Abstract
In most bacteria, the ferric uptake regulator (Fur) is a global regulator that controls iron homeostasis and other cellular processes, such as oxidative stress defense. In this work, we apply a combination of bioinformatics, in vitro and in vivo assays to identify the Caulobacter crescentus Fur regulon. A C. crescentus fur deletion mutant showed a slow growth phenotype, and was hypersensitive to H2O2 and organic peroxide. Using a position weight matrix approach, several predicted Fur-binding sites were detected in the genome of C. crescentus, located in regulatory regions of genes not only involved in iron uptake and usage but also in other functions. Selected Fur-binding sites were validated using electrophoretic mobility shift assay and DNAse I footprinting analysis. Gene expression assays revealed that genes involved in iron uptake were repressed by iron-Fur and induced under conditions of iron limitation, whereas genes encoding iron-using proteins were activated by Fur under conditions of iron sufficiency. Furthermore, several genes that are regulated via small RNAs in other bacteria were found to be directly regulated by Fur in C. crescentus. In conclusion, Fur functions as an activator and as a repressor, integrating iron metabolism and oxidative stress response in C. crescentus.
INTRODUCTION
Iron is an essential nutrient for most organisms. It is a highly versatile cofactor of many iron-using enzymes, which are involved in pivotal biological processes (1). However, levels of free iron in the environment are very low because of its poor solubility under aerobic conditions at neutral pH or due to iron sequestration by eukaryote hosts as a defense mechanism against microbial pathogens (2). On the other hand, excessive intracellular unincorporated iron catalyzes, via the Fenton reaction, generation of hydroxyl radical, a reactive oxygen species (ROS) extremely destructive for biological molecules (3). Thus, bacteria and other organisms have evolved a range of strategies to maintain iron levels within a physiological range.
Sophisticated iron acquisition systems are used to scavenge iron from the environment under iron-restricted conditions. In many cases, bacteria solubilize iron (Fe3+) with secreted siderophores, low-molecular-weight compounds that chelate iron with high affinity. Some bacteria also use iron present in heme, hemoglobin, transferrin and lactoferrin of eukaryotic hosts (4). In Gram-negative bacteria, the active transport across the outer membrane of iron-siderophore complexes involve outer membrane proteins called TonB-dependent receptors and is driven by an inner membrane energy-transducing TonB–ExbB–ExbD protein complex. Once inside the cell, the metal is deposited into Fe-S proteins, heme, or iron-storage proteins (ferritins) such as bacterioferritin (5).
The most-studied system of iron homeostasis in bacteria is mediated by the ferric uptake regulator (Fur) transcriptional regulator. Fur protein utilizes Fe2+ as a cofactor and binds to specific sequence elements in the promoter regions of its target genes, called Fur boxes, inhibiting gene expression under iron-replete conditions (6). Besides its role as a direct repressor of bacterial iron uptake systems, Fur can also activate genes encoding iron-using proteins (Escherichia coli sdhCDAB, acnA, fumA, ftnA, bfr and sodB), often by an indirect mechanism. Fur represses small RNAs (for example RyhB in E. coli and PrrF1 and PrrF2 in Pseudomonas aeruginosa), that facilitate degradation of the mRNAs positively regulated by Fur (7–9). However, in Neisseria meningitidis Fur can also act as a direct transcriptional activator (10). Fur also regulates some iron regulatory cascades, such as the specialized iron starvation subfamily of extracytoplasmic function (ECF) sigma factors in E. coli and Pseudomonas aeruginosa that are positively required for expression of genes involved in siderophore synthesis and uptake (11–13).
The α-subdivision of proteobacteria includes several important genera, such as Caulobacter, Rhodobacter, Brucella, Agrobacterium, Bradyrhizobium, Sinorhizobium and Mesorhizobium. Despite the importance of this widespread group of organisms, very few studies have been carried out to understand how they regulate their genes in response to iron availability. Most of the experimental studies are limited to three species of the Rhizobiales (Rhizobium leguminosarum, Sinorhizobium meliloti and Bradyrhizobium japonicum) (14). These studies clearly demonstrate that in rhizobia Fur is not the main regulator of iron homeostasis but other regulators such as RirA and Irr accomplish the role of global iron-responsive transcriptional regulators (14,15). Bioinformatic and phylogenetic analyses suggest that this substitution of Fur by RirA and Irr appear to take place in other members of the Rhizobiales and Rhodobacterales orders (15,16). In other α-proteobacteria, outside of the Rhizobiales and Rhodobacterales (such as Caulobacter), Fur seems to maintain its more conventional role as an iron-responsive regulator (16), although no experimental work has been done to confirm this in silico prediction.
Caulobacter crescentus is a freshwater oligotrophic α-proteobacterium that divides asymmetrically to produce a stalked sessile cell and a motile swarmer cell (17). Genome sequence analysis revealed 67 TonB-dependent receptors, presumably important for Caulobacter to grow in a dilute aquatic environment (18). Recently, it was demonstrated that two of these TonB-dependent receptors, named MalA and NagA, are required for the transport of specific carbohydrates (19,20), and at least in part this transport is TonB–ExbB–ExbD-dependent (19,21). However, the mechanisms that C. crescentus utilizes to scavenge iron from its low nutrient environment and to maintain iron homeostasis in the face of its aerobic metabolism have not yet been determined.
In this work, we analyze the role of Fur in C. crescentus, using an in silico approach combined with experimental data to describe the Fur regulon. Fur has an important role in oxidative stress resistance generated by hydroperoxides, given that a C. crescentus fur mutant was highly sensitive to H2O2 and tert-butyl hydroperoxide. Several Fur-binding sites identified in the C. crescentus genome are associated with genes involved in iron homeostasis indicating that Fur is the main regulator of the iron starvation response in this α-proteobacterium. These Fur-binding sites were found at promoter regions of both Fur-repressed genes as well as Fur-activated genes, suggesting that C. crescentus Fur protein acts as dual transcriptional regulator. Furthermore, several genes that are regulated via small RNAs in other bacteria were shown to be directly regulated by Fur in C. crescentus.
MATERIALS AND METHODS
Bacterial strains and growth conditions
Bacterial strains and plasmids are described in Table 1. Caulobacter crescentus strains were grown in PYE medium (22) at 30°C with shaking. When necessary, the medium was supplemented with kanamycin (5 μg/ml), tetracycline (1 μg/ml), chloramphenicol (1 μg/ml) or nalidixic acid (20 μg/ml). Iron-replete and iron-restricted conditions were achieved by supplementing PYE medium with 100 μM FeSO4 and 100 μM 2,2-dipyridyl (Sigma), respectively. Escherichia coli strains were grown at 37°C in Luria-Bertani medium supplemented with ampicillin (100 μg/ml), kanamycin (50 μg/ml), tetracycline (12.5 μg/ml) or chloramphenicol (30 μg/ml) as required. Plasmids were introduced into C. crescentus by conjugation with E. coli strain S17-1. All primers used in this work are listed in Supplementary Data (Table S1).
Table 1.
Bacterial strains and plasmids
| Strain or plasmid | Description | Reference or source |
|---|---|---|
| E. coli | ||
| DH5α | E. coli strain for cloning purposes | (23) |
| S17-1 | E. coli strain for plasmid mobilization | (24) |
| C. crescentus | ||
| NA1000 | Synchronizable derivative of wild-type CB15 | (25) |
| SP0057 | NA1000 (Δfur) | This study |
| SP0057 (pMRFur) | NA1000 (Δfur, pMRFur) | This study |
| Plasmids | ||
| pGEM-T easy | Cloning vector, Ampr | Promega |
| pProEX HTa | Overexpression vector, Ampr | Gibco BRL |
| pNPTS138 | Suicide vector, containing oriT sacB, Kanr | D. Alley |
| pMR20 | Broad-host-range low-copy vector, Tetr | (26) |
| pUJ142 | Xylose-inducible promoter, Chlr | (27) |
| pRKlacZ290 | pRK2-derived vector with a promoterless lacZ gene, Tetr | (28) |
| pPROFur | fur coding region cloned in pProEX HTa | This study |
| pMRFur | pMR20 containing the fur gene | This study |
| pUJFur | pUJ142 containing the fur gene | This study |
| pNPTΔFur | fur flanking regions cloned ‘in tandem’ in PnpTS138 | This study |
| pLAC0028 | PRKlacZ290 with CC0028 promoter | This study |
| pLAC0139 | PRKlacZ290 with CC0139 promoter | This study |
| pLAC0711 | PRKlacZ290 with CC0711 promoter | This study |
| pLAC1956 | PRKlacZ290 with CC1956 promoter | This study |
| pLAC2194 | PRKlacZ290 with CC2194 promoter | This study |
| pLAC2928 | PRKlacZ290 with CC2928 promoter | This study |
| pLAC3529 | PRKlacZ290 with CC3529 promoter | This study |
| pLAC3667 | PRKlacZ290 with CC3667 promoter | This study |
| pLAC1956* | PRKlacZ290 with CC1956 promoter containing a mutated Fur-binding site | This study |
| PLAC3529* | PRKlacZ290 with CC3529 promoter containing a mutated Fur binding site | This study |
Construction and complementation of a C. crescentus fur mutant
A fur deletion mutant strain was constructed from C. crescentus NA1000 strain by allelic exchange. Two fragments containing the regions downstream and upstream of the fur gene (801 bp and 679 bp, respectively) were amplified by PCR with primers Fur1/Fur2 and Fur3/Fur4 and cloned sequentially into pGEM-T Easy vector. The 1480-bp EcoRI/HindIII resultant fragment was then cloned into the pNTPS138 suicide vector. The obtained plasmid, pNPTΔfur, contains the 5′- and 3′-flanking regions of fur, with entire fur gene (501 bp) removed. The pNPTΔfur vector was then introduced into C. crescentus NA1000 by conjugation, and clones with fur deletion after double recombination events were selected as previously described (29). The fur mutant strain (SP0057) was confirmed by PCR and Southern blot. For complementation of the fur mutant, a 1981-bp DNA fragment containing the entire fur gene including the promoter region was amplified by PCR with primers Fur1/Fur4 and cloned in the low-copy-number pMR20 vector (pMRFur). In the case of strains carrying the pRKlacZ290, pUJFur was used to complement the fur mutant strain since it carries chloramphenicol resistance marker instead of tetracycline.
Growth curves and survival tests
Overnight C. crescentus cultures were diluted to an optical density at 600 nm (OD600) of 0.1 in PYE medium. Growth rates were determined by measuring OD600 at regular intervals from cultures grown in liquid PYE medium at 30°C. Cell viability was determined after exposure to stress conditions by counting the number of colony forming units (CFU) after incubation of plates at 30°C. Aliquots of cells were taken before and after exposure to stress conditions at indicated time points and serial dilutions were plated on PYE plates. To assess the sensitivity to oxidative stress, C. crescentus cultures were grown until mid-log phase and then 5-mM paraquat (a superoxide generator) or 5-mM tert-butyl hydroperoxide was added. Resistance to hydrogen peroxide was determined by disk inhibition assay. C. crescentus cultures were grown until either mid-log phase or stationary phase and 0.5-ml samples were spread on top of PYE plates. Then, 6-mm-diameter paper disks soaked with 3% hydrogen peroxide were applied on the agar surface. Diameters of clearing zones were measured after incubation of the plates for 48 h at 30°C.
Computational prediction of Fur-binding sites
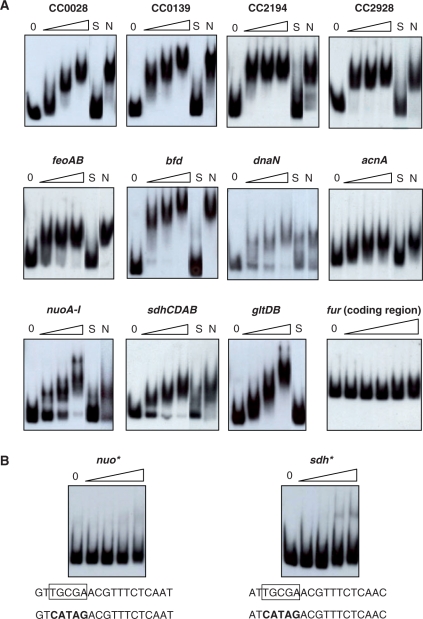
A set of 10 predicted C. crescentus Fur-binding sites (16) was used as training set for weight matrix construction with the CONSENSUS algorithm (30) from the Regulatory Sequence Analysis Tools (RSAT) website (31). This initial matrix was used to perform a genome-wide screening for putative Fur-binding sites in the C. crescentus genome sequence (18) with the PATSER module from the RSAT (32,33). A group of 11 predicted Fur-binding sites identified in this preliminary analysis was chosen for validation by gel shift assays in this study (Figure 3). A more accurate matrix was constructed by searching these Fur-binding promoters for sequence motifs with MEME (34) and CONSENSUS algorithms. The same 19-bp motif detected in all 11 sequences by both analyses was used to generate our final weight matrix for an iterative screening of the C. crescentus genome sequence as described above. The search for putative Fur-binding sites was performed between regions –400 to +200 of the start codon on the coding strand of all annotated genes. DNA sequence logos were generated using the WebLogo package (35).
Figure 3.
Fur binding to promoter regions was assessed by gel electrophoresis mobility shift assay. (A) DNA fragments corresponding to each promoter was 32P-labeled and incubated or not (0) with increasing concentrations of purified Fur protein (50, 200 and 500 nM, respectively). As controls, the binding of Fur (250 nM) was carried out in the presence of 30-fold of unlabeled fragments of the same region (S) or the fur coding region (N) as competitors. The last panel shows a control experiment using as probe the fur coding region, and the DNA was incubated with the following Fur concentrations: 50-, 100-, 250-, 500- and 1000-nM protein, respectively. (B) Fur-binding sites at the promoter regions of operons sdh and nuo were mutagenized (indicated by an asterisk) substituting the conserved TGCGA motif by the sequences in bold type. The fragments were incubated or not (0) with increasing concentrations of purified Fur protein (50, 200, 500 and 1000 nM, respectively).
Expression of the Fur protein, obtention of the anti-Fur antiserum and immunoblots
To express Fur as a poly-histidine tail fusion protein (His-Fur), the coding region of the C. crescentus NA1000 fur gene (CC0057) was amplified by PCR with primers FurExp1/FurExp2 and the 408-bp EcoRI/HindIII fragment was cloned into vector pProEX HTa (Gibco BRL). The resultant plasmid was transformed into E. coli DH5α and the His-Fur protein was induced with 0.5-mM IPTG from midlog cultures grown in LB media and purified from the soluble extract by NTA-resin affinity chromatography (Qiagen). Polyclonal antiserum against C. crescentus Fur was obtained by immunizing a New Zealand rabbit with two subcutaneous injections of 1 mg of purified His-Fur protein following standard procedures (36) approved by the Biomedical Sciences Institute Ethics Committee. Immunoblots were performed essentially as described (37). Whole-cell lysates of C. crescentus were separated by SDS–PAGE (15% gel), proteins were transferred to nitrocellulose membranes and incubated with anti-Fur antiserum using a 1:1,000 dilution.
Site-directed mutagenesis of Fur-binding sites
The promoter regions of CC1956 and CC3529 were mutagenized by PCR amplification using primers indicated in Table S1. Briefly, initial PCR reactions were carried out using one of two complementary primers with the altered sequence combined with the corresponding external primers. All the PCRs were carried out with the high-fidelity Pfx enzyme (Invitrogen), using the cloned regions of each gene as template. A mix of the two products generated was used in a second amplification reaction with the external primers to generate full-length fragments containing the mutation. The final fragments obtained were analyzed by DNA sequencing to confirm that they were altered only in the Fur-binding site as shown in Figure 3B.
Electrophoretic mobility shift assays (EMSAs)
Probes (representing 190–400-bp regions upstream of the start codon of the selected genes) were obtained by PCR amplification with appropriate primers (Table S1) and were end-labeled with [γ32P]-ATP using T4 polynucleotide kinase (Invitrogen). Unincorporated nucleotides were removed with Qiaquick PCR purification kit (Qiagen). DNA binding was performed in a 20-μl reaction volume containing binding buffer (10 M Tris–HCl pH 7.5, 40 mM KCl, 1 mM MgCl2, 1 mM dithiothreitol, 0.1 mM MnCl2, 0.1 mg/ml bovine serum albumin, 5% glycerol), competitor salmon sperm DNA (0.1 mg/ml), labeled DNA probes and increasing amounts of purified His-Fur protein (0, 50, 200 and 500 nM). In competition assays, a 30-fold excess of cold probe was used to challenge each of the labeled probes. After incubation at 30°C for 30 min, the samples were loaded onto a native 5% polyacrylamide gel and electrophoresed in 0.5 X Tris-borate (TB) buffer containing 5 mM MnCl2 for 3 h at 40 mA. Radioactive species were detected by autoradiography.
DNase I footprinting
The same primers used for amplification of the DNA fragments for EMSA (Table S1) were used for PCR amplification of the CC2194 and CC3529 promoter regions. PCR amplifications were performed only with the reverse primers end labeled, to obtain probes with a single 32P-labeled end. The probes were incubated with increasing amounts of purified His-Fur protein (0, 50, 100, 250, 500 and 1000 nM) exactly in the same buffer and conditions used for EMSA. Before DNA digestion, 20 μl of CaCl2/MgCl2 solution (5 mM CaCl2 and 10 mM MgCl2) was added, followed by the incubation for 1 min at room temperature. Then, RQ1 RNase-Free DNase I (Promega) was added to the reaction mixture and incubated for exactly 1 min. The reaction was stopped by addition of stop solution (200 mM NaCl, 30 mM EDTA and 1% SDS), extracted with phenol:chloroform:isoamyl alcohol (25:24:1) and precipitated with ethanol. Reaction mixtures were run on a 6% polyacrylamide-urea sequencing gel alongside sequencing ladders generated with the same DNA fragments of DNase I footprinting, using the Thermosequenase Cycle Sequencing kit (USB).
Construction of transcriptional lacZ fusions and β-galactosidase assays
The upstream regions of genes CC0028, CC0139, CC0711, CC1956, CC2194, CC2928, CC3529 and CC3667 were PCR amplified with appropriately designed primers (Table S1), as well as the mutagenized promoter regions of CC1956 and CC3529 described above. These PCR products (comprising regions 190–350-bp upstream of the start codon of the genes) were cloned into plasmid pGEM-T easy, sequenced and then subcloned as BamHI/HindIII or KpnI/HindIII fragments into the pRKlacZ290 vector to generate transcriptional fusions to the lacZ gene. C. crescentus cultures containing these reporter plasmids were grown in PYE medium up to mid-log phase and supplemented with iron (100 μM FeSO4) or an iron chelator (100 μM 2,2-dipyridyl). The cells were further incubated during two hours and assayed for β-galactosidase activity as described previously (38).
RESULTS
Construction and characterization of a fur mutant strain
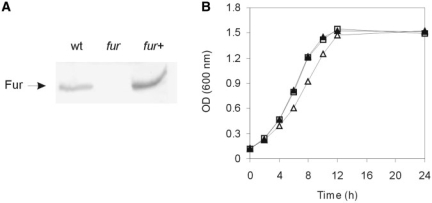
The C. crescentus genome contains two members of the Fur family of metalloregulators, Fur and Zur, putatively involved in iron and zinc homeostasis, respectively, and a Fe-S cluster biogenesis repressor IscR (18); however, no orthologs of iron-responsive regulators present in other α-proteobacteria, such as Irr and RirA are found (15). To determine the role of Fur in C. crescentus, the fur-deleted strain SP0057 was generated by allelic exchange using a suicide vector after a two-step recombination into C. crescentus NA1000 strain. The absence of the Fur protein in SP0057 was confirmed by immunoblot analysis with an anti-Fur antiserum, and expression was restored after complementation with the gene carried in plasmid pMR20-Fur (Figure 1A). The fur mutant formed smaller colonies than wild-type strain when grown on PYE solid medium for two days at 30°C and the size of the colonies was restored in the complemented mutant strain (data not shown). To further analyze this apparent slower growth of SP0057, we have evaluated the growth curves of the three strains. The fur mutant displayed a slower growth rate at exponential phase in PYE medium than the wild-type strain and reached similar cell density at stationary phase. The complemented strain revealed the same growth characteristics as the wild-type strain, demonstrating that the growth deficiency of the fur mutant was totally complemented (Figure 1B).
Figure 1.
(A) Immunoblot analysis of the fur mutant strain using a polyclonal anti-Fur antiserum. Wt, wild-type strain NA1000; fur, SP0057 strain; fur+, SP0057(pMRFur). (B) Growth curve of strains NA1000 (white square), SP0057 (white triangle) or SP0057(pMRFur) (black triangle). The figure shows one representative experiment of three independent replicates.
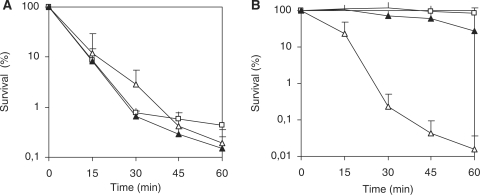
The absence of proper control over iron metabolism can cause as a secondary effect a difficulty in coping with oxidative stress, not only because this is generated in the presence of iron via the Fenton reaction, but also because several oxidative stress response enzymes need iron as a cofactor. The oxidative stress response of SP0057 was analyzed after exposure to the oxidative agents H2O2, paraquat and tert-butyl hydroperoxide. Increased sensitivity of SP0057 to H2O2 was demonstrated by larger zones of inhibition compared to strain NA1000 in a plate assay (Table 2). This difference was evident for lawns starting with cells from both exponential and stationary phase cells, and the phenotype was complemented by the fur gene in trans. The response to superoxide was not affected, as determined by cell viability assay in the presence of paraquat (Figure 2A), but the mutant was extremely sensitive to tert-butyl hydroperoxide (Figure 2B), indicating that it may be impaired in responding to organic hydroperoxides. These results suggest that the absence of fur in C. crescentus affects more notably the oxidative stress response generated by hydroperoxides than by superoxide.
Table 2.
Zone of inhibition assay for H2O2
| Strain | Diameter of zone of clearing (cm)a |
|
|---|---|---|
| Exponential | Stationary | |
| NA1000 | 3.02 ± 0.12 | 2.74 ± 0.14 |
| SP0057 | 4.53 ± 0.19 | 3.50 ± 0.01 |
| SP0057 (pMRFur) | 3.51 ± 0.17 | 2.88 ± 0.08 |
aNumbers represent the average and standard deviation of the growth inhibition halos from four independent experiments.
Figure 2.
Survival test of strains NA1000 (white square), SP0057(white triangle) or SP0057(pMRFur) (black triangle) after addition of 5 mM paraquat (A) or 5 mM tert-butyl hydropheroxide (B). Cultures grown at 30°C in PYE medium up to midlog phase and the oxidative agents were added at time zero. At the indicated time points, aliquots were removed and plated for counting colonies. Survival was determined relative to the colony number at time 0. The results shown are the average of three independent experiments.
Weight matrix prediction of C. crescentus candidate Fur-binding sites
As an effort to identify genes directly regulated by Fur, we implemented an iterative approach using different weight matrices to locate potential Fur-binding sites in the C. crescentus genome. Initially, the C. crescentus genome sequence was scanned using a weight matrix derived from a set of predicted C. crescentus Fur-binding sites detected in an ab initio comparative genomic analysis of multiple alpha-proteobacteria genomes (16). Of the several potential Fur-binding sites detected in this initial analysis (data not shown), 11 sequences were experimentally validated (Figure 3) and used to obtain a more confident motif model. The complete list of the predictions obtained by searching the C. crescentus genome with this refined weight matrix is in Supplementary Table S2. This data set contained almost all predicted sites of the initial search and all experimentally confirmed sites used for matrix construction. Predicted Fur-binding sites with scores >10 and their associated genes are summarized in Table 3. To obtain a more realistic estimative of the total of genes regulated by Fur, the genes associated with Fur-binding sites were analyzed with regard to their organization in putative operons (Table 3).
Table 3.
Predicted Fur-binding sites in C. crescentus genome
| Gene IDa | Gene or gene clusterb | d ATGc | Predicted Fur-binding sites | Score | Product |
|---|---|---|---|---|---|
| CC_3529 | sdhCDAB | –101 | ATTGCGAACGTTTCTCAAC | 17.85 | succinate dehydrogenase, cytochrome b556 subunit |
| CC_1956 | nuoABCDEFGHIJKLMN | –87 | GTTGCGAACGTTTCTCAAT | 17.41 | NADH dehydrogenase subunit A |
| CC_0155d,e | –32 | ATTGAGAACCATTCTCAAA | 16.50 | Hypothetical protein | |
| CC_0710d | –83 | ATTGCGAACGATTATCAGT | 16.32 | Hypothetical protein, EAL domain | |
| CC_3606d | gltDB | –144 | ATTGCGACAGGTTCTCAAT | 16.30 | Glutamate synthase iron-sulfur flavoprotein |
| CC_0139d,e | –72 | ATTGCGAGTGACACTCAGA | 14.96 | TonB-dependent receptor | |
| CC_2194d,e | –118 | ATTGCGATTGATTCTGAGA | 14.39 | TonB-dependent receptor | |
| CC_3667d | acnA | –204 | AATGAGAACAGCTCTCAAC | 14.32 | Aconitate hydratase |
| CC_2928e | CC2928-CC2927-CC2926 | –33 | ATTGCGACGCACTCGCAAT | 14.05 | TonB-dependent receptor |
| CC_3263d,e | bfd | –38 | GATGAGAATGACACTCAAT | 13.96 | Hypothetical protein, BFD-like [2Fe-2S] binding domain |
| CC_2193d | –131 | CATGCGAATGGCTCGCAAC | 13.57 | Hypothetical protein | |
| CC_0028d,e | 15 | CAGGCGAACGGCTCTGAAA | 13.44 | TonB-dependent receptor | |
| CC_0156d | dnaN-CC0157-CC0158 | –164 | TTTGAGAATGGTTCTCAAT | 13.39 | DNA polymerase III subunit beta CLAMP |
| CC_2329 | CC2329-CC2328 | –291 | CTTGCGACGAATTCTCAGC | 12.10 | D-Amino acid oxidase family protein 2328 |
| CC_2367e | –95 | CTTGCGAAGCATTCTCAAG | 11.98 | Hypothetical protein | |
| CC_3421 | ubiA | –380 | ATTGAGAGCGGCTCGCAAG | 11.95 | 4-Hydroxybenzoate octaprenyltransferase UbiA |
| CC_2367 | –129 | TTTGCGATTGACTCGCAAT | 11.72 | Hypothetical protein | |
| CC_3208 | CC3208-moeA-CC3210 | –397 | AATGCGAAAAACTCGCAGC | 11.65 | Ferredoxin-NADP reductase |
| CC_1461 | fljK | –192 | ATGGCGAAAGGCTCTCGGC | 10.92 | Flagellin FljK |
| CC_3605d | bacA | –105 | ATTGAGAACCTGTCGCAAT | 10.91 | Undecaprenyl pyrophosphate phosphatase |
| CC_2193d | –68 | ATTAAGATTGATTCTCAGA | 10.48 | Hypothetical protein | |
| CC_0028d,e | 132 | ATTGCGAATAATTATCATT | 10.27 | TonB-dependent receptor | |
| CC_3408 | CC3408-CC3409-CC3410 | –126 | GTCGCGAATGCCTCTCAGA | 10.25 | Hypothetical protein |
| CC_0138d | CC0138-CC0137 | –144 | GATGCGACTGACTTGCAAT | 10.20 | Sensor histidine kinase/response regulator |
| CC_3675 | 96 | CTGGCGAACGGCTATCAGG | 10.12 | Hypothetical protein | |
| CC_0682d | –108 | TTTGCGAACGCCACGCAAT | 10.04 | Hypothetical protein | |
| CC_1362 | –108 | ATCGAGAAGCGCTCTGAAT | 10.04 | Hypothetical protein |
aGene ID of the gene immediately downstream of the predicted Fur-binding site. Sites that were experimentally validated are indicated in bold letters.
bGenes probably cotranscribed.
cDistance to the annotated ATG.
dSites located between divergent genes.
eSites previously identified in (16).
As a rule, we find one predicted Fur-binding site for each gene; however, some present more than one, such as genes CC0001, CC0029, CC0179, CC0228, CC0682, CC1063, CC1362, CC2193, CC2194, CC2367, CC3208 and CC3264 that possess two sites each and gene CC0028 with four putative Fur-binding sites in its promoter region (Table S2). It is also worth noticing that at least 12 predicted Fur-binding sites were detected in promoter regions of divergent genes (as indicated in Table 3) and this binding might or not affect expression of both genes simultaneously.
Consistent with the definition for a global regulator, Fur-binding sites with high scores are present in genes belonging to diverse functional categories, such as transport and iron uptake systems, energy metabolism (iron-containing enzymes) and transcription regulation among others. Some of these categories will be discussed below.
Many of the genes associated with Fur-binding sites are involved with iron uptake systems. Four genes encoding TonB-dependent receptors (CC0028, CC0139, CC2194 and CC2928), as well as others encoding proteins of transport systems belonging to different families showed high scores for predicted Fur-binding sites, suggesting that they may be involved in iron transport (Table 3). Additionally, low-score Fur-binding sites (scores >5 and <7.5) were also found in front of four more genes encoding TonB-dependent receptors (CC0185, CC1136, CC1666 and CC3161) and of the exbBD-tonB1 (CC2336) and feoAB (CC0711) operons (Table S2). A possible Fur-binding site is also present in the promoter region of its own gene (CC0057, score 7.26), suggesting that the C. crescentus fur gene is transcriptionally autoregulated, as it has been reported for other bacteria (1).
Predicted Fur-binding sites were identified upstream of several genes and/or operons encoding important iron-containing enzymes that are involved in energy metabolism, such as NADH dehydrogenase (nuo), succinate dehydrogenase (sdh), aconitate hydratase (acnA), cytochrome c oxidase (CC3402) and ATP synthase (CC0367 and CC3450). Fur-binding sites were also detected upstream of three genes encoding putative ferredoxins: CC3208 (ferredoxin-NADP reductase), CC0068 (ferredoxin, Rieske 2Fe-2S family) and CC3263 (ferredoxin bfd). Although the bfd gene is adjacent to bfr gene (CC3262, encoding the iron storage bacterioferritin), they are not probably cotranscribed. In fact, our search did not detect Fur-binding sites in none of the two ferritins (Bfr and Dps) encoded in the C. crescentus genome.
Experimental validation of predicted Fur-binding sites by EMSA and footprinting analysis
To evaluate the functionality of the predicted Fur-binding sites detected by our in silico analysis, 11 regulatory regions of selected genes were PCR-amplified and used in a gel mobility shift assay with the C. crescentus purified Fur protein. As it can be observed in Figure 3A, all the tested probes were shifted in the presence of up to 500 nM Fur in a dose-dependent manner. The specificity of Fur binding was demonstrated, since complete loss of the shift was observed when excess unlabeled DNA probe was used as specific competitor (Figure 3A, S lanes) but not with an unlabeled non-specific DNA (Figure 3A, N lanes). As an additional control, it was demonstrated that the Fur protein did not bind to a DNA probe of similar size corresponding to the fur-coding region even if 1 μM Fur was used. The assay also allowed a rough estimate of the relative Fur-binding affinity for each fragment, with genes dnaN, nuo and sdh showing the lowest affinities. Therefore, EMSA experiments confirmed in vitro specific binding of the Fur regulator to all 11 tested DNA fragments containing predicted Fur-binding sites.
As a second validation of the specific binding of Fur, the predicted Fur-binding sites present at the nuo and sdh regulatory regions were mutated, exchanging five of the conserved base pairs for a different sequence. As shown in Figure 3B, the fragments containing the altered sites were no longer retarded in EMSA, confirming that this conserved region is essential for Fur binding to these fragments.
Most of these validated Fur-binding sites are located in regulatory regions of divergent genes (Table 3). Admitting its control on each pair of divergent genes, we have confirmed Fur-binding sites controlling expression of at least 19 different genes and/or operons. Among them are genes known to be regulated by Fur in other organisms such as the ferrous iron transporter feoAB, several TonB-dependent receptors for ferri–siderophore transport (CC0028, CC0139, CC2194 and CC2928), the ferredoxin bfd and a variety of genes encoding iron-using enzymes (acnA, sdhCDAB, nuoA-N and gltDB).
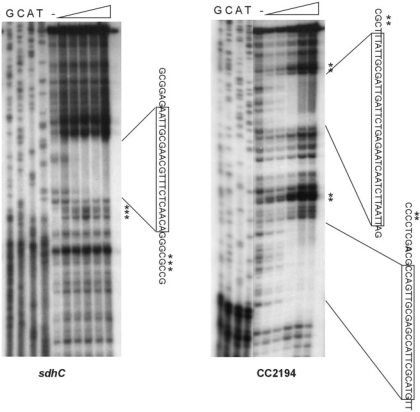
The sdh and CC2194 regulatory regions were further analyzed by DNase I footprinting assay to determine the actual sequence of Fur binding (Figure 4). Probes consisting of the sdh and CC2194 promoter regions were radiolabelled at one extremity, incubated with increasing amounts of Fur protein and digested with DNase I. A region corresponding to positions –102 to –80 with respect to translational start site of the sdh gene was protected by the Fur protein. Interestingly, for the CC2194 gene, two regions encompassing positions –120 to –87 and –46 to –22 with respect to the start codon were protected. Consistent with these two protected regions, two Fur-binding sites were predicted by the in silico analysis for the CC2194 gene, although only the most upstream one was highly scored. In conclusion, the nucleotide sequences of these three protected regions correspond to the three predicted Fur-binding sites, confirming a correct prediction of the C. crescentus Fur-binding site by our in silico analysis.
Figure 4.
DNase I footprinting assays of Fur in the sdhC and CC2194 promoter regions. Probes containing each promoter region were end-labeled and incubated in the presence or absence of increasing concentrations of purified Fur (50, 100, 250, 500 and 1000 nM, respectively). The DNA–protein complexes were treated with DNase I as described in ‘Materials and Methods’ section. The protected regions are boxed in the respective sequences. A minus sign indicates no protein. Asterisks indicate hypersensitive sites.
The role of Fur on controlling expression of genes with predicted Fur-binding sites
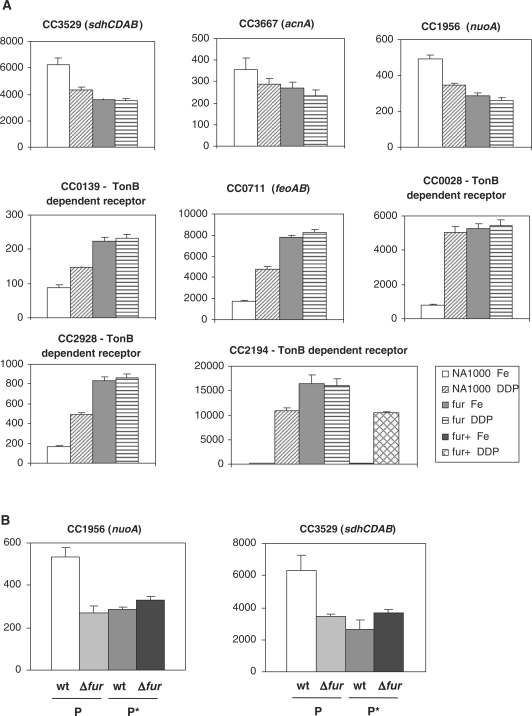
To determine the effect of Fur in gene expression in vivo, some of the promoter regions of genes with validated Fur-binding sites were cloned in front of a lacZ reporter gene and plasmids containing each transcriptional fusion were introduced into the wild-type NA1000 strain and into the fur mutant strain. Expression driven by each promoter was assessed by measuring β-galactosidase activity in the presence of either 100 μM FeSO4 or 100 μM of the iron chelator 2,2-dipyridyl (Figure 5A). The results showed that the expression driven by the sdhC, acnA and nuoA promoters was diminished in the presence of dipyridyl and in the fur mutant strain, indicating that these genes are activated by Fur in the presence of iron. The TonB-dependent receptors-encoding genes CC0139, CC0028, CC2928 and CC2194, as well as the feoAB operon, were induced in the presence of dipyridyl in the wild-type strain and constitutively derepressed in the fur mutant strain independently of iron, indicating that Fur represses these genes in response to high iron level. As a control, expression of CC2194 was also determined in the Fur complemented strain. The iron-dependent repression of the CC2194 gene was completely restored in this strain.
Figure 5.
Analysis of expression of selected genes in response to iron and Fur. (A) Expression was determined from cells harboring the respective promoter fusions to a lacZ reporter gene after incubation at 30°C for 2 h in presence of either 100 μM FeSO4 (Fe) or 100 μM 2′-dipyridyl (DDP). The last panel shows as a control the expression of CC2194 in NA1000, the fur mutant (fur) and fur complemented with the gene in trans (fur+), indicating that the presence of Fur restores the wild-type expression. (B) Expression driven by the nuoA and sdhC promoters was determined from cells harboring the respective promoter fusions to a lacZ reporter gene after incubation at 30°C for 2 h in presence of 100 μM FeSO4. The assays were carried out using either the wild-type promoters (P) or the mutagenized promoters (P*) as described in Figure 3, introduced into the NA1000 or SP0057 strains. β-Galactosidase activity is expressed in Miller units (38) and is the average of at least three independent assays.
The results indicated that the sdh, nuo and acnA genes were activated by Fur, showing about 2-fold higher expression in the NA1000 strain than in the SP0057 strain. In order to determine whether this increase in expression was directly driven by the binding of Fur to the promoter, lacZ expression was determined from transcriptional fusions carrying the nuo and sdh promoter regions with mutated Fur binding sites (Figure 5B). The results showed that the mutated promoters were unable to generate the levels of expression observed with the original promoters in the wild-type strain. In fact, those levels were similar to those obtained with the original promoters in the fur mutant strain. These results confirm that the activation of the nuo and sdh genes in vivo is dependent on Fur binding to the predicted Fur-binding sites.
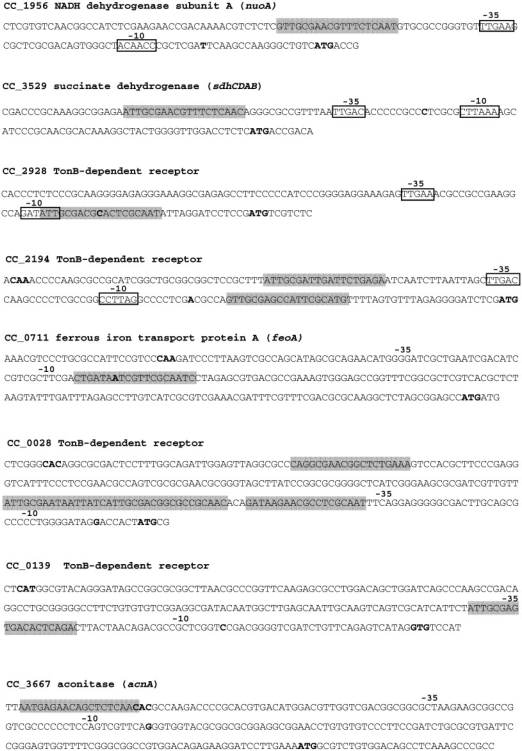
Transcriptional start sites previously determined (39) were used to analyze the position of some verified Fur-binding sites in relation to the promoter region of the respective genes (Figure 6). The Fur-binding sites of genes nuoA, sdhC and acnA are found upstream of the deduced –35 promoter regions, consistent with them being activated by Fur, as verified by gene expression assays (Figure 5). On the other hand, the Fur-binding sites of genes CC0139, CC0028, CC2928, C2194 and feoA are located overlapping or downstream of the RNA polymerase binding site, suggesting transcriptional repression by steric hindrance. In fact, all these genes were repressed by Fur in response to high iron level (Figure 5A). Thus, transcription start sites correlated well with –35 and –10 promoter elements and with predicted Fur-binding sites, except for sdhC. In this gene, the proposed transcriptional start site was found between the –35 and –10 elements of an excellent C. crescentus σ70 consensus (TTGAC-16-CCTANA) and the role of Fur as activator indicates that the Fur-binding site is correctly positioned with regard to this consensus, indicating that the transcriptional start site is probably a few base pairs downstream.
Figure 6.
Promoter sequences of selected genes, indicating the Fur-binding sites (shaded) predicted in silico or experimentally demonstrated (sdhC and CC2194). Boxes indicate conserved –35 and –10 sequences of Caulobacter σ70 promoters (TTGAC-16 bp-G/CCTANA) and previously identified transcription start sites (39) are indicated in bold. Annotated start codons are also indicated in bold letters, except for the putative start codon of CC0028, which was proposed in this work.
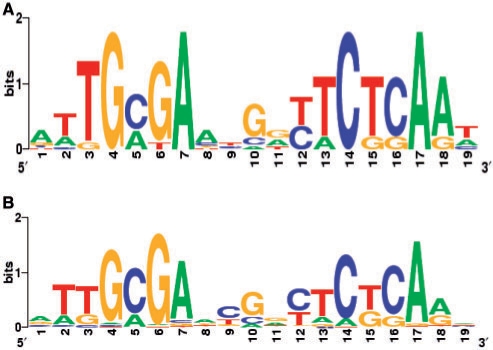
DNA sequence logos were derived from the 11 Fur-binding sites verified in vitro and from all predicted Fur-binding sites with score >7.5, and are represented in Figure 7. The consensus sequence contains a 19-bp palindromic motif (9-1-9 inverted repeat) that is highly similar to the previously described Furα-box predicted for several α-proteobacteria (16). Interestingly, this Fur-binding motif resembles two unknown motifs (motif cc_13 and motif m_3) identified in a global analysis for novel regulatory signals in C. crescentus (39). The cc_13 motif was identified in a cluster of genes presenting the same cell cycle pattern of expression (39). In fact, almost all the genes belonging to motif cc_13 were also identified in our search and were shown to be negatively regulated by Fur. These genes showed an increase in expression in the late predivisional cell (39), which is consistent with the fact that synthesis of Fur was reported to be higher in the stalked cell and decreasing in the predivisional cell (40). The motif m_3 was found in genes with similar metal response pattern of expression (39), but in this case some genes belonging to that cluster (CC1956, CC3529 and CC3667) were positively regulated by Fur.
Figure 7.
DNA sequence logos representing C. crescentus Fur-binding site. Experimentally validated Fur-binding sites (11 sites) (A) and computationally predicted Fur binding sites (62 sites with score >7.5 shown in Table S2) (B) were used to create the logos with the WebLogo generator (http://weblogo.berkeley.edu/).
DISCUSSION
In this paper, we initiate to reveal the mechanisms employed by the α-proteobacterium C. crescentus to maintain iron homeostasis taking into account its oligotrophic and obligatory aerobic lifestyle. Bioinformatic predictions followed by in vitro and in vivo experimental validation provided reliable evidence that the Fur protein is the master transcriptional regulator controlling iron metabolism in C. crescentus. More specifically, this assertion is sustained by the following results: (i) prediction of Fur-binding sites upstream of genes controlling essential aspects in iron metabolism, for instance, iron uptake systems and iron-using enzymes; (ii) confirmation of 11 predicted Fur-binding sites by EMSA that were subsequently used to construct a more confident C. crescentus Fur position weight matrix. The regions of Fur binding within two of the fragments used in EMSA were revealed by DNase I footprinting assay; (iii) demonstration in vivo that C. crescentus Fur functions as an iron-dependent transcriptional repressor of genes encoding iron uptake proteins (four genes encoding TonB-dependent receptors and feoAB) as well as an iron-dependent activator of genes encoding iron-using enzymes (acnA, sdhCDAB and nuoA-N operons); and (iv) the sensitivity of the C. crescentus fur mutant to ROS, demonstrating interplay between iron homeostasis and oxidative stress defenses.
To address the role of Fur and identify its regulon in C. crescentus, a fur mutant was obtained by complete deletion of locus CC0057 from the C. crescentus NA1000 genome. Null mutations of fur have been isolated in many other bacteria, such as E. coli (41), Vibrio cholerae (42) and Staphylococcus aureus (43). However, Fur seems to be essential for some bacteria, such as P. aeruginosa (44) and Burkholderia pseudomallei (45), where only point mutations have been isolated by the manganese selection method (46). As it has been observed in many other bacteria, the C. crescentus fur mutant presents a slow growth phenotype and is hypersensitive to oxidative stress. Escherichia coli and P. aeruginosa fur mutants have been shown to be sensitive to ROS, due to an increase in intracellular iron and reduction in antioxidant enzyme activities, presumably causing formation of hydroxyl radicals in the presence of hydrogen peroxide (41,44). The sensitivity of C. crescentus SP0057 to ROS is specific for hydrogen peroxide and organic hydroperoxide, given that the resistance to superoxide was not affected. As no obvious Fur-binding sites were detected in the regulatory regions of genes encoding antioxidant enzymes, such as katG, ahpCD, ohr, sodA and sodB, more studies are necessary to determine whether Fur directly or indirectly regulates transcription of these genes and which mechanisms could explain the susceptibility of the C. crescentus fur mutant to ROS.
Numerous computational strategies have been developed to predict transcription factors binding sites in the regulatory regions of genes (47,48). A plethora of motif discovery algorithms have been used to reveal unknown binding sites by searching for overrepresented DNA patterns upstream of functionally related genes (47–50). These sequence motifs associated with experimentally determined binding sites can be used on the construction of probabilistic models, in the form of position weight matrices. These strategies have been applied to predict regulons in many organisms. For example, Fur-binding sites have been discovered in E. coli (51), Acidithiobacillus ferrooxidans (52), Anabaena sp. (53) and Yersinia pestis (54). We searched the C. crescentus genome for sites matching the position weight matrix constructed from 11 Fur-binding sites experimentally confirmed in this work. Some aspects of the predicted C. crescentus Fur regulon that emerge from this analysis will be discussed below.
In diverse bacterial groups Fur controls genes encoding ferrous iron and ferri–siderophore transporting systems (1). Analysis of the C. crescentus genome did not show any obvious gene involved in siderophore synthesis, and very little is known about ferri–siderophore uptake in this bacterium (18,19). Fur-binding sites were detected and confirmed in the promoter regions of feoAB operon (a ferrous iron transporter) and four genes encoding TonB-dependent receptors (CC0028, CC0139, CC2194 and CC2928) and we showed that these five genes are upregulated under iron-limiting conditions and repressed by Fur under iron sufficiency. Additionally, low-score Fur-binding sites were predicted upstream of four more genes encoding TonB-dependent receptors (CC0185, CC1136, CC1666 and CC3161) and of the exbBD-tonB1 operon. Therefore, among the 67 TonB-dependent receptors identified in the C. crescentus genome, at least eight seem to be iron and Fur regulated, although their involvement in ferri–siderophore complexes transport remain to be determined. In fact, the function of only two TonB-dependent receptors have been demonstrated in C. crescentus, MalA and NagA, that are required for the transport of specific carbohydrates in a TonB–ExbB–ExbD-dependent manner (19–21). Similarly, in other bacteria that possess overrepresented number of TonB-dependent receptors, such as P. aeruginosa and Xanthomonas campestris, only a small part of them appear to be related with iron transport functions (55,56).
One subclass of TonB-dependent receptors containing an N-terminal extension is known as TonB-dependent transducers because they compose, together with anti-sigma and iron starvation ECF sigma factors, trans-envelope signaling pathways. In general, the genes encoding these FecI-type ECF sigma factors are regulated by Fur and iron availability (13,57). Unexpectedly, our search did not detect Fur-binding sites in any of the four TonB-dependent signaling systems predicted in C. crescentus genome (CC0560-61-62, CC0981-82-83, CC1129-30-31 and CC2707-08-09). The role of these regulatory systems and their predicted involvement in the expression of genes encoding siderophore synthesis and uptake has not yet been determined in C. crescentus.
Interestingly, Fur-binding sites were found upstream of genes encoding iron-using proteins that were not expected to undergo direct negative regulation by Fur. More detailed analysis, performed in the promoter regions of the genes acnA (aconitase), sdhCBAD (succinate dehydrogenase) and nuoA-N operon (NADH dehydrogenase), confirmed that C. crescentus Fur directly binds to these predicted sites and mediates positive regulation of these three genes in response to iron. We have shown here that the 2-fold increase in expression observed for the nuo and sdh operons is directly dependent on Fur-binding to its conserved binding site. In E. coli, several genes encoding iron-using enzymes (acnA, fumA, ftnA, bfr, sodB and sdhCBAD) are positively regulated by Fur via the small regulatory RNA RhyB (7). The mechanism of positive regulation by direct binding of Fur upstream of promoters was first described in Neisseria meningitidis for the genes norB, nuoA and pan1 (10), although subsequent investigations demonstrated that other genes (sdhA and sdhC) are positively regulated by Fur via the small regulatory RNA NrrF (58). Therefore, further research is needed to ascertain whether C. crescentus Fur can function as an activator only by directly binding to its recognition site upstream of genes such as acnA, sdhCBAD and nuoA-N and/or whether in certain cases it can mediate positive regulation through repression of some of the small RNAs recently described in C. crescentus (59).
Most of the research related to iron homeostasis in α-proteobacteria has been done in Rhizobiales (14,15). In this group, Fur has almost no physiological role in iron homeostasis, since its regulatory targets are restricted to genes encoding manganese uptake systems, and thus Fur was renamed Mur (60–62). Recently, comparative genomics and computational approaches were used for genomic reconstruction of the iron (Fur, Irr and RirA), manganese (Mur and MntR) and Fe-S biogenesis (IscR) regulons in several sequenced genomes of α-proteobacteria (16). Briefly, the results showed that, in the Rhizobiales and Rhodobacteraceae, RirA and Irr have dominant role in iron homeostasis, while in several genera outside of Rhizobiales and Rhodobacterales (Caulobacter, Parvularcula, Novosphingobium, Gluconobacter, Rhodospirillum, Magnetospirillum and Pelagibacter), Fur seems to be the main iron-responsive regulator. In these organisms, a similar Fur-binding site was predicted and named Furα-box (16). Iron-responsive gene regulation of this latter group was so far still unknown. In this work, we have obtained experimental confirmation for the C. crescentus Fur-binding site and so provided, for the first time, direct evidence for the in silico prediction of the Furα-box.
We have also found that the C. crescentus Fur-binding site is very similar to the cc_13 and m_3 motifs, identified using the MEME motif finder by searching clusters of coexpressed genes from cell cycle and heavy metal stress response microarray data sets (39). Most of the genes containing the cc_13 and/or m_3 motifs were detected in our in silico analyses and some of them were experimentally validated. Thus, we propose that these two novel motifs, which share no similarity to the characterized motifs of C. crescentus transcriptional regulators, correspond to the Fur-binding site. The fact of genes belonging to the cc_13 motif to be cell cycle regulated, combined with the variation on Fur protein synthesis during cell cycle (40) could suggest an involvement of Fur in C. crescentus gene regulation according to the cell cycle. This possibility is currently being investigated.
In summary, this study has initiated the characterization of the Fur transcriptional regulator and the identification of its regulon in C. crescentus. By validating a subset of predicted Fur-binding sites upstream of representative genes involved in iron homeostasis, we provide experimental evidence that Fur acts as a dual transcriptional regulator controlling the expression of genes involved both in iron uptake systems (classical iron-dependent repression) as well as in the iron-sparing response by direct positive regulation of genes encoding iron using enzymes.
SUPPLEMENTARY DATA
Supplementary Data are available at NAR Online.
FUNDING
The Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq); Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP); postdoctoral fellowships from FAPESP (J.F.S.N., V.C.S.I. and V.S.B); CNPq (to M.V.M.). Funding for open access charge: Fundação de Amparo à Pesquisa do Estado de São Paulo.
Conflict of interest statement. None declared.
Supplementary Material
ACKNOWLEDGEMENTS
The authors wish to thank Patrícia Barco and Letícia Bisson for helping with the fur mutant strain construction and Dr Regina Baldini for critical reading of the manuscript.
REFERENCES
- 1.Andrews SC, Robinson AK, Rodriguez-Quinones F. Bacterial iron homeostasis. FEMS Microbiol. Rev. 2003;27:215–237. doi: 10.1016/S0168-6445(03)00055-X. [DOI] [PubMed] [Google Scholar]
- 2.Schaible UE, Kaufmann SHE. Iron and microbial infection. Nat. Rev. Microbiol. 2004;2:946–953. doi: 10.1038/nrmicro1046. [DOI] [PubMed] [Google Scholar]
- 3.Imlay JA. Cellular defenses against superoxide and hydrogen peroxide. Annu. Rev. Biochem. 2008;77:755–776. doi: 10.1146/annurev.biochem.77.061606.161055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wandersman C, Delepelaire P. Bacterial iron sources: from siderophores to hemophores. Annu. Rev. Microbiol. 2004;58:611–647. doi: 10.1146/annurev.micro.58.030603.123811. [DOI] [PubMed] [Google Scholar]
- 5.Braun V, Braun M. Active transport of iron and siderophore antibiotics. Curr. Opin. Microbiol. 2002;5:194–201. doi: 10.1016/s1369-5274(02)00298-9. [DOI] [PubMed] [Google Scholar]
- 6.Lee JW, Helmann JD. Functional specialization within the Fur family of metalloregulators. Biometals. 2007;20:485–499. doi: 10.1007/s10534-006-9070-7. [DOI] [PubMed] [Google Scholar]
- 7.Masse E, Gottesman S. A small RNA regulates the expression of genes involved in iron metabolism in Escherichia coli. Proc. Natl Acad. Sci. USA. 2002;99:4620–4625. doi: 10.1073/pnas.032066599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wilderman PJ, Sowa NA, FitzGerald DJ, FitzGerald PC, Gottesman S, Ochsner UA, Vasil ML. Identification of tandem duplicate regulatory small RNAs in Pseudomonas aeruginosa involved in iron homeostasis. Proc. Natl Acad. Sci. USA. 2004;101:9792–9797. doi: 10.1073/pnas.0403423101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Masse E, Salvail H, Desnoyers G, Arguin M. Small RNAs controlling iron metabolism. Curr. Opin. Microbiol. 2007;10:140–145. doi: 10.1016/j.mib.2007.03.013. [DOI] [PubMed] [Google Scholar]
- 10.Delany I, Rappuoli R, Scarlato V. Fur functions as an activator and as a repressor of putative virulence genes in Neisseria meningitidis. Mol. Microbiol. 2004;52:1081–1090. doi: 10.1111/j.1365-2958.2004.04030.x. [DOI] [PubMed] [Google Scholar]
- 11.Crosa JH. Signal transduction and transcriptional and posttranscriptional control of iron-regulated genes in bacteria. Microbiol. Mol. Biol. Rev. 1997;61:319–336. doi: 10.1128/mmbr.61.3.319-336.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Visca P, Leoni L, Wilson MJ, Lamont IL. Iron transport and regulation, cell signalling and genomics: lessons from Escherichia coli and Pseudomonas. Mol. Microbiol. 2002;45:1177–1190. doi: 10.1046/j.1365-2958.2002.03088.x. [DOI] [PubMed] [Google Scholar]
- 13.Braun V, Mahren S, Ogierman M. Regulation of the FecI-type ECF sigma factor by transmembrane signalling. Curr. Opin. Microbiol. 2003;6:173–180. doi: 10.1016/s1369-5274(03)00022-5. [DOI] [PubMed] [Google Scholar]
- 14.Rudolph G, Hennecke H, Fischer HM. Beyond the Fur paradigm: Iron- controlled gene expression in rhizobia. FEMS Microbiol. Rev. 2006;30:631–648. doi: 10.1111/j.1574-6976.2006.00030.x. [DOI] [PubMed] [Google Scholar]
- 15.Johnston AWB, Todd JD, Curson AR, Lei S, Nikolaidou-Katsaridou N, Gelfand MS, Rodionov DA. Living without Fur: the subtlety and complexity of iron-responsive gene regulation in the symbiotic bacterium Rhizobium and other α-proteobacteria. Biometals. 2007;20:501–511. doi: 10.1007/s10534-007-9085-8. [DOI] [PubMed] [Google Scholar]
- 16.Rodionov DA, Gelfand MS, Todd JD, Curson ARJ, Johnston AWB. Computational reconstruction of iron- and manganese-responsive transcriptional networks in α-proteobacteria. PLoS Comput. Biol. 2006;2:e163. doi: 10.1371/journal.pcbi.0020163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Laub MT, Shapiro L, McAdams HH. Systems biology of Caulobacter. Annu. Rev. Genet. 2007;41:429–441. doi: 10.1146/annurev.genet.41.110306.130346. [DOI] [PubMed] [Google Scholar]
- 18.Nierman WC, Feldblyum TV, Laub MT, Paulsen IT, Nelson KE, Eisen JA, Heidelberg JF, Alley MR, Ohta N, Maddock JR, et al. Complete genome sequence of Caulobacter crescentus. Proc. Natl Acad. Sci. USA. 2001;98:4136–4141. doi: 10.1073/pnas.061029298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Neugebauer H, Herrmann C, Kammer W, Schwarz G, Nordheim A, Braun V. ExbBD-dependent transport of maltodextrins through the novel MalA protein across the outer membrane of Caulobacter crescentus. J. Bacteriol. 2005;187:8300–8311. doi: 10.1128/JB.187.24.8300-8311.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eisenbeis S, Lohmiller S, Valdebenito M, Leicht S, Braun V. NagA-dependent uptake of N-acetyl-glucosamine and N-acetyl-chitin oligosaccharides across the outer membrane of Caulobacter crescentus. J. Bacteriol. 2008;190:5230–5238. doi: 10.1128/JB.00194-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lohmiller S, Hantke K, Patzer SI, Braun V. TonB-dependent maltose transport by Caulobacter crescentus. Microbiology. 2008;154:1748–1754. doi: 10.1099/mic.0.2008/017350-0. [DOI] [PubMed] [Google Scholar]
- 22.Ely B. Genetics of Caulobacter crescentus. Methods Enzymol. 1991;204:372–384. doi: 10.1016/0076-6879(91)04019-k. [DOI] [PubMed] [Google Scholar]
- 23.Hanahan D. Studies on transformation of Escherichia coli with plasmids. J. Mol. Biol. 1983;166:557. doi: 10.1016/s0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- 24.Simon R, Prieffer U, Puhler A. A broad host range mobilization system for in vivo genetic engineering: transposon mutagenesis in gram negative bacteria. Biotechnology. 1983;1:784–790. [Google Scholar]
- 25.Evinger M, Agabian N. Envelope-associated nucleoid from Caulobacter crescentus stalked and swarmer cells. J. Bacteriol. 1977;132:294–301. doi: 10.1128/jb.132.1.294-301.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Roberts RC, Toochinda C, Avedissian M, Baldini RL, Gomes SL, Shapiro L. Identification of a Caulobacter crescentus operon encoding hrcA, involved in negatively regulating heat-inducible transcription and the chaperone gene grpE. J. Bacteriol. 1996;178:1829–1841. doi: 10.1128/jb.178.7.1829-1841.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Meisenzahl AC, Shapiro L, Jenal U. Isolation and characterization of the xylose-dependent promoter from Caulobacter crescentus. J. Bacteriol. 1997;179:592–600. doi: 10.1128/jb.179.3.592-600.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gober JW, Shapiro L. A developmentally regulated Caulobacter flagellar promoter is activated by 3′ enhancer and IHF binding elements. Mol. Biol. Cell. 1992;3:913–916. doi: 10.1091/mbc.3.8.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Galhardo RS, Rocha RP, Marques MV, Menck CFM. An SOS- regulated operon involved in damage-inducible mutagenesis in Caulobacter crescentus. Nucleic Acids Res. 2005;33:2603–2614. doi: 10.1093/nar/gki551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hertz GZ, Hartzell GW, III, Stormo GD. Identification of consensus patterns in unaligned DNA sequences known to be functionally related. Comput. Appl. Biosci. 1990;6:81–92. doi: 10.1093/bioinformatics/6.2.81. [DOI] [PubMed] [Google Scholar]
- 31.Thomas-Chollier M, Sand O, Turatsinze JV, Janky R, Defrance M, Vervisch E, Brohée S, van Helden J. RSAT: regulatory sequence analysis tools. Nucleic Acids Res. 2008;36:W119–W127. doi: 10.1093/nar/gkn304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stormo GD, Hartzell GW., III Identifying protein-binding sites from unaligned DNA fragments. Proc. Natl Acad. Sci. USA. 1989;86:1183–1187. doi: 10.1073/pnas.86.4.1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hertz GZ, Stormo GD. Identifying DNA and protein patterns with statistically significant alignments of multiple sequences. Bioinformatics. 1999;15:563–577. doi: 10.1093/bioinformatics/15.7.563. [DOI] [PubMed] [Google Scholar]
- 34.Bailey TL, Elkan C. Fitting a mixture model by expectation maximization to discover motifs in biopolymers. In: Altman RB, Brutlag DL, Karp PD, Lathrop RH, Searls DB, editors. Proceedings of the Second International Conference on Intelligent Systems for Molecular Biology, August. Menlo Park, CA: AAAI Press; 1994. pp. 28–36. [PubMed] [Google Scholar]
- 35.Crooks GE, Hon G, Chandonia JM, Brenner SE. WebLogo: a sequence logo generator. Genome Res. 2004;14:1188–1190. doi: 10.1101/gr.849004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Da Silva Neto JF, Koide T, Abe CM, Gomes SL, Marques MV. Role of σ54 in the regulation of genes involved in type I and type IV pili biogenesis in Xylella fastidiosa. Arch. Microbiol. 2008;189:249–261. doi: 10.1007/s00203-007-0314-x. [DOI] [PubMed] [Google Scholar]
- 37.Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc. Natl Acad. Sci. USA. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Miller JH. Experiments in Molecular Genetics. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1972. [Google Scholar]
- 39.McGrath PT, Lee H, Zhang L, Iniesta AA, Hottes AK, Tan MH, Hillson NJ, Hu P, Shapiro L, McAdams HH. High-throughput identification of transcription start sites, conserved promoter motifs and predicted regulons. Nat. Biotechnol. 2007;25:584–592. doi: 10.1038/nbt1294. [DOI] [PubMed] [Google Scholar]
- 40.Grünenfelder B, Rummel G, Vohradsky J, Röder D, Langen H, Jenal U. Proteomic analysis of the bacterial cell cycle. Proc. Natl Acad. Sci. USA. 2001;98:4681–4686. doi: 10.1073/pnas.071538098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Touati D, Jacques M, Tardat B, Bouchard L, Despied S. Lethal oxidative damage and mutagenesis are generated by iron in Δfur mutants of Escherichia coli: protective role of superoxide dismutase. J. Bacteriol. 1995;177:2305–2314. doi: 10.1128/jb.177.9.2305-2314.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Litwin CM, Calderwood SB. Analysis of the complexity of gene regulation by Fur in Vibrio cholerae. J. Bacteriol. 1994;176:240–248. doi: 10.1128/jb.176.1.240-248.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Horsburgh MJ, Ingham E, Foster SJ. In Staphylococcus aureus, Fur is an interactive regulator with PerR, contributes to virulence, and is necessary for oxidative stress resistance through positive regulation of catalase and iron homeostasis. J. Bacteriol. 2001;183:468–475. doi: 10.1128/JB.183.2.468-475.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hassett DJ, Sokol PA, Howell ML, Ma JF, Schweizer HT, Ochsner U, Vasil ML. Ferric uptake regulator (Fur) mutants of Pseudomonas aeruginosa demonstrate defective siderophore-mediated iron uptake, altered aerobic growth, and decreased superoxide dismutase and catalase activities. J. Bacteriol. 1996;178:3996–4003. doi: 10.1128/jb.178.14.3996-4003.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Loprasert S, Sallabhan R, Whangsuk W, Mongkolsuk S. Characterization and mutagenesis of fur gene from Burkholderia pseudomallei. Gene. 2000;254:129–137. doi: 10.1016/s0378-1119(00)00279-1. [DOI] [PubMed] [Google Scholar]
- 46.Hantke K. Selection procedure for deregulated iron transport mutants (fur) in Escherichia coli K 12: fur not only affects iron metabolism. Mol. Gen. Genet. 1987;210:135–139. doi: 10.1007/BF00337769. [DOI] [PubMed] [Google Scholar]
- 47.D’haeseleer P. What are DNA sequence motifs? Nat. Biotechnol. 2006;24:423–425. doi: 10.1038/nbt0406-423. [DOI] [PubMed] [Google Scholar]
- 48.Rodionov DA. Comparative genomic reconstruction of transcriptional regulatory networks in bacteria. Chem. Rev. 2007;107:3467–3497. doi: 10.1021/cr068309+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tompa M, Li N, Bailey TL, Church GM, De Moor B, Eskin E, Favorov A, Frith MC, Fu Y, Kent WJ, et al. Assessing computational tools for the discovery of transcription factor binding sites. Nat. Biotechnol. 2005;23:137–144. doi: 10.1038/nbt1053. [DOI] [PubMed] [Google Scholar]
- 50.Das MK, Dai HK. A survey of DNA motif finding algorithms. BMC Bioinformatics. 2007;8(Suppl. 7):S21. doi: 10.1186/1471-2105-8-S7-S21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chen Z, Lewis KA, Shultzaberger RK, Lyakhov IG, Zheng M, Doan B, Storz G, Schneider TD. Discovery of Fur binding site clusters in Escherichia coli by information theory models. Nucleic Acids Res. 2007;35:6762–6777. doi: 10.1093/nar/gkm631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Quatrini R, Lefimil C, Veloso FA, Pedroso I, Holmes DS, Jedlicki E. Bioinformatic prediction and experimental verification of Fur-regulated genes in the extreme acidophile Acidithiobacillus ferrooxidans. Nucleic Acids Res. 2007;35:2153–2166. doi: 10.1093/nar/gkm068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.López-Gomollón S, Hernández JA, Pellicer S, Angarica VE, Peleato ML, Fillat MF. Cross-talk between iron and nitrogen regulatory networks in Anabaena (Nostoc) sp. PCC 7120: identification of overlapping genes in FurA and NtcA regulons. J. Mol. Biol. 2007;374:267–281. doi: 10.1016/j.jmb.2007.09.010. [DOI] [PubMed] [Google Scholar]
- 54.Gao H, Zhou D, Li Y, Guo Z, Han Y, Song Y, Zhai J, Du Z, Wang X, Lu J, et al. The iron-responsive Fur regulon in Yersinia pestis. J. Bacteriol. 2008;190:3063–3075. doi: 10.1128/JB.01910-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Llamas MA, Sparrius M, Kloet R, Jimenez CR, Vandenbroucke-Grauls C, Bitter W. The heterologous siderophores ferrioxamine B and ferrichrome activate signaling pathways in Pseudomonas aeruginosa. J. Bacteriol. 2006;188:1882–1891. doi: 10.1128/JB.188.5.1882-1891.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Blanvillain S, Meyer D, Boulanger A, Lautier M, Guynet C, Deancé N, Vasse J, Lauber E, Arlat. M. Plant carbohydrate scavenging through TonB-dependent receptors: a feature shared by phytopathogenic and aquatic bacteria. PLoS ONE. 2007;2:e224. doi: 10.1371/journal.pone.0000224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Koebnik R. TonB-dependent trans-envelope signalling: the exception or the rule? Trends Microbiol. 2005;13:343–347. doi: 10.1016/j.tim.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 58.Mellin JR, Goswami S, Grogan S, Tjaden B, Genco CA. A novel Fur- and iron-regulated small RNA, NrrF, is required for indirect Fur-mediated regulation of the sdhA and sdhC genes in Neisseria meningitides. J. Bacteriol. 2007;189:3686–3694. doi: 10.1128/JB.01890-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Landt SG, Abeliuk E, McGrath PT, Lesley JA, McAdams HH, Shapiro L. Small non-coding RNAs in Caulobacter crescentus. Mol. Microbiol. 2008;68:600–614. doi: 10.1111/j.1365-2958.2008.06172.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wexler M, Todd JD, Kolade O, Bellini D, Hemmings AM, Sawers G, Johnston AWB. Fur is not the global regulator of iron uptake genes in Rhizobium leguminosarum. Microbiology. 2003;149:1357–1365. doi: 10.1099/mic.0.26130-0. [DOI] [PubMed] [Google Scholar]
- 61.Chao TC, Becker A, Buhrmester J, Puhler A, Weidner S. The Sinorhizobium meliloti fur gene regulates, with dependence on Mn(II), transcription of the sitABCD operon, encoding a metal-type transporter. J. Bacteriol. 2004;186:3609–3620. doi: 10.1128/JB.186.11.3609-3620.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Diaz-Mireles E, Wexler M, Sawers G, Bellini D, Todd JD, Johnston AW. The Fur-like protein Mur of Rhizobium leguminosarum is a Mn2+-responsive transcriptional regulator. Microbiology. 2004;150:1447–1456. doi: 10.1099/mic.0.26961-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.