Abstract
Individualized, model-based, target-oriented optimal concentration-controlled dosing of HIV medications can be beneficial to patients for whom there are limited dosing guidelines, such as children, adolescents, or patients with altered physiologic function. Barriers to this approach include lack of training, expertise, and access to appropriate software to assist the clinician. The authors present 4 illustrative clinical cases of HIV-infected patients whose therapy was optimized using population pharmacokinetic models (here generated from published studies) and supplemented by individualized Bayesian adaptive control of dosage regimens as implemented in the MM-USCPACK software. These 4 cases illustrate how clinicians can maximize therapeutic success in (1) patients with reduced drug clearance, (2) young adolescents transitioning to adult physiology, (3) patients with dose-dependent toxicity, and (4) adolescents with limited therapeutic options.
Keywords: HIV, dose optimization, concentration control
Antiretroviral drug therapy for HIV infection is typically initiated with a standard dose, which is generally fixed for adults regardless of patient size and, for children, is indexed to body size either by weight or surface area. Except for dose adjustments related to the growth of children or significant organ failure, such as the kidneys, further consideration of the actual dose amount has rarely taken place, despite considerable evidence documenting the very wide variability among individuals in their drug behavior and resultant drug exposure.1 Such a standard one-dose-fits-all approach for a given patient population, which is the goal of drug development and licensure, can cause significant problems for individual patients for numerous reasons.
To begin, the usual assumption is made that the patient is enough like the average patient so as to warrant the average dose. For patients who are not average, the recommended dose either may not achieve the desired outcome or may not do it safely. This in turn can result in the use of additional or different agents. Because antiretroviral drug concentrations are not often obtained in routine clinical practice, at least in the United States, there exists no systematic, rational method to adjust the dose should clinical circumstances suggest the need for it (eg, when to change from pediatric to adult dosing regimens). Because children and adolescents generally require higher drug doses than adults do, when adjusted for body weight,2,3 simply capping a pediatric size-based dose at the adult fixed dose may result in inadequate drug exposure in a larger child.
In the absence of therapeutic drug management, individual concentration measurements, and Bayesian individualized modeling of drug behavior, HIV-infected adolescents and young adults or other atypical patients are placed at a significant risk of developing resistance from subtherapeutic dosing or toxicity from supratherapeutic dosing. Doses that result in excessive plasma concentrations are unlikely to be detected unless and until clinical toxicity develops, and without such monitoring of concentration data, dose-dependent versus dose-independent toxicity cannot be distinguished. Therefore, the usual remedy for toxicity has been not dose adjustment, even if this might be the appropriate response, but rather a change to another agent, with all its attendant risks of toxic dosing or subtherapeutic dosing leading to unnecessary emergence of viral resistance. Finally, therapeutic alliances between the patient and the health care provider may be damaged by erroneous assumptions of behaviorally motivated suboptimal adherence to drug regimens as a cause of therapeutic failure, when in fact the failure was caused by either unusually low drug exposure or even by excessive exposure resulting in toxicity and undisclosed reluctance to take the drug.
Carefully studied therapeutic drug management has been shown to improve efficacy, reduce complications, and/or reduce hospitalization costs significantly.4,5 Within the realm of HIV therapy, there are now several clinical investigations6–13 and numerous reviews and position papers or guidelines14–20 that affirm the usefulness of incorporating measurement of antiretroviral drug concentrations into the clinical management of HIV-infected patients. However, evidence does not suggest a universal benefit,21–24 primarily because not all patients require such intervention, and target concentrations may be ill-defined. Furthermore, clinicians may lack training and the required expertise to recognize a potential need for dose modification as well as the skills and clinical software to implement it when necessary. Particularly in the outpatient setting, where most HIV care occurs, there are numerous barriers to individualized dosage optimization that have prevented widespread adoption of the practice. These are summarized in Figure 1.
Figure 1.
Barriers to the use of therapeutic drug management.
Our laboratory has been working for more than 40 years to address many of the obstacles shown in Figure 1 by developing software for population pharmacokinetic modeling and clinically oriented software for Bayesian individualized dose optimization.25 Here, we report techniques to use published pharmacokinetic data that may have been previously unusable for dose optimization. Use of these population models, supplemented by Bayesian individualized target-oriented dosage regimens, is illustrated by 4 relevant clinical examples of HIV-infected patients whose therapy was optimized using our approach and software.26,27 These case examples illustrate the practical benefit of dose individualization and optimization in an outpatient clinic.
METHODS
Population Pharmacokinetic Models
A population pharmacokinetic model is a set of descriptive equations with parameter values (clearance, volume of distribution, etc). When parameter values are reasonably estimated, concentrations of a drug may be predicted at any time during a dosage regimen in an individual with a set of characteristics (eg, weight or creatinine clearance) whose effects on drug disposition are accounted for by the model equations. Population pharmacokinetic modeling deals with many of the obstacles associated with therapeutic optimization, even in the outpatient setting. Chiefly, population pharmacokinetic models allow for discrimination between the sources of error between observed (measured) and predicted drug concentrations (ie, variability between patients, between occasions in a single patient, and residual variability due to model misspecifications or environmental factors that also influence drug disposition28). Moreover, because models provide information on the pharmacokinetic parameter values in a population of patients, the parameter values of an individual patient who belongs to that population, even if not among the original contributors to the model, can usually be estimated fairly well even with only a single measured drug concentration, although precision and accuracy improve with multiple samples from the individual.25,27,29
Once a set of therapeutic drug pharmacokinetic parameter estimates exists for an individual patient, the clinician can predict likely blood concentrations in the patient at any given time after a dose. More important, the opportunity arises to control the dose in a systematic manner to achieve clinically selected target concentrations with maximum precision and therefore to better control the patient’s expected clinical response.27
In our clinical practice, and in this report, to overcome the patient-based obstacles in Figure 1, a population pharmacokinetic model for each drug to be optimized was first necessary. Because of the costs and difficulties associated with conducting a phase 1 trial de novo, simulation was used to make population pharmacokinetic models from available pharmacokinetic data in the peer-reviewed literature, using different modules within the MM-USCPACK software collection.30 This entire software package is available by license from the University of Southern California at http://www.lapk.org. Clinical use of such models to analyze and interpret random measurements of drug concentrations, correlating them with virologic outcomes, was validated in a prior 2-year pilot research study. Summarized below, additional model-building details are included in the appendix.31
Mean pharmacokinetic parameter estimates with standard deviations were obtained or derived for each drug of interest from published pediatric and adult pharmacokinetic trials for 3 parameters: absorption rate constant (ka), volume of distribution (Vd), and clearance (CL). One model was constructed as needed for patients who were Tanner stage 3 or less (children) and one for patients who were Tanner stage 4 or 5 (adults). The Tanner breakpoint was chosen as one attempt to differentiate pharmacophysiologic children from adults. It was adapted from national guidelines.17 For each model, 50 sets of parameter values were simulated randomly by Monte Carlo sampling.32 Each parameter set therefore comprised randomly sampled values for ka, Vd, and CL, from which drug concentrations were calculated at standardized times after an age-appropriate dose. This collection of simulated drug concentrations was then used by the Non-Parametric Adaptive Grid (NPAG) program in the MM-USCPACK collection33 to make the population pharmacokinetic model, comprising a collection of estimated points and associated probability of each point in the population, where a point represents a given set of parameter estimates. The graphical representation of a model is shown in Figure 2. In summary, with this method, a population pharmacokinetic model for each anti-retroviral drug was generated from published parameter estimates without recapitulating multiple clinical trials. Final model parameters for those used in this report are shown in Table I.
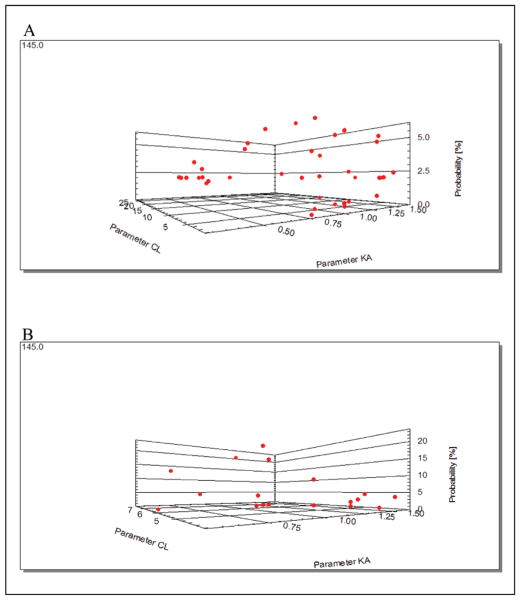
Figure 2.
Nonparametric population model for atazanavir with clearance and absorption on the horizontal plane and the associated probability of each set of estimates on the vertical axis. There is at most 1 point per simulated individual in the population, although individuals with similar parameter estimates within the context of model error will not be resolvable as separate points. The population model (the Bayesian prior) is shown in (A), and the individualized Bayesian-posterior model for patient 4 is shown in (B) fitted to that patient’s measured atazanavir concentrations. Some points in the model have risen to higher probabilities (note the changed vertical axis scale), other points are lower, and half are so unlikely as to not be plotted. The more probable sets of parameter estimates are now accorded more weight in selecting the dose that minimizes the least-squared error between the predicted and observed concentrations for the patient.
Table I.
Mean (SD) Parameter Values From the Models Used to Optimize Antiretroviral Therapy in This Report
| Drug | Type | ka | Vd | CL |
|---|---|---|---|---|
| Nelfinavir34 | Pediatric | 0.4 (0.2) h−1 | 6.4 (2.5) L/kg | 1.0 (0.2) L/h · kg |
| Efavirenz35,36 | Pediatric | 0.3 (0.1) h−1 | 4.2 (1.1) L/kg | 0.1 (0.03) L/h · kg |
| fos-Amprenavir37,38 | Adult | 3.0 (1.3) h−1 | 5.7 (1.5) L/kg | 0.7 (0.2) L/h · kg |
| Atazanavir39 | Adult | 1.0 (0.3) h−1 | 86.2 (14.9) L | 8.1 (0.9) L/h |
Models based on pediatric studies were used for patients with a Tanner Sexual Maturity Rating stage ≤3. References indicate the source articles for initial parameter estimates.
For interpretation of serum drug concentrations obtained randomly from each of the patients, the MM-USCPACK clinical software was used to fit the appropriate model to all of the patients’ available data points (including measured drug concentrations) to obtain Bayesian posterior distributions for each parameter. When adjustment of a dosage regimen was necessary, this was accomplished using the software’s multiple model (MM) dosage design30 based on the entire distribution of posterior parameter values, to hit the target goals with maximum precision (minimum predicted weighted squared error).
Patients
All patients were seen at a clinic that delivers comprehensive, family-centered care primarily to minority HIV-infected women during and after pregnancy and to HIV-infected children and youth. Approximately 500 infected individuals come to the clinic for care. One of the authors (M.N.) provides HIV care and therapeutic drug management to these patients. In the 3 years that the program has been available, consultations have been requested by providers on approximately 40 patients, which is about 8% of the clinic population. It is difficult to quantify success, as each patient is unique. In all cases, therapy was positively affected, whether to indicate a change in dose or to be reassured that the dose regimen in unusual clinical situations was achieving the expected concentration profile. Clinician feedback has been positive, and the number of consultation requests is increasing. We have selected 4 patients who represent clinical problem settings typically appropriate for use of therapeutic drug management and dosage individualization.
Drug Assays
Concentrations of antiretroviral protease inhibitors and nonnucleoside reverse transcriptase inhibitors for therapeutic drug management were obtained from commercial laboratories with a 1- to 2-week turnaround time. Efavirenz measurement was performed at the Pharmacokinetics Laboratory of the National Jewish Medical and Research Center (Denver, Colorado), through Focus Diagnostics, Inc (Cypress, California). Serum concentrations of efavirenz were determined using a validated high-performance liquid chromatography (HPLC) assay. Samples were measured using a system consisting of a ThermoFinnegan P4000 HPLC pump (San Jose, California) with model AS3000 fixed-volume autosampler, a model UV2000 ultraviolet detector, a Gateway Series e computer (Poway, California), and the Chromquest HPLC data management system. The plasma standard curve for efavirenz ranged from 0.05 to 10 μg/mL. The absolute recovery of efavirenz from plasma was 102%, reflecting differential solubility in the water-organic solvent mixture versus plasma. The within-sample precision (percentage coefficient of variation) of a single-validation 0.50 μg/mL standard concentration was 5.6%, and the overall validation precision across all quality control samples was 2.3% to 7.2%. No interferences were observed with the measurement of efavirenz with 90 different commonly used medications.
Amprenavir and nelfinavir were assayed on site at Focus. The amprenavir assay was conducted on an Agilent 1050 Series HPLC equipped with a UV-VIS detector set to 265 nm and column heater housing an Agilent Hypersil BDS C-18 column (4.0 × 125 mm, 5 μm) that was heated to 38°C, through which a mobile phase consisting of acetonitrile and an aqueous sodium heptane sulfonate buffer (pH 2.7) was passed. A solution of sulindac in water was added as the internal standard to each sample at a fixed concentration. The assay is linear for amprenavir over the range of 0.1 to 40.0 μg/mL in serum and has demonstrated both precision (1.4% at 15.0 μg/mL, 5.2% at 1.0 μg/mL, N = 20) and accuracy (96.7% at 15.0 μg/mL, 94.0% at 1.0 μg/mL, N = 20). The nelfinavir assay was conducted on an Agilent 1050 Series HPLC equipped with a UV-VIS detector set to 254 nm and column heater housing an Agilent Hypersil BDS C-18 column (4.0 × 125 mm, 5 μm) that was heated to 38°C, through which a mobile phase consisting of acetonitrile and an aqueous potassium phosphate buffer (pH 7.2) was passed. A solution of 6,7-dimethyl-2,3-di(2-pyridyl)-quinoxaline in DMSO was added as the internal standard to each sample at a fixed concentration. The assay is linear for nelfinavir over the range of 0.1 to 20.0 μg/mL in serum and has demonstrated both precision (1.0% at 15.0 μg/mL, 3.1% at 1.0 μg/mL, N = 20) and accuracy (97.3% at 15.0 μg/mL, 100.0% at 1.0 μg/mL, N = 20).
Atazanavir measurement, using a published method,40 was provided by the University of California San Diego Pediatric Pharmacology Laboratory, a CLIA-certified laboratory that participates in an international quality control program for the measurement of antiretroviral agents.41
Problem 1: Unsuspected Impaired Clearance—Patients Needing Smaller Doses Than Usual
Patient 1
Patient 1 was a 13-year-old, 26-kg, anti-retroviral-naive African boy (Tanner stage 2), started on highly active antiretroviral therapy including efavirenz 350 mg once daily in the evening, which was the recommended dose for his age and weight.42 After 2 weeks, his mother reported that he had become much too somnolent to attend school since starting the medication, which was a significantly more severe manifestation than the transient drowsiness that may occur after starting efavirenz.42 Suspecting that he had impaired drug clearance, possibly due to a polymorphism in his cytochrome P450 2B6 gene,43 his dose was empirically reduced to 200 mg once daily, and a week after this reduction, a serum concentration of 1.37 mg/L was measured 22 hours after his previous dose. Using the MM-USCPACK software, his 24-hour trough concentration was predicted to remain greater than a target of 1 mg/L,44 as shown in Figure 3A. He continued on this dose, and a follow-up sample obtained several months later on the same low dose, also shown in Figure 3A, confirmed his therapeutic concentrations. He has maintained an undetectable HIV viral load with no further somnolence for the past 2 years.
Figure 3.
MM-USC*PACK output for each patient. Black lines are weighted average Bayesian-posterior predicted concentrations. Dots are measured serum concentrations. Vertical lines at the bottom are dose events. Intervals between measured serum concentrations have been compressed for clarity. Target concentrations have been added as a reference. (A) Patient 1, efavirenz. (B) Patient 2, nelfinavir. (C) Patient 3, amprenavir given as fos-amprenavir. (D) Patient 4, atazanavir.
Problem 2: Adolescents—Patients Needing Larger Doses Than Usual
Patient 2
Patient 2 was a 10-year-old girl (Tanner stage 2) who weighed 30.7 kg. Based on the standard pediatric dose of 55 mg/kg,45 and within formulation limitations, she was prescribed 1875 mg twice daily. Because the recommended maximum nelfinavir dose is the smaller adult dose of only 1250 mg twice daily, a random serum concentration of 4.9 mg/L was measured 4 hours after her most recent dose to ensure that she was not in a toxic range. From this concentration, her predicted peak concentration on the 1875-mg dose was 5.5 mg/L, and her 12-hour trough was 2.1 mg/L (Figure 3B). By using the software to simulate the results on the lower dose of 1250 mg, given her estimated pharmacokinetic parameter distributions, her predicted trough concentration would have been 1.4 mg/L (not shown). Because she was tolerating the higher dose, and because her predicted most likely nelfinavir profile was within a suggested therapeutic range of 1 to 6 mg/L,44 we elected to continue that larger dose to give her a greater margin above the minimum trough target of 1 mg/L. She did not develop gastrointestinal or any other toxicity, despite the larger dose, and she continued to have an undetectable viral load.
Problem 3: The Need for Nonstandard Dosage Schedules
Patient 3
Patient 3 was a 45-year-old woman (Tanner stage 5) with a long history of medication intolerance. She was started on a regimen containing fos-amprenavir (without ritonavir), 2 × 700 mg tablets twice daily (total 2800 mg/d). After starting the new regimen, she complained of daytime fatigue, which she attributed to the morning dose, and she inquired about taking the entire dose at night. Prior to making changes, a serum amprenavir concentration of 1.4 mg/L was measured 4.5 hours after her previous dose. Modeling suggested that 2800 mg once daily would not maintain her trough concentration above the minimum target of 0.23 mg/L.44 However, a regimen of 1 tablet at 8 AM followed by 3 tablets at 6 PM (a 10- to 14-hour schedule) would be 90% likely to achieve this goal. After careful and thorough discussion of her probable dose-dependent toxicity, she was changed to the latter regimen. Her fatigue resolved, and she achieved an undetectable viral load. A follow-up level of 0.9 mg/L obtained 4 hours after the morning dose several weeks after beginning the new regimen confirmed that her predicted troughs were likely to be therapeutic (Figure 3C). She has maintained an undetectable viral load for 11 months, as of this writing.
Problem 4: Extrapolating From Adults to Children
Patient 4
Patient 4 was a 14-year-old boy (Tanner stage 4) with poor medication adherence and previous resistance to nonnucleoside reverse transcriptase inhibitor. To encourage better adherence, he was changed to a once-daily regimen that included atazanavir given in combination with low-dose ritonavir. The usual adult dose of atazanavir is 300 mg when given with ritonavir, so he was started on 200 mg based on his age and small size (height and weight in the 25th percentile). Because there were no published pediatric pharmacokinetic data at the time, we obtained a random atazanavir concentration of 780 ng/mL 18 hours after his previous dose. His predicted trough concentration was 380 ng/mL (Figure 3D), greater than the minimum target of 150 ng/mL,46 so the dose was continued. He initially achieved an undetectable viral load. However, self-disclosed persistently poor adherence allowed his viral load to rebound partially to about 2000 copies/mL, despite a second measured therapeutic concentration (660 ng/mL) of atazanavir (Figure 3D). By having concentration data available, we were able to accurately ascertain that the cause of his virologic failure was indeed due solely to suboptimal adherence and not because we had underdosed him.
DISCUSSION
By its very nature, dose optimization is an individual, not a group, process. Because of this, publication of illustrative individual case reports such as these provides important supportive evidence in a manner that does not obscure this essentially individual nature of therapy, as may often happen in large clinical trials with limited or no actual individual dose modification.21,22,24 Our method for converting reported pharmacokinetic data into population models can be used locally to optimize safety and efficacy in individual patients. Successful and practical therapeutic drug management using Bayesian adaptive control and individualization of the dosage regimen to achieve desired target goals has been illustrated by 4 specific clinical examples.
The first patient in problem 1 illustrated the situation in which drug clearance differed substantially from clearance reported in the package literature. In this case, we suspected (but did not prove) that it was due to a polymorphism in cytochrome P450 2B6, a key drug-metabolizing enzyme with clinical significance for both efavirenz and nevirapine therapy.47,48 However, there has not been universal agreement about the clinical significance of polymorphisms in this gene with regard to efavirenz pharmacokinetics,49 and for many drugs, compensatory or alternative pathways exist that mitigate effects of mutations in the gene product primarily responsible for metabolism.
Because of this, we feel that it is necessary to measure and interpret drug exposure followed by individualized Bayesian dosage adjustment when necessary. This is currently more feasible and practical than genomic profiling, which must still always be linked to a population pharmacokinetic model of drug behavior. Furthermore, such measurement and analysis of drug concentrations account for the overall quantitative effect of all relevant known or as yet undiscovered genetic polymorphisms and also for any other relevant factors that may also be present, such as drug interactions. Moreover, patients with changes in hepatic or renal function that also affect therapeutic drug exposure, especially in the absence of specific dose recommendations under such circumstances, will be equally likely to benefit from knowledge of the measured drug concentrations and Bayesian adaptive dosage individualization.
Problem 2 is a common one in adolescent medicine. Adolescents may weigh enough so that, according to the weight-based pediatric dosing guidelines, their absolute dose amount exceeds the typical maximum recommended adult dose. Clinicians are left in a quandary about whether the published dosing cap should be respected or whether their individual adolescent patient is still a child in pharmacophysiological respects. Children typically have higher weight-adjusted volumes of distribution and more rapid clearances than adults do,2,3 which reduce drug concentrations after a given dose in these younger patients relative to adults and thus require higher doses when adjusted for body weight. Although guidelines recommend pediatric dosing for those who are Tanner Sexual Maturity Stage 3 and less,17,50 lack of concentration data and therapeutic drug management increases the risk of improper dosing. Furthermore, the reason for therapeutic failure can be misconstrued to be an adherence problem: on one hand, patients who are underdosed may be incorrectly labeled as poorly adherent. Alternatively, overdosed patients who are experiencing medication-related adverse effects may be reluctant to admit that they avoid them. The clinician will rightly detect the nonadherence when therapeutic failure is observed, but the true source of the nonadherence (toxicity) may go unrecognized. This example shows clearly that dosage individualization does not simply mean dose reduction to minimize toxicity. It also means giving larger doses than usual when they are warranted in an individual patient, to obtain appropriate benefit.
The patient in problem 3 went beyond the issue not only of changing the dose but also of changing the dosing schedule. While we do not advocate this practice routinely, for some patients it is the only way to achieve a useful therapeutic alliance with the physician. If sufficient published data become available to enable construction of a reasonable pharmacokinetic model, and if concentration targets exist, a clinician may at least thoughtfully depart from the usual standard dosing regimen in a manner that now can be evidence based. As always, using therapeutic drug management tools to develop such an individualized regimen does not relieve the clinician of the responsibility to closely monitor his or her patient, preferably with repeated drug concentrations and Bayesian dosage individualization, to ensure optimal therapeutic efficacy and safety.
Use of repeated measurements is important for another reason. Substantial within-individual variability in orally administered drug concentrations may arise in the outpatient setting, due, for example, to variation in dose times, gastric pH, gastric contents, or drug-drug interactions.51 Nonetheless, this variability does not preclude therapeutic drug management.52 However, it must be recognized for appropriate interpretation of results. It is rare in modern medicine to substantially alter any therapy on the basis of a single laboratory result, especially if the information is not immediately life threatening or corroborated by findings on physical examination. In our practice, repeated measurements are an important component of successful therapeutic drug management. We do not react to a single high or low measurement by immediately changing the dose. Rather, we use that information as a guided opportunity to carefully screen for toxicity if necessary and to review the patient’s medication-taking behavior, such as appropriate separation from food, concomitant medications such as proton-pump inhibitors, missed doses, and regularity of dosing. Repeat testing may demonstrate that therapeutic exposure is possible on the given dose. Although not employed for the patients in this report, on the rare occasions that we are unable to reconcile a patient’s self-reported behavior with measured drug concentrations, or if we have been unable to document a single concentration within our targeted exposure despite 2 or 3 samples, we use witnessed dosing in the clinic, preceded by a blood sample and followed by 1 or 2 more samples for drug measurement over a 4-hour period. In this setting, we have recently begun to set the initial conditions of the model equal to the predose concentration, thereby bypassing the need for any dosing history whatsoever and eliminating the confounding effects of adherence on the interpretation of results.53,54
Finally, the patient in problem 4 is all too familiar to pediatricians who are commonly forced to prescribe medications to children without Food and Drug Administration (FDA) approval to do so. According to information available from the FDA, of the 32 currently licensed unique and combination HIV medications, only 15 (47%) are approved for children younger than 16 years, and only 3 (9%) have been approved since 2000.55 Although it is preferable to prescribe medications at doses that have already been tested for safety and efficacy, by using therapeutic drug management and Bayesian dosage individualization when necessary, the clinician can ensure that a child’s drug exposure is similar to that of safe and effective adult exposures, which is the foundational approach to pediatric phase 1–2 labeling trials.
In addition to other patients who fit the clinical situations described here, we have used the same techniques to adjust therapy in many other settings. Pregnant HIV-infected women may have altered anti-retroviral drug exposure, particularly during the third trimester, and many drugs remain to be studied.56–59 With an average of 3 HIV-related deliveries per month at our center, we have been able to verify appropriate third-trimester exposure for what have become our typical doses of nelfinavir (1250 mg twice daily), lopinavir (600 mg + 150 mg twice daily of the new formulation), and atazanavir (300 mg + ritonavir 100 mg once daily) in a handful of women. Another setting for therapeutic drug management is to compensate for and manage the problem of drug-drug interactions. One has only to look at a package insert to know that drug interactions among antiretroviral agents and between them and other agents are complex and often competing.60 We used the same techniques reported here, for example, to establish that the combination of fos-amprenavir and lopinavir resulted in dramatically reduced amprenavir concentrations in a multidrug-resistant adolescent. We immediately stopped that combination prior to published recommendations against using the regimen because of that interaction.37 Finally, on numerous occasions, we have established some estimate of patient adherence to outpatient drug regimens either by documenting absent drug, using the software to estimate the probability of having taken the dose at the reported time given the measured concentration, or documenting adequate concentrations after witnessed dosing. When suboptimal adherence is suspected, our standard practice is now to inform patients that “we are going to measure the amount of drug in your blood, but if you did not take it within the past 24 to 48 hours, then we are unlikely to find it. Should we proceed?” In several cases, patients have then recanted their prior claims of perfect adherence by admitting that they, in fact, missed at least 1 dose. One young adult woman, for example, after more than 5 years of high viral loads, declining CD4+ counts, changing regimens, and continued claims of good adherence, when confronted with her absent atazanavir after a self-reported dose and therapeutic concentrations after a witnessed dose, finally admitted that her pills made her nauseous. That breakthrough enabled us, within a year, to find a tolerable regimen and to fully suppress her viral replication.
Taking advantage of published pharmacologic data and a tool such as the MM-USCPACK software, which runs on a laptop PC, puts Bayesian adaptive control in the hands of physicians and pharmacists who recognize special patients requiring a more specific and individualized approach to therapy than one size fits all. It is not possible to know with absolute certainty how patients take their drugs and how to interpret randomly obtained levels. However, we argue that in appropriate settings such as those presented here, with a judicious clinician, therapeutic drug management can be quite practical and is better than blindly trusting the one-size-fits-all dose. Why wait until virologic failure or dose-dependent toxicity to find out, at the patient’s medical and financial cost, that such blind trust was misplaced?
APPENDIX
Details of the model-building process, using the nelfinavir model in children as an example, are given here. The first step was to search PubMed using the terms nelfinavir, pharmacokinetics, and (child OR children). Compartmental models were preferred because non-compartmental analyses do not include some estimate of absorption (ie, ka). In this case, at the time we were making the model, 1 reference was available.34
The mean parameter values from the reference for absorption (ka), volume of distribution per kilogram of body weight (Vd), and clearance per kilogram (CL) were passed to the Monte Carlo simulator. Standard deviations were reduced to 25% of the mean. This reduction served 2 purposes: (1) it prevented generation of negative parameter values during random sampling, which could have also been accomplished with log transformation, and (2) more important, in the absence of covariance information (eg, how Vd and CL might vary together), it excluded extreme points from the model (eg, a combination of extremely low ka, high Vd, and high CL), which would result in simulated drug concentrations close to zero, more likely due to poor adherence in reality. The simulator generated 50 random sets of parameter values from the given normal distributions, with each value corrupted by an additive process noise to simulate real-world residual error due to unmeasurable effects. Noise for each concentration was a random draw from a normal distribution around a mean of 0, with a variance of σ2, where σ = (0.1 * the simulated drug concentration). For each set of simulated pharmacokinetic (PK) parameters (ie, a simulated patient), drug concentrations were calculated at 3, 6, 9, and 12 hours after the fifth dose (to simulate steady-state conditions) of 1250 mg given every 12 hours to a 40-kg child. A population PK model was then constructed from the simulated data set using the NPAG program in the MM-USCPACK collection.33 After being converted into the form used by the MM-USCPACK software, the output of NPAG was a collection of points, each comprising an estimated value for the 3 PK parameters and the associated estimated probability that those 3 parameter values were the true values for the population. An example model for atazanavir is shown graphically in Figure 2A. In summary, with these techniques, we generated a population PK model for each antiretroviral drug from published point parameter estimates without recapitulating multiple PK clinical trials.
The MM-USCPACK software uses the nonparametric population model as a Bayesian prior distribution. Given a real patient’s nelfinavir dose amount and times, body weight, and measured nelfinavir concentration(s), the software computes the Bayesian posterior distribution of parameter values given the patient’s data. The probability of each point in the model is adjusted up or down depending on the ability of that point to minimize the squared error between predicted and observed concentrations. This can be seen in Figure 2B as the probability that each point has changed (the z-axis). The plots in Figure 3 are the weighted mean of the 50 concentration-time curves, each based on 1 of the points in the Bayesian posterior distribution. These plots are useful for predicting an individual patient’s concentration at any time within the dose interval and knowing the certainty of that prediction.
The other important use of the MM-USCPACK software is for dose finding. This function finds the dose regimen, within user-specified constraints, that minimizes the weighted squared error between predicted and target concentrations (eg, a specified trough) when given to every point in the posterior distribution (ie, multiple models). By using the entire posterior distribution, rather than, say, the mean, we can know the expected error with which the dose regimen predicts the desired goal. For patients 2 and 3, we used this function to explore alternate regimens, estimating the likelihood of hitting target concentrations for each.
Footnotes
Financial disclosure: None declared.
References
- 1.Fraaij PL, van Kampen JJ, Burger DM, de Groot R. Pharmacokinetics of antiretroviral therapy in HIV-1-infected children. Clin Pharmacokinet. 2005;44(9):935–956. doi: 10.2165/00003088-200544090-00004. [DOI] [PubMed] [Google Scholar]
- 2.Reed MD, Besunder JB. Developmental pharmacology: ontogenic basis of drug disposition. Pediatr Clin North Am. 1989;36(5):1053–1074. doi: 10.1016/s0031-3955(16)36757-8. [DOI] [PubMed] [Google Scholar]
- 3.Kearns GL, Abdel-Rahman SM, Alander SW, Blowey DL, Leeder JS, Kauffman RE. Developmental pharmacology—drug disposition, action, and therapy in infants and children. N Engl J Med. 2003;349(12):1157–1167. doi: 10.1056/NEJMra035092. [DOI] [PubMed] [Google Scholar]
- 4.Lent-Evers NA, Mathot RA, Geus WP, van Hout BA, Vinks AA. Impact of goal-oriented and model-based clinical pharmacokinetic dosing of aminoglycosides on clinical outcome: a cost-effectiveness analysis. Ther Drug Monit. 1999;21(1):63–73. doi: 10.1097/00007691-199902000-00010. [DOI] [PubMed] [Google Scholar]
- 5.Bleyzac N, Souillet G, Magron P, et al. Improved clinical outcome of paediatric bone marrow recipients using a test dose and Bayesian pharmacokinetic individualization of busulfan dosage regimens. Bone Marrow Transplant. 2001;28(8):743–751. doi: 10.1038/sj.bmt.1703207. [DOI] [PubMed] [Google Scholar]
- 6.Fletcher CV, Anderson PL, Kakuda TN, et al. Concentration-controlled compared with conventional antiretroviral therapy for HIV infection. AIDS. 2002;16(4):551–560. doi: 10.1097/00002030-200203080-00006. [DOI] [PubMed] [Google Scholar]
- 7.Best BM, Goicoechea M, Witt MD, et al. A randomized controlled trial of therapeutic drug monitoring in treatment-naive and -experienced HIV-1-infected patients. J Acquir Immune Defic Syndr. 2007;46(4):433–442. doi: 10.1097/QAI.0b013e318156f029. [DOI] [PubMed] [Google Scholar]
- 8.Boyd MA, Siangphoe U, Ruxrungtham K, et al. The use of pharmacokinetically guided indinavir dose reductions in the management of indinavir-associated renal toxicity. J Antimicrob Chemother. 2006;57(6):1161–1167. doi: 10.1093/jac/dkl112. [DOI] [PubMed] [Google Scholar]
- 9.Burger D, Hugen P, Reiss P, et al. Therapeutic drug monitoring of nelfinavir and indinavir in treatment-naive HIV-1-infected individuals. AIDS. 2003;17(8):1157–1165. doi: 10.1097/00002030-200305230-00007. [DOI] [PubMed] [Google Scholar]
- 10.Cleijsen RM, van de Ende ME, Kroon FP, et al. Therapeutic drug monitoring of the HIV protease inhibitor atazanavir in clinical practice. J Antimicrob Chemother. 2007;60:897–900. doi: 10.1093/jac/dkm298. [DOI] [PubMed] [Google Scholar]
- 11.Durant J, Clevenbergh P, Garraffo R, et al. Importance of protease inhibitor plasma levels in HIV-infected patients treated with genotypic-guided therapy: pharmacological data from the Viradapt Study. AIDS. 2000;14:1333–1339. doi: 10.1097/00002030-200007070-00005. [DOI] [PubMed] [Google Scholar]
- 12.Wasmuth JC, Lambertz I, Voigt E, et al. Maintenance of indinavir by dose adjustment in HIV-1-infected patients with indinavir-related toxicity. Eur J Clin Pharmacol. 2007;63:901–908. doi: 10.1007/s00228-007-0343-z. [DOI] [PubMed] [Google Scholar]
- 13.Fletcher CV, Brundage RC, Fenton T, et al. Pharmacokinetics and pharmacodynamics of efavirenz and nelfinavir in HIV-infected children participating in an area-under-the-curve controlled trial. Clin Pharmacol Ther. 2008;83(2):300–306. doi: 10.1038/sj.clpt.6100282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dahri K, Ensom MH. Efavirenz and nevirapine in HIV-1 infection: is there a role for clinical pharmacokinetic monitoring? Clin Pharmacokinet. 2007;46:109–132. doi: 10.2165/00003088-200746020-00002. [DOI] [PubMed] [Google Scholar]
- 15.Fraaij PL, Rakhmanina N, Burger DM, de GR. Therapeutic drug monitoring in children with HIV/AIDS. Ther Drug Monit. 2004;26:122–126. doi: 10.1097/00007691-200404000-00006. [DOI] [PubMed] [Google Scholar]
- 16.Wertheimer BZ, Freedberg KA, Walensky RP, Yazdanapah Y, Losina E. Therapeutic drug monitoring in HIV treatment: a literature review. HIV Clin Trials. 2006;7(2):59–69. doi: 10.1310/hct.2006.7.2.004. [DOI] [PubMed] [Google Scholar]
- 17.US Department of Health and Human Services. [Accessed July 7, 2006];Guidelines for the Use of Antiretroviral Agents in HIV-Infected Adults and Adolescents. 2006 May 4; http://www.aidsinfo.nih.gov. [PubMed]
- 18.Acosta EP, Gerber JG. Position paper on therapeutic drug monitoring of antiretroviral agents. AIDS Res Hum Retroviruses. 2002;18(12):825–834. doi: 10.1089/08892220260190290. [DOI] [PubMed] [Google Scholar]
- 19.Acosta EP, King JR. Methods for integration of pharmacokinetic and phenotypic information in the treatment of infection with human immunodeficiency virus. Clin Infect Dis. 2003;36(3):373–377. doi: 10.1086/345993. [DOI] [PubMed] [Google Scholar]
- 20.European AIDS Clinical Society (EACS) [Accessed May 15, 2008.];Guidelines for the Clinical Management and Treatment of HIV-Infected Adults in Europe. 2007 December 1; doi: 10.1111/j.1468-1293.2007.00533.x. http://www.eacs.eu/guide/index.htm. [DOI] [PubMed]
- 21.Bossi P, Peytavin G, it-Mohand H, et al. GENOPHAR: a randomized study of plasma drug measurements in association with genotypic resistance testing and expert advice to optimize therapy in patients failing antiretroviral therapy. HIV Med. 2004;5(5):352–359. doi: 10.1111/j.1468-1293.2004.00234.x. [DOI] [PubMed] [Google Scholar]
- 22.Clevenbergh P, Garraffo R, Durant J, Dellamonica P. PharmAdapt: a randomized prospective study to evaluate the benefit of therapeutic monitoring of protease inhibitors: 12 week results. AIDS. 2002;16:2311–2315. doi: 10.1097/00002030-200211220-00011. [DOI] [PubMed] [Google Scholar]
- 23.Khoo SH, Lloyd J, Dalton M, et al. Pharmacologic optimization of protease inhibitors and nonnucleoside reverse transcriptase inhibitors (POPIN)—a randomized controlled trial of therapeutic drug monitoring and adherence support. J Acquir Immune Defic Syndr. 2006;41:461–467. doi: 10.1097/01.qai.0000218345.65434.21. [DOI] [PubMed] [Google Scholar]
- 24.Torti C, Quiros-Roldan E, Regazzi M, et al. RADAR-MASTER Study Group. A randomized controlled trial to evaluate antiretro-viral salvage therapy guided by rules-based or phenotype-driven HIV-1 genotypic drug-resistance interpretation with or without concentration-controlled intervention: the Resistance and Dosage Adapted Regimens (RADAR) study. Clin Infect Dis. 2005;40(12):1828–1836. doi: 10.1086/429917. [DOI] [PubMed] [Google Scholar]
- 25.Jelliffe R, Schumitzky A, Bayard D, Van Guilder M, Leary RH. The USC*PACK programs for parametric and nonparametric population PK/PD modeling, for multiple model adaptive control of drug dosage regimens, and for IMM Bayesian posterior individual models with changing parameter distributions during the period of data analysis [abstract]. Population Analysis Group in Europe; Paris, France. June 2002. [Google Scholar]
- 26.Bayard DS, Jelliffe RW. A Bayesian approach to tracking patients having changing pharmacokinetic parameters. J Pharmacokinet Pharmacodyn. 2004;31(1):75–107. doi: 10.1023/b:jopa.0000029490.76908.0c. [DOI] [PubMed] [Google Scholar]
- 27.Jelliffe R, Bayard D, Milman M, Van Guilder M, Schumitzky A. Achieving target goals most precisely using nonparametric compartmental models and “multiple model” design of dosage regimens. Ther Drug Monit. 2000;22(3):346–353. doi: 10.1097/00007691-200006000-00018. [DOI] [PubMed] [Google Scholar]
- 28.Ette EI, Williams PJ. Population pharmacokinetics I: background, concepts, and models. Ann Pharmacother. 2004;38(10):1702–1706. doi: 10.1345/aph.1D374. [DOI] [PubMed] [Google Scholar]
- 29.Jelliffe RW, Maire P. Goal-oriented, model-based drug regimens. Comput Biol Med. 2001;31(3):145–146. doi: 10.1016/s0010-4825(00)00030-5. [DOI] [PubMed] [Google Scholar]
- 30.Bayard D, Jelliffe R, Schumitzky A, Milman M, Van Guilder M. Precision drug dosage regimens using multiple model adaptive control: theory and application to simulated vancomycin therapy. In: Sridhar R, Rao KS, Lakshminarayanan V, editors. Selected Topics in Mathematical Physics, Prof. R. Vasudevan Memorial Volume. Madras, India: World Scientific Publishing; 1995. [Google Scholar]
- 31.Neely MN, Lam J, Kovacs A, Van Guilder M, Jelliffe R, Louie S. Novel modeling strategy using random antiretroviral drug levels to measure adherence in HIV-infected patients. Abstract presented at: 7th Annual HIV Pharmacology Workshop; Lisbon, Portugal. 2006. [Google Scholar]
- 32.Metropolis N, Ulam S. The Monte Carlo method. J Am Stat Assoc. 1949;44(247):335–341. doi: 10.1080/01621459.1949.10483310. [DOI] [PubMed] [Google Scholar]
- 33.Bustad A, Terziivanov D, Leary R, Port R, Schumitzky A, Jelliffe R. Parametric and nonparametric population methods: their comparative performance in analysing a clinical dataset and two Monte Carlo simulation studies. Clin Pharmacokinet. 2006;45(4):365–383. doi: 10.2165/00003088-200645040-00003. [DOI] [PubMed] [Google Scholar]
- 34.Hirt D, Urien S, Jullien V, et al. Age-related effects on nelfinavir and M8 pharmacokinetics: a population study with 182 children. Antimicrob Agents Chemother. 2006;50(3):910–916. doi: 10.1128/AAC.50.3.910-916.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Csajka C, Marzolini C, Fattinger K, et al. Population pharmacokinetics and effects of efavirenz in patients with human immunodeficiency virus infection. Clin Pharmacol Ther. 2003;73(1):20–30. doi: 10.1067/mcp.2003.22. [DOI] [PubMed] [Google Scholar]
- 36.Starr SE, Fletcher CV, Spector SA, et al. Efavirenz liquid formulation in human immunodeficiency virus-infected children. Pediatr Infect Dis J. 2002;21(7):659–663. doi: 10.1097/00006454-200207000-00011. [DOI] [PubMed] [Google Scholar]
- 37.Kashuba AD, Tierney C, Downey GF, et al. Combining fosam-prenavir with lopinavir/ritonavir substantially reduces amprenavir and lopinavir exposure: ACTG protocol A5143 results. AIDS. 2005;19(2):145–152. doi: 10.1097/00002030-200501280-00006. [DOI] [PubMed] [Google Scholar]
- 38.Wire MB, Shelton MJ, Studenberg S. Fosamprenavir: clinical pharmacokinetics and drug interactions of the amprenavir pro-drug. Clin Pharmacokinet. 2006;45(2):137–168. doi: 10.2165/00003088-200645020-00002. [DOI] [PubMed] [Google Scholar]
- 39.Colombo S, Buclin T, Cavassini M, et al. Population pharmacokinetics of atazanavir in patients with human immunodeficiency virus infection. Antimicrob Agents Chemother. 2006;50(11):3801–3808. doi: 10.1128/AAC.00098-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Keil K, Hochreitter J, DiFrancesco R, et al. Integration of atazanavir into an existing liquid chromatography UV method for protease inhibitors: validation and application. Ther Drug Monit. 2007;29:103–109. doi: 10.1097/FTD.0b013e3180318ef3. [DOI] [PubMed] [Google Scholar]
- 41.Holland DT, DiFrancesco R, Connor JD, Morse GD. Quality assurance program for pharmacokinetic assay of antiretrovirals: ACTG proficiency testing for pediatric and adult pharmacology support laboratories, 2003 to 2004: a requirement for therapeutic drug monitoring. Ther Drug Monit. 2006;28:367–374. doi: 10.1097/01.ftd.0000211817.58052.b8. [DOI] [PubMed] [Google Scholar]
- 42.Sustiva [prescribing information] New York, NY: Bristol-Myers Squibb; 2007. [Accessed December 18, 2007]. http://packageinserts.bms.com/pi/pi_sustiva.pdf. [Google Scholar]
- 43.Haas DW, Ribaudo HJ, Kim RB, et al. Pharmacogenetics of efavirenz and central nervous system side effects: an Adult AIDS Clinical Trials Group study. AIDS. 2004;18(18):2391–2400. [PubMed] [Google Scholar]
- 44.Kappelhoff BS, Crommentuyn KM, de Maat MM, Mulder JW, Huitema AD, Beijnen JH. Practical guidelines to interpret plasma concentrations of antiretroviral drugs. Clin Pharmacokinet. 2004;43(13):845–853. doi: 10.2165/00003088-200443130-00002. [DOI] [PubMed] [Google Scholar]
- 45.Viracept prescribing information. New York, NY: Pfizer; 2007. [Accessed December 18, 2007]. www.PfizerPro.com/Viracept. [Google Scholar]
- 46.Gonzalez de Requena D, Bonora S, Canta F, et al. Atazanavir Ctrough is associated with efficacy and safety: definition of therapeutic range. Poster Abstracts, 12th Conference on Retroviruses and Opportunistic Infections; Boston, MA. 2005. [Google Scholar]
- 47.Saitoh A, Sarles E, Capparelli E, et al. CYP2B6 genetic variants are associated with nevirapine pharmacokinetics and clinical response in HIV-1-infected children. AIDS. 2007;21:2191–2199. doi: 10.1097/QAD.0b013e3282ef9695. [DOI] [PubMed] [Google Scholar]
- 48.Gatanaga H, Hayashida T, Tsuchiya K, et al. Successful efavirenz dose reduction in HIV type 1-infected individuals with cytochrome P450 2B6 *6 and *26. Clin Infect Dis. 2007;45:1230–1237. doi: 10.1086/522175. [DOI] [PubMed] [Google Scholar]
- 49.Burger D, van der Heiden I, la Porte C, et al. Interpatient variability in the pharmacokinetics of the HIV non-nucleoside reverse transcriptase inhibitor efavirenz: the effect of gender, race, and CYP2B6 polymorphism. Br J Clin Pharmacol. 2006;61(2):148–154. doi: 10.1111/j.1365-2125.2005.02536.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.US Department of Health and Human Services. . [Accessed March 6, 2008.];Guidelines for the Use of Antiretroviral Agents in Pediatric HIV Infection. February 28, 2008. http://www.aidsinfo.nih.gov.
- 51.Nettles R, Kieffer T, Parsons T, et al. Marked intraindividual variability in antiretroviral concentrations may limit the utility of therapeutic drug monitoring. Clin Infect Dis. 2006;42(8):1189–1196. doi: 10.1086/501458. [DOI] [PubMed] [Google Scholar]
- 52.Haas D. Editorial commentary: can responses to antiretroviral therapy be improved by therapeutic drug monitoring? Clin Infect Dis. 2006;42(8):1197–1199. doi: 10.1086/501464. [DOI] [PubMed] [Google Scholar]
- 53.Neely MN, van den Anker J, Soldin SJ, Williams K, Baghdassarian A, Rakhmanina N. Population kinetics and dynamics of lopinavir in HIV-infected children. Abstract presented at: 17th Population Approach Group in Europe Meeting; Jun, 2008. Abstract 1340. [Google Scholar]
- 54.Neely MN, Jelliffe R. Population pharmacokinetic models and individualized Bayesian dose optimization in HIV patients. Abstract presented at: First American Conference on Pharmacometrics; Tucscon, AZ. Mar, 2008. [Google Scholar]
- 55. [Accessed March 15, 2008];Drugs used in the treatment of HIV infection. 2008 January; http://www.fda.gov/oashi/aids/virals.html.
- 56.Villani P, Floridia M, Pirillo MF, et al. Pharmacokinetics of nelfinavir in HIV-1-infected pregnant and nonpregnant women. Br J Clin Pharmacol. 2006;62:309–315. doi: 10.1111/j.1365-2125.2006.02669.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bryson YJ, Mirochnick M, Stek A, et al. Pharmacokinetics and safety of nelfinavir when used in combination with zidovudine and lamivudine in HIV-infected pregnant women: Pediatric AIDS Clinical Trials Group (PACTG) Protocol 353. HIV Clin Trials. 2008;9:115–125. doi: 10.1310/hct0902-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Stek AM, Mirochnick M, Capparelli E, et al. Reduced lopinavir exposure during pregnancy. AIDS. 2006;20:1931–1939. doi: 10.1097/01.aids.0000247114.43714.90. [DOI] [PubMed] [Google Scholar]
- 59.Mirochnick M, Capparelli E. Pharmacokinetics of antiretrovirals in pregnant women. Clin Pharmacokinet. 2004;43(15):1071–1087. doi: 10.2165/00003088-200443150-00002. [DOI] [PubMed] [Google Scholar]
- 60.Robertson SM, Penzak SR, Pau A. Drug interactions in the management of HIV infection: an update. Expert Opin Pharmacother. 2007;8(17):2947–2963. doi: 10.1517/14656566.8.17.2947. [DOI] [PubMed] [Google Scholar]