Abstract
Body mass index (BMI) is often used as a surrogate estimate of percent body fat in epidemiological studies. This study tested the hypothesis that BMI is representative of body fatness independent of age, sex, ethnicity, and geographic location in prepubertal children. The study sample included a total of 605 prepubertal children (275 girls and 330 boys) of which 247 were Chinese from Jinan, Shandong, Mainland China, and 358 children were from various ethnic backgrounds in New York City (NYC): 121 Caucasians, 94 African Americans, and 143 Asians (Chinese and Korean). In this cross-sectional study, dual energy X-ray absorptiometry was used to quantify total body fat (TBF) and percent body fat (PBF). Prepubertal status was assessed by the criteria of Tanner. Multiple regression models were developed with TBF and PBF as the dependent variables and BMI, age, sex, and ethnicity as independent variables. Multiple regression analysis showed that BMI alone explained 85% and 69% of between-subject variance for TBF and PBF, respectively. Sex was a significant contributor to the models (P < 0.001) with girls having higher TBF and PBF than boys. Ethnicity and geographic location were significant contributors to the model (P < 0.0001) with Asians (Jinan and NYC Asians) having higher PBF than all non-Asian groups (P < 0.0001), and Jinan Asians having higher TBF and PBF than NYC-Asians. Among prepubertal children, for the same BMI, Asians have significantly higher PBF compared with African Americans and Caucasians. Caution is warranted when applying BMI across sex and ethnic prepubertal groups.
Keywords: body mass index, percent body fat, African American, Asian, Caucasian
childhood obesity has emerged as a worldwide epidemic and has become a serious public health problem in the United States and in developing countries undergoing fast economic transition (33). This rising prevalence of obesity in children is a concern given the associated health risks later in life. Although there are many accurate body fat measurement methods available to measure pediatric body composition in the research laboratory (27), their usage is limited due to high costs. The most frequently used tools in field settings in public health research are anthropometric-based measurements such as the body mass index (BMI).
Over the past 30 years, BMI, which is body weight (kg) divided by height squared (m2), has been used to diagnose obesity in adults and children in a myriad of epidemiological studies because of its simplicity and low cost (26). This measurement was first described by Adolphus Quetelet in the mid-19th century based on the observation that body weight or mass was proportional to height or stature squared in adults with normal body frames (26). This simple, inexpensive index has been widely recommended for individual use in clinical practice to guide recommendations for weight loss and weight control. A major assumption in adults, however, is that BMI represents adiposity independent of age, sex, and ethnicity.
It has been suggested that racial and ethnic differences affect the relationship between BMI and body fatness. An increasing number of studies have proposed the need to redefine the BMI overweight and obesity cutoff points for Asian populations (4, 9, 29, 42). The rationale is based on findings that Asian populations have different associations between BMI, percent body fat (PBF), and health risks than do European populations (9, 11, 17, 23, 28, 37, 40).
Based on the different relationships between BMI and body fat percent in adults from different ethnic groups, it is questionable whether the uniform cut-off points recommended for BMI by the International Obesity Taskforce in children are appropriate (4). Limited data exist on the relationship between BMI and body fat in prepubertal children from different ethnic backgrounds. This time period, just before puberty (adiposity rebound), is hypothesized to be one of the three critical periods of obesity development in children and is probably the least understood (12). To our knowledge no previous study has looked at the relationship between body fat and BMI across this wide prepubertal age range (3–12 yr).
The primary aim of this study was to establish whether BMI is an appropriate surrogate measure of body fat in prepubertal children. A secondary aim was to determine whether sex and ethnicity differences exist in the body fat-BMI relationship in this prepubertal period. A third aim was to compare the body fat-BMI relationship between Asian children from urban settings on two separate continents—Asian (Chinese) children raised in Jinan City, Mainland China, and Asian (Chinese and Korean) children raised in New York City (NYC). With respect to the latter, this study aimed to compare populations of similar genetic background from distinctly different environments. Overall, this study was undertaken to test the hypothesis that BMI is representative of body fatness independent of age, sex, ethnicity, and environment in prepubertal children.
METHODS
Protocol
All body composition evaluations were carried out on the same day at least 1 h after a light meal, with the subject clothed in a hospital gown and wearing foam slippers. The studies were approved by the Institutional Review Board of St. Luke's-Roosevelt Hospital (SLRHC) and by the Institutional Review Board of Jinan Maternity and Childcare Hospital (JMCH).
Subjects
Included in the study were a total of 605 prepubertal children (275 girls and 330 boys) of whom 247 were Asians from Jinan, Shandong, Mainland China (Jinan Asians), and 358 children were from various ethnic backgrounds in New York City (NYC)–121 Caucasians, 94 African Americans, and 143 Asians. The Asian volunteers from Jinan were Chinese and those from NYC were of Chinese and Korean background. The children ranged in age from 3 to 12 yr. The Jinan Asians were recruited (2003–2004) through local schools or were children of hospital employees. The NYC children were recruited (1995–2000) through local newspaper notices, announcements at schools and after-school centers, and word of mouth. Consistent Asian, non-Hispanic African American, or non-Hispanic Caucasian background of both parents and all four grandparents by questionnaire established ethnicity in the NYC group.
After physical examination, only prepubertal children were included in the study. Prepubertal status (breast stage 1 and pubic hair stage 1 for female subjects; and genital stage 1 and pubic hair stage 1 for male subjects) was established according to the criteria of Tanner (38) by a pediatric endocrinologist or nurse. There were no body weight or height limitations to enrollment, and inclusion criteria required that participants be healthy and free from any diagnosed medical condition that could potentially affect the variables under investigation. Consent was obtained from each volunteer's parent or guardian, and assent was obtained from each volunteer as well.
Anthropometrics
A physical examination was performed including body weight, height, and pubertal staging. All measurements were standardized between SLRHC and JMCH; a pediatrician from JMCH received training in the Body Composition Unit of SLRHC in the methods used for data collection before initiation of the study. Body weight was measured to the nearest 0.1 kg (SLRHC: Weight Tronix, New York, NY; JMCH: Wuxi Weigher Factory, Wuxi City, China) and height to the nearest 0.5 cm using a stadiometer (SLRHC: Holtain, Crosswell, UK; JMHC: Shanghai Drawing Stationery Factory, Shanghai, China).
Dual-Energy X-Ray Absorptiometry
Total body fat (TBF) was measured with a whole body dual-energy X-ray absorptiometry (DXA) scanner (SLRHC: DPX, Lunar, Madison, WI, using pediatric software version 3.8G; JMCH: Prodigy, GE Lunar, Madison, WI, using pediatric software version 4.0).
DXA Quality Control
A standard soft-tissue calibration method for DXA developed in NYC and used daily since 1987 was employed in both the JMCH and SLRHC laboratories and has been previously described (25). During the study setup phase, ethanol and water bottles (8-liter volume, Fischer Scientific, Pittsburgh, PA), simulating fat and fat-free soft tissues, respectively, were scanned as soft-tissue quality control markers three times per day for 7 consecutive days in JMCH. These data were sent to the Body Composition Unit in NYC for quality control verification. The purpose of this calibration procedure was twofold: 1) to monitor the stability of a DXA machine over time, and 2) to allow for the pooling of DXA soft-tissue data (i.e., PBF) collected on different machines. The latter is essential when data collected at different locations on similar machines from the same manufacturer are compared, because machines do not read the same exact soft-tissue values. During the course of the data collection phase, the soft-tissue quality control was performed weekly (when no subject was studied) and each day that a subject was studied. Reproducibility of DXA in children has been reported (15); however, because of concerns surrounding unnecessary radiation exposure in healthy children, scan reproducibility in children was not performed in this study. All DXA data were sent to NYC and analyzed by a single technician in the Body Composition Unit.
PBF Conversion Equation
To answer the question of whether, for a similar BMI, Asian children from JMCH have a greater PBF compared with NYC Caucasians, African Americans, and Asians, the PBF readings (derived from the ratio of the attenuation at 2 energy peaks) from the methanol and water bottles were used to generate a regression equation where PBF for the JMHC sample was converted to NYC values. The conversion equation was: PBF Jinan Asian = 0.9644218 × JMHC + 3.536583.
Statistical Analysis
ANOVA was used to test the effects of sex, race, and sex-by-race interaction on the demographic variables age, body mass, stature, BMI, TBF, and PBF. To test the main hypothesis, multiple linear regression analysis was used to investigate the possible influence of age, sex, and ethnicity on the relation between TBF and BMI and PBF and BMI. In these analyses TBF and PBF were used as the dependent variables, and BMI, sex, age, ethnic group, and their interaction terms were used as independent variables. Gender and ethnic groups were coded as dummy variables (sex: 1 = male, 0 = female; ethnicity: c = 1 if Caucasian, 0 otherwise; b = 1 if African American, 0 otherwise; a = 1 if Asian, 0 otherwise, making the Jinan group the reference group). Two-sided P values were considered significant at P < 0.05. Pooled subject data are expressed as the mean with SD. Data were analyzed using the SAS statistical program version 9.1 (35).
RESULTS
Group Characteristics
Characteristics for the 605 prepubertal subjects are presented in Table 1. The Jinan Asian children were younger (P < 0.001), shorter (P < 0.001), and had higher TBF and PBF (P < 0.001). Across groups, girls in general weighed less (P < 0.001), were shorter (P < 0.001), and had a lower BMI (P < 0.01) compared with boys. A correlation matrix describing the relationships between the main variables is presented in Table 2.
Table 1.
Descriptive characteristics of subjects
| Jinan Asian |
NYC Asian | NYC Caucasian | NYC African American | |||||
|---|---|---|---|---|---|---|---|---|
| Boys (n = 148) | Girls (n = 99) | Boys (n = 74) | Girls (n = 69) | Boys (n = 66) | Girls (n = 55) | Boys (n = 42) | Girls (n = 52) | |
| Age, yr | 7.11±2.23 | 5.99±1.94 | 8.11±1.54 | 7.77±1.42 | 7.88±1.59 | 8.16±1.46 | 7.76±1.61 | 7.77±1.49 |
| Weight, kg | 32.46±13.53 | 25.05±8.23 | 31.84±8.57 | 27.67±6.85 | 30.77±9.99 | 29.64±9.18 | 32.43±12.14 | 30.34±8.40 |
| Height, cm | 128.79±14.43 | 120.51±13.70 | 131.55±9.93 | 127.17±8.69 | 131.34±10.84 | 130.53±10.97 | 131.06±11.26 | 129.46±9.99 |
| BMI, kg/m2 | 18.76±4.63 | 16.86±2.92 | 18.11±2.94 | 16.90±2.56 | 17.40±3.17 | 17.03±3.13 | 18.44±4.66 | 17.93±3.65 |
| DXA | ||||||||
| TBF, kg | 10.64±8.49 | 7.57±5.03 | 7.19±4.50 | 6.53±4.14 | 5.87±5.12 | 6.64±5.10 | 6.60±7.45 | 7.07±5.76 |
| PBF, % | 28.43±11.89 | 27.81±9.06 | 20.96±8.55 | 22.00±8.46 | 16.87±8.68 | 20.51±7.71 | 16.68±11.36 | 21.19±9.96 |
All values are given as means ± SD (unadjusted); n = 330 boys and 275 girls. NYC, New York City; BMI, body mass index; DXA, dual-energy X-ray absorptiometry; TBF, total body fat; PBF, percent body fat.
Table 2.
Pearson correlation matrix between main variables
| Variable | PBF | TBF | Height | Weight | BMI | Age |
|---|---|---|---|---|---|---|
| PBF | 1.0000 | 0.9011 | 0.3367 | 0.7094 | 0.8303 | 0.1790 |
| TBF | 0.9011 | 1.0000 | 0.5643 | 0.9140 | 0.9287 | 0.4129 |
| Height | 0.3367 | 0.5643 | 1.0000 | 0.8070 | 0.4353 | 0.8746 |
| Weight | 0.7094 | 0.9140 | 0.8070 | 1.0000 | 0.8701 | 0.6528 |
| BMI | 0.8303 | 0.9287 | 0.4353 | 0.8701 | 1.0000 | 0.2966 |
| Age | 0.1790 | 0.4129 | 0.8746 | 0.6528 | 0.2966 | 1.0000 |
All correlations are significantly different from zero (P < 0.0001).
Fatness-BMI Regression Models
TBF vs. BMI.
Multiple regression analysis was performed for all subjects with TBF as the dependent variable and BMI, age, sex, ethnicity, and their interactions as independent variables (Table 3). TBF was first regressed onto BMI alone. Results indicated that BMI was significantly associated with TBF (R2 = 0.85; P < 0.0001). Age was next added to the model as a covariate, and the variance increased to 87% (P < 0.0001). To test whether the association between BMI and TBF was consistent across the age range, the BMI × age interaction was included. The interaction was significant (P < 0.0001) but increased the explained variance by only 1%, suggesting that the association between BMI and TBF is generally consistent across the age range in these prepubertal children. When sex was added as a covariate, the variance increased modestly to 89% (P < 0.0001), with girls having significantly higher TBF (adjusted) than boys. With the addition of ethnicity to the model, the explained variance increased to 94% (P < 0.0001). Jinan Asians had significantly higher TBF (adjusted) than all NYC groups (P < 0.0001). When groups within NYC were compared, African Americans had lower TBF (adjusted) than Caucasians (P < 0.01) and Asians (P < 0.0002), while no difference was seen between Caucasians and Asians.
Table 3.
Multiple regression analysis of TBF vs. BMI, age, sex, and ethnicity for the total study population
| Coefficient ± SE |
SEE | R2 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| BMI | Age | BMI × age | Sex‡ | NYC Caucasian§ | NYC African American§ | NYC Asian§ | Intercept | ||
| 1.599±0.027† | −20.612±0.496† | 2.470 | 0.85 | ||||||
| 1.531±0.027† | 0.440±0.052† | −22.664±0.528† | 2.336 | 0.87 | |||||
| 0.571±0.101† | −1.729±0.226† | 0.122±0.012† | −5.809±1.787* | 2.17 | 0.88 | ||||
| 0.559±0.100† | −1.780±0.224† | 0.125±0.012† | −0.761±0.178† | −5.261±1.767* | 2.140 | 0.89 | |||
| 0.675±0.076† | −1.110±0.172† | 0.103±0.009† | −1.071±0.136† | −2.967±0.190† | −3.568±0.203† | −2.693±0.180† | −7.439±1.342† | 1.620 | 0.94 |
R2 is the explained variance of the model; SE is standard error; SEE is standard error of estimate.
P < 0.001,
P < 0.0001.
1 = male, 0 = female.
Reference group: Jinan Asian.
PBF vs. BMI.
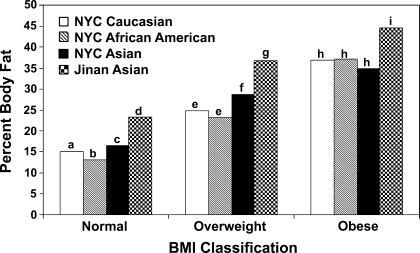
The overall regression models with PBF as a dependent variable are shown in Table 4. PBF was first regressed onto BMI alone. Results indicate that BMI was significantly associated with PBF (R2 = 0.69; P < 0.0001). Age was next added to the multiple regression model as a covariate, and even though it was significant, it did not add to the model (R2 = 0.69). To test whether the association was consistent across the entire age range, the BMI × age interaction was tested, which was not statistically significant (not shown in Table 4). The addition of sex significantly added to the model (R2 = 0.72), with girls having higher PBF than boys (P < 0.0001). Finally when ethnicity was added to the model, it resulted in a marked significant increase in the explained variance (R2 = 0.86). Significant differences were seen between PBF in all four ethnic groups: African American had the lowest PBF (adjusted), followed by NYC Caucasians, NYC Asians, and Jinan Asians (P < 0.001). The PBF (adjusted for age and sex) in Asians was significantly higher than the NYC Caucasians and African Americans (Fig. 1). The positive age coefficient in the final model indicates that after controlling for BMI, sex, and ethnicity, TBF increases with age.
Table 4.
Multiple regression analysis of PBF vs. BMI, age, sex, and ethnicity for the total study population
| Coefficient ± SE |
SEE | R2 | ||||||
|---|---|---|---|---|---|---|---|---|
| BMI | Age | Sex‡ | NYC Caucasian§ | NYC African American§ | NYC Asian§ | Intercept | ||
| 2.417±0.066† | −19.660±1.198† | 5.970 | 0.69 | |||||
| 2.481±0.069† | −0.412±0.132* | −17.743±1.338† | 5.927 | 0.69 | ||||
| 2.553±0.066† | −0.364±0.126* | −3.616±0.469† | −17.404±1.278† | 5.658 | 0.72 | |||
| 2.409±0.048† | 0.409±0.096† | −4.547±0.335† | −8.601±0.469† | −10.535±0.501† | −6.477±0.443† | −15.170±0.913† | 4.013 | 0.86 |
P < 0.001,
P < 0.0001.
1 = male, 0 = female.
Reference group: Jinan Asian.
Fig. 1.
Percent body fat (adjusted for sex and age) by ethnic groups and body mass index (BMI) classifications. Bars within each BMI category not sharing the same letter are significantly different (P < 0.05). NYC, New York City.
Other analytical approaches.
When TBF was used as the dependent variable with body weight and height (as opposed to BMI) used as separate independent variables in the model, results were similar to those obtained when BMI was used as the independent variable. The influence of sex and ethnicity was highly significant (P < 0.001). The variance explained in TBF was higher when body weight and height were used as separate independent variables (R2 = 0.91) compared with the model in which BMI was an independent variable (R2 = 0.85). The explained variance for PBF as the dependent variable was, however, lower when body weight and height were used as separate independent variables (R2 = 0.66) compared with the model in which BMI was an independent variable (R2 = 0.69).
DISCUSSION
The purpose of this study was to test the hypothesis that BMI is representative of body fatness independent of age, sex, ethnicity, and geographic location in prepubertal children. While BMI and sex were not surprisingly found to be significant predictors of TBF, ethnicity and geographic location were found to be significant contributors to the model with Asians (Jinan and NYC Asians) having higher PBF than all non-Asian groups, and Jinan Asians having higher TBF and PBF than NYC Asians. The latter are important and novel findings of this study.
BMI As a Measure of Body Fatness
The results of this study carried out in four different groups over a wide prepubertal age range with an established body composition reference method indicate that BMI accounts for a large proportion of the between-individual differences in fatness. Specifically, in the pooled sample BMI alone explained 85% and 69% of the between-individual differences in TBF and PBF, respectively. The finding that BMI is closely related to TBF and PBF derived from DXA is compatible with several previous investigations in children over a wide age range and in adults (7, 13, 14, 16, 22, 24, 30, 32, 36). Results were similar although quantitatively different when PBF was examined as the dependent variable rather than TBF. The stronger association observed between BMI and TBF than between BMI and PBF is consistent with several previous investigations (7, 21, 32). For the present data, weight and height as independent variables performed better than BMI for predicting TBF, whereas BMI as an independent variable performed better than weight and height for predicting PBF.
BMI-Ethnicity Relations
The International Obesity Taskforce (IOTF) recommends BMI as an index of fatness in children and has developed international cutoff points for BMI for obesity by sex between 2 and 18 yr, defined to pass through BMI of 30 at age 18, and were obtained by averaging data from Brazil, Great Britain, Hong Kong, Netherlands, Singapore, and United States (4). These cutoff points were used to calculate the cutoffs for normal, overweight, and obese children in the present study. Even though the mean BMI among the ethnic groups was not significantly different within each subgroup, the PBF (adjusted for age and sex) in Asians was significantly higher than the NYC Caucasians and African Americans (Fig. 1). This extends earlier findings in which a different relationship between BMI and PBF has been reported in Indonesian (23), Koreans (3), and in Singaporean Chinese (10, 11). Differences have also been observed across Black populations living on different continents (18) and between Caucasians and African Americans (6) and between Caucasians and Polynesians (37). The present study confirms that the relationship between BMI and PBF in Asians is different from that in Caucasians and African Americans. The elevated TBF and PBF at low levels of BMI suggest that the BMI cutoff values recommended by IOTF are too high for Asian prepubertal children and may need to be lowered. With the lowered values, the overweight and obesity rates will be much higher in Asian children, and this could have important implications on public health policy.
BMI-Environmental Relations
This study also offered a unique opportunity to compare populations of similar genetic background from distinctly different environments or geographic locations. A comparison of Asian children from urban settings on two separate continents showed the Jinan Asians have significantly higher PBF compared with the NYC Asians (P < 0.001). Urbanization has been suggested as an important obesity risk factor in developing nations undergoing fast economic transition (33). It has been reported that many urban Chinese residents have high family incomes, easier access to energy-dense fast food, and are also adopting more sedentary lifestyles (41). With one-child per family in China, there is also an increased chance of overnutrition (2). All of these could contribute to the greater PBF in Jinan Asians compared with NYC Asians.
BMI-Age Relations
An important consideration in this study was whether age contributed to fatness predictions in prepubertal children after controlling for BMI. After adjusting for BMI, increasing age was associated with a significantly greater TBF but lower PBF. Although these fat-related observations may seem contradictory, they are nonetheless consistent with each other. If a young and an older subject has an equivalent BMI and PBF, the older and thus heavier and taller subject would have a greater TBF. Children in this study, after controlling for BMI, had greater TBF with greater age. Evidently, the relative increase in fat-free body mass with increasing age was greater than that for TBF, and therefore the proportion of body mass as fat (i.e., PBF) decreased. These observations highlight the differing nature of TBF and PBF as measures of fatness. Similar findings were reported in healthy Italian children between 5 and 19 yr of age (32).
BMI-Sex Relations
While it is known that sex differences are consistently present in adolescents (5, 8, 31), greater subcutaneous fat (5, 20, 34) and greater TBF (39) have been reported in girls than in boys before puberty. The results of the present study using DXA confirm significantly greater amounts of TBF and PBF in prepubertal girls compared with boys for an equivalent BMI. The difference in PBF between boys and girls of similar BMI were maintained throughout the prepubertal age range of 3–12 yr (P < 0.05) and is consistent with previous reports in prepubertal children (1, 7, 19).
Study Limitations
Although this study was based on a large population of children, as with all convenience samples, the results should be considered sample specific. This study was also cross-sectional; there is a clear need to explore the observed relations between BMI, fatness, age, sex, and ethnicity in longitudinally evaluated cohorts. Last, the purpose of this study was to evaluate the relationship between BMI and body fat measurements and not to establish reference values for prepubertal children from various ethnicities.
Conclusion
While the results of this study support the recommendation that BMI be used as a screening tool for pediatric obesity in Asian and non-Asian prepubertal children, we conclude that for comparisons of fatness/obesity across ethnic groups, universal BMI cutoff points would be misleading. For the same BMI, Asian prepubertal children both in the United States and China have higher PBF than Caucasians and African Americans, which puts them at a higher risk of developing chronic diseases.
GRANTS
This study was supported in part by National Institutes of Health Grants P30-DK-26687, RO1-DK-37352, RO1-HD-42187, and RR-024156.
REFERENCES
- 1.Arfai K, Pitukcheewanont PD, Goran MI, Tavare CJ, Heller L, Gilsanz V. Bone, muscle, and fat: sex-related differences in prepubertal children. Radiology 224: 338–344, 2002. [DOI] [PubMed] [Google Scholar]
- 2.Cheng TO One-child policy and increased mechanization are additional risk factors for increased coronary artery disease in modern China. Int J Cardiol 100: 333, 2005. [DOI] [PubMed] [Google Scholar]
- 3.Chung S, Song M, Shin H, Kim D, He Q, Heshka S, Wang J, Thornton J, Laferrère B, Pi-Sunyer FX, Gallagher D. Korean and Caucasian overweight premenopausal women have different relationship of body mass index to percent body fat with age. J Appl Physiol 99: 103–107, 2005. [DOI] [PubMed] [Google Scholar]
- 4.Cole TJ, Bellizzi MC, Flegal KM, Dietz WH. Establishing a standard definition for child overweight and obesity worldwide: international survey. BMJ 320: 1240–1243, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cowell CT, Briody J, Lloyd-Jones S, Smith C, Moore B, Howman-Giles R. Fat distribution in children and adolescents–the influence of sex and hormones. Horm Res 48, Suppl 5: 93–100, 1997. [DOI] [PubMed] [Google Scholar]
- 6.Daniels SR, Khoury PR, Morrison JA. The utility of body mass index as a measure of body fatness in children and adolescents: differences by race and gender. Pediatrics 99: 804–807, 1997. [DOI] [PubMed] [Google Scholar]
- 7.Dencker M, Thorsson O, Linden C, Wollmer P, Andersen LB, Karlsson MK. BMI and objectively measured body fat and body fat distribution in prepubertal children. Clin Physiol Funct Imaging 27: 12–16, 2007. [DOI] [PubMed] [Google Scholar]
- 8.Deurenberg P, Pieters JJ, Hautvast JG. The assessment of the body fat percentage by skinfold thickness measurements in childhood and young adolescence. Br J Nutr 63: 293–303, 1990. [DOI] [PubMed] [Google Scholar]
- 9.Deurenberg P, Yap M, van Staveren WA. Body mass index and percent body fat: a meta analysis among different ethnic groups. Int J Obes Relat Metab Disord 22: 1164–1171, 1998. [DOI] [PubMed] [Google Scholar]
- 10.Deurenberg-Yap M, Chew SK, Lin VF, Tan BY, van Staveren WA, Deurenberg P. Relationships between indices of obesity and its co-morbidities in multi-ethnic Singapore. Int J Obes Relat Metab Disord 25: 1554–1562, 2001. [DOI] [PubMed] [Google Scholar]
- 11.Deurenberg-Yap M, Schmidt G, van Staveren WA, Deurenberg P. The paradox of low body mass index and high body fat percentage among Chinese, Malays and Indians in Singapore. Int J Obes Relat Metab Disord 24: 1011–1017, 2000. [DOI] [PubMed] [Google Scholar]
- 12.Dietz WH Critical periods in childhood for the development of obesity. Am J Clin Nutr 59: 955–959, 1994. [DOI] [PubMed] [Google Scholar]
- 13.Eisenmann JC, Heelan KA, Welk GJ. Assessing body composition among 3- to 8-year-old children: anthropometry, BIA, and DXA. Obes Res 12: 1633–1640, 2004. [DOI] [PubMed] [Google Scholar]
- 14.Ellis KJ, Shypailo RJ, Pratt JA, Pond WG. Accuracy of dual-energy x-ray absorptiometry for body-composition measurements in children. Am J Clin Nutr 60: 660–665, 1994. [DOI] [PubMed] [Google Scholar]
- 15.Figueroa-Colon R, Mayo MS, Treuth MS, Aldridge RA, Weinsier RL. Reproducibility of dual-energy X-ray absorptiometry measurements in prepubertal girls. Obes Res 6: 262–267, 1998. [DOI] [PubMed] [Google Scholar]
- 16.Fors H, Gelander L, Bjarnason R, Albertsson-Wikland K, Bosaeus I. Body composition, as assessed by bioelectrical impedance spectroscopy and dual-energy X-ray absorptiometry, in a healthy paediatric population. Acta Paediatr 91: 755–760, 2002. [DOI] [PubMed] [Google Scholar]
- 17.Gallagher D, Heymsfield SB, Heo M, Jebb SA, Murgatroyd PR, Sakamoto Y. Healthy percentage body fat ranges: an approach for developing guidelines based on body mass index. Am J Clin Nutr 72: 694–701, 2000. [DOI] [PubMed] [Google Scholar]
- 18.Gallagher D, Visser M, Sepúlveda D, Pierson RN, Harris T, Heymsfield SB. How useful is body mass index for comparison of body fatness across age, sex, and ethnic groups? Am J Epidemiol 143: 228–239, 1996. [DOI] [PubMed] [Google Scholar]
- 19.Garnett SP, Hogler W, Blades B, Baur LA, Peat J, Lee J, Cowell CT. Relation between hormones and body composition, including bone, in prepubertal children. Am J Clin Nutr 80: 966–972, 2004. [DOI] [PubMed] [Google Scholar]
- 20.Gasser T, Ziegler P, Largo RH, Molinari L, Prader A. A longitudinal study of lean and fat areas at the arm. Ann Hum Biol 21: 303–314, 1994. [DOI] [PubMed] [Google Scholar]
- 21.Goran MI, Driscoll P, Johnson R, Nagy TR, Hunter G. Cross-calibration of body-composition techniques against dual-energy X-ray absorptiometry in young children. Am J Clin Nutr 63: 299–305, 1996. [DOI] [PubMed] [Google Scholar]
- 22.Goulding A, Taylor RW, Gold E, Lewis-Barned NJ. Regional body fat distribution in relation to pubertal stage: a dual-energy X-ray absorptiometry study of New Zealand girls and young women. Am J Clin Nutr 64: 546–551, 1996. [DOI] [PubMed] [Google Scholar]
- 23.Gurrici S, Hartriyanti Y, Hautvast JG, Deurenberg P. Relationship between body fat and body mass index: differences between Indonesians and Dutch Caucasians. Eur J Clin Nutr 52: 779–783, 1998. [DOI] [PubMed] [Google Scholar]
- 24.Gutin B, Litaker M, Islam S, Manos T, Smith C, Treiber F. Body-composition measurement in 9–11-y-old children by dual-energy X-ray absorptiometry, skinfold-thickness measurements, and bioimpedance analysis. Am J Clin Nutr 63: 287–292, 1996. [DOI] [PubMed] [Google Scholar]
- 25.He Q, Zhang X, He S, Gong L, Sun Y, Heshka S, Deckelbaum RJ, Gallagher D. Higher insulin, triglycerides, and blood pressure with greater trunk fat in Tanner 1 Chinese. Obesity (Silver Spring) 15: 1004–1011, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Keys A, Fidanza F, Karvonen MJ, Kimura N, Taylor HL. Indices of relative weight and obesity. J Chronic Dis 25: 329–343, 1972. [DOI] [PubMed] [Google Scholar]
- 27.Lee SY, Gallagher D. Assessment methods in human body composition. Curr Opin Clin Nutr Metab Care 11: 566–572, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Luke A, Durazo-Arvizu R, Rotimi C, Prewitt TE, Forrester T, Wilks R, Ogunbiyi OJ, Schoeller DA, McGee D, Cooper RS. Relation between body mass index and body fat in black population samples from Nigeria, Jamaica, and the United States. Am J Epidemiol 145: 620–628, 1997. [DOI] [PubMed] [Google Scholar]
- 29.McKeigue PM, Shah B, Marmot MG. Relation of central obesity and insulin resistance with high diabetes prevalence and cardiovascular risk in South Asians. Lancet 337: 382–386, 1991. [DOI] [PubMed] [Google Scholar]
- 30.Mei Z, Grummer-Strawn LM, Pietrobelli A, Goulding A, Goran MI, Dietz WH. Validity of body mass index compared with other body-composition screening indexes for the assessment of body fatness in children and adolescents. Am J Clin Nutr 75: 978–985, 2002. [DOI] [PubMed] [Google Scholar]
- 31.Ogle GD, Allen JR, Humphries IR, Lu PW, Briody JN, Morley K, Howman-Giles R, Cowell CT. Body-composition assessment by dual-energy x-ray absorptiometry in subjects aged 4–26 y. Am J Clin Nutr 61: 746–753, 1995. [DOI] [PubMed] [Google Scholar]
- 32.Pietrobelli A, Faith MS, Allison DB, Gallagher D, Chiumello G, Heymsfield SB. Body mass index as a measure of adiposity among children and adolescents: a validation study. J Pediatr 132: 204–210, 1998. [DOI] [PubMed] [Google Scholar]
- 33.Popkin BM, Doak CM. The obesity epidemic is a worldwide phenomenon. Nutr Rev 56: 106–114, 1998. [DOI] [PubMed] [Google Scholar]
- 34.Prader A, Largo RH, Molinari L, Issler C. Physical growth of Swiss children from birth to 20 years of age. First Zurich longitudinal study of growth and development. Helv Paediatr Acta Suppl 52: 1–125, 1989. [PubMed] [Google Scholar]
- 35.SAS Institute. SAS User's Guide: Statistics. Cary, NC: SAS, 2008.
- 36.Steinberger J, Jacobs DR, Raatz S, Moran A, Hong CP, Sinaiko AR. Comparison of body fatness measurements by BMI and skinfolds vs. dual energy X-ray absorptiometry and their relation to cardiovascular risk factors in adolescents. Int J Obes (Lond) 29: 1346–1352, 2005. [DOI] [PubMed] [Google Scholar]
- 37.Swinburn BA, Craig PL, Daniel R, Dent DP, Strauss BJ. Body composition differences between Polynesians and Caucasians assessed by bioelectrical impedance. Int J Obes Relat Metab Disord 20: 889–894, 1996. [PubMed] [Google Scholar]
- 38.Tanner JM Growth at Adolescence. Oxford, UK: Blackwell, 1962.
- 39.Taylor RW, Gold E, Manning P, Goulding A. Gender differences in body fat content are present well before puberty. Int J Obes Relat Metab Disord 21: 1082–1084, 1997. [DOI] [PubMed] [Google Scholar]
- 40.Wang J, Thornton JC, Russell M, Burastero S, Heymsfield S, Pierson RN, Jr. Asians have lower body mass index (BMI) but higher percent body fat than do whites: comparisons of anthropometric measurements. Am J Clin Nutr 60: 23–28, 1994. [DOI] [PubMed] [Google Scholar]
- 41.Wang Y, Lobstein T. Worldwide trends in childhood overweight and obesity. Int J Pediatr Obes 1: 11–25, 2006. [DOI] [PubMed] [Google Scholar]
- 42.WHO Expert Consultation. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet 363: 157–163, 2004. [DOI] [PubMed] [Google Scholar]