Abstract
Elevated level of homocysteine (Hcy), known as hyperhomocysteinemia (HHcy), is associated with end-stage renal diseases. Hcy metabolizes in the body to produce hydrogen sulfide (H2S), and studies have demonstrated a protective role of H2S in end-stage organ failure. However, the role of H2S in HHcy-associated renal diseases is unclear. The present study was aimed to determine the role of H2S in HHcy-associated renal damage. Cystathionine-β-synthase heterozygous (CBS+/−) and wild-type (WT, C57BL/6J) mice with two kidney (2-K) were used in this study and supplemented with or without NaHS (30 μmol/l, H2S donor) in the drinking water. To expedite the HHcy-associated glomerular damage, uninephrectomized (1-K) CBS(+/−) and 1-K WT mice were also used with or without NaHS supplementation. Plasma Hcy levels were elevated in CBS(+/−) 2-K and 1-K and WT 1-K mice along with increased proteinuria, whereas, plasma levels of H2S were attenuated in these groups compared with WT 2-K mice. Interestingly, H2S supplementation increased plasma H2S level and normalized the urinary protein secretion in the similar groups of animals as above. Increased activity of matrix metalloproteinase (MMP)-2 and -9 and apoptotic cells were observed in the renal cortical tissues of CBS(+/−) 2-K and 1-K and WT 1-K mice; however, H2S prevented apoptotic cell death and normalized increased MMP activities. Increased expression of desmin and downregulation of nephrin in the cortical tissue of CBS(+/−) 2-K and 1-K and WT 1-K mice were ameliorated with H2S supplementation. Additionally, in the kidney tissues of CBS(+/−) 2-K and 1-K and WT 1-K mice, increased superoxide (O2•−) production and reduced glutathione (GSH)-to-oxidized glutathione (GSSG) ratio were normalized with exogenous H2S supplementation. These results demonstrate that HHcy-associated renal damage is related to decreased endogenous H2S generation in the body. Additionally, here we demonstrate with evidence that H2S supplementation prevents HHcy-associated renal damage, in part, through its antioxidant properties.
Keywords: cystathionine-β synthase, cystathionine-γ lyase, homocysteine, matrix metalloproteinase
hydrogen sulfide (h2s) is known as a toxic gas with a very strong repulsive odor. Despite its toxicity, many prokaryotic and eukaryotic organisms thrive in sulfidic habitats (11). Recently, the presence of tissue H2S from sulfidic organisms to the animals who live in a sulfur-free environment has been confirmed by several independent investigations (10, 49). These findings unambiguously suggest that H2S is a constituent of cellular milieu. However, to date, very little is known about the physiological role of H2S in normal animal and during pathological stages of renal diseases.
Endogenously, H2S is generated in the mammalian tissue from a nonprotein amino acid, homocysteine (Hcy). This is a sulfur-containing amino acid, which is an intermediate product of methionine metabolism. In the body, two enzymes, cystathionine β-synthase (CBS) and cystathionine γ-lyase (CSE), of the transulfuration pathway of methionine metabolism catalyze conversion of H2S from Hcy. Studies from independent laboratories reported that, at low level, H2S defends organs from several pathophysiological conditions, such as oxidative stress, ischemia-reperfusion, and hypertension (20, 52, 59). Recently, Yan et al. (51) postulated that, being a reducing molecule, H2S may modulate redox tone and redox cell signaling. These authors, however, reported that at high levels H2S induces reactive oxygen species (ROS) and reactive nitrogen species (RNS) formation but at low levels decreases hydrogen peroxide (H2O2), peroxinitrite (ONOO•−), and superoxide anion (O2•−) generation induced by Hcy in a cell culture model (51). These antioxidant properties were partly mediated by increasing the antioxidant properties of cellular antioxidant molecules. Nevertheless, the endogenous status of H2S and its role in hyperhomocysteinemia (HHcy)-associated renal failure remain unknown.
Previously, we have reported that reduction in renal function leads to an increased plasma Hcy level (36, 38). The elevated plasma Hcy, in turn, causes renal insufficiency, which leads to a vicious cycle (22). Accumulated evidences from independent laboratories, including our own, suggested that, at an elevated level, Hcy is an independent and graded risk factor for cardiorenovascular diseases (7, 27, 38). Reports are also available that it may contribute to the pathogenesis of atherosclerosis (2, 26), including glomerulosclerosis, cellular apoptosis, podocyte injury, and proteinuria (22, 56). These pathophysiological effects of Hcy are mediated through generation of ROS, including O2•− and H2O2, and reduction in endothelial nitric oxide (NO) bioavailability (41, 60).
It is well known that ROS plays a major role in the extracellular matrix (ECM) remodeling during various pathophysiological conditions. One of the early events of the ECM remodeling process is the activation of matrix metalloproteinases (MMPs) (8, 39). Among MMPs, elastinases and collagenases are particularly important of matrix degradation in fibrotic occlusive diseases including renal fibrosis (5, 21). We have previously reported that MMP-2 and MMP-9 play an important role in matrix accumulation associated with diabetic nephropathy and HHcy (39). However, the role of H2S, if any, in the regulation of these two MMPs in HHcy-associated renal diseases was not defined. Also, the physiological status of glomerular podocytes, injured podocyte marker, desmin, as well as normal slit diaphragm component nephrin during HHcy is not clear. Therefore, the present study aimed to determine whether HHcy was associated with decreased plasma H2S level and increased oxidative stress during chronic renal failure by modulating MMP-2 and -9 activities, glomerular cell apoptosis, and increased urinary protein secretion. Additionally, the possible preventive role of H2S, if any, has been explored to these deleterious effects in the failing kidney.
MATERIALS AND METHODS
Animals.
Wild-type (WT, C57BL/6J) male mice aged 8 wk were obtained from Jackson Laboratories (Bar Harbor, ME) and housed in the animal care facility at University of Louisville. Mice were acclimatized for 2 wk before the start of experiments. WT (C57BL/6J) and heterozygous CBS(+/−), a model for hyperhomocysteinemic mice, were used for this study. Mice were divided into two sets, the first set of mice had two kidneys (2-K), and the groups were as follows: 1) WT, 2) CBS(+/−), 3) WT + NaHS (30 μmol/l, H2S donor), and 4) CBS(+/−) + NaHS (30 μmol/l). To speed up the renal damaging effects, the second set of mice was uninephrectomized (1-K) with all above four groups. NaHS were supplied for 8 wk to the appropriate groups. At the end of the experiments, mice were deeply anesthetized, blood was collected, and the animals were killed to harvest the tissues. All animal procedures were in accordance with the National Institute of Health Guidelines for animal research and were approved by the Institutional Animal Care and Use Committee of the University Of Louisville School Of Medicine.
Rationale for NaHS (H2S donor) dose.
The physiological concentration of H2S ranges from 10–100 μmol/l (59). NaHS in the aqueous phase produces exactly equal concentration of H2S gas in the solution. Therefore, we used 30 μmol/l NaHS in the drinking water to supplement animals with 30 μmol/l of H2S.
Antibodies and reagents.
Rabbit polyclonal antibodies to desmin and nephrin were purchased from Abcam (Cambridge, MA). Anti-β-actin antibody, NaHS, and other analytical reagents were from Sigma-Aldrich (St. Louis, MO). Horseradish peroxidase-linked anti-rabbit IgG antibody was from Santa Cruz Biotechnology (Santa Cruz, CA). PVDF membrane was from Bio-Rad (Hercules, CA).
Measurement of plasma Hcy.
The high-performance liquid chromatography (HPLC) apparatus, chromatographic conditioning, and sample preparation for plasma Hcy measurement were adopted from a previously reported procedure (25) with modifications as described earlier (39).
Measurement of plasma H2S.
To measure the H2S concentration in the plasma of each of the experimental groups, 100 μl of aliquots were mixed with 50 μl of water in microcentrifuge tubes containing 300 μl of zinc acetate (1% wt/vol) to trap H2S. The reaction was stopped after 5 min by adding 200 μl of N,N-dimethyl-p-phenylenediamine sulfate (20 mM in 7.2 M HCl), immediately followed by addition of 200 μl of FeCl3 (30 mM in 1.2 M HCl). The mixture was kept in the dark for 20 min. To precipitate protein from the plasma, 150 μl of trichloroacetic acid (10% wt/vol) was added. The mixture was then centrifuged at 10,000 g for 10 min, and the absorbance of the resulting supernatant was determined at 670 nm (53) in a 96-well plate using a spectrophotometer (Spectramax M2; Molecular Devices, Sunnyvale, CA). All samples were assayed in duplicate, and H2S concentration in the plasma was calculated against a calibration curve of NaHS (3.125–100 μM).
Urinary protein measurement.
Mice were housed in metabolic cages to collect 24-h urine samples for quantitative determination of protein content in the urine. Quantitative urinary protein concentration was determined using the Bio-Rad protein assay reagent on the basis of the Bradford dye-binding procedure (4). Protein concentrations were calculated using the calibration curve prepared from a standard solution (0–2 mg/ml) of BSA (Sigma Chemical).
In vitro MMP-2, -9 activities assay.
Gelatin zymography was performed using 1.5% in gel gelatin as described elsewhere (28). In brief, glomerular tissues were minced into small pieces in ice-cold extraction buffer (1:3 wt/vol) containing (in mmol/l) 10 cacodylic acid, 20 ZnCl, 1.5 NaN3, and 0.01% Triton X-100 (pH 5.0) and incubated overnight at 4°C with gentle shaking. The homogenate was then centrifuged for 10 min at 800 g, and supernatant was collected. Protein concentration in the sample was measured using Bradford method, and 100 μg of the protein was electrophoretically resolved for each sample in 8% SDS-PAGE containing 1.5% gelatin as MMP substrate. Gels were washed in 2.5% Triton X-100 for 30 min to remove SDS, rinsed in water, and incubated for at least 24 h in activation buffer (50 mmol/l Tris·HCl, 5 mmol/l CaCl2, and 0.02% NaN3, pH 7.5) at 37°C in a water bath with gentle shaking. Gels were then transferred to staining solution (acetic acid:methanol:water, 10:50:40) containing 0.5% Coomassie blue for 1 h at room temperature. MMP activity in the gel was detected in a dark blue background with white bands.
Cryosectioning.
The kidneys were excised, and appropriate portions of the kidney were cryopreserved in Peel-A-Way disposable plastic tissue embedding molds (Polysciences, Warrington, PA) containing tissue freezing media (Triangle Biomedical Sciences, Durham, NC). These molds were kept frozen (−70°C) until serial 5-μm tissue sections were made in Cryocut 1800 (Reichert-Jung). Cryosections were placed on Superfrost/plus microscope slides and air dried.
TUNEL staining.
Apoptotic cells were detected and quantified using an in situ Apoptosis Detection Kit [TACS Terminal deoxynucleotidyl Transferase (TdT) kit; R&D Systems, Minneapolis, MN] following manufacturer instructions. Briefly, 5-μm kidney tissue cryosections were fixed with 3.7% formaldehyde solution and the permeabilized with proteinase K. Endogenous peroxidase was quenched using hydrogen peroxide (H2O2). Next, biotinylated nucleotides were incorporated into 3′-OH ends of the DNA fragments by TdT. Positive controls were generated by treating samples with TACS-Nuclease before TdT labeling. The biotinylated nucleotides were detected using streptavidin-horseradish peroxidase conjugate followed by the substrate, TACS Blue Label. Apoptotic cells were detected by those that exhibit blue nuclear staining.
Western blots.
The kidney tissue homogenates were prepared using protein extraction buffer (0.01 M cacodylic acid pH 5.0, 0.15 M NaCl, 1 μM ZnCl2, 0.02 M CaCl2, 0.0015 M NaN3, and 0.01% vol/vol Triton X-100). The extracted proteins were collected, and pH rose to 7.5 by adding 0.1 M Tris. Equal amounts of protein were analyzed on 10% SDS-PAGE, transferred to PVDF membrane, and probed with appropriate antibodies following the earlier adopted method (39).
Detection of ROS.
The method to detect ROS, specifically superoxide, was adopted from Dayal et al. (9). The oxidative fluorescent dye, dihydroethidium (DHE; Invitrogen, Carlsbad, CA), was used in frozen kidney sections, and the intensity of the fluorescent was measured by laser scanning confocal microscopy (Fluo View 1000, Olympus). Control sections were preincubated for 30 min with 250 U/ml polyethylene glycol-superoxide dismutase (PEG-SOD; Sigma-Aldrich) before incubation with DHE. Fluorescent images were analyzed with ImagePro software (Media Cybernetics, Bethesda, MD).
Glutathione assay.
Kidney tissue levels of reduced glutathione (GSH) and oxidized glutathione (GSSG) were measured using a commercially available kit (Cayman Chemicals, Ann Arbor, MI). The GSH-to-GSSG ratio was calculated for each sample according to manufacturer's instructions.
Statistical analysis.
Values are given as means ± SE from n number of animals in each group as mentioned in each of the figure legends. Differences between groups were tested with the use of two-way ANOVA for repeated measures. Comparisons between groups were made with the use of Student's independent t-test. Significance was accepted at P < 0.05.
RESULTS
Total plasma Hcy significantly increased in CBS(+/−) mice.
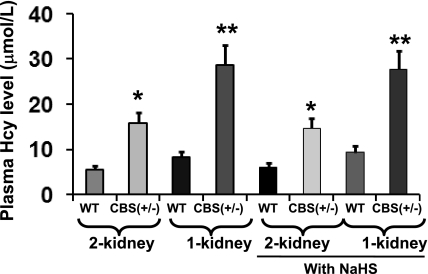
HPLC was performed to measure plasma levels of Hcy from the experimental and control samples. Hcy from the samples were identified according to the retention times and cochromatography with standards. In CBS(+/−) 2-K mice, the plasma Hcy level was found to be significantly higher compared with WT 2-K mice (Fig. 1). This increase of Hcy level in 8-wk-postsurgery mice was even higher and more dramatic in CBS(+/−) 1-K mice compared with age-matched WT 1-K mice. Although there was a tendency of higher plasma Hcy level in WT 1-K mice compared with WT 2-K mice, the difference was not significant. Additionally, there were no further changes of plasma Hcy levels in the similar groups of mice supplemented with NaHS (Fig. 1). These results clearly suggest that, although 2-K CBS(+/−) mice develop high Hcy, this effect was more acute in CBS(+/−) 1-K mice, and H2S supplementation does not have a role in the plasma Hcy level.
Fig. 1.
Effect of uninephrectomy (1-K) and H2S supplementation on plasma homocysteine (Hcy) level. Total homocysteine was extracted from plasma and analyzed by high-performance liquid chromatography as described in materials and methods using our previously adopted procedure. Data represent the means ± SE, n = 4; NaHS, 30 μmol/l. *Significant difference (P < 0.05) compared with respective wild-type (WT) two kidney mice (2-K). **Significant difference (P < 0.05) compared with respective WT 1-K. CBS, cystathionine-β-synthase.
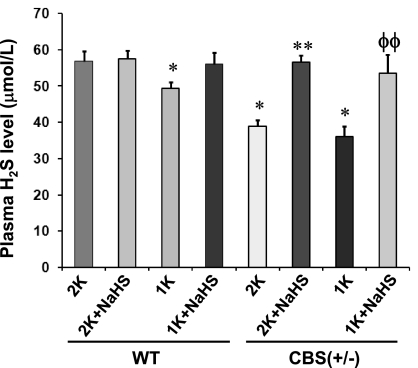
NaHS supplementation increased total plasma H2S level.
Plasma total H2S levels were decreased ∼12% in WT 1-K mice compared with WT 2-K mice, whereas there was virtually no difference between WT 2-K mice supplemented with or without NaHS (Fig. 2). However, a significant increase in plasma H2S was observed in WT 1-K mice supplemented with NaHS, which was comparable to WT 2-K mice. H2S level in CBS(+/−) 2-K mice was found significantly lower than in WT 2-K littermates. This difference was even higher in CBS(+/−) 1-K mice. Interestingly, NaHS treatment in CBS(+/−) 2-K mice showed a significant increase (∼32%, compared with its 2-K littermates without NaHS supplementation) in plasma H2S level, which was comparable to WT 2-K mice. Similar results were observed (∼29% increase of plasma H2S level, compared with its 1-K littermates without NaHS supplementation) in CBS(+/−) 1-K mice treated with NaHS (Fig. 2). These results suggest that HHcy is associated with decreased plasma H2S level and that exogenous supplementation of NaHS increases plasma total H2S level.
Fig. 2.
Effect of NaHS supplementation on plasma H2S level. Reduced H2S production was observed in CBS(+/−) 2-K, as well as CBS(+/−) 1-K and WT 1-K mice. Interestingly, NaHS supplementation increased total plasma H2S level in these mice. *Significant differences (P < 0.05) compared with WT 2-K mice. **P < 0.05 vs. CBS(+/−) 2-K mice; ΦΦP < 0.05 vs. CBS(+/−) 1-K. Data represent the means ± SE, n = 6 animals in each group.
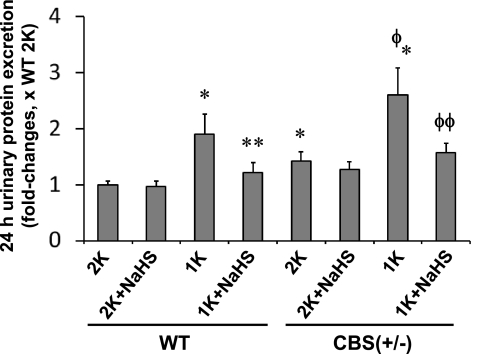
Hydrogen sulfide prevented proteinuria in 1-K mice.
Urinary protein concentration was higher in CBS(+/−) 2-K mice compared with WT 2-K mice (Fig. 3). In these animals, however, NaHS did not affect total urinary protein excretion. Compared with WT 2-K mice, WT 1-K mice showed significant hyperproteinuria. This effect was even more acute in CBS(+/−) 1-K mice. Interestingly, in response to NaHS, the increased proteinuria in both the WT 1-K and CBS 1-K mice was normalized. This result suggests that increased proteinuria was attributable to HHcy-associated glomerular damage, and this damage can be partially prevented by H2S supplementation.
Fig. 3.
Role of H2S in homocysteine-associated proteinuria. Urinary protein was measured using Bradford method. *Significant difference (P < 0.05) compared with WT 2-K. **P < 0.05 vs. WT 1-K mice; Φ P < 0.05 vs. CBS(+/−) 2-K; ΦΦP < 0.05 vs. CBS(+/−) 1-K. Data represent the means ± SE, n = 8 animals per group.
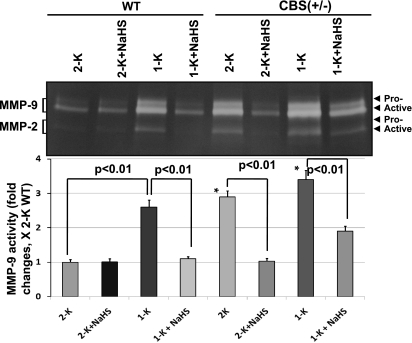
MMP activities attenuated by H2S.
MMPs are involved in the remodeling process in the normal and diseased vascular beds. Hence, we examined the MMP-2 and -9 activities in the kidney because these two MMPs are critically involved in the glomerular matrix remodeling process. As shown in the Fig. 4, WT 2-K mice showed basal level of the proform and active form of MMP-9 activity as well as active form of MMP-2 activity in the cortical tissue extract. The activities of these MMPs were elevated in WT 1-K mice. Contrary to WT 2-K mice, CBS(+/−) 2-K mice showed a very high level of these MMP activities, which were further elevated in CBS(+/−) 1-K mice (Fig. 4). Interestingly, H2S supplementation almost normalized these MMP activities in WT 1-K and both in CBS(+/−) 2-K and 1-K mice. These results suggest that both the MMP-2 and -9 play a major role in high Hcy-associated renopathy. Importantly, modulation of these two MMPs by H2S suggests a possible trigger of both these of MMPs during HHcy in the renal cortex.
Fig. 4.
H2S regulates matrix metalloproteinase (MMP)-2 and -9 activities during hyperhomocysteinemia. In gel, gelatin zymography was performed to measure MMP-2 and -9 activities in the kidney cortex tissue-extracted protein. Both proforms and active forms of MMP-9 and active form of MMP-2 in WT 1-K, CBS(+/−) 2-K, and 1-K mice were found to be significantly higher than WT 2-K littermates. These MMP activities were attenuated by H2S supplementation (NaHS, 30 μmol/l) for the period of 8 wk postsurgery; data represent means ± SE, n = 5 for WT mice per group, and n = 6 for CBS(+/−) mice per group; *P < 0.01 vs. WT 2-K.
H2S prevented HHcy-associated glomerular cell death.
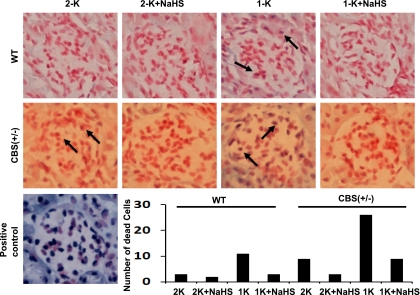
We determined the glomerular cell apoptosis by TUNEL staining. Representative images of TUNEL staining showed that, in the glomerulus of WT 2-K kidney, there were very few apoptotic podocytic cells (Fig. 5). Similarly, WT 2-K mice treated with NaHS showed very few dead cells. WT 1-K mice showed a relatively high number of apoptotic cells (Fig. 5). These increases in apoptotic cells were absent when WT 1-K mice were supplemented with NaHS.
Fig. 5.
Effect of H2S on glomerular cell death. Histological kidney sections were analyzed for in situ apoptosis as described in materials and methods. Numbers of dead cells were counted under the microscope from 15 randomized fields in each group, quantitated, and plotted as bar diagram as shown; n = 7 in each group; ×200 magnification.
In the CBS(+/−) 2-K mice, however, although a very minimal number of apoptotic cells was identified, the number of apoptotic cells was higher than in WT 2-K littermates (Fig. 5). This number was dramatically increased in CBS(+/−) 1-K mice, which was almost completely prevented in the mice treated with NaHS. Likewise, in the kidney of CBS(+/−) 2-K mice treated with NaHS, a minimal number of apoptotic cells was observed.
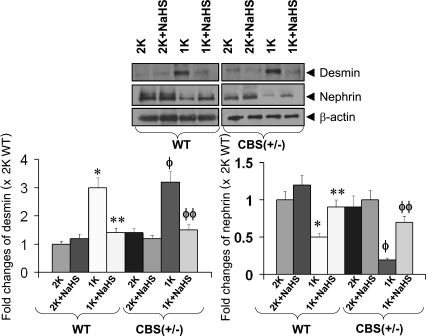
Expression of desmin and nephrin during HHcy.
A basal level of desmin expression was observed in CBS(+/−) 2-K mice. This expression was almost comparable with WT 2-K mice; however, the expression was increased significantly in both WT 1-K and CBS(+/−) 1-K mice although the expression was higher in CBS(+/−) 1-K mice (Fig. 6). WT 1-K and CBS(+/−)1-K mice supplemented with NaHS prevented the increased expression of desmin, and the levels were comparable to their respective 2-K littermates. Contrary to the desmin expression, the expression of nephrin in the kidney was found to be opposite in the respective experimental groups. NaHS treatment, however, prevented the changes of nephrin expressions in WT 1-K and both the CBS(+/−) 2-K and CBS(+/−)1-K mice (Fig. 6).
Fig. 6.
H2S normalized tissue expression of desmin and nephrin. Protein was extracted from the kidney tissue and was analyzed by Western blot. Equal amount of protein was loaded in each well, and the expression of each of the proteins was normalized with β-actin. *Significant difference (P < 0.05) compared with WT 2-K. **P < 0.05 vs. WT 1-K mice; ΦP < 0.05 vs. CBS(+/−) 2-K; ΦΦP < 0.05 vs. CBS(+/−) 1-K. Data represent the means ± SE, n = 6 per group.
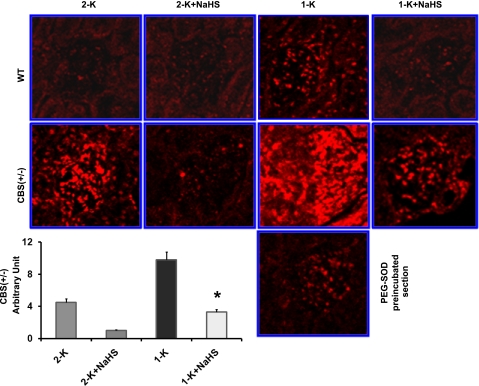
Increased ROS production was associated with HHcy.
Production of ROS in the glomerulus was measured using oxidative fluorescent dye, DHE (Fig. 7). The DHE fluorescence indicates ROS production. When CBS(+/−) mice were supplemented with exogenous H2S, a significant decrease in DHE fluorescence was observed. The fluorescence intensity in CBS(+/−) 2-K mice was higher compared with WT 2-K age-matched littermates. Interestingly, in CBS(+/−) 1-K mice, a robust increase of DHE fluorescence was observed. PEG, which is covalently linked to SOD (PEG-SOD) and a potent superoxide (O2•−) anion scavenger, dramatically diminished DHE fluorescence, suggesting that the increased DHE fluorescence observed in CBS(+/−)1-K was attributable to increased production of O2•−.
Fig. 7.
H2S mitigated reactive oxygen species (ROS) production. Production of ROS in the frozen kidney sections was measured using oxidative fluorescent dye, dihydroethidium (DHE), as described in materials and methods. DHE fluorescence was detected at a higher level in the glomerulus of CBS(+/−) 1-K mice. Interestingly, CBS(+/−) 1-K mice supplemented with exogenous H2S showed a significant decrease in DHE fluorescence. In addition, preincubation of CBS(+/−) 1-K kidney sections with polyethylene glycol-superoxide dismutase (PEG-SOD) dramatically diminished DHE fluorescence, suggesting that the major portions of ROS are superoxide (O2•−). *Significant difference (P < 0.05) vs. CBS(+/−) 1-K (means ± SE, n = 5–6 animals in each group). Magnification, ×200.
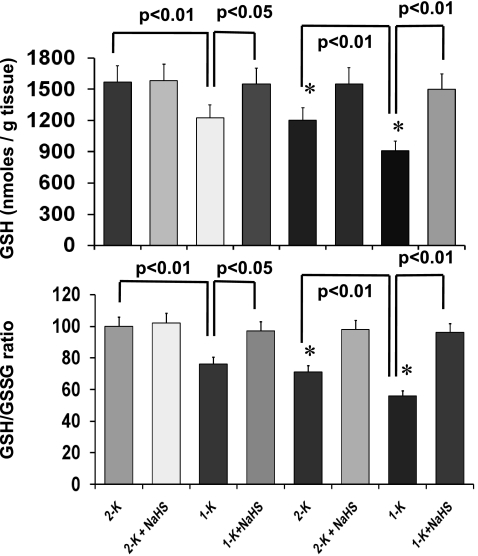
H2S increased cortical tissue GSH-to-GSSG ratio.
GSH is a potent intracellular antioxidant, and GSH-to-GSSG ratio corresponds to the capacity of a cell to attenuate oxidative stress (13). To assess the oxidative redox state in our experimental groups, we measured the cortical-tissue reduced GSH and GSH-to-GSSG ratio, and the calculated data are shown in Fig. 8. There was a significant decrease of GSH level in WT 1-K and both in CBS(+/−) 2-K and CBS(+/−)1-K mice compared with WT 2-K control groups. Also, a significant decrease was observed in GSH/GSSG ratio in these groups. Interestingly, H2S supplementation normalized the GSH and GSH/GSSG ratio, suggesting that H2S maintained the intracellular antioxidant capacity.
Fig. 8.
Decreased glutathione (GSH)/oxidized glutathione (GSSG) ratio was normalized by H2S. Cortical tissue reduced GSH, and GSH-to-GSSG ratio was measured and calculated as described in materials and methods. H2S therapy significantly improved GSH level in WT 1-K and in CBS(+/−)2-K and CBS(+/−)1-K mice. Additionally, H2S preserved the GSH/GSSG ratio compared with respective nontreated groups. Data represent means ± SE, n = 7 per group. *P < 0.05 vs. 2-K WT.
DISCUSSION
The present study clearly demonstrates that plasma Hcy was increased in CBS(+/−) mice, and this increase was further elevated when renal function was reduced by the removal of one kidney. The plasma Hcy was also correlated with the extent of renal damage as detected by proteinuria. Plasma H2S levels were found to be the opposite to that of the Hcy levels, and this decrease in H2S level was elevated back toward normal with exogenous supplementation of NaHS, a donor of H2S. The increased MMP-2 and -9 activities and the occurrence of glomerular cell death were associated with the increased oxidative stress, which has been shown to be associated with HHcy. In addition to that, the increased proteinuria was corroborated with the renal damage, as evidenced by increased expression of podocyte injury marker, desmin, and decreased expression of glomerular slit diaphragm protein, nephrin. Interestingly, the extent of HHcy-associated renal damage was ameliorated by H2S supplementation. Although it is not completely understood, it appears that the defense mechanism of H2S was exhibited, in part, through reducing oxidative stress and maintaining intracellular GSH-to-GSSG ratio. This mechanism, in addition to normalizing the expression of desmin and nephrin, prevented glomerular cell apoptosis and restored basal MMP activities. These resulted in reversals of proteinuria in our hyperhomocysteinemic experimental animal model treated with H2S.
HHcy is a risk factor for chronic kidney disease and is associated with end-stage renal disease. In disease condition, such as in diabetic nephropathy, Hcy disposal and clearance are impaired and therefore accumulate in the body, resulting in increase of plasma and tissue levels of Hcy (43). This, in turn, causes renal microvascular impairment and vasoconstriction (37), which lead to renal volume retention and further accumulation of Hcy (38). This is a vicious cycle and is often associated with chronic renal failure. The purpose of uninephrectomy (1-K), in the present study, was to impair the renal function to subnormal level, which further accumulates Hcy through increased renal volume retention. The results from our study suggest that 1-K mice have more Hcy in the plasma than their respective 2-K control groups. Therefore, this model was created and used to investigate Hcy-associated renal damage and remodeling process in the kidney.
In the body, Hcy is metabolized by two enzymes, CBS and CSE, and produces a gaseous substance, H2S (16, 42). CBS is a predominant H2S-generating enzyme in the brain and nervous tissues (1, 12), and CSE is mainly expressed in the liver, kidney, and vascular smooth muscle cells (3, 61). Although Hcy has been shown to promote CSE activity at its lower concentrations, inhibition of CSE activity was reported at its higher concentration in the rat liver (54). At pathological conditions, elevated levels of Hcy alter the transulfuration pathway by inhibiting CSE enzyme activity (6), thereby reducing endogenous production of H2S in the body. In the present study, we investigated the effect of an increased level of plasma Hcy, not the glomerular tissue level of Hcy, on the glomerulus and its consequences in the renal remodeling process. CBS(+/−) mice show higher levels of plasma Hcy and a very reliable model to test HHcy-related disease process. Additionally, it is also reported that HHcy inhibits CSE (6); therefore, the use of CBS(+/−) mice is advantageous over CSE(+/−). Hence, we used CBS(+/−) mice for the present study to investigate the HHcy-related renal-damaging effect.
The growing body of evidence suggests that, despite its past reputation as a noxious gas, H2S is rapidly emerging as a third gaseous transmitter, in addition to nitric oxide and carbon monoxide (16, 17, 46); the physiological function of endogenous H2S, however, is not clear. It is reported that H2S is involved in the regulation of vascular tone (24) and hypertension (52) and protects neuronal cells from oxidative stress by increasing the intracellular concentration of antioxidant and GSH (19, 48). Recently, Tripatara et al. (44) demonstrated that generation of endogenous H2S limits renal ischemia/reperfusion injury and dysfunction. In the present study, to test any protective effects of H2S in HHcy-associated renal damage, we used CBS(+/−) mutant mice. These mice have an ∼50% reduction in CBS mRNA and enzyme activity in the liver and have twice the normal plasma Hcy levels than WT littermates (35). Thus the CBS(+/−) mice develop mild HHcy and are a very good model to study HHcy-related disease processes, including cardiovascular-renal diseases. Reports are available that CBS inhibitors hydroxylamine and amino-oxyacetate suppress the production of H2S and that a CBS activator, S-adenosyl-l-methionine, enhances H2S production in the brain tissue (1). A similar trend was observed by Xia et al. (50) where inhibition of CBS reduced endogenous generation of H2S in the kidney tissues. These reports indicated the regulatory role of CBS enzyme in the production of endogenous H2S. Our present result (Fig. 1) suggested that HHcy was associated with CBS deficiency, where CBS(+/−) mutant mice exhibited a higher level of plasma Hcy. This increase of plasma Hcy level was even higher in CBS(+/−) mice after uninephrectomy (1-K). Thus the results clearly indicated a strong relationship between renal insufficiency and plasma Hcy level. Because Hcy is one of the precursors of endogenous H2S generation, it was expected that increase of plasma Hcy level would eventually be elevated in the plasma H2S level. Contrary to this mechanism, the data presented in Fig. 2 showed that the plasma H2S level was, in fact, inversely regulated by plasma Hcy level, where increased Hcy repressed H2S level in the plasma. This may be due to a negative feedback mechanism, where increased plasma Hcy inhibited its metabolizing enzyme, CSE and/or CBS, resulting in low production of H2S. We previously reported that stress, such as volume overload, decreased CSE expression and subsequent H2S generation in the cardiac tissue (40). Whether this mechanism is still applicable to HHcy-associated oxidative stress and regulates the plasma H2S level needs to be verified. Nevertheless, the results from our present study indicated the possibility of such mechanisms that may be involved in part, if not the only mechanism, which triggered endogenous generation of H2S and subsequent plasma level of this gaseous substance.
Along the same line, Wei et al. (48) showed that severe oxidative stress was present in a model of hypoxic pulmonary hypertension and was accompanied by a decrease in the endogenous production of H2S in the lung tissue. H2S, however, acted as an antioxidant during this oxidative stress, which was a result of the attenuated GSSG content. More recently, perfusion of H2S in ischemia-reperfusion-injured lung has been shown to reduce malondialdehyde production, potentiated SOD, and catalase (CAT) activities and restrain superoxide (O2•−) production in the lung, resulting in an attenuated oxidative lung injury (15). This emerging evidence suggests the potential antioxidant properties of H2S in normal and pathophysiological conditions. To determine the antioxidant role of H2S, we measured the reduced GSH content and reduced GSH-to-GSSG ratio in our study. Our results suggested that H2S supplementation normalized the reduced GSH and reduced-GSH-to-GSSG ratio associated with HHcy. In our experiments, although we have found that H2S supplementation increased GSH/GSSG ratio in the kidney tissues, the exact mechanisms, however, were not elucidated. Recently, Liu et al. (23) reported that H2S protected intestinal ischemia-reperfusion injury by increasing serum and intestinal level of SOD and GSH peroxidase (23). Our laboratory has previously shown that, in vitro, H2S enhanced the inhibitory effects of CAT and SOD in methionine-loaded oxidative stress in mouse brain endothelial cells (45). This mechanism has clearly indicated the antioxidant property of H2S and was partly mediated by increasing intracellular CAT and SOD. The present study did not aim to elucidate this mechanism; however, whether or not the same mechanism applies to HHcy-associated renal remodeling needs to be explored comprehensively. Interestingly, in the present study, increased superoxide (O2•−) production was attenuated with H2S supplementation in the mice that exhibited high Hcy. These results suggest that H2S protects glomerular tissue, at least in part, through its antioxidant properties.
H2S had been reported to be a general protective mechanism for degenerative organ damages. Herein, we have focused our study to ameliorate HHcy-associated kidney damages, if any, through exogenous H2S supplementation. It is a need of future investigation to elucidate whether H2S could be such a protective factor in the hypertensive kidney damages. For example, does H2S protect DOCA salt-induced kidney damage? It is reported that DOCA salt-induced hypertension decreased SOD in the rat aorta and that antioxidant therapy increased SOD activity (31). Given the fact that both DOCA salt and Hcy induce oxidative stress through inhibition of SOD, and H2S is an enhancer of intracellular SOD, it is possible that H2S may also play a role to increase SOD activity in the kidney of DOCA salt-induced hypertensive rat. Although this is a very interesting area to study antioxidant properties of H2S, the scope of the present investigation, however, was limited to investigate the protective role of H2S in HHcy-associated renal damages.
Clinical studies have implicated proteinuria as a key prognostic factor for renal complications in hypertension; however, the pathogenesis causing proteinuria is poorly understood (30). It is reported that dysfunction of podocytes, the final filtration barrier in the glomerulus, plays a pivotal role in proteinuria (29, 32, 47). Yi et al. (55) have shown that urinary albumin excretion increased at the second week of methionine, the precursor of Hcy, treatment. These reports established a strong correlation between high Hcy and kidney disease. In our present study, we have found that proteinuria was strongly correlated with high Hcy and attenuated H2S in the plasma. Increased Hcy levels were associated with apoptosis of podocytes, in part attributable to reduction of H2S and increased oxidative stress, and therefore damaged the final filtration barrier of the kidney. These changes in the glomerulus allowed excessive protein excretion in the urine. On the contrary to this mechanism, H2S supplementation prevented podocyte apoptosis, and this provided protection to the kidney against HHcy-associated renal damage and proteinuria. It is important to mention that, although the level of protein excretion was high in CBS (+/−) 2-K mice compared with WT 2-K mice, the difference was not significant even though the reduction of plasma H2S levels was significant in CBS(+/−) 2-K mice compared with WT 2-K mice. Herein, it is possible that other antioxidants in the tissue, such as CAT, SOD, lipid peroxidase, etc., may have played a similar role as of H2S, to repress the oxidative stress, and therefore ameliorated proteinuria in CBS(+/−) 2-K mice. Thus the observed proteinuria in CBS(+/−) mice was not parallel with the reduction of H2S levels. Further understanding of the regulatory mechanisms of proteinuria by H2S in HHcy-associated renal failure may provide a new insight into the molecular mechanisms of this disease process.
Proteinuria is a hallmark of renal complication and a major deteriorating factor for the progression to end-stage renal diseases (34). The outer aspect of glomerular basement membrane is lined up with very specialized visceral epithelial cells, named podocytes, and these podocytes serve as the final defense against urinary protein loss in the normal glomerulus (30). Any damage to the podocytes and their slit diaphragm is intimately associated with proteinuria (32). Biochemical assessment of normal slit diaphragm component, such as nephrin (18), and injured podocyte marker desmin (14) are now therefore considered as two major sensitive markers of podocyte injury and subsequently glomerulopathy in renal diseases. Therefore, we measured desmin and nephrin in the kidney tissue extract to asses whether the kidney injury is associated with a high level of plasma Hcy and whether H2S supplementation can ameliorate this change. In our present study, we have found a conspicuous increase in the expression of desmin, whereas expression of nephrin was decreased in the mice showing high Hcy levels and decreased plasma H2S level. H2S supplementation reversed the effect on these two protein expressions associated with high Hcy, suggesting the regulatory role of H2S on these two proteins during HHcy.
High Hcy has been reported to produce a sustained and abnormal elevation of glomerular arterial wall stress through generation of ROS (57, 58). This stress initiates a complex and progressive glomerular remodeling, including activation of MMPs, collagen degradation, glomerular hypertrophy, and dysfunction (22, 37). One of the major causes of glomerular sclerosis and renal dysfunction is the increase of glomerular ECM. Glomerular ECM, which is composed of mesangial matrix and basement membrane, plays an important role in physical, mechanical, and structural functions of the glomerulus. MMPs degrade both the collagenous and noncollagenous components of the ECM and are thereby actively involved in matrix turnover. In pathological conditions, collagenases initiate the degradation process of ECM and denature collagen into nonhelical gelatin derivatives. Gelatinases, which are a member of the family of MMPs, digest these products into smaller peptides. Of particular interest are gelatinases MMP-2 and MMP-9, which have potential capability to disrupt the kidney architecture by virtue of their specificity for various components of basement membrane (33). Therefore, the measurement of MMP-2 and -9 activities allows for an estimation of the remodeling process during HHcy-associated glomerulopathies. Data from our present report suggested that high Hcy induced elevation of superoxide (O2•−) production in the CBS(+/−) kidney, and this production of O2•− was even greater in 1-K mice. The level of MMP-2 and -9 activities followed the increasing trend of O2•− production in both 2-K and 1-K CBS(+/−) mice. H2S supplementation normalized both MMP activity level and the increased level of O2•− production in these mice. This result suggests that the activities of MMP-2 and -9 are associated with increased O2•− production and that H2S scavenges O2•− production, thereby regulating MMP activities in our experimental condition. This regulatory mechanism of O2•− by H2S prevented renal damage and subsequent renal failure associated with HHcy.
In summary, we have shown that elevation of plasma Hcy level causes a decrease in the level of plasma H2S and is associated with renal impairment. This increase of plasma Hcy level induced glomerular oxidative stress, resulting in augmented MMP activities and induction in glomerular cell apoptosis. Additionally, HHcy altered expression of desmin and the final filtration barrier regulatory protein, nephrin. These changes in cellular and protein level indicated damage in the kidney, which was exhibited by proteinuria, a marker of renal failure. H2S supplementation, however, showed the reversal of these deleterious changes associated with HHcy and is therefore protective to the kidney.
Acknowledgments
This study was supported, in part, by NIH grants HL-71010, HL-88012, HL-74185, and NS-51568.
REFERENCES
- 1.Abe K, Kimura H. The possible role of hydrogen sulfide as an endogenous neuromodulator. J Neurosci 16: 1066–1071, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Austin RC, Lentz SR, Werstuck GH. Role of hyperhomocysteinemia in endothelial dysfunction and atherothrombotic disease. Cell Death Differ 11, Suppl 1: S56–S64, 2004. [DOI] [PubMed] [Google Scholar]
- 3.Barber T, Triguero A, Martinez-Lopez I, Torres L, Garcia C, Miralles VJ, Vina JR. Elevated expression of liver gamma-cystathionase is required for the maintenance of lactation in rats. J Nutr 129: 928–933, 1999. [DOI] [PubMed] [Google Scholar]
- 4.Bradford MM A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72: 248–254, 1976. [DOI] [PubMed] [Google Scholar]
- 5.Chang HR, Yang SF, Li ML, Lin CC, Hsieh YS, Lian JD. Relationships between circulating matrix metalloproteinase-2 and -9 and renal function in patients with chronic kidney disease. Clin Chim Acta 366: 243–248, 2006. [DOI] [PubMed] [Google Scholar]
- 6.Chang L, Geng B, Yu F, Zhao J, Jiang H, Du J, Tang C. Hydrogen sulfide inhibits myocardial injury induced by homocysteine in rats. Amino Acids 34: 573–585, 2008. [DOI] [PubMed] [Google Scholar]
- 7.Clarke R, Daly L, Robinson K, Naughten E, Cahalane S, Fowler B, Graham I. Hyperhomocysteinemia: an independent risk factor for vascular disease. N Engl J Med 324: 1149–1155, 1991. [DOI] [PubMed] [Google Scholar]
- 8.Cruz CI, Ruiz-Torres P, del Moral RG, Rodriguez-Puyol M, Rodriguez-Puyol D. Age-related progressive renal fibrosis in rats and its prevention with ACE inhibitors and taurine. Am J Physiol Renal Physiol 278: F122–F129, 2000. [DOI] [PubMed] [Google Scholar]
- 9.Dayal S, Wilson KM, Leo L, Arning E, Bottiglieri T, Lentz SR. Enhanced susceptibility to arterial thrombosis in a murine model of hyperhomocysteinemia. Blood 108: 2237–2243, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Doeller JE, Grieshaber MK, Kraus DW. Chemolithoheterotrophy in a metazoan tissue: thiosulfate production matches ATP demand in ciliated mussel gills. J Exp Biol 204: 3755–3764, 2001. [DOI] [PubMed] [Google Scholar]
- 11.Doeller JE, Isbell TS, Benavides G, Koenitzer J, Patel H, Patel RP, Lancaster JR Jr, Darley-Usmar VM, Kraus DW. Polarographic measurement of hydrogen sulfide production and consumption by mammalian tissues. Anal Biochem 341: 40–51, 2005. [DOI] [PubMed] [Google Scholar]
- 12.Eto K, Ogasawara M, Umemura K, Nagai Y, Kimura H. Hydrogen sulfide is produced in response to neuronal excitation. J Neurosci 22: 3386–3391, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 13.Exner R, Wessner B, Manhart N, Roth E. Therapeutic potential of glutathione. Wien Klin Wochenschr 112: 610–616, 2000. [PubMed] [Google Scholar]
- 14.Floege J, Kriz W, Schulze M, Susani M, Kerjaschki D, Mooney A, Couser WG, Koch KM. Basic fibroblast growth factor augments podocyte injury and induces glomerulosclerosis in rats with experimental membranous nephropathy. J Clin Invest 96: 2809–2819, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fu Z, Liu X, Geng B, Fang L, Tang C. Hydrogen sulfide protects rat lung from ischemia-reperfusion injury. Life Sci 82: 1196–1202, 2008. [DOI] [PubMed] [Google Scholar]
- 16.Geng B, Yang J, Qi Y, Zhao J, Pang Y, Du J, Tang C. H2S generated by heart in rat and its effects on cardiac function. Biochem Biophys Res Commun 313: 362–368, 2004. [DOI] [PubMed] [Google Scholar]
- 17.Hosoki R, Matsuki N, Kimura H. The possible role of hydrogen sulfide as an endogenous smooth muscle relaxant in synergy with nitric oxide. Biochem Biophys Res Commun 237: 527–531, 1997. [DOI] [PubMed] [Google Scholar]
- 18.Kawachi H, Koike H, Kurihara H, Yaoita E, Orikasa M, Shia MA, Sakai T, Yamamoto T, Salant DJ, Shimizu F. Cloning of rat nephrin: expression in developing glomeruli and in proteinuric states. Kidney Int 57: 1949–1961, 2000. [DOI] [PubMed] [Google Scholar]
- 19.Kimura Y, Dargusch R, Schubert D, Kimura H. Hydrogen sulfide protects HT22 neuronal cells from oxidative stress. Antioxid Redox Signal 8: 661–670, 2006. [DOI] [PubMed] [Google Scholar]
- 20.Kimura Y, Kimura H. Hydrogen sulfide protects neurons from oxidative stress. FASEB J 18: 1165–1167, 2004. [DOI] [PubMed] [Google Scholar]
- 21.Lelongt B, Legallicier B, Piedagnel R, Ronco PM. Do matrix metalloproteinases MMP-2 and MMP-9 (gelatinases) play a role in renal development, physiology and glomerular diseases? Curr Opin Nephrol Hypertens 10: 7–12, 2001. [DOI] [PubMed] [Google Scholar]
- 22.Li N, Chen YF, Zou AP. Implications of hyperhomocysteinemia in glomerular sclerosis in hypertension. Hypertension 39: 443–448, 2002. [DOI] [PubMed] [Google Scholar]
- 23.Liu H, Bai XB, Shi S, Cao YX. Hydrogen sulfide protects from intestinal ischaemia-reperfusion injury in rats. J Pharm Pharmacol 61: 207–212, 2009. [DOI] [PubMed] [Google Scholar]
- 24.Lowicka E, Beltowski J. Hydrogen sulfide (H2S)—the third gas of interest for pharmacologists. Pharmacol Rep 59: 4–24, 2007. [PubMed] [Google Scholar]
- 25.Malinow MR, Kang SS, Taylor LM, Wong PW, Coull B, Inahara T, Mukerjee D, Sexton G, Upson B. Prevalence of hyperhomocyst(e)inemia in patients with peripheral arterial occlusive disease. Circulation 79: 1180–1188, 1989. [DOI] [PubMed] [Google Scholar]
- 26.McCully KS Chemical pathology of homocysteine. I. Atherogenesis. Ann Clin Lab Sci 23: 477–493, 1993. [PubMed] [Google Scholar]
- 27.McCully KS Homocysteine and vascular disease. Nat Med 2: 386–389, 1996. [DOI] [PubMed] [Google Scholar]
- 28.Moshal KS, Rodriguez WE, Sen U, Tyagi SC. Targeted deletion of MMP-9 attenuates myocardial contractile dysfunction in heart failure. Physiol Res 57: 379–384, 2008. [DOI] [PubMed] [Google Scholar]
- 29.Mundel P, Shankland SJ. Podocyte biology and response to injury. J Am Soc Nephrol 13: 3005–3015, 2002. [DOI] [PubMed] [Google Scholar]
- 30.Nagase M, Shibata S, Yoshida S, Nagase T, Gotoda T, Fujita T. Podocyte injury underlies the glomerulopathy of Dahl salt-hypertensive rats and is reversed by aldosterone blocker. Hypertension 47: 1084–1093, 2006. [DOI] [PubMed] [Google Scholar]
- 31.Ndisang JF, Lane N, Jadhav A. Crosstalk between the heme oxygenase system, aldosterone, and phospholipase C in hypertension. J Hypertens 26: 1188–1199, 2008. [DOI] [PubMed] [Google Scholar]
- 32.Pavenstadt H, Kriz W, Kretzler M. Cell biology of the glomerular podocyte. Physiol Rev 83: 253–307, 2003. [DOI] [PubMed] [Google Scholar]
- 33.Rao VH, Lees GE, Kashtan CE, Nemori R, Singh RK, Meehan DT, Rodgers K, Berridge BR, Bhattacharya G, Cosgrove D. Increased expression of MMP-2, MMP-9 (type IV collagenases/gelatinases), and MT1-MMP in canine X-linked Alport syndrome (XLAS). Kidney Int 63: 1736–1748, 2003. [DOI] [PubMed] [Google Scholar]
- 34.Remuzzi G, Bertani T. Pathophysiology of progressive nephropathies. N Engl J Med 339: 1448–1456, 1998. [DOI] [PubMed] [Google Scholar]
- 35.Robert K, Chasse JF, Santiard-Baron D, Vayssettes C, Chabli A, Aupetit J, Maeda N, Kamoun P, London J, Janel N. Altered gene expression in liver from a murine model of hyperhomocysteinemia. J Biol Chem 278: 31504–31511, 2003. [DOI] [PubMed] [Google Scholar]
- 36.Rodriguez WE, Sen U, Tyagi N, Kumar M, Carneal G, Aggrawal D, Newsome J, Tyagi SC. PPAR gamma agonist normalizes glomerular filtration rate, tissue levels of homocysteine, and attenuates endothelial-myocyte uncoupling in alloxan induced diabetic mice. Int J Biol Sci 4: 236–244, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rodriguez WE, Tyagi N, Joshua IG, Passmore JC, Fleming JT, Falcone JC, Tyagi SC. Pioglitazone mitigates renal glomerular vascular changes in high-fat, high-calorie-induced type 2 diabetes mellitus. Am J Physiol Renal Physiol 291: F694–F701, 2006. [DOI] [PubMed] [Google Scholar]
- 38.Sen U, Rodriguez WE, Tyagi N, Kumar M, Kundu S, Tyagi SC. Ciglitazone, a PPARγ agonist, ameliorates diabetic nephropathy in part through homocysteine clearance. Am J Physiol Endocrinol Metab 295: E1205–E1212, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sen U, Tyagi N, Kumar M, Moshal KS, Rodriguez WE, Tyagi SC. Cystathionine-β-synthase gene transfer and 3-deazaadenosine ameliorate inflammatory response in endothelial cells. Am J Physiol Cell Physiol 293: C1779–C1787, 2007. [DOI] [PubMed] [Google Scholar]
- 40.Sen U, Vacek TP, Hughes WM, Kumar M, Moshal KS, Tyagi N, Metreveli N, Hayden MR, Tyagi SC. Cardioprotective role of sodium thiosulfate on chronic heart failure by modulating endogenous H2S generation. Pharmacology 82: 201–213, 2008. [DOI] [PubMed] [Google Scholar]
- 41.Stamler JS, Osborne JA, Jaraki O, Rabbani LE, Mullins M, Singel D, Loscalzo J. Adverse vascular effects of homocysteine are modulated by endothelium-derived relaxing factor and related oxides of nitrogen. J Clin Invest 91: 308–318, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Swaroop M, Bradley K, Ohura T, Tahara T, Roper MD, Rosenberg LE, Kraus JP. Rat cystathionine beta-synthase. Gene organization and alternative splicing. J Biol Chem 267: 11455–11461, 1992. [PubMed] [Google Scholar]
- 43.Tessari P, Coracina A, Kiwanuka E, Vedovato M, Vettore M, Valerio A, Zaramella M, Garibotto G. Effects of insulin on methionine and homocysteine kinetics in type 2 diabetes with nephropathy. Diabetes 54: 2968–2976, 2005. [DOI] [PubMed] [Google Scholar]
- 44.Tripatara P, Patel NS, Collino M, Gallicchio M, Kieswich J, Castiglia S, Benetti E, Stewart KN, Brown PA, Yaqoob MM, Fantozzi R, Thiemermann C. Generation of endogenous hydrogen sulfide by cystathionine gamma-lyase limits renal ischemia/reperfusion injury and dysfunction. Lab Invest 88: 1038–1048, 2008. [DOI] [PubMed] [Google Scholar]
- 45.Tyagi N, Moshal KS, Sen U, Vacek TP, Kumar M, Hughes WM Jr, Kundu S, Tyagi SC. H2S protects against methionine-induced oxidative stress in brain endothelial cells. Antioxid Redox Signal 11: 25–33, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang R Two's company, three's a crowd: can H2S be the third endogenous gaseous transmitter? FASEB J 16: 1792–1798, 2002. [DOI] [PubMed] [Google Scholar]
- 47.Wartiovaara J, Ofverstedt LG, Khoshnoodi J, Zhang J, Makela E, Sandin S, Ruotsalainen V, Cheng RH, Jalanko H, Skoglund U, Tryggvason K. Nephrin strands contribute to a porous slit diaphragm scaffold as revealed by electron tomography. J Clin Invest 114: 1475–1483, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wei HL, Zhang CY, Jin HF, Tang CS, Du JB. Hydrogen sulfide regulates lung tissue-oxidized glutathione and total antioxidant capacity in hypoxic pulmonary hypertensive rats. Acta Pharmacol Sin 29: 670–679, 2008. [DOI] [PubMed] [Google Scholar]
- 49.Wohlgemuth SE, Taylor AC, Grieshaber MK. Ventilatory and metabolic responses to hypoxia and sulphide in the lugworm Arenicola marina (L.). J Exp Biol 203: 3177–3188, 2000. [DOI] [PubMed] [Google Scholar]
- 50.Xia M, Chen L, Muh RW, Li PL, Li N. Production and actions of hydrogen sulfide, a novel gaseous bioactive substance, in the kidneys. J Pharmacol Exp Ther 329: 1056–1062, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yan SK, Chang T, Wang H, Wu L, Wang R, Meng QH. Effects of hydrogen sulfide on homocysteine-induced oxidative stress in vascular smooth muscle cells. Biochem Biophys Res Commun 351: 485–491, 2006. [DOI] [PubMed] [Google Scholar]
- 52.Yang G, Wu L, Jiang B, Yang W, Qi J, Cao K, Meng Q, Mustafa AK, Mu W, Zhang S, Snyder SH, Wang R. H2S as a physiologic vasorelaxant: hypertension in mice with deletion of cystathionine gamma-lyase. Science 322: 587–590, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yang G, Yang W, Wu L, Wang R. H2S, endoplasmic reticulum stress, and apoptosis of insulin-secreting beta cells. J Biol Chem 282: 16567–16576, 2007. [DOI] [PubMed] [Google Scholar]
- 54.Yao K Effects of several unusual sulfur-containing amino acids on rat liver cystathionine-gamma-lyase. Physiol Chem Phys 7: 401–408, 1975. [PubMed] [Google Scholar]
- 55.Yi F, dos Santos EA, Xia M, Chen QZ, Li PL, Li N. Podocyte injury and glomerulosclerosis in hyperhomocysteinemic rats. Am J Nephrol 27: 262–268, 2007. [DOI] [PubMed] [Google Scholar]
- 56.Yi F, Li PL. Mechanisms of homocysteine-induced glomerular injury and sclerosis. Am J Nephrol 28: 254–264, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yi F, Zhang AY, Janscha JL, Li PL, Zou AP. Homocysteine activates NADH/NADPH oxidase through ceramide-stimulated Rac GTPase activity in rat mesangial cells. Kidney Int 66: 1977–1987, 2004. [DOI] [PubMed] [Google Scholar]
- 58.Yi F, Zhang AY, Li N, Muh RW, Fillet M, Renert AF, Li PL. Inhibition of ceramide-redox signaling pathway blocks glomerular injury in hyperhomocysteinemic rats. Kidney Int 70: 88–96, 2006. [DOI] [PubMed] [Google Scholar]
- 59.Yonezawa D, Sekiguchi F, Miyamoto M, Taniguchi E, Honjo M, Masuko T, Nishikawa H, Kawabata A. A protective role of hydrogen sulfide against oxidative stress in rat gastric mucosal epithelium. Toxicology 241: 11–18, 2007. [DOI] [PubMed] [Google Scholar]
- 60.Zhang X, Li H, Jin H, Ebin Z, Brodsky S, Goligorsky MS. Effects of homocysteine on endothelial nitric oxide production. Am J Physiol Renal Physiol 279: F671–F678, 2000. [DOI] [PubMed] [Google Scholar]
- 61.Zhao W, Zhang J, Lu Y, Wang R. The vasorelaxant effect of H(2)S as a novel endogenous gaseous K(ATP) channel opener. EMBO J 20: 6008–6016, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]