Abstract
In pulmonary emphysema, the alveolar structure progressively breaks down via a three-dimensional (3D) process that leads to airspace enlargement. The characterization of such structural changes has, however, been based on measurements from two-dimensional (2D) tissue sections or estimates of 3D structure from 2D measurements. In this study, we developed a novel silver staining method for visualizing tissue structure in 3D using micro-computed tomographic (CT) imaging, which showed that at 30 cmH20 fixing pressure, the mean alveolar airspace volume increased from 0.12 nl in normal mice to 0.44 nl and 2.14 nl in emphysematous mice, respectively, at 7 and 14 days following elastase-induced injury. We also assessed tissue structure in 2D using laser scanning confocal microscopy. The mean of the equivalent diameters of the alveolar airspaces was lower in 2D compared with 3D, while its variance was higher in 2D than in 3D in all groups. However, statistical comparisons of alveolar airspace size from normal and emphysematous mice yielded similar results in 2D and 3D: compared with control, both the mean and variance of the equivalent diameters increased by 7 days after treatment. These indexes further increased from day 7 to day 14 following treatment. During the first 7 days following treatment, the relative change in SD increased at a much faster rate compared with the relative change in mean equivalent diameter. We conclude that quantifying heterogeneity in structure can provide new insight into the pathogenesis or progression of emphysema that is enhanced by improved sensitivity using 3D measurements.
Keywords: heterogeneity, alveolar airspace diameter, confocal microscopy, silver staining
pulmonary emphysema is characterized by the progressive breakdown of alveolar walls and the subsequent appearance of abnormally enlarged airspaces (1, 47). Quantifying such structural changes in the lung plays a vital role in detecting and assessing the extent of emphysema. Currently two-dimensional (2D) histological sections are used to visualize the three-dimensional (3D) structure of the parenchyma. Such 2D approaches have important limitations when it comes to understanding the true 3D structure of the parenchyma (41) and also in relation to the interpretation of disease progression. For instance, on a 2D histological section, it may be difficult to ascertain whether a larger than average airspace belongs to a duct or a true alveolar airspace that has been enlarged by the disease. These drawbacks can be circumvented by imaging lung tissue structure in 3D.
Direct measurement of 3D structural properties of the parenchyma has always been limited by the inability to visualize the structure in 3D. Consequently, current methods employ measurements made on 2D histological sections to estimate 3D structural parameters using stereological techniques (3, 17, 23, 33, 58, 59). Previous attempts at direct measurement of 3D structure of lung tissue have been limited to reconstructions of structures such as alveoli from serial histological sections (7, 36–38). However, gaps between sections and deformation as well as shrinkage during sectioning make these methods unreliable for the quantitative characterization of the structure in 3D. Advances in micro-focal computed tomographic (μ-CT) imaging have made it possible to visualize anatomical structures in 3D with micrometer resolution (13, 44). Recently, μ-CT imaging has been used to visualize the 3D structure of human, pig, and rat lung tissue (28, 29, 56). This has opened up the possibility to directly measure structural properties of the lung parenchyma in 3D. One such property that is of ubiquitous interest, is the real size of an alveolar airspace (7, 11, 18, 31, 50). However, no direct measurement of alveolar airspace volumes has been reported.
In this study, we used μ-CT imaging to visualize normal and emphysematous mouse lung tissue. To image the small alveoli and thin septal walls of the mouse lung, we developed a simple, but sensitive technique for staining lung tissue with silver. Using this technique, we were able to reconstruct the tissue structure in 3D, identify individual alveolar airspaces, and measure their volumes during the course of emphysema. To validate the μ-CT-based measurements, we compared 2D sections from μ-CT images with those obtained with a high-resolution confocal microscopy.
METHODS
Animal preparation.
We imaged lungs from three groups of 10-wk-old male C57BL/6 mice (Charles River, Boston, MA) weighing 24–26 g: a normal group (n = 4) and two emphysematous groups (n = 4 each). The mice were initially anesthetized with isoflurane and treated with oropharyngeal instillation of 50 μg (0.25 IU) of porcine pancreatic elastase (PPE; Sigma-Aldrich, St. Louis, MO) dissolved in 100 μl of phosphate buffered saline (14, 16, 26, 46, 48). Mice were treated on day 0 and again on day 2. One week following initial treatment, animals in the first group (day 7) were anesthetized by intraperitoneal injection of pentobarbital sodium (70 mg/kg), tracheostomized, and cannulated in the supine position. The cannula was connected to a computer-controlled ventilator (Flexivent, SCIREQ, Montreal, Canada) and lung mechanics were measured. The animals were then killed by exsanguination, and the lungs were fixed tracheally with 10% neutral buffered formalin at an airway pressure of 30 cmH2O. The same procedure was repeated on the second group of treated mice 2 wk after initial treatment (day 14). After fixation, tissue samples were cut manually from the lower right lobe for imaging. The protocol was approved by Boston University's Institutional Animal Care and Use Committee.
Measurement of respiratory mechanics.
Mice were mechanically ventilated with room air using a tidal volume (VT) of 8 ml/kg at a frequency of 240 breaths/min. After stabilization, dynamic respiratory mechanics were measured at 3 cmH2O positive end-expiratory pressure (PEEP) in the closed chest condition using the forced oscillation technique combined with the optimal ventilator waveform (OVW; Ref. 32). The OVW is a composite waveform consisting of five sine waves so that in the time domain the waveform is similar to a tidal breath while allowing a smooth estimation of the impedance. During measurement, the peak-to-peak OVW amplitude was matched to the VT delivered by the mechanical ventilator. To standardize volume history, each measurement was preceded by two consecutive inflations of the lungs to total lung capacity (TLC) as recruitment maneuvers, defined as a tracheal pressure of 25 cmH2O. The pressure was maintained for 3 s. The impedance spectra were then fitted to the constant phase model (19) to obtain dynamic elastance of the respiratory system (H).
Confocal microscopy.
Autofluorescence was used to visualize alveolar structure in 2D. The samples were excited using a 488 nm laser and the fluorescence between 500 and 600 nm was collected with an Olympus FV-1000 laser scanning confocal microscope (LSCM). The images had an in-plane resolution of 1.2 μm and an axial resolution 2.2 μm. During imaging, sampling of the same alveolus more than once was carefully avoided. This was done by making sure that all images were from the same focal plane and there was no overlap in the fields of view corresponding to two images. Also, these nonoverlapping fields of view were chosen from uniformly random locations in the sample. Individual airspaces were identified from the confocal images and their area measured by tracing the outline of the alveolar walls along an airspace using an operator-assisted method (Amira 4.0, Mercury Computer Systems, Chelmsford, MA). The area bounded by the closed contour was then taken as the area of the airspace. A minimum of 120 airspaces were identified from the LSCM images in each treatment group. The confocal microscopic images in this study were taken at the Whitaker imaging facility, Boston University.
Silver staining.
Silver staining (4, 39, 40) is a method commonly used for detecting proteins in polyacramide gels. We started with a commercially available staining kit (BioRad Silver Stain Plus, BioRad, Hercules, CA) based on the photochemical method described by Merril et al. (39). As it was intended for detecting proteins in gels, the original protocol only stained the exterior of tissue and had to be modified to obtain a uniform stain throughout the tissue piece so as to make it suitable for imaging using μ-CT.
The samples were first washed twice in distilled deionized water for 20 min to remove the excess fixative. A staining solution consisting of 5% silver complex solution, 5% reduction moderator solution, 5% image development reagent, and 35% distilled deionized water was prepared immediately prior to use. The washed samples were immersed in a staining solution for 5 min. To facilitate diffusion of the staining solution into the small airspaces of the mouse lung, the solution was gently stirred using an orbital shaker. Next, a development accelerator solution was added and the reaction was allowed to continue for another 5 min. The staining reaction was then stopped by transferring the tissue specimens to 5% acetic acid. The method produced a consistent stain throughout the tissue specimen.
μ-CT imaging.
The silver-stained tissue specimens were imaged using a small angle cone beam system (μ-CT 40, Scanco Medical, Brüttisellen, Switzerland) with isotropic resolution of 6–72 μm (45kV tube potential, 177 μA tube current, and a 300 ms integration time for each projection). Continuous slices were obtained at an isotropic resolution of 6 μm. The scanning was repeated three times and averaged to reduce noise. Continuous slices obtained using μ-CT were stacked up to reconstruct the tissue structure in 3D.
3D reconstruction.
A connected component labeling method (Amira, Mercury computer systems, Chelmsford, MA) was used to isolate airspaces connected to the largest airway in a sample. First, a seed point with a CT value Cs was chosen. All points that were connected to the seed with CT values Ci such that Ci - Cs ≤ T, where T is a threshold, were given a unique label. From this labeled set, which included alveolar ducts, alveoli, and small airways, alveoli were manually identified.
Measurement of alveolar airspace volumes.
Following 3D reconstruction, alveolar airspaces close to the edge of the silver-stained tissue specimen were examined to identify individual alveolar airspaces. The process of manual identification can be best explained via an example. Shown in Fig. 1A are three orthogonal planes intersecting a piece of tissue that was imaged using μ-CT. We focus our attention on the airspace that is marked by the red arrow. To examine the 3D structure of this airspace, we move the three slicing planes such that the point of intersection of these three planes falls inside the airspace of interest.
Fig. 1.
A: 3 orthogonal planes intersecting the 3-dimensional (3D) structure reconstructed from micro-computed tomographic (μ-CT) slices of an emphysematous mouse lung tissue (day 14 group). The dark regions represent airspaces and the lighter regions represent silver-stained tissue. We focus our attention on the airspace that is marked by the red arrow. The 3 planes are moved to intersect this airspace and the 3 orthogonal views of this airspace are shown in axial (A; horizontal), coronal (C; vertical), and sagittal (D; vertical) view. The 2 perpendicular lines indicate the other 2 orthogonal planes. The mouth of the airspace where it opens to the duct was manually identified using these 3 views. E and F: a 3D reconstruction, showing the branching from the top of the largest airway to an airspace (in blue and arrow). All other branches have been eliminated for ease of visualization.
The 2D views created by three orthogonal planes passing through the alveolus of interest are shown in Fig. 1, B-D, where Fig. 1B corresponds to the view generated by the horizontal plane and Fig. 1, C and D, correspond to the two vertical planes. In one of these 2D views (Fig. 1C), we can see that the alveolus actually opens into a duct. On this view, we first draw a line marking the junction between the alveolus and the duct. We then move the slicing planes around in 3D and repeat the process of identifying the joint between the duct and the alveolus on the other 2D sections. However, from these 2D sections alone, it is difficult to appreciate the 3D structure of the junction. Therefore, we also visualize and keep track of the 3D surface formed by junction identified on the 2D sections. This 3D surface that separates the alveolus from the duct is what is commonly referred to as the alveolar mouth. Once the 3D labeling of the mouth is complete, the alveolus becomes a closed 3D compartment whose volume can be measured by counting the number of voxels it contains. A minimum of 65 alveoli were measured in each treatment group.
Equivalent diameters.
To compare 2D area measurements from confocal microscopic images to 3D measurements of alveolar airspace volume from μ-CT and to measurements from existing literature, we used the cross-sectional areas measured from confocal microscopic images and alveolar airspace volumes measured from μ-CT to calculate equivalent diameters. In 2D, the equivalent diameter of a single alveolar airspace with cross-sectional area A identified from confocal images was calculated as:
 |
(1) |
which is equal to the diameter of a circle with area A. In 3D, the equivalent diameter of an alveolar airspace with volume V was calculated as:
 |
(2) |
which is the diameter of a sphere with volume V. It should be noted that d2D and d3D depend, respectively, only on the measured area in 2D or volume in 3D and are shape independent.
Statistical analysis.
One-way ANOVA was used to examine whether there was any significant differences between the different animals within each of the three groups: normal, 7 day, and 14 day (Sigmastat, Systat Software, San Jose, CA). Since there was no statistical difference between the different animals within any of the groups, the data from all animals in each group were combined to form three distinct data sets corresponding to normal, 7 day, and 14 day groups. To isolate the group or groups that differed from each other, the second one-way ANOVA was followed by pairwise comparison tests between groups using the Tukey test when data were normally distributed or the Dunn's test when they were not normally distributed. The F-test was used to evaluate differences in variance (Matlab, Mathworks, Natick, MA). The Kolmogrov-Smirnoff test (KS test) was used to detect differences between distributions (Matlab, Mathworks). The level of significance was 0.05.
RESULTS
Mechanics measurements.
Preliminary analysis of equivalent diameters from LSCM showed that one of the animals in the normal group had 30% higher mean diameter, which was statistically different from that in the other three mice. On further investigation it was found that this mouse was not age/weight matched to the rest of the population, therefore, both the mechanics and the morphological data from this mouse were discarded from subsequent analysis. The mean ± SD of dynamic elastance (H) for normal mice was found to be 20.7±1.2 cmH2O/ml. Animals in the day 7 group had significantly decreased H values with mean and SD of 15.1±1.6 cmH2O/ml (P < 0.005). Animals in the day 14 group had even lower H values with mean and SD of 14.2±3.9 cmH2O/ml, which, however, was not different from the mean H in the day 7 group.
μ-CT imaging of silver-stained lung tissue.
A typical μ-CT scan provided ∼300–500 continuous slices, which were stacked together to make 3D measurements of alveolar airspace volume (Fig. 1). Typical slices are shown in Fig. 2 with images from the three groups of mice. Images in Fig. 2, A–C, correspond to parenchymal tissues from representative animals in the normal, day 7, and day 14 groups, respectively. Note the visually apparent larger airspace sizes following treatment.
Fig. 2.
μ-CT images of silver-stained mouse lung tissue fixed at 30 cmH2O. A: normal lung tissue. B: lung tissue 7 days after elastase treatment. C: lung tissue 14 days after elastase treatment.
Fluorescent microscopic imaging of lung tissue.
Representative autofluorescent images obtained with the LSCM are shown in Fig. 3. Images in Fig. 3, A–C, correspond to parenchymal tissues from representative animals in the normal, day 7, and day 14 groups, respectively. With the increased resolution compared with the μ-CT, differences in airspace sizes among the groups are even more evident than in Fig. 2. Perhaps the most striking difference is, however, the appearance of heterogeneity in airspace structure at 7 days following treatment, which becomes even more substantial at 14 days after treatment.
Fig. 3.
Laser scanning confocal microscopic (LSCM) images of mouse lung tissue fixed at 30 cmH2O. A: normal lung tissue. B: lung tissue 7 days after elastase treatment. C: lung tissue 14 days after elastase treatment.
Measurement of 2D equivalent diameter.
Individual airspaces were manually identified from the confocal images and their areas were measured. From these measurements, equivalent diameters were calculated using Eq. 1. The results are summarized in Table 1, which lists the mean diameter, SD of diameters, and the coefficient of variation (CV), which is defined as the ratio of SD to the mean. These statistics were calculated for each animal in the group and are reported in Table 1 as mean ± SD across animals. The bar chart in Fig. 5 summarizes the mean and SD of each group.
Table 1.
Mean, SD, and CV of equivalent diameters of alveolar airspaces from 2D confocal microscopic images
| Mean, μm | SD, μm | CV, % | |
|---|---|---|---|
| Normal | 39±2 | 10±2 | 25±5 |
| Day 7 | 59±3 | 30±2 | 51±5 |
| Day 14 | 117±2 | 78±8 | 66±6 |
The variability indicated by the ± is the inter-animal SD of mean, SD, and coefficient of variance (CV). 2D, 2 dimensional.
One-way ANOVA on the equivalent diameters of alveolar airspaces was performed to test for differences between the three treatment groups. A statistically significant difference (P < 0.001) was found between the median value of equivalent diameters among the treatment groups, indicating that the effect of elastase treatment on equivalent diameters measured from 2D sections was significant. Pairwise comparisons of the three groups revealed significant differences between all three groups (P < 0.05). There was also a significant increase in the variance of equivalent diameters between the normal and the day 7 groups (P < 0.001) and also between the day 7 and 14 groups (P < 0.001). The difference in mean equivalent diameter was also significant between the normal and the day 7 groups (P < 0.001), and also between the day 7 group and day 14 group (P < 0.001).
Comparing 2D measurements from μ-CT and confocal microscopy.
The mean ± SD of 2D equivalent diameters measured from μ-CT images for normal mouse lungs were found to be 39 ± 10 μm. The cumulative probability distribution of 2D diameter measurements from μ-CT and LSCM images are shown in Fig. 4. As can be seen from the figure, the 2D diameters from both methods have nearly identical distributions. Indeed, the KS test showed that the two distributions were identical with a probability of 0.98.
Fig. 4.
The probability distribution of 2D equivalent diameters of normal mouse lungs measured using μ-CT and LSCM. Kolmogrov-Smirnoff test shows no statistical differences between the 2 distributions.
Measurement of alveolar airspace volumes and diameters.
The alveolar airspace volumes directly measured in 3D from the stacked μ-CT images are summarized in Table 2. For comparison with our 2D measurements from confocal images, we converted the volume measurements to equivalent diameters using Eq. 2. The results are listed in Table 3 and plotted as a bar chart in Fig. 5.
Table 2.
Mean, SD, and CV of alveolar airspace volumes measured in 3D from μ-CT
| Mean, nl | SD, nl | CV, % | |
|---|---|---|---|
| Normal | 0.12±0.02 | 0.08±0.01 | 63±8 |
| Day 7 | 0.44±0.14 | 0.50±0.3 | 108±44 |
| Day 14 | 2.14±0.32 | 1.62±0.5 | 75±14 |
The variability indicated by the ± is the inter-animal SD of mean, SD, and CV. μ-CT, micro-computed tomography.
Table 3.
Mean, SD, and CV of equivalent diameters of alveolar airspaces identified from 3D volume measurements from μ-CT
| Mean, μm | SD, μm | CV, % | |
|---|---|---|---|
| Normal | 59±2 | 12±1 | 20±2 |
| Day 7 | 84±5 | 28±12 | 33±13 |
| Day 14 | 150±6 | 39±9 | 26±6 |
The variability indicated by the ± is the inter-animal SD of mean, SD, and CV.
Fig. 5.
Comparison of 3D and 2D mean equivalent diameters of alveolar airspaces calculated from alveolar volumes measured from μ-CT and cross-sectional areas measured from LSCM. The bar and the error indicate the mean and SD of diameters in each treatment group. *3D mean diameter was significantly higher than the corresponding 2D mean diameter. § and +Significant increases in mean and SD of equivalent diameters between treatment groups (statistical significance of the difference in mean and SD was the same in both 2D and 3D).
A statistically significant difference (P < 0.001) was found between the mean value of equivalent diameters among the treatment groups, indicating that the effect of elastase treatment on 3D equivalent diameters of alveolar airspaces was significant. Pairwise comparison of the three groups revealed significant differences between all three groups (P < 0.05). There was a significant increase in the variance of the equivalent diameters between the normal and the day 7 groups (P < 0.001) and also between the day 7 and 14 groups (P < 0.001). The difference in mean equivalent diameter was also significant between the normal and the day 7 groups (P < 0.001) and also between the day 7 group and day 14 group (P < 0.001). These statistical tests were repeated on the alveolar airspace volumes and they yielded identical results.
DISCUSSION
The mouse is perhaps the most commonly used species as an animal model of pulmonary emphysema (8, 12, 46). However, all available quantitative information about the mouse lung structure, and the changes that occur in emphysema, come from measurements made on 2D lung tissue sections (22, 31) or estimates of 3D structure made from 2D measurements (21). In this study, we used μ-CT imaging to visualize the 3D structure of mouse lung tissue at the level of individual alveoli. To our knowledge, the measurements reported here represent the first direct 3D measurement of the volume of a single alveolus in the normal mouse lung without resorting to stereological techniques that estimate average 3D properties from 2D measurements. Additionally, we examined how alveolar airspace dimensions changed during the progression of elastase-induced emphysema in mice. Specifically, we found that, compared with the normal mice, elastase treatment led to a significant increase in the mean and SD of alveolar airspace volumes at day 7. These indexes showed a further significant increase from day 7 to day 14. While the findings from direct 3D measurements appeared to be consistent with those from 2D measurements, there are important differences. Before examining these issues, we first discuss the methodological factors associated with μ-CT imaging and the 3D processing.
Methodological factors.
A major challenge in μ-CT imaging of soft porous tissue such as the lung parenchyma is the low contrast between the tissue and the surrounding medium. To overcome this limitation, Watz et al. (56) proposed a method for staining human lung tissue with silver. Our initial efforts at using this method for staining mouse lung tissue did not result in a uniform deposition of silver. Comparatively better results were obtained using Osmium tetroxide (OsO4) as a staining agent (29). However, controlling the reaction step involving OsO4 was tenuous and getting the excess staining solution out of the small airspaces of the mouse lung proved difficult.
In this paper, we present a novel method for staining lung tissue with silver that consistently produced a uniform deposition of silver. Our staining method is based on a photochemical reaction (39) that leaves silver bound to proteins in the lung tissue. The method, originally proposed as a way to detect proteins in gels, is very sensitive and can detect trace amounts of protein. However, the original protocol stained only the exterior of the tissue samples and hence it had to be modified to obtain a uniform staining suitable for imaging the tissue with μ-CT. Since there is an abundant quantity of protein in lung tissue, the concentration of the silver in the staining solution was reduced and the addition of the catalyst that accelerates the staining reaction was delayed. This delay, which allows the staining solution to diffuse deep into the small airspaces of the mouse lung, was a function of the size of the tissue specimen. The samples were irregularly shaped pieces that fit in a bounding cube of 5 mm in dimensions. The small size of the stained samples was not a limitation of the staining method, rather it was dictated by the memory constraints during the 3D reconstruction of the image data. We imaged our silver-stained samples in water to avoid tissue deformation associated with drying. The image quality obtained was suitable for 3D reconstruction despite the reduced contrast when imaging the tissue in water.
In addition to μ-CT, currently there are several other techniques available that can be used to visualize and quantify lung tissue structure in 3D (35, 55). However, to our knowledge, automated techniques for identifying and measuring alveolar structures in 3D do not exist. In this study, we manually identified alveolar cross-sections from 2D LSCM images and entire alveoli and airspaces from 3D reconstruction of the parenchyma. In contrast to automated measurement techniques, manual measurement may be a source for potential bias. Additionally, manual identification of alveoli was a time consuming process and the number of alveoli that could be measured was much smaller compared with automated techniques that exist for assessing 2D features from histological slices (42). This is certainly a limitation of the current technique and future studies should develop automated analysis. As explained in the methods, to close off the alveoli for measurement, we found it extremely useful to simultaneously visualize the 3D rendering of the alveolus and the ductal structure connected to it. For airspaces that were close to the exterior surface of the tissue block being imaged, it was easy to rotate and zoom into the 3D rendering. However, for alveoli that were away from the edge in the interior of the sample, space became very crowded with surrounding alveoli and ducts so that rotating and examining the selected alveolus in 3D was difficult. For this reason, we limited our measurements to alveoli near the surface of the tissue piece. In most cases, the surface of the tissue specimen corresponded to the pleural surface, and hence most of our measurements, included subpleural alveoli. However, occasionally we also measured alveoli that were up to 1.5 mm away from the pleura. Furthermore, we only imaged tissue samples that were chosen in a uniformly random manner from the lower right lobe of normal and treated mouse. The samples were chosen blindly, without any selection bias. Nevertheless, it is not known how the elastase is distributed among the lobes immediately following treatment and how emphysematous lesions develop later, both of which warrant further investigation.
There are other imaging modalities that can be used to visualize alveolar structure in 3D. Confocal microscopy (10, 43) uses spatial filters to eliminate the detection of fluorescence emissions from points that are not in the plane of focus. This allows a tissue specimen to be optically sectioned along serial focal planes. We investigated the possibility of using such serial optical sections to reconstruct alveolar structure in 3D. Since elastin and collagen fibers are autofluorescent, no special stain was necessary. Optical sections 0.7 μm in thickness and 0.7 μm apart were used to reconstruct the alveolar structure of normal mice in 3D. One example is shown in Fig. 6. Unfortunately, the low depth of penetration and recovery of photons due to multiple scattering events caused the image quality to deteriorate considerably beyond ∼100 μm, which made the LSCM unsuitable for quantitative 3D measurements especially in the emphysematous lung with considerably increased airspace sizes.
Fig. 6.
LSCM was used to image the same region of lung tissue at different depths and these images were used to reconstruct the tissue structure in 3D. A: one typical slice from such a stack of images obtained from normal lung tissue. B: the block of tissue that was reconstructed from the slices. Note the increased noise at the bottom of the block. This is due to the multiple scattering events that restrict the detection and recovery of photons beyond ∼100 μm. C: a view from inside the lung tissue showing opening of an alveolus. The opening is ∼40 μm wide.
Although μ-CT has the advantage that it provides 3D structural data, it does not have the resolution offered by microscopes used to image 2D histologic sections. To examine the effect of reduced resolution of μ-CT, we compared cross-sectional areas of alveolar airspaces from LSCM and μ-CT images. The resolution of an LSCM image was 1.2 μm compared with a resolution of 6 μm for μ-CT. The errors in the measurement due to the lower resolution of μ-CT should be higher in normal lungs with smaller alveoli than in the emphysematous lung. Therefore, the comparison was only made in normal lungs. The distributions of cross-sectional areas of alveolar airspaces identified in LSCM and μ-CT images of normal mouse lungs were not different, indicating that the errors due to the lower resolution of μ-CT compared with LSCM were not significant. Furthermore, since the samples imaged using μ-CT were stained with silver and the LSCM images were unstained, this also implies that the presence of silver stain did not influence the measurements.
In this study, the lungs were fixed by tracheal aspiration of 10% neutral buffered formalin. Although, this method of fixation is widely used (15, 27, 30, 54), it can potentially cause tissue shrinkage (20, 30). Such artifacts cannot be completely eliminated, but their effects can be minimized by using better fixation techniques (2, 34, 52). Using our experimental setup, it was not possible to accurately quantify the degree of local tissue shrinkage so that its effect on our measurements could not be estimated. While we have not examined the effects of shrinkage, it is important to note that our method does avoid two other important sources of error in traditional histological analysis associated with embedding in paraffin as well as sectioning. Paraffin embedding can cause larger shrinkage than fixation (6) while the sectioning process can also lead to broken alveolar septa (9).
Comparing 2D and 3D measurements of alveolar airspace size.
To compare our results to those in the literature, we converted our 2D area and 3D volume measurements to equivalent diameters using Eqs. 1 and 2, respectively. The mean equivalent diameter of alveolar airspaces in C57BL/6 mouse lungs fixed at 30 cmH2O calculated from 2D measurements (39 μm) is in agreement with previously reported data in 2D (44.7 μm) obtained lungs of C57BL/6 mice fixed also at 30 cmH2O (49). However, it is important to notice that this value is significantly lower than the 3D mean equivalent diameter of 59 μm calculated from measurements of volume (see Fig. 5). This apparent discrepancy is a result of sectioning. In the appendix, we calculated the difference between 2D and 3D assessments of size for a single sphere. The results show that the expected value of the diameter of 2D cross sections is only 78% of the 3D diameter of the sphere (Eq. A4c in appendix). The smaller 2D diameter is a consequence of the fact that sectioning of a sphere with a series of planes will produce circles, most of which have a diameter smaller than the sphere's great circle. Since the shape and size of the airspaces vary in the parenchyma, the above 78% correction worked out for a sphere can only be taken as approximate. Thus the true size of an alveolus can only be determined from direct 3D measurements.
As Table 3 demonstrates, the variability of the 3D equivalent diameters was always consistently lower than that of the 2D equivalent diameters even after allowing for the differences in mean (P < 0.005). This is especially apparent with the progression of emphysema because the coefficient of variation of the equivalent diameters from 3D measurements (CV3D) for the day 14 group was less than half of the coefficient of variation of the equivalent diameters from 2D measurements (CV2D). To better understand this, we calculated the relationship between CV3D and CV2D for a collection of spheres (Eq. A5 in appendix). While the relationship is complex, it is apparent that even for a collection of identical spheres (CV3D=0), sectioning will result in a nonzero CV2D (28%). The implication of this result is important. First, note that the SD in 2D increased by a factor of 3 and 7.8 from normal to day 7 and day 14, respectively. The same numbers are much smaller for the SD in 3D (2.3 and 3.3, respectively). The increase in the equivalent diameter heterogeneity slows down significantly in 3D from day 7 to day 14. Thus the mean equivalent diameter directly measured in 3D with considerably less heterogeneity should be significantly more sensitive to subtle alterations in structure than that assessed in 2D. We thus conclude that due to the increased variability introduced by the sectioning, mean airspace size measurements in 2D might not be able to detect early alterations caused by the pathogenesis of a disease such as emphysema. Alternatively, 2D measurements might miss, for example, the potentially important but mild effect of a drug treatment to slow down the progression of the disease.
Effect of elastase treatment: early changes.
Comparing the 3D equivalent diameters in all groups (Table 3), we found significant increases in the mean and SD of equivalent diameters between normal and day 7 as well as between day 7 and day 14 groups. At 14 days, the signs of destruction are visually evident (Fig. 2C and 3C), however, this is not so clear at 7 days (Fig. 2B and 3B). To better understand the effect of early destruction on alveolar airspace size, we examined the rate of increase of the mean and the SD with elastase treatment. We note from Table 3 that the percentage increase in mean equivalent diameter from normal to day 7 was 42% while the SD, during the same time period, increased by 133%. To examine whether the %increase was significantly higher in the SD than the mean, we compared the fractional changes in mean equivalent diameter and SD of 3D diameters between the normal and the day 7 groups using a paired t-test. The difference was found to be statistically significant (P < 0.005), suggesting that the heterogeneity of the structure increases faster than the mean diameter. However, from day 7 to day 14 there was no difference between the %change in mean and SD. This result is reflected in the CV3D (Table 3), which increases first and then decreases. It should be noted that as Eq. A5 in the appendix suggests, CV2D (Table 1) need not mirror the changes in CV3D as the former is also influenced by changes in 3D skewness. This analysis then indicates that the early stages of destruction are marked by a faster rate of increase in the variability of airspace sizes compared with the mean, suggesting that the variance may be a more sensitive indicator of early structural changes due to emphysema, a finding similar to what we reported previously in 2D (24).
Before concluding, we note that the elastance of the respiratory system, H, did not always mirror the changes in structure. H was significantly smaller in the day 7 group than in the normal mice, but there was no difference between the H values in the day 7 and day 14 groups. While anomalous, the disconnect between structural and functional changes in emphysema observed here is consistent with previous reports that structural changes follow a different course from functional changes in emphysema (5, 45, 51, 53). It is possible that different mechanical behavior would be seen at higher PEEP levels or using more complex models that incorporate the effects of heterogeneities (25). Nevertheless, it is important to note that since the changes in alveolar airspace structural parameters between the day 7 and day 14 groups were smaller in 3D than in 2D, one would expect to find a better structure-function correlation when using structural parameters derived from 3D analysis of the parenchyma.
To summarize, we introduced a novel staining method for imaging the microstructure of mouse lung tissue in 3D using μ-CT. We found that the 2D measurements significantly underestimated the true alveolar airspace size in 3D while significantly overestimating its variability. This has implications for the detection of subtle structural changes in the lung as a result of disease progression or in response to medications. Our results also showed that there is a significant increase in the heterogeneity of tissue structure during the early stages of the disease, which is followed by an increase in the mean size of airspaces only at a later stage. Thus quantifying heterogeneity in tissue structure may provide new insights into the pathogenesis or progression of emphysema and potentially other diseases, which is enhanced by improved sensitivity using 3D measurements.
GRANTS
This study was supported by National Institutes of Health Grants HL-059215 and RR-021072.
Acknowledgments
The authors thank Dr. E. F. Morgan and Z. Mason, Orthopaedic and Developmental Biomechanics Laboratory, Department of Mechanical Engineering, Boston University for their contributions.
APPENDIX A: RELATING 2D AND 3D DIAMETERS FOR SPHERES
The purpose of this appendix is to derive relations between the 2D and 3D expressions of equivalent diameters and their variability. To do this, we examine a population of spheres with radii distributed according to a probability density distribution function Π3D. The spheres are sectioned with a set of planes uniformly spaced at a distance Δ. The goal is to relate the statistics of the 2D radii (r) of the resulting circles to those of the 3D radii (R) of the spheres. We note that the distribution of 2D radii of circular cross sections resulting from uniformly sectioning a population of spheres (Eq. A2 below) has been described by Weibel (Chapter 6 of Ref. 57), which, for the sake of completeness, is also reproduced here. However, to our knowledge, the expressions for the general moments (Eq. A3), the relations between 2D and 3D variances (Eq. A4b), diameters (Eq. A4c), and CVs (Eq. A5) are novel.
The number of planes and hence circular sections obtained from a single sphere of radius R is given by 2R/Δ. If the total number of spheres is N, then the number of spheres with radius between R and R+dR is NΠ3D(R)dR. Therefore, the number of circular sections obtained from all spheres with radius between R and R+dR, is given by n(R)dR, where 2NΠ3D(R)/RΔ. The total number of circular sections from all spheres is ∫dR n(R). For the following calculations, we assume that N→∞ and Δ→0.
For a given sphere of radius R, the conditional probability K(r|R) of finding circular sections with 2D radii r can be calculated (42) for a uniformly spaced set of sectioning planes as the separation Δ between the planes tend to 0, as
 |
(A1) |
where Θ(.) is the unit-step function.
We can thus express the distribution Π2D(r) of 2D radii r of circular sections in terms of the Π3D(R) of 3D radii R of spheres as follows:
 |
(A2) |
where 〈…〉3D represents the average over 3D radii. This equation is the same as Eq. 6.40 in Ref. 57.
We can now express the k-th moment of r as
 |
(A3) |
where Γ(.) represents the average over 2D radii and Qk is given by
 |
where Γ(.) is the standard Gamma function. Since Q1(R) = π R/4 and Q2(R) = 2R2/3, the moments in 2D can be related to those in 3D as:
 |
(A4a) |
 |
(A4b) |
For a single sphere, this relationship reduces to  which is identical to Eq. 6.16 in Ref. 57. Eq. A4a can also be expressed in terms of diameters:
which is identical to Eq. 6.16 in Ref. 57. Eq. A4a can also be expressed in terms of diameters:
 |
(A4c) |
where d = 2r is the diameter of circular 2D sections and D = 2R is the diameter of the spheres in 3D. If all 3D spheres have identical diameters D = D0, the corresponding mean diameter in 2D, 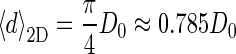 Similarly, the second moment
Similarly, the second moment  and the standard deviation
and the standard deviation  Thus the coefficient of variation (CV2D) in 2D,
Thus the coefficient of variation (CV2D) in 2D, although the CV in three-dimensions (CV3D) is 0. When the diameters of the spheres are not identical, the CV2D can be related to the CV3D from Eq. A4a and A4b as:
although the CV in three-dimensions (CV3D) is 0. When the diameters of the spheres are not identical, the CV2D can be related to the CV3D from Eq. A4a and A4b as:
 |
(A5) |
where γ is the skewness of the 3D diameter distribution.
REFERENCES
- 1.American Thoracic Society. Standards for the diagnosis and care of patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 152: S77–121, 1995. [PubMed] [Google Scholar]
- 2.Bachofen H, Ammann A, Wangensteen D, Weibel ER. Perfusion fixation of lungs for structure-function analysis: credits and limitations. J Appl Physiol 53: 528–533, 1982. [DOI] [PubMed] [Google Scholar]
- 3.Baddeley AJ, Gundersen HJ, Cruz-Orive LM. Estimation of surface area from vertical sections. J Microsc 142: 259–276, 1986. [DOI] [PubMed] [Google Scholar]
- 4.Bassam BJ, Caetano-Anollés G, Gresshoff PM. Fast and sensitive silver staining of DNA in polyacrylamide gels. Anal Biochem 196: 80–83, 1991. [DOI] [PubMed] [Google Scholar]
- 5.Bates JH, Davis GS, Majumdar A, Butnor KJ, Suki B. Linking parenchymal disease progression to changes in lung mechanical function by percolation. Am J Respir Crit Care Med 176: 617–623, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bishai JM, Fields MJ, Mitzner W. Comparison of mouse lung volumes inflated with air and instilled fixatives. In: American Thoracic Society Meeting. San Diego, CA, 2006.
- 7.Blanco LN, Massaro GD, Massaro D. Alveolar dimensions and number: developmental and hormonal regulation. Am J Physiol Lung Cell Mol Physiol 257: L240–L247, 1989. [DOI] [PubMed] [Google Scholar]
- 8.Campbell EJ Animal models of emphysema: the next generations. J Clin Invest 106: 1445–1446, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cookson MJ, Entwistle A. Application of a laser scalpel to sectioning fragile tissues prior to optical sectioning microscopy and 3D reconstruction. J Microsc 200: 174–176, 2000. [DOI] [PubMed] [Google Scholar]
- 10.Davidovits P, Egger MD. Scanning laser microscope for biological investigations. Appl Opt 10: 1615–1619, 1971. [DOI] [PubMed] [Google Scholar]
- 11.Fehrenbach A, Ochs M, Wittwer T, Cornelius J, Fehrenbach H, Wahlers T, Richter J. Stereological estimation of the volume weighted mean volumes of alveoli and acinar pathways in the rat lung to characterise alterations after ischaemia/reperfusion. J Anat 194 : 127–135, 1999. [DOI] [PubMed] [Google Scholar]
- 12.Fehrenbach H Animal models of pulmonary emphysema: a stereologist's perspective. Eur Respir Rev 15: 136–147, 2006. [Google Scholar]
- 13.Feldkamp LA, Davis LC, Kress JW. Practical cone-beam algorithm. J Opt Soc Am A 1: 612–619, 1984. [Google Scholar]
- 14.Foster WM, Walters DM, Longphre M, Macri K, Miller LM. Methodology for the measurement of mucociliary function in the mouse by scintigraphy. J Appl Physiol 90: 1111–1117, 2001. [DOI] [PubMed] [Google Scholar]
- 15.Gajic O, Lee J, Doerr CH, Berrios JC, Myers JL, Hubmayr RD. Ventilator-induced cell wounding and repair in the intact lung. Am J Respir Crit Care Med 167: 1057–1063, 2003. [DOI] [PubMed] [Google Scholar]
- 16.Gross P, Pfitzer EA, Tolker E, Babyak MA, Kaschak M. Experimental emphysema: its production with papain in normal and silicotic rats. Arch Environ Health 11: 50–58, 1965. [DOI] [PubMed] [Google Scholar]
- 17.Gundersen HJ, Jensen EB. Stereological estimation of the volume-weighted mean volume of arbitrary particles observed on random sections. J Microsc 138: 127–142, 1985. [DOI] [PubMed] [Google Scholar]
- 18.Haber PS, Colebatch HJ, Ng CK, Greaves IA. Alveolar size as a determinant of pulmonary distensibility in mammalian lungs. J Appl Physiol 54: 837–845, 1983. [DOI] [PubMed] [Google Scholar]
- 19.Hantos Z, Daroczy B, Suki B, Nagy S, Fredberg JJ. Input impedance and peripheral inhomogeneity of dog lungs. J Appl Physiol 72: 168–178, 1992. [DOI] [PubMed] [Google Scholar]
- 20.Hayatdavoudi G, Crapo JD, Miller FJ, O'Neil JJ. Factors determining degree of inflation in intratracheally fixed rat lungs. J Appl Physiol 48: 389–393, 1980. [DOI] [PubMed] [Google Scholar]
- 21.Heemskerk-Gerritsen BA, Dijkman JH, Ten Have-Opbroek AA. Stereological methods: a new approach in the assessment of pulmonary emphysema. Microsc Res Tech 34: 556–562, 1996. [DOI] [PubMed] [Google Scholar]
- 22.Huang Y, Mitzner W. Distribution analysis of alveolar size by digital imaging. J Comp Assist Microsc 1: 397–409, 1989. [Google Scholar]
- 23.Hyde DM, Tyler NK, Putney LF, Singh P, Gundersen HJ. Total number and mean size of alveoli in mammalian lung estimated using fractionator sampling and unbiased estimates of the Euler characteristic of alveolar openings. Anat Rec A Discov Mol Cell Evol Biol 277: 216–226, 2004. [DOI] [PubMed] [Google Scholar]
- 24.Ito S, Bartolak-Suki E, Shipley JM, Parameswaran H, Majumdar A, Suki B. Early emphysema in the tight skin and the pallid mice: roles of microfibril associated glycoproteins, collagen and mechanical forces. Am J Respir Cell Mol Biol 34: 688–694, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ito S, Ingenito EP, Arold SP, Parameswaran H, Tgavalekos NT, Lutchen KR, Suki B. Tissue heterogeneity in the mouse lung: effects of elastase treatment. J Appl Physiol 97: 204–212, 2004. [DOI] [PubMed] [Google Scholar]
- 26.Janoff A, Sloan B, Weinbaum G, Damiano V, Sandhaus RA, Elias J, Kimbel P. Experimental emphysema induced with purified human neutrophil elastase: tissue localization of the instilled protease. Am Rev Respir Dis 115: 461–478, 1977. [DOI] [PubMed] [Google Scholar]
- 27.Ladha F, Bonnet S, Eaton F, Hashimoto K, Korbutt G, Thébaud B. Sildenafil improves alveolar growth and pulmonary hypertension in hyperoxia-induced lung injury. Am J Respir Crit Care Med 172: 750–756, 2005. [DOI] [PubMed] [Google Scholar]
- 28.Langheinrich AC, Leithauser B, Greschus S, von Gerlach S, Breithecker A, Matthias FR, Rau WS, Bohle RM. Acute rat lung injury: feasibility of assessment with micro-CT. Radiology 233: 165–171, 2004. [DOI] [PubMed] [Google Scholar]
- 29.Litzlbauer HD, Neuhaeuser C, Moell A, Greschus S, Breithecker A, Franke FE, Kummer W, Rau WS. Three-dimensional imaging and morphometric analysis of alveolar tissue from microfocal X-ray-computed tomography. Am J Physiol Lung Cell Mol Physiol 291: L535–L545, 2006. [DOI] [PubMed] [Google Scholar]
- 30.Lum H, Mitzner W. Effects of 10% formalin fixation on fixed lung volume and lung tissue shrinkage. A comparison of eleven laboratory species. Am Rev Respir Dis 132: 1078–1083, 1985. [DOI] [PubMed] [Google Scholar]
- 31.Lum H, Mitzner W. A species comparison of alveolar size and surface forces. J Appl Physiol 62: 1865–1871, 1987. [DOI] [PubMed] [Google Scholar]
- 32.Lutchen KR, Yang K, Kaczka DW, Suki B. Optimal ventilation waveforms for estimating low-frequency respiratory impedance. J Appl Physiol 75: 478–488, 1993. [DOI] [PubMed] [Google Scholar]
- 33.Matthias O A brief update on lung stereology. J Microsc 222: 188–200, 2006. [DOI] [PubMed] [Google Scholar]
- 34.Mazzone RW, Kornblau S, Durand CM. Shrinkage of lung after chemical fixation for analysis of pulmonary structure-function relations. J Appl Physiol 48: 382–385, 1980. [DOI] [PubMed] [Google Scholar]
- 35.Meissner S, Knels L, Mertens M, Wendel M, Tabuchi A, Kuebler WM, Koch T, Koch E. Three-dimensional imaging of subpleural alveoli by fourier domain optical coherence tomography. In: 4th European Conference of the International Federation for Medical and Biological Engineering 2009, p. 2035–2039.
- 36.Mercer RR, Crapo JD. Three-dimensional reconstruction of the rat acinus. J Appl Physiol 63: 785–794, 1987. [DOI] [PubMed] [Google Scholar]
- 37.Mercer RR, Laco JM, Crapo JD. Three-dimensional reconstruction of alveoli in the rat lung for pressure-volume relationships. J Appl Physiol 62: 1480–1487, 1987. [DOI] [PubMed] [Google Scholar]
- 38.Mercer RR, Russell ML, Crapo JD. Alveolar septal structure in different species. J Appl Physiol 77: 1060–1066, 1994. [DOI] [PubMed] [Google Scholar]
- 39.Merril CR, Goldman D, Sedman SA, Ebert MH. Ultrasensitive stain for proteins in polyacrylamide gels shows regional variation in cerebrospinal fluid proteins. Science 211: 1437–1438, 1981. [DOI] [PubMed] [Google Scholar]
- 40.Merril CR, Switzer RC, Van Keuren ML. Trace polypeptides in cellular extracts and human body fluids detected by two-dimensional electrophoresis and a highly sensitive silver stain. Proc Natl Acad Sci USA 76: 4335–4339, 1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Oldmixon EH, Butler JP, Hoppin FGJ. Lengths and topology of alveolar septal borders. J Appl Physiol 67: 1930–1940, 1989. [DOI] [PubMed] [Google Scholar]
- 42.Parameswaran H, Majumdar A, Ito S, Alencar AM, Suki B. Quantitative characterization of airspace enlargement in emphysema. J Appl Physiol 100: 186–193, 2006. [DOI] [PubMed] [Google Scholar]
- 43.Pawley JB, Masters BR. Handbook of biological confocal microscopy. Appl Opt 31: 2398–2399, 1992.20725158 [Google Scholar]
- 44.Ritman EL Micro-computed tomography-current status and developments. Annu Rev Biomed Eng 6: 185–208, 2004. [DOI] [PubMed] [Google Scholar]
- 45.Sashidhar K, Gulati M, Gupta D, Monga S, Suri S. Emphysema in heavy smokers with normal chest radiography. Detection and quantification by HCRT. Acta Radiol 43: 60–65, 2002. [DOI] [PubMed] [Google Scholar]
- 46.Shapiro SD Animal models for COPD. Chest 117: 223S–227S, 2000. [DOI] [PubMed] [Google Scholar]
- 47.Snider G, Kleinerman J, Thurlbeck W, Bengali. Z. The definition of emphysema. Report of a National Heart, Lung, and Blood Institute, Division of Lung Diseases workshop. Am Rev Respir Dis 132: 182–185, 1985. [DOI] [PubMed] [Google Scholar]
- 48.Snider GL, Lucey EC, Stone PJ. Animal models of emphysema. Am Rev Respir Dis 133: 149–169, 1986. [DOI] [PubMed] [Google Scholar]
- 49.Soutiere SE, Mitzner W. On defining total lung capacity in the mouse. J Appl Physiol 96: 1658–1664, 2004. [DOI] [PubMed] [Google Scholar]
- 50.Soutiere SE, Tankersley CG, Mitzner W. Differences in alveolar size in inbred mouse strains. Respir Physiol Neurobiol 140: 283–291, 2004. [DOI] [PubMed] [Google Scholar]
- 51.Srinakarin J, Thammaroj J, Boonsawat W. Comparison of high-resolution computed tomography with pulmonary function testing in symptomatic smokers. J Med Assoc Thai 86: 522–528, 2003. [PubMed] [Google Scholar]
- 52.Staub NC, Storey WF. Relation between morphological and physiological events in lung studied by rapid freezing. J Appl Physiol 17: 381–390, 1962. [DOI] [PubMed] [Google Scholar]
- 53.Takasugi JE, Godwin JD. Radiology of chronic obstructive pulmonary disease. Radiol Clin North Am 36: 29–55, 1998. [DOI] [PubMed] [Google Scholar]
- 54.Tsao PN, Su YN, Li H, Huang PH, Chien CT, Lai YL, Lee CN, Chen CA, Cheng WF, Wei SC, Yu CJ, Hsieh FJ, Hsu SM. Overexpression of placenta growth factor contributes to the pathogenesis of pulmonary emphysema. Am J Respir Crit Care Med 169: 505–511, 2004. [DOI] [PubMed] [Google Scholar]
- 55.Tsuda A, Filipovic N, Haberthur D, Dickie R, Matsui Y, Stampanoni M, Schittny JC. Finite element 3D reconstruction of the pulmonary acinus imaged by synchrotron X-ray tomography. J Appl Physiol 105: 964–976, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Watz H, Breithecker A, Rau WS, Kriete A. Micro-CT of the human lung: imaging of alveoli and virtual endoscopy of an alveolar duct in a normal lung and in a lung with centrilobular emphysema-initial observations. Radiology 236: 1053–1058, 2005. [DOI] [PubMed] [Google Scholar]
- 57.Weibel ER Stereological Methods. London: Academic, 1980.
- 58.Weibel ER, Gomez DM. A principle for counting tissue structures on random sections. J Appl Physiol 17: 343–348, 1962. [DOI] [PubMed] [Google Scholar]
- 59.Weibel ER, Hsia CC, Ochs M. How much is there really? Why stereology is essential in lung morphometry. J Appl Physiol 102: 459–467, 2007. [DOI] [PubMed] [Google Scholar]








