Abstract
Animal and human studies support an untoward effect of excess dietary NaCl (salt) intake on cardiovascular and renal function and life span. Recent work has promoted the concept that the endothelium, in particular, reacts to changes in dietary salt intake through a complex series of events that are independent of blood pressure and the renin-angiotensin-aldosterone axis. The cellular signaling events culminate in the intravascular production of transforming growth factor-β (TGF-β) and nitric oxide in response to increased salt intake. Plasticity of the endothelium is integral in the vascular remodeling consequences associated with excess salt intake, because nitric oxide serves as a negative regulator of TGF-β production. Impairment of nitric oxide production, such as occurs with endothelial dysfunction in a variety of disease states, results in unopposed excess vascular TGF-β production, which promotes reduced vascular compliance and augmented peripheral arterial constriction and hypertension. Persistent alterations in vascular function promote the increase in cardiovascular events and reductions in renal function that reduce life span during increased salt intake.
Keywords: transforming growth factor-β, nitric oxide, vascular remodeling, arterial compliance
investigators studying dietary NaCl (salt) intake observed more than a half century ago a dose-dependent decrease in survival of rats fed a standard diet containing increasing amounts of salt (Fig. 1) (38). The authors suggested that a high-salt diet increased mortality by accelerating arteriosclerosis, arteriolosclerosis, and renal parenchymal damage. These outcomes were associated with changes in blood pressure, which increased in a dose-dependent fashion after 9 mo on the diets, although some animals were remarkably resistant to the hypertensive response to salt in the diet. This dramatic illustration of the effect of excess salt intake on mammalian cardiovascular and renal physiology has not been stressed, but recent developments have revitalized these original findings. With emphasis on the role of the renin-angiotensin-aldosterone system (RAAS) in cardiovascular disease states, an early concern was that salt restriction in the general population promotes significant, but small, decreases in systolic and diastolic blood pressure while producing a physiological, but large (>300%), increase in circulating renin and aldosterone levels (31). These issues were resolved when Cook et al. (11) performed a large prospective clinical trial and showed that, despite the small reductions in systolic (1.7 mmHg) and diastolic (0.8 mmHg) blood pressure, chronic salt restriction to ∼2–2.6 g/day promoted cardiovascular health and reduced cardiovascular event rate by 25% in the intervention group.
Fig. 1.
Survival of 679 male rats fed formulated diets containing varying amounts of NaCl. Life span was progressively reduced as dietary salt content increased beyond 2.0% NaCl. [Reproduced from Meneely and Ball (38), with permission from Elsevier.]
In contrast to the physiological upregulation of the RAAS during low salt intake, pathological changes in the RAAS, as well as other genetic and acquired disorders that facilitate sodium retention (51), promote salt sensitivity, which accentuates the alterations in cardiovascular/renal function induced by salt excess (39, 63). An excellent example of the consequence of excess salt intake on end-organ damage in the setting of salt sensitivity induced by dysfunction of the RAAS comes from the study by Pimenta et al. (46), who observed that proteinuria correlated with urinary sodium excretion rates in patients with resistant hypertension and pathological aldosterone excess, but not in subjects with normal urinary aldosterone levels. The intent of this review is to delineate how excess salt intake alters cardiovascular function through mechanisms that involve, in particular, transforming growth factor (TGF)-β1 and nitric oxide (NO).
DIETARY SALT INCREASES PRODUCTION OF TGF-β
After the discovery of the initial member of the family of TGFs in 1980 (47), these important molecules have been shown to have complex effects on organ development and cell growth and differentiation, but they are particularly important in the expression of extracellular matrix proteins (49). Numerous studies have shown that these fibrogenic or prosclerotic growth factors participate integrally in vascular and renal fibrosis in a variety of disease states (1, 7, 13, 33, 54, 65, 66, 76, 77). Mammals express three isoforms of TGF-β: TGF-β1, -β2, and -β3. Although some differences exist, most evidence supports similar functions among these three TGF-β isoforms, with TGF-β1 considered the most important mammalian isoform. TGF-β1 is synthesized by many cell types, including endothelium, and is secreted as a latent dimeric ∼75-kDa protein complex (3, 18). A latency-associated peptide is cleaved from the active TGF-β molecule by the enzyme furin, during intracellular processing, but remains noncovalently complexed to the mature peptide after secretion. In addition, latent TGF-β-binding proteins, which are members of the fibrillin/latent TGF-β-binding protein family, bind this complex and direct it to the adjacent interstitium. Once in the extracellular space, removal of latent TGF-β frees the mature, ∼24-kDa biologically active form of TGF-β (3). Thus endothelium-derived TGF-β1 is typically a locally acting molecule with autocrine and paracrine actions on neighboring endothelium and vascular smooth muscle. Although there are several known mechanisms of activation of TGF-β1 (3, 48), thrombospondin-1 appears to be a major regulatory factor involved in TGF-β1 activation following secretion by endothelial cells (53) and in vivo in a model of mesangial proliferative glomerulonephritis (14).
TGF-β is involved in blood pressure regulation. Elastin microfibril interface-located protein 1 (Emilin1), a glycoprotein expressed in the vascular tree, binds the TGF-β precursor and prevents processing by furin. EMILIN1-knockout mice, therefore, display increased TGF-β1 signaling in the vessel wall. These animals develop peripheral vasoconstriction and arterial hypertension, which were prevented by inactivation of one TGFB1 allele (75). In another study, parenteral administration of an anti-TGF-β antibody to Dahl salt-sensitive rats every other day for 2 wk significantly reduced blood pressure and the associated proteinuria, glomerulosclerosis, and interstitial and vascular fibrosis observed in this model of salt-sensitive hypertension (13).
Vascular pathology encompasses remodeling not only of resistance vessels, but also compliance vessels. Arterial stiffness, an important determinant of systolic blood pressure, may also stimulate hypertrophy and remodeling of the microcirculation. The effect of reduced vascular compliance on the microcirculation is dramatic in the brain and kidney, two organs that are perfused at high flow rates and pulsatile pressures (43). Isolated systolic hypertension is also associated with subsequent development of heart failure (20). Pulse pressure, another marker of arterial stiffening, correlated with decline in renal function during treatment of essential hypertension (21). TGF-β is considered important in the development of arterial stiffness, by promoting hypertrophy of vascular smooth muscle (44) and by increasing the local production of extracellular matrix proteins (28) and inhibiting activity of those metalloproteinases involved in collagen degradation and remodeling (40).
Excess dietary salt intake increases vascular collagen deposition and TGF-β1 production. After the studies by Meneely and Ball (38), Tobian and Hanlon (58) suggested that a high-salt diet produced arterial lesions without increasing blood pressure. Deoxycorticosterone acetate and 1% salt were administered to uninephrectomized Dahl salt-resistant rats for 6 wk; then the animals were allowed to recover for 4 mo, during which deoxycorticosterone acetate and the salt additive were not administered. Determination of blood pressure at the end of the recovery period confirmed that the rats were hypertensive. The animals were then fed a diet that contained 0.3% or 8.0% NaCl. Although no further increases in blood pressure were observed, animals fed the high-salt diet had a very high mortality rate, which was attributed to cerebrovascular accidents (58). Yu and associates (74) demonstrated collagen deposition in the arteries, arterioles, glomeruli, and interstitium of the hearts and kidneys of normotensive (Wistar-Kyoto) and spontaneously hypertensive rats fed an 8.0% salt diet; upregulation of TGF-β1 mRNA was observed in the kidney and heart during administration of the high-salt diet in both strains. An 8.0% NaCl diet also increased albuminuria and accelerated progression of renal failure in a rodent model of chronic allograft nephropathy (52).
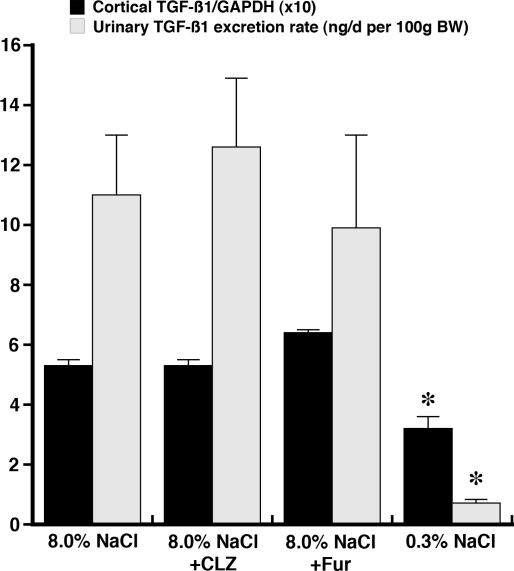
A major interest of our laboratory has been, in general, the effect of salt intake on endothelial cell function and, in particular, TGF-β1. Initial studies used Sprague-Dawley (SD) rats, in which blood pressure did not increase during the period of observation, to demonstrate that expression of all three mammalian TGF-β isoforms increased in cortical tissue of rats fed a formulated diet containing 8.0% NaCl, compared with tissue from rats maintained on a 0.3% NaCl diet (69). Increased expression was observed 1 day after institution of the diet and persisted over the 15 days of the experiment. Although serum levels of TGF-β1 did not change, urinary excretion of TGF-β1 increased on the 8.0% NaCl diet, indicating an intrarenal generation of TGF-β1. Administration of furosemide (5 mg/kg) and chlorothiazide (4 mg/kg) to rats maintained on the high-salt diet did not diminish the intrarenal generation of TGF-β1 (Fig. 2). Additional studies confirmed that an increase in salt intake increased the production of total and active TGF-β1 throughout the arterial tree, including the aorta (67), intrarenal arteries and arterioles (unpublished observations), and glomeruli (69). Removal of the aortic endothelium confirmed that this was the source of augmented TGF-β1 production during increased salt intake. Total and active TGF-β1 increased in the SD rats fed the high-salt diet.
Fig. 2.
Effect of addition of chlorothiazide (CLZ) and furosemide (Fur) on renal cortical expression of transforming growth factor (TGF)-β1, relative to GAPDH, and urinary excretion of TGF-β1 in rats fed 8.0% NaCl diet for 4 days. Neither diuretic altered these parameters compared with data obtained from rats maintained on the high-salt diet, suggesting that diuretics are ineffective in reducing salt-induced intrarenal production of TGF-β1. *P < 0.05 vs. other groups. [Data obtained from Ying and Sanders (69).]
ENDOTHELIAL CELL SIGNALING MECHANISMS AND DIETARY SALT
Hemodynamic forces are strong activators of the endothelium and modulate expression of numerous genes (23). By virtue of the location and response to shear stress, endothelial cells serve as biomechanical sensors that detect and respond to changes in blood flow (15), such as the change that occurs after expansion of blood volume when dietary salt is increased. Exposure of bovine aortic endothelial cells in culture to shear promoted TGFB1 gene transcription and the release of biologically active TGF-β; these effects were inhibited by addition of tetraethylammonium (42). An increase in shear induced by creation of an aortocaval fistula produced an acute increase in the expression of TGF-β1 and -β3 in the aorta (64). Endothelium-dependent NO production induced by flow is also inhibited by tetraethylammonium and charybdotoxin (12).
Consistent with a shear effect, the initial event that stimulates TGF-β1 production by aortic ring and glomerular preparations from SD rats fed a high-salt diet was the opening of a tetraethylammonium-sensitive potassium channel (67, 69). Downstream of the cell surface events, shear force activates the p38 MAPK (5, 29, 36) and p42/44 MAPK (29, 60, 61) pathways in endothelial cells in culture. Dietary salt activated, in a dose-dependent fashion, p38 MAPK and p42/44 MAPK pathways in aortic ring segments and preparations of isolated glomeruli (68, 70). Intravenous injection of tetraethylammonium just before harvesting of the tissue inhibited the increase in aortic and glomerular p38 and p42/44 MAPK activities that occurred with the high salt intake (68, 70). Thus these MAPK pathways were downstream of the tetraethylammonium-inhibitable potassium channel. Nuclear accumulation of phosphorylated p38 MAPK and p42/44 MAPK in the endothelial cells lining the aorta of SD rats on the 8.0% NaCl diet suggested activation of nuclear transcription. Additional experiments identified increased amounts of phosphorylated (activated) nuclear transcription factors that included activating transcription factor-2, which is activated by p38 MAPK, and Elk-1, which is a substrate of p42/44 MAPK, in SD rats fed the high-salt diet (70). Selective MAPK inhibitors demonstrated the essential role of both MAPK pathways in salt-induced increases in expression of TGF-β1 in aorta and glomeruli of SD rats (68, 70, 71).
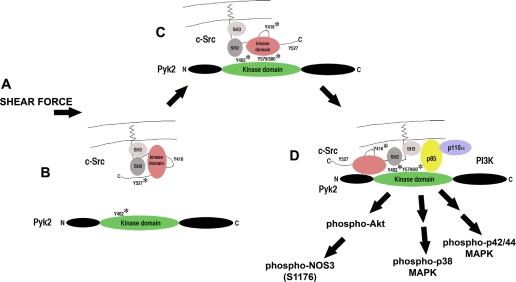
Recent studies focused on salt-induced activation of proline-rich tyrosine kinase 2 (Pyk2, also known as RAFTK, CAK-β, and CADTK), which is a member of the focal adhesion protein tyrosine kinase family (4). Pyk2 is activated by extracellular stress signals, such as shear stress (57), but also by G protein-coupled receptors, such as the angiotensin type 1 (AT1) receptor (4, 27). Increasing dietary salt intake between 0.3% and 8.0% NaCl induced a dose-dependent increase in activation of endothelial Pyk2, which recruited and activated c-Src (73). Complex formation between Pyk2 and c-Src was necessary to activate p38 and p42/44 MAPK and generate TGF-β1 (Fig. 3). The combined data fit a working hypothesis that dietary salt intake affects endothelial cell activity through shear force.
Fig. 3.
Working model of salt-induced endothelial cell activation. A: introduction of shear force activates the endothelium by opening a tetraethylammonium-inhibitable potassium channel. B: Pyk2 is autophosphorylated at Y402 and activated. C: c-Src is recruited and activated by Pyk2. Activity of Pyk2 is increased by c-Src-mediated phosphorylation at Y579/580 in the kinase domain. D: phosphatidylinositol 3-kinase (PI3K) is recruited to the complex, permitting activation of Akt and calcium-independent activation of nitric oxide synthase isoform 3 (NOS3) by phosphorylation at S1176 in rats. This complex also activates p38 and p42/44 MAPK pathways, resulting in augmented endothelial cell production of TGF-β1.
ROLE OF NO
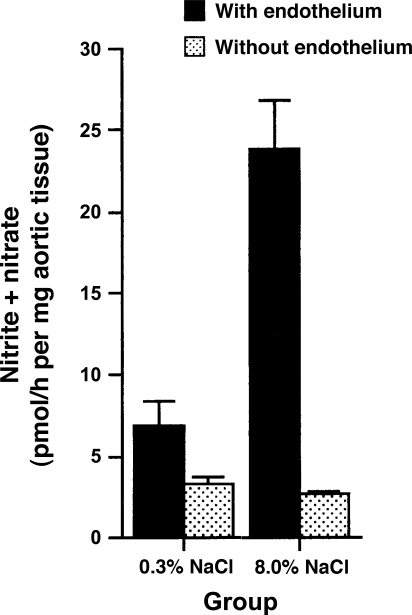
NO production becomes a central feature in the vascular response to salt intake. Increased salt intake increased NO production in SD and Dahl/Rapp salt-resistant (R) rats (8, 9, 17, 55) and healthy humans (6). Bech et al. (6) showed that increased dietary salt intake increased renal plasma flow and glomerular filtration rate and, further, demonstrated that the associated increase in NO production was integrally involved in this renal hemodynamic response as well as sodium excretion. Although the source of the increase in NO could not be determined in that study, the endothelium was the vascular source of the salt-induced augmented NO production in rats (Fig. 4) (67). Increased NO production promotes vasorelaxation of the afferent arteriole (30), augments glomerular filtration rate, and improves the pressure-natriuresis curve, facilitating salt excretion (16, 45). Inhibition of NO produces salt retention and salt-sensitive hypertension (59) and, if protracted, results in renal injury, particularly if the animals are maintained on a high-salt diet (22).
Fig. 4.
Aortic ring sections from Sprague-Dawley rats fed 0.3% NaCl diet produced less nitrite, a metabolite of nitric oxide (NO), than ring sections from Sprague-Dawley rats fed 8.0% NaCl diet. Effect was lost with removal of the endothelium. [Data obtained from Ying and Sanders (67).]
In addition to the hemodynamic effects, NO also modulates salt-induced TGF-β1 production. Glomerular and vascular ring preparations incubated with Nω-nitro-l-arginine methyl ester produced increased amounts of TGF-β1, whereas incubation with an NO donor, (±)-(E)-4-ethyl-2-[(E)-hydroxyimino]-5-nitro-3-hexenamide, decreased production of TGF-β1 (71). Thus NO limits the production of TGF-β1. The mechanism was independent of blood pressure and flow, since the experiments were performed in vitro. The proposed signaling mechanism of increased NO production is that shear forces generated by increased salt intake stimulate Akt-mediated phosphorylation of NO synthase isoform 3 (NOS3) at amino acid residue 1,176 in rats. Activation of Akt occurred with the formation of a Pyk2-c-Src-phosphatidylinositol 3-kinase complex (72), and the findings are consistent with the known modulatory effect of Pyk2 on NOS3 activity in other conditions (37). These data are also consistent with the original description of shear-induced phosphorylation of NOS3 by Akt in endothelial cells (19) and with experiments that used isolated aortic ring segments (56). Interestingly, Soucy et al. (56) showed that aging promoted a decrease in flow-mediated activation of Akt and NO production and an associated increase in pulse-wave velocity compared with vascular tissue from young animals. Mice lacking NOS3 demonstrated higher mean pulse-wave velocities than wild-type mice. The authors suggested that impaired Akt-mediated NOS3 activation contributes to age-associated vascular stiffness (56).
During increased salt intake, NO production serves as a countervailing influence that mitigates the consequences of production of TGF-β1. Conceivably, endothelial dysfunction associated with aging promotes vascular stiffness from unopposed TGF-β1 production; this effect would be enhanced by the addition of an increased-salt diet, which stimulates intravascular production of TGF-β1 (73). Limiting salt intake might therefore improve arterial stiffness, a risk factor associated with cardiovascular events (35). In a double-blind, placebo-controlled, crossover study of older adults, a low salt intake increased carotid arterial compliance by 27% by the end of the 1st wk, and the improvement stabilized at 46% by the 2nd wk (24). These findings suggest that although conduit artery compliance is a function of aging, it is not irreversible, and decreased salt intake improves arterial stiffness.
NO production regulates blood pressure not only through vasorelaxation of resistance vessels but also by limitation of TGF-β1 production. The Dahl/Rapp salt-sensitive (S) rat strain rapidly develops hypertension and severe renal failure related to tissue hypoxia from a progressive arteriopathic process involving the interlobular arteries and preglomerular arterioles (8–10, 62). S rats manifest impaired NO production that is exacerbated by increased salt intake (8, 9). Vascular and glomerular production of active TGF-β1 and NO metabolites, nitrite and nitrate, were directly correlated in R and S rats, but production of TGF-β1 was increased in prehypertensive S rats, and differences in endothelial production of TGF-β1 and NO between S and R rats were further exaggerated with institution of the increased-salt diet (71). Along with the demonstrated antihypertensive action of anti-TGF-β in S rats (13), the augmented vascular and glomerular production of TGF-β1, exacerbated by impaired NO, may contribute integrally to the development of hypertension and hypertensive nephrosclerosis in this salt-sensitive strain.
In addition to aging, there are other factors that promote endothelial dysfunction. Evidence supports a direct effect of salt intake on endothelial function mediated through plasma sodium concentration. Endothelial cells in culture stiffened and produced less shear-induced NO when the medium concentration of sodium increased from 135 to 145 meq/l. Altered endothelial cell function from reduction of medium sodium concentration was observed only in the presence of aldosterone (41). However, because reduction of salt intake to very low levels (10 mmol/day) produced a small, but significant, reduction in plasma sodium concentration (∼3 meq/l) compared with values obtained from the same patients on a 350 mmol/day salt diet (26), the overall significance of the in vitro studies is uncertain. Other disease states, including hypertension per se, promote oxidative stress and inflammation, which promote endothelial dysfunction and diminished production of NO (2, 25, 34, 50).
CONCLUSIONS
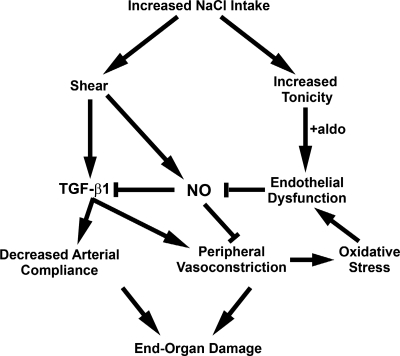
Dietary salt intake promotes endothelial cell production of TGF-β1 and NO (Fig. 5). NO release promotes vascular relaxation and inhibits TGF-β1 production. When NO production is impaired, such as with aging, hypertension, and other systemic diseases, unopposed excess vascular TGF-β1 production results in reduced vascular compliance and augmented peripheral arterial constriction and hypertension. Cerebrovascular and renovascular diseases are likely the proximate causes of reduced life span associated with excess salt intake. Meneely and Ball (38) reported that “salt is rough on rats,” and recent findings support adverse effects of salt on the vascular biology of humans as well. The obvious answer might be to simply reduce the content of salt in the diet, as demonstrated by Cook et al. (11). However, exactly how a low-salt diet promotes cardiovascular health when circulating angiotensin II levels are increased is a conundrum, particularly since angiotensin II can stimulate the production of TGF-β in vitro (32). TGF-β production in vivo is not increased with salt restriction. Diminished tissue responsiveness to angiotensin II seems an unlikely explanation, since adrenal production of aldosterone is similarly increased by a low-salt diet, although vascular expression of the AT1 receptor in response to changes in dietary salt intake has not been explored. Finally, another interesting observation from these early research pioneers is that increased dietary potassium intake exerted a protective action that diminished the mortality rate associated with excess salt intake in rats (38). Although the mechanism is forthcoming, these studies provide support for additional experiments that examine the mitigating role of dietary potassium in salt intake.
Fig. 5.
Proposed mechanistic link between dietary salt intake and end-organ damage mediated through alteration in vascular structure and function. Excess dietary salt intake induces endothelial shear forces that increase production of TGF-β1 and NO (67, 69, 72, 73). In the setting of salt-induced increased tonicity and aldosterone (aldo), endothelial cells in culture become dysfunctional and produce less NO (41). Oxidative stress, induced by hypertension associated with peripheral vasoconstriction or by other disease states (2, 25, 34, 50), also promotes endothelial dysfunction and diminished NO production. These factors become critical determinants of outcome, since NO serves a vasodilator function and inhibits endothelial TGF-β1 production (71). Fibrogenic effects of TGF-β1 promote a decrease in arterial compliance, and the role of TGF-β1 as a peripheral vasoconstrictor facilitates hypertension (75). Both processes contribute to end-organ damage.
GRANTS
P. W. Sanders is supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant R01 DK-46199 and George M. O'Brien Kidney and Urological Research Centers Program Grant P30 DK-079337 and the Medical Research Service of the Department of Veterans Affairs.
REFERENCES
- 1.Akagi Y, Isaka Y, Arai M, Kaneko T, Takenaka M, Moriyama T, Kaneda Y, Ando A, Orita Y, Kamada T, Ueda N, Imai E. Inhibition of TGF-β1 expression by antisense oligonucleotides suppressed extracellular matrix accumulation in experimental glomerulonephritis. Kidney Int 50: 148–155, 1996. [DOI] [PubMed] [Google Scholar]
- 2.Alp NJ, Channon KM. Regulation of endothelial nitric oxide synthase by tetrahydrobiopterin in vascular disease. Arterioscler Thromb Vasc Biol 24: 413–420, 2004. [DOI] [PubMed] [Google Scholar]
- 3.Annes JP, Munger JS, Rifkin DB. Making sense of latent TGFβ activation. J Cell Sci 116: 217–224, 2003. [DOI] [PubMed] [Google Scholar]
- 4.Avraham H, Park SY, Schinkmann K, Avraham S. RAFTK/Pyk2-mediated cellular signalling. Cell Signal 12: 123–133, 2000. [DOI] [PubMed] [Google Scholar]
- 5.Azuma N, Akasaka N, Kito H, Ikeda M, Gahtan V, Sasajima T, Sumpio BE. Role of p38 MAP kinase in endothelial cell alignment induced by fluid shear stress. Am J Physiol Heart Circ Physiol 280: H189–H197, 2001. [DOI] [PubMed] [Google Scholar]
- 6.Bech JN, Nielsen CB, Ivarsen P, Jensen KT, Pedersen EB. Dietary sodium affects systemic and renal hemodynamic response to NO inhibition in healthy humans. Am J Physiol Renal Physiol 274: F914–F923, 1998. [DOI] [PubMed] [Google Scholar]
- 7.Border WA, Okuda S, Languino LR, Sporn MB, Ruoslahti E. Suppression of experimental glomerulonephritis by antiserum against transforming growth factor β1. Nature 346: 371–374, 1990. [DOI] [PubMed] [Google Scholar]
- 8.Chen PY, Sanders PW. l-Arginine abrogates salt-sensitive hypertension in Dahl/Rapp rats. J Clin Invest 88: 1559–1567, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen PY, Sanders PW. Role of nitric oxide synthesis in salt-sensitive hypertension in Dahl/Rapp rats. Hypertension 22: 812–818, 1993. [DOI] [PubMed] [Google Scholar]
- 10.Chen PY, St. John PL, Kirk KA, Abrahamson DR, Sanders PW. Hypertensive nephrosclerosis in the Dahl/Rapp rat. Initial sites of injury and effect of dietary l-arginine administration. Lab Invest 68: 174–184, 1993. [PubMed] [Google Scholar]
- 11.Cook NR, Cutler JA, Obarzanek E, Buring JE, Rexrode KM, Kumanyika SK, Appel LJ, Whelton PK. Long-term effects of dietary sodium reduction on cardiovascular disease outcomes: observational follow-up of the Trials of Hypertension Prevention (TOHP). BMJ 334: 885, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cooke JP, Rossitch E Jr, Andon NA, Loscalzo J, Dzau VJ. Flow activates an endothelial potassium channel to release an endogenous nitrovasodilator. J Clin Invest 88: 1663–1671, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dahly AJ, Hoagland KM, Flasch AK, Jha S, Ledbetter SR, Roman RJ. Antihypertensive effects of chronic anti-TGF-β antibody therapy in Dahl S rats. Am J Physiol Regul Integr Comp Physiol 283: R757–R767, 2002. [DOI] [PubMed] [Google Scholar]
- 14.Daniel C, Wiede J, Krutzsch HC, Ribeiro SM, Roberts DD, Murphy-Ullrich JE, Hugo C. Thrombospondin-1 is a major activator of TGF-β in fibrotic renal disease in the rat in vivo. Kidney Int 65: 459–468, 2004. [DOI] [PubMed] [Google Scholar]
- 15.Davies PF Flow-mediated endothelial mechanotransduction. Physiol Rev 75: 519–560, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Deng A, Baylis C. Locally produced EDRF controls preglomerular resistance and ultrafiltration coefficient. Am J Physiol Renal Fluid Electrolyte Physiol 264: F212–F215, 1993. [DOI] [PubMed] [Google Scholar]
- 17.Deng X, Welch WJ, Wilcox CS. Renal vasoconstriction during inhibition of NO synthase: effects of dietary salt. Kidney Int 46: 639–646, 1994. [DOI] [PubMed] [Google Scholar]
- 18.Derynck R, Jarrett JA, Chen EY, Eaton DH, Bell JR, Assoian RK, Roberts AB, Sporn MB, Goeddel DV. Human transforming growth factor-β complementary DNA sequence and expression in normal and transformed cells. Nature 316: 701–705, 1985. [DOI] [PubMed] [Google Scholar]
- 19.Dimmeler S, Fleming I, Fisslthaler B, Hermann C, Busse R, Zeiher AM. Activation of nitric oxide synthase in endothelial cells by Akt-dependent phosphorylation. Nature 399: 601–605, 1999. [DOI] [PubMed] [Google Scholar]
- 20.Ekundayo OJ, Allman RM, Sanders PW, Aban I, Love TE, Arnett D, Ahmed A. Isolated systolic hypertension and incident heart failure in older adults: a propensity-matched study. Hypertension 53: 458–465, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fesler P, Safar ME, du Cailar G, Ribstein J, Mimran A. Pulse pressure is an independent determinant of renal function decline during treatment of essential hypertension. J Hypertens 25: 1915–1920, 2007. [DOI] [PubMed] [Google Scholar]
- 22.Fujihara CK, Michellazzo SM, De Nucci G, Zatz R. Sodium excess aggravates hypertension and renal parenchymal injury in rats with chronic NO inhibition. Am J Physiol Renal Fluid Electrolyte Physiol 266: F697–F705, 1994. [DOI] [PubMed] [Google Scholar]
- 23.Garcia-Cardeña G, Comander J, Anderson KR, Blackman BR, Gimbrone MA Jr. Biomechanical activation of vascular endothelium as a determinant of its functional phenotype. Proc Natl Acad Sci USA 98: 4478–4485, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gates PE, Tanaka H, Hiatt WR, Seals DR. Dietary sodium restriction rapidly improves large elastic artery compliance in older adults with systolic hypertension. Hypertension 44: 35–41, 2004. [DOI] [PubMed] [Google Scholar]
- 25.Gu JW, Tian N, Shparago M, Tan W, Bailey AP, Manning RD Jr. Renal NF-κB activation and TNF-α upregulation correlate with salt-sensitive hypertension in Dahl salt-sensitive rats. Am J Physiol Regul Integr Comp Physiol 291: R1817–R1824, 2006. [DOI] [PubMed] [Google Scholar]
- 26.He FJ, Markandu ND, Sagnella GA, de Wardener HE, MacGregor GA. Plasma sodium: ignored and underestimated. Hypertension 45: 98–102, 2005. [DOI] [PubMed] [Google Scholar]
- 27.Hunyady L, Catt KJ. Pleiotropic AT1 receptor signaling pathways mediating physiological and pathogenic actions of angiotensin II. Mol Endocrinol 20: 953–970, 2006. [DOI] [PubMed] [Google Scholar]
- 28.Ignotz RA, Endo T, Massague J. Regulation of fibronectin and type I collagen mRNA levels by transforming growth factor-β. J Biol Chem 262: 6443–6446, 1987. [PubMed] [Google Scholar]
- 29.Jo H, Sipos K, Go YM, Law R, Rong J, McDonald JM. Differential effect of shear stress on extracellular signal-regulated kinase and N-terminal Jun kinase in endothelial cells. J Biol Chem 272: 1395–1401, 1997. [DOI] [PubMed] [Google Scholar]
- 30.Juncos LA, Garvin J, Carretero OA, Ito S. Flow modulates myogenic responses in isolated microperfused rabbit afferent arterioles via endothelium-derived nitric oxide. J Clin Invest 95: 2741–2748, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jurgens G, Graudal NA. Effects of low sodium diet versus high sodium diet on blood pressure, renin, aldosterone, catecholamines, cholesterols, and triglyceride. Cochrane Database Syst Rev: CD004022, 2004. [DOI] [PubMed]
- 32.Kagami S, Border WA, Miller DE, Noble NA. Angiotensin II stimulates extracellular matrix protein synthesis through induction of transforming growth factor-β expression in rat glomerular mesangial cells. J Clin Invest 93: 2431–2437, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kopp JB, Factor VM, Mozes M, Nagy P, Sanderson N, Bottinger EP, Klotman PE, Thorgeirsson SS. Transgenic mice with increased plasma levels of TGF-β1 develop progressive renal disease. Lab Invest 74: 991–1003, 1996. [PubMed] [Google Scholar]
- 34.Landmesser U, Dikalov S, Price SR, McCann L, Fukai T, Holland SM, Mitch WE, Harrison DG. Oxidation of tetrahydrobiopterin leads to uncoupling of endothelial cell nitric oxide synthase in hypertension. J Clin Invest 111: 1201–1209, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Laurent S, Boutouyrie P. Recent advances in arterial stiffness and wave reflection in human hypertension. Hypertension 49: 1202–1206, 2007. [DOI] [PubMed] [Google Scholar]
- 36.Li YS, Shyy JYJ, Li S, Lee J, Su B, Karin M, Chien S. The Ras-JNK pathway is involved in shear-induced gene expression. Mol Cell Biol 16: 5947–5954, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Matsui A, Okigaki M, Amano K, Adachi Y, Jin D, Takai S, Yamashita T, Kawashima S, Kurihara T, Miyazaki M, Tateishi K, Matsunaga S, Katsume A, Honshou S, Takahashi T, Matoba S, Kusaba T, Tatsumi T, Matsubara H. Central role of calcium-dependent tyrosine kinase PYK2 in endothelial nitric oxide synthase-mediated angiogenic response and vascular function. Circulation 116: 1041–1051, 2007. [DOI] [PubMed] [Google Scholar]
- 38.Meneely GR, Ball COT. Experimental epidemiology of chronic sodium chloride toxicity and the protective effect of potassium chloride. Am J Med 25: 713–725, 1958. [DOI] [PubMed] [Google Scholar]
- 39.Morimoto A, Uzu T, Fujii T, Nishimura M, Kuroda S, Nakamura S, Inenaga T, Kimura G. Sodium sensitivity and cardiovascular events in patients with essential hypertension. Lancet 350: 1734–1737, 1997. [DOI] [PubMed] [Google Scholar]
- 40.Mozes MM, Bottinger EP, Jacot TA, Kopp JB. Renal expression of fibrotic matrix proteins and of transforming growth factor-β (TGF-β) isoforms in TGF-β transgenic mice. J Am Soc Nephrol 10: 271–280, 1999. [DOI] [PubMed] [Google Scholar]
- 41.Oberleithner H, Riethmuller C, Schillers H, MacGregor GA, de Wardener HE, Hausberg M. Plasma sodium stiffens vascular endothelium and reduces nitric oxide release. Proc Natl Acad Sci USA 104: 16281–16286, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ohno M, Cooke JP, Dzau VJ, Gibbons GH. Fluid shear stress induces endothelial transforming growth factor β1 transcription and production. Modulation by potassium channel blockade. J Clin Invest 95: 1363–1369, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.O'Rourke MF, Safar ME. Relationship between aortic stiffening and microvascular disease in brain and kidney: cause and logic of therapy. Hypertension 46: 200–204, 2005. [DOI] [PubMed] [Google Scholar]
- 44.Owens GK, Geisterfer AA, Yang YW, Komoriya A. Transforming growth factor-β-induced growth inhibition and cellular hypertrophy in cultured vascular smooth muscle cells. J Cell Biol 107: 771–780, 1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Patel A, Layne S, Watts D, Kirchner KA. l-Arginine administration normalizes pressure natriuresis in the hypertensive Dahl rats. Hypertension 22: 863–869, 1993. [DOI] [PubMed] [Google Scholar]
- 46.Pimenta E, Gaddam KK, Pratt-Ubunama MN, Nishizaka MK, Aban I, Oparil S, Calhoun DA. Relation of dietary salt and aldosterone to urinary protein excretion in subjects with resistant hypertension. Hypertension 51: 339–344, 2008. [DOI] [PubMed] [Google Scholar]
- 47.Roberts AB, Lamb LC, Newton DL, Sporn MB, De Larco JE, Todaro GJ. Transforming growth factors: isolation of polypeptides from virally and chemically transformed cells by acid/ethanol extraction. Proc Natl Acad Sci USA 77: 3494–3498, 1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Roberts AB, Sporn MB. Transforming growth factor β. Adv Cancer Res 51: 107–145, 1988. [PubMed] [Google Scholar]
- 49.Roberts AB, Sporn MB, Assoian RK, Smith JM, Roche NS, Wakefield LM, Heine UI, Liotta LA, Falanga V, Kehrl JH, et al. Transforming growth factor type β: rapid induction of fibrosis and angiogenesis in vivo and stimulation of collagen formation in vitro. Proc Natl Acad Sci USA 83: 4167–4171, 1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rodriguez-Iturbe B, Vaziri ND, Herrera-Acosta J, Johnson RJ. Oxidative stress, renal infiltration of immune cells, and salt-sensitive hypertension: all for one and one for all. Am J Physiol Renal Physiol 286: F606–F616, 2004. [DOI] [PubMed] [Google Scholar]
- 51.Sanders PW Dietary salt intake, salt sensitivity and cardiovascular health. Hypertension 53: 442–445, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sanders PW, Gibbs CL, Akhi KM, MacMillan-Crow LA, Zinn KR, Chen YF, Young CJ, Thompson JA. Increased dietary salt accelerates chronic allograft nephropathy in rats. Kidney Int 59: 1149–1157, 2001. [DOI] [PubMed] [Google Scholar]
- 53.Schultz-Cherry S, Lawler J, Murphy-Ullrich JE. The type 1 repeats of thrombospondin 1 activate latent transforming growth factor-β. J Biol Chem 269: 26783–26788, 1994. [PubMed] [Google Scholar]
- 54.Shihab FS, Andoh TF, Tanner AM, Noble NA, Border WA, Franceschini N, Bennett WM. Role of transforming growth factor-β1 in experimental chronic cyclosporine nephropathy. Kidney Int 49: 1141–1151, 1996. [DOI] [PubMed] [Google Scholar]
- 55.Shultz PJ, Tolins JP. Adaptation to increased dietary salt intake in the rat: role of endogenous nitric oxide. J Clin Invest 91: 642–650, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Soucy KG, Ryoo S, Benjo A, Lim HK, Gupta G, Sohi JS, Elser J, Aon MA, Nyhan D, Shoukas AA, Berkowitz DE. Impaired shear stress-induced nitric oxide production through decreased NOS phosphorylation contributes to age-related vascular stiffness. J Appl Physiol 101: 1751–1759, 2006. [DOI] [PubMed] [Google Scholar]
- 57.Tai LK, Okuda M, Abe J, Yan C, Berk BC. Fluid shear stress activates proline-rich tyrosine kinase via reactive oxygen species-dependent pathway. Arterioscler Thromb Vasc Biol 22: 1790–1796, 2002. [DOI] [PubMed] [Google Scholar]
- 58.Tobian L, Hanlon S. High sodium chloride diets injure arteries and raise mortality without changing blood pressure. Hypertension 15: 900–903, 1990. [DOI] [PubMed] [Google Scholar]
- 59.Tolins JP, Shultz PJ. Endogenous nitric oxide synthesis determines sensitivity to the pressor effect of salt. Kidney Int 46: 230–236, 1994. [DOI] [PubMed] [Google Scholar]
- 60.Traub O, Monia BP, Dean NM, Berk BC. PKC-ɛ is required for mechano-sensitive activation of ERK1/2 in endothelial cells. J Biol Chem 272: 31251–31257, 1997. [DOI] [PubMed] [Google Scholar]
- 61.Tseng H, Peterson TE, Berk BC. Fluid shear stress stimulates mitogen-activated protein kinase in endothelial cells. Circ Res 77: 869–878, 1995. [DOI] [PubMed] [Google Scholar]
- 62.Wang PX, Sanders PW. Mechanism of hypertensive nephropathy in the Dahl/Rapp rat: a primary disorder of vascular smooth muscle. Am J Physiol Renal Physiol 288: F236–F242, 2005. [DOI] [PubMed] [Google Scholar]
- 63.Weinberger MH, Fineberg NS, Fineberg SE, Weinberger M. Salt sensitivity, pulse pressure, and death in normal and hypertensive humans. Hypertension 37: 429–432, 2001. [DOI] [PubMed] [Google Scholar]
- 64.Xu C, Lee S, Shu C, Masuda H, Zarins CK. Expression of TGF-β1 and -β3 but not apoptosis factors relates to flow-induced aortic enlargement. BMC Cardiovasc Disord 2: 11, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yamamoto T, Nakamura T, Noble NA, Ruoslahti E, Border WA. Expression of transforming growth factor β is elevated in human and experimental diabetic nephropathy. Proc Natl Acad Sci USA 90: 1814–1818, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yamamoto T, Noble NA, Miller DE, Border WA. Sustained expression of TGF-β1 underlies development of progressive kidney fibrosis. Kidney Int 45: 916–927, 1994. [DOI] [PubMed] [Google Scholar]
- 67.Ying WZ, Sanders PW. Dietary salt increases endothelial nitric oxide synthase and TGF-β1 in rat aortic endothelium. Am J Physiol Heart Circ Physiol 277: H1293–H1298, 1999. [DOI] [PubMed] [Google Scholar]
- 68.Ying WZ, Sanders PW. Dietary salt intake activates MAP kinases in the rat kidney. FASEB J 16: 1683–1684, 2002. [DOI] [PubMed] [Google Scholar]
- 69.Ying WZ, Sanders PW. Dietary salt modulates renal production of transforming growth factor-β in rats. Am J Physiol Renal Physiol 274: F635–F641, 1998. [DOI] [PubMed] [Google Scholar]
- 70.Ying WZ, Sanders PW. Increased dietary salt activates rat aortic endothelium. Hypertension 39: 239–244, 2002. [DOI] [PubMed] [Google Scholar]
- 71.Ying WZ, Sanders PW. The interrelationship between TGF-β1 and nitric oxide is altered in salt-sensitive hypertension. Am J Physiol Renal Physiol 285: F902–F908, 2003. [DOI] [PubMed] [Google Scholar]
- 72.Ying WZ, Aaron K, Sanders PW. Dietary salt activates an endothelial proline-rich tyrosine kinase 2/c-Src/phosphatidylinositol 3-kinase complex to promote endothelial nitric oxide synthase phosphorylation. Hypertension 52: 1134–1141, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ying WZ, Aaron K, Sanders PW. Mechanism of dietary salt-mediated increase in intravascular production of TGF-β1. Am J Physiol Renal Physiol 295: F406–F414, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yu HCM, Burrell LM, Black MJ, Wu LL, Dilley RJ, Cooper ME, Johnston CI. Salt induces myocardial and renal fibrosis in normotensive and hypertensive rats. Circulation 98: 2621–2628, 1998. [DOI] [PubMed] [Google Scholar]
- 75.Zacchigna L, Vecchione C, Notte A, Cordenonsi M, Dupont S, Maretto S, Cifelli G, Ferrari A, Maffei A, Fabbro C, Braghetta P, Marino G, Selvetella G, Aretini A, Colonnese C, Bettarini U, Russo G, Soligo S, Adorno M, Bonaldo P, Volpin D, Piccolo S, Lembo G, Bressan GM. Emilin1 links TGF-β maturation to blood pressure homeostasis. Cell 124: 929–942, 2006. [DOI] [PubMed] [Google Scholar]
- 76.Zhu L, Herrera GA, Murphy-Ullrich JE, Huang ZQ, Sanders PW. Pathogenesis of glomerulosclerosis in light chain deposition disease: role for transforming growth factor-β. Am J Pathol 147: 375–385, 1995. [PMC free article] [PubMed] [Google Scholar]
- 77.Ziyadeh FN, Hoffman BB, Han DC, Iglesias-de la Cruz MC, Hong SW, Isono M, Chen S, McGowan TA, Sharma K. Long-term prevention of renal insufficiency, excess matrix gene expression, and glomerular mesangial matrix expansion by treatment with monoclonal anti-transforming growth factor-β antibody in db/db diabetic mice. Proc Natl Acad Sci USA 97: 8015–8020, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]