Abstract
Nitric oxide (NO), a neurotransmitter in the lower urinary tract, stimulates soluble guanylyl cyclase (sGC) and in turn cGMP-dependent protein kinase G (PKG) to modulate a number of downstream targets. NO donors reduce bladder hyperactivity in some pathological models but do not affect normal bladder activity in the adult rat. In this study, the NO donor S-nitroso-N-acetyl-dl-penicillamine (SNAP; 100 μM) decreased the amplitude and frequency of spontaneous and carbachol-enhanced contractions in neonatal rat bladder strips, which are intrinsically hyperactive. This effect was blocked by inhibition of sGC and mimicked by application of a membrane-permeable cGMP analog (8-bromo-cGMP, 100 μM). Inhibition of PKG prevented or reversed the inhibitory effects of 8-bromo-cGMP. A portion of the SNAP-mediated inhibition was also dependent upon PKG; however, a short-lasting, sGC-dependent inhibitory effect of SNAP was still present after PKG inhibition. Inhibition of NO synthase with l-NAME (100 μM) did not change the amplitude or frequency of contractions. However, inhibition of endogenous phosphodiesterase (PDE)-5 with zaprinast (25 μM) reduced the amplitude and frequency of phasic contractions and increased the magnitude of inhibition produced by maximal concentrations of SNAP, suggesting that endogenous PDEs are constitutively active and regulate cGMP production. These results suggest that the NO-cGMP-PKG pathway may be involved in inhibitory control of the neonatal rat bladder.
Keywords: smooth muscle, spontaneous activity, phosphodiesterase, carbachol
nitric oxide (NO) has an important physiological role as a neurotransmitter in the peripheral and central nervous systems and as a vasodilator substance released from endothelial cells. NO, which is synthesized by nitric oxide synthase (NOS), activates soluble guanylyl cyclase (sGC) to produce cGMP. cGMP in turn activates cGMP-dependent protein kinase (PKG), which regulates many cellular processes including smooth muscle relaxation and ion channel function (20).
In the lower urinary tract, NO has the potential to function as a transmitter at various sites because NOS is expressed in afferent and efferent nerves, as well as in the urothelium, smooth muscle, and striated muscle (19). NO acts as an inhibitory neurotransmitter in the urethra, where it is released from parasympathetic nerves; however, its function in the urinary bladder is uncertain and seems to vary in different species. For example, NO donors abolish rhythmic activity induced by muscarinic stimulation in the mouse whole-bladder preparation (23), but increase the frequency of phasic activity in a guinea pig whole-bladder preparation (14). On the other hand, the contractile activity of the rat and rabbit bladder seems to be resistant to NO donors (1, 9, 25). Exposure to NO donors increases the levels of cGMP in serosal and intramuscular interstitial cells in the mouse bladder, and in interstitial cells in the guinea pig bladder located near the serosal surface, between muscle bundles and in the suburothelium (15–17, 22, 23).
In adult rats, where the detrusor muscle is thought to be comparatively insensitive to NO (32), several studies have demonstrated an effect of NO on reflex bladder activity in vivo. For example, in rats, intravesical administration of an NO donor suppresses cyclophosphamide-induced bladder hyperactivity (29), while the NO scavenger oxyhemoglobin induces bladder overactivity (30), likely to be due to a reduction of an inhibitory effect of NO generated in the urothelium (3, 4). In addition, the amplitude and number of nonvoiding contractions in chronic spinal cord-injured rats are decreased by treatment with an arginase inhibitor (35). This effect is prevented by treatment with a NOS inhibitor, demonstrating that the effect of the arginase inhibitor is due to increased NO production.
The neonatal rat bladder is intrinsically overactive during the first 3–4 wk of life (37). During this time, bladder activity is characterized by high-amplitude low-frequency spontaneous contractions that are likely to be myogenic, as they occur in the absence of nerve stimulation and are not blocked by inhibiting nerve activity with TTX (39). Later, during postnatal development, the spontaneous bladder activity is reduced to a low-amplitude high-frequency pattern characteristic of the normal adult rat. Spontaneous activity in the neonatal rat bladder is hypothesized to be initiated at the bladder dome (21) or the bladder neck region (39) and propagated throughout the detrusor muscle by a network of interstitial cells interconnected by gap junctions (21). Activity arising in the urothelium and/or lamina propria may contribute to the generation of the spontaneous contractions. These spontaneous contractions are thought to be under tonic inhibitory control by the central nervous system because in the neonatal rat spinal cord-bladder preparation, application of TTX or removal of the spinal cord increases spontaneous activity (38). This inhibition may be important for maintaining urinary continence in the pup and allowing the mother to control voiding via the perineal-to-bladder excitatory reflex. However, the neurotransmitter mediating the inhibition has yet to be identified.
This study investigated the effect of the NO-cGMP pathway on spontaneous bladder contractions in neonatal rat bladder strips to evaluate the possibility that NO has a role in the putative neural inhibition of the neonatal bladder. The results demonstrate that activation of the NO-cGMP pathway inhibits spontaneous and carbachol-enhanced contractions in neonatal rat bladder strips, suggesting that NO may be an inhibitory neurotransmitter in the bladder during early postnatal development.
METHODS
Bladder strip preparation.
All experimental procedures were approved and performed in accordance with the Institutional Animal Care and Use Committee of the University of Pittsburgh. Bladder strips from male and female neonatal (10–21 days old) Sprague-Dawley rats were prepared as described previously (39). Briefly, the bladder was removed from isoflurane (4% in O2)-anesthetized rats, placed in warm Krebs solution [composition (in mM): 118 NaCl, 4.7 KCl, 1.9 CaCl2, 1.2 MgSO4, 24.9 NaHCO3, 1.2 KH2PO4, and 11.7 dextrose, pH. 7.4, bubbled with 95% O2-5% CO2], and cut into two to four longitudinal strips (∼1.5 × 8–10 mm). Strips were tied with fine thread at each end and mounted in a vertical double-jacketed organ bath in oxygenated Krebs solution (15-ml volume) kept at 37°C via a circulating warm water bath. The tissue was allowed to equilibrate for 1–2 h before drug testing. Drugs from concentrated stock solutions were added directly to the organ bath. Because the amplitude and frequency of spontaneous contractions vary over several hours, in most experiments, 50–200 nM carbachol was applied to each strip to enhance spontaneous contractions without increasing baseline tension (28). This stabilized bladder strip activity so that contractions and the effects of drugs could be measured over several hours. After setting of baseline tension to 10 mN (1 g), spontaneous and carbachol-enhanced contractions were measured with a force displacement transducer (Grass, Astromed, West Warwick, RI). Data, including baseline tone and amplitude and frequency of spontaneous contractions, were recorded for offline analysis using Windaq software (DATAQ Instruments, Akron, OH) and analyzed using Excel (Microsoft, Redmond, WA) and Origin (version 7; Origin Lab, Northampton, MA).
Drugs used in this study include S-nitroso-N-acetyl-dl-penicillamine (SNAP), an NO donor; 8-bromo-cGMP, a membrane-permeable, phophodiesterase-resistant cGMP analog; NG-nitro-l-arginine methyl ester hydrochloride (l-NAME), a NOS inhibitor; 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one (ODQ), an inhibitor of soluble guanylyl cyclase; Rp-8-bromo-PET-cGMPS hydrate (Rp-cGMPS), a cGMP-dependent protein kinase (PKG) inhibitor; zaprinast, a phosphodiesterase (PDE) 5 inhibitor; and carbachol, a cholinergic receptor agonist. Zaprinast, Rp-cCMPS and 8-bromo-cGMP were obtained from Tocris Bioscience (Ellisville, MO). All other reagents were obtained from Sigma (St. Louis, MO). SNAP was prepared fresh daily in H2O at 100× final concentration. All other drugs were prepared at 1,000× final concentration and stored at −20°C. Drug stocks were thawed and added directly to the bath to achieve the desired final concentration. Vehicles (0.1% EtOH for ODQ and 0.1% DMSO for zaprinast) had no effect on bladder strip contractions.
Data analysis.
All data are presented as means ± SE. Bladder strip activity was measured in 5-min intervals. The effects of drugs were measured as change in amplitude or frequency of contractions (contraction threshold was set at 0.15 g) and reported as percentage of control. Baseline tone was not appreciably changed by various treatments and therefore was not subjected to detailed analysis. Statistical significance was tested with a paired two-tailed t-test with a layered Bonferroni post hoc test for multiple comparisons when appropriate, using Excel and Origin 7 software. Data were considered statistically significant when P < 0.05.
RESULTS
Activation of the NO-cGMP pathway inhibits carbachol-enhanced spontaneous contractions in neonatal rat bladder strips.
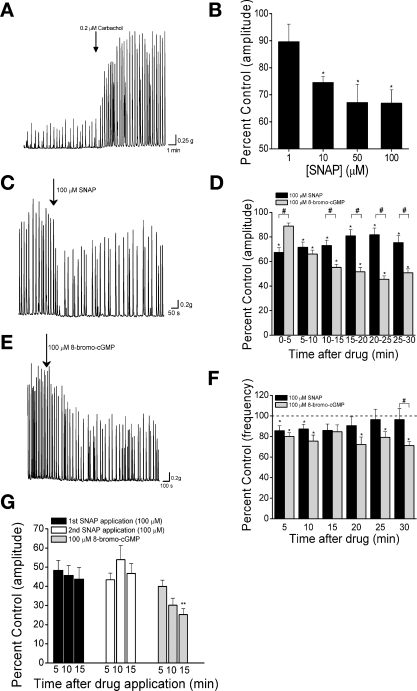
Bladder strips from neonatal rats (10–21 days of age) exhibited high-amplitude, low-frequency spontaneous contractions typical of neonatal rat detrusor (Fig. 1A) (39). Application of low concentrations of carbachol (50–200 nM) increased the amplitude and frequency of spontaneous contractions without increasing baseline tension (Fig. 1A). The amplitude and frequency of carbachol-enhanced spontaneous contractions (SCcarb) were stable over the course of an experiment (1–3 h). The NO donor SNAP reduced the amplitude of SCcarb in a concentration-dependent manner up to 10 μM with no additional reduction with higher concentrations (Fig. 1B). The effect of SNAP occurred within 5 min of application (Table 1, Fig. 1, C and D). Inhibition lasted for up to 30 min, although the contraction amplitude partially recovered 15–20 min after drug application (Fig. 1D). SNAP (100 μM) reduced the frequency of SCcarb 5 min after application (Fig. 1F), while lower concentrations of SNAP had no significant effect on SCcarb frequency. SCcarb frequency began to recover after 10 min and returned to control levels 25–30 min after SNAP application (Fig. 1F). To test for effects of endogenous NO, 100 μM l-NAME was applied to inhibit NOS (n = 6). l-NAME did not alter the amplitude or frequency of SCcarb (data not shown), suggesting that SCcarb were not tonically modulated by a nitrergic mechanism under the conditions of our experiments.
Fig. 1.
S-nitroso-N-acetyl-dl-penicillamine (SNAP) and 8-bromo-cGMP reduce the amplitude and frequency of carbachol-enhanced spontaneous contractions (SCcarb). A: carbachol-induced enhancement of spontaneous activity in a bladder strip from neonatal rat. Arrow indicates time of carbachol (100 nM) application. B: concentration dependence of SNAP-mediated inhibition of SCcarb amplitude (n = ≥7 for each concentration). C and E: inhibition of SCcarb by 100 μM SNAP (C) and 100 μM 8-bromo-cGMP (E). D and F: summary data showing average effect of 100 μM SNAP (n = 15) and 100 μM 8-bromo-cGMP (n = 13) on SCcarb amplitude (D) and frequency (F). G: summary of inhibition of SCcarb amplitude by 2 consecutive applications of 100 μM SNAP followed by 1 application of 100 μM 8-bromo-cGMP (n = 12). *P < 0.05 compared with control. #P < 0.05 between groups. **P < 0.05 compared with maximum SNAP inhibition by 2-tailed t-test with layered Bonferroni correction.
Table 1.
Effects of SNAP and 8-bromo-cGMP on carbachol-enhanced neonatal bladder strip activity
| Amplitude of SCcarb | Frequency of SCcarb | Inhibition of Amplitude After 30 min | Inhibition of Frequency After 30 min | |
|---|---|---|---|---|
| 100 μM SNAP (n =15) | 33.2±4%* | 10.25±6.2%* | 14.9±4.7%* | 3.6±10.8% |
| 100 μM 8-bromo-cGMP (n =13) | 55.2±2.5%* | 28.2±3.8%* | 49.2±3.2%* | 28.8±3.9%* |
Values are means ± SE expressed as percentage of drug-induced reduction measured as percentage of control; n = no. of strips. SNAP, S-nitroso-N-acetyl-dl-penicillamine; SCcarb, carbachol-enhanced spontaneous contractions.
P < 0.05 vs. control by Student's t-test with layered Bonferroni corrections when multiple comparisons were made.
8-bromo-cGMP at 100 μM, (a concentration that has been shown to relax mouse urethral smooth muscle strips) (33) also reduced SCcarb amplitude (Fig. 1, D and E). However, the effect developed more slowly than the effect of SNAP, becoming significant 10 min after application, and reaching a maximum in an additional 10–15 min. Contraction frequency was also reduced by 100 μM 8-bromo-cGMP within 5 min and was maximally reduced 25–30 min after application (Fig. 1F). The magnitude and duration of 8-bromo-cGMP effects were greater than those of SNAP. Thirty minutes after application of 8-bromo-cGMP, SCcarb were still significantly reduced in amplitude and frequency while the effects of SNAP had diminished appreciably (Table 1, Fig. 1, D, and G).
In some experiments, strips were washed, allowed to recover for at least 1 h, and carbachol (50–200 nM) was reapplied. Application of 100 μM SNAP or 100 μM 8-bromo-cGMP inhibited the SCcarb amplitude to a similar degree as during the first application (Table 2).
Table 2.
Effects of SNAP and 8-bromo-cGMP on neonatal bladder strip activity
| Amplitude of SCcarb, 2nd Drug Application | Amplitude of Spontaneous Contractions | Frequency of Spontaneous Contractions | |
|---|---|---|---|
| 100 μM SNAP | 38.6±7.6%*(n=13) | 54.5±10.5%*(n=6) | 38.7±13.6%*(n=6) |
| 100 μM 8-bromo-cGMP | 53.3±9.15%*(n=12) | 59.2±7.4%*(n=5) | 39.9±11.1%*(n=5) |
Values are means ± SE expressed as percentage of drug-induced reduction measured as percentage of control; n = no. of strips.
P < 0.05 vs. control by Student's t-test with layered Bonferroni corrections when multiple comparisons were made.
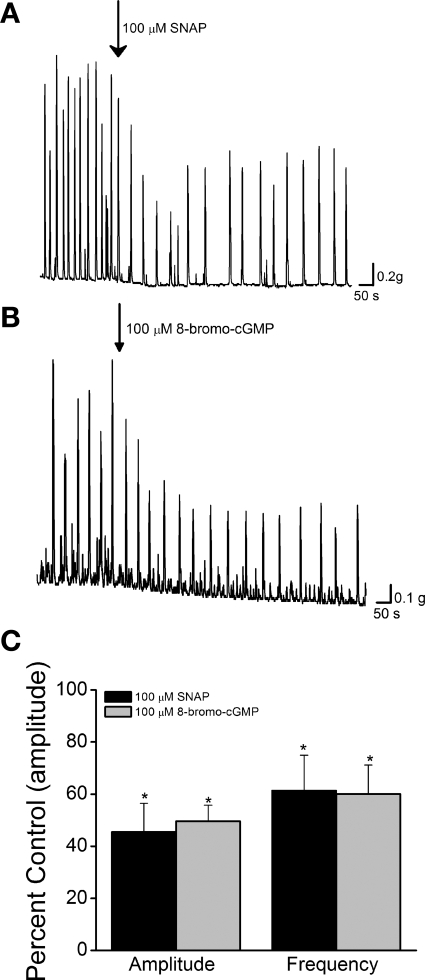
To determine whether the NO-inhibitory effect was selective for muscarinic-induced activity, we tested the agents on spontaneous contractions in untreated preparations. Application of SNAP (100 μM) or 8-bromo-cGMP (100 μM) significantly reduced the amplitude and frequency of spontaneous contractions for 5–15 min after drug application to a similar extent as with carbachol-enhanced activity (Table 2, Fig. 2).
Fig. 2.
Inhibition of spontaneous contractions by SNAP and 8-bromo-cGMP. A and B: spontaneous contractions (not enhanced with carbachol) inhibited by 100 μM SNAP (A) or 100 μM 8-bromo-cGMP (B). C: summary data showing inhibition of spontaneous contraction amplitude and frequency by 100 μM SNAP (n = 6) or 100 μM 8-bromo-cGMP (n = 5). *P < 0.05 compared with control by 2-tailed t-test.
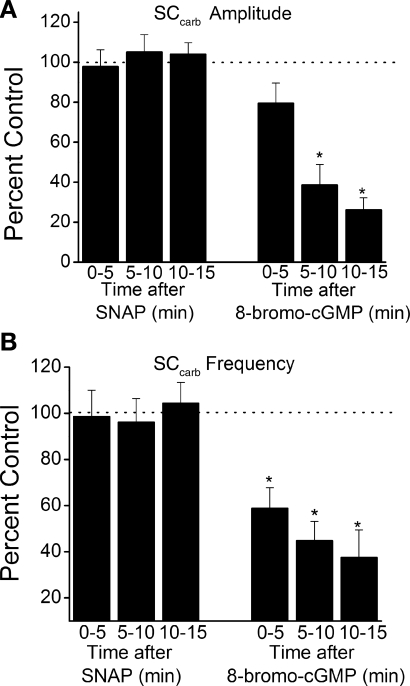
To determine whether the effects of SNAP were mediated by stimulation of sGC, bladder strips were treated for 15 min before SNAP application with 1 μM ODQ, a sCG inhibitor which has been shown to be selective at concentrations ≤10 μM (12). ODQ, which alone did not affect contraction amplitude (97.8 ± 8.4% of EtOH control; P > 0.05) or frequency (104.3 ± 9.1% of EtOH control; P > 0.05), completely prevented the effect of SNAP on the amplitude and frequency of SCcarb, but did not alter the effects of 8-bromo-cGMP (Fig. 3).
Fig. 3.
1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one (ODQ) prevents effects of SNAP. Summary data from strips pretreated with 1 μM ODQ are shown. A and B: effects of 100 μM SNAP (n = 10) or 100 μM 8-bromo-cGMP (n = 8) on SCcarb amplitude (A) and frequency (B) in rat neonatal bladder strips pretreated for 15 min with 1 μM ODQ. *P < 0.05 vs. control by 2-tailed t-test.
NO and 8-bromo-cGMP-induced inhibition of SCcarb is mediated primarily by cGMP-dependent protein kinase.
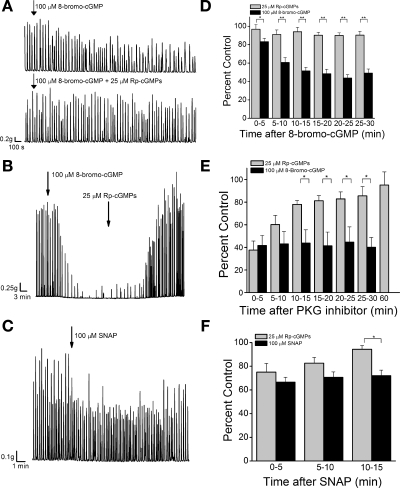
Pretreatment of bladder strips with the membrane-permeable PKG inhibitor Rp-cGMPS (25 μM, a concentration that is selective for PKG over PKA) (6) prevented the inhibitory effect of subsequent 8-bromo-cGMP application on SCcarb (Fig. 4, A and D). In addition, in bladder strips treated with 8-bromo-cGMP for 30 min, application of 25 μM Rp-cGMPS gradually reversed the effect of 8-bromo-cGMP (Fig. 4, B and E). The reversal of 8-bromo-cGMP-mediated inhibition was significant 10 min after Rp-cGMPS application, and after 1 h the contraction amplitude was completely restored (97.3 ± 11.6% of control).
Fig. 4.
Role of PKG in SNAP- and 8-bromo-cGMP-mediated inhibition of SCcarb. A: 100 μM 8-bromo-cGMP was added to a bladder strip without (top) or with (bottom) PKG inhibitor (25 μM Rp-cGMPS). B: PKG inhibition reversed the inhibitory effect of 100 μM 8-bromo-cGMP. C: 100 μM SNAP added to a bladder strip pretreated with 25 μM Rp-cGMPS. D: summary data showing 100 μM 8-bromo-cGMP inhibition of SCcarb contraction in the presence and absence of 25 μM Rp-cGMPS (n = 9). E: summary data showing 25 μM Rp-cGMPS reversal of 100 μM 8-bromo-cGMP inhibition of SCcarb contraction (n = 4). E and F: summary data showing 100 μM SNAP inhibition of SCcarb amplitude in the presence and absence of 25 μM Rp-cGMPS (n = 10). *P < 0.05, **P < 0.01 between groups by 2-tailed t-test with layered Bonferroni correction.
In contrast, pretreatment of strips with Rp-cGMPS (25 μM) reduced, but did not completely prevent the inhibitory effect of SNAP on SCcarb amplitude (22.6 ± 4.9% inhibition in the presence of PKG inhibitor vs. 33.2 ± 4.0% in control) during the first 5 min after application (Fig. 4, C and F). However, the inhibitory effect of SNAP in the presence of the PKG inhibitor was briefer than in untreated strips. In control strips, contraction amplitude was significantly inhibited 15 min after SNAP application (21.7 ± 8.8% inhibition), while in Rp-cGMPS-pretreated strips, contraction amplitude recovered within 15 min of SNAP application (94.4 ± 3.1% of control, Fig. 4F).
Role of endogenous PDEs in NO-mediated inhibition of SCcarb.
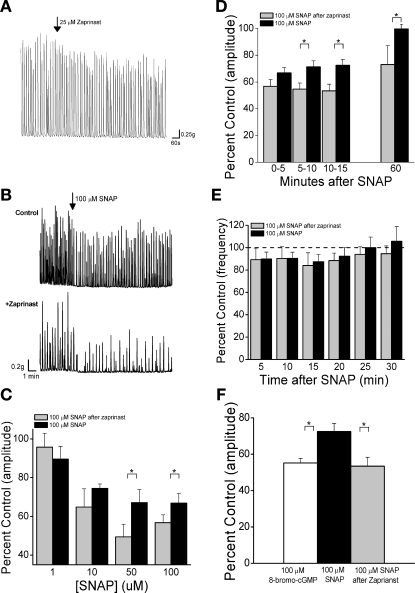
To determine the influence of endogenous PDEs on SCcarb and the effects of inhibitory agents, strips were treated with zaprinast (a potent PDE-5-specific inhibitor that at 25 μM has been shown to increase cGMP and relax penile smooth muscle) (5, 13). Zaprinast (25 μM) significantly (P < 0.05) reduced SCcarb amplitude by 17.6 ± 2.4% 10–15 min after application (n = 25, Fig. 5A). Zaprinast also significantly increased, by 30–60%, the peak inhibition of SCcarb amplitude induced 10–15 min after 50 and 100 μM SNAP (Fig. 5, B and C). In addition, zaprinast increased the duration of SNAP-mediated inhibition. In the presence of zaprinast, contraction amplitude was inhibited by 41.5 ± 5.6% 60 min after SNAP application, while in the absence of zaprinast contraction amplitude had completely recovered at this time (Fig. 5D, P < 0.05). Zaprinast pretreatment did not alter the 8-bromo-cGMP-mediated inhibition of SCcarb amplitude (50.8 ± 6.7 inhibition alone vs. 44.7 ± 8.7% inhibition after zaprinast, P > 0.05). The maximal inhibition elicited by SNAP after zaprinast was similar to that induced by 8-bromo-cGMP (Fig. 5F). Zaprinast (25 μM) alone elicited a small but significant reduction in SCcarb frequency (11.8 ± 2.3%, P < 0.05) but did not change the effect of SNAP on SCcarb frequency (Fig. 5E).
Fig. 5.
Inhibition of phosphodiesterase reduces the amplitude and frequency of SCcarb and enhances the inhibitory effect of SNAP. A: effect of 25 μM zaprinast on SCcarb. B: SNAP-mediated reduction of SCcarb in a vehicle-treated strip (0.1% DMSO; top) and in a strip pretreated with 25 μM zaprinast (bottom). C: concentration-response data for SNAP-mediated inhibition of SCcarb amplitude in control strips (without zaprinast) and in strips pretreated with 25 μM zaprinast (n = ≥6 for each concentration and treatment). D and E: summary data showing 100 μM SNAP-mediated inhibition of SCcarb amplitude (D) and frequency (E) in vehicle-treated control strips and strips pretreated with 25 μM zaprinast (n = 13). F: summary data comparing SCcarb amplitude with 100 μM SNAP, 100 μM 8-bromo-cGMP, and 100 μM SNAP after zaprinast application (n = ≥8 for each condition). *P < 0.05 between groups by 2-tailed t-test with layered Bonferroni correction.
DISCUSSION
The present results indicate that the frequency and amplitude of rhythmic smooth muscle activity in bladder strips of neonatal rats are sensitive to the inhibitory effects of an intracellular sGC-cGMP-PKG signaling pathway that can be activated by an exogenously administered agent that generates NO. The inhibitory pathway is modulated by PDE-5 activity. Zaprinast, a PDE-5 inhibitor, enhanced the inhibitory effect of SNAP and produced a small inhibitory effect when applied alone, suggesting that low levels of cGMP are present in the tissue. However, the full inhibitory pathway does not seem to be tonically active under the conditions of our experiments, in which bladder nerves are quiescent. Still, bladder nerves that express neuronal NOS are presumably able to synthesize and release NO when activated. Thus the present experiments raise the possibility that NO might play a role in the previously identified neural-inhibitory pathway that regulates spontaneous contractions in the in vitro spinal cord-urinary bladder preparation of the neonatal rat (38).
Signaling pathway.
In the present study, an NO donor, SNAP, reduced the amplitude and frequency of spontaneous contractions as well as carbachol-enhanced contractions in neonatal rat bladder strips. ODQ completely blocked the effects of SNAP, indicating that the effects of NO were mediated via activation of sGC. The cGMP analog 8-bromo-cGMP mimicked the inhibitory effect of SNAP but elicited a slower-onset and longer-lasting response, consistent with the slow passage of the large molecule across the plasma membrane, and its resistance to metabolism by endogenous phosphodiesterases. Treatment with zaprinast, a PDE-5 inhibitor, increased the efficacy and duration of action of SNAP such that it produced effects similar to those of 8-bromo-cGMP. This suggests a role for endogenous PDE-5 in regulating the NO signaling pathway in the neonatal bladder.
Pretreatment of bladder strips with a PKG inhibitor reduced SNAP inhibition and completely prevented or reversed 8-bromo-cGMP inhibition of SCcarb amplitude, suggesting that the effects of cGMP are dependent upon PKG. An early component of SNAP-induced inhibition that was resistant to PKG inhibition was short-lived, lasting only 10 min. Taken together, these data suggest that the classic NO-cGMP-PKG pathway can regulate bladder activity in the neonatal rat and provide evidence for a second inhibitory pathway initiated by NO that is sGC dependent but PKG independent.
The inhibitory effect of NO on neonatal bladder strips contrasts with the lack of effect of NO on in vitro bladder preparations from adult rats and other species including human, pig, and rabbit (2, 9, 25). Thus the sensitivity to NO-mediated inhibition may be lost during postnatal development. This suggests that either the sGC-PKG signaling pathway is downregulated or that the smooth muscle contractile mechanisms become resistant to this pathway. The data in this paper are insufficient to distinguish between these two possibilities. Therefore, further studies of the mechanisms underlying NO-mediated inhibition in the neonatal rat during development and adult bladder are needed to address this question.
Site of action of NO.
There are several possible targets of NO-cGMP signaling in the neonatal rat bladder preparation. Experiments in other species (e.g., guinea pig and mouse) in which bladder activity is sensitive to NO might provide insights into the mechanism of action of NO. For example, in guinea pig bladder, an NO donor increases the levels of cGMP in interstitial cells/myofibroblasts (15) and 8-bromo-cGMP reduces contractile activity (40). Interstitial cells, which communicate via gap junctions, are thought to underlie the propagation of rhythmic contractile activity through the bladder wall. Since NO can cause uncoupling of neuronal gap junctions (31), it is possible that NO suppresses phasic contractions by inhibiting the propagation of signals through the interstitial cell network in the bladder. Our data are consistent with an effect on propagation as the frequency as well as the amplitude of spontaneous activity were reduced by NO.
NO might also act directly on the smooth muscle. One possible NO target within smooth muscle is Rho kinase. PKG phosphorylates and inactivates RhoA, a G protein that activates Rho kinase (36). Rho kinase inhibition reduces the peak and sustained components of carbachol-induced contractions in mouse bladder (8) and decreases bladder tone (34). Alternatively, NO may target various types of potassium channels. For example, in guinea pig bladder myocytes, an NO donor opens ATP-sensitive K channels in a cGMP-dependent manner (7). Large-conductance Ca2+-activated K channels, which are known to regulate spontaneous activity in the neonatal rat bladder (18, 28) and which are activated by NO in vascular smooth muscle (24), are also potential targets for NO in bladder smooth muscle.
NO could also alter phasic activity in the bladder indirectly by acting on intramural nerves. Although phasic activity in the neonatal rat bladder is myogenic and occurs in the presence of TTX, which blocks nerve action potentials, spontaneous release of a neurotransmitter from nerves can, under certain conditions, modulate phasic contractile activity in the neonatal rat bladder (28). NO is known to inhibit transmitter release from efferent autonomic nerves (41) and to inhibit calcium channels in bladder afferent neurons that are important for release of afferent neurotransmitters (42). Thus NO could modulate phasic contractile activity by suppressing spontaneous release of excitatory neurotransmitters.
Species differences in effects of NO.
Effects of NO in the neonatal rat bladder might be different from effects in the guinea pig and mouse bladder because the latter contain a more complex intramural nervous system composed of autonomic ganglion cells and local reflex pathways (10) that are not present in the rat bladder (11). This might account for some of the divergent findings reported in these species. For example, in guinea pig detrusor strips, SNAP increased the amplitude and frequency of spontaneous bladder activity and baseline tone, but in a sGC-independent manner, while in the same strips, 8-bromo-cGMP reduced contractile activity (40). In the mouse bladder, the peak amplitude and plateau of carbachol-induced contractions were suppressed by 8-bromo-cGMP, but the phasic contractions were not suppressed (8). Another study of the mouse bladder found no effect of NO donors, but 8-bromo-cGMP relaxed carbachol-precontracted bladder strips (9). Thus differences in the species, age of animal, types of contractile activity, and pharmacological agents should all be considered when the effects of NO on bladder activity are evaluated.
Physiological role of NO-sGC-cGMP signaling pathway.
In neonatal rat bladder strips, inhibition of endogenous PDE-5 with zaprinast reduced the amplitude and frequency of SCcarb, indicating that sufficient cGMP is generated under the conditions of our experiments to inhibit bladder contractions. Furthermore, zaprinast increased the maximal effect of an exogenous NO donor, suggesting that there is sufficient endogenous PDE to limit NO efficacy. PDE-5 inhibitors such as sildenafil, which are used clinically to treat erectile dysfunction by increasing cGMP levels in penile smooth muscle, seem to also improve lower urinary tract symptoms associated with benign prostatic hyperplasia (26, 27). This suggests that the NO-cGMP-inhibitory pathway may be active under conditions of bladder dysfunction. In some pathological conditions, such as spinal cord injury or bladder outlet obstruction, the normal pattern of low-amplitude spontaneous activity is converted to a large-amplitude neonatal-like pattern of activity (39). This raises the possibility that pathology may cause relatively quiescent adult bladders to revert to a spontaneous neonatal pattern and initiate the reemergence of NO-mediated inhibitory effects. Therefore, the neonatal rat bladder may be a useful model for detrusor overactivity in adults.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney DiseasesGrants DK-49430 to William C. de Groat and K08-DK-5759 to Hsi-Yang Wu.
Acknowledgments
Present address of Hsi-Yang Wu: Dept. of Urology, Stanford University School of Medicine, Stanford, CA 94305.
REFERENCES
- 1.Andersson KE, Persson K. Nitric oxide synthase and nitric oxide-mediated effects in lower urinary tract smooth muscles. World J Urol 12: 274–280, 1994. [DOI] [PubMed] [Google Scholar]
- 2.Andersson KE, Persson K. Nitric oxide synthase and the lower urinary tract: possible implications for physiology and pathophysiology. Scand J Urol Nephrol Suppl 175: 43–53, 1995. [PubMed] [Google Scholar]
- 3.Birder LA, Apodaca G, De Groat WC, Kanai AJ. Adrenergic- and capsaicin-evoked nitric oxide release from urothelium and afferent nerves in urinary bladder. Am J Physiol Renal Physiol 275: F226–F229, 1998. [DOI] [PubMed] [Google Scholar]
- 4.Birder LA, Nealen ML, Kiss S, de Groat WC, Caterina MJ, Wang E, Apodaca G, Kanai AJ. Beta-adrenoceptor agonists stimulate endothelial nitric oxide synthase in rat urinary bladder urothelial cells. J Neurosci 22: 8063–8070, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bowman A, Drummond AH. Cyclic GMP mediates neurogenic relaxation in the bovine retractor penis muscle. Br J Pharmacol 81: 665–674, 1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Butt E, van Bemmelen M, Fischer L, Walter U, Jastorff B. Inhibition of cGMP-dependent protein kinase by (Rp)-guanosine 3′,5′-monophosphorothioates. FEBS Lett 263: 47–50, 1990. [DOI] [PubMed] [Google Scholar]
- 7.Deka DK, Brading AF. Nitric oxide activates glibenclamide-sensitive K+ channels in urinary bladder myocytes through a c-GMP-dependent mechanism. Eur J Pharmacol 492: 13–19, 2004. [DOI] [PubMed] [Google Scholar]
- 8.Ekman M, Andersson KE, Arner A. Developmental regulation of nerve and receptor mediated contractions of mammalian urinary bladder smooth muscle. Eur J Pharmacol 532: 99–106, 2006. [DOI] [PubMed] [Google Scholar]
- 9.Fujiwara M, Andersson K, Persson K. Nitric oxide-induced cGMP accumulation in the mouse bladder is not related to smooth muscle relaxation. Eur J Pharmacol 401: 241–250, 2000. [DOI] [PubMed] [Google Scholar]
- 10.Gabella G Intramural neurons in the urinary bladder of the guinea-pig. Cell Tissue Res 261: 231–237, 1990. [DOI] [PubMed] [Google Scholar]
- 11.Gabella G, Uvelius B. Urinary bladder of rat: fine structure of normal and hypertrophic musculature. Cell Tissue Res 262: 67–79, 1990. [DOI] [PubMed] [Google Scholar]
- 12.Garthwaite J, Southam E, Boulton CL, Nielsen EB, Schmidt K, Mayer B. Potent and selective inhibition of nitric oxide-sensitive guanylyl cyclase by 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one. Mol Pharmacol 48: 184–188, 1995. [PubMed] [Google Scholar]
- 13.Gibson A Phosphodiesterase 5 inhibitors and nitrergic transmission-from zaprinast to sildenafil. Eur J Pharmacol 411: 1–10, 2001. [DOI] [PubMed] [Google Scholar]
- 14.Gillespie JI, Drake MJ. The actions of sodium nitroprusside and the phosphodiesterase inhibitor dipyridamole on phasic activity in the isolated guinea-pig bladder. BJU Int 93: 851–858, 2004. [DOI] [PubMed] [Google Scholar]
- 15.Gillespie JI, Markerink-van Ittersum M, de Vente J. cGMP-generating cells in the bladder wall: identification of distinct networks of interstitial cells. BJU Int 94: 1114–1124, 2004. [DOI] [PubMed] [Google Scholar]
- 16.Gillespie JI, Markerink-van Ittersum M, de Vente J. Endogenous nitric oxide/cGMP signalling in the guinea pig bladder: evidence for distinct populations of sub-urothelial interstitial cells. Cell Tissue Res 325: 325–332, 2006. [DOI] [PubMed] [Google Scholar]
- 17.Gillespie JI, Markerink-van Ittersum M, de Vente J. Expression of neuronal nitric oxide synthase (nNOS) and nitric-oxide-induced changes in cGMP in the urothelial layer of the guinea pig bladder. Cell Tissue Res 321: 341–351, 2005. [DOI] [PubMed] [Google Scholar]
- 18.Herrera GM, Heppner TJ, Nelson MT. Regulation of urinary bladder smooth muscle contractions by ryanodine receptors and BK and SK channels. Am J Physiol Regul Integr Comp Physiol 279: R60–R68, 2000. [DOI] [PubMed] [Google Scholar]
- 19.Ho MH, Bhatia NN, Khorram O. Physiologic role of nitric oxide and nitric oxide synthase in female lower urinary tract. Curr Opin Obstet Gynecol 16: 423–429, 2004. [DOI] [PubMed] [Google Scholar]
- 20.Hofmann F, Ammendola A, Schlossmann J. Rising behind NO: cGMP-dependent protein kinases. J Cell Sci 113: 1671–1676, 2000. [DOI] [PubMed] [Google Scholar]
- 21.Kanai A, Roppolo J, Ikeda Y, Zabbarova I, Tai C, Birder L, Griffiths D, de Groat W, Fry C. Origin of spontaneous activity in neonatal and adult rat bladders and its enhancement by stretch and muscarinic agonists. Am J Physiol Renal Physiol 292: F1065–F1072, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lagou M, De Vente J, Kirkwood TB, Hedlund P, Andersson KE, Gillespie JI, Drake MJ. Location of interstitial cells and neurotransmitters in the mouse bladder. BJU Int 97: 1332–1337, 2006. [DOI] [PubMed] [Google Scholar]
- 23.Lagou M, Drake MJ, Markerink-Van Ittersum M, de Vente J, Gillespie JI. Interstitial cells and phasic activity in the isolated mouse bladder. BJU Int 98: 643–650, 2006. [DOI] [PubMed] [Google Scholar]
- 24.Lunardi CN, Vercesi JA, da Silva RS, Bendhack LM. Vasorelaxation induced by the new nitric oxide donor cis-[Ru(Cl)(bpy)(2)(NO)](PF(6)) is due to activation of K(Ca) by a cGMP-dependent pathway. Vascul Pharmacol 47: 139–144, 2007. [DOI] [PubMed] [Google Scholar]
- 25.Mamas MA, Reynard JM, Brading AF. Nitric oxide and the lower urinary tract: current concepts, future prospects. Urology 61: 1079–1085, 2003. [DOI] [PubMed] [Google Scholar]
- 26.McVary KT, Monnig W, Camps JL Jr, Young JM, Tseng LJ, van den Ende G. Sildenafil citrate improves erectile function and urinary symptoms in men with erectile dysfunction and lower urinary tract symptoms associated with benign prostatic hyperplasia: a randomized, double-blind trial. J Urol 177: 1071–1077, 2007. [DOI] [PubMed] [Google Scholar]
- 27.McVary KT, Roehrborn CG, Kaminetsky JC, Auerbach SM, Wachs B, Young JM, Esler A, Sides GD, Denes BS. Tadalafil relieves lower urinary tract symptoms secondary to benign prostatic hyperplasia. J Urol 177: 1401–1407, 2007. [DOI] [PubMed] [Google Scholar]
- 28.Ng YK, de Groat WC, Wu HY. Muscarinic regulation of neonatal rat bladder spontaneous contractions. Am J Physiol Regul Integr Comp Physiol 291: R1049–R1059, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ozawa H, Chancellor MB, Jung SY, Yokoyama T, Fraser MO, Yu Y, de Groat WC, Yoshimura N. Effect of intravesical nitric oxide therapy on cyclophosphamide-induced cystitis. J Urol 162: 2211–2216, 1999. [DOI] [PubMed] [Google Scholar]
- 30.Pandita RK, Mizusawa H, Andersson KE. Intravesical oxyhemoglobin initiates bladder overactivity in conscious, normal rats. J Urol 164: 545–550, 2000. [PubMed] [Google Scholar]
- 31.Patel LS, Mitchell CK, Dubinsky WP, O'Brien J. Regulation of gap junction coupling through the neuronal connexin Cx35 by nitric oxide and cGMP. Cell Commun Adhes 13: 41–54, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Persson K, Igawa Y, Mattiasson A, Andersson KE. Effects of inhibition of the l-arginine/nitric oxide pathway in the rat lower urinary tract in vivo and in vitro. Br J Pharmacol 107: 178–184, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Persson K, Pandita RK, Aszodi A, Ahmad M, Pfeifer A, Fassler R, Andersson KE. Functional characteristics of urinary tract smooth muscles in mice lacking cGMP protein kinase type I. Am J Physiol Regul Integr Comp Physiol 279: R1112–R1120, 2000. [DOI] [PubMed] [Google Scholar]
- 34.Peters SL, Schmidt M, Michel MC. Rho kinase: a target for treating urinary bladder dysfunction? Trends Pharmacol Sci 27: 492–497, 2006. [DOI] [PubMed] [Google Scholar]
- 35.Sasatomi K, Hiragata S, Miyazato M, Chancellor MB, Morris SM Jr, Yoshimura N. Nitric oxide-mediated suppression of detrusor overactivity by arginase inhibitor in rats with chronic spinal cord injury. Urology 72: 696–700, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sawada N, Itoh H, Yamashita J, Doi K, Inoue M, Masatsugu K, Fukunaga Y, Sakaguchi S, Sone M, Yamahara K, Yurugi T, Nakao K. cGMP-dependent protein kinase phosphorylates and inactivates RhoA. Biochem Biophys Res Commun 280: 798–805, 2001. [DOI] [PubMed] [Google Scholar]
- 37.Sugaya K, de Groat WC. Influence of temperature on activity of the isolated whole bladder preparation of neonatal and adult rats. Am J Physiol Regul Integr Comp Physiol 278: R238–R246, 2000. [DOI] [PubMed] [Google Scholar]
- 38.Sugaya K, de Groat WC. Inhibitory control of the urinary bladder in the neonatal rat in vitro spinal cord-bladder preparation. Brain Res Dev Brain Res 138: 87–95, 2002. [DOI] [PubMed] [Google Scholar]
- 39.Szell EA, Somogyi GT, de Groat WC, Szigeti GP. Developmental changes in spontaneous smooth muscle activity in the neonatal rat urinary bladder. Am J Physiol Regul Integr Comp Physiol 285: R809–R816, 2003. [DOI] [PubMed] [Google Scholar]
- 40.Yanai Y, Hashitani H, Hayase M, Sasaki S, Suzuki H, Kohri K. Role of nitric oxide/cyclic GMP pathway in regulating spontaneous excitations in detrusor smooth muscle of the guinea-pig bladder. Neurourol Urodyn 27: 446–453, 2008. [DOI] [PubMed] [Google Scholar]
- 41.Yoshida M, Akaike T, Inadome A, Takahashi W, Seshita H, Yono M, Goto S, Maeda H, Ueda S. The possible effect of nitric oxide on relaxation and noradrenaline release in the isolated rabbit urethra. Eur J Pharmacol 357: 213–219, 1998. [DOI] [PubMed] [Google Scholar]
- 42.Yoshimura N, Seki S, de Groat WC. Nitric oxide modulates Ca2+ channels in dorsal root ganglion neurons innervating rat urinary bladder. J Neurophysiol 86: 304–311, 2001. [DOI] [PubMed] [Google Scholar]