Abstract
Previous studies showed that unilateral denervation (DNV) of the rat diaphragm muscle (DIAm) results in loss of myosin heavy chain protein by 1 day after DNV. We hypothesize that DNV decreases net protein balance as a result of activation of the ubiquitin-proteasome pathway. In DIAm strips, protein synthesis was measured by incorporation of 3H-Tyr, and protein degradation was measured by Tyr release at 1, 3, 5, 7, and 14 days after DNV. Total protein ubiquitination, caspase-3 expression/activity, and actin fragmentation were analyzed by Western analysis. We found that, at 3 days after DNV, protein synthesis increased by 77% relative to sham controls. Protein synthesis remained elevated at 5 (85%), 7 (53%), and 14 days (123%) after DNV. At 5 days after DNV, protein degradation increased by 43% relative to sham controls and remained elevated at 7 (49%) and 14 days (74%) after DNV. Thus, by 5 days after DNV, net protein balance decreased by 43% compared with sham controls and was decreased compared with sham at 7 (49%) and 14 days (72%) after DNV. Protein ubiquitination increased at 5 days after DNV and remained elevated. DNV had no effect on caspase-3 activity or actin fragmentation, suggesting that the ubiquitin-proteasome pathway rather than caspase-3 activation is important in the DIAm response to DNV. Early loss of contractile proteins, such as myosin heavy chain, is likely the result of selective protein degradation rather than generalized protein breakdown. Future studies should evaluate this selective effect of DNV.
Keywords: innervation, protein balance, skeletal muscle
maintenance of skeletal muscle structure and function requires precise control of protein synthesis and degradation. Numerous studies showed that denervation (DNV) strongly affects expression of contractile proteins and mechanical properties of skeletal muscles, including the diaphragm (DIAm) (4, 12, 25, 28, 35, 36, 43–46). DNV caused an increase in cross-sectional area in myosin heavy chain slow (MHCslow)- and MHC2A-expressing fiber types and a decrease in MHC2X- and MHC2B-expressing fiber types, with no net change in mass (33, 35, 45). However, total MHC protein content in the rat DIAm decreased significantly 1 day after DNV (10) and decreased even further by 3 days after DNV, remaining fairly constant from 3 to 14 days after DNV (10). Furthermore, DIAm-specific force was reduced 14 days after DNV (12), reflecting a loss of contractile proteins and an increase in filament lattice spacing (11).
Changes in contractile proteins, such as MHC, could reflect a generalized effect on protein synthesis and/or degradation, causing negative protein turnover, or selective protein degradation. Studies by Buse et al. (3) and Turner and Garlick (39) reported increased protein synthesis by 1 day after rat DIAm DNV, but longer term studies are not available. In rat hindlimb muscles, DNV resulted in a generalized increase in protein breakdown by 1 day (13, 14), but gravitational unloading of hindlimb muscles can confound the effect of DNV on protein balance. Early studies in rat hindlimb muscles showed that loss of cell proteins was primarily due to enhanced proteolysis, especially of myofibrillar proteins (13). It is presently unknown if similar effects occur in the DIAm after DNV. Concurrent measurements of both protein synthesis and degradation in DIAm after DNV would provide important insight into pathways affecting total protein turnover; unfortunately, these measurements have not been previously reported.
The present study was undertaken to determine the effects of DIAm DNV on protein synthesis and degradation over time, thus permitting assessment of net protein balance after DNV. We hypothesized that DIAm DNV decreases net protein balance as a result of activation of the ubiquitin-proteasome pathway. The majority of intracellular protein is degraded by the ubiquitin-proteasome pathway, which marks proteins for degradation by the covalent attachment of multiple ubiquitin molecules (26). Caspase-3 yields protein fragments that are degraded by the ubiquitin-proteasome pathway (7). Thus the possible involvement of proteasomal pathways was assessed by measuring ubiquitination of total proteins and activation of caspase-3. These results may have important implications for the treatment of peripheral nerve injuries and associated muscle atrophy. For example, if DNV induces a decrease in net protein balance rather than selective loss of contractile proteins, future studies should investigate the use of proteasome inhibitors in the treatment of DNV-induced muscle dysfunction.
METHODS
Experiments were performed on adult male Sprague-Dawley rats (body weight ∼300 g). Animals were assigned to one of five DNV groups (n = 6 for each time point; n = 30 total) with sham control surgeries performed in parallel (n = 6 for each time point; n = 30 total). Aseptic conditions were maintained during all surgical procedures, and recovery from surgery was carefully monitored. The Institutional Animal Care and Use Committee of the Mayo Clinic approved all procedures.
Unilateral DNV.
The procedure for unilateral DNV has previously been described (16, 25, 44, 46). Briefly, intramuscular injection of ketamine (60 mg/kg) and xylazine (2.5 mg/kg) was used to anesthetize the animals. At a point beneath the sternomastoid muscle in the lower neck, the right phrenic nerve was exposed and transected. To minimize trophic effects emanating from the remaining nerve stump and to prevent reinnervation of the DIAm, a portion (∼10–20 mm) of the distal end was removed. The wound was sutured and treated with topical antibiotics. Periods of DNV were maintained for 1, 3, 5, 7, or 14 days before tissue collection, when the right hemidiaphragm was excised and cut into eight strips. Samples from sham control surgery animals were collected at these same postsurgical time points.
Protein synthesis assays.
Protein synthesis was measured using a previously described method with minor modifications (19, 38). Briefly, DIAm strips were pinned at approximately two times resting length and incubated for 3 h in the presence of either 5 μCi/ml 35S-Met or 5 μCi/ml 3H-Tyr (Amersham Biosciences, Piscataway, NJ) diluted in 0.4 mM unlabeled Tyr. Experiments were terminated by quickly rinsing the DIAm in phosphate-buffered saline and snap freezing in liquid N2. Samples were mechanically homogenized by mortar and pestle in 0.5 ml 5% trichloroacetic acid (TCA), and homogenization chambers were rinsed with 0.5 ml TCA. After incubating on ice for 30 min, proteins were precipitated by centrifugation at 10,000 g for 10 min and washed three times with TCA, with each wash followed by centrifugation at 10,000 g for 10 min. The precipitate was resuspended in 0.1 N NaOH and 0.1% SDS at 70°C for 2 h. Protein concentration was determined using the Bio-Rad DC protein assay, according to manufacturer's protocol (Bio-Rad Laboratories, Hercules, CA). Incorporation of 35S-Met or 3H-Tyr was determined using a Beckman LS5000TD liquid scintillation counter (Beckman Coulter, Fullerton, CA) and expressed as counts per minute (cpm)/μg of protein. Specific activity was used to calculate nanomoles of Tyr incorporated into protein. At least three DIAm strips were averaged per animal.
Protein degradation assay.
Protein degradation was measured independently of protein synthesis using a previously described method with minor modifications (32, 42). Briefly, DIAm strips were pinned at approximately two times resting length and incubated in Krebs-Ringer buffer containing dextrose (13.9 mM). Cycloheximide (0.5 mM; Tocris Bioscience, Ellisville, MO) was added to the medium to block protein synthesis and prevent reuse of the Tyr released by degradation of muscle protein. After 3 h, the buffer was removed from the samples and was then mixed with an equal volume of 50:50 nitrosonaphthol-nitric acid reagent and incubated at 55°C for 30 min. Ethylene dichloride was then added to extract a fluorescent Tyr derivative. Tyr content was determined by spectrophotofluorimetry with excitation at 460 nm and emission at 570–630 nm, using appropriate standards. DIAm protein content was determined using BioRad's DC protein analysis assay, according to the manufacturer-supplied technique, as described above. Tyrosine release was expressed relative to total DIAm protein content (nmol/μg). At least three DIAm strips were averaged per animal.
Western analysis.
Two DIAm strips from each animal were immediately frozen in liquid N2 after being excised from the rat. DIAm strips were placed in lysis buffer (Cell Signaling Technology, Beverly, MA) with 5 mM sodium fluoride, 5 mM sodium orthovanadate, 2.5 mM sodium pyrophosphate, and complete mini protease inhibitor (Roche, Indianapolis, IN). The homogenate was centrifuged (10,000 g for 10 min), and the supernatant was diluted in Laemmli sample buffer (Bio-Rad) for ubiquitin and caspase-3 protein analyses. The pellet was resuspended in Laemmli sample buffer for actin fragmentation analysis of the insoluble myofibrils. Samples were electrophoretically separated under denaturing conditions on 4–15, 10, or 15% SDS-PAGE Criterion gels (Bio-Rad) and transferred to polyvinylidene difluoride membranes (Bio-Rad). Membranes were blocked in Tris-buffered saline containing 5% milk and 0.1% Tween 20 (Sigma-Aldrich, St. Louis, MO), followed by an overnight incubation with primary antibodies for ubiquitin, caspase-3 (both from Cell Signaling), and actin (Sigma). Antibodies were diluted 1:1,000 in Tris-buffered saline containing 5% milk and 0.1% Tween 20. After three washes, membranes were incubated with horseradish peroxidase-conjugated secondary antibodies (Santa Cruz Biotechnology, Santa Cruz, CA) for 1 h. Immunodetection was performed using enhanced chemiluminescence, according to the manufacturer's protocol (Pierce Biotechnology, Rockford, IL). Membranes were reprobed with antibodies for β-tubulin (Santa Cruz) or actin (Sigma) to serve as loading controls. Protein amounts were semiquantitated by measuring the density of autoradiographs with Kodak MM4000 Image Station software (Kodak Molecular Imaging Systems, New Haven, CT). The ubiquitination profile was verified using proteasome inhibitor MG-132 (Tocris) treatment (data not shown). For analysis of molecular weight distribution of ubiquitinated proteins, the pixel intensities of each lane were plotted against molecular weight using Analyze software (Biomedical Imaging Resource, Rochester, MN). The area under each profile curve was found using Image J software (National Institutes of Health), and the average area of each treatment group was calculated.
Caspase activity assay.
Caspase-3 activity was measured using the Caspase-3 Colorimetric Assay Kit (BioVision, Mountain View, CA), which is based on spectrophotometric detection of the chromophore p-nitroanilide (pNA) after cleavage from the labeled substrate DEVD-pNA. Briefly, cell lysates (100 μg protein) were mixed with 200 μM DEVD-pNA substrate. Assays were conducted at 37°C for 120 min, according to the recommended protocol. The release of pNA was monitored by measuring absorbance at 405 nm.
Statistical analyses.
Statistical analyses were performed with JMP software (SAS Institute, Cary, NC). Protein synthesis and degradation in the sham control and DNV DIAm were compared using one-way ANOVA at each time point to control for interassay differences related to the radioactive or fluorescence technique. The effect of DNV on total protein ubiquitination, caspase-3 activity, and actin fragmentation was assessed by one-way ANOVA. Significant differences between individual treatment conditions were determined post hoc by the Tukey-Kramer honestly significant difference test. Statistical significance was indicated by a P value < 0.05. All values are reported as means ± SE, unless otherwise specified.
RESULTS
The surgical DNV procedure had no effect on subsequent body weight gain during the 2-wk experimental period (initial body weight: 280–300 g). Final body weights were comparable across groups over time [nonsignificant (NS) for all comparisons].
Protein concentration after DIAm DNV.
Protein content for each DIAm strip was measured after the 3-h incubation period and normalized to mass of the strip. Protein content was not significantly affected at 1 day (sham: 117 ± 11 vs. DNV: 106 ± 4 μg protein/mg muscle), 3 days (sham: 129 ± 8 vs. DNV: 134 ± 12 μg protein/mg muscle), 5 days (sham: 109 ± 5 vs. DNV: 108 ± 5 μg protein/mg muscle), and 7 days (sham: 97 ± 6 vs. DNV: 96 ± 7 μg protein/mg muscle) after DNV compared with sham controls (NS for all comparisons). By 14 days after DNV, protein content decreased 18% compared with sham controls (sham: 146 ± 4 vs. DNV: 119 ± 2 μg protein/mg muscle; P < 0.05).
Protein synthesis after DIAm DNV.
Protein synthesis, as measured by incorporation of 35S-Met per total amount of precipitated protein, increased at all time points after DNV compared with sham controls. Protein synthesis increased 56% at 1 day after DNV (sham: 5.63 ± 0.32 vs. DNV: 8.78 ± 0.72 cpm/μg protein). Protein synthesis remained elevated, with 3 days after DNV resulting in an 81% increase (sham: 4.86 ± 0.25 vs. DNV: 8.79 ± 0.41 cpm/μg protein), 7 days after DNV resulting in a 64% increase (sham: 5.86 ± 0.33 vs. DNV: 9.60 ± 0.47 cpm/μg protein), and 14 days after DNV resulting in a 52% increase relative to sham controls (sham: 2.73 ± 0.08 vs. DNV: 4.15 ± 0.20 cpm/μg protein; P < 0.05 for all comparisons). Minimal nonspecific radioactivity levels were found when cycloheximide was added to block protein synthesis (sham: 1.56 ± 0.11; DNV: 1.43 ± 0.09 cpm/μg protein, respectively; NS).
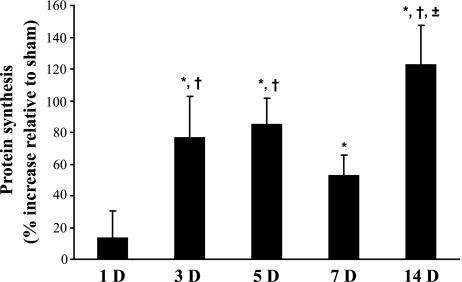
Additional experiments using 3H-Tyr were performed to concurrently measure incorporation (i.e., synthesis; Fig. 1) and release (i.e., degradation; Fig. 2) in the same animal. Results related to protein synthesis obtained with 3H-Tyr were qualitatively similar to those obtained using 35S-Met. Incorporation of 3H-Tyr at each time point after DNV was compared with its own time-matched sham control (Fig. 1). Protein synthesis was unchanged 1 day after DNV (sham: 0.18 ± 0.01 vs. DNV: 0.21 ± 0.03 pmol Tyr/μg protein). Incorporation of 3H-Tyr increased 77% by 3 days after DNV (sham: 0.19 ± 0.03 vs. DNV: 0.33 ± 0.05 pmol Tyr/μg protein). Protein synthesis remained elevated, with 5 days after DNV resulting in an 85% increase (sham: 0.27 ± 0.02 vs. DNV: 0.51 ± 0.05 pmol Tyr/μg protein), 7 days after DNV resulting in a 53% increase (sham: 0.17 ± 0.01 vs. DNV: 0.26 ± 0.02 pmol Tyr/μg protein), and 14 days after DNV resulting in a 123% increase relative to time-matched sham controls (sham: 0.17 ± 0.01 vs. DNV: 0.39 ± 0.04 pmol Tyr/μg protein; P < 0.05 for all comparisons).
Fig. 1.
Unilateral denervation (DNV)-induced increase in rat diaphragm muscle (DIAm) protein synthesis, as determined by incorporation of 3H-Tyr. Mean and SE of the percent increase relative to sham controls are plotted over time (in days, D) after DNV. *Significantly different from the sham control group at the same DNV time point; †significantly different from 1 day after DNV; ±significantly different from 7 days after DNV (P < 0.05 for all comparisons).
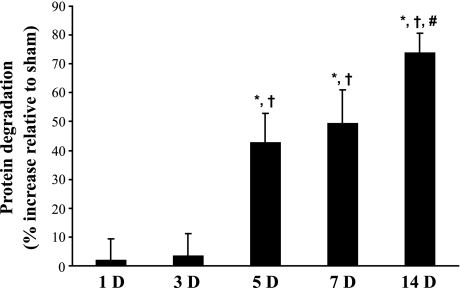
Fig. 2.
DNV-induced increase in rat DIAm protein degradation, in the presence of cycloheximide, as determined by Tyr release. Mean and SE of the percent increase relative to sham controls are plotted over time after DNV. *Significantly different from the sham control group at the same DNV time point; †significantly different from 1 and 3 days after DNV; #significantly different from 5 days after DNV (P < 0.05 for all comparisons).
Protein degradation after DIAm DNV.
Protein degradation was estimated from the rate of free Tyr release per total amount of precipitated protein in DIAm samples from sham control and DNV animals (9, 14, 42). Release of free Tyr at each time point after DNV was compared with its own time-matched sham control (Fig. 2). Protein degradation was unchanged 1 day after DNV (sham: 5.7 ± 0.7 vs. DNV: 5.8 ± 0.4 pmol Tyr/μg protein) and 3 days after DNV (sham: 5.7 ± 0.3 vs. DNV: 5.9 ± 0.4 pmol Tyr/μg protein). Increased Tyr release was evident by 5 days after DNV, with a 43% increase (sham: 4.9 ± 0.4 vs. DNV: 7.1 ± 0.5 pmol Tyr/μg protein). Protein degradation remained elevated, with 7 days after DNV resulting in a 49% increase (sham: 5.8 ± 0.5 vs. DNV: 8.7 ± 0.7 pmol Tyr/μg protein), and 14 days after DNV resulting in a 74% increase relative to time-matched sham controls (sham: 4.9 ± 0.2 vs. DNV: 8.6 ± 0.3 pmol Tyr/μg protein; P < 0.05 for all comparisons).
Net protein balance after DIAm DNV.
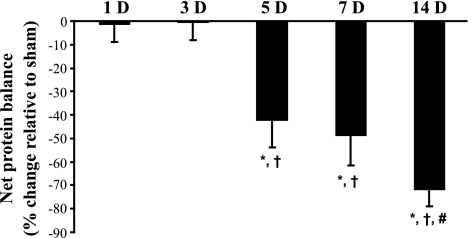
By measuring both Tyr incorporation and release, net protein balance could be estimated for each animal. The net protein balance was calculated directly as the difference between protein synthesis and protein degradation in the same muscle and compared with its own time-matched sham control (Fig. 3). At all time points, protein degradation was greater than protein synthesis for both sham control and DNV animals. Net protein balance was unchanged at 1 day after DNV (sham: −5.5 ± 0.7 vs. DNV: −5.6 ± 0.4 pmol Tyr/μg protein) and 3 days after DNV (sham: −5.5 ± 0.3 vs. DNV: −5.6 ± 0.4 pmol Tyr/μg protein). By 5 days after DNV, net protein balance decreased by 43% compared with sham controls (sham: −4.6 ± 0.4 vs. DNV: −6.6 ± 0.5 pmol Tyr/μg protein). Net protein balance was decreased compared with sham controls at subsequent time points after DNV, with 7 days after DNV resulting in a 49% decrease (sham: −5.6 ± 0.5 vs. DNV: −8.4 ± 0.7 pmol Tyr/μg protein), and 14 days after DNV resulting in a 72% decrease relative to time-matched sham controls (sham: −4.8 ± 0.2 vs. DNV: −8.2 ± 0.3 pmol Tyr/μg protein; P < 0.05 for all comparisons).
Fig. 3.
DNV-induced change in rat DIAm net protein balance as determined by performing parallel, but separate, incubations of strips from the same diaphragm for protein synthesis and protein degradation measurements. Mean and SE of the percent change relative to sham controls are plotted over time after DNV. *Significantly different from the sham control group at the same DNV time point; †significantly different from 1 and 3 days after DNV; #significantly different from 5 days after DNV (P < 0.05 for all comparisons).
Protein ubiquitination after DIAm DNV.
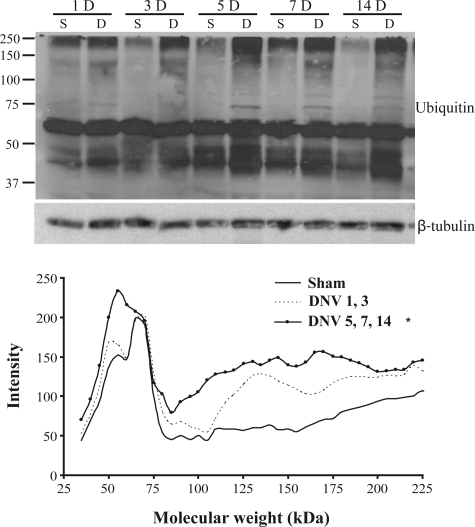
To determine mechanisms underlying the decrease in net protein balance after unilateral DIAm DNV, total protein ubiquitination was evaluated. A strong band was detected at ∼70 kDa, with the same intensity across all sham control or DNV groups. Intensity profiles were measured for each lane to evaluate changes in total protein ubiquitination across molecular weight. Area under the profile curve was also measured to compare ubiquitination across time after DNV. Based on intensity profiles and area under the profile curve measurements, ubiquitination was not significantly different at 1 or 3 days after DNV compared with sham controls (Fig. 4). Ubiquitination increased by 5 days after DNV (65% increase) and remained elevated at 7 days (90% increase) and 14 days (76% increase) after DNV compared with sham controls (P < 0.05 for all comparisons; Fig. 4).
Fig. 4.
DNV-induced increase in total protein ubiquitination, as detected by Western analyses. Top: representative immunoblot of ubiquitin and β-tubulin for each DNV time point (S, sham control; D, DNV). Bottom: mean intensity profile of protein ubiquitination across molecular weight, separated by groups determined by measuring area under the intensity profile curve. *Significantly different (P < 0.05) from the sham controls and from 1 and 3 days after DNV.
Caspase-3 protein expression and activity after DIAm DNV.
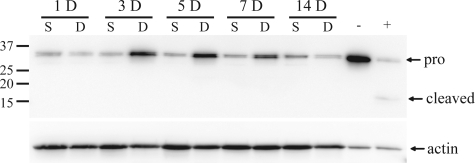
DNV caused a time-dependent increase in procaspase-3 expression compared with sham controls, starting at 3 days after DNV (Fig. 5). Only the pro-form (∼35 kDa) was measurable in experimental samples, whereas the cleaved/active form (∼17 kDa) was below the limit of detection of Western blot analysis at all time points after DNV. Caspase-3 activity assays showed that activity did not significantly change at 1, 3, 5, 7, or 14 days after DNV compared with sham controls (Table 1).
Fig. 5.
DNV-induced change in procaspase-3 (pro) and cleaved capase-3 protein expression in rat DIAm, as detected by Western analyses. Representative immunoblots are shown of procaspase-3 (35 kDa), cleaved caspase-3 (17 kDa), and actin (43 kDa) for each DNV time point. Cytochrome c-treated Jurkat cell extract was used as positive control.
Table 1.
DNV-induced change in caspase-3 activity as determined by a colorimetric caspase-3 activity assay
| Day | Caspase-3 Activity |
|
|---|---|---|
| Sham | DNV | |
| 1 | 2.2±0.1 | 2.1±0.1 |
| 3 | 2.2±0.1 | 2.0±0.1 |
| 5 | 2.5±0.1 | 2.2±0.1 |
| 7 | 2.1±0.1 | 1.9±0.2 |
| 14 | 2.1±0.1 | 2.1±0.3 |
| Positive control | 2.6±0.4 | |
Values are means ± SE in fold increase relative to negative control. Camptothecin-treated Jurkat cell extract was used as positive control. DNV, denervation.
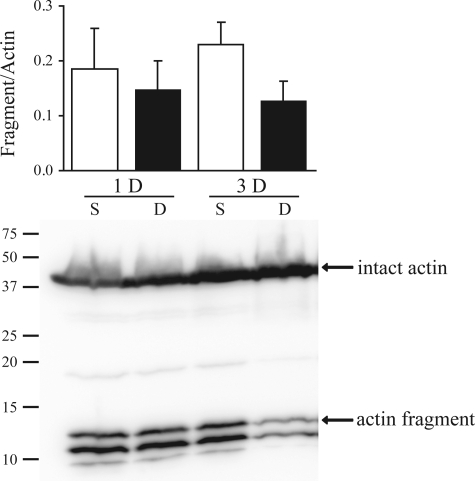
Actin fragmentation after DIAm DNV.
To determine lack of caspase-3 involvement in the DIAm response to DNV, actin fragmentation was also evaluated. Caspase-3 produces a characteristic 14-kDa actin fragment on activation (7). Expression of the actin fragment relative to intact actin did not change at 1 or 3 days after DNV compared with sham controls (Fig. 6).
Fig. 6.
DNV-induced change in actin fragmentation as detected by Western analyses. Top: ratio of actin fragment to total actin for 1 and 3 days after DNV. Bottom: representative immunoblot of intact actin and actin fragment for 1 and 3 days after DNV.
DISCUSSION
The results of this study support the hypothesis that DNV increases net protein breakdown in DIAm, as a result of increased protein ubiquitination. Past studies have focused on either protein synthesis or degradation across several models of inactivity (3, 13, 14, 39). This study measured both synthesis and degradation, allowing measurements of net protein breakdown after DIAm DNV, and provides novel information regarding signaling pathways involved in this process.
Net protein balance after DNV.
Measurement of protein synthesis with two different amino acid tracers showed an increase in synthesis after DNV, which is consistent with previous literature (3, 33). Buse et al. (3) showed a 50% increase in synthesis by 1 day after DIAm DNV, as measured by increased 14C-Leu incorporation. We found a similar increase for 3, 5, 7, and 14 days after DNV. Turner and Garlick (39) performed unilateral phrenicectomy and measured synthesis by 14U-Tyr incorporation. They reported that, by 3 days after DNV, there was a 200% increase in incorporation compared with controls. By 5 and 10 days after DNV, protein synthesis increased 50% compared with control. While our study used different amino acid tracers than those in these previous studies, DNV consistently caused an increase in protein synthesis. In the present study, measurements with 35S-Met showed an increase in synthesis starting 1 day after DNV, while measurements with 3H-Tyr showed a significant increase starting at 3 days after DNV. Differences in the magnitude and timing of changes in protein synthesis may relate to differential incorporation of Leu, Met, and Tyr into newly synthesized proteins.
Protein degradation increased by 5 days after DIAm DNV. Increased protein degradation also occurs in hindlimb muscles following DNV. Goldberg reported a 29% increase in 3H-Leu release from soleus and plantaris muscles at 10 days after sciatic DNV (13). Furuno et al. (9) found that Tyr release from soleus muscle was twice that of control at 3 days after sciatic DNV. However, neither study performed a detailed time course analysis of protein degradation following DNV. It is also important to note that hindlimb muscles exhibit considerable metabolic and functional differences compared with the DIAm. Whereas the DIAm is of mixed fiber composition, the soleus is predominantly composed of MHCslow-expressing fibers (29). In addition, the daily duty cycle (percentage of time being active) for the soleus muscle is ∼14% (20), compared with ∼40% for the DIAm (30). The differences in onset for increased protein degradation after DNV in the DIAm relative to hindlimb muscles may relate to muscle- or fiber-type-specific adaptations (e.g., genetically determined) or changes in gravitational load in hindlimb muscles. To the best of our knowledge, no previous study has measured protein degradation after DIAm DNV.
In the present study, rates of Tyr incorporation and Tyr release were measured in DIAm strips in vitro. It is possible that the amino acid-depleted medium (necessary for these measurements) and/or in vitro preparation itself alter baseline rates of protein turnover. However, tissues obtained from sham and DNV animals were compared under identical conditions. Furthermore, Tyr release is not affected by incubation in a Tyr-free media at 37°C (18). Thus we are confident that the differences in protein synthesis and degradation observed reflect differences in protein turnover associated with DIAm DNV.
The results of the present study highlight the importance of protein degradation as a cause of a negative protein balance. Based on a previous study by Geiger et al. (10), MHC mRNA increased at 1 day after DIAm DNV, but decreased significantly at 3, 7, and 14 days after DNV. The correlation between MHC protein and mRNA levels after DNV was not straightforward, with decreased MHC2B protein expression 14 days after DNV associated with unchanged MHC2B mRNA levels, for example. Thus, although MHC gene transcription and translation may change differentially after DNV, posttranslational changes, including increased protein degradation, are likely responsible for reductions in DIAm MHC protein expression.
Selective loss of proteins after DNV may occur before a generalized change in net protein balance. We confirmed previous findings by Geiger et al. (10), showing that there is a rapid loss in total MHC protein content by 1 day after DNV. In contrast, total protein content in the DIAm does not decrease until 14 days after DNV. Using an estimate of MHC content per muscle mass of 40 mg MHC/g tissue (8), MHC content would be 33% of total muscle protein, and thus changes in MHC content may be obscured by concomitant changes in abundance of other muscle proteins (e.g., as a result of increased protein synthesis after DNV). Regardless, these results suggest that DNV results in selective breakdown of proteins, with contractile proteins such as MHC being degraded preferentially at early time points after DNV. Indeed, sarcomere disassembly is likely one of the first steps induced by proteasomal degradation, before MHC degradation. Proteasomal degradation of other intracellular proteins might be delayed, and future studies should explore fractional synthesis and degradation rates for both contractile and structural proteins after DNV.
Net protein balance was negative for both sham control and DNV groups. The negative values for sham control groups could relate to the in vitro assay, where the DIAm is acutely denervated. In this regard, cutting muscle fibers alters rates of protein synthesis and degradation in soleus muscle, and this could have contributed to net protein breakdown in sham control groups (31). Regardless, in this study, changes in protein synthesis and degradation in DIAm muscle were compared in DNV and sham control muscles under identical in vitro conditions, and thus any changes likely reflect in vivo changes in protein balance. Indeed, Goldspink et al. (15) found that in vivo and in vitro measurements of protein synthesis and degradation gave good agreement for changes in protein turnover after tenotomy of the gastrocnemius muscle. Although every in vitro assay has limitations, measurements for sham control and DNV DIAm were performed in parallel in this study, and thus comparisons of sham control vs. DNV DIAm were highly reliable.
Involvement of the ubiquitin-proteasome pathway after DIAm DNV.
Although proteolytic pathways such as calpain activation and lysosomal autophagy may be involved in protein degradation, skeletal muscle protein degradation is likely regulated by the ubiquitin-proteasome pathway (23). In fact, hindlimb DNV is associated with upregulation of ubiquitin ligases and proteasome activity (2). The cellular signaling mechanisms that activate the ubiquitin-proteasome pathway and cause loss of muscle proteins remain unknown. Caspase-3 expression and total protein ubiquitination, however, are key regulators of proteolysis (7). MHC is degraded by the ubiquitin-proteasome pathway (5, 37). In fact, the E3 ligase muscle RING finger protein 1 was identified as a key modulator of MHC protein levels (5).
In the present study, total protein ubiquitination increased by 5 days after DNV, which is when a change in net protein balance became evident. The results of the present study indicate that activation of the caspase-3 pathway was not necessary for skeletal muscle degradation and, specifically, MHC degradation. Cleaved caspase-3 was not detectable after DNV, and capase-3 activity remained unchanged compared with time-matched sham controls. Furthermore, the insoluble 14-kDa actin fragment, which is regarded as a reliable index of actomyosin degradation through caspase-3, did not change at 1 or 3 days after DNV (7).
The accumulation of ubiquitinated proteins following DNV may result from increased E3 ligase activity (5) or reduced proteasome activity, and the exact mechanisms underlying protein degradation after DNV deserve further study. However, sciatic DNV-induced atrophy of the soleus muscle was attenuated by 7-day treatment with bortezomib, a direct inhibitor of the proteasome (1). Importantly, bortezomib treatment had minimal effects on DNV-induced atrophy of the extensor digitorum longus muscle, indicating that muscle- and/or injury-specific effects of proteasome inhibition may be present. Whether proteasome inhibitors can be used to mitigate muscle protein degradation following DIAm DNV may be relevant to patients with DIAm dysfunction following cardiac surgery, for example (6, 22). Consistent with this hypothesis, the proteasome inhibitor bortezomib improves DIAm contractility by restoring MHC content and decreasing caspase-3 activity in a rat model of congestive heart failure and DIAm dysfunction (40). Future studies are needed to elucidate the role of proteasome inhibition in DIAm adaptations to DNV.
Mechanisms of DNV-induced DIAm adaptations.
Adaptations in skeletal muscle after DNV may result from muscle inactivity or removal of a trophic influence. Because of its unique activation pattern (30), the DIAm may be particularly susceptible to inactivity induced by DNV. Indeed, several models of DIAm inactivity have been developed. For example, mechanical ventilation-induced disuse of DIAm results in atrophy within 18 h of ventilation in both rats (24) and humans (24, 34), despite minimal changes in MHC protein (27). However, mechanical ventilation may induce a rapid inflammatory response (41), and whether inactivity itself is responsible for the injury is not clear. Inactivity is likely not the cause of protein imbalance after DIAm DNV. In previous studies, we found that both DIAm DNV and tetrodotoxin-induced paralysis of the phrenic nerve cause a selective decrease in cross-sectional area of MHC2X- and MHC2B-expressing fiber types (25, 45, 46). In contrast, DIAm paralysis induced by C2 spinal hemisection caused little if any change in fiber size (25). The difference in these models may reflect the fact that, after DNV and tetrodotoxin-induced DIAm paralysis, a positive neural influence is removed, but in C2 hemisection this trophic influence remains intact.
Neural influences are likely essential in maintaining DIAm contractile properties (17). For example, neuregulin (NRG), a nerve-derived trophic factor, contributes to the regulation of muscle fiber growth by increasing protein synthesis and possibly altering the balance between protein synthesis and degradation (19). It is possible that DNV results in loss of neural-derived NRG and thus shifts the balance of protein synthesis and degradation toward net protein breakdown. Whether NRG has selective effects on synthesis of specific muscle proteins is not known. During development, NRG is necessary for synthesis of ACh receptor (21), but its effect on contractile proteins such as MHC has not been reported. Future studies should examine the potential beneficial effect of NRG or other nerve-derived trophic factors in preventing DNV-induced protein breakdown.
In summary, both protein synthesis and protein degradation increase after unilateral DIAm DNV, with the increase in degradation outweighing the increase in synthesis and leading to a decrease in net protein balance by 5 days after DNV. Total protein ubiquitination, an important intracellular signaling event implicated in muscle protein loss, also increases after DNV. In contrast, DNV has no effect on caspase-3 activity, suggesting that the ubiquitin-proteasome pathway is primarily responsible for the DIAm response to DNV. Early loss of contractile proteins such as MHC suggests that protein degradation may be a selective rather than generalized response to DNV. Additional studies are needed to determine the mechanisms responsible for selective loss of contractile proteins following DIAm DNV.
GRANTS
This study was supported by National Institute of Arthritis and Musculoskeletal and Skin Diseases grant R01 AR51173 and the Mayo Foundation.
REFERENCES
- 1.Beehler BC, Sleph PG, Benmassaoud L, Grover GJ. Reduction of skeletal muscle atrophy by a proteasome inhibitor in a rat model of denervation. Exp Biol Med (Maywood) 231: 335–341, 2006. [DOI] [PubMed] [Google Scholar]
- 2.Bodine SC, Latres E, Baumhueter S, Lai VK, Nunez L, Clarke BA, Poueymirou WT, Panaro FJ, Na E, Dharmarajan K, Pan ZQ, Valenzuela DM, DeChiara TM, Stitt TN, Yancopoulos GD, Glass DJ. Identification of ubiquitin ligases required for skeletal muscle atrophy. Science 294: 1704–1708, 2001. [DOI] [PubMed] [Google Scholar]
- 3.Buse MG, McMaster J, Buse J. The effect of denervation and insulin on protein synthesis in the isolated rat diaphragm. Metabolism 14: 1220–1232, 1965. [DOI] [PubMed] [Google Scholar]
- 4.Carraro U, Morale D, Mussini I, Lucke S, Cantini M, Betto R, Catani C, Libera LD, Betto DD, Noventa D. Chronic denervation of rat hemidiaphragm: maintenance of fiber heterogeneity with associated increasing uniformity of myosin isoforms. J Cell Biol 100: 161–174, 1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clarke BA, Drujan D, Willis MS, Murphy LO, Corpina RA, Burova E, Rakhilin SV, Stitt TN, Patterson C, Latres E, Glass DJ. The E3 ligase MuRF1 degrades myosin heavy chain protein in dexamethasone-treated skeletal muscle. Cell Metab 6: 376–385, 2007. [DOI] [PubMed] [Google Scholar]
- 6.Dimopoulou I, Daganou M, Dafni U, Karakatsani A, Khoury M, Geroulanos S, Jordanoglou J. Phrenic nerve dysfunction after cardiac operations: electrophysiologic evaluation of risk factors. Chest 113: 8–14, 1998. [DOI] [PubMed] [Google Scholar]
- 7.Du J, Wang X, Miereles C, Bailey JL, Debigare R, Zheng B, Price SR, Mitch WE. Activation of caspase-3 is an initial step triggering accelerated muscle proteolysis in catabolic conditions. J Clin Invest 113: 115–123, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Everett AW, Prior G, Clark WA, Zak R. Quantitation of myosin in muscle. Anal Biochem 130: 102–107, 1983. [DOI] [PubMed] [Google Scholar]
- 9.Furuno K, Goodman MN, Goldberg AL. Role of different proteolytic systems in the degradation of muscle proteins during denervation atrophy. J Biol Chem 265: 8550–8557, 1990. [PubMed] [Google Scholar]
- 10.Geiger PC, Bailey JP, Zhan WZ, Mantilla CB, Sieck GC. Denervation-induced changes in myosin heavy chain expression in the rat diaphragm muscle. J Appl Physiol 95: 611–619, 2003. [DOI] [PubMed] [Google Scholar]
- 11.Geiger PC, Cody MJ, Han YS, Hunter LW, Zhan WZ, Sieck GC. Effects of hypothyroidism on maximum specific force in rat diaphragm muscle fibers. J Appl Physiol 92: 1506–1514, 2002. [DOI] [PubMed] [Google Scholar]
- 12.Geiger PC, Cody MJ, Macken RL, Bayrd ME, Sieck GC. Effect of unilateral denervation on maximum specific force in rat diaphragm muscle fibers. J Appl Physiol 90: 1196–1204, 2001. [DOI] [PubMed] [Google Scholar]
- 13.Goldberg AL Protein turnover in skeletal muscle. II. Effects of denervation and cortisone on protein catabolism in skeletal muscle. J Biol Chem 244: 3223–3229, 1969. [PubMed] [Google Scholar]
- 14.Goldspink DF The effects of denervation on protein turnover of rat skeletal muscle. Biochem J 156: 71–80, 1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goldspink DF, Garlick PJ, McNurlan MA. Protein turnover measured in vivo and in vitro in muscles undergoing compensatory growth and subsequent denervation atrophy. Biochem J 210: 89–98, 1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gosselin LE, Brice G, Carlson B, Prakash YS, Sieck GC. Changes in satellite cell mitotic activity during acute period of unilateral diaphragm denervation. J Appl Physiol 77: 1128–1134, 1994. [DOI] [PubMed] [Google Scholar]
- 17.Gundersen K Determination of muscle contractile properties: the importance of the nerve. Acta Physiol Scand 162: 333–341, 1998. [DOI] [PubMed] [Google Scholar]
- 18.Guroff G, Udenfriend S. The uptake of tyrosine by isolated rat diaphragm. J Biol Chem 235: 3518–3522, 1960. [PubMed] [Google Scholar]
- 19.Hellyer NJ, Mantilla CB, Park EW, Zhan WZ, Sieck GC. Neuregulin-dependent protein synthesis in C2C12 myotubes and rat diaphragm muscle. Am J Physiol Cell Physiol 291: C1056–C1061, 2006. [DOI] [PubMed] [Google Scholar]
- 20.Hensbergen E, Kernell D. Daily durations of spontaneous activity in cat's ankle muscles. Exp Brain Res 115: 325–332, 1997. [DOI] [PubMed] [Google Scholar]
- 21.Jo SA, Zhu X, Marchionni MA, Burden SJ. Neuregulins are concentrated at nerve-muscle synapses and activate ACh-receptor gene expression. Nature 373: 158–161, 1995. [DOI] [PubMed] [Google Scholar]
- 22.Laub GW, Muralidharan S, Chen C, Perritt A, Adkins M, Pollock S, Bailey B, McGrath LB. Phrenic nerve injury. A prospective study. Chest 100: 376–379, 1991. [DOI] [PubMed] [Google Scholar]
- 23.Lecker SH, Goldberg AL, Mitch WE. Protein degradation by the ubiquitin-proteasome pathway in normal and disease states. J Am Soc Nephrol 17: 1807–1819, 2006. [DOI] [PubMed] [Google Scholar]
- 24.Levine S, Nguyen T, Taylor N, Friscia ME, Budak MT, Rothenberg P, Zhu J, Sachdeva R, Sonnad S, Kaiser LR, Rubinstein NA, Powers SK, Shrager JB. Rapid disuse atrophy of diaphragm fibers in mechanically ventilated humans. N Engl J Med 358: 1327–1335, 2008. [DOI] [PubMed] [Google Scholar]
- 25.Miyata H, Zhan WZ, Prakash YS, Sieck GC. Myoneural interactions affect diaphragm muscle adaptations to inactivity. J Appl Physiol 79: 1640–1649, 1995. [DOI] [PubMed] [Google Scholar]
- 26.Rock KL, Gramm C, Rothstein L, Clark K, Stein R, Dick L, Hwang D, Goldberg AL. Inhibitors of the proteasome block the degradation of most cell proteins and the generation of peptides presented on MHC class I molecules. Cell 78: 761–771, 1994. [DOI] [PubMed] [Google Scholar]
- 27.Sassoon CS, Zhu E, Caiozzo VJ. Assist-control mechanical ventilation attenuates ventilator-induced diaphragmatic dysfunction. Am J Respir Crit Care Med 170: 626–632, 2004. [DOI] [PubMed] [Google Scholar]
- 28.Schiaffino S, Gorza L, Pitton G, Saggin L, Ausoni S, Sartore S, Lomo T. Embryonic and neonatal myosin heavy chain in denervated and paralyzed rat skeletal muscle. Dev Biol 127: 1–11, 1988. [DOI] [PubMed] [Google Scholar]
- 29.Schiaffino S, Reggiani C. Myosin isoforms in mammalian skeletal muscle. J Appl Physiol 77: 493–501, 1994. [DOI] [PubMed] [Google Scholar]
- 30.Schlenker EH, Goldman M. Ventilatory responses of aged male and female rats to hypercapnia and to hypoxia. Gerontology 31: 301–308, 1985. [DOI] [PubMed] [Google Scholar]
- 31.Seider MJ, Kapp R, Chen CP, Booth FW. The effects of cutting or of stretching skeletal muscle in vitro on the rates of protein synthesis and degradation. Biochem J 188: 247–254, 1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shanely RA, Zergeroglu MA, Lennon SL, Sugiura T, Yimlamai T, Enns D, Belcastro A, Powers SK. Mechanical ventilation-induced diaphragmatic atrophy is associated with oxidative injury and increased proteolytic activity. Am J Respir Crit Care Med 166: 1369–1374, 2002. [DOI] [PubMed] [Google Scholar]
- 33.Sieck GC Physiological effects of diaphragm muscle denervation and disuse. Clin Chest Med 15: 641–659, 1994. [PubMed] [Google Scholar]
- 34.Sieck GC, Mantilla CB. Effect of mechanical ventilation on the diaphragm. N Engl J Med 358: 1392–1394, 2008. [DOI] [PubMed] [Google Scholar]
- 35.Sieck GC, Zhan WZ. Denervation alters myosin heavy chain expression and contractility of developing rat diaphragm muscle. J Appl Physiol 89: 1106–1113, 2000. [DOI] [PubMed] [Google Scholar]
- 36.Sola OM, Martin AW. Denervation hypertrophy and atrophy of the hemidiaphragm of the rat. Am J Physiol 172: 324–332, 1953. [DOI] [PubMed] [Google Scholar]
- 37.Solomon V, Goldberg AL. Importance of the ATP-ubiquitin-proteasome pathway in the degradation of soluble and myofibrillar proteins in rabbit muscle extracts. J Biol Chem 271: 26690–26697, 1996. [DOI] [PubMed] [Google Scholar]
- 38.Strle K, Broussard SR, McCusker RH, Shen WH, Johnson RW, Freund GG, Dantzer R, Kelley KW. Proinflammatory cytokine impairment of insulin-like growth factor I-induced protein synthesis in skeletal muscle myoblasts requires ceramide. Endocrinology 145: 4592–4602, 2004. [DOI] [PubMed] [Google Scholar]
- 39.Turner LV, Garlick PJ. The effect of unilateral phrenicectomy on the rate of protein synthesis in rat diaphragm in vivo. Biochim Biophys Acta 349: 109–113, 1974. [DOI] [PubMed] [Google Scholar]
- 40.Van Hees HW, Li YP, Ottenheijm CA, Jin B, Pigmans CJ, Linkels M, Dekhuijzen PN, Heunks LM. Proteasome inhibition improves diaphragm function in congestive heart failure rats. Am J Physiol Lung Cell Mol Physiol 294: L1260–L1268, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vaneker M, Joosten LA, Heunks LM, Snijdelaar DG, Halbertsma FJ, van Egmond J, Netea MG, van der Hoeven JG, Scheffer GJ. Low-tidal-volume mechanical ventilation induces a toll-like receptor 4-dependent inflammatory response in healthy mice. Anesthesiology 109: 465–472, 2008. [DOI] [PubMed] [Google Scholar]
- 42.Waalkes TP, Udenfriend S. A fluorometric method for the estimation of tyrosine in plasma and tissues. J Lab Clin Med 50: 733–736, 1957. [PubMed] [Google Scholar]
- 43.Yellin H Changes in fiber types of the hypertrophying denervated hemidiaphragm. Exp Neurol 42: 412–428, 1974. [DOI] [PubMed] [Google Scholar]
- 44.Zhan WZ, Farkas GA, Schroeder MA, Gosselin LE, Sieck GC. Regional adaptations of rabbit diaphragm muscle fibers to unilateral denervation. J Appl Physiol 79: 941–950, 1995. [DOI] [PubMed] [Google Scholar]
- 45.Zhan WZ, Miyata H, Prakash YS, Sieck GC. Metabolic and phenotypic adaptations of diaphragm muscle fibers with inactivation. J Appl Physiol 82: 1145–1153, 1997. [DOI] [PubMed] [Google Scholar]
- 46.Zhan WZ, Sieck GC. Adaptations of diaphragm and medial gastrocnemius muscles to inactivity. J Appl Physiol 72: 1445–1453, 1992. [DOI] [PubMed] [Google Scholar]