Abstract
The mammalian kidney is particularly vulnerable to hypoperfusion, because the O2 supply to the renal medulla barely exceeds its O2 requirements. In this study, we examined the impact of the complex structural organization of the rat outer medulla (OM) on O2 distribution. We extended the region-based mathematical model of the rat OM developed by Layton and Layton (Am J Physiol Renal Physiol 289: F1346–F1366, 2005) to incorporate the transport of RBCs, Hb, and O2. We considered basal cellular O2 consumption and O2 consumption for active transport of NaCl across medullary thick ascending limb epithelia. Our model predicts that the structural organization of the OM results in significant Po2 gradients in the axial and radial directions. The segregation of descending vasa recta, the main supply of O2, at the center and immediate periphery of the vascular bundles gives rise to large radial differences in Po2 between regions, limits O2 reabsorption from long descending vasa recta, and helps preserve O2 delivery to the inner medulla. Under baseline conditions, significantly more O2 is transferred radially between regions by capillary flow, i.e., advection, than by diffusion. In agreement with experimental observations, our results suggest that 79% of the O2 supplied to the medulla is consumed in the OM and that medullary thick ascending limbs operate on the brink of hypoxia.
Keywords: region-based model, red blood cells, medullary thick ascending limbs, active sodium transport
renal oxygen tension (Po2) in the rat kidney ranges from 40–50 mmHg in the cortex to 20 and 10 mmHg in the outer medulla (OM) and inner medulla (IM), respectively (40). Low medullary Po2 results from the high metabolic requirements of medullary thick ascending limbs (mTALs) and the countercurrent arrangement of blood vessels in the medulla. The countercurrent architecture, combined with low medullary blood flow [IM blood flow is <1% of total renal blood flow (12)], serves to preserve the corticomedullary tubular and vascular fluid osmolality gradient that is necessary for the production of concentrated urine, but the concomitant shunting of O2 between descending and ascending vessels and limbs significantly reduces O2 supply to the deep medulla. The production of concentrated urine is also contingent on the active transport of NaCl in mTALs, a process that is highly energy dependent. Thus the O2 supply to the renal medulla barely exceeds its O2 requirements (16).
The kidney is therefore particularly vulnerable to hypoperfusion, inasmuch as the associated hypoxia leads to localized injury. Under experimental conditions resulting in reduced medullary Po2, mTALs are the most injured segments; lesions may also spread to surrounding structures such as thin limbs, interstitium, and collecting ducts (CDs) (16, 18). The mTAL is prominently susceptible to decreases in medullary O2 supply as a result of its high metabolic requirements, as well as its distance from descending vasa recta (DVR), i.e., the source of O2 delivery. Indeed, the highly structured organization of tubules and vessels in the rat OM (27, 29), in which DVR and ascending vasa recta (AVR) form tightly packed vascular bundles that appear to dominate the histotopography of the OM, especially in the inner stripe, and in which CDs and mTALs are found distant from the vascular bundles, likely increases the vulnerability of mTALs to hypoxia.
In this study, we examined the impact of the structural organization of the rat OM on O2 distribution. We extended the region-based model of the urine-concentrating mechanism of the rat OM developed by Layton and Layton (33), which was originally formulated for two solutes, Na+ and urea, the principal solutes in the mammalian urine-concentrating mechanism. To investigate O2 distribution in the OM, we incorporated RBCs, as well as Hb and O2.
In this study, we discuss our baseline results. In a companion study (8), we investigate model sensitivity to fundamental structural assumptions and to parameters whose values are uncertain.
GLOSSARY
Acronyms
- AVR/DVR
Ascending vas rectum/descending vas rectum
- CD
Collecting duct
- IMCD
Inner medullary collecting duct
- IM/OM
Inner medulla/outer medulla
- iRBC
Interstitial RBC
- LAL/LDL
Long ascending limb/long descending limb
- LAV/LDV
Long ascending vas rectum/long descending vas rectum
- LAVa/LAVb
LAV in R1/LAV in R2
- mTAL
Medullary thick ascending limb
- R1, R2, R3, R4
Concentric regions 1–4
- SAL/SDL
Short ascending limb/short descending limb
- SAV/SDV
Short ascending vas rectum/short descending vas rectum
- SAVa/SAVb
SAV in R3/SAV in R4
- SDVa/SDVb
SDV in R1/SDV in R2
- SDLa/SDLb
SDL with a prebend segment/SDL without a prebend segment
Indexes
- i
Tubule, vas rectum
- j
a, b or 1, 2, 3, 4
- k
Solute Na+, urea, O2, Hb, or HbO2
- R
Region R1, R2, R3, or R4
Independent Variables
- x or y
Medullary depth, in axial direction
- z
iRBC length, in radial direction
Dependent Variables
- Ci,k
Concentration of solute k in tubule i
- CR,k
Concentration of solute k in concentric region R
- Ci,kP
Concentration of solute k in vessel i plasma
- Ci,kR
Concentration of solute k in vessel i RBC
- Fi,V
Water flow rate in tubule or vessel i
- Fi,VP
Water flow rate in vessel i plasma
- Fi,VR
Water flow rate in vessel i RBC or in iRBC tube
- Ji,V
Transmural water flux into tubule or vessel i
- Ji,VP
Transmural water flux into vessel i plasma
- Ji,VR
Transmural water flux into vessel i RBC or iRBC tube
- Ji,k
Transmural flux of solute k into tubule or vessel i
- Ji,kP
Transmural flux of solute k into vessel i plasma
- Ji,kR
Transmural flux of solute k into vessel i RBC or iRBC tube
- JR,diff
O2 flux from region R into neighboring regions via diffusion
- JR,adv
O2 flux from region R into neighboring regions via capillary advection
- J̄iRBCj,R,k
Transmural flux of solute k from iRBC tube j into region R
Parameters
- Ai,cell
Cross-sectional area of surrounding cell layer in tubule or vas rectum i
- AR
Area of region R
- ĀR
Area occupied by interstitial cells in region R
- Fi,O2B
Total amount of O2 in vessel i
- FO2OM,ascending
Total amount of O2 flowing back to OM via LAL and LAV
- KM,Na
Michaelis-Menten constant for Na+ active transport
- KM,O2
Michaelis-Menten constant for O2 basal consumption
- k1/k−1
Dissociation/association constant of HbO2
- L
Length of OM
- ni
Number of tubules or vessels i per nephron
- PO2C
Overall O2 permeability of capillary
- PO2R
O2 permeability of RBC membrane
- PO2W
O2 permeability of capillary wall
- PR,R′,k
Permeability of boundary between regions R and R′ to solute k
- QO2IM
Total O2 consumption in IM
- QO2OM,active
Overall O2 consumption for active transport in OM
- QO2OM,basal
Overall O2 consumption for basal metabolism in OM
- QR,R′
Transregion capillary flow from region R into R′
- ri
Inner radius of tubule or vas rectum i
- rR
Radius of region R
- Ri,O2active
O2 volumetric consumption rate for active transport in tubule i
- Ri,O2basal
O2 volumetric consumption rate for basal metabolism in tubule or vas rectum i
- Rmax,O2basal
Maximum volumetric rate of O2 basal consumption
- RRBC,O2scav
Volumetric rate of O2 scavenging by RBCs
- SO2OM
Overall O2 supply to OM
- Ti,Naactive
Volumetric rate of Na+ reabsorption in tubule i
- Vmax,i,Na
Maximum transport rate of Na+ by tubule i
- αSDVa
Fraction of plasma flow from terminating SDVa into region R1
- σk
Reflection coefficient of solute k
- φk
Osmotic coefficient of solute k
- ωSAV/ωSDV
Fraction of SAV/SDV reaching a given medullary level
- Ψi,Naactive
Rate of Na+ active transport in tubule i (in mol·m−2·s−1)
- θcell
Fraction of solute consumed in epithelial or endothelial cell layer that is taken from the lumen
MODEL DESCRIPTION
The region-based model of the urine-concentrating mechanism in the rat OM developed by Layton and Layton (33) accounts for the three-dimensional architecture of the OM and preferential interactions among tubules and vessels by representing four concentric regions centered on a vascular bundle. Tubules and vasa recta that reach into the IM are represented by rigid cylinders that extend from the corticomedullary junction (x = 0) to the OM-IM boundary (x = L). Vasa recta that supply the OM, i.e., short descending and ascending vasa recta (SDV and SAV), are represented by continuously decreasing distributions of vasa recta that reach different levels in the OM.
The radial organization is incorporated by assignment of appropriate tubules and vasa recta (or fractions thereof) to each concentric region. Model positions of tubules and vasa recta are shown in Fig. 1 for outer and inner stripes. Two distinct populations of SDV are represented in the two central-most regions (R1 and R2) to allow us the potential to distinguish vasodilatory effects in different regions. Distinct populations of AVR are used to avoid straddling of region boundaries by the highly permeable AVR, which introduces excessive mixing between regions. Vasa recta that supply the IM, i.e., long descending and ascending vasa recta (LDV, LAVa, and LAVb), are assumed to form the vascular bundle, which is contained within the two central-most regions (R1 and R2). SDVa and SDVb are assumed to reside in R1 and R2, respectively, consistent with immunolabeling results, which indicate that DVR are distributed centrally within the vascular bundle (24), and also with SDV peeling off to supply the capillary plexus of the inner stripe. CDs, which are located at a distance from the vascular bundle, are assigned to R4. Distinct populations of SAV, labeled SAVa and SAVb, ascend through R3 and R4, respectively. Thick ascending limbs from short loops of Henle (i.e., SALs), which are near the CD throughout the OM, are assumed to straddle R3 and R4. At the beginning of the outer stripe, the long loops of Henle are situated near the vascular bundle, in R2 and R3, where the long ascending limbs (LALs) maintain their position throughout the OM.
Fig. 1.
Schematic representation of the region-based model: cross sections through outer stripe (A) and inner stripe (B) of rat outer medulla (OM). In the model formulation, the 4 regions (R1–R4) have coincident centers; display is intended to minimize the figure area. LDV, long descending vas rectum; SDVa and SDVb, 2 populations of short descending vasa recta; LAVa and LAVb, 2 populations of long ascending vasa recta; SAVa and SAVb, 2 populations of short ascending vasa recta; LDL, long descending limb of Henle's loop; SDL, short descending limb; LAL, long ascending limb; SAL, short ascending limb; CD, collecting duct. Relative weight of interaction between a type of vessel or tubule and a given region (i.e., the parameter κi,R in Eqs. A31, A32, A41, and A42) is represented by 0.25, 0.5, and 0.75.
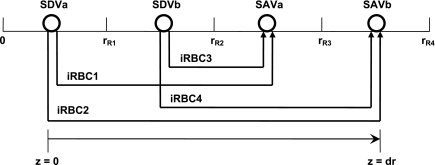
The portion of each concentric region that is exterior to tubules and vessels represents interstitial spaces, interstitial cells, and capillary plasma flow (given the highly fenestrated nature of the capillaries). This exterior compartment is designated the “interstitial region.” Capillary RBC flow is modeled following the approach used by Layton (32): RBCs within capillaries [referred to as interstitial RBCs (iRBCs)] are represented by rigid tubes extending perpendicular to the medullary axis (i.e., in the z direction) at each medullary level x. The iRBC tubes at level x form at the end of the SDV that terminate at x and extend to the SAV originating at x. Since we consider two distinct populations of SDV (SDVa and SDVb, which descend through R1 and R2, respectively) and two distinct populations of SAV (SAVa, and SAVb, which ascend through R3 and R4, respectively), four different populations of iRBC tubes are represented at each level x (Fig. 2). In the model developed by Layton and Layton (33), the SDV were represented as straddling R1 and R2. In the present study, the SDVa-to-SDVb number ratio was taken as 1:99 in the base case, to account for the fact that the vessels that irrigate the interbundle region peel off from the outer edge of the vascular bundles.
Fig. 2.
Schematic representation of interstitial RBC (iRBC) tubes that carry capillary RBC flow from the SDV (SDVa in R1, SDVb in R2) that terminate at a given level x, along the radial (z) direction, to the SAV (SAVa in R3, SAVb in R4) that originate at that level. RBCs travel a radial distance dr before being taken up by SAV. rRi, outer radius of region Ri (i = 1, 2, 3, and 4). We assume that each population of short vasa recta is, on average, localized in the center of the region where it is distributed.
Conservation equations that describe fluid flow and solute concentrations at steady state in each tubule, vas rectum, and interstitial region are summarized in the appendix. Boundary conditions (BCs) prescribe flows and concentrations in tubules and vessels at the corticomedullary junction and the OM-IM boundary. Below we describe the representation of O2-related processes in the model.
Rate of Active Na+ Reabsorption
The active transport rate in tubule i (Eq. A41) is usually characterized with the assumption of CNa-dependent Michaelis-Menten kinetics (33, 56)
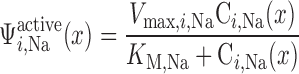 |
(1) |
where Ci,Na is Na+ concentration in tubule i, Vmax,i,Na (in mol Na+·m−2·s−1) is the maximum rate of Na+ transport in tubule i, and KM,Na is the Michaelis-Menten constant, taken as 70 mM (35). The critical Po2, below which O2 consumption becomes limited by O2 concentration, was found to be very low, <1 mmHg (10). Hence, we assume that, at <1 mmHg luminal Po2, the active transport rate is a linearly decreasing function of Po2, that is
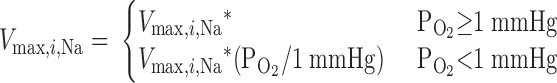 |
(2) |
The results are indistinguishable if we replace luminal Po2 with interstitial Po2 in Eq. 2. Vmax,i,Na is estimated as 10.5 and 25.9 nmol Na+·cm−2·s−1 in mTALs in the outer and inner stripes, respectively (33).
O2 Consumption
O2 is carried to the medulla by DVR blood, in free form (O2) and as HbO2. In RBCs, the dissociation of HbO2 into O2 and Hb is expressed as a one-step reaction
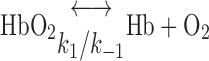 |
where Hb denotes one of the four heme groups in each Hb molecule. Thus the volumetric rate of O2 scavenging by Hb in RBCs (R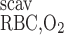 ) is calculated as
) is calculated as
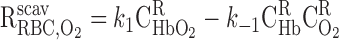 |
(3) |
Given that the kinetics of HbO2 dissociation are significantly faster than O2 diffusion, the reaction is generally taken to be at equilibrium, such that
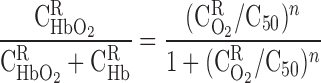 |
(4) |
where C50 is the O2 concentration at half-saturation (equivalent to 26.4 mmHg) and n is the Hill equation parameter, taken as 2.6 (9). The solubility coefficient for O2 (i.e., the C -to-Po2 conversion factor) is taken as 1.34 μM/mmHg in plasma and tubular fluid and 1.56 μM/mmHg in RBCs. Following the approach of Clark et al. (9), which is suitable for low Po2, the dissociation constant k1 is fixed (49 s−1), and the association constant is calculated at each level as a function of local O2 concentrations, to yield compatibility with the Hill equation for saturation
-to-Po2 conversion factor) is taken as 1.34 μM/mmHg in plasma and tubular fluid and 1.56 μM/mmHg in RBCs. Following the approach of Clark et al. (9), which is suitable for low Po2, the dissociation constant k1 is fixed (49 s−1), and the association constant is calculated at each level as a function of local O2 concentrations, to yield compatibility with the Hill equation for saturation
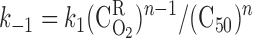 |
(5) |
We consider basal O2 consumption by interstitial cells, vascular endothelial cells, and tubular epithelial cells (Eqs. A2–A4 and A62). The volumetric rate of basal O2 consumption in tubule or vas rectum i (R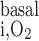 , in mol O2·m−3·s−1) is calculated as
, in mol O2·m−3·s−1) is calculated as
 |
(6) |
Since epithelial or endothelial cells are situated at the interface between the interstitium and the lumen of tubule or vessel i, the fraction θcell of R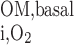 that is attributed to the lumen (Eq. A2) is calculated on the basis of the luminal C
that is attributed to the lumen (Eq. A2) is calculated on the basis of the luminal C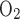 , whereas the remaining fraction (1 − θcell), which is attributed to the interstitium (Eq. A62), is calculated on the basis of the interstitial C
, whereas the remaining fraction (1 − θcell), which is attributed to the interstitium (Eq. A62), is calculated on the basis of the interstitial C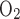 .
.
The maximum volumetric rate of O2 consumption (R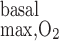 ) is assumed to be the same in each compartment and taken as 10 μM/s, as discussed in the companion study (8). The Michaelis-Menten constant (K
) is assumed to be the same in each compartment and taken as 10 μM/s, as discussed in the companion study (8). The Michaelis-Menten constant (K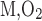 ) is taken as 5.4 μM, or 4 mmHg, under basal conditions for nitric oxide (7). The overall basal consumption of O2 in the OM (Q
) is taken as 5.4 μM, or 4 mmHg, under basal conditions for nitric oxide (7). The overall basal consumption of O2 in the OM (Q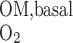 ) is then estimated as follows
) is then estimated as follows
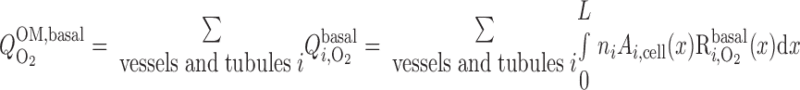 |
(7) |
where ni is the number of tubules or vessels i per nephron and Ai,cell is the cross-sectional area of the epithelial or endothelial cells. Po2 is expected to be significantly >4 mmHg in most OM segments; hence, R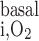 ≈ R
≈ R , and Eq. 7 can be simplified as
, and Eq. 7 can be simplified as
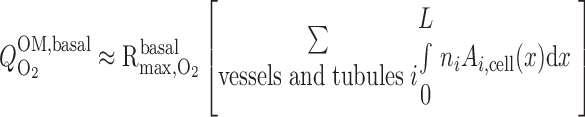 |
(8) |
The term in brackets on the right-hand side of Eq. 8 is calculated as 2.0 × 10−12 m3/nephron. If R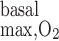 = 10 μM/s, then Q
= 10 μM/s, then Q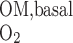 ≈ 2.0 × 10−14 mol O2·s−1·nephron−1.
≈ 2.0 × 10−14 mol O2·s−1·nephron−1.
Under maximal efficiency, moles of Na+ reabsorbed through the transcellular pathway per mole of O2 consumed [also known as the TNa-to-Q ratio (TNa/Q
ratio (TNa/Q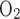 )] is 18, as suggested by Na+-K+-ATPase stoichiometry. Under favorable thermodynamic conditions, additional moles of Na+ are reabsorbed in parallel through the paracellular route, namely, when the driving force imparted by the lumen-negative transepithelial potential is not counterbalanced by too large an interstitium-to-lumen Na+ concentration gradient. This may explain why measurements in thick ascending limbs (TALs) yielded TNa/Q
)] is 18, as suggested by Na+-K+-ATPase stoichiometry. Under favorable thermodynamic conditions, additional moles of Na+ are reabsorbed in parallel through the paracellular route, namely, when the driving force imparted by the lumen-negative transepithelial potential is not counterbalanced by too large an interstitium-to-lumen Na+ concentration gradient. This may explain why measurements in thick ascending limbs (TALs) yielded TNa/Q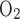 = 36 (11). To account for paracellular transport, which is not explicitly represented in the present model, we set TNa/Q
= 36 (11). To account for paracellular transport, which is not explicitly represented in the present model, we set TNa/Q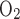 = 24. Using baseline parameters, our model predicts a lumen-to-interstitium Na+ concentration ratio that is sufficiently large along most of the mTAL length to support significant paracellular reabsorption. That result is consistent with our choice for TNa/Q
= 24. Using baseline parameters, our model predicts a lumen-to-interstitium Na+ concentration ratio that is sufficiently large along most of the mTAL length to support significant paracellular reabsorption. That result is consistent with our choice for TNa/Q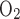 . The rate of O2 consumption for active transport in tubule i (R
. The rate of O2 consumption for active transport in tubule i (R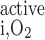 , in mol O2·m−3·s−1) is thus determined as
, in mol O2·m−3·s−1) is thus determined as
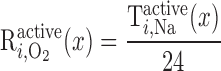 |
(9) |
where T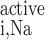 (in mol Na+·m−3·s−1) is the rate of Na+ reabsorption in tubule i. T
(in mol Na+·m−3·s−1) is the rate of Na+ reabsorption in tubule i. T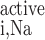 and Ψ
and Ψ are related as follows (Eqs. 9, A2, and A41)
are related as follows (Eqs. 9, A2, and A41)
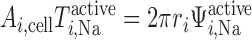 |
(10) |
where ri is the inner radius of tubule i. The overall O2 consumption for active transport in the OM (Q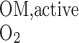 ) is then calculated as
) is then calculated as
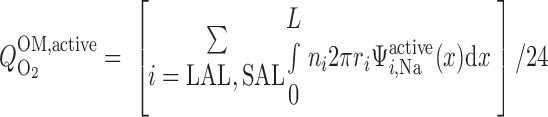 |
(11) |
Using baseline parameters, we estimate Q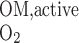 to be 5.1 × 10−13 mol O2·s−1·nephron−1.
to be 5.1 × 10−13 mol O2·s−1·nephron−1.
BCs at the OM-IM Boundary
As described in the appendix, BCs for O2 in LAV and LAL at the OM-IM boundary are determined on the basis of O2 consumption in the IM (Q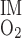 ). The basal component of Q
). The basal component of Q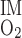 is estimated as ∼0.9 × 10−14 mol·s−1·nephron−1 if R
is estimated as ∼0.9 × 10−14 mol·s−1·nephron−1 if R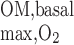 = 10 μM/s in the IM (as in the OM). Total Na+-K+-ATPase activity and active Na+ transport are significantly lower in inner medullary CDs (IMCDs) than in mTALs. Moreover, a significant proportion of energy is provided by anaerobic glycolysis in the IM (52). We therefore assume that O2 consumption in the IM is 5% of O2 supply to the OM.
= 10 μM/s in the IM (as in the OM). Total Na+-K+-ATPase activity and active Na+ transport are significantly lower in inner medullary CDs (IMCDs) than in mTALs. Moreover, a significant proportion of energy is provided by anaerobic glycolysis in the IM (52). We therefore assume that O2 consumption in the IM is 5% of O2 supply to the OM.
We postulate that the architecture of the IM plays a determinant role in the distribution of O2 between LAL and LAV. The dominating organizing structural elements in the IM for arrangement of the tubules and vasa recta are the clearly distinguishable clusters of CDs (43, 44). Loops of Henle that turn within the first millimeter of the IM are located within the CD clusters, whereas DVR and LDL that are associated with the remainder of the loops of Henle are nearly always located outside these clusters. In contrast, LAL and AVR are arranged nearly uniformly across the IM when viewed in transverse sections (44). Recall that we distinguish between two populations of LAV in this model: a model LAV, denoted LAVa, lies within the vascular bundle in the OM and is assumed to be close to DVR in the IM; another model LAV, denoted LAVb, lies on the periphery of the bundle in the OM and is assumed to be distant from DVR in the IM. Thus the flow of O2 in LAVa at the OM-IM boundary is likely to be significantly greater, per vessel, than that in LAVb. In addition, LAL do not carry HbO2, the concentration of which is much greater than that of O2. Given the position of the two populations of LAV and also taking into consideration that 17 LAVa and 33 LAVb are represented per vascular bundle, we assume, as a baseline, that 38.8% and 60.2% of the O2 and HbO2 at the OM-IM boundary that flow back in the OM via ascending limbs and vessels (F ), respectively, enters via the LAVa and LAVb at the OM-IM junction (i.e., 25% more O2 in each LAVa than LAVb), whereas the remaining 1% enters via the LAL.
), respectively, enters via the LAVa and LAVb at the OM-IM junction (i.e., 25% more O2 in each LAVa than LAVb), whereas the remaining 1% enters via the LAL.
Parameter Values
Parameters related to the transport of water, Na+, and urea across tubules and vessels are taken from the study of Layton and Layton (33). Those that concern the transport of O2 across vasa recta are taken from the study of Zhang and Edwards (57). The permeability of medullary tubules and vessels to O2 has not been measured directly, to the best of our knowledge. The (overall) capillary permeability to O2 (P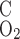 ) can be expressed as
) can be expressed as
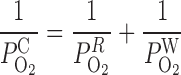 |
(12) |
where P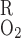 and P
and P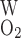 denote the O2 permeability of the RBC membrane and the capillary wall, respectively. On the basis of literature estimates (for review see Ref. 57), the P
denote the O2 permeability of the RBC membrane and the capillary wall, respectively. On the basis of literature estimates (for review see Ref. 57), the P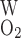 -to-P
-to-P ratio is taken as 3, and P
ratio is taken as 3, and P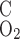 is taken as 0.01 cm/s (i.e., P
is taken as 0.01 cm/s (i.e., P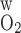 = 0.04 cm/s). For simplicity, we assume that the permeability of O2 across vascular endothelial cells and tubular epithelial cells is the same. The baseline values of parameters related to O2 transport are summarized in Table 1.
= 0.04 cm/s). For simplicity, we assume that the permeability of O2 across vascular endothelial cells and tubular epithelial cells is the same. The baseline values of parameters related to O2 transport are summarized in Table 1.
Table 1.
O2 transport parameter values
| Parameter | Value | Ref. |
|---|---|---|
| Half-saturation in Hill equation (C50) | 26.4 mmHg | 9 |
| Hill equation parameter (n) | 2.6 | 9 |
| Dissociation constant (k1) | 49 s−1 | 2† |
| O2 solubility coeff in plasma | 1.34 μM/mmHg | 23 |
| O2 solubility coeff in RBC | 1.56 μM/mmHg | 9 |
| Initial overall heme concn | 20.3 mM | 9 |
| Diffusivity of O2 in tissue (DO2) | 2,800 μm2/s | 7 |
| Maximum volumetric rate of basal O2 consumption (Rmax,O2basal) | 10 μM/s | Present study |
| Michaelis-Menten constant for basal O2 consumption (KM,O2) | 4 mmHg | 7‡ |
| Maximum rate of Na+ active transport (Vmax,i,Na) | 33 | |
| Outer stripe | 10.5 nmol·m−2·s−1* | |
| Inner stripe | 25.9 nmol·m−2·s−1* | |
| Michaelis-Menten constant for Na+ active transport (KM,mTAL,Na) | 70 mM | 33 |
| O2 permeability of capillary and tubule wall (PO2W) | 0.04 cm/s | Present study |
Assuming that k−1 = 3.5×10−6 M−1·s−1 at x = 0.
Assuming a resting NO concentration of ≈ 80 nM.
Short and long ascending limbs.
The study of Rasmussen (47) suggests that blood hematocrit in the OM (30–40%) is comparable to that in the cortex and significantly higher than that in the IM. The baseline value of the OM hematocrit is taken as 35% in our simulations.
Inner diameters and other dimensions are taken from the study of Layton and Layton (see Tables 1 and 2 in Ref. 33). The thickness of the vascular endothelial layer is estimated as 1 μm (T. L. Pallone, personal communication); that of the epithelial layer is taken as 8 μm in the mTAL (26), 1.1 μm in the descending limb (the average of the 0.4- to 1.8-μm range reported in Ref. 41), and 9 μm in the outer medullary CD (OMCD) (55).
Table 2.
Boundary conditions for descending tubules and vessels at x = 0
| SDL | LDL | CD | DVR |
||
|---|---|---|---|---|---|
| Plasma | RBC | ||||
| Fv,† nl/min | 10 | 12 | 6.1 | 6 | 2 |
| CNa, mM | 160 | 160 | 88.2* | 163.7 | 0 |
| Curea, mM | 15 | 15 | 151.2* | 8 | 8 |
| CO2, mM | 48.2×10−3 (36 mmHg) | 48.2×10−3 (36 mmHg) | 48.2×10−3 (36 mmHg) | 80.4×10−3 (60 mmHg) | 80.4×10−3 |
| CHb, mM | 0 | 0 | 0 | 0 | 3.0‡ |
| CHbO2, mM | 0 | 0 | 0 | 0 | 17.3‡ |
Boundary conditions for water, Na+, and urea are taken from the study of Layton and Layton (33).
Collecting duct (CD) inflow concentrations are obtained as described in appendix (Eq. A10).
Flow rates (FV) are given for per individual tubule or vessel. We assume that hematocrit (i.e., RBC-to-blood flow ratio) is 35%.
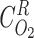 and assuming kinetic equilibrium,
and assuming kinetic equilibrium, 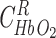 and
and 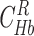 can be obtained by solving the following equations:
can be obtained by solving the following equations:
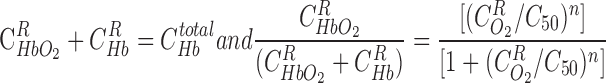 |
Areas occupied by interstitial cells (ĀR) are needed to estimate basal O2 consumption in the regions (Eq. A62). We estimate interstitial cell areas by assuming that, in the outer stripe, 3.71% of region areas are occupied by interstitial cells (45). In the inner stripe, interstitial cells are sparse within the vascular bundles (36) but less sparse in the interbundle regions. We thus assume that 3.71% of the region areas are occupied by interstitial cells in the vascular bundle (i.e., R1 and R2); however, in R3 and R4, that percentage increases linearly from 3.71% at the outer stripe-inner stripe junction to 10.07% at the mid-inner stripe. The latter value (10.07%) is chosen so that, at the mid-inner stripe, 8.27% of the region areas are occupied by interstitial cells (45). In other words, for R = R3 and R4
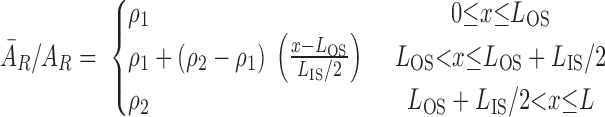 |
(13) |
where AR is the area of the whole region, LOS, LIS, and L denote the length of the outer stripe, inner stripe, and OM, respectively, and ρ1 and ρ2 are taken as 0.0371 and 0.1007, respectively.
As described above, the model assumes that capillary plasma is well mixed with the local interstitium, whereas capillary RBCs flow within iRBC tubes. In this representation, RBCs in capillary tubes exchange directly with the interstitium, whereas RBCs in vasa recta are surrounded by plasma. To account for the additional resistance of interstitial cells and capillary walls, the permeability of capillary RBCs to water, Na+, and urea is taken as one-tenth of that of RBCs in vasa recta.
Numerical Methods
The steady-state differential equations are discretized to form a system of nonlinear algebraic equations. A spatial discretization of 200–400 grid points along the medullary axis and 1,000 grid points in the radial direction (for iRBCs) is used. The system of coupled, nonlinear conservation equations at each medullary level is solved using MINPACK, a numerical package implemented in FORTRAN for solving nonlinear equations with a modification of Powell's hybrid algorithm. For computation of steady-state solutions, the following steps are repeated until the convergence criterion is satisfied: an initial guess is made for interstitial solute concentrations; concentration profiles in descending and ascending tubules and vessels, as well as in the horizontal RBC tubes, are obtained by solving the nonlinear algebraic equations along the x and z axes; and water and solute conservation equations in the four regions are solved, and interstitial solute concentrations are updated accordingly. Iterations are stopped when the largest relative difference in solute concentration from one iteration to the next is <10−6.
RESULTS
Overall O2 Supply in the Rat OM
The overall supply of O2 to the OM (S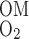 ) is determined as the total molar flow rate per nephron of O2 and HbO2 entering descending vessels and tubules at the corticomedullary junction (i.e., x = 0)
) is determined as the total molar flow rate per nephron of O2 and HbO2 entering descending vessels and tubules at the corticomedullary junction (i.e., x = 0)
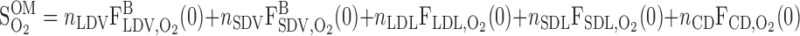 |
(14) |
where
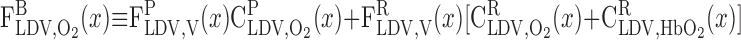 |
(15) |
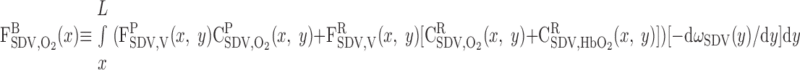 |
(16) |
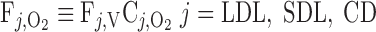 |
(17) |
where Fi,V is the volumetric flow rate in tubule or vessel i, Ci,k is the concentration of solute k in tubule or vessel i, and the superscripts B, P, and R denote blood, plasma compartment, and RBC compartment, respectively. In Eq. 16, ωSDV(y) denotes the fraction of SDV that reaches medullary depth y (Eq. A22); the negative of its derivative, −dωSDV(y)/dy, is the rate at which SDV terminate along the OM, reckoned in the positive x direction. With the BCs specified in Table 2, S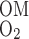 is calculated as 6.6 × 10−13 mol·s−1·nephron−1, 97% of which is delivered to the OM in the form of HbO2; we thus ignore the amount of O2 supplied by aqueous fluids relative to that supplied by RBCs in the remainder of this study.
is calculated as 6.6 × 10−13 mol·s−1·nephron−1, 97% of which is delivered to the OM in the form of HbO2; we thus ignore the amount of O2 supplied by aqueous fluids relative to that supplied by RBCs in the remainder of this study.
Interstitial, Intratubular, and Intravascular Po2 Gradient
Using the base-case configuration, parameter set, and BCs, we solved the model equations to yield flow and concentration profiles. Figure 3 shows Po2 profiles in each class of tubules and vessels and in each concentric region. Figure 3, A, B, C, and D, shows results for tubules and vessels associated with regions R1, R2, R3, and R4, respectively; tubules and vessels are assigned to the region with which they are in contact for half or more of their inner stripe length. Figure 3E shows Po2 profiles in each concentric region. As described in model description, the SDV are represented by a continuous distribution, with SDV terminating at all depths of R1 and R2 and with SAV originating at all depths of R3 and R4. However, Fig. 3 contains only the profiles corresponding to the longest SDVa, SDVb, SAVa, and SAVb (i.e., those that reach the OM-IM boundary). Figure 4 shows O2 flow, per nephron, in each class of tubules and vessels. Because Fig. 4 portrays directed flows, flow toward the cortex is considered to be negative.
Fig. 3.
Po2 in tubules, vasa recta, and concentric regions. A, B, C, and D: regions R1, R2, R3, and R4, respectively; tubules are assigned to the region with which they are in contact for ≥50% of their inner stripe (IS) length. E: Po2 profiles in the interstitium of the 4 regions. Vertical dotted lines mark boundary between outer stripe (OS) and IS; x/L, ratio of axial coordinate to total length of OM.
Fig. 4.
A–D: O2 flow in regions, tubules, and vasa recta. Notation is analogous to that in Fig. 3. E: HbO2 flow in LDV RBCs.
Interstitial Po2 gradients and their generation.
As described below, our model predicts that O2 availability varies greatly from one region to another. In this section, interstitial Po2 profiles in each region are depicted consecutively. Figure 3E shows a decreasing Po2 gradient along the model's corticomedullary axis in R1, the core of the vascular bundle, and in LDV and LAVa, both of which lie within the central-most region through the OM (Fig. 3A). In R1, interstitial Po2 decreases from 58.2 mmHg at the corticomedullary junction to 48.3 mmHg at the OM-IM boundary, as O2 diffuses radially outward to O2-poor regions. Because interstitial Po2 in R1 lags behind that of the LDV, O2 is continually reabsorbed from the LDV, the O2 flow of which decreases from 0.0707 to 0.0494 pmol·min−1·nephron−1 (Fig. 4A).
Similarly, the R2 interstitial Po2 profile exhibits a general decrease along the corticomedullary axis. The O2 demand in R2 is at its highest when the prebend segments (which, similar to the mTALs, actively transport Na+) begin, at ∼50 μm from the OM-IM boundary. Closer to the OM-IM boundary, however, the SDL move away from R2 to occupy a position between R3 and R4. The consequent decrease in O2 demand results in a rise in R2 interstitial Po2.
Po2 is predicted to be low in the interbundle regions, i.e., in R3 and R4, where Po2 hovers between 5.7 and 8.7 mmHg between the mid-outer stripe and the mid-inner stripe. These outer regions do not contain O2-supplying vessels but, instead, contain most of the mTALs, which impose the largest O2 demand. (In the outer stripe, one-half of the LAL and one-half of the SAL reside in R3, and the remainder of the SAL resides in R4; in the inner stripe, one-half of the LAL and three-fourths of the SAL reside in R3, and the remainder of the SAL resides in R4.) Interstitial Po2 in R3 and R4 decreases in the lower inner stripe, where the mTAL Na+ active transport rates are the highest.
Because of the radial organization in the OM, in particular, the localization of DVR in the vascular bundles and of mTAL away from the vascular bundles, the model predicts substantial R1-R4 Po2 gradients. Because the vessels are tightly packed in the vascular bundle core, diffusion of O2 out of R1 is limited. Also, O2 flow via capillaries from R1 to R2 is assumed to be small (only 5% of net fluid accumulation in R1 is directed to R2 as capillary plasma flow). These factors contribute to the significant Po2 gradient, a difference of about two-thirds of R1 interstitial Po2, between R1 and R2. Because R2, R3, and R4 contain different tubules and vessels, all of which have different metabolic needs, which may also vary axially, the Po2 profiles of these three regions differ significantly. In most of the inner stripe, Po2 does not decrease monotonically in the radial direction from R1 to R4.
Near the cortex, interstitial Po2 in R2, R3, and R4 decreases sharply along the corticomedullary axis. This boundary layer effect can be attributed, in large part, to the rapid decrease in the LDL tubular fluid Po2 near the cortex. The model assumes that the content of the fluid entering the descending limbs at the corticomedullary boundary approximates that of plasma. The underlying assumption is that the cortex is largely homogeneous and highly oxygenated. Because Po2 is substantially higher at the LDL boundary than in the ascending limbs and SAV, with which LDL share a position in R3, and because of the high O2 permeability of LDL, there is a large efflux of O2 from the LDL near the corticomedullary junction and, thus, a precipitous drop of Po2 in LDL and a subsequent decrease in interstitial Po2 in R3 and in the neighboring regions R2 and R4. This boundary layer effect might be reduced by an explicit representation of the cortex, into which the regions continue their dissolution. Such a model would give a better prediction of the LDL Po2 at the corticomedullary boundary and would also allow O2 to diffuse into the upper outer stripe from the O2-rich cortex, increasing the interstitial Po2 and smoothing any boundary layer.
Intravascular and intratubular Po2 gradients and their generation.
Basal metabolism of all tubules and vessels and active transport metabolism in mTALs consume O2. Taken in isolation, this consumption should result in decreasing O2 flows in all tubules and vessels along their flow directions. However, tubules and vessels are assumed to be highly permeable to O2 (0.04 cm/s), which results in significant O2 diffusion across epithelial or endothelial walls when an O2 gradient is established. Thus tubular and vascular plasma Po2 profiles generally approximate those of the surrounding interstitium. Figure 3, A–D, shows Po2 profiles for each class of tubules and vessels; the corresponding O2 flow rates, per nephron, are shown in Fig. 4. The flow rates are taken to be positive when flow is in the direction of increasing medullary depth. Composite flow rates are given for SDV and SAV. Because short vasa recta turn back along the OM, the per-nephron SDV O2 flow rapidly decreases, from 0.2573 pmol·min−1·nephron−1 at the corticomedullary boundary to 0 at the OM-IM boundary, where the longest SDV is assumed to terminate.
LDV and LAVa, which lie within the core of the vascular bundle (R1), have the highest Po2. As Po2 in DVR plasma decreases, within RBCs O2 dissociates from HbO2 and, subsequently, diffuses down a concentration gradient into the surrounding plasma (see HbO2 flow profiles for LDV in Fig. 4E). Along the LDV, 7.4% of the HbO2 that enters at the corticomedullary boundary has dissociated before reaching the IM.
The Po2 profiles of LAVb and SDL have similar gradients in the inner stripe but diverge in the outer stripe (Fig. 3B). This occurs because, although LAVb stays in R2 throughout the OM, SDL occupies an outer stripe position farther from the vascular bundle, between R3 and R4, where Po2 is significantly lower.
Intratubular fluid Po2 of SAL and LAL is lowest near the OM-IM boundary. In the mTAL, basolateral Na+ reabsorption is carried out by Na+-K+-ATPase pumps, which hydrolyze ATP, thereby requiring O2 consumption. As a result, Na+ concentrations of intratubular fluid in the prebend segments and in the mTALs are progressively reduced along the luminal flow direction (Fig. 5). Taken in isolation, the active transport processes would decrease mTAL Po2 along the direction of their flow. However, Po2 is lower within the mTAL lumen than in the surrounding interstitium, so that O2 diffuses into the mTAL. These two competing factors result in a general increase in mTAL O2 flow in the SAL in the inner stripe and in the LAL along their flow direction and a decrease in SAL O2 flow in the outer stripe.
Fig. 5.
Na+ concentration (CNa) profiles in tubules (A) and vessels (B) along the corticomedullary axis. Fluid CNa increases in all tubules and vessels along the corticomedullary axis, except along the prebend segments of the SDL and along the SAV near the OM-IM boundary.
Radial O2 Transport: Diffusion vs. Capillary Transport
We then examined the relative contribution of different O2 transport mechanisms. O2 exchange between regions can occur in two ways: advection and diffusion. Advection takes place via capillary flow. At each medullary level, a fraction of the SDV population breaks up into capillaries and releases their plasma content, including O2, into R1 and R2 interstitium (see Eqs. A52a and A52b). The RBCs associated with those SDVs become iRBCs that traverse in the direction perpendicular to the corticomedullary axis toward R4 (see Eqs. A29a–A30b). These capillaries form some SAV in R3 and the rest in R4. As the capillaries traverse from R1 and R2 toward R3 and R4, some O2 also diffuses out of the iRBC tubes into the interstitium. Interregion diffusive O2 exchange takes place as O2 permeates out of the LDV and SDVa into R1 and out of the SDVb into R2. A fraction of that O2, which depends on the interregion permeability to O2 (P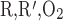 ), then diffuses radially toward the surrounding regions.
), then diffuses radially toward the surrounding regions.
To compare the contributions of diffusion vs. advection to interregion O2 transport, we computed the fluxes of O2 exchanged between neighboring concentric regions that take place via interregion boundary diffusion and via capillary advection, respectively, given by
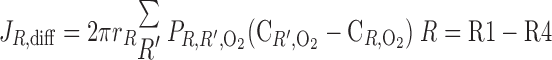 |
(18) |
 |
(19a) |
 |
(19b) |
 |
(19c) |
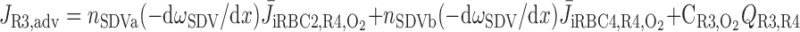 |
(19d) |
where J̄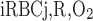 is the O2 flux from the iRBCj tubes into region R (Eqs. A58–A61), αSDVa denotes the fraction of the capillary plasma flow from terminating SDVa that is directed into R1 (10%), C
is the O2 flux from the iRBCj tubes into region R (Eqs. A58–A61), αSDVa denotes the fraction of the capillary plasma flow from terminating SDVa that is directed into R1 (10%), C is the interstitial O2 concentration in region R, and QR,R′ represents the interregional flow from region R to R′.
is the interstitial O2 concentration in region R, and QR,R′ represents the interregional flow from region R to R′.
Figure 6 shows interregion diffusive and advective O2 fluxes as a function of medullary depth. In all regions, advective O2 fluxes are significantly larger in the inner stripe than in outer stripe, since capillary flow is almost negligible in the outer stripe, where there are very few capillaries (28). [The model assumes that only 3% of SDV break up above the outer stripe-inner stripe junction (see Eq. A22).]
Fig. 6.
Diffusive and advective interregion O2 fluxes, taken positive into a region. A–D: regions R1–R4, respectively.
Throughout R1, O2 is reabsorbed mainly from LDV; the number of SDVa, and thus the flow across iRBC1 and iRBC2, is negligible in the base case. Most of that reabsorbed plasma O2 is carried away by LAVa, but a small fraction (5%) enters R2 via plasma capillary flow (i.e., the last term in Eq. 19b). Because interstitial Po2 in R1 is more than twice that in R2, O2 also diffuses into R2. Since capillary flow is small and R1 Po2 is high, diffusion exceeds advection throughout the OM.
Because capillary flows are assumed to be more abundant outside the vascular core, advection exceeds diffusion in R2, R3, and R4 throughout most of the OM (except in portions of the outer stripe for R4). For R3, advective and diffusive fluxes are positive throughout the OM; this indicates that O2 enters R3, driven by capillary flows (advection) and by a favorable interregion O2 gradient (diffusion). The diffusive fluxes in R1 are approximately one order of magnitude lower than the advective fluxes in R2–R4: the fact that R1-to-R2 O2 diffusion remains relatively low, despite a significant Po2 gradient, can be attributed to the small interstitial area in R1 (Fig. 7), which limits diffusion.
Fig. 7.
Interstitial area (×10−6 cm2) in regions R1–R4.
Basal and Active Transport O2 Consumption
We distinguish in this study between the basal and active components of O2 consumption. As defined by Mandel and Balaban (38), the basal rate comprises 1) the energy required for active transport processes not linked to Na+ transport and 2) the energy required for biochemical reactions in renal cells (such as synthetic activity and interconversion of substrates, but not glycolysis). The active O2 consumption rate corresponds to the energy required for the active transport of Na+ across renal cells. The relative contribution of each component is discussed below.
The molar flow of O2 entering descending vessels and tubules at x = 0 (i.e., the corticomedullary junction) is calculated as 6.6 × 10−13 mol·s−1·nephron−1, or 39.6 pmol·min−1·nephron−1, as described above. Most of that O2 is consumed in the renal medulla, mostly in the OM. The molar flow of O2 returned to the cortex at x = 0 via ascending vessels and tubules (i.e., SAL, SAVa, SAVb, LAL, LAVa, and LAVb) is predicted to be 6.2 pmol·min−1·nephron−1. Since O2 consumption in the IM is taken as 5% of medullary O2 supply, overall O2 consumption in the OM is calculated as 0.95(39.6) − 6.2 = 31.4 pmol·min−1·nephron−1, that is, 79.3% of the supply.
Table 3 shows total basal and active transport O2 consumption, per nephron, along each class of tubules and vessels and in each concentric region. The basal (or active) O2 consumption reported for a given type i of tubules or vessels reflects the total basal (or active) consumption of the epithelial or endothelial cells surrounding the lumen of i. The basal O2 consumption reported for region Ri reflects the basal consumption of the interstitial cells in Ri. Basal O2 consumption is by far the lowest in R1, because the core of the vascular bundles has a small area and is assumed to be tightly packed, with only a small number of interstitial cells (45). In contrast, R3, which has the largest area (thus, the largest number of interstitial cells) and contains the greatest proportion of tubules and vessels, has the highest basal consumption among all regions.
Table 3.
Basal and active transport O2 consumption
| O2 consumption, pmol·min−1·nephron−1 |
|||
|---|---|---|---|
| Basal | Active | Total | |
| R1 | 0.003121 | 0 | 0.003121 |
| R2 | 0.03219 | 0 | 0.03219 |
| R3 | 0.05676 | 0 | 0.05676 |
| R4 | 0.03539 | 0 | 0.03539 |
| SDL | 0.01878 | 0.2292 | 0.2480 |
| SDL2 | 0.06558 | 0 | 0.06558 |
| SAL | 0.01906 | 8.2740 | 8.2931 |
| SAL2 | 0.06556 | 8.2947 | 8.3603 |
| CD | 0.1219 | 0 | 0.1219 |
| LDL | 0.03571 | 0 | 0.03571 |
| LAL | 0.1133 | 13.8334 | 13.9467 |
| LDV | 0.007109 | 0 | 0.007109 |
| LAVa | 0.01580 | 0 | 0.01580 |
| LAVb | 0.02617 | 0 | 0.02617 |
| SDVa | 1.6615e-4 | 0 | 1.6615e-4 |
| SDVb | 0.01526 | 0 | 0.01526 |
| SAVa | 0.1047 | 0 | 0.1047 |
| SAVb | 0.03515 | 0 | 0.03515 |
Different basal O2 consumption rates are reported for LAVa and LAVb (0.01580 and 0.02617 pmol·min−1·nephron−1), in large part because the model represents almost twice as many LAVb as LAVa (there are 17 LAVa per 71 nephrons and 33 LAVb per 71 nephrons). Indeed, because LAVa resides in the O2-rich R1, the basal O2 consumption, which depends on luminal Po2, of each LAVa actually exceeds that of each LAVb. The discrepancies among basal consumption among the different populations of SDV and SAV are also attributable to their number ratios and luminal Po2.
The total OM O2 consumption due to basal metabolism, by all epithelial, endothelial, and interstitial cells, is computed to be 0.8789 pmol·min−1·nephron−1, whereas the consumption due to active transport is 30.63 pmol·min−1·nephron−1. The latter accounts for 97%, a majority, of the total OM O2 consumption. Of the active transport O2 consumption, 54% and 45% are attributable to SAL and LAL, respectively. Although the metabolic needs of each SAL are less than those of each LAL (see above), there are also twice as many SAL as LAL; thus SAL O2 consumption is greater.
mTAL Active Na+ Transport
The amount of ATP produced by glycolysis in mTALs is a small proportion of that produced by aerobic metabolism (53), even though mTALs have a high capacity for glycolysis (54). Studies suggest that only 2–3% of the ATP produced in the kidney originates from glycolysis and that only in the OMCD and IMCD can glycolysis maintain a substantial fraction of tubular function (11, 21). In the base case, anaerobic metabolism is not represented.
To simulate the effects of anaerobic metabolism on Na+ concentration profiles in the OM, we assume that as Po2 decreases between 1 and 0 mmHg, anaerobic metabolism supplies an increasingly larger fraction of the energy needed to actively transport NaCl across mTALs, so that, in the absence of any O2 supply, anaerobic metabolism supplies enough energy to sustain an active Na+ transport rate that is half of the maximum rate when O2 supply is abundant. With this assumption, Eq. 2 becomes
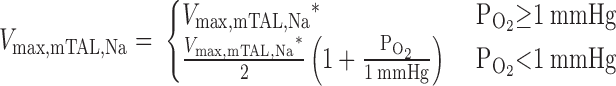 |
(20) |
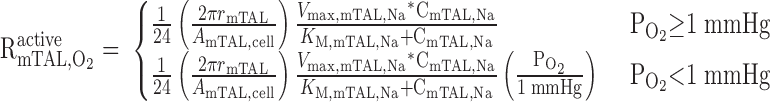
The rate of active O2 consumption is then calculated as
 |
(21) |
The model predicts that anaerobic metabolism could significantly increase the concentrating capability of the OM. Figure 8 shows transmural Na+ fluxes driven by aerobic and anaerobic active transport along the SAL and LAL. In the early OM (for x < 0.3L), luminal Po2 in SAL and LAL is sufficiently high that anaerobic active Na+ transport does not take place. Closer to the OM-IM boundary, where interstitial and mTAL luminal Po2 are very low, anaerobic active Na+ transport becomes significant. At x = 0.95L, where luminal Po2 in SAL and LAL is near its minimum, anaerobic active Na+ transport accounts for 66.8% and 70.0% of the mTAL Na+ reabsorption in LAL and SAL, respectively. The trough in the LAL profile corresponds to an analogous trough in the R2 interstitial Po2 profile (Fig. 3E).
Fig. 8.
Na+ reabsorption along SAL (A) and LAL (B) driven by aerobic (solid line) and anaerobic (dashed line) metabolism, with the assumption that in medullary thick ascending limbs (mTALs), glycolysis provides significant energy at low Po2 (Eqs. 20 and 21). In the outer stripe, where mTAL luminal Po2 is sufficiently high, all Na+ active transport is oxidative.
With the inclusion of anaerobic active Na+ transport, 848 pmol·min−1·nephron−1 of Na+ is reabsorbed from the mTAL compared with 735 pmol·min−1·nephron−1 in the base case without anaerobic active transport. This 15.4% increase in total Na+ reabsorption has the effect of raising the tubular and vascular fluid osmolality gradient along the corticomedullary axis. With anaerobic active transport, CD, LDL, and LDV tubular fluid osmolalities are predicted to be 665, 666, and 488 mosmol/kgH2O, respectively, at the OM-IM boundary compared with baseline values of 580, 581, and 450 mosmol/kgH2O, respectively. These results suggest that because the mTALs operate near the brink of hypoxia, anaerobic active Na+ transport along the mTAL, if it does occur, may contribute significantly to generation of the OM osmolality gradients.
To assess the degree to which mTAL active Na+ transport is limited by its luminal Po2, we computed the total mTAL active Na+ transport (in pmol·min−1·nephron−1) under different scenarios. For the base case without anaerobic transport, total mTAL active transport is calculated to be 735 pmol·min−1·nephron−1. For the idealized case in which Vmax,mTAL,Na is assumed to be constant (i.e., independent of mTAL luminal Po2), total mTAL active transport is calculated to be 961 pmol·min−1·nephron−1, which corresponds to a 30.7% increase. With the inclusion of anaerobic transport, total mTAL active transport is computed to be 848 pmol·min−1·nephron−1, which is 15.4% higher than the base case and 11.8% less than the idealized case.
DISCUSSION
Summary of Main Results
We have developed a highly detailed mathematical model for O2 transport in the OM of the rat kidney. The model uses a region-based configuration (33) to represent radial organization of renal tubules and vessels with respect to the vascular bundles. In addition to renal tubules and vessels, the model also explicitly represents RBCs. The model predicts, at steady state, in all represented structures the concentrations of Na+, urea, and O2 (and also Hb in RBCs), intratubular or intravascular flow rates of water and solutes, and transmural fluxes of water and solutes.
Our results suggest that the structural organization of the OM results in significant Po2 gradients in axial and radial directions. Because almost four-fifths of the DVR turn within the OM, ∼80% of the O2 supplied to the medulla bypasses the IM. The decreasing DVR population, together with the large metabolic requirements of Na+ active transport across mTAL cells, results in a Po2 drop along the corticomedullary axis in most structures. Moreover, the segregation of the DVR (i.e., the main supply of O2) at the center and immediate periphery of the vascular bundles creates large regional differences in Po2 in the radial direction. In the context of a urine-concentrating mechanism, the vascular bundles promote countercurrent exchange by bringing LDV and LAV in close proximity and enhance the concentrating capability of the OM by shielding the relatively dilute LDV from other structures, thereby reducing the osmotic load on the concentrating mechanism (33). In the context of O2 transport, by isolation of LDV within the relatively O2-rich vascular bundle core, O2 reabsorption from LDV is reduced. As a result, 92.4% of the O2 supplied to LDV at the corticomedullary junction reaches the IM.
In addition, our model predicts, in agreement with experimental observations (5, 17), that the mTALs operate on the brink of hypoxia. The mTALs are localized far from the vascular bundles, an arrangement that improves the OM concentrating effect, inasmuch as the Na+ that is actively transported out of the mTALs can be used more effectively to raise the osmolality of the CD (33). However, because of the significant distance between the mTALs and the O2-carrying DVR, together with the high O2 consumption of the mTALs, mTAL luminal Po2 is very low (<10 mmHg) near the OM-IM boundary, so that mTALs are particularly vulnerable to hypoxic injury.
Comparison With Experimental Results
Measurements of Po2 in the renal OM generally range from 20 to 30 mmHg. Po2 was measured as 20 mmHg at a depth of 3.7 mm below the cortex, that is, ∼0.7 mm below the corticomedullary junction (13), 21 mmHg at a depth of 4.0–4.5 mm (4), and 23 mmHg at depth of 4 mm (42). Higher values, between 30 and 36 mmHg, were obtained by other investigators (14, 37) at depths of 3–4 mm.
Our predictions in the base case are in agreement with the data in the lower range. As shown in Fig. 3, within the first millimeter below the corticomedullary junction (i.e., at 3–4 mm below the cortex), interstitial Po2 is predicted to vary between 35 and 14 mmHg in the periphery of the vascular bundles (R2) and between 17 and 5 mmHg in the interbundle regions (R3 and R4). Po2 is predicted to be significantly greater (>48 mmHg) in the core of the vascular bundles (R1), but it is unlikely that measurements would have been performed in the very center of the vascular bundle, where very few interstitial cells are present (36).
Furthermore, the model predicts that the O2 consumption-to-delivery ratio is 79.3% in the base case (Fig. 3), in excellent agreement with the 79% estimate reported by Brezis et al. (5). The basal metabolic rate has been estimated as 3–18% of the O2 consumption rate in the mammalian kidney (11). Our model predicts a corresponding percentage of 3% in the OM, that is, at the lower end of the reported range. It is possible that basal O2 consumption was underestimated in our simulations.
O2 distribution affects the urine-concentrating mechanism, inasmuch as the active transport rate of Na+ may decrease when O2 availability becomes limited, which indeed occurs in the interbundle regions in the late OM. Because the OM urine-concentrating mechanism is driven by the active transport of Na+ by the mTAL, when that transport is reduced, the concentrating effect of the model OM is reduced. Nonetheless, the model predicts a CD fluid osmolality of 580 mosmol/kgH2O at the OM-IM boundary, which is a 1.9-fold increase over the inflow osmolality (309 mosmol/kgH2O). Such an increase is consistent with experimental measurements (49).
Comparison With Previous Models
O2 transport in the microcirculation has been the focus of mathematical modeling for several decades following the pioneering work of Krogh (30). Kinetic expressions for the formation of HbO2 were developed by Adair (1) and Moll (39). The model of O2 transport developed by Hellums (23) and further extended by Baxley and Hellums (2) was among the first to consider RBCs and plasma separately, instead of treating the blood as a continuum. In contrast with previous approaches, these studies showed that the resistance to O2 transport in the capillary is a significant fraction of the overall resistance. The discrete cell model was further advanced by the work of Federspiel and Popel (19) and Groebe (20). The history and present challenges of O2 transport simulations are reviewed in greater detail by Hellums et al. (22) and Popel (46).
In our previous model of O2 transport across vasa recta (57), the tubular system was not explicitly included; instead, we specified the volumetric O2 consumption rate along the corticomedullary axis. The model was used to predict the overall O2 consumption rate on the basis of measurements of the medullary interstitial Po2 gradient. That model predicted an upper estimate of overall consumption, 5 × 10−6 mmol·s−1·kidney−1 (i.e., 1.7 × 10−13 mol·s−1·nephron−1, with the assumption of 423 bundles per kidney and 71 nephrons per bundle), which is comparable to the predictions of the present model, 5.3 × 10−13 mol·s−1·nephron−1. In the previous study, the fraction of O2 supplied to DVR at the corticomedullary junction that reaches the OM-IM boundary was predicted to be approximately equal to the relative number of DVR assumed to reach that medullary depth, a prediction that is consistent with the present study. However, in the previous study, we assumed that fewer DVR reach into the IM. Thus the present model predicts a significantly higher O2 delivery rate into the IM, at 1.3 × 10−13 mol·s−1·nephron−1, compared with 4.8 × 10−14 mol·s−1·nephron−1 in the previous model.
Another noteworthy difference between the present study and the work of Zhang and Edwards (57) is that they did not represent the complex radial organization of the OM. The interstitial Po2 profile that was assumed in the previous model was based on OM measurements that likely correspond to averages of intra- and interbundle Po2. Thus the previous model predicted a DVR-interstitium Po2 difference that remained <10 mmHg throughout the medulla. In contrast, in the present model, DVR are surrounded by the O2-rich R1 interstitium; thus the present model predicts larger DVR-R4 Po2 differences of 44 mmHg at x = 0 to 46 mmHg at x = L but significantly smaller DVR-R1 Po2 differences of 2 mmHg at x = 0 to 0.1 mmHg at x = L.
The present model's representation of the OM structural organization and the transport of Na+ and urea is based on the region-based urine-concentrating mechanism model of Layton and Layton (33) (hereafter referred to as the L & L model). In the L & L model, the active Na+ transport is approximated by a saturable expression having the form of Michaelis-Menten kinetics, with the maximum transport rate Vmax,i,Na assumed constant. In the present model, Vmax,i,Na is assumed to decrease when luminal Po2 dips below 1 mmHg. Thus the total active Na+ transport is predicted to be less in the present model than in the L & L model: the present model predicts that SAL and LAL tubular fluid enters the cortex with an Na+ concentration of 144 and 107 mM, respectively, whereas in the L & L model (33), the analogous Na+ concentrations were predicted to be 122 and 93 mM, respectively. In part because of the reduced Na+ reabsorption in the present model, a somewhat lower osmolality gradient is predicted: at the OM-IM boundary, CD, LDL, and LDV tubular fluid osmolalities are predicted to be 580, 581, and 450 mosmol/kgH2O, respectively, compared with 851, 822, and 586 mosmol/kgH2O, respectively, in the L & L model (33). Nonetheless, the 1.9-fold increase in fluid osmolality along the OMCD is consistent with micropuncture measurements (49). When anaerobic metabolism is included, the model predicts higher mTAL Na+ active transport, resulting in more concentrated fluid entering the IM: CD, LDL, and LDV tubular fluid osmolalities are predicted to be 665, 666, and 488 mosmol/kgH2O, respectively; those values somewhat better approximate the predictions of the L & L model, but remain significantly lower. The remaining difference can be attributed, in large part, to the different blood flow representation of the two models. In the L & L model, which does not explicitly represent RBCs, 95% and 5% of the net fluid reabsorption in R1 and R2, part of which arises from the break-up of SDV and, thus, contains RBCs, is taken up by LAVa and LAVb, respectively, leaving only a small amount of fluid to be shunted to the neighboring regions. In contrast, the present model assumes that 95% and 5% of the reabsorbed plasma, but not RBCs, in R1 and R2, respectively, are taken up by LAV; the remainder of the plasma and all the RBCs are shunted to R2 and R3, respectively. As a result, water reabsorption from the RBC in R3 and R4 lowers the interstitial fluid osmolality in those outer regions and in tubules that reside in those regions.
O2 Consumption by mTAL Cells
Na+-K+-ATPase stoichiometry indicates that 1 mol of ATP is needed to actively carry 3 mol of Na+ across the pump, which corresponds to TNa/Q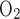 = 18 under maximal efficiency (38). However, measurements in the kidney and in various segments of the nephrons have yielded higher Na+-to-ATP ratios, which implies higher TNa/Q
= 18 under maximal efficiency (38). However, measurements in the kidney and in various segments of the nephrons have yielded higher Na+-to-ATP ratios, which implies higher TNa/Q (21). In particular, measurements in the thick ascending limbs yielded TNa/Q
(21). In particular, measurements in the thick ascending limbs yielded TNa/Q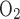 = 36 (11). The discrepancy in these measurements may be attributable to the transport processes of Na+ (or, in some cases, Cl− or its counterions), which are facilitated by the Na+-K+-ATPase pump but do not consume O2 (see discussion in Ref. 48). Such transport processes, which may proceed through transcellular (via transporters other than the Na+-K+-ATPase pump) or paracellular pathways, are not explicitly discussed in this model because of its simple single-barrier representation of transepithelial transport processes. To account for these additional routes of Na+ reabsorption, we use a higher TNa/Q
= 36 (11). The discrepancy in these measurements may be attributable to the transport processes of Na+ (or, in some cases, Cl− or its counterions), which are facilitated by the Na+-K+-ATPase pump but do not consume O2 (see discussion in Ref. 48). Such transport processes, which may proceed through transcellular (via transporters other than the Na+-K+-ATPase pump) or paracellular pathways, are not explicitly discussed in this model because of its simple single-barrier representation of transepithelial transport processes. To account for these additional routes of Na+ reabsorption, we use a higher TNa/Q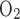 = 24.
= 24.
Na+-K+-ATPase pumps must hydrolyze ATP to export Na+ and import K+. As reviewed by Gullans and Mandel (21), in all renal segments, most of the ATP appears to originate from mitochondrial oxidative metabolism. Inhibition of mitochondrial oxidative metabolism markedly stimulates glycolysis in mTALs; however, even under anaerobic conditions, the amount of ATP generated by glycolysis is a small fraction of that produced by oxidative metabolism (21). Thus only aerobic active Na+ transport is represented in the base case. In a separate simulation, we included anaerobic active Na+ transport along the mTAL. Our results suggest that if anaerobic transport were able to account for ∼38.9% of the total mTAL active Na+ transport, the osmolality gradient along the corticomedullary axis would be significantly enhanced, and CD fluid osmolality at the OM-IM junction would increase by 14.7% from the baseline value of 580 to 665 mosmol/kgH2O.
Regulation of O2 Supply and Demand
The high rate of active NaCl reabsorption and the low availability of O2 in their vicinity render the mTALs particularly susceptible to anoxic damage. As described by Brezis et al. (6), perfusion of isolated rat kidneys with cell-free medium produces an anatomic lesion sharply localized to mTAL cells; the lesion appears first in mTALs most removed from vascular bundles and near the IM. Other studies (for review see Ref. 3) have shown that inhibition of NaCl transport with furosemide or ouabain reduces tubular necrosis, whereas increases in Na+-K+-ATPase activity markedly enhance hypoxic damage. To protect itself against hypoxic injury, the kidney uses two types of mechanisms: medullary vasodilation to augment blood supply and reduction of transport work to lower O2 consumption (3). Schurek and Johns (50) suggested that tubuloglomerular feedback, which reduces the glomerular filtration rate, may serve as a tool to adapt the tubular energy demand to its actual O2 supply.
Comparison With Other Tissues
As discussed by Cohen (10), the mammalian kidney, which constitutes ∼0.5% of the total body weight, consumes ∼10% of the body's O2 uptake. Thus, on a tissue weight basis, O2 consumption by the kidney (5 ml O2·min−1·100 g−1) is second only to that of the working heart (8 and 70 ml O2·min−1·100 g−1 at rest and during heavy exercise, respectively). O2 consumption in the brain, another work-intensive tissue, is slightly lower (3 ml O2·min−1·100 g−1), whereas that in the skin is on the order of 0.2 O2·min−1·100 g−1 (25). Yet, since the renal blood flow rate is so high [678 ml·min−1·100 g−1 (31)], the extraction of O2 from each millimeter of blood is significantly lower in the kidney than in other tissues. Blood flow rate per 100 g of tissue is 5 times lower in the heart (10), 15 times lower in the brain, and 55 times lower in the skin (31).
In conclusion, our new model of O2 transport in the rat OM suggests that the structural organization of the OM results in significant Po2 gradients in the axial and radial directions. The segregation of DVR, the main supply of O2, at the center and immediate periphery of the vascular bundles gives rise to large radial differences in Po2 between regions, limits O2 reabsorption from long DVR, and helps preserve O2 delivery to the IM.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant DK-53775 to A. Edwards and A. T. Layton and National Science Foundation Grant DMS-0701412 to A. T. Layton.
APPENDIX
The general framework for the region-based model is that developed by Layton and Layton (33). We expanded their model to include RBCs, O2, Hb, and HbO2, as well as metabolic reactions.
Water and Solute Conservation in Tubules and Vessels
Steady-state conservation equations are used to predict the volumetric flow rate (Fi,v) and the concentration of solute k (Ci,k) in a tubule or vessel i
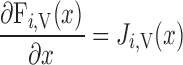 |
(A1) |
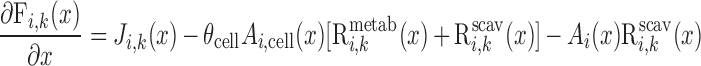 |
(A2) |
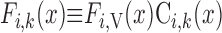 |
where x is the position along the OM, ranging from 0 at the corticomedullary junction to L at the OM-IM boundary; Ji,V and Ji,k denote the transmural line flux of water and solute k into tubule or vessel i; and Ai designates the cross-sectional area of the lumen of i (i.e., based on its inner diameter), whereas Ai,cell denotes that of the surrounding cell layer (i.e., epithelium for tubules and endothelium for vessels). When Eqs. A1 and A2 are applied specifically to distributed short vasa recta (i.e., SDV and SAV), the number of which changes along the corticomedullary axis, variables must be expressed as a function of x and y, where x denotes the medullary depth at which the flow or flux is evaluated and y denotes the medullary depth reached by the terminus of the short vas rectum being considered (0 ≤ x ≤ y ≤ L).
We consider two types of consumption rates: R is the volumetric rate of metabolic consumption of solute k by the epithelial or endothelial cells surrounding tubule or vessel i, and R
is the volumetric rate of metabolic consumption of solute k by the epithelial or endothelial cells surrounding tubule or vessel i, and R is the volumetric rate at which solute k is scavenged by other solutes (such as the reaction between Hb and O2). By convention, all the rate terms are taken to be positive. Since we do not explicitly distinguish solute concentrations in the endothelial or epithelial layer surrounding the lumen of tubule or vessel i, we assume that a fraction θcell (taken as 0.5) of the amount of solute consumed in that layer is taken from the lumen, with the rest (1 − θcell) being taken from the interstitium (Eqs. A2 and A62).
is the volumetric rate at which solute k is scavenged by other solutes (such as the reaction between Hb and O2). By convention, all the rate terms are taken to be positive. Since we do not explicitly distinguish solute concentrations in the endothelial or epithelial layer surrounding the lumen of tubule or vessel i, we assume that a fraction θcell (taken as 0.5) of the amount of solute consumed in that layer is taken from the lumen, with the rest (1 − θcell) being taken from the interstitium (Eqs. A2 and A62).
O2 is consumed by endothelial, epithelial, and interstitial cells for their basal metabolism and by epithelial cells in ascending and descending limbs for the active transport of Na+. Thus
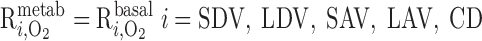 |
(A3) |
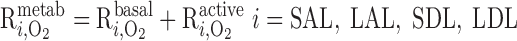 |
(A4) |
where R and R
and R are given in Eqs. 6 and 9, respectively. The rate of reaction between O2 and Hb (R
are given in Eqs. 6 and 9, respectively. The rate of reaction between O2 and Hb (R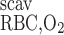 ), which is nonzero in RBCs only, is given in Eq. 3.
), which is nonzero in RBCs only, is given in Eq. 3.
Conservation equations and BCs for individual tubules.
For i = SDL, LDL, SAL, LAL, or CD, the solute conservation equation (Eq. A2) can be rewritten as
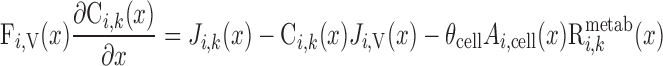 |
(A5) |
R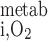 is given in Eqs. A3 and A4, and R
is given in Eqs. A3 and A4, and R is set to 0 for all other solutes.
is set to 0 for all other solutes.
The BCs at x = 0 for water flow and solute concentrations in SDL and LDL are summarized in Table 2. The BCs at x = L for SAL are obtained by continuity with SDL
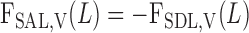 |
(A6a) |
 |
(A6b) |
The BCs at x = L for LAL are as follows for water flow, Na+, and urea (33)
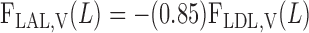 |
(A7a) |
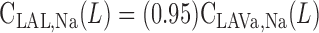 |
(A7b) |
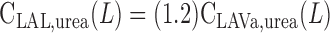 |
(A7c) |
O2 BCs are based on the following quantities: S is the overall supply of O2 (in the form of O2 and HbO2) per nephron to the (outer) medulla, Q
is the overall supply of O2 (in the form of O2 and HbO2) per nephron to the (outer) medulla, Q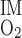 denotes the O2 consumption rate in the IM per nephron, taken to be 5% of S
denotes the O2 consumption rate in the IM per nephron, taken to be 5% of S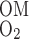 , and F
, and F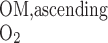 is the amount of O2 (including that bound to Hb) per nephron that is returned to the OM from the IM via ascending limbs and vessels
is the amount of O2 (including that bound to Hb) per nephron that is returned to the OM from the IM via ascending limbs and vessels
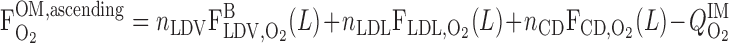 |
(A8a) |
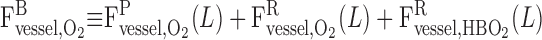 |
(A8b) |
where the superscripts B, P, and R denote blood, plasma, and RBCs, respectively. With this notation, the O2 BC for LAL is given by
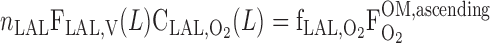 |
(A9) |
where ni denotes the number of tubules of vessels of type i per nephron and f is the fraction of O2 returning from the IM to the OM that flows within LAL (taken as 1%).
is the fraction of O2 returning from the IM to the OM that flows within LAL (taken as 1%).
The BCs for water flow and Po2 in CDs are given in Table 2. Conservation of urea in the cortex, with the fractional absorption δurea (taken as 0.25) taken into account, can be written as (33)
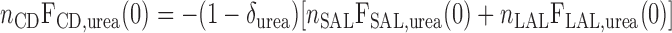 |
(A10a) |
We assume that the osmolality of the CD fluid at x = 0 is equal to that of plasma, so that the Na+ concentration in the CD at x = 0 is calculated as
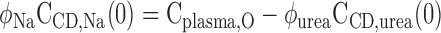 |
(A10b) |
where Cplasma,O denotes plasma osmolality (taken to be 309 mosmol/kgH2O) and φk is the osmotic coefficient of solute k (taken as 1.84 for NaCl and 0.97 for urea).
Conservation equations and BCs for individual vessels.
In the model blood vessels, plasma and RBCs are treated as two separate compartments. F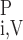 and F
and F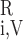 denote the plasma and RBC water flow rate, respectivelly, in vessel i, so that the total water flow rate in vessel i is Fi,V = F
denote the plasma and RBC water flow rate, respectivelly, in vessel i, so that the total water flow rate in vessel i is Fi,V = F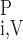 + F
+ F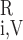 . Similarly, C
. Similarly, C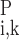 and C
and C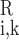 denote the respective plasma and RBC concentration of solute k in vessel i.
denote the respective plasma and RBC concentration of solute k in vessel i.
The transmural line flux entering the plasma from the interstitium is designated by the superscript “wall” and that entering the RBCs from the plasma is designated by the superscript “R”; therefore, the net water and solute fluxes entering the plasma of vessel i are given by
 |
(A11) |
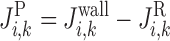 |
(A12) |
Water conservation in plasma and RBCs can be expressed as
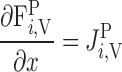 |
(A13) |
 |
(A14) |
Similarly, solute conservation in plasma and RBCs is expressed as
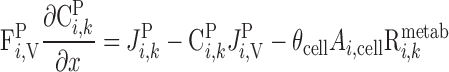 |
(A15) |
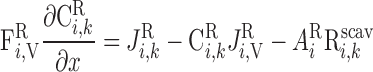 |
(A16) |
The cross-sectional area of the RBC compartment is calculated as
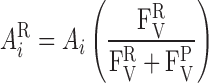 |
(A17) |
The BCs at x = 0 for water flow and solute concentrations in SDV and LDV are summarized in Table 2.
BCs for LAV.
The BCs at x = L for the two populations of LAV (LAVa and LAVb) are as follows.
water.
 |
(A18a) |
 |
(A18b) |
where Furine,V denotes the urine flow per nephron (taken to be 0.065 nl·min−1·kidney−1) and FLAL,V(L) is given in Eq. A9. Once the total blood flow FLAVj,V(L) is calculated, the RBC flow is determined assuming that the LAV-to-LDV hematocrit ratio at x = L (fhem,L) is known
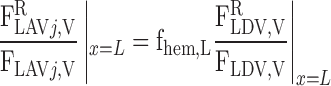 |
(A19c) |
On the basis of baseline results from the study of Layton (32), fhem,L is taken as 10.8:16.9.
sodium and urea.
We assume that plasma (and RBC) concentrations of Na+ and urea are equal in LAVa and LAVb at x = L; that is, C (L) = C
(L) = C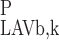 (L) and C
(L) and C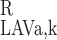 (L) = C
(L) = C (L) (k = Na+ and urea). We also assume that the RBC-to-plasma concentration ratio is equal in LAV and LDV at x = L, so that C
(L) (k = Na+ and urea). We also assume that the RBC-to-plasma concentration ratio is equal in LAV and LDV at x = L, so that C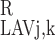 (L)/C
(L)/C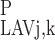 (L) = C
(L) = C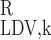 (L)/C
(L)/C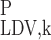 (L). The plasma concentrations of Na+ and urea at x = L can then be found by solving the following conservation equation
(L). The plasma concentrations of Na+ and urea at x = L can then be found by solving the following conservation equation
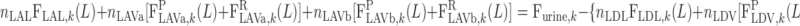 |
(A20) |
where Furine,k denotes the urine flow of solute k per nephron. The ratios CLAL,Na(L)/CLAVa,Na(L) and CLAL,urea(L)/CLAVa,urea(L) are given in Eqs. A7b and A7c.
oxygen.
The fractions of O2 (O2 + HbO2) returning from the IM to the OM that flows within LAVa (f ) and LAVb (f
) and LAVb (f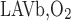 ) are taken to be fixed
) are taken to be fixed
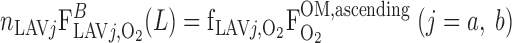 |
(A21a) |
where F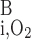 is the total amount of O2 in blood in vessel i (Eq. A8b) and F
is the total amount of O2 in blood in vessel i (Eq. A8b) and F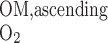 is the total amount of O2 that flows back into the OM via ascending limbs and vessels (Eq. A8a). As noted above, we set f
is the total amount of O2 that flows back into the OM via ascending limbs and vessels (Eq. A8a). As noted above, we set f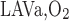 = 38.8% and f
= 38.8% and f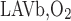 = 60.2%. In addition, conservation of Hb and reaction equilibrium at the OM-IM boundary can be expressed as
= 60.2%. In addition, conservation of Hb and reaction equilibrium at the OM-IM boundary can be expressed as
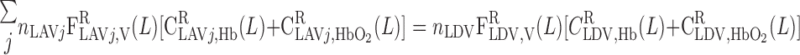 |
(A21b) |
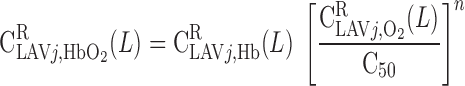 |
(A21c) |
These nonlinear equations are solved simultaneously to yield C (L), C
(L), C (L), and C
(L), and C (L). We also assume that O2 concentration in LAVj plasma at x = L is equal to that in the surrounding region.
(L). We also assume that O2 concentration in LAVj plasma at x = L is equal to that in the surrounding region.
BCs for SAV.
The fractional population of SDV (ωSDV) and SAV (ωSAV) that reaches medullary depth x is given by (33)
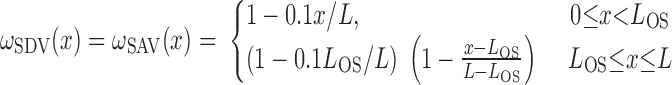 |
(A22) |
where LOS is the outer stripe thickness. The plasma water flow of SAVj originating at y = x is given by
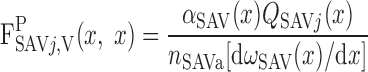 |
(A23) |
where j = a or b, QSAVj is the total fluid accumulation carried away by SAVj at a medullary level x, and αSAV is the fraction of QSAVj that enters the lumen of the SAVj that originate at x. The remaining fraction enters through the endothelial pores of the SAVj that originate at y > x. The plasma concentration of solute k in SAVj originating at y = x is taken as that of the region in which the SAVj are located, i.e.
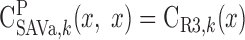 |
(A24a) |
 |
(A24b) |
Water and solute flows in the RBCs associated with the SAV are obtained by continuity with the iRBC tubes at medullary level x that empty into the SAVj originating at that level
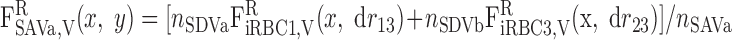 |
(A25a) |
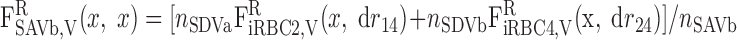 |
(A25b) |
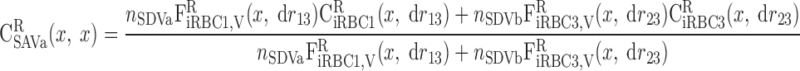 |
(A25c) |
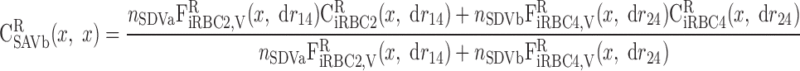 |
(A25d) |
where drmn (m = 1, 2 and n = 3, 4) denotes the length of the corresponding iRBC tube (Fig. 2) and is given by
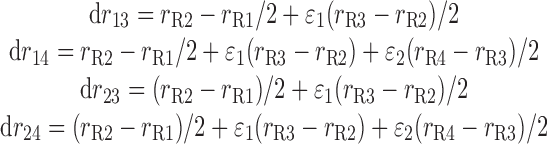 |
(A26) |
 |
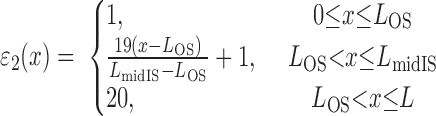 |
where rRi is the outer radius of region Ri and the factors ɛ1 and ɛ2 account for the tortuosity of the capillaries in the outer regions of inner stripe, where the interbundle capillary plexus was found to be significantly denser than in the outer stripe and the vascular bundles in the inner stripe (28).
Conservation equations and BCs for iRBC tubes.
At a given medullary level x, water and solute conservation equations in the iRBC tubes that form at the end of SDV terminating at y = x can be written as
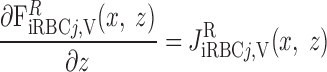 |
(A27) |
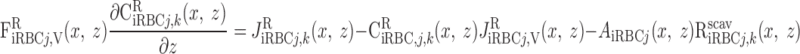 |
(A28) |
where z is the radial distance from the position of terminating SDV. The BCs at z = 0 are obtained by continuity with the terminating SDV
 |
(A29a) |
 |
(A29b) |
 |
(A30a) |
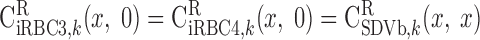 |
(A30b) |
Water and Solute Flux Calculations
Water flux into tubules and vessels.
The transmural flux of water into tubule i is given by
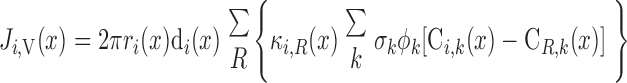 |
(A31) |
where di(x) is the product of the water partial molar volume and the water permeability of i, κi,R is the fraction of tubule i in contact with region R, and σk is the reflection coefficient for solute k (taken to be 1 for all solutes). Similarly, the flux of water flowing from the interstitium into DVR plasma is given by
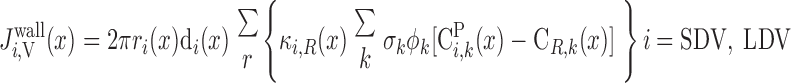 |
(A32) |
When Eq. A32 is applied to SDV, variables must be expressed as a function of x and y.
Since AVR are fenestrated, we assume that their transmural water flux is driven by hydraulic pressure and enters by advection; that is, we neglect osmotic and oncotic pressures. Hence, the transmural flux of water into the plasma of LAVj is given by
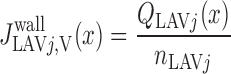 |
(A33) |
where QLAVj is the fluid accumulation carried into LAVj (and is calculated as shown below). The composite transmural flux of water into the plasma of SAVj originating at y > x is given by
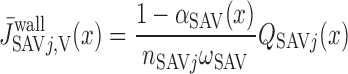 |
(A34) |
Following the approach of Layton (32), the equivalent RBC radius that gives the total surface area of the RBCs as a cylinder is calculated as
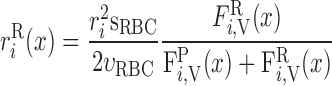 |
(A35) |
where ri is the inner radius of vessel i; the surface area (sRBC) and volume (υRBC) of a single RBC are taken as 129 μm2 and 61 μm3, respectively (15). Similarly, the equivalent radius for iRBC tube j formed by termination of SDVj is given by
 |
(A36a) |
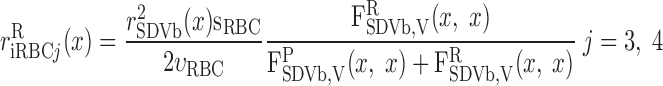 |
(A36b) |
Equation 35 implicitly assumes that the entire surface of all the RBCs is exposed to plasma. Since RBCs are tightly packed within a central core in the vessel lumen, it is possible that Eq. 35 overestimates the surface area available for exchange between the RBCs and the surrounding plasma.
The flux of water from plasma into RBCs is then expressed as
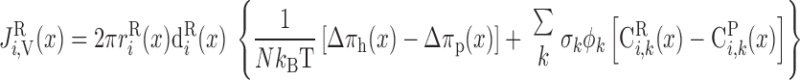 |
(A37) |
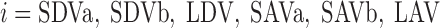 |
where Δπh and Δπp are the oncotic pressures due to Hb in RBCs and to albumin and other proteins in plasma and interstitium, respectively (see below), N is Avogadro's number, kB is the Boltzmann constant, and T is the absolute temperature (the product NkBT equals 19.3 mmHg/mM). When Eq. A37 is applied to SDV and SAV, variables must be expressed as a function of x and y.
Similarly, the transmural flux of water from the interstitium into iRBCs can be expressed as
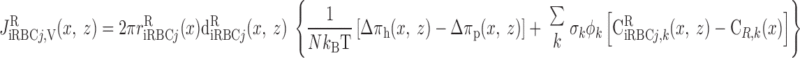 |
(A38) |
where R in CR,k depends on j and z.
For j = 1, z varies between rR1/2 and rR2 + ɛ1(rR3 − rR2)/2, so that rR1/2 ≤ z ≤ rR1, R = R1; rR1 ≤ z ≤ rR2, R = R2; and rR2 ≤ z ≤ rR2 + ɛ1(rR3 − rR2)/2, R = R3.
For j = 2, z varies between rR1/2 and rR2 + ɛ1(rR3 − rR2) + ɛ2(rR4 − rR3)/2, so that rR1/2 ≤ z ≤ rR1, R = R1; rR1 ≤ z ≤ rR2, R = R2; rR2 ≤ z ≤ rR2 + ɛ1(rR3 − rR2), R = R3; and rR2 + ɛ1(rR3 − rR2) ≤ z ≤ rR2 + ɛ1(rR3 − rR2) + ɛ2(rR4 − rR3)/2, R = R4.
For j = 3, z varies between (rR1 + rR2)/2 and rR2 + ɛ1(rR3 − rR2)/2, so that (rR1 + rR2)/2 ≤ z ≤ rR2, R = R2; and rR2 ≤ z ≤ rR2 + ɛ1(rR3 − rR2)/2, R = R3.
For j = 4, z varies between (rR1 + rR2)/2 and rR2 + ɛ1(rR3 − rR2) + ɛ2(rR4 − rR3)/2, so that (rR1 + rR2)/2 ≤ z ≤ rR2, R = R2; rR2 ≤ z ≤ rR2 + ɛ1(rR3 − rR2), R = R3; and rR2 + ɛ1(rR3 − rR2) ≤ z ≤ rR2 + ɛ1(rR3 − rR2) + ɛ2(rR4 − rR3)/2, R = R4.
On the basis of the theoretical framework developed by Sidel and Solomon (51), the oncotic pressure due to Hb is calculated as (15)
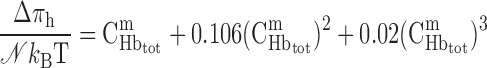 |
(A39) |
 |
(A40) |
where C and C
and C are the molal and molar concentrations of Hb (i.e., one-fourth of the total concentration of heme groups), respectively, and ν̄ is the partial specific volume of Hb (0.75 ml/g). The oncotic pressure due to albumin and other proteins is calculated as (15)
are the molal and molar concentrations of Hb (i.e., one-fourth of the total concentration of heme groups), respectively, and ν̄ is the partial specific volume of Hb (0.75 ml/g). The oncotic pressure due to albumin and other proteins is calculated as (15)
 |
where Cpr is the total protein concentration (in g/dl). Since we do not explicitly include the transport of plasma proteins in this study, Cpr is fixed at 6.8 g/dl (32).
Solute flux into tubules and vessels.
The transmural flux of solute k into tubule i is given by
 |
(A41) |
where Ψ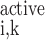 is the rate of active transport of solute k in tubule i (Eq. 1). That term is zero everywhere, except for Na+ in SAL and LAL.
is the rate of active transport of solute k in tubule i (Eq. 1). That term is zero everywhere, except for Na+ in SAL and LAL.
The transmural flux of solute k flowing from the interstitium into DVR plasma is given by
 |
(A42a) |
 |
(A42b) |
The AVR transmural solute fluxes are assumed to arise from solute advection passing through fenestrations and from solute diffusion through fenestrations, so that
 |
(A43a) |
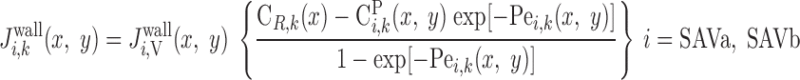 |
(A43b) |
where Pei,k ≡ J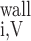 /Pi,k is the Péclet number for solute k associated with i, and Pi,k is the effective permeability of i to solute k.
/Pi,k is the Péclet number for solute k associated with i, and Pi,k is the effective permeability of i to solute k.
The flux of solute k from plasma into RBCs in vessel i is expressed as
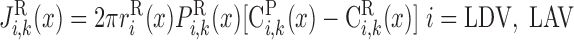 |
(A44a) |
 |
(A44b) |
The transmural flux of solute k from the interstitium into iRBCs is expressed as
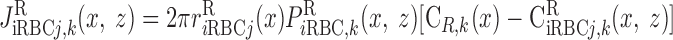 |
(A45) |
where R in CR,k is defined after Eq. A38.
Water Conservation Equation in the Concentric Regions
Water fluxes into regions.
The water flux from tubules and LDV into region R is calculated as
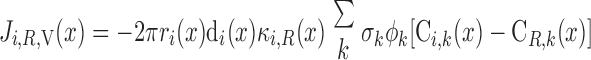 |
(A46) |
The composite water flux from SDV terminating at y > x is given by
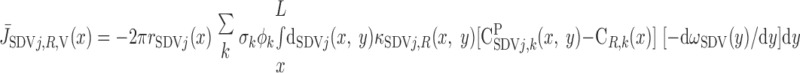 |
(A47) |
Water flux from iRBC tube j into region R is assumed to be osmotically driven and depends on the distance over which the iRBC tube traverses through region R. In R1, SDVa is assumed to terminate and give rise to iRBC1 and iRBC2 midway between the center and perimeter of R1 (Fig. 2). Thus
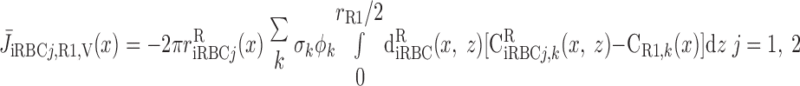 |
(A48) |
The iRBC3 and iRBC4 tubes, which arise from SDVb that is located in R2, contribute no water flux into R1. In R2, iRBC1 and iRBC2 traverse through the whole length, whereas SDVb terminates and gives rise to iRBC3 and iRBC4 midway between the perimeters of R1 and R2 (Fig. 2)
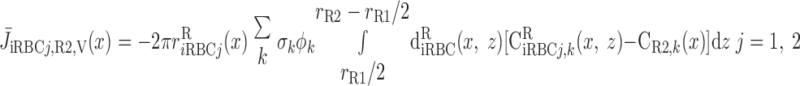 |
(A49a) |
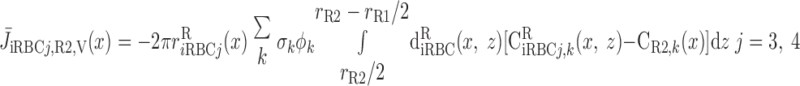 |
(A49b) |
In R3, iRBC1 and iRBC3 combine to form SAVa midway between R2 and R3, whereas iRBC2 and iRBC4 traverse through R3 into R4
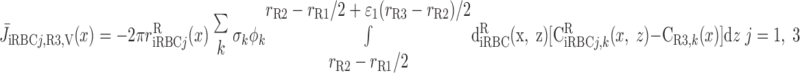 |
(A50a) |
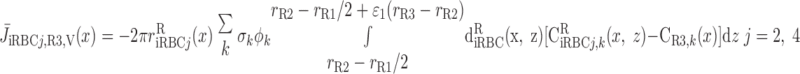 |
(A50b) |
In R4, iRBC2 and iRBC4 combine to form SAVb
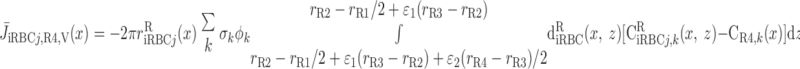 |
(A51) |
iRBC1 and iRBC3 have no contribution.
Interstitial water conservation equations.
Following the approach of Layton and Layton (33), fluid accumulation in each region Rj (j = 1–4) is calculated by solving the water conservation equation in that region
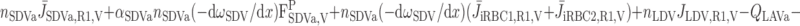 |
(A52a) |
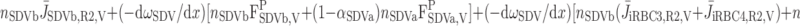 |
(A52b) |
 |
(A52c) |
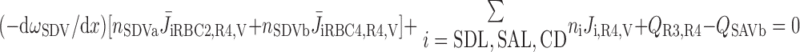 |
(A52d) |
In Eqs. A52a and A52b, the first term represents the composite water flux from SDVj terminating at y > x into R1 and R2, respectively. The second term represents the water flux from SDVj terminating at y = x (αSDVa denotes the fraction of the capillary plasma flow from terminating SDVa that is directed into R1, taken as 10%). These two terms are absent from Eqs. A46c and A46d, because there are no SDV in regions R3 and R4. The third term corresponds to the water flux from iRBCs formed by the terminating SDVj into region R. The fourth term is the total water flux from the vessel or limb i into R (i = LDL, LAL, SDL, SAL, and CD). The remaining terms represent the disposition of fluid accumulation by means of fluid entry into AVR and by interregional flow; QR,R′ represents the interregional flow from region R to R′. A fraction βLAVa (taken as 95%) of the net fluid accumulation in region R1 is taken up by LAVa through its fenestrations, and the remainder enters R2 as capillary flow. Similarly, a fraction βLAVb (taken as 5%) of the net fluid accumulation in R2 is taken up by LAVb, and the remainder enters R3. A fraction βSAVa (taken as 80%) of the net fluid accumulation R3 is taken up by SAVa, and the remainder enters R4. Hence
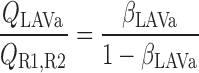 |
(A53a) |
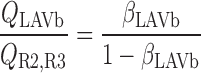 |
(A53b) |
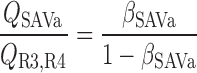 |
(A53c) |
Solute Conservation Equation in the Concentric Regions
Solute fluxes into regions.
The solute flux from tubule i into region R is given by
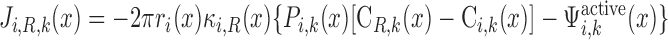 |
(A54) |
The solute flux from LDV into region R is given by
 |
(A55) |
For SDV terminating at y > x and R = R1 and R2, we have
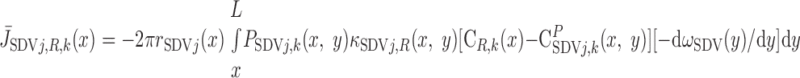 |
(A56) |
For SAV originating at y > x and R = R3 and R4, we have
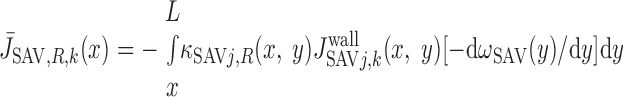 |
(A57) |
The solute flux from iRBC tubes into region R depends on the region being considered. In R1
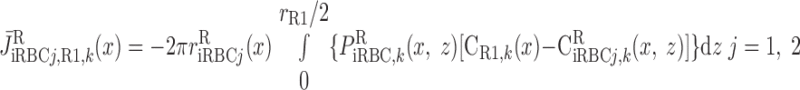 |
(A58) |
In R2
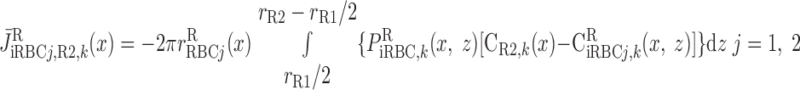 |
(A59a) |
 |
(A59b) |
In R3
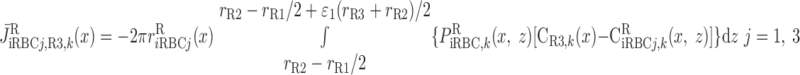 |
(A60a) |
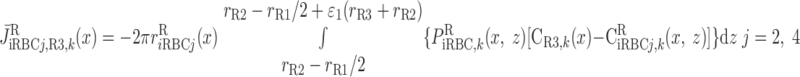 |
(A60b) |
In R4
 |
(A61) |
Interstitial solute conservation equation.
Interstitial concentrations in each region R can be obtained by solving the solute conservation equation in R
 |
(A62) |
The first term represents the diffusive solute into region R from adjacent regions R′. The second term is the sum of solute fluxes from tubules and long vasa recta into R. The third and fourth terms denote the composite solute fluxes at level x from all SDV and SAV, respectively, that are present in region R and that reach to medullary level y > x. The first term in the first pair of square brackets represents the solute flux from SDV terminating at level y = x into region R; the coefficients αSDVj,R are given by αSDVa,R1 = αSDVa, αSDVa,R2 = 1 − αSDVa, αSDVb,R1 = 0, αSDVb,R2 = 1, and αSDVj,R3 = αSDVj,R4 = 0 (j = a, b). The next term in the first pair of square brackets is the solute flux from iRBC tubes into R. The term CR′,kQR′,R − CR,k(QAVR + QR,R′) represents the solute that is carried by flow at the local concentration into AVR or into an adjoining region R′. The next term involving (1 − θcell) represents the fraction of the net amount of solute produced by endothelial and epithelial cells that is released into the interstitium (Eq. A2). The last two terms denote the consumption rates of solute k by interstitial cells in region R, by metabolic and scavenging reactions, respectively; ĀR is the area, per nephron, occupied by interstitial cells in region R.
We neglect interstitial diffusion in the axial direction, inasmuch as its effects on water flow and solute concentration are predicted to be small (34).
REFERENCES
- 1.Adair GS The hemoglobin system. VI. The oxygen dissociation curve of hemoglobin. J Biol Chem 63: 529–545, 1925. [Google Scholar]
- 2.Baxley P, Hellums J. A simple model for simulation of oxygen transport in the microcirculation. Ann Biomed Eng 11: 401–416, 1983. [DOI] [PubMed] [Google Scholar]
- 3.Brezis M, Dinour D, Greenfeld Z, Rosen S. Susceptibility of Henle's loop to hypoxic and toxic insults. Adv Nephrol Necker Hosp 20: 41–56, 1991. [PubMed] [Google Scholar]
- 4.Brezis M, Heyman SN, Epstein FH. Determinants of intrarenal oxygenation. II. Hemodynamic effects. Am J Physiol Renal Fluid Electrolyte Physiol 267: F1063–F1068, 1994. [DOI] [PubMed] [Google Scholar]
- 5.Brezis M, Rosen S, Silva P, Epstein F. Renal ischemia: a new perspective. Kidney Int 26: 375–383, 1984. [DOI] [PubMed] [Google Scholar]
- 6.Brezis M, Rosen S, Silva P, Epstein F. Selective vulnerability of the medullary thick ascending limb to anoxia in the isolated perfused rat kidney. J Clin Invest 73: 182–190, 1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Buerk D, Lamkin-Kennard K, Jaron D. Modeling the influence of superoxide dismutase on superoxide and nitric oxide interactions, including reversible inhibition of oxygen consumption. Free Radic Biol Med 34: 1488–1503, 2003. [DOI] [PubMed] [Google Scholar]
- 8.Chen J, Edwards A, Layton AT. A mathematical model of oxygen transport in the rat outer medulla. II. Impacts of outer medullary architecture. Am J Physiol Renal Physiol 297: F537–F548, 2009. [DOI] [PMC free article] [PubMed]
- 9.Clark A, Federspiel WJ, Clark PA, Cokelet GR. Oxygen delivery from red cells. Biophys J 47: 171–181, 1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cohen JJ Is the function of the renal papilla coupled exclusively to an anaerobic pattern of metabolism? Am J Physiol Renal Fluid Electrolyte Physiol 236: F423–F433, 1979. [DOI] [PubMed] [Google Scholar]
- 11.Cohen JJ, Kamm DE. Renal metabolism: relation to renal function. In: The Kidney (2nd ed.), edited by B. M. Brenner and F. C. Rector. Philadelphia: Saunders, 1981, p. 144–248.
- 12.Cowley AW, Mattson DL, Lu S, Roman RJ. The renal medulla and hypertension. Hypertension 25: 663–673, 1995. [DOI] [PubMed] [Google Scholar]
- 13.Dinour D, Brezis M. Effects of adenosine on intrarenal oxygenation. Am J Physiol Renal Fluid Electrolyte Physiol 261: F787–F791, 1991. [DOI] [PubMed] [Google Scholar]
- 14.dos Santos E, Li LP, Ji L, Prasad P. Early changes with diabetes in renal medullary hemodynamics as evaluated by fiberoptic probes and BOLD magnetic resonance imaging. Invest Radiol 42: 157–162, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Edwards A, Pallone TL. Facilitated transport in vasa recta: theoretical effects on solute exchange in the medullary microcirculation. Am J Physiol Renal Physiol 272: F505–F514, 1997. [DOI] [PubMed] [Google Scholar]
- 16.Epstein F Oxygen and renal metabolism. Kidney Int 51: 381–385, 1997. [DOI] [PubMed] [Google Scholar]
- 17.Epstein F, Balaban R, Ross B. Redox state of cytochrome aa3 in isolated perfused rat kidney. Am J Physiol Renal Fluid Electrolyte Physiol 243: F356–F363, 1982. [DOI] [PubMed] [Google Scholar]
- 18.Epstein F, Brezis M, Silva P, Rosen S. Physiological and clinical implications of medullary hypoxia. Artif Organs 11: 463–467, 1987. [DOI] [PubMed] [Google Scholar]
- 19.Federspiel WJ, Popel AS. A theoretical analysis of the effect of the particulate nature of blood on oxygen release in capillaries. Microvasc Res 32: 164–189, 1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Groebe K A versatile model of steady state O2 supply to tissue. Application to skeletal muscle. Biophys J 57: 485–498, 1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gullans S, Mandel L. Coupling of energy to transport in proximal and distal nephron. In: The Kidney: Physiology and Pathophysiology (3rd ed.), edited by D. Seldin and G. Giebisch. Philadelphia: Lippincott Williams & Wilkins, 2000, p. 443–482.
- 22.Hellums J, Nair P, Huang N, Ohshima N. Simulation of intraluminal gas transport processes in the microcirculation. Ann Biomed Eng 24: 1–24, 1996. [DOI] [PubMed] [Google Scholar]
- 23.Hellums JD The resistance to oxygen transport in the capillaries relative to that in the surrounding tissue. Microvasc Res 13: 131–136, 1977. [DOI] [PubMed] [Google Scholar]
- 24.Hu MC, Bankir L, Michelet S, Rousselet G, Trinh-Trang-Tan MM. Massive reduction of urea transporters in remnant kidney and brain of uremic rats. Kidney Int 58: 1202–1210, 2000. [DOI] [PubMed] [Google Scholar]
- 25.Klabunde RE Cardiovascular Physiology Concepts. Philadelphia: Lippincott Williams & Williams, 2005.
- 26.Knepper M, Danielson R, Saiderl G, Post R. Quantitative analysis of renal medullary anatomy in rats and rabbits. Kidney Int 12: 313–323, 1977. [DOI] [PubMed] [Google Scholar]
- 27.Kriz W Der architektonische and funktionelle Aufbau der Ratttenniere. Z Zellforsch 82: 495–535, 1967. [PubMed] [Google Scholar]
- 28.Kriz W Structural organization of the renal medulla: comparative and functional aspects. Am J Physiol Regul Integr Comp Physiol 241: R3–R16, 1981. [DOI] [PubMed] [Google Scholar]
- 29.Kriz W, Schnermann J, Koepsell H. The position of short and long loops of Henle in the rat kidney. Z Anat Entwicklungsgesch 138: 301–319, 1972. [DOI] [PubMed] [Google Scholar]
- 30.Krogh A The number and distribution of capillaries in muscles with calculations of the oxygen pressure head. J Physiol 52: 409–415, 1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kuwahira I, Gonzalez NC, Heisler N, Piiper J. Regional blood flow in conscious resting rats determined by microsphere distribution. J Appl Physiol 74: 203–210, 1993. [DOI] [PubMed] [Google Scholar]
- 32.Layton A Role of UTB urea transporters in the urine concentrating mechanism of the rat kidney. Bull Math Biol 67: 887–929, 2007. [DOI] [PubMed] [Google Scholar]
- 33.Layton A, Layton H. A region-based mathematical model of the urine concentrating mechanism in the rat outer medulla. I. Formulation and base-case results. Am J Physiol Renal Physiol 289: F1346–F1366, 2005. [DOI] [PubMed] [Google Scholar]
- 34.Layton AT, Layton HE. A region-based mathematical model of the urine concentrating mechanism in the rat outer medulla. II. Parameter sensitivity and tubular inhomogeneity. Am J Physiol Renal Physiol 289: F1367–F1381, 2005. [DOI] [PubMed] [Google Scholar]
- 35.Layton HE, Pitman EB, Moore LC. Bifurcation analysis of TGF-mediated oscillations in SNGFR. Am J Physiol Renal Fluid Electrolyte Physiol 261: F904–F919, 1991. [DOI] [PubMed] [Google Scholar]
- 36.Lemley K, Kriz W. Anatomy of the renal interstitium. Kidney Int 39: 370–381, 1991. [DOI] [PubMed] [Google Scholar]
- 37.Liss P, Nygren A, Erikson U, Ulfendahl HR. Injection of low and iso-osmolar contrast medium decreases oxygen tension in the renal medulla. Kidney Int 53: 698–702, 1998. [DOI] [PubMed] [Google Scholar]
- 38.Mandel LJ, Balaban RS. Stoichiometry and coupling of active transport to oxidative metabolism in epithelial tissues. Am J Physiol Renal Fluid Electrolyte Physiol 240: F357–F371, 1981. [DOI] [PubMed] [Google Scholar]
- 39.Moll W The influence of hemoglobin diffusion on oxygen uptake and release by red cells. Respir Physiol 6: 1–15, 1968. [DOI] [PubMed] [Google Scholar]
- 40.Neuhofer W, Beck FX. Cell survival in the hostile environment of the renal medulla. Annu Rev Physiol 67: 531–555, 2005. [DOI] [PubMed] [Google Scholar]
- 41.Osvaldo L, Latta H. The thin limbs of the loop of Henle. J Ultrastruct Res 15: 144–168, 1966. [DOI] [PubMed] [Google Scholar]
- 42.Palm F, Friederich M, Carlsson PO, Hansell P, Teerlink T, Liss P. Reduced nitric oxide in diabetic kidneys due to increased hepatic arginine metabolism: implications for renomedullary oxygen availability. Am J Physiol Renal Physiol 294: F30–F37, 2008. [DOI] [PubMed] [Google Scholar]
- 43.Pannabecker TL, Dantzler WH. Three-dimensional architecture of inner medullary vasa recta. Am J Physiol Renal Physiol 290: F1355–F1366, 2006. [DOI] [PubMed] [Google Scholar]
- 44.Pannabecker TL, Dantzler WH. Three-dimensional lateral and vertical relationships of inner medullary loops of Henle and collecting ducts. Am J Physiol Renal Physiol 287: F767–F774, 2004. [DOI] [PubMed] [Google Scholar]
- 45.Pfaller W Structure Function Correlation on Rat Kidney: Quantitative Correlation of Structure and Function in the Normal and Injured Rat Kidney. New York: Springer-Verlag, 1982. [PubMed]
- 46.Popel AS Theory of oxygen transport to tissue. Crit Rev Biomed Eng 17: 257–321, 1989. [PMC free article] [PubMed] [Google Scholar]
- 47.Rasmussen SN Red cell and plasma volume flows to the inner medulla of the rat kidney. Determinations by means of a step function input indicator technique. Pflügers Arch 373: 153–159, 1978. [DOI] [PubMed] [Google Scholar]
- 48.Reeves WB, Andreoli TE. Sodium chloride transport in the loop of Henle, distal convoluted tubule, and collecting duct. In: The Kidney (4th ed.), edited by R. J. Alpern and S. C. Hebert. Philadelphia: Lippincott Williams & Wilkins, 2008, p. 849–889.
- 49.Sands J, Layton H. concentrating mechanism and its regulation. In: The Kidney: Physiology and Pathophysiology (3rd ed.), edited by D. Seldin and G. Giebisch. Philadelphia: Lippincott Williams & Wilkins, 2000, p. 1175–1216.
- 50.Schurek HJ, Johns O. Is tubuloglomerular feedback a tool to prevent nephron oxygen deficiency? Kidney Int 51: 386–392, 1997. [DOI] [PubMed] [Google Scholar]
- 51.Sidel VW, Solomon AK. Entrance of water into human red cells under an osmotic gradient. J Gen Physiol 41: 243–257, 1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Thomas SR Inner medullary lactate production and accumulation: a vasa recta model. Am J Physiol Renal Physiol 279: F468–F481, 2000. [DOI] [PubMed] [Google Scholar]
- 53.Uchida S, Endou H. Substrate specificity to maintain cellular ATP along the mouse nephron. Am J Physiol Renal Fluid Electrolyte Physiol 255: F977–F983, 1988. [DOI] [PubMed] [Google Scholar]
- 54.Vandewalle A, Wirthensohn G, Heidrich HG, Guder WG. Distribution of hexokinase and phosphoenolpyruvate carboxykinase along the rabbit nephron. Am J Physiol Renal Fluid Electrolyte Physiol 240: F492–F500, 1981. [DOI] [PubMed] [Google Scholar]
- 55.Weinstein AM A mathematical model of the outer medullary collecting duct of the rat. Am J Physiol Renal Physiol 279: F24–F45, 2000. [DOI] [PubMed] [Google Scholar]
- 56.Wexler A, Kalaba R, Marsh D. Three-dimensional anatomy and renal concentrating mechanism. Am J Physiol Renal Fluid Electrolyte Physiol 260: F369–F383, 1991. [DOI] [PubMed] [Google Scholar]
- 57.Zhang W, Edwards A. Oxygen transport across vasa recta in the renal medulla. Am J Physiol Heart Circ Physiol 283: H1042–H1055, 2002. [DOI] [PubMed] [Google Scholar]