Abstract
Patellofemoral pain (PFP) is thought to be related to patellar maltracking due to imbalances in the knee extensor. However, no study has evaluated the in vivo biomechanical properties of the quadriceps tendon in PFP syndrome. Our purpose was to compare the biomechanical properties of the quadriceps tendons in vivo and noninvasively in patients with PFP syndrome to those of control subjects. The null hypothesis was that the quadriceps tendons of PFP subjects would have significantly decreased strain compared with control subjects. Fourteen subjects (7 control, 7 PFP) performed voluntary ramp isometric contractions to a range of torque levels, while quadriceps tendon elongation was measured using ultrasonography. Tendon strain was calculated for the vastus medialis obliquus (VMO) and vastus lateralis (VL) portion of the quadriceps tendon and compared between subjects (control vs. PFP) and within subjects (VMO vs. VL). PFP subjects showed significantly less VMO tendon strain than control subjects (P < 0.001), but there was no difference in VL tendon strain between PFP and control subjects (P = 0.100). Relative weakness of the VMO is the most likely cause of the decreased tendon strain seen in subjects with PFP. VMO weakness not only explains the decreased medial tendon strain but also explains the presence of increased lateral patellar translation and lateral patellar spin (distal pole rotates laterally) reported in the literature in this population. This technique can potentially be used in a clinical setting to evaluate quadriceps tendon properties and infer the presence of muscle weakness in PFP.
Keywords: mechanical properties, ultrasound, patellar kinematics, vastus medialis obliquus, muscle weakness
patellofemoral pain (PFP) syndrome is defined as pain originating from the patellofemoral articulation and associated structures that excludes other intra-articular and peripatellar pathology (12, 17). PFP syndrome is common, affecting up to 25% of the general population (11, 21, 42). Participation in sports and daily activities may be substantially affected by the pain, and chronic PFP is associated with joint degeneration and osteoarthritis (16).
Several authors have proposed that static malalignment and abnormal patellar tracking are associated with PFP syndrome (14, 16, 20, 48, 54). Excess lateral patellar translation in subjects with PFP syndrome has been shown in numerous previous studies (8, 38, 44, 48, 54). Fulkerson (16) suggested that imbalance of the extensor mechanism may lead to overload of the retinaculum and subchondral bone, resulting in pain due to activation of nociceptive fibers in the bone, synovium, or retinaculum. This imbalance may be due to imbalances in the forces vectors from the individual quadriceps components (2), moment arm imbalance (18), or an imbalance in the material properties of the medial vs. lateral portions of the quadriceps tendon.
Ultrasonography technology has made it practical to conduct in vivo investigations of tendon biomechanical properties (7, 23, 25, 31, 33, 39, 40). No study has compared the in vivo biomechanical properties of the medial vs. lateral quadriceps tendon structures or assessed changes in the biomechanical properties of the quadriceps tendons associated with PFP syndrome. The primary purpose of this study was to compare the biomechanical properties of the quadriceps tendons in vivo and noninvasively in patients with PFP to those of control subjects. An additional purpose was to compare the biomechanical properties of the portions of the quadriceps tendon associated with the vastus medialis obliquus (VMO) and vastus lateralis (VL) muscles, in both populations. We proposed three null hypotheses: 1) the quadriceps tendon strain is significantly decreased in subjects with PFP compared with control subjects; 2) in control subjects, there is no significant difference in medial quadriceps tendon strain compared with the lateral quadriceps tendon; 3) in subjects with PFP, medial quadriceps tendon strain is significantly decreased compared with lateral quadriceps tendon strain.
MATERIALS AND METHODS
Seven control subjects and seven subjects with PFP syndrome volunteered to participate in this study. The study protocol was approved by the Northwestern University Institutional Review Board, and all volunteers gave informed consent before participation. All subjects with PFP had a clinical diagnosis of PFP provided by a clinician based on a physical examination and the patient's history (6, 22). For diagnosis each patient had to have a positive result for at least one of the following: “J” sign (20), squat test (43), or glide test (6, 16, 43). In addition, all patients had to have active PFP at the time of testing, evidenced by reproducible anterior knee pain in at least two of the following activities: squatting, stair ascent/descent, or seated knee extension. These tested activities were selected because of their association with PFP (12). Subjects were excluded if they had positive signs of a meniscal, ligamentous, or iliotibial band lesion, traumatic onset of PFP, patellar instability [for example, a positive apprehension test (43)], or a past history of any other injury or pathological condition involving the lower extremity. All control participants had no current or past symptoms of anterior knee pain.
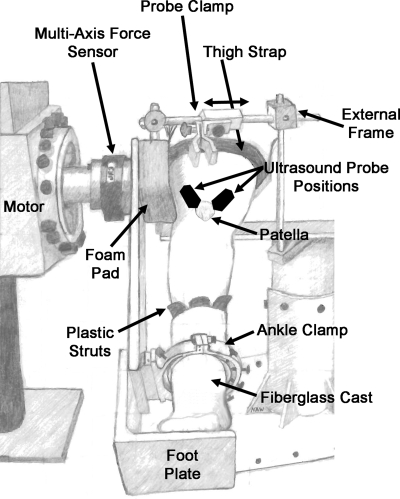
The biomechanical properties of the tendons associated with the VMO and VL components of the quadriceps muscle were examined. Isometric knee extension moments were measured with the subject seated in a custom chair with their hip flexed to 85° and their knee flexed to 60°. This position was selected because 60° of knee flexion lies within the range that voluntary activation of the quadriceps muscle is not dependent on knee flexion angle (5). The thigh was strapped to the seat, and the plantar surface of the foot was supported on a foot plate (Fig. 1). Rigid plastic struts were placed on the medial, lateral, and anterior aspects of the subject's ankle joint inside a fiberglass cast. The fiberglass cast covered the midfoot and ankle with the ankle in the neutral position and was secured to the footplate using a custom clamp. The clamp was tightened to ensure no translation or rotation of the tibia occurred during isometric knee extension (Fig. 1). The ankle clamp and foot plate were attached to a six-axis force/torque sensor (JR3, Woodland, CA) through an aluminum beam with the flexion/extension axis of the knee aligned with the center of the sensor. The fixation system for the foot and knee prevented observable movement of the lower extremity during isometric knee extension.
Fig. 1.
Experimental setup. The subject was seated in a custom chair with the hip at 85° and the knee at 60°. The plantar surface of the foot was supported on a foot plate. Rigid plastic struts were placed on the medial, lateral, and anterior aspects of the ankle joint inside a fiberglass cast. The fiberglass cast covered the midfoot and ankle and was secured to the footplate using a custom clamp. The clamp was tightened to ensure no translation or rotation of the tibia during isometric knee extension. The ankle clamp and foot plate were attached to a six degree-of-freedom torque sensor through an aluminum beam. An external frame was used to fix the ultrasound probe over the tendon of interest. The black ovals on the knee represent the approximate position of the ultrasound probe for measurement of vastus medialis obliquus (VMO) and vastus lateralis (VL) tendon elongation, slightly overlapping the proximal patella.
The angle of orientation, defined as the angle in the frontal plane between the anatomic axis of the femur and the longitudinal orientation of the tendon fibers, was determined manually for each subject. A 14-MHz linear array B-mode ultrasound probe (Linear M12L, GE LOGIQ-9, GE Healthcare, Waukesha, WI), with a 39-mm scanning window, was placed over the distal aspect of the VMO or VL muscle, and the myotendinous junction (MTJ) was identified. The probe was then positioned parallel to the femoral axis over the MTJ of the tendon of interest, and small adjustments in the angle were made until the characteristic fibrillar structure of the tendon was visible in the image. The longitudinal orientation of the tendon fibers was defined as the angle at which the fibrillar structure was most clear in the image (as determined by a single investigator). With the ultrasound probe positioned parallel to the direction of the tendon fibers, a small mark was made on the skin at the distal and proximal poles of the ultrasound probe. The angle of orientation was then measured between the skin markings and the femoral axis (with the center of the patella as the vertex) using a handheld goniometer.
Tendon resting length was measured with the ultrasound probe positioned parallel to the tendon fiber direction for each tendon. For the VMO tendon, the entire length of the tendon could be visualized in a single image, so both the MTJ and the proximal portion of the patella were visible in each image. To determine resting length, three sagittal plane still-frame images were captured with the quadriceps at rest, and the entire length of the tendon (along the curvilinear path from MTJ to patella) was digitized using ImageJ. The mean resting length of the three images was used for further analysis. Both the MTJ and proximal patella could not be visualized in a single frame for the VL tendon. Therefore, LOGIQView, a technique of extended field-of-view imaging implemented in the GE LOGIQ-9 ultrasound machine, was used to overcome the limited field of view and register the entire length of the VL tendon. For extended field-of-view imaging, the probe was positioned over the VL tendon (oriented along the fiber direction) with the proximal patella visible in the image. The probe was then slowly moved proximally along the fiber direction until the MTJ was visible in the image. A proprietary GE algorithm based on spatial averaging and feature consistency stitched successive image frames together creating a single image of the entire length of the tendon. Three images were taken of the VL tendon, the entire curvilinear length of the tendon was digitized using ImageJ, and the mean resting length was used for further analysis. For cross-sectional area measurements, the length of each tendon was estimated during testing from resting length images. Three still-frame axial plane images (perpendicular to tendon fiber direction) were taken at 50% of the length of the tendon (23). The perimeter of the tendon cross-sectional area was manually digitized using ImageJ, and the mean cross-sectional area from the three images was used for further analysis.
Subjects were asked to contract their quadriceps muscle to match a specified target torque level (1, 3, 5, 7, 9, 15, and 20 N·m) for 30 s while minimizing the off-axis torque in internal/external rotation and abduction/adduction. Visual and auditory feedback was provided to aid subjects in matching the torque level. Each subject was allowed to practice the torque-matching procedure until they were capable of generating torques within ±1 N·m of the target extension torque. Practice time served as preconditioning of the tendon before recording elongation data.
An external frame mounted on the custom chair was used to fix the ultrasound probe over the distal portion of the tendon of interest during isometric extension (Fig. 1). Preliminary experiments with a thin tube fixed between the ultrasound probe and the skin showed that no movement of the probe occurred during isometric extension. The probe was fixed such that the angle of orientation was maintained and the proximal portion of the patella was visible in the image (Fig. 2). The elongation of the tendon was recorded continuously and simultaneously with torque during isometric contraction from rest to the specified torque level. The ultrasound scanner produced a movie file containing a sequence of image frames (30 frames/s, pixel dimension 100 × 100 μm) that were saved for subsequent analysis. The position of the patella and a point on the tendon in the proximal portion of the image were digitized in each frame of the ultrasound recording, and tendon elongation was calculated for each frame of the recording (ImageJ, National Institutes of Health, Bethesda, MD). For the medial- side measurements, the location of the patella and the position of the MTJ were used for the calculation of tendon elongation. Each torque-matching trial lasted 30 s, which involved an initial ramp- up to the target torque followed by a brief period of hysteresis around the target torque until the subject could comfortably hold the target torque (Fig. 3). Three 30-s trials were collected at each torque level, and the best of the three trials (based on the visibility and clarity of landmark features in all frames of the ultrasound images) was chosen for analysis. Tendon elongation was tracked for each frame of the ultrasound images, but strain and elongation values were calculated using only the resting length and the average tendon elongation during the initial plateau (1 s) following the hysteresis of the torque signal. All digitization measurements on ultrasound images were made three times by the same investigator, and average values were used for data analysis. Tendon strain was calculated as ɛ=Δl/l0, where l0 is the tendon length at rest, and Δl is the change in tendon length during isometric contraction.
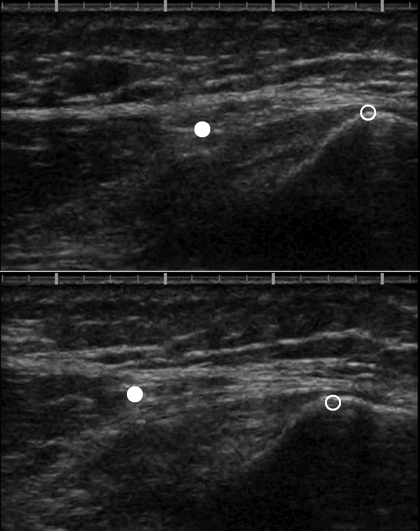
Fig. 2.
Measurement of VMO tendon elongation. The ultrasound probe was positioned such that the angle of orientation was maintained and the proximal portion of the patella (open circle) was visible in the image. For the VMO tendon, the myotendinous junction (MTJ; filled circle) was also visible in the image. Top: ultrasound image before isometric quadriceps contraction. Bottom: image during isometric quadriceps contraction (7 N·m). Notice the proximal shift of both the patella and the MTJ during contraction; larger proximal shift of MTJ indicates tendon elongation. Proximal shift of the patella is due to patellar tendon elongation.
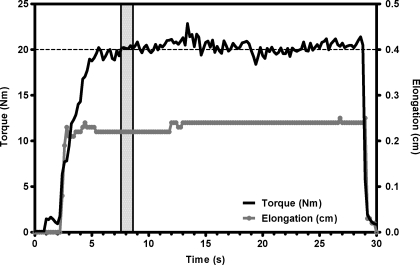
Fig. 3.
Sample torque and elongation curves over a single 30-s trial. Torque is plotted in black on the left axis and elongation is plotted in gray on the right axis. The horizontal dashed line represents the target torque for this trial (20 N·m), and the vertical shaded area represents the 1-s period from which the tendon strain values were calculated.
Differences in the tendon biomechanical properties between control subjects and subjects with PFP were analyzed using a two-factor ANOVA (condition × torque) with post hoc Bonferroni comparisons. The torque factor had seven levels. VMO vs. VL tendon properties within subjects were analyzed using a two-factor ANOVA with repeated measures (side × torque) and post hoc Bonferroni comparisons. Resting length and cross-sectional area were compared using Student's t-tests; α was preset to 0.05.
RESULTS
There were no significant differences between the two groups for sex, age, height, or weight (Table 1). Both the MTJ and the patella shifted proximally during isometric contraction (Fig. 2); however, the MTJ consistently shifted further in the proximal direction than the patella, indicating quadriceps tendon elongation. Control subjects showed greater VMO tendon elongation than PFP subjects (P < 0.001); however, there was no difference between PFP and control subjects for VL tendon elongation (P = 0.198). In PFP subjects, the VL tendon elongated more than the VMO tendon (P = 0.026). However, in control subjects, there was no significant difference between VMO tendon elongation and VL tendon elongation (P = 0.950).
Table 1.
Demographic characteristics of the subject population
| Healthy Subjects | PFP Subjects | P Value | |
|---|---|---|---|
| Sex | 3 male, 4 female | 2 male, 5 female | 1.000 |
| Age, yr | 29.43±7.7 | 31.71±14.1 | 0.712 |
| Height, cm | 170.90±7.3 | 169.82±10.7 | 0.828 |
| Weight, kg | 65.80±14.5 | 71.93±20.5 | 0.531 |
Values are means ±SD. PFP, patellofemoral pain.
There were no differences between control and PFP subjects for tendon length or cross-sectional area (Table 2). VL tendons were significantly longer than VMO tendons in both PFP (P < 0.001) and control subjects (P < 0.001); however, the cross-sectional area of VL tendons did not differ from VMO tendons for either PFP or control subjects (P = 0.407, P = 0.565, respectively).
Table 2.
Tendon resting length and cross-sectional area
| Healthy Subjects | PFP Subjects | P Value | |
|---|---|---|---|
| VMO tendon | |||
| Length, mm | 18.66±3.40 | 18.69±2.66 | 0.985 |
| Cross-sectional area, mm2 | 56.14±16.87 | 59.14±11.81 | 0.707 |
| VL tendon | |||
| Length, mm | 52.32±5.71 | 55.17±5.46 | 0.360 |
| Cross-sectional area, mm2 | 48.71±20.25 | 52.57±14.95 | 0.692 |
Values are means ±SD. VMO, vastus medialis obliquus portion of quadriceps tendon; VL, vastus lateralis portion of quadriceps tendon.
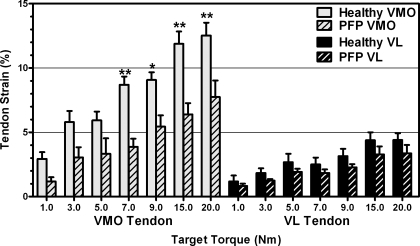
Control subjects showed significantly more VMO tendon strain than PFP subjects (P < 0.001, Fig. 4), but there was no difference in VL tendon strain between control and PFP subjects (P = 0.100, Fig. 4). For both PFP and control subjects, VMO tendon strain was significantly greater than VL tendon strain (P < 0.001, P < 0.001, respectively).
Fig. 4.
Mean (± SE) strain in the VMO and VL tendons. Bonferroni post hoc comparisons indicate significantly decreased strain in the VMO tendon in subjects with patellofemoral pain (PFP) compared with control subjects at the 7, 9, 15, and 20 N·m torque levels: *P < 0.05, **P < 0.01.
DISCUSSION
The literature suggests that the etiology of PFP is multifactorial (10, 47) and may be related to extensor mechanism imbalance (16, 54). Null hypothesis 1 was partially rejected because quadriceps tendon strain was significantly decreased in subjects with PFP, compared with control subjects, only for the medial portion of the quadriceps tendon. Additionally, null hypotheses 2 and 3 were rejected because for both control and subjects with PFP, strain in the medial quadriceps tendon was greater than lateral quadriceps tendon strain. Since there were no differences in tendon morphology (resting length and cross-sectional area) between control and PFP subjects, the difference in strains can be attributed to differences in tendon elongation. Decreased tendon elongation in subjects with PFP has two potential causes: 1) increased stiffness of the medial portion of the quadriceps tendon, or 2) relative weakness of the vastus medialis component of the quadriceps muscle.
Increased stiffness of the medial portion of the quadriceps tendon may be the result of adaptational changes in the tendon. It has been shown that the cytoskeleton of tendon cells is mechanoresponsive and controls reciprocal anabolic and catabolic gene expression (34, 35). Furthermore, it has been suggested that mechanoresponsive cells in tendon operate with set points or within a homeostatic range (4, 34). Mechanical perturbations outside this range cause alterations in gene expression and lead to morphological and material property alterations. In a recent in vivo study, Arampatzis et al. (3) showed that the Achilles tendon altered its material and morphological properties only after high-strain-magnitude exercise. Chronic alterations in these set points or an artificial narrowing of the homeostatic range is a potential explanation for the chronic nature of most idiopathic patellofemoral pain.
An alternate hypothesis for decreased VMO tendon elongation in PFP is weakness of the vastus medialis muscle. Weakness of the vastus medialis, particularly the obliquus portion, is commonly thought to play a role in the pathogenesis of PFP (1, 19, 38, 52). Vastus medialis weakness is further supported by results from our previous study in which patellar kinematics were measured in the same subjects with PFP (54). PFP subjects exhibited excess lateral patellar translation and lateral patellar spin (distal pole rotates laterally) while squatting. Lieb and Perry (36) showed that insufficient force (<20% of VL force) on the VMO tendon, perhaps from VMO weakness, resulted in lateral patellar translation during knee extension and an inability of the patella to maintain the neutral position in the trochlear groove. Weakness of the VMO component of the quadriceps muscle may cause the extensor mechanism to be incapable of generating effective forces in opposition to the action of the VL quadriceps component.
The data from the present study cannot distinguish between increased tendon stiffness and VM weakness as the cause of decreased medial tendon strain in PFP. It is unlikely that VMO weakness directly results in increased tendon stiffness. Several studies have found that strength training (increased demand) leads to increased tendon stiffness (30, 32, 46). Furthermore, Kubo et al. (29) found significantly decreased tendon stiffness after 20 days of bed rest in young healthy subjects. However, VMO weakness or potential reduced VMO contribution to the knee extension torque during the experiment could directly result in decreased VMO tendon elongation and therefore an apparent increase in tendon stiffness.
The relationship between tendon mechanical/material properties and muscle weakness is complex in PFP and may cascade from an initial injury. It has been shown that the biomechanics of the knee are altered in subjects with PFP syndrome (16). However, what initiates the shift from healthy to pathological knee biomechanics remains unknown. The VMO component of the quadriceps muscle weakens first in response to pain or swelling in the patellofemoral joint (1, 53, 55). In idiopathic PFP, pain or swelling in the patellofemoral joint may result from an initial subclinical injury to the joint or surrounding structures. This initial injury may lead to VMO weakness and abnormal patellar tracking. Initial injury, VMO weakness, and/or patellar tracking changes may move mechanoresponsive tendon cells beyond their homeostatic set points, triggering healing. Increases in collagen content and associated increases in Young's modulus (stiffness) of the tendon have been shown to be associated with tendon healing (45). These biomechanical changes may lead to overload of the subchondral bone in the lateral patellofemoral compartment, triggering pain (16). All these factors may trigger additional adaptational changes in tendon material properties causing a vicious cycle and chronic PFP.
The strain values reported in the present study, particularly for the VMO tendon, are larger than most found in previous studies of free tendon. Most studies have focused on the biomechanical properties of isolated tendons such as the patellar tendon (24–28, 46, 51), which has been shown to be a less compliant tendon than the quadriceps tendon (51). Only a single study has investigated the material properties of the quadriceps tendon (51). This study tested quadriceps tendon from human cadavers and reported a failure strain of 14.7% (± 3.7%). As strain is dependent on the initial length of the tendon, much of the difference between the present results and these previous results can be ascribed to the length of the tendon being studied. Staubli et al. (51) reported an initial length of 4.13 cm for the central portion of the quadriceps tendon. The higher strains for the VMO portion of the quadriceps tendon in the present study reflect an initial length of only 1.87 cm. In addition, each quadriceps muscle has a broad MTJ, which was represented as a single point in this study. A range of geometry and material properties exist as the tendon transitions from insertion at the patella to aponeurosis and muscle fibers proximal to the MTJ. For example, it is known that the aponeurosis is more compliant than free tendon (40), and that muscle fibers exhibit larger strain values than tendon, in vivo (41). Therefore, it is possible that tracking the MTJ for VMO tendon elongation caused an overestimation of tendon strain due to the inclusion of more compliant structures at the proximal end of the tendon. However, this potential difference in absolute strain magnitude would not likely affect the conclusions drawn between subjects with PFP and control subjects. Finally, factors such as tissue preservation and hydration status have been shown to affect the mechanical properties of cadaver tissue (26, 49) and may lead to differences in strain.
Accuracy and precision have been reported for using ultrasound to track muscle contractile length (37) and tendon length (23) in vivo. For the present study the highest available probe frequency (14 MHz) was used to maximize spatial resolution. Limited depth of view was not a concern as all the quadriceps tendons are located relatively close to the surface of the knee. Previous studies have demonstrated that movements as small as 5 μm can be registered by an ultrasound scanner, despite a much larger pixel resolution (185 × 185 μm) (37). However, the magnitude of tendon elongation in the present study ranged from 600 μm (mean elongation at 1 N·m knee extension torque) to 2,300 μm (mean elongation at 20 N·m). Therefore because the smallest tendon elongation measured was 6 times larger than the pixel resolution, the accuracy of the system was adequate to resolve tendon elongations.
Measurement precision is limited by the repeatability of probe placement and the repeatability of manually tracking points within the images. Due to the limited field of view available for dynamic ultrasound imaging, VL tendon elongation was measured by tracking a point on the tendon in each frame of the ultrasound images, instead of the MTJ. This may have introduced error into the measurement due to portions of the tendon moving in and out of the image plane during isometric contraction. Off-plane observations will underestimate tendon elongations with an error of magnitude 1 − cosθ, where θ is the difference in angle between the ultrasound probe plane and the tendon line of action (37). Based on cadaver dissection, the line of action of the VMO muscle is 42–70° from the anatomic axis of the femur in the frontal plane, and the VL 10–17° (13). Assuming a 10° error, tendon elongation would be underestimated by 6.9%. Careful orientation of the ultrasound probe along the tendon fiber direction minimized this potential source of error.
The external joint torques tested in this study were low (10–20%) compared with joint torques generated during maximal voluntary contraction. While this may limit the ability to extrapolate the strains reported here to functional tasks involving higher levels of muscle activation, torque values were standardized among trials and subjects ensuring that the relative comparisons (PFP vs. control, and medial vs. lateral) are valid. The low external joint torques also ensured that the magnitude of tendon motion within a single trial was small (<0.3 cm), and the experimental setup was designed to minimize off-axis torques, effectively limiting the direction of tendon motion to the image plane.
The repeatability of manually tracking points within the images was also investigated. A single investigator manually tracked each point three times for each trial. The average absolute difference in tendon elongation between successive analyses was 0.2 mm (∼1% strain for the VL tendon), or a difference of 2 pixels when manually tracking points. Similarly, the reproducibility of manual digitization for cross-sectional area measurements was investigated. The average absolute difference in tendon cross-sectional area between successive analyses was 1.9 mm2 (3.9% of cross-sectional area), suggesting manual digitization of ultrasound images yields reproducible results. These results match well with previously published results showing a typical error of 1.5 mm2 and an intraclass correlation coefficient of 0.99 for tendon cross-sectional area measurements taken on different days (46).
The distal tendon attachment on the patella is difficult to locate from the ultrasound image. Therefore tendon length at rest was defined as that from the MTJ to the insertion of the posterior tendon surface on the patella. This definition may have produced a slight underestimation of tendon length and thus an increase in calculated strain. However, all relative comparisons are valid, and an increase in strain would not change any of the conclusions in the present study.
A wide range of values for tendon properties has been previously reported (9, 15, 24, 39, 40, 46), making it is difficult to estimate the magnitude of change that would represent a clinically significant alteration. Values for tendon strain at failure are typically 14–15% (27, 51), and values for in vivo tendon strain are typically 7.5–9.9% (28, 50). Therefore, assuming a minimum detectable difference of 5%, and choosing a common SD of 3%, a preliminary power analysis showed that two groups of 10 subjects each would be needed to yield a power of 90%; an effect size of 1.5 could be detected with two groups of 10 subjects. Since the former assumptions were made somewhat a priori, a post hoc power analysis was also conducted. Based on a difference in strain between PFP subjects and control controls of 2% (VL tendon, 20 N·m torque), and a calculated pooled SD of 1.11%, a revised effect size of 1.82 was found—two groups of seven subjects each were adequate to obtain 90% power. Therefore, this study has sufficient power to reveal significant differences even with the smaller group size presented.
This study presents an initial investigation with the long-term goal of in vivo, noninvasive measurement of tendon material properties, such as Young's modulus. Current technology is not able to distinguish between the force contributions from the separate quadriceps components using noninvasive in vivo methods (7). Therefore, material properties such as tendon stress and Young's modulus cannot be measured directly. This study offers possible pathways for future investigation.
GRANTS
We acknowledge receipt of grant funds from the National Institutes of Health in support of this research. The authors have full control of all primary data and agree to allow the journal to review the data, if requested.
Acknowledgments
Present address for N. A. Wilson: Rehabilitation Medicine Dept., National Institutes of Health, Bldg. 10 CRC, Rm 1-1469, 10 Center Dr., MSC 1604, Bethesda, MD 20892-1604.
REFERENCES
- 1.Amis AA, Senavongse W, Bull AM. Patellofemoral kinematics during knee flexion-extension: an in vitro study. J Orthop Res 24: 2201–2211, 2006. [DOI] [PubMed] [Google Scholar]
- 2.Andriacchi TP, Andersson GB, Ortengren R, Mikosz RP. A study of factors influencing muscle activity about the knee joint. J Orthop Res 1: 266–275, 1984. [DOI] [PubMed] [Google Scholar]
- 3.Arampatzis A, Karamanidis K, Albracht K. Adaptational responses of the human Achilles tendon by modulation of the applied cyclic strain magnitude. J Exp Biol 210: 2743–2753, 2007. [DOI] [PubMed] [Google Scholar]
- 4.Arnoczky SP, Lavagnino M, Egerbacher M, Caballero O, Gardner K, Shender MA. Loss of homeostatic strain alters mechanostat “set point” of tendon cells in vitro. Clin Orthop Relat Res 466: 1583–1591, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Becker R, Awiszus F. Physiological alterations of maximal voluntary quadriceps activation by changes of knee joint angle. Muscle Nerve 24: 667–672, 2001. [DOI] [PubMed] [Google Scholar]
- 6.Boden BP, Pearsall AW, Garrett WE Jr, Feagin JA Jr. Patellofemoral instability: evaluation and management. J Am Acad Orthop Surg 5: 47–57, 1997. [DOI] [PubMed] [Google Scholar]
- 7.Bojsen-Moller J, Magnusson SP, Rasmussen LR, Kjaer M, Aagaard P. Muscle performance during maximal isometric and dynamic contractions is influenced by the stiffness of the tendinous structures. J Appl Physiol 99: 986–994, 2005. [DOI] [PubMed] [Google Scholar]
- 8.Brossmann J, Muhle C, Schroder C, Melchert UH, Bull CC, Spielmann RP, Heller M. Patellar tracking patterns during active and passive knee extension: evaluation with motion-triggered cine MR imaging. Radiology 187: 205–212, 1993. [DOI] [PubMed] [Google Scholar]
- 9.Cooper DE, Deng XH, Burstein AL, Warren RF. The strength of the central third patellar tendon graft. A biomechanical study. Am J Sports Med 21: 818–823, 1993. [DOI] [PubMed] [Google Scholar]
- 10.Csintalan RP, Schulz MM, Woo J, McMahon PJ, Lee TQ. Gender differences in patellofemoral joint biomechanics. Clin Orthop Relat Res 402: 260–269, 2002. [DOI] [PubMed] [Google Scholar]
- 11.Devereaux MD, Lachmann SM. Patello-femoral arthralgia in athletes attending a Sports Injury Clinic. Br J Sports Med 18: 18–21, 1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dixit S, DiFiori JP, Burton M, Mines B. Management of patellofemoral pain syndrome. Am Fam Physician 75: 194–202, 2007. [PubMed] [Google Scholar]
- 13.Farahmand F, Senavongse W, Amis AA. Quantitative study of the quadriceps muscles and trochlear groove geometry related to instability of the patellofemoral joint. J Orthop Res 16: 136–143, 1998. [DOI] [PubMed] [Google Scholar]
- 14.Fitzgerald GK, McClure PW. Reliability of measurements obtained with four tests for patellofemoral alignment. Phys Ther 75: 84–90, 1995. [DOI] [PubMed] [Google Scholar]
- 15.Flahiff CM, Brooks AT, Hollis JM, Vander Schilden JL, Nicholas RW. Biomechanical analysis of patellar tendon allografts as a function of donor age. Am J Sports Med 23: 354–358, 1995. [DOI] [PubMed] [Google Scholar]
- 16.Fulkerson JP Diagnosis and treatment of patients with patellofemoral pain. Am J Sports Med 30: 447–456, 2002. [DOI] [PubMed] [Google Scholar]
- 17.Fulkerson JP, Arendt EA. Anterior knee pain in females. Clin Orthop Relat Res 69–73, 2000. [DOI] [PubMed]
- 18.Gage JR, Novacheck TF. An update on the treatment of gait problems in cerebral palsy. J Pediatr Orthop 10: 265–274, 2001. [PubMed] [Google Scholar]
- 19.Goh JC, Lee PY, Bose K. A cadaver study of the function of the oblique part of vastus medialis. J Bone Joint Surg Br 77: 225–231, 1995. [PubMed] [Google Scholar]
- 20.Grelsamer RP, Stein DA. Rotational Malalignment of the Patella. Rosemont, IL: American Academy of Orthopaedic Surgeons, 2005.
- 21.Hahn T, Foldspang A. Prevalent knee pain and sport. Scand J Soc Med 26: 44–52, 1998. [DOI] [PubMed] [Google Scholar]
- 22.Haim A, Yaniv M, Dekel S, Amir H. Patellofemoral pain syndrome: validity of clinical and radiological features. Clin Orthop Relat Res 451: 223–228, 2006. [DOI] [PubMed] [Google Scholar]
- 23.Hansen P, Bojsen-Moller J, Aagaard P, Kjaer M, Magnusson SP. Mechanical properties of the human patellar tendon, in vivo. Clin Biomech (Bristol, Avon) 21: 54–58, 2006. [DOI] [PubMed] [Google Scholar]
- 24.Haraldsson BT, Aagaard P, Krogsgaard M, Alkjaer T, Kjaer M, Magnusson SP. Region-specific mechanical properties of the human patella tendon. J Appl Physiol 98: 1006–1012, 2005. [DOI] [PubMed] [Google Scholar]
- 25.Hashemi J, Chandrashekar N, Slauterbeck J. The mechanical properties of the human patellar tendon are correlated to its mass density and are independent of sex. Clin Biomech (Bristol, Avon) 20: 645–652, 2005. [DOI] [PubMed] [Google Scholar]
- 26.Haut TL, Haut RC. The state of tissue hydration determines the strain-rate-sensitive stiffness of human patellar tendon. J Biomech 30: 79–81, 1997. [DOI] [PubMed] [Google Scholar]
- 27.Johnson GA, Tramaglini DM, Levine RE, Ohno K, Choi NY, Woo SL. Tensile and viscoelastic properties of human patellar tendon. J Orthop Res 12: 796–803, 1994. [DOI] [PubMed] [Google Scholar]
- 28.Kongsgaard M, Aagaard P, Roikjaer S, Olsen D, Jensen M, Langberg H, Magnusson SP. Decline eccentric squats increases patellar tendon loading compared to standard eccentric squats. Clin Biomech (Bristol, Avon) 21: 748–754, 2006. [DOI] [PubMed] [Google Scholar]
- 29.Kubo K, Akima H, Kouzaki M, Ito M, Kawakami Y, Kanehisa H, Fukunaga T. Changes in the elastic properties of tendon structures following 20 days bed-rest in humans. Eur J Appl Physiol 83: 463–468, 2000. [DOI] [PubMed] [Google Scholar]
- 30.Kubo K, Ikebukuro T, Yaeshima K, Yata H, Tsunoda N, Kanehisa H. Effects of static and dynamic training on the stiffness and blood volume of tendon in vivo. J Appl Physiol 106: 412–417, 2009. [DOI] [PubMed] [Google Scholar]
- 31.Kubo K, Kanehisa H, Fukunaga T. Gender differences in the viscoelastic properties of tendon structures. Eur J Appl Physiol 88: 520–526, 2003. [DOI] [PubMed] [Google Scholar]
- 32.Kubo K, Kanehisa H, Ito M, Fukunaga T. Effects of isometric training on the elasticity of human tendon structures in vivo. J Appl Physiol 91: 26–32, 2001. [DOI] [PubMed] [Google Scholar]
- 33.Kubo K, Ohgo K, Takeishi R, Yoshinaga K, Tsunoda N, Kanehisa H, Fukunaga T. Effects of series elasticity on the human knee extension torque-angle relationship in vivo. Res Q Exerc Sport 77: 408–416, 2006. [DOI] [PubMed] [Google Scholar]
- 34.Lavagnino M, Arnoczky SP. In vitro alterations in cytoskeletal tensional homeostasis control gene expression in tendon cells. J Orthop Res 23: 1211–1218, 2005. [DOI] [PubMed] [Google Scholar]
- 35.Lavagnino M, Arnoczky SP, Egerbacher M, Gardner KL, Burns ME. Isolated fibrillar damage in tendons stimulates local collagenase mRNA expression and protein synthesis. J Biomech 39: 2355–2362, 2006. [DOI] [PubMed] [Google Scholar]
- 36.Lieb FJ, Perry J. Quadriceps function. An anatomical and mechanical study using amputated limbs. J Bone Joint Surg Am 50: 1535–1548, 1968. [PubMed] [Google Scholar]
- 37.Loram ID, Maganaris CN, Lakie M. Use of ultrasound to make noninvasive in vivo measurement of continuous changes in human muscle contractile length. J Appl Physiol 100: 1311–1323, 2006. [DOI] [PubMed] [Google Scholar]
- 38.MacIntyre NJ, Hill NA, Fellows RA, Ellis RE, Wilson DR. Patellofemoral joint kinematics in individuals with and without patellofemoral pain syndrome. J Bone Joint Surg Am 88: 2596–2605, 2006. [DOI] [PubMed] [Google Scholar]
- 39.Maganaris CN, Paul JP. In vivo human tendon mechanical properties. J Physiol 521: 307–313, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Maganaris CN, Paul JP. Load-elongation characteristics of in vivo human tendon and aponeurosis. J Exp Biol 203: 751–756, 2000. [DOI] [PubMed] [Google Scholar]
- 41.Magnusson SP, Hansen P, Aagaard P, Brond J, Dyhre-Poulsen P, Bojsen-Moller J, Kjaer M. Differential strain patterns of the human gastrocnemius aponeurosis and free tendon, in vivo. Acta Physiol Scand 177: 185–195, 2003. [DOI] [PubMed] [Google Scholar]
- 42.Malek MM, Mangine RE. Patellofemoral pain syndromes: a comprehensive and conservative approach. J Orthop Sports Phys Ther 2: 108–116, 1981. [DOI] [PubMed] [Google Scholar]
- 43.Nijs J, Van Geel C, Van der Auwera C, Van de Velde B. Diagnostic value of five clinical tests in patellofemoral pain syndrome. Man Ther 11: 69–77, 2006. [DOI] [PubMed] [Google Scholar]
- 44.Powers CM Patellar kinematics, part I: the influence of vastus muscle activity in subjects with and without patellofemoral pain. Phys Ther 80: 956–964, 2000. [PubMed] [Google Scholar]
- 45.Reddy GK, Gum S, Stehno-Bittel L, Enwemeka CS. Biochemistry and biomechanics of healing tendon. II. Effects of combined laser therapy and electrical stimulation. Med Sci Sports Exerc 30: 794–800, 1998. [DOI] [PubMed] [Google Scholar]
- 46.Reeves ND, Maganaris CN, Narici MV. Effect of strength training on human patella tendon mechanical properties of older individuals. J Physiol 548: 971–981, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schepsis AA, Watson FJ. Patellofemoral Arthritis with Malalignment. Rosemont, IL: American Academy of Orthopaedic Surgeons, 2005.
- 48.Sheehan FT, Derasari A, Brindle TJ, Alter KE. Understanding patellofemoral pain with maltracking in the presence of joint laxity: complete 3D in vivo patellofemoral and tibiofemoral kinematics. J Orthop Res 27: 561–570, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Smith CW, Young IS, Kearney JN. Mechanical properties of tendons: changes with sterilization and preservation. J Biomech Eng 118: 56–61, 1996. [DOI] [PubMed] [Google Scholar]
- 50.Stafilidis S, Karamanidis K, Morey-Klapsing G, DeMonte G, Bruggemann GP, Arampatzis A. Strain and elongation of the vastus lateralis aponeurosis and tendon in vivo during maximal isometric contraction. Eur J Appl Physiol 94: 317–322, 2005. [DOI] [PubMed] [Google Scholar]
- 51.Staubli HU, Schatzmann L, Brunner P, Rincon L, Nolte LP. Mechanical tensile properties of the quadriceps tendon and patellar ligament in young adults. Am J Sports Med 27: 27–34, 1999. [DOI] [PubMed] [Google Scholar]
- 52.Stokes M, Young A. Investigations of quadriceps inhibition: implications for clinical practice. Physiotherapy 70: 425–428, 1984. [Google Scholar]
- 53.Thomee R, Renstrom P, Karlsson J, Grimby G. Patellofemoral pain syndrome in young women. II. Muscle function in patients and healthy controls. Scand J Med Sci Sports 5: 245–251, 1995. [PubMed] [Google Scholar]
- 54.Wilson NA, Press JM, Koh JL, Hendrix RW, Zhang L. In vivo noninvasive evaluation of abnormal patellar tracking during squatting in patients with patellofemoral pain. J Bone Joint Surg Am 91: 558–566, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Young A, Stokes M, Iles JF. Effects of joint pathology on muscle. Clin Orthop Relat Res 21–27, 1987. [PubMed]