Abstract
we extended the region-based mathematical model of the urine-concentrating mechanism in the rat outer medulla (OM) developed by Layton and Layton (Am J Physiol Renal Physiol 289: F1346–F1366, 2005) to examine the impact of the complex structural organization of the OM on O2 transport and distribution. In the present study, we investigated the sensitivity of predicted Po2 profiles to several parameters that characterize the degree of OM regionalization, boundary conditions, structural dimensions, transmural transport properties, and relative positions and distributions of tubules and vessels. Our results suggest that the fraction of O2 supplied to descending vasa recta (DVR) that reaches the inner medulla, i.e., a measure of the axial Po2 gradient in the OM, is insensitive to parameter variations as a result of the sequestration of long DVR in the vascular bundles. In contrast, O2 distribution among the regions surrounding the vascular core strongly depends on the radial positions of medullary thick ascending limbs (mTALs) relative to the vascular core, the degree of regionalization, and the distribution of short DVR along the corticomedullary axis. Moreover, if it is assumed that the mTAL active Na+ transport rate decreases when mTAL Po2 falls below a critical level, O2 availability to mTALs has a significant impact on the concentrating capability of the model OM. The model also predicts that when the OM undergoes hypertrophy, its concentrating capability increases significantly only when anaerobic metabolism supports a substantial fraction of the mTAL active Na+ transport and is otherwise critically reduced by low interstitial and mTAL luminal Po2 in a hypertrophied OM.
Keywords: region-based model, medullary thick ascending limbs, red blood cells, active sodium transport
in a companion paper (7), we formulated a region-based mathematical model of O2 transport in the outer medulla (OM) of the rat kidney. In that model, the complex structural organization of the OM (13, 14) was represented by four concentric regions, centered around a vascular bundle, following the region-based approach of Layton and Layton (15). The radial positions of structures were incorporated by assignment of appropriate tubules and vasa recta (or fractions thereof) to each concentric region. Of particular importance is the prediction that regionalization has a significant impact on radial distribution of O2 and, therefore, the active Na+ transport rate of the medullary thick ascending limbs (mTALs).
In the present study, we investigate model sensitivity to fundamental structural assumptions and to parameters that are not well characterized. The model incorporates a large number of parameters that characterize regionalization, boundary conditions, structural dimensions, transmural transport properties, and relative positions and distributions of tubules and vessels. Most of these parameters were based on, or estimated from, the experimental literature. However, considerable uncertainty remains in the specification of appropriate values for some other parameters. In the companion study (7), we made assumptions that appeared reasonable to us; in the present study, we examine how variations in these parameters affect model results.
Given the radial organization of tubules and vessels in the OM, we also assess, in the context of O2 transport, the role of diffusion, capillary transport, basal O2 consumption, and active Na+ transport-related O2 consumption. We also study O2 distribution under special conditions, in which an appropriate stimulus is applied to induce hyperfiltration, hypertrophy in the OM inner stripe, and increased Na+ transport rates in the mTAL (3, 5, 12, 17, 22, 23), and we study the synergy between these factors and the impact of these factors on the concentrating capability of the hypertrophied OM.
MODEL DESCRIPTION
The O2 transport model used in the present study and in the companion study (7) was obtained by extending the region-based model of the urine-concentrating mechanism of the rat OM developed by Layton and Layton (15). The region-based formulation represents the structural organization of the OM by means of four concentric regions centered on a vascular bundle: an innermost region containing the central vascular bundle (R1), a peripheral region of the vascular bundle (R2), a region near the vascular bundle (R3), and the region most distant from the vascular bundle (R4). The radial organization of tubules and vasa recta with respect to vascular bundles is represented by specification of the fractions of the tubules and vasa recta assigned to each concentric region at each medullary level (see Fig. 1 in Ref. 7).
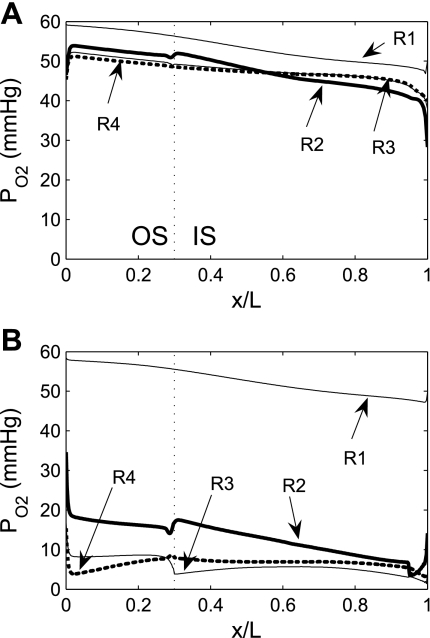
Fig. 1.
Interstitial Po2 in the 4 concentric regions in the absence of outer medulla (OM) O2 consumption (A) and in the base case (B). Vertical dashed lines, boundary between outer stripe (OS) and inner stripe (IS); x/L, ratio of axial coordinate to total length of OM.
The region-based model was originally formulated for Na+ and urea only (15). The present model incorporates RBCs, Hb, and O2. As described in detail in the companion study (7), fluid flow and solute concentrations are determined by solving conservation equations in each tubule, vas rectum, and interstitial region. Boundary conditions prescribe flows and concentrations in tubules and vessels at the corticomedullary junction and the OM-inner medulla (IM) boundary. Acronyms and symbols are given in the Glossary in the companion study (7).
RESULTS
We conducted simulations to study the individual effects of O2 transport and consumption processes; to assess model sensitivity to variations in selected parameters; to examine the effects of OM structural organization on O2 distribution; and to investigate the synergy between OM hypertrophy, increased single nephron glomerular filtration rate (SNGFR), increased mTAL active Na+ transport, and O2 distribution and the overall effects on the OM urine-concentrating mechanism. Model results summarized in Table 1 show the fractional O2 delivery to the IM, which is a measure of the axial gradient of Po2, and the interstitial Po2 difference between the core of the vascular bundle (R1) and the outermost region (R4) at the OM-IM boundary, which is a measure of the radial Po2 gradient.
Table 1.
Fraction of O2 supplied at the corticomedullary junction that reaches the IM
| ftotal,IM, % | fLDV,IM, % | fLDL,IM, % | fCD,IM, % |
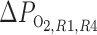 , mmHg , mmHg |
|
|---|---|---|---|---|---|
| Baseline* | 19.5 | 92.4 | 5.5 | 4.0 | 45.7 |
| No O2 consumption in OM | 20.1 | 94.1 | 50.3 | 45.6 | 10.2 |
| No basal O2 consumption in OM | 19.6 | 92.7 | 5.5 | 4.2 | 46.0 |
| No active transport-related O2 consumption in OM | 20.0 | 93.7 | 48.0 | 43.1 | 11.8 |
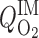 = 0.1 = 0.1 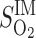
|
17.3 | 81.8 | 3.7 | 3.9 | 36.1 |
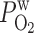 = 0.02 cm/s = 0.02 cm/s |
19.9 | 94.2 | 4.7 | 6.4 | 46.2 |
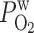 = 0.06 cm/s = 0.06 cm/s |
19.4 | 91.7 | 6.1 | 2.7 | 46.1 |
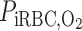 × 2 × 2 |
19.5 | 92.4 | 7.7 | 4.5 | 45.2 |
| Po2 = 70 mmHg in DVR at x = 0 | 20.2 | 95.8 | 6.0 | 4.0 | 50.4 |
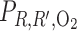 × 10 × 10 |
18.7 | 88.4 | 5.5 | 4.2 | 40.4 |
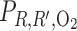 × 100 × 100 |
15.4 | 73.1 | 5.5 | 4.8 | 21.2 |
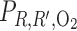 × 1,000 × 1,000 |
11.2 | 53.0 | 5.0 | 4.2 | 3.5 |
| All LAL in R4 | 19.6 | 92.7 | 7.1 | 10.7 | 39.9 |
| All SAL in R2 | 19.5 | 92.2 | 10.7 | 11.5 | 42.2 |
| All SAL and LAL in R2 | 19.9 | 93.9 | 17.1 | 16.0 | 43.4 |
| SDVa/SDVb = 20:80 | 20.0 | 94.6 | 6.1 | 4.5 | 47.6 |
| SDVa/SDVb = 50:50 | 20.3 | 96.1 | 7.1 | 5.3 | 48.7 |
| SDVa/SAVb = 80:20 | 20.5 | 96.9 | 8.1 | 6.3 | 49.0 |
ftotal,IM, Fraction of total O2 + HbO2 supplied at corticomedullary junction that reaches inner medulla (IM); fLDV,IM, fLDL,IM, and fCD,IM, fraction of O2 + HbO2 supplied to long descending vas rectum; long descending limb and collective duct, respectively at the corticomedullary junction that reaches the IM; 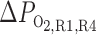 , R1–R4
, R1–R4 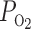 difference at the outer medulla-IM boundary.
difference at the outer medulla-IM boundary.
Baseline case corresponds to the following parameter values: 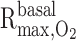 = 10 μM/s, TNa/
= 10 μM/s, TNa/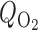 =24,
=24, 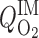 = (0.05)
= (0.05) 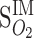 ,
, 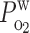 = 0.04 cm/s, Hct = 35%, Po2 = 60 mmHg in descending vas rectum at x = 0, SDVa/SDVb = 1:99.
= 0.04 cm/s, Hct = 35%, Po2 = 60 mmHg in descending vas rectum at x = 0, SDVa/SDVb = 1:99.
Isolated Effects of O2 Transport and Consumption Processes
Several factors contribute to O2 distribution in the OM: passive diffusion and capillary flows, which facilitate O2 exchanges between tubules and vessels in different radial positions, and O2 consumption due to basal and active transport-related metabolism. To assess the individual effects of each of these processes, we considered three scenarios in which one or both of the consumption processes are eliminated.
Elimination of OM O2 consumption.
We first simulated O2 transport in the OM in the absence of O2 consumption in the OM for metabolic and active transport purposes [i.e., we set the rates of O2 consumption for basal and active transport processes (R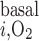 and R
and R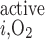 , respectively) to 0]. The total consumption of O2 in the IM was taken as 5% of the overall supply of O2 to the OM, as in the base case (7).
, respectively) to 0]. The total consumption of O2 in the IM was taken as 5% of the overall supply of O2 to the OM, as in the base case (7).
Interstitial Po2 profiles in the four regions in the absence of O2 consumption are shown in Fig. 1A; the corresponding base-case Po2 profiles, in which basal and active transport-related O2 consumption are included, are shown in Fig. 1B. Interstitial Po2 profiles obtained for the no-consumption case exhibit strictly decreasing gradients along the corticomedullary axis; these gradients are generated by IM O2 consumption, which results in relatively O2-poor fluid returned via the long ascending limbs (LALs) and the long ascending vasa recta (LAV). The luminal Po2 of tubules and vessels closely tracks local interstitial Po2: Po2 in descending fluid slightly exceeds, and Po2 in ascending fluid slightly lags behind, that of the local interstitium (results not shown).
The model predicts that elimination of OM O2 consumption has negligible effects on the R1 interstitial Po2, which decreases from 59.3 mmHg at the corticomedullary boundary to 50.1 mmHg at the OM-IM boundary; these values correspond to <4% differences from the base-case values of 58.2 and 48.3 mmHg at the same junctions. The effects of consumption are much greater in the three outer regions: in the no-OM consumption case, predicted interstitial Po2 values in these regions are more than double those in the base case. The vastly different effects of O2 consumption in the vascular core and in the outer regions can be attributed to the relatively small basal O2 consumption in R1 and the absence of active transport processes in that same region. The differences in interstitial Po2 among the three outer regions are substantially smaller because of the greater interregional fluxes, which are proportional to the radius of these regions.
Although the model predicts that elimination of OM O2 consumption reduces R1-R2 interstitial Po2 differences by about two-thirds, significant radial interstitial Po2 gradients remain (Fig. 1A). Throughout the OM, interstitial Po2 in R1, where all the O2-rich long descending vasa recta (LDV) are located, is ≥4.1 mmHg greater than interstitial Po2 in R2. The isolation of LDV within the vascular bundle core, where much O2 is sequestered, serves to preserve O2 supply to the IM. The fraction of O2 supplied to LDV at the corticomedullary junction that reaches the IM is predicted to be 94.1%, which corresponds to a 1.8% increase over the base case. (However, in large part because the LDV-to-SDV number ratio is 12:44, only 20.1% of the total amount of O2 supplied to the OM reaches the IM.) O2 reabsorption is significantly higher along descending tubules located in the outer regions (R2–R4): the fraction of O2 supplied to long descending limbs (LDLs) and collecting ducts (CDs) at the corticomedullary junction that reaches the IM is calculated as 50.3% and 45.6%, respectively, in the absence of O2 consumption in the OM (Table 1).
The following calculations also illustrate the effects of seclusion of the descending vasa recta (DVR) located in R1. As previously noted, 97% of the O2 supplied by DVR is bound to HbO2 at the corticomedullary junction. As blood flows toward the IM along DVR, some O2 dissociates from HbO2 and, subsequently, diffuses across RBCs into plasma and then into the interstitium. In the absence of O2 consumption in the OM, our calculations indicate that only 5.6% of the O2 that enters LDV (all of which are in R1) at the corticomedullary junction bound to Hb has dissociated at the OM-IM boundary; in contrast, 10.5% of the O2 that enters the longest SDVb (all of which are in R2) bound to Hb has dissociated at the termination of the vessel. The difference in the amount of HbO2 that dissociates along LDV, on one hand, and the SDVb on the other hand, provides evidence that the localization of LDV within the tightly packed vascular bundles preserves O2 supply to the IM.
Basal O2 consumption.
We then examined the impact of basal O2 consumption on medullary Po2 profiles. We included basal O2 consumption as described in the companion study (7), but we did not include active transport-related O2 consumption (i.e., R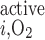 = 0). The maximum volumetric rate of O2 consumption (R
= 0). The maximum volumetric rate of O2 consumption (R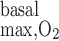 ) was taken to be the same for OM endothelial, epithelial, and interstitial cells, in the absence of specific data. Reported values of R
) was taken to be the same for OM endothelial, epithelial, and interstitial cells, in the absence of specific data. Reported values of R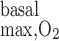 in the arterial wall range from 130 nl O2·s−1·cm tissue−3 (24), or 5.4 μM/s (assuming 755 ml O2/g), to 3,000 nl O2·s−1·cm tissue−3 (9), or 124 μM/s. Measurements in the mesentery vary between 8 and 240 nl O2·s−1·cm tissue−3 (9), or between 0.3 and 10 μM/s. A value of 116 μM/s was reported for the rat liver (20). For the base case in this study, we chose an intermediate R
in the arterial wall range from 130 nl O2·s−1·cm tissue−3 (24), or 5.4 μM/s (assuming 755 ml O2/g), to 3,000 nl O2·s−1·cm tissue−3 (9), or 124 μM/s. Measurements in the mesentery vary between 8 and 240 nl O2·s−1·cm tissue−3 (9), or between 0.3 and 10 μM/s. A value of 116 μM/s was reported for the rat liver (20). For the base case in this study, we chose an intermediate R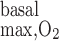 value of 10 μM/s. We also performed simulations assuming higher values, that is, 25 and 50 μM/s.
value of 10 μM/s. We also performed simulations assuming higher values, that is, 25 and 50 μM/s.
Interstitial Po2 profiles in the four regions, in the absence of active transport-related O2 consumption, are exhibited in Fig. 2, A, B, and C, which correspond to R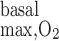 = 10, 25, and 50 μM/s, respectively. The model predicts that, relative to the no-consumption case, basal O2 consumption reduces Po2 much more significantly in the three outer regions than in R1. With the base-case value of 10 μM/s, Po2 at the mid-inner stripe is 0.6 mmHg lower in R1, 2.0 mmHg lower in R2, 2.4 mmHg lower in R3, and 2.8 mmHg lower in R4. The small effect of basal O2 consumption on R1 Po2 profiles can be attributed to the model's assumption that R1 represents a tightly packed vascular bundle, the interstitial area of which is significantly smaller than that in other regions (see Fig. 7 in Ref. 7). As shown in Table 3 in the companion study (7), basal O2 consumption is at least one order of magnitude lower in R1 (0.003121 pmol·min−1·nephron−1) than in the other regions (from 0.03219 pmol·min−1·nephron−1 in R2 to 0.05676 pmol·min−1·nephron−1 in R3).
= 10, 25, and 50 μM/s, respectively. The model predicts that, relative to the no-consumption case, basal O2 consumption reduces Po2 much more significantly in the three outer regions than in R1. With the base-case value of 10 μM/s, Po2 at the mid-inner stripe is 0.6 mmHg lower in R1, 2.0 mmHg lower in R2, 2.4 mmHg lower in R3, and 2.8 mmHg lower in R4. The small effect of basal O2 consumption on R1 Po2 profiles can be attributed to the model's assumption that R1 represents a tightly packed vascular bundle, the interstitial area of which is significantly smaller than that in other regions (see Fig. 7 in Ref. 7). As shown in Table 3 in the companion study (7), basal O2 consumption is at least one order of magnitude lower in R1 (0.003121 pmol·min−1·nephron−1) than in the other regions (from 0.03219 pmol·min−1·nephron−1 in R2 to 0.05676 pmol·min−1·nephron−1 in R3).
Fig. 2.
Po2 in the 4 concentric regions, including basal O2 consumption by interstitial, endothelial, and epithelial cells, but not Na+ active transport-related O2 consumption. Maximal rate of basal consumption (R 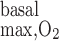 ) is varied between 10 (A), 25 (B), and 50 μM/s (C). Compared with the no-OM consumption case (Fig. 1A), Po2 is generally lower in outer regions.
) is varied between 10 (A), 25 (B), and 50 μM/s (C). Compared with the no-OM consumption case (Fig. 1A), Po2 is generally lower in outer regions.
For these same reasons, increasing R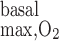 from 10 to 25 or 50 μM/s significantly lowers interstitial Po2 profiles in R2, R3, and R4, but not in R1 (Fig. 2). With R
from 10 to 25 or 50 μM/s significantly lowers interstitial Po2 profiles in R2, R3, and R4, but not in R1 (Fig. 2). With R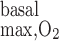 = 25 and 50 μM/s, Po2 at the mid-inner stripe is ∼3 and 7 mmHg lower, respectively, in R2–R4 than in the no-consumption case.
= 25 and 50 μM/s, Po2 at the mid-inner stripe is ∼3 and 7 mmHg lower, respectively, in R2–R4 than in the no-consumption case.
Variations in R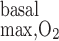 have less impact on OM Po2 profiles when basal and active transport-related O2 consumption are considered. With both included, interstitial Po2 in R2 at the mid-inner stripe is predicted to be 10.8 mmHg with R
have less impact on OM Po2 profiles when basal and active transport-related O2 consumption are considered. With both included, interstitial Po2 in R2 at the mid-inner stripe is predicted to be 10.8 mmHg with R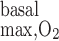 = 10 μM/s (i.e., the base case) and 10.7 and 10.4 mmHg with R
= 10 μM/s (i.e., the base case) and 10.7 and 10.4 mmHg with R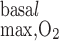 = 25 and 50 μM/s, respectively (results not shown). In parallel, CD osmolality at the OM-IM boundary is predicted to decrease from 580 to 563 and 548 mosmol/kgH2O as R
= 25 and 50 μM/s, respectively (results not shown). In parallel, CD osmolality at the OM-IM boundary is predicted to decrease from 580 to 563 and 548 mosmol/kgH2O as R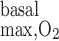 increases from 10 to 25 and 50 μM/s, respectively.
increases from 10 to 25 and 50 μM/s, respectively.
Active transport-related O2 consumption.
Given that basal metabolism is predicted to represent 3% of overall O2 consumption, as described in the companion study (7), O2 consumption for active Na+ reabsorption has a much greater impact on radial Po2 gradients than basal cell metabolism. To examine the isolated effect of active transport on O2 distribution, we conducted simulations with active Na+ transport-related O2 consumption but without basal consumption (i.e., R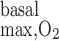 = 0). As previously noted, the active transport rate is characterized by Michaelis-Menten kinetics (see Eq. 1 in Ref. 7), where the maximum transport rate (Vmax,i,Na) decreases when mTAL luminal Po2 drops below 1 mmHg. The moles of Na+ reabsorbed per mole of O2 consumed [the baseline TNa-to-Q
= 0). As previously noted, the active transport rate is characterized by Michaelis-Menten kinetics (see Eq. 1 in Ref. 7), where the maximum transport rate (Vmax,i,Na) decreases when mTAL luminal Po2 drops below 1 mmHg. The moles of Na+ reabsorbed per mole of O2 consumed [the baseline TNa-to-Q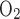 ratio (TNa/Q
ratio (TNa/Q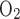 )] is set to 24 in the base case.
)] is set to 24 in the base case.
The model assumes that LALs straddle the boundary of R2 and R3 and that short ascending limbs (SALs) lie between R3 and R4. Hence, metabolic needs are greatest in R3, where O2 supply is limited (there are no DVR in R3; see also Fig. 1 in Ref. 7). Because mTAL tubular Na+ concentration is largest near the OM-IM boundary and because Vmax,i,Na is higher in the inner stripe when O2 supply is abundant, O2 consumption is generally higher in the inner stripe than in the outer stripe.
Figure 3A shows Po2 profiles in the four regions obtained with active O2 consumption only, with the assumption that TNa/Q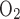 = 24. The profiles show a generally decreasing trend along the corticomedullary axis. Our results suggest that active O2 consumption lowers Po2 by 11–46 mmHg in R2, R3, and R4 but has negligible effects in R1 (compare with Fig. 1A). At the mid-inner stripe, Po2 is 34.6 mmHg lower in R2, 41.1 mmHg lower in R3, and 39.5 mmHg lower in R4 than in the no-consumption case and 32.6 mmHg lower in R2, 38.6 mmHg lower in R3, and 36.7 mmHg lower in R4 than in the basal-consumption-only case.
= 24. The profiles show a generally decreasing trend along the corticomedullary axis. Our results suggest that active O2 consumption lowers Po2 by 11–46 mmHg in R2, R3, and R4 but has negligible effects in R1 (compare with Fig. 1A). At the mid-inner stripe, Po2 is 34.6 mmHg lower in R2, 41.1 mmHg lower in R3, and 39.5 mmHg lower in R4 than in the no-consumption case and 32.6 mmHg lower in R2, 38.6 mmHg lower in R3, and 36.7 mmHg lower in R4 than in the basal-consumption-only case.
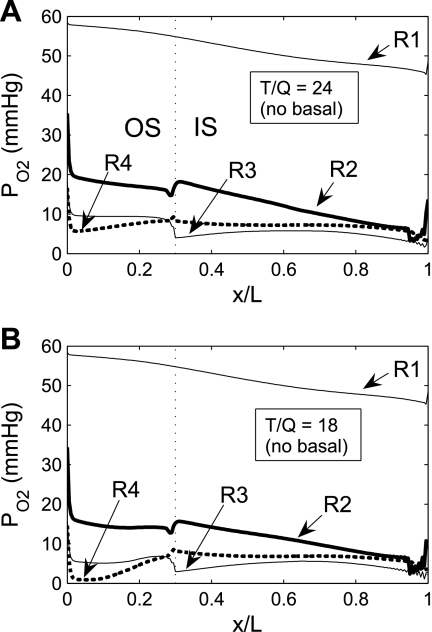
Fig. 3.
Po2 in the 4 concentric regions, including Na+ active transport-related O2 consumption, without basal O2 consumption at TNa-to-Q
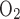 ratio [i.e., moles of Na+ reabsorbed per mole of O2 consumed (T/Q)] of 24 (A) and 18 (B). At T/Q = 18, results with and without basal O2 consumption are indistinguishable.
ratio [i.e., moles of Na+ reabsorbed per mole of O2 consumed (T/Q)] of 24 (A) and 18 (B). At T/Q = 18, results with and without basal O2 consumption are indistinguishable.
We then examined the effect of variations in TNa/Q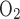 . As described in the companion study (7), the baseline value of this ratio, taken as 24, was chosen to account for paracellular reabsorption. If there were no net Na+ reabsorption through the paracellular pathway, TNa/Q
. As described in the companion study (7), the baseline value of this ratio, taken as 24, was chosen to account for paracellular reabsorption. If there were no net Na+ reabsorption through the paracellular pathway, TNa/Q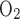 would be 18 under conditions of maximal efficiency in mitochondrial ATP formation. At a lower TNa/Q
would be 18 under conditions of maximal efficiency in mitochondrial ATP formation. At a lower TNa/Q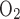 of 18, the model predicts very low Po2 in R3 (Fig. 3B). With or without basal O2 consumption, mTAL Po2 remains below 10 mmHg just below the corticomedullary junction and below 0.9 mmHg throughout the inner stripe. This is hardly surprising, since the total amount of O2 consumed for active NaCl reabsorption (Q
of 18, the model predicts very low Po2 in R3 (Fig. 3B). With or without basal O2 consumption, mTAL Po2 remains below 10 mmHg just below the corticomedullary junction and below 0.9 mmHg throughout the inner stripe. This is hardly surprising, since the total amount of O2 consumed for active NaCl reabsorption (Q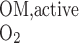 ) when TNa/Q
) when TNa/Q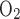 = 18 is estimated as 5.3 × 10−13 mol O2·s−1·nephron−1 if Vmax,i,Na depends linearly on Po2 below the critical value of 1 mmHg (our baseline assumption). This estimate is, in fact, greater than the amount of O2 supplied to the short DVR, that is, 5.1 × 10−13 mol·s−1·nephron−1.
= 18 is estimated as 5.3 × 10−13 mol O2·s−1·nephron−1 if Vmax,i,Na depends linearly on Po2 below the critical value of 1 mmHg (our baseline assumption). This estimate is, in fact, greater than the amount of O2 supplied to the short DVR, that is, 5.1 × 10−13 mol·s−1·nephron−1.
If it is assumed that TNa/Q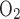 = 18, the model also predicts that the low Po2 in the outer regions significantly reduces active NaCl reabsorption. As a result, CD osmolality is predicted to be 497 mosmol/kgH2O at the OM-IM boundary (vs. 580 mosmol/kgH2O in the base case), which is only a 1.6-fold increase over the inflow osmolality (309 mosmol/kgH2O). Thus it appears that with TNa/Q
= 18, the model also predicts that the low Po2 in the outer regions significantly reduces active NaCl reabsorption. As a result, CD osmolality is predicted to be 497 mosmol/kgH2O at the OM-IM boundary (vs. 580 mosmol/kgH2O in the base case), which is only a 1.6-fold increase over the inflow osmolality (309 mosmol/kgH2O). Thus it appears that with TNa/Q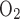 = 18, aerobic metabolism in the OM is unable to sustain near-optimal mTAL active Na+ transport; as a result, the OM concentrating capability is substantially reduced.
= 18, aerobic metabolism in the OM is unable to sustain near-optimal mTAL active Na+ transport; as a result, the OM concentrating capability is substantially reduced.
Active transport along descending limbs.
In the base case, we assumed that active NaCl transport occurs only along mTAL and the prebend segment. Na+-K+-ATPase activity along the proximal tubule and descending limb has been reported by Garg et al. (8). In the next set of simulations, we investigated the effects of incorporating active NaCl transport along these tubules. The maximum rate of Na+ transport (i.e., V*max,i,Na in Eq. 2 in Ref. 7) was taken as 0.43 nmol·cm−2·s−1 in the short descending limbs (SDLs) and LDLs and 2.1 nmol·cm−2·s−1 in the proximal straight tubule (15) vs. 10.5 nmol·cm−2·s−1 in mTALs in the outer stripe and 25.9 nmol·cm−2·s−1 in mTALs in the inner stripe.
Given that V*max,i,Na is 5–50 times lower in SDLs and LDLs than in mTALs, our results suggest that active NaCl reabsorption along descending limbs has a negligible effect on Po2 profiles. The model predicts that luminal Po2 in SDLs and LDLs is 6.2 and 2.0 mmHg lower, respectively, at the mid-outer stripe and 0.7 and 5.6 mmHg lower, respectively, at the mid-inner stripe than in the base case. Since SDLs and LDLs straddle R3 and R4 in the outer stripe and move toward the periphery of the vascular bundle (i.e., R2) in the inner stripe, active transport in the descending limbs does not significantly affect Po2 in R1 (results not shown).
Parameter Sensitivity Studies
Variations in transepithelial and transendothelial O2 permeabilities.
The wall permeability of vasa recta and tubules to O2 (P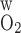 ) has not been measured experimentally, to the best of our knowledge; our baseline estimate (0.04 cm/s) is based on measurements in other capillaries (for review see Ref. 26). To assess the effects of variations in O2 permeabilities on Po2 profiles, we conducted simulations in which P
) has not been measured experimentally, to the best of our knowledge; our baseline estimate (0.04 cm/s) is based on measurements in other capillaries (for review see Ref. 26). To assess the effects of variations in O2 permeabilities on Po2 profiles, we conducted simulations in which P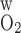 was reduced to 0.02 cm/s or increased to 0.06 cm/s. As expected, an increase in P
was reduced to 0.02 cm/s or increased to 0.06 cm/s. As expected, an increase in P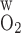 reduces transepithelial and transendothelial O2 concentration gradients and, consequently, among the tubules and vessels within each region. Increasing P
reduces transepithelial and transendothelial O2 concentration gradients and, consequently, among the tubules and vessels within each region. Increasing P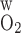 from 0.04 to 0.06 cm/s reduces the Po2 difference between SDVa and LAVa, which are located in R1, from 2.7 to 1.8 mmHg at the OM midpoint (i.e., x = 0.5L) and the Po2 difference between SDVb and LAVb in R2 from 8.2 to 7.7 mmHg. Conversely, decreasing P
from 0.04 to 0.06 cm/s reduces the Po2 difference between SDVa and LAVa, which are located in R1, from 2.7 to 1.8 mmHg at the OM midpoint (i.e., x = 0.5L) and the Po2 difference between SDVb and LAVb in R2 from 8.2 to 7.7 mmHg. Conversely, decreasing P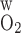 from 0.04 to 0.02 cm/s raises the Po2 difference between SDVa and LAVa from 2.7 to 3.9 mmHg at x = 0.5L and the Po2 difference between SDVb and LAVb from 8.2 to 8.9 mmHg.
from 0.04 to 0.02 cm/s raises the Po2 difference between SDVa and LAVa from 2.7 to 3.9 mmHg at x = 0.5L and the Po2 difference between SDVb and LAVb from 8.2 to 8.9 mmHg.
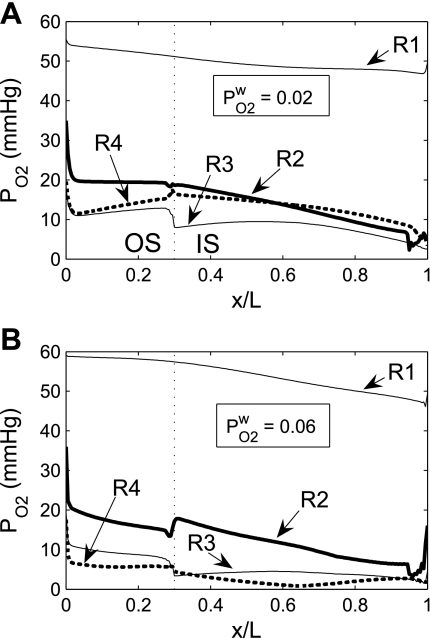
A larger P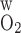 allows O2 to more easily diffuse out of the LDV lumen, thereby raising the Po2 in the local (R1) interstitium (Fig. 4). The higher O2 reabsorption from LDV also means that the amount of O2 supplied to the IM is reduced. Thus the fraction of O2 supplied to LDV at the corticomedullary junction that reaches the IM is calculated as 94.2% and 91.7% if P
allows O2 to more easily diffuse out of the LDV lumen, thereby raising the Po2 in the local (R1) interstitium (Fig. 4). The higher O2 reabsorption from LDV also means that the amount of O2 supplied to the IM is reduced. Thus the fraction of O2 supplied to LDV at the corticomedullary junction that reaches the IM is calculated as 94.2% and 91.7% if P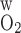 equals 0.02 and 0.06 cm/s, respectively (vs. 92.4% in the base case). Since isolated increases in transcellular O2 permeability do not directly affect interregion O2 fluxes, an increase in interstitial Po2 in R1 does not subsequently raise Po2 in the adjacent regions (Fig. 4).
equals 0.02 and 0.06 cm/s, respectively (vs. 92.4% in the base case). Since isolated increases in transcellular O2 permeability do not directly affect interregion O2 fluxes, an increase in interstitial Po2 in R1 does not subsequently raise Po2 in the adjacent regions (Fig. 4).
Fig. 4.
Po2 in concentric regions obtained for O2 permeability of vessel and tubule wall (P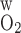 ) = 0.02 cm/s (A) and 0.06 cm/s (B). Relative to the base case, a small P
) = 0.02 cm/s (A) and 0.06 cm/s (B). Relative to the base case, a small P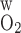 lowers R1 Po2 and increases the Po2 differences between the other, outer regions. A higher P
lowers R1 Po2 and increases the Po2 differences between the other, outer regions. A higher P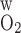 increases the Po2 gradient between R1 and R2 and decreases the Po2 differences between the outer regions.
increases the Po2 gradient between R1 and R2 and decreases the Po2 differences between the outer regions.
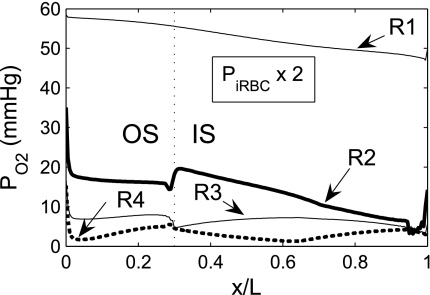
Given the important role of capillaries in delivering O2 to the interbundle regions, we examined the effects of higher O2 permeability of capillaries. Recall that because of the fenestrated nature of the capillaries, we assume that capillary plasma is well mixed with interstitial cells and interstitial spaces in each region; solute permeabilities are assigned to the capillary RBCs, represented as radially running interstitial RBC (iRBC) tubes. Figure 5 shows the Po2 profiles in the regions obtained with the assumption that the iRBC O2 permeability is twice that of the base case; baseline interregion, transepithelial, and transendothelial O2 permeability values were used. Doubling the iRBC O2 permeability has only a minor impact on medullary Po2 profiles, the most significant effect being a small (∼1 mmHg) increase in R2 Po2 (and in all the tubules and vessels therein) throughout the OM, with a noticeable jump at the outer stripe-inner stripe boundary. That a larger iRBC O2 permeability has only negligible effects on model predictions can be attributed to the model's assumption that capillaries are highly tortuous in the interbundle region in the inner stripe, which implies a large surface area for transcapillary exchange, even in the base case. Thus further increases in iRBC O2 permeability yielded only minimal improvement in O2 delivery. Indeed, a 10-fold increase in iRBC O2 permeability produced no noticeable effects on medullary Po2 profiles. The jump in the R2 Po2 profile at the outer stripe-inner stripe boundary (Fig. 5) stems from changes in the population distribution of SDV. The rate at which SDV terminate and form iRBC tubes exhibits a jump discontinuity at the outer stripe-inner stripe boundary (see Eq. A22 in Ref. 7) resulting in a sudden increase in capillary plasma (which mixes with the interstitium) and in capillary RBCs (which form iRBC tubes). Both deliver O2 to all interstitial regions, but mostly to R2 (see Fig. 2 in Ref. 7). When the iRBC permeability to O2 is increased by a factor of 2, O2 diffusion from the iRBC tubes into R2 is enhanced, so that interstitial Po2 increases significantly more abruptly in R2 at the boundary between the outer and inner stripes. However, the increased O2 reabsorption from the early iRBC tubes reduces O2 supply to R4 via capillary flow, so that interstitial Po2 in R4 falls below that in R3 throughout the OM (Fig. 5).
Fig. 5.
Po2 in concentric regions obtained for a 2-fold increase in the O2 permeability of interstitial RBC (iRBC) tubes. Note small increase in R2 Po2 relative to the base case.
RBC kinetics.
Since the reaction between O2 and Hb is much faster than the diffusion of O2, models of O2 transport generally assume that, even during O2 unloading, the reaction is at equilibrium (10, 11). As described in the companion study (7), the association constant (k−1) is calculated on the basis of the dissociation constant (k1) to obtain compatibility with the equilibrium curve (see Eqs. 3 and 4 in Ref. 7). We conducted simulations in which we increased or decreased k1 by a factor of 5. The model predicted that such variations have negligible effects on Po2 profiles in the OM (results not shown).
Boundary conditions.
Po2 in plasma DVR is taken as 60 mmHg at the corticomedullary junction. If it were increased to 70 mmHg (with no change in O2 consumption in the IM) interstitial and luminal Po2 in R1–R4 would increase by 0.2–1.8 mmHg in the mid-outer stripe and by 0.6–5.3 mmHg in the mid-inner stripe. The fraction of O2 supplied to LDV at the corticomedullary junction that reaches the IM would increase from 92.4% to 95.8% (Table 1).
The model's boundary conditions at the OM-IM boundary reflect our assumption that most of the O2 (on a per-vessel basis) returns from the IM via LAVa, which in the IM remain closer to DVR than do LAVb and LALs. In the base case, we assume that LAVa carry 25% more O2 per vessel than LAVb at x = L.
If LAVa and LAVb were carrying the same amount of O2 per vessel, Po2 in LAVa and R1 interstitium would decrease by 4 mmHg, i.e., by 8.3%, at the OM-IM boundary. The predicted Po2 in R2, R3, and R4 would not be significantly affected by this change in boundary conditions.
Variations in the fraction of O2 returning to the OM via LAL, from 1% in the base case to 0.1% and 2%, have negligible effects on Po2, except near the OM-IM boundary in LAL, SAL, and R3 interstitium; in the inner stripe, portions of SAL and LAL are positioned in R3. The model predicts that if the fraction were 0.1%, at x = L Po2 would be 3.3 mmHg lower in R3 interstitium than in the base case. Conversely, increasing the fraction to 2% would raise Po2 at the OM-IM boundary by 3.8 mmHg in R3 interstitium relative to the base case.
Effects of OM Architecture
Interregion permeability.
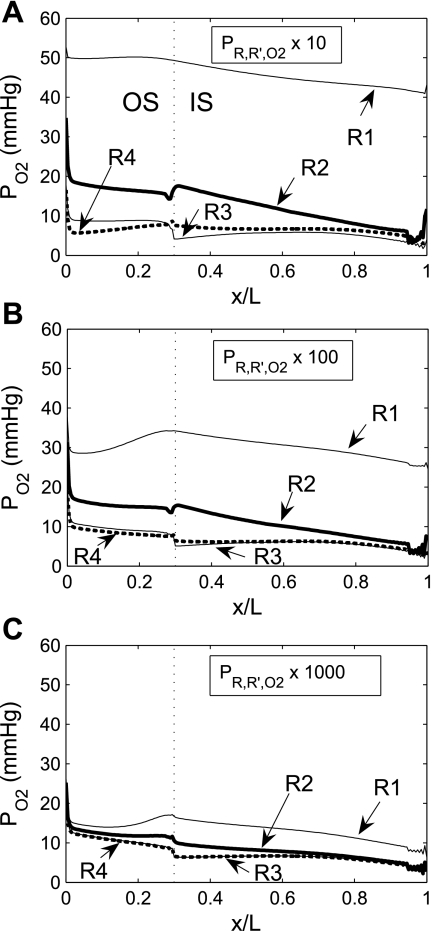
To assess the impact of regionalization on model results, we varied the O2 permeabilities of the region boundaries. The O2 permeabilities of region boundaries (P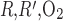 ) used in the base case are founded on estimates of the fractional areas of the regions that consist of interstitium, diffusion resistance arising from macromolecules and interstitial cells in the interstitium, and tortuosity of the diffusion path around tubules and vessels (7, 15). The degree of regionalization increases as the region boundary permeabilities decrease. In our sensitivity study, the base-case boundary O2 permeabilities were scaled by factors of 10, 100, and 1,000.
) used in the base case are founded on estimates of the fractional areas of the regions that consist of interstitium, diffusion resistance arising from macromolecules and interstitial cells in the interstitium, and tortuosity of the diffusion path around tubules and vessels (7, 15). The degree of regionalization increases as the region boundary permeabilities decrease. In our sensitivity study, the base-case boundary O2 permeabilities were scaled by factors of 10, 100, and 1,000.
Interstitial Po2 profiles for different degrees of regionalization are shown in Fig. 6. A 10-fold increase in P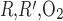 does not significantly affect interstitial Po2 profiles, because, as predicted by the baseline model (see Fig. 6 in Ref. 7), O2 is transferred between regions mostly by capillary flow, rather than by diffusion, when P
does not significantly affect interstitial Po2 profiles, because, as predicted by the baseline model (see Fig. 6 in Ref. 7), O2 is transferred between regions mostly by capillary flow, rather than by diffusion, when P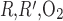 is sufficiently low (≤10 times the baseline value).
is sufficiently low (≤10 times the baseline value).
Fig. 6.
Po2 in the concentric regions as a function of interregion O2 permeability (P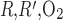 ) for 10 (A), 100 (B), and 1,000 (C) times baseline value. When regionalization is reduced by increasing boundary O2 permeabilities, differences between interstitial Po2 become smaller, although the effect is noticeable only for a ≥100-fold increase, owing to the predominance of capillary flows in the interregion O2 exchange at sufficiently low boundary O2 permeabilities.
) for 10 (A), 100 (B), and 1,000 (C) times baseline value. When regionalization is reduced by increasing boundary O2 permeabilities, differences between interstitial Po2 become smaller, although the effect is noticeable only for a ≥100-fold increase, owing to the predominance of capillary flows in the interregion O2 exchange at sufficiently low boundary O2 permeabilities.
As P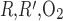 is further increased, the interregion diffusive fluxes become increasingly predominant and Po2 gradients among the four regions decrease: significantly more O2 diffuses from the center of the vascular bundles to the periphery, so that, in R1, interstitial and DVR Po2 drops by as much as 32.4 mmHg at the OM-IM boundary when P
is further increased, the interregion diffusive fluxes become increasingly predominant and Po2 gradients among the four regions decrease: significantly more O2 diffuses from the center of the vascular bundles to the periphery, so that, in R1, interstitial and DVR Po2 drops by as much as 32.4 mmHg at the OM-IM boundary when P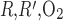 is increased 1,000-fold. The fraction of O2 supplied to LDV at the corticomedullary junction that reaches the IM is calculated as 88.4%, 73.1%, and 53.0% if P
is increased 1,000-fold. The fraction of O2 supplied to LDV at the corticomedullary junction that reaches the IM is calculated as 88.4%, 73.1%, and 53.0% if P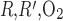 is increased by a factor of 10, 100, and 1,000, respectively (vs. 92.4% with the baseline P
is increased by a factor of 10, 100, and 1,000, respectively (vs. 92.4% with the baseline P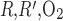 ; Table 1).
; Table 1).
Radial positions of thick ascending limbs.
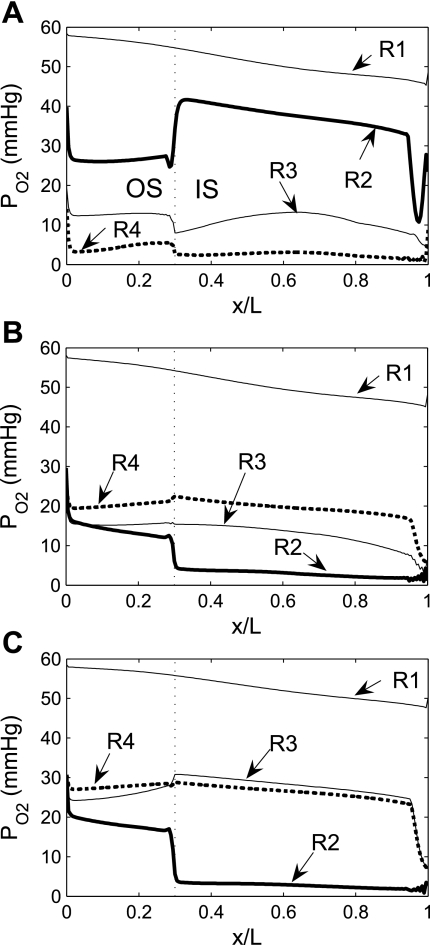
In the context of the urine-concentrating mechanism, it is beneficial for the mTALs to be near the CDs, so that the salt that is actively transported out of the mTALs can efficiently raise the tubular fluid osmolality of the CD by osmotic water removal. However, the CD is also located far from the main source of O2, i.e., the LDV and SDV. Thus, to examine the impact of structural organization on O2 distribution in the OM, we conducted simulations in which the radial positions of the model SALs and LALs were varied. Specifically, we considered three cases: throughout the OM 1) all LALs are in R4, 2) all SALs are in R2, and 3) all LALs and SALs are in R2.
We first considered the effects of varying the radial positions of mTALs on O2 distribution. If all LALs were positioned in the outermost region R4, rather than equally distributed between R2 and R3, O2 demand would increase in R4, resulting in a decrease in R4 interstitial Po2 by 4.7 mmHg, or 60.3%, at the mid-inner stripe. The corresponding decrease in O2 demand in R2 would raise interstitial R2 Po2 by 26.6 mmHg, or 246.3%, at the mid-inner stripe (Fig. 7A). Conversely, if all SALs were positioned in R2, instead of straddling R3 and R4, interstitial Po2 in R2 would fall below that of the other three regions; at the mid-inner stripe, it would be 8.0 mmHg (i.e., 73.8%) lower than in the base case (Fig. 7B). If SALs and LALs were localized in R2 only, interstitial Po2 would drop even further in R2; at the mid-inner stripe, it would be 8.3 mmHg (i.e., 76.9%) lower than in the base case. The reduced O2 demand in the interbundle regions would raise the interstitial Po2 in R3 and R4 (Fig. 7C). These results indicate that radial positions of LALs have a significant impact on interstitial Po2.
Fig. 7.
Po2 in concentric regions for different radial distributions of short and long ascending limbs (SAL and LAL): all LAL in R4 (A), all SAL in R2 (B), and all SAL and LAL in R2 (C).
Because we assume that the mTAL active Na+ transport rate (Vmax,mTAL,Na) decreases when luminal Po2 drops below 1 mmHg, the radial positions of the mTALs affect Na+ transport through their effect on interstitial Po2. If all LALs were positioned in R4 (Fig. 7A), local interstitial Po2, as well as mTAL luminal Po2, would fall below 1 mmHg in approximately the second half of the inner stripe, thus reducing Na+ transport rate there. However, with the LALs occupying a position neighboring the CD, the concentrating effect on CD tubular fluid would be highly effective, such that, despite a reduction in active Na+ transport rate in the deep inner stripe, CD tubular fluid osmolality at the OM-IM boundary would increase to 621 mosmol/kgH2O, which corresponds to a 7.1% increase over the base case. Conversely, if SAL and LAL were closer to the vascular bundle (Fig. 7, B and C), the lower active Na+ transport owing to subcritical mTAL luminal Po2 and the reduced water reabsorption from CD owing to the larger separation from mTAL would decrease the CD osmolality gradient: at the OM-IM boundary, CD tubular fluid osmolality would decrease to 447 and 377 mosmol/kgH2O, respectively. In other words, if LALs and SALs were located in the vascular bundle periphery (R2), the model predicts that the OM would barely concentrate. The distribution of mTALs among the regions would not, however, significantly affect Po2 profiles in the secluded R1 or the fraction of O2 supplied to LDV that reaches the IM (Table 1).
As described in the companion study (7), the difference between the total mTAL active Na+ transport (TAT, in pmol·min−1·nephron−1) under actual conditions (i.e., Vmax,mTAL,Na is assumed to decrease when mTAL luminal Po2 falls below 1 mmHg) and idealized conditions (i.e., Vmax,mTAL,Na is assumed to be constant and independent of mTAL luminal Po2) can be used to assess the degree to which mTAL active Na+ transport is limited by its luminal Po2. In the base case, actual and idealized TAT are equal to 735 and 961 pmol·min−1·nephron−1, respectively, meaning that mTAL Na+ active reabsorption would be 30.8% higher if it were not limited by O2 availability. When all LALs are positioned in R4 (Fig. 7A), the actual and idealized TATs = 677 and 959 pmol·min−1·nephron−1, respectively. Despite the reduction in actual TAT, the CD osmolality gradient increases because of the close proximity of the LALs to the CD (see above). When all SALs are positioned in R2 (Fig. 7B), the actual and idealized TATs = 602 and 952 pmol·min−1·nephron−1, respectively; when all SALs and LALs are positioned in R2 (Fig. 7C), the actual and idealized TATs = 430 and 947 pmol·min−1·nephron−1, respectively. In this latter case, interstitial Po2 is so low in R2 that the active transport of NaCl would be more than twice as high if it were not limited by O2 availability.
Radial position of SDV.
In the next set of simulations, we investigated the effects of varying the radial positions of SDV on O2 distribution. In the model, SDVb are located in R2, which represents the immediate periphery of the vascular bundles. Thus, SDVb have significant interactions with neighboring vessels and tubules (LAVb, LDL, and LAL). The SDVa occupy a more secluded position in R1, within the vascular bundle. In the base case, the SDVa-to-SDVb ratio is taken as 1:99, i.e., the number of SDVa is negligible. An increase in the SDVa-to-SDVb ratio (i.e., the R1-to-R2 SDV ratio) can be interpreted as an indication of a more tightly packed vascular bundle, with less peripheral interactions. To better understand the effect of that number ratio, we conducted a simulation for a SDVa-to-SDVb number ratio of 50:50, instead of the baseline value of 1:99. The model predicts that slightly more O2 supplied to LDV would reach the IM (96.1% vs. 92.4%), because the additional SDVa that reside in R1 help maintain a high interstitial Po2 in that region and reduce O2 loss from the LDV (Table 1). The R2 interstitial Po2 would be lower throughout the OM (4.4 mmHg lower at the junction between the outer and inner stripe) than in the base case. In R3 and R4, interstitial Po2 would be ∼2–3 mmHg lower than in the base case in the outer stripe but ∼1 mmHg higher in the inner stripe, where most SDV break into capillaries; since a larger fraction of O2 would then reach the inner stripe, more O2 would diffuse outwardly to R3 and R4.
Distribution of short vasa recta.
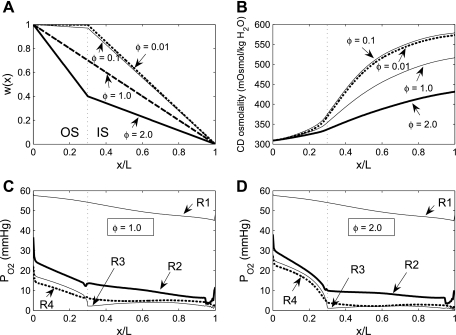
In the base-case simulation, the number of short vasa recta was assumed to decrease piecewise linearly with increasing OM depth. To assess the impact of the short vasa recta population distribution, we conducted simulations using alternative distributions of the form
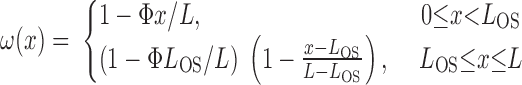 |
(1) |
where L is OM thickness, LIS is inner stripe thickness, LOS is outer stripe thickness, and ω(x) denotes the fraction of SDV or short ascending vasa recta (SAV) that reaches to level x. In Eq. 1, the case of Φ = 0.1 corresponds to the baseline distribution, in which 3% of the SDV break up within the outer stripe. We conducted simulations where Φ = 0.01, 0.05, 1.0, and 2.0. Selected population distributions are illustrated in Fig. 8A.
Fig. 8.
Po2 in concentric regions obtained with different distribution of short vasa recta. Parameter Φ (Eq. 1), which characterizes the rate at which short descending vasa recta (SDV) break up in the outer stripe, is taken as 0.01, 0.1 (base case), 1.0, and 2.0, respectively. A: fraction of SDV that reaches medullary depth x [ωSDV(x)] for Φ = 0.01, 0.1, 1.0, and 2.0. B: collecting duct (CD) osmolality profiles for Φ = 0.01, 0.1, 1.0, and 2.0. C: Po2 in concentric regions for Φ = 1.0. D: Po2 in concentric regions for Φ = 2.0.
Whereas a 5- or 10-fold decrease in Φ has a negligible effect on interstitial and luminal Po2 profiles, 10- and 20-fold increases in Φ significantly affect corticomedullary Po2 gradients in all regions except R1 (Fig. 8). With more SDV breaking up into capillaries in the outer stripe, interstitial Po2 in the outer regions increases in the initial outer stripe; e.g., with Φ = 1.0, interstitial Po2 in R3 and R4 is 23.1 and 22.0 mmHg, respectively, at the corticomedullary junction (vs. 17.5 and 15.9 mmHg in the base case); with Φ = 2.0, R3 and R4 Po2 at the corticomedullary junction further increases to 28.3 and 27.3 mmHg, respectively. However, because the higher Φ values also generate lower Po2 in the inner stripe (see below), Po2 for these cases also exhibits a greater decreasing axial gradient in the outer stripe.
With a higher Φ value, fewer SDV terminate to form capillaries in the inner stripe. In large part because capillary flows account for the majority of the interregion O2 exchange, inner stripe interstitial Po2 decreases in the three outer regions with higher Φ values. For Φ = 1.0, interstitial Po2 in R3 and R4 is 4.1 and 4.9 mmHg, respectively, at the mid-inner stripe (vs. 5.7 and 6.9 mmHg in the base case); with Φ = 2.0, R3 and R4 Po2 at the mid-inner stripe further decreases to 2.2 and 2.5 mmHg, respectively.
The lower Po2 in the inner stripe among the interbundle regions, arising from the slower break-up of SDV in the inner stripe, leads to a reduction in active Na+ transport in the SALs and LALs. As a result, interstitial fluid osmolality decreases, water reabsorption from CDs decreases, and CD tubular fluid osmolality decreases. For Φ = 1.0 and 2.0, CD fluid osmolality at the OM-IM boundary is 517 and 432 mosmol/kgH2O, respectively, which correspond to 10.9% and 25.5% decreases from the baseline osmolality of 580 mosmol/kgH2O.
These results suggest that since SDV constitute the main O2 supply to the OM, their distribution has a large impact on O2 profiles in the regions surrounding the vascular bundles. In contrast, the segregation of LDV within the vascular bundle core ensures that R1 Po2 profiles and the supply of O2 to the IM are much less sensitive to the rate at which SDV break up.
Hyperfiltration and OM Hypertrophy in Rats Fed a High-Protein Diet
It has long been observed that a high-protein diet induces hypertrophy in the OM of the rat kidney (5, 17), increases SNGFR (3, 22), and increases the urine-concentrating capability (12). In particular, the epithelial volume of mTALs increases, which may be accompanied by a corresponding increase in Na+-K+-ATPase activity (21) and active Na+ transport. Given that mTALs normally operate on the brink of hypoxia, one might wonder whether there is sufficient O2 to support the increased active transport resulting from the hypertrophy. Will the corresponding increase in SNGFR satisfy the increased O2 demand?
To answer these questions, we simulated OM hypertrophy for varying mTAL active Na+ transport rates and SNGFR. In these simulations, LOS and LIS were increased by 30% and 54%, respectively; i.e., they were set to 0.78 and 2.16 mm (5). In the outer stripe, the cross-sectional areas of mTAL epithelial cells were assumed to increase by 6.8%; in the inner stripe, SAL cell areas were increased by 45% and LAL cell areas by 38% (5). Region radii were assumed to remain unchanged in the outer stripe but were slightly increased in the inner stripe: rR1 = 42.4 μm, rR2 = 125 μm, rR3 = 201 μm, and rR4 = 237 μm.
Measurements in mTAL cells in antidiuretic hormone-treated Brattleboro rats suggest that Na+-K+-ATPase activity may increase to the same extent as epithelial volume (23). Bankir et al. (2) reported that, in the antidiuretic hormone-treated Brattleboro rat, mTAL epithelial volume increased by 15.6% in the outer stripe and by an average of 45.1% in the inner stripe. On the basis of these measurements, we conducted simulations in which Vmax,mTAL,Na values were increased by factors of 0.156a and 0.451a in the outer and inner stripes, respectively, where a = 0.5, 0.75, and 1.0.
SNGFR in rats fed a high-protein diet has been found to increase by up to 50% (2, 22). Thus we conducted simulations in which fluid flow rates at the corticomedullary junction in descending limbs, DVR, and CDs increased by 0%, 15%, 30%, and 45%. Because the IM is not believed to be hypertrophied, IM O2 consumption (Q ) was kept at baseline, 3.3 × 10−14 mol O2·s−1·nephron−1.
) was kept at baseline, 3.3 × 10−14 mol O2·s−1·nephron−1.
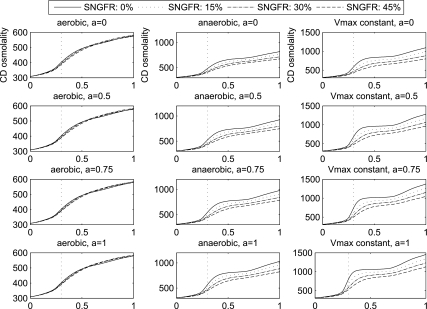
We considered three assumptions regarding the O2 requirements of mTAL active Na+ transport: 1) the “aerobic-only” case, in which anaerobic metabolism is assumed to play no role and Vmax,mTAL,Na decreases to a minimum of 0 at critically low mTAL luminal Po2; 2) the “anaerobic” case, in which, at low mTAL luminal Po2, anaerobic metabolism is assumed to supply enough energy to sustain a Vmax,mTAL,Na that is half of the maximum rate when O2 supply is abundant (see Eq. 20 in Ref. 7); and 3) the “ideal” case, in which Vmax,mTAL,Na is assumed constant and independent of mTAL luminal Po2. For each of these cases, simulations were conducted for each value of a and for each increase in SNGFR. OMCD tubular fluid osmolality profiles are shown in Fig. 9.
Fig. 9.
Profiles of OMCD tubular fluid osmolality (in mosmol/kgH2O) under hypertrophic conditions: aerobic, anaerobic, and “ideal” [maximum medullary thick ascending limb active Na+ transport rate is kept constant (Vmax constant)]. Note difference in y-axis scale among the 3 conditions. Each panel corresponds to a different value a (a = 0, 0.5, 0.75, and 1). Single-nephron glomerular filtration rate (SNGFR) is increased by 0%, 15%, 30%, and 45%.
For the ideal case, a larger a, which corresponds to a higher Vmax,mTAL,Na, yields a substantially larger OM concentrating effect. In the cases where SNGFR is assumed not to increase, a = 0.5, 0.75, and 1.0 generate 18%, 26%, and 33% increases, respectively, in CD tubular fluid osmolality at the OM-IM boundary, relative to the a = 0 case. However, for a fixed a, higher SNGFR has a diluting effect on the OM concentrating mechanism. For a = 1.0, CD tubular fluid osmolality at the OM-IM boundary decreases from 1,455 to 1,130 mosmol/kgH2O when SNGFR is increased by 45%. A higher SNGFR corresponds to higher tubular fluid flow rates along the descending limbs, CD, and DVR, which impose a larger load on the OM concentrating mechanism. The resulting reduction in the CD tubular fluid osmolality gradient is particularly prominent in the ideal case, because the increase in O2 supply has no direct effect on the mTAL active Na+ transport rates. However, at a = 1.0 and when SNGFR is not increased, CD tubular fluid flow rate at the OM-IM boundary is predicted to be 1.3 nl·min−1·nephron−1, which may not be high enough to deliver sufficient urea to the IM to drive the concentrating mechanism there. When SNGFR is increased by 45%, CD tubular fluid flow rate at the OM-IM boundary is predicted to be 2.5 nl·min−1·nephron−1, which appears to be more reasonable.
With an elongated OM and, in cases where a > 0, a higher Vmax,mTAL,Na, mTAL O2 consumption is substantially higher than in the base case. For a = 1.0, mTAL luminal Po2 falls below 1 mmHg (the value below which Vmax,mTAL,Na is assumed to decrease) for most of its length. For the anaerobic case, when SNGFR is assumed not to increase, LAL luminal Po2 is predicted to be 7.1 and 0.94 mmHg at the mid-outer stripe and mid-inner stripe, respectively, compared with baseline (no hypertrophy) values of 10.1 and 0.97 mmHg; SAL luminal Po2 is even lower, 0.75 mmHg at the mid-outer stripe and 0.2 mmHg at the mid-inner stripe, compared with baseline values of 4.7 and 0.71 mmHg. The lower Vmax,mTAL,Na results in a reduced OM concentrating effect compared with the ideal case: CD tubular fluid osmolality is predicted to be 1,034 mosmol/kgH2O for a = 1.0 without an increase in SNGFR, which is 29% lower than the corresponding osmolality value in the ideal case. A higher SNGFR increases O2 supply and raises interstitial and mTAL luminal Po2. A 45% increase in SNGFR at a = 1.0 raises R4 interstitial Po2 from 2.6 (at a = 1.0, but baseline SNGFR) to 7.4 mmHg at the mid-inner stripe; such an increase leads to a higher, although still suboptimal, Vmax,mTAL,Na. However, such an increase in mTAL active Na+ transport rate is offset by the increase in concentrating load imposed by the higher tubular fluid flow rates. Thus, as in the ideal case, for a given a, increases in SNGFR decrease the CD fluid osmolality gradient in the anaerobic case.
In the aerobic-only case, the dependence of Vmax,mTAL,Na on mTAL luminal Po2 is more critical. Thus the improvement in the OM concentrating effect by the increased O2 supply becomes more significant. In contrast to the ideal and anaerobic cases, increasing SNGFR no longer yields monotonic decreases in CD fluid osmolality gradient. Indeed, the opposing effects of higher Vmax,mTAL,Na and larger concentrating load almost balance each other, and increasing SNGFR yields only minor changes in CD fluid osmolality profiles. For a = 0.0, 0.5, 0.75, and 1.0, maximum CD fluid osmolality is predicted at SNGFR increases of 15%, 30%, 30%, and 30%, respectively. However, because mTAL luminal Po2 dips below 1 mmHg along much of the OM, even with higher SNGFR, the OM concentrating effect remains low in the absence of anaerobic metabolism. The higher CD fluid osmolality value at the OM-IM boundary, obtained at a = 1.0 with a 30% SNGFR increase, is only 582 mosmol/kgH2O (vs. 580 at a = 0 and no SNGFR increase).
DISCUSSION
Summary of Main Results
We have conducted a number of parameter studies to determine the impact of assumptions that were needed for model formulation, but for which experimental data are lacking. In addition, we used the model to assess the degree to which O2 transport and consumption processes contribute to the radial distribution of O2 and the sequestration of O2 within the vascular core; we used the model to investigate, in the context of O2 distribution, the role of the structural organization of the rat OM; and we studied OM O2 distribution under special conditions in which an appropriate stimulus is applied to induce hyperfiltration, hypertrophy in the OM inner stripe, and increased Na+ transport rates in the mTAL. Our results indicate or predict that 1) the radial organization of the OM, with respect to vascular bundles, promotes O2 sequestration within the vascular core, thereby preserving O2 delivery to the IM; 2) O2 distribution among the regions surrounding the vascular core is strongly determined by the distance that separates SAL and LAL from the core, by the degree of regionalization, and by the population distribution of short vasa recta along the corticomedullary axis; 3) because the model assumes that mTAL active Na+ transport rate decreases when mTAL luminal Po2 falls below a critical level, O2 distribution, in particular its availability to mTALs, has a significant impact on the concentrating capability of the model OM; and 4) in the absence of anaerobic metabolism, which maintains mTAL active Na+ transport at low luminal Po2, OM hypertrophy, brought on by appropriate stimuli, does not substantially increase the concentrating capability of the OM.
Radial Organization of Tubules and Vessels
The fraction of O2, in the form of O2 and HbO2, supplied to LDV at the corticomedullary junction that reaches the OM-IM boundary can be used as a measure of the steepness of the axial Po2 gradient in the OM. Since there are no tubules in the core region (R1) and relatively few interstitial cells, basal and active O2 consumption are low in R1; moreover, given the small cross-sectional area of R1, very little O2 diffuses out toward the surrounding regions. Hence, the fraction of O2 supplied to LDV at the corticomedullary junction that reaches the IM is very large, 92.4% under baseline conditions. An increase in the interregion O2 permeability significantly reduces this fraction (Table 1), which strongly suggests that the segregation of LDV within tight vascular bundles serves to preserve O2 supply to the IM.
As illustrated in Table 1, the main determinants of the axial Po2 gradient in the rat OM are predicted to be 1) the metabolic requirements of the medulla, 2) the interregion O2 permeability, 3) the distribution and localization of short vasa recta, and 4) the permeability of vessels and tubules to O2. Determinants 2–4 affect the magnitude of O2 exchange between vessels, tubules, and interstitium at different medullary levels. Nevertheless, even when corresponding parameters are varied by as much as 10-fold, the amount of O2 that is predicted to reach the IM remains approximately equal to 92% of that supplied to LDV at the corticomedullary junction. This suggests that the O2 supply to the IM is not too vulnerable to small parameter variations, owing to the specific OM architecture.
The radial Po2 gradient, as quantified by the R1-R4 interstitial Po2 difference at the OM-IM boundary, is similarly strongly dependent on the metabolic requirements of the medulla and the interregion and transcellular permeability to O2 (Table 1). In contrast with the axial Po2 gradient, however, the radial Po2 gradient varies over a much greater range: in the absence of O2 consumption in the OM, interstitial Po2 differences between the vascular bundle core and the outermost region would decrease from 45.7 to 10.2 mmHg. Thus, although the supply of O2 to the IM via LDV is carefully protected, the oxygenation of the peripheral regions in the OM is much more sensitive to variations in metabolic needs.
Boundary Conditions
Because the IM is implicitly represented via boundary conditions, a limitation of this model may be that IM O2 consumption and O2 distribution among the LAL and the different populations of LAV, used in the OM-IM boundary conditions, are assumed to be known a priori and are independent of OM flows and concentrations. Our parameter studies suggest that model results are affected to a small extent by variations in the OM-IM boundary conditions. The three-dimensional architecture of the IM likely plays a role in the O2 distribution in the IM. Anatomic studies by Pannabecker and Dantzler (18, 19) showed that, in the first 3 mm of the IM, CDs form clearly distinguishable clusters, outside of which the O2-delivering DVR are located. This organization likely results in a radial Po2 gradient, especially given the metabolic needs of the IMCDs, which actively transport NaCl (25).
Short Vas Rectum Population Distribution
By conducting simulations using alternative vasa recta distributions along the corticomedullary axis, we found that when a smaller fraction of SDV terminate in the inner stripe, Po2 in the interbundle regions becomes sufficiently low that the active salt transport rate of the mTALs decreases. This has a negative impact on the concentrating capability of the OM, as assessed by the CD osmolality at the OM-IM boundary. It is noteworthy that active salt transport by anaerobic metabolism was not represented in those studies in which we varied SDV population distribution; if it were, the OM concentrating capability would likely have improved.
Our results contradict the findings of Layton and Layton (16), who conducted a similar study in essentially the same four-region model without including O2. They found that when a larger fraction of SDV terminate in the portion of the OM near the cortex, the load is reduced in the deep OM and concentrating capability increases. These studies supported the following intuitive principle: when a smaller fraction of the short vasa recta extend into the deep OM, the fraction of DVR flow that must be raised to the higher concentrations of the OM is reduced; consequently, the effectiveness of short vasa recta as countercurrent exchangers is enhanced and the osmolality of CD fluid at the OM-IM boundary is increased. However, that intuitive principle ignores the effects of O2 and hypoxia and thus, as indicated by results of the present studies, fails to capture the whole picture. Indeed, the population distribution of short vasa recta has likely evolved, as have other aspects of the countercurrent exchange phenomenon, as a compromise between reducing the concentrating load in the deep OM and ensuring a sufficient O2 supply.
mTAL Active Transport-Related O2 Consumption
Brezis et al. (6) found that inhibition of mTAL active transport by furosemide raises medullary Po2 from 21 to 40 mmHg. Similarly, our results suggest that eliminating active transport-related O2 consumption would raise Po2 to ∼45–50 mmHg in the outer regions: at the boundary between the outer and inner stripes, interstitial Po2 is predicted to be 51.6 mmHg in R2, 49.2 mmHg in R3, and 48.5 mmHg in R4, in the absence of O2 consumption for active NaCl reabsorption.
Our model suggests that the structural organization of the rat renal medulla has developed in ways that satisfy competing demands. The metabolic needs of the deeper medulla require that enough O2 be preserved for consumption in the IM. Hence, LDV are segregated within vascular bundles. Meanwhile, the urine-concentrating mechanism requires that OMCD osmolality be efficiently raised by active (i.e., O2-consuming) Na+ transport out of mTALs. Hence, mTALs are localized between the DVR and the OMCDs; the latter need to be sufficiently far from vasa recta to prevent dissipation of the OMCD corticomedullary osmolality gradient by blood flow.
Our simulation results suggest that the radial positions of the mTAL may optimize or nearly optimize the CD axial fluid osmolality gradient and the mTAL luminal Po2. In the base case, luminal Po2 in LAL and SAL is predicted to be 21.2 and 0.43 mmHg, respectively, near the OM-IM boundary. Interestingly, if LAL and SAL were located in the periphery of the vascular bundles (R2), the model predicts that LAL luminal Po2 at the OM-IM boundary would actually decrease to 15.6 mmHg (Fig. 7), despite their proximity to the DVR. In that case, the OM concentrating effect, as measured by the CD fluid osmolality axial gradient, would be nearly eliminated, with the CD osmolality at the OM-IM boundary decreasing from the base-case value of 580 to 377 mosmol/kgH2O. Conversely, if all mTALs were located in R4, near the CD, at the OM-IM boundary Po2 would be 15.3 mmHg in LAL (i.e., 27.8% lower than in the base case) and 8.2 mmHg in SAL (i.e., ∼20 times higher than in the base case); CD osmolality would also be lower than in the base case, 493 mosmol/kgH2O at the OM-IM boundary.
OM Hypertrophy
Experiments in rats and mice have reported that a high-protein diet induces hypertrophy in the OM (5, 17) and increases SNGFR (3, 22). Hendrikx and Epstein (12) reported that water-deprived rats fed a high-protein diet produced urine that was 36% more concentrated than that of rats fed a low-protein diet. Similar observations were made for rats fed a urea diet (29% increase in urine osmolality). It has been hypothesized that a significant fraction of this increase in urine osmolality arises from the enhanced concentrating capability of the OM. Our model simulations, however, suggest that, along much of the mTAL in a hypertrophied OM, luminal Po2 is below the level at which active Na+ transport can be supported solely by aerobic metabolism. Thus, unless there is sufficient anaerobic metabolism to compensate for the reduction in aerobic active transport, the concentrating capability of a hypertrophied OM is not significantly improved.
Our model did not predict a clear role for the SNGFR increase in raising the OM concentrating effect, as measured by the CD tubular fluid osmolality gradient. Indeed, when anaerobic respiration is assumed to support a significant fraction of the mTAL active transport, increasing SNGFR reduces the CD fluid osmolality gradient by imposing a larger load on the OM concentrating mechanism. Nonetheless, without the SNGFR increase, the urea and Na+ supply to the IM, via the CD and LDL, respectively, may not be sufficient to drive the IM concentrating mechanism.
It is also noteworthy that a higher SNGFR may be accompanied by capillary proliferation, which protects the capillaries from the higher upstream fluid pressure and increases the surface area available for diffusive transport. Capillary proliferation that accompanies cellular hypertrophy has been reported in the left ventricle during the development of spontaneous hypertension in rats (1), although we are not aware of experimental evidence in the case of OM hypertrophy induced by a high-protein diet. If indeed the diffusive surface area is increased by capillary proliferation during OM hypertrophy, O2 delivery to the mTAL will likely be enhanced, which may in turn increase active Na+ transport and the concentrating effect of the OM.
Model Limitations
Our model does not account for the numerous neurohumoral factors that regulate oxidative metabolism and O2 consumption, as reviewed by Blantz and Weir (4). In particular, angiotensin II and nitric oxide affect not only renal hemodynamics (i.e., blood supply and distribution) but also tubular epithelial cell function. A more complete understanding of the determinants of medullary oxygenation would require incorporation of these factors.
In summary, our results suggest that O2 distribution among the regions surrounding the vascular core strongly depends on the radial positions of mTALs relative to the vascular core, the degree of regionalization, and the population distribution of short DVR along the corticomedullary axis. If it is assumed that mTAL active Na+ transport is limited below a critical Po2, O2 availability to mTALs has a significant impact on the concentrating capability of the model OM.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant DK-53775 to A. Edwards and A. T. Layton and National Science Foundation Grant DMS-0701412 to A. T. Layton.
REFERENCES
- 1.Anversa P, Melissari M, Beghi C, Olivetti G. Structural compensatory mechanisms in rat heart in early spontaneous hypertension. Am J Physiol Heart Circ Physiol 246: H739–H746, 1984. [DOI] [PubMed] [Google Scholar]
- 2.Bankir L, Fischer C, Fischer S, Jukkala K, Speeht HC, Kriz W. Adaptation of the rat kidney to altered water intake and urine concentration. Pflügers Arch 412: 42–53, 1988. [DOI] [PubMed] [Google Scholar]
- 3.Bankir L, Kriz W. Adaptation of the kidney to protein intake and to urine concentrating activity: similar consequences in health and CRF. Kidney Int 47: 7–24, 1995. [DOI] [PubMed] [Google Scholar]
- 4.Blantz R, Weir M. Are the oxygen costs of kidney function highly regulated? Curr Opin Nephrol Hypertens 13: 67–71, 2004. [DOI] [PubMed] [Google Scholar]
- 5.Bouby N, Trinh-Trang-Tan MM, Laouari D, Kleinknecht C, Grunfeld JP, Kriz W, Bankir L, Doute M, Hahnel B, Coutaud C. Role of the urinary concentrating process in the renal effects of high protein intake. Kidney Int 34: 4–12, 1988. [DOI] [PubMed] [Google Scholar]
- 6.Brezis M, Heyman SN, Epstein FH. Determinants of intrarenal oxygenation. II. Hemodynamic effects. Am J Physiol Renal Fluid Electrolyte Physiol 267: F1063–F1068, 1994. [DOI] [PubMed] [Google Scholar]
- 7.Chen J, Layton AT, Edwards A. A mathematical model of oxygen transport in the rat outer medulla. I. Model formulation and baseline results. Am J Physiol Renal Physiol 297: F517–F536, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Garg LC, Mackie S, Tisher CC. Effect of low potassium-diet on Na-K-ATPase in rat nephron segments. Pflügers Arch 394: 113–117, 1982. [DOI] [PubMed] [Google Scholar]
- 9.Golub AS, Barker MC, Pittman RN. Po2 profiles near arterioles and tissue oxygen consumption in rat mesentery. Am J Physiol Heart Circ Physiol 293: H1097–H1106, 2007. [DOI] [PubMed] [Google Scholar]
- 10.Groebe K An easy-to-use model for O2 supply to red muscle. Validity of assumptions, sensitivity to errors in data. Biophys J 68: 1246–1269, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hellums J, Nair P, Huang N, Ohshima N. Simulation of intraluminal gas transport processes in the microcirculation. Ann Biomed Eng 24: 1–24, 1996. [DOI] [PubMed] [Google Scholar]
- 12.Hendrikx A, Epstein FH. Effect of feeding protein and urea on renal concentrating ability in the rat. Am J Physiol 195: 539–542, 1958. [DOI] [PubMed] [Google Scholar]
- 13.Kriz W, Kaissling B. Structural organization of the mammalian kidney. In: The Kidney: Physiology and Pathophysiology (3rd ed.), edited by D. Seldin and G. Giebisch. Philadelphia: Lippincott Williams & Wilkins, 2000, p. 587–654.
- 14.Kriz W, Schnermann J, Koepsell H. The position of short and long loops of Henle in the rat kidney. Z Anat Entwicklungsgesch 138: 301–319, 1972. [DOI] [PubMed] [Google Scholar]
- 15.Layton AT, Layton HE. A region-based mathematical model of the urine concentrating mechanism in the rat outer medulla. I. Formulation and base-case results. Am J Physiol Renal Physiol 289: F1346–F1366, 2005. [DOI] [PubMed] [Google Scholar]
- 16.Layton AT, Layton HE. A region-based mathematical model of the urine concentrating mechanism in the rat outer medulla. II. Parameter sensitivity and tubular inhomogeneity. Am J Physiol Renal Physiol 289: F1367–F1381, 2005. [DOI] [PubMed] [Google Scholar]
- 17.MacKay EM, MacKay LL, Addis T. Factors which determine renal weight. V. The protein intake. Am J Physiol 86: 459–465, 1928. [Google Scholar]
- 18.Pannabecker TL, Dantzler WH. Three-dimensional architecture of inner medullary vasa recta. Am J Physiol Renal Physiol 290: F1355–F1366, 2006. [DOI] [PubMed] [Google Scholar]
- 19.Pannabecker TL, Dantzler WH. Three-dimensional lateral and vertical relationships of inner medullary loops of Henle and collecting ducts. Am J Physiol Renal Physiol 287: F767–F774, 2004. [DOI] [PubMed] [Google Scholar]
- 20.Porter RK Allometry of mammalian cellular oxygen consumption. Cell Mol Life Sci 58: 815–822, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Seney F Jr, Marver D. Effect of protein intake on medullary thick ascending limb NaK-ATPase activity (Abstract). Clin Res 34: 609A, 1986. [Google Scholar]
- 22.Seney F Jr, Wright F. Dietary protein suppresses feedback control of glomerular filtration in rats. J Clin Invest 75: 558–568, 1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Trinh-Trang-Tan M, Bankir L, Doucet A, El Mernissi G, Imbert-Teboul M, Montegut M, Siaume S, Morel F. Influence of chronic ADH treatment on adenylate cyclase and ATPase activity in distal nephron segments of diabetes insipidus Brattleboro rats. Pflügers Arch 405: 216–222, 1984. [DOI] [PubMed] [Google Scholar]
- 24.Vaidya VS, Back LH, Banerjee RK. Coupled oxygen transport analysis in the avascular wall of a post-angioplasty coronary artery stenosis. Biorheology 42: 249–269, 2005. [PubMed] [Google Scholar]
- 25.Weinstein AM A mathematical model of the inner medullary collecting duct of the rat: pathways for Na and K transport. Am J Physiol Renal Physiol 274: F841–F855, 1998. [DOI] [PubMed] [Google Scholar]
- 26.Zhang W, Edwards A. Oxygen transport across vasa recta in the renal medulla. Am J Physiol Heart Circ Physiol 283: H1042–H1055, 2002. [DOI] [PubMed] [Google Scholar]