Abstract
Calcineurin (PP2B) has recently been shown to be upregulated in the medullary thick ascending limb (mTAL) during diabetes. The mTAL expresses all three isoforms of nitric oxide synthase (NOS), which are subject to phosphoregulation and represent substrates for PP2B. Therefore, we hypothesized that diabetes induces PP2B-dependent upregulation of NOS activity and NO production in the mTAL. Three weeks after injection of streptozotocin (STZ rats) or vehicle (sham rats), mTAL suspensions were prepared for use in functional and biochemical assays. PP2B activity and expression were increased in mTALs from STZ rats compared with sham. Nitrite production was significantly reduced in mTALs from STZ rats compared with sham. However, incubation with the free radical scavenger, tempol, unmasked a significant increase in nitrite production by mTALs from STZ rats. Inhibition of PP2B attenuated the increase in nitrite production and NOS activity evident in mTALs from STZ rats. Analysis of specific NOS isoform activity revealed increased NOS1 and NOS2 activities in mTALs from STZ rats. All three NOS isoform activities were regulated in a PP2B-dependent manner. Western blot analysis detected no differences in NOS isoform expression, although phosphorylation of pThr495-NOS3 was significantly reduced in mTALs from STZ rats. Phosphorylation of pSer852-NOS1, pSer633-NOS3, and pSer1177-NOS3 was similar in mTALs from STZ and sham rats. Inhibition of PP2B did not alter the phosphorylation of NOS1 or NOS3 at known sites. In conclusion, while NO bioavailability in mTALs is reduced during diabetes, free radical scavenging with tempol unmasks increased NO production that involves PP2B-dependent activation of NOS1 and NOS2.
Keywords: diabetes mellitus, nitric oxide, nitric oxide synthase, protein phosphatase 2B, calcineurin
gooch et al. (11) reported increased expression and activity of the serine/threonine protein phosphatase 2B (PP2B; calcineurin) in the renal cortex and medulla of rats with streptozotocin (STZ)-induced diabetes mellitus (STZ rats; 2 wk after onset). The most prominent increase in PP2B immunostaining in kidneys from STZ rats is evident in the medullary thick ascending limb (mTAL) (12). STZ rats treated chronically with the selective PP2B inhibitor, cyclosporin A (CsA), exhibit increased transforming growth factor (TGF)-β and fibronectin mRNA levels and extracellular matrix accumulation in the renal tubulointerstitium, as well as exaggerated diabetic hyperfiltration relative to untreated STZ rats (11). Thus, upregulation of PP2B may represent a protective mechanism to limit renal damage during diabetes.
Among the myriad substrates of PP2B, nitric oxide synthases (NOS) are widely expressed in the kidney and changes in their phosphorylation status represent a major mechanism controlling NO production. There are known serine/threonine phosphorylation sites on NO synthase 1 (NOS1; neuronal NOS) and NO synthase 3 (NOS3; endothelial NOS). PP2B has been shown to dephosphorylate pSer852NOS1 in hypothalamic neurons (50), as well as pSer116NOS3 and pThr495NOS3 in endothelial cells (15). Dephosphorylation of the NOS1 site and the threonine NOS3 site results in increased NOS activity and NO production. Thus, increased PP2B activity in the mTAL during diabetes may promote increased NOS activity and NO production. Although limited investigation focused on the NO pathway in the renal medulla during diabetes, our laboratory documented increased NOS3 activity without concomitant changes in NOS3 protein levels in renal medullary homogenates from STZ rats (22). Shin et al. (42) found increased NOS1 and NOS3 mRNA expression in the outer medulla of STZ rats, but no change in NO synthase 2 (NOS2; inducible NOS) mRNA, with the greatest increase in immunostaining intensity for NOS1 and NOS3 located in the proximal straight tubule and the mTAL. Interestingly, very little is known regarding the regulation of NOS2 phosphorylation. Currently, there is no evidence in the literature suggesting that PP2B may regulate NOS2 phosphorylation.
Despite immunohistochemical evidence suggesting that both PP2B and NOS protein levels may be upregulated in the mTAL during diabetes, there is no specific information available regarding the impact of diabetes on the activities of these enzymes in the mTAL or the role of PP2B in regulating NOS activity and NO production by the mTAL under these conditions. The overall hypothesis guiding our study states that diabetes induces PP2B-dependent NO production and NOS activity. Accordingly, the aims of this study were 1) to verify the upregulation of PP2B activity and expression in mTAL suspensions from diabetic rats, 2) to test the hypothesis that NO production and NOS activity are increased in mTALs from diabetic rats and to determine which NOS isoform(s) are involved, and 3) to test the hypothesis that increased activity of one or more NOS isoforms in the mTAL during diabetes is PP2B dependent.
METHODS
Animal model.
All animal studies were approved by the Medical College of Georgia Institutional Animal Care and Use Committee (IACUC) and performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Experiments utilized male Sprague-Dawley rats (250 g body wt, Harlan Laboratories). On the first day of the study, each rat was randomly divided into two groups: sham rats (vehicle treatment) and STZ rats (STZ-induced diabetes). The rats were weighed and initial blood glucose measurements were obtained (Accu-Check III model 766; Boehringer Mannheim, Indianapolis, IN). Under isoflurane anesthesia, STZ (65 mg/kg; Sigma, St. Louis, MO) or vehicle (saline) was injected intravenously. Upon recovery from anesthesia, the rats were returned to the animal facility and provided free access to food and water. The following day, a 2.3 × 2.0-mm sustained-release insulin implant (Linplant; Linshin Canada, Scarborough, Ontario) was inserted subcutaneously in STZ rats to maintain afternoon blood glucose levels within 400–500 mg/dl range. Sham rats received a vehicle (palmitic acid) implant. Blood glucose and body weights were measured approximately every 3 days. Terminal studies were performed 3 wk after STZ or vehicle injection.
mTAL suspensions and incubations.
mTAL suspensions were prepared following the method originally described by Chamberlin et al. (3), with slight modifications. Briefly, under pentobarbital sodium anesthesia (50 mg/kg ip), the kidneys were flushed via retrograde perfusion from the aorta below the renal arteries with 20 ml of 95% O2-5% CO2-equilibrated HBSS (MediaTech, Manassas, VA) containing 1 mg/ml collagenase (Sigma), protease inhibitors (1 μM aprotinin, 2 μM leupeptin, 1 μM pepstatin A, 1 mM PMSF; Sigma), a panel of phosphatase inhibitors targeting acid and alkaline phosphatases and protein tyrosine phosphatases but not serine/threonine phosphatases (2 mM imidazole, 1 mM NaF, 1.15 mM Na2MoO4, 1 mM Na3VO4, 4 mM Na2C4H4O6·2H2O; from Calbiochem Phosphatase Inhibitor Cocktail Set II) and either 5.5 mM glucose (sham rats) or 20 mM glucose (STZ rats). The inner stripe of the outer medulla was dissected from coronal slices of the kidneys and minced into ∼1-mm3 pieces using a McIlwain tissue chopper (Warner Instruments, Hamden, CT). Tissue pieces were digested in HBSS/collagenase with protease and phosphatase inhibitors at 37°C for 30 min while bubbling with 95% O2-5% CO2, with gentle agitation every 5 min. After centrifugation, pellets were resuspended in ice-cold HBSS without collagenase with protease and phosphatase inhibitors and continuously agitated on ice for 20 min. The nephron segments were filtered through a 250-μm nylon mesh and washed twice with ice-cold HBSS. For most experiments, the resulting mTAL suspensions were incubated at 37°C for 30 min in the presence of one or more of the following: 250 μM l-arginine (NOS substrate; Sigma), 10 mM 4-hydroxy-TEMPO (tempol; free radical scavenger; Sigma), 200 U/ml PEG-SOD (polyethylene glycol-superoxide dismutase; cell-permeable superoxide scavenger; Sigma), 100 ng/ml CsA (PP2B inhibitor; Sigma), 100 μM calcineurin auto-inhibitory peptide (CAIP; PP2B inhibitor, Calbiochem/EMD, Gibbstown, NJ), 100 μM scrambled peptide (SP; Genscript, Piscataway, NJ), 100 μM poly-arginine peptide (Genscript), 1 μM N5-(1-imino-3-butenyl)-l-ornithine (VNIO; NOS1 inhibitor; Alexis/Enzo, Plymouth Meeting, PA), 100 nM 1400W dihydrochloride (1400W; NOS2 inhibitor; Cayman, Ann Arbor, MI), or vehicle (HBSS). Following this incubation, mTAL suspensions were pelleted by centrifugation. The incubation buffer and mTAL pellets were flash-frozen separately in liquid nitrogen for storage at −80°C. Nitrite levels were measured in the incubation buffer; the mTAL pellets were utilized for Western blot analysis, as well as measurement of PP2B activity and NOS activity.
CAIP is composed of the calcineurin autoinhibitory domain fused to a poly-arginine-based protein transduction domain and acts as an inhibitor of PP2B activity (45). The poly-arginine domain allows the peptide to be cell permeable. The CAIP sequence is as follows: Ac-Arg-Arg-Arg-Arg-Arg-Arg-Arg-Arg-Arg-Arg-Arg-Gly-Gly-Gly-Arg-Met-Ala-Pro-Pro-Arg-Arg-Asp-Ala-Met-Pro-Ser-Asp-Ala-NH2. The control SP sequence and the poly-arginine peptide sequence are Ac-Arg-Arg-Arg-Arg-Arg-Arg-Arg-Arg-Arg-Arg-Arg-Gly-Gly-Gly-Arg-Met-Arg-Asp-Arg-Pro-Ala-Pro-Ala-Met-Asp-Pro-Ser-Ala-NH2 and Ac-Arg-Arg-Arg-Arg-Arg-Arg-Arg-Arg-Arg-Arg-Arg-NH2, respectively.
PP2B activity.
PP2B activity was measured using a commercially available, colorimetric Calcineurin Activity Kit (Calbiochem) that utilizes the RII phosphopeptide substrate. Briefly, mTALs were lysed with manufacturer's supplied lysis buffer and centrifuged at 150,000 g. The soluble fraction was desalted to remove excess phosphate. To discriminate between the activity of PP2B and other phosphatases, activity was measured in the presence and absence of EGTA (Ca2+ chelator) and okadaic acid (PP1 and PP2A inhibitor). Free phosphate released was detected using the malachite green reagent, with absorbance read at 620 nm. Protein concentration was determined using the standard Bradford assay. Activity is reported as nanomoles of phosphate per milligram of protein per 30 min.
NOS activity.
NOS activity was determined by the conversion of [3H]arginine to [3H]citrulline, as previously described (22). Briefly, mTALs incubated in the presence/absence of CsA, but in the absence of exogenous l-arginine, were lysed by sonication in homogenization buffer (39) containing protease inhibitors. Total NOS activity was defined as the [3H]arginine to [3H]citrulline conversion inhibited by the nonselective NOS inhibitor Nω-nitro-l-arginine (l-NNA; 1 mM). The activity of each NOS isoform was determined based on the impact of isoform-selective inhibitors of NOS1 (1 μM VNIO) and NOS2 (100 nM 1400W). NOS3 activity was calculated as total NOS activity minus NOS1 and NOS2 activities. Results were normalized to protein concentration determined by the Bradford method and expressed as picomoles per nanogram of protein per 30 min.
Nitrite production.
After incubation of mTAL suspensions for 30 min in the presence of exogenous l-arginine and in the absence/presence of various inhibitors, the incubation buffer was collected for determining nitrite production. The concentration of nitrite, a metabolite of NO, in the incubation buffer was measured using a sensitive HPLC system (ENO-20; EiCom, Kyoto, Japan), according to the manufacturer's protocol. Briefly, the nitrite present in 80 μl of mTAL incubation buffer was separated from other substances on a unique separation column and mobile phase. Nitrite was reacted with Griess reagent postcolumn to generate diazo compounds that were measured by absorbance at 540 nm with an in-line detection system. The sensitivity limit of this assay is 0.1 pmol nitrite and sample quantification was achieved using a nitrite standard curve. Nitrite was normalized to protein concentration determined by the Bradford method (Bio-Rad).
Western blot analysis.
Western blot analysis was performed as described previously (39) with modifications. Briefly, mTALs were lysed by sonication in homogenization buffer (39) containing protease and phosphatase inhibitors. Proteins (80 μg per lane) were separated by SDS-PAGE and transferred to PVDF. Membranes were blocked with Blocking One-P buffer (Nacalai Tesque, Kyoto, Japan) for 1 h at room temperature and incubated overnight with primary antibodies directed against one or more of the following proteins: PP2B-Aβ (1:1,000; Upstate/Millipore, Billerica, MA), PP2B-B (1:1,000; Upstate), NOS1 (1:250; BD Transduction Laboratories, San Jose, CA), NOS2 (1:100; Santa Cruz Biotechnology, Santa Cruz, CA), NOS3 (1:500; BD Transduction Laboratories), and β-actin (1:10,000; Sigma). Some assays employed phosphorylation site-specific primary antibodies targeting pSer1177NOS3 (1:500; BD Transduction Laboratories), pSer633NOS3 (1:500; Upstate), pSer617NOS3 (1:500; Upstate), pThr495NOS3 (1:500; Upstate), pSer116NOS3 (1:500; Upstate), or pSer852NOS1 (1:100; Santa Cruz Biotechnology). After incubation with secondary antibody (IRDye 800-conjugated affinity-purified anti-mouse IgG, 1:2,000 or AlexaFluor 680-conjugated affinity-purified anti-rabbit IgG, 1:2,000), the membrane was scanned and the intensity of specific bands was quantified using the Odyssey Infrared Imaging System (LI-COR Biosciences, Lincoln, NE).
Live/dead assay.
The Molecular Probes LIVE/DEAD Viability/Cytotoxicity Kit (Invitrogen, Carlsbad, CA) was used to measure cell viability, according to the manufacturer's standard protocol. Briefly, mTALs were incubated for 30 min in the presence of l-arginine (250 μM), tempol (10 mM), and in the presence/absence of CsA (100 ng/ml) or CAIP (100 μM). Following incubation, 100 μl of mTAL suspensions were loaded onto a 96-well plate in triplicate. Calcein AM fluorescent dye (2 μM) was added to each well for 30 min. Fluorescence was determined using an automated plate reader at the manufacturer's suggested settings. Results are expressed as percent viable cells.
Statistics.
All values are reported as means ± SE (n = number of animals). Statistical comparisons were performed using an unpaired t-test or two-way ANOVA followed by the Holm-Sidek Multiple-Comparison Test, as appropriate. In instances in which the normality test failed due to groups with undetectable activity, a Kruskal-Wallis one-way ANOVA on Ranks was employed. Values of P < 0.05 were considered significant.
RESULTS
During the 3-wk period between injection of STZ (or vehicle) and the terminal study, blood glucose values were significantly greater in STZ rats (473 ± 15 mg/dl; n = 22) than in sham rats (94 ± 3 mg/dl; n = 20; P < 0.05). Rats in both groups gained weight during this period, although the STZ rats did so a slower rate. Accordingly, at the time of the terminal study, body weight of sham rats (320 ± 7 g; n = 20) significantly exceeded that of STZ rats (284 ± 4 g; n = 22; P < 0.05).
PP2B expression and activity.
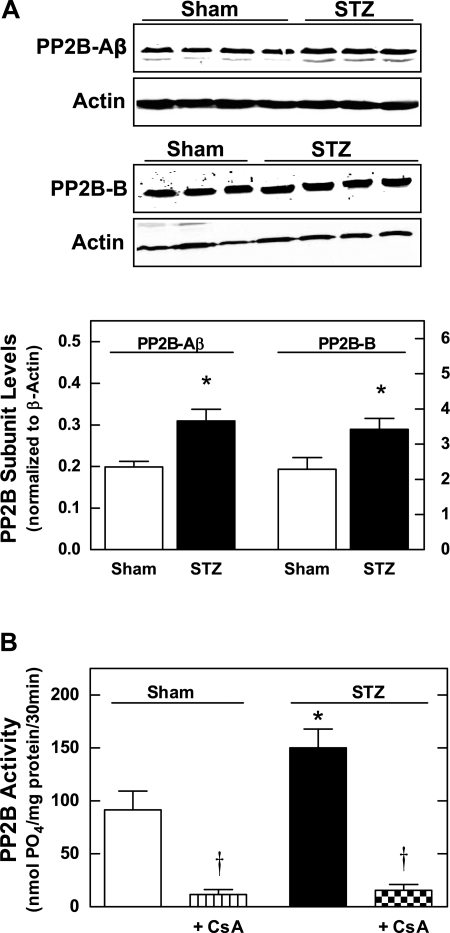
PP2B protein levels were measured by Western blot analysis of the regulatory (PP2B-B) and catalytic (PP2B-Aβ) subunits in mTAL homogenates from sham and STZ rats. As shown in Fig. 1A, both subunits of PP2B were significantly upregulated in mTALs from STZ rats compared with sham. In addition, PP2B activity was increased by ∼40% in mTALs from STZ rats compared with sham (Fig. 1B). Furthermore, 30-min incubation of mTALs with 100 ng/ml CsA significantly reduced PP2B activity by ∼90% (P < 0.05 vs. untreated) in both sham and STZ groups, thus validating the efficacy of the acute CsA treatment as a PP2B inhibitor in our experimental setting.
Fig. 1.
Calcineurin (PP2B) subunit protein expression and PP2B activity in medullary thick ascending limbs (mTALs) from streptozotocin (STZ) and sham rats. A, top: representative Western blots of PP2B-Aβ (cytosolic subunit) and PP2B-B (regulatory subunit). A, bottom: summary of relative intensity data from Western blots (normalized to β-actin). *P < 0.05 vs. sham. B: PP2B activity measured in the presence and absence of the PP2B inhibitor, cyclosporin A (CsA; 100 ng/ml). Values are means ± SE (n = 5–8 rats). *P < 0.05 vs. sham. †P < 0.05 vs. untreated.
NOS activity.
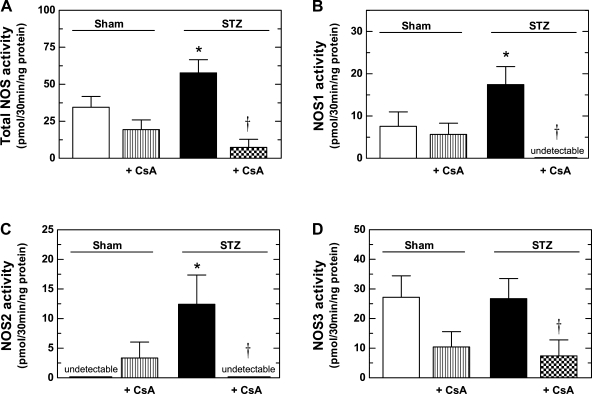
Total NOS activity was significantly greater in mTAL homogenates from STZ rats than in homogenates prepared from sham rats (Fig. 2A). Moreover, incubation of the mTALs with CsA substantially reduced total NOS activity in mTALs from STZ rats, but had no significant effect on total NOS activity in mTALs from sham rats (Fig. 2A). NOS1 and NOS2 activities were found to be increased in mTALs from STZ rats, compared with sham (Fig. 2, B and C). Incubation in the presence of CsA reduced both NOS1 and NOS2 activities to levels below detection in mTALs from STZ rats, but did not significantly alter NOS1 or NOS2 activities in mTALs from sham rats. NOS3 activity in mTAL homogenates did not differ between STZ and sham rats (Fig. 2D); however, CsA treatment significantly reduced NOS3 activity in STZ rats (P < 0.05 vs. untreated). Thus, although NOS3 activity in the mTAL is PP2B dependent, it is not increased during diabetes. Rather, NOS1 and NOS2 activities are significantly increased in the mTAL during diabetes in a PP2B-dependent manner.
Fig. 2.
Effect of PP2B inhibition (100 ng/ml CsA) on nitric oxide synthase (NOS) activity in mTALs from STZ and sham rats. A: total NOS activity. B: NOS1 activity. C: NOS2 activity. D: NOS3 activity. Values are means ± SE (n = 5–14 rats). *P < 0.05 vs. sham. †P < 0.05 vs. untreated.
Nitrite production.
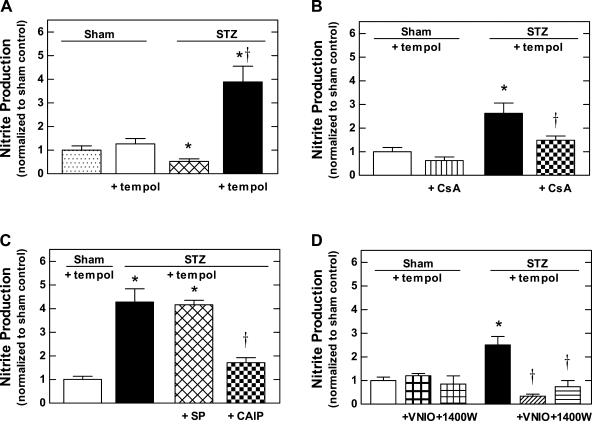
Nitrite production by mTALs was measured based on accumulation of nitrite, an NO metabolite, in the buffer during a 30-min incubation period. Results were normalized to sham control (256 ± 74 pmol nitrite·mg protein−1·30 min−1; n = 9). In preliminary experiments, nitrite production was not affected by the presence or absence of l-arginine in the incubation media (data not shown); nevertheless, because NO production can be substrate limited in mTALs studied in vitro (37), the nitrite production experiments presented here were all conducted in the presence of 250 μM l-arginine. Under these conditions and in the absence of pharmacological agents, nitrite production by mTALs from STZ rats was ∼50% less than that of mTALs from sham rats (P < 0.05; Fig. 3A). As the mTAL is a primary renal site of superoxide anion (O2•−) production (23, 53), and because of the ability of O2•− to rapidly react with NO to form peroxynitrite (which is not subsequently metabolized to nitrite), we also measured nitrite production by mTALs incubated in the presence of 10 mM tempol (free radical scavenger) or 200 U/ml PEG-SOD (superoxide scavenger). Nitrite production by mTALs from STZ rats was increased more than sevenfold in the presence of tempol compared with that evident in the absence of tempol (Fig. 3A; P < 0.05). Moreover, in the presence of tempol, nitrite production by mTALs from STZ rats was three times higher than that of mTALs from sham rats (Fig. 3A; P < 0.05). Similarly, nitrite production by mTALs from STZ rats incubated in the presence of PEG-SOD was 3.3 ± 0.4 (n = 6) times higher than mTALs from sham rats (P < 0.05). Nitrite production by mTALs from sham rats was unaltered by tempol (Fig. 3A; P > 0.05). Based on these observations, nitrite production measured in the presence of tempol is considered to represent a reasonable index of NO production.
Fig. 3.
Nitrite production by mTALs from STZ and sham rats. A: effect of 10 mM tempol on nitrite production. B: effect of PP2B inhibition (100 ng/ml CsA) on nitrite production measured in the presence of 10 mM tempol. C: effect of PP2B inhibition [100 μM calcineurin auto-inhibitory peptide (CAIP)] and scrambled peptide (100 μM SP) on nitrite production measured in the presence of 10 mM tempol. D: effect of inhibiting NOS1 (1 μM VNIO) or NOS2 (100 nM 1400W) on nitrite production measured in the presence of 10 mM tempol. Values are normalized to sham control (256 ± 74 pmol nitrite·mg protein−1·30 min−1), expressed as means ± SE (n = 6–14 rats). *P < 0.05 vs. sham. †P < 0.05 vs. untreated.
To determine the impact of PP2B on NO production during diabetes, we measured nitrite production by mTALs from sham and STZ rats incubated with tempol in the presence or absence of CsA (100 ng/ml), CAIP (100 μM), SP (100 μM), or poly-arginine peptide (100 μM). As shown in Fig. 3B, there was no significant effect of CsA on mTALs from sham rats; however, incubation with CsA reduced nitrite production under these conditions by ∼44% in mTALs from STZ rats (P < 0.05 vs. STZ untreated/vehicle), achieving values that did not differ significantly from sham. As shown in Fig. 3C, CAIP reduced nitrite production by ∼59% in mTALs from STZ rats (P < 0.05 vs. STZ untreated/vehicle), while the SP (a control for CAIP) did not change nitrite production. The 11 amino acid poly-arginine peptide also failed to alter nitrite production (data not shown). CAIP inhibits PP2B by mimicking the endogenous auto-inhibitory domain of the PP2B-A subunit, while CsA must form a complex with cyclophilin, with the CsA-cyclophilin complex binding to the interface between the catalytic and regulatory subunits of the PP2B. CsA and CAIP are mechanistically different inhibitors of PP2B, thus these results indicate that accelerated NO production by mTALs from STZ rats is PP2B dependent. These results are similar to the effect of CsA on total NOS activity shown in Fig. 2.
Additional experiments were performed to determine which NOS isoform(s) represent the enzymatic source of accelerated NO production by mTALs during diabetes. Nitrite production by mTALs from STZ rats, measured in the presence of tempol, was found to be significantly reduced by isoform-selective NOS1 and NOS2 inhibitors (VNIO and 1400W, respectively; Fig. 3D). These inhibitors did not alter nitrite production by mTALs from sham rats measured under identical conditions. Thus, the increased NO production by mTALs from STZ rats is NOS1 and NOS2 dependent.
NOS protein expression.
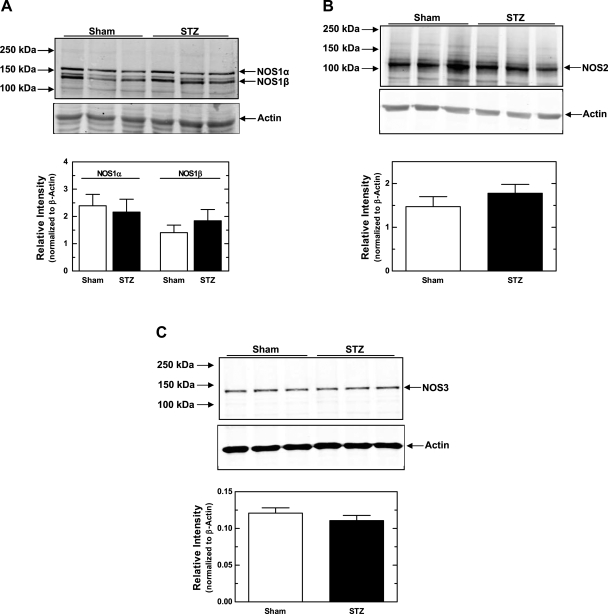
Protein levels for each NOS isoform were measured by Western blot analysis and normalized to β-actin. As shown in Fig. 4A, we detected NOS1-immunoreactive bands at 155 and 130 kDa. NOS1α (155 kDa) is the predominant NOS1 isoform expressed in all organisms. The other band likely represents NOS1β, which is reported to be a 130-kDa protein. We did not observe any difference in the expression of either NOS1 variant in mTALs from sham vs. STZ rats. We were also able to detect NOS2 in mTALs from both sham and STZ rats (Fig. 4B), but no difference was apparent between groups. The specificity of the NOS2 antibody was confirmed by antigen blockade with the specific NOS2 peptide antigen (Santa Cruz Biotechnology; data not shown). Moreover, as shown in Fig. 4C, NOS3 protein levels were similar in mTALs from sham and STZ rats. Thus, there were no significant differences in the expression of any NOS isoform in mTAL suspensions from sham vs. STZ rats.
Fig. 4.
NOS1, NOS2, and NOS3 protein expression from mTAL homogenates from STZ and sham rats. A, top: representative Western blots of NOS1α, NOS1β, and β-actin. A, bottom: summary of Western blot data for NOS1α and NOS1β, each normalized to β-actin. B, top: representative Western blots of NOS2 and β-actin. B, bottom: summary of Western blot data for NOS2 normalized to β-actin. C, top: representative Western blots of NOS3 and β-actin. C, bottom: summary of Western blot data for NOS3 normalized to β-actin. Values are means ± SE (n = 7–14 rats).
Phospho-specific NOS protein expression.
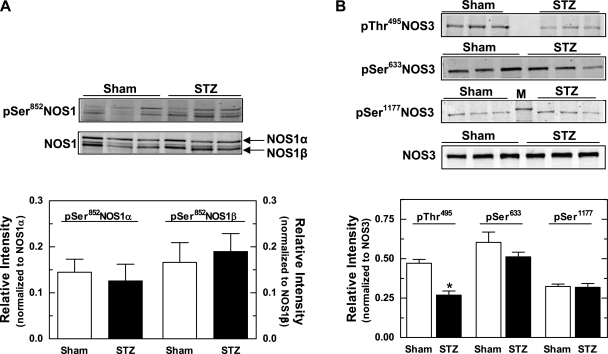
We examined phosphorylation of NOS1 and NOS3 at known serine and threonine regulatory sites, quantified based on the intensity of the phospho-specific band normalized to the NOS isoform detected with an antibody to a nonphospho-specific site. Two-color detection with the phospho-specific and nonphospho-specific immunoblots viewed as a merged image confirmed the specificity of each phosphorylation site within NOS1 and NOS3.
There is one known serine phosphorylation site on NOS1, Ser852, but our data failed to reveal any difference between mTALs from sham vs. STZ rats with regard to pSer852NOS1/NOS1 (Fig. 5A). This finding holds true for both NOS1α and NOS1β. We also probed the phosphorylation sites on NOS3: Ser116, Thr495, Ser617, Ser633, and Ser1177. As shown in Fig. 5B, pThr495NOS3/NOS3 in homogenates of mTALs from STZ rats was reduced by more than 40% compared with that detected in sham rats (P < 0.05). In contrast, mTAL homogenates from sham and STZ rats did not differ with regard to pSer633NOS3/NOS3 and pSer1177NOS3/NOS3 levels. We were unable to detect pSer116NOS3 and pSer617NOS3 in mTALs from either group (data not shown), despite the ability of the phospho-specific primary antibodies to detect phosphorylation at these sites in endothelial cells.
Fig. 5.
Effect of diabetes on NOS1 and NOS3 phosphorylation in mTAL homogenates from sham and STZ rats. A, top: representative Western blot of pSer852NOS1 and total NOS1 (α and β variants). A, bottom: pSer852NOS1α normalized to NOS1α and pSer852NOS1β normalized to NOS1β, as determined by 2-color Western blot analysis. B, top: representative Western blots of pThr495NOS3, pSer633NOS3, pSer1177NOS3, and total NOS3. B, bottom: summary of Western blot data for pThr495NOS3, pSer633NOS3, and pSer1177NOS3 (each normalized to total NOS3). In pThr495NOS3 blot, lane 4 was left empty. In the pSer1177NOS3 blot, lane 4 contains a marker band that represents 150 kDa. Values are means ± SE (n = 5–13 rats). *P < 0.05 vs. sham.
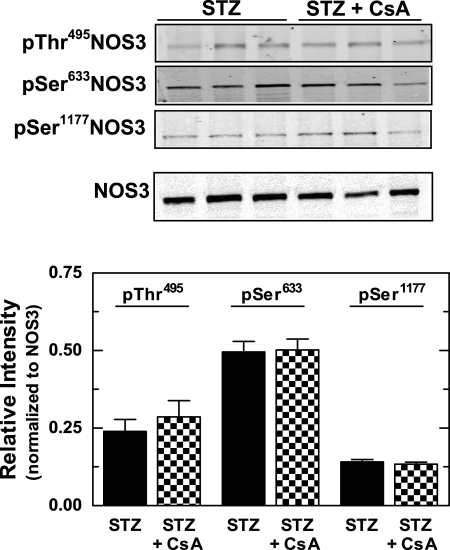
Finally, we determined whether PP2B inhibition alters the phosphorylation status of NOS1 and NOS3 in mTALs from STZ rats. As shown in Fig. 6, Western blot analysis revealed no effect of CsA on Ser852NOS1/NOS1, pThr495NOS3/NOS3, pSer633NOS3/NOS3, or pSer1177NOS3/NOS3 in mTALs from either sham or STZ rats. These results indicate that PP2B does not directly alter NOS1 or NOS3 phosphorylation at these known regulatory sites.
Fig. 6.
Effect of PP2B inhibition (100 ng/ml CsA) on NOS3 phosphorylation in mTAL homogenates from STZ rats. Top: representative Western blots of pThr495NOS3, pSer633NOS3, pSer1177NOS3, and total NOS3. Bottom: summary of NOS3 phosphorylation data showing pThr495NOS3, pSer633NOS3, and pSer1177NOS3 (each normalized to total NOS3). Values are means ± SE (n = 5–7 rats).
Effect of CsA and CAIP on cell viability.
The concentration of CsA (100 ng/ml) used in all experiments was based on the literature demonstrating that this concentration was not cytotoxic in murine cultured mTAL cells (47, 49). However, to ensure that the changes in NO production induced by CsA and CAIP were not the result of cytotoxicity, cell viability was measured in mTAL suspensions incubated for 30 min in the presence of l-arginine (250 μM), tempol (10 mM), and CsA (100 ng/ml) or CAIP (100 μM). There were no significant differences between the percentage of viable cells between vehicle control, CsA-, or CAIP-treated tubules (vehicle control: 97 ± 2%, CsA: 96 ± 3%, CAIP: 108 ± 5%, n = 6 for all groups).
DISCUSSION
In type 1 diabetes mellitus, net sodium retention results in a positive sodium balance (32, 43). DiPetrillo et al. (7) found that diabetic rats were not able to excrete sodium to the level needed to match the rise in dietary sodium intake due to hyperphagia. While the mTAL is an important region of the nephron for maintenance of sodium homeostasis, normally reabsorbing ∼20% of the filtered sodium, the mechanisms regulating sodium reabsorption by the mTAL during diabetes remain unclear. NO directly inhibits sodium reabsorption at various sites along the nephron (10, 13, 25, 51), including the mTAL (37), under normal physiological conditions. The mTAL expresses all three isoforms of NOS; however, few studies examined the regulation of the NO/NOS pathway in the mTAL under diabetic conditions. The activity of each NOS isoform is regulated, in part, by alterations in phosphorylation status. PP2B has been postulated to be upregulated as a compensatory mechanism in the kidney during diabetes and is one of the phosphatases that can act on NOS. The major findings of the present study are 1) PP2B catalytic and regulatory subunit protein expression and PP2B activity are increased in mTALs from diabetic rats, 2) increased NOS activity and nitrite production in mTALs from diabetic rats are attributable to NOS1 and NOS2 activities, although diabetes does not alter protein expression of either NOS isoform in the mTAL, 3) diabetes increases PP2B-dependent nitrite production and NOS activity in the mTAL; however, net NO bioavailability is reduced, 4) phosphorylation of NOS3 at Thr495 is reduced in mTALs from diabetic rats while phosphorylation at other known regulatory sites on NOS1 and NOS3 is unchanged, and 5) PP2B regulation of NOS activity does not appear to directly alter the phosphorylation status of known NOS1 or NOS3 sites.
It is well-known that diabetes reflects a state of oxidative stress characterized by accumulation of reactive oxygen species, specifically O2•−. Reaction of O2•− with NO results in a decrease in NO bioavailability, which has been documented following exposure to high glucose in human aortic endothelial cells (6) and human glomerular endothelial cells (19). Indeed, reduced NO bioavailability has been shown to be the primary mechanism involved in endothelial dysfunction in the vasculature in diabetes (17). As the mTAL is a prominent source of renal O2•− production (23, 53), which can be accelerated by acute exposure to high glucose levels (30), we reasoned that mTALs may produce sufficient O2•− to reduce NO bioavailability. (Examination of the regulation of O2•− production by mTALs under diabetic conditions is the focus of another study in our laboratory.) Indeed, incubation in the presence of tempol, a free radical scavenger, or PEG-SOD, superoxide scavenger, unmasked a significant increase in nitrite production by mTALs from diabetic rats that was not observed in sham rats. This observation is interpreted as evidence of reduced NO bioavailability in mTALs from STZ rats. We postulate that the increase in NO generation is an adaptive mechanism in the mTAL to compensate for the activation of reactive oxygen species production during diabetes, blunting the magnitude of the decrease in NO bioavailability.
A previous study from our laboratory demonstrated an increase in renal medullary NOS3 activity in STZ rats compared with sham (22). The present study utilized mTAL suspensions from a similar model of diabetes, yet we found NOS1 and NOS2 activities were increased during diabetes, with no increase in NOS3-specific activity. The obvious difference in these two studies from our laboratory is the tissue source that was evaluated. Lee et al. (22) examined homogenates from whole medullary tissue containing various nephron segments and vascular structures with several cell types, while the present study focused solely on the mTAL. We also previously reported similar NOS3 protein levels in renal medullary homogenates from sham and STZ rats, yet we demonstrated reduced pThr495NOS3 staining in the mTAL from STZ rats by immunohistochemical analysis (22). Indeed, the present study confirms these phenomena in mTAL suspensions.
Results of the present study revealed increased NOS1 and NOS2 activities in mTALs from diabetic rats, although we did not detect any change in NOS1 or NOS2 protein levels following 3 wk of diabetes. NOS1 is regulated by alternative splicing and expression of these splice variants has been shown in the kidney (33). NOS1 splicing results in proteins with NH2-terminal variants; thus, we utilized a COOH-terminal-specific NOS1 primary antibody to detect expression of all possible NOS1 variants by Western blot. NOS1-specific bands were detected at 155 kDa (NOS1α) and 130 kDa (likely NOS1β). NOS1β is reported to have 80% of the enzymatic activity of NOS1α. Neither NOS1 variant exhibited altered protein levels in mTALs from STZ rats. While Shin et al. (42) found increased immunostaining for NOS1 in the mTAL following 6 wk of diabetes, it is not known which NOS1 splice variants were detected in their report. Possibly, a more prolonged diabetic state (6 wk) induces NOS1 protein expression, as well as the increased NOS1 activity that was evident in the present study (3 wk after onset).
NOS2 is known to be transcriptionally upregulated through activation of cytokine pathways under proinflammatory conditions. There is consistent evidence of increased NOS2 mRNA and protein expression by whole kidney, macrophages, mesangial cells, or glomeruli under proinflammatory conditions such as ischemia-reperfusion injury (21, 36) or lipopolysaccharide stimulation (21, 29, 31). Furthermore, NOS2 activity is upregulated in murine macrophages and glomerular mesangial cells exposed to high glucose (40). It is also well-known that NOS2 is constitutively expressed in the kidney, although little has been reported about the regulation of constitutive NOS2. Two previous studies found that the site of highest basal expression of NOS2 mRNA in the kidney is the mTAL (29, 31). While our data reveal constitutive expression of NOS2 protein in mTALs from sham rats, it does not seem to be enzymatically active. Although NOS2 protein levels were similar in mTALs from sham and STZ rats, increased NOS2 activity was apparent in mTALs from STZ rats. Previous to this study, there was little evidence in the literature to suggest that diabetes induces NOS2 mRNA, protein expression, or activity in the mTAL. We cannot rule out the possibility that the upregulated NOS2 activity evident in our study originates from inflammatory cells residing within the mTAL segments. Indeed, we observed macrophage infiltration in the renal medulla of diabetic rats (unpublished observations, Foster JM and Pollock JS). Further analysis will be necessary to determine the specific cellular source(s) of the NOS2 activity evident in mTAL suspensions from diabetic rats.
PP2B is a calcium/calmodulin-dependent serine/threonine phosphatase that plays a critical role in Ca2+-mediated signal transduction in many tissues. PP2B contains a catalytic subunit (PP2B-A) and a regulatory subunit (PP2B-B). The catalytic subunit (PP2B-A) has three related isoforms: α, β, and γ. A previous study (2) demonstrated PP2B-Aα mRNA expression in the kidney, primarily in the proximal tubules, while PP2B-Aβ mRNA was primarily in the medulla, with highest expression in the mTAL. Immunohistochemical analysis indicates that PP2B-Aβ protein expression is upregulated specifically in the mTAL following 2 wk of STZ-induced diabetes (12). This agrees with our findings that protein levels of PP2B-Aβ, as well as the regulatory subunit PP2B-B, are increased in mTAL suspensions from diabetic rats. Our results also extend previous observations by revealing that PP2B activity is increased in mTAL lysates from STZ rats.
NOS isoform protein expression is unchanged while NOS activity is increased in mTALs during diabetes. Thus, we focused on posttranslational phosphorylation mechanisms of NOS isoform activation. We hypothesized that the phosphatase, PP2B, activates NO production and NOS activity in the mTAL. To examine this hypothesis, we primarily utilized the PP2B inhibitor, CsA. Notably, CsA is one of the most potent, specific, and well-known inhibitors of PP2B (4, 14, 27, 34, 38, 41). Furthermore, CsA does not inhibit members of the PP1, PP2A, or PP2C classes of serine/threonine phosphatases and PP2B is the exclusive cellular target of CsA (26). We also utilized the mechanistically distinct PP2B inhibitor, CAIP, in some of our studies (45), yielding effects similar to those evoked by CsA. The data reveal that NOS activity and NO production (nitrite production measured in the presence of tempol) are increased in mTALs from diabetic rats in a PP2B-dependent manner. In contrast, PP2B inhibition does not affect NOS activity or nitrite production by mTALs under normal physiological conditions, despite the fact that CsA blocked PP2B activity in mTALs from both sham and STZ rats. Thus, while constitutive PP2B activity has little impact on NOS activity in the mTAL under normal conditions, it is likely that the increase in PP2B activity that accompanies diabetes is responsible for the increase in NOS activity and NO production evident in mTALs from diabetic rats.
To our knowledge, no other study showed a role for PP2B regulation of NOS1 activity in diabetes. In a recent study, PP2B was reported to dephosphorylate NOS1 at Ser852 in primary rat hypothalamic neurons (50). However, we found no effect of CsA on phosphorylation of Ser852NOS1, suggesting that the PP2B-dependent NOS1 activation in the mTAL during diabetes is not the result of direct PP2B effects at this NOS1 site and possibly arises via an indirect mechanism.
The specific mechanisms regarding PP2B regulation of NOS2 are poorly understood. It has been demonstrated previously that PP2B inhibition leads to reduced NOS activity and NO production in cultured rat macrophages (5). Reports showed that PP2B inhibition by CsA inhibits NOS2 gene transcription and associated NO production in porcine proximal tubule cells (18) and vascular smooth muscle cells (28). Interestingly, CsA has been shown to reduce NOS2 mRNA and NO production in cultured mouse mTAL cells (48). However, none of these studies assessed the effects of CsA and NOS2 under diabetic conditions. Posttranslational regulation of NOS2 activity by phosphorylation has been reported (52); however, as specific phosphorylation sites have not been identified, we did not explore NOS2 phosphorylation in this study.
Phosphorylation of NOS3 has been examined primarily in cultured endothelial cells under conditions of shear stress (8) or in mTALs under nondiabetic conditions such as increased flow (35) or a high-salt diet (16). In cultured endothelial cells, Thr495NOS3 is a negative regulatory site, such that reduced phosphorylation indicates increased NO production. Furthermore, pThr495NOS3/NOS3 levels were reduced in mTALs from diabetic rats [in accord with our previously published immunohistochemical observations (22)], suggesting that NOS3-derived NO production may be increased. Surprisingly, NOS3 activity was found to be similar in mTALs from STZ and sham rats. Further research is needed to resolve this finding. The inability to detect pSer116NOS3 and pSer617NOS3 in mTALs, despite the fact that NOS3 is highly expressed in this nephron segment, would suggest that the Ser116 and Ser617 sites on NOS3 are phosphorylated at very low levels in mTALs, both in normal rats and during diabetes. In cultured bovine aortic endothelial cells, PP2B dephosphorylates the Thr495 residue on NOS3 resulting in increased bradykinin stimulation of NO release (15). In porcine aortic endothelial cells, CsA fails to affect phosphorylation of NOS3 at the Thr495 and Ser1177 sites (9). In the present study, however, CsA did not affect any of the detectable phosphorylation sites, including pThr495NOS3, in the mTAL under diabetic conditions.
Our study reveals that diabetes induces PP2B-dependent activation of NO production, without demonstrating a direct regulation of known phosphorylation sites on NOS isoforms. Thus, we suspect that PP2B may regulate NO production indirectly via a NOS-interacting protein and/or by regulating the production of a NOS cofactor. Two possible candidates that are regulated by phosphorylation/dephosphorylation pathways are caveolin and GTP cyclohydrolase. A protein constitutent of caveolae, caveolin has been shown to interact with and function as a negative regulator of NOS activity in endothelial cells (20). Caveolin is also known to be regulated by phosphorylation (24), although no information is available with regards to caveolin regulation of NOS activity in the mTAL. GTP cyclohydrolase is the rate-limiting enzyme in the production of tetrahydrobiopterin (BH4), a critical cofactor necessary for NOS activity. In endothelial cells, shear stress activates phosphorylation of GTP cyclohydrolase, increasing production of BH4 and subsequent NO production (46). Future studies will address whether these processes underlie the PP2B-dependent increase in NOS activity and NO production in the mTAL during diabetes.
In summary, while NO bioavailability in mTALs is reduced during diabetes, free radical scavenging with tempol unmasks an increase in NO production involving PP2B-dependent activation of NOS1 and NOS2. In addition, PP2B regulates NOS3 activity in the mTAL under both normal and diabetic conditions. We propose that the upregulation of PP2B and NOS activity in the mTAL during diabetes is an adaptive compensatory mechanism. Under diabetic conditions, the balance of NO and O2•− is altered, with a dominance of O2•− production. O2•− has been shown to promote sodium reabsorption in the mTAL, while NO inhibits sodium reabsorption in the mTAL. We propose that the increased NO production may be an adaptive mechanism allowing the rat to remain normotensive with a positive sodium balance under the high O2•−, diabetic conditions. Of further interest are the clinical implications of the use of CsA in transplant patients. CsA is a potent and widely used immunosuppressant and its use is complicated by the development of nephropathy, nephrotoxicity, and substantial hypertension (1, 44). We propose that CsA reduces the PP2B-dependent increase in NOS activity and NO production resulting in enhanced sodium reabsorption in the mTAL contributing to the development of hypertension. Further work is needed to address this hypothesis.
GRANTS
The authors acknowledge the support of the American Heart Association (J. M. Foster: Southeast Predoctoral Fellowship; J. S. Pollock: Established Investigator Award) and National Institutes of Health Grant HL-60653.
Acknowledgments
We thank J. Musall, H. Socha, and F. Spradley for expert technical assistance.
REFERENCES
- 1.Andoh TF, Bennet WM. Chronic cyclosporine nephrotoxicity. Curr Opin Nephrol Hypertens 7: 265–270, 1998. [DOI] [PubMed] [Google Scholar]
- 2.Buttini M, Limonta S, Luyten M, Boddeke H. Distribution of calcineurin A isoenzyme mRNAs in rat thymus and kidney. Histochem J 27: 291–299, 1995. [DOI] [PubMed] [Google Scholar]
- 3.Chamberlin ME, LeFurgey A, Mandel LJ. Suspension of medullary thick ascending limb tubules from the rabbit kidney. Am J Physiol Renal Fluid Electrolyte Physiol 247: F955–F964, 1984. [DOI] [PubMed] [Google Scholar]
- 4.Clipstone NA, Crabtree GR. Calcineurin is a key signaling enzyme in T lymphocyte activation and the target of the immunosuppressive drugs cyclosporin A and FK506. Ann NY Acad Sci 696: 20–30, 1993. [DOI] [PubMed] [Google Scholar]
- 5.Conde M, Andrade J, Bedoya FJ, Santa Maria C, Sobrino F. Inhibitory effect of cyclosporin A and FK506 on nitric oxide production by cultured macrophages. Evidence of a direct effect on nitric oxide synthase activity. Immunology 84: 476–481, 1995. [PMC free article] [PubMed] [Google Scholar]
- 6.Cosentino F, Hishikawa K, Katusic ZS, Luscher TF. High glucose increases nitric oxide synthase expression and superoxide anion generation in human aortic endothelial cells. Circulation 96: 25–28, 1997. [DOI] [PubMed] [Google Scholar]
- 7.DiPetrillo K, Coutermarsh B, Gesek FA. Urinary tumor necrosis factor contributes to sodium retention and renal hypertrophy during diabetes. Am J Physiol Renal Physiol 284: F113–F121, 2003. [DOI] [PubMed] [Google Scholar]
- 8.Fleming I, Busse R. Molecular mechanisms involved in the regulation of the endothelial nitric oxide synthase. Am J Physiol Regul Integr Comp Physiol 284: R1–R12, 2003. [DOI] [PubMed] [Google Scholar]
- 9.Fleming I, Fisslthaler B, Dimmeler S, Kemp BE, Busse R. Phosphorylation of Thr495 regulates Ca2+/calmodulin-dependent endothelial nitric oxide synthase activity. Circ Res 88: 68–75, 2001. [DOI] [PubMed] [Google Scholar]
- 10.Garcia NH, Stoos BA, Carretero OA, Garvin JL. Mechanism of the nitric oxide-induced blockade of collecting duct water permeability. Hypertension 27: 679–683, 1996. [DOI] [PubMed] [Google Scholar]
- 11.Gooch JL, Barnes JL, Garcia S, Abboud HE. Calcineurin is activated in diabetes and is required for glomerular hypertrophy and ECM accumulation. Am J Physiol Renal Physiol 284: F144–F154, 2003. [DOI] [PubMed] [Google Scholar]
- 12.Gooch JL, Pablo PE, Guler RL, Abboud HE, Barnes JL. Differential expression of calcineurin A isoforms in the diabetic kidney. J Am Soc Nephrol 15: 1421–1429, 2004. [DOI] [PubMed] [Google Scholar]
- 13.Guzman NJ, Fang MZ, Tang SS, Ingelfinger JR, Garg LC. Autocrine inhibition of Na+-K+-ATPase by nitric oxide in mouse proximal tubule epithelial cells. J Clin Invest 95: 2083–2088, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Halloran PF, Kung L, Noujaim J. Calcineurin and the biological effect of cyclosporine and tacrolimus. Transplant Proc 30: 2167–2170, 1998. [DOI] [PubMed] [Google Scholar]
- 15.Harris MD, Ju H, Venema VJ, Liang H, Zou R, Michell BJ, Chen ZP, Kemp BE, Venema RC. Reciprocal phosphorylation and regulation of endothelial nitric oxide synthase in response to bradykinin stimulation. J Biol Chem 276: 16587–16591, 2001. [DOI] [PubMed] [Google Scholar]
- 16.Herrera M, Silva GB, Garvin JL. A high salt diet dissociates NO synthase-3 expression and NO production by the THAL. Hypertension 47: 95–101, 2006. [DOI] [PubMed] [Google Scholar]
- 17.Hink U, Li H, Mollnau H, Oelze M, Matheis E, Hartmann M, Skatchkov M, Thaiss F, Stahl RA, Warnholtz A, Meinertz T, Griendling K, Harrison DG, Forstermann U, Münzel T. Mechanisms underlying endothelial dysfunction in diabetes mellitus. Circ Res 88: E14–E22, 2001. [DOI] [PubMed] [Google Scholar]
- 18.Hortelano S, Castilla M, Torres A, Tejedor A, Bosca L. Potentiation by nitric oxide of cyclosporin A and FK506-induced apoptosis in renal proximal tubule cells. J Am Soc Nephrol 11: 2315–2323, 2000. [DOI] [PubMed] [Google Scholar]
- 19.Hoshiyama M, Li B, Yao J, Harada T, Morioka T, Oite T. Effect of high glucose on nitric oxide production and endothelial nitric oxide synthase protein expression in human glomerular endothelial cells. Nephron Exp Nephrol 95: 62–68, 2003. [DOI] [PubMed] [Google Scholar]
- 20.Ju H, Zou R, Venema VJ, Venema RC. Direct interaction of endothelial nitric oxide synthase and caveolin-1 inhibits synthase activity. J Biol Chem 272: 18522–18525, 1997. [DOI] [PubMed] [Google Scholar]
- 21.Kosaka H, Yoneyama H, Zhang L, Fujii S, Yamamoto A, Igarashi J. Induction of LOX-1 and iNOS expressions by ischemia-reperfusion of rat kidney and the opposing effect of l-arginine. FASEB J 17: 636–643, 2003. [DOI] [PubMed] [Google Scholar]
- 22.Lee DL, Sasser JM, Hobbs JL, Boriskie A, Pollock DM, Carmines PK, Pollock JS. Posttranslational regulation of NO synthase activity in the renal medulla of diabetic rats. Am J Physiol Renal Physiol 288: F82–F90, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li N, Yi FX, Spurrier JL, Bobrowitz CA, Zou AP. Production of superoxide through NAPH oxidase in thick ascending limb of Henle's loop in rat kidney. Am J Physiol Renal Physiol 282: F1111–F1119, 2002. [DOI] [PubMed] [Google Scholar]
- 24.Li S, Seitz R, Lisanti MP. Phosphorylation of caveolin by Src tryosine kinases. The α-isoform of caveolin is selectively phosphorylated by vSrc in vivo. J Biol Chem 271: 3863–3868, 1996. [PubMed] [Google Scholar]
- 25.Liang M, Knox FG. Nitric oxide reduces the molecular activity of Na+-K+-ATPase in opossum kidney cells. Kidney Int 56: 627–634, 1999. [DOI] [PubMed] [Google Scholar]
- 26.Liu J, Albers MW, Wandless TJ, Luan S, Alberg DG, Belshaw PJ, Cohen P, MacKintosh C, Klee CB, Schreiber SL. Inhibition of T cell signaling by immunophilin-ligand complexes correlates with loss of calcineurin phosphatase activity. Biochemistry 31: 3896–3901, 1992. [DOI] [PubMed] [Google Scholar]
- 27.Liu J, Farmer JD Jr, Lane WS, Friedman J, Weissman I, Schreiber SL. Calcineurin is a common target of cyclophilin-cyclosporin A and FKBP-FK506 complexes. Cell 66: 807–815, 1991. [DOI] [PubMed] [Google Scholar]
- 28.Marumo T, Nakaki T, Hishikawa K, Suzuki H, Kato R, Saruta T. Cyclosporin A inhibits nitric oxide synthase induction in vascular smooth muscle cells. Hypertension 25: 764–768, 1995. [DOI] [PubMed] [Google Scholar]
- 29.Mohaupt MG, Elzie JL, Ahn KY, Clapp WL, Wilcox CS, Kone BC. Differential expression and induction of mRNAs encoding two inducible nitric oxide synthases in rat kidney. Kidney Int 46: 653–665, 1994. [DOI] [PubMed] [Google Scholar]
- 30.Mori T, Cowley AW Jr. Renal oxidative stress in medullary thick ascending limbs produced by elevated NaCl and glucose. Hypertension 43: 341–346, 2004. [DOI] [PubMed] [Google Scholar]
- 31.Morrissey J, McCracken R, Kaneto H, Vehaskari M, Montani D, Klahr S. Location of inducible nitric oxide synthase mRNA in the normal kidney. Kidney Int 45: 998–1005, 1994. [DOI] [PubMed] [Google Scholar]
- 32.Norgaard K, Feldt-Rasmussen B. Sodium retention and insulin treatment in insulin-dependent diabetes mellitus. Acta Diabetol 31: 19–25, 1994. [DOI] [PubMed] [Google Scholar]
- 33.Oberbäumer I, Moser D, Bachmann S. Nitric oxide synthase 1 mRNA: tissue-specific variants from rat with alternative first exons. Biol Chem 379: 913–919, 1998. [PubMed] [Google Scholar]
- 34.O'Keefe SJ, O'Neill EA. Cyclosporin A and FK-506: immunosuppression, inhibition of transcription and the role of calcineurin. Perspect Drug Disc Design 2: 85–102, 1994. [Google Scholar]
- 35.Ortiz PA, Hong NJ, Garvin JL. Luminal flow induces eNOS activation and translocation in the rat thick ascending limb. Am J Physiol Renal Physiol 287: F274–F280, 2004. [DOI] [PubMed] [Google Scholar]
- 36.Park KM, Byun JY, Kramers C, Kim JI, Huang PL, Bonventre JV. Inducible nitric oxide synthase is an important contributor to prolonged protective effects of ischemic preconditioning in the mouse kidney. J Biol Chem 278: 27256–27266, 2003. [DOI] [PubMed] [Google Scholar]
- 37.Plato CF, Stoos BA, Wang D, Garvin JL. Endogenous nitric oxide inhibits chloride transport in the thick ascending limb. Am J Physiol Renal Physiol 276: F159–F163, 1999. [DOI] [PubMed] [Google Scholar]
- 38.Rusnak F, Mertz P. Calcineurin: form and function. Physiol Rev 80: 1483–1521, 2000. [DOI] [PubMed] [Google Scholar]
- 39.Sasser JM, Sullivan JC, Hobbs JL, Yamamoto T, Pollock DM, Carmines PK, Pollock JS. Endothelin A receptor blockade reduces diabetic renal injury via an anti-inflammatory mechanism. J Am Soc Nephrol 18: 143–154, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sharma K, Danoff TM, DePiero A, Ziyadeh F. Enhanced expression of inducible nitric oxide synthase in murine macrophages and glomerular mesangial cells by elevated glucose levels: possible mediation via protein kinase C. Biochem Biophys Res Commun 207: 80–88, 1995. [DOI] [PubMed] [Google Scholar]
- 41.Shenolikar S Protein phosphatase regulation by endogenous inhibitors. Semin Cancer Biol 6: 219–227, 1995. [DOI] [PubMed] [Google Scholar]
- 42.Shin SJ, Lai FJ, Wen JD, Hsiao PJ, Hsieh MC, Tzeng TF, Chen HC, Guh JY, Tsai JH. Neuronal and endothelial nitric oxide synthase expression in outer medulla of streptozotocin-induced diabetic rat kidney. Diabetologia 43: 649–659, 2000. [DOI] [PubMed] [Google Scholar]
- 43.Strojek K, Grzeszczak W, Lacka B, Gorska J, Keller CK, Ritz E. Increased prevalence of salt sensitivity of blood pressure in IDDM with and without microalbuminuria. Diabetologia 38: 1443–1448, 1995. [DOI] [PubMed] [Google Scholar]
- 44.Taler SJ, Textor SC, Canzanello VJ, Schwartz L. Cyclosporin-induced hypertension: incidence, pathogenesis and management. Drug Saf 20: 437–449, 1999. [DOI] [PubMed] [Google Scholar]
- 45.Terada H, Matsushita M, Lu YF, Shirai T, Li ST, Tomizawa K, Moriwaki A, Nishio S, Date I, Ohmoto T, Matsui H. Inhibition of excitatory neuronal cell death by cell-permeable calcineurin autoinhibitory peptide. J Neurochem 87: 1145–1151, 2003. [DOI] [PubMed] [Google Scholar]
- 46.Widder JD, Chen W, Li L, Dikalov S, Thöny B, Hatakeyama K, Harrison DG. Regulation of tetrahydrobiopterin biosynthesis by shear stress. Circ Res 101: 830–838, 2007. [DOI] [PubMed] [Google Scholar]
- 47.Wu MS, Yang CW, Bens M, Peng KC, Yu HM, Vandewalle A. Cyclosporine stimulates Na+-K+-Cl− cotransport activity in cultured mouse medullary thick ascending limb cells. Kidney Int 58: 1652–1663, 2000. [DOI] [PubMed] [Google Scholar]
- 48.Wu MS, Yang CW, Bens M, Yu HM, Huang JY, Wu CH, Huang CC, Vandewalle A. Cyclosporin inhibits nitric oxide production in medullary thick ascending limb cultured cells. Nephrol Dial Transplant 13: 2814–2820, 1998. [DOI] [PubMed] [Google Scholar]
- 49.Wu MS, Yang CW, Chang CT, Bens M, Vandewalle A. Cyclosporin increases the density of angiotensin II subtype 1 (AT1) receptors in mouse medullary thick ascending limb cells. Nephrol Dial Transplant 18: 1458–1465, 2003. [DOI] [PubMed] [Google Scholar]
- 50.Xu Y, Krukoff TL. Adrenomedullin stimulates nitric oxide production from primary rat hypothalamic neurons: roles of calcium and phosphatases. Mol Pharmacol 72: 112–120, 2007. [DOI] [PubMed] [Google Scholar]
- 51.Zeidel ML, Silva P, Brenner BM, Seifter JL. cGMP mediates effects of atrial peptides on medullary collecting duct cells. Am J Physiol Renal Fluid Electrolyte Physiol 252: F551–F559, 1987. [DOI] [PubMed] [Google Scholar]
- 52.Zhang Y, Brovkovych V, Brovkovych S, Tan F, Lee BS, Sharma T, Skidgel RA. Dynamic receptor-dependent activation of inducible nitric oxide synthase by ERK-mediated phosphorylation of Ser745. J Biol Chem 282: 32453–32461, 2007. [DOI] [PubMed] [Google Scholar]
- 53.Zou AP, Li N, Cowley AW Jr. Production and actions of superoxide in the renal medulla. Hypertension 37: 547–553, 2001. [DOI] [PubMed] [Google Scholar]