Abstract
Renal dopamine and nitric oxide contribute to natriuresis during high-salt intake which maintains sodium and blood pressure homeostasis. We wanted to determine whether concurrent inhibition of these natriuretic factors increases blood pressure during high-sodium intake. Male Sprague-Dawley rats were divided into the following groups: 1) vehicle (V)-tap water, 2) NaCl-1% NaCl drinking water, 3) 30 mM l-buthionine sulfoximine (BSO), an oxidant, 4) BSO plus NaCl, and 5) BSO plus NaCl with 1 mM tempol (antioxidant). Compared with V, NaCl intake for 10 days doubled sodium intake and increased urinary dopamine level but reduced urinary nitric oxide content. NaCl intake also reduced basal renal proximal tubular Na-K-ATPase activity with no effect on blood pressure. However, NaCl intake in BSO-treated rats failed to reduce basal Na-K-ATPase activity despite higher urinary dopamine levels. Also, dopamine failed to inhibit proximal tubular Na-K-ATPase activity and these rats exhibited reduced urinary nitric oxide levels and high blood pressure. Tempol supplementation in NaCl plus BSO-treated rats reduced blood pressure. BSO treatment alone did not affect the urinary nitric oxide and dopamine levels or blood pressure. However, dopamine failed to inhibit proximal tubular Na-K-ATPase activity in BSO-treated rats. BSO treatment also increased basal protein kinase C activity, D1 receptor serine phosphorylation, and oxidative markers like malondialdehyde and 8-isoprostane. We suggest that NaCl-mediated reduction in nitric oxide does not increase blood pressure due to activation of D1 receptor signaling. Conversely, oxidative stress-provoked inhibition of D1 receptor signaling fails to elevate blood pressure due to presence of normal nitric oxide. However, simultaneously decreasing nitric oxide levels with NaCl and inhibiting D1 receptor signaling with BSO elevated blood pressure.
Keywords: dopamine, G proteins, Na-K-ATPase, l-buthionine sulfoximine
dopamine acts as an intrarenal natriuretic factor and plays an important role in sodium homeostasis (28). The effects of dopamine are mediated through G protein-coupled receptors which are divided into two subfamilies, D1-like (D1 and D5) and D2-like (D2, D3, and D4) (28). The activation of D1 receptors via stimulation of adenylyl cyclase and phospholipase C inhibits Na-K-ATPase and Na/H exchanger 3, two transporters involved in sodium uptake in the renal proximal tubules (1, 15). During sodium-replete conditions, nearly 60% of sodium excreted is accounted for by the actions of newly synthesized renal dopamine (11, 20). There is evidence that defect in D1 receptor signaling may be a contributing factor in salt-sensitive hypertension. As an example, the defect in D1 receptor signaling has been demonstrated in salt-sensitive hypertensive models such as Dahl salt-sensitive and spontaneously hypertensive rats (30, 37). Although the mechanism for defective D1 receptor signaling is not clear, antioxidant treatment in animal studies has been reported to restore the D1 receptor function indicating that oxidative stress can lead to D1 receptor dysfunction (8, 27). Another important regulator of sodium excretion is renal nitric oxide system. In animal models, the increase in blood pressure after salt loading is characterized by decrease in nitric oxide production (13, 35). Salt loading is also associated with decreased levels of nitric oxide metabolites in salt-sensitive humans (16). The mechanisms for this phenomenon are not known; however, Kitiyakara et al. (21) showed that high-salt intake increases generation of superoxide by enhancing the renal expression and activity of NAD(P)H oxidase with diminished renal expression of superoxide dismutase. The increased superoxide generation in renal cortex could cause bioinactivation of nitric oxide by its conversion to peroxynitrite.
Although defects in D1 receptor signaling or nitric oxide production pose an independent risk for salt-sensitive hypertension, the defect in these natriuretic hormones is not always associated with hypertension. As an example, animal models like Fisher 344 old rats and streptozotocin-induced diabetic rats have normal blood pressure despite the failure of renal D1 receptor to inhibit Na-K-ATPase and induce natriuresis (5, 27). Similarly, high-salt diet does not always produce hypertension despite diminished nitric oxide production and signaling (14, 33, 34). Interestingly, evidence to date indicates that in humans and animal models of hypertension, inheritable or salt induced, there is increased oxidative stress which coexists with defective D1 receptor function and impaired nitric oxide production or signaling (7, 9, 17, 37). It is reported that antioxidant supplementation lowers oxidative stress, blood pressure, and restores nitric oxide signaling and D1 receptor function (7, 9, 17, 26, 37). Collectively, these observations suggest that dysfunction of both renal nitric oxide and dopamine systems presumably contributes to salt-sensitive hypertension. Previously, we and others showed that oxidative stress reduces D1 receptor function and high-salt loading leads to suppression of renal nitric oxide synthases in Sprague-Dawley rats (27, 29). Therefore, we hypothesized that in Sprague-Dawley rats increased salt intake causes decrease in nitric oxide levels accompanied by l-buthionine sulfoximine (BSO)-induced oxidative stress and D1 receptor inhibition most likely results in decreased sodium excretion and hypertension. To test this hypothesis, different groups of animals were given tap water supplemented with BSO, NaCl, and BSO plus NaCl with and without antioxidant tempol for 10 days. Oxidative markers, nitric oxide production, D1 receptor function, and blood pressure were studied at the end of each treatment.
METHODS
Animal treatment.
Male Sprague-Dawley rats (200–250 g) were obtained from Harlan (Indianapolis, IN). The animals were divided into the following experimental groups: V, rats were provided with tap water; NaCl, rats were provided with 1% NaCl; BSO, rats were provided with 30 mM bso (a prooxidant); and BSO plus NaCl, rats were treated with 30 mM BSO plus 1% NaCl. NaCl plus BSO-treated rats were also provided with 1 mM tempol (an antioxidant). All the rats had free access to tap water or water supplemented with BSO/NaCl/tempol for 10 days. The experiments were approved by Institutional Animal Care and Use Committee.
Blood pressure was measured as published earlier (27). Briefly, rats were anesthetized with Inactin (100 mg/kg ip) and tracheotomy was performed to facilitate breathing. To measure blood pressure and heart rate, the left carotid artery was catheterized with PE-50 tubing, connected to a Statham P23AC pressure transducer, and blood pressure and heart rate were recorded on a Grass polygraph (model 7D, Grass Instrument, Quincy, MA). Urinary sodium excretion was measured as detailed earlier (27). Urinary nitric oxide levels (nitrate/nitrite) were determined by a colorimetric method using commercially available kit (R&D Systems, Minneapolis, MN) and asymmetric dimethylarginine levels were assayed by ELISA (Diagnostika, GMBH, Germany). Dopamine was measured by high-performance liquid chromatography equipped with a C18 reverse-phase 3 μM Luna column. Malondialdehyde was determined in renal proximal tubular homogenates. Tubular homogenate (1–1.5 mg protein) was incubated with thiobarbituric acid and trichloroacetic acid solution for 15 min in boiling water bath. The color was extracted with butanol and read at 535 nM (27). 8-Isoprostane was measured by RIA kit (516351; Cayman, Ann Arbor, MI). Protein kinase C activity was determined by a commercially available protein kinase C assay kit (Promega, Madison, WI) as detailed in our previous study (8).
Preparation of renal proximal tubular suspension.
Renal proximal tubular suspension was prepared as described earlier (8, 27). Briefly, rats were anesthetized with pentobarbital sodium (50 mg/kg ip) and after a midline incision, the aorta was cannulated below the kidneys and an in situ digestion was accomplished by perfusing an enzyme solution of collagenase and hyaluronidase. Enrichment of proximal tubules was carried out using 20% ficoll gradient in Krebs buffer. The band at ficoll interface was collected and washed by centrifugation at 250 g for 5 min. Protein was determined by bicinchoninic acid method (Pierce, Rockford, IL) using bovine serum albumin as a standard (8, 27).
Na-K-ATPase assay.
Na-K-ATPase activity was determined as reported earlier (8, 27). Briefly, dopamine-induced Na-K-ATPase inhibition was determined in proximal tubular suspensions (1 mg protein/ml) incubated with or without 1 mM ouabain (Sigma) at 37°C for 15 min.
Immunoprecipitation of D1 receptor.
Proximal tubules were homogenized in a buffer containing 10 mM Tris·HCl, pH 7.6, 250 mM sucrose, 0.2 mM phenylmethylsulfonyl fluoride (PMSF), and protease inhibitor cocktail (Roche, Nutley, NJ) and centrifuged at 2,500 g for 10 min. The supernatant was centrifuged at 24,000 g for 20 min. The upper fluffy layer of the pellet was resuspended in the same buffer, homogenized in a Dounce Homogenizer (Wheaton, Millville, NJ), and centrifuged at 24,000 g for 20 min. The pellet was washed with buffer containing 100 mM KCl, 100 mM mannitol, and 5 mM HEPES, pH 7.2, and centrifuged at 34,000 g for 30 min. The membranes (pellet) were suspended in immunoprecipitation (IP) buffer containing (in mM) 140 NaCl, 3 KCl, 10 Na2HPO4, 2 KH2PO4, 1 orthovanadate, and 1 PMSF and 1% NP-40, 0.5% sodium cholate, 0.1% SDS, and protease inhibitor cocktail, pH 7.4. The membranes (1.5 mg protein/ml) were incubated with 10 μg of D1 receptor antibody (Chemicon) for 2 h. The D1 receptor-antibody complex was incubated overnight with protein A/G covalently bound to agarose beads (protein A/G-agarose). The D1 receptor-antibody-protein A/G complex settled down and washed with IP buffer and 50 mM Tris·HCl, pH 8.0. The D1A receptor-antibody-protein A/G complex was dissociated with 2× Laemmeli buffer containing 125 mM Tris·HCl, 4% SDS, 5% β-mercaptoethanol, and 20% glycerol at 37°C for 1 h (22). The samples (10 μl) were resolved by SDS-PAGE electrophoresis, and the proteins were electrotransferred on a polyvinylidene difluoride membrane. The membrane was blocked with 4% bovine serum albumin in PBS with 0.1% Tween 20 and probed with phosphoserine antibody (Calbiochem, Gibson, NJ) and D1 receptor antibody to detect serine phosphorylation on D1 receptors and D1 receptor, respectively. Both antiphosphoserine and D1 receptor have been characterized previously for their antigenic specificity (3, 6). Horseradish peroxidase-conjugated secondary antibody was used to probe the antibodies, and the bands were visualized with electrochemiluminescence using enhanced chemiluminescence reagent kit (Santa Cruz Biotechnology, Santa Cruz, CA). D1 receptor phosphorylation was normalized with D1 receptor protein.
Immunoblotting.
Renal proximal tubular homogenates were used to immunoblot nitric oxide synthase 3 (50 μg protein), GAPDH (10 μg protein), and dimethylarginine dimethylaminohydrolase (60 μg protein). Protein was solubilized in Laemmli buffer, separated by SDS-PAGE, and transferred to polyvinylidene difluoride. The membranes were blocked and incubated with antisera directed against nitric oxide synthase 3 (BD Biosciences, San Jose, CA), dimethylarginine dimethylaminohydrolase (BD Biosciences), and GAPDH (Calbiochem) in 0.1% Tris-buffered saline, followed by incubation with horseradish peroxidase-conjugated secondary antibodies. GAPDH was used as an internal control to normalize the nitric oxide synthase 3 and dimethylarginine dimethylaminohydrolase bands.
To ascertain the linearity of detection system, we used varying protein concentrations (1 to 10 μg protein) to detect GAPDH band while keeping the antibody concentration constant. We also confirmed the specificity of immunoreactivity by preincubating the antibody with blocking peptide.
Statistical analysis.
Differences between means were evaluated using the unpaired t-test or ANOVA with Newman-Keuls multiple comparison test, as appropriate; P < 0.05 was considered statistically significant.
RESULTS
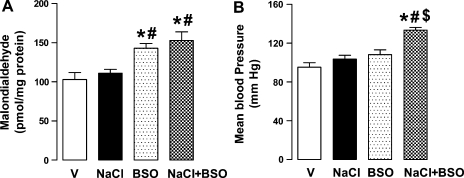
NaCl (1%) intake increased urinary dopamine [in pmol/min, vehicle (V): 4.3 ± 0.3; NaCl: 8.1 ± 0.4, P < 0.05] and sodium excretion (in μmol/min, V: 0.2 ± 0.03; NaCl: 0.42 ± 0.04, P < 0.05) and decreased urinary nitric oxide levels (nitrate/nitrite; in nmol/min, V: 4.1 ± 0.2; NaCl: 2.8 ± 0.1, P < 0.05). NaCl did not affect urinary 8-isoprostane (in pg/mg creatine, V: 1.2 ± 0.1; NaCl: 1.4 ± 0.2), proximal tubular malondialdehyde, and blood pressure (Fig. 1, A and B). BSO treatment caused oxidative stress as evidenced by increased urinary 8-isoprostane excretion (2.3 ± 0.2, P < 0.05 vs. V or NaCl) and renal malondialdehyde (Fig. 1A). BSO did not affect blood pressure (Fig. 1B), urinary nitric oxide level (BSO: 3.8 ± 0.3), dopamine level (BSO: 3.9 ± 0.2), or sodium excretion (0.18 ± 0.02). Compared with vehicle, rats cosupplemented with NaCl and BSO had lower urinary nitric oxide levels (2.5 ± 0.2) and higher dopamine level (7.9 ± 0.4) and sodium excretion (0.38 ± 0.05). These rats also had increased 8-isoprostane (2.6 ± 0.2) and malondialdehyde levels and exhibited high blood pressure (Fig. 1, A and B). Supplementation of BSO plus NaCl-treated rats with antioxidant tempol normalized 8-isoprostane (1.5 ± 0.3), malondialdehyde (110.3 ± 7.2 pmol/mg protein), and blood pressure (106.3 ± 5.8 mmHg). The body weight and food intake were similar in all experimental groups (data not shown).
Fig. 1.
Oxidative stress and blood pressure in vehicle (V)-, 1% NaCl (NaCl)-, l-buthionine sulfoximine (BSO)-, and BSO + NaCl-treated rats. Renal proximal tubular malondialdehyde levels (A) and mean arterial pressure (B). Bars represent means ± SE from 6–8 animals. *P < 0.05 vs. V, #P < 0.05 vs. NaCl, and $P < 0.05 vs. BSO, using 1-way ANOVA followed by post hoc Newman-Keuls multiple comparison test.
Effect of NaCl and BSO on nitric oxide synthase 3 expression in renal proximal tubules.
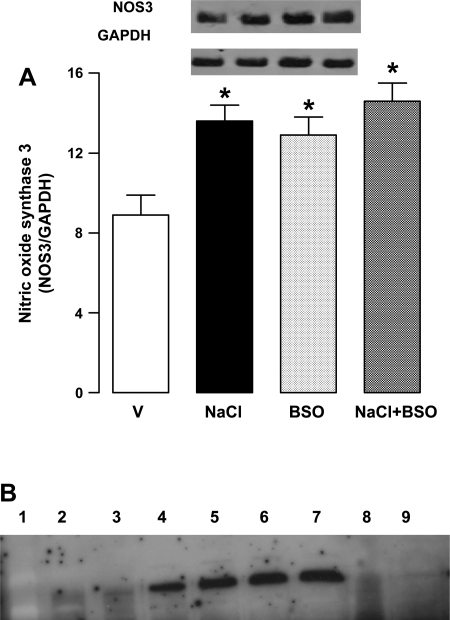
NaCl intake reduced nitric oxide levels and one possible mechanism for this could be a decrease in nitric oxide synthase protein expression. However, rats supplemented with NaCl showed significant increase in nitric oxide synthase 3 expression compared with vehicle-treated rats (Fig. 2). Nitric oxide synthase 3 protein expression was also higher in BSO and BSO plus NaCl-treated rats compared with vehicle (Fig. 2A).
Fig. 2.
A: endothelial nitric oxide synthase 3 (NOS3) expression in renal proximal tubules from V-, 1% NaCl-, BSO-, and BSO + NaCl-treated rats. Blots (NOS3 ∼140 kDa and GAPDH ∼45 kDa) shown are a single experiment, representative of 6–8 individual experiments, and bars (NOS3/GAPDH) represent means ± SE. Proximal tubular homogenate (50 μg protein for NOS3 and 10 μg protein for GAPDH) was used for immunobloting. *P < 0.05 vs. V, using 1-way ANOVA followed by post hoc Newman-Keuls multiple comparison test. B: GAPDH band intensity with varying protein concentrations. Lane 1: markers; lane 2: 1 μg protein; lane 3: 2 μg protein; lane 4: 4 μg protein; lane 5: 6 μg protein; lane 6: 8 μg protein; lane 7: 10 μg protein; lane 8: 10 μg protein but antibody preincubated with antigen; lane 9: blank well.
As shown in Fig. 2B, there was a linear increase in GAPDH band intensity with increasing protein concentrations (lane 1: markers, lane 2: 1 μg protein, lane 3: 2 μg protein, lane 4: 4 μg protein, lane 5: 6 μg protein, lane 6: 8 μg protein, lane 7: 10 μg protein, lane 8: 10 μg protein but antibody preincubated with antigen, and lane 9: blank well).
Effect of NaCl and BSO on urinary asymmetric dimethylarginine.
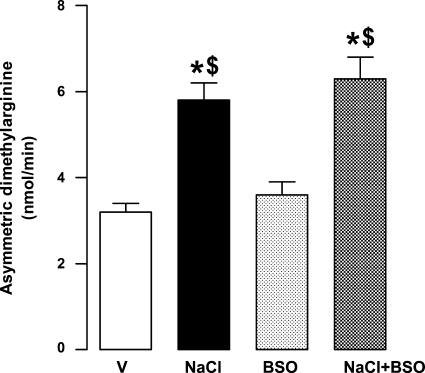
To further identify the mechanisms responsible for decreased nitric oxide production in NaCl-supplemented rats, we measured urinary asymmetric dimethylarninine, an endogenous inhibitor of nitric oxide synthase. Treatment of rats with BSO had no effect on urinary asymmetric dimethylarninine levels (Fig. 3). However, NaCl per se or in combination with BSO significantly increased the asymmetric dimethylarginine content compared with vehicle (Fig. 3).
Fig. 3.
Urinary asymetric dimethylarginine levels in V-, 1% NaCl-, BSO-, and BSO + NaCl-treated rats. Bars represent means ± SE from 6–8 experiments performed in triplicate. *P < 0.05 vs. V and $P < 0.05 vs. BSO, using 1-way ANOVA followed by post hoc Newman-Keuls multiple comparison test.
Effect of NaCl and BSO on dimethylarginine dimethylaminohydrolase expression in renal proximal tubules.
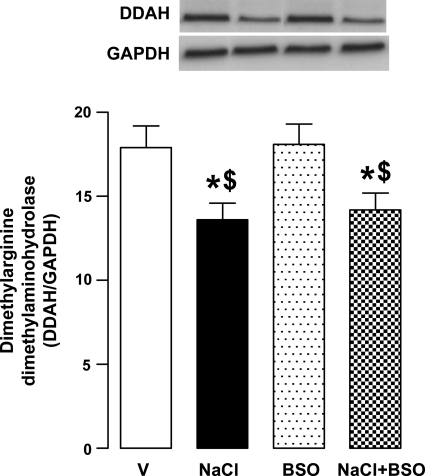
Dimethylarginine dimethylaminohydrolase hydrolyzes asymmetric dimethylarginine and thus helps to maintain normal nitric oxide level. In here, NaCl intake significantly reduced the protein expression of dimethylarginine dimethylaminohydrolase in renal proximal tubules (Fig. 4). The expression of this enzyme was also low in BSO plus NaCl-treated rats (Fig. 4). However, BSO alone had no effect on protein expression of dimethylarginine dimethylaminohydrolase (Fig. 4).
Fig. 4.
Dimethylarginine dimethylaminohydrolase expression in renal proximal tubules from V-, 1% NaCl-, BSO-, and BSO + NaCl-treated rats. Blots (dimethylarginine dimethylaminohydrolase-DDAH ∼38 kDa and GAPDH) shown are a single experiment, representative of 6–8 individual experiments, and bars (DDAH/GAPDH) represent means ± SE. Proximal tubular homogenate (60 μg protein for DDAH and 10 μg protein for GAPDH) was used for immunobloting. *P < 0.05 vs. V and $P < 0.05 vs. BSO, using 1-way ANOVA followed by post hoc Newman-Keuls multiple comparison test.
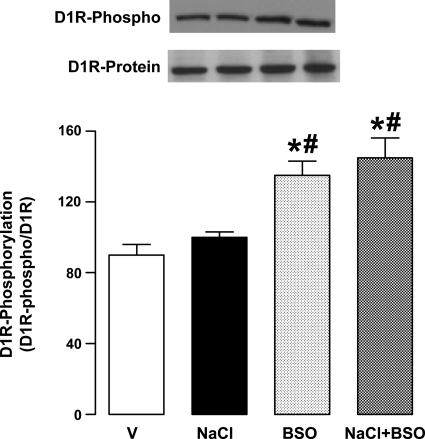
Effect of NaCl and BSO on renal proximal tubular membrane D1 receptor serine phosphorylation.
Treatment of rats with BSO increased proximal tubular membrane D1 receptor serine phosphorylation (Fig. 5). Compared with vehicle, NaCl intake did not affect the D1 receptor phosphorylation (Fig. 5). However, the membrane D1 receptor serine phosphorylation was significantly higher in BSO and BSO plus NaCl-treated rats compared with rats provided with vehicle or NaCl alone (Fig. 5). The membrane D1 receptor protein expression was similar in all experimental groups (Fig. 5).
Fig. 5.
Dopamine D1 receptor (D1R) phosphorylation in renal proximal tubular membranes from V-, 1% NaCl-, BSO-, and BSO + NaCl-treated rats. Membrane D1 receptors were immunoprecipitated, followed by immunoblotting for D1 receptor protein (D1R ∼52 kDa) and serine phosphorylation (D1R-phospho). Blots shown are a single experiment, representative of 6–8 individual experiments, and bars (D1R-phospho/D1R protein) represent means ± SE. *P < 0.05 vs. V and #P < 0.05 vs. NaCl, using 1-way ANOVA followed by post hoc Newman-Keuls multiple comparison test.
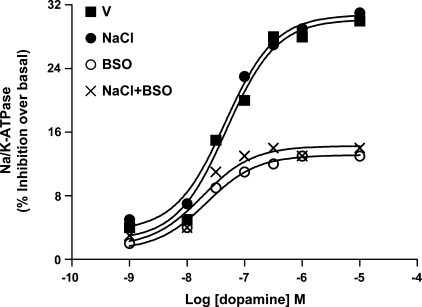
Effect of NaCl and BSO on dopamine-induced Na-K-ATPase inhibition in renal proximal tubules.
Supplementation of rats with NaCl caused a significant decrease in proximal tubular basal Na-K-ATPase activity (V: 11.3 ± 0.3; NaCl: 6.6 ± 0.2 mmol Pi·mg protein−1·h−1). The decrease in basal activity was blunted in rats treated with NaCl plus BSO (10.3 ± 0.2). BSO alone had no effect on basal Na-K-ATPase activity (10.5 ± 0.4). Dopamine caused a concentration-dependent inhibition of Na-K-ATPase activity in proximal tubules from vehicle- and NaCl-treated rats, but failed to inhibit Na-K-ATPase activity in BSO and BSO plus NaCl rats (Fig. 6).
Fig. 6.
Dopamine-induced Na-K-ATPase inhibition in renal proximal tubules from V-, 1% NaCl-, BSO-, and BSO + NaCl-treated rats. Proximal tubules were challenged with the indicated doses of dopamine for 20 min and data are expressed as a percentage of inhibition produced by the indicated concentration of dopamine. A single experiment is shown, representative of 6–8 individual experiments, performed in triplicate.
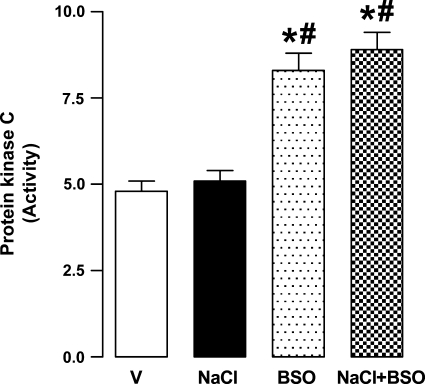
Effect of NaCl and BSO on protein kinase C activity in renal proximal tubules.
As shown in Fig. 7, the basal protein kinase C activity was significantly higher in BSO and BSO plus NaCl-treated rats compared with vehicle. NaCl alone had no effect on basal protein kinase C activity (Fig. 7).
Fig. 7.
Protein kinase C activity (ng peptide phosphorylated·mg protein−1·min−1) in renal proximal tubular homogenates from V-, 1% NaCl-, BSO-, and BSO + NaCl-treated rats. Bars represent means ± SE from 6–8 animals performed in triplicate. *P < 0.05 vs. V and #P < 0.05 vs. NaCl, using 1-way ANOVA followed by post hoc Newman-Keuls multiple comparison test.
DISCUSSION
Our results show that concomitant inhibition of renal D1 receptor signaling and nitric oxide bioavailability increases blood pressure in Sprague-Dawley rats. The decrease in nitric oxide level alone or an inhibition of renal D1 receptor did not affect the blood pressure. We found that NaCl intake increased urinary dopamine level, reduced basal Na-K-ATPase activity, and evoked sodium natriuresis. However, NaCl intake reduced urinary nitric oxide level despite increased nitric oxide synthase 3 protein expression. NaCl also increased asymmetric dimethylarginine levels and reduced dimethylarginine dimethylaminohydrolase expression. BSO treatment caused oxidative stress, protein kinase C activation, D1 receptor hyperphosphorylation, and failure of dopamine to inhibit proximal tubular Na-K-ATPase activity. Rats cosupplemented with NaCl and BSO had impaired D1 receptor function and nitric oxide system and exhibited significant increase in blood pressure. Oxidative stress may also have contributed to high blood pressure in BSO plus NaCl rats as antioxidant tempol reduced oxidative stress and normalized blood pressure.
Under physiological conditions, nitric oxide promotes sodium excretion and nitric oxide-induced natriuresis and diuresis can occur in the absence of hemodynamic changes, indicating that nitric oxide directly inhibits Na and water absorption along the nephron (18, 25, 31, 32, 36). In vitro studies showed that nitric oxide blunts sodium transport in isolated proximal tubules, cortical collecting ducts, inner medullary collecting ducts, and thick ascending limbs (23, 24, 32, 36). Additionally, nitric oxide is reported to inhibit Na transport in several cultured renal cells (23, 24). Impaired nitric oxide production in response to salt loading has been implicated in the pathogenesis of salt sensitivity (10, 12, 35). In animal models of salt-sensitive hypertension, the increase in blood pressure after salt loading is characterized by reduced nitric oxide production (10, 12, 35). Inhibition of nitric oxide synthesis in salt-resistant rats induces hypertension when the animals are exposed to a high intake of salt (10, 12, 35). Together, these observations point to the role of defective nitric oxide response in salt-sensitive hypertension. However, decreased nitric oxide production in response to salt loading in non-salt-sensitive Sprague-Dawley rats did not lead to hypertension (14). We found that in Sprague-Dawley rats, NaCl intake increased urinary dopamine level, decreased basal Na-K-ATPase activity, and caused marked natriuresis. Therefore, we reasoned that Sprague-Dawley rats remain normotensive during NaCl intake despite decreased urinary nitric oxide because of the functional renal D1 receptor signaling.
There are remarkable parallels in abnormal D1 receptor signaling in rodent hypertensive models and in human essential hypertension (2, 7, 19, 20). In both, there is a failure of dopamine to activate D1 receptors and cause inhibition of Na/H exchanger and Na-K-ATPase activities in renal proximal tubules (2, 7, 19, 20). Defect in D1 receptor is also implicated in salt-sensitive and obesity-associated hypertension (2, 7, 19, 20). Although there exists a direct correlation between oxidative stress and D1 receptor dysfunction in hypertensive animal models, we found that old rats and rats treated with streptozotocin exhibited oxidative stress but remained normotensive despite an inhibition of renal D1 receptor function (4, 27). In agreement with the later finding, the present data also show that BSO-induced oxidative stress failed to increase blood pressure despite D1 receptor inhibition. Although the mechanism for the absence of hypertension in BSO-treated rats is not clear, it is possible that the intact nitric oxide system in these animals may be compensating for inhibited D1 receptor function.
To further clarify these issues, we designed an animal model with inhibited renal D1 receptor function and nitric oxide system. It is well-recognized that in most hypertensive models the defect in D1 receptor function coexists with impaired nitric oxide signaling or production. Since both dopamine and nitric oxide are known to inhibit renal sodium transporters and increase sodium excretion, it is conceivable that simultaneous failure of both the pathways may be causing decreased sodium excretion and hypertension. Our results show that this is indeed the case in Sprague-Dawley rats treated with BSO plus NaCl as concomitant decrease in nitric oxide level and inhibition of D1 receptor function increased blood pressure. It is of interest that antioxidant treatment of BSO plus NaCl rats normalized the blood pressure, suggesting that oxidative stress could be another important contributing factor to salt sensitivity.
The observation that NaCl intake reduced renal nitric oxide production in Sprague-Dawley rats shows that these effects are not specific to genetically salt-sensitive hypertension, but could be observed in salt-resistant animal models as well. The exact mechanism responsible for high salt-induced decrease in urinary nitric oxide is not known. It is reported that high-salt intake increases renal superoxide production which could convert nitric oxide to peroxynitrite and thus reduce its bioavailability (21, 38). However, in the present study, NaCl intake failed to increase the 8-isoprostane or malondialdehyde levels thus it does not support the idea that bioavailability of nitric oxide was reduced due to oxidative stress. We found that NaCl intake decreased dimethylarginine dimethylaminohydrolase expression which may have contributed to increased production of asymmetric dimethylarginine. The asymmetric dimethylarginine is an endogenous inhibitor of nitric oxide synthases and could be responsible for decreased nitric oxide level. It is worth noting that decrease in urinary nitric oxide cannot be attributed to reduced nitric oxide synthase expression as there was increased nitric oxide synthase 3 protein content in both NaCl- and BSO-treated rats. The increase in nitric oxide synthase 3 expression could be a compensatory mechanism to increased asymmetric dimethylarginine level in NaCl-treated rats and increased free radical production in BSO-treated rats.
In present study, we observed that BSO caused inability of dopamine to inhibit renal Na-K-ATPase. Although the detailed mechanism was not studied, we found that BSO treatment increased basal protein kinase C activity and D1 receptor phosphorylation. Previous reports from our and other laboratories show that D1 receptor hyperphosphorylation via protein kinase C activation can lead to D1 receptor uncoupling from effector complex and failure to inhibit Na transporters (4, 8, 20).
In conclusion, our studies provide substantial evidence that renal natriuretic factors play an important role in maintaining sodium homeostasis and blood pressure in conditions associated with high-salt intake. However, under situations in which both renal nitric oxide (high salt) and dopamine (oxidative stress) systems are compromised, animals develop hypertension.
GRANTS
This study was supported by Scientist Development Grant 0835428N from the American Heart Association (to A. A. Banday) and National Institutes of Health Grant AG-25056 from the National Institute on Aging (to M. F. Lokhandwala).
REFERENCES
- 1.Aperia A, Bertorello A, Seri I. Dopamine causes inhibition of Na+-K+-ATPase activity in rat proximal convoluted tubule segments. Am J Physiol Renal Fluid Electrolyte Physiol 252: F39–F45, 1987. [DOI] [PubMed] [Google Scholar]
- 2.Aperia AC Intrarenal dopamine: a key signal in the interactive regulation of sodium metabolism. Annu Rev Physiol 62: 621–647, 2000. [DOI] [PubMed] [Google Scholar]
- 3.Asghar M, Hussain T, Lokhandwala MF. Activation of dopamine D1-like receptor causes phosphorylation of alpha1 subunit of Na+,K+-ATPase in rat renal proximal tubules. Eur J Pharmacol 411: 61–66, 2001. [DOI] [PubMed] [Google Scholar]
- 4.Asghar M, Hussain T, Lokhandwala MF. Higher basal serine phosphorylation of D1A receptors in proximal tubules of old Fischer 344 rats. Am J Physiol Renal Physiol 283: F350–F355, 2002. [DOI] [PubMed] [Google Scholar]
- 5.Asghar M, Kansra V, Hussain T, Lokhandwala MF. Hyperphosphorylation of Na pump contributes to defective renal dopamine response in old rats. J Am Soc Nephrol 12: 226–232, 2001. [DOI] [PubMed] [Google Scholar]
- 6.Banday AA, Hussain T, Lokhandwala MF. Renal dopamine D1 receptor dysfunction is acquired and not inherited in obese Zucker rats. Am J Physiol Renal Physiol 287: F109–F116, 2004. [DOI] [PubMed] [Google Scholar]
- 7.Banday AA, Lokhandwala MF. Dopamine receptors and hypertension. Curr Hypertens Rep 10: 268–275, 2008. [DOI] [PubMed] [Google Scholar]
- 8.Banday AA, Marwaha A, Tallam LS, Lokhandwala MF. Tempol reduces oxidative stress, improves insulin sensitivity, decreases renal dopamine D1 receptor hyperphosphorylation, and restores D1 receptor G protein coupling and function in obese Zucker rats. Diabetes 54: 2219–2226, 2005. [DOI] [PubMed] [Google Scholar]
- 9.Bayorh MA, Ganafa AA, Socci RR, Silvestrov N, Abukhalaf IK. The role of oxidative stress in salt-induced hypertension. Am J Hypertens 17: 31–36, 2004. [DOI] [PubMed] [Google Scholar]
- 10.Bloch J, Qiu C, Erdely A, Baylis C. Inhibition of inducible nitric oxide synthase during high dietary salt intake. Am J Hypertens 15: 230–235, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen CJ, Lokhandwala MF. Role of endogenous dopamine in the natriuretic response to various degrees of iso-osmotic volume expansion in rats. Clin Exp Hypertens 13: 1117–1126, 1991. [DOI] [PubMed] [Google Scholar]
- 12.Chen PY, Gladish RD, Sanders PW. Vascular smooth muscle nitric oxide synthase anomalies in Dahl/Rapp salt-sensitive rats. Hypertension 31: 918–924, 1998. [DOI] [PubMed] [Google Scholar]
- 13.Chen PY, Sanders PW. l-Arginine abrogates salt-sensitive hypertension in Dahl/Rapp rats. J Clin Invest 88: 1559–1567, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Debinski W, Kuchel O, Buu NT, Nemer M, Tremblay J, Hamet P. Effect of prolonged high salt diet on atrial natriuretic factor in rats. Proc Soc Exp Biol Med 194: 251–257, 1990. [DOI] [PubMed] [Google Scholar]
- 15.Felder CC, Campbell T, Albrecht F, Jose PA. Dopamine inhibits Na+-H+ exchanger activity in renal BBMV by stimulation of adenylate cyclase. Am J Physiol Renal Fluid Electrolyte Physiol 259: F297–F303, 1990. [DOI] [PubMed] [Google Scholar]
- 16.Fujiwara N, Osanai T, Kamada T, Katoh T, Takahashi K, Okumura K. Study on the relationship between plasma nitrite and nitrate level and salt sensitivity in human hypertension: modulation of nitric oxide synthesis by salt intake. Circulation 101: 856–861, 2000. [DOI] [PubMed] [Google Scholar]
- 17.Gill PS, Wilcox CS. NADPH oxidases in the kidney. Antioxid Redox Signal 8: 1597–1607, 2006. [DOI] [PubMed] [Google Scholar]
- 18.Herrera M, Ortiz PA, Garvin JL. Regulation of thick ascending limb transport: role of nitric oxide. Am J Physiol Renal Physiol 290: F1279–F1284, 2006. [DOI] [PubMed] [Google Scholar]
- 19.Jose PA, Eisner GM, Felder RA. Renal dopamine and sodium homeostasis. Curr Hypertens Rep 2: 174–183, 2000. [DOI] [PubMed] [Google Scholar]
- 20.Jose PA, Eisner GM, Felder RA. Renal dopamine receptors in health and hypertension. Pharmacol Ther 80: 149–182, 1998. [DOI] [PubMed] [Google Scholar]
- 21.Kitiyakara C, Chabrashvili T, Chen Y, Blau J, Karber A, Aslam S, Welch WJ, Wilcox CS. Salt intake, oxidative stress, and renal expression of NADPH oxidase and superoxide dismutase. J Am Soc Nephrol 14: 2775–2782, 2003. [DOI] [PubMed] [Google Scholar]
- 22.Laemmli UK Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227: 680–685, 1970. [DOI] [PubMed] [Google Scholar]
- 23.Liang M, Knox FG. Nitric oxide activates PKCα and inhibits Na+-K+-ATPase in opossum kidney cells. Am J Physiol Renal Physiol 277: F859–F865, 1999. [DOI] [PubMed] [Google Scholar]
- 24.Liang M, Knox FG. Nitric oxide reduces the molecular activity of Na+,K+-ATPase in opossum kidney cells. Kidney Int 56: 627–634, 1999. [DOI] [PubMed] [Google Scholar]
- 25.Liang M, Knox FG. Production and functional roles of nitric oxide in the proximal tubule. Am J Physiol Regul Integr Comp Physiol 278: R1117–R1124, 2000. [DOI] [PubMed] [Google Scholar]
- 26.Manning RD, Tian N, Meng S. Oxidative stress and antioxidant treatment in hypertension and the associated renal damage. Am J Nephrol 25: 311–317, 2005. [DOI] [PubMed] [Google Scholar]
- 27.Marwaha A, Lokhandwala MF. Tempol reduces oxidative stress and restores renal dopamine D1-like receptor G protein coupling and function in hyperglycemic rats. Am J Physiol Renal Physiol 291: F58–F66, 2006. [DOI] [PubMed] [Google Scholar]
- 28.Missale C, Nash SR, Robinson SW, Jaber M, Caron MG. Dopamine receptors: from structure to function. Physiol Rev 78: 189–225, 1998. [DOI] [PubMed] [Google Scholar]
- 29.Ni Z, Vaziri ND. Effect of salt loading on nitric oxide synthase expression in normotensive rats. Am J Hypertens 14: 155–163, 2001. [DOI] [PubMed] [Google Scholar]
- 30.Nishi A, Eklof AC, Bertorello AM, Aperia A. Dopamine regulation of renal Na+,K+-ATPase activity is lacking in Dahl salt-sensitive rats. Hypertension 21: 767–771, 1993. [DOI] [PubMed] [Google Scholar]
- 31.Ortiz PA, Garvin JL. Intrarenal transport and vasoactive substances in hypertension. Hypertension 38: 621–624, 2001. [DOI] [PubMed] [Google Scholar]
- 32.Ortiz PA, Garvin JL. Role of nitric oxide in the regulation of nephron transport. Am J Physiol Renal Physiol 282: F777–F784, 2002. [DOI] [PubMed] [Google Scholar]
- 33.Osborn JW, Hornfeldt BJ. Arterial baroreceptor denervation impairs long-term regulation of arterial pressure during dietary salt loading. Am J Physiol Heart Circ Physiol 275: H1558–H1566, 1998. [DOI] [PubMed] [Google Scholar]
- 34.Tojo A, Kimoto M, Wilcox CS. Renal expression of constitutive NOS and DDAH: separate effects of salt intake and angiotensin. Kidney Int 58: 2075–2083, 2000. [DOI] [PubMed] [Google Scholar]
- 35.Tolins JP, Shultz PJ. Endogenous nitric oxide synthesis determines sensitivity to the pressor effect of salt. Kidney Int 46: 230–236, 1994. [DOI] [PubMed] [Google Scholar]
- 36.Varela M, Herrera M, Garvin JL. Inhibition of Na-K-ATPase in thick ascending limbs by NO depends on O2− and is diminished by a high-salt diet. Am J Physiol Renal Physiol 287: F224–F230, 2004. [DOI] [PubMed] [Google Scholar]
- 37.White BH, Sidhu A. Increased oxidative stress in renal proximal tubules of the spontaneously hypertensive rat: a mechanism for defective dopamine D1A receptor/G protein coupling. J Hypertens 16: 1659–1665, 1998. [DOI] [PubMed] [Google Scholar]
- 38.Wilcox CS Oxidative stress and nitric oxide deficiency in the kidney: a critical link to hypertension? Am J Physiol Regul Integr Comp Physiol 289: R913–R935, 2005. [DOI] [PubMed] [Google Scholar]