Abstract
Renal Na+ and K+ excretion was measured in rats with varying dietary K+ intake. The requirement for channel-mediated distal nephron Na+ reabsorption was assessed by infusing the animals with the K+-sparing diuretic amiloride via osmotic minipumps. At infusion rates of 2 nmol/min, the concentration of amiloride in the urine was 38 μM, corresponding to concentrations of 9–23 μM in the distal tubular fluid, sufficient to block >98% of Na+ transport through apical Na+ channels (ENaC). With a control K+ intake (0.6% KCl), amiloride reduced K+ excretion rates (UKV) from 0.85 ± 0.15 to 0.05 ± 0.01 μmol/min during the first 2 h of infusion, suggesting that distal nephron K+ secretion was completely dependent on the activity of Na+ channels. When K+ intake was increased by feeding overnight with a diet containing 10% KCl, amiloride reduced UKV from 7.5 ± 0.7 to 1.3 ± 0.1 μmol/min despite an increased plasma K+ of 9 mM, again suggesting a major but not exclusive role for the Na+ channel-dependent pathway of K+ secretion. The maximal measured rates of amiloride-sensitive K+ excretion correspond well with estimates based on apical K+ channel activity in distal nephron segments. However, when the animals were adapted to the high-K+ diet for 7–9 days, the diuretic decreased UKV less, from 6.1 ± 0.6 to 3.0 ± 0.8 μmol/min, indicating an increasing fraction of K+ excretion that was independent of Na+ channels. This indicates the upregulation of a Na+ channel-independent mechanism for secreting K+.
Keywords: amiloride, K+ adaptation, K+ channels
in the classic model of renal K+ secretion by the distal nephron, K+ exits the cells across the apical membrane through K+-selective ion channels (16). The driving force for this passive efflux is generated largely by depolarization of the apical membrane voltage resulting from the conductance of apical membrane Na+ channels (10, 34). Quantitative analysis based on measured activities of apical Na+ and K+ channels in the rat CNT suggests that this mechanism can account for a substantial fraction of urinary K+ excretion (9).
To assess more completely the role of this classic route of K+ secretion, we used a recently developed protocol for administering amiloride, a blocker of the epithelial Na+ channel, to rats at constant rates to achieve a controlled concentration of the drug in the luminal fluid of the distal nephron (7). In this communication, we have used this protocol to assess the dependence of K+ secretion on the presence of an apical Na+ conductance and on the delivery of Na+ to the Na+ channel-containing (aldosterone-sensitive) part of the distal nephron, or ASDN. We find that under normal conditions K+ excretion is almost entirely dependent on Na+ channel activity. However, in some circumstances an amiloride-insensitive (presumably Na+ channel-insensitive) pathway can become a significant, and in some cases predominant, factor in K+ elimination.
METHODS
Animals
All animal procedures were approved by the Institutional Animal Care and Use Committee of the Weill Medical College of Cornell University. Female Sprague-Dawley rats (150–200 g body wt, Charles River) were raised free of pathogens. To vary Na+ and K+ intake, animals were fed synthetic diets (Harlan Teklad, Madison, WI). The control diet (CA170555) contained 150 mmol K+/kg and 143 mmol Na+/kg. The matched high-K+ diet (TD76448) contained 1,342 mmol K+/kg and was also high in Cl− (Table 1). For Na+ depletion, the low-Na+ diet contained 2 mmol Na+/kg and 235 mmol K+/kg, and the matched control diet had 164 mmol Na+/kg. In some experiments, animals were implanted with osmotic minipumps (model 2002, Alza) that delivered aldosterone at rates of 1 μg/h. The rats were kept in metabolic cages overnight, starting at 18:00 h with free access to food and water. Food intake was measured to calculate Na+ and K+ intakes. They were then maintained for 4 h without food (∼8 a.m.-12 noon) for the collection of urine. During this period, they were offered water with 3% sucrose to increase fluid intake and facilitate accurate urine collections.
Table 1.
Electrolyte contents of diets
| Na+ | K+ | Cl− | |
|---|---|---|---|
| Control K+ | 143 | 150 | 247 |
| High K+ | 142 | 1,342 | 1,430 |
| Control Na+ | 164 | 235 | 325 |
| Low Na+ | 2 | 240 | 154 |
Values are expressed as mmol/kg.
Amiloride Infusion
Amiloride (Sigma-Aldrich) was dissolved in polyethylene glycol 300 at a concentration of 240 mM. This solution was used to fill osmotic minipumps (Alzet model 2002) delivering fluid at a rate of 0.5 μl/h. The pumps were preincubated for 1–2 h in warm saline so that infusion would begin immediately after implantation. Rats were briefly anesthetized with isoflurane, and the pumps were implanted subcutaneously at the upper back at ∼8 a.m. Control animals had a sham implantation, but no minipump was implanted. Urine was collected over two periods of ∼2 h each. Data presented in the figures are from the first collection period. At the end of the last collection, the rats were anesthetized with an intraperitoneal injection of 100 mg/kg inactin to collect a blood sample from the abdominal aorta before euthanasia.
Amiloride Measurement
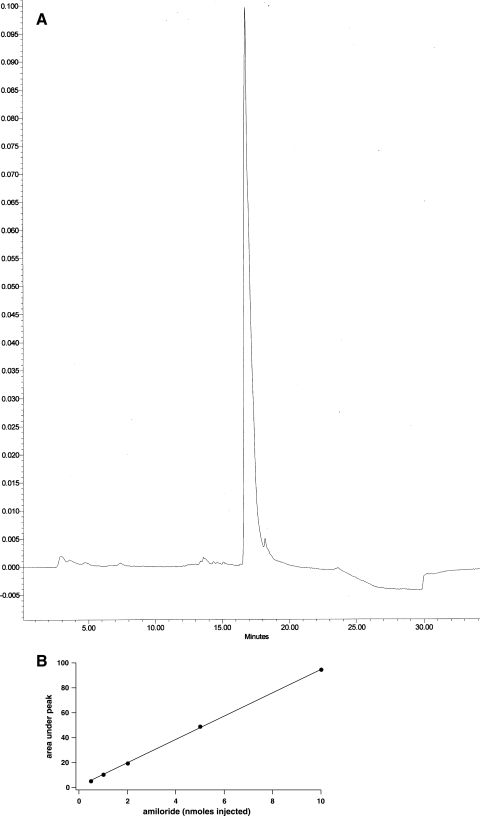
Urine amiloride concentration was measured using HPLC. The instrument (Waters, Milford, MA) included a system controller (model 600E), a photodiode array detector (model 996), and an injector (model U16K) and was operated under Millenium software. A reverse-phase C18 column (Vydac 201TP54) was used as the stationary phase. A concentration gradient of 20 mM ammonium acetate (solvent A), pH 5.4, and acetonitrile (solvent B) was used as the mobile phase. The column was equilibrated at 100% solvent A before injection. After injection, solvent B was increased linearly to 30% over 15 min at a flow rate of 1 ml/min. Amiloride had a retention time of 16.7 min and was well separated from other compounds present in urine in chromatographs obtained at 361 nm. A calibration curve of peak area vs. amiloride amount was linear over the range of 0.5–10 nmol (Fig. 1).
Fig. 1.
Measurement of amiloride concentration in urine. A: typical HPLC trace of absorbance at 361 nm vs. time for a urine sample. Amiloride eluted in a well-defined peak at 16.7 min. B: calibration curve for amiloride standards. The area under the curve was linear with amiloride concentration.
Analytic Measurements
Na+ and K+ in urine and plasma were measured using a flame photometer (model 943, Instrumentation Laboratories, Lexington, MA). Plasma and urine creatinine were measured by an enzyme assay using a commercial kit (Thermo Fisher Scientific, Pittsburgh, PA). Urine chloride was measured with the thiocyanate method using a commercial kit (Thermo Electron, Louisville, CO).
Statistical significance was assessed using the unpaired Student's t-test.
RESULTS
Measurement of Amiloride in Urine
Amiloride was chosen as a Na+ channel blocker for in vivo experiments. Although some analogs such as benzamil have a higher affinity for the channels and a higher specificity for the channels vs. some, but not all, other transporters (14), we preferred using amiloride itself because it is known to be excreted mainly in the urine (12, 15, 28, 35), it has a higher water solubility reducing uptake into cells, and because we had previous experience with infusing the drug through osmotic minipumps (7). Figure 1 shows a typical trace in rat urine and a standard curve of amiloride as measured by HPLC. There was a single peak at the same retention time as that of the amiloride standards, indicating that the drug reaching the urine was not modified or metabolized. The mean concentration in urine collected in the first 2 h after implantation of the pumps was 38 ± 8 μM. The rate of amiloride excretion in the urine was 1.5 ± 0.2 nmol/min and was ∼75% of the nominal rate of infusion (2 nmol/min). These values were similar with different pretreatments of the rats (see Figs. 2–4).
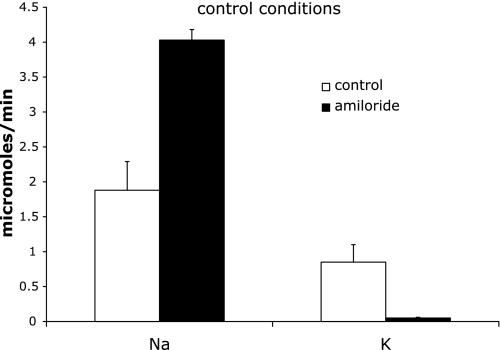
Fig. 2.
Effect of acute amiloride administration on Na+ and K+ excretion in animals on the control K+ diet. Excretion was measured 0–2 h after amiloride infusion began. Control indicates sham-operated rats. Amiloride indicates rats infused with amiloride. Values are means ± SE for 4 animals in each group.
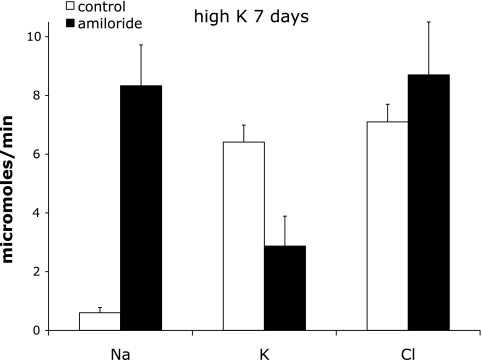
Fig. 4.
Effect of acute amiloride administration on Na+, K+ and Cl− excretion in animals on a high-K+ diet for 7–10 days. Excretion was measured 0–2 h after amiloride infusion began. Control indicates sham-operated rats. Amiloride indicates rats infused with amiloride. Values are means ± SE for 5 animals in each group.
The concentrations of drug in distal tubular fluid were estimated assuming that amiloride was neither secreted into nor reabsorbed from the urine beyond the connecting tubule. Thus the delivery of the drug to the distal segments (in nmol/min) equals the urinary excretion rate. We further assume a GFR of 1 ml/min (Table 2) and a delivery rate of fluid of 200 μl/min (20% of GFR) to the connecting tubule and 80 μl/min (8% of GFR) to the cortical collecting duct. Thus from mass balance
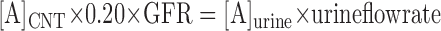 |
where [A]CNT is the concentration of amiloride in the lumen of the CNT. This permits the estimation of drug concentrations in the luminal fluid, which averaged 7.5 μM in the CNT and 18 μM in the CCD. Since the Ki for amiloride block of Na+ channels is ∼100 nM (23), this means that the Na+ conductance would be reduced by at least 98%. Similar results were obtained previously (7).
Table 2.
Effect of amiloride on Na+ and K+ excretion with altered dietary K+
| Condition | n | BW, g | Plasma, mM |
O/N Intake, mmol | GFR, ml/min | Urine Flow, μl/min | Excretion, μmol/min | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Na+ | K+ | Na+ | K+ | Na+ | K+ | |||||
| Control K+ diet | ||||||||||
| Control | 4 | 145±1 | 144±6 | 3.5±0.2 | 1.32±0.12 | 1.39±0.13 | 0.87±0.15 | 30±3 | 1.88±0.41 | 0.85±0.15 |
| +Amiloride | 4 | 155±3 | 142±2 | 4.5±0.2* | 1.45±0.11 | 1.52±0.11 | 1.08±0.10 | 65±20 | 4.03±0.26* | 0.05±0.01* |
| High-K+ diet O/N | ||||||||||
| Control | 9 | 149±6 | 141±3 | 3.9±0.1 | 1.19±0.06 | 11.4±0.6 | 0.94±0.10 | 42±10 | 0.88±0.26 | 7.51±0.74 |
| +Amiloride | 5 | 137±6 | 137±3 | 8.9±0.8* | 1.24±0.10 | 11.9±0.9 | 0.91±0.17 | 47±9 | 7.44±1.15* | 1.33±0.12* |
| High-K+ diet 1 wk | ||||||||||
| Control | 5 | 186±10 | 142±1 | 3.4±0.1 | 1.85±0.05 | 17.8±0.50 | 1.36±0.24 | 66±20 | 0.57±0.14 | 6.08±0.55 |
| +Amiloride | 3 | 189±16 | 139±2 | 5.5±0.6* | 1.90±0.12 | 18.2±1.1 | 1.26±0.18 | 88±22 | 8.50±1.09* | 2.99±0.80* |
Values are means ± SE; n = no. of rats; BW, body wt; O/N, overnight (6:00 p.m–8:00 a.m.); GFR, glomerular filtration rate. Excretion, urine flow, and GFR were measured between 8:00 and 10:00 a.m.
P < 0.05 vs. control for same conditions.
We could not detect amiloride in plasma using the HPLC method due to its low level. If amiloride is freely filtered and neither reabsorbed nor secreted into the urine in any part of the nephron, then, again assuming a GFR of 1 ml/min, the plasma concentration would be 1.5 μM. This is likely to be an overestimate since amiloride is probably secreted by the proximal tubule (12, 28, 35).
Control K+ Diet
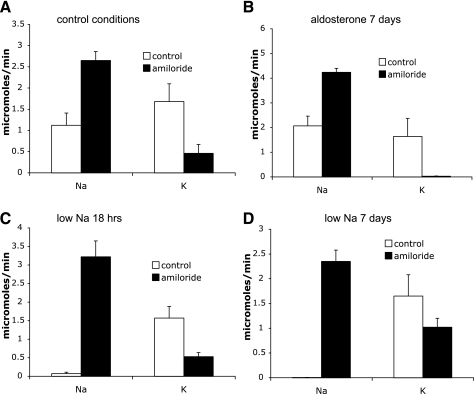
Animals on a control K+ diet excreted Na+ at a rate of 1.9 μmol/min and K+ at a rate of 0.85 μmol/min during the first 2-h test period. When the rats were treated with amiloride, UNaV increased to 4.03 and UKV decreased to 0.05 μmol/min (Fig. 2). During the second 2-h period, K+ excretion rates of controls decreased to 0.77 and that of amiloride-treated animals increased slightly to 0.07 μmol/min. Plasma K+ levels in the amiloride-treated animals increased from 3.5 to 4.5 mM (Table 2). Thus under these conditions virtually all of K+ excretion, and by extension K+ secretion, depends on the activity of the apical Na+ channels. The relative changes in the absolute rates of Na+ and K+ excretion indicate that in the distal nephron where Na+ channels are expressed ∼40% of Na+ reabsorption is balanced by K+ secretion. The data also indicate that Na+ channels are active in the distal nephron in vivo in these animals. This finding contrasts to measurements of Na+ transport in isolated perfused rat CCDs (26, 32), of Na+ channel abundance in isolated CCDs (20), and of amiloride-sensitive conductance in isolated CCDs and CNTs (6, 8) that failed to detect any evidence of Na+ channel activity unless the animals were salt deprived or treated with aldosterone. It suggests that factors present in the animal but absent in vitro contribute to maintaining channel activity in vivo.
Acute High K+ Intake
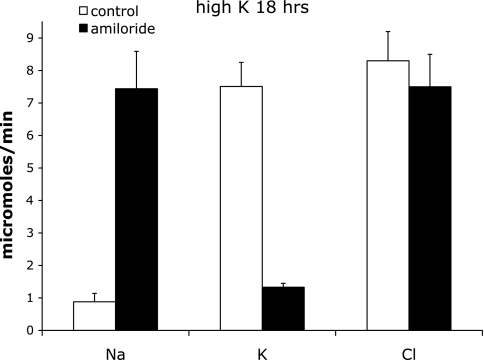
In a second series of experiments, rats were fed a high-K+ diet from 6 p.m. the night before the amiloride challenge. K+ intake during this period was 11.4 ± 0.6 mmol in controls and 11.9 ± 0.9 mmol in the amiloride-treated group (Table 2). We then asked to what extent excretion of this acute K+ load depends on Na+ channel activity. The amiloride excretion rate was 1.7 ± 0.5 nmol/min in the treated animals. From 8 to 10 a.m., there was an enhanced amiloride-induced natriuresis (from 0.88 to 7.44 μmol/min) and also a decrease in K+ excretion (from 7.5 to 1.3 μmol/min) that was much larger in magnitude than was seen under control K+ intake conditions. However, the residual, amiloride-insensitive K+ excretion was larger (Fig. 3). In these animals, plasma K+ increased dramatically from 3.9 to 8.9 mM with amiloride infusion (Table 2). We conclude that K+ secretion under these conditions is still strongly dependent on apical Na+ conductance. In addition, nearly all of the amiloride-sensitive Na+ reabsorption appears to be balanced by K+ secretion. The residual excretion could reflect either amiloride-insensitive K+ secretion or an inhibition of K+ reabsorption in other nephron segments. In the second 2-h period, K+ excretion in untreated animals decreased from 7.5 to 4.4 μmol/min. Over the same time period, amiloride-insensitive K+ excretion increased from 1.3 to 2.4 μmol/min, indicating a rapid adaptation to the high plasma K+ through an amiloride-insensitive mechanism.
Fig. 3.
Effect of acute amiloride administration on Na+, K+ and Cl− excretion in animals on a high-K+ diet overnight. Excretion was measured 0–2 h after amiloride infusion began. Control indicates sham-operated rats. Amiloride indicates rats infused with amiloride. Values are means ± SE for 9 and 6 animals, respectively.
Chronic High K+ Intake
A third group of animals was fed the high-K+ diet for 6–8 days before challenge with amiloride. The amiloride excretion rate was 1.7 ± 0.5 nmol/min in the treated animals. As shown in Fig. 4, in this group the natriuresis induced by amiloride was similar to that of the acute high-K+ protocol (UNaV increased from 0.57 to 8.50 μmol/min) while the K+-sparing effect was significantly less (UKV decreased from 6.08 to 2.99 μmol/min). Thus under these conditions only about half the K+ excretion was amiloride sensitive. This suggests the development of an alternative pathway for K+ secretion. Consistent with this idea, plasma K+ rose much less (to 5.5 mM) in response to amiloride than it did in the group that was given only an acute K+ load (Table 2).
Aldosterone Infusion and a Low-Na+ Diet
We further examined the effects of amiloride with two additional conditions: aldosterone administration and dietary Na+ restriction. The low-Na+ diet and its matched control diet had 60% more K+ than the control food used in the previous experiments. With this diet, amiloride increased Na+ excretion to a similar extent as in Fig. 2, but basal K+ excretion was higher and amiloride did not fully inhibit it (Fig. 5A). This situation is intermediate between the control and chronic K+-loading conditions described above. Infusion of rats with aldosterone for 6–8 days resulted in a new steady state in which Na+ excretion matches Na+ intake despite the continued presence of the hormone (“aldosterone escape”) and K+ excretion matches intake albeit at a substantially reduced plasma K+ concentration (Table 3). Acute infusion with amiloride increased Na+ excretion and decreased K+ excretion nearly to zero (Fig. 5B). We also studied animals fed a low-Na+ diet both acutely and chronically. After the overnight Na+ depletion, amiloride decreased K+ excretion although a significant fraction remained (Fig. 5C). This fraction increased substantially after 1 wk on the low-Na+ diet (Fig. 5D). Here, only about one-third of K+ excretion was amiloride sensitive.
Fig. 5.
Effect of acute amiloride on rats with altered Na+ balance. Control indicates sham-operated rats. Amiloride indicates rats infused with amiloride. Excretion was measured 0–2 h after amiloride infusion began. A: control conditions with 235 mmol K+/kg diet. B: animals infused with aldosterone for 7 days. C: animals fed a low-Na+ diet overnight. D: animals fed a low-Na+ diet for 7 days. Values are means ± SE for 5–8 animals/group.
Table 3.
Effect of amiloride on Na+ and K+ excretion with altered dietary Na+ and aldosterone
| Condition | n | BW, 5 | Plasma, mM |
O/N Intake, mmol | Urine Flow, μl/min | Excretion, μmol/min | |||
|---|---|---|---|---|---|---|---|---|---|
| Na+ | K+ | Na+ | K+ | Na+ | K+ | ||||
| Control Na+ diet | |||||||||
| Control | 5 | 207±11 | 141±2 | 3.4±0.2 | 2.1±0.2 | 3.0±0.3 | 26±5 | 1.11±0.28 | 1.68±0.18 |
| +Amiloride | 5 | 204±13 | 141±1 | 3.9±0.1 | 1.9±0.2 | 2.8±0.2 | 30±9 | 2.62±0.42* | 0.46±0.21* |
| +Aldosterone | |||||||||
| Control | 4 | 207±11 | 144±1 | 2.02±0.08 | 2.1±0.1 | 3.0±0.1 | 49±29 | 2.00±0.33 | 1.67±0.21 |
| +Amiloride | 4 | 220±18 | 143±1 | 2.32±0.02 | 2.2±0.1 | 3.2±0.2 | 30±5 | 4.33±0.67* | 0.03±0.01* |
| Low-Na+ diet O/N | |||||||||
| control | 5 | 173±6 | 142±2 | 3.2±0.1 | 0.022±0.003 | 2.7±0.3 | 18±4 | 0.07±0.04 | 1.41±0.36 |
| +Amiloride | 6 | 168±7 | 144±1 | 4.6±0.2* | 0.026±0.002 | 3.1±0.2 | 21±1 | 3.22±0.31* | 0.48±0.11* |
| Low-Na+ diet 1 wk | |||||||||
| Control | 7 | 173±6 | 141±1 | 3.9±0.1 | 0.024±0.001 | 2.85±0.05 | 9.3±1.1 | 0.006±0.001 | 1.71±0.25 |
| +Amiloride | 8 | 170±3 | 140±1 | 4.4±0.1* | 0.025±0.001 | 3.04±0.12 | 20±2* | 2.33±0.42* | 1.08±0.17* |
Values are means ± SE; n = no. of rats. Excretion and urine flow were measured between 8:00 and 10:00 a.m.
P < 0.05 vs. control for same conditions.
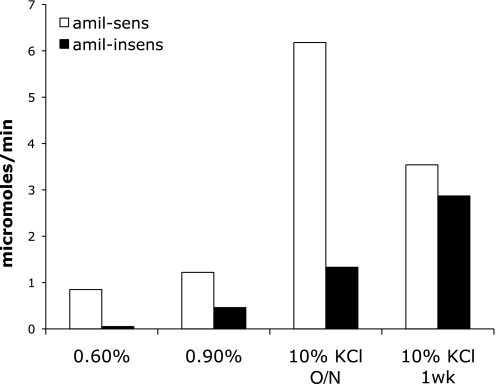
The amiloride-sensitive and amiloride-insensitive portions of UKV for different magnitudes of K+ intake are summarized in Fig. 6. Under different conditions, the amiloride-insensitive component of K+ excretion varies from 0.05 to 2.9 μmol/min. The amiloride-sensitive component ranges from 0.8 to 6.2 μmol/min. As shown in Tables 2 and 3, the animals in the various groups had somewhat different body weights, which will affect the absolute K+ excretion rates, but presumably not the fractions that are amiloride sensitive and amiloride insensitive.
Fig. 6.
Amiloride-sensitive and amiloride-insensitive K+ excretion under different conditions as shown in Figs. 2–5.
High K+ Intake and Na+ Reabsorption
Both an overnight and a sustained high intake of K+ led to the excretion of a larger fraction of Na+ intake during the night (Table 4). By 8 a.m., the animals on a high K+ intake had excreted practically all the overnight Na+ intake, while control animals retained ∼20% of the ingested Na+. Similar observations of natriuresis accompanying a high K+ intake have been reported previously (1, 39). Stokes (29) proposed that a K+ load reduces rates of Na+ reabsorption in the thick ascending limb with subsequent increased delivery of Na+ to more distal segments. Consistent with this hypothesis, we found that amiloride-induced natriuresis was much larger with a high intake of K+ (Table 4). Again assuming complete inhibition of Na+ reabsorption in the connecting tubule and collecting duct by amiloride, the excretion rate in the presence of the drug represents a minimum estimate of the delivery of Na+ to the ASDN. Using measured GFRs and plasma Na+ levels for each condition, these delivery rates were 2.1% of the filtered load of Na+ under control conditions, increasing to 4.8 and 4.7%, respectively, with acute and chronic K+ loads.
Table 4.
Overnight Na excretion with increased K+ intake
| Overnight Intake, μmol |
Na Excretion, % of intake | n | ||
|---|---|---|---|---|
| Na+ | K+ | |||
| Control K+ | 1,500±120 | 1,580±120 | 78±7 | 9 |
| Acute high K+ | 1,150±50 | 11,000±400 | 105±7* | 9 |
| Chronic high K+ | 1,880±60 | 18,000±600 | 99±3† | 10 |
Values are means ± SE; n = no. of rats.
P < 0.005 vs. control K+.
P < 0.05 vs. control K+.
DISCUSSION
Amiloride Blocks K+ Secretion by a Classic Scheme
We have used a protocol that delivers the K+-sparing diuretic amiloride to rats at a constant rate by infusion through osmotic minipumps. We have shown that the drug reaches the urine quickly at concentrations that are sufficient to almost completely block epithelial Na+ channels; the estimated concentration in tubular fluid in the ASDN or ENaC-expressing parts of the nephron is ∼50 times the Ki of the drug for the channels (23). A major assumption of this paper is that this will also completely inhibit distal tubular K+ secretion via the classic pathway involving uptake of K+ from interstitial fluid into principal cells by the Na-K-ATPase and exit into the urine through apical membrane K+ channels (16). In the absence of an apical Na+ conductance, K+ channels will dominate the conductance of the apical membrane. The membrane potential will approach the equilibrium potential for K+, and there will be little or no driving force for secretion. Furthermore, the activity of the Na/K pump will diminish, since Na+ will no longer enter the cell across the apical membrane. The cell will therefore not accumulate K+ to concentrations much above those of electrochemical equilibrium. Finally, since the lumen-negative transepithelial potential will be abolished or turned positive (30), the apical and basolateral membrane potentials will be similar. Any K+ that does enter the cell through the pump will tend to recycle across the basolateral membrane, rather than be secreted into the lumen, because the K+ conductance of the basolateral membrane is considerably higher than that of the apical membrane (11, 19, 27). Mathematical models of transport by distal nephron epithelia quantitatively support these qualitative arguments. In a detailed model of the connecting tubule, reducing apical Na+ conductance to zero effectively abolished K+ secretion (34). A similar result was reported for a simplified model of the CCD that involved only Na+, K+, and Cl− fluxes (10).
Magnitude of Amiloride-Sensitive K+ Excretion
Under quasi-steady-state conditions, the amiloride-sensitive excretion of K+ (measured between 8 and 10 a.m.) ranged from 1 to 3.1 μmol/min. Under extreme conditions of acute K+ loading, the value reached 6 μmol/min. Employing a simple model of ion fluxes across the CNT with measured values of apical Na+ and K+ conductances (9), we calculated that this segment could contribute up to 3.2 μmol/min. Given the uncertainties involved in the extrapolation of in vitro measurements to the in vivo calculation, we consider this quite good agreement. The differences could arise from circulating factors that activate K+ (or Na+) channels in vivo perhaps being lost in vitro. Furthermore, amiloride-sensitive K+ secretion could be augmented by the presence of BK channels, not included in the calculations, and/or by flow-dependent stimulation of ion conductances (31, 38).
Development of Amiloride-Insensitive K+ Excretion
The ability of amiloride to virtually abolish K+ excretion at low to moderate levels of K+ intake is consistent with the dependence of K+ secretion in the ASDN on Na+ channel activity. Our interpretation is that most filtered K+, and well as K+ secreted by the descending limb of Henle's loop, is reabsorbed by segments proximal to the distal convoluted tubule and that downstream segments are no longer able to add significant amounts of K+ to the urine in the presence of the drug. Some of the K+ remaining in the collecting duct may be reabsorbed by H-K-ATPases (16). However, under conditions of elevated K+ intake, a significant fraction of K+ excretion becomes amiloride insensitive. This can be already observed after an acute (overnight) K+ load (Fig. 3). A similar result was reported in dogs treated with amiloride and given an acute K+ load (40). The effect becomes more prominent with longer periods of elevated K+ intake (Figs. 4 and 5A). With K+ loading, urine flow rates tended to be higher (Tables 2 and 3), and these high flows may have facilitated both amiloride-sensitive and amiloride-insensitive K+ excretion. However, these flows were variable and not correlated with excretion rates. Amiloride did not increase flow rates significantly in these groups.
We suggest that this is an adaptive response of the kidney, by which a second pathway for K+ secretion into the urine is recruited to help eliminate a large K+ load. Chronic reduction in dietary Na+ (Fig. 5D) also augments amiloride-insensitive K+ excretion. The latter case may reflect the inability to secrete K+ in the distal nephron due to a reduced Na+ delivery to the distal nephron. In both of these conditions, plasma K+ (Table 2) as well as plasma aldosterone (20, 22) will be elevated. The effect on amiloride-insensitive excretion correlates better with high plasma K+, since aldosterone infusion per se did not induce the phenomenon (Fig. 5).
Nature of Amiloride-Insensitive Excretion
The finding of high rates of K+ excretion, even in the presence of amiloride, under conditions of high K+ intake or chronic low Na+ intake implies that other renal mechanisms must be involved in the control of K+ balance. We consider several candidate mechanisms below.
Reduced reabsorption.
The simplest such mechanism involves increased delivery of K+ to the distal nephron due to either increased filtration or inhibition of K+ reabsorption by more proximal segments. GFR was not greatly affected by dietary K+ or by amiloride (Table 2); the larger values measured with a chronic high-K+ diet probably reflect the somewhat larger size of the animals. It is more likely that K+ reabsorption in the thick ascending limb of Henle's loop might be affected. It has been previously shown that a high K+ intake increases the K+ concentration in the medullary interstitium through a recycling process in the collecting duct (3). In the thick ascending limb of Henle's loop, the high peritubular K+ inhibits Na+ and Cl− reabsorption and reverses K+ absorption (29). This inhibition appears to involve depolarization of the basolateral membrane of the cells of the thick ascending limb of Henle's loop, reducing the driving force for Cl− exit from the cell through basolateral Cl− channels. The resulting accumulation of Cl− in the cell will slow Na+, Cl−, and K+ entry via the apical Na-K-2Cl cotransporter. More recently, an increased delivery of K+ to the distal nephron was observed in mice lacking ROMK channels, another case in which the classic K+ secretion mechanism is impaired (2). However, such an inhibition of absorption is not likely to fully account for the observed results, since the K+ recycling process ultimately still depends on K+ secretion in the cortical segments of the distal nephron (29). It is also possible that absorption of K+ in the thick ascending limb of Henle's loop is directly inhibited by amiloride. This also seems unlikely since the Na-K-2Cl cotransporter is insensitive to amiloride (5).
KCl cotransport.
Wright and colleagues proposed that K+ can exit the cells of the late distal convoluted tubule via cotransport with Cl− (33). Wingo observed a similar Cl-dependent K+ secretion in the isolated perfused rabbit CCD (36, 37). This electroneutral mechanism would not require depolarization of the apical cell membrane and would therefore be insensitive to amiloride. However, this transport was observed mainly when Cl− concentrations in the luminal fluid were low, creating a driving force for Cl− efflux into the urine (33). In our experiments, Cl− excretion rates were high under conditions of K loading, reflecting the high Cl− content of the food (Figs. 3 and 4). This would tend to inhibit the cotransport process. However, we do not know the Cl− concentration in the lumen of the DCT, and such a mechanism may contribute to amiloride-insensitive excretion.
Basolateral Na+ entry.
Early studies of the perfused rabbit CCD indicated that luminal amiloride eliminated both net Na+ reabsorption and net K+ secretion (30). Recently, however, Muto et al. (18) reported that the isolated perfused rabbit CCD can secrete K+ in the absence of luminal Na+, a condition similar to that of a maximal dose of amiloride. Because this secretion was reduced by the Na/H exchange inhibitor EIPA added to the bath solution, they suggested that it was driven, at least in part, by Na+ entry into the cells through the exchanger, followed by K+ entry through the Na/K pump as Na+ was extruded. Although in the absence of apical Na+ entry, this process will be limited by the low driving force for K+ secretion into the lumen (see above), it could contribute to the observed amiloride-insensitive K+ excretion that we observe.
Intercalated cells.
Although intercalated cells are not generally considered to be sites of Na+ or K+ transport, we found a high abundance of Ca2+-activated “maxi” or BK channels in the apical membranes of these cells (21, 24). These intercalated cells have a relatively depolarized basolateral membrane potential, due to a high intracellular Cl− concentration together with a high Cl− conductance and small K+ conductance of this membrane (17, 24). This will also lead to a depolarized apical membrane potential, even when the transepithelial potential is abolished by amiloride, providing a driving force for K+ exit across this membrane through the K+-selective channels. Secretion through these cells may therefore contribute to the observed amiloride-insensitive excretion. Consistent with this idea, Bailey and coworkers (2) recently observed that in mice fed a high-K+ diet or lacking ROMK channels a significant component of K+ secretion by the distal tubule could be blocked by luminal iberiotoxin, a specific inhibitor of BK channels. A major problem with this mechanism, however, is the low activity of the Na/K pump in the basolateral membrane of these cells (4, 24). This will limit the rate of transepithelial transport of K+ that can be sustained by these cells.
Conclusions
In summary, the kidney responds to the challenge of a K+ load in various ways. The activities of apical membrane K+ and Na+ channels increase in distal nephron segments (13, 25). These will enhance the conductance and driving force for K+ secretion, respectively. Furthermore, reabsorption of filtered K+ may be inhibited (2), and the delivery of Na+ to the distal nephron may increase (Ref. 29, Table 4), further stimulating K+ secretion through the classic channel-mediated mechanism (16). Increased K+ excretion through pathways that are independent of Na+ channels may reflect another independent mechanism for maintaining K+ homeostasis.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant DK-27847.
Acknowledgments
We thank Drs. Jochen Buck and Anthony Sauve for use of HPLC apparatus and Dr. Y. Q. Chen for help with HPLC measurements.
REFERENCES
- 1.Aizman RI, Rabinowitz L, Mayer-Harnisch C. Early effects of uninephrectomy on K homeostasis in unanesthetized rats. Am J Physiol Regul Integr Comp Physiol 270: R434–R442, 1996. [DOI] [PubMed] [Google Scholar]
- 2.Bailey MA, Cantone A, Yan Q, MacGregor GG, Leng Q, Amorim JB, Wang T, Hebert SC, Giebisch G, Malnic G. Maxi-K channels contribute to urinary potassium excretion in the ROMK-deficient mouse model of type II Bartter's syndrome and in adaptation to a high-K diet. Kidney Int 70: 51–59, 2006. [DOI] [PubMed] [Google Scholar]
- 3.Battilana CA, Dobyan DC, Lacy FB, Bhattacharya J, Johnston PA, Jamison RL. Effect of chronic potassium loading on potassium secretion by the pars recta or descending limb of the juxtamedullary nephron in the rat. J Clin Invest 62: 1093–1103, 1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beck FX, Dorge A, Blumner E, Giebisch G, Thurau K. Cell rubidium uptake: a method for studying functional heterogeneity in the nephron. Kidney Int 33: 642–651, 1988. [DOI] [PubMed] [Google Scholar]
- 5.Eveloff J, Bayerdörffer E, Silva P, Kinne R. Sodium-chloride transport in the thick ascending limb of Henle's loop. Oxygen consumption studies in isolated cells. Pflügers Arch 389: 263–270, 1981. [DOI] [PubMed] [Google Scholar]
- 6.Frindt G, Masilamani S, Knepper MA, Palmer LG. Activation of epithelial Na channels during short-term Na deprivation. Am J Physiol Renal Physiol 280: F112–F118, 2001. [DOI] [PubMed] [Google Scholar]
- 7.Frindt G, McNair T, Dahlmann A, Jacobs-Palmer E, Palmer LG. Epithelial Na channels and the short-term renal response to salt deprivation. Am J Physiol Renal Physiol 283: F717–F726, 2002. [DOI] [PubMed] [Google Scholar]
- 8.Frindt G, Palmer LG. Na channels in the rat connecting tubule. Am J Physiol Renal Physiol 286: F669–F674, 2004. [DOI] [PubMed] [Google Scholar]
- 9.Frindt G, Shah A, Edvinsson JM, Palmer LG. Dietary K regulates ROMK channels in connecting tubule and cortical collecting duct of rat kidney. Am J Physiol Renal Physiol 296: F347–F354, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gray DA, Frindt G, Palmer LG. Quantification of K+ secretion through apical low-conductance K channels in the CCD. Am J Physiol Renal Physiol 289: F117–F1126, 2005. [DOI] [PubMed] [Google Scholar]
- 11.Gray DA, Frindt G, Zhang YY, Palmer LG. Basolateral K+ conductance in principal cells of rat CCD. Am J Physiol Renal Physiol 288: F493–F504, 2005. [DOI] [PubMed] [Google Scholar]
- 12.Grayson MF, Smith AJ, Smith RN. Absorption, distribution and elimination of 14C-amiloride in normal human subjects. Br J Pharmacol 43: 734P–744P, 1971. [PMC free article] [PubMed] [Google Scholar]
- 13.Hebert SC, Desir G, Giebisch G, Wang W. Molecular diversity and regulation of renal potassium channels. Physiol Rev 85: 319–371, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kleyman TR, Cragoe EJJ. Amiloride and its analogs as tools in the study of ion transport. J Membr Biol 105: 1–21, 1988. [DOI] [PubMed] [Google Scholar]
- 15.Knowles MR, Church NL, Waltner WE, Gatzy JT, Boucher RC. Amiloride in cystic fibrosis: safety, pharmacokinetics and efficacy in the treatment of pulmonary disease. In: Amiloride and its Analogs: Unique Cation Transport Inhibitors, edited by Cragoe EJJ, Kleyman TR, and Simchowitz L. New York: VCH, 1992, p. 301–316.
- 16.Malnic G, Muto S, Giebisch G. Regulation of potassium excretion. In: The Kidney: Physiology and Pathophysiology, edited by Alpern RJ and Hebert SC. Burlington, MA: Academic, 2008.
- 17.Muto S, Giebisch G, Sansom S. Effects of adrenalectomy on CCD: evidence for differential responses of two cell types. Am J Physiol Renal Fluid Electrolyte Physiol 253: F724–F752, 1987. [DOI] [PubMed] [Google Scholar]
- 18.Muto S, Tsuruoka S, Miyata Y, Fujimura A, Kusano E, Wang WH, Seldin D, Giebisch G. Basolateral Na+/H+ exchange maintains potassium secretion during diminished sodium transport in the rabbit cortical collecting duct. Kidney Int 75: 25–30, 2009. [DOI] [PubMed] [Google Scholar]
- 19.O'Neil RG, Sansom SC. Electrophysiological properties of cellular and paracellular conductive pathways of the rabbit cortical collecting duct. J Membr Biol 82: 281–295, 1984. [DOI] [PubMed] [Google Scholar]
- 20.Pácha J, Frindt G, Antonian L, Silver R, Palmer LG. Regulation of Na channels of the rat cortical collecting tubule by aldosterone. J Gen Physiol 102: 25–42, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pácha J, Frindt G, Sackin H, Palmer LG. Apical maxi K channels in intercalated cells of CCT. Am J Physiol Renal Fluid Electrolyte Physiol 261: F696–F705, 1991. [DOI] [PubMed] [Google Scholar]
- 22.Palmer LG, Antonian L, Frindt G. Regulation of apical K and Na channels and Na/K pumps in rat cortical collecting tubule by dietary K. J Gen Physiol 104: 693–710, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Palmer LG, Frindt G. Amiloride-sensitive Na+ channels from the apical membrane of the rat cortical collecting tubule. Proc Natl Acad Sci USA 83: 2767–2770, 1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Palmer LG, Frindt G. High-conductance K channels in intercalated cells of the rat distal nephron. Am J Physiol Renal Physiol 292: F966–F973, 2007. [DOI] [PubMed] [Google Scholar]
- 25.Palmer LG, Frindt G. Na+ and K+ transport by the renal connecting tubule. Curr Opin Nephrol Hypertens 16: 477–483, 2007. [DOI] [PubMed] [Google Scholar]
- 26.Reif MC, Troutman SL, Schafer JA. Sodium transport by rat cortical collecting tubule. Effects of vasopressin and desoxycorticosterone. J Clin Invest 77: 1291–1298, 1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schlatter E, Schafer JA. Electrophysiological studies in principal cells of rat cortical collecting tubules. ADH increases the apical membrane Na+ conductance. Pflügers Arch 409: 81–92, 1987. [DOI] [PubMed] [Google Scholar]
- 28.Smith AJ, Smith RN. Kinetics and bioavailability of two formulations of amiloride in man. Br J Pharmacol 48: 646–649, 1973. [PMC free article] [PubMed] [Google Scholar]
- 29.Stokes JB Consequences of potassium recycling in the renal medulla. Effects on ion transport by the medullary thick ascending limb of Henle's loop. J Clin Invest 70: 219–229, 1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stoner LC, Burg MB, Orloff J. Ion transport in cortical collecting tubule: effect of amiloride. Am J Physiol 227: 453–459, 1974. [DOI] [PubMed] [Google Scholar]
- 31.Taniguchi J, Imai M. Flow-dependent activation of maxi K+ channels in apical membrane of rabbit connecting tubule. J Membr Biol 164: 35–45, 1998. [DOI] [PubMed] [Google Scholar]
- 32.Tomita K, Pisano JJ, Knepper MA. Control of sodium and potassium transport in the cortical collecting tubule of the rat. Effects of bradykinin, vasopressin, and deoxycorticosterone. J Clin Invest 76: 132–136, 1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Velázquez H, Ellison DH, Wright FS. Chloride-dependent potassium secretion in early and late renal distal tubules. Am J Physiol Renal Fluid Electrolyte Physiol 253: F555–F562, 1987. [DOI] [PubMed] [Google Scholar]
- 34.Weinstein AM A mathematical model of rat distal convoluted tubule. II. Potassium secretion along the connecting segment. Am J Physiol Renal Physiol 289: F721–F741, 2005. [DOI] [PubMed] [Google Scholar]
- 35.Weiss P, Hersey RM, Dujovne CA, Bianchine JR. The metabolism of amiloride hydrochloride in man. Clin Pharmacol Ther 10: 401–406, 1969. [DOI] [PubMed] [Google Scholar]
- 36.Wingo CS Potassium secretion by the cortical collecting tubule: effect of Cl gradients and ouabain. Am J Physiol Renal Fluid Electrolyte Physiol 256: F306–F313, 1989. [DOI] [PubMed] [Google Scholar]
- 37.Wingo CS Reversible chloride-dependent potassium flux across the rabbit cortical collecting tubule. Am J Physiol Renal Fluid Electrolyte Physiol 256: F697–F704, 1989. [DOI] [PubMed] [Google Scholar]
- 38.Woda CB, Bragin A, Kleyman TR, Satlin LM. Flow-dependent K+ secretion in the cortical collecting duct is mediated by a maxi-K channel. Am J Physiol Renal Physiol 280: F786–F793, 2001. [DOI] [PubMed] [Google Scholar]
- 39.Wright FS, Strieder N, Fowler NB, Giebisch G. Potassium excretion by distal tubule after potassium adaptation. Am J Physiol 221: 437–448, 1971. [DOI] [PubMed] [Google Scholar]
- 40.Yeyati NL, Etcheverry JC, Adrogué HJ. Kaliuretic response to potassium loading in amiloride-treated dogs. Ren Physiol Biochem 13: 190–199, 1990. [DOI] [PubMed] [Google Scholar]