Abstract
The dendritic tree of layer 5 (L5) pyramidal neurons spans the neocortical layers, allowing the integration of intra- and extracortical synaptic inputs. Here we investigate the postnatal development of the integrative properties of rat L5 pyramidal neurons using simultaneous whole cell recording from the soma and distal apical dendrite. In young (P9-10) neurons, apical dendritic excitatory synaptic input powerfully drove action potential output by efficiently summating at the axonal site of action potential generation. In contrast, in mature (P25-29) neurons, apical dendritic excitatory input provided little direct depolarization at the site of action potential generation but was integrated locally in the apical dendritic tree leading to the generation of dendritic spikes. Consequently, over the first postnatal month the fraction of action potentials driven by apical dendritic spikes increased dramatically. This developmental remodeling of the integrative operations of L5 pyramidal neurons was controlled by a >10-fold increase in the density of apical dendritic Hyperpolarization-activated cyclic nucleotide (HCN)-gated channels found in cell-attached patches or by immunostaining for the HCN channel isoform HCN1. Thus an age-dependent increase in apical dendritic HCN channel density ensures that L5 pyramidal neurons develop from compact temporal integrators to compartmentalized integrators of basal and apical dendritic synaptic input.
INTRODUCTION
Layer 5 pyramidal neurons are the output neurons of the neocortex and so represent the final site of neocortical processing. The dendritic tree of layer 5 pyramidal neurons spans all cortical layers with a prominent dendritic field in layer 1, referred to as the apical dendritic tuft and a basal dendritic field in layers 5 and 6. In addition, the ascending apical dendritic trunk of layer 5 pyramidal neurons gives rise to a number of oblique dendrites that sample synaptic inputs in layers 2–4 (Wise et al. 1979). This anatomical arrangement suggests that layer 5 pyramidal neurons integrate divergent intra- and extracortical streams of synaptic input to form an action potential output. As action potentials are initiated in the axon of layer 5 pyramidal neurons (Palmer and Stuart 2006), classical models of neuronal function suggest that synaptic input must be funneled to this site to influence neuronal output (Rall 1967). The dendritic tree of layer 5 pyramidal neurons, however, enlarges during postnatal development (Miller 1981; Wise et al. 1979), suggesting that synaptic inputs may undergo increased voltage attenuation as they spread from dendritic site of generation to the axonal site of action potential initiation (Berger et al. 2001; Nevian et al. 2007; Stuart and Spruston 1998; Williams and Stuart 2002). Indeed, in mature layer 5 pyramidal neurons, the amplitude of dendritic synaptic inputs generated at apical and basal dendritic sites are heavily attenuated as they spread through the dendritic tree (Berger et al. 2001; Nevian et al. 2007; Stuart and Spruston 1998; Williams and Stuart 2002). This arises partly because of passive cable filtering and partly because of the interaction of synaptic potentials with voltage-activated channels (Berger et al. 2001; Nevian et al. 2007; Stuart and Spruston 1998; Williams and Stuart 2002).
In mature cortical pyramidal neurons, the spread of synaptic potentials through the apical dendritic tree is powerfully controlled by hyperpolarization-activated cyclic nucleotide (HCN)- gated channels (Berger et al. 2001; Magee 1998, 1999; Stuart and Spruston 1998; Williams and Stuart 2000, 2003). In addition, HCN channels control the resting membrane potential and apparent input resistance of layer 5 pyramidal neurons (Berger et al. 2001; Spain et al. 1991; Williams and Stuart 2000). In adult CA1 and neocortical layer 5 pyramidal neurons, electrophysiological and immunocytochemical approaches have shown a highly compartmentalized subcellular distribution of HCN channels with little or no surface expression at somatic, axonal, or basal dendritic sites but high expression at apical dendritic sites, where HCN channel density progressively increases with distance from the soma (Berger et al. 2001; Brewster et al. 2007; Kole et al. 2006; Lorincz et al. 2002; Magee 1998; Williams and Stuart 2000).
Functionally, HCN channels have been shown to control the time course of synaptic potentials in pyramidal neurons, both at dendritic sites of generation and following their spread to the soma, by acting as a shunt conductance and through voltage-dependent activation and deactivation properties (Angelo et al. 2007; Magee 1999; Nicoll et al. 1993; van Brederode and Spain 1995; Williams and Stuart 2000, 2003). Interestingly, the interaction between synaptic potentials and HCN channels acts to compensate for the distance-dependent effects of cable filtering on the somatic time course of excitatory synaptic potentials in hippocampal CA1 and neocortical layer 5 pyramidal neurons (Magee 1999; Williams and Stuart 2000). In CA1 pyramidal neurons, this interaction has been suggested to simplify integrative operations by normalizing the temporal summation of dendritic excitatory postsynaptic potentials (EPSPs) at the soma (Magee 1999). This finding together with the site independence of the somatic amplitude of apical dendritic EPSPs, mediated by scaling of dendritic excitatory synaptic conductance (Magee and Cook 2000; Nicholson et al. 2006), suggests that in CA1 pyramidal neurons excitatory synaptic inputs generated across the apical dendritic tree will have a similar impact on axonal action potential output (Magee 2000). In contrast, in layer 5 pyramidal neurons, the somatic amplitude of apical and basal dendritic EPSPs are not normalized but are determined by the site of generation in the dendritic tree (Nevian et al. 2007; Williams and Stuart 2002). EPSPs generated at proximal apical dendritic sites therefore have a larger somatic amplitude than those generated distally in the dendritic tree (Williams and Stuart 2002). The dendritic site dependence of EPSP amplitude in layer 5 pyramidal neurons suggests that the functional role of HCN channels may not only be to normalize the somatic time course of dendritically generated synaptic potentials. One alternative function may be electrical compartmentalization.
In mature layer 5 pyramidal neurons, HCN channels ensure that trains of distal apical dendritic EPSPs are heavily attenuated and do not summate as they spread from apical dendritic sites of generation to the soma (Berger et al. 2001; Kole et al. 2007; Williams and Stuart 2000). High-frequency barrages of distal apical dendritic EPSPs therefore provide a weak direct drive for action potential initiation (Williams 2005). To have a salient signaling role in layer 5 neocortical pyramidal neurons, distal apical dendritic excitatory input must be locally integrated in the apical dendritic tree (Larkum and Zhu 2002; Williams 2004; Williams and Stuart 2002; Zhu 2000). Indeed, in mature layer 5 pyramidal neurons, single or barrages of apical dendritic EPSPs evoke dendritic spikes that robustly forward propagate through the dendritic tree to initiate action potential firing (Larkum and Zhu 2002; Williams 2004; Williams and Stuart 2002; Zhu 2000). Mature layer 5 pyramidal neurons are therefore compartmentalized into axo-somatic and distal apical dendritic integration compartments, where each integration compartment has unique properties (Larkum and Zhu 2002; Williams and Stuart 2002). Such compartmentalized synaptic integration has been suggested to allow the independent integration of synaptic input arriving at basal and apical dendritic sites (Williams 2004). Here we investigate if this pattern of synaptic integration is a feature of layer 5 pyramidal neurons and so present throughout the development of the neocortical network or if compartmentalized integration develops in response to the postnatal maturation of the neocortical circuit. Interestingly, a previous study has shown that early postnatal pyramidal neurons are electrotonically compact and become increasingly electrically distributed with postnatal age (Zhu 2000). We have therefore investigated the postnatal development of the somatic impact of single and barrages of simulated excitatory synaptic input delivered to the soma or distal apical dendritic sites of layer 5 pyramidal neurons and their role in the control of action potential firing. We find an age-dependent switch in the integrative operations of pyramidal neurons, from one analogous to a single-compartment temporal integrator early in postnatal development to a highly compartmentalized integrator after the first postnatal month. This transformation of the integrative operations of layer 5 pyramidal neurons was underpinned by a dramatic age-dependent increase in the density of apical dendritic HCN channels.
METHODS
Brain-slice preparation
Coronal neocortical brain-slices from male Wistar rats (Postnatal days 9–42) were prepared following Institutional and UK. Home Office guidelines. Brain slices were cut in ice-cold solution of composition (in mM) 125 NaCl, 25 NaHCO3, 3 KCl, 1.25 NaH2PO4, 1 CaCl2, 6 MgCl2, 3 Na pyruvate, and 25 glucose bubbled with 95% O2-5% CO2 at a thickness of 300 μm for electrophysiological or 60 μm for immunostaining experiments. For electrophysiological experiments, brain-slices were stored in this media for 30 min at 34–35°C and then at room temperature.
Electrophysiological recording
Brain-slices were transferred to a recording chamber perfused with a solution of composition (mM) 125 NaCl, 25 NaHCO3, 3 KCl, 1.25 NaH2PO4, 2 CaCl2, 1 MgCl2, 3 Na pyruvate, and 25 glucose at 35 to 37°C bubbled with 95% O2-5% CO2. The postnatal development of the electrophysiological properties of visually identified layer 5B pyramidal neurons was investigated using simultaneous whole cell current-clamp recordings from the soma and apical dendrite. For each neuron, the apical dendritic recording pipette was positioned as close as possible to the nexus of the apical dendritic trunk [58 ± 5 (SE) μm from the layer 1–layer 2 border; n = 57]. Whole cell recording pipettes were filled with (mM) 135 K-gluconate, 7 NaCl, 10 HEPES, 2 Na2-ATP, 0.3 Na-GTP, 2 MgCl2, and 0.01 Alexa Fluor 568 (Molecular Probes, Eugene, OR; pH 7.3–7.4; KOH). Somatic recording pipettes had a resistance of between 3 and 6 MΩ and pipettes for apical dendritic recordings were between 10 and 12 MΩ. Voltage responses were recorded using identical current-clamp amplifiers (BVC-700A, Dagan, MN). Voltage recordings were corrected for an experimentally determined 11-mV liquid junction potential. In all current-clamp experiments, antagonists of excitatory and inhibitory amino acid receptors were included in the extracellular media [6-cyano-7-nitroquinoxaline-2,3-dione (10 μM); 6-imino-3-(4-methoxyphenyl)-1(6H)-pyridazinebutanoic acid hydrobromide (10 μM); d-(−)-2-amino-5-phosphonopentanoic acid (50 μM)]. In some experiments, 4-ethylphenylamino-1,2-dimethyl-6-methylaminopyrimidinium chloride (ZD 7288; 20 μM) was added to the perfusion media. Tetrodotoxin (TTX; 1 μM) was dissolved in extracellular solution and locally applied to the region of the apical dendrite by pressure application (100–200 mmHg, 2 s) from a pipette with similar characteristics to those used for dendritic recordings, positioned 20–30 μm from the apical dendritic recording site. At the termination of each whole cell recording, the location of recording pipettes and neuronal morphology was examined by fluorescence microscopy and digitally recorded (Retiga EXI, QImaging, Burnaby, BC, Canada).
Single excitatory postsynaptic currents (EPSCs) were generated as an ideal current source (peak amplitude: 50–100 pA) as the sum of two exponential processes with rise and decay time constants of between 0.2 and 3 ms and 2 and 30 ms, respectively. Barrages of simulated EPSCs were generated as trains of randomly occurring unitary inputs (peak amplitude, 50–500 pA) with rise and decay time constants of 0.2 and 2 ms, respectively, generated at mean frequencies of between 50 and 500 Hz (Williams 2005). Current and voltage signals were low-pass filtered (DC to 10 kHz) and acquired at 30–50 kHz. Data were acquired and analyzed using AxographX software (AxographX, Sydney, Australia).
Cell-attached patch-clamp recordings were made from either proximal (112.3 ± 3.6 μm from the soma; n = 84 patches) or distal (45 ± 3 μm from the layer 1–layer 2 border; n = 191 patches) apical dendritic sites using pipettes of similar resistance (10–12 MΩ) filled with (in mM) 120 KCl, 20 tetraethyammonium, 10 HEPES, 5 4-aminopyridine, 3 BaCl, 2 CaCl, 1 MgCl, 1 NiCl, 0.5 CdCl, and 0.001 tetrodotoxin (pH 7.4; KOH). Ensemble HCN channel activity was evoked by the delivery of positive voltage steps from an intra-pipette potential set to −50 mV using an Axopatch 200B amplifier (Molecular Devices, Union City, CA). To map channel density, ensemble HCN currents were generated in response to a 100-mV test step, interleaved with a 20-mV step used for off-line leak subtraction, an approach previously used to map the subcellular distribution of HCN channels (Berger et al. 2001; Kole et al. 2006; Williams and Stuart 2000). Current-voltage relationship were constructed by the delivery of an incremental series of positive voltage steps (40–110 mV), interleaved with 10-mV steps for off-line leak subtraction. After gathering ensemble HCN channel data, a whole cell recording was obtained from the neuron, usually at the same apical dendritic site or occasional at the soma, and the neuron was filled with Alexa Fluor 568 to ensure channel data were obtained from the apical dendrite of a layer 5B pyramidal neuron. In no instance was a recording made from the neurite of another neuronal class. In some experiments, simultaneous whole cell and cell-attached recordings were made from closely spaced (<10 μm) apical dendritic sites. In these experiments, the local apical dendritic membrane potential was controlled by the injection of DC current.
Immunostaining
Brain-slices prepared in an identical manner to those used for electrophysiological experiments were fixed in 4% paraformaldehyde immediately after preparation to investigate the subcellular distribution of HCN channel isoforms in preparations that allowed direct comparison with the results of electrophysiological experiments. Following fixation, brain-slices were blocked and permeabalized (1% donkey serum/0.2% Triton) and incubated in 10% dilution of blocking solution with either Anti-HCN1 N-terminus (host: rabbit; 1:200, Alomone Labs), Anti-HCN1 C-terminus (host: rabbit; 1:200, Abcam, Cambridge, UK), or Anti-HCN2 (host: guinea pig; final concentration 4 μg/ml) (Notomi and Shigemoto 2004) for 36 h at 4°C. Brain-slices were then incubated with Alexa Fluor 488 (1:1,000) for 2 h at room temperature and mounted using Vectorshield mounting medium (Vector, Burlingame, CA). C- and N-terminus HCN1 antibodies gave similar results, and presented data were gathered using the N-terminus HCN1 antibody.
Images of HCN immunostaining of layer 5 pyramidal neurons were obtained using a laser confocal scanning system (Radiance Plus, Bio-Rad) equipped with a ×40 oil-immersion objective lens. Scanning parameters including laser power [2.4% of maximal output (5 mW)], iris area (0.32 mm2), gain (30%), and offset (0) were kept constant, and confocal stacks of 1 μm optical sections were taken for each neuron (Kalman = 8, resolution = 1,024 pixels2). Eleven optical sections containing the selected neuron were summed to form a Z projection. To image the entire apical dendritic tree at ×40 magnification, overlapping areas of the neuron were scanned and landmarks common to each adjacent image used to form a complete montage. Scanned images were visualized using a 12-band color LUT to ensure that the full range of fluorescence intensity was detected. Z projections were transformed into grey scale images and regions of interest (ROIs) centered on the soma or apical dendrite (46 μm along the axis of the dendrite, 3,512 ± 145 pixels) were selected from the neuron and neighboring background areas (Fig. 6A). Image analysis was performed using ImageJ (National Institutes of Health) and numerical data analyzed using AxographX. A measure of HCN immunostaining was calculated by subtracting the median pixel intensity in background regions from the median pixel intensity in dendritic regions of interest (Fig. 6, A and B). ROIs throughout the apical dendritic tree were selected to detect any regional changes in HCN immunostaining. To reduce variability caused by changes in scanning conditions, such as changes in slice thickness, neuronal and corresponding background ROIs were in close proximity to each other, approximately the same rectangular shape and in the same orientation (Fig. 6, A and B). To build montages, overlapping areas of each high-resolution scan were imaged twice to allow correct alignment. To check whether fluorescence in these areas had been reduced, selected ROIs were exposed to a series of four scan sequences (Kalman of 8) and the average pixel intensity after each sequence calculated. No significant reduction in average pixel intensity was found.
Statistical analysis
Pooled data are presented as means ± SE. Statistical analysis included ANOVA. Student's t-test and Wilcoxon matched pairs test. All curve fitting was performed using AxographX. The evolution of the activation of ensemble HCN channel activity was fit with a single-exponential function. The voltage-dependent activation of ensemble HCN channel activity was fit with a single Boltzmann function of the form: y = 1/[I + e(V1/2–V/k)], where V1/2 is the voltage of half-maximal activation and k is a constant. The average amplitude of barrages of simulated EPSCs and evoked voltage responses was calculated by measuring the mean amplitude throughout the time course of the injected simulated synaptic current.
RESULTS
Developmental control of the somatic efficacy of dendritic synaptic input
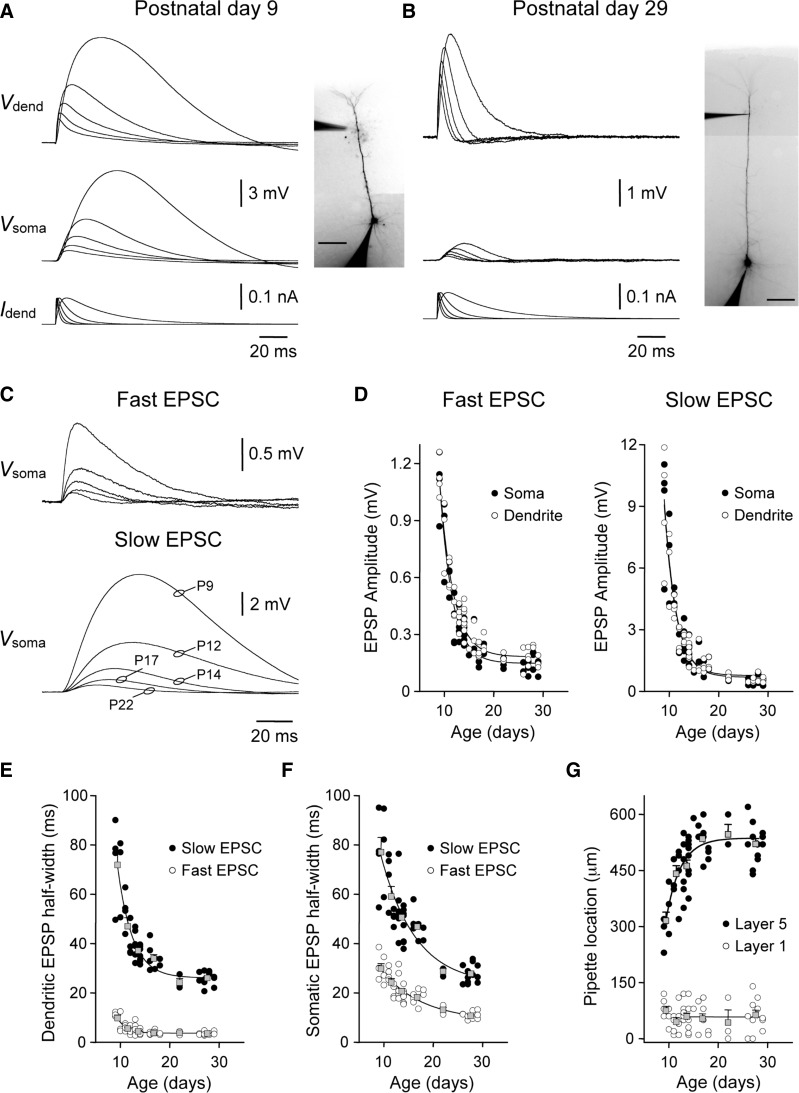
The majority of synaptic inputs to layer 5 pyramidal neurons are formed at dendritic sites. To directly influence neuronal output, synaptic potentials must spread from their dendritic site of generation to the site of action potential initiation in the axon (Rall 1967). As the spread of synaptic potentials is controlled by the passive and active properties of the dendritic tree, we first investigated how the spread of synaptic potentials through layer 5 pyramidal neurons changes during postnatal development. Simulated EPSPs (sEPSPs) were generated at somatic or distal apical dendritic sites (58 ± 5 μm from the layer 1–layer 2 border; n = 57) by the injection of a series of EPSC-shaped current signals and recorded simultaneously at their site of generation and following spread through the dendritic tree (τrise = 0.2–3 ms; τdecay = 2–30 ms, peak current = 100 pA; Fig. 1, A and B). Targeted dendritic recording allowed us to generate and record sEPSPs at equally remote apical dendritic sites across postnatal development (Fig. 1G, ○, average distance from the layer 1–layer 2 border was not different between age groups; ANOVA; P = 0.71). In early postnatal layer 5 pyramidal neurons, sEPSPs were of large amplitude with slow kinetics both at their dendritic site of generation and following spread to the soma (P9; Fig. 1A). In older animals, however, dendritic sEPSPs generated in response to identical EPSCs were of smaller amplitude with faster kinetics and provided modest excitation of the soma (P29; Fig. 1B). Indeed, pooled data showed a steep age-dependent decrease in the somatic amplitude of dendritically generated sEPSPs (Fig. 1, C and D), a relationship that was apparent for sEPSPs generated across a wide range of EPSC kinetics (Fig. 1, A–D). Furthermore, the spread of sEPSPs from the soma back into the apical dendritic tree was developmentally regulated with a similar age dependence (Fig. 1D). The developmental decrease in the somatic amplitude of dendritic sEPSPs was accompanied by an age-dependent speeding of sEPSPs kinetics, both at dendritic site of generation and following the spread of sEPSPs to the soma (Fig. 1, E and F). Taken together, these data indicate that single excitatory synaptic inputs generated at remote apical dendritic sites powerfully excite the soma of early postnatal layer 5 pyramidal neurons but have a progressively weaker somatic impact as animals mature.
FIG. 1.
Developmental decrease in the somatic impact of apical dendritic excitatory postsynaptic potentials (EPSPs). A and B: simultaneous somatic (Vsoma) and dendritic (Vdend) recording of simulated EPSPs in a postnatal day 9 (P9, A) and P29 (B) layer 5 pyramidal neurons. Simulated EPSPs were generated by the injection of a series of simulated excitatory postsynaptic currents (EPSCs) at the distal apical dendritic recording site (Idend; bottom traces). The amplitude and kinetics of simulated EPSPs are transformed with development. Neuronal morphology and the placement of recording electrodes are shown in the inset photomicrographs (scale bars = 100 μm). C: age-dependent reduction in the somatic amplitude of distal dendritically generated EPSPs. Overlain traces show simulated EPSPs recorded at the indicated postnatal ages. Fast and slow EPSCs had τrise of 0.2 and 3 ms and τdecay of 2 and 30 ms, respectively. D: pooled data show the age-dependent decrease in the amplitude of EPSPs, generated with fast and slow EPSC kinetics, when generated at the soma and recorded at an apical dendritic site (○) or vice versa (•). Data have been fit with single exponential functions (—). E and F: developmental speeding of the kinetics of simulated EPSPs generated by fast or slow EPSCs. Simulated EPSPs were recorded at their site of generation (E) and following spread to the soma (F). Overlain gray symbols show results pooled into 6 age groups (means ± SE). Grouped data have been fit with single-exponential functions (—). G: position of recording electrodes. •, the distance of the apical dendritic recording electrode from the soma of layer 5 pyramidal neurons; ○, the distance of the dendritic recording electrode from the layer 1–layer 2 border. Grouped data have been fit with a single-exponential function (layer 5) or linear regression (layer 1).
Modeling and experimental studies have indicated that in a passive neuron, the somatic time course of EPSPs is determined by their site of generation within the dendritic tree, increasing as synapses are activated more remotely (Rall 1967; Williams and Stuart 2002). One consequence of this behavior is that trains of EPSPs generated at relatively distal dendritic sites are predicted to show greater temporal summation at the soma (Magee 1999; Rall 1967; Williams and Stuart 2000). In mature cortical pyramidal neurons, however, the somatic time course of EPSPs generated at the soma and in the apical dendritic tree are similar, a process described as time-course normalization (Magee 1999; Williams and Stuart 2000). This behavior cannot arise in a passive system but is shaped by the interaction of EPSPs with dendritic voltage-activated conductances, in particular HCN channels (Magee 1999; Williams and Stuart 2000). We therefore investigated if this relationship held throughout postnatal development, by comparing the somatic time course of sEPSPs generated at the soma with those generated distally in the apical dendritic tree (Fig. 2). In early postnatal pyramidal neurons, the somatic time course of dendritically generated sEPSPs was not normalized, but rather dendritic synaptic inputs produced somatic sEPSPs that were slower in time course than those generated at the soma (Fig. 2A). Importantly, the disparity between the somatic time course of somatically and dendritically generated sEPSPs was dependent on EPSC kinetics and was greatest for fast EPSCs (τdecay = 2 ms; somatic half-width: soma EPSC: 18.3 ± 1.2 ms; dendritic EPSC: 26.2 ± 1.6 ms; paired t-test, P < 0.01; n = 15) but near unity for slow EPSCs (τdecay = 30 ms; somatic half-width: soma EPSC: 69.2 ± 4.2 ms; dendritic EPSC: 67.5 ± 4.2 ms; paired t-test, P = 0.24; n = 15; Fig. 2B). This relationship was refined during postnatal development so that in mature pyramidal neurons, the somatic time course of fast but not slow dendritic EPSPs were similar (P 26–29; fast EPSC: somatic half-width: soma EPSC: 10.9 ± 0.4 ms; dendritic EPSC: 10.8 ± 0.4 ms; paired t-test, P = 0.81; slow EPSC: somatic half-width: soma EPSC: 39.2 ± 0.6 ms; dendritic EPSC: 27.8 ± 1.0 ms; paired t-test, P < 0.001; n = 12; Fig. 2B). Indeed, across the first postnatal month, the kinetics of EPSCs that produced sEPSPs that showed time-course normalization accelerated from 30 to 2 ms (Fig. 2B). The somatic time course of fast sEPSPs that accurately replicate the time course of spontaneously occurring EPSPs in layer 5 neurons (Hausser and Roth 1997; Williams and Stuart 2002) is therefore only normalized in layer 5 pyramidal neurons older than ∼1 month of age. As a consequence, in these mature neurons, the somatic time course of sEPSPs generated with slow EPSC kinetics were heavily constrained and significantly briefer than those generated at the soma (groups significantly different for dendritic EPSCs with τdecay of 5, 10, and 30 ms; paired t-test, P < 0.01; n = 12; Fig. 2B). These data suggest that the properties and subcellular distribution of the voltage-activated conductances that control the time course of dendritic EPSPs are regulated during postnatal development. Furthermore at a functional level, these data suggest that trains of dendritic EPSPs in early postnatal layer 5 pyramidal neurons are more likely to show temporal summation at the soma than those in mature neurons.
FIG. 2.
Postnatal development of EPSP time course normalization. A: overlain somatic recording of simulated EPSPs (sEPSPs) evoked by the injection of fast EPSCs (τrise = 0.2 ms, τdecay = 2 ms) at somatic (black traces) or distal apical dendritic (gray traces) sites in a P10 and a P29 layer 5 pyramidal neuron. In each case, the dendritic EPSC generates smaller-amplitude EPSPs at the soma. The marked gray traces represent the digital scaling of the dendritically generated sEPSP. Note in the P10 neuron that the time course (time at half-amplitude) of the scaled dendritic sEPSPs is longer than the somatically generated sEPSP. B: developmental refinement of time-course normalization. Relationship between the somatic half-width (time at half-amplitude) of sEPSPs generated at dendritic and somatic sites with EPSCs of different kinetics (deacy time constant) in the indicated age groups. Note that simulated EPSPs with similar somatic time course are generated by EPSCs with faster kinetics in older neurons. Values represent means ± SE; the horizontal reference line indicates a ratio of 1.
To directly test this idea, we generated random barrages of simulated EPSCs across a wide range of frequencies (frequency = 50–500 Hz, duration = 2 s; unitary amplitude = 50 or 100 pA; τrise = 0.2 ms; τdecay 2 ms) and injected this barrage of excitatory input either at somatic or apical dendritic recording sites. This procedure allowed us to directly record how effectively barrages of dendritic excitatory synaptic input summate at the soma. In early postnatal pyramidal neurons, barrages of dendritic sEPSPs effectively depolarized the soma, with higher frequency barrages producing progressively greater somatic depolarization (Fig. 3 A). When we compared the average somatic depolarization produced by identical barrages of synaptic input delivered to the soma or remotely in the dendritic tree, we found that in early postnatal neurons dendritic input produced voltage responses that were comparable in magnitude to those evoked at the soma (P 9–10: dendritic response 20.3 ± 4.6% less than somatic; n = 6; Fig. 3A). Across the first postnatal month, however, the ability of dendritic excitatory input to depolarize the soma decreased dramatically (Fig. 3B) so that at P26-29, dendritic excitatory input only weakly excited the soma (Fig. 3B). Indeed, in older animals, dendritic excitatory input did not summate at the soma and generated only a shallow relationship between somatic depolarization and injected dendritic synaptic current (2.9 ± 0.7 mV/nA; n = 8; Fig. 3B). This is in stark contrast to the steep relationship generated when synaptic input was delivered to the soma in older animals (28.9 ± 3.2 mV/nA; n = 8; Fig. 3B). During the first postnatal month, therefore barrages of apical dendritic excitatory synaptic input become progressively weaker sources of somatic excitation. To quantify this, we compared the slope of the relationship between somatic depolarization and injected somatic or apical dendritic synaptic current for neurons recorded in six age groups (Fig. 4 A). The subthreshold efficacy of dendritic excitatory input decreased with postnatal age according to an exponential relationship with an age constant of 5 days (Fig. 4A). This refinement of the electrophysiological properties of layer 5 pyramidal neurons was accompanied by an increase in the degree of attenuation of voltage signals as they spread from the soma to the apical dendrite. A dramatic age-dependent increase in somato-dendritic voltage attenuation was apparent for both single EPSPs (Fig. 1D) and for steady-state voltage responses (Fig. 4, B and C). Indeed when steps of negative current were injected at the soma to evoke hyperpolarizing responses of 19.9 ± 0.1 mV (n = 40), the degree of somato-dendritic voltage attenuation was found to increase during postnatal development (somatic voltage responses were of similar amplitude in each age group; ANOVA; P > 0.05; Fig. 4, B and C). Interestingly this effect was accompanied by an age-dependent decrease in the somatic apparent input resistance of layer 5 pyramidal neurons (Fig. 4C). Taken together, these data show that the electrotonic architecture of layer 5 pyramidal neurons is significantly remodeled over the first postnatal month.
FIG. 3.
Developmental reduction of EPSP temporal summation. A and B: somatic voltage responses evoked by the injection of identical patterns of simulated EPSCs (bottom traces) at somatic and apical dendritic sites. Note in the P10 layer 5 pyramidal neuron (A) that the somatic voltage response evoked by EPSCs injected at somatic and dendritic sites are similar in amplitude. In contrast, in the P27 neuron (B) dendritic EPSCs only weakly depolarized the soma. Barrages of simulated EPSCs of different frequency and the corresponding voltage responses are delineated by gray scale for clarity. Inset: photomicrographs show the morphology of the illustrated neurons (scale bars = 100 μm). Summary graphs show the relationship between the magnitude of injected simulated synaptic current and evoked somatic voltage responses, when identical barrages of simulated EPSCs were generated at somatic (○) or apical dendritic sites (•). Data has been pooled (means ± SE) for neurons from the indicated age groups. Values of injected current and evoked voltage responses represent the average amplitude throughout the barrage. Note the similar slope of these relationships in P9-10 neurons (n = 6), but the weak somatic impact of dendritic input in P26-29 neurons (n = 8). Relationships were fit for subthreshold voltage responses by linear regression (lines).
FIG. 4.
Developmental refinement of the electrotonic properties of layer 5 pyramidal neurons. A: subthreshold dendritic efficacy decreases with postnatal age. Subthreshold dendritic efficacy was calculated as the fraction of somatic depolarization produced by identical barrages of synaptic inputs generated at somatic and distal apical dendritic sites across a broad range of injected synaptic current (Fig. 3). Data have been pooled for the indicated age groups (means ± SE). —, a fit to grouped data by a single-exponential function. B: simultaneous somato-dendritic recording of voltage responses evoked by the injection of a current step at the soma in P10 and P27 layer 5 pyramidal neurons. Note the minimal somato-dendritic voltage attenuation at the negative peak (○) and at steady state (•) in the P10 neuron. In contrast in the P27 neuron, dramatic somato-dendritic voltage attenuation is evident. In each neuron, a similar amplitude somatic voltage response was generated in response to a step of negative current, the smaller current step was injected in the P10 neuron (bottom traces). C: summary data describing the age-dependent increase in somato-dendritic voltage attenuation when measured at peak and steady state. The grey symbols show the age-dependent increase in apparent input conductance. Data are pooled for 6 age groups (means ± SE). Lines represent fits with single-exponential functions.
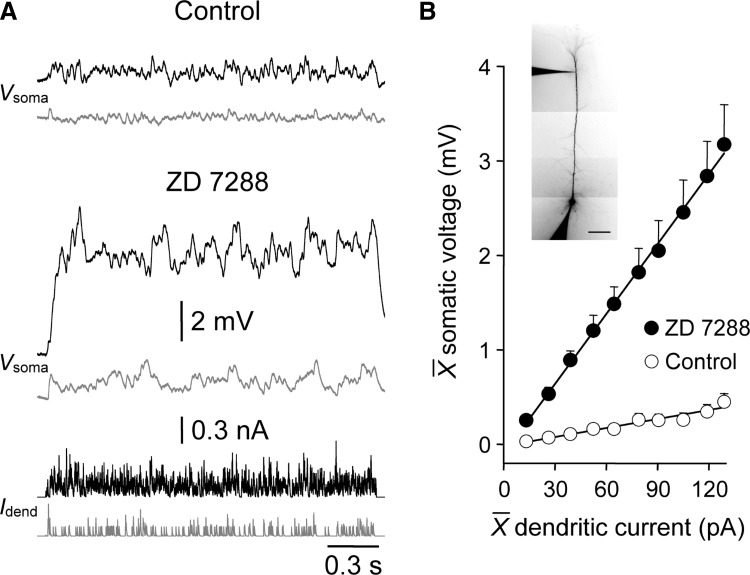
The electrotonic architecture of mature cortical pyramidal neurons is critically controlled by the subcellular distribution and density of voltage-activated channels (Migliore and Shepherd 2005). Previous observations have indicated in mature layer 5 pyramidal neurons that HCN channels play a major role in controlling the attenuation of synaptic potentials as they spread through the apical dendritic tree (Berger et al. 2001; Stuart and Spruston 1998; Williams and Stuart 2000, 2003). To highlight the constraint of the somatic impact of dendritic excitatory inputs by HCN channels in mature pyramidal neurons, we explored the influence of blocking HCN channels on the somatic efficacy of dendritic excitatory synaptic input (Fig. 5 A). The somatic depolarization produced by barrages of distal apical dendritic sEPSPs was transformed by the pharmacological block of HCN channels with ZD 7288 (P26-29; dendritic recordings 84 ± 17 μm from the layer 1–layer 2 border; n = 6; Fig. 5A). Indeed when HCN channels were blocked, the slope of the relationship between somatic depolarization and dendritic current injection was similar to that obtained under control when sEPSPs were injected at the level of the soma (dendritic: control: 3.2 ± 0.7 mV/nA; ZD 7288: 24.9 ± 3.4 mV/nA; n = 6; soma: control: 28.9 ± 3.2 mV/nA; n = 8; compare Fig. 5B with Fig. 3B). These values were calculated from similar somatic and dendritic membrane potentials, following the delivery of positive DC current through recordings electrodes to offset the membrane potential hyperpolarization produced by the blockade of HCN channels (Williams and Stuart 2000, 2003). HCN channels therefore powerfully control the subthreshold somatic efficacy of dendritic excitation in mature layer 5 pyramidal neurons. This finding prompted us to investigate how the density and sub-cellular distribution of this important class of voltage-activated ion channel was refined during postnatal development.
FIG. 5.
Blockade of HCN channels increases the somatic impact of dendritic excitatory input. A: somatic voltage traces (Vsoma) evoked by barrages of simulated EPSCs injected at a distal apical dendritic site (Idend) under control and in the presence of ZD 7288 (20 μM). Traces are gray-scaled for clarity. The morphology of the neuron is shown in the inset (scale bar = 100 μm). B: summary data (P26-29; n = 6; means ± SE) describing the somatic depolarization generated by dendritic simulated synaptic current under control (○) and in the presence of ZD 7288 (•). Values of injected current and evoked voltage responses represent the average amplitude throughout the barrage. Data were fit by linear regression (—).
Development of HCN channel expression and targeting
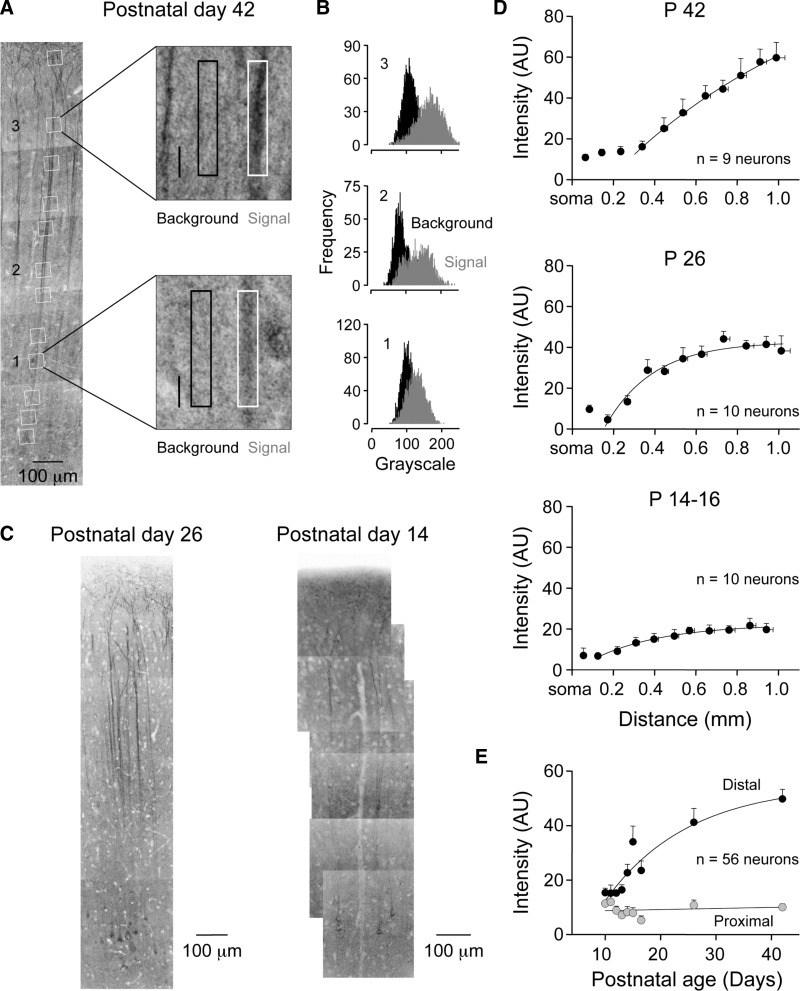
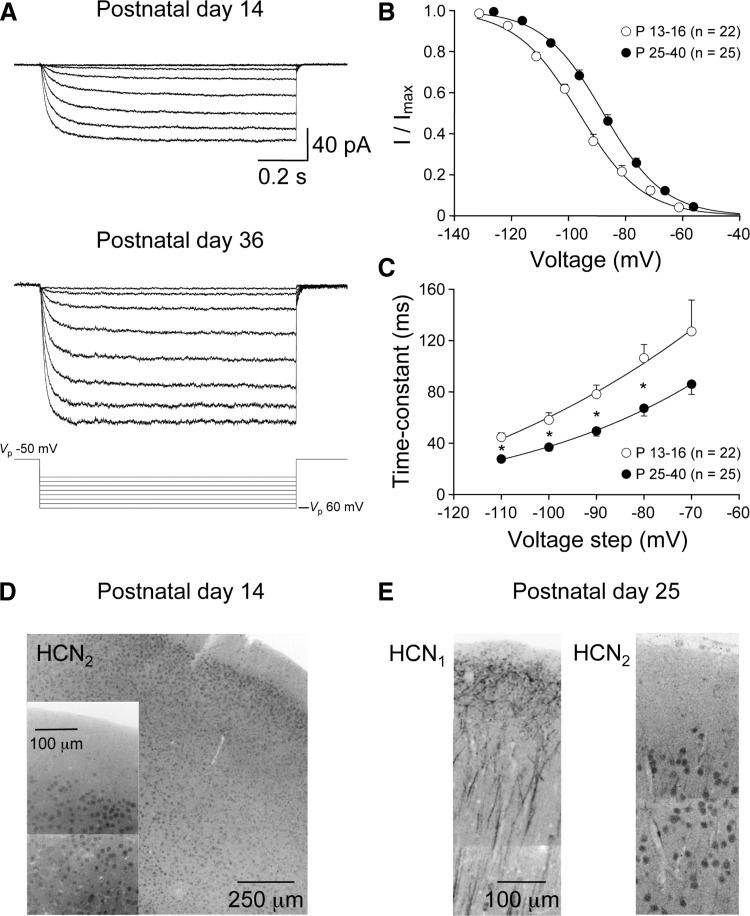
We examined the postnatal refinement of HCN channel expression in layer 5 pyramidal neurons using a combination of immunostaining of HCN channel isoforms and cell-attached patch-clamp recording of functional ensemble HCN channel activity. In confirmation of a previous study (Lorincz et al. 2002), we observed in adult animals (P42) a striking apical dendritic distribution of HCN channels in confocal montages of layer 5 neurons resolved by anti-HCN1/Alexa 488 immunostaining. We calculated the optical density of HCN1 immunostaining in regions of interest placed at apical dendritic sites progressively distal from the soma of individual layer 5 pyramidal neurons (Fig. 6A).
FIG. 6.
Developmental increase in the density of apical dendritic HCN1 channels in layer 5 pyramidal neurons. A: the density of HCN1 immunostaining (dark reaction) increases at distal apical dendritic sites in P42 animals. The montage is formed from a series of high-resolution confocal stacks. Regions of interest (ROIs) are highlighted by white squares. Two ROIs from proximal and distal apical dendritic sites are magnified and pixel intensities calculated from dendritic (white border) and background areas (black border). B: intensity histograms calculated from background and dendritic (gray) ROIs. In this P42 neuron, there is clear separation between intensity measured from background and distal apical dendritic sites (2 and 3). In contrast, at a proximal apical dendritic site (1), a large overlap of fluorescent intensity between background and dendrite is apparent. C: confocal montage illustrating HCN1 immunostaining of P26 and P14 layer 5 pyramidal neurons. D: summary graphs describe the increase in apical dendritic HCN1 optical density with distance from the soma. Values were calculated by subtracting pixel intensity of background area from dendritic regions of interests. Data in the 3 indicated age groups have been pooled from the indicated number of neurons (means ± SE). Lines represent fits to the data with single bounded exponentials. E: summary data describing the age-dependent increase in the density of HCN1 immunostaining at distal apical dendritic sites (black symbols). In contrast, at proximal apical dendritic sites HCN1 immunostaining does not increase with age (gray symbols). Lines represent fits to the data with single bounded exponential function (distal) or linear regression (proximal).
The subtraction of autofluorescence from adjacent background areas revealed an increase in HCN1 channel density at progressively distal apical dendritic sites (Fig. 6, A and B). Next we charted the postnatal development of apical dendritic HCN1 channel expression. At early postnatal ages (P10), we observed diffuse and low levels of HCN1 expression with no significant apical dendritic targeting (n = 5 neurons; Supplementary Fig. S11 ). A prominent apical dendritic gradient of HCN1 channel expression was first apparent around P14-16 (Fig. 6, C and D). At this age background-subtracted average pixel intensity from dendritic regions of interest showed a distance-dependent increase in the level of HCN1 immunostaining at progressively distal apical dendritic sites (P14-16: proximal ROI: 125.1 ± 6.8 μm from the soma; 6.8 ± 1.1 AU; distal ROI: 942.0 ± 19.7 μm from the soma; 19.7 ± 3.0 AU; ANOVA P < 0.001; n = 10). As animals matured, the apical dendritic polarization of HCN1 immunostaining was refined, a process characterized by an age-dependent increase in the density of channels at distal but not proximal apical dendritic sites (P42: proximal: 143.91 ± 7.6 μm from the soma; 13.2 ± 1.7 AU; distal: 989.0 ± 42.0 μm from the soma; 59.7 ± 7.6 AU; n = 9 neurons; Fig. 6, C and D). When we plotted the intensity of HCN1 immunostaining at apical dendritic sites as a function of postnatal age, we observed a steep age-dependent increase in density at distal but not proximal sites (Fig. 6E). At distal apical dendritic sites, this age-dependent relationship was well described by a bounded exponential function with an age constant of 15 days (Fig. 6E).
As the HCN1 antibodies we used target intracellular sites of the channel, and so required tissue permeabilization, we explored if HCN1 immunostaining was located at or close to the plasma membrane or at intracellular sites. In single confocal sections (1 μm), the optical density along line profiles drawn through the soma and apical dendrites of HCN1 immunostained layer 5 pyramidal neurons was plotted (Supplementary Fig. S2). For comparison, we filled layer 5 pyramidal neurons with the fluorescent dye Alexa 568, following somatic whole cell recording, a dye that does not partition into the neuronal membrane (Supplementary Fig. S2). At distal apical dendritic sites of P 42 layer 5 pyramidal neurons, HCN1 immunostaining was restricted to sites close to the plasma membrane, as shown by the clear peaks in the line profiles (>500 μm from the soma, n = 6; Supplementary Fig. S2A, left). This line profile was in contrast to the unimodal intracellular peak in optical density observed at distal apical dendritic sites in Alexa 568 filled neurons (Supplementary Fig. S2A, right). In somatic regions, however, line profiles revealed that HCN1 immunostaining was both intracellular and membrane-bound, judged by the similar, but broader, line profiles obtained for Alexa-568-filled and HCN1-stained neurons (n = 5; Supplementary Fig. S2B). This intracellular accumulation of HCN1 immunostaining rapidly dissipated at proximal apical dendritic sites (Fig. 6, A and C). These data suggest that HCN1 immunostaining at apical dendritic sites reports the surface density of HCN channels.
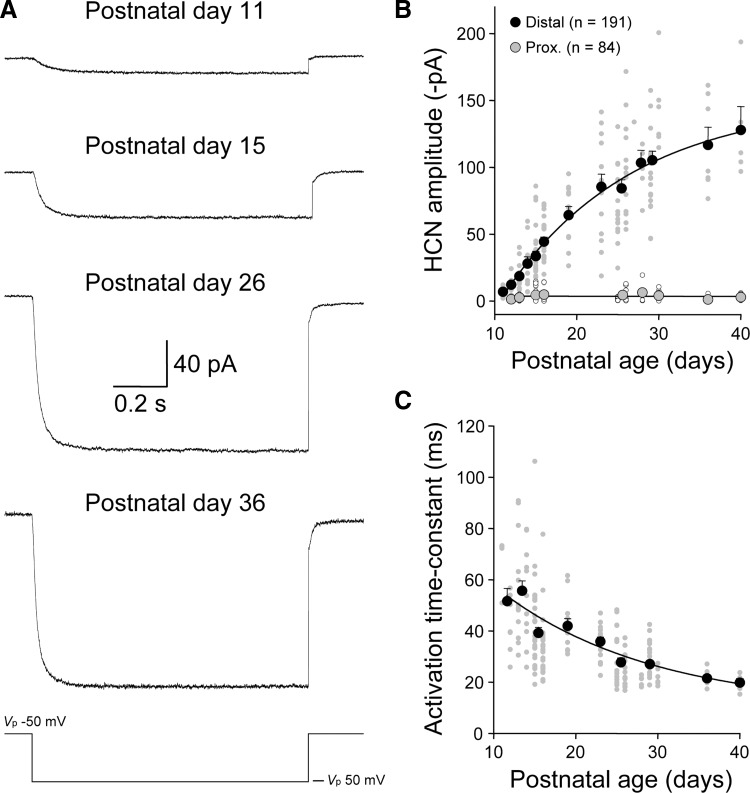
Electrophysiological techniques were used to independently map the postnatal development of the subcellular distribution of functional HCN channels. In P11-12 animals, the amplitude of ensemble HCN channel activity in cell-attached patches from distal apical dendritic sites was small (current amplitude at steady state = −10.3 ± 1.9 pA; distance from layer 1–layer 2 border: 79 ± 10 μm; n = 11; Fig. 7, A and B). The steady-state amplitude of ensemble HCN channel activity in patches made at distal apical dendritic sites, however, increased with postnatal age (P36-40: −121 ± 10 pA; distance from layer 1–layer 2 border: 49 ± 8 μm; n = 12; Fig. 7, A and B). Indeed the age-dependent increase in the density of ensemble HCN channel activity evoked in response to a standard 100-mV test step could be well fit by a single bounded exponential function with an age-constant of 15.7 days (P11 to P40; 45 ± 3 μm from the layer 1–layer 2 border; n = 191; Fig. 7B). In contrast, we found that the amplitude of ensemble HCN channel activity in patches made from proximal apical dendritic recordings sites remained at low levels throughout this period of postnatal development (P12–P40; 112 ± 4 μm from layer 5; n = 84; linear regression: −0.006 pA/day; Fig. 7B).
FIG. 7.
Developmental increase in the density of functional HCN channels at distal apical dendritic sites. A: cell-attached recording of leak-subtracted ensemble HCN channel activity evoked by a 100-mV voltage step (bottom trace). Note the increase in current amplitude and acceleration of activation kinetics with development [postnatal age is indicated, apical dendritic recordings were made at 55 (P11), 70 (P15); 15 (P26) and 30 μm (P36) from the layer 1–layer 2 border]. Leak subtraction was performed off-line by the subtraction of scaled responses generated by interleaved 20-mV voltage steps. B: summary data describing the age-dependent increase in HCN channel density with age at distal but not proximal apical dendritic sites. Each data point is represented by the smaller symbols (distal dendritic, gray; proximal dendritic, open). The number of recorded patches is indicated. Pooled data are represented by the large symbols (distal, black; proximal, gray; means ± SE). Pooled data have been fit by a bounded exponential function for distal and linear regression for proximal apical dendritic recordings. C: pooled data describing the acceleration of ensemble HCN channel kinetics with postnatal development. Each gray data point represents the kinetics of ensemble activity generated in an apical dendritic patch, approximated by a single-exponential fit to the evolution of the current, in response to a 100-mV voltage step. Large black symbols show results pooled into age groups (means ± SE). Pooled data have been fit with an exponential function (line).
Although we found a close parallel between the age-dependent increase in the density of functional HCN channels and immunostaining for the HCN1 channel isoform at distal apical dendritic sites, the activation properties of functional HCN channels were found to change during postnatal development (Fig. 7, A and C). In response to a 100-mV voltage step, the activation kinetics of ensemble HCN channel activity accelerated with postnatal development (Fig. 7A). Indeed when we plotted the relationship between postnatal age and the activation kinetics of ensemble channel activity, we observed an age-dependent speeding of current responses that could be approximated by an exponential function with an age-constant of 18.6 days (Fig. 7C).
In expression systems, the activation kinetics of ensemble homomeric HCN channel activity is determined by channel isoform, with HCN1 channels exhibiting fast and HCN2 channels relatively slow activation kinetics (Ludwig et al. 1998; Santoro and Tibbs 1999; Santoro et al. 1998). In the neocortex, high HCN1 and HCN2 mRNA and protein levels have been detected (Kole et al. 2007; Notomi and Shigemoto 2004; Santoro et al. 2000). The developmental speeding of the activation kinetics of ensemble HCN channel activity in layer 5 pyramidal neurons may therefore reflect a developmental change in the subunit composition of HCN channels. To explore this in more detail, we investigated the voltage-dependent properties of ensemble HCN channel activity in two developmental epochs: young: P13-16 (n = 22) and mature: P25-40 (n = 25). As cell-attached recordings do not control the neurons membrane potential, we first measured the distal apical dendritic resting membrane potential in young and mature neurons, a value that will be influenced by the age-dependent increase in HCN channel density. Dendritic whole cell recording revealed that the local distal apical dendritic membrane potential of young layer 5 pyramidal neurons was significantly hyperpolarized compared with mature neurons (P13–P16: −71.3 ± 0.8 mV; n = 23; P25–P30: −66.2 ± 0.8 mV; n = 17; t-test; P < 0.001). Tail current analysis revealed a negative shift in the voltage at half-maximal activation of ensemble HCN channel activity between young and mature groups when activation curves were fit with a single Boltzmann function (young: V1/2 = −96.3 mV; slope = 11.2; old: V1/2 = −87.8 mV; slope = 10.6; Fig. 8, A and B). Furthermore, when we fit the activation kinetics of ensemble channel activity generated across the current-voltage relationship, we found a significant speeding of activation across a wide voltage range (Fig. 8C). The observed difference in apical dendritic resting membrane potential is unlikely to explain the developmental speeding of ensemble HCN channel activation kinetics. The delivery of positive voltage steps in a cell-attached recording from a relatively hyperpolarized membrane potential would be expected to lead to the activation of faster ensemble current responses, as the activation kinetics of ensemble HCN channels accelerate with membrane hyperpolarization (Fig. 8, A and C). To confirm this intuition, we made simultaneous whole cell current-clamp and cell-attached voltage-clamp recordings from closely spaced (<10 μm) sites in the distal apical dendrite of layer 5 pyramidal neurons (P25-29; 39 ± 12 μm from the layer 1–layer 2 border; n = 11). The activation time constant of ensemble HCN channel activity generated in response to a 100-mV test step was accelerated by 23.3 ± 8.3% following a 9.5 ± 0.7 mV tonic hyperpolarization of the local dendritic membrane (membrane potential hyperpolarized from −64.2 ± 0.5 to −74.0 ± 0.8 mV by the injection of −0.65 ± 0.05 nA DC current, data not shown). Similarly, membrane hyperpolarization led to an increase in the steady-state amplitude of current responses (23.9 ± 3.1%; data not shown). These data suggest that differences in the resting membrane potential of layer 5 pyramidal neurons cannot explain the age-dependent changes in the activation properties of ensemble HCN channel activity.
FIG. 8.
Developmental refinement of the activation properties of distal apical dendritic HCN channels. A: families of leak-subtracted cell-attached current responses evoked by an incremental series of voltage steps (bottom traces) recorded from an apical dendritic site of a P14 (70 μm from the layer 1–layer 2 border) and P36 layer 5 pyramidal neuron (30 μm from the layer 1–layer 2 border). Leak subtraction was performed off-line by the subtraction of scaled responses generated by interleaved 10-mV voltage steps. B: voltage-dependent activation curves of HCN channel activity calculated from tail currents in cell-attached patches made from young (P13-16, ○; means ± SE) and mature (P25–40, •) layer 5 pyramidal neurons. Voltage refers to calculated membrane potential (see results for details). Relationship have been fit with single Boltzmann functions (—). Note the relatively hyperpolarized voltage at half-maximal activation in the young group. C: activation kinetics of ensemble HCN channel activity are different between young and mature layer 5 pyramidal neurons across a wide voltage range. Note that in each group activation kinetics speed as the size of the pipette negative voltage step increases. Data are fit by single-exponential functions (—). The statistical significance between groups is indicated by stars (P values <0.01, 2-tailed t-test). D: HCN2 immunostaining (dark reaction) of the neocortex from a P14 animal; inset: the cell bodies of layer 2/3 neurons at a higher magnification. E: HCN1 and HCN2 immunostaining (dark reaction) in consecutive neocortical slices from a P25 animal. Note the prominent HCN1 immunostaining of the apical dendrites of layer 5 pyramidal neurons, and the somatic HCN2 immunostaining of layer 2/3 neurons. Montages were formed from a series of high-resolution confocal stacks.
We therefore explored if this behavior arose because of a change in the subunit composition of HCN channels. Immunostaining for HCN2 channels in both young (P13-14) and mature (P25) slices revealed somatic labeling throughout neocortical layers (Fig. 8, D and E). However, we did not observe dendritic immunostaining in either young or mature groups, despite the clear immunostaining of the apical dendritic tree of layer 5 pyramidal neurons by HCN1 antibodies in adjacent sections (Fig. 8E). Immunostaining results therefore do not support a prominent alteration in the subunit composition of HCN channels with postnatal development in the neocortex.
Dendritic synaptic integration
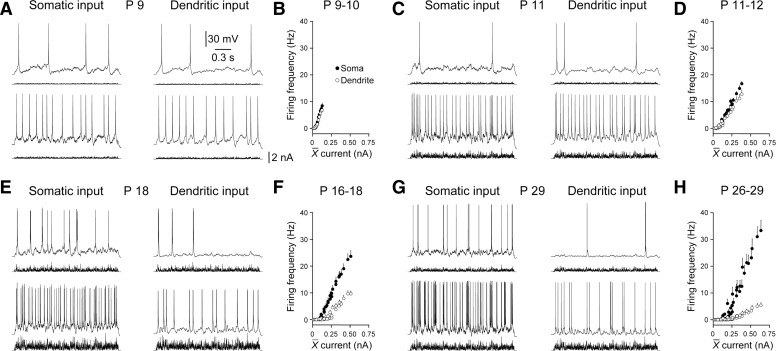
The age-dependent increase in the density and apical dendritic polarization of HCN channels provides a basis for the refinement of the electrotonic structure of layer 5 pyramidal neurons. Classical models of synaptic integration indicate that the direct impact of dendritic synaptic inputs at the site of action potential initiation solely determines their influence on neuronal output (Rall 1967). However, recent evidence suggests that in mature neocortical pyramidal neurons, distal apical dendritic excitatory inputs influence action potential output not only by providing direct depolarization at the site of action potential initiation but also by local integration in the dendritic tree that leads to the initiation of regenerative dendritic electrical activity termed apical dendritic spikes (Kim and Connors 1993; Larkum and Zhu 2002; Williams and Stuart 2002). In mature layer 5 pyramidal neurons, apical dendritic spikes have been shown to forward propagate through the dendritic tree to trigger axonal action potential firing (Larkum and Zhu 2002; Williams 2004; Williams and Stuart 2002; Zhu 2000). However, previous observations have indicated that apical dendritic spikes are absent or only manifest weakly in early postnatal neurons (Zhu 2000). Moreover, once generated in immature neurons, dendritic spikes have been shown to be unreliable triggers for the initiation of action potential output and are often confined to the distal apical dendritic tree, failing to faithfully forward propagate (Schiller et al. 1997; Zhu 2000). We therefore investigated how efficaciously barrages of distal apical dendritic excitatory synaptic input drove axonal action potential firing across the first month of postnatal development. To do this, we directly compared how effectively barrages of EPSCs generated at somatic or distal apical dendritic sites drove action potential output, calculating the number of action potentials evoked by the delivery of series of identical 2-s-long barrages of EPSCs generated at each site (Fig. 9). In early postnatal layer 5 pyramidal neurons, the delivery of barrages of EPSCs to the soma or apical dendrite resulted in the generation of a similar number of action potentials across a wide range of injected synaptic current (P9-10; dendritic recording 78 ± 13 μm from the layer 1–layer 2 border; n = 6; Fig. 9A). Therefore at this age, current-discharge relationship had similar slopes, when synaptic input was generated at the soma or distal apical dendrite (Fig. 9B). In progressively older animals, the number of action potentials generated by the apical dendritic synaptic input became less than those generated by the delivery of identical simulated synaptic input to the soma (Fig. 9, C, E, and G). This resulted in a disparity between the slope of current-discharge relationship (Fig. 9, D, F, and H). Interestingly, in mature pyramidal neurons, robust action potential output could be evoked by distal dendritic barrages of sEPSPs, a finding that could not be predicted by the heavy constraint of subthreshold voltage responses (Fig. 9, G and H). We therefore explored if barrages of dendritic excitatory input triggered action potential output not only by directly spreading to the site of action potential initiation but also by the generation of dendritic spikes. To do this, we overlaid voltage recordings for each action potential generated by somatic or dendritic barrages of simulated synaptic input (Fig. 10, A and B). When excitatory input was delivered to the soma, each action potential was first recorded from the somatic recording electrode and then subsequently at the dendritic site, consistent with the axonal initiation of action potential firing and subsequent back-propagation into the apical dendritic tree (data not shown) (Stuart and Sakmann 1994). Similarly, in early postnatal neurons when synaptic excitation was generated at distal apical dendritic sites, the vast majority of action potentials were recorded first at somatic sites (P9–10; 92.7 ± 4.6%; n = 6; Fig. 10, A–C). In neurons older than P11, the majority of somatically recorded action potentials were however preceded by dendritic spikes (Fig. 10B). The fraction of somatically recorded action potentials that were preceded by a dendritic spike increased in an age-dependent manner. Indeed in mature pyramidal neurons, the vast majority of action potentials were driven by dendritic spikes (Fig. 10C). This relationship could be fit with a bounded exponential function with an age-constant of just 2.4 days (Fig. 10C). These data indicate that after postnatal day 11, dendritic synaptic integration significantly enhances the impact of distal apical dendritic synaptic inputs on action potential output. To quantify this, we plotted the relative efficacy of dendritic synaptic inputs in the sub- and suprathreshold range (Fig. 10D). Subthreshold efficacy was calculated as the ratio of the slope between somatic depolarization and injected current when barrages of EPSCs were injected at the soma or distal apical dendritic sites (Figs. 3 and 4A). Suprathreshold efficacy was calculated as the ratio of the current-discharge relationship evoked by barrages of somatic or distal apical dendritic EPSCs (Fig. 9). This comparison revealed a significant increase in the efficacy of dendritic synaptic inputs in the suprathreshold domain after postnatal day 13, indicating that after the first 2 wk of age, dendritic synaptic integration profoundly contributes to the output of layer 5 pyramidal neurons (Fig. 10D).
FIG. 9.
Developmental refinement of the suprathreshold efficacy of apical dendritic excitation. A, C, E, and G: somatic voltage recordings of the action potential output generated in response to identical barrages of EPSCs injected at somatic (left) and apical dendritic sites (right). Recordings were made from layer 5 pyramidal neurons of the indicated postnatal age. B, D, F, and H: current-discharge relationship generated when barrages of EPSCs were delivered at somatic (•) and distal apical dendritic sites (○). Data have been pooled in the indicated postnatal age groups. Each value represents means ± SE Note that in early postnatal neurons the action potential output is similar when synaptic current is injected at somatic and apical dendritic sites.
FIG. 10.
Developmental increase in apical dendritic synaptic integration. A: simultaneous somatic and dendritic voltage recordings of action potential output evoked by the injection of barrages of EPSCs at distal apical dendritic sites. For neurons of the indicated postnatal age, somatic (black trace) and dendritic (gray) voltage records have been overlain. B: action potential waveforms recorded from somatic (black) and apical dendritic sites (gray). Action potentials were aligned at the peak of somatically recorded events and averaged for all events generated across current-discharge relationship. Note in the P9 neuron that the average action potential waveform is recorded first at the soma and then backpropagates to the dendritic recording site. In contrast, recordings from older animals indicate that somatically recorded action potentials are preceded by dendritic spikes (P12–29). C: summary graph describing the percentage of somatically recorded action potentials that were preceded by a dendritic spike. Results have been pooled in the indicated age groups (means ± SE). The line represents a fit to the data with a single bounded exponential function. Note the age-dependent increase in dendritic spike generation. D: comparison of the efficacy of dendritic inputs in the sub- and supra-threshold domain (see results for details). Significant differences are indicated by stars (P values <0.05, Wilcoxon matched pairs test).
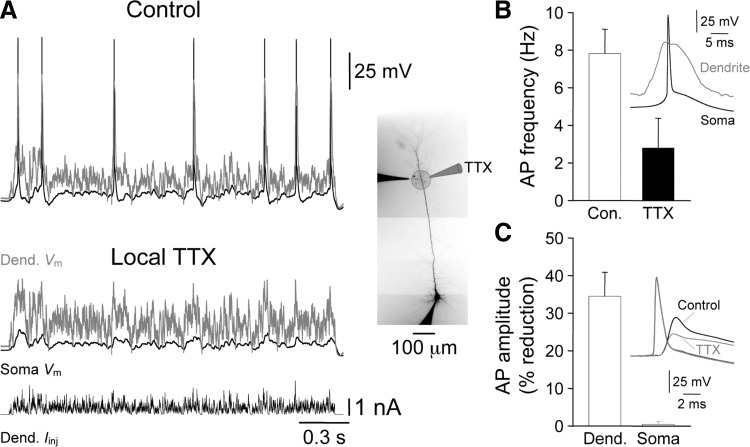
To directly test if apical dendritic spikes in layer 5 pyramidal neurons from immature animals drove action potential output, we locally pharmacologically blocked apical dendritic spikes by the brief pressure application of TTX (1 μM, in puffer pipette) delivered from a pipette placed close to (20–30 μm) the apical dendritic recording site in P13-14 layer 5 pyramidal neurons (Fig. 11, inset). To ensure that TTX had effects only locally, we measured the amplitude of somatic and back-propagating action potentials (BPAP) generated by somatic current injection and found that somatic action potentials were unaffected, but BPAPs were significantly attenuated by the local apical dendritic application of TTX (soma: control = 87.3 ± 1.3 mV; TTX = 86.9 ± 1.8 mV, n = 6; not significantly different; dendrite: control = 41.2 ± 4.7 mV; TTX = 25.7 ± 1.5 mV, n = 6, P < 0.01; paired t-test; Fig. 11C). In response to a barrage of apical dendritic simulated synaptic input (535 ± 17 μm from the soma; n = 6), 87.0 ± 8.0% of action potentials were preceded by an apical dendritic spike (Fig. 11, A and B). The local application of TTX blocked apical dendritic spikes and resulted in a 71.4 ± 11.7% reduction in the number of action potentials evoked by the distal apical dendritic excitatory input (Fig. 11, A and B). In neurons recorded from older animals, however, the local apical dendritic application of TTX blocked the generation of dendritic spikes evoked by distal apical dendritic excitatory input, and resulted in somatic voltage responses that were sub-threshold for the generation of axonal action potentials (P27–29; n = 3; not shown). Taken together, these data indicate that apical dendritic spikes powerfully drive neuronal output in layer 5 pyramidal neurons.
FIG. 11.
Dendritic spikes drive action potential output in immature layer 5 pyramidal neurons. A: the local apical dendritic application of TTX (1 μM in a puffer pipette) blocks the action potential output evoked by apical dendritic excitatory input in a P13 layer 5 pyramidal neuron. Simultaneously recorded somatic (black traces) and dendritic (gray traces) voltage responses evoked by the injection of a barrage of EPSCs (bottom trace) at distal apical dendritic sites under control and following the local apical dendritic application of TTX. Note that under control dendritic spikes preceded action potential output, the sequence of regenerative events is shown in B, inset. The photomicrograph shows the morphology of this pyramidal neuron and the placement of pipettes. B: pooled data describing the reduction in the frequency of action potential output produced by the local apical dendritic application of TTX (n = 6; P13- 14). C: pooled data describing the selective attenuation of the amplitude of back-propagating action potentials by local apical dendritic TTX (n = 6). Inset: the influence of TTX on the waveforms of the somatic and dendritic action potentials, same neuron as shown in A.
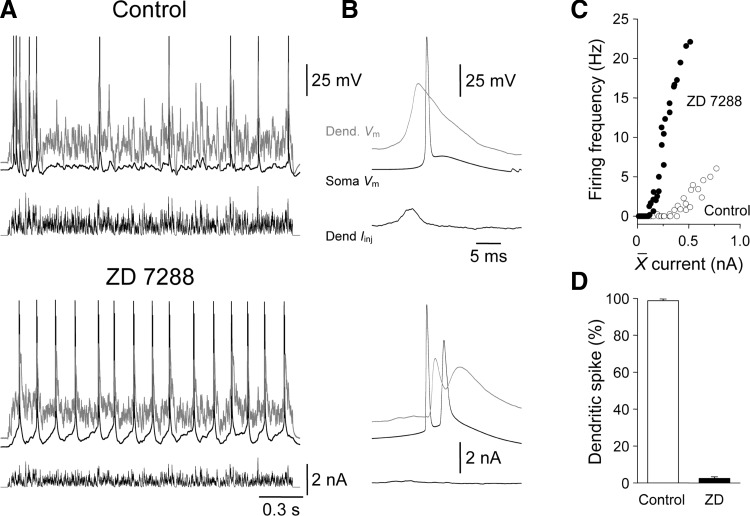
The proceeding results suggest that the development increase in the density of apical dendritic HCN channels sculpts the integrative operations of layer 5 pyramidal neurons. Early in postnatal development (P9) excitatory synaptic input to the apical dendrite is funneled to the soma and axon to influence action potential firing. In contrast, in mature layer 5 pyramidal neurons little direct depolarization reaches the soma and axon, and apical dendritic excitatory inputs are locally integrated to evoke dendritic spikes that in turn drive action potential output. We therefore explored if the blockade of HCN channels in mature layer 5 pyramidal neurons could transform this pattern of compartmentalized integration and result in behavior reminiscent of early postnatal neurons where synaptic input to the apical dendrite is integrated at the axon. When the distal apical dendrites of mature layer 5 pyramidal neurons was depolarized by barrages of simulated excitatory synaptic input, prominent apical dendritic spikes were evoked that drove 98.7 ± 1.0% of action potential output (n = 237 action potentials; 533 ± 8 μm from the soma; n = 6; P27–29; Fig. 12, A–D). This behavior was transformed by the pharmacological blockade of HCN channels with ZD 7288 (20 μM; Fig. 12, A–D). When HCN channels were blocked, and the somatic and apical dendritic membrane potential repolarized to control levels by the delivery of tonic DC through the recording electrodes, the vast majority of apical dendritic excitatory input was integrated at the axon, with only a small fraction of action potentials preceded by dendritic spikes (2.4 ± 0.9%; n = 916 action potentials; Fig. 12, A–D). Moreover, in the presence of ZD 7288 the structure of the current-discharge relationship generated by distal apical dendritic barrages of simulated EPSCs resembled that produced by excitation delivered to the soma under control conditions (compare Fig. 12C with Fig. 9H). Taken together these data indicate that the high-density of apical dendritic HCN channels enforces compartmentalized integration in mature layer 5 pyramidal neurons.
FIG. 12.
Hyperpolarization-activated cyclic nucleotide (HCN)-gated channels enforce dendritic synaptic integration. A: simultaneous somatic (black traces) and dendritic (550 μm from the soma, gray traces) voltage recordings of action potential output evoked by the injection of barrages of EPSCs (bottom traces) at distal apical dendritic sites under control and in the presence of ZD 7288 (20 μM) in a P27 layer 5 pyramidal neuron. B: averaged action potential waveforms recorded from somatic (black) and apical dendritic sites (gray) under control (top traces) and in ZD 7288. Action potentials were aligned at the peak of somatically recorded events and averaged for all events generated across current-discharge relationship. Under control the somatic action potential was preceded by a dendritic spike, while in ZD 7288 somatic action potentials were followed by dendritic back-propagating action potentials. Data from the same cell as A. C: blockade of HCN channels transforms the current-discharge relationship evoked by barrages of distal apical dendritic simulated synaptic current. Data has been averaged from 6 neurons (P27–29). D: the percentage of action potentials driven by dendritic spikes under control and in the presence of ZD 7288.
DISCUSSION
In this study, we find an age-dependent switch in the integrative operations of layer 5 pyramidal neurons, from one analogous to a single-compartment temporal-integrator early in postnatal development to a highly compartmentalized integrator after the first postnatal month. This transformation of the integrative operations of pyramidal neurons was underpinned by a dramatic age-dependent increase in the density of apical dendritic HCN channels.
Functional classes of layer 5 pyramidal neurons are identifiable morphologically around the first postnatal week (Kasper et al. 1994). After this age, thick/tufted layer 5 pyramidal neurons can be differentiated by the presence of an apical dendritic tuft in layer 1 from slender/untufted layer 5 pyramidal neurons (Kasper et al. 1994). This morphological division correlates with the cell body position of layer 5 pyramidal neurons in sublayer 5B and layer 5A, respectively, in the somatosensory cortices (Frick et al. 2008; Wise and Jones 1977). In this study, we visualized the apical dendritic tree of layer 5 pyramidal neurons under infra-red differential interference contrast microscopy prior to electrophysiological recording, allowing us to target layer 5 pyramidal neurons that exhibited a prominent apical dendritic tuft in layer 1. Thus the presented data represents the postnatal development of thick/tufted layer 5B pyramidal neurons.
Key morphological features of the postnatal development of layer 5 pyramidal neurons are the age-dependent increases in the size of the dendritic tree and the density of dendritic spines, which reach adult levels by postnatal day 30 (Miller 1981; Wise et al. 1979). Analysis of Golgi-impregnated layer 5 pyramidal neurons has shown a low spine density at basal and apical dendritic sites of early postnatal (P3-5) neurons consistent with a low number of excitatory synaptic inputs (Miller 1981; Wise et al. 1979). Dendritic spine density, however, increases with age in abrupt steps around P15 and P21 in layer 5 pyramidal neurons of the visual cortices (Miller 1981). Interestingly, until P21, the density of spines is uniform across the apical dendritic trunk of layer 5 pyramidal neurons (Miller 1981). However, after P21, spine density is low at proximal but high at relatively distal apical dendritic sites (Miller 1981). Our electrophysiological results provide a functional framework in which to interpret these morphological data.
In early postnatal layer 5 neurons (P9-10), the somatic amplitude of dendritically generated simulated EPSPs was large. Moreover, barrages of apical dendritic sEPSPs powerfully summated at the soma, depolarizing the soma only slightly less efficiently than identical barrages of EPSPs generated somatically. Distal apical dendritic and somatic excitatory synaptic input therefore drove action potential firing with almost equal efficacy where each action potential was initiated axonally. These properties indicate that a relatively small number of excitatory inputs are required to generate action potential firing in early postnatal layer 5 pyramidal neurons consistent with the low dendritic spine density at this age. Thus in early postnatal neurons, excitatory synaptic activity produces prominent voltage and conductance changes that spread throughout the neuron. Excitatory synaptic activity generated at basal and apical dendritic sites is therefore expected to interact by decreasing the driving force at even distant excitatory synapses. Similarly, the compact electrotonic structure will allow the wide spatial distribution of synaptic conductance as conductance is compartmentalized with a length constant on the order of half the voltage length constant in the apical dendrite of layer 5 pyramidal neurons (Williams 2004). Voltage interactions will, however, be more influential in early postnatal neurons because the time course of sEPSPs greatly outlasts that of the underlying synaptic current. Early postnatal layer 5 pyramidal neurons can therefore be considered electrotonically compact temporal integrators, allowing, on one hand, remote synaptic inputs to powerfully influence action potential output but on the other, promoting voltage interaction between distant excitatory synapses. If this situation was maintained during development as spine density increases, intersynaptic voltage interactions would decrease the efficacy of synaptic signaling, Moreover, intersynaptic depolarization would enhance the NMDA receptor-dependent calcium entry at activated excitatory synapses (Markram et al. 1997; Thomson et al. 1989), hampering the input-specificity of synaptic plasticity (Andersen et al. 1977; Engert and Bonhoeffer 1997), and potentially leading to neurotoxic levels of calcium entry (Lipton and Rosenberg 1994). Therefore as dendritic spine density increases, postsynaptic mechanisms must be in place to ensure translation of dendritic excitatory input into neuronal output and damp undesirable intersynaptic interactions. The physical expansion of the dendritic tree in part allows for this, by creating longer dendritic cables between areas of the tree, for example basal and apical dendrites. Similarly the relatively low numbers of excitatory synapses at proximal apical dendritic sites in mature (> P 21) layer 5 pyramidal neurons will help to minimize direct interaction between basal and apical dendritic excitatory synapses. The electrical compartmentalization of areas of the dendritic tree will also be increased by the age-dependent increase in the density of HCN channels.
HCN channel density was found to increase by >10-fold at distal apical dendritic sites of layer 5 pyramidal neurons from P11 to P40. This dramatic rise in apical dendritic channel density had a profound influence on the spread of synaptic potential through the dendritic tree. As postnatal age increased, single or barrages of distal apical dendritic sEPSPs become progressively weaker sources of somatic excitation. Indeed we found an age-dependent constraint of the somatic time course of apical dendritically generated sEPSPs that resulted in a progressive reduction in the temporal summation of barrages of dendritic sEPSPs at the soma. Consequently in mature layer 5 pyramidal neurons, the pharmacological blockade of HCN channels dramatically increased the somatic depolarization produced by apical dendritic sEPSPs. The age-dependent increase in HCN channel density therefore profoundly increases electrical compartmentalization. At a functional level, such compartmentalization ensures that synaptic activity in different areas of the dendritic tree have minimal voltage and conductance interactions (Williams 2004). However, the high density of apical dendritic HCN channels also ensures that distal apical dendritic synaptic inputs provide only a weak direct source of axo-somatic excitation.
Interestingly, we find that the activation properties of ensemble HCN channel activity in cell-attached patches from distal apical dendritic sites changes with age; with the activation, kinetics speeding and the voltage dependence of activation become relatively depolarized as the channel density increases with age. Whole cell recordings from the somata of CA1 hippocampal pyramidal neurons have similarly shown that the properties of IH, the macroscopic manifestation of HCN channel activity, are developmentally regulated, with activation kinetics speeding and the voltage dependence of activation becoming more depolarized with age (Surges et al. 2006; Vasilyev and Barish 2002). Informatively, in area CA1, an age-dependent increase in HCN1 mRNA, protein level and apical dendritic HCN1 immunostaining has been found (Brewster et al. 2007). This increase in HCN1 expression is accompanied by an age-dependent reduction in HCN4 mRNA and protein levels (Brewster et al. 2007). In contrast to the apical dendritic expression of HCN1, however, HCN4 immunostaining is restricted to the somata of CA1 pyramidal neurons (Brewster et al. 2007), that together with the predominately somatic HCN2 isoform acts to slow the kinetics of somatically recorded IH in early postnatal neurons (Brewster et al. 2007; Surges et al. 2006; Vasilyev and Barish 2002). Our cell-attached recording approach, however, directly indicates an age-dependent modification of the properties of ensemble channel activity at apical dendritic sites and is not complicated by voltage-clamp errors apparent during somatic whole cell recording (Williams and Mitchell 2008) or by the subcellular distribution of HCN channel isoforms. Combined electrophysiological and immunostaining showed that the age-dependent increase in the density of functional channels at distal apical dendritic sites of layer 5 pyramidal neurons was paralleled by an increase in the density of HCN1 immunostaining. In the neocortex, little HCN4 expression is detectable with HCN1 and HCN2 being the abundant channel isoforms (Notomi and Shigemoto 2004; Santoro et al. 2000). As HCN2 channels produce macroscopic currents with relatively slow kinetics (Santoro et al. 2000), we explored if the HCN2 channel isoform was expressed at distal apical dendritic sites preferentially during early postnatal development. However, we were unable to detect significant apical dendritic HCN2 immunostaining in either young or mature animals. A previous study has, however, reported relatively weak HCN2 expression at distal apical dendritic sites in 5- to 10-wk-old layer 5 pyramidal neurons (Notomi and Shigemoto 2004). Interestingly, in HCN1 knockout mice, somatic whole cell recordings of layer 5 pyramidal neurons have shown a prominent IH current with slower activation kinetics and relatively hyperpolarized voltage dependence compared with wild-type mice consistent with a functional role of HCN2 isoforms (Chen et al. 2008). We suggest therefore that the age-dependent refinement of the properties of ensemble HCN channels at distal apical dendritic sites is consistent with an age-dependent alteration in HCN channel subunit composition (Santoro and Tibbs 1999). Alternatively, our data are consistent with a developmental regulation of HCN channel properties by interacting proteins (Santoro et al. 2004; Yu et al. 2001) and/or by the channels phosphorylation state (Huang et al. 2008). Interestingly, a putative β subunit of the HCN channel, MiRP1 has been shown to regulate the activation kinetics of HCN channel responses (Yu et al. 2001). We consider it unlikely that differences in basal intracellular levels of cyclic nucleotides, which modulate the activation properties of HCN channels, underlie the developmental regulation of HCN channel function because HCN1 channels are weakly influenced by cyclic nucleotide levels (Santoro et al. 1998) and age-dependent differences in the activation properties of ensemble HCN channel activation in cell-attached patches were apparent during simultaneous whole cell and cell-attached recordings where intracellular nucleotide levels are dominated by the intra-pipette filling solution (data not shown).
The electrical compartmentalization of layer 5 pyramidal neurons imposed by the high density of HCN channels ensures that distal apical dendritic excitatory and inhibitory synaptic input evoke minimal voltage responses at the axonal site of action potential generation in mature animals (Berger et al. 2001; Stuart and Spruston 1998; Williams and Stuart 2000, 2003). Indeed modeling studies have shown that single EPSPs generated in the apical tuft, are attenuated by >100-fold as they spread to the soma (Stuart and Spruston 1998). Here we find that even a high-frequency barrage of distal apical dendritic sEPSPs provides less than 500 μV of somatic depolarization in mature layer 5 pyramidal neurons. As both cortico-cortical and subcortical synaptic input is conveyed in layer 1 of the neocortex (Bernander et al. 1994; Zhu and Zhu 2004), these properties suggest that such distal apical dendritic excitatory inputs are ineffective direct drivers of action potential output. We however, found an age-dependent switch in the way distal apical dendritic synaptic input influenced neuronal output. As postnatal age increased, the fraction of action potentials that were driven by apical dendritic spikes increased. Therefore we find that active dendritic synaptic integration, leading to the initiation and forward propagation of dendritic spikes, acts to compensate for the distance-dependent attenuation of apical dendritic input. Interestingly, in comparison with a previous study that probed the excitability of distal apical dendrites of layer 5 neurons by the injection of simple positive current steps (Zhu 2000), we find that apical dendritic integration becomes dominant after postnatal day 11, when studied in response to more in vivo-like, time-varying barrages of simulated synaptic input. Indeed during a development period between P11 and P22, we find that the apical dendrite can be considered to be hyperexcitable; electrical compartmentalization is incomplete at this age, resulting in a significant spread of depolarization from the apical dendrite to the soma and axon that is complemented by the robust forward prorogation of apical dendritic spikes. An idea supported by the reduction, but not abolition, of action potential output evoked by distal apical dendritic excitatory input when dendritic spike generation was blocked by local apical dendritic application of TTX in P13–14 neurons. In mature layer 5 pyramidal neurons, however, the high density of apical dendritic HCN channels ensures that little synaptic depolarization flows from the apical dendrite to the soma, and integration is largely restricted to distal apical dendritic sites, resulting in the initiation of dendritic spikes. A conclusion supported by the transformation of the site of integration of distal apical dendritic synaptic input from apical dendritic to axonal following the pharmacological blockade of HCN channels in mature neurons. Functionally, the high density of HCN channels provides a platform for compartmentalized synaptic integration (Williams 2004) as well as allowing interaction between axo-somatic and apical dendritic integration compartments through regenerative activity, such as BAC firing (Larkum et al. 1999; Williams 2005). During the first month of postnatal development therefore, the integrative properties of layer 5 pyramidal neurons are transformed, from electronically compact temporal integrators of synaptic input to highly compartmentalized integrators of basal and apical dendritic synaptic input.
GRANTS
This work was supported by the Medical Research Council.
Supplementary Material
Acknowledgments
We are grateful to Prof. R. Shigemoto for the gift of an HCN2 antibody. S. E. Atkinson performed immunostaining and S. R. Willliams performed electrophysiological experiments.
Footnotes
The online version of this article contains supplemental data.
REFERENCES
- Andersen et al. 1977.Andersen P, Sundberg SH, Sveen O, Wigstrom H. Specific long-lasting potentiation of synaptic transmission in hippocampal slices. Nature 266: 736–737, 1977. [DOI] [PubMed] [Google Scholar]
- Angelo et al. 2007.Angelo K, London M, Christensen SR, Hausser M. Local and global effects of I(h) distribution in dendrites of mammalian neurons. J Neurosci 27: 8643–8653, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger et al. 2001.Berger T, Larkum ME, Luscher HR. High I(h) channel density in the distal apical dendrite of layer V pyramidal cells increases bidirectional attenuation of EPSPs. J Neurophysiol 85: 855–868, 2001. [DOI] [PubMed] [Google Scholar]
- Bernander et al. 1994.Bernander O, Koch C, Douglas RJ. Amplification and linearization of distal synaptic input to cortical pyramidal cells. J Neurophysiol 72: 2743–2753, 1994. [DOI] [PubMed] [Google Scholar]
- Brewster et al. 2007.Brewster AL, Chen Y, Bender RA, Yeh A, Shigemoto R, Baram TZ. Quantitative analysis and subcellular distribution of mRNA and protein expression of the hyperpolarization-activated cyclic nucleotide-gated channels throughout development in rat hippocampus. Cereb Cortex 17: 702–712, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen et al. 2008.Chen X, Shu S, Kennedy DP, Willcox SC, Bayliss DA. Subunit-specific effects of isoflurane on neuronal Ih in HCN1 knockout mice. J Neurophysiol 29: 129–140, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engert and Bonhoeffer 1997.Engert F, Bonhoeffer T. Synapse specificity of long-term potentiation breaks down at short distances. Nature 388: 279–284, 1997. [DOI] [PubMed] [Google Scholar]
- Frick et al. 2008.Frick A, Feldmeyer D, Helmstaedter M, Sakmann B. Monosynaptic connections between pairs of L5A pyramidal neurons in columns of juvenile rat somatosensory cortex. Cereb Cortex 18: 397–406, 2008. [DOI] [PubMed] [Google Scholar]
- Hausser and Roth 1997.Hausser M, Roth A. Estimating the time course of the excitatory synaptic conductance in neocortical pyramidal cells using a novel voltage jump method. J Neurosci 17: 7606–7625, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang et al. 2008.Huang J, Huang A, Zhang Q, Lin YC, Yu HG. Novel mechanism for suppression of hyperpolarization-activated cyclic nucleotide-gated pacemaker channels by receptor-like tyrosine phosphatase-alpha. J Biol Chem 283: 29912–29919, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasper et al. 1994.Kasper EM, Larkman AU, Lubke J, Blakemore C. Pyramidal neurons in layer 5 of the rat visual cortex. II. Development of electrophysiological properties. J Comp Neurol 339: 475–494, 1994. [DOI] [PubMed] [Google Scholar]
- Kim and Connors 1993.Kim HG, Connors BW. Apical dendrites of the neocortex: correlation between sodium- and calcium-dependent spiking and pyramidal cell morphology. J Neurosci 13: 5301–5311, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kole et al. 2007.Kole MH, Brauer AU, Stuart GJ. Inherited cortical HCN1 channel loss amplifies dendritic calcium electrogenesis and burst firing in a rat absence epilepsy model. J Physiol 578: 507–525, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kole et al. 2006.Kole MH, Hallermann S, Stuart GJ. Single IH channels in pyramidal neuron dendrites: properties, distribution, and impact on action potential output. J Neurosci 26: 1677–1687, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larkum and Zhu 2002.Larkum ME, Zhu JJ. Signaling of layer 1 and whisker-evoked Ca2+ and Na+ action potentials in distal and terminal dendrites of rat neocortical pyramidal neurons in vitro and in vivo. J Neurosci 22: 6991–7005, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larkum et al. 1999.Larkum ME, Zhu JJ, Sakmann B. A new cellular mechanism for coupling inputs arriving at different cortical layers. Nature 398: 338–341, 1999. [DOI] [PubMed] [Google Scholar]
- Lipton and Rosenberg 1994.Lipton SA, Rosenberg PA. Excitatory amino acids as a final common pathway for neurologic disorders. N Engl J Med 330: 613–622, 1994. [DOI] [PubMed] [Google Scholar]
- Lorincz et al. 2002.Lorincz A, Notomi T, Tamas G, Shigemoto R, Nusser Z. Polarized and compartment-dependent distribution of HCN1 in pyramidal cell dendrites. Nat Neurosci 5: 1185–1193, 2002. [DOI] [PubMed] [Google Scholar]
- Ludwig et al. 1998.Ludwig A, Zong X, Jeglitsch M, Hofmann F, Biel M. A family of hyperpolarization-activated mammalian cation channels. Nature 393: 587–591, 1998. [DOI] [PubMed] [Google Scholar]
- Magee 1998.Magee JC Dendritic hyperpolarization-activated currents modify the integrative properties of hippocampal CA1 pyramidal neurons. J Neurosci 18: 7613–7624, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magee 1999.Magee JC Dendritic lh normalizes temporal summation in hippocampal CA1 neurons. Nat Neurosci 2: 508–514, 1999. [DOI] [PubMed] [Google Scholar]
- Magee 2000.Magee JC Dendritic integration of excitatory synaptic input. Nat Rev Neurosci 1: 181–190, 2000. [DOI] [PubMed] [Google Scholar]
- Magee and Cook 2000.Magee JC, Cook EP. Somatic EPSP amplitude is independent of synapse location in hippocampal pyramidal neurons. Nat Neurosci 3: 895–903, 2000. [DOI] [PubMed] [Google Scholar]
- Markram et al. 1997.Markram H, Lubke J, Frotscher M, Roth A, Sakmann B. Physiology and anatomy of synaptic connections between thick tufted pyramidal neuroes in the developing rat neocortex. J Physiol 500: 409–440, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Migliore and Shepherd 2005.Migliore M, Shepherd GM. Opinion: an integrated approach to classifying neuronal phenotypes. Nat Rev Neurosci 6: 810–818, 2005. [DOI] [PubMed] [Google Scholar]
- Miller 1981.Miller M Maturation of rat visual cortex. I. A quantitative study of Golgi-impregnated pyramidal neurons. J Neurocytol 10: 859–878, 1981. [DOI] [PubMed] [Google Scholar]
- Nevian et al. 2007.Nevian T, Larkum ME, Polsky A, Schiller J. Properties of basal dendrites of layer 5 pyramidal neurons: a direct patch-clamp recording study. Nat Neurosci 10: 206–214, 2007. [DOI] [PubMed] [Google Scholar]
- Nicholson et al. 2006.Nicholson DA, Trana R, Katz Y, Kath WL, Spruston N, Geinisman Y. Distance-dependent differences in synapse number and AMPA receptor expression in hippocampal CA1 pyramidal neurons. Neuron 50: 431–442, 2006. [DOI] [PubMed] [Google Scholar]
- Nicoll et al. 1993.Nicoll A, Larkman A, Blakemore C. Modulation of EPSP shape and efficacy by intrinsic membrane conductances in rat neocortical pyramidal neurons in vitro. J Physiol 468: 693–710, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Notomi and Shigemoto 2004.Notomi T, Shigemoto R. Immunohistochemical localization of Ih channel subunits, HCN1-4, in the rat brain. J Comp Neurol 471: 241–276, 2004. [DOI] [PubMed] [Google Scholar]
- Palmer and Stuart 2006.Palmer LM, Stuart GJ. Site of action potential initiation in layer 5 pyramidal neurons. J Neurosci 26: 1854–1863, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rall 1967.Rall W Distinguishing theoretical synaptic potentials computed for different soma-dendritic distributions of synaptic input. J Neurophysiol 30: 1138–1168, 1967. [DOI] [PubMed] [Google Scholar]
- Santoro et al. 2000.Santoro B, Chen S, Luthi A, Pavlidis P, Shumyatsky GP, Tibbs GR, Siegelbaum SA. Molecular and functional heterogeneity of hyperpolarization-activated pacemaker channels in the mouse CNS. J Neurosci 20: 5264–5275, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santoro et al. 1998.Santoro B, Liu DT, Yao H, Bartsch D, Kandel ER, Siegelbaum SA, Tibbs GR. Identification of a gene encoding a hyperpolarization-activated pacemaker channel of brain. Cell 93: 717–729, 1998. [DOI] [PubMed] [Google Scholar]
- Santoro and Tibbs 1999.Santoro B, Tibbs GR. The HCN g9ne family: molecular basis of the hyperpolarization-activated pacemaker channels. Ann N[Y Acad Sci 868: 741–764, 1999. [DOI] [PubMed] [Google Scholar]
- Santoro et al. 2004.Santoro B, Wainger BJ, Siegelbaum SA. Regulation of HCN channel surface expression by a novel C-terminal protein-protein interaction. J Neurosci 24: 10750–10762, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiller et al. 1997.Schiller J, Schiller Y, Stuart G, Sakmann B. Calcium action potentials restricted to distal apical dendrites of rat neocortical pyramidal neurons. J Physiol 505: 605–616, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spain et al. 1991.Spain WJ, Schwindt PC, Crill WE. Post-inhibitory excitation and inhibition in layer V pyramidal neurons from cat sensorimotor cortex. J Physiol 434: 609–626, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuart and Sakmann 1994.Stuart GJ, Sakmann B. Active propagation of somatic action potentials into neocortical pyramidal cell dendrites. Nature 367: 69–72, 1994. [DOI] [PubMed] [Google Scholar]
- Stuart and Spruston 1998.Stuart G, Spruston N. Determinants of voltage attenuation in neocortical pyramidal neuron dendrites. J Neurosci 18: 3501–3510, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surges et al. 2006.Surges R, Brewster AL, Bender RA, Beck H, Feuerstein TJ, Baram TZ. Regulated expression of HCN channels and cAMP levels shape the properties of the h current in developing rat hippocampus. Eur J Neurosci 24: 94–104, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson et al. 1989.Thomson AM, Walker VE, Flynn DM. Glycine enhances NMDA-receptor mediated synaptic potentials in neocortical slices. Nature 338: 422–424, 1989. [DOI] [PubMed] [Google Scholar]
- van Brederode and Spain 1995.van Brederode JF, Spain WJ. Differences in inhibitory synaptic input between layer II-III and layer V neurons of the cat neocortex. J Neurophysiol 74: 1149–1166, 1995. [DOI] [PubMed] [Google Scholar]
- Vasilyev and Barish 2002.Vasilyev DV, Barish ME. Postnatal development of the hyperpolarization-activated excitatory current Ih in mouse hippocampal pyramidal neurons. J Neurosci 22: 8992–9004, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams 2004.Williams SR Spatial compartmentalization and functional impact of conductance in pyramidal neurons. Nat Neurosci 7: 961–967, 2004. [DOI] [PubMed] [Google Scholar]
- Williams 2005.Williams SR Encoding and decoding of dendritic excitation during active states in pyramidal neurons. J Neurosci 25: 5894–5902, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams and Mitchell 2008.Williams SR, Mitchell SJ. Direct measurement of somatic voltage clamp errors in central neurons. Nat Neurosci 11: 790–798, 2008. [DOI] [PubMed] [Google Scholar]
- Williams and Stuart 2000.Williams SR, Stuart GJ. Site independence of EPSP time course is mediated by dendritic I(h) in neocortical pyramidal neurons. J Neurophysiol 83: 3177–3182, 2000. [DOI] [PubMed] [Google Scholar]
- Williams and Stuart 2002.Williams SR, Stuart GJ. Dependence of EPSP efficacy on synapse location in neocortical pyramidal neurons. Science 295: 1907–1910, 2002. [DOI] [PubMed] [Google Scholar]
- Williams and Stuart 2003.Williams SR, Stuart GJ. Voltage- and site-dependent control of the somatic impact of dendritic IPSPs. J Neurosci 23: 7358–7367, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise et al. 1979.Wise SP, Fleshman JW Jr, Jones EG. Maturation of pyramidal cell form in relation to developing afferent and efferent connections of rat somatic sensory cortex. Neuroscience 4: 1275–1297, 1979. [DOI] [PubMed] [Google Scholar]
- Wise and Jones 1977.Wise SP, Jones EG. Cells of origin and terminal distribution of descending projections of the rat somatic sensory cortex. J Comp Neurol 175: 129–157, 1977. [DOI] [PubMed] [Google Scholar]
- Yu et al. 2001.Yu H, Wu J, Potapova I, Wymore RT, Holmes B, Zuckerman J, Pan Z, Wang H, Shi W, Robinson RB, El-Maghrabi MR, Benjamin W, Dixon J, McKinnon D, Cohen IS, Wymore R. MinK-related peptide 1: a beta subunit for the HCN ion channel subunit family enhances expression and speeds activation. Circ Res 88: 84–87, 2001. [DOI] [PubMed] [Google Scholar]
- Zhu 2000.Zhu JJ Maturation of layer 5 neocortical pyramidal neurons: amplifying salient layer 1 and layer 4 inputs by Ca2+ action potentials in adult rat tuft dendrites. J Physiol 526: 571–587, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu and Zhu 2004.Zhu Y, Zhu JJ. Rapid arrival and integration of ascending sensory information in layer 1 nonpyramidal neurons and tuft dendrites of layer 5 pyramidal neurons of the neocortex. J Neurosci 24: 1272–1279, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.