Abstract
Olfactory glomeruli are the loci where the first odor-representation map emerges. The glomerular layer comprises exquisite local synaptic circuits for the processing of olfactory coding patterns immediately after their emergence. To understand how an odor map is transferred from afferent terminals to postsynaptic dendrites, it is essential to directly monitor the odor-evoked glomerular postsynaptic activity patterns. Here we report the use of a transgenic mouse expressing a Ca2+-sensitive green fluorescence protein (GCaMP2) under a Kv3.1 potassium-channel promoter. Immunostaining revealed that GCaMP2 was specifically expressed in mitral and tufted cells and a subpopulation of juxtaglomerular cells but not in olfactory nerve terminals. Both in vitro and in vivo imaging combined with glutamate receptor pharmacology confirmed that odor maps reported by GCaMP2 were of a postsynaptic origin. These mice thus provided an unprecedented opportunity to analyze the spatial activity pattern reflecting purely postsynaptic olfactory codes. The odor-evoked GCaMP2 signal had both focal and diffuse spatial components. The focalized hot spots corresponded to individually activated glomeruli. In GCaMP2-reported postsynaptic odor maps, different odorants activated distinct but overlapping sets of glomeruli. Increasing odor concentration increased both individual glomerular response amplitude and the total number of activated glomeruli. Furthermore, the GCaMP2 response displayed a fast time course that enabled us to analyze the temporal dynamics of odor maps over consecutive sniff cycles. In summary, with cell-specific targeting of a genetically encoded Ca2+ indicator, we have successfully isolated and characterized an intermediate level of odor representation between olfactory nerve input and principal mitral/tufted cell output.
INTRODUCTION
How odors are represented at different stages of the olfactory central projection pathway is critical for understanding the system's superb capability of discriminating a large variety of odorants. Odor maps initially emerge from specific activation of distinct sets of sensory neurons as well as convergence of these neurons' axonal projections into a unique pattern of activated glomeruli (Mombaerts et al. 1996). Immediately after its emergence, the odor map is transferred, via the first synapse in the olfactory pathway, to the mitral and tufted cell dendritic branches distributed within the glomeruli. Such an initial coding-pattern transformation involves several intricate local synaptic processing circuits.
Each glomerulus is surrounded by a large and diverse population of neurons (Kosaka et al. 1998), which not only make dendrodendritic reciprocal synapses with mitral and tufted cell glomerular tuft branches (Christie et al. 2001; Pinching and Powell 1971) but also provide presynaptic feedback inhibition to olfactory nerve terminals (Aroniadou-Anderjaska et al. 2000; Ennis et al. 2001; Hsia et al. 1999; McGann et al. 2005; Wachowiak et al. 2005). In addition, some of these neuronal types form an extensive network within the glomerular layer which mediates center-surround inter-glomerular inhibition (Aungst et al. 2003; Vucinic et al. 2006). Furthermore, glomeruli also receive centrifugal fibers from deep brain structures for modulation of local synaptic circuits (Gomez et al. 2005; Hardy et al. 2005; Nickell and Shipley 1988).
One major unexplored question is to what extent these local glomerular circuits can regulate the transfer of odor maps from presynaptic sensory nerve terminals to postsynaptic mitral/tufted cell dendrites. Answering this question requires direct analysis of the postsynaptic odor representation within the glomerular layer. Among a wide range of functional mapping approaches used to study olfactory glomerular patterns, 2-deoxyglucose autoradiography (Johnson et al. 1998; Sharp et al. 1975), functional MRI (Xu et al. 2003), and intrinsic optical signal imaging (Meister and Bonhoeffer 2001; Mori et al. 2006; Rubin and Katz 1999; Uchida et al. 2000) are based on odor-induced metabolic changes. A recent in vivo pharmacological analysis suggests that the signals detected by these methods are primarily due to olfactory-nerve presynaptic activity with little contribution from postsynaptic neurons. (Gurden et al. 2006; Nawroth et al. 2007; but see Soucy et al. 2001). Ca2+ imaging of anterogradely labeled sensory terminals (Fried et al. 2002; Friedrich and Korsching 1997; Wachowiak and Cohen 2001) and imaging of afferent synaptic-vesicle release with genetically targeted synaptopHluorin (Bozza et al. 2004) are specifically designed to reflect only the presynaptic input pattern. On the other hand, odor responses imaged by voltage-sensitive dyes comprise a mixture of both pre- and postsynaptic components, as the binding of dyes to membrane is not cell-specific (Friedrich and Korsching 1998; Spors and Grinvald 2002). While a few recent studies have begun to investigate postsynaptic odor responses in vivo by visualizing mitral/tufted cell somatic odor responses through the used of Ca2+-sensitive dyes (Li et al. 2005; Nagayama et al. 2007; Yaksi et al. 2007), the general principles underlying the glomerular postsynaptic odor representation have only begun to be explored.
Recently glomerular postsynaptic activity has been successfully isolated with an optical imaging approach by taking advantage of a transgenic mouse expressing a genetically encoded Ca2+ indicator [Ca2+-sensitive green fluorescence protein (GCaMP2)] under the Kv3.1 K+-channel promoter Chaigneau et al. 2007; Fletcher et al. 2006, 2007a,b). Using this animal model, we present a comprehensive analysis of the basic properties of such purely postsynaptic glomerular odor maps. More specifically, we have explored systematically how odorant identity and concentration are encoded at this intermediate level of representation and how these postsynaptic odor maps evolve dynamically across consecutive respiration cycles.
METHODS
Animal surgery and slice preparation
Most experiments were performed using transgenic mice expressing a genetically encoded Ca2+ indicator (GCaMP2) (Nakai et al. 2001; Tallini et al. 2006) under the regulatory sequences of the Kv3.1 potassium-channel promoter as described previously (Diez-Garcia et al. 2005). For comparing the sizes of GCaMP2-responsive spots and olfactory glomeruli, a few mice with synaptopHluorin (SpH) genetically targeted to olfactory nerve terminals were also used (Bozza et al. 2004). All experimental protocols were approved by the Yale University Institutional Animal Care and Use Committee in accordance with the National Institutes of Health guidelines.
For in vivo imaging, adult mice 4- to 24-wk old were anesthetized with injections of sodium pentobarbital (Nembutal, 50 mg/kg ip) and atropine (5 mg/kg ip). Animals were placed in a stereotaxic apparatus with a heating pad underneath to maintain body temperature. After local application of 2% lidocaine, a skin incision was made over the dorsal surface of the mouse head. In most experiments, the bone overlying the olfactory bulb was thinned with a dental drill. In cases in which in vivo pharmacological manipulation was carried out, both the skull and dura mater were removed with the exposed olfactory bulb covered with Ringer solution. All animals breathed freely with respiratory activity monitored by a piezoelectric device strapped around the chest.
For in vitro slice imaging, GCaMP2 mice were anesthetized with urethan (1.2 g/kg ip) and decapitated after losing toe-pinch reflex. The mouse head was quickly immersed in 4°C artificial cerebral spinal fluid (ACSF) oxygenated with 95% O2-5% CO2. The olfactory bulbs on both sides were carefully dissected and then cut horizontally into 400-μm-thick slices using a rotary slicer (Dosaka, Japan). Slices were initially incubated in ACSF at 34°C for 30 min and then maintained at room temperature for ≤8 h. The ACSF contained (in mM, pH 7.4) 124 NaCl, 3 KCl, 1.3 MgSO4, 2 CaCl2, 26 NaHCO3, 1.25 NaH2PO4, and 10 glucose.
In vivo imaging of GCaMP2 response patterns
Imaging was performed using an Olympus BX50WI microscope equipped with ×4 (0.10 NA), ×4 (0.28 NA), and ×10 (0.3 NA) objectives. The dorsal olfactory bulb was illuminated with a Polychrome II monochromator (TILL Photonics GmbH, Munich, Germany) through a dichroic mirror (Chroma, Q505LP). The excitation wavelength was centered at 480 nm. The GCaMP2 fluorescence signal was band-passed filtered with a Chroma emission filter (HQ535/50) and recorded with a back-illuminated NeuroCCD SM-256 camera (RedShirtImaging, Fairfield, CT) at a 256 × 256 resolution and a frame rate of 25 Hz. Image acquisition and initial stage of analysis were performed using NeuroPlex software (RedShirtImaging, Fairfield, CT).
For in vivo electric stimulation, a single current pulse (500 μs, 1 mA) was delivered to the olfactory bulb dorsal surface using a Ringer-filled glass pipette. The pipette was usually positioned on the anterio-lateral portion of the bulb, where olfactory nerve bundles wrapped around the dorsal surface in a posterio-medial direction so that electric stimuli were able to activate a long string of glomeruli (see Fig. 5A). All drugs were dissolved in the ACSF and directly bath-applied to the exposed dorsal bulbar surface. 2,3-dihydroxy-6-nitro-7-sulfamoyl-benzo[f]quinoxaline-2,3-dione (NBQX), CGP 37849 and LY 341495 were obtained from Tocris (Ellisville, MO).
In vitro imaging analysis of GCaMP2 Ca2+ signals
For in vitro imaging experiments, olfactory bulb slices were transferred into a recording chamber mounted on an Olympus BX50WI upright microscope (Tokyo, Japan). Different layers of the olfactory bulb were visualized with infrared DIC video-microscopy. A bipolar concentric stimulating electrode connected to an electric current isolator was placed in the olfactory nerve layer. While in some cases, a response could be seen to a single shock, the GCaMP2 response was much more reliably induced by a 40-Hz pulse train. A ×20 objective (0.50 NA) was used to image the olfactory nerve layer, glomerular layer, and external plexiform layer within a single field of view.
The slice was illuminated at a wavelength of 460–500 nm (HQ480/40x) (Chroma, Rockingham, VT) with a 150W Xenon arc lamp (Opti-Quip, Highland Mills, NY) through a dichroic mirror (Chroma, Q505LP). Fluorescence signal was collected through a 510- to 560-nm emission filter (Chroma, HQ535/50m) and with a PentaMax frame-transfer cooled CCD camera (Princeton Instrument, Trenton, NJ). For each imaging trial, electric pulses of 0.2 ms width were delivered to the olfactory nerve layer at 40 Hz for 500 ms. Image acquisition was carried out at a 10-Hz frame rate using Axon Imaging Workbench 2.2 (Axon Instruments, Union City, CA). Fluorescence response traces were first corrected for photobleaching via monoexponential curve fitting and subtraction, and then averaged over multiple trials. Glutamate receptor antagonists, d(−)-2-amino-5-phosphonopentanoic acid (APV), and 6-cyano-7-nitroquinoxaline-2,3-dione disodium salt (CNQX,(Sigma-Aldrich, St. Louis, MO) and bath-applied to olfactory bulb slices.
For the quantification of the extent of signal blockade induced by the NMDA receptor antagonist APV, the ON-evoked GCaMP2 response under different stimulus intensities was first measured. One weak stimulus and one strong stimulus were chosen to elicit two different amplitudes of GCaMP2 response. The peak signal amplitude was measured for each stimulus condition in ACSF alone and compared with the peak signal amplitude in the presence of APV. From this, APV's blocking efficiency was determined for each stimulus condition by calculating the percent change in peak signal amplitude relative to the controls. A larger blocking efficiency indicates a greater reduction in response amplitude following APV application.
Odorant presentation
Odorants were delivered using a flow-dilution-based olfactometer in which the saturated vapor of an odorant was diluted into a main stream of clean air. The clean air stream was fixed at 0.7 l/min throughout the experiment, and the odor vapor stream was adjusted to give the final concentration delivered to the animal. Filtered nitrogen was used as the odor vapor carrier to avoid oxidation. The saturated vapor was mixed with a filtered air stream in a chamber right before the opening of the delivery system. The tube opening was positioned <1 cm from the animal's nostrils. The mixing chamber was normally under vacuum suction to prevent odorants from reaching the nose. For odor delivery, the suction was suddenly stopped using a solenoid valve, and the diluted odorant was blown toward the nose. Two separate mass flow controllers were used to control the flow rate of clean air and odor vapor, allowing for fine volume adjustment to achieve a desired odorant concentration. To avoid cross-contamination, separate Teflon tubing was used in parallel for delivery of different odorants. Odorants were usually presented with a pulse duration of 2 s and interstimulus interval of ≥60 s to avoid potential sensory adaptation. A constant suction system was used to quickly remove remnant odorants. The odorants used in this study included propanal, butanal, pentanal, hexanal, heptanal, methyl valerate, butyl acetate, amyl acetate, and 2-heptanone (Sigma-Aldrich). The deliverable concentration range of our custom-made olfactometer was from 0.06–10% of saturated vapor.
Imaging analysis of odor response patterns
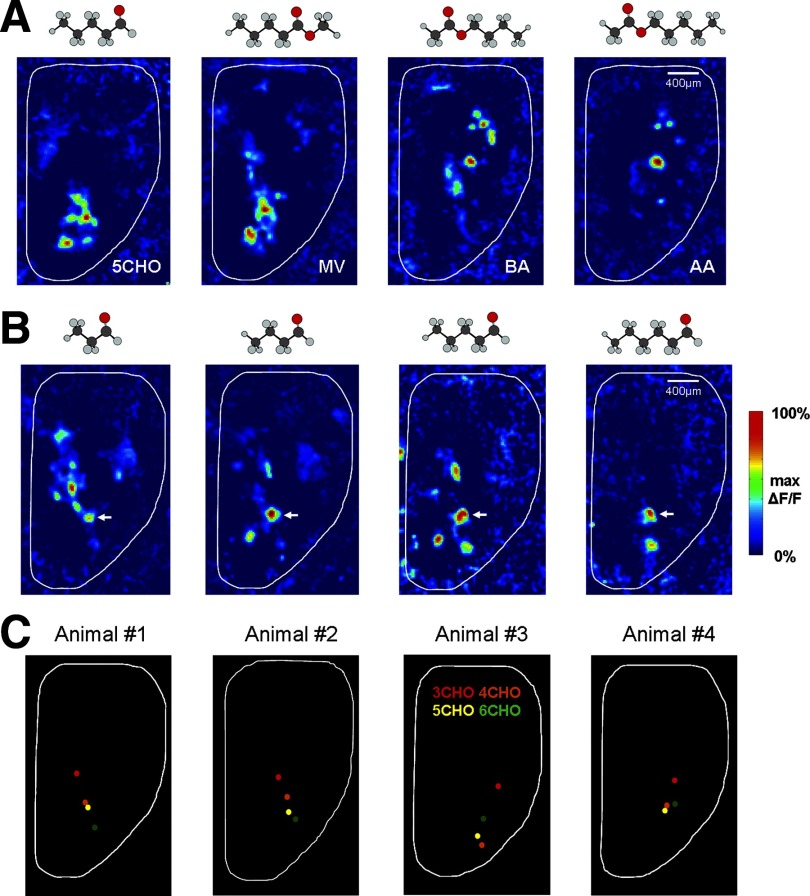
Raw fluorescence traces from individual glomeruli were sampled by spatial averaging of 4–9 pixels located near the center of each glomerulus. Photo-bleaching was corrected for either by subtracting a no-odor imaging trial or by a built-in function in NeuroPlex software to carry out exponential curve fitting and subtraction. To directly visualize the glomerular odor response pattern, a spatial band-pass filter using a Gaussian function (low-pass: σ = 25 μm; high-pass: σ = 300 μm) was applied to all images during an odor response trial (Spors et al. 2006). For each pixel, the odorant-evoked fluorescence change (ΔF) was calculated by subtracting the average of five image frames taken immediately before odorant delivery from an average of five frames taken from the peak of odor-evoked fluorescence response. The ΔF/F was then calculated by dividing this change by the pixel's resting fluorescence level. The calculated negative values were set to zero, and all spatial response maps were presented in pseudocolor. For comparing the odor maps across animals in response to a series of aliphatic aldehydes, odorant-evoked activity patterns were aligned using landmarks such as the sagittal suture and the olfactory bulb caudal sinus. We treated each aldehyde response map as a “mountain” range and calculated its overall mass center using MATLAB (Mathworks). The mass centers acquired for the series of aldehydes were then overlaid to test for the topographic organization of olfactory coding. The near threshold concentration for each odorant was defined as the minimal concentration that reliably elicited a clear response. A glomerulus was considered to respond if its signal amplitude was five times greater than the rms noise level calculated from an adjacent nonresponsive area. The concentrations used for the four animals shown in Fig. 5 were: mouse 1: 3CHO-0.06%, 4CHO-0.06%, 5CHO-0.06%, 6CHO-0.25%; mouse 2: 3CHO-0.06%, 4CHO-0.06%, 5CHO-0.25%, 6CHO-0.50%; mouse 3: 3CHO-0.06%, 4CHO-0.06%, 5CHO-0.13%, 6CHO- 0.06%; mouse 4: 3CHO-0.06%, 4CHO-0.06%, 5CHO-0.13%, 6CHO-0.13%.
For all quantitative analysis of odor responses, the ΔF/F was calculated directly from raw images before any spatial filtering was applied. In each odor trial, the glomerular responses were obtained by averaging the fluorescence signals measured in the first two respiration cycles following the odor pulse onset. To plot the glomerular concentration-response curves, two to four imaging trials were averaged for each odorant at a given concentration. Half-widths of GCaMP2- and SpH-responsive glomeruli were measured by fitting the signal intensity profile of individual foci viewed under a ×10 objective to a Gaussian function and calculating the sigma value (Meister and Bonhoeffer 2001).
For analysis of response patterns to different odor concentrations, a glomerulus was considered to respond if its signal amplitude was five times greater than the rms noise level, which was calculated from an adjacent nonresponsive area (Wachowiak and Cohen 2001). Concentration-response curves were calculated from the ΔF/F measured at the center of each glomerulus and fitted to a sigmoid dose-response function
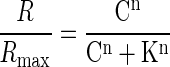 |
where R is the measured ΔF/F at a given odor concentration of C, and Rmax is the maximal response to the highest delivered concentration. K is the odorant concentration that induces a half-maximum response, and n is the Hill coefficient reflecting the slope of the concentration-response curve. For plotting the number of activated glomeruli with odorant concentration, the glomerular count at a given concentration was normalized to the number of glomeruli activated by 1% odorant for each animal. Such a concentration of 1% saturated vapor was chosen for normalization because it was the highest concentration at which individual glomeruli could be readily identified. With higher concentrations, the spatially diffuse nonglomerular GCaMP2 response became much more evident (see following text in results), which potentially masked some weakly responding glomeruli. From the fitted glomerular concentration-response curve, the maximal slope or Hill coefficient was derived. All data were presented as means ± SD. Statistical analysis was carried out using Statview.
For comparison of responses maps across respiratory cycles, odor maps were generated from the odorant-evoked fluorescence change (ΔF/F) calculated from a three frame average centered on the peak of each respiratory cylce. All negative values were set to zero and the spatial response maps were low-pass filtered with a 3 × 3 mean spatial low-pass filter. All traces were calculated from the ΔF/F response measured at the center of each activated glomerulus and temporally low-pass filtered at 6 Hz. Response map correlations were calculated using Matlab.
The relationship between concentration and respiratory modulation was quantified by first measuring the peak and trough signal amplitude for each respiratory cycle obtained from each glomerulus during the odor presentation. For each glomerulus, the amount of respiratory modulation was defined as the average trough-to-peak ratio across all respirations. In this way, a value near one would indicate strong respiratory modulation, whereas a value near zero would indicate no respiratory modulation. Respiratory modulation data were obtained from three different animals (31 total glomeruli) that were presented the same odorant at a relatively low concentration (0.25% s.v.) and a high concentration (5–10% s.v.).
To compare the number of odor-evoked glomeruli per sniff cycle, responding glomeruli were counted at the peak of each respiration cycle during the odorant presentation. Glomerular counts were taken from 20 odor presentations in 13 mice using six different odorants (concentrations range: 0.13–6% s.v.). For each animal, the number of activated glomeruli per respiratory cycle was normalized to the maximum number of activated glomeruli observed through all cycles. The number of activated glomeruli across cycles was compared using an ANOVA.
Immunohistochemistry
Adult GCaMP2 mice were deeply anesthetized with 150 mg/kg Nembutal and perfused transcardially with 0.9% NaCl followed by 4% paraformaldehyde in 0.1 M phosphate buffer (PB). Brains were postfixed in the same fixative overnight. Fixed tissue were cryo-preserved in 30% sucrose in 0.1M PB (pH 7.4) and embedded in O.C.T. compound (Sakura Finetek; Torrance, CA). The olfactory tissue were cut on a cryostat (Reichert-Jung 2800 Frigocut E cryostat) into 20-μm slices, and stored at −20°C until use. On the day of immunohistochemistry, the slices were first rinsed with TBS-T [10 mM Tris-HCl (pH 7.4), 100 mM NaCl with 0.3% Triton-X], blocked with 3% bovine serum albumin and 5% normal donkey serum in TBS-T (blocking buffer) at room temperature for 60 min and incubated with primary antibodies diluted in blocking buffer overnight at room temperature. Sections were washed with TBS-T, then incubated with secondary antibodies with 4′,6-diamino-2-phenylindole dihydrochrolide (DAPI, Invitrogen; Carlsbad, CA), and DRAQ5 (Biostatus; Leicestershire, UK) for nuclei staining for 60 min at room temperature. The immunoreacted sections were washed, mounted with PermaFluor mounting medium (Thermo Fisher Scientific; Fremont, CA), and imaged with a laser scanning confocal microscope (Leica TCS SL, Leica Microsystems; Wetzlar, Germany) or with an epifluorescent microscope (BX51, Olympus).
Primary antibodies used were as follows: goat anti-olfactory marker protein (anti-OMP, 1:1,000; Wako; Richmond, VA); mouse anti-NeuN (1:300; Millipore; Billerica, MA); mouse anti-calretinin (1:400; Millipore); mouse anti-calbindin (1:1,000; Sigma); mouse anti-parvalbumin (1:1,000; Sigma); mouse anti-GAD67 (1:1,000; Millipore); rabbit anti-TH (1:1,000; Millipore); rabbit anti-Tbx21 (1:10,000; kindly provided by Dr. Yoshihara at RIKEN, Japan) (Yoshihara et al. 2005); rabbit anti-GFP (1:1,000; Invitrogen).
Secondary antibodies used were as follows: donkey anti-rabbit Alexa 488 (Invitrogen); donkey anti-rabbit Alexa 555 (Invitrogen); goat anti-mouse Alexa 555 (Invitrogen). All secondary antibodies were used with 1:500 dilution.
RESULTS
GCaMP2 expression patterns in the olfactory bulb
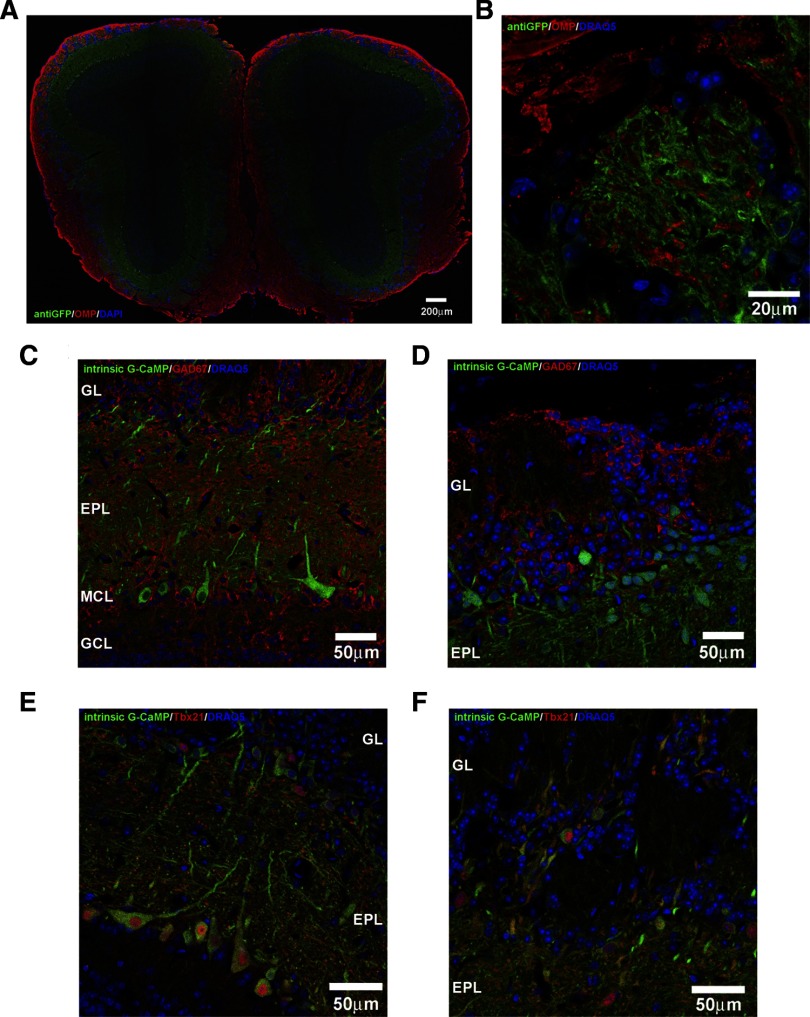
To verify the identity and location of the olfactory bulb cell types expressing GCaMP2, we performed immunofluorescence labeling of fixed olfactory bulb sections. GCaMP2 expression appeared to be the strongest in the glomerular (GL), the external plexiform (EPL), and mitral cell layers (MCL) with no expression found in the olfactory nerve layer (ONL) or granule cell layer (GCL) (Fig. 1A). OMP immunoreactivity, a protein that is expressed only in olfactory sensory neurons, did not co-localize with GCaMP2-positive neurons. Under high magnification, no overlap between the GCaMP2 and OMP signals were observed in any glomeruli (Fig. 1B). Overall the intrinsic GCaMP2 fluorescence observed in the fixed tissue was quite strong and clearly visible without GFP antibody staining (Fig. 1, C–F). These results clearly show that the GCaMP2 expression is confined solely to olfactory bulb neurons and is not expressed in any olfactory sensory neurons.
FIG. 1.
Ca2+-sensitive green fluorescence protein (GCaMP2) is specifically expressed in olfactory bulb neurons that are postsynaptic to olfactory sensory terminals. A: an olfactory bulb section triple stained with anti-olfactory marker protein (OMP, red), anti-GFP (green), and 4′,6-diamino-2-phenylindole dihydrochrolide (DAPI, blue) reveals GCaMP2 expression throughout the glomerular (GL), external plexiform (EPL), and mitral cell layers (MCL). No GCaMP2 expression was seen in the olfactory nerve layer (ONL) or granule cell layer (GCL). B: high-magnification image of a single glomerulus shows no overlap between GCaMP2-positive cell dendrites (green) and olfactory sensory axons (red). C and D: intrinsic GCaMP2 fluorescence in olfactory bulb sections double stained with anti-GAD67 (green) and DRAQ5 (blue). The somata and dendrites of mitral cells, tufted cells, and a subset of juxtaglomerular cells express GCaMP2, whereas no GCaMP2-positive granule cells can be seen. No GAD67-positive GCaMP2 expressing cells were observed in any layer, suggesting GCaMP2 is not expressed in the inhibitory neurons of the olfactory bulb. E and F: co-expression of Tbx21 (red), a mitral/tufted cell marker, in many GCaMP2 cells (green) indicates that a majority of GCaMP2-positive cells are mitral and tufted cells.
Based on their location and soma size, GCaMP2-positive cells appeared to be comprised of mitral and tufted cells and a small subset of juxtaglomerular (JG) cells with no labeling observed in inhibitory granule cells or inhibitory periglomerular cells (PG) (Fig. 1, C and D). In GCaMP2-positive cells, the indicator appeared to be expressed throughout the soma and the dendrites. However, GCaMP2-positive cell somata displayed an unlabeled nucleus, suggesting that GCaMP2 was predominantly distributed in the cytosol. To identify GCaMP2-positive cells, olfactory bulb sections were labeled with antibodies to Tbx21, a known mitral/tufted cell marker (Faedo et al. 2002; Yoshihara et al. 2005). Within the EPL and MCL, nearly all of the GCaMP2-positive cells expressed Tbx21 immunoreactivity (Fig. 1E).
In addition to mitral/tufted cells, a population of JG cells also expressed GCaMP2. Many of these GCaMP2-postive JG cells were also Tbx21-positive, suggesting that they are external tufted cells (Fig. 1F). However, some GCaMP2-positive JG cells were Tbx21-negative. Previous immunohistochemical studies have shown that a large percentage of JG cells can be identified as PG cells based on four different chemical markers: glutamic acid decarboxylase (GAD), tyrosine hydroxylase (TH), calretinin, and calbindin (Halasz et al. 1977; Kosaka and Kosaka 2007; Kosaka et al. 1998; Parrish-Aungst et al. 2007). Using these markers, we attempted to identify GCaMP2-positive JG cells as PG cells through the use of immunofluorescence labeling. Overall no GCaMP2-positive cells were GAD67-positive, suggesting that the GCaMP2 cells of the glomerular layer are not GABAergic (Fig. 1, C and D). No GCaMP2-positive cells were observed to be TH-positive, suggesting that the GCaMP2 cells of the GL are also not dopaminergic (Supplementary Fig. S1A).1 Finally, no GCaMP2-positive cells were found to express either calbindin or calretinin. (Supplementary Fig. S1, B and C). However, a small population of GCaMP2-positive JG neurons were found to be parvalbumin-positive, another calcium binding protein that is expressed in a small percentage of GL and EPL cells (Kosaka et al. 1994; Kosaka and Kosaka 2008; Parrish-Aungst et al. 2007) (Supplementary Fig. S1D).
Glomerular components in odor-evoked GCaMP2 response map
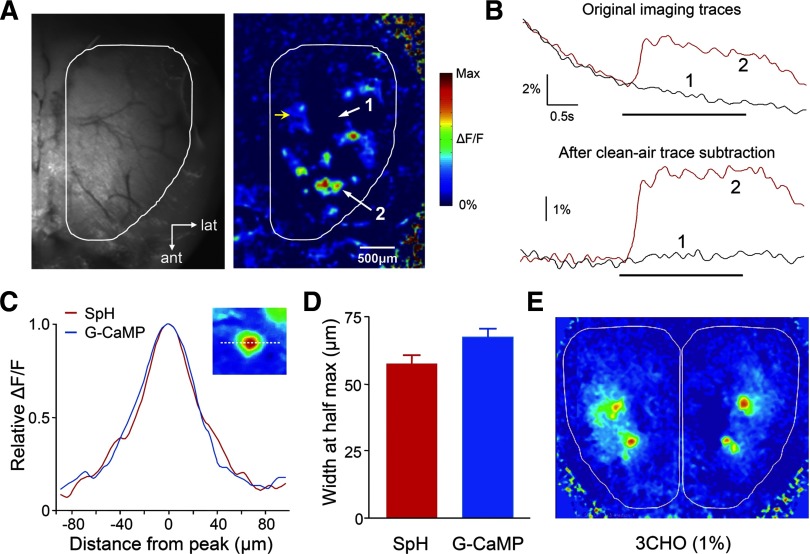
To characterize the GCaMP2 olfactory bulb signal, we carried out in vivo optical imaging of odor responses in the dorsal olfactory bulbs of GCaMP2 mice. Odor presentation was found to induce GCaMP2 fluorescence increase across a large area of the olfactory bulb surface. In general, there were two different types of spatial response signals in a GCaMP2 odor map. The first type of signal was relatively weak and more diffusely distributed (as indicated by a yellow arrow in Fig. 2A). The second type of response took the form of many discrete foci that were scattered across the bulbar surface (as indicated by white arrow 2 in Fig. 2A). These odor-evoked “hot spots” showed strong increases in fluorescence (≤11% ΔF/F).
FIG. 2.
Odorant-evoked GCaMP2 Ca2+ response in the olfactory bulb. A left: an in vivo resting fluorescence image of the olfactory bulb dorsal surface observed through thinned skull in a GCaMP2 mouse. The visible range of dorsal bulbar surface is outlined in white. Right: a single-trial odor response map induced by butyraldehyde (0.25% of saturated vapor). Two numbered white arrows indicate the locations where the odor-evoked response traces shown in B were obtained. The yellow arrow points to the weak and diffusive GCaMP2 response profile, as compared with more focalized hot spots (exemplified by white arrow 2). B, top: raw fluorescence traces without correcting for photo-bleaching. The red and gray traces are the GCaMP2 signals measured, respectively, at odor activated and nonactivated regions, as indicated in A by the two white arrows. A black horizontal bar indicates a 2-sec odor delivery. Bottom: the same odor-responses with photo-bleaching subtracted by using a no-odor imaging trial. C: comparison of the averaged ΔF/F response profile obtained from odor-evoked hot spots in the GCaMP2 mice (cyan) and from synaptopHluorin (SpH)-labeled olfactory glomeruli in the OMP-SpH mice (red). Inset: the profile was measured along a transverse line through the center of a typical responsive spot. For each spot, signals at different sites were normalized to the peak of the profile. The profiles from different GCaMP2 hot spots or SpH-labeled glomeruli were then averaged, respectively, by aligning their response peaks. D: quantitative comparison of the mean widths measured at half-maximum in the GCaMP2 and OMP-SpH mice. E: odor-evoked GCaMP2 response maps were bilaterally symmetrical.
Due to photo-bleaching, the overall fluorescence signal decreased on average by 8.67 ± 2.1% over a time lapse of 20 s (n = 9 animals; Fig. 2B, the upper black trace). Two methods were routinely used to correct for photo-bleaching. One was to subtract a no-odor imaging trial from an odor stimulus trial (Fig. 2B, bottom traces). The other was to carry out exponential fitting of fluorescence decay prior to the odor delivery and then to subtract the fitted line from the original odor response trace.
As no correlation was observed between the level of GCaMP2 resting fluorescence and the response amplitude of odor-evoked hot spots (r = 0.20, P > 0.5, n = 40 hot spots from 5 animals), the appearance of responsive foci was not due to inhomogeneous expression of GCaMP2. To gain insights into whether these focal odor responses actually reflect individual glomerular activation, we plotted the ΔF/F signal profile along a transverse line through the center of a typical GCaMP2 odor-responsive hot spot. The mean diameter of hot spots measured at half-peak amplitude was 67.3 ± 25.6 μm (n = 55, from 6 animals), which was comparable to the mean half-peak diameter of the glomeruli which were visualized morphologically in the OMP-SpH mice [57.5 ± 21.6 μm, n = 42, from 4 animals; unpaired t-test: t (95) = 1.99, P = 0.049; Fig. 2D]. Furthermore, these measurements were consistent with a previous more detailed analysis of the mouse glomerular dimensions (Royet et al. 1988), suggesting that the GCaMP2 hot spots indeed reflected the activation of individual olfactory glomeruli. Additional evidence came from the symmetric distribution of GCaMP2 hot spots in bilateral olfactory bulbs (Fig. 2E), a feature predicted by the glomerulus-targeted nose-to-bulb projection. In the rest of this paper, our analysis will focus on the glomerular components in a GCaMP2 odor map exclusively. Finally, it is important to point out that while the baseline fluorescence measured from the dorsal surface of the olfactory bulb was significantly higher in GCaMP2 animals as compared with wild-type mice [G-CaMP2: 0.47 ± 0.05V, wild-type: 0.29 ± 0.03V, t-test: t (34) = 11.9, P < 0.001] individual glomeruli were not visible under normal resting in vivo conditions.
In vitro characterization of glomerular GCaMP2 Ca2+ signals
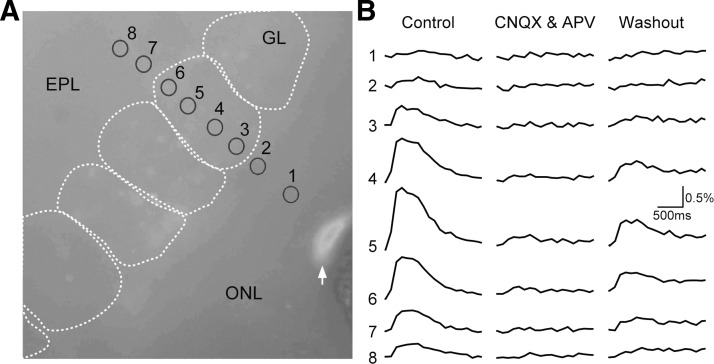
To characterize the functional nature and origin of GCaMP2-reported Ca2+ signals in the olfactory bulb, we used an in vitro slice preparation to image the GCaMP2 fluorescence response to electric stimulation of the olfactory nerve layer. Twenty electric pulses of 0.2 ms width and 0.05-mA amplitude were delivered at 40 Hz to stimulate the olfactory-nerve axon fibers for inducing a GCaMP2 fluorescence increase (control column, Fig. 3). The response amplitude was always maximal in the glomerular layer, but nearly absent in the olfactory nerve layer. Bath application of a mixture of ionotropic glutamatergic receptor antagonists (20 μM CNQX and 50 μM APV) abolished the GCaMP2 response in a reversible manner, further confirming a postsynaptic origin of the GCaMP2 signal in response to ON activation. Such a blocking effect was observed in all slices tested (n = 7), with glomerular GCaMP2 responses reduced to 1.16 ± 1.2% of the control amplitude (paired t-test: t (6) = 4.23, P < 0.01).
FIG. 3.
In vitro pharmacological identification of the postsynaptic origin of GCaMP2 fluorescence response signals. A: a resting fluorescence image of the ONL, GL, and EPL in a horizontally-cut olfactory bulb slice preparation. Individual glomeruli are outlined by white dashed cycles. Regions of interest (ROIs) are illustrated as small solid black circles from which signal traces in B were measured. A white arrow indicates the tip of a concentric bipolar stimulating electrode. B: control, a train of electric stimuli (50 μA, 20 pulses at 40 Hz) was delivered to the ONL and evoked GCaMP2 fluorescence response both in the glomerular and external plexiform layer. The traces numbered 1–8 correspond to the ROIs labeled in A. 6-cyano-7-nitroquinoxalene-2,3-dione (CNQX) and d(−)-2-amino-5-phosphonopentanoic acid (APV), bath application of ionotropic glutamate receptor antagonists (20 μM CNQX and 50 μM APV) blocked the GCaMP2 responses, indicative of a postsynaptic origin of signals. Washout, the GCaMP2 response was partially recovered with washout.
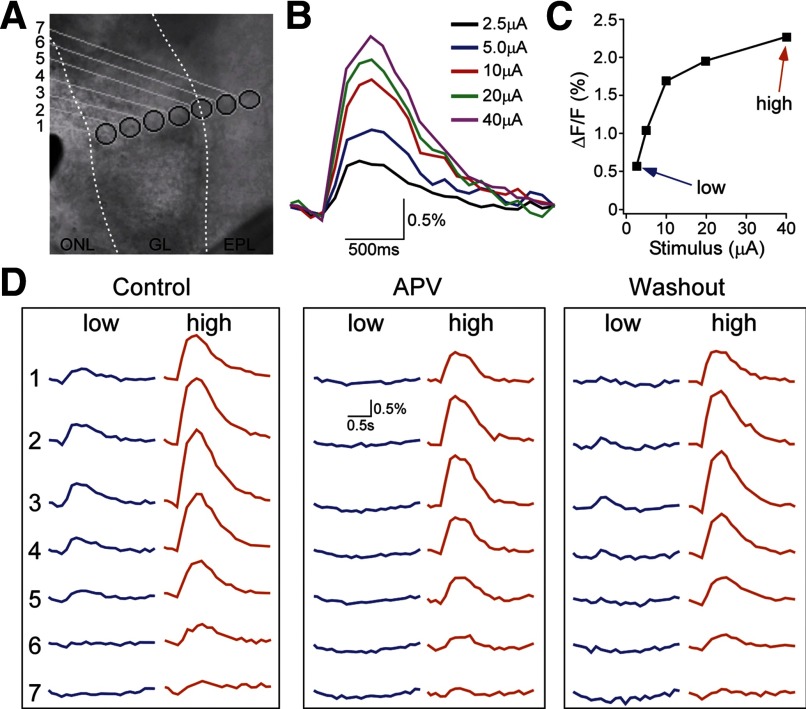
As GCaMP2 is a Ca2+ indicator, the signal it reported is most likely due to Ca2+ influx through glutamate NMDA receptors and voltage-gated Ca2+ channels. It has been previously reported that the mitral/tufted cell glomerular dendritic tufts express NMDA receptors (Giustetto et al. 1997) and also that the ON-evoked excitatory postsynaptic potentials in these neurons display an NMDA component (Berkowicz et al. 1994; Chen and Shepherd 1997; Ennis et al. 1996). To understand the nature of GCaMP2 glomerular signal, we tested the efficiency of signal blockade by NMDA receptor antagonist (50 μM APV) alone. Under the same conditions as the previous experiment, a portion of the response remained even in the presence of APV (n = 6; Fig. 4).
FIG. 4.
Pharmacological analysis of the ON-evoked GCaMP2 response. A: an infrared DIC imaging of an olfactory bulb slice prepared from a GCaMP2 mouse. The borders between the ONL, GL and EPL are delineated with 2 white dashed lines. The black shade located near the left margin of the image is the tip of a concentric bipolar stimulating electrode used to activate olfactory nerve input. The numbered circles mark the ROIs from which GCaMP2 signal traces in D were measured. B: electric stimulation-evoked GCaMP2 response increased with stimulus intensity. Data were sampled from ROI 3. C: plot of ΔF/F versus the stimulus current. The blue and red arrows denote the low and high stimulus intensities with which the data in D were imaged. D: spatial profile and pharmacology of GCaMP2 signals evoked by the 2 different stimulus intensities. Left: control responses. Middle: bath application of 50 μM APV selectively blocked the glomerular GCaMP2 responses to the weak stimulus, but only partially suppressed the strong stimulus-evoked responses. Right: wash-out.
To quantify the extent of the GCaMP2 signal blocked by APV, we measured the ON-evoked GCaMP2 response under different stimulus intensities. To do this, we first gradually increased the stimulus current to plot a curve of GCaMP2 response amplitude versus ON stimulus intensity (n = 4; Fig. 4, B and C). With this curve, two different ON stimuli, one weak and the other strong, were chosen to stimulate the olfactory input alternatively to elicit two different amplitudes of GCaMP2 response (Fig. 4D). At the low stimulus intensity, the glomerular GCaMP2 signal was almost completely blocked by APV (blocking efficiency = 94.7 ± 5.3%, effect observed in 3 of 4 slices). One slice displayed increased responses when APV was applied at the low intensity and was not included. However, the trend still remained if this slice was included (blocking efficiency = 73.2 ± 20.6%). Contrarily, with the strong ON stimulus, APV only partially reduced the GCaMP2 response (blocking efficiency = 30.9 ± 14.9%, effect observed in all 4 slices). This effect was completely abolished by the addition of the AMPA receptor antagonist CNQX (20 μM; Supplementary Fig. S3). These results suggest that the NMDA receptor-mediated Ca2+ increase dominates in a low range of afferent input, while with strong ON excitation, the majority of the glomerular GCaMP2 signal reflects Ca2+ entry driven by other mechanisms such as spike activation of voltage-gated Ca2+ channels (Yuan and Knöpfel 2006a; Zhou et al. 2006b), activation of metabotropic glutamate receptors (Ferraguti et al. 1998; Heinbockel et al. 2004; Yuan and Knöpfel 2006b), or calcium influx through Ca2+-permeable AMPA receptors (Blakemore et al. 2006; Ma and Lowe 2007). Thus while glomerular GCaMP2 responses are caused by several calcium pathways, they only reflect postsynaptic neural activity, and can thereby shed light on both the overall glomerular output and local dendritic processing of olfactory signals.
In vivo characterization of glomerular GCaMP2 Ca2+ signals
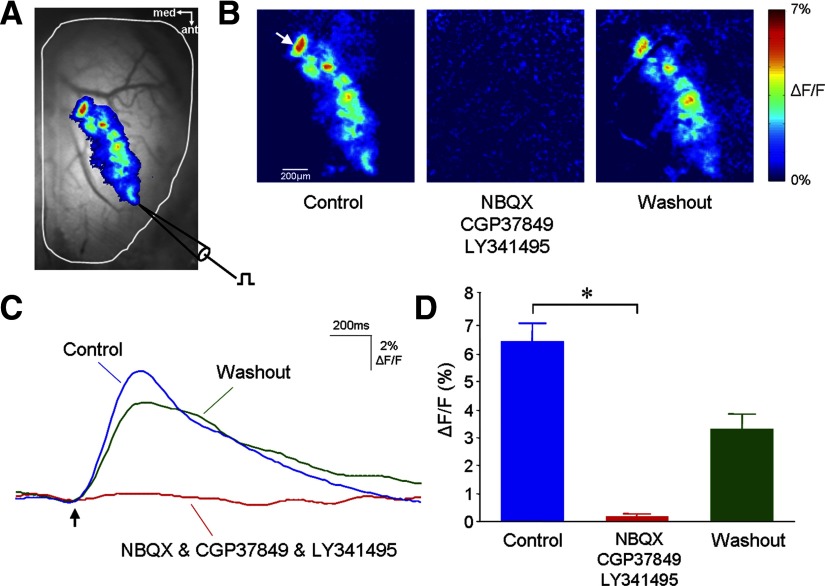
To further confirm that in vivo GCaMP2 Ca2+ imaging reports postsynaptic odor representation, we electrically evoked an artificial GCaMP2 response pattern on the dorsal olfactory bulb glomerular layer. As shown in Fig. 5, a saline-filled glass pipette was positioned on the dorsal bulbar surface close to the anterolateral edge, where olfactory nerve bundles wrapped around in a mediocaudal direction so that electric stimuli delivered by the pipette was able to activate a long string of glomeruli (Fig. 5A). This elongated activation pattern ruled out a possible direct stimulating action of electric current on the glomerular postsynaptic dendrites. The electrically evoked glomerular GCaMP2 response had a large amplitude (mean: 6.42 ± 2.2% ΔF/F). Simultaneous in vivo application of both ionotropic and metabotropic glutamate receptor antagonists (0.5 mM NBQX or CNQX, 2.5 mM CGP 37849, and 1 mM LY 341495) to the dorsal olfactory bulb surface completely eliminated the electrically evoked glomerular response pattern [0.16 ± 0.09% ΔF/F; paired t-test: t (9) = 9.12, P < 0.001); Fig. 5, B and C, D], with the blocking effect reversed after drug washout. Together, these results strongly suggest that the GCaMP2-reported Ca2+ response is of postsynaptic origin and indeed suitable for imaging of postsynaptic odor maps.
FIG. 5.
In vivo pharmacology of the odor-evoked GCaMP2 response signal. A: electric stimulation of the ONL-induced GCaMP2 Ca2+ response in a string of glomeruli located on the dorsal olfactory bulb surface. Here a pseudocolor response map is superimposed on a resting fluorescence image. B: olfactory nerve stimulation-induced GCaMP2 response patterns before (control), during, and after (washout) bath application of both ionotropic and metabotropic glutamate receptor antagonists (0.5 mM NBQX, 2.5 mM CGP 37849, and 1.0 mM LY 341495). C: stimulation-evoked GCaMP2 fluorescence increase in individual glomeruli was abolished by the drug application and with washout recovered to a near control level. The traces shown here were obtained from a glomerulus indicated by a white arrow in B. A black arrow indicates a single electric-pulse stimulus. D: statistics of evoked GCaMP2 responses under the control, drug application and washout conditions. An asterisk indicates a significant difference between the control and drug groups (P < 0.001).
Postsynaptic glomerular codes for odor identity
Previous studies of olfactory presynaptic maps, either with anterograde Ca2+-dye loading of olfactory nerve terminals or OMP-driven expression of a presynaptic release reporter (SpH), have established that odor identity is encoded as distinct combinations of activated glomeruli (Bozza et al. 2004; Fried et al. 2002; Friedrich and Korsching 1997; Wachowiak and Cohen 2001). By taking advantage of the GCaMP2 mice, we examined how different chemical structures of odorants are represented in the glomerular postsynaptic map. Not surprisingly, odor-evoked postsynaptic glomerular activation was found to be broadly distributed across the olfactory bulb surface, and there existed a clear relationship between odorant structures and activated glomeruli. Odorants with similar chemical structures tended to activate a common gross area in the bulb, as illustrated in Fig. 6A by the maps for pentanal versus methyl valerate and for butyl acetate versus amyl acetate. On the contrary, odorants with quite different structures usually activated different regions. For example, the pentanal- and hexanal-activated glomeruli were both located in the anterior part of the olfactory bulb (Fig. 6B, right 2 panels), whereas glomerular activation by butyl acetate and amyl acetate was more confined to the posterior region (Fig. 6A).
FIG. 6.
Spatial coding of odorant identity. A: odor response maps in the same animal to pentanal (5CHO), methyl valerate (MV), butyl acetate (BA), and amyl acetate (AA), each at 0.5% of saturated vapor. The molecular structure of each odorant is displayed above each map. B: response maps from a different animal to a homologous series of aliphatic aldehydes (3CHO-6CHO) at near-threshold concentrations. A white arrow indicates a glomerulus that was responsive to all odorants in the series. Each map is scaled to its own maximum ΔF/F. For comparison, the molecular structure of each odorant is shown above the map. C: Disstribution of the centers of “mass” for each glomerular activation pattern evoked by 1 of the 4 aldehydes at near-threshold concentration. Left-most panel: the response centers computed from the odor maps in B; other 3 panels:obtained from 3 different animals.
Response maps from a homologous series of aliphatic aldehydes also exhibited a clear spatial specificity. To compare the distributions of the most sensitive glomeruli in response to each aldehyde, we focused our analysis on near-threshold concentrations. Aldehydes with different carbon-chain lengths activated distinct but overlapping sets of glomeruli. Interestingly, there were always one or two glomeruli that responded to all tested aldehydes (Fig. 6B, white arrows). One controversial issue regarding the chemotopic representation of the aldehyde series is whether there exists a systematic progression of odor maps with increasing carbon-chain lengths. In most animals tested, there appeared to be a trend toward longer carbon-chain lengths activating more anterior and lateral portions of the dorsal olfactory bulb (Fig. 6B). For a more quantitative comparison, we calculated the center of mass for each odor map as weighted by the pixel response amplitude. In this way, topographic organization of representations of carbon chain length would be observed if the calculated center of mass for each odorant was observed to systematically shift from anterior to posterior as carbon chain length increased. The calculated results for Fig. 6B are illustrated in Fig. 6C, far left. For this animal, a progressive shift of the odor-map “mass” center was observed when the carbon-chain length was increased. The same trend of shift was displayed by a second animal in Fig. 6C. However, only about half of the animals tested in our study showed such kind of precise, systematic topographic organization; while the other half did not exhibit clear progression of odor maps, as exemplified in Fig. 6C, right two panels.
Postsynaptic glomerular concentration coding
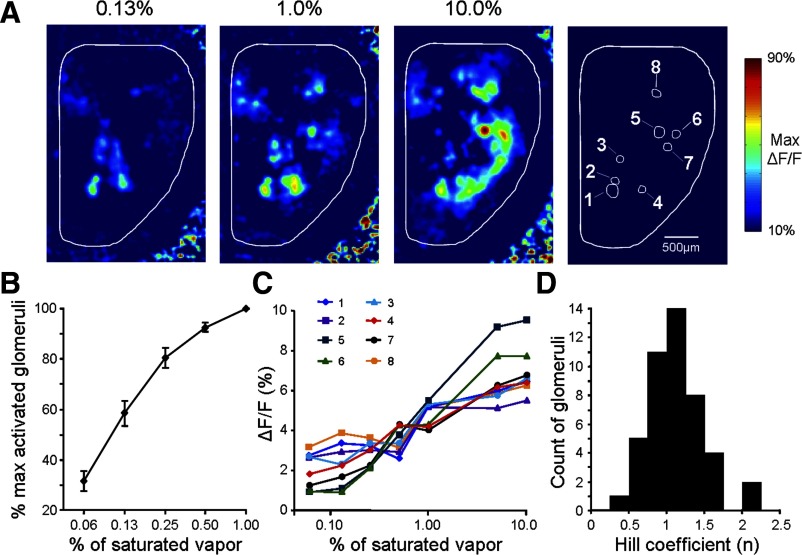
Odorant-induced GCaMP2 fluorescence increase was detected in olfactory glomeruli at a concentration as low as 0.06% of saturated vapor, which was the lowest concentration that could be reliably delivered with our flow-dilution based olfactometer. As the concentration increased, the total number of activated glomeruli increased correspondingly (Fig. 7A), indicating a process of recruiting more glomeruli for coding higher concentrations. To quantify such a process of glomerulus recruitment, we first counted the total number of identifiable glomeruli activated by 1% (s.v.) pentanal and then normalized the number of responsive glomeruli at a given lower concentration as a percentage of this total number. On average, 32% of the total glomeruli activated by 1% pentanal were also responsive to the lowest concentration tested in our analysis (0.06% s.v.), suggesting that a significant portion of glomeruli had a much higher sensitivity that was beyond our tested concentration range (Fig. 7B).
FIG. 7.
Coding of odor concentration at the postsynaptic glomerular level. A: odor maps in response to pentanal at 0.13, 1.0, and 10% of saturated vapor. Increasing odor concentration increased both individual glomerular response amplitudes and the total number of responding glomeruli. For comparison, all three maps are scaled to the same range (10–90%) of the maximum ΔF/F recorded in the 10% odor concentration map. Right-most panel: the glomeruli used for generating the plots in C. At high concentrations such as 10%, spatial filtering caused some glomerular responses appearing weaker in the pseudo-color map, as high odor concentrations tended to induce a diffuse response signal that was distributed broadly across the bulbar surface. B: plot of the mean percentage of activated glomeruli vs. the odorant concentration. The data were pooled from 4 different animals in response to pentanal. For each animal, the percentage was calculated by normalizing the number of activated glomeruli at a given concentration to the total number of glomeruli activated by 1% of saturated vapor. C: odor concentration-response curves for the eight glomeruli labeled in A. All data were measured from raw images before spatial filter was applied. D: histogram of the Hill coefficients (n) calculated by fitting the concentration-response curves of 45 glomeruli.
Besides the number of activated glomeruli, increasing odorant concentration also enhanced the response amplitude of GCaMP2 signal in each activated glomerulus. To assess the dynamic range of such glomerular postsynaptic concentration codes, we analyzed the concentration-response relationship for 57 glomeruli (from 4 animals) to a series of pentanal concentrations from 0.06 to 10.0% of saturated vapor (Fig. 7C, 8 glomeruli from a single animal). Seventy-nine percent of tested glomeruli displayed a continuous increase in GCaMP2 signal over the entire concentration range, suggesting that GCaMP2 Ca2+ response itself was not saturated under these conditions. The remaining glomeruli displayed a large GCaMP2 response at a low concentration but did not show further increase across a wide range of higher concentrations. These glomeruli could not be reasonably fitted to a concentration-response curve and were thus excluded from further analysis.
A Hill function was used to fit the concentration-response curves of 45 glomeruli. The derived Hill coefficients varied from 0.44 to 2.06 with an average of 1.12 ± 0.36 (Fig. 7D). These values are very similar to those reported by Ca2+ imaging of odor responses in olfactory nerve presynaptic terminals (Wachowiak and Cohen 2001) but lower than those acquired with a genetically encoded reporter of presynaptic transmitter release (SpH) (Bozza et al. 2004). Therefore the dynamic range estimated from the Hill coefficient varied across different glomeruli but in general spanned at least two log concentration units.
Temporal dynamics of glomerular postsynaptic odor maps
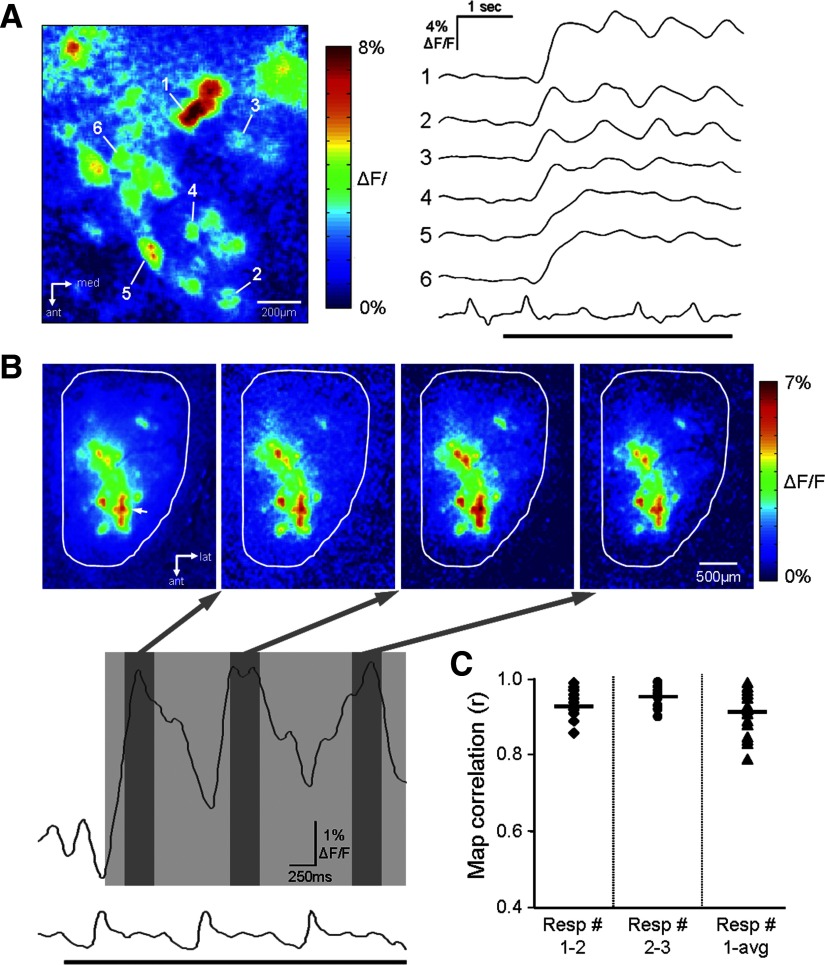
The odor-evoked glomerular GCaMP2 signals displayed a fast time course with the mean rise time of 241.4 ± 80.1 ms (measured from 10 to 90% of the peak response amplitude; n = 58 glomeruli from 5 animals; methyl valerate and pentanal: 0.06–1% s.v.). This mean rise time for the odor-evoked signal is slower than that reported by presynaptic calcium indicators (143 ± 99 ms) (Spors et al. 2006). The odor-evoked GCaMP2 signals were often correlated with respiration with peak responses phase-locked to individual respiratory cycles (Fig. 8A). In some cases, respiration-driven GCaMP2 signals were observed in the absence of odor stimulation, although this modulation was small and in most cases difficult to distinguish from noise. In many glomeruli, the odor-evoked respiration-coupled phasic response was most prominent at intermediate odorant concentrations specific to that glomerulus. Higher concentrations usually elicited a response with much weaker respiratory modulation. Across all glomeruli observed, there was overall significant reduction in respiratory modulation at high concentration odorant stimulation (mean respiratory modulation = 0.31) when compared with the intermediate concentration (mean respiratory modulation = 0.71) [paired t-test: t (30) = 11.45, P < 0.001]. This effect was observed in 94% all glomeruli tested (n = 31).
FIG. 8.
Correlation between odor-evoked GCaMP2 glomerular response signals with animal respiration cycles. A, left: a glomerular odor response map evoked by 0.25% propanal. Right: traces are the response time courses of six different glomeruli aligned with respiratory cycles. Note that glomeruli 1–3 displayed strong phasic responses that closely followed each inhalation. The upward deflections of the recorded respiratory trace represent inhalation, and a black horizontal bar indicates odorant delivery. B: odor response maps in 3 consecutive respiratory cycles (resp 1–3) following the onset of 0.25% methyl valerate delivery. The 2 traces below the maps reflect the odor-evoked GCaMP2 fluorescence signal in an activated glomerulus (white arrowhead) as well as its correlation with the animal's respiration activity. The overall pattern of activated glomeruli varied very little across different inhalation cycles. The response maps generated from each cycle (as indicated by 3 dark gray boxes) strongly resembled the left-most map, which was an average of GCaMP2 response signals across the entire first three respirations (light gray box). C: quantitative analysis via cross-correlation to test for the odor-map stability across the 1st 3 respiration cycles following odorant delivery. Data set was taken from 13 animals, from which 18 odor maps were imaged in response to 5 different odorants.
Given such a correlation between odorant responses and respiration cycles, we analyzed the temporal dynamics of postsynaptic odor maps to test whether they were stable across successive sniff cycles. As our analyses were performed on anesthetized freely breathing animals, the respiration frequency was usually at 1–3 cycle/s. Under these conditions, odor maps were found to be quite stable, with little variation across different sniffs. No significance difference was observed in the number of activated glomeruli across respiratory cycles [ANOVA: F(2,19) = 0.804, P = 0.46]. Figure 8B shows the postsynaptic response maps acquired in each of three consecutive breathing cycles during a 2-s presentation of methyl valerate (0.13% s.v.). The maps were obtained by averaging the GCaMP2 signal over a 120-ms time window centered on each response peak (dark gray boxes). When these individual-sniff maps were compared with the map generated by averaging the signal across the entire 2 s of odorant presentation (light gray box), there were no real differences. To quantify this, we calculated the correlation between the response map obtained from each respiratory cycle and the map obtained from the entire odorant presentation (Fig. 8C). Maps were obtained from 13 mice using five different odorants. In all cases, the maps were highly correlated across respiratory cycles [resp cycle 1 − resp cyle 2: r = 0.93 (median); resp cycle 2 − resp cyle 3: r = 0.95 (median); resp cycle 1 − entire presentation: r = 0.91 (median)]. Although the maps were generally quite stable across respiratory cycles, in some cases there were individual glomeruli that displayed varying responses across respiratory cycles although they did not have a significant effect on the overall map observed on the dorsal surface.
DISCUSSION
To date, almost all glomerular odor maps revealed by in vivo optical imaging reflect either olfactory nerve activity, that is presynaptic afferent responses (Bozza et al. 2004; Friedrich and Korsching 1997; Gurden et al. 2006; Wachowiak and Cohen 2001) or a combination of both pre- and postsynaptic responses (Friedrich and Korsching 1998; Spors and Grinvald 2002). In this study, we have established a mammalian animal model in which we have described, for the first time, the odor-evoked sensory map reflecting exclusively the activity of olfactory bulb neurons postsynaptic to sensory afferents. Immunohistological and pharmacological analysis confirmed that these maps reflect synaptic activation mainly of the major excitatory neurons of the bulb, mitral, and tufted cells.
Compared with the previously analyzed olfactory sensory neuron input-based odor maps, postsynaptic representation of odorants within the glomerular layer appeared to share many common features. The identity of an odorant was encoded by a distinct set of glomeruli, while concentration coding involved both the graded response of each activated glomerulus and the total number of responsive glomeruli. The odor-evoked glomerular GCaMP2 signal usually displayed a dynamic range that spanned at least two log units of concentration. Finally, postsynaptic odor maps phase-locked with respiration showed little variation or adaptation across consecutive sniff cycles at a regular breathing rate of 1∼3 Hz.
Postsynaptic origin of glomerular GCaMP2 signals
In our transgenic mice, the expression of Ca2+-sensitive GCaMP2 was driven by the Kv3.1 K+-channel promoter. Previous studies using in situ hybridization and antibody detection to characterize the distribution pattern of Kv3.1 K+ channels reported that Kv3.1 is expressed in mitral/tufted cells and a number of unidentified JG cells (Ozaita et al. 2002; Weiser et al. 1994). To identify the cell types expressing GCaMP2 in our mice, we performed immunoflourescent labeling using markers of known olfactory bulb cell types. Overall the extent of GCaMP2 labeling is consistent with the previously reported Kv3.1 expression patterns (Ozaita et al. 2002; Weiser et al. 1994). GCaMP2 expression was confined to the upper layers of the olfactory bulb with no expression detected in the ONL. We found that nearly all mitral/tufted cells express GCaMP2. The GCaMP2 appeared to be evenly distributed throughout mitral/tufted cell dendrites and somata, allowing the indicator to report calcium increases at all possible sites within the cell. The lack of GAD-positive GCaMP2 cells in the any layer of the olfactory bulb suggests that none of the cells are inhibitory.
GCaMP2 expression was also observed in some JG cells. In general, JG cells have been classified into three cell types: PG cells, short-axon cells, and external tufted cells (Shepherd et al. 2004). In rodents, PG cells express one or a combination of several different cell markers including GAD, TH, calbindin, and calretinin (Halasz et al. 1977; Kosaka et al. 1998; Kosaka and Kosaka 2008; Parrish-Aungst et al. 2007). As no GCaMP2-positive JG cells were found that express any of these markers, it is believed that the GCaMP2-postive JG cells are most likely not PG cells. This, combined with the fact that most GCaMP2-postive JG cells were co-labeled with the mitral/tufted cell marker Tbx21, suggests that most of the GCaMP2 expressing neurons of the glomerular layer are external tufted cells. It is important to point out, however, that some GCaMP2-postive JG cells could not be identified (e.g., Tbx21-negative cells, PV-positive cells) and may represent an unknown JG cell subtype. Overall it is believed that a vast majority of the GCaMP2-postive cells in the olfactory bulb are excitatory mitral and tufted cells.
Our in vitro and in vivo pharmacological analyses have provided further direct evidence on the postsynaptic origin of GCaMP2 signals by demonstrating that the evoked GCaMP2 signal can be effectively blocked with glutamate antagonists. Despite this, the mechanisms underlying the GCaMP2 glomerular response appear to be complex. Both NMDA receptors and other mechanisms appear to be involved in signal generation. At relatively weak olfactory nerve input, NMDA receptors appeared to be a major contributor to the GCaMP2-reported Ca2+ increase, whereas other mechanisms, such as voltage-gated Ca2+ channels, may play a more dominant role as sensory input increased. Because the mitral/tufted cell primary dendrites can reliably support action potential back-propagation into distal glomerular branches (Chen et al. 1997, 2002; Yuan and Knöpfel 2006a; Zhou et al. 2006b), the GCaMP2 signal in the glomerulus could reflect, at least to a certain degree, the spike activity of these output neurons (Supplementary Fig. S2). It remains to be analyzed whether spike bursts or plateau potentials discharged in certain external tufted cells contribute significantly to the GCaMP2 glomerular signal (Zhou et al. 2006a).
As this study used wide-field fluorescence imaging, a major portion of detected GCaMP2 signal originated from the superficial glomerular layer. Our analysis of signal intensity profiles through individual odor-responsive foci confirmed that these hot spots reflected the activation of single glomeruli. The slightly larger diameter at half-maximum (67.3 ± 25.6 μm for GCaMP2 signal spots vs. 57.5 ± 21.6 μm for SpH-labeled glomeruli) may be due to the GCaMP2 expression in some JG cell somata. In addition to the focal glomerular responses, odor maps also contained areas of diffuse signal, especially in response to high concentrations. This more broad response signal appears to be originating in deeper layers as judged by the time course of in vivo pharmacological manipulations of odor responses (Xing et al. 2007). The most likely source of this signal is GCaMP2 expression in mitral/tufted cell dendrites and soma. Currently, additional studies are underway that aim at fully investigating this phenomenon. Finally, this deeper, more diffuse signal may obscure the glomerular boundaries in the resting condition and could explain why individual glomeruli cannot be visualized at resting conditions in vivo.
Glomerular postsynaptic odor identity and concentration codes
Previous studies have established that different odorants activate distinct, but overlapping sets of glomeruli on the olfactory bulb surface (Belluscio and Katz 2001; Leon and Johnson 2003; Mori et al. 2006; Xu et al. 2003). Not surprisingly, such a combinatorial coding strategy continues to hold for the GCaMP2-reported postsynaptic odor map. As shown in Fig. 6A, glomeruli activated by structurally similar odorants tended to be clustered in the same region, while dramatically different chemicals activated separate regions.
One controversial issue regarding the glomerular spatial codes is to what extent odor maps are topographically organized (Cleland and Sethupathy 2006; Laurent et al. 2001; Leon and Johnson 2003; Mori et al. 2006; Soucy et al. 2009;). In response to a homologous series of aliphatic compounds, some studies have reported a systematic shift of glomerular activation pattern as the backbone carbon-chain length gradually changes (Belluscio and Katz 2001; Johnson et al. 1999; Meister and Bonhoeffer 2001; Uchida et al. 2000). However, a few studies using presynaptic imaging methods found no evidence for such a progression of glomerular codes across the odorant series (Bozza et al. 2004; Fried et al. 2002). In the postsynaptic odor maps analyzed here, we did observe an overall shift of glomerular activation patterns toward the anterior-lateral direction with increasing the carbon-chain length. However, when a quantitative analysis was carried out by calculating the center of each odor map, only about half of the tested animals displayed a precise topographic shift (Fig. 6C). Our results suggest that there is a certain degree of topography in coding a homologous series of odor compounds but with considerable variation across different animals.
Odor responses detected with GCaMP2 as a sensor displayed a sensitivity comparable to that reported previously with synthetic Ca2+-sensitive dyes (Wachowiak and Cohen 2001). In fact, the limiting factor in our analysis of concentration coding was not the GCaMP2 sensitivity but rather the concentration-delivery range of our flow-rate based olfactometer (0.06∼10% s.v.). A significant portion of the glomeruli activated at the highest concentration were also responsive to the lowest deliverable concentration. For these glomeruli, our analysis was likely to be artificially constrained toward the high end of their actual concentration responding range. Even so, the majority of tested glomeruli showed a postsynaptic dynamic range of at least two log units. Because we cannot exclude saturation of the GCaMP2 Ca2+ response at high spiking frequency, the real range of coded concentration can be even larger. However, at high odor concentrations, a spatially diffuse GCaMP2 response signal became evident and was superimposed on the glomerular signal. This could lead to an overestimation of glomerular dynamic range.
Overall, it appears that the changes observed in postsynaptic odor maps with increasing odorant concentration resemble those reported by other methods (Bozza et al. 2004; Johnson and Leon 2000). In this case, increasing concentration lead to both stronger responses in individual glomeruli as well as an increase in the overall glomerular representation of that odorant. Given that the GCaMP2 glomerular signal represents a population response, the increase in fluorescence with increasing concentration most likely reflects both the recruitment of more neurons as well as each neuron being activated more strongly. To further address this issue, experiments at single-cell resolution are needed.
Temporal dynamics of postsynaptic odor map
Odor representation is a dynamic process with responses evolving over the time course of sensory input (Cinelli et al. 1995; Spors and Grinvald 2002; Spors et al. 2006; Verhagen et al. 2007). Previous studies (Diez-Garcia et al. 2005, 2007) have indicated that the GCaMP2 Ca2+ probe had a response time course fast enough for exploring the temporal dynamics of odor maps within and across individual respiratory cycles. Analysis of odor maps sampled across consecutive peaks of respiratory cycles revealed very similar odor representations. No differences were observed either in the number of activated glomeruli or the spatial response pattern. It thus appears that the overall postsynaptic pattern of activated glomeruli is established during the first sniff cycle and repeated across subsequent cycles. These data fit well with recent behavioral experiments demonstrating that rodents are capable of identifying different odorants within time frames as short as a single sniff (Abraham et al. 2004; Rinberg et al. 2006; Uchida and Mainen 2003).
Dissecting sensory maps with genetically targeted functional indicators
Genetic targeting of functional indicators to specific neuronal types promises a powerful approach to isolate sensory maps within the central projection pathway. Transgenic mice expressing inverse pericam and camgaroo-2 have been reported previously, but the odor response patterns imaged in these mice are spatially diffusive, lacking single glomerulus resolution (Hasan et al. 2004). The present study, together with work in the cerebellum (Diez-Garcia et al. 2005, 2007), has established one of the first mammalian models that employ genetically encoded Ca2+ indicators to explore neural functions at the systems level (Heim et al. 2007). Unlike the SpH in the OMP-SpH mice, both the sensitivity and fast time course of GCaMP2 signal appear to be comparable to those of synthetic Ca2+ dyes (Bozza et al. 2004; Wachowiak and Cohen 2001). Furthermore, the GCaMP2 expressed in the cerebellar parallel fibers has demonstrated an unprecedented capability to report the Ca2+ increase induced by a single population action potential (Diez-Garcia et al. 2005), despite a nonlinear Ca2+ response of GCaMP2 (Pologruto et al. 2004).
Overall, the GCaMP2 animal reported here promises to be an important tool in our understanding of the transformation of odor representation from the receptor input to mitral/tufted cell output within the olfactory bulb. While the odor representations revealed in this study appear to be similar to those of presynaptic afferent patterns, many differences are possible given the abundance of local synaptic processing circuits within the glomerular layer (Aungst et al. 2003; Olsen et al. 2007). This processing could be studied further by using the GCaMP2 mice to investigate lateral inhibition of mitral/tufted cell dendritic responses within the glomerular layer itself. While an interglomerular inhibitory center-surround circuit has been demonstrated in vitro (Aungst et al. 2003), its function in shaping glomerular odor representations has only begun to be explored (Vucinic et al. 2006). Another exciting perspective is to carry out in vivo awake-animal imaging (Verhagen et al. 2007), which can provide a practical approach to assess the behavioral and learning-induced plasticity of glomerular odor maps.
GRANTS
The work was supported by National Institute of Deafness and Other Communication Disorders Grants DC-003918 and DC-009666 to W. R. Chen and DC-009853 to M. L. Fletcher), a RIKEN BSI intramural fund (T. Knópfel), an National Institutes of Health training grant (NS-007224) and Yale Brown-Coxe Postdoctoral Fellowship to M. L. Fletcher, the National Institutes of Health Medical Scientist Training Program to A.V. Masurkar, and a Patterson Trust Postdoctoral Fellowship in Brain Circuitry to S. Nagayama.
Supplementary Material
Acknowledgments
We thank Drs. Gordon Shepherd and Lawrence Cohen for reading the manuscript critically.
Footnotes
The online version of this article contains supplemental data.
REFERENCES
- Abraham 2004.Abraham NM, Spors H, Carleton A, Margrie TW, Kuner T, Schaefer AT. Maintaining accuracy at the expense of speed: stimulus similarity defines odor discrimination time in mice. Neuron 44: 865–876, 2004. [DOI] [PubMed] [Google Scholar]
- Aroniadou-Anderjaska 2000.Aroniadou-Anderjaska V, Zhou FM, Priest CA, Ennis M, Shipley MT. Tonic and synaptically evoked presynaptic inhibition of sensory input to the rat olfactory bulb via GABA(B) heteroreceptors. J Neurophysiol 84: 1194–1203, 2000. [DOI] [PubMed] [Google Scholar]
- Aungst 2003.Aungst JL, Heyward PM, Puche AC, Karnup SV, Hayar A, Szabo G, Shipley MT. Centre-surround inhibition among olfactory bulb glomeruli. Nature 426: 623–629, 2003. [DOI] [PubMed] [Google Scholar]
- Belluscio 2001.Belluscio L, Katz LC. Symmetry, stereotypy, and topography of odorant representations in mouse olfactory bulbs. J Neurosci 21: 2113–2122, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkowicz 1994.Berkowicz DA, Trombley PQ, Shepherd GM. Evidence for glutamate as the olfactory receptor cell neurotransmitter. J Neurophysiol 71: 2557–2561, 1994. [DOI] [PubMed] [Google Scholar]
- Blakemore 2006.Blakemore LJ, Resasco M, Mercado MA, Trombley PQ. Evidence for Ca(2+)-permeable AMPA receptors in the olfactory bulb. Am J Physiol Cell Physiol 290: 925–935, 2006. [DOI] [PubMed] [Google Scholar]
- Bozza 2004.Bozza T, McGann JP, Mombaerts P, Wachowiak M. in vivo imaging of neuronal activity by targeted expression of a genetically encoded probe in the mouse. Neuron 42: 9–21, 2004. [DOI] [PubMed] [Google Scholar]
- Chaigneau 2007.Chaigneau E, Tiret P, Lecoq J, Ducros M, Knöpfel T, Charpak S. The relationship between blood flow and neuronal activity in the rodent olfactory bulb. J Neurosci 27: 6452–6460, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen 1997.Chen WR, Midtgaard J, Shepherd GM. Forward and backward propagation of dendritic impulses and their synaptic control in mitral cells. Science 278: 463–467, 1997. [DOI] [PubMed] [Google Scholar]
- Chen 2002.Chen WR, Shen GY, Shepherd GM, Hines ML, Midtgaard J. Multiple modes of action potential initiation and propagation in mitral cell primary dendrite. J Neurophysiol 88: 2755–2764, 2002. [DOI] [PubMed] [Google Scholar]
- Chen 1997.Chen WR, Shepherd GM. Membrane and synaptic properties of mitral cells in slices of rat olfactory bulb. Brain Res 745: 189–196, 1997. [DOI] [PubMed] [Google Scholar]
- Christie 2001.Christie JM, Schoppa NE, Westbrook GL. Tufted cell dendrodendritic inhibition in the olfactory bulb is dependent on NMDA receptor activity. J Neurophysiol 85: 169–173, 2001. [DOI] [PubMed] [Google Scholar]
- Cinelli 1995.Cinelli AR, Hamilton KA, Kauer JS. Salamander olfactory bulb neuronal activity observed by video rate, voltage-sensitive dye imaging. III. Spatial and temporal properties of responses evoked by odorant stimulation. J Neurophysiol 73: 2053–2071, 1995. [DOI] [PubMed] [Google Scholar]
- Cleland 2006.Cleland TA, Sethupathy P. Non-topographical contrast enhancement in the olfactory bulb. BMC Neurosci 7: 7, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diez-Garcia 2007.Diez-Garcia J, Akemann W, Knöpfel T. in vivo calcium imaging from genetically specified target cells in mouse cerebellum. Neuroimage 34: 859–869, 2007. [DOI] [PubMed] [Google Scholar]
- Diez-Garcia 2005.Diez-Garcia J, Matsushita S, Mutoh H, Nakai J, Ohkura M, Yokoyama J, Dimitrov D, Knöpfel T. Activation of cerebellar parallel fibers monitored in transgenic mice expressing a fluorescent Ca2+ indicator protein. Eur J Neurosci 22: 627–635, 2005. [DOI] [PubMed] [Google Scholar]
- Ennis 2001.Ennis M, Zhou FM, Ciombor KJ, Aroniadou-Anderjaska V, Hayar A, Borrelli E, Zimmer LA, Margolis F, Shipley MT. Dopamine D2 receptor-mediated presynaptic inhibition of olfactory nerve terminals. J Neurophysiol 86: 2986–2997, 2001. [DOI] [PubMed] [Google Scholar]
- Ennis 1996.Ennis M, Zimmer LA, Shipley MT. Olfactory nerve stimulation activates rat mitral cells via NMDA and non-NMDA receptors in vitro. Neuroreport 7: 989–992, 1996. [DOI] [PubMed] [Google Scholar]
- Faedo 2002.Faedo A, Ficara F, Ghiani M, Aiuti A, Rubenstein JL, Bulfone A. Developmental expression of the T-box transcription factor T-bet/Tbx21 during mouse embryogenesis. Mech Dev 116: 157–160, 2002. [DOI] [PubMed] [Google Scholar]
- Ferraguti 1998.Ferraguti F, Conquet F, Corti C, Grandes P, Kuhn R, Knöpfel T. Immunohistochemical localization of the mGluR1beta metabotropic glutamate receptor in the adult rodent forebrain: evidence for a differential distribution of mGluR1 splice variants. J Comp Neurol 400: 391–407, 1998. [PubMed] [Google Scholar]
- Fletcher 2006.Fletcher ML, Masurkar AV, Xiong W, Nagayama S, Mutoh H, Cohen LB, Knöpfel T, Chen WR. Optical imaging of olfactory bulb postsynaptic odor representation. Soc Neurosci Abstr 32: 406.3, 2006. [Google Scholar]
- Fletcher 2007a.Fletcher ML, Masurkar AV, Xing J, Xiong W, Nagayama S, Mutoh H, Homma R, Cohen LB, Knöpfel T, Chen WR. Optical imaging of postsynaptic odorant representations in the olfactory bulb. Chem Abstr 29: 81, 2007a. [Google Scholar]
- Fletcher 2007b.Fletcher ML, Masurkar AV, Xing J, Xiong W, Nagayama S, Mutoh H, Homma R, Cohen LB, Knöpfel T, Chen WR. Mapping odor representation with a genetically encoded calcium indicator (G-CaMP2) (Abstract). Cold Spring Harbor, Imaging Neurons and Neural Activity: New Methods, New Results: 67, 2007b.
- Fried 2002.Fried HU, Fuss SH, Korsching SI. Selective imaging of presynaptic activity in the mouse olfactory bulb shows concentration and structure dependence of odor responses in identified glomeruli. Proc Natl Acad Sci USA 99: 3222–3227, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedrich 1997.Friedrich RW, Korsching SI. Combinatorial and chemotopic odorant coding in the zebrafish olfactory bulb visualized by optical imaging. Neuron 18: 737–752, 1997. [DOI] [PubMed] [Google Scholar]
- Friedrich 1998.Friedrich RW, Korsching SI. Chemotopic, combinatorial, and noncombinatorial odorant representations in the olfactory bulb revealed using a voltage-sensitive axon tracer. J Neurosci 18: 9977–9988, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giustetto 1997.Giustetto M, Bovolin P, Fasolo A, Bonino M, Cantino D, Sassoe-Pognetto M. Glutamate receptors in the olfactory bulb synaptic circuitry: heterogeneity and synaptic localization of N-methyl-d-aspartate receptor subunit 1 and AMPA receptor subunit 1. Neuroscience 76: 787–798, 1997. [DOI] [PubMed] [Google Scholar]
- Gomez 2005.Gomez C, Brinon JG, Barbado MV, Weruaga E, Valero J, Alonso JR. Heterogeneous targeting of centrifugal inputs to the glomerular layer of the main olfactory bulb. J Chem Neuroanat 29: 238–254, 2005. [DOI] [PubMed] [Google Scholar]
- Gurden 2006.Gurden H, Uchida N, Mainen ZF. Sensory-evoked intrinsic optical signals in the olfactory bulb are coupled to glutamate release and uptake. Neuron 52: 335–345, 2006. [DOI] [PubMed] [Google Scholar]
- Halasz 1977.Halasz N, Hokfelt T, Ljungdahl A, Johansson O, Goldstein M. Dopamine neurons in the olfactory bulb. Adv Biochem Psychopharmacol 16: 169–177, 1977. [PubMed] [Google Scholar]
- Hardy 2005.Hardy A, Palouzier-Paulignan B, Duchamp A, Royet JP, Duchamp-Viret P. 5-Hydroxytryptamine action in the rat olfactory bulb: in vitro electrophysiological patch-clamp recordings of juxtaglomerular and mitral cells. Neuroscience 131: 717–731, 2005. [DOI] [PubMed] [Google Scholar]
- Hasan 2004.Hasan MT, Friedrich RW, Euler T, Larkum ME, Giese G, Both M, Duebel J, Waters J, Bujard H, Griesbeck O, Tsien RY, Nagai T, Miyawaki A, Denk W. Functional fluorescent Ca2+ indicator proteins in transgenic mice under TET control. PLoS Biol 2: e163, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heim 2007.Heim N, Garaschuk O, Friedrich MW, Mank M, Milos RI, Kovalchuk Y, Konnerth A, Griesbeck O. Improved calcium imaging in transgenic mice expressing a troponin C-based biosensor. Nat Methods 4: 127–129, 2007. [DOI] [PubMed] [Google Scholar]
- Heinbockel 2004.Heinbockel T, Heyward P, Conquet F, Ennis M. Regulation of main olfactory bulb mitral cell excitability by metabotropic glutamate receptor mGluR1. J Neurophysiol 92: 3085–3096, 2004. [DOI] [PubMed] [Google Scholar]
- Hsia 1999.Hsia AY, Vincent JD, Lledo PM. Dopamine depresses synaptic inputs into the olfactory bulb. J Neurophysiol 82: 1082–1085, 1999. [DOI] [PubMed] [Google Scholar]
- Johnson 2000.Johnson BA, Leon M. Modular representations of odorants in the glomerular layer of the rat olfactory bulb and the effects of stimulus concentration. J Comp Neurol 422: 496–509, 2000. [DOI] [PubMed] [Google Scholar]
- Johnson 1999.Johnson BA, Woo CC, Hingco EE, Pham KL, Leon M. Multidimensional chemotopic responses to n-aliphatic acid odorants in the rat olfactory bulb. J Comp Neurol 409: 529–548, 1999. [PubMed] [Google Scholar]
- Johnson 1998.Johnson BA, Woo CC, Leon M. Spatial coding of odorant features in the glomerular layer of the rat olfactory bulb. J Comp Neurol 393: 457–471, 1998. [DOI] [PubMed] [Google Scholar]
- Kosaka 1994.Kosaka K, Heizmann CW, Kosaka T. Calcium-binding protein parvalbumin-immunoreactive neurons in the rat olfactory bulb. I. Distribution and structural features in adult rat. Exp Brain Res 99: 191–204, 1994. [DOI] [PubMed] [Google Scholar]
- Kosaka 1998.Kosaka K, Toida K, Aika Y, Kosaka T. How simple is the organization of the olfactory glomerulus?: the heterogeneity of so-called periglomerular cells. Neurosci Res 30: 101–110, 1998. [DOI] [PubMed] [Google Scholar]
- Kosaka 2007.Kosaka K, Kosaka T. Chemical properties of type 1 and type 2 periglomerular cells in the mouse olfactory bulb are different from those in the rat olfactory bulb. Brain Res 1167: 42–55, 2007. [DOI] [PubMed] [Google Scholar]
- Kosaka 2008.Kosaka T, Kosaka K. Heterogeneity of parvalbumin-containing neurons in the mouse main olfactory bulb, with special reference to short-axon cells and betaIV-spectrin positive dendritic segments. Neurosci Res 60: 56–72, 2008. [DOI] [PubMed] [Google Scholar]
- Laurent 2001.Laurent G, Stopfer M, Friedrich RW, Rabinovich MI, Volkovskii A, Abarbanel HD. Odor encoding as an active, dynamical process: experiments, computation, and theory. Annu Rev Neurosci 24: 263–297, 2001. [DOI] [PubMed] [Google Scholar]
- Leon 2003.Leon M, Johnson BA. Olfactory coding in the mammalian olfactory bulb. Brain Res Brain Res Rev 42: 23–32, 2003. [DOI] [PubMed] [Google Scholar]
- Li 2005.Li J, Mack JA, Souren M, Yaksi E, Higashijima S, Mione M, Fetcho JR, Friedrich RW. Early development of functional spatial maps in the zebrafish olfactory bulb. J Neurosci 25: 5784–5795, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma 2007.Ma J, Lowe G. Calcium permeable AMPA receptors and autoreceptors in external tufted cells of rat olfactory bulb. Neuroscience 144: 1094–1108, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGann 2005.McGann JP, Pirez N, Gainey MA, Muratore C, Elias AS, Wachowiak M. Odorant representations are modulated by intra- but not interglomerular presynaptic inhibition of olfactory sensory neurons. Neuron 48: 1039–1053, 2005. [DOI] [PubMed] [Google Scholar]
- Meister 2001.Meister M, Bonhoeffer T. Tuning and topography in an odor map on the rat olfactory bulb. J Neurosci 21: 1351–1360, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mombaerts 1996.Mombaerts P, Wang F, Dulac C, Chao SK, Nemes A, Mendelsohn M, Edmondson J, Axel R. Visualizing an olfactory sensory map. Cell 87: 675–686, 1996. [DOI] [PubMed] [Google Scholar]
- Mori 2006.Mori K, Takahashi YK, Igarashi KM, Yamaguchi M. Maps of odorant molecular features in the Mammalian olfactory bulb. Physiol Rev 86: 409–433, 2006. [DOI] [PubMed] [Google Scholar]
- Nagayama 2007.Nagayama S, Zeng S, Xiong W, Fletcher ML, Masurkar AV, Davis DJ, Pieribone VA, Chen WR. in vivo simultaneous tracing and Ca2+ imaging of local neuronal circuits. Neuron 53: 789–803, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakai 2001.Nakai J, Ohkura M, Imoto K. A high signal-to-noise Ca2+ probe composed of a single green fluorescent protein. Nat Biotechnol 19: 137–141, 2001. [DOI] [PubMed] [Google Scholar]
- Nawroth 2007.Nawroth JC, Greer CA, Chen WR, Laughlin SB, Shepherd GM. An energy budget for the olfactory glomerulus. J Neurosci 27: 9790–9800, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickell 1988.Nickell WT, Shipley MT. Neurophysiology of magnocellular forebrain inputs to the olfactory bulb in the rat: frequency potentiation of field potentials and inhibition of output neurons. J Neurosci 8: 4492–4502, 1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen 2007.Olsen SR, Bhandawat V, Wilson RI. Excitatory interactions between olfactory processing channels in the Drosophila antennal lobe. Neuron 54: 89–103, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozaita 2002.Ozaita A, Martone ME, Ellisman MH, Rudy B. Differential subcellular localization of the two alternatively spliced isoforms of the Kv3.1 potassium channel subunit in brain. J Neurophysiol 88: 394–408, 2002. [DOI] [PubMed] [Google Scholar]
- Parrish-Aungst 2007.Parrish-Aungst S, Shipley MT, Erdelyi F, Szabo G, Puche AC. Quantitative analysis of neuronal diversity in the mouse olfactory bulb. J Comp Neurol 501: 825–836, 2007. [DOI] [PubMed] [Google Scholar]
- Pinching 1971.Pinching AJ, Powell TP. The neuropil of the glomeruli of the olfactory bulb. J Cell Sci 9: 347–377, 1971. [DOI] [PubMed] [Google Scholar]
- Pologruto 2004.Pologruto TA, Yasuda R, Svoboda K. Monitoring neural activity and [Ca2+] with genetically encoded Ca2+ indicators. J Neurosci 24: 9572–9579, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinberg 2006.Rinberg D, Koulakov A, Gelperin A. Speed-accuracy tradeoff in olfaction. Neuron 51: 351–358, 2006. [DOI] [PubMed] [Google Scholar]
- Royet 1988.Royet JP, Souchier C, Jourdan F, Ploye H. Morphometric study of the glomerular population in the mouse olfactory bulb: numerical density and size distribution along the rostrocaudal axis. J Comp Neurol 270: 559–568, 1988. [DOI] [PubMed] [Google Scholar]
- Rubin 1999.Rubin BD, Katz LC. Optical imaging of odorant representations in the mammalian olfactory bulb. Neuron 23: 499–511, 1999. [DOI] [PubMed] [Google Scholar]
- Sharp 1975.Sharp FR, Kauer JS, Shepherd GM. Local sites of activity-related glucose metabolism in rat olfactory bulb during olfactory stimulation. Brain Res 98: 596–600, 1975. [DOI] [PubMed] [Google Scholar]
- Shepherd 2004.Shepherd GM, Chen WR, Greer CA. Olfactory Bulb. In: The Synaptic Organization of the Brain, edited by Shepherd GM. New York: Oxford, 2004, p. 165–216.
- Soucy 2001.Soucy ER, Fantana AL, Meister M. Mapping chemical space onto the olfactory bulb. Soc Neurosci Abstr 27: 622–628, 2001. [Google Scholar]
- Soucy 2009.Soucy ER, Albeanu DF, Fantana AL, Murthy VN, Meister M. Precision and diversity in an odor map on the the olfactory bulb. Nat Neurosci 12: 210–220, 2009. [DOI] [PubMed] [Google Scholar]
- Spors 2002.Spors H, Grinvald A. Spatio-temporal dynamics of odor representations in the mammalian olfactory bulb. Neuron 34: 301–315, 2002. [DOI] [PubMed] [Google Scholar]
- Spors 2006.Spors H, Wachowiak M, Cohen LB, Friedrich RW. Temporal dynamics and latency patterns of receptor neuron input to the olfactory bulb. J Neurosci 26: 1247–1259, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tallini 2006.Tallini YN, Ohkura M, Choi BR, Ji G, Imoto K, Doran R, Lee J, Plan P, Wilson J, Xin HB. Imaging cellular signals in the heart in vivo: Cardiac expression of the high-signal Ca2+ indicator GCaMP2. Proc Natl Acad Sci USA 103: 4753–4758, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida 2003.Uchida N, Mainen ZF. Speed and accuracy of olfactory discrimination in the rat. Nat Neurosci 6: 1224–1229, 2003. [DOI] [PubMed] [Google Scholar]
- Uchida 2000.Uchida N, Takahashi YK, Tanifuji M, Mori K. Odor maps in the mammalian olfactory bulb: domain organization and odorant structural features. Nat Neurosci 3: 1035–1043, 2000. [DOI] [PubMed] [Google Scholar]
- Verhagen 2007.Verhagen JV, Wesson DW, Netoff TI, White JA, Wachowiak M. Sniffing controls an adaptive filter of sensory input to the olfactory bulb. Nat Neurosci 10: 631–639, 2007. [DOI] [PubMed] [Google Scholar]
- Vucinic 2006.Vucinic D, Cohen LB, Kosmidis EK. Interglomerular center-surround inhibition shapes odorant-evoked input to the mouse olfactory bulb in vivo. J Neurophysiol 95: 1881–1887, 2006. [DOI] [PubMed] [Google Scholar]
- Wachowiak 2001.Wachowiak M, Cohen LB. Representation of odorants by receptor neuron input to the mouse olfactory bulb. Neuron 32: 723–735, 2001. [DOI] [PubMed] [Google Scholar]
- Wachowiak 2005.Wachowiak M, McGann JP, Heyward PM, Shao Z, Puche AC, Shipley MT. Inhibition of olfactory receptor neuron input to olfactory bulb glomeruli mediated by suppression of presynaptic calcium influx. J Neurophysiol 94: 2700–2712, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiser 1994.Weiser M, Vega-Saenz de Miera E, Kentros C, Moreno H, Franzen L, Hillman D, Baker H, Rudy B. Differential expression of Shaw-related K+ channels in the rat central nervous system. J Neurosci 14: 949–972, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing 2007.Xing J, Xiong W, Fletcher ML, Nagayama S, Zeng S, Masurkar A, Knopfel T, Chen WR. in vivo block of dendrodendritic inhibition unleashes widely spread lateral propagation of odor-evoked calcium signals. Soc Neurosci Abstr 33: 503.13, 2007. [Google Scholar]
- Xu 2003.Xu F, Liu N, Kida I, Rothman DL, Hyder F, Shepherd GM. Odor maps of aldehydes and esters revealed by functional MRI in the glomerular layer of the mouse olfactory bulb. Proc Natl Acad Sci USA 100: 11029–11034, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaksi 2007.Yaksi E, Judkewitz B, Friedrich RW. Topological reorganization of odor representations in the olfactory bulb. PLoS Biol 5: e178, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshihara 2005.Yoshihara S, Omichi K, Yanazawa M, Kitamura K, Yoshihara Y. Arx homeobox gene is essential for development of mouse olfactory system. Development 132: 751–762, 2005. [DOI] [PubMed] [Google Scholar]
- Yuan 2006a.Yuan Q, Knöpfel T. Olfactory nerve stimulation-induced calcium signaling in the mitral cell distal dendritic tuft. J Neurophysiol 95: 2417–2426, 2006a. [DOI] [PubMed] [Google Scholar]
- Yuan 2006b.Yuan Q, Knöpfel T. Olfactory nerve stimulation-evoked mGluR1 slow potentials, oscillations, and calcium signaling in mouse olfactory bulb mitral cells. J Neurophysiol 95: 3097–3104, 2006b. [DOI] [PubMed] [Google Scholar]
- Zhou 2006a.Zhou Z, Xiong W, Masurkar AV, Chen WR, Shepherd GM. Dendritic calcium plateau potentials modulate input-output properties of juxtaglomerular cells in the rat olfactory bulb. J Neurophysiol 96: 2354–2363, 2006a. [DOI] [PubMed] [Google Scholar]
- Zhou 2006b.Zhou Z, Xiong W, Zeng S, Xia A, Shepherd GM, Greer CA, Chen WR. Dendritic excitability and calcium signalling in the mitral cell distal glomerular tuft. Eur J Neurosci 24: 1623–1632, 2006b. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.