Abstract
Purpose
Use of partial nephrectomy for renal cortical tumors appears unacceptably low in the United States according to population-based data. We examined the use of partial nephrectomy at our tertiary care facility in the contemporary era.
Methods
Using our prospectively maintained nephrectomy database, we identified 1,533 patients treated for a sporadic and localized renal cortical tumor between 2000 and 2007. Patients with bilateral disease or solitary kidneys were excluded and an elective operation required an estimated GFR ≥45 ml/min/1.73m2. Predictors of partial nephrectomy were evaluated using logistic regression models.
Results
Overall, 854 (56%) and 679 (44%) patients were treated with partial and radical nephrectomy, respectively. Among the 820 patients treated electively for a tumor ≤4cm, the frequency of partial nephrectomy use steadily increased from 69% in the year 2000 to 89% in 2007. Among the 365 patients treated electively for a tumor 4–7cm, the frequency of partial nephrectomy use also steadily increased from 20% in the year 2000 to 60% in 2007. In a multivariate analysis, male gender (p=0.021), later year of surgery (p<0.001), younger age (p=0.004), smaller tumor size (p<0.001), and open surgery (p<0.001) were significant predictors of receiving a partial nephrectomy. ASA score, race, and body mass index were not significantly associated with type of treatment.
Conclusions
Use of partial nephrectomy is increasing and is now utilized for ~90% of patients with T1a tumors at our institution. For reasons that remain unclear, certain groups of patients are less likely to be treated with partial nephrectomy.
Keywords: Kidney neoplasms, Nephrectomy, Carcinoma, renal cell, Survival, Treatment outcome
INTRODUCTION
During the last decade, partial nephrectomy has been accepted as an effective and safe alternative to radical nephrectomy for small renal cortical tumors. In fact, many urologists suggest that partial nephrectomy should be the standard of care for most small renal tumors even in the setting of a normal contralateral kidney.1–4 Reasons supporting partial nephrectomy, as opposed to radical nephrectomy, include a reduction in the incidence of chronic renal disease,1, 5 a potential reduction in the risk for morbidity related to renal insufficiency including hip fractures and cardiovascular morbidity, and a possible reduction in the risk of death from any cause.6, 7 Additionally, evidence to date strongly supports that cancer control and risk of cancer-specific death is not compromised when partial nephrectomy is utilized in lieu of the more traditional radical nephrecomy.8, 9
However, recent observations suggest that partial nephrectomy is clearly underutilized in the United States.10, 11 Data from the National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) program demonstrates that partial nephrectomy in this century (2000 – 2002) was performed in less than 10% of all surgical patients11 including only 20% of patients with tumors 2–4cm in size.10 Thus, one can conclude that despite published data supporting partial nephrectomy for small renal masses, radical nephrectomy remains the procedure most often performed in the United States for reasons that are not completely understood. Nevertheless, recent observations from tertiary care centers1, 6 suggests that the underutilization of partial nephrectomy is not uniform. Consistent with this, urban location, nephrectomy volume, and teaching hospitals were features significantly associated with partial nephrectomy use in the SEER datablase.11 Thus, we evaluated our experience with renal mass patients and report contemporary partial nephrectomy rates at a tertiary care facility along with predictors for overutilization of radical nephrectomy at our institution.
MATERIALS AND METHODS
Patient Selection
Upon approval from the Institutional Review Board, we reviewed the Memorial Sloan-Kettering nephrectomy database and identified 1,533 patients treated with radical or partial nephrectomy between 2000 and 2007. Patients were selected based on the presence of a sporadic, unilateral, localized, enhancing renal cortical tumor with either benign histology or any renal cell carcinoma histologic subtype. Patients with nodal or distant metastasis at time of surgery were excluded from analysis. In addition, patients with bilateral disease, an atrophic contralateral kidney, or solitary kidney were also excluded. Among the 1,533 patients studied, their surgical procedures were performed by 20 different surgeons; 15 surgeons performed only open surgery while 5 surgeons utilized both open and laparoscopic approaches.
The estimated glomerular filtration rate (GFR) was calculated using the modified MDRD equation [GFR expressed in ml/min/1.73m2 = 186 * (serum creatinine in mg/dl)− 1.154 * (age in years)− 0.203 * (0.742 if female) * (1.210 if black)].12 A GFR <45 ml/min/1.73m2 was arbitrarily considered an imperative indication for partial nephrectomy; for patients with a GFR ≥45 ml/min/1.73m2, a partial nephrectomy was considered elective. Therefore, since patients with solitary kidneys or bilateral disease were excluded, an elective operation required a normal appearing contralateral kidney and a GFR >45 ml/min/1.73m2. For reference, median serum creatinine (range) for all patients with a GFR ≥45 was 1.1 (0.4 – 1.7) mg/dl.
Statistical Methods
Features associated with partial nephrectomy use were evaluated using logistic regression models adjusting for age (binary, separated at the median age), sex, year of surgery, tumor size (continuous variable), surgical approach (open versus laparoscopic), American Society of Anesthesiology (ASA) score (1+2 vs 3+4), and race (black vs non-black- similar to MDRD equation). In the logistic regression analyses, all 1,533 patients had the above variables coded in the database. In effort to evaluate the potential impact of body mass index (BMI), a variable which was missing in 56 patients, the logistic regression analyses was also performed incorporating this feature as a continuous variable with 1,477 patients. Statistical analyses were performed using the Stata software package 8.2 (Stata Corp., Cellege Station, TX) and p-values <0.05 were considered statistically significant.
RESULTS
Over the entire study duration, partial nephrectomy was more frequently performed compared with radical nephrectomy; 854 (55.7%) and 679 (44.3%) patients underwent partial and radical nephrectomy, respectively. Histology was benign in 200 (13%) patients and renal cell carcinoma in 1,333 (87%) patients. Median (IQR) age was 62 (52, 70) years and median (IQR) tumor size was 3.7 (2.5–6.0) cm. Median (IQR) serum creatinine was 1.1 (1.0, 1.3) mg/dl and median GFR was 66 (57, 76) ml/min/1.73m2. A comparison of clinical features for patients according to type of operation is demonstrated in Table 1.
Table 1.
Clinical Features Stratified by Treatment Type for 1,533 Patients Treated Surgically Between 2000 and 2007
| Feature | Partial Nephrectomy N=854 | Radical Nephrectomy N=679 |
|---|---|---|
| Median (IQ Range) | ||
| Age at Surgery in Years | 61 (52 – 70) | 62 (53 – 71) |
| Preoperative Serum Creatinine (mg/dL) | 1.1 (1.0 – 1.3) | 1.1 (1.0 – 1.3) |
| Estimated GFR | 66 (58 – 76) | 65 (56 – 76) |
| Tumor Size (cm) | 2.7 (2.0 – 3.7) | 6.0 (4.3 – 9.0) |
| N (%) | ||
| Age | ||
| <62 years | 445 (52%) | 332 (49%) |
| 62+ years | 409 (48%) | 347 (51%) |
| ASA score | ||
| 1–2 | 529 (62%) | 394 (58%) |
| 3–4 | 318 (38%) | 283 (42%) |
| Sex | ||
| Female | 329 (39%) | 257 (38%) |
| Male | 525 (61%) | 422 (62%) |
| Race | ||
| Black | 33 (4%) | 36 (5%) |
| Not-black | 821 (96%) | 643 (95%) |
| Surgical Approach | ||
| Open | 753 (88%) | 575 (85%) |
| Laparoscopic | 101 (12%) | 104 (15%) |
Analyzing the subset of 820 patients with a renal tumor 4cm or less and treated in an elective situation (i.e. GFR ≥45 ml/min/1.73m2), the use of partial nephrectomy increased with time (Figure 1). For example, in the year 2000, 69% of patients in this T1a subset were treated with partial nephrectomy which steadily increased to 89% in the year 2007. We found similar trends for the 365 patients with a renal tumor >4cm and ≤7cm and treated in an elective situation (Figure 2). For example, in the year 2000, 20% of patients within this T1b subset were treated with partial nephrectomy which steadily increased to 60% in the year 2007. The use of a laparoscopic surgical approach began in 2002 and also increased with time; 3% of cases in the year 2002 were performed laparoscopically compared with 22% of cases in 2007.
Figure 1.
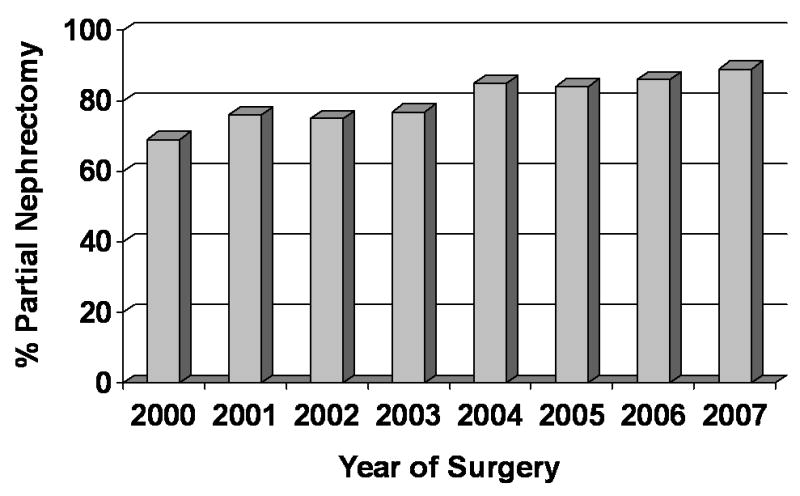
Partial nephrectomy use by year for subset of patients with tumors ≤4cm and treated in an elective situation
Figure 2.
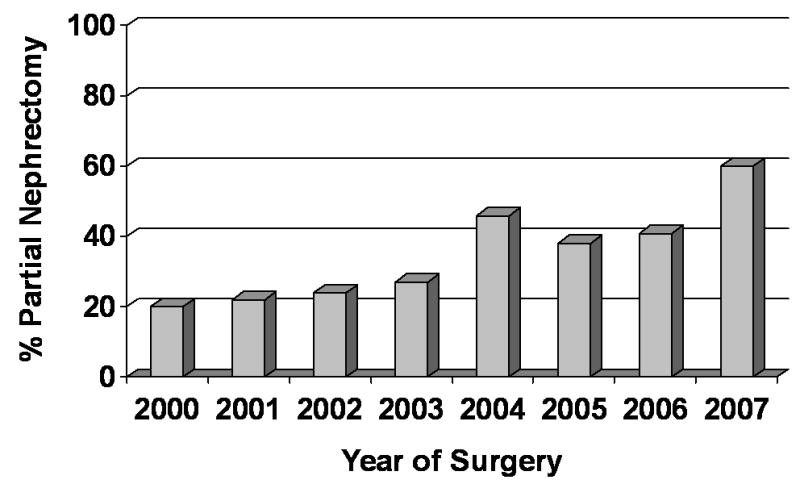
Partial nephrectomy use by year for subset of patients with tumors >4 – 7cm and treated in an elective situation
Attempting to identify predictors of type of procedure, we performed a logistic regression analysis adjusting for age, gender, year of surgery, surgical approach, tumor size, race, and ASA score. Table 2 demonstrates that male gender, later year in diagnosis, open surgical approach, smaller tumor size, and younger age are each significant predictors of partial nephrectomy. Therefore, significant independent predictors of radical nephrectomy included female gender, laparoscopic surgical approach, and older age. Race and ASA score were not significantly associated with treatment type. Similar results were obtained if the multivariate analysis is performed in the subset of patients with tumors ≤ 4cm and treated in an elective situation. For example, in this subset partial nephrectomy was performed in 81 (74%) patients treated laparoscopically compared with 587 (83%) patients treated with open surgery. However, if we limit the analysis to the 659 patients treated after 2004, surgical approach (open vs laparoscopic) no longer remains significantly associated with treatment type (odds ratio 1.32; 95% CI 0.78 – 2.24; p=0.3 for open surgery to predict use of partial nephrectomy).
Table 2.
Features predictive of partial nephrectomy use in a multivariable logistic regression analysis
| Feature | Odds Ratio | 95% CI | P-value |
|---|---|---|---|
| Male Gender | 1.39 | 1.04 – 1.85 | 0.025 |
| Year of surgery | 1.27 | 1.19 – 1.36 | <0.001 |
| Open (vs. Laparoscopic Surgery) | 2.55 | 1.69 – 3.84 | <0.001 |
| Tumor Size | 0.43 | 0.39 – 0.47 | <0.001 |
| Black Race | .77 | 0.40 – 1.49 | 0.4 |
| ASA score | 1.01 | 0.75 – 1.36 | 0.9 |
| Age (62+ vs <62 years old) | .65 | 0.49 – 0.88 | 0.005 |
We also evaluated BMI as a potential predictor of partial nephrectomy in the 1,477 patients with BMI recorded. When adding BMI to the logistic regression analysis, male gender (p=0.020), later year of surgery (p<0.001), open surgical approach (p<0.001), smaller tumor size (p<0.001), and younger age (p=0.006) remained significantly associated with partial nephrectomy while BMI (odds ratio 0.98; 95% CI 0.96 – 1.01; p=0.2) was not significantly associated with treatment type.
DISCUSSION
Radical nephrectomy including ipsilateral adrenalectomy became standard of care for renal cortical tumors approximately 40 years ago. This was subsequently challenged in the 1980’s by several reports demonstrating favorable results with partial nephrectomy in imperative situations.13, 14 During the last decade, partial nephrectomy has been accepted as a safe and preferable alternative to radical nephrectomy for most small renal tumors even in the setting of a normal contralateral kidney.1–4 However, data from the 2000–2002 SEER cancer registry clearly demonstrates that across the United States, partial nephrectomy is underutilized in the surgical management of renal tumors. With the widespread use of cross-sectional imaging, approximately 2/3 of all renal masses today are small and incidentally detected. Thus, it remains concerning as to why so few patients are treated with partial nephrectomy in the United States.
Our contemporary experience with surgical management of renal tumors demonstrates a few important points. First, the “emerging quality of care concern” reported by Miller et al10 is being addressed as evidenced by the steady increase in the frequency of partial nephrectomy use at our institution. In the year 2007 alone, nearly 90% of patients with T1a tumors were treated with partial nephrectomy despite an elective situation. Furthermore, most (60%) patients with T1b tumors were also treated with partial nephrectomy and we expect these trends to continue to increase for surgically managed patients. Second, we confirm previous population based observations that younger patient age, smaller tumor size, male gender, and more recent diagnostic year are independent determinants of partial nephrectomy use.7, 10 While tumor size and diagnostic year seem logical as predictors of partial nephrectomy, knowledge that females and older patients are at risk for overtreatment should prove helpful in limiting the underutilization of partial nephrectomy. Third, our results suggest that while integration of laparoscopy into renal surgery is initially associated with performing a radical nephrectomy, increased experience facilitates proper patient selection such that surgical approach does not affect the procedure performed.
Recent observations from our institution and others demonstrate a significantly increased risk of chronic renal insufficiency among patients treated with radical compared with partial nephrectomy for renal tumors in elective situations.1, 5 In a graded fashion, chronic renal failure places patients at increased risks of hospitalization, cardiovascular morbidity, and death.15 It is important to emphasize that these risks occur in a graded and escalating fashion beginning when GFR declines below 60. We have previously shown that among all renal mass patients with a “normal” contralateral kidney on imaging and a “normal” serum creatinine, more than 25% actually have baseline chronic kidney disease (GFR <60) if their GFR is estimated using the abbreviated MDRD equation.1 Thus, treatment of contemporary renal mass patients should focus on minimizing the risk of chronic renal disease and not simply attempting to limit the rare progression to dialysis. Additionally, recent observations with mid term clinical follow-up suggest that overall survival is diminished if patients with small renal masses are treated with a radical compared with partial nephrectomy.6, 7 Collectively, these observations suggest that there are serious potential consequences for overutilization of radical nephrectomy for patients with small renal masses. In this report, we demonstrate that this quality of care concern is being addressed at our tertiary care center. We also surmise that our results are applicable to most academic centers, and thus, we believe that the improvement in partial nephrectomy use is likely occurring across the United States and abroad. We also confirm important clinical features that predict for overutilization of radical nephrectomy which should prove useful when additional centers similarly address the national quality of care concern.
This study is not without limitations. Our analysis represents a retrospective, single institution experience that may not be reflective of other institutions. Additionally, we would like to emphasize that the limited number of surgeons who performed laparoscopic surgery at our institution, coupled with the strong preference for open partial nephrectomy by the senior author (who performed 50% of the operations), suggests that the results we observed between surgical approach and type of procedure clearly need external validation or populated based confirmation prior to embracing as a valid association. Furthermore, our results are limited by a referral bias to our tertiary care facility. Patients who request partial nephrectomy or those with imperative indications may be more likely to be referred to our institution. However, we attempted to minimize this bias by limiting the analyses to patients with normal contralateral kidneys and an estimated GFR >45 which we defined as an elective situation. Nonetheless, our results may not be applicable or indicative of all hospitals or surgeons in the United States.
Compared with radical nephrectomy, partial nephrectomy is associated with more procedure related complications; however, the majority of these are minor and there does not appear to be a difference in the frequency of serious complications or the presence of any early complication.16 Additionally, with increasing experience, complications from open partial nephrectomy have significantly decreased to the point where risk of urine leak or hemorrhage are less than 5%.2, 17 In elective situations, health related quality of life is improved with partial compared with radical nephrectomy.18 Hospital costs and length of stay are similar for partial and radical nephrectomy.19, 20 Additionally, laparoscopic partial nephrectomy has now been investigated with excellent mid-term oncologic and functional outcomes.21 Partial nephrectomy reduces the risk of chronic renal failure1, 5 and recent data suggest that partial nephrectomy may reduce the risk of subsequent overall mortality compared with radical nephrectomy.6, 7 Collectively, these observations support that partial nephrectomy is standard of care for most small renal tumors even in the setting of a normal contralateral kidney. Patients with small and often incidental renal tumors should be offered partial nephrectomy or referred to a center that performs the procedure with efficiency. Radical nephrectomy for low grade, indolent, and frequently benign tumors may have long-term adverse consequences including renal failure, cardiovascular morbidity, and death. Our data suggest that these quality of care concerns are currently being addressed to improve the long-term care for renal mass patients.
CONCLUSION
At our tertiary care center, use of partial nephrectomy is increasing and is now utilized for ~90% of patients with T1a tumors even in an elective situation. These results demonstrate that use of partial nephrectomy at an academic institution is much higher than has been reported in the population as a whole. For reasons that remain unclear, certain groups in the population have lower rates of partial nephrectomy compared with others.
Acknowledgments
We are indebted to the Stephen Hanson Family Fellowship for their support. This project was also supported by NIH T32 CA82088.
References
- 1.Huang WC, Levey AS, Serio AM, Snyder M, Vickers AJ, Raj GV, et al. Chronic kidney disease after nephrectomy in patients with renal cortical tumours: a retrospective cohort study. Lancet Oncol. 2006;7:735. doi: 10.1016/S1470-2045(06)70803-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thompson RH, Leibovich BC, Lohse CM, Zincke H, Blute ML. Complications of contemporary open nephron sparing surgery: a single institution experience. J Urol. 2005;174:855. doi: 10.1097/01.ju.0000169453.29706.42. [DOI] [PubMed] [Google Scholar]
- 3.Patard JJ, Shvarts O, Lam JS, Pantuck AJ, Kim HL, Ficarra V, et al. Safety and efficacy of partial nephrectomy for all T1 tumors based on an international multicenter experience. J Urol. 2004;171:2181. doi: 10.1097/01.ju.0000124846.37299.5e. [DOI] [PubMed] [Google Scholar]
- 4.Becker F, Siemer S, Humke U, Hack M, Ziegler M, Stockle M. Elective nephron sparing surgery should become standard treatment for small unilateral renal cell carcinoma: Long-term survival data of 216 patients. Eur Urol. 2006;49:308. doi: 10.1016/j.eururo.2005.10.020. [DOI] [PubMed] [Google Scholar]
- 5.Lau WK, Blute ML, Weaver AL, Torres VE, Zincke H. Matched comparison of radical nephrectomy vs nephron-sparing surgery in patients with unilateral renal cell carcinoma and a normal contralateral kidney. Mayo Clin Proc. 2000;75:1236. doi: 10.4065/75.12.1236. [DOI] [PubMed] [Google Scholar]
- 6.Thompson RH, Boorjian SA, Lohse CM, Leibovich BC, Kwon ED, Cheville JC, et al. Radical nephrectomy for pT1a renal masses may be associated with decreased overall survival compared with partial nephrectomy. J Urol. 2008;179:468. doi: 10.1016/j.juro.2007.09.077. [DOI] [PubMed] [Google Scholar]
- 7.Miller DC, Schonlau M, Litwin MS, Lai J, Saigal CS. Renal and cardiovascular morbidity after partial or radical nephrectomy. Cancer. 2008;112:511. doi: 10.1002/cncr.23218. [DOI] [PubMed] [Google Scholar]
- 8.Leibovich BC, Blute ML, Cheville JC, Lohse CM, Weaver AL, Zincke H. Nephron sparing surgery for appropriately selected renal cell carcinoma between 4 and 7 cm results in outcome similar to radical nephrectomy. J Urol. 2004;171:1066. doi: 10.1097/01.ju.0000113274.40885.db. [DOI] [PubMed] [Google Scholar]
- 9.Dash A, Vickers AJ, Schachter LR, Bach AM, Snyder ME, Russo P. Comparison of outcomes in elective partial vs radical nephrectomy for clear cell renal cell carcinoma of 4–7 cm. BJU Int. 2006;97:939. doi: 10.1111/j.1464-410X.2006.06060.x. [DOI] [PubMed] [Google Scholar]
- 10.Miller DC, Hollingsworth JM, Hafez KS, Daignault S, Hollenbeck BK. Partial nephrectomy for small renal masses: an emerging quality of care concern? J Urol. 2006;175:853. doi: 10.1016/S0022-5347(05)00422-2. [DOI] [PubMed] [Google Scholar]
- 11.Hollenbeck BK, Taub DA, Miller DC, Dunn RL, Wei JT. National utilization trends of partial nephrectomy for renal cell carcinoma: a case of underutilization? Urology. 2006;67:254. doi: 10.1016/j.urology.2005.08.050. [DOI] [PubMed] [Google Scholar]
- 12.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 13.Zincke H, Engen DE, Henning KM, McDonald MW. Treatment of renal cell carcinoma by in situ partial nephrectomy and extracorporeal operation with autotransplantation. Mayo Clin Proc. 1985;60:651. doi: 10.1016/s0025-6196(12)60739-3. [DOI] [PubMed] [Google Scholar]
- 14.Novick AC, Streem S, Montie JE, Pontes JE, Siegel S, Montague DK, et al. Conservative surgery for renal cell carcinoma: a single-center experience with 100 patients. J Urol. 1989;141:835. doi: 10.1016/s0022-5347(17)41026-3. [DOI] [PubMed] [Google Scholar]
- 15.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351:1296. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- 16.Stephenson AJ, Hakimi AA, Snyder ME, Russo P. Complications of radical and partial nephrectomy in a large contemporary cohort. J Urol. 2004;171:130. doi: 10.1097/01.ju.0000101281.04634.13. [DOI] [PubMed] [Google Scholar]
- 17.Gill IS, Matin SF, Desai MM, Kaouk JH, Steinberg A, Mascha E, et al. Comparative analysis of laparoscopic versus open partial nephrectomy for renal tumors in 200 patients. J Urol. 2003;170:64. doi: 10.1097/01.ju.0000072272.02322.ff. [DOI] [PubMed] [Google Scholar]
- 18.Lesage K, Joniau S, Fransis K, Van Poppel H. Comparison between open partial and radical nephrectomy for renal tumours: perioperative outcome and health-related quality of life. Eur Urol. 2007;51:614. doi: 10.1016/j.eururo.2006.10.040. [DOI] [PubMed] [Google Scholar]
- 19.Uzzo RG, Wei JT, Hafez K, Kay R, Novick AC. Comparison of direct hospital costs and length of stay for radical nephrectomy versus nephron-sparing surgery in the management of localized renal cell carcinoma. Urology. 1999;54:994. doi: 10.1016/s0090-4295(99)00348-9. [DOI] [PubMed] [Google Scholar]
- 20.McKiernan JM, Teschendorf B, Katz J, Herr HW, Russo P. A comparison of hospital-based charges following partial and radical nephrectomy. Urol Oncol. 2002;7:3. doi: 10.1016/s1078-1439(01)00135-1. [DOI] [PubMed] [Google Scholar]
- 21.Lane BR, Gill IS. 5-Year outcomes of laparoscopic partial nephrectomy. J Urol. 2007;177:70. doi: 10.1016/j.juro.2006.08.093. [DOI] [PubMed] [Google Scholar]


