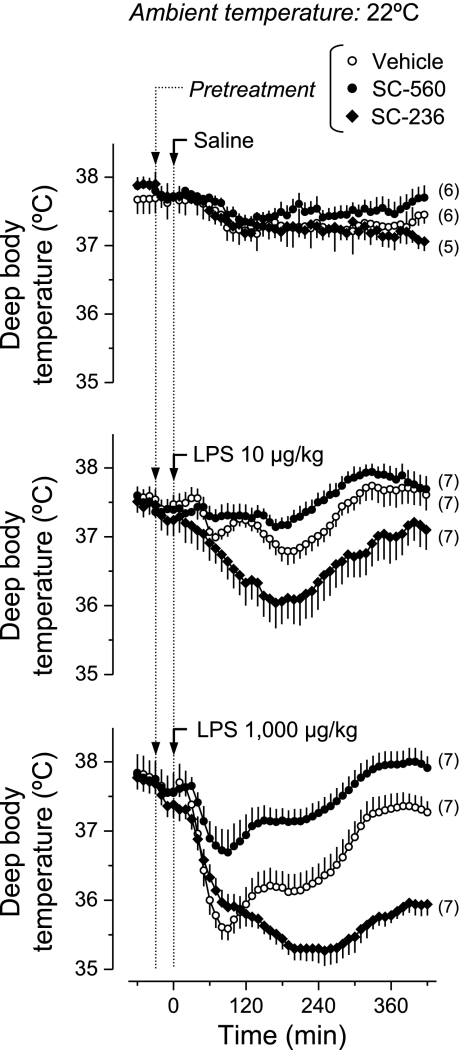
Fig. 2.
Effects of COX-1 and COX-2 inhibitors on the thermal responses to LPS at a subneutral Ta. Rats kept at a Ta of 22°C were pretreated with a COX-1 inhibitor (SC-560, 5 mg/kg iv), a COX-2 inhibitor (SC-236, 2.5 mg/kg iv), or their vehicle before the intravenous administration of LPS (doses indicated) or saline. Compared with the vehicle pretreatment, SC-560 blocked the hypothermic response to LPS (P < 2.2 × 10−5, 50–420 min for either LPS dose), whereas SC-236 enhanced the hypothermic response (P < 2.2 × 10−5, 80–420 min for the lower LPS dose, 130–420 min for the higher LPS dose).
At the subneutral Ta (Fig. 2), hypothermia was the prevailing response to LPS. The hypothermic response was characterized by two subsequent drops in Tb: a prominent early drop (nadir at 70–90 min post-LPS) and a later drop (nadir at ∼200 min) in Tb were consistently observed. The time course of the change in Tb was not dependent on the LPS dose, but the magnitude of the hypothermic response was greater at the higher dose. SC-560 largely attenuated the hypothermic responses to both doses of LPS (P < 2.2 × 10−5, 50–420 min for either dose). On the other hand, SC-236 exaggerated the hypothermic responses to LPS (P < 2.2 × 10−5, 80–420 min for the lower LPS dose, 130–420 min for the higher LPS dose).
LPS-Induced Hypotension Is Blocked by SC-560 or SC-236
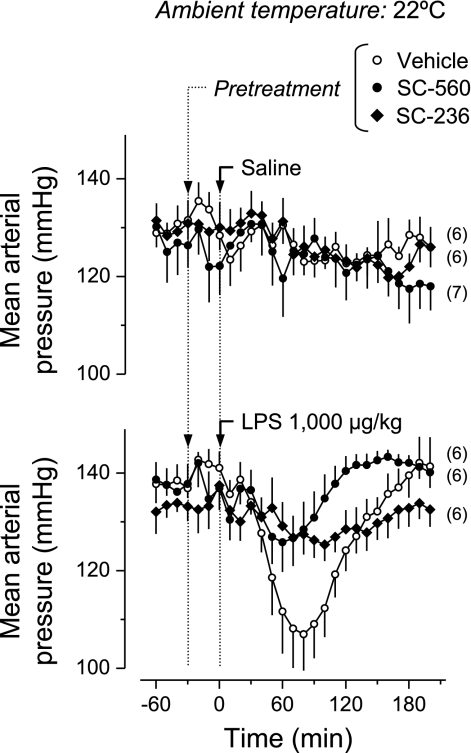
Because the hypothermic response to LPS has been reported to be associated with hypotension (46, 70), we studied the effects of SC-560 and SC-236 on the blood pressure changes caused by the higher dose of LPS. This experiment was performed at the subneutral Ta (22°C), because the effects of SC-560 and SC-236 on LPS hypothermia were most manifest at this Ta (Fig. 2). Baseline mean arterial pressure was ∼130 mmHg, a value similar to those recorded by others in rats exposed to a subneutral Ta (15, 64). No significant change in mean arterial pressure was observed in the saline-treated rats pretreated with SC-560, SC-236, or their vehicle (Fig. 3). Injection of LPS (1,000 μg/kg) to the vehicle-pretreated rats evoked a decrease (∼30 mmHg) in blood pressure. The blood pressure reached a nadir at ∼80 min after LPS injection, corresponding in time to the first phase of the hypothermic response. LPS-induced hypotension was largely attenuated by pretreatment with either SC-560 (P < 2.2 × 10−5, 50–170 min) or SC-236 (P < 1.2 × 10−4, 50–120 min), despite the fact that these drugs had opposite effects on LPS-induced hypothermia.
Fig. 3.
Effects of COX-1 and COX-2 inhibitors on LPS-induced hypotension. Rats kept at a Ta of 22°C were pretreated with a COX-1 inhibitor (SC-560, 5 mg/kg iv), a COX-2 inhibitor (SC-236, 2.5 mg/kg iv), or their vehicle before the intravenous administration of LPS (dose indicated) or saline. LPS-induced hypotension was blocked by pretreatment with either SC-560 (P < 2.2 × 10−5, 50–170 min) or SC-236 (P < 1.2 × 10−4, 50–120 min), compared with the vehicle pretreatment.
The Functional Activity of the COX-1 Pathway (but not COX-1 Expression) Is Increased by LPS
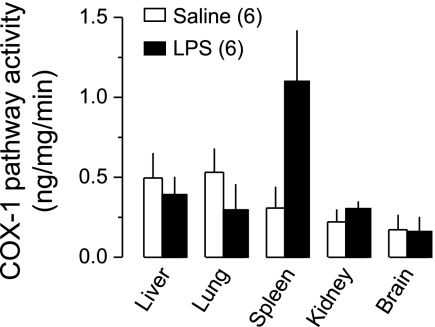
The functional activity of the COX-1 pathway (COX-1-mediated PGE2 synthesis ex vivo) was measured in tissues collected 50 min after administration of LPS (1,000 μg/kg) or saline at Ta of 22°C; this time corresponds to the onset of LPS-induced hypothermia (and also hypotension). In the controls (saline-treated rats), the COX-1 pathway was active in all organs investigated: liver, lung, spleen, kidney, and brain (Fig. 4). Compared with saline, LPS did not significantly change the activity of the COX-1 pathway in the liver, lung, kidney, or brain. However, it produced a 3.6-fold increase in the COX-1 pathway activity in the spleen (P < 7.5 × 10−3).
Fig. 4.
Functional activity of the COX-1 pathway at the onset of LPS-induced hypothermia. The activity was measured in homogenates of tissues collected 50 min after intravenous administration of LPS (1,000 μg/kg) or saline at a subneutral Ta (22°C). The COX-1 pathway activity (ng/mg/min) was determined as a SC-560-sensitive portion of the ex vivo production of PGE2 expressed as PGE2 concentration (ng/ml) divided by the total protein concentration (mg/ml) and the duration of incubation with arachidonic acid (min). Compared with saline, LPS significantly increased the COX-1 pathway activity in the spleen (P < 7.5 × 10−3) but not in the liver, lung, kidney, or brain.
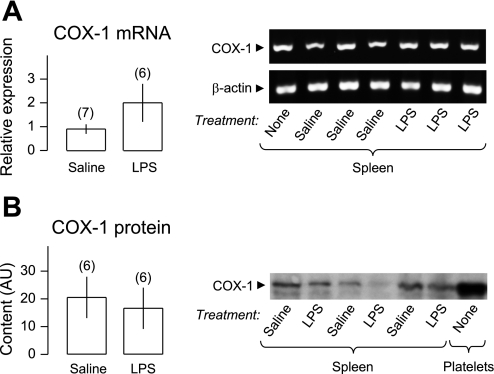
We next evaluated whether the increased activity of the COX-1 pathway in the spleen was associated with changes in the splenic expression of COX-1 measured at the mRNA and protein levels. For both COX-1 and β-actin, a single mRNA product of the expected size (196 bp for COX-1 and 106 bp for β-actin) was amplified (Fig. 5A). No product was amplified in the absence of primers or when water was used instead of RNA (data not shown). The spleen mRNA expression of COX-1 (relative to β-actin) did not differ (P = 0.16) between LPS- and saline-treated rats (Fig. 5A). The Western blot analysis of COX-1 revealed a protein with a molecular mass between 70 and 80 kDa in the Western blot membranes of all spleen samples (Fig. 5B). The same molecular mass protein was markedly expressed in platelets (positive control for COX-1), and no such protein was revealed when the Western blot membranes were incubated without the anti-COX-1 antibody (negative control; data not shown). The intensity of the COX-1 bands did not differ (P = 0.69) between samples obtained from the spleens of LPS- and saline-treated rats (Fig. 5B). Taken together, the data indicate that the expression of COX-1 in the spleen did not change at the onset of LPS hypothermia (and hypotension).
Fig. 5.
Expression of COX-1 at the onset of LPS-induced hypothermia. COX-1 mRNA (A) and protein (B) were measured in spleen samples collected 50 min after intravenous administration of LPS (1,000 μg/kg) or saline at a subneutral Ta (22°C). A: mean relative expressions determined by quantitative real-time RT-PCR are shown on the left, whereas agarose-gel bands of amplicons obtained at the exponential phase of PCR amplification are shown on the right. B: mean contents (arbitrary units, AU) of COX-1 protein determined by densitometry of Western blot bands are shown on the left, whereas representative images of Western blot bands for spleen samples and platelet lysate (positive control) are shown on the right. Although β-actin expression was not examined in the Western blot analysis, it should be noted that an identical amount of total protein (35 μg) was loaded into each gel lane. Compared with saline, LPS did not increase the level of COX-1 mRNA or protein.
DISCUSSION
Effects of COX-1 and COX-2 Inhibitors on LPS-Induced Responses
The present study was carried out to clarify which isoform of COX mediates hypothermia in systemic inflammation. First, we evaluated the effects of SC-560 (COX-1 inhibitor) and SC-236 (COX-2 inhibitor) on the thermoregulatory responses to LPS. SC-236 blocked all phases of LPS-induced fever in experiments conducted at a neutral Ta, whereas it enhanced LPS-induced hypothermia at a subneutral Ta. These findings indicate that COX-2 not only mediates fever but also limits the hypothermic response to LPS. It is possible that COX-2-mediated production of the same febrigenic mediator, possibly PGE2 (57), underlies the involvement of this enzyme in both the development of fever and the limitation of hypothermia. Indeed, the complexity of the thermoregulatory response to LPS is thought to result from a balance between a febrile component (driven by febrigenic mediators) and a hypothermic component (driven by cryogenic mediators) (42, 82, 84).
The COX-1 inhibitor, SC-560, consistently blocked LPS hypothermia (but not fever) under all experimental conditions tested, thus indicating that COX-1 is required for the development of LPS hypothermia. The COX-1 product involved in LPS hypothermia has yet to be identified, but PGD2 is a potential candidate, as it induces hypothermia when injected in rats either intracerebroventricularly (86) or intravenously: as an albumin complex (A. A. Steiner, A. S. Dragic, J. Pan, A. A. Romanovsky, unpublished observation) or as a hydroalcoholic solution (A. Garami, E. Pakai, A. A. Romanovsky; unpublished observation). It should be noted, however, that Krueger et al. (44) have reported that high doses of PGD2 cause fever (rather than hypothermia) in rabbits; the same authors have suggested that effects of PGD2 on thermoregulation (and sleep) may be species specific. While this study was in preparation, Gao et al. (27) reported that intracisternal PGD2 did not cause hypothermia in rats and, in fact, caused a delayed fever, possibly by interfering with the transport of the febrigenic PGE2 in the brain. Clearly, the search for the COX-1-derived mediator of hypothermia should continue.
In contradiction with the present findings are studies by Dogan et al. (20) and Zhang et al. (90), which reported that SC-236 blocked LPS hypothermia. In the study by Dogan et al. (20), such a blockade occurred only when the dose of SC-236 was substantially higher (40 mg/kg) than the doses (1–15 mg/kg) known to selectively block COX-2 in vivo (31, 53). At such a high dose, SC-236 might have inhibited COX-1, or it might have exerted COX-unrelated effects (39). Although the study by Zhang et al. (90) employed a lower dose of SC-236 (5 mg/kg), it was limited by marked stress hyperthermia following intraperitoneal drug administration, which overlapped substantially with the early hypothermic response to LPS, thus making the results difficult to interpret. Zhang et al. (90) reported another observation that seemingly contradicts the present results, i.e., that SC-560 might have prolonged LPS hypothermia. It should be considered, however, that Zhang et al. (90) used a low dose of LPS, which resulted in only minimal decreases in Tb (∼0.5°C) in both SC-560-treated and SC-560-untrerated rats. In the present study, the usage of higher doses of LPS in a tightly controlled thermal environment allowed us to produce a much stronger hypothermic response (∼2°C) and, therefore, to study effects on the hypothermic response at a higher “resolution.”
Although we found opposite effects of SC-560 and SC-236 on LPS-induced hypothermia, both drugs attenuated LPS-induced hypotension. The fact that SC-236 enhanced hypothermia while blocking hypotension is of interest, as it is currently the strongest piece of evidence suggesting that hypothermia is not a consequence of hypotension-associated hypoperfusion. The present results are also the first demonstration that both COX isoforms are required for the hypotensive response to LPS to develop. With a single exception (33), all previous studies found that COX-2 is not required for the development of LPS hypotension (47, 87), and one study (87) found that COX-1 is also uninvolved in this response. However, it should be considered that all previous studies were performed in anesthetized rats receiving fluid resuscitation. Under these conditions, rats respond to high doses of LPS with a slowly developing, progressive fall in blood pressure (47, 87). The present study was conducted in unanesthetized rats not receiving fluid resuscitation, conditions under which rats respond to high doses of LPS with a rapid drop in blood pressure that corresponds in time to the first phase of the hypothermic response (70).
Functional Activity of the COX-1 Pathway During LPS-Induced Responses
The involvement of COX-2 in the LPS-induced responses is consistent with the expression of this isozyme being upregulated as early as 30 min after intravenous administration of LPS (34, 80). Expressional upregulation is widely accepted as a principal mechanism for activation of COX-2 in inflammation (35, 58). On the other hand, the molecular mechanisms underlying the involvement of COX-1 in LPS-induced responses are unknown. We now report that the functional activity of the COX-1 pathway (COX-1-mediated PGE2 synthesis ex vivo) is increased in the spleen at the onset of LPS-induced hypothermia and hypotension. This increase in the activity of the COX-1 pathway occurred without upregulation of COX-1 mRNA or protein. Although the observed activation of the COX-1 pathway in the spleen does not imply that it is the splenic COX-1 that mediates LPS hypothermia (and hypotension), the association between COX-1 pathway activation in the spleen and LPS hypothermia should not be dismissed. Furthermore, our recent data (E. Pakai, A. Garami, T. B. Nucci, A. A. Romanovsky, unpublished data) show that LPS hypothermia is attenuated in splenectomized rats, thus suggesting a role for factors synthesized in the spleen.
A possible mechanism for COX-1 activation involves peroxide species, such as hydroperoxide and peroxynitrate. These species serve as activators for both COX isozymes by promoting the formation of a tyrosyl radical near the enzyme's active sites (51). Hence, it is possible that the increased production of hydroperoxide and peroxynitrate during inflammation (26, 63) promotes activation of COX. The fact that higher peroxide levels are required to activate COX-1 than COX-2 (14, 45) is consistent with the observation that the hypothermic response (dependent on COX-1) occurs in the most severe forms of systemic inflammation, e.g., in the response to high doses of LPS (9, 52, 70). It should also be noted that mechanisms upstream or downstream from COX-1 may affect the activity of the COX-1 pathway, since these mechanisms determine substrate availability to COX-1 and the fate of the COX-1 product, respectively (see Perspectives and Significance).
A splicing variant of COX-1 has been identified in canine tissues (16). Originally named COX-3, this variant is now most commonly referred to as COX-1b. Might COX-1b have contributed to the increased COX-1 pathway activity found in the present study? At present, the answer to this question appears to be negative. Although a COX-1b transcript (which retains intron-1) has been found in rat tissues (40, 41), a functional COX-1b protein has not yet been identified in this species (77). The rat intron-1 (unlike the canine intron-1) contains an incomplete codon sequence consisting of 32 codons plus 2 extra nucleotides; the extra nucleotides are available to form a codon with the nucleotide of the exon that follows, thus shifting the reading frame of the exonic portion of the transcript (77).
Study Limitations and Design Considerations
In the present study, baseline colonic temperature in confined rats ranged from 37.5 to 38.5°C. These values are higher (by ∼0.5°C) than baseline values of abdominal temperature recorded telemetrically in freely moving rats (see, for example, Refs. 2 and 90). However, this expected difference likely stems from two factors (for detailed analysis, see Ref. 71). First, colonic temperature is one of the highest temperatures in the rat's body. It is 0.1–0.8°C higher than aortic temperature (22), and it is very likely higher than abdominal temperature, especially if the latter is measured near the abdominal wall (71). Second, the perception that “normal” values of abdominal temperature range from 37.0 to 37.5°C originated from experiments performed at room temperature (see, e.g., Refs. 2, 90), which are usually subneutral for rats (67). At a subneutral Ta, basal Tb of rats in our experiment rarely exceeded 38.0°C. Only when rats were exposed to a neutral Ta (30°C) did their basal Ta reach 38.5°C, an observation that agrees with the known influence of Ta on basal Tb (28, 67, 71, 73, 89). Nevertheless, the presence of a low-grade confinement stress in our experiments cannot be ruled out completely, especially because the rats had a somewhat elevated basal arterial pressure (∼130 mmHg). One can argue, however, that the increased blood pressure, which was observed at a subneutral Ta only, was due to mild cold exposure (15, 64), rather than confinement, to which the animals were well adapted.
In spite of the fact that we carefully chose the doses of SC-560 and SC-236 so that each drug was administered at the minimal dose that has been demonstrated to maximally inhibit one COX isoform without affecting the other in vivo (31, 53, 54), the effectiveness of our approach is subject to limitations inherent to any pharmacological approach, including the possibility that a drug may produce COX-unrelated (off-target) effects (12). The limitations of pharmacological approaches can be offset by the use of complementary approaches such as genetically modified mice. Indeed, mice genetically deficient of COX-1 or COX-2 have been instrumental in studies that established the role of COX-2 in the mediation of fever (49, 83). However, the early hypothermia, which was observed in the present study and that has been reported to occur in response to LPS in rats (70, 79), rabbits (88), and chicken (18), does not occur in mice (73). The early (starts within minutes) hypothermic response of rats occurring at LPS doses of 10 μg/kg and higher clearly differs from the late (starts at 4 h or later) and prolonged (lasts 12 h or more) hypothermic response that develops in mice injected with extremely high (10,000 μg/kg and higher) doses of LPS (38, 73, 75). Hence, mice cannot be used to study the early LPS hypothermia that is common for other species. Another limitation of our study is that it is unknown to what extent the data obtained can be generalized beyond the experimental conditions used: an LPS model of systemic inflammation in the rat.
Conclusion
In conclusion, by conducting a differential analysis of the effects of two highly selective COX inhibitors in several models of LPS fever and hypothermia, the present study indicates that COX-1, and not COX-2, is the isoform that mediates the hypothermic response to LPS, at least in the rat. By investigating the functional activity of the COX-1 pathway (COX-1-mediated PGE2 synthesis ex vivo) and COX-1 expression at the mRNA and protein levels, this study has also found that COX-1 pathway activity increases in the spleen at the onset of LPS hypothermia via mechanisms that do not involve transcriptional upregulation of COX-1. By evaluating the effects of COX inhibitors on blood pressure, this study has further shown that both COX isoforms are required for the hypotensive response to LPS to develop.
Perspectives and Significance
In the absence of transcriptional upregulation of COX-1 in the spleen, an increased splenic COX-1 pathway activity during LPS hypothermia may involve not only posttranslational activation of COX-1 (discussed above), but also the activation of mechanisms upstream or downstream from COX-1. The COX substrate, arachidonic acid, derives from the breakdown of membrane phospholipids by enzymes of the phospholipase (PL) A2 superfamily (35, 58). It is generally accepted that certain PLA2 enzymes may couple more favorably with one COX isoform than with the other. For example, cytosolic PLA2-α colocalizes with COX-1, but not with COX-2, in the Golgi apparatus of activated epithelial cells in vitro (29). Therefore, the activation [possibly by phosphorylation (50)] of cytosolic PLA2-α may direct arachidonic acid to COX-1 in preference to COX-2. The phosphorylated form of cytosolic PLA2 has been detected in the lungs as early as 40 min after intravenous administration of LPS (80).
The mechanisms downstream from COX determine which bioactive prostanoids are formed (35, 85). Recent studies (23, 34, 74) have shown that microsomal PGE synthase-1 (mPGES-1) is the enzyme responsible for the conversion of PGH2 into febrigenic PGE2. This enzyme is functionally coupled with COX-2 in marked preference to COX-1, presumably because both COX-2 and mPGES-1 are primarily compartmentalized in the perinuclear envelope (60). Massive (more than 1,000 fold) transcriptional upregulation of mPGES-1 occurs in the course of LPS-induced fever (23, 34). The terminal synthases involved in the hypothermia of systemic inflammation remain to be identified, but given the ability of PGD2 to cause hypothermia, at least according to some reports (86), it is reasonable to suspect that a PGD synthase (PGDS) may be involved. There are two known PGDS isoforms: lipocalin PGDS (L-PGDS) and hematopoietic PGDS (H-PGDS) (58). Whereas the LPS-induced production of PGD2 in neural tissue appears to be dependent on COX-2 and L-PGDS (30), the early production of PGD2 by activated macrophages and mast cells seems to depend on COX-1 and H-PGDS (59, 61). Interestingly, large amounts of H-PGDS are present in the rat spleen (37), the organ in which the COX-1 pathway was activated at the onset of LPS hypothermia. Furthermore, Feleder et al. (24, 25) have recently shown that splenectomy or splenic vein ligation enhances LPS fever, and they have proposed that LPS causes the synthesis of a cryogenic lipid in the spleen. Our recent experiments (E. Pakai, A. Garami, T. B. Nucci, A. A. Romanovsky, unpublished data) further show that splenectomy attenuates LPS hypothermia. Whether the activation of a COX-1 → H-PGDS pathway in the spleen and the consequent production of PGD2 or some other COX-1-mediated mechanism underlies LPS-induced hypothermia is a subject of future studies.
GRANTS
This study has been supported by grant R01NS41233 from the National Institute of Neurological Disorders and Stroke to A. A. Romanovsky.
Acknowledgments
The authors thank Jason Curtis for his help with radioimmunoassays and Jacob Orme for the preparation of Western blot images. The SC-236 and SC-560 compounds were generously provided by Pharmacia & Upjohn, a division of Pfizer. The reported data on COX-1 expression are part of the work by T. B. Nucci toward obtaining a doctorate degree through the Arizona State University Graduate Program in Neuroscience.
REFERENCES
- 1.Abercrombie ED, Jacobs BL. Single-unit response of noradrenergic neurons in the locus coeruleus of freely moving cats. II. Adaptation to chronically presented stressful stimuli. J Neurosci 7: 2844–2848, 1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akarsu ES, Mamuk S. Escherichia coli lipopolysaccharides produce serotype-specific hypothermic response in biotelemetered rats. Am J Physiol Regul Integr Comp Physiol 292: R1846–R1850, 2007. [DOI] [PubMed] [Google Scholar]
- 3.Almeida MC, Steiner AA, Branco LG, Romanovsky AA. Cold-seeking behavior as a thermoregulatory strategy in systemic inflammation. Eur J Neurosci 23: 3359–3367, 2006. [DOI] [PubMed] [Google Scholar]
- 4.Almeida MC, Steiner AA, Branco LG, Romanovsky AA. Neural substrate of cold-seeking behavior in endotoxin shock. PLoS One 1: e1, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aronoff DM, Neilson EG. Antipyretics: mechanisms of action and clinical use in fever suppression. Am J Med 111: 304–315, 2001. [DOI] [PubMed] [Google Scholar]
- 6.Arons MM, Wheeler AP, Bernard GR, Christman BW, Russell JA, Schein R, Summer WR, Steinberg KP, Fulkerson W, Wright P, Dupont WD, Swindell BB. Effects of ibuprofen on the physiology and survival of hypothermic sepsis. Ibuprofen in Sepsis Study Group. Crit Care Med 27: 699–707, 1999. [DOI] [PubMed] [Google Scholar]
- 7.Azab AN, Kaplanski J. Involvement of eicosanoids in the hypothermic response to lipopolysaccharide during endotoxemia in rats. Prostaglandins Leukot Essent Fatty Acids 70: 67–75, 2004. [DOI] [PubMed] [Google Scholar]
- 8.Bjork DS, Engstrom L, Blomqvist A, Engblom D. COX-2 rather than mPGES-1 is rate-limiting for fever. In: Proceedings of the Society for Neuroscience Meeting, Washington DC: Society for Neuroscience, 2008, Program # 83.1.
- 9.Blanque R, Meakin C, Millet S, Gardner CR. Hypothermia as an indicator of the acute effects of lipopolysaccharides: comparison with serum levels of IL1β, IL6, and TNFα. Gen Pharmacol 27: 973–977, 1996. [DOI] [PubMed] [Google Scholar]
- 10.Blatteis CM Endotoxic fever: new concepts of its regulation suggest new approaches to its management. Pharmacol Ther 111: 194–223, 2006. [DOI] [PubMed] [Google Scholar]
- 11.Bone RC, Balk RA, Cerra FB, Dellinger RP, Fein AM, Knaus WA, Schein RM, Sibbald WJ. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee American College of Chest Physicians/Society of Critical Care Medicine. Chest 101: 1644–1655, 1992. [DOI] [PubMed] [Google Scholar]
- 12.Brenneis C, Maier TJ, Schmidt R, Hofacker A, Zulauf L, Jakobsson PJ, Scholich K, Geisslinger G. Inhibition of prostaglandin E2 synthesis by SC-560 is independent of cyclooxygenase 1 inhibition. FASEB J 20: 1352–1360, 2006. [DOI] [PubMed] [Google Scholar]
- 13.Cao C, Matsumura K, Yamagata K, Watanabe Y. Involvement of cyclooxygenase-2 in LPS-induced fever and regulation of its mRNA by LPS in the rat brain. Am J Physiol Regul Integr Comp Physiol 272: R1712–R1725, 1997. [DOI] [PubMed] [Google Scholar]
- 14.Capdevila JH, Morrow JD, Belosludtsev YY, Beauchamp DR, DuBois RN, Falck JR. The catalytic outcomes of the constitutive and the mitogen inducible isoforms of prostaglandin H2 synthase are markedly affected by glutathione and glutathione peroxidase(s). Biochemistry 34: 3325–3337, 1995. [DOI] [PubMed] [Google Scholar]
- 15.Carnio EC, Branco LG. Participation of the nitric oxide pathway in cold-induced hypertension. Life Sci 60: 1875–1880, 1997. [DOI] [PubMed] [Google Scholar]
- 16.Chandrasekharan NV, Dai H, Roos KL, Evanson NK, Tomsik J, Elton TS, Simmons DL. COX-3, a cyclooxygenase-1 variant inhibited by acetaminophen and other analgesic/antipyretic drugs: cloning, structure, and expression. Proc Natl Acad Sci USA 99: 13926–13931, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Clemmer TP, Fisher CJ Jr, Bone RC, Slotman GJ, Metz CA, and Thomas FO. Hypothermia in the sepsis syndrome and clinical outcome. The Methylprednisolone Severe Sepsis Study Group. Crit Care Med 20: 1395–1401, 1992. [DOI] [PubMed] [Google Scholar]
- 18.De Boever S, Beyaert R, Vandemaele F, Baert K, Duchateau L, Goddeeris B, De Backer P, Croubels S. The influence of age and repeated lipopolysaccharide administration on body temperature and the concentration of interleukin-6 and IgM antibodies against lipopolysaccharide in broiler chickens. Avian Pathol 37: 39–44, 2008. [DOI] [PubMed] [Google Scholar]
- 19.Derijk RH, Berkenbosch F. Hypothermia to endotoxin involves the cytokine tumor necrosis factor and the neuropeptide vasopressin in rats. Am J Physiol Regul Integr Comp Physiol 266: R9–R14, 1994. [DOI] [PubMed] [Google Scholar]
- 20.Dogan MD, Ataoglu H, Akarsu ES. Effects of selective cyclooxygenase enzyme inhibitors on lipopolysaccharide-induced dual thermoregulatory changes in rats. Brain Res Bull 57: 179–185, 2002. [DOI] [PubMed] [Google Scholar]
- 21.Dogan MD, Ataoglu H, Akarsu ES. Nimesulide and diclofenac inhibit lipopolysaccharide-induced hypothermia and tumour necrosis factor-α elevation in rats. Fundam Clin Pharmacol 16: 303–309, 2002. [DOI] [PubMed] [Google Scholar]
- 22.Donhoffer S Homeothermia of the Brain (Cerebral Blood Flow, Metabolic Rate, and Brain Temperature in the Cold: the Possible Role of Neuroglia). Budapest, Hungary: Akadémiai Kiadó, 1980.
- 23.Engblom D, Saha S, Engstrom L, Westman M, Audoly LP, Jakobsson PJ, Blomqvist A. Microsomal prostaglandin E synthase-1 is the central switch during immune-induced pyresis. Nat Neurosci 6: 1137–1138, 2003. [DOI] [PubMed] [Google Scholar]
- 24.Feleder C, Li Z, Perlik V, Evans A, Blatteis CM. The spleen modulates the febrile response of guinea pigs to LPS. Am J Physiol Regul Integr Comp Physiol 284: R1466–R1476, 2003. [DOI] [PubMed] [Google Scholar]
- 25.Feleder C, Perlik V, Tang Y, Blatteis CM. Putative antihyperpyretic factor induced by LPS in spleen of guinea pigs. Am J Physiol Regul Integr Comp Physiol 289: R680–R687, 2005. [DOI] [PubMed] [Google Scholar]
- 26.Forman HJ, Torres M. Redox signaling in macrophages. Mol Aspects Med 22: 189–216, 2001. [DOI] [PubMed] [Google Scholar]
- 27.Gao W, Schmidtko A, Lu R, Brenneis C, Angioni C, Schmidt R, Geisslinger G. Prostaglandin D2 sustains the pyrogenic effect of prostaglandin E2. Eur J Pharmacol 608: 28–31, 2009. [DOI] [PubMed] [Google Scholar]
- 28.Gordon CJ, Yang Y. Contribution of spontaneous motor activity to the 24-hour control of body temperature in male and female rats. J Therm Biol 22: 59–68, 1997. [Google Scholar]
- 29.Grewal S, Ponnambalam S, Walker JH. Association of cPLA2-α and COX-1 with the Golgi apparatus of A549 human lung epithelial cells. J Cell Sci 116: 2303–2310, 2003. [DOI] [PubMed] [Google Scholar]
- 30.Grill M, Peskar BA, Schuligoi R, Amann R. Systemic inflammation induces COX-2 mediated prostaglandin D2 biosynthesis in mice spinal cord. Neuropharmacology 50: 165–173, 2006. [DOI] [PubMed] [Google Scholar]
- 31.Gross GJ, Moore J. Effect of COX-1/COX-2 inhibition versus selective COX-2 inhibition on coronary vasodilator responses to arachidonic acid and acetylcholine. Pharmacology 71: 135–142, 2004. [DOI] [PubMed] [Google Scholar]
- 32.Hashimoto K, Suemaru S, Takao T, Sugawara M, Makino S, Ota Z. Corticotropin-releasing hormone and pituitary-adrenocortical responses in chronically stressed rats. Regul Pept 23: 117–126, 1988. [DOI] [PubMed] [Google Scholar]
- 33.Hocherl K, Dreher F, Kurtz A, Bucher M. Cyclooxygenase-2 inhibition attenuates lipopolysaccharide-induced cardiovascular failure. Hypertension 40: 947–953, 2002. [DOI] [PubMed] [Google Scholar]
- 34.Ivanov AI, Pero RS, Scheck AC, Romanovsky AA. Prostaglandin E2-synthesizing enzymes in fever: differential transcriptional regulation. Am J Physiol Regul Integr Comp Physiol 283: R1104–R1117, 2002. [DOI] [PubMed] [Google Scholar]
- 35.Ivanov AI, Romanovsky AA. Prostaglandin E2 as a mediator of fever: synthesis and catabolism. Front Biosci 9: 1977–1993, 2004. [DOI] [PubMed] [Google Scholar]
- 36.Ivanov AI, Scheck AC, Romanovsky AA. Expression of genes controlling transport and catabolism of prostaglandin E2 in lipopolysaccharide fever. Am J Physiol Regul Integr Comp Physiol 284: R698–R706, 2003. [DOI] [PubMed] [Google Scholar]
- 37.Jowsey IR, Thomson AM, Flanagan JU, Murdock PR, Moore GB, Meyer DJ, Murphy GJ, Smith SA, Hayes JD. Mammalian class Sigma glutathione S-transferases: catalytic properties and tissue-specific expression of human and rat GSH-dependent prostaglandin D2 synthases. Biochem J 359: 507–516, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Juttler E, Inta I, Eigler V, Herrmann O, Maegele I, Maser-Gluth C, Schwaninger M. Neuronal NF-κB influences thermoregulation and survival in a sepsis model. J Neuroimmunol 189: 41–49, 2007. [DOI] [PubMed] [Google Scholar]
- 39.Kim SJ, Jeong HJ, Choi IY, Lee KM, Park RK, Hong SH, Kim HM. Cyclooxygenase-2 inhibitor SC-236 [4-[5-(4-chlorophenyl)-3-(trifluoromethyl)-1-pyrazol-1-l] benzenesulfonamide] suppresses nuclear factor-κB activation and phosphorylation of p38 mitogen-activated protein kinase, extracellular signal-regulated kinase, and c-Jun N-terminal kinase in human mast cell line cells. J Pharmacol Exp Ther 314: 27–34, 2005. [DOI] [PubMed] [Google Scholar]
- 40.Kis B, Snipes A, Bari F, Busija DW. Regional distribution of cyclooxygenase-3 mRNA in the rat central nervous system. Brain Res Mol Brain Res 126: 78–80, 2004. [DOI] [PubMed] [Google Scholar]
- 41.Kis B, Snipes JA, Isse T, Nagy K, Busija DW. Putative cyclooxygenase-3 expression in rat brain cells. J Cereb Blood Flow Metab 23: 1287–1292, 2003. [DOI] [PubMed] [Google Scholar]
- 42.Kluger MJ Fever: role of pyrogens and cryogens. Physiol Rev 71: 93–127, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kluger MJ, Ringler DH, Anver MR. Fever and survival. Science 188: 166–168, 1975. [PubMed] [Google Scholar]
- 44.Krueger JM, Kapas L, Opp MR, Obal F Jr. Prostaglandins E2 and D2 have little effect on rabbit sleep. Physiol Behav 51: 481–485, 1992. [DOI] [PubMed] [Google Scholar]
- 45.Kulmacz RJ, Wang LH. Comparison of hydroperoxide initiator requirements for the cyclooxygenase activities of prostaglandin H synthase-1 and -2. J Biol Chem 270: 24019–24023, 1995. [DOI] [PubMed] [Google Scholar]
- 46.Lang CH, Bagby GJ, Spitzer JJ. Glucose kinetics and body temperature after lethal and nonlethal doses of endotoxin. Am J Physiol Regul Integr Comp Physiol 248: R471–R478, 1985. [DOI] [PubMed] [Google Scholar]
- 47.Leach M, Hamilton LC, Olbrich A, Wray GM, Thiemermann C. Effects of inhibitors of the activity of cyclo-oxygenase-2 on the hypotension and multiple organ dysfunction caused by endotoxin: a comparison with dexamethasone. Br J Pharmacol 124: 586–592, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Levy MM, Fink MP, Marshall JC, Abraham E, Angus D, Cook D, Cohen J, Opal SM, Vincent JL, Ramsay G. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Crit Care Med 31: 1250–1256, 2003. [DOI] [PubMed] [Google Scholar]
- 49.Li S, Wang Y, Matsumura K, Ballou LR, Morham SG, Blatteis CM. The febrile response to lipopolysaccharide is blocked in cyclooxygenase-2−/−, but not in cyclooxygenase-1−/− mice. Brain Res 825: 86–94, 1999. [DOI] [PubMed] [Google Scholar]
- 50.Lin LL, Wartmann M, Lin AY, Knopf JL, Seth A, Davis RJ. cPLA2 is phosphorylated and activated by MAP kinase. Cell 72: 269–278, 1993. [DOI] [PubMed] [Google Scholar]
- 51.Lu G, Tsai AL, Van Wart HE, Kulmacz RJ. Comparison of the peroxidase reaction kinetics of prostaglandin H synthase-1 and -2. J Biol Chem 274: 16,162–16,167, 1999. [DOI] [PubMed] [Google Scholar]
- 52.Martin SM, Malkinson TJ, Veale WL, Pittman QJ. Fever in pregnant, parturient, and lactating rats. Am J Physiol Regul Integr Comp Physiol 268: R919–R923, 1995. [DOI] [PubMed] [Google Scholar]
- 53.Masferrer JL, Koki A, Seibert K. COX2 inhibitors. A new class of antiangiogenic agents. Ann NY Acad Sci 889: 84–86, 1999. [DOI] [PubMed] [Google Scholar]
- 54.Masferrer JL, Leahy KM, Koki AT, Zweifel BS, Settle SL, Woerner BM, Edwards DA, Flickinger AG, Moore RJ, Seibert K. Antiangiogenic and antitumor activities of cyclooxygenase-2 inhibitors. Cancer Res 60: 1306–1311, 2000. [PubMed] [Google Scholar]
- 55.Matsumura K, Kobayashi S. Signaling the brain in inflammation: the role of endothelial cells. Front Biosci 9: 2819–2826, 2004. [DOI] [PubMed] [Google Scholar]
- 56.Melia KR, Ryabinin AE, Schroeder R, Bloom FE, Wilson MC. Induction and habituation of immediate early gene expression in rat brain by acute and repeated restraint stress. J Neurosci 14: 5929–5938, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Milton AS, Wendlandt S. Effects on body temperature of prostaglandins of the A, E, and F series on injection into the third ventricle of unanaesthetized cats and rabbits. J Physiol 218: 325–336, 1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Murakami M, Kudo I. Recent advances in molecular biology and physiology of the prostaglandin E2-biosynthetic pathway. Prog Lipid Res 43: 3–35, 2004. [DOI] [PubMed] [Google Scholar]
- 59.Murakami M, Matsumoto R, Urade Y, Austen KF, Arm JP. c-kit ligand mediates increased expression of cytosolic phospholipase A2, prostaglandin endoperoxide synthase-1, and hematopoietic prostaglandin D2 synthase and increased IgE-dependent prostaglandin D2 generation in immature mouse mast cells. J Biol Chem 270: 3239–3246, 1995. [DOI] [PubMed] [Google Scholar]
- 60.Murakami M, Naraba H, Tanioka T, Semmyo N, Nakatani Y, Kojima F, Ikeda T, Fueki M, Ueno A, Oh S, Kudo I. Regulation of prostaglandin E2 biosynthesis by inducible membrane-associated prostaglandin E2 synthase that acts in concert with cyclooxygenase-2. J Biol Chem 275: 32,783–32,792, 2000. [DOI] [PubMed] [Google Scholar]
- 61.Naraba H, Murakami M, Matsumoto H, Shimbara S, Ueno A, Kudo I, and Oh-ishi S. Segregated coupling of phospholipases A2, cyclooxygenases, and terminal prostanoid synthases in different phases of prostanoid biosynthesis in rat peritoneal macrophages. J Immunol 160: 2974–2982, 1998. [PubMed] [Google Scholar]
- 62.Ohashi K, Saigusa T. Sympathetic nervous responses during cytokine-induced fever in conscious rabbits. Pflügers Arch 433: 691–698, 1997. [DOI] [PubMed] [Google Scholar]
- 63.Ohshima H, Tatemichi M, Sawa T. Chemical basis of inflammation-induced carcinogenesis. Arch Biochem Biophys 417: 3–11, 2003. [DOI] [PubMed] [Google Scholar]
- 64.Papanek PE, Wood CE, Fregly MJ. Role of the sympathetic nervous system in cold-induced hypertension in rats. J Appl Physiol 71: 300–306, 1991. [DOI] [PubMed] [Google Scholar]
- 65.Penning TD, Talley JJ, Bertenshaw SR, Carter JS, Collins PW, Docter S, Graneto MJ, Lee LF, Malecha JW, Miyashiro JM, Rogers RS, Rogier DJ, Yu SS, AndersonGd Burton EG, Cogburn JN, Gregory SA, Koboldt CM, Perkins WE, Seibert K, Veenhuizen AW, Zhang YY, Isakson PC. Synthesis and biological evaluation of the 1,5-diarylpyrazole class of cyclooxygenase-2 inhibitors: identification of 4-[5-(4-methylphenyl)-3-(trifluoromethyl)-1H-pyrazol-1-yl]benze nesulfonamide (SC-58635, celecoxib). J Med Chem 40: 1347–1365, 1997. [DOI] [PubMed] [Google Scholar]
- 66.Romanovsky AA, Almeida MC, Aronoff DM, Ivanov AI, Konsman JP, Steiner AA, Turek VF. Fever and hypothermia in systemic inflammation: recent discoveries and revisions. Front Biosci 10: 2193–2216, 2005. [DOI] [PubMed] [Google Scholar]
- 67.Romanovsky AA, Ivanov AI, Shimansky YP. Ambient temperature for experiments in rats: a new method for determining the zone of thermal neutrality. J Appl Physiol 92: 2667–2679, 2002. [DOI] [PubMed] [Google Scholar]
- 68.Romanovsky AA, Kulchitsky VA, Akulich NV, Koulchitsky SV, Simons CT, Sessler DI, Gourine VN. First and second phases of biphasic fever: two sequential stages of the sickness syndrome? Am J Physiol Regul Integr Comp Physiol 271: R244–R253, 1996. [DOI] [PubMed] [Google Scholar]
- 69.Romanovsky AA, Kulchitsky VA, Simons CT, Sugimoto N. Methodology of fever research: why are polyphasic fevers often thought to be biphasic? Am J Physiol Regul Integr Comp Physiol 275: R332–R338, 1998. [DOI] [PubMed] [Google Scholar]
- 70.Romanovsky AA, Shido O, Sakurada S, Sugimoto N, Nagasaka T. Endotoxin shock: thermoregulatory mechanisms. Am J Physiol Regul Integr Comp Physiol 270: R693–R703, 1996. [DOI] [PubMed] [Google Scholar]
- 71.Romanovsky AA, Simons CT, Kulchitsky VA. “Biphasic” fevers often consist of more than two phases. Am J Physiol Regul Integr Comp Physiol 275: R323–R331, 1998. [DOI] [PubMed] [Google Scholar]
- 72.Romanovsky AA, Szekely M. Fever and hypothermia: two adaptive thermoregulatory responses to systemic inflammation. Med Hypotheses 50: 219–226, 1998. [DOI] [PubMed] [Google Scholar]
- 73.Rudaya AY, Steiner AA, Robbins JR, Dragic AS, Romanovsky AA. Thermoregulatory responses to lipopolysaccharide in the mouse: dependence on the dose and ambient temperature. Am J Physiol Regul Integr Comp Physiol 289: R1244–R1252, 2005. [DOI] [PubMed] [Google Scholar]
- 74.Saha S, Engstrom L, Mackerlova L, Jakobsson PJ, Blomqvist A. Impaired febrile responses to immune challenge in mice deficient in microsomal prostaglandin E synthase-1. Am J Physiol Regul Integr Comp Physiol 288: R1100–R1107, 2005. [DOI] [PubMed] [Google Scholar]
- 75.Saia RS, Carnio EC. Thermoregulatory role of inducible nitric oxide synthase in lipopolysaccharide-induced hypothermia. Life Sci 79: 1473–1478, 2006. [DOI] [PubMed] [Google Scholar]
- 76.Smith CJ, Zhang Y, Koboldt CM, Muhammad J, Zweifel BS, Shaffer A, Talley JJ, Masferrer JL, Seibert K, Isakson PC. Pharmacological analysis of cyclooxygenase-1 in inflammation. Proc Natl Acad Sci USA 95: 13,313–13,318, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Snipes JA, Kis B, Shelness GS, Hewett JA, Busija DW. Cloning and characterization of cyclooxygenase-1b (putative cyclooxygenase-3) in rat. J Pharmacol Exp Ther 313: 668–676, 2005. [DOI] [PubMed] [Google Scholar]
- 78.Stamp JA, Herbert J. Multiple immediate-early gene expression during physiological and endocrine adaptation to repeated stress. Neuroscience 94: 1313–1322, 1999. [DOI] [PubMed] [Google Scholar]
- 79.Steiner AA, Chakravarty S, Robbins JR, Dragic AS, Pan J, Herkenham M, Romanovsky AA. Thermoregulatory responses of rats to conventional preparations of lipopolysaccharide are caused by lipopolysaccharide per se—not by lipoprotein contaminants. Am J Physiol Regul Integr Comp Physiol 289: R348–R352, 2005. [DOI] [PubMed] [Google Scholar]
- 80.Steiner AA, Ivanov AI, Serrats J, Hosokawa H, Phayre AN, Robbins JR, Roberts JL, Kobayashi S, Matsumura K, Sawchenko PE, Romanovsky AA. Cellular and molecular bases of the initiation of fever. PLoS Biol 4: e284, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Steiner AA, Li S, Llanos QJ, Blatteis CM. Differential inhibition by nimesulide of the early and late phases of intravenous- and intracerebroventricular-LPS-induced fever in guinea pigs. Neuroimmunomodulation 9: 263–275, 2001. [DOI] [PubMed] [Google Scholar]
- 82.Steiner AA, Romanovsky AA. Leptin: at the crossroads of energy balance and systemic inflammation. Prog Lipid Res 46: 89–107, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Steiner AA, Rudaya AY, Robbins JR, Dragic AS, Langenbach R, Romanovsky AA. Expanding the febrigenic role of cyclooxygenase-2 to the previously overlooked responses. Am J Physiol Regul Integr Comp Physiol 289: R1253–R1257, 2005. [DOI] [PubMed] [Google Scholar]
- 84.Szekely M, Romanovsky AA. Pyretic and antipyretic signals within and without fever: a possible interplay. Med Hypotheses 50: 213–218, 1998. [DOI] [PubMed] [Google Scholar]
- 85.Ueno N, Takegoshi Y, Kamei D, Kudo I, Murakami M. Coupling between cyclooxygenases and terminal prostanoid synthases. Biochem Biophys Res Commun 338: 70–76, 2005. [DOI] [PubMed] [Google Scholar]
- 86.Ueno R, Narumiya S, Ogorochi T, Nakayama T, Ishikawa Y, Hayaishi O. Role of prostaglandin D2 in the hypothermia of rats caused by bacterial lipopolysaccharide. Proc Natl Acad Sci USA 79: 6093–6097, 1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Vayssettes-Courchay C, Bouysset F, Verbeuren TJ. Involvement of COX and NOS induction in the sympatho-activation during sepsis. Auton Neurosci 98: 33–36, 2002. [DOI] [PubMed] [Google Scholar]
- 88.Weinberg JR, Wright DJ, Guz A. Interleukin-1 and tumour necrosis factor cause hypotension in the conscious rabbit. Clin Sci (Lond) 75: 251–255, 1988. [DOI] [PubMed] [Google Scholar]
- 89.Yang Y, Gordon CJ. Ambient temperature limits and stability of temperature regulation in telemetered male and female rats. J Therm Biol 21: 353–363, 1996. [Google Scholar]
- 90.Zhang YH, Lu J, Elmquist JK, Saper CB. Specific roles of cyclooxygenase-1 and cyclooxygenase-2 in lipopolysaccharide-induced fever and Fos expression in rat brain. J Comp Neurol 463: 3–12, 2003. [DOI] [PubMed] [Google Scholar]