Abstract
Concepts of brain-steroid signaling have traditionally placed emphasis on the gonads and adrenals as the source of steroids, the strict dichotomy of early developmental (“organizational”) and mature (“activational”) effects, and a relatively slow mechanism of signaling through intranuclear receptors. Continuing research shows that these concepts are not inaccurate, but they are certainly incomplete. In this review, we focus on the song control circuit of songbird species to demonstrate how each of these concepts is limited. We discuss the solid evidence for steroid synthesis within the brain (“neurosteroidogenesis”), the role of neurosteroids in organizational events that occur both early in development and later in life, and how neurosteroids can act in acute and non-traditional ways. The songbird model therefore illustrates how neurosteroids can dramatically increase the diversity of steroid-sensitive brain functions in a behaviorally-relevant system. We hope this inspires further research and thought into neurosteroid signaling in songbirds and other animals.
Introduction: Re-evaluating core principles
Traditional concepts about sex steroid-brain signaling are framed by three core principles. First, it is the gonads, and to a lesser extent the adrenals, that synthesize steroids. Second, these steroids either permanently organize growth of some neural structures during development or activate neural structures transiently to control behavior and physiology after sexual maturation. Third, steroids act predominantly by binding to intranuclear receptors that regulate gene expression through relatively slow mechanisms. These core principles largely summarize how steroids act on the brain to ensure appropriate sex-and season-specific reproduction in animals. Therefore, these three principles have become the foundation of neural sex steroid biology.
Contemporary research, however, shows that each of these ideas is limited and as a consequence, in need of updating. For example, we now know that steroids can be intracranial signals, synthesized in partial or complete independence from the periphery. We also now know that steroids can influence a vast array of neural targets and functions across an animal’s lifespan, blurring their ontogenetic (e.g., developmental or adult) and functional (e.g., reproductive) classifications. Lastly, we now know that steroids can influence brain and behavior by binding to several classes of receptors and ion channels in neuronal cells, expanding the range of steroid-dependent cellular changes. Therefore, the group of chemicals that have been called the ‘sex steroids’ must now be viewed as multi-functional, ageless and, in some cases, genderless neurochemical signals.
New insights on neural steroid action arose from extensive neuromolecular and neurochemical studies combined with neuroanatomical and behavioral examination in a diverse set of vertebrate models. Perhaps surprising to many, songbirds have emerged from this set as one of the most valuable systems for advancing understanding of brain-steroid biology. Across a bird’s life, sex steroids facilitate growth and function of neural structures that subserve the recognition, learning and production of song. In many cases, the three traditional core principles fail to explain how these occur. Therefore, while it is true that gonadal steroids can act by traditional mechanisms to influence song as a reproductive behavior, to fully describe songbird brain-steroid biology, it is necessary to incorporate new findings into the old conceptual framework.
In this review, we focus on the sex steroids and their actions on the songbird brain. We do not intend to question the numerous and well-documented actions of gonadally-derived steroids on song (reviewed comprehensively elsewhere [1; 2; 3; 4; 5; 6; 7; 8]. Rather, we focus here on findings that do not conform to the classical concepts of steroid action, though we will in some cases invoke the traditional core principles to frame our points.
We will also focus on the zebra finch, a common laboratory songbird model. It does not in all cases represent the patterns of steroidogenic synthesis and action, or all of the behavioral diversity, found among songbirds. It does, however, have a striking sex difference in song system anatomy and behavior. And, while steroids are clearly involved in masculinizing the song circuit, it remains a mystery as to how this circuit becomes masculine. The zebra finch and the puzzle of its song circuit has, therefore, motivated many investigations into steroid synthesis and action that do represent well the complexity of steroids in the brain – investigations that have even expanded beyond the boundaries of the sexually dimorphic song circuit itself.
We will start with a general introduction to songbird brains and behavior. Then, we will briefly describe the process of steroidogenesis and introduce the idea that important steroidal signals may be synthesized within the brain itself. Next, we will discuss the potential for neurosteroids to contribute to the early developmental organization of the sexually dimorphic song control circuit in zebra finches, and how neurosteroids might also contribute to “organizational” events in the adult song circuit in other songbirds. Finally, we will highlight studies illustrating that steroids can be synthesized at synaptic terminals and, like other neurotransmitters and neuromodulators, are subject to rapid local synthesis and/or release in behaviorally-relevant contexts. Each of the following sections describes songbird research that fundamentally shifts how steroid-brain interactions must be conceived. This relationship is no longer simple, but it is much more interesting for its richness and complexity. We hope the ideas presented here stimulate research and discussion of steroids as fascinating neurochemical signals not only in songbirds, but in other vertebrate species as well.
Songbirds and the neural song control circuit
With the exception of Antarctica, virtually every landmass on the planet teems with songbirds, birds of the Order of the Passeriformes, suborder Oscine. These small birds, unlike other familiar “perching” birds like pigeons, hawks and owls, are able to produce songs that are learned. In some species, young birds copy adult vocalizations they hear during a sensitive developmental period. In other species, birds retain the ability to learn new songs throughout life [9]. Songs can be simple in frequency, amplitude and structure, or unbelievably rich and complex [10; 11]. The diversity represented within the songbird species therefore offers an abundance of models for investigating the interactions of steroids and neural systems in the context of a naturally-occurring behavior.
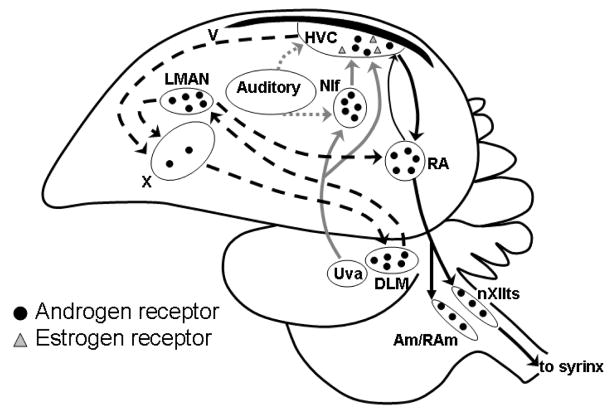
Songbirds possess a conspicuous neural circuit, or “song system,” that is dedicated to song learning, song recognition, and the motor output of song (Figure 1). Details of this circuit’s connectivity are described elsewhere [12]. For our purposes here, it is important to recognize a few general features of the song system. Auditory input is essential, and is processed within the telencephalon primarily through specialized sensory areas: Field L, the caudomedial nidopallium (NCM), and the caudomedial mesopallium (CMM). At least partially through another telencephalic area, nucleus interface of the nidopallium (Nif), auditory information is transmitted to HVC (used as a proper name) and becomes integrated into sensorimotor and motor processes within two other major interconnected neuroanatomical loops. HVC connects to a circuit involved in fine motor control and developmental song learning (HVC → area X → medial dorsolateral nucleus of the thalamus (DLM) → lateral magnocellular nucleus of the anterior nidopallium (LMAN) and from LMAN to both the robust nucleus of the arcopallium (RA) and back to area X), and to a pathway controlling motor production of vocalizations (HVC→ RA → tracheosyringeal nucleus of the XII cranial nerve (nXIIts)). This latter nucleus contains motoneurons that innervate and control muscle contractions of the syrinx, the avian vocal organ [13].
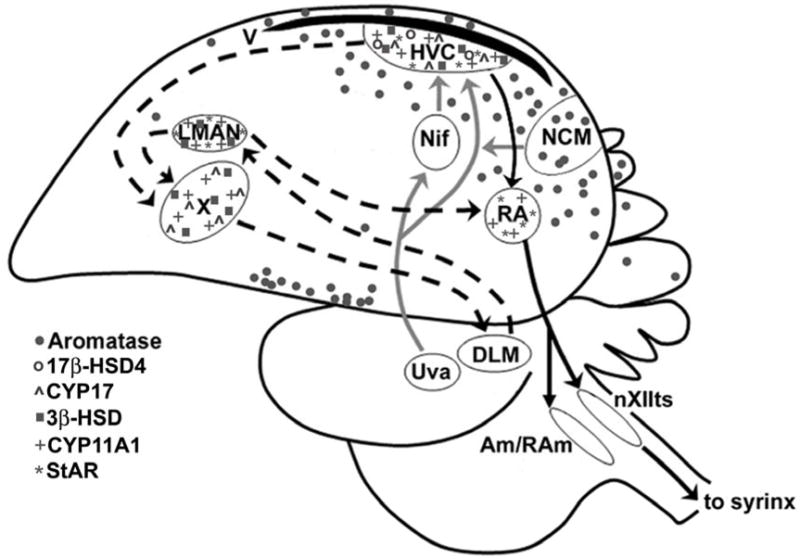
Figure 1.
A schematic sagittal drawing of the songbird brain showing projections of major nuclei in the song system, and the distribution of steroid receptors. The descending motor pathway (black and dark gray arrows) controls the production of song. The dark gray arrows indicate inputs to HVC from the thalamic nucleus Uva and the nidopallial nucleus Nif. The black arrows indicate the descending projections from HVC in the nidopallium to RA in the arcopallium and thence to the vocal nucleus nXIIts, the respiratory nucleus RAm, and the laryngeal nucleus Am in the medulla. The white arrows indicate the anterior forebrain pathway that is essential for song learning. It indirectly links HVC to RA, via area X in the medial striatum, DLM in the thalamus, and LMAN in the nidopallium. LMAN also projects to area X. Field L is an auditory region in the nidopallium that projects to HVC (light grey arrow). Abbreviations: Am, nucleus ambiguous; DLM, medial portion of the dorsolateral nucleus of the thalamus; LMAN, lateral portion of the magnocellular nucleus of the anterior nidopallium; NIf, nucleus interface of the nidopallium; RA, robust nucleus of the arcopallium; RAm, nucleus retroambigualis; Uva, nucleus uvaeformis; V, ventricle; X, area X; nXIIts, tracheosyringeal nucleus of the hypoglossal nerve. Taken from [12].
Elements of this neural circuitry are shared by other bird species such as parrots and hummingbirds that also exhibit learned vocalizations [14; 15; 16]. Songbirds, however, have evolved a particularly robust song system that is the focus of extensive investigation into many core aspects of neurobiology. Songbirds therefore are important models for investigating mechanisms of behaviorally-relevant neural circuits [17; 18]. For example, in some species such as the Australian zebra finch (Taeniopygia guttata), only males sing, and the complexity of the neural song circuit mirrors sex differences in behavior [19]. Many other songbird species alter their singing behavior seasonally, and the song control circuit partially deteriorates and regenerates in parallel with the behavioral changes [20; 21; 22]. This is arguably the most extraordinary case of adult neural plasticity that occurs in the absence of trauma, making songbirds invaluable for investigations into the natural flux of neuronal birth, death, migration, and connectivity [23]. Lastly, many songbirds are highly social animals that depend on generating clear vocalizations, and quickly and accurately perceiving the vocalizations of others. Songbirds are therefore excellent models for investigating the many rapid changes throughout the song system that are likely involved in these processes. Sex steroids have roles in all of this neurobiology.
Core Principle 1: Neurosteroidogenesis; steroid synthesis within the brain
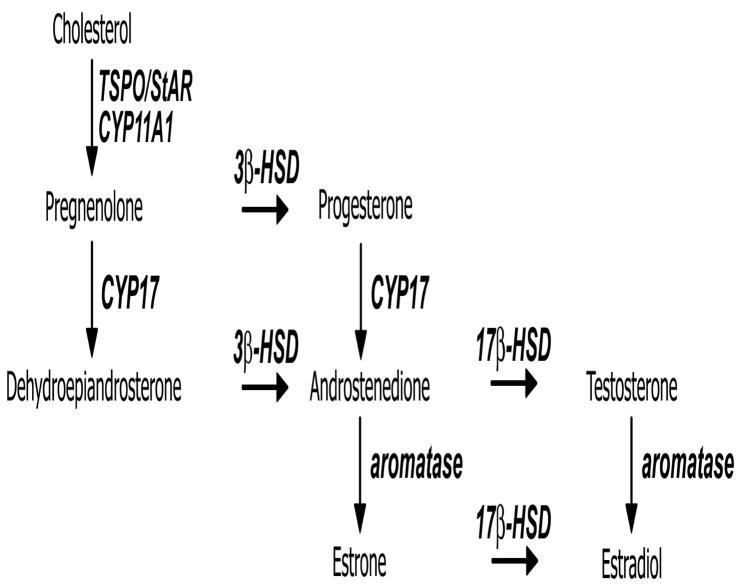
Steroid synthesis (steroidogenesis) is abundant in the gonads and adrenals of virtually all vertebrate species by a process [24] illustrated in Figure 2. Leydig and theca cells in the testes and ovaries, respectively, and cortical cells of the adrenals, are specialized to initiate, and in some cases complete, the synthesis of the biologically important sex steroids. These cells possess mitochondria equipped with transport proteins, the Steroidogenic Acute Regulatory protein (StAR) or the mitochondrial translocator protein (TSPO; previously known as the peripheral-type benzodiazepine receptor, PBR) [25; 26; 27; 28; 29; 30; 31; 32], that move cholesterol across the mitochondrial membranes. This transport places cholesterol in contact with the side-chain cleavage enzyme (Cytochrome P450 SCC; CYP11A1) that produces the first steroidal molecule, pregnenolone. As a lipophilic steroid, pregnenolone passively diffuses out of the mitochondria into the cytoplasm where it can interact with additional steroidogenic enzymes embedded in smooth endoplasmic reticulum. One of these enzymes is 3β-hydroxysteroid dehydrogenase/Δ5-Δ4 isomerase (3β-HSD), which converts pregnenolone into the transcriptionally-active progestin progesterone. Pregnenolone can also be converted into the androgen dehydroepiandrosterone (DHEA) via the activity of 17α-hydroxylase/17,20 lyase (Cytochrome P450 17A1; CYP17). CYP17 also converts progesterone into the androgen androstenedione (AE); AE is also the product of 3β-HSD metabolism of DHEA. AE is converted into the androgen testosterone (T) by an isoform of the 17β-HSD enzyme. T is converted into the potent estrogen estradiol (E2) by the enzyme aromatase (Cytochrome P450 19A1; CYP19). Alternatively, testosterone can be converted into the potent androgen 5α-dihydrotesterone by the enzyme 5α-reductase, or the inactive androgen 5β-dihydrotestosterone by the enzyme 5β-reductase (not pictured in Figure 2). The synthesis of the glucocorticoids cortisol and corticosterone also occur via a similar pregnenolone/progesterone precursor pathway. In general, steroidogenic enzymes display very high substrate specificity, though there is evidence that non-steroidal compounds can bind to and in some cases, modify activity of, at least a subset of these enzymes [e.g. [33; 34; 35]]. The focus of this review is on the neural production of androgens and estrogens via the main cascade of cholesterol transport and enzymatic conversions described above.
Figure 2.
Steroidogenesis pathway: Steroids are in bold; enzymes and cholesterol transport proteins are in italics. Enzyme abbreviations: cytochrome P450 side-chain cleavage=CYP11A1; cytochrome P450 17α-hydroxylase/C17,20 lyase=CYP17; 3β–hydroxysteroid dehydrogenase/Δ5-Δ4 isomerase=3β-HSD; 17β-hydroxysteroid dehydrogenase=17β-HSD; cytochrome P450 19A1 =CYP19 (“aromatase”). Cholesterol transport protein abbreviations: translocator protein (previously known as the peripheral-type benzodiazepine receptor)=TSPO; steroidogenic acute regulatory protein = StAR.
The capacity for the nervous system to synthesize steroids de novo, ”neurosteroidogenesis,” was first proposed based on work from the laboratory of Etienne Baulieu after they discovered that steroids were detectable in animals that had been gonadectomized and adrenalectomized [36; 37; 38]. Unlike in the gonads and adrenals, the enzymes and transporters necessary for steroid synthesis are present in minute, not abundant, quantities in the brain. This made their identification difficult, and interpretation of their biological significance challenging. Nevertheless, experiments from many labs have now confirmed the potential for neurosteroidogenesis in a variety of vertebrate models [39; 40; 41] leaving little doubt that neurosteroids are elemental components of the complex neurochemistry of the vertebrate brain.
The term “neurosteroid” is used to distinguish steroids that are produced by steroidogenic activity within the nervous system from those synthesized in the periphery. Here, we consider neurosteroids to be steroids that are synthesized in the brain de novo from cholesterol substrate, as well as those that are metabolized in the brain from steroid substrates supplied from the periphery. The ability of the brain to synthesize its own steroids has profound implications for how we think about neural steroid action. If all of the enzymes and transporters required for de novo steroidogenesis are expressed in the brain, then it can control the “steroidogenic throttle” independently from the gonads and adrenals. Enzymes and transporters can be present in different quantities, in different locations, and at different times to regulate the quantity and mixture of steroids within specific brain regions. It is not that peripheral steroids have no function; indeed it has been shown that circulating steroids can alter neural expression of steroidogenic enzymes in the songbird brain [42; 43]. This effect, however, is not equivalent in all brain regions. The brain itself can therefore regulate steroidogenic activity to supplement or modify peripherally-synthesized steroids, dramatically increasing their potential functional complexity.
In all songbird species examined, the song control circuit expresses intracellular steroid receptors [3; 44; 45; 46; 47; 48; 49]. Thus, the song circuit shares a primary characteristic with reproductive neural circuits: steroid sensitivity. Song is not, however, exclusively a reproductive behavior; songbirds sing in both reproductive and non-reproductive contexts. Anatomically, there are connections between the song circuitry and phylogenetically conserved brain regions that control appetitive and consummatory copulatory behaviors e.g.[50; 51; 52; 53]. Therefore, song represents a complex behavior that has some relationship to reproductive behaviors and relies on sex steroid signaling, but singing is not necessarily under strict reproductive control. This suggests that instead of being wholly regulated by the gonadally-synthesized steroids so strongly implicated in control of reproductive function, brain-specific steroid regulation is essential to the song control circuit.
Core Principle 2: Neurosteroidogenesis and brain organization throughout life
Background
In the zebra finch, one of the most striking organizational processes is the construction of the sexually dimorphic song control circuit during posthatch development. The mechanisms by which individual components of this system become sexually dimorphic may not be exactly the same [54; 55; 56; 57; 58; 59; 60; 61; 62], but two factors are common: steroids can significantly alter every one [18; 61; 63; 64; 65], and the cells that comprise each nucleus likely arise from the proliferative zone surrounding the lateral ventricles [66; 67; 68].
Male songbirds generally have more pronounced (i.e., masculinized) song circuitry than females and in zebra finches sex-steroids profoundly masculinize the song system. Young females systemically administered steroids, especially estradiol, developed masculinized song circuits [64; 69; 70; 71; 72; 73]. In some cases, early exposure to high systemic estradiol levels alone was sufficient to support singing in females [74]. The masculinizing effect was measurable when estradiol was administered during the first three weeks of posthatch development, but particularly potent during the first week of posthatch life [73].
It is during the first week or so after hatching that major neuroanatomical changes occur, too. Major song nuclei are first identifiable 7–10 days after hatching [75; 76]. At this early age (songbirds are altricial), these nuclei are already sexually dimorphic [75; 76]. Thus, the time of greatest steroid influence is consistent with the emergence of brain areas that show significant sex differences.
The traditional dogma of sexual differentiation would postulate that sex-specific secretions from the gonads, in particular sex steroids, directed the male and female song system to organize differentially. However, no consistent sex differences in circulating steroid levels were measured during the first few weeks of posthatch life in zebra finches [77; 78; 79], and testes do not express aromatase [80; 81; 82]. Consequently, two alternative and non-competing mechanisms for the sexual differentiation of the song system were put forth.
One was that the genetic differences between males and females, i.e. the difference in sex chromosome complement, drive sexual differentiation of the brain. The idea that differential expression of sex chromosome genes could influence at least some aspects of “brain sex” has been well supported in mammals [83; 84; 85]. It might have special significance in songbirds because dosage compensation of sex chromosome genes is not as widespread in birds as in mammals [86; 87].
Experimentally, this direct genetic theory is gaining traction in songbirds. Notably, examination of sex chromosome gene expression in a naturally occurring gynandromorph, a bird that was morphologically male on one side and female on the other, showed that only one brain hemisphere expressed a marker for the W chromosome while the other expressed significantly more of a Z chromosome marker [88]. This was consistent with one side being genetically female (ZW) and the other being genetically male (ZZ). Interestingly, the genetically male side had larger, more masculinized song nuclei than the female side [88]. This suggested that the presence of two copies of Z-linked genes in males might promote masculinization of the song nuclei, or alternatively, that the presence of a W-linked gene present only in females might promote demasculinization of the song nuclei (default growth of the zebra finch song system seems to be feminine). One or both of these genetic differences could therefore contribute to the sex differences in the song system. Other sex chromosome genes, for example the trkB receptor, also show sex differences in expression levels within the song control circuit [89]. Experimental evidence for a genetic foundation of behaviorally-relevant brain sexual differentiation comes from a study performed in a non-songbird: embryonic male quail transplanted with female hypothalamic tissue prior to gonadal development fail to display male-typical reproductive behaviors [90]. All of this evidence suggests that it is likely that sex differences in the expression levels of genes localized to sex chromosomes are relevant to the song system.
The other mechanism by which the zebra finch song system may become sexually dimorphic is through the production and action of sex steroids within the brain. This idea in fact combines both the principles of the dogma of sexual differentiation – the necessity of sex steroids, and the direct genetic theory – the sex-specific regulation of neural gene expression. Even in the gynandromorph discussed above, the genetic female hemisphere had larger song nuclei than typical in female zebra finches [88]. Circulating testosterone and estradiol levels in the gynandromorph were below detection limits, more similar to control females than males, supporting the idea that gonadally-derived steroids did not masculinize the song circuit. Therefore, it is possible that the song nuclei in the genetically female hemisphere were masculinized by the actions of a masculine factor diffusing from the male hemisphere into the female hemisphere. Though a mechanism by which steroids could travel between hemispheres is still untested, it is possible that a lipophilic neurosteroid could be this diffusible factor.
Guided by the aromatization hypothesis that explained mammalian brain masculinization [91], it was first postulated that the neural aromatization of gonadally-derived androgens directed the estradiol-mediated masculinization of the song system. As a consequence, aromatase in the zebra finch brain was intensively examined at quantified at the mRNA, protein, and activity level [92; 93; 94; 95; 96; 97; 98]. Though aromatase is abundant in the zebra finch brain, it is essentially absent from cells bodies within the major song nuclei [95; 99; 100] (but see discussion below) and no distinct sex difference in somal aromatase measures emerged from these studies (but see [95; 101]).
Still, evidence mounted for the potential of neurosteroids to impact developmental organization of the song control circuit. Intracerebral steroid implants had greater masculinizing effects the closer they were located to song nuclei [102], demonstrating that steroids synthesized in close proximity – or within – a brain region have a greater likelihood of impacting those song nuclei. Biochemical measurements of several steroidogenic enzymes from neural preparations and homogenates demonstrated the presence of multiple enzymes that produced a variety of steroids [103; 104; 105; 106; 107; 108; 109; 110; 111; 112; 113]. To further the idea that neurosteroidogenesis could contribute to early events in song system construction, a comprehensive examination of each component of the estradiol-synthetic pathway, and the ability to spatially identify neuroanatomical locations of potential neurosteroidogenesis, was needed.
Early development
The discovery that StAR, CYP11A1, 3β-HSD, CYP17, aromatase, and 17β-HSD are expressed in the zebra finch brain as early as posthatch days 1 and 5 demonstrated that the brain could synthesize estradiol de novo at the earliest stages of posthatch development [107; 108; 114; 115]. In situ hybridization mapping of these genes revealed how neurosteroids might exert their effects on the developmental organization of song nuclei: by producing steroids in the cell proliferative zone surrounding the lateral ventricle (Figure 3).
Figure 3.
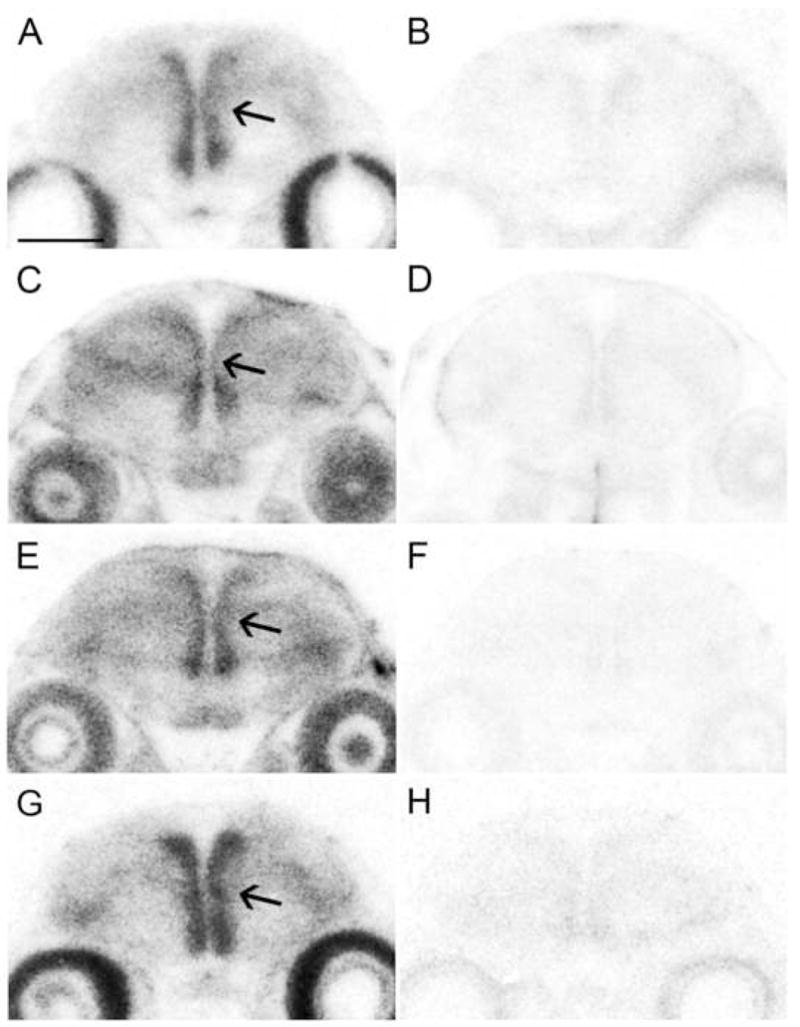
StAR, CYP11A1, 3β-HSD, and CYP17 expression along the lateral ventricles in birds at posthatch day 1. Film images of coronal sections illustrate the intense hybridization of all four genes overlapping with the major proliferative zone. StAR (A), CYP11A1 (C), 3β-HSD (E), and CYP17 (G) hybridized along the lateral ventricle only with antisense (A,C,E,G) but not sense (B,D,F,H) configured riboprobes. Arrows point to the intermediate portion of right hemisphere lateral ventricle. Quantitation of hybridization patterns for all four genes showed that StAR and CYP11A1 had the most precisely overlapping expression, likely because CYP11A1 activity is dependent upon cholesterol transporters. Otherwise, each gene had some unique patterns of hybridization, suggesting the potential for steroid microenvironments along the major cell proliferative zone. Scale bar = 1mm. Figure from [108].
The lateral ventricle is the largest ventricle in the avian brain. It is situated at the midline, and spans almost the entire rostral-caudal and dorsal-ventral extents of the telencephalon. The ventricular zone along the wall of the lateral ventricle is the major site of cell proliferation in the avian brain. From this zone, cells that end up populating all brain areas migrate. In other words, the region surrounding the lateral ventricle is the birthplace of most of the cells that make up the brain, including the song control circuit. Abundant StAR, CYP11A1, 3β-HSD, CYP17, and 17β-HSD expression along the lateral ventricles at early ages provided an explanation for how neurosteroids might impact the anatomy of the song nuclei within the first week of posthatch life when sex differences in the system first emerge [75; 76], and when the effects of steroids are most potent [73].
Cell proliferation is not uniform across the rostral-caudal or the dorsal-ventral dimensions of the lateral ventricles [68; 116; 117]. Hybridization patterns for StAR, CYP11A1, 3β-HSD, and CYP17 – the steroidogenic genes for which quantification was performed – are also not uniformly distributed along the lateral ventricles [107; 108]. In a young songbird, cell proliferation is greatest at the dorsal and ventral aspects of the ventricle [117]. Further, proliferation is greater in the ventral compared to the dorsal aspect of the lateral ventricle [117]. These patterns are most obvious at the rostral-caudal level of the anterior commissure and least noticeable in the most caudal portion of the lateral ventricles [117]. In all four of these dimensions, hybridization patterns of StAR, CYP11A1, 3β-HSD, and CYP17 at posthatch days 1 and 5 mimicked those of cell proliferation [108]. While no definitive marker for the proliferative zone was utilized in conjunction with the in situ hybridization, Nissl-stained brain sections and the qualitative convergence of proliferative regions and hybridization patterns suggested that indeed, steroidogenic genes were present in the cell-dense region characteristic of the ventricular zone.
What significance do these data have to indicate how neurosteroids could direct the earliest stages of song control system organization? One sex difference was detected in the hybridization patterns of StAR, CYP11A1, 3β-HSD, and CYP17 along the lateral ventricles: males showed a wider spread of hybridization than females at the dorsal aspect of the ventricles at the rostral-caudal level of the anterior commissure [105]. A sex difference in proliferative activity was also reported in this region in juvenile songbirds [108]. Cells from the lateral ventricle proliferative zone migrate into HVC, and the number of newly-divided cells migrating from this zone is already greater in males than females before they arrived at the position of HVC [49]. Therefore, while it is unlikely that the sex difference in steroidogenic capacity in this region causes the sex difference in proliferative rates [67; 118], it is possible that due to this juxtaposition, neurosteroidogenesis along the lateral ventricle might have the greatest impact on HVC by affecting cells that ultimately become incorporated into it.
While the potential for neurosteroids to impact newly-divided cells along the lateral ventricle in a sex-specific manner exists, their ability to make a robust impact on the song nucleus cells is limited with the existing data. No large sex differences in steroidogenic gene expression levels or distributions were detected [108], as might be expected if neurosteroidogenesis along the lateral ventricle was the sole mechanism for sexual differentiation of the song circuit. Second, the migration patterns of newly-divided cells from the lateral ventricle to all song nuclei are undescribed in young songbirds, thus the precise origin of those neurons is still unknown. Further, sex differences in major song nuclei other than HVC and area X arise later in development due to the loss of cells in females, or a re-distribution of cells in males, not because of an early accumulation of neurons in males [61; 119; 120]. This mode of sexual differentiation may limit the impact of early neurosteroidogenesis on these nuclei, though it is possible that neurosteroids could impact these later events (see below).
Nevertheless, the presence of multiple steroidogenic factors along the lateral ventricles at the earliest ages of posthatch life indicates an enormous potential for neurosteroid-mediated influence on early brain organization. For instance, neurosteroids could influence cells in close proximity by diffusing though cell membranes, potentially having long-term impacts on the cells that eventually become incorporated into various brain regions. Neurosteroids could also possibly affect cells at distal sites if bound to steroid binding proteins and transported through the ventricles or blood [121; 122; 123]. Therefore, much more work will be required to understand the exact role of neurosteroids on early events in neural development, including that of the song system. What is clear is that the traditional model that considers only the gonadally-derived steroids in organizational actions in the brain must now consider the dramatic capacity for the developing songbird brain to express the steroidogenic enzymes that enable it to metabolize its own set of active steroids.
Late development and adult
Inasmuch as neural organization is the construction of brain areas permissive for a behavior, this process is not limited to early stages in development. Instead, like in the case of the song control circuit in several species of songbirds, it can persist into later developmental stages and adulthood. Neurosteroids could therefore continue to influence organization of the song control circuit throughout life.
Notably, cell proliferation within the ventricular zone of the lateral ventricles continues into adulthood in songbirds [66; 68; 116; 124; 125; 126; 127; 128; 129]. Newly-divided cells can be found throughout the telencephalon, including in at least two major song nuclei, HVC and area X [56; 57; 62; 66; 67; 130; 131; 132]. Proliferative activity in zebra finches is much lower in older birds than in young ones, and the ventricular zone is shallower. Consequently, it was much more difficult to conclude that StAR, CYP11A1, 3β-HSD, and CYP17 were specifically expressed within the proliferative zones of the lateral ventricle in adults [107; 108; 115]. In adult zebra finches and other songbirds, however, rates of proliferation and recruitment of new neurons into existing brain nuclei are not static and are correlated with changes in learned behavior, including song [133; 134]; this may in part explain the differences in adult cell proliferation rates between birds that do not learn song as adults (e.g. zebra finches) and those that do (e.g. canary) e.g. [116; 134]. It may be that levels of steroidogenesis along the lateral ventricle might also fluctuate under the same circumstances. If this were found to be true, it would strengthen the postulation that neurosteroids impact newly divided cells that are relevant to neural function.
Indeed, steroids have been shown to impact newly-divided cells in adult songbirds. Both testosterone and estradiol can increase the volume of and change the complement of neurons within major song nuclei in some adult songbirds such as canaries and song sparrows [135; 136; 137; 138]. This is likely because steroids influence postmitotic events [138; 139; 140; 141; 142] that promote cells that can be functionally incorporated into song control nuclei [143]. It is still unclear if all of these effects are directly on the newly-divided cells themselves. For example, estradiol can promote migration of new neurons by acting on estrogen-sensitive cells that gate their departure from the ventricular zone [142]. Further, the effect of testosterone seems to be largely mediated by BDNF [144; 145; 146], not a direct effect of the steroid itself. In canaries and sparrows, seasonal changes in the song system and neurogenesis parallel changes in circulating testosterone levels [134; 136; 138; 147; 148; 149; 150]. This suggests that gonadally-synthesized testosterone controls these neural changes. It is important to note, however, that free-living canaries maintain a fully-developed song system in spite of seasonal changes in circulating testosterone levels [151]. Thus, it is still unclear if there might also be seasonal regulation of neurosteroid production that supports the song system when circulating testosterone levels are low.
More direct evidence that neurosteroids interact directly with changes in newly-divided cells in adult songbirds comes from studies of neuronal injury. In the zebra finch, a penetrating injury can induce an increase in aromatase expression concomitant with an increase in cells labeled with bromo-deoxyUridine (BrdU), a marker of mitosis [152; 153; 154]. This effect is also sensitive to changes in circulating steroid levels [152], but the clear colocalization of aromatase and the proliferative zone in this injury model (Figure 4; aromatase is not present along the lateral ventricle in uninjured adult zebra finches [49; 155]) provides a great opportunity to investigate the functional role of neurosteroids and newly-divided cells in the adult brain.
Figure 4.
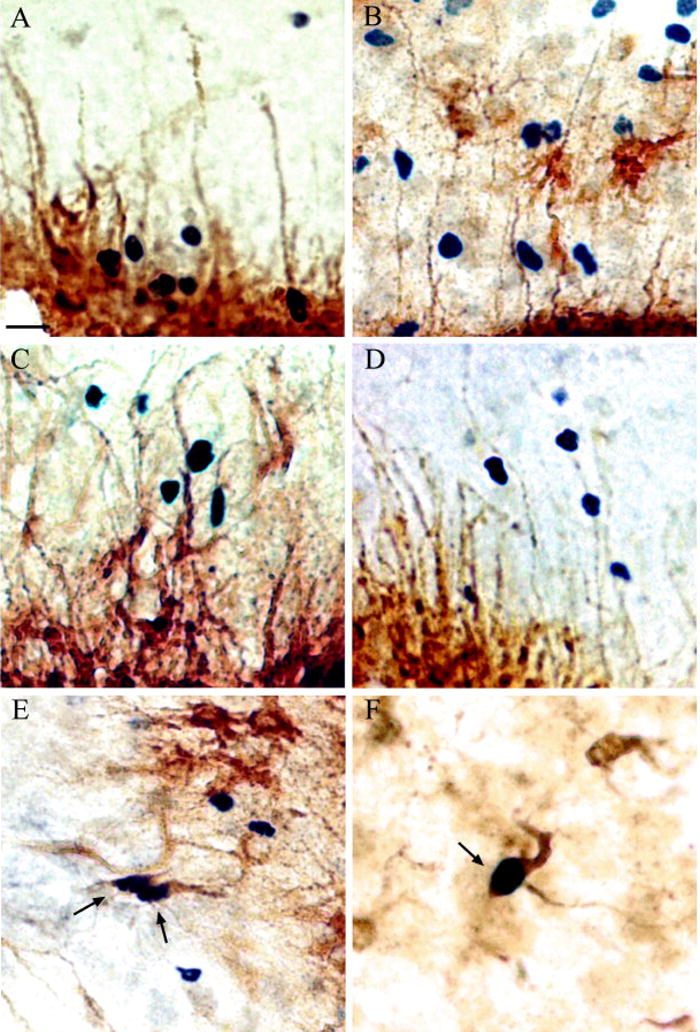
In the adult brain, BrdU-labeled cells can be observed in close juxtaposition with aromatase-positive cells along the lateral ventricle after injury. The newly-divided cells can be seen in the same ventricular zone as the aromatase positive (aromatase represented by DAB-brown stain) cell bodies in (A). Aromatase positive cells morphologically are radial glia. The potential for the estrogen-producing radial glia to guide newly divided cells from the ventricular zone is demonstrated in (B–D), where BrdU-labeled cells (black chromagen-stained cell bodies) appear to migrate along the processes of the glia towards the lesion site (E, F) show aromatase-and BrdU-immunoreactivity colocalized in cells of undetermined types, possibly in the process of differentiation. The lateral ventricle is out of view at the bottom of images A–D. Position of lesion is out of view at top of images A–D; radial glia are extending towards the site of lesion, and restricted to the portion of the lateral ventricle proximal to the lesion site. Scale bar = 20 μm. Figure from [153].
The best evidence that neurosteroids can organize the brain in late development, after other major components are constructed, was an experiment tracking masculinization of the projection from HVC to RA. The connection from HVC to RA is required for vocalizations, and takes place between approximately posthatch days 21–35 [156; 157; 158]. Axons in this projection functionally innervate RA neurons in males, but do not penetrate RA in females [156; 157; 158]. Holloway and Clayton (2001)[65] used a brain slice preparation to show not only that estradiol would masculinize the innervation in female slices – demonstrated previously – but that simply co-culturing a female brain slice with a male brain slice could masculinize the HVC to RA projection in the female slice [65]. This strongly implicated a diffusible factor in masculinization of this song system component. That this factor could be estradiol was supported by the fact that aromatase inhibitors and estrogen receptor antagonists prevented masculinization of the projection in male slices [65]. It was strengthened by the fact that estradiol could be measured in the media of the slices even though they had been cultured in media essentially free of steroids, and that the media from male slices contained more estradiol than females [65]. This study also bolstered the possibility that the putative diffusible masculinizing factor in the gynandromorph was estradiol, emphasizing how neurosteroids might be essential components in shaping the song control circuit.
The potential for a complex milieu of neurosteroids to impact the juvenile and adult brain and song system was further demonstrated by a later investigation of male and female brain slices that contained either the lateral ventricles or the region containing RA, HVC, and its projection neurons in P20 and adult zebra finches [111]. This study measured the activity levels of four steroidogenic enzymes in these slices. Consistent with previous studies, no sex differences in aromatase levels were detected, nor was there a significant difference in aromatase levels between the two medial-lateral regions studied in either age [111]. Regional, age, and sex differences were, however, detected for 3β-HSD, 5α-reductase, and 5β-reductase. In male but not female brain slices from both juvenile and adult birds, slices containing HVC and RA produced more steroids synthesized from a combination of 3β-HSD, 5α-reductase, and 5β-reductase than the lateral ventricle–containing slices. In juvenile slices only, females had higher levels of steroid synthesized by coordinated action of 3β-HSD and 5β-reductase than males in slices containing the lateral ventricle. This study did not measure a neuroanatomical change within the slices. Thus, it is not possible at this time to directly link the differences in enzyme activity levels to measures of the song circuit masculinization or cell proliferation, migration, or differentiation. This study did, however, demonstrate that the brain region surrounding at least one major component of the male zebra finch song system has a greater capacity to synthesize neurosteroids than a non-song circuit area (the ventricular region) that in later development and adulthood shows, at best, a very low capacity for neurosteroidogenesis in zebra finches. Further, there is indication that enzymes along the P20 female lateral ventricle and surrounding brain may be acting to produce 5β-reduced androgens more so than in males. These steroids do not activate the androgen receptor and cannot be aromatized to estrogens. Therefore, it is possible that a combination of steroidogenic enzymes along the estrogen-synthetic pathway could work in concert to differentially regulate steroid production in the juvenile and adult, male and female, songbird brain in spatially distinct ways.
The organization of behaviorally relevant neural circuits persists throughout the life of many songbirds. Even after the majority of the brain is constructed, neurosteroids can masculinize song system components in juvenile zebra finches. In adults, steroids clearly promote neural plasticity, but the evidence that steroids synthesized within the brain impact adult song system organization is less certain in the zebra finch, though better supported in other songbirds. It may be that in adult zebra finches, the role of neurosteroids shifts from being primarily organizational to largely modulatory of neural circuit function in healthy animals or inducible when neural plasticity is required after damage.
Core Principle 3: Neurosteroidogenesis and non-traditional modes of action
Background
The capacity for the adult songbird brain to synthesize neurosteroids is not limited to the injured brain or the ventricular region. In fact, StAR, CYP11A1, 3β-HSD, CYP17, 17β-HSD and aromatase are expressed throughout the adult zebra finch brain (Figure 5). All of the song control nuclei themselves express at least a subset of these factors [95; 101; 107; 114]. Generally, the finding of steroidogenic factors within major song nuclei implicate neurosteroids as integral signals in the modulation of song production or auditory processing; specific predictions of how neurosteroids impact each nucleus have been presented elsewhere [114] but have yet to be tested.
Figure 5.
Schematic showing StAR, CYP11A1, 3β-HSD, CYP17, 17βHSD, and aromatase expression patterns in and around the adult male zebra finch song control circuit, based on results of examining somata only (not including projections). Most steroidogenic enzymes and StAR are expressed within the major telencephalic song nuclei; aromatase is largely expressed in cell bodies located outside of these areas, but is found within synaptic terminals contained with song nuclei. These data suggest that neurosteroids could continue to influence the function of the song control circuit into adulthood, and that each brain area could be impacted by a distinct mixture of neurosteroids.
Since the most detailed information is currently available for aromatase and estrogen-mediated actions in songbirds, the focus in this section will be on aromatase and testosterone/estradiol actions. As discussed above, the distribution of aromatase in the songbird brain is region-dependent (Figure 5), which suggests that local concentrations of estrogens can be modulated within circuits involved in complex behaviors such as song, audition, learning and courtship.
Adding to the complexity of how neurosteroids might act on the song system and other brain areas is the fact that there are regions of the songbird brain that exhibit rich expression of steroidogenic enzymes, but little or no ‘classical’ nuclear steroid receptors. Perhaps the most glaring example is the NCM region, which has abundant aromatase, but a paucity of nuclear estrogen receptors – though it does express nuclear androgen receptors [[49]]. Similarly, in the zebra finch HVC, cells that express estrogen receptors are virtually undetectable, restricted only to a few cells in medial HVC [48; 49], but there are detectable levels of aromatase expression and activity. This appears to reflect the presence of aromatase within presynaptic terminals, not cell bodies (see below). If the enzymatic activity of aromatase produces estradiol that acts locally within these brain areas, this strongly suggests the possibility of alternative mechanisms by which estradiol could act via binding to non-classical estrogen sites (e.g., ion channels), and requires consideration of the possibility that classical estrogen receptors might act through alternative, ligand-independent or non-estradiol mediated, mechanisms. Interestingly, in other songbirds, both aromatase and estrogen receptors are colocalized within HVC; perhaps the zebra finch has additional or alternative steroid signaling pathways not utilized in these other birds.
In the traditional mode-of-action, steroids must first bind to intracellular receptors, then translocate to the nucleus, then regulate gene expression, and finally affect changes in protein production and regulation. These combined processes take place on the order of hours to days in most cases. By contrast, the time course of acute changes in steroid levels and steroid production can occur on the order of seconds to minutes. If the temporal precision of steroid-encoded information is important in the regulation neural circuits, there must be alternative ‘rapid’ modes of neurosteroid action. Non-traditional (e.g. nongenomic) sites-of-action within the avian brain are poorly understood, but their existence have been suggested by the acute behavioral actions of steroids [159; 160; 161].
Steroid production in synaptic terminals
In mammals, aromatase is found in both soma as well as within synaptic terminals of neurons specifically within hypothalamus [162] and hippocampus [163]. This cellular compartmentalization suggests that aromatase could serve different modulatory purposes when it is active in the neuronal cell body versus at the synapse. In zebra finch brain, aromatase is also contained in synaptic terminals of the forebrain including hippocampus, and in the song system (HVC and NCM) [101]. For biochemical analysis, the subcellular compartments can be separated via centrifugation into a microsomal fraction (somal aromatase) and a synaptosomal fraction (synaptic aromatase). Subcellular fractions from avian brain have been analyzed for biochemical activity of the aromatase enzyme [164]. In adult zebra finches, aromatase activity within the posterior telencephalon is higher in males than in females, and this sex difference in aromatase activity is most pronounced in synaptosomes [165]. This sex difference may solely reflect a previously-described sex difference in fibers that contain aromatase within NCM [95], but it is intriguing to consider that the sex difference in the posterior telencephalon could occur within HVC, and therefore relate to the sex difference in song production.
A subsequent set of biochemistry experiments set out to test the proposed link between singing and synaptic aromatase [166]. Male zebra finches were individually exposed to females for 30 minutes and were divided into two groups based on their behavior. Males that sang at least one song bout during the 30-minute trial (‘singers’) had elevated aromatase activity in the posterior telencephalon as compared to males that did not sing during the 30 minute trial (‘nonsingers’). This experiment was repeated, and the elevated aromatase activity in singers was localized specifically to synaptic terminals in the posterior telencephalon [166]. Playback of zebra finch song vs. white noise did not elicit differential changes in aromatase activity in synaptic terminals, indicating that the link between song production and aromatase activity is not exclusively dependent on auditory self-stimulation. It therefore appears that courtship singing involves an up-regulation of aromatase activity specifically in synaptic terminals (Figure 6). It remains to be seen whether synaptosomal aromatase is rapidly upregulated during singing behavior, or is constitutively upregulated only in males that choose to sing (‘prior condition’ hypothesis, see [167]). However, the localization of aromatase to pre-synaptic terminals suggests a dynamic mode of action for locally-produced estradiol that could be modulated by rapid events in the neuronal synapse.
Figure 6.
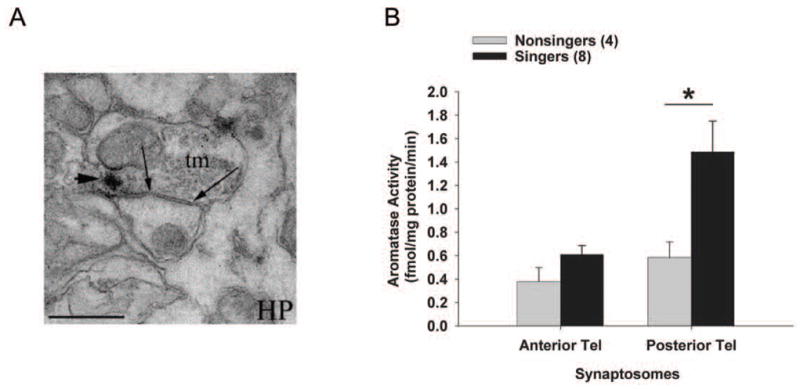
Synaptosomal aromatase expression and activity in adult zebra finches. Ultraphotomicrograph in (A) shows aromatase immune-product (arrowhead) expressed in presynaptic terminals (tm; arrows) of the zebra finch hippocampus (HP). Similar results were obtained in song nuclei, such as HVC (picture from [92]). Scale bar = 1 μm. The biochemical activity of aromatase (B) specifically within synaptosomes is elevated in males that have been singing vs. nonsingers after 30 min. Singers have higher synaptic aromatase activity is only within the posterior telencephalon (containing song and auditory nuclei, see Fig. 5), and not within the anterior telencephalon (* p = 0.008). Data from [166].
The above results for synaptic aromatase are from posterior telencephalon, which contains both auditory and motor components of the song system. Moreover, the mechanisms for acute changes in synaptic aromatase activity are unclear. Several classes of modulatory neurotransmitters are present in the song system, most notably catecholamines [168; 169; 170; 171]. Synaptic aromatase activity is therefore most likely regulated by acute interactions between steroids and modulatory neurotransmitters. Aromatase is colocalized with NMDA-receptors [172] and both aromatase enzyme activity [173] and forebrain estradiol levels (see below) are acutely regulated by glutamate. The most concrete evidence for acute regulation of the aromatase protein comes from quail hypothalamic in vitro slices. Changes in aromatase activity are regulated via rapid Ca++-dependent phosphorylation events in vitro [175] and have been linked to the expression of copulatory behavior in vivo in Japanese quail [160; 174]. It remains to be seen whether comparable acute mechanisms for steroidogenic enzyme activity occur in or around the avian song system.
Rapid changes in neurosteroids
Despite the overwhelming evidence for rapid (seconds-to-minutes) effects of estrogens and other steroids on neuronal and neural circuit activity in vertebrate systems [176; 177; 178; 179], there is no evidence to date for rapid actions of steroids on neural circuits in the avian song system. In addition to the work in quail mentioned above, the concentration of progesterone and the activity of 3β-HSD within the diencephalon are both increased in brooding males versus non-breeding male quail [180] though it is unclear whether this is mediated over a rapid timescale. Estradiol exerts rapid effects on the biochemical activity of steroidogenic enzymes, including 3β-HSD, in zebra finch telencephalic tissue [181] but whether this occurs specifically within the song system is unknown.
The activity of 3β-HSD was shown to be rapidly responsive to stress in the telencephalon of zebra finches [182]. Baseline 3β-HSD activity was found to be lower in the brain of male versus female zebra finches, especially within the telencephalon. This sex difference was reversed following a brief period of stress (10 minutes), such that males showed higher 3β-HSD activity than females following acute stress. Collectively, the time course of these changes in enzyme activity (< 30 minutes) indicated that local concentrations of steroids can shift in a region-specific manner over the course of minutes in the songbird brain.
The recent advancement of neurosteroid microdialysis allows unprecedented precision in determining the local regulation of steroids within specific brain regions in awake animals. Microdialysis relies on passive diffusion of in vivo neurochemicals across an implanted micro-membrane into fluid that can then be analyzed for time-dependent changes. Using a recently-adapted system for neurosteroid microdialysis, it was observed that a forebrain circuit in zebra finches exhibits acute changes in local steroid concentrations in vivo [183]. The presence of the neurosteroid estradiol in the brain of males was verified using highly-sensitive gas chromatography/mass spectrometry. The passive diffusion of steroids across the dialysis membrane was confirmed using in vitro methods with radiolabelled estradiol. When microdialyzed males were exposed to females in an adjacent cage for 30 minutes, local estradiol levels increased two-fold within NCM, while local testosterone levels remained unchanged (steroid levels were quantified in microdialysates via optimized ELISAs). Males were observed singing courtship song during these female presentation trials, and local estradiol levels then returned to baseline immediately during the 30 minute period after the females were removed. Furthermore, during brief periods of auditory activation, forebrain estradiol levels increased and testosterone levels decreased within NCM when males were exposed to other males’ song, but not white noise or female chirps (Table 1). These rapid fluctuations were specific to the forebrain NCM region, as they occurred independently from peripheral steroid changes. Rapid changes in steroid levels outside of the forebrain were not measured, but local fluctuations in forebrain steroids were also exceedingly restricted, as acute neurosteroid changes that occurred within the auditory NCM region were not observed in adjacent forebrain areas outside the NCM region [183].
Table 1.
Summary of results from in vivo neurosteroid microdialysis experiments in male zebra finches. Up-arrows and down-arrows indicate significant increases and decreases, respectively, in local steroid levels, as measured by ELISA. Neurosteroids were measured in an auditory region, the caudo-medial nidopallium (NCM) in response to behavioral manipulations (interactions with females or song playback) or in response to reverse-microdialysis (retrodialysis) of neurotransmitters. Data based on results from [166]
| Local steroid | Social interactions | Song playback | Glutamate retrodialysis | GABA retrodialysis | NMDA retrodialysis |
|---|---|---|---|---|---|
| 17-β-Estradiol | ↑ | ↑ | ↓ | ↔ | ↔ |
| Testosterone | ↔ | ↓ | ↔ | ↑ | ↔ |
The acute regulation of forebrain steroid levels, as measured by in vivo microdialysis, and forebrain steroidogenic enzyme activity, together provide a direct impetus to revise our thinking of how steroids act and are regulated within the brain. Instead of being a passive target of seasonal or developmental changes in circulating steroids, the avian song system should now be considered a source of steroids that change on a moment-by-moment basis. Moreover, these and other findings make it difficult to categorize sex steroids like estradiol as purely ‘masculinizing’ or ‘feminizing’ agents acting through intranuclear receptors, since they can be involved in momentary alterations in neural circuit function after gross sex-specific organization has occurred.
Neurotransmitter regulation of neurosteroids
The proximate mechanisms for acute regulation of steroids within forebrain circuits are poorly understood, especially in the avian song system. However, the advent of in vivo microdialysis has provided a means to explore the regulation of local steroid levels by modulatory neurotransmitters. Neurotransmitters such as glutamate and GABA were delivered via retrodialysis (reverse delivery through the microdialysis system) to NCM, and subsequent effects on local steroid levels were monitored [183]. The excitatory amino acid transmitter glutamate significantly and acutely suppressed the levels of estradiol, while having no detectable effects on local testosterone levels. Conversely, infusion of inhibitory transmitter GABA caused robust increases in local testosterone levels, while having no detectable effects on local estradiol levels (Table 1). Therefore, there appear to be differential, neurotransmitter-dependent mechanisms for regulating local steroid levels in the forebrain, and these mechanisms could allow a good deal of precision in activating local androgen-versus estrogen-mediated signaling pathways. The divergent control mechanisms of androgens versus estrogens could be important in light of the observations that, in songbirds, estradiol is associated with increases in neuronal excitability and song system plasticity, while androgens are associated with decreased excitability (i.e. 5β-reduced androgens) and ‘crystallization’ of the song system [184; 185; 186; 187]. These effects could be mediated by either classical or non-classical mechanisms, as there are androgen receptors present in NCM [49].
The increases in local forebrain testosterone levels in response to GABA retrodialysis suggests two novel mechanisms. GABA could inhibit the activity of local testosterone-metabolizing enzymes, such as 5α-reductase, and thereby secondarily cause a ‘buildup’ of local testosterone. Alternatively, GABA could increase the activity of testosterone-synthesizing enzymes, such as CYP17, which is expressed in the avian song system [107]. Further experiments are required to distinguish among these possibilities, but it is intriguing to note that testosterone appears to be a neurosteroid, synthesized by enzymes present and active in the songbird brain [103; 104; 105; 106; 107; 109; 111; 112; 113; 114; 115], not simply a gonadally-synthesized steroid. Moreover, applying neurosteroid microdialysis to juvenile songbirds during critical song-learning periods could identify acute, modulatory actions for brain steroids on song pathways, that might facilitate their functional masculine development.
Conclusions
The brain has traditionally been considered a passive target of steroids synthesized within the gonads and adrenals. In this paradigm, the primary role for sex steroids was in regulating brain structures that controlled sex-specific reproductive behaviors. Here, we described how this view of steroid production is limited, focusing on evidence from the song control circuit and related brain areas in songbirds.
From this model system, it has become clear that it is difficult to neatly categorize the sources and actions of steroids into existing “Core Principles” as described in the Introduction. Investigations with songbirds have shown that the brain itself can synthesize a variety of steroids independently from the periphery, that the role of steroids is much broader than in the control of reproduction, that steroid effects on brain morphology are not restricted to developmental life stages, that steroids can be rapidly synthesized in response to experience, and that steroids can influence multiple cellular functions by virtue of being synthesized in different subcellular compartments. It is hard to understate the implications of these findings. They demonstrate that neurosteroids potentially impact numerous functions of the brain throughout life, with measurable effects on behavior; neurosteroids in the songbird do not merely tweak the system, they could shape, re-shape, and modulate it with exquisite specificity. The greatest advantage to the capacity to synthesize steroids within the brain is the ability to regulate the mix and concentration of steroids in defined populations of cells at precise times.
Although we are far from a comprehensive understanding how neurosteroids influence the avian song system, songbirds have provided powerful evidence of how neurosteroid mechanisms can directly impact the brain, and have paved a path towards understanding the role of steroids in the brains of all animals.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Arnold AP, Gorski RA. Gonadal steroid induction of structural sex differences in the central nervous system. Ann Rev Neurosci. 1984;7:413–42. doi: 10.1146/annurev.ne.07.030184.002213. [DOI] [PubMed] [Google Scholar]
- 2.Ball GF. Neuroendocrine basis of seasonal changes in vocal behavior among songbirds. In: Hauser M, Konishi M, editors. Neural Mechanisms of Communication. MIT Press; Cambridge, MA: 2000. pp. 213–253. [Google Scholar]
- 3.Ball GF, Riters LV, Balthazart J. Neuroendocrinology of song behavior and avian brain plasticity: Multiple sites of action of sex steroid hormones. Frontiers in Neuroendocrinology. 2002;23:137–178. doi: 10.1006/frne.2002.0230. [DOI] [PubMed] [Google Scholar]
- 4.Balthazart J, Ball GF. Sexual differentiation of brain and behavior in birds. Trends Endocrinol Metab. 1995;6:21–29. doi: 10.1016/1043-2760(94)00098-o. [DOI] [PubMed] [Google Scholar]
- 5.Bottjer SW, Johnson F. Circuits, hormones, and learning: vocal behavior in songbirds. J Neurobiol. 1997;33:602–18. doi: 10.1002/(sici)1097-4695(19971105)33:5<602::aid-neu8>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 6.Fusani L, Gahr M. Hormonal influence on song structure and organization: the role of estrogen. Neurosci. 2006;138 doi: 10.1016/j.neuroscience.2005.08.041. [DOI] [PubMed] [Google Scholar]
- 7.Schlinger BA, Brenowitz EA. Neural and hormonal control of birdsong. In: Pfaff DW, Arnold AP, Etgen AM, Fahrbach SE, Rubin RT, editors. Hormones, Brain and Behavior. Academic Press; Amsterdam: 2002. pp. 799–839. [Google Scholar]
- 8.Wingfield JC, Farner D. Endocrinology of Reproduction in Wild Species. In: Farner DS, King JR, Parkes KC, editors. Avian Biology. Academic Press; New York: 1993. pp. 163–327. [Google Scholar]
- 9.Marler P. Three models of song learning: evidence from behavior. Journal of Neurobiology. 1997;33:501–16. [PubMed] [Google Scholar]
- 10.Brenowitz EA, Kroodsma DE. The neuroethology of birdsong. In: Kroodsma DE, Miller EH, editors. Ecology and Evolution of Acoustic Communication in Birds. Cornell University Press; Ithaca: 1996. pp. 269–281. [Google Scholar]
- 11.Catchpole CK. The evolution of bird sounds in relation to mating and spacing behavior. In: Kroodsma DEMEH, Ouellet H, editors. Acoustic Communication in Birds. Academic Press; New York: 1982. pp. 297–319. [Google Scholar]
- 12.Schlinger B, Brenowitz EA. Neural and Hormonal Control of Birdsong. In: Pfaff DW, editor. Hormones, Brain and Behavior. Elsevier; 2009. pp. 799–838. [Google Scholar]
- 13.Suthers RA, Goller F, Pytte C. The neuromuscular control of birdsong. Proc Roy Soc, London B. 1999;354:927–39. doi: 10.1098/rstb.1999.0444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gahr M. Neural song control system of hummingbirds: comparison to swifts, vocal learning (Songbirds) and nonlearning (Suboscines) passerines, and vocal learning (Budgerigars) and nonlearning (Dove, owl, gull, quail, chicken) nonpasserines. J Comp Neurol. 2000;426:182–96. [PubMed] [Google Scholar]
- 15.Jarvis ED, Ribeiro S, da Silva ML, Ventura D, Vielliard J, Mello CV. Behaviourally driven gene expression reveals song nuclei in hummingbird brain. Nature. 2000;406:628–32. doi: 10.1038/35020570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Streidter G. The vocal control pathways in budgerigars differ from those in songbirds. J Comp Neurol. 1994;343:35–56. doi: 10.1002/cne.903430104. [DOI] [PubMed] [Google Scholar]
- 17.Arnold AP. Sex chromosomes and brain gender. Nat Rev Neurosci. 2004;5:701–708. doi: 10.1038/nrn1494. [DOI] [PubMed] [Google Scholar]
- 18.Wade J, Arnold AP. Sexual differentiation of the zebra finch song system. Ann N Y Acad Sci. 2004;1016:540–59. doi: 10.1196/annals.1298.015. [DOI] [PubMed] [Google Scholar]
- 19.Nottebohm F, Arnold AP. Sexual dimorphism in vocal control areas of the songbird brain. Science. 1976;194:211–3. doi: 10.1126/science.959852. [DOI] [PubMed] [Google Scholar]
- 20.Brenowitz EA. Plasticity of the adult avian song control system. Ann N Y Acad Sci. 2004;1016:560–85. doi: 10.1196/annals.1298.006. [DOI] [PubMed] [Google Scholar]
- 21.Nottebohm F. The King Solomon Lectures in Neuroethology. A white canary on Mount Acropolis. J Comp Physiol [A] 1996;179:149–56. doi: 10.1007/BF00222782. [DOI] [PubMed] [Google Scholar]
- 22.Nottebohm F. Behavioral Neurobiology of Birdsong. 2004. The road we traveled - Discovery, choreography, and significance of brain replaceable neurons; pp. 628–658. [DOI] [PubMed] [Google Scholar]
- 23.Tramontin AD, Brenowitz EA. Seasonal plasticity in the adult brain. Trends Neurosci. 2000;23:251–8. doi: 10.1016/s0166-2236(00)01558-7. [DOI] [PubMed] [Google Scholar]
- 24.Payne AH, Hales DB. Overview of steroidogenic enzymes in the pathway from cholesterol to active steroid hormones. Endocr Revs. 2004;25:947–970. doi: 10.1210/er.2003-0030. [DOI] [PubMed] [Google Scholar]
- 25.Culty M, Luo L, Yao ZX, Chen H, Papadopoulos V, Zirkin BR. Cholesterol transport, peripheral benzodiazepine receptor, and steroidogenesis in aging Leydig cells. J Androl. 2002;23:439–47. [PubMed] [Google Scholar]
- 26.Hauet T, Yao ZX, Bose HS, Wall CT, Han Z, Li W, Hales DB, Miller WL, Culty M, Papadopoulos V. Peripheral-type benzodiazepine receptor-mediated action of steroidogenic acute regulatory protein on cholesterol entry into leydig cell mitochondria. Mol Endocrinol. 2005;19:540–54. doi: 10.1210/me.2004-0307. [DOI] [PubMed] [Google Scholar]
- 27.Miller WL. Steroidogenic acute regulatory protein (StAR), a novel mitochondrial cholesterol transporter. Biochim Biophys Acta. 2007;1771:663–76. doi: 10.1016/j.bbalip.2007.02.012. [DOI] [PubMed] [Google Scholar]
- 28.Miller WL. Mechanism of StAR’s regulation of mitochondrial cholesterol import. Mol Cell Endocrinol. 2007;265–266:46–50. doi: 10.1016/j.mce.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 29.Papadopoulos V, Amri H, Li H, Yao Z, Brown RC, Vidic B, Culty M. Structure, function and regulation of the mitochondrial peripheral-type benzodiazepine receptor. Therapie. 2001;56:549–56. [PubMed] [Google Scholar]
- 30.Papadopoulos V, Liu J, Culty M. Is there a mitochondrial signaling complex facilitating cholesterol import? Mol Cell Endocrinol. 2007;265–266:59–64. doi: 10.1016/j.mce.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 31.Papadopoulos V, Widmaier EP, Amri H, Zilz A, Li H, Culty M, Castello R, Philip GH, Sridaran R, Drieu K. In vivo studies on the role of the peripheral benzodiazepine receptor (PBR) in steroidogenesis. Endocr Res. 1998;24:479–87. doi: 10.3109/07435809809032636. [DOI] [PubMed] [Google Scholar]
- 32.Stocco DM, Clark BJ. Regulation of acute production of steroids in steroidogenic cells. Endocr Revs. 1996;17:221–244. doi: 10.1210/edrv-17-3-221. [DOI] [PubMed] [Google Scholar]
- 33.Sanderson JT. The steroid hormone biosynthesis pathway as a target for endocrine-disrupting chemicals. Toxicol Sci. 2006;94:3–21. doi: 10.1093/toxsci/kfl051. [DOI] [PubMed] [Google Scholar]
- 34.Vanden Bossche HV, Moereels H, Koymans LM. Aromatase inhibitors--mechanisms for non-steroidal inhibitors. Breast Cancer Res Treat. 1994;30:43–55. doi: 10.1007/BF00682740. [DOI] [PubMed] [Google Scholar]
- 35.Baillien M, Balthazart J. A direct dopaminergic control of aromatase activity in the quail preoptic area. J Ster Biochem Mol Biol. 1997;63:99–113. doi: 10.1016/s0960-0760(97)00080-0. [DOI] [PubMed] [Google Scholar]
- 36.Baulieu EE, Robel P, Schumacher M. Neurosteroids: Beginning of the story. Neurosteroids and Brain Function. 2001:1–32. doi: 10.1016/s0074-7742(01)46057-0. [DOI] [PubMed] [Google Scholar]
- 37.Corpechot C, Robel P, Axelson M, Sjovall J, Baulieu EE. Characterization and measurement of dehydroepiandrosterone sulfate in rat brain. Proc Natl Acad Sci U S A. 1981;78:4704–7. doi: 10.1073/pnas.78.8.4704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Corpechot C, Synguelakis M, Talha S, Axelson M, Sjovall J, Vihko R, Baulieu EE, Robel P. Pregnenolone and its sulfate ester in the rat brain. Brain Res. 1983;270:119–25. doi: 10.1016/0006-8993(83)90797-7. [DOI] [PubMed] [Google Scholar]
- 39.Compagnone NA, Mellon SH. Neurosteroids: Biosynthesis and function of these novel neuromodulators. Front Neuroendocrinol. 2000;21:1–56. doi: 10.1006/frne.1999.0188. [DOI] [PubMed] [Google Scholar]
- 40.Mensah-Nyagan AG, Do-Rego JL, Beaujean D, Luu-The V, Pelletier G, Vaudry H. Neurosteroids: expression of steroidogenic enzymes and regulation of steroid biosynthesis in the central nervous system. Pharmacol Rev. 1999;51:63–81. [PubMed] [Google Scholar]
- 41.Tsutsui K, Matsunaga M, Miyabara H, Ukena K. Neurosteroid biosynthesis in the quail brain: A review. J Exp Zool A. 2006;305A:733–742. doi: 10.1002/jez.a.302. [DOI] [PubMed] [Google Scholar]
- 42.Fusani L, Hutchison JB, Gahr M. Testosterone regulates the activity and expression of aromatase in the canary neostriaturn. J Neurobio. 2001;49:1–8. doi: 10.1002/neu.1061. [DOI] [PubMed] [Google Scholar]
- 43.Vockel A, Prove E, Balthazart J. Effects of Castration and Testosterone Treatment on the Activity of Testosterone-Metabolizing Enzymes in the Brain of Male and Female Zebra Finches. J Neurobio. 1990;21:808–825. doi: 10.1002/neu.480210514. [DOI] [PubMed] [Google Scholar]
- 44.Arnold AP. Quantitative analysis of sex differences in hormone accumulation in the zebra finch brain: methodological and theoretical issues. J Comp Neurol. 1980;189:421–36. doi: 10.1002/cne.901890302. [DOI] [PubMed] [Google Scholar]
- 45.Bernard DJ, Bentley GE, Balthazart J, Turek FW, Ball GF. Androgen receptor, estrogen receptor alpha, and estrogen receptor beta show distinct patterns of expression in forebrain song control nuclei of European starlings. Endocrinol. 1999;140:4633–43. doi: 10.1210/endo.140.10.7024. [DOI] [PubMed] [Google Scholar]
- 46.Brenowitz EA, Arnold AP, Loesche P. Steroid accumulation in song nuclei of a sexually dimorphic duetting bird, the rufous and white wren. J Neurobiol. 1996;31:235–44. doi: 10.1002/(SICI)1097-4695(199610)31:2<235::AID-NEU8>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 47.Gahr M, Metzdorf R. Distribution and dynamics in the expression of androgen and estrogen receptors in vocal control systems of songbirds. Brain Res Bull. 1997;44:509–517. doi: 10.1016/s0361-9230(97)00233-5. [DOI] [PubMed] [Google Scholar]
- 48.Jacobs EC, Arnold AP, Campagnoni AT. Zebra finch estrogen receptor cDNA: cloning and mRNA expression. J Ster Biochem Mol Biol. 1996;59:135–45. doi: 10.1016/s0960-0760(96)00096-9. [DOI] [PubMed] [Google Scholar]
- 49.Metzdorf R, Gahr M, Fusani L. Distribution of aromatase, estrogen receptor, and androgen receptor mRNA in the forebrain of songbirds and nonsongbirds. J Comp Neurol. 1999;407:115–29. [PubMed] [Google Scholar]
- 50.Riters LV, Ball GF. Lesions to the medial preoptic area affect singing in the male European starling (Sturnus vulgaris) Horm Behav. 1999;36:276–86. doi: 10.1006/hbeh.1999.1549. [DOI] [PubMed] [Google Scholar]
- 51.Riters LV, Alger SJ. Neuroanatomical evidence for indirect connections between the medial preoptic nucleus and the song control system: possible neural substrates for sexually motivated song. Cell Tissue Res. 2004;316:35–44. doi: 10.1007/s00441-003-0838-6. [DOI] [PubMed] [Google Scholar]
- 52.Appeltants D, Absil P, Balthazart J, Ball GF. Identification of the origin of catecholaminergic inputs to HVc in canaries by retrograde tract tracing combined with tyrosine hydroxylase immunocytochemistry. J Chem Neuroanat. 2000;18:117–33. doi: 10.1016/s0891-0618(99)00054-x. [DOI] [PubMed] [Google Scholar]
- 53.Appeltants D, Ball GF, Balthazart J. The origin of catecholaminergic inputs to the song control nucleus RA in canaries. Neuroreport. 2002;13:649–53. doi: 10.1097/00001756-200204160-00023. [DOI] [PubMed] [Google Scholar]
- 54.Bottjer SW. Ontogenetic changes in the pattern of androgen accumulation in song- control nuclei of male zebra finches [published erratum appears in J Neurobiol 1987 Jul;18(4):391] J Neurobiol. 1987;18:125–39. doi: 10.1002/neu.480180202. [DOI] [PubMed] [Google Scholar]
- 55.Bottjer SW, Glaessner SL, Arnold AP. Ontogeny of brain nuclei controlling song learning and behavior in zebra finches. J Neurosci. 1985;5:1556–62. doi: 10.1523/JNEUROSCI.05-06-01556.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bottjer SW, Miesner EA, Arnold AP. Changes in neuronal number, density and size account for increases in volume of song-control nuclei during song development in zebra finches. Neurosci Letts. 1986;67:263–8. doi: 10.1016/0304-3940(86)90319-8. [DOI] [PubMed] [Google Scholar]
- 57.Burek MJ, Nordeen KW, Nordeen EJ. Ontogeny of sex differences among newly-generated neurons of the juvenile avian brain. Dev Brain Res. 1994;78:57–64. doi: 10.1016/0165-3806(94)90009-4. [DOI] [PubMed] [Google Scholar]
- 58.Burek MJ, Nordeen KW, Nordeen EJ. Initial sex differences in neuron growth and survival within an avian song nucleus develop in the absence of afferent input. J Neurobiol. 1995;27:85–96. doi: 10.1002/neu.480270109. [DOI] [PubMed] [Google Scholar]
- 59.Burek MJ, Nordeen KW, Nordeen EJ. Estrogen promotes neuron addition to an avian song-control nucleus by regulating post-mitotic events. Dev Brain Res. 1995;85:220–4. doi: 10.1016/0165-3806(94)00215-l. [DOI] [PubMed] [Google Scholar]
- 60.Burek MJ, Nordeen KW, Nordeen EJ. Sexually dimorphic neuron addition to an avian song-control region is not accounted for by sex differences in cell death. J Neurobiol. 1997;33:61–71. [PubMed] [Google Scholar]
- 61.Konishi M, Akutagawa E. Growth and atrophy of neurons labeled at their birth in a song nucleus of the zebra finch. Proc Natl Acad Sci U S A. 1990;87:3538–41. doi: 10.1073/pnas.87.9.3538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nordeen EJ, Nordeen KW. Sex and regional differences in the incorporation of neurons born during song learning in zebra finches. J Neurosci. 1988;8:2869–74. doi: 10.1523/JNEUROSCI.08-08-02869.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gurney M, Konishi M. Hormone-induced sexual differentiation of brain and behavior in zebra finches. Science. 1980;208:1380–1383. doi: 10.1126/science.208.4450.1380. [DOI] [PubMed] [Google Scholar]
- 64.Gurney ME. Hormonal control of cell form and number in the zebra finch song system. J Neurosci. 1981;1:658–73. doi: 10.1523/JNEUROSCI.01-06-00658.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Holloway CC, Clayton DE. Estrogen synthesis in the male brain triggers development of the avian song control pathway in vitro. Nat Neurosci. 2001;4:170–175. doi: 10.1038/84001. [DOI] [PubMed] [Google Scholar]
- 66.Alvarez-Buylla A, Nottebohm F. Migration of young neurons in adult avian brain. Nature. 1988;335:353–4. doi: 10.1038/335353a0. [DOI] [PubMed] [Google Scholar]
- 67.Goldman SA, Nottebohm F. Neuronal production, migration, and differentiation in a vocal control nucleus of the adult female canary brain. Proc Natl Acad Sci U S A. 1983;80:2390–4. doi: 10.1073/pnas.80.8.2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Alvarez-Buylla A, Theelen M, Nottebohm F. Proliferation “hot spots” in adult avian ventricular zone reveal radial cell division. Neuron. 1990;5:101–9. doi: 10.1016/0896-6273(90)90038-h. [DOI] [PubMed] [Google Scholar]
- 69.Grisham W, Arnold AP. A direct comparison of the masculinizing effects of testosterone, androstenedione, estrogen, and progesterone on the development of the zebra finch song system. J Neurobio. 1995;26:163–70. doi: 10.1002/neu.480260202. [DOI] [PubMed] [Google Scholar]
- 70.Grisham W, Lee J, Park SH, Mankowski JL, Arnold AP. A dose-response study of estradiol’s effects on the developing zebra finch song system. Neurosci Lett. 2008;445:158–61. doi: 10.1016/j.neulet.2008.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Konishi M, Akutagawa E. Hormonal control of cell death in a sexually dimorphic song nucleus in the zebra finch. Ciba Found Symp. 1987;126:173–85. doi: 10.1002/9780470513422.ch11. [DOI] [PubMed] [Google Scholar]
- 72.Simpson HB, Vicario DS. Early estrogen treatment of female zebra finches masculinizes the brain pathway for learned vocalizations. J Neurobio. 1991;22:777–93. doi: 10.1002/neu.480220711. [DOI] [PubMed] [Google Scholar]
- 73.Adkins-Regan E, Mansukhani V, Seiwert C, Thompson R. Sexual differentiation of brain and behavior in the zebra finch: critical periods for effects of early estrogen treatment. J Neurobio. 1994;25:865–77. doi: 10.1002/neu.480250710. [DOI] [PubMed] [Google Scholar]
- 74.Simpson HB, Vicario DS. Early estrogen treatment alone causes female zebra finches to produce learned, male-like vocalizations. J Neurobio. 1991;22:755–76. doi: 10.1002/neu.480220710. [DOI] [PubMed] [Google Scholar]
- 75.Gahr M, Metzdorf R. The sexually dimorphic expression of androgen receptors in the song nucleus hyperstriatilis ventrale pars caudale of the zebra finch develops independently of gonadal steroids. J Neurosci. 1999;19:2628–2636. doi: 10.1523/JNEUROSCI.19-07-02628.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kim YH, Perlma WR, Arnold AP. Expression of androgen receptor mRNA in zebra finch song system: developmental regulation by estrogen. J Comp Neurol. 2004;469:535–547. doi: 10.1002/cne.11033. [DOI] [PubMed] [Google Scholar]
- 77.Adkins-Regan E, Abdelnabi M, Mobarak M, Ottinger MA. Sex steroid levels in developing and adult male and female zebra finches (Poephila guttata) Gen Comp End. 1990;78:93–109. doi: 10.1016/0016-6480(90)90051-m. [DOI] [PubMed] [Google Scholar]
- 78.Hutchison JB, Wingfield JC, Hutchison RE. Sex differences in plasma concentrations of steroids during the sensitive period for brain differentiation in the zebra finch. J Endocrinol. 1984;103:363–9. doi: 10.1677/joe.0.1030363. [DOI] [PubMed] [Google Scholar]
- 79.Schlinger BA, Arnold AP. Plasma Sex Steroids and Tissue Aromatization in Hatchling Zebra Finches - Implications For the Sexual Differentiation of Singing Behavior. Endocrinol. 1992;130:289–299. doi: 10.1210/endo.130.1.1727704. [DOI] [PubMed] [Google Scholar]
- 80.Freking F, Nazairians T, Schlinger BA. The expression of the sex steroid-sythesizing enzymes CYP11A1, 3β-HSD, CYP17, and CYP 19 in gonads and adrenals of adult and developing zebra finches. Gen Comp Endocrinol. 2000;119:140–151. doi: 10.1006/gcen.2000.7503. [DOI] [PubMed] [Google Scholar]
- 81.Schlinger BA, Arnold AP. Brain Is the Major Site of Estrogen Synthesis in a Male Songbird. Proc Nat Acad Sci U S A. 1991;88:4191–4194. doi: 10.1073/pnas.88.10.4191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Schlinger BA, Arnold AP. Circulating estrogens in a male songbird originate in the brain. Proc Nat Acad Sci USA. 1992;89:7650–3. doi: 10.1073/pnas.89.16.7650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Arnold AP, Burgoyne PS. Are XX and XY brain cells intrinsically different? Trends Endocrinol Metab. 2004;15:6–11. doi: 10.1016/j.tem.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 84.Arnold AP, Rissman EF, De Vries GJ. Two perspectives on the origin of sex differences in the brain. Ann N Y Acad Sci. 2003;1007:176–88. doi: 10.1196/annals.1286.018. [DOI] [PubMed] [Google Scholar]
- 85.Arnold AP, Xu J, Grisham W, Chen X, Kim YH, Itoh Y. Minireview: Sex chromosomes and brain sexual differentiation. Endocrinol. 2004;145:1057–62. doi: 10.1210/en.2003-1491. [DOI] [PubMed] [Google Scholar]
- 86.Arnold AP, Itoh Y, Melamed E. A bird’s-eye view of sex chromosome dosage compensation. Annu Rev Hum Genet. 2008;9:109–27. doi: 10.1146/annurev.genom.9.081307.164220. [DOI] [PubMed] [Google Scholar]
- 87.Itoh Y, Melamed E, Yang X, Kampf K, Wang S, Yehya N, Van Nas A, Replogle K, Band MR, Clayton DF, Schadt EE, Lusis AJ, Arnold AP. Dosage compensation is less effective in birds than in mammals. J Biol. 2007;6:2. doi: 10.1186/jbiol53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Agate RJ, Grisham W, Wade J, Mann S, Wingfield J, Schanen C, Palotie A, Arnold AP. Neural, not gonadal, origin of brain sex differences in a gynandromorphic finch. Proc Natl Acad Sci USA. 2003;100:4873–4878. doi: 10.1073/pnas.0636925100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Chen X, Agate RJ, Itoh Y, Arnold AP. Sexually dimorphic expression of trkB, a Z-linked gene, in early posthatch zebra finch brain. Proc Nat Acad Sci USA. 2005;102:7730–7735. doi: 10.1073/pnas.0408350102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Gahr M. Male Japanese quails with female brains do not show male sexual behaviors. Proc Natl Acad Sci U S A. 2003;100:7959–64. doi: 10.1073/pnas.1335934100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Naftolin F, Ryan KJ, Davies IJ, Reddy VV, Flores F, Petro Z, Kuhn M, White RJ, Takaoka Y, Wolin L. The formation of estrogens by central neuroendocrine tissues. Rec Prog Horm Res. 1975;31:295–319. doi: 10.1016/b978-0-12-571131-9.50012-8. [DOI] [PubMed] [Google Scholar]
- 92.Jacobs EC, Arnold AP, Campagnoni AT. Developmental regulation of the distribution of aromatase- and estrogen-receptor-mRNA-expressing cells in the zebra finch. Dev Neurosci. 1999;21:453–472. doi: 10.1159/000017413. [DOI] [PubMed] [Google Scholar]
- 93.Perlman WR, Arnold AP. Expression of estrogen receptor and aromatase mRNAs in embryonic and posthatch zebra finch brain. J Neurobio. 2003;55:204–219. doi: 10.1002/neu.10190. [DOI] [PubMed] [Google Scholar]
- 94.Ramachandran B, Schlinger BA, Arnold AP, Campagnoni AT. Zebra finch aromatase gene expression is regulated in the brain through an alternate promoter. Gene. 1999;240:209–216. doi: 10.1016/s0378-1119(99)00399-6. [DOI] [PubMed] [Google Scholar]
- 95.Saldanha CJ, Tuerk MJ, Kim YH, Fernandes AO, Arnold AP, Schlinger BA. Distribution and regulation of telencephalic aromatase expression in the zebra finch revealed with a specific antibody. J Comp Neurol. 2000;423:619–630. doi: 10.1002/1096-9861(20000807)423:4<619::aid-cne7>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 96.Schlinger BA, Arnold AP. Androgen effects on the development of the zebra finch song system. Brain Res. 1991;561:99–105. doi: 10.1016/0006-8993(91)90754-j. [DOI] [PubMed] [Google Scholar]
- 97.Shen P, Campagnoni CW, Kampf K, Schlinger BA, Arnold AP, Campagnoni AT. Isolation and characterization of a zebra finch aromatase cDNA: in situ hybridization reveals high aromatase expression in brain. Mol Brain Res. 1994;24:227–37. doi: 10.1016/0169-328x(94)90136-8. [DOI] [PubMed] [Google Scholar]
- 98.Vockel A, Pröve E, Balthazart J. Sex- and age-related differences in the activity of testosterone-metabolizing enzymes in microdissected nuclei of the zebra finch brain. Brain Res. 1990;511:291–302. doi: 10.1016/0006-8993(90)90174-a. [DOI] [PubMed] [Google Scholar]
- 99.Balthazart J, Foidart A, Surlemont C, Vockel A, Harada N. Distribution of aromatase in the brain of the Japanese quail, ring dove, and zebra finch: an immunocytochemical study. J Comp Neurol. 1990;301:276–88. doi: 10.1002/cne.903010210. [DOI] [PubMed] [Google Scholar]
- 100.Shen P, Schlinger BA, Campagnoni AT, Arnold AP. An Atlas of Aromatase Messenger-Rna Expression in the Zebra Finch Brain. J Comp Neurol. 1995;360:172–184. doi: 10.1002/cne.903600113. [DOI] [PubMed] [Google Scholar]
- 101.Peterson RS, Yarram L, Schlinger BA, Saldanha CJ. Aromatase is pre-synaptic and sexually dimorphic in the adult zebra finch brain. Proc Roy Soc B. 2005;272:2089–2096. doi: 10.1098/rspb.2005.3181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Grisham W, Mathews GA, Arnold AP. Local intracerebral implants of estrogen masculinize some aspects of the zebra finch song system. J Neurobio. 1994;25:185–96. doi: 10.1002/neu.480250209. [DOI] [PubMed] [Google Scholar]
- 103.Cam V, Schlinger BA. Activities of aromatase and 3beta-hydroxysteroid dehydrogenase/delta4-delta5 isomerase in whole organ cultures of tissues from developing zebra finches. Horm Behav. 1998;33:31–9. doi: 10.1006/hbeh.1998.1434. [DOI] [PubMed] [Google Scholar]
- 104.Freking F, Ramachandran B, Schlinger BA. Regulation of aromatase, 5 alpha- and 5 beta-reductase in primary cell cultures of developing zebra finch telencephalon. J Neurobiol. 1998;36:30–40. doi: 10.1002/(sici)1097-4695(199807)36:1<30::aid-neu3>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 105.Lane N, Grisham W, Brown S, Thompson L, Arnold A, Schlinger B. Plasma testosterone and tissue 17alpha-hydroxylase activity in castrated and fadrozole-treated male zebra finches. Soc Neurosci Absts. 1996;22 [Google Scholar]
- 106.Schlinger BA, Amur Umarjee S, Campagnoni AT, Arnold AP. 5 beta-reductase and other androgen-metabolizing enzymes in primary cultures of developing zebra finch telencephalon. J Neuroendocrinol. 1995;7:187–92. doi: 10.1111/j.1365-2826.1995.tb00746.x. [DOI] [PubMed] [Google Scholar]
- 107.London SE, Boulter J, Schlinger BA. Cloning of the zebra finch androgen synthetic enzyme CYP17: A study of its neural expression throughout posthatch development. J Comp Neurol. 2003;467:496–508. doi: 10.1002/cne.10936. [DOI] [PubMed] [Google Scholar]
- 108.London SE, Schlinger BA. Steroidogenic enzymes along the ventricular proliferative zone in the developing songbird brain. J Comp Neuro. 2007;502:507–521. doi: 10.1002/cne.21335. [DOI] [PubMed] [Google Scholar]
- 109.Schlinger BA, Vanson AM, Amur-Umjaree S, Camppagnoni A, Arnold AP. 3β-Hydroxysteroid dehydrogenase/isomerase activity in primary cultures from developing zebra finch telecephalon. Soc Neurosci Abst. 1992b;18:231. [Google Scholar]
- 110.Schlinger BA, Lane NI, Grisham W, Thompson L. Androgen synthesis in a songbird: A study of Cyp17 (17 alpha-hydroxylase/C17,20-lyase) activity in the zebra finch. Gen Comp Endocrinol. 1999;113:46–58. doi: 10.1006/gcen.1998.7179. [DOI] [PubMed] [Google Scholar]
- 111.Tam H, Schlinger BA. Activities of 3B-HSD and aromatase in slices of the adult and developing zebra finch brain. Gen Comp Endocrinol. 2007;150:26–33. doi: 10.1016/j.ygcen.2006.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Vanson A, Arnold AP, Schlinger BA. 3β-hydroxysteroid dehydrogenase/isomerase and aromatase activity in primary cultures of developing zebra finch telencephalon: Dehydroepiandrosterone as substrate for synthesis of androstenedione and estrogens. Gen Comp Endocrinol. 1996;102:342–350. doi: 10.1006/gcen.1996.0077. [DOI] [PubMed] [Google Scholar]
- 113.Wade J, Schlinger BA, Arnold AP. Aromatase and 5 beta-reductase activity in cultures of developing zebra finch brain: an investigation of sex and regional differences. J Neurobio. 1995;27:240–51. doi: 10.1002/neu.480270210. [DOI] [PubMed] [Google Scholar]
- 114.London S, Monks DA, Wade J, Schlinger BA. Widespread capacity for steroid synthesis in the avian brain and song system. Endocrinol. 2006;147:5975–5987. doi: 10.1210/en.2006-0154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.London SE, Itoh Y, Lance VA, Wise P, Ekanayake PS, Oyama R, Arnold AP, Schlinger BA. Neural expression and post-transcriptional dosage compensation of the steroid metabolic enzyme 17b-HSD type 4. doi: 10.1186/1471-2202-11-47. (submitted) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.DeWulf V, Bottjer SW. Age and sex differrences in mitotic activity within the zebra finch telencephalon. J Neurosci. 2002;22:4080–4094. doi: 10.1523/JNEUROSCI.22-10-04080.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.DeWulf V, Bottjer SW. Neurogenesis within the juvenile zebra finch telencephalic ventricular zone: a map of proliferative activity. J Comp Neurol. 2005;481:70–83. doi: 10.1002/cne.20352. [DOI] [PubMed] [Google Scholar]
- 118.Brown SD, Johnson F, Bottjer SW. Neurogenesis in adult canary telencephalon is independent of gonadal hormone levels. The Journal of neuroscience. 1993;13:2024–32. doi: 10.1523/JNEUROSCI.13-05-02024.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Nixdorf-Bergweiler BE. Divergent and parallel development in volume sizes of telencephalic song nuclei in male and female zebra finches. J Comp Neurol. 1996;375:445–56. doi: 10.1002/(SICI)1096-9861(19961118)375:3<445::AID-CNE7>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 120.Nixdorf-Bergweiler BE. Enlargement of neuronal somata in the LMAN coincides with the onset of sensorimotor learning for song. Neurobiol Learn Mem. 1998;69:258–73. doi: 10.1006/nlme.1998.3819. [DOI] [PubMed] [Google Scholar]
- 121.Wada H, Salvante KG, Stables C, Wagner E, Williams TD, Breuner CW. Adrenocortical responses in zebra finches (Taeniopygia guttata): individual variation, repeatability, and relationship to phenotypic quality. Horm Behav. 2008;53:472–80. doi: 10.1016/j.yhbeh.2007.11.018. [DOI] [PubMed] [Google Scholar]
- 122.Breuner CW, Orchinik M. Plasma binding proteins as mediators of corticosteroid action in vertebrates. J Endocrinol. 2002;175:99–112. doi: 10.1677/joe.0.1750099. [DOI] [PubMed] [Google Scholar]
- 123.Swett MB, Breuner CW. Interaction of testosterone, corticosterone and corticosterone binding globulin in the white-throated sparrow (Zonotrichia albicollis) Comp Biochem Physiol A Mol Integr Physiol. 2008;151:226–31. doi: 10.1016/j.cbpa.2008.06.031. [DOI] [PubMed] [Google Scholar]
- 124.Alvarez-Buylla A, Garcia-Verdugo JM. Neurogenesis in adult subventricular zone. Journal of Neuroscience. 2002;22:629–634. doi: 10.1523/JNEUROSCI.22-03-00629.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Alvarez-Buylla A, Garcia-Verdugo JM, Mateo AS, Merchant-Larios H. Primary neural precursors and intermitotic nuclear migration in the ventricular zone of adult canaries. Journal of Neuroscience. 1998;18:1020–1037. doi: 10.1523/JNEUROSCI.18-03-01020.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Alvarez-Buylla A, Kirn JR. Birth, migration, incorporation, and death of vocal control neurons in adult songbirds. J Neurobiol. 1997;33:585–601. [PubMed] [Google Scholar]
- 127.Nottebohm F. From bird song to neurogenesis. Sci Am. 1989;260:74–9. doi: 10.1038/scientificamerican0289-74. [DOI] [PubMed] [Google Scholar]
- 128.Nottebohm F. Neuronal replacement in adult brain. Brain Res Bull. 2002;57:737–749. doi: 10.1016/s0361-9230(02)00750-5. [DOI] [PubMed] [Google Scholar]
- 129.Tramontin AD, Garcia-Verdugo JM, Lim DA, Alvarez-Buylla A. Postnatal development of radial glia and the ventricular zone (VZ): a continuum of the neural stem cell compartment. Cereb Cortex. 2003;13:580–7. doi: 10.1093/cercor/13.6.580. [DOI] [PubMed] [Google Scholar]
- 130.Nordeen KW, Nordeen EJ. Projection neurons within a vocal motor pathway are born during song learning in zebra finches. Nature. 1988;334:149–51. doi: 10.1038/334149a0. [DOI] [PubMed] [Google Scholar]
- 131.Nottebohm F. Neuronal replacement in adulthood. Ann N Y Acad Sci. 1985;457:143–61. doi: 10.1111/j.1749-6632.1985.tb20803.x. [DOI] [PubMed] [Google Scholar]
- 132.Scott BB, Lois C. Developmental origin and identity of song system neurons born during vocal learning in songbirds. J Comp Neurol. 2007;502:202–14. doi: 10.1002/cne.21296. [DOI] [PubMed] [Google Scholar]
- 133.Barkan S, Ayali A, Nottebohm F, Barnea A. Neuronal recruitment in adult zebra finch brain during a reproductive cycle. Dev Neurobiol. 2007;67:687–701. doi: 10.1002/dneu.20379. [DOI] [PubMed] [Google Scholar]
- 134.Kirn J, O’Loughlin B, Kasparian S, Nottebohm F. Cell death and neuronal recruitment in the high vocal center of adult male canaries are temporally related to changes in song [see comments] Proc Natl Acad Sci U S A. 1994;91:7844–8. doi: 10.1073/pnas.91.17.7844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Nottebohm F. A brain for all seasons: cyclical anatomical changes in song control nuclei of the canary brain. Science. 1981;214:1368–70. doi: 10.1126/science.7313697. [DOI] [PubMed] [Google Scholar]
- 136.Nottebohm F. Plasticity in adult avian central nervous system: possible relation between hormones, learning, and brain repair. In: Plum F, editor. Handbook of Physiology, Section 1. Willimas & Wilkins; Baltimore: 1987. pp. 85–108. [Google Scholar]
- 137.Smith TG, Brenowitz EA, Beecher MD, Wingfield JC. Seasonal changes in testosterone, neural attributes of song control nuclei, and song structure in wild songbirds. The Journal of Neuroscience. 1997;17:6001–6010. doi: 10.1523/JNEUROSCI.17-15-06001.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Tramontin AD, Brenowitz EA. A field study of seasonal neuronal incorporation into the song control system of a songbird that lacks adult song learning. J Neurobiol. 1999;40:316–26. doi: 10.1002/(sici)1097-4695(19990905)40:3<316::aid-neu4>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 139.Hidalgo A, Barami K, Iversen K, Goldman SA. Estrogens and non-estrogenic ovarian influences combine to promote the recruitment and decrease the turnover of new neurons in the adult female canary brain. J Neurobiol. 1995;27:470–87. doi: 10.1002/neu.480270404. [DOI] [PubMed] [Google Scholar]
- 140.Nottebohm F. Testosterone triggers growth of brain vocal control nuclei in adult female canaries. Brain Research. 1980;189:429–36. doi: 10.1016/0006-8993(80)90102-x. [DOI] [PubMed] [Google Scholar]
- 141.Rasika S, Nottebohm F, Alvarezbuylla A. Testosterone Increases the Recruitment and/or Survival of New High Vocal Center Neurons in Adult Female Canaries. Proceedings of the National Academy of Sciences of the United States of America. 1994;91:7854–7858. doi: 10.1073/pnas.91.17.7854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Williams S, Leventhal C, Lemmon V, Nedergaard M, Goldman SA. Estrogen promotes the initial migration and inception of NgCAM-dependent calcium-signaling by new neurons of the adult songbird brain. Molecular and Cellular Neuroscience. 1999;13:41–55. doi: 10.1006/mcne.1998.0729. [DOI] [PubMed] [Google Scholar]
- 143.Goldman SA, Nedergaard M. Newly generated neurons of the adult songbird brain become functionally active in long-term culture. Brain Res Dev Brain Res. 1992;68:217–23. doi: 10.1016/0165-3806(92)90063-3. [DOI] [PubMed] [Google Scholar]
- 144.Alvarez-Borda B, Haripal B, Nottebohm F. Timing of brain-derived neurotrophic factor exposure affects life expectancy of new neurons. Proc Natl Acad Sci U S A. 2004;101:3957–61. doi: 10.1073/pnas.0308118101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Li XC, Jarvis ED, Alvarez-Borda B, Lim DA, Nottebohm F. A relationship between behavior, neurotrophin expression, and new neuron survival. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:8584–8589. doi: 10.1073/pnas.140222497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Rasika S, Alvarez-Buylla A, Nottebohm F. BDNF mediates the effects of testosterone on the survival of new neurons in an adult brain. Neuron. 1999;22:53–62. doi: 10.1016/s0896-6273(00)80678-9. [DOI] [PubMed] [Google Scholar]
- 147.Nottebohm F. A Brain for All Seasons - Cyclical Anatomical Changes in Song Control. Science. 1981;214:1368–1370. doi: 10.1126/science.7313697. [DOI] [PubMed] [Google Scholar]
- 148.Brenowitz EA, Baptista LF, Lent K, Wingfield JC. Seasonal plasticity of the song control system in wild Nuttall’s white-crowned sparrows. J Neurobiol. 1998;34:69–82. doi: 10.1002/(sici)1097-4695(199801)34:1<69::aid-neu6>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 149.Smith GT, Brenowitz EA, Wingfield JC, Baptista LF. Seasonal changes in song nuclei and song behavior in Gambel’s white-crowned sparrows. Journal of Neurobiology. 1995;28:114–25. doi: 10.1002/neu.480280110. [DOI] [PubMed] [Google Scholar]
- 150.Soma KK, Hartman VN, Wingfield JC, Brenowitz EA. Seasonal changes in androgen receptor immunoreactivity in the song nucleus HVc of a wild bird. J Comp Neurol. 1998;409:224–236. [PubMed] [Google Scholar]
- 151.Leitner S, Voigt C, Garcia-Segura LM, Van’t Hof T, Gahr M. Seasonal activation and inactivation of song motor memories in wild canaries is not reflected in neuroanatomical changes of forebrain song areas. Horm Behav. 2001;40:160–8. doi: 10.1006/hbeh.2001.1700. [DOI] [PubMed] [Google Scholar]
- 152.Lee DW, Fernando G, Peterson RS, Allen TA, Schlinger BA. Estrogen mediation of injury-induced cell birth in neuroproliferative regions of the adult zebra finch brain. Dev Neurobio. 2007;67:1107–1117. doi: 10.1002/dneu.20399. [DOI] [PubMed] [Google Scholar]
- 153.Peterson RS, Lee DW, Fernando G, Schlinger BA. Radial glia express aromatase in the injured zebra finch brain. J Comp Neurol. 2004;480:261–269. doi: 10.1002/cne.20157. [DOI] [PubMed] [Google Scholar]
- 154.Peterson RS, Fernando G, Day L, Allen TA, Chapleau JD, Menjivar J, Schlinger BA, L DW. Aromatase expression and cell proliferation following injury of the adult zebra finch Hippocampus. Dev Neurobio. 2007;67:1867–1878. doi: 10.1002/dneu.20548. [DOI] [PubMed] [Google Scholar]
- 155.Shen P, Schlinger BA, Campagnoni AT, Arnold AP. An atlas of aromatase mRNA expression in the zebra finch brain. J Comp Neurol. 1995;360:172–84. doi: 10.1002/cne.903600113. [DOI] [PubMed] [Google Scholar]
- 156.Foster EF, Bottjer SW. Axonal connections of the high vocal center and surrounding cortical regions in juvenile and adult male zebra finches [In Process Citation] J Comp Neurol. 1998;397:118–38. [PubMed] [Google Scholar]
- 157.Konishi M, Akutagawa E. Neuronal growth, atrophy and death in a sexually dimorphic song nucleus in the zebra finch brain. Nature. 1985;315:145–7. doi: 10.1038/315145a0. [DOI] [PubMed] [Google Scholar]
- 158.Mooney R, Rao M. Waiting periods versus early innervation: the development of axonal connections in the zebra finch song system. J Neurosci. 1994;14:6532–43. doi: 10.1523/JNEUROSCI.14-11-06532.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Breuhner CW, Greenberg AL, Wingfield JC. Noninvasive corticosterone treatment rapidly increases activity in Gambel’s white-crowned sparrows (Zonotrichia leucophrys gambelli) Gen Comp Endocrinol. 1998;111:386–394. doi: 10.1006/gcen.1998.7128. [DOI] [PubMed] [Google Scholar]
- 160.Cornil CA, Dalla C, Papadopoulou-Daifoti Z, Baillien M, Balthazart J. Estradiol rapidly activates male sexual behavior and affects brain monoamine levels in the quail brain. Behavioural Brain Research. 2006;166:110–123. doi: 10.1016/j.bbr.2005.07.017. [DOI] [PubMed] [Google Scholar]
- 161.Evrard H, Balthazart J. Rapid regulation of pain by estrogens synthesized in spinal dorsal horn neurons. J Neurosci. 2004;24:7225–7229. doi: 10.1523/JNEUROSCI.1638-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Naftolin F, Horvath TL, Jakab RL, Leranth C, Harada N, Balthazart J. Aromatase immunoreactivity in axon terminals of the vertebrate brain. An immunocytochemical study on quail, rat, monkey and human tissues. Neuroendocrinol. 1996;63:149–55. doi: 10.1159/000126951. [DOI] [PubMed] [Google Scholar]
- 163.Hojo Y, Hattori TA, Enami T, Furukawa A, Suzuki K, Ishii HT, Mukai H, Morrison JH, Janssen WG, Kominami S, Harada N, Kimoto TKS. Adult male rat hippocampus synthesizes estradiol from pregnenolone by cytochromes P45017alpha and P450 aromatase localized in neurons. Proc Natl Acad Sci USA. 2004;101:865–870. doi: 10.1073/pnas.2630225100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Schlinger BA, Callard GV. Localization of Aromatase in Synaptosomal and Microsomal Subfractions of Quail (Coturnix-Coturnix-Japonica) Brain. Neuroendocrinology. 1989;49:434–441. doi: 10.1159/000125149. [DOI] [PubMed] [Google Scholar]
- 165.Rohman KN, Schlinger BA, Saldanha CJ. The subcellular compartmentalization of aromatase is sexually dimorphic in the adult zebra finch brain. J Neurobio. 2006;67:1–9. doi: 10.1002/dneu.20303. [DOI] [PubMed] [Google Scholar]
- 166.Remage-Healey L, Oyama RK, Schlinger BA. Elevated aromatase activity in forebrain synaptic terminals during song. J Neuroendocrinol. 2009;21:191–9. doi: 10.1111/j.1365-2826.2009.01820.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Schlinger BAaGVCAiqbcwa. Aromatase in quail brain: correlations with aggressiveness. Endocrinology. 1989;124:437–443. doi: 10.1210/endo-124-1-437. [DOI] [PubMed] [Google Scholar]
- 168.Balthazart J, Baillien M, Ball GF. Interactions between aromatase (estrogen synthase) and dopamine in the control of male sexual behavior in quail. Comparative Biochemistry and Physiology B-Biochemistry & Molecular Biology. 2002;132:37–55. doi: 10.1016/s1096-4959(01)00531-0. [DOI] [PubMed] [Google Scholar]
- 169.Barclay SR, Harding CF, Waterman SA. Central DSP-4 treatment decreases norepinephrine levels and courtship behavior in male zebra finches. Pharmacol Biochem Behav. 1996;53:213–20. doi: 10.1016/0091-3057(95)00183-2. [DOI] [PubMed] [Google Scholar]
- 170.Mello CV, Pinaud R, Ribeiro S. Noradrenergic system of the zebra finch brain: immunocytochemical study of dopamine-beta-hydroxylase. J Comp Neurol. 1998;400:207–28. [PubMed] [Google Scholar]
- 171.Sizemore M, Perkel DJ. Noradrenergic and GABA B receptor activation differentially modulate inputs to the premotor nucleus RA in zebra finches. J Neurophysiol. 2008;100:8–18. doi: 10.1152/jn.01212.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Saldanha CJ, Schlinger BA, Micevych PE, Horvath TL. Presynaptic N-methyl-D-aspartate receptor expression is increased by estrogen in an aromatase-rich area of the songbird hippocampus. J Comp Neurol. 2004;469:522–534. doi: 10.1002/cne.11035. [DOI] [PubMed] [Google Scholar]
- 173.Balthazart J, Baillien M, Ball GF. Rapid control of brain aromatase activity by glutamatergic inputs. Endocrinology. 2006;147:359–366. doi: 10.1210/en.2005-0845. [DOI] [PubMed] [Google Scholar]
- 174.Cornil CA, Dalla C, Papadopoulou-Daifoti Z, Baillien M, Dejace C, Ball GF, Balthazart J. Rapid decreases in preoptic aromatase activity and brain monoamine concentrations after engaging in male sexual behavior. Endocrinology. 2005;146:3809–3820. doi: 10.1210/en.2005-0441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Balthazart J, Baillien M, Charlier TD, Ball GF. Calcium-dependent phosphorylation processes control brain aromatase in quail. European Journal of Neuroscience. 2003;17:1591–1606. doi: 10.1046/j.1460-9568.2003.02598.x. [DOI] [PubMed] [Google Scholar]
- 176.Dewing P, Boulware MI, Sinchak K, Christensen A, Mermelstein PG, Micevych P. Membrane estrogen receptor-alpha interactions with metabotropic glutamate receptor 1a modulate female sexual receptivity in rats. J Neurosci. 2007;27:9294–300. doi: 10.1523/JNEUROSCI.0592-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Remage-Healey L, Bass AH. Rapid, hierarchical modulation of vocal patterning by steroid hormones. Journal of Neuroscience. 2004;24:5892–5900. doi: 10.1523/JNEUROSCI.1220-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Remage-Healey L, Bass AH. Plasticity in brain sexuality is revealed by the rapid actions of steroid hormones. J Neurosci. 2007;27:1114–1122. doi: 10.1523/JNEUROSCI.4282-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Woolley CS. Acute effects of estrogen on neuronal physiology. Ann Rev Pharmacol Toxicol. 2007;47:657–680. doi: 10.1146/annurev.pharmtox.47.120505.105219. [DOI] [PubMed] [Google Scholar]
- 180.Lea RW, Clark JA, Tsutsui K. Changes in central steroid receptor expression, steroid synthesis, and dopaminergic activity related to the reproductive cycle of the ring dove. Microscopy Research and Technique. 2001;55:12–26. doi: 10.1002/jemt.1152. [DOI] [PubMed] [Google Scholar]
- 181.Pradhan DS, Yu Y, Soma KK. Rapid estrogen regulation of DHEA metabolism in the male and female songbird brain. J Neurochem. 2008;104:244–253. doi: 10.1111/j.1471-4159.2007.04953.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Soma KK, Alday NA, Hau M, Schlinger BA. Dehydroepiandrosterone metabolism by 3β-hydroxysteroid dehydrogenase/Delta 5-Delta 4 isomerase in adult zebra finch brain: Sex difference and rapid effect of stress. Endocrinology. 2004;145:1668–1677. doi: 10.1210/en.2003-0883. [DOI] [PubMed] [Google Scholar]
- 183.Remage-Healey L, Maidment N, Schlinger BA. Forebrain steroid levels fluctuate rapidly during social interactions. Nat Neurosci. 2008:1327–1334. doi: 10.1038/nn.2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Carlisle HJ, Hales TG, Schlinger BA. Characterization of Neuronal Zebra Finch GABAA Receptors. J Comp Physiology A. 1998;182:531–538. [Google Scholar]
- 185.Titus RC, Ketterson ED, Nolan V., Jr High testosterone prior to song crystallization inhibits singing behavior in captive yearling dark-eyed juncos (Junco hyemalis) Horm Behav. 1997;32:133–40. doi: 10.1006/hbeh.1997.1414. [DOI] [PubMed] [Google Scholar]
- 186.Marler P, Peters S, Ball GF, Dufty AM, et al. The role of sex steroids in the acquisition and production of birdsong. Nature. 1988;336:770–772. doi: 10.1038/336770a0. [DOI] [PubMed] [Google Scholar]
- 187.Gardner TJ, Naef F, Nottebohm F. Freedom and rules: the acquisition and reprogramming of a bird’s learned song. Science. 2005;308:1046–9. doi: 10.1126/science.1108214. [DOI] [PubMed] [Google Scholar]