Abstract
The RNA sequences boxA, boxB and boxC constitute the nut regions of phage λ. They nucleate the formation of a termination-resistant RNA polymerase complex on the λ chromosome. The complex includes E. coli proteins NusA, NusB, NusG and NusE, and the λ N protein. A complex that includes the Nus proteins and other factors forms at the rrn leader. Whereas RNA-binding by NusB and NusE has been described in quantitative terms, the interaction of NusA with these RNA sequences is less defined. Isotropic as well as anisotropic fluorescence equilibrium titrations show that NusA binds only the nut spacer sequence between boxA and boxB. Thus, nutR boxA5-spacer, nutR boxA16-spacer and nutR boxA69-spacer retain NusA binding, whereas a spacer mutation eliminates complex formation. The affinity of NusA for nutL is 50% higher than for nutR. In contrast, rrn boxA, which includes an additional U residue, binds NusA in the absence of spacer. The Kd values obtained for rrn boxA and rrn boxA-spacer are 19-fold and 8-fold lower, respectively, than those for nutR boxA-spacer. These differences may explain why λ requires an additional protein, λ N, to suppress termination. Knowledge of the different affinities now describes the assembly of the anti-termination complex in quantitative terms.
INTRODUCTION
Gene expression in Escherichia coli and its phage can be controlled at the level of transcription termination. The best-studied examples of this mechanism are the ribosomal operons (rrn) and the bacteriophage λ (1–3). Transcription of the E. coli rrn operons is in part regulated by suppression of termination (anti-termination) (4). Anti-termination in rrn is mediated by an RNA recognition sequence (AT) located just distal to the promoters, close to the 5′ end of the pre-rRNA transcript (Figure 1A). A number of factors, including NusA, NusB, NusE (ribosomal protein S10) and NusG, modify RNA polymerase (RNAP) at AT. The modified RNAP is insensitive to termination by Rho-dependent terminators that occur throughout the long pre-rRNA transcript. AT includes a highly conserved sequence (boxA) that binds NusB, NusE and NusB–NusE complex (5,6). Distal to AT is an additional conserved sequence (boxC) that is less well characterized, but is a specific binding site for NusA in Mycobacterium tuberculosis rrn (7). Two short oligo ribonucleotides derived from the boxC stem–loop motif bind exclusively to the two KH domains of NusA in a completely extended conformation, and adenine-backbone interactions with the trinucleotide sequence AUA are particularly critical for this interaction (8).
Figure 1.
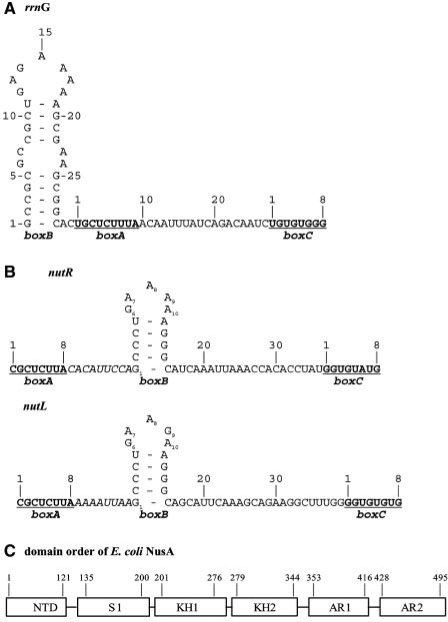
Different anti-terminator signal sequences. (A) rrnG leader sequence of E. coli. boxB, boxA and boxC refer to the λ-like anti-terminator (AT) features. Each box sequence is numbered separately; boxA and boxC are underlined. (B) Phage λ nut anti-termination sequence. λnutR and λnutL differ in the sequence and length of the spacer between boxA and boxB, as well as position 9 in the loop region of boxB. (C) Domain order of E. coli NusA. The numbers show the boarders of the six domains: N-terminal domain (NTD), S1 domain (S1), K-homologous domain (KH), acidic repeat (AR).
Gene expression in lambdoid phages is also controlled by anti-termination. The Nus proteins form a complex with and modify RNAP at the λnutL and nutR sequences. nutL and nutR consist of boxA, a spacer, a stem–loop element (boxB), and boxC (Figure 1B). The rrn boxA (5′-UGCUCUUUA-3′) and the λ boxA (5′-CGCUCUUA-3′) differ; the CUUUA of rrn boxA is thought to enhance anti-termination efficiency (9). λ and other lambdoid phages express N, an RNA-binding protein of the arginine-rich motif (ARM) family, that binds boxB (10–13). N is required for anti-termination on the λ chromosome. (14). In both the rrn and λ anti-termination systems, the modified RNAP retains the ability to transcribe through multiple terminators. However, rrn anti-termination is effective only at Rho-dependent terminators, whereas λ anti-termination complexes are highly resistant to both Rho-dependent and Rho-independent terminators (15).
The nut sequences and the Nus factors are also utilized by the phage HK022 Nun protein, an ARM protein related to N, to arrest transcription on the λ chromosome (11,16,17).
NusA is essential in wild-type E. coli (18,19) but not in E. coli deleted for cryptic prophage (20). In addition to promoting anti-termination, it enhances RNAP pausing (21,22) and termination (23,24). These reactions may be promoted by contacts between NusA and the 3′OH end of nascent RNA (25). NusA consists of five functional subdomains: an N-terminal domain that interacts with RNAP (26), three RNA-binding domains, S1, KH1 and KH2 (8,27,28) and two C-terminal acidic domains, AR1 and AR2, that interact with λ N and the α subunit of RNAP, respectively (Figure 1C) (17,29,30). AR2 masks one or more of the RNA-binding domains, thereby preventing NusA interaction with RNA (31). Structures of homologous NusA proteins from Thermotoga maritima (T. maritima) and from M. tuberculosis were determined in the absence and presence of RNA, respectively. Both structures show NusA to be highly elongated (8,27,28). Although knowledge of NusA has increased in recent years, several key questions are still open: Does E. coli NusA bind specifically or non-specifically to RNA? What rrn or nut sequences are critical for NusA binding? Are there structural differences between the NusA-rrn and NusA–nut RNA complexes?
MATERIALS AND METHODS
Buffers and reagents
All fluorescence titrations were performed in 50 mM potassium phosphate, 100 mM NaCl, 10 mM β-mercaptoethanol, pH 7.6, unless otherwise stated. Oligodeoxynucleotides as well as fluorescently-labeled oligoribonucleotides were obtained from biomers.net (Ulm, Germany; Table 1) and used according to the manufacturer's instructions.
Table 1.
3′6-carboxyfluorescein (6-Fam)-labeled RNA oligonucleotides used in this study
| Oligonucleotide | Sequence | Kd for NusA–SKK (µM) |
|---|---|---|
| nutR boxA-spacer | 5′-cgcucuuacacauucca-3′ | 126 ± 4 |
| nutL boxA-spacer | 5′-cgcucuuaaaaauuaa-3′ | 71 ± 4 |
| rrn cac-boxA-spacer | 5′-CACugcucuuuaacaauuua-3′ | 14 ± 0.2 |
| nutR boxA | 5′-cgcucuua-3′ | n.d. |
| nutR spacer | 5′-cacauucca-3′ | 137 ± 17 |
| nutL spacer | 5′-aaaauuaa-3′ | 24 ± 2.2 |
| nutR boxA5-spacer | 5′-cucucuuacacauucca-3′ | 124 ± 7 |
| nutR boxA16-spacer | 5′-cgcuauuacacauucca-3′ | 106 ± 4 |
| nutR boxA69-spacer | 5′-auagcggccacauucca-3′ | n.d. |
| nutL boxA-spacer (mut) | 5′-cgcucuuaaaaaggaa-3′ | n.d. |
| rrn boxA | 5′-ugcucuuua-3′ | 194 ± 38 |
| rrn-upstream-boxA’ (I) | 5′-CACugcuc-3′ | 26 ± 0.8 |
| rrn-upstream (II) | 5′-AGCGGCAC-3′ | 30 ± 1.9 |
| rrn spacer (III) | 5′-acaauuua-3′ | 71 ± 3.3 |
| nutR boxB | 5′-agcccugaaaaagggc-3′ | n.d. |
boxA nucleotides are shown in bold. Mutated nucleotides are underlined. Flanking regions of rrn-boxA are in capital letters. The spacer is shown in italic.
Plasmid construct, expression and protein purification
The DNA sequence of the NusA RNA-binding domains from amino acid 132 to 348 (NusA–SKK) was cloned via the BamHI and NdeI restriction sites into the E. coli expression vector pET11a (Novagen). The soluble recombinant NusA-SKK protein contained an N-terminal 5×His tag. NusA-SKK was expressed and purified according to published procedures (31). Briefly, E. coli strain BL21 (DE3) (Novagen) harboring the recombinant plasmid was grown at 37°C in LB medium (Luria-Bertani) containing ampicillin (100 µg/ml) until OD600 = 0.5 and then induced with 0.1 mM isopropyl 1-thio-β-d-galactopyranoside (IPTG). Cells were harvested 4 h after induction, lysed and purified as described (31). Finally, the protein was dialyzed against buffer as used for fluorescence measurements. The dialyzed protein was concentrated with Vivaspin concentrators (Vivascience, MWCO 10 000 Da). The identity and structural integrity of purified protein was analyzed by 19% SDS–PAGE as well as by CD- and NMR spectroscopy.
NMR spectroscopy
NMR spectra were recorded on Bruker DRX 600 MHz spectrometers with triple-resonance probes equipped with pulsed field-gradient capabilities. The sample temperature was 298 K. 1D 1H spectra were collected with water suppression using a 1-1 spin-echo pulse sequence including gradients.
Fluorescence equilibrium measurements
We used various RNA sequences corresponding to λ nut to rrnG boxA sequence (rrn BoxA) of the E. coli genome (Table 1). Fluorescence equilibrium titrations were performed using an L-format Jobin-Yvon Horiba Fluoromax fluorimeter equipped with an automatic titration device (Hamilton). Extrinsic fluorescence measurements with 3′ 6-carboxy-fluorescein (6-FAM)-labeled RNA were performed in fluorescence buffer as above in a total volume of 1 ml using a 10 × 4 mm quartz cuvette (Hellma GmbH, Mühlheim, Germany). The excitation wavelength was 492 nm, and the emission intensity was measured at 516 nm applying a 500 nm cutoff filter. For anisotropic measurements, slit widths were set at 4.5 nm and 3.5 nm for excitation and emission, respectively. All titration measurements were performed at 25°C with 50 nM of fluorescently-labeled RNA. Following sample equilibration, at least six data points with an integration time of 0.8 s were collected for each titration point in the case of anisotropic measurements.
Data fitting
Isotropic as well as anisotropic data were fitted to a two-state binding equation to determine the equilibrium dissociation constant (Kd) using standard software. The anisotropy was calculated from:
| 1 |
where A, Acomplex and ARNA are the anisotropy values and fcomplex, fRNA are the fractional intensities. The change in fluorescence intensity has to be taken into account, so that the bound fraction is given by
| 2 |
with
 |
3 |
where A is the anisotropy; ARNA is the initial free anisotropy, Acomplex is the anisotropy of the protein–RNA complex and P0 and RNA0 represent the total protein and RNA concentrations, respectively. R is the ratio of intensities of the bound and free forms.
RESULTS
The E. coli NusA protein includes a C-terminal domain that masks the RNA-binding region (17,26,29,31). To determine the interaction of E. coli NusA with different RNA substrates, we used a NusA construct (NusA–SKK) lacking the two acidic-repeat C-terminal domains AR1 and AR2, as well as the N-terminal domain (Figure 1C). These regions are not directly involved in RNA binding. Thus, E. coli NusA416, deleted for AR2, forms complexes with the rrnG leader region as well as with the M. tuberculosis nut RNA. Electrophoretic mobility-shift assays (EMSAs) showed that the truncated E. coli NusA protein bound nut-like RNA species with high affinity, whereas the specificity was significantly lower than that of the M. tuberculosis NusA (7). This prompted us to investigate the affinity of different RNA species to E. coli NusA using fluorescence measurements. To avoid possible false negatives due to protein binding too distal to the fluorescence dye to alter fluorescence signal intensity, we used anisotropic fluorescence titrations instead of isotropic fluorescence measurements. Fluorescence anisotropy can detect molecular interactions even when an isotropic fluorescence signal change is weak or absent (32). Furthermore, changes of the fluorophore environment can be neglected with anisotropic measurements since the results are related to the rotational correlation time of a macromolecule with a rigidly attached fluorophore (33).
An extended rrn boxA sequence has the highest affinity to NusA–SKK
We first turned our attention to three different RNA species, the rrnG anti-terminator region, λnutL and λ nutR, all of which interact with NusA and the other Nus factors (4). rrn carries a stem–loop structure (boxB), boxA and boxC sequences. The boxB and boxC sequences of rrn are not required for anti-termination (1).
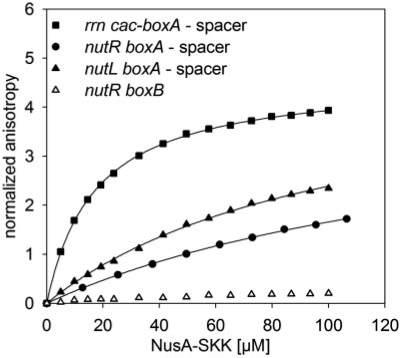
The boxA sequence of rrn differs from that of λ at the initial base and by the insertion of an additional U residue at the penultimate site, converting the rrn boxA to a consensus site. Conversion of λboxA to consensus enhances N activity (34). The spacer sequence of rrn differs from both λnutL and λnutR, but all three spacers carry a conserved sequence of AUU (Figure 1). Interestingly, we find that the rrn cac-boxA-spacer sequence, which includes a CAC sequence just upstream to boxA, binds with higher affinity to NusA–SKK (Kd = 14 µM; Figure 2; Table 1) than either the λnutR boxA-spacer (126 µM) or the λnutL boxA-spacer (71 µM; Figure 2; Table 1).
Figure 2.
Fluorescence anisotropy measurements with homologous nut-RNAs. 50 nM rrn boxA-spacer (filled square), λnutR boxA-spacer (filled circles), λnutL boxA-spacer (filled triangles) and λnutR boxB (open triangles) were titrated with NusA–SKK. The extrinsic fluorescence of the 3′ 6-FAM label of the RNAs was determined. The curves show the best fit to Equation (3) (see ‘Materials and Methods’ section). Kd values of 14 µM, 126 µM, 71 µM were determined, respectively (solid line; see Table 1). No Kd values could be fitted to λnutR boxB.
Role of boxA flanking sequences in binding of NusA–SKK
In these experiments, we tested boxA sequences with flanking regions (Table 1). In the case of rrn, these included sequences between boxB and boxA (in capital letter), as well as sequences between boxA and boxC (spacer, in italics). In the case of phage λnutL and λnutR, the spacer separates boxA from boxB. We proceeded to further define the NusA–SKK interaction regions at λnutR, λnutL and rrn.
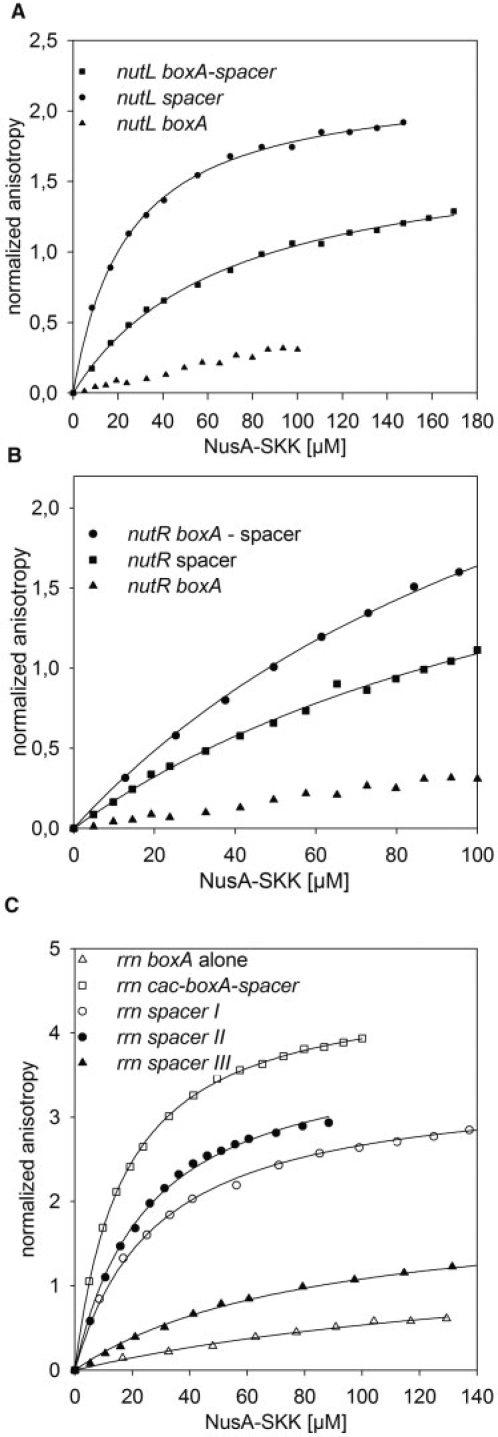
In the case of the λnut sites, we find that the λnutL spacer binds to NusA–SKK (24 µM), whereas boxA alone shows no association with the protein (Figure 3A). Similarly, the nutR spacer binds NusA-SKK with an affinity nearly identical to that of nutR boxA-spacer (Kd value ∼137 µM; Figure 3B), whereas NusA–SKK binding to boxA could not be detected.
Figure 3.
Fluorescence anisotropy measurements with seperated RNAs regions. In each titration 50 nM of 6-FAM-labeled RNA was used. (A) 50 nM of 6-FAM-labeled λnutL boxA-spacer (squares), λnutL spacer (circles), λnutL boxA (triangles) were titrated with NusA-SKK. Kd values of 71 µM and 24 µM were determined for λnutL boxA-spacer, λnutL spacer, respectively (solid lines). No Kd value could be fitted to λnutL boxA (see Table 1). (B) 50 nM of 6-FAM-labeled λnutR boxA-spacer (circles), λnutR spacer (squares), λnutR boxA (triangles) were titrated with NusA-SKK. Kd values of 126 µM and 137 µM were determined for λnutR boxA-spacer, λnutR spacer, respectively (solid lines). No Kd value could be fitted to λnutR boxA (see Table 1). (C) 50 nM of 6-FAM-labeled rrn boxA alone (open triangle), rrn cac-boxA-spacer (open square), rrn spacer I (open circle), rrn spacer II (filled circle), rrn spacer III (filled triangle) were titrated with NusA-SKK. Kd values can be seen in Table 1. No Kd value could be fitted to rrn boxA (see Table 1).
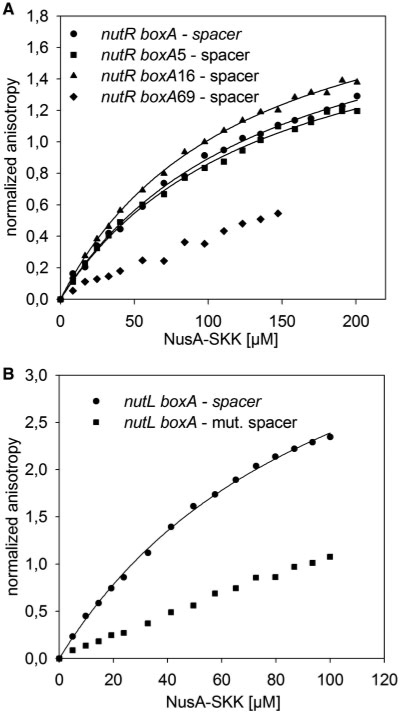
To validate this result, we analyzed nutR boxA-spacer sequences with mutations in the boxA region (34,35). The boxA5 and boxA16 mutations decrease N activity, whereas the boxA69 mutation has little effect on anti-termination (36). Fluorescence titrations of the three mutant RNAs indicate that only boxA69 significantly increased the Kd value (>200 µM) for NusA–SKK complex formation, whereas boxA5 and boxA16 exhibited Kd values similar to that of wild-type boxA (∼120 µM; Figure 4A). These data demonstrate that boxA mutations that affect anti-termination have a very limited effect on NusA–SKK binding. Their phenotype instead may reflect a failure to bind NusB (37). Why λnutR boxA69-spacer binds NusA–SKK less efficiently than λnutR spacer alone is unclear, although a similar result was reported by Mah et al. (31) for a λnut containing a reversed boxA. Furthermore, we also tested a mutation in the λnutL spacer that replaces residues U13 and U14 that are conserved at both λnutR and λnutL (Figure 1B). This conservation suggests that these bases are important for binding of interaction partners. Indeed, transversion of these residues to G completely abolished NusA binding to nutL-spacer (Figure 4B).
Figure 4.
Fluorescence anisotropy measurements with mutated λnut boxA RNA. (A) 50 nM of 6-FAM-labeled λnutR boxA-spacer (circles), λnutR boxA5-spacer (squares), λnutR boxA16-spacer (triangles), or λnutR boxA69-spacer (diamonds) were titrated with NusA-SKK. Kd values of 126 µM, 124 µM, 106 µM were determined, respectively (solid lines; see Table 1). No Kd values could be fitted to λnutR boxA69 (see Table 1). (B) 50 nM of 6-FAM-labeled λnutL boxA-spacer (circles), λnutL mut. spacer (squares) were titrated with NusA-SKK. Kd value of 71 µM was determined for nutL boxA-spacer (solid lines). No Kd value could be fitted to nutL boxA-mut- spacer (see Table 1).
We extended our analysis to the rrn anti-termination region, examining the binding affinities of RNA sequences upstream and downstream to boxA as well as boxA itself (Table 1). First, we found that rrn boxA showed no binding to NusA–SKK (Table 1). However, RNA that included an upstream CAC, as well as the first five bases of boxA was bound with high affinity (26 ± 0.8 μM), as was the 8 bases upstream of boxA (AGCGGCAC, 30 ± 1.9 μM; Figure 3C). The rrn spacer also bound NusA-SKK with an affinity intermediate between that of λnutR spacer and λnutL spacer (71 ± 3.3 μM). Alignment with ClustalW2 of rrn, λnutL, and λnutR shows a conserved sequence, 5′-auu-3′, in all three spacers.
λboxB does not interact with NusA–SKK
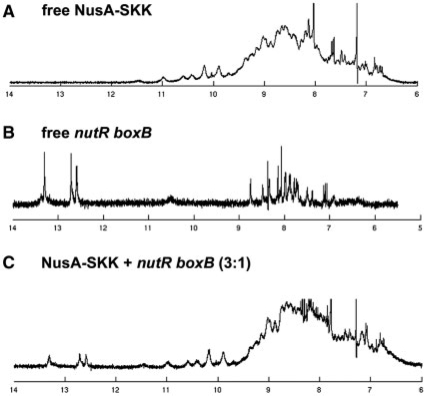
In contrast to boxA and flanking sequences, titration of λnutR boxB with NusA-SKK, showed no, or only very weak, nonspecific protein–RNA interactions (Figure 5). To confirm that λnutR boxB does not interact with NusA-SKK, even at higher concentrations, we analyzed a sample containing both species with 1D-NMR. In contrast to λnutR boxA, λnutR boxB forms a stable stem–loop structure allowing the detection of the slowly exchanging imino protons in the double-stranded stem region. The 1D-NMR spectrum of NusA-SKK in the absence of RNA shows a well-dispersed amide proton signal region, indicating a stably folded, highly structured protein (Figure 5A). The λnutR boxB 1D-NMR spectrum reveals signals in the range of 12–14 p.p.m., corresponding to the imino protons of the stem region (Figure 5B). Interaction between the stem region of λnutR boxB and NusA–SKK, would affect these readily observable imino proton signals. The observable signals, however, of both protein and RNA, were unchanged when incubated together, clearly indicating that no complex forms between NusA–SKK and λnutR boxB even at NusA concentrations in the high micromolar range (Figure 5C). The observed signal increase is due to the lower concentration of λnutR RNA after addition of NusA–SKK.
Figure 5.
1D-NMR analysis. Imino proton region of NusA–SKK (175 µM; A), nutR boxB (100 µM; B) and NusA-SKK+λnutR boxB (3:1; C).
CONCLUSIONS
Mutational studies indicated that NusA as well as boxA play an important role in anti-termination (38,39). boxA forms a complex with NusB/NusE (5,6,37) and it was suggested that NusA links λnut boxA and λnut boxB by binding to both (37,40). Oddly, however, and in contrast to boxA point mutations, anti-termination was still efficient, and NusB-independent, in a boxA deletion mutant (36,41). Additionally, deletion of the initial three bases (cac) of the λnutR spacer did not affect anti-termination, whereas deletion of the initial six bases (cacauu) led to complete loss of anti-termination activity. In agreement with the X-ray structure of M. tuberculosis NusA with RNA and deletion studies, our fluorescence analyses revealed that NusA recognizes a spacer sequence that includes the critical bases AUU (8,36). NusA interaction with the rrn, λnutL and λnutR boxA-spacer motif was demonstrated by mutational studies and in vitro binding assays (1,37,39,42), and NusA was also suggested to recognize RNA outside the λnut region (19,27,37,43). Complex formation between NusA and spacer might promote binding of NusE/NusB to the adjacent boxA sequence, and the notion that NusA binds to the λnut spacer region is now strongly supported by the present fluorescence titration data.
Differences between the rrn anti-terminator regions and λnut have already been described (5,37). Berg et al. (1) showed that rrn boxA-spacer plus seven upstream residues were sufficient to suppress termination at Rho-dependent terminators. We show here that the upstream CAC sequence as well as the downstream spacer, bind NusA–SKK. This redundancy may be related to the fact that λnut-dependent anti-termination, which suppresses both Rho-dependent and Rho-independent terminators, requires λN and boxB, whereas rrn anti-termination requires neither (37,44). In addition to greater anti-termination efficiency, the requirement for λN and BoxB allows regulation of λ anti-termination. Thus, λ N levels are controlled at the levels of transcription, translation and protein stability (45,46).
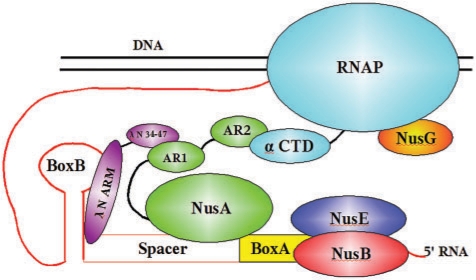
Note that the NusE/NusB complex binds to boxA with affinities in the nanomolar range (5), whereas the Kd values for NusA–SKK are in the micromolar range. We suggest that tight RNA binding by NusA may not be required since it is already bound to RNAP and thus in close vicinity to nascent RNA. The λnutL spacer sequence differs from that of λnutR spacer (Table 1), and this difference is thought to account in part for the enhanced efficiency of Nun-mediated termination at λnutL relative to λnutR (Washburn, R.S. and Gottesman,M.E., unpublished data), and λnutL boxA-spacer binds with significantly higher affinity to NusA–SKK than does λnutR boxA-spacer. Both spacer sequences contain U's at residues 13 and 14, implying that these bases are important interaction partners. As shown above, replacement of the U's with G's completely abolished NusA–SKK binding to λnutL-spacer. From this and other data, the following picture of the assembly of the anti-termination complex at the λnut RNA has evolved (Figure 6): After RNAP has synthesized λnut RNA, NusE and NusB bind to boxA, and NusA binds to spacer facilitated by NusA AR2 interaction with the C-terminal domain of the α subunit of RNAP. λN protein binds to AR1 of NusA as demonstrated for N(34-47) (17,30), forming a weak helix at the protein's N-terminus (17). This weak helix facilitates recognition of boxB (17). NusA interaction with RNA is thus stabilized by the AR2:RNAP interaction as well as by the AR1:N:boxB interaction, relieving the requirement for tight binding of NusA to λnut.
Figure 6.
Model of the anti-termination network. The interaction of the RNAP with various factors important for anti-termination (see text for details).
ACKNOWLEDGEMENTS
P.R. would like to thank the Columbia University Microbiology Department for their patience during his sabbatical stay in New York.
FUNDING
The Deutsche Forschungsgemeinschaft DFG (Ro617/16-1 to B.M.W. and P.R.); and the NIH (NIGMS R01/GM37219 to M.E.G.).
Conflict of interest statement. None declared.
REFERENCES
- 1.Berg KL, Squires C, Squires CL. Ribosomal RNA operon anti-termination. function of leader and spacer region box B-box A sequences and their conservation in diverse micro-organisms. J. Mol. Biol. 1989;209:345–358. doi: 10.1016/0022-2836(89)90002-8. [DOI] [PubMed] [Google Scholar]
- 2.Friedman DI, Court DL. Transcription antitermination: the lambda paradigm updated. Mol. Microbiol. 1995;18:191–200. doi: 10.1111/j.1365-2958.1995.mmi_18020191.x. [DOI] [PubMed] [Google Scholar]
- 3.Weisberg RA, Gottesman ME. Processive antitermination. J. Bacteriol. 1999;181:359–367. doi: 10.1128/jb.181.2.359-367.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Squires CL, Greenblatt J, Li J, Condon C, Squires CL. Ribosomal RNA antitermination in vitro: requirement for nus factors and one or more unidentified cellular components. Proc. Natl Acad. Sci. USA. 1993;90:970–974. doi: 10.1073/pnas.90.3.970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Greive SJ, Lins AF, von Hippel PH. Assembly of an RNA-protein complex. binding of NusB and NusE (S10) proteins to boxA RNA nucleates the formation of the antitermination complex involved in controlling rRNA transcription in escherichia coli. J. Biol. Chem. 2005;280:36397–36408. doi: 10.1074/jbc.M507146200. [DOI] [PubMed] [Google Scholar]
- 6.Luo X, Hsiao HH, Bubunenko M, Weber G, Court DL, Gottesman ME, Urlaub H, Wahl MC. Structural and functional analysis of the E. coli NusB-S10 transcription antitermination complex. Mol. Cell. 2008;32:791–802. doi: 10.1016/j.molcel.2008.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arnvig KB, Pennell S, Gopal B, Colston MJ. A high-affinity interaction between NusA and the rrn nut site in mycobacterium tuberculosis. Proc. Natl Acad. Sci. USA. 2004;101:8325–8330. doi: 10.1073/pnas.0401287101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beuth B, Pennell S, Arnvig KB, Martin SR, Taylor IA. Structure of a mycobacterium tuberculosis NusA-RNA complex. EMBO J. 2005;24:3576–3587. doi: 10.1038/sj.emboj.7600829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Friedman DI, Olson ER, Johnson LL, Alessi D, Craven MG. Transcription-dependent competition for a host factor: the function and optimal sequence of the phage lambda boxA transcription antitermination signal. Genes Dev. 1990;4:2210–2222. doi: 10.1101/gad.4.12a.2210. [DOI] [PubMed] [Google Scholar]
- 10.Friedman DI, Baron LS. Genetic characterization of a bacterial locus involved in the activity of the N function of phage lambda. Virology. 1974;58:141–148. doi: 10.1016/0042-6822(74)90149-4. [DOI] [PubMed] [Google Scholar]
- 11.Nudler E, Gottesman ME. Transcription termination and anti-termination in E. coli. Genes Cells. 2002;7:755–768. doi: 10.1046/j.1365-2443.2002.00563.x. [DOI] [PubMed] [Google Scholar]
- 12.Schärpf M, Sticht H, Schweimer K, Boehm M, Hoffmann S, Rösch P. Antitermination in bacteriophage lambda. the structure of the N36 peptide-boxB RNA complex. Eur. J. Biochem. 2000;267:2397–408. doi: 10.1046/j.1432-1327.2000.01251.x. [DOI] [PubMed] [Google Scholar]
- 13.Legault P, Li J, Mogridge J, Kay LE, Greenblatt J. NMR structure of the bacteriophage lambda N peptide/boxB RNA complex: recognition of a GNRA fold by an arginine-rich motif. Cell. 1998;93:289–299. doi: 10.1016/s0092-8674(00)81579-2. [DOI] [PubMed] [Google Scholar]
- 14.Das A, Wolska K. Transcription antitermination in vitro by lambda N gene product: requirement for a phage nut site and the products of host nusA, nusB, and nusE genes. Cell. 1984;38:165–173. doi: 10.1016/0092-8674(84)90537-3. [DOI] [PubMed] [Google Scholar]
- 15.Condon C, Squires C, Squires CL. Control of rRNA transcription in escherichia coli. Microbiol. Rev. 1995;59:623–645. doi: 10.1128/mr.59.4.623-645.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Robert J, Sloan SB, Weisberg RA, Gottesman ME, Robledo R, Harbrecht D. The remarkable specificity of a new transcription termination factor suggests that the mechanisms of termination and antitermination are similar. Cell. 1987;51:483–492. doi: 10.1016/0092-8674(87)90644-1. [DOI] [PubMed] [Google Scholar]
- 17.Prasch S, Schwarz S, Eisenmann A, Wöhrl BM, Schweimer K, Rösch P. Interaction of the intrinsically unstructured phage lambda N protein with escherichia coli NusA. Biochemistry. 2006;45:4542–4549. doi: 10.1021/bi0523411. [DOI] [PubMed] [Google Scholar]
- 18.Zhou Y, Mah TF, Yu YT, Mogridge J, Olson ER, Greenblatt J, Friedman DI. Interactions of an arg-rich region of transcription elongation protein NusA with NUT RNA: implications for the order of assembly of the lambda N antitermination complex in vivo. J. Mol. Biol. 2001;310:33–49. doi: 10.1006/jmbi.2001.4722. [DOI] [PubMed] [Google Scholar]
- 19.Zhou Y, Mah TF, Greenblatt J, Friedman DI. Evidence that the KH RNA-binding domains influence the action of the E. coli NusA protein. J. Mol. Biol. 2002;318:1175–1188. doi: 10.1016/s0022-2836(02)00238-3. [DOI] [PubMed] [Google Scholar]
- 20.Cardinale CJ, Washburn RS, Tadigotla VR, Brown LM, Gottesman ME, Nudler E. Termination factor rho and its cofactors NusA and NusG silence foreign DNA in E. coli. Science. 2008;320:935–938. doi: 10.1126/science.1152763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Landick R, Yanofsky C. Isolation and structural analysis of the escherichia coli trp leader paused transcription complex. J. Mol. Biol. 1987;196:363–377. doi: 10.1016/0022-2836(87)90697-8. [DOI] [PubMed] [Google Scholar]
- 22.Chan CL, Landick R. Dissection of the his leader pause site by base substitution reveals a multipartite signal that includes a pause RNA hairpin. J. Mol. Biol. 1993;233:25–42. doi: 10.1006/jmbi.1993.1482. [DOI] [PubMed] [Google Scholar]
- 23.Farnham PJ, Greenblatt J, Platt T. Effects of NusA protein on transcription termination in the tryptophan operon of escherichia coli. Cell. 1982;29:945–951. doi: 10.1016/0092-8674(82)90457-3. [DOI] [PubMed] [Google Scholar]
- 24.Schmidt MC, Chamberlin MJ. nusA protein of escherichia coli is an efficient transcription termination factor for certain terminator sites. J. Mol. Biol. 1987;195:809–818. doi: 10.1016/0022-2836(87)90486-4. [DOI] [PubMed] [Google Scholar]
- 25.Liu K, Hanna MM. NusA contacts nascent RNA in escherichia coli transcription complexes. J. Mol. Biol. 1995;247:547–558. doi: 10.1006/jmbi.1994.0161. [DOI] [PubMed] [Google Scholar]
- 26.Mah TF, Li J, Davidson AR, Greenblatt J. Functional importance of regions in escherichia coli elongation factor NusA that interact with RNA polymerase, the bacteriophage lambda N protein and RNA. Mol. Microbiol. 1999;34:523–537. doi: 10.1046/j.1365-2958.1999.01618.x. [DOI] [PubMed] [Google Scholar]
- 27.Worbs M, Bourenkov GP, Bartunik HD, Huber R, Wahl MC. An extended RNA binding surface through arrayed S1 and KH domains in transcription factor NusA. Mol. Cell. 2001;7:1177–1189. doi: 10.1016/s1097-2765(01)00262-3. [DOI] [PubMed] [Google Scholar]
- 28.Gopal B, Haire LF, Gamblin SJ, Dodson EJ, Lane AN, Papavinasasundaram KG, Colston MJ, Dodson G. Crystal structure of the transcription elongation/anti-termination factor NusA from mycobacterium tuberculosis at 1.7 A resolution. J. Mol. Biol. 2001;314:1087–1095. doi: 10.1006/jmbi.2000.5144. [DOI] [PubMed] [Google Scholar]
- 29.Eisenmann A, Schwarz S, Prasch S, Schweimer K, Rösch P. The E. coli NusA carboxy-terminal domains are structurally similar and show specific RNAP- and λ N interaction. Protein Sci. 2005;14:2018–2029. doi: 10.1110/ps.051372205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bonin I, Muhlberger R, Bourenkov GP, Huber R, Bacher A, Richter G, Wahl MC. Structural basis for the interaction of Escherichia coli NusA with protein N of phage lambda. Proc. Natl Acad. Sci. USA. 2004;101:13762–13767. doi: 10.1073/pnas.0405883101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mah TF, Kuznedelov K, Mushegian A, Severinov K, Greenblatt J. The alpha subunit of E. coli RNA polymerase activates RNA binding by NusA. Genes Dev. 2000;14:2664–2675. doi: 10.1101/gad.822900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Takahashi M, Sakumi K, Sekiguchi M. Interaction of ada protein with DNA examined by fluorescence anisotropy of the protein. Biochemistry. 1990;29:3431–3436. doi: 10.1021/bi00466a002. [DOI] [PubMed] [Google Scholar]
- 33.Cantor CR, Schimmel PR. Biophysical Chemistry. San Francisco: Freeman; 1981. pp. 454–465. [Google Scholar]
- 34.Olson ER, Tomich CS, Friedman DI. The nusA recognition site. alteration in its sequence or position relative to upstream translation interferes with the action of the N antitermination function of phage lambda. J. Mol. Biol. 1984;180:1053–1063. doi: 10.1016/0022-2836(84)90270-5. [DOI] [PubMed] [Google Scholar]
- 35.Robledo R, Gottesman ME, Weisberg RA. Lambda nutR mutations convert HK022 nun protein from a transcription termination factor to a suppressor of termination. J. Mol. Biol. 1990;212:635–643. doi: 10.1016/0022-2836(90)90226-c. [DOI] [PubMed] [Google Scholar]
- 36.Zuber M, Patterson TA, Court DL. Analysis of nutR, a site required for transcription antitermination in phage lambda. Proc. Natl Acad. Sci. USA. 1987;84:4514–4518. doi: 10.1073/pnas.84.13.4514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mogridge J, Mah TF, Greenblatt J. A protein-RNA interaction network facilitates the template-independent cooperative assembly on RNA polymerase of a stable antitermination complex containing the lambda N protein. Genes Dev. 1995;9:2831–2845. doi: 10.1101/gad.9.22.2831. [DOI] [PubMed] [Google Scholar]
- 38.Olson ER, Flamm EL, Friedman DI. Analysis of nutR: a region of phage lambda required for antitermination of transcription. Cell. 1982;31:61–70. doi: 10.1016/0092-8674(82)90405-6. [DOI] [PubMed] [Google Scholar]
- 39.Friedman DI, Olson ER. Evidence that a nucleotide sequence, ‘boxA,’ is involved in the action of the NusA protein. Cell. 1983;34:143–149. doi: 10.1016/0092-8674(83)90144-7. [DOI] [PubMed] [Google Scholar]
- 40.Mogridge J, Legault P, Li J, Van Oene MD, Kay LE, Greenblatt J. Independent ligand-induced folding of the RNA-binding domain and two functionally distinct antitermination regions in the phage lambda N protein. Mol. Cell. 1998;1:265–275. doi: 10.1016/s1097-2765(00)80027-1. [DOI] [PubMed] [Google Scholar]
- 41.Patterson TA, Zhang Z, Baker T, Johnson LL, Friedman DI, Court DL. Bacteriophage lambda N-dependent transcription antitermination: competition for an RNA site may regulate antitermination. J. Mol. Biol. 1994;236:217–228. doi: 10.1006/jmbi.1994.1131. [DOI] [PubMed] [Google Scholar]
- 42.Schauer AT, Carver DL, Bigelow B, Baron LS, Friedman DI. Lambda N antitermination system: functional analysis of phage interactions with the host NusA protein. J. Mol. Biol. 1987;194:679–690. doi: 10.1016/0022-2836(87)90245-2. [DOI] [PubMed] [Google Scholar]
- 43.Tsugawa A, Kurihara T, Zuber M, Court DL, Nakamura Y. E. coli NusA protein binds in vitro to an RNA sequence immediately upstream of the boxA signal of bacteriophage lambda. EMBO J. 1985;4:2337–2342. doi: 10.1002/j.1460-2075.1985.tb03935.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Morgan EA. Antitermination mechanisms in rRNA operons of Escherichia coli. J. Bacteriol. 1986;168:1–5. doi: 10.1128/jb.168.1.1-5.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wilson HR, Zhou JG, Yu D, Court DL. Translation repression by an RNA polymerase elongation complex. Mol. Microbiol. 2004;53:821–828. doi: 10.1111/j.1365-2958.2004.04170.x. [DOI] [PubMed] [Google Scholar]
- 46.Gottesman S, Gottesman M, Shaw JE, Pearson ML. Protein degradation in E. coli: the lon mutation and bacteriophage lambda N and cII protein stability. Cell. 1981;24:225–233. doi: 10.1016/0092-8674(81)90518-3. [DOI] [PubMed] [Google Scholar]