Abstract
Proper targeting of the aquaporin-2 (AQP2) water channel to the collecting duct apical plasma membrane is critical for the urine concentrating mechanism and body water homeostasis. However, the trafficking mechanisms that recruit AQP2 to the plasma membrane are still unclear. Snapin is emerging as an important mediator in the initial interaction of trafficked proteins with target soluble N-ethylmaleimide-sensitive factor attachment protein (SNAP) receptor (t-SNARE) proteins, and this interaction is functionally important for AQP2 regulation. We show that in AQP2-Madin-Darby canine kidney cells subjected to adenoviral-mediated expression of both snapin and syntaxins, the association of AQP2 with both syntaxin-3 and syntaxin-4 is highly enhanced by the presence of snapin. In pull-down studies, snapin detected AQP2, syntaxin-3, syntaxin-4, and SNAP23 from the inner medullary collecting duct. AQP2 transport activity, as probed by AQP2's urea permeability, was greatly enhanced in oocytes that were coinjected with cRNAs of SNARE components (snapin+syntaxin-3+SNAP23) over those injected with AQP2 cRNA alone. It was not enhanced when syntaxin-3 was replaced by syntaxin-4 (snapin+syntaxin-4+SNAP23). On the other hand, the latter combination significantly enhanced the transport activity of the related AQP3 water channel while the presence of syntaxin-3 did not. This AQP-syntaxin interaction agrees with the polarity of these proteins' expression in the inner medullary collecting duct epithelium. Thus our findings suggest a selectivity of interactions between different aquaporin and syntaxin isoforms, and thus in the regulation of AQP2 and AQP3 activities in the plasma membrane. Snapin plays an important role as a linker between the water channel and the t-SNARE complex, leading to the fusion event, and the pairing with specific t-SNAREs is essential for the specificity of membrane recognition and fusion.
Keywords: urea transport, UT-A1, lithium, cAMP, MDCK cells
to maintain constant body fluid osmolality, the kidney tightly regulates the amount of water to be excreted in the urine by reabsorbing up to 99% of the water that was filtered in the glomerulus. In the renal collecting duct, the water is transported by three different water-selective channels or aquaporins (AQPs): AQP2 in the apical plasma membrane (17, 24, 36) and AQP3 and AQP4 in the basolateral plasma membrane (6, 35). AQP2 is the primary renal water channel that is regulated by antidiuretic hormone or arginine vasopressin (AVP) (10, 30, 39). It resides in intracellular vesicles near the apical membrane of the collecting duct principal cell. AVP stimulates transcellular water transport by causing the AQP2-bearing vesicles to move to and fuse with the apical membrane, increasing the apical water permeability many times over its resting value (15, 16). The basolateral membrane in turn possesses constitutively active water channels (AQP3 and AQP4) which render the basolateral membrane constitutively water permeable, so that the apical membrane is the rate-limiting membrane in the transepithelial movement of water (30, 34).
Vesicular fusion and membrane trafficking depend on the soluble N-ethylmaleimide-sensitive factor attachment protein (SNAP) receptor (SNARE) family of proteins, which consists of integral membrane proteins on the vesicle (v-SNARE) and on the target membrane (t-SNARE) (32, 33). The specificity of this process is achieved by pairing v-SNAREs, such as VAMP2, in the carrier vesicles with their cognate t-SNAREs in the target membrane in the process of initial docking. The t-SNARE complex is made up of a syntaxin and a synaptosome-associated protein (SNAP23 or SNAP25). Both v- and t-SNAREs have been identified in the collecting duct principal cells. The v-SNARE protein VAMP2 is found in collecting duct principal cells and is colocalized with AQP2 in the same vesicles (7, 12, 31). The t-SNARE syntaxin-3 resides in the apical membrane of collecting duct principal cells, and syntaxin-4 is found in the basolateral membrane of the collecting duct principal cells and Madin-Darby canine kidney (MDCK) cells (19, 21), although the two syntaxins have also been reported on the opposite side of epithelial cells (25, 26). Another major t-SNARE, SNAP23, has been found in collecting duct principal cells, both at the apical membrane and in AQP2-bearing vesicles (11). Therefore, all components of the SNARE system are present in the collecting duct. Procino and coworkers (31a) presented an extensive study of the functional role of the SNAREs VAMP2 and 3, SNAP23, and syntaxin-3 in the recruitment of AQP2 to the apical membrane. However, to date there is no functional study of the SNAREs' ultimate role, namely, to mediate the activation of the AQP2-mediated water permeability. We have recently published the first characterization of a physical association between the t-SNARE complex (SNAP23/syntaxin-4) and the urea transporter/channel UT-A1 via another SNARE-associated protein, snapin (28). Snapin has been identified as a binding protein of syntaxin-4 and SNAP23 (3, 28). Collectively, these observations suggest that snapin is an important intermediate scaffolding molecule that provides the link between the SNARE machinery and the AQP2 or UT-A1 channels, a prerequisite for vesicular docking and fusion. Recently, a similar observation was reported with adipocytes, suggesting a modulator role for snapin in GLUT4 trafficking via the SNARE system (1).
The purpose of this study was to expand on the previous findings of snapin as a binding partner with SNAP23 and a syntaxin in directing UT-A1 vesicle trafficking (28) and to test snapin's role in directing aquaporin trafficking to the apical or basolateral membrane of medullary collecting duct cells. In investigating the modulatory role of snapin in AQP2 and AQP3 trafficking to the apical or basolateral membrane, respectively, we found that snapin interacts with both AQP2 and AQP3 as well as with the syntaxin/SNAP23 binary complex in inner medullary tissue, in a cultured renal cell line (MDCK cells) and in Xenopus laevis oocytes. We show that snapin plays an important role as a linker between the aquaporin protein and the t-SNARE complex and that the specific pairing with t-SNAREs is essential for the specificity of AQP2 or AQP3 recognition. We conclude that snapin mediates AQP2 trafficking from storage vesicle to the apical plasma membrane by the specific association with the t-SNAREs syntaxin-3 and SNAP23 and that this assembling in the SNARE machinery may be functionally important in body water homeostasis.
METHODS
Antibodies.
We used our rabbit polyclonal antibody to the COOH terminus of the rat renal urea transporter UT-A1 (14). Rabbit anti-snapin antibody, anti-syntaxin-3, and anti-syntaxin-4 were purchased from Synaptic Systems (Göttingen, Germany). Mouse monoclonal anti-actin, anti-glutathione-S-transferase (GST), and nonspecific rabbit serum (as a control) were from Sigma-Aldrich (St. Louis, MO) and rabbit polyclonal antibodies from BD Biosciences (Franklin Lakes, NJ). Rabbit polyclonal anti-SNAP-23 and anti-VAMP2 were the kind gifts of Dr. Mark Knepper (Laboratory of Kidney and Electrolyte Metabolism, National Heart, Lung, and Blood Institute, National Institutes of Health, Bethesda, MD).
Plasmid construction.
The full-length human snapin cDNA was isolated from a human kidney cDNA library (BD Biosciences Clontech). The coding region (411 bp) of human snapin was amplified by PCR using sequence-specific primer pairs and subcloned into the multiple cloning sites of oocyte expression vector pGH19 and several other expression vectors (e.g., pGEX-4T-snapin, pShuttle-CMV-snapin, and pAdEasy-snapin). Full-length AQP2 and its mutants, AQP3, snapin, syntaxin-4, syntaxin-3, and SNAP23, were cloned into the oocyte expression vector pGH19. The insert sequences and reading frames were verified by sequencing.
Preparation of GST fusion proteins and pull-down assays.
The full-length open reading frame encoding snapin was cloned into GST fusion vector pGEX-4T-2 and then expressed in Escherichia. coli BL21-Codon plus-(DE3) (Stratagene) cells, followed by induction with 1 mM isopropyl β-d-thiogalactopyranoside, essentially as described previously (28). Bacterial pellets were sonicated and lysed in binding buffer (20 mM HEPES, pH 7.4, 140 mM KCl, 20 mM NaCl, 0.5% Triton X-100). GST-snapin was immobilized on glutathione-Sepharose (Amersham Biosciences) and washed six times with binding buffer. The quantity and quality of the GST fusion protein were checked by Bradford protein assay and SDS-PAGE analysis using Coomassie brilliant blue staining. Rat kidney inner medulla tissue (0.5 mg) was homogenized in the lysis/binding buffer containing 1.5% Triton X-100 with a glass teflon homogenizer (12 strokes at 900 rpm), sheared by passing the extract through a 25-gauge needle (5 times), and centrifuged at 12,000 g for 45 min. The supernatant was diluted with binding buffer to reduce Triton X-100 to 0.6%, kept under gyration overnight, and centrifuged at 30,000 g for 45 min at 4°C. The resultant supernatant was incubated with the immobilized GST-snapin resin. After extensive washing, the bound protein complexes were resolved on a 4–15% SDS-polyacrylamide gel and transferred to a polyvinylidene difluoride membrane. The blots were probed with the indicated antibodies.
Cell culture and transfection.
The AQP2-MDCK cells, which express AQP2 by functional assay and Western blot analysis (4), were the kind gift of Dr. Peter Deen (University of Nijmegen, The Netherlands). The AQP2-MDCK cells were cultured in Dulbecco's modified Eagle's medium containing 10% fetal bovine serum, 100 U/ml penicillin, and 100 μg/ml streptomycin at 37°C in 5% CO2.
Generation of recombinant adenovirus and infection of AQP2-MDCK cells.
A recombinant adenovirus (Ad-snapin and Ad-GFP) was constructed using the Ad-Easy-1 system (Clontech) through multiple rounds of subcloning of PCR products or of restriction endonuclease fragments as described previously (28). Ad-syntaxin-3 and Ad-syntaxin-4 (human) have been described previously (22). Ad-snapin, Ad-syntaxin-4, and Ad-GFP were expanded, purified, and titered as described previously (28). AQP2-MDCK cells were grown to 90% confluence in Dulbecco's modified Eagle's medium containing 10% fetal bovine serum as above. For virus infection, cells were incubated with serum-free medium containing 10–60 pfu/cell for 2–6 h, washed twice with PBS, and further cultured in fresh medium containing 10% fetal bovine serum, 100 U/ml penicillin, and 100 μg/ml streptomycin in 5% CO2-95% air at 37°C for 18–20 h to allow transgene expression of recombinant protein. Ad-GFP was used as a control adenovirus.
Coimmunoprecipitation and Western blotting.
Adenovirus-infected snapin-overexpressing AQP2-MDCK cells, grown on 12-well plates, were washed with cold PBS containing 1 mM EDTA and then scraped into 5 ml of lysis buffer (50 mM Tris, pH 7.4, 50 mM NaCl, 0.5% Triton X-100, and 1% protease inhibitor mixture). Cells were homogenized with a glass teflon homogenizer (10 strokes at 900 rpm) and lysed by passing the extract through a 25-gauge needle six times. The extract was centrifuged at 12,000 g for 30 min at 4°C to remove insoluble material, and the supernatants were solubilized in binding buffer (50 mM Tris, pH 7.4, 50 mM NaCl, 0.1% bovine serum albumin, and 0.1% Triton X-100) and centrifuged at 20,000 g for 30 min at 4°C. Finally, the resultant supernatant was incubated with anti-snapin, anti-AQP2, or rabbit IgG (as a control) at 4°C for 2 h with continuous mixing. Protein A beads were added, and incubation was continued for an additional 2 h. Subsequently, the bead-immobilized antibody-protein complexes were washed five times with binding buffer. Immunoprecipitated proteins were resuspended in SDS-PAGE loading buffer (60 mM Tris, 2% SDS, 10% glycerol, 5 mM EDTA, 2% β-mercaptoethanol, 0.01% bromophenol blue, pH 6.8), heated at 85°C for 5 min, and separated on a 4–15% SDS-PAGE polyacrylamide gels. Following transfer to polyvinylidene difluoride membranes, bound proteins were identified by immunoblotting. Experiments were performed in duplicate or triplicate.
Animal preparation and tissue collection.
All animal protocols were approved by the Emory University Institutional Animal Care and Use Committee. Male Sprague-Dawley rats, weighing 250–350 g, were kept in cages with autoclaved bedding and received free access to water and a standard diet (diet 5001, Purina). Immediately after the rats were killed, their kidneys were dissected to separate the cortex, outer medulla, and inner medulla. These tissues were placed in an ice-cold isolation buffer [10 mM triethanolamine, 250 mM sucrose (pH 7.6), 1 μg/ml leupeptin, and 2 mg/ml phenylmethylsulfonyl fluoride], homogenized, SDS was added to a final concentration of 1%, and samples were sheared with a 25-gauge needle. Total protein concentration in each sample was measured by a modified Lowry assay (DC Protein Assay Kit, Bio-Rad).
X. laevis oocyte injections.
Linearized plasmids encoding AQP2, AQP3, UT-A1, snapin, syntaxin-3, syntaxin-4, and SNAP23 in the oocyte expression vector pGH19 cRNAs served as templates to synthesize cRNAs with T7 polymerase using a mMessage mMachine T7 Ultra kit (Ambion, Austin, TX). The cRNAs (2 ng/oocyte in 23 nl of water) were microinjected into collagenase-treated stage V–VI oocytes obtained from two to three animals for each experimental series. Control oocytes were injected with the same volume (23 nl) of water. Injected oocytes were maintained in OR3 medium at 18°C, and after 2–3 days healthy oocytes were selected for urea influx measurements.
Functional analysis of AQP2 and AQP3 in X. laevis oocytes.
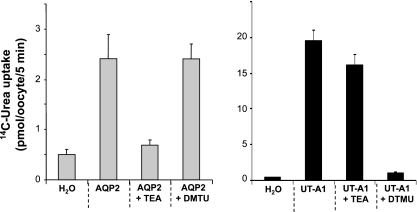
To functionally characterize aquaporins, researchers have relied on measuring osmotic water movements by following the rates of volume change of the injected oocyte in the presence of an osmotic gradient. The reason was that tracer measurements with [3H]H2O are not feasible due to the high hydrogen ion (relative to water) permeability of the plasma membrane. As an alternative to water fluxes, we therefore made use of our discovery that AQP2 is also permeable to urea. AQP3, AQP7, and AQP9 are known to mediate urea movements (20, 27), but AQP2 has not previously been reported to do so. Figure 1 demonstrates that this is actually the case: Urea influx into AQP2-injected oocytes could be inhibited by TEA, which blocks the AQP1 and AQP2 channels (5), but not by the urea channel blocker dimethylthiourea (DMTU) (8). Conversely, urea influx into UT-A1-injected oocytes was blocked by DMTU but not TEA. The methods of [14C]urea uptake into oocytes were described previously (28). Oocytes were removed from the OR3 medium and washed with 1 ml of uptake solution (200 mM mannitol, 2 mM KCl, 1 mM MgCl2, 1 mM CaCl2, 10 mM HEPES buffer, 5 mM Tris·HCl, pH 7.4). UT-A1-injected cells were further preincubated in the uptake solution for 30 min, whereas AQP2-injected cells were immediately used in flux experiments. The urea uptake experiment consisted of transferring the oocytes into uptake solution containing 2 μCi of [14C]urea/ml and 1 mM cold urea. After 1.5, 3, or 6 min, they were washed four times with ice-cold uptake solution containing 1 mM cold urea. Each individual cell was then dissolved in 10% SDS, followed by scintillation counting. Except where noted otherwise, the bar graphs of the urea fluxes represent means ± SD for 10 oocytes in each group.
Fig. 1.
The aquaporin-2 (AQP2) water channel mediates urea movements. Stage V-VI oocytes were injected with cRNA for AQP2 or urea transporter UT-A1 (2 ng of cRNA/oocyte in 23 nl of H2O) or only H2O, then maintained for 2–3 days at 18°C. To initiate tracer urea uptake, the oocytes were transferred into flux medium containing 2 μCi of [14C]urea/ml and 1 mM cold urea, plus the indicated inhibitors [0.1 mM TEA or 10 mM dimethylthiourea (DMTU)]. Urea uptake in AQP2-injected oocytes was inhibited by TEA but not by DMTU, as expected for AQP2-mediated urea transport. In contrast, the urea uptake in UT-A1-injected oocytes was unaffected by TEA but blocked by DMTU. Values are means ± SD for 12–18 oocytes in each group. Note the different scales on the ordinates of the left vs. right panels; as expected, UT-A1 is much more efficient in moving urea than AQP2.
Isolation of total membranes from X. laevis oocytes.
Six to eight oocytes were rinsed in uptake solution and homogenized in 1 ml of the same solution supplemented with a protease inhibitor cocktail (Sigma-Aldrich). Homogenates were centrifuged at 200 g for 10 min at 4°C to discard cell debris, and the supernatant was centrifuged at 15,000 g for 30 min at 4°C to pellet down total membrane. The pellets were collected and resuspended in ice-cold homogenization buffer (1% Triton X-100, 1 mM EDTA, and 150 mM NaCl, 20 mM HEPES, pH 7.4) containing the protease inhibitor mixture at 4°C. SDS was added to a final concentration of 1%, and samples were sheared with a 25-gauge needle. Total protein concentration in each sample was measured by a modified Lowry assay (DC Protein Assay Kit, Bio-Rad) and adjusted to ∼1 μg/μl with the same homogenization buffer as for Western blot analysis.
Statistical methods.
The bar graphs of the urea fluxes show means ± SD obtained from at least 10 oocytes per experimental condition. Comparisons of flux rates were performed by one-way ANOVA, followed by Tukey's honestly significant difference test.
RESULTS
Snapin detects AQP2 and t-SNARE components in kidney inner medulla by pull-down assay.
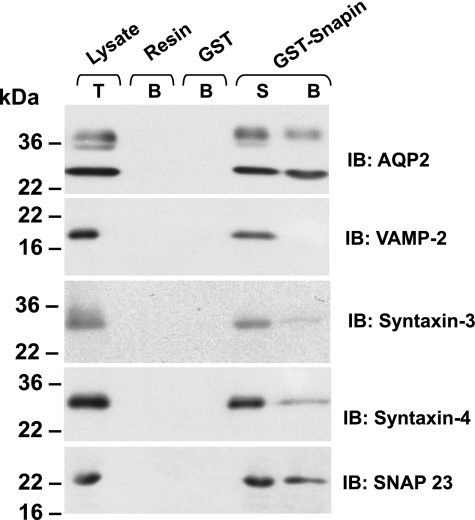
To investigate the interaction of snapin with AQP2 and other integral membrane proteins of the SNARE complex in the inner medulla, we generated GST-snapin, a fusion of full-length snapin with GST, and immobilized it on glutathione beads. Then, we incubated these beads with rat inner medullary tissue lysate and analyzed the bound proteins by immunoblotting. As shown in Fig. 2, snapin pulled down AQP2, syntaxin-3, syntaxin-4, and SNAP23 from the inner medulla, but not VAMP2. This result indicates that snapin is an interacting partner protein that can link to t-SNARE components and to AQP2 in the inner medullary collecting duct (IMCD).
Fig. 2.
Snapin interacts in vitro with AQP2 and soluble N-ethylmaleimide-sensitive fusion attachment receptor (SNARE) proteins. Rat kidney inner medullary tissue lysate was incubated with bead-immobilized glutathione-S-transferase (GST)-snapin. Snapin-bound proteins were resolved by SDS-PAGE and immunoblotted with indicated antibodies. Glutathione-agarose beads (Resin) and bead-bound GST (GST) were used as controls. S, supernatant remaining after pulldown; B, bound material; T, total protein lysate.
Coimmunoprecipitation of AQP2 and snapin.
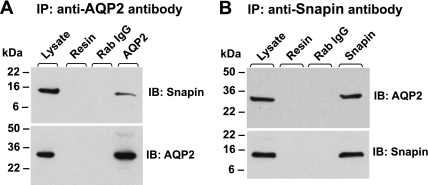
We also tested AQP2-snapin interaction by performing coimmunoprecipitation studies using Ad-snapin-infected AQP2-MDCK cells. AQP2 and snapin were immunoprecipitated from Triton X-100-solubilized cell lysate using anti-AQP2 and anti-snapin antibodies, respectively. Snapin was coimmunoprecipitated by the anti-AQP2 antibody (Fig. 3A), and AQP2 was coimmunoprecipitated by the anti-snapin antibody (Fig. 3B). Neither snapin nor AQP2 were precipitated with nonspecific rabbit IgG and protein-A beads (“Resin”), and neither were they immunoprecipitated from Ad-GFP-infected AQP2-MDCK cells (data not shown). Thus our data suggest that snapin and AQP2 coexist in a core complex in AQP2-MDCK cells.
Fig. 3.
Snapin interacts with AQP2 in situ. Recombinant adenovirus (Ad)-snapin-infected AQP2-Madin-Darby canine kidney (MDCK) cell lysate was used to perform the coimmunoprecipitation studies. A: snapin was coimmunoprecipitated with anti-AQP2 antibody. B: AQP2 was coimmunoprecipitated with anti-snapin antibody. The immunoprecipitated protein complexes were immunoblotted with the indicated antibodies. Protein A beads alone (Resin) were used as control for nonspecific association. Nonspecific rabbit serum (IgG) was used as the serum control.
Snapin links AQP2 to t-SNARE.
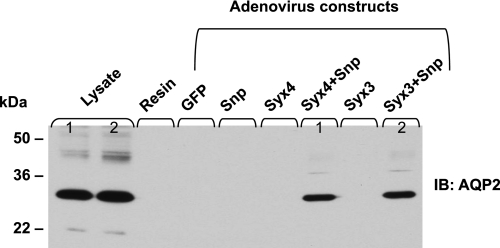
To investigate the snapin link between AQP2 and t-SNARE proteins, we infected AQP2-MDCK cells with Ad-snapin, Ad-syntaxin-3 (Ad-syx3), and Ad-syntaxin-4 (Ad-syx4). A His6-tag was added to the COOH terminus of both syntaxins, but not to snapin. We infected the cells with either Ad-snapin, Ad-syx-3 or Ad-syx4 alone or with snapin plus either syntaxin. Then, we performed His pull-down experiments and identified the pulled down t-SNARE proteins by subsequent immunoblotting. Figure 4 shows that both syntaxin-3 and syntaxin-4 pulled down AQP2 in AQP2-MDCK cells when they were coexpressed with snapin, but they did not pull down AQP2 if snapin was not coexpressed. Snapin was previously identified as a binding protein of syntaxin-4 and SNAP23 (3), in that these two proteins form a cargo complex at the target membrane. The data in Fig. 4 suggest that AQP2 associates indirectly with the SNARE machinery, via snapin as bridging mediator.
Fig. 4.
Snapin links AQP2 to target SNARE (t-SNARE). AQP2-MDCK cells grown in 6-well plates were infected by the indicated Ad constructs at about 20 pfu/cell and grown to confluence for 30 h before the assay. Only the two Ad-syntaxin constructs, Ad-Syx-3 and Ad-Syx-4, contained an (His6) epitope tag. The cell lysates were incubated with Ni-NTA agarose in a pull-down (His6) assay. Ni-nitrilotriacetic acid (NTA)-agarose-bound syntaxin-protein complexes were immunoblotted with anti-AQP2 antibody. Neither syntaxin was able to pull down AQP2 by itself but required the presence of snapin. Although snapin and AQP2 did bind to each other without syntaxin present (as demonstrated in Fig. 3), their binary complex was not pulled down since it did not contain the (His6) epitope tag. Ad-green fluorescent protein (GFP) was used as a control adenovirus.
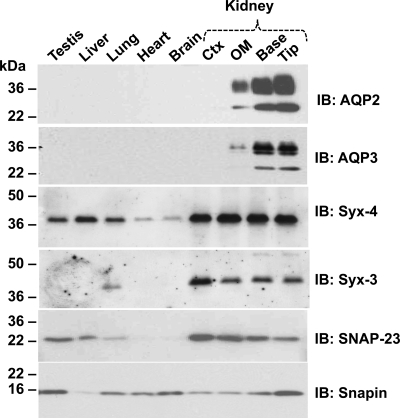
Distribution of SNARE proteins in rat tissues.
We investigated the distribution of SNARE proteins in several rat tissues using Western blotting. Our results demonstrate that SNARE proteins are present in the renal inner medulla. AQP2, AQP3, and SNAP23 were almost exclusively confined to the medulla, syntaxin-3 was found primarily in the kidney, while snapin, syntaxin-4, and SNAP23 were expressed in multiple tissues (Fig. 5).
Fig. 5.
Abundance of SNARE proteins in rat tissues. SDS-PAGE gels were loaded with equal amounts (10 μg) of total protein from the homogenates of different rat tissues. Blots were probed with indicated antibodies. AQP2, AQP3, VAMP2, and syntaxin-3 are predominantly expressed in the kidney inner medulla. Snapin, synaptosome-associated protein (SNAP) 23 and syntaxin-4 are expressed ubiquitously in multiple tissues. Ctx, cortex; OM, outer medulla; Base, base of inner medulla; Tip, tip of inner medulla.
Snapin and t-SNARE specificity modulates AQP2 and AQP3 transport activity in X. laevis oocytes.
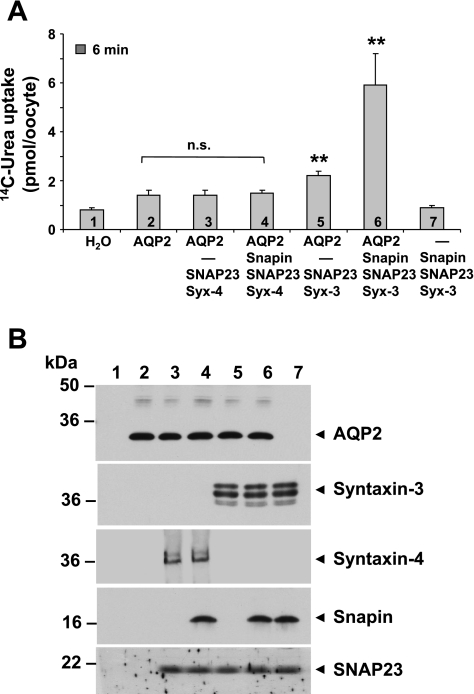
To test the functional importance of snapin for AQP2 transport activity via trafficking to the plasma membrane, we performed tracer fluxes in cRNA-injected X. laevis oocytes, using urea fluxes as a marker for AQP2-mediated water movements. We found that injection with AQP2 cRNA moderately enhanced the urea permeability, but coinjection with syntaxin-4+SNAP23, with or without snapin, had no additional effect, despite the association we found in Fig. 4. However, when we replaced syntaxin-4 with syntaxin-3, we found a moderate (50%) enhancement by the two t-SNAREs (syntaxin-3 + SNAP23) alone and a strong (4-fold) stimulation when snapin was included in the coinjection mixture (Fig. 6).
Fig. 6.
Snapin enhances AQP2 transport activity via syntaxin-3. A: tracer urea uptake was measured into oocytes injected with the indicated cRNAs. Values are means ± SD obtained from 10 individual oocytes; the results were all the same in 3 different experimental series. There was no stimulating effect of syntaxin-4 (with or without snapin present) on AQP2 activity. However, in the absence of snapin, syntaxin-3 enhanced AQP2 activity by ∼50%, and in combination, syntaxin-3 and snapin enhanced AQP2 activity 4-fold. Also, no stimulation was observed in the presence of SNAP23/syntaxin alone or snapin alone (data not shown). B: confirmation of expressed proteins by Western blotting.
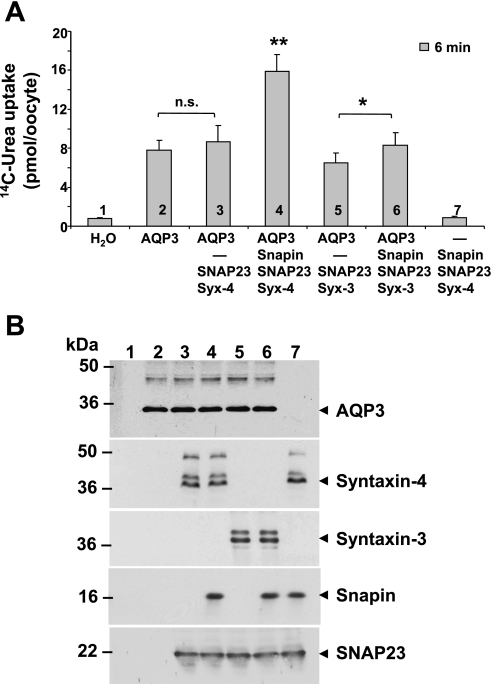
As AQP2 is known to move to the apical membrane, our finding is consistent with the published report of apically located syntaxin-3 (19, 21). To test the counterpart, namely, whether syntaxin-4 associates with basolaterally targeted proteins, we tested the effect of snapin and syntaxin-4 on AQP3. AQP3 is found primarily on the basolateral membrane of the IMCD, and it is known to be permeable to urea as well. Using the same oocyte assay as for AQP2, we found that in the case of AQP3 the snapin-mediated stimulation required syntaxin-4 and that syntaxin-3 had no effect (Fig. 7).
Fig. 7.
Snapin enhances AQP3 transport activity via syntaxin-4. The experimental conditions were the same as in Fig. 6. A: in contrast to AQP2, AQP3-mediated urea fluxes were specifically enhanced by syntaxin-4 instead of syntaxin-3. There was no stimulating effect of syntaxin-3 (with or without snapin present) on AQP3 activity. In the presence of snapin, syntaxin-4 enhanced AQP3 activity by ∼80%. B: confirmation of expressed proteins by Western blotting.
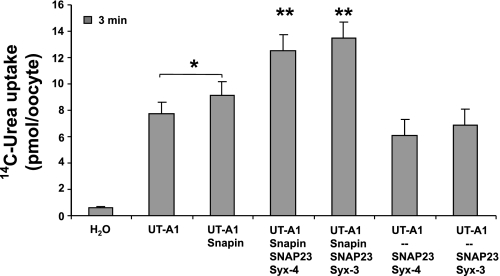
The selectivity of AQP2 for syntaxin-3 over syntaxin-4 in oocytes appears to contradict an observation we previously made with UT-A1 (28). In that study, syntaxin-4, coinjected with snapin and SNAP23, weakly stimulated UT-A1-mediated urea flux in oocytes. We had performed these experiments under the assumption that syntaxin-4, like UT-A1, was located at the apical membrane (25) and because snapin had been shown to form a ternary complex with syntaxin-4 and SNAP23 (3). Because of contradicting reports according to which syntaxin-4 was actually located at the basolateral and syntaxin-3 at the apical membrane (19, 21), and in light of our observation that the apical AQP2 was selectively stimulated in the presence of syntaxin-3 instead of syntaxin-4, we repeated and expanded on our previous experiments with UT-A1. We confirmed our previous observation that syntaxin-4 (plus SNAP23) weakly stimulated urea flux compared with snapin alone (Fig. 8). However, in contrast to its strong effect on AQP2, syntaxin-3 had the same weakly stimulating effect on UT-A1 as syntaxin-4, and in the absence of added syntaxin the UT-A1 activity was no greater than with snapin alone. Thus our data suggest that there is a fundamental difference in the way snapin and syntaxins participate in the trafficking of UT-A1 and AQP2 to the plasma membrane.
Fig. 8.
UT-A1 activation exhibits no selectivity for syntaxins. In contrast to AQP2, in the presence of snapin both syntaxin-3 and syntaxin-4 weakly stimulated UT-A1 activity (P < 0.01) to the same extent. In the absence of snapin, this stimulation disappeared.
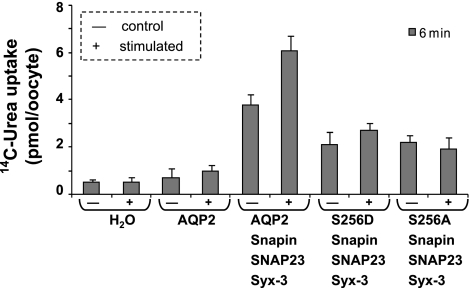
Agonists that increase cAMP levels in IMCD cells such as vasopressin or forskolin stimulate the transfer of AQP2 into the apical membrane (29). Protein kinase A is known to phosphorylate AQP2 during this process (18). In the experiment shown in Fig. 9, we tested the effect of a cocktail of cAMP agonists on urea influx in oocytes. The results show that the snapin-enhanced urea influx was further stimulated by cAMP. We also tested the action of AQP2-S256D and AQP2-S256A, a phosphomimetic and a phospho-null mutant of aquaporin, respectively. With both mutants, coexpression with snapin+syntaxin-3+SNAP23 resulted in less stimulation compared with wild-type AQP2, and in neither case was there an additional stimulation by cAMP agents.
Fig. 9.
cAMP activation enhances AQP2 transport activity in Xenopus laevis oocytes. Tracer urea uptake was measured into cRNA-injected oocytes in the absence of stimulators of cAMP or after incubation with a cocktail of 500 μM cyclopentyltheophylline (CPT)-cAMP, 500 μM IBMX, and 50 μM forskolin for 6 h. S256D and S256 are the phosphomimetic and phosphorylation-null mutants of AQP2, respectively.
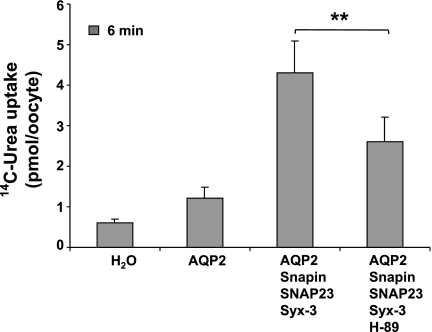
To examine why AQP2 activity (in the presence of snapin/syntaxin-3) is relatively high even in the absence of externally added cAMP agonist, we tested the effect of the protein kinase inhibitor H-89 on the basal AQP2 activity. We found that preincubation of the oocytes with 10 μM H-89 reduced AQP2 activity by ∼40% (Fig. 10). This suggests that under these conditions, protein kinase was constitutively active, possibly by elevated intracellular cAMP levels.
Fig. 10.
AQP2-mediated urea transport is activated by protein kinase A in unstimulated oocytes. Cells were preincubated with or without 10 μM H89 for 30 min before the start of the flux experiment. Urea flux was inhibited 40% by H89 pretreatment.
DISCUSSION
In our oocyte experiments, we performed tracer urea flux experiments to characterize the activities of the water channels AQP2 and AQP3. The reason for this approach was technical. Measuring tracer urea movement is technically simpler than measuring osmotic water movement. While the aquaporin isoforms AQP3, AQP7, and AQP9 are known to be permeable to urea (20, 27), there is no report of a urea permeability through AQP2. The earlier inability to detect urea movement through AQP2 may be due to differences in flux protocols. In previous reports, the oocytes were customarily preincubated for 30–60 min in mannitol-containing uptake solution, whereas in our present studies we only rinsed them in the mannitol-containing uptake solution immediately before the flux experiment. In control experiments (not shown), we confirmed that after prolonged incubation in uptake solution the rate of urea uptake by AQP2 was drastically reduced. We did not systematically study which component(s) in the two media (OR3 vs. uptake medium) or other factors were responsible for the greater ease of detecting AQP2-mediated urea movement. In addition, we demonstrated that the urea movement in AQP2-injected oocytes was mediated by AQP2: it was inhibited by TEA (5) but not by the urea flux inhibitor DMTU (9). In contrast, the urea movement in UT-A1-injected cells was unaffected by TEA and blocked by DMTU (Fig. 1).
To maintain the body water content, the kidney regulates the rate of water (and urea) reabsorption in the collecting duct by recruiting AQP2 from intracellular vesicles to the apical membrane. Several SNARE-mediated vesicle fusion proteins are known to be present in the same kidney cells that also express AQP2 and UT-A1 in the apical and AQP3, AQP4, and UT-A3 in the basolateral membrane (2, 11, 12, 17, 30, 31, 37). The SNARE proteins are understood to function mainly in the vesicle-docking/fusion events of protein trafficking in neuronal and non-neuronal cells. Snapin, a SNARE-associated protein, could provide a link between the vesicle and the fusion machinery, having originally been described as a syntaxin-binding protein with a function in regulated exocytosis of synaptic vesicles (38). Our previous studies provided evidence that snapin recruits UT-A1 to the apical plasma membrane in snapin-expressing UT-A1-MDCK cells, a process that is enhanced by arginine vasopressin (AVP) and forskolin stimulation (40).
We extended our previous studies to the aquaporins AQP2 and AQP3. AQP2 is found in the apical and AQP3 in the basolateral membrane of the collecting duct (6, 30), with AQP2's insertion into the apical membrane being subject to regulation by vasopressin. We show here that in addition to binding to syntaxin-4, syntaxin-3, and SNAP23, snapin also interacts with AQP2. AQP2 binding to the SNARE complex requires snapin, as we failed to observe a direct interaction between AQP2 and syntaxin-3 or syntaxin-4. The fact that AQP2/syntaxin complex formation required the presence of snapin suggests that the AQP2 vesicle fusion event is made possible by snapin forming a link between SNAREs and AQP2, similar to snapin's role as a linker in other neuronal or non-neuronal cells. A good example is the insulin-regulated translocation of GLUT4-containing vesicles in adipose cells (1). GLUT4 translocation also requires specific pairing of t-SNAREs to achieve the necessary fidelity of membrane recognition and fusion (33). The t-SNAREs that mediate GLUT4 exocytosis, syntaxin-4 and SNAP23, form stable and SDS-resistant complexes with VAMP2 (47). Similarly, in this study we demonstrated that immobilized GST-snapin binds AQP2 and t-SNARE components (syntaxin-3, syntaxin-4, and SNAP23) in the kidney inner medulla.1 In agreement with Buxton et al. (3), we also found that snapin did not interact detectably with the v-SNARE VAMP2. Thus it is possible that the association with snapin plays an important role in joining AQP2 with syntaxin-3 and in guiding the AQP2-containing intracellular vesicle to the SNARE complex of the apical membrane.
Our data clearly demonstrate that, as a minimal function, snapin links aquaporin with syntaxin, but they suggest a function beyond being a just nonspecific linker. If the snapin merely formed a bridge between aquaporin and syntaxin, one would expect no distinction as to which aquaporin would be paired with which syntaxin. Instead, we found that snapin enhanced AQP2-mediated fluxes only in the presence of syntaxin-3 and AQP3-mediated fluxes only in the presence of syntaxin-4. The role of snapin most likely is not simply one of bringing aquaporin and syntaxin into the vicinity of each other and then leaving them alone. Rather, there has to be a ternary complex between snapin, aquaporin, and syntaxin. First, our data give no indication that snapin would be released, leaving aquaporin and syntaxin alone; and second, they show (Fig. 4) that without snapin there is no detectable interaction between AQP2 and syntaxin-4, even after prolonged incubation that should establish binding equilibrium between aquaporin and syntaxin, with or without the aid of snapin.
Thus the specificity of syntaxin-3 for AQP2 and of syntaxin-4 for AQP3 requires the existence of a complex that contains the aquaporin, its cognate syntaxin (and possibly SNAP23), and snapin. Snapin is a required component in this complex because only in its presence were AQP2 and AQP3 activities greatly enhanced. In the simplest mechanism to explain this phenomenon, snapin brings aquaporin and syntaxin close to each other in a way that enables specific interactions between these two components and, by forming the additional bridge between aquaporin and syntaxin, it adds to the stability of the proposed ternary complex. As an alternative, snapin could, by binding to syntaxin, induce a conformational change in the syntaxin that opens up a binding site for its cognate aquaporin. In either mechanism, an additional binding component is created beyond snapin simply forming a bridge between aquaporin and syntaxin; the aquaporin-syntaxin interaction provides the necessary additional binding energy (to the AQP2 in the vesicle) to stabilize the membrane-specific complex and enable the fusion process with the bilayer.
In the past, oocytes have been a convenient expression system for proteins that are normally found in the apical or basolateral membrane. One possible explanation is that the plasma membrane of the nonpolar oocytes acts as a default membrane irrespective of the protein's native targeting mechanism. Our findings suggest that the oocyte system can be a useful model for studying interactions between trafficked proteins and the target membrane, be they apical or basolateral, as long as the necessary components are coexpressed. Nevertheless, the specificity between aquaporins and syntaxins (Figs. 6 and 7) is surprising in the face of the binding experiments using MDCK cell lysates where we did not observe a selectivity of AQP2 for syntaxin-3 over syntaxin-4 (Fig. 4). One possibility is that overexpression of AQP2, syntaxin (-3 or -4), and snapin in the cultured cells permitted a situation of reduced selectivity in the binding among aquaporins and syntaxins. One might argue that these proteins were also overexpressed in the oocytes; however, one could expect that, compared with the pull-down assay, the functional urea flux assay poses more stringent requirements for the status of the snapin-SNARE complex. For example, the MDCK cell lysates were obtained from unstimulated cells whereas it is likely that cAMP in oocytes moderately stimulated AQP2 activity oocytes (see discussion below). Alternatively, the oocytes may have possessed an additional component that existed only in limited amounts in the MDCK cells. For example, no exogenous SNAP23 was included in the pull-down assay whereas it was coexpressed in the oocyte flux assay. It could be interesting to evaluate the role the SNAP proteins play in the observed specificity between AQP2 and syntaxin-3.
We interpret a snapin-mediated stimulation of aquaporin-mediated urea fluxes in oocytes as being due to increased incorporation of open aquaporin channels into the plasma membrane, as opposed to an effect on the open channel probability of already present channels. With this assumption and in view of the tight hormonal regulation of AQP2 activity in the kidney, the observed activity of AQP2 in unstimulated oocytes (Fig. 9) might be considered high. In fact, all AQP2-mediated water permeabilities in nonstimulated oocytes that have been reported in the literature reports could be considered high; only, in this study, the AQP2-mediated urea fluxes observed are enhanced by the presence of snapin and syntaxin, rendering the constitutive AQP2 activity more noticeable.
As a possible explanation for this high apparent constitutive AQP2 activity, the high levels of snapin and syntaxin in the oocytes could help overcome a kinetic barrier to trafficking in the absence of cAMP, constituting a cAMP-independent means of moving AQP2 into the plasma membrane. It is also possible that some of the observed AQP2 activity is due to cAMP present even in the absence of external stimulation. Our observation that H-89 reduced AQP2 activity by ∼40% (Fig. 10) supports the notion that PKA activity is active in these oocytes, probably maintained by intrinsic cAMP levels.
In the experiments in Fig. 8, we also observed that the phosphomimetic AQP2 mutant S265D was as active as the phospho-null S265A, and both had a lower activity than wild-type AQP2. This confirms a published observation that both mutants mediate a lower water permeability in oocytes than wild-type AQP2 (18) and stands in apparent contrast to other published observations that the S265D mutant is constitutively found enriched near the apical membrane (13). A possible explanation for this phenomenon is that, while phosphorylation of the serine-265 site is important for getting the AQP2-containing vesicles moved to the subapical space, other phosphorylation steps may be involved in the ultimate incorporation step of the AQP2-containing vesicle into the plasma membrane (23).
In summary, our binding and functional data suggest that snapin plays an important role as a link between the AQP channels and the t-SNARE complex in preparation for or during the fusion event and that the specific pairing with t-SNAREs is essential for the specificity of membrane recognition and fusion. The essential role of t-SNARE specificity in linking snapin to the fusion machinery is a new pathway for AQP vesicle trafficking.
GRANTS
This work was supported by National Institutes of Health Grants P01-DK-61521 and R01-DK-41707 to J. M. Sands and GM-66785 to T. Weimbs and a grant from the Emory University Research Council to A. C. Mistry.
Footnotes
The negative result we had previously obtained for GST-snapin and syntaxin-3 (28) was due to the use of a weak commercial antibody.
REFERENCES
- 1.Bao Y, Lopez JA, James DE, Hunziker W. Snapin interacts with the Exo70 subunit of the exocyst and modulates GLUT4 trafficking. J Biol Chem 283: 324–331, 2008. [DOI] [PubMed] [Google Scholar]
- 2.Blount MA, Klein JD, Martin CF, Tchapyjnikov D, Sands JM. Forskolin stimulates phosphorylation and membrane accumulation of UT-A3. Am J Physiol Renal Physiol 293: F1308–F1313, 2007. [DOI] [PubMed] [Google Scholar]
- 3.Buxton P, Zhang XM, Walsh B, Sriratana A, Schenberg I, Manickam E, Rowe T. Identification and characterization of Snapin as a ubiquitously expressed SNARE-binding protein that interacts with SNAP23 in non-neuronal cells. Biochem J 375: 433–440, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Deen PM, Rijss JP, Mulders SM, Errington RJ, van Baal J, van Os CH. Aquaporin-2 transfection of Madin-Darby canine kidney cells reconstitutes vasopressin-regulated transcellular osmotic water transport. J Am Soc Nephrol 8: 1493–1501, 1997. [DOI] [PubMed] [Google Scholar]
- 5.Detmers FJ, de Groot BL, Muller EM, Hinton A, Konings IB, Sze M, Flitsch SL, Grubmuller H, Deen PM. Quaternary ammonium compounds as water channel blockers. Specificity, potency, and site of action. J Biol Chem 281: 14207–14214, 2006. [DOI] [PubMed] [Google Scholar]
- 6.Ecelbarger CA, Terris J, Frindt G, Echevarria M, Marples D, Nielsen S, Knepper MA. Aquaporin-3 water channel localization and regulation in rat kidney. Am J Physiol Renal Fluid Electrolyte Physiol 269: F663–F672, 1995. [DOI] [PubMed] [Google Scholar]
- 7.Franki N, Macaluso F, Gao Y, Hays RM. Vesicle fusion proteins in rat inner medullary collecting duct and amphibian bladder. Am J Physiol Cell Physiol 268: C792–C797, 1995. [DOI] [PubMed] [Google Scholar]
- 8.Fröhlich O, Aggarwal D, Klein JD, Kent KJ, Yang Y, Gunn RB, Sands JM. Stimulation of UT-A1-mediated transepithelial urea flux in MDCK cells by lithium. Am J Physiol Renal Physiol 294: F518–F524, 2008. [DOI] [PubMed] [Google Scholar]
- 9.Fröhlich O, Klein JD, Smith PM, Sands JM, Gunn RB. Regulation of UT-A1-mediated transepithelial urea flux in MDCK cells. Am J Physiol Cell Physiol 291: C600–C606, 2006. [DOI] [PubMed] [Google Scholar]
- 10.Fushimi K, Uchida S, Hara Y, Hirata Y, Marumo F, Sasaki S. Cloning and expression of apical membrane water channel of rat kidney collecting tubule. Nature 361: 549–552, 1993. [DOI] [PubMed] [Google Scholar]
- 11.Inoue T, Nielsen S, Mandon B, Terris J, Kishore BK, Knepper MA. SNAP-23 in rat kidney: colocalization with aquaporin-2 in collecting duct vesicles. Am J Physiol Renal Physiol 275: F752–F760, 1998. [DOI] [PubMed] [Google Scholar]
- 12.Jo I, Harris HW, Amendt-Raduege AM, Majewski RR, Hammond TG. Rat kidney papilla contains abundant synaptobrevin protein that participates in the fusion of antidiuretic hormone-regulated water channel-containing endosomes in vitro. Proc Natl Acad Sci USA 92: 1876–1880, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kamsteeg EJ, Heijnen I, van Os CH, Deen PM. The subcellular localization of an aquaporin-2 tetramer depends on the stoichiometry of phosphorylated and nonphosphorylated monomers. J Cell Biol 151: 919–930, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Klein JD, Price SR, Bailey JL, Jacobs JD, Sands JM. Glucocorticoids mediate a decrease in AVP-regulated urea transporter in diabetic rat inner medulla. Am J Physiol Renal Physiol 273: F949–F953, 1997. [DOI] [PubMed] [Google Scholar]
- 15.Klussmann E, Maric K, Rosenthal W. The mechanisms of aquaporin control in the renal collecting duct. Rev Physiol Biochem Pharmacol 141: 33–95, 2000. [DOI] [PubMed] [Google Scholar]
- 16.Knepper MA, Inoue T. Regulation of aquaporin-2 water channel trafficking by vasopressin. Curr Opin Cell Biol 9: 560–564, 1997. [DOI] [PubMed] [Google Scholar]
- 17.Kuwahara M, Asai T, Terada Y, Sasaki S. The C-terminal tail of aquaporin-2 determines apical trafficking. Kidney Int 68: 1999–2009, 2005. [DOI] [PubMed] [Google Scholar]
- 18.Kuwahara M, Fushimi K, Terada Y, Bai L, Marumo F, Sasaki S. cAMP-dependent phosphorylation stimulates water permeability of aquaporin-collecting duct water channel protein expressed in Xenopus oocytes. J Biol Chem 270: 10384–10387, 1995. [DOI] [PubMed] [Google Scholar]
- 19.Li X, Low SH, Miura M, Weimbs T. SNARE expression and localization in renal epithelial cells suggest mechanism for variability of trafficking phenotypes. Am J Physiol Renal Physiol 283: F1111–F1122, 2002. [DOI] [PubMed] [Google Scholar]
- 20.Litman T, Sogaard R, Zeuthen T. Ammonia and urea permeability of mammalian aquaporins. Handb Exp Pharmacol 327–358, 2009. [DOI] [PubMed]
- 21.Low SH, Chapin SJ, Weimbs T, Komuves LG, Bennett MK, Mostov KE. Differential localization of syntaxin isoforms in polarized Madin-Darby canine kidney cells. Mol Biol Cell 7: 2007–2018, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Low SH, Vasanji A, Nanduri J, He M, Sharma N, Koo M, Drazba J, Weimbs T. Syntaxins 3 and 4 are concentrated in separate clusters on the plasma membrane before the establishment of cell polarity. Mol Biol Cell 17: 977–989, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lu HJ, Matsuzaki T, Bouley R, Hasler U, Qin QH, Brown D. The phosphorylation state of serine 256 is dominant over that of serine 261 in the regulation of AQP2 trafficking in renal epithelial cells. Am J Physiol Renal Physiol 295: F290–F294, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Magni F, Sarto C, Ticozzi D, Soldi M, Bosso N, Mocarelli P, Kienle MG. Proteomic knowledge of human aquaporins. Proteomics 6: 5637–5649, 2006. [DOI] [PubMed] [Google Scholar]
- 25.Mandon B, Chou CL, Nielsen S, Knepper MA. Syntaxin-4 is localized to the apical plasma membrane of rat renal collecting duct cells: possible role in aquaporin-2 trafficking. J Clin Invest 98: 906–913, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mandon B, Nielsen S, Kishore BK, Knepper MA. Expression of syntaxins in rat kidney. Am J Physiol Renal Physiol 273: F718–F730, 1997. [DOI] [PubMed] [Google Scholar]
- 27.Meinild AK, Klaerke DA, Zeuthen T. Bidirectional water fluxes and specificity for small hydrophilic molecules in aquaporins 0–5. J Biol Chem 273: 32446–32451, 1998. [DOI] [PubMed] [Google Scholar]
- 28.Mistry AC, Mallick R, Fröhlich O, Klein JD, Rehm A, Chen G, Sands JM. The UT-A1 urea transporter interacts with snapin, a SNARE-associated protein. J Biol Chem 282: 30097–30106, 2007. [DOI] [PubMed] [Google Scholar]
- 29.Nielsen S, Chou CL, Marples D, Christensen EI, Kishore BK, Knepper MA. Vasopressin increases water permeability of kidney collecting duct by inducing translocation of aquaporin-CD water channels to plasma membrane. Proc Natl Acad Sci USA 92: 1013–1017, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nielsen S, Frokiaer J, Marples D, Kwon TH, Agre P, Knepper MA. Aquaporins in the kidney: from molecules to medicine. Physiol Rev 82: 205–244, 2002. [DOI] [PubMed] [Google Scholar]
- 31.Nielsen S, Marples D, Birn H, Mohtashami M, Dalby NO, Trimble M, Knepper M. Expression of VAMP-2-like protein in kidney collecting duct intracellular vesicles. Colocalization with aquaporin-2 water channels. J Clin Invest 96: 1834–1844, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31a.Procino G, Barbieri C, Tamma G, De Benedictis L, Pessin JE, Svelto M, Valenti G. AQP2 exocytosis in the renal collecting duct - involvement of SNARE isoforms and the regulatory role of Munc18b. J Cell Sci 121: 2097–2106, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Söllner T, Bennett MK, Whiteheart SW, Scheller RH, Rothman JE. A protein assembly-disassembly pathway in vitro that may correspond to sequential steps of synaptic vesicle docking, activation, and fusion. Cell 75: 409–418, 1993. [DOI] [PubMed] [Google Scholar]
- 33.Söllner T, Whiteheart SW, Brunner M, Erdjument-Bromage H, Geromanos S, Tempst P, Rothman JE. SNAP receptors implicated in vesicle targeting and fusion. Nature 362: 318–324, 1993. [DOI] [PubMed] [Google Scholar]
- 34.Star RA Apical membrane limits urea permeation across the rat inner medullary collecting duct. J Clin Invest 86: 1172–1178, 1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Terris J, Ecelbarger CA, Marples D, Knepper MA, Nielsen S. Distribution of aquaporin-4 water channel expression within rat kidney. Am J Physiol Renal Fluid Electrolyte Physiol 269: F775–F785, 1995. [DOI] [PubMed] [Google Scholar]
- 36.van Balkom BW, Graat MP, van Raak M, Hofman E, van der Sluijs P, Deen PM. Role of cytoplasmic termini in sorting and shuttling of the aquaporin-2 water channel. Am J Physiol Cell Physiol 286: C372–C379, 2004. [DOI] [PubMed] [Google Scholar]
- 37.van Balkom BW, Hoffert JD, Chou CL, Knepper MA. Proteomic analysis of long-term vasopressin action in the inner medullary collecting duct of the Brattleboro rat. Am J Physiol Renal Physiol 286: F216–F224, 2004. [DOI] [PubMed] [Google Scholar]
- 38.Widberg CH, Bryant NJ, Girotti M, Rea S, James DE. Tomosyn interacts with the t-SNAREs syntaxin4 and SNAP23 and plays a role in insulin-stimulated GLUT4 translocation. J Biol Chem 278: 35093–35101, 2003. [DOI] [PubMed] [Google Scholar]
- 39.Yamamoto T, Sasaki S. Aquaporins in the kidney: emerging new aspects. Kidney Int 54: 1041–1051, 1998. [DOI] [PubMed] [Google Scholar]
- 40.Zhang C, Sands JM, Klein JD. Vasopressin rapidly increases phosphorylation of UT-A1 urea transporter in rat IMCDs through PKA. Am J Physiol Renal Physiol 282: F85–F90, 2002. [DOI] [PubMed] [Google Scholar]