Abstract
Hyperuricemia has recently been recognized to be a risk factor for nephropathy in the diabetic subject. We tested the hypothesis that lowering uric acid with a xanthine oxidase inhibitor might reduce renal injury in the diabetic mouse. Diabetic (db/db) mice were treated with allopurinol or no treatment for 8 wk. Serum uric acid, renal function, and histology were assessed at death. The direct effect of uric acid in human proximal tubular epithelial cells was also evaluated under normal or high glucose condition. We found that db/db mice developed hyperuricemia, albuminuria, mesangial matrix expansion, and mild tubulointerstitial disease. Allopurinol treatment significantly lowered uric acid levels, reduced albuminuria, and ameliorated tubulointerstitial injury, but it did not prevent mesangial expansion. The mechanism for protection was shown to be due to a reduction in inflammatory cells mediated by a reduction in ICAM-1 expression by tubular epithelial cells. Interestingly, allopurinol did not reduce oxidative stress in the kidney. An inflammatory role of uric acid on tubular cells was also confirmed by our in vitro evidence that uric acid directly induced ICAM-1 expression in the human proximal tubular cell. In conclusion, hyperuricemia has a pathogenic role in the mild tubulointerstitial injury associated with diabetic nephropathy but not glomerular damage in db/db mice. Lowering uric acid may reduce tubulointerstitial injury in diabetes.
Keywords: inflammation, monocyte chemoattractant protein-1, intercellular adhesion molecule-1
diabetic nephropathy is currently the major cause of end stage renal disease worldwide. While renin-angiotensin blockade is considered the gold standard in therapy, this treatment tends to slow renal progression rather than arrest or reverse the process. Thus we need additional therapeutic strategies to halt this epidemic.
Our group has been studying the causal role of uric acid on hypertension and renal disease (14, 19, 23). We found that experimentally induced hyperuricemia could cause hypertension and renal disease in the rat (14, 19, 23). Recently, a prospective randomized controlled trial also suggested that lowering uric acid with allopurinol could slow the progression of renal disease in patients with hyperuricemia and chronic kidney disease (31). Several epidemiological studies (2, 29, 33) have also demonstrated that serum uric acid is associated with diabetic nephropathy, suggesting a potential role for uric acid in the disease pathogenesis.
Given these facts, we hypothesized that uric acid plays a pathological role in the development of diabetic nephropathy in type 2 diabetic db/db mice. To address this hypothesis, we examined the effect of lowering uric acid with allopurinol on renal histology and function in this diabetic model.
MATERIALS AND METHODS
Animals and experimental design.
Diabetic db/db (BKS.Cg- m+/+Leprdb/J) mice and age-matched background strain C57BLKS/J mice (8 wk old, male) were purchased from the Jackson Laboratory (Bar Harbor, ME). The total of 4 groups with 10 mice per each group included 1) control C57BLKS/J mice, 2) control with allopurinol, 3) db/db mice, and 4) db/db mice with allopurinol. Allopurinol (30 mg·kg−1·day−1) in drinking water was administered. Systolic blood pressure was assessed using a tail cuff sphygmomanometer (Visitech BP2000; Visitech Systems, Apex, NC). Mice were killed at 8 wk after treatment. Blood samples were collected at 2, 4, and 8 wk. Urinary samples were obtained at the end of study. Blood urea nitrogen (BUN), serum uric acid levels, urinary albumin-to-creatinine ratio and uric acid were measured as described previously (24). The mean serum uric acid was calculated by using values of 2-, 6-, and 8-wk time points. All of the animal experiments were performed in accordance with the Animal Care and Use Committee of the University of Florida.
Renal histology.
The removed kidneys were fixed in 10% formalin, embedded in paraffin, and then cut into 2-μm sections. The sections were used for periodic acid-Schiff (PAS) staining and immunohistochemistry. Indirect immunoperoxidase staining was performed by using antibodies to collagen IV deposition with a polyclonal rabbit anti-mouse collagen IV antibody (Chemicon International, Temecula, CA), to tubular damage with a polyclonal rabbit anti-mouse osteopontin antibody (Cosmo Bio, Tokyo, Japan), to collagen III deposition with a polyclonal goat anti-human collagen III antibody (Southern Biotechnology Associates, Birmingham, AL), and to transforming growth factor (TGF)-β expression with a rabbit polyclonal TGF-β antibody (Santa Cruz Biotechnology, Santa Cruz, CA). The staining was visualized with 3,3′-diaminobenzidine (Dako, Carpinteria, CA) for brown color development. These stained areas in cortical fields were measured by using the AxioVision image analysis computer program (Carl Zeiss, Thrornwood, NY). To assess macrophage infiltration into the tubulointerstitium, we performed immunostaining with a monoclonal anti-rat monocyte-macrophage marker F4/80 (Serotec, Oxford, UK). Macrophages positive for F4/80 were counted by examining 10 fields of the cortex under a light microscope at ×200 magnification in a blind manner. Negative controls were performed by the replacement of primary antibodies with species-matched antibodies.
Quantification of morphology.
The percentage of atrophic tubules (tubular dilation, detachment of tubular epithelial cells, and condensation of tubular nuclei) was assessed by scoring 800 renal cortical tubules in randomly selected fields for each subject (17). The extent of the mesangial expansion was determined by assessing the PAS-positive and nuclei-free area in the mesangium on 40 glomeruli, as previously described (16). The glomerular area was also treated along the outline of capillary loop. Their areas were measured using the AxioVision image analysis computer program (Carl Zeiss). All quantifications were performed in a blinded manner.
Western blot analysis.
Mouse kidney tissues were snap-frozen in liquid nitrogen for protein isolation. Western blot analysis was performed as described previously (18). The blots were subsequently incubated with a polyclonal goat anti-mouse ICAM-1 (R&D Systems, Minneapolis, MN), a monoclonal anti-mouse β-actin antibody (Sigma-Aldrich, St. Louis, MO), a polyclonal rabbit anti-mouse heme oxygenase (HO)-1 antibody (Stressgen, Ann Arbor, MI), or a polyclonal rabbit anti-xanthine oxidoreductase antibody (Santa Cruz Biotechnology), followed by incubation with peroxidase-conjugated goat IgG, mouse IgG, or rabbit IgG (Dako). Proteins were visualized with an ECL detection system (Amersham Pharmacia, Piscataway NJ). The density of each band was measured using the public domain National Institutes of Health Image program.
ELISA assay.
ELISA assay was performed by using commercial kits for monocyte chemotactic protein (MCP-1; BD Biosciences, San Diego, CA), 8-hydroxy-2′-deoxyguanosine (OHdG; Japan Institute for the Control of Aging, Fukuroi, Shizuoka, Japan), and soluble (s)ICAM-1/CD54 (R&D Systems) according to the manufacturer's instructions.
Cell culture.
Human renal proximal tubular epithelial cells (HK-2) were obtained from the American Type Culture Collection (Manassas, VA) and maintained in DMEM containing 5.4 mM d-glucose supplemented with 10% FBS. HK-2 cells were seeded on 60-mm plastic dishes. After 24 h starvation in serum-free medium, subconfluent HK-2 cells were stimulated with different concentrations (0, 7.5, or 15 mg/dl) of uric acid (Sigma-Aldrich) in the presence of 5.4 or 25 mM d-glucose (Sigma-Aldrich) for 24 or 48 h. To examine the effect of osmotic pressure of 25 mM d-glucose, 25 mM l-glucose (Sigma-Aldrich) was used as a control. The supernatants were then subjected to ELISA assay. Experiments were repeated three times.
Statistical analysis.
All values are expressed as means ± SD. Statistical analysis was performed with unpaired, two-tailed Student t-tests for single comparisons or ANOVA with post hoc test using Tukey's method for multiple comparisons. A P value of <0.05 was taken to indicate a significant difference.
RESULTS
General characteristics.
The db/db mice exhibited hyperglycemia and an increase in body weight during the 8 wk of the study period compared with control C57BLKS/J mice (Table 1). All mice survived. In contrast, the kidney weights in db/db mice were not different from those in control C57BLKS/J mice. Allopurinol treatment was well tolerated and was not associated with any difference in body weight or hyperglycemia compared with control db/db mice (Table 1). Allopurinol also did not alter weight or other parameters in control C57BLKS/J mice.
Table 1.
Genaral characteristics at 8 wk after treatment
| C57BLKS/J |
db/db | |||
|---|---|---|---|---|
| Control | Allopurinol | Control | Allopurinol | |
| Body weight, g | 25.2±1.5 | 24.6±1.2 | 49.1±4.2* | 47.3±4.5* |
| Blood sugar, mg/dl | 91±14 | 97±14 | 287±95* | 348±112* |
| Blood pressure, mmHg | 108±4.5 | 108±10 | 105±8.8 | 97±6†‡ |
| Blood urea nitrogen, mg/dl | 24±9.3 | 24±5.3 | 31±4.9 | 26±3.3‡ |
| Urinary albumin-to-urinary creatinine ratio, 10−1 | 0.28±0.18 | 0.21±0.14 | 3.7±3.0§ | 1.3±1.1‡ |
Values are means ± SE.
P < 0.0001 vs. C57BLKS/J control;
P < 0.01 vs. C57BLKS/J control;
P <0.05 vs. db/db control;
P < 0.05 vs. C57BLKS/J control.
Uric acid levels.
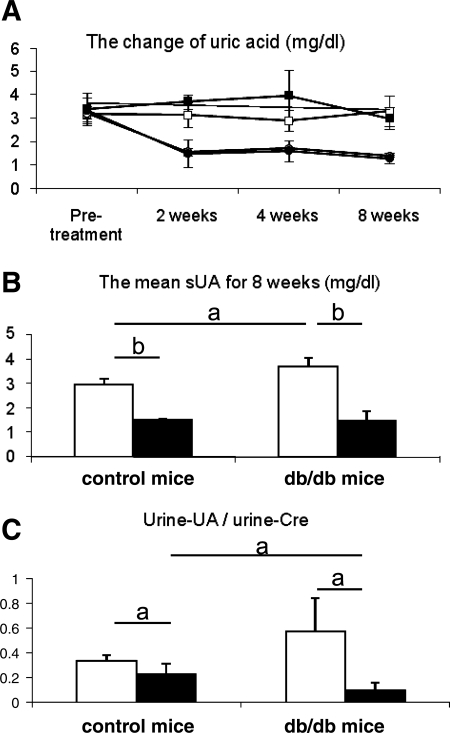
Serum uric acid levels tended to be higher in db/db mice than in control mice at 2 and 4 wk, although this was not statistically significant (Fig. 1A). However, mean serum uric acid for 8 wk was significantly higher in db/db mice compared with that in control mice, and this elevation of serum uric acid was significantly reduced by allopurinol (Fig. 1B). Urinary uric acid excretion (measured as the urine uric acid-to-creatinine ratio) was also increased in db/db mice at wk 8. The urine uric acid-to-creatinine ratio was also significantly reduced by allopurinol (Fig. 1C).
Fig. 1.
Uric acid level in serum and urine in db/db mouse. A: change of serum uric acid levels. Serum uric acid tends to be high in db/db mouse compared with control mouse during study period (□, control mice with no treatment; ○, control with allopurinol treatment; ▪, db/db mice with no treatment; •, db/db mice with allopurinol treatment). However, the mean of serum uric acid levels for 8 wk (from 8 to 16 wk age) is significantly high in db/db mouse compared with control mouse. B: allopurinol treatment significantly reduced mean serum uric acid (sUA) level. C: urinary uric acid (UA) excretion is expressed the urine uric acid-to-urine creatinine (Cre) ratio. Urinary uric acid excretion is also significantly high in db/db mouse compared with control mouse. However, it is reduced by allopurinol treatment. □, No treatment; ▪, allopurinol treatment. Data are means ± SD. aP < 0.05; bP < 0.001; n = 10/each group.
Renal function and blood pressure.
BUN and urinary albumin excretion mice were significantly elevated in db/db compared with control mice, whereas systolic blood pressure was not different (Table 1). The db/db mice receiving allopurinol treatment had significantly lower systemic blood pressure, BUN, and albuminuria compared with untreated db/db mice at 8 wk. Allopurinol treatment did not affect any parameters in the control C57BLKS/J mice (Table 1).
Glomerular changes.
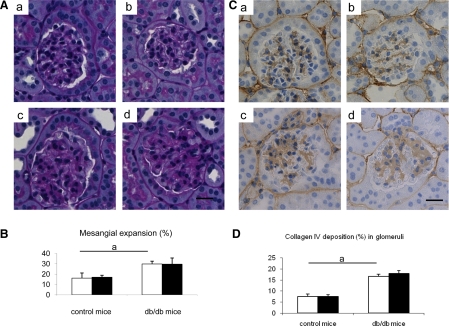
PAS staining documented glomerular mesangial expansion, an early feature of diabetic nephropathy, in db/db mice compared with control C57BLKS/J mice. The mesangial expansion was not prevented by allopurinol treatment in db/db mice (Fig. 2, A and B). Compatible with PAS staining, glomerular collagen IV deposition increased in db/db mice, but allopurinol had no effect on this deposition (Fig. 2, C and D).
Fig. 2.
Glomerular changes in db/db mouse. A: glomerular structure in periodic acid-Schiff staining. Compared with control C57BLKS/J mouse (a), db/db mouse exhibits mesangial expansion (c). allopurinol has no effect in glomerulus on both control (b) and db/db mouse (d). B: quantitative analysis for mesangial expansion. C: immunohistochemical staining shows glomerular of collagen IV depositions. Compared with glomeruli in control C57BLKS/J mouse (a), collagen IV deposition (brown color) increased in db/db mouse (c). Allopurinol has no effect on glomerular collagen IV deposition in both control (b) and db/db mouse (d). Bar = 20 μm. D: quantitative data for collagen IV deposition. Open bars, no treatment; filled bars, allopurinol treatment. Data are means ± SD. aP < 0.01; n = 10/each group.
Tubulointerstitial changes.
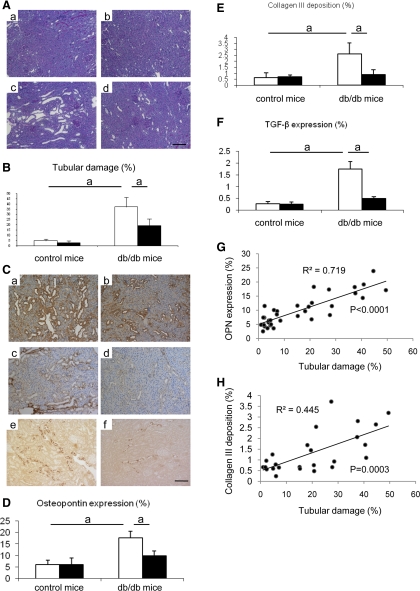
The db/db mice also developed mild tubular damage as evidenced predominantly by tubular dilatation. However, allopurinol significantly reduced the tubular injury in spite of the presence of high glucose (Fig. 3, A and B). To confirm the tubulointerstitial injury and the beneficial effect of allopurinol, we examined osteopontin expression, collagen III deposition, and TGF-β expression (Fig. 3C). Tubular osteopontin expression was significantly induced in tubules of db/db mice (Fig. 3C, a), whereas this expression was reduced by allopurinol treatment (Fig. 3, C, b and D). Similarly, interstitial collagen III deposition (Fig. 3, C, c and E) and tubular TGF-β expression (Fig. 3, C, e and F) also increased in db/db mice and were blocked by allopurinol (Fig. 3, C, d and C, f, respectively). The tubular damage was significantly correlated with tubular osteopontin expression (Fig. 3G) and interstitial collagen III deposition (Fig. 3H). In contrast, nondiabetic mice with allopurinol did not show any pathological changes (Fig. 3, D, E, and F). Consistent with these findings, we found a positive correlation of tubular osteopontin expression with serum uric acid (r = 0.52; P = 0.039) and urine uric acid levels (r = 0.65; P < 0.01).
Fig. 3.
Tubulointerstitial changes in db/db mouse. A: representative tubularinterstitial injuries are shown in. Compared with tubules in control mouse (a) and in control mouse with allopurinol treatment (b), db/db mouse (c) shows tubularinterstitial injury, as characterized by ballooning tubules and detachment of tubular epithelial cells from tubules. However, allopurinol treatment prevents the development of these lesions (d). B: quantitative analysis for tubulointerstitial injury. C: immunohistochemistry. Nontreated db/db mouse is shown in (a, c, e). db/db mouse with allopurinol treatment is shown in (b, d, f). Compared with nontreated db/db mouse, allopurinol treatment reduces osteopontin (OPN) expression (brown color) in tubules (b), collagen III deposition (brown color) in interstitium (d) and tranforming growth factor-β (TGF-β) expression (brown color) in tubules (f). Bar = 50 μm. Quantitative analysis is shown for OPN expression (D), collagen III deposition (E), and TGF-β expression (F). Also, shown is the correlation of tubular damage with OPN expression (G) and interstitial collagen III deposition (H). Open bars, no treatment; filled bars, allopurinol treatment. Data are means ± SD. aP < 0.01; n = 10/each group.
Inflammatory changes.
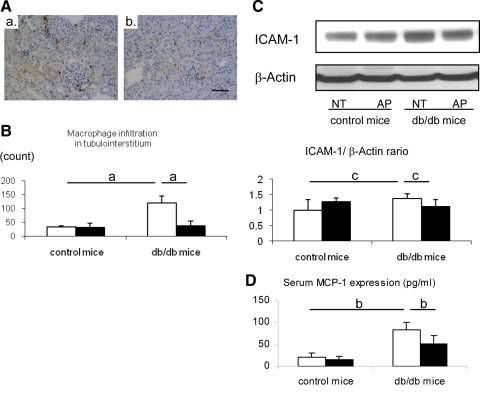
We next attempted to elucidate the mechanism by which allopurinol prevented the progression of tubulointerstitial injury in db/db mice. Since macrophage infiltration is known to be involved in the pathogenesis of the early stage of diabetic nephropathy (8, 30) and macrophage infiltration is a hallmark of tubulointerstitial injury, we evaluated the effect of allopurinol in tubulointerstitial inflammation. As expected, macrophage infiltration was prominent in the tubulointerstitum of db/db mice compared with control mice. Allopurinol treatment completely blocked macrophage infiltration in db/db mice (Fig. 4, A and B). Compatibly, renal ICAM-1, a cell adhesion protein that is important in the inflammatory response, was also induced in db/db mice and was significantly reduced by allopurinol treatment (Fig. 4D). The db/db mice also exhibited an elevation in serum MCP-1 levels, which was also blocked by allopurinol treatment (Fig. 4C).
Fig. 4.
Hyperuricemia-induced inflammatory response in db/db mouse. A: macrophage infiltration is examined by using immunohistochemistry for F4/80. Interstitial macrophage infiltration (brown color) is prominent in db/db (a), whereas allopurinol significantly reduces intrarenal macrophage infiltration in db/db mouse (b; bar = 50 μm). Quantitative analysis for F4/80 (+) macrophage infiltration shows that db/db mouse displays significantly increased macrophage infiltration in the renal cortex of db/db mouse. However, it is markedly prevented by allopurinol treatment (B). Western blotting demonstrates that ICAM-1 protein level is significantly increased in the whold kidney of db/db mouse, compared with control mouse. C: allopurinol (AP) significantly blocks the elevation of renal ICAM-1 expression compared with nontreatment mice (NT); quantitative data are expressed as the relative ratio of ICAM-1 to β-actin. D: ELISA assay demonstrates an elevation of serum MCP-1 levels in db/db mice compared with control mice and was blocked by allopurinol treatment. Open bars, no treatment; filled bars, allopurinol treatment. Data are means ± SD. aP < 0.001; bP < 0.005; cP < 0.05; n = 10/each group.
Oxidative stress.
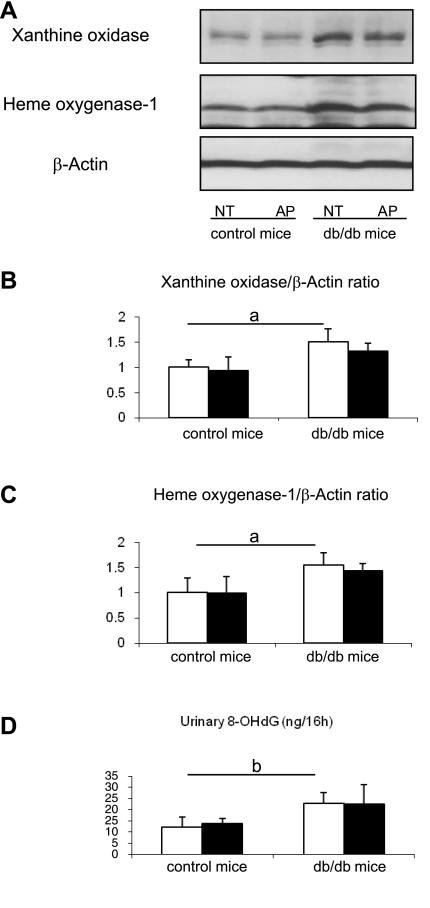
Since allopurinol can also block xanthine oxidase-induced oxidants, we measured oxidative stress in the kidneys of the control and allopurinol-treated mice. Xanthine oxidoreductase expression was higher in db/db mice compared with control mice (Fig. 5, A and B). Other markers of oxidative stress, including HO-1 and urinary 8-OHdG excretion, were also significantly higher in db/db mice. However, these markers of oxidative stress were not reduced by allopurinol treatment (Fig. 5, A, C, and D).
Fig. 5.
Oxidative stress in db/db mouse. Hemoxygenase (HO)-1 and xanthine oxidase proteins in whole kidney are examined by western blotting (A). Quantitative results are expressed as the relative ratio of HO-1 (B) or xanthine oxidase (C) to β-actin, respectively. Both HO-1 and xanthine oxidase are significantly high in db/db mice compared with control mice, but these increments are not blocked by allopurinol treatment compared with nontreatment mice. ELISA assay shows that urinary 8-hydroxy-2′-deoxyguanosine (8-OHdG) levels in db/db mice is significantly high compared with control mice, but this induction is not inhibited by allopurinol treatment (D). Open bars, no treatment; filled bars, allopurinol treatment. Data are means ± SD. aP < 0.01; bP < 0.05; n = 10/each group.
Induction of ICAM-1 protein on human proximal tubular epithelial cells in culture.
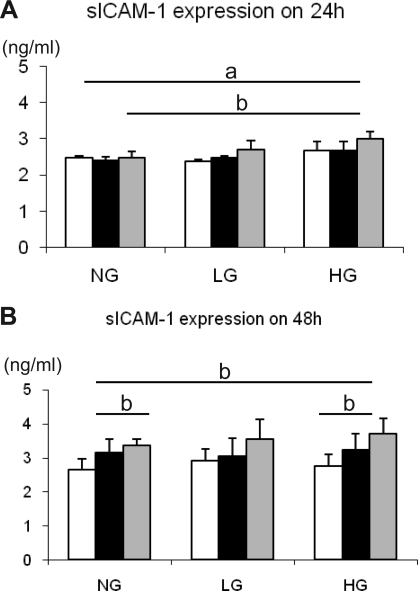
We next determined if uric acid can directly induce inflammatory changes in cultured HK-2 cells. The expression of the ICAM-1 was measured under normal (5.4 mM d-glucose) and high glucose (25 mM d-glucose) conditions. We used 25 mM of l-glucose, which exhibits the same level of osmotic pressure as 25 mM d-glucose, as a control. At 24 h, 15 mg/dl of uric acid significantly induced sICAM-1 release under high glucose condition compared with 5 mM d-glucose, while a lower concentration (0 and 7.5 mg/dl) of uric acid did had no effect under any glucose concentrations (Fig. 6A), suggesting that the combination of uric acid and high glucose induced ICAM-1 expression in HK-2 cells. In contrast, at 48 h, higher concentration of uric acid significantly stimulated sICAM-1 release under normal as well as high glucose conditions (Fig. 6B). l-glucose tended to exhibit similar response, but it was not significant.
Fig. 6.
Soluble intercellular adhesion molecule-1 (sICAM-1) expression in response to uric acid in HK-2 cells. HK-2 cells are stimulated by a various concentration of uric acid (0, 7.5, and 15 mg/dl) in the presence of normal d-glucose (5.4 mM; NG) or high d-glucose (HG; 25 mM) at 1 (A) and 2 days (B). l-glucose (LG) was used to examine the same level of osmotic pressure as 25 mM d-glucose loading. Open bars, 0 mg/dl uric acid; filled bars, 7.5 mg/dl uric acid; grey bars, 15 mg/dl uric acid. Data are means ± SD. aP < 0.01; bP < 0.05; n = 10/each group.
DISCUSSION
We found that type 2 diabetic db/db mice exhibit mild hyperuricemia, likely secondary due in part to increased expression of xanthine oxidoreductase within the kidney. Treatment with allopurinol lowered the serum and urinary uric acid levels, and this was associated with lower blood pressure, reduced urinary albumin excretion, improved renal function, and less tubulointerstitial injury. Interestingly, allopurinol did not improve glomerular damage. The mechanism for the renal injury might be due to the proinflammatory effects of uric acid, as renal injury was associated with increased macrophage infiltration, tubular ICAM-1 expression, and serum MCP-1 level, importantly all of which were reduced in allopurinol-treated rats. The inflammatory role of uric acid in tubular epithelial cell was also confirmed by an in vitro study in which uric acid directly induced ICAM-1 expression in human proximal tubular cells. Finally, we could not show an effect of allopurinol on urinary 8-OHdG excretion, suggesting that the beneficial effect of allopurinol might not be due to its antioxidant action. These studies suggest that treatment of diabetic nephropathy may be benefited by treatment with a xanthine oxidase inhibitor and that the mechanism may relate to preventing uric acid-induced renal inflammation.
In this study, we documented mild tubulointerstitial injury in db/db mice. The tubulointerstitial injury was confirmed with sensitive markers for tubular injury (osteopontin) and fibrosis (collagen III deposition). A previous study (26) of db/db mice also documented the presence of mild tubulointerstitial injury as evidenced by interstitial macrophage infiltration, tubular cell proliferation, and loss of peritubular capillaries, all of which were further deteriorated in nephrectomized db/db mice. While glomerular damage is a major feature of diabetic nephropathy, it is known that diabetic nephropathy is also associated with tubulointerstitial damage (reviewed in Refs. 10, 34), which can occasionally be a major manifestation of the disease process (6). In this case, the tubulointersisial injury was mild. While it is likely that the injury was a consequence of the diabetic state, it remains possible that other mechanisms such as leptin resistance could have a role in the injury.
Hypeuricemia is known to be a feature of insulin resistance syndrome (21). However, to our knowledge no investigators have yet addressed the role of hyperuricemia in diabetic nephropathy. Bo et al. (2) examined 2,113 patients with type 2 diabetes in Italy and found that hyperuricemia was associated with insulin resistance and with early onset or deterioration of diabetic nephropathy. Tseng (33) also demonstrated the association between serum uric acid and microalbuminuria in Taiwanese type 2 diabetic patients. Recently, a study (29) from the Joslin clinic documented that high normal serum uric acid was associated with a decreased renal function in type 1 diabetic patients by using serum cystain-C-based estimates glomerular filtration rates. Recently, we (11) have also collaborated with the Steno group and found that an elevated serum uric acid early in the course of type 1 diabetes can predict the development of diabetic nephropathy.
Our group has been studying the role of uric acid on hypertension and renal disease for a decade. We originally demonstrated that mild hyperuricemia causes glomerular injury and mild tubulointerstitial disease in both normal rats and rats with chronic kidney disease (14, 23). The mechanisms by which uric acid exerts renal injury involve uric acid-mediated endothelial dysfunction (15), an activation of renin angiogensin system (19), and an induction of intrarenal inflammation (13, 28). Importantly, in these hyperuricemic rats, increased macrophage infiltration is commonly observed in tubulointerstitium (14, 20, 23), suggesting a potential role of hyperuricemia on tubulointerstitial inflammation and injury.
We (28) previously reported that the potential mechanism for interstitial macrophage infiltration is attributed to the induction of intrarenal MCP-1 expression in these hyperuricemic animals. In addition, we also found that uric acid directly stimulates MCP-1 expression in vascular smooth muscle cells (13), which could account for the systemic elevation of MCP-1 levels observed in this study. In addition, we newly discovered in this study that uric acid was capable of inducing ICAM-1 in tubular epithelial cell, which could contribute to the tubulointerstitial macrophage infiltration in db/db mice. Interestingly, our in vitro study indicated that uric acid likely induces tubular ICAM-1 expression in concert with hyperglycemia in the early time point, whereas uric acid itself turns to be potent to stimulate ICAM-1 in proximal tubular cells later. We also found that urinary excretion of uric acid was also higher in db/db mice. Therefore, we might need to consider the potential role of urinary uric acid in stimulating the proximal tubular cell to entry via the apical membrane.
One of the interesting findings in this study is that allopurinol improved tubulointerstitial injury but not glomerular disease in db/db mice. These data suggest that other factors, including high glucose or oxidative stress, could be responsible for the diabetic glomerular disease at this stage of db/db mice. In fact, although high glucose is well known to be one of the major stimuli to accelerate extracellular matrix deposition in diabetic glomeruli (12, 35), several studies indicated the causal role of oxidative stress in the development of diabetic glomerular injury. Suszatak et al. (32) showed that glucose-induced oxidative stress causes podocyte injury, which could initiate the development of diabetic glomerulopathy in db/db mice. Similarly, it was also demonstrated that the downregulation of NOX4, a subunit of NADPH oxidase, is associated with the beneficial effect of statin in experimental diabetic nephropathy (7). In this study, we confirmed that an increase in oxidative stress was associated with renal injury in db/db mice by using several parameters, including HO-1, xanthine oxidoreductase, and urinary OHdG. However, none of these markers was suppressed by allopurinol treatment. Thus oxidative stress may not be responsible for the tubulointerstitial injury, whereas it might contribute to the development of glomerular damage in this model.
Since allopurinol exerts both lowering uric acid and reducing oxidative stress, it is often unclear whether the beneficial effect of allopurinol can be attributed to either effect. In addition, the anti-oxidative role of allopurinol in diabetic patients is still controversial in clinical settings. Afshari et al. (1) showed that allopurinol did not reduce oxidative stress in diabetic patients, whereas another study showed a significant reduction of oxidative stress with allopurinol in type 1 diabetic patients (3). In contrast, our study is consistent with a causal role of hyperuricemia, but not oxidative stress, in the development of the tubulointerstitial injury in db/db mice. The failure of allopurinol to reduce oxidative stress could be due in part to its dose. While many studies used 150 mg/l of allopurinol in animal models (19, 22, 27), a recent study (9) suggests that a high dose of allopurinol may be required to reduce oxidative stress. Hence, a higher dose may be required to reduce oxidative stress, while uric acid could be lowered at lower concentration of allopurinol as demonstrated in this study.
An interesting finding was the lack of effect of allopurinol in healthy control mice. This is not dissimilar with the situation in humans in which the association of uric acid with hypertension, insulin resistance, and kidney disease occurs only when uric acid levels are greater than 5.2–5.5 mg/dl (4, 5, 25). This suggests that uric acid may require a threshold level for toxicity.
In conclusion, we demonstrated that db/db develop hyperuricemia, which may have a role in mediating tubulointerstitial injury associated with diabetes. Lowering uric acid improved renal function, proteinuria, and tubulointerstitial damage, and the mechanism is likely due to blocking uric acid-induced intrarenal inflammation.
GRANTS
This study was supported by the Juvenile Diabetes Research Foundation, by National Institute of Diabetes and Digestive and Kidney Diseases Grant DK-52121, by National Heart, Lung, and Blood Institute Grant HL-68607, and by generous funds from Gatorade.
DISCLOSURES
Dr. T. Nakagawa and Dr. R. J. Johnson are listed as inventors on several patent applications related to lowering uric acid as a means to reduce cardiovascular disease and diabetes.
REFERENCES
- 1.Afshari M, Larijani B, Rezaie A, Mojtahedi A, Zamani MJ, Astanehi-Asghari F, Mostafalou S, Hosseinnezhad A, Heshmat R, Abdollahi M. Ineffectiveness of allopurinol in reduction of oxidative stress in diabetic patients; a randomized, double-blind placebo-controlled clinical trial. Biomed Pharmacother 58: 546–550, 2004. [DOI] [PubMed] [Google Scholar]
- 2.Bo S, Cavallo-Perin P, Gentile L, Repetti E, Pagano G. Hypouricemia and hyperuricemia in type 2 diabetes: two different phenotypes. Eur J Clin Invest 31: 318–321, 2001. [DOI] [PubMed] [Google Scholar]
- 3.Desco MC, Asensi M, Marquez R, Martinez-Valls J, Vento M, Pallardo FV, Sastre J, Vina J. Xanthine oxidase is involved in free radical production in type 1 diabetes: protection by allopurinol. Diabetes 51: 1118–1124, 2002. [DOI] [PubMed] [Google Scholar]
- 4.Feig DI, Johnson RJ. Hyperuricemia in childhood primary hypertension. Hypertension 42: 247–252, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Feig DI, Kang DH, Johnson RJ. Uric acid and cardiovascular risk. N Engl J Med 359: 1811–1821, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fioretto P, Mauer M, Brocco E, Velussi M, Frigato F, Muollo B, Sambataro M, Abaterusso C, Baggio B, Crepaldi G, Nosadini R. Patterns of renal injury in NIDDM patients with microalbuminuria. Diabetologia 39: 1569–1576, 1996. [DOI] [PubMed] [Google Scholar]
- 7.Fujii M, Inoguchi T, Maeda Y, Sasaki S, Sawada F, Saito R, Kobayashi K, Sumimoto H, Takayanagi R. Pitavastatin ameliorates albuminuria and renal mesangial expansion by downregulating NOX4 in db/db mice. Kidney Int 72: 473–480, 2007. [DOI] [PubMed] [Google Scholar]
- 8.Galkina E, Ley K. Leukocyte recruitment and vascular injury in diabetic nephropathy. J Am Soc Nephrol 17: 368–377, 2006. [DOI] [PubMed] [Google Scholar]
- 9.George J, Carr E, Davies J, Belch JJ, Struthers A. High-dose allopurinol improves endothelial function by profoundly reducing vascular oxidative stress and not by lowering uric acid. Circulation 114: 2508–2516, 2006. [DOI] [PubMed] [Google Scholar]
- 10.Gilbert RE, Cooper ME. The tubulointerstitium in progressive diabetic kidney disease: more than an aftermath of glomerular injury? Kidney Int 56: 1627–1637, 1999. [DOI] [PubMed] [Google Scholar]
- 11.Hovind P, Possing P, Tarnow L, Johnson RJ, Parving HH. Serum uric acid as a predictor for development of diabetic nephropathy in type 1 diabetes–an inception cohort study. Diabetes 2009. May 1. [Epub ahead of print]. [DOI] [PMC free article] [PubMed]
- 12.Isono M, Cruz MC, Chen S, Hong SW, Ziyadeh FN. Extracellular signal-regulated kinase mediates stimulation of TGF-beta1 and matrix by high glucose in mesangial cells. J Am Soc Nephrol 11: 2222–2230, 2000. [DOI] [PubMed] [Google Scholar]
- 13.Kanellis J, Watanabe S, Li JH, Kang DH, Li P, Nakagawa T, Wamsley A, Sheikh-Hamad D, Lan HY, Feng L, Johnson RJ. Uric acid stimulates monocyte chemoattractant protein-1 production in vascular smooth muscle cells via mitogen-activated protein kinase and cyclooxygenase-2. Hypertension 41: 1287–1293, 2003. [DOI] [PubMed] [Google Scholar]
- 14.Kang DH, Nakagawa T, Feng L, Watanabe S, Han L, Mazzali M, Truong L, Harris R, Johnson RJ. A role for uric acid in the progression of renal disease. J Am Soc Nephrol 13: 2888–2897, 2002. [DOI] [PubMed] [Google Scholar]
- 15.Khosla UM, Zharikov S, Finch JL, Nakagawa T, Roncal C, Mu W, Krotova K, Block ER, Prabhakar S, Johnson RJ. Hyperuricemia induces endothelial dysfunction. Kidney Int 67: 1739–1742, 2005. [DOI] [PubMed] [Google Scholar]
- 16.Kosugi T, Heinig M, Nakayama T, Connor T, Yuzawa Y, Li Q, Hauswirth WW, Grant MB, Croker BP, Campbell-Thompson M, Zhang L, Atkinson MA, Segal MS, Nakagawa T. Lowering blood pressure blocks mesangiolysis and mesangial nodules, but not tubulointerstitial injury, in diabetic eNOS knockout mice. Am J Pathol 174: 1221–1229, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kosugi T, Yuzawa Y, Sato W, Arata-Kawai H, Suzuki N, Kato N, Matsuo S, Kadomatsu K. Midkine is involved in tubulointerstitial inflammation associated with diabetic nephropathy. Lab Invest 87: 903–913, 2007. [DOI] [PubMed] [Google Scholar]
- 18.Kosugi T, Yuzawa Y, Sato W, Kawai H, Matsuo S, Takei Y, Muramatsu T, Kadomatsu K. Growth factor midkine is involved in the pathogenesis of diabetic nephropathy. Am J Pathol 168: 9–19, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mazzali M, Hughes J, Kim YG, Jefferson JA, Kang DH, Gordon KL, Lan HY, Kivlighn S, Johnson RJ. Elevated uric acid increases blood pressure in the rat by a novel crystal-independent mechanism. Hypertension 38: 1101–1106, 2001. [DOI] [PubMed] [Google Scholar]
- 20.Mazzali M, Kanellis J, Han L, Feng L, Xia YY, Chen Q, Kang DH, Gordon KL, Watanabe S, Nakagawa T, Lan HY, Johnson RJ. Hyperuricemia induces a primary renal arteriolopathy in rats by a blood pressure-independent mechanism. Am J Physiol Renal Physiol 282: F991–F997, 2002. [DOI] [PubMed] [Google Scholar]
- 21.Modan M, Halkin H, Karasik A, Lusky A. Elevated serum uric acid–a facet of hyperinsulinaemia. Diabetologia 30: 713–718, 1987. [DOI] [PubMed] [Google Scholar]
- 22.Nakagawa T, Hu H, Zharikov S, Tuttle KR, Short RA, Glushakova O, Ouyang X, Feig DI, Block ER, Herrera-Acosta J, Patel JM, Johnson RJ. A causal role for uric acid in fructose-induced metabolic syndrome. Am J Physiol Renal Physiol 290: F625–F631, 2006. [DOI] [PubMed] [Google Scholar]
- 23.Nakagawa T, Mazzali M, Kang DH, Kanellis J, Watanabe S, Sanchez-Lozada LG, Rodriguez-Iturbe B, Herrera-Acosta J, Johnson RJ. Hyperuricemia causes glomerular hypertrophy in the rat. Am J Nephrol 23: 2–7, 2003. [DOI] [PubMed] [Google Scholar]
- 24.Nakagawa T, Sato W, Glushakova O, Heinig M, Clarke T, Campbell-Thompson M, Yuzawa Y, Atkinson M, Johnson RJ, Croker B. Diabetic eNOS knockout mice develop advanced diabetic nephropathy. J Am Soc Nephrol 18: 539–550, 2007. [DOI] [PubMed] [Google Scholar]
- 25.Nakagawa T, Tuttle KR, Short RA, Johnson RJ. Hypothesis:fructose-induced hyperuricemia as a causal mechanism for the epidemic of the metabolic syndrome. Nat Clinical Practice Nephorlogy 1: 80–86, 2005. [DOI] [PubMed] [Google Scholar]
- 26.Ninichuk V, Khandoga AG, Segerer S, Loetscher P, Schlapbach A, Revesz L, Feifel R, Khandoga A, Krombach F, Nelson PJ, Schlondorff D, Anders HJ. The role of interstitial macrophages in nephropathy of type 2 diabetic db/db mice. Am J Pathol 170: 1267–1276, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reungjui S, Roncal CA, Mu W, Srinivas TR, Sirivongs D, Johnson RJ, Nakagawa T. Thiazide diuretics exacerbate fructose-induced metabolic syndrome. J Am Soc Nephrol 18: 2724–2731, 2007. [DOI] [PubMed] [Google Scholar]
- 28.Roncal CA, Mu W, Croker B, Reungjui S, Ouyang X, Tabah-Fisch I, Johnson RJ, Ejaz AA. Effect of elevated serum uric acid on cisplatin-induced acute renal failure. Am J Physiol Renal Physiol 292: F116–F122, 2007. [DOI] [PubMed] [Google Scholar]
- 29.Rosolowsky ET, Ficociello LH, Maselli NJ, Niewczas MA, Binns AL, Roshan B, Warram JH, Krolewski AS. High-normal serum uric acid is associated with impaired glomerular filtration rate in nonproteinuric patients with type 1 diabetes. Clin J Am Soc Nephrol 3: 706–713, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sato W, Kosugi T, Zhang L, Roncal CA, Heinig M, Campbell-Thompson M, Yuzawa Y, Atkinson MA, Grant MB, Croker BP, Nakagawa T. The pivotal role of VEGF on glomerular macrophage infiltration in advanced diabetic nephropathy. Lab Invest 88: 949–961, 2008. [DOI] [PubMed] [Google Scholar]
- 31.Siu Y, Leung K, Tong M, Kwan T. Use of allopurinol in slowing the progression of renal disease through its ability to lower serum uric acid level. Am J Kidney Dis 47: 51–59, 2006. [DOI] [PubMed] [Google Scholar]
- 32.Susztak K, Raff AC, Schiffer M, Bottinger EP. Glucose-induced reactive oxygen species cause apoptosis of podocytes and podocyte depletion at the onset of diabetic nephropathy. Diabetes 55: 225–233, 2006. [PubMed] [Google Scholar]
- 33.Tseng CH Correlation of uric acid and urinary albumin excretion rate in patients with type 2 diabetes mellitus in Taiwan. Kidney Int 68: 796–801, 2005. [DOI] [PubMed] [Google Scholar]
- 34.Ziyadeh FN Significance of tubulointerstitial changes in diabetic renal disease. Kidney Int Suppl 54: S10–S13, 1996. [PubMed] [Google Scholar]
- 35.Ziyadeh FN, Hoffman BB, Han DC, Iglesias-De La Cruz MC, Hong SW, Isono M, Chen S, McGowan TA, Sharma K. Long-term prevention of renal insufficiency, excess matrix gene expression, and glomerular mesangial matrix expansion by treatment with monoclonal antitransforming growth factor-beta antibody in db/db diabetic mice. Proc Natl Acad Sci USA 97: 8015–8020, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]