Abstract
Mammalian swallowing involves the coordinated and sequential activity of many oropharyngeal muscles. Using synchronous electromyography (EMG) and videofluorography, we recorded the pattern of EMG activity for 12 muscles during swallowing in neurologically intact suckling pigs. We tested the hypothesis that this EMG pattern corresponded to the established pattern of activity for the isolated, reflexive pharyngeal swallow of the decerebrate infant pig. The EMG activity associated with the normal swallow of the intact animal had two components: a staggered pattern of single EMG bursts that were prominent in the stylohyoid, thyrohyoid, cricothyroid, and omohyoid muscles and double bursts of activity in some muscles, including geniohyoid and genioglossus, with the same underlying periodicity as suckling. Most of the staggered activity pattern, a linear sequence of progressively delayed activities in different muscles, was not statistically different from that previously found in the reflexive pharyngeal swallow of the decerebrate. However, not all components of the linear sequence of the reflexive swallow were inserted unchanged into the intact swallow. Some components appeared to be delayed or advanced, bringing them into phase with the underlying rhythmic activity. The difference between swallows of intact and of decerebrate animals was not solely due to the presence of rhythmic activity in the former. The timing of some EMG activities in intact animals also differed from the same activities in the few decerebrates that exhibited rhythmic tongue and jaw activity. These results suggest cerebral function influences the EMG pattern of the pharyngeal swallow, which has traditionally been considered a purely reflex pattern.
INTRODUCTION
In non-human mammals, fluids are transported through the oral cavity by consecutive cycles of rhythmic oral activity. The transported material gradually accumulates as a bolus in the oropharynx (primarily in the valleculae), and the space is then periodically emptied into the esophagus. The process of emptying the valleculae corresponds to the pharyngeal stage of the classical swallow, and this action is periodically inserted into one of the fluid transporting cycles to form a combined transport/swallow cycle (German et al. 1992; Hiiemae and Crompton 1985).
In the decerebrate infant animal, the pharyngeal swallow can be elicited as an isolated, “reflexive” event with a distinctive core pattern of EMG activity (Thexton et al. 2007). However, it is not known to what extent the temporal pattern of EMG activity is retained when the pharyngeal swallow is incorporated into cycles of normal suckling activity. Previous results suggest that the event might be incorporated in a relatively unchanged form. EMG components of the isolated pharyngeal swallow (Thexton et al. 2007) can be found within the more complex EMG pattern of swallowing that occurs during the occasional periods of rhythmic tongue and jaw movement (RTJM) in the decerebrate (Thexton et al. 2008). In RTJM-associated swallows, many of the muscles maintain the same order and relative timing of activation as in the isolated pharyngeal swallow. These results suggest the hypothesis that, in the conscious suckling animal, the EMG activity of the complex suck/swallow cycle is separable into two components, one reflecting the ongoing rhythm and the other reflecting an inserted nonrhythmic reflex component.
Besides being a significant component of the rhythmic (nonswallowing) activity during suckling or drinking in the intact pig (Thexton et al. 1998), geniohyoid activity occurs in the swallows of decerebrates, both in the isolated pharyngeal swallow (Thexton et al. 2007) and also when that swallow is associated with rhythmic activity (Thexton et al. 2008). However, the geniohyoid activity has a different timing in the two swallows. The timing of the activity in this muscle is of particular interest because geniohyoid activity can cause either protraction of the hyoid/tongue complex or it can cause jaw opening, depending on whether there is a simultaneous contraction of the serially connected jaw elevator muscles or of the sternohyoid muscles.
The main aim of this study was to establish a statistically robust measure of the temporal pattern of EMG activity during swallowing in the conscious, naturally feeding animal to facilitate comparison with the known pattern of the isolated pharyngeal swallow in the decerebrate. Using this pattern, we tested the hypothesis that the EMG pattern of the reflexive swallow of the decerebrate (Thexton et al. 2007) was partly or totally contained within the swallow of the conscious intact animal.
The secondary aim was to compare the EMG pattern of swallowing in the conscious animal with comparable activity in those decerebrates that are capable of rhythmic oral activity and swallowing (Thexton et al. 2008). This allowed testing of the hypothesis that the pattern of EMG activity in the pharyngeal swallow, when incorporated into RTJM, differed between the intact and the decerebrate animal; any difference would be due primarily to the presence/absence of cerebral function.
METHODS
Except for the feeding and handling of the conscious intact animals, the methods used were identical to those used previously in a study of swallowing in decerebrate animals (Thexton et al. 2008).
Animals and surgical procedures
All experiments that generated data for this paper were approved by the Harvard University IACUC (23-05). Videoradiographic and EMG data were collected in a series of experiments using seven infant mini-pigs between 20 and 30 days of age (preweaning). Surgical procedures to implant electrodes and radio-opaque markers were carried out on each animal under Isoflurane anesthesia (2–5% in oxygen administered by face mask).
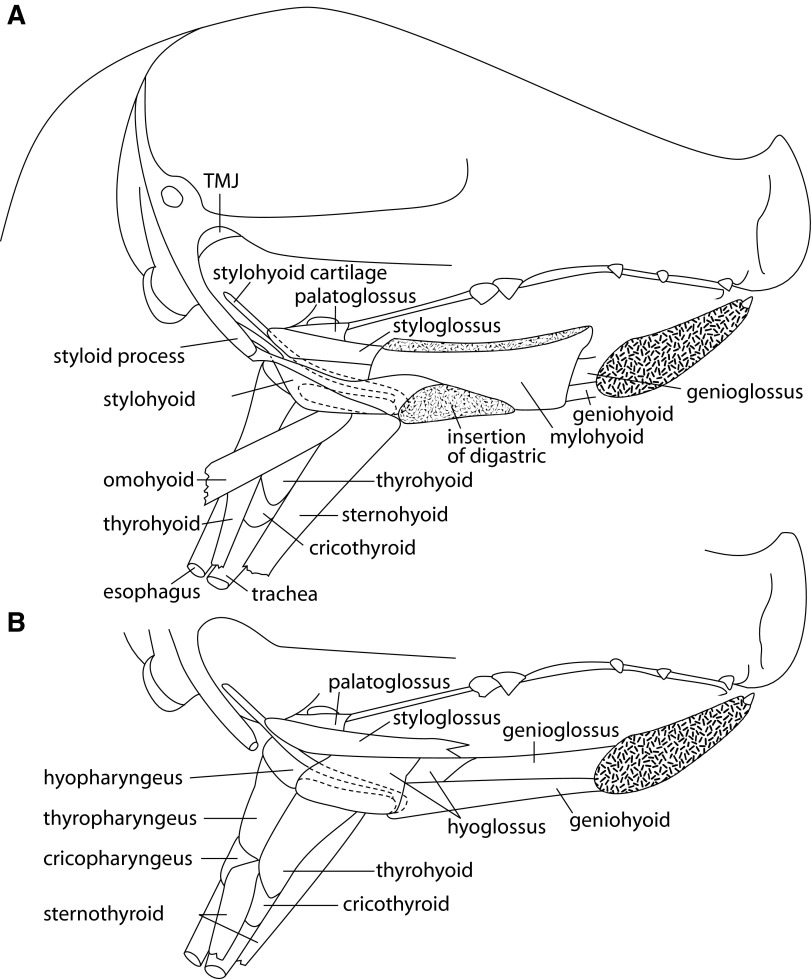
The relevant anatomy of the oro-pharyngeal region, in piglets of the age used in this study, is shown in sagittal section in Fig. 1, A (superficial) and B (deeper). The activities, in the muscles that connect the hyoid to the mandible (mylohyoid, geniohyoid), to the skull (stylohyoid), and to the thyroid cartilage (thyrohyoid), are consequently important in characterizing generation of the swallow (Miller 2002; Spiro et al. 1994; Thexton et al. 1998; Uchida et al. 1994).
FIG. 1.
Sagittal section of head of a young pig with mandible removed to show underlying muscles at superficial (A) and deeper (B) levels. TMJ, tempromandibular joint. ···, the hyoid bone.
Under general anesthesia and aseptic conditions, a micro-surgical hematological clip was placed on the tip of the epiglottis as a radio-opaque marker. This technique was previously used in several studies (Laitman and Crelin 1980; Laitman et al. 1977; Larson and Herring 1996), including some by the present authors (Crompton et al. 2008; Thexton et al. 2007) without any adverse effects.
Then, the supra- and infrahyoid muscles were exposed and identified in accordance with established pig anatomy (Sack 1982). Intramuscular bipolar wire electrodes (Basmajian and Stecko 1962) were implanted with the separation between the recording surfaces oriented parallel to the long axis of the muscle fibers, in: genioglossus (at its mandibular origin), geniohyoid (close to its hyoid insertion), omohyoid (superior belly), stylohyoid, anterior digastric, thyrohyoid, cricothyroid, hyoglossus (close to its origin from the hyoid), sternohyoid, and sternothyroid as previously described (Thexton et al. 1998, 2007). The bipolar electrodes were duplicated in most muscles; however, we inserted triplicate electrodes in the mylohyoid and, because of the difficulty of accurate insertion, in the putative palatopharyngeus. In all cases, the bipolar electrodes were inserted into each muscle so as to be laterally displaced from each other, relative to the long axis of the muscle fibers. Patch electrodes (Loeb and Gans 1986) were sutured onto the surface of the mylohyoid at incremental rostrocaudal positions along a line halfway between the midline and the mandibular attachment of the muscle, i.e., neither overlying the geniohyoid nor close to the anterior digastric.
After surgery, the animals were left for a minimum of 5 h to eliminate the inhalational anesthetic agent before offering food and recording swallows. The experimental period lasted 1–3 days, after which the animals were killed following IACUC standards. Workers who did not originally place the electrodes performed a post mortem to confirm the position and physical state of the electrodes.
Experimental feeding methodology and data recording
From their time of arrival at the Harvard University vivarium, animals were fed infant pig formula from a standard baby bottle, fitted with a special pig nipple (Nasco, Fort Atkinson, WI); in some cases, the pressure in the teat was recorded using a microtransducer (Thexton et al. 2004). The bottle was held in a clamp in the end wall of a Plexiglas box designed to orient the animal at right angles to the X-ray beam. In the experimental situation, a small amount of barium was added to the milk to increase its radiographic visibility. The movements of suckling (suck cycles interspersed with suck/swallow cycles) were recorded in the lateral view (sagittal plane) using digital video-radiography (Siemens Tridoros 150G3 cineradiographic apparatus with a Sony DCR-VX1000 digital video camera). EMG signals from the selected muscles were amplified (×400 to ×10,000) using an MA-300 EMG System (Motion Lab Systems, Los Angeles, CA) with a band-pass of 20 Hz to 2 kHz and a 60-Hz notch filter. The EMG signals were then recorded on a TEAC RD-145T digital data recorder together with the synchronization signals from the X-ray apparatus; an overall digitization rate of 6 kHz was used. Sections of EMG data that corresponded to cineradiographic recordings of sequences of swallows were then transferred to a computer.
Recording protocol and data selection
For the purposes of data analysis, the timing of the swallow was defined radiographically by the start of the rapid, caudally directed movement of the epiglottal tip; the reliability of this timing measure depends on statistical factors and has previously been considered in detail (Thexton et al. 2007). In this paper, the first caudal movement of the epiglottis is for convenience referred to as the epiglottal flexion. The procedure for data selection, in the first stage of analysis, was based primarily on the presence of adequate videoradiographic visualization of successive individual swallows, and secondarily on the matching EMG record also being artifact- and noise-free. Although duplicate or triplicate electrodes were inserted into most of the selected muscles in each animal, the number of electrode recordings at any one time was limited to 15 because 1 of the 16 channels on the digital tape recorder was reserved for the synchronization signal from the digital camera. Consequently, duplicate recordings were possible in only seven muscles at a time or six muscles when triplicate recordings were made from either mylohyoid or palatopharyngeus.
Data analysis
Previous experience with bipolar wire electrodes (1-mm bare tips separated by 2 mm), including specific checks for cross-talk, indicated a high level of selectivity (Thexton et al. 2007) and consequently also indicated that the detection of EMG signals in anything other than the target muscles did not occur to any discernable extent. The amplified EMG activity was processed and analyzed as previously described (Thexton et al. 2007). Briefly, band-pass filtered and amplified EMGs were processed by rectification and 10-ms constant time reset integration after which a noise threshold was defined statistically and background activity was rejected (Thexton 1996). These processes were combined in a computer program that also presented a display of the quantified EMG signals and allowed swallow related sections of the data to be indexed for further analysis.
When swallows were identified based on radiographic criteria, the data were indexed with reference to the onset of the epiglottal flexion. The timing of epiglottal flexion-onset during each swallow was identified from the frame count on the videotape and the corresponding time measured in the EMG data stream, using the common synchronization signal. Because swallows were identified videoradiographically, there was an uncertainty of one frame (∼30 ms) in establishing the epiglottal flexion-onset and therefore a potential error of the same order in delimiting the analysis period. However, the principle has been established that, when timing signals are derived from large data sets with image movement, the error around the timing of the visual/radiographic event tends to have a mean of zero (Khalili et al. 2005). Consequently, when the corresponding EMG data were processed statistically, they also had a timing error that tended to zero (Thexton et al. 2007).
The subsequent steps in analysis used the processed EMG signals, occurring in the 400-ms period that started 100 ms before the onset of epiglottal flexion and ended 300 ms after that event. For convenience, the data were first linearly interpolated to generate 100 time bins and the EMG data, recorded by each electrode, were then arranged to generate a matrix with 100 time bins and n swallows. Each row of these matrices is referred to as an “electrode-swallow” dataset, and the numbers recorded for each muscle are given in Table 1. The EMG activity recorded by each electrode in each swallow was then scaled to a maximum amplitude of 100 units. This procedure was adopted because the dominant effect on signal amplitude is the distance of an electrode from the nearest active muscle fiber (Buchthal and Schmalbruch 1980; Ertas et al. 1995). In this way maximum signal amplitude was equalized, across both electrodes and swallows for each muscle, yielding a sample of EMG activities that had identical peak amplitude for each electrode and for each swallow.
TABLE 1.
Sample size and variability for all muscles
| Percentage Overlap of Signals From Duplicate Electrodes |
||||||
|---|---|---|---|---|---|---|
| Muscles | Delay Relative to Leading Complex, ms | No. of Electrode Swallows | No. Animals | Overall Index of Temporal Variability, % | Median | Range |
| Mylohyoid | −36 | 366 | 3 | 234.1 | 47 | 31–80 |
| Stylohyoid | 0 | 339 | 2 | 74.9 | 80 | 70–89 |
| Hyoglossus | 0 | 542 | 5 | 273.4 | 66 | 5–87 |
| Palatopharyngeus | 20 | 169 | 2 | 185.6 | 62 | (1 value) |
| Omohyoid | 20 | 329 | 3 | 118.0 | 86 | 84–88 |
| Digastric | 20 | 361 | 3 | 258.2 | 41 | 7–68 |
| Thyrohyoid | 36 | 513 | 5 | 73.1 | 73 | 63–91 |
| Genioglossus | 84 | 361 | 3 | 227.9 | 52 | 28–64 |
| Sternothyroid | 116 | 238 | 2 | 155.8 | 66 | 56–77 |
| Cricothyroid | 128 | 224 | 3 | 126.7 | 87 | 75–90 |
| Sternohyoid | 120 | 152 | 2 | 202.5 | No duplicates | |
| Geniohyoid | 144 | 224 | 3 | 238.5 | No duplicates | |
The experimental design of this study stems from the hierarchical nature of the data (German et al. 2008). The unit of analysis for these analyses is the electrode swallow, that is the EMG signal measured by a single electrode for a single swallow. There were duplicate electrodes in the same muscle in most of the individual animals, which permitted analysis of within muscle variability of the EMG signals. A sequence is a series of swallows recorded in one feeding session for one individual animal, thus the electrode swallows were nested within sequences, and these sequences were nested within animals. To summarize the EMG activity for each muscle, we calculated the median level of activity in each of the 100 time bins, over all electrodes, sequences and individuals. A plot of these medians against time produced a “typical” profile of EMG activity for each muscle.
We derived a single measure of variability of the data over the 400-ms analysis period by summing the interquartile range (IQR, the difference between the 25th and 75th percentiles) over the 100 time bins and scaling it to the sum of the median values over the same 100 time bins. This measure, which is a nonparametric analog of the coefficient of variation, was calculated for the “within sequence” data, as a measure of the level of variation of that data (Thexton et al. 2007). When measured over all sequences and all animals for a named muscle, it is referred to as an “index of overall variability.” Within each sequence, an IQR of 200% was set as the maximum allowable measure of variation or noise, to be consistent with previous work (Thexton et al. 2007). If the sequence data for a muscle had an IQR above the 200% level, it was excluded from the analysis as being excessively noisy.
When signals were obtained from duplicate electrodes, their similarity was estimated from the area of overlap of the two median profiles, expressed as a percentage of the area of the combined profiles of the two median profiles (Thexton et al. 2007). Thus 100% indicates a perfect overlap of the two signals.
The final analysis of the data used the profiles of median activity, over the 100 time bins, to establish timing differences between the bursts of activity in different muscles. The lag between the EMG activities, of the 16 muscles studied, was determined by cross-correlation analysis of the median activity profiles (Loeb et al. 1987; Thexton et al. 2007).
The data from the intact animals were quantitatively compared with data from decerebrate animals (Thexton et al. 2008). The decerebrate animals swallowed more slowly than the intact animals. However, within each model the swallow events were scaled to 100 time units. Although the events were of different durations, this scaling permitted both within and between model comparisons. The timing of muscle activities were based on the median profiles of the EMG signals, and the lags between the muscle activities were calculated from the CCF of each muscle with hyoglossus as the reference muscle. The time lags of the various muscles relative to the hyoglossus formed three vectors of timing for the swallow: intact animals (this paper), isolated pharyngeal swallows in decerebrates (Thexton et al. 2007) and pharyngeal swallows in decerebrates with rhythmic activity (Thexton et al. 2008). We compared each of the decerebrate vectors to the intact timings using a regression analysis with a 95% CI. This tested the hypothesis that the temporal patterns of EMG activity during swallowing were similar in the intact and in the decerebrate animals.
RESULTS
General suckling behavior
All animals readily suckled from identical feeding bottles delivering the same milk formula through identical teats. Between one and six suck cycles, transporting milk into the oropharynx, preceded each radiographically observed swallow cycle. In the swallow cycles, the oropharyngeal space (containing the valleculae) was emptied but, as previously described (German et al. 1992; Thexton et al. 2004), a wavelike tongue motion also carried an aliquot of milk into the valleculae in the same cycle, In all swallows, a caudally directed epiglottal flexion (followed later by a rapid return movement) was associated with the emptying of the valleculae into the hypopharynx and esophagus.
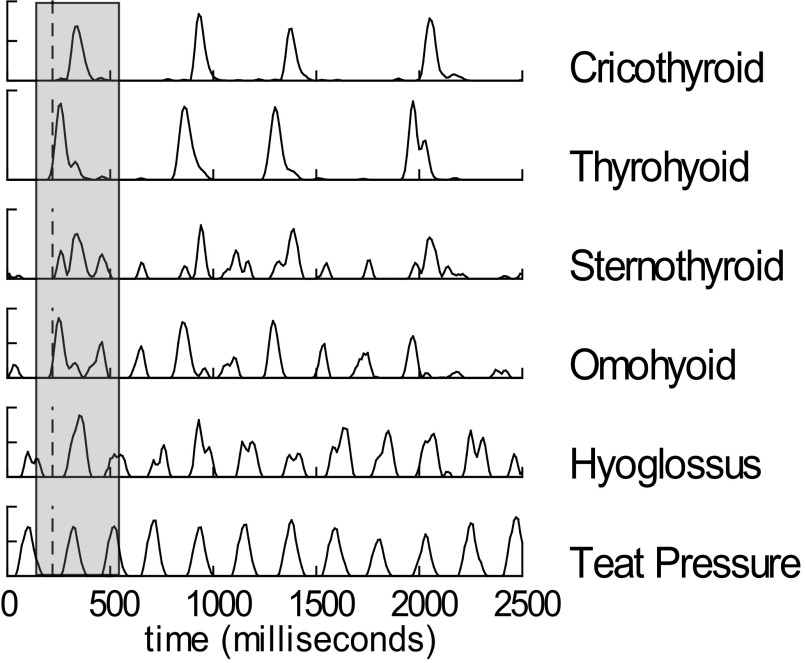
During suckling, rhythmic bursts of EMG activity in several muscles occurred in synchrony with the pressure changes in the teat (Fig. 2). These cycles of increased pressure correlated with radiographically recorded compressions of the teat due to tongue and jaw movements. The radiographically defined swallows (emptying of the valleculae) coincided with a set of discreet high-amplitude bursts of EMG activity, occurring with slightly different timings in several muscles (Fig. 2), particularly the thyrohyoid, cricothyroid, and stylohyoid.
FIG. 2.
Electromyographic (EMG) activity and teat pressures during sucking and suck/swallow cycles in the intact infant pig. Some muscles are activated mainly during swallow cycles, whereas others are activated with every suck cycle. Amplitude is relative to signal maximum over an individual feeding sequence. A total of 12 suck cycles and 4 swallow cycles are shown. Grey bar indicates swallow cycle.
Variability of EMG signal
Over successive swallows, the temporal variability of EMG activity was low in the stylohyoid, thyrohyoid, omohyoid, and cricothyroid as indicated by a restricted separation between the median and upper and lower quartile profiles of EMG activity (Fig. 3) or quantitatively by the IQR%, which for these muscles was <127% (Table 1). In contrast, over successive swallows, the variation in palatopharyngeal and sternohyoid recordings frequently exceeded the IQR% acceptance threshold of 200%. Rejection of such data led to a large reduction in the data pool available for the final analysis of the activity pattern in these two muscles (Table 1).
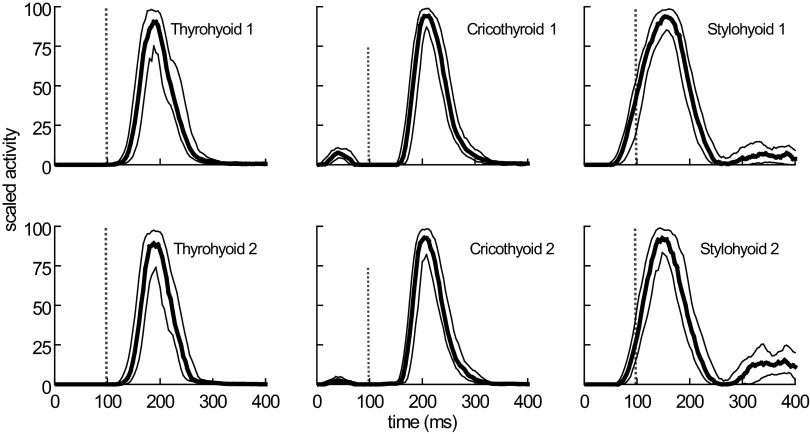
FIG. 3.
EMG activity, during successive swallows, recorded simultaneously by duplicate electrodes in 3 muscles. The EMG signals have been processed and the median, upper quartile, and lower quartile profiles of activity derived. The small separation between the quartile profiles is an indication of the low temporal variability of the signals recorded in these 3 muscles.
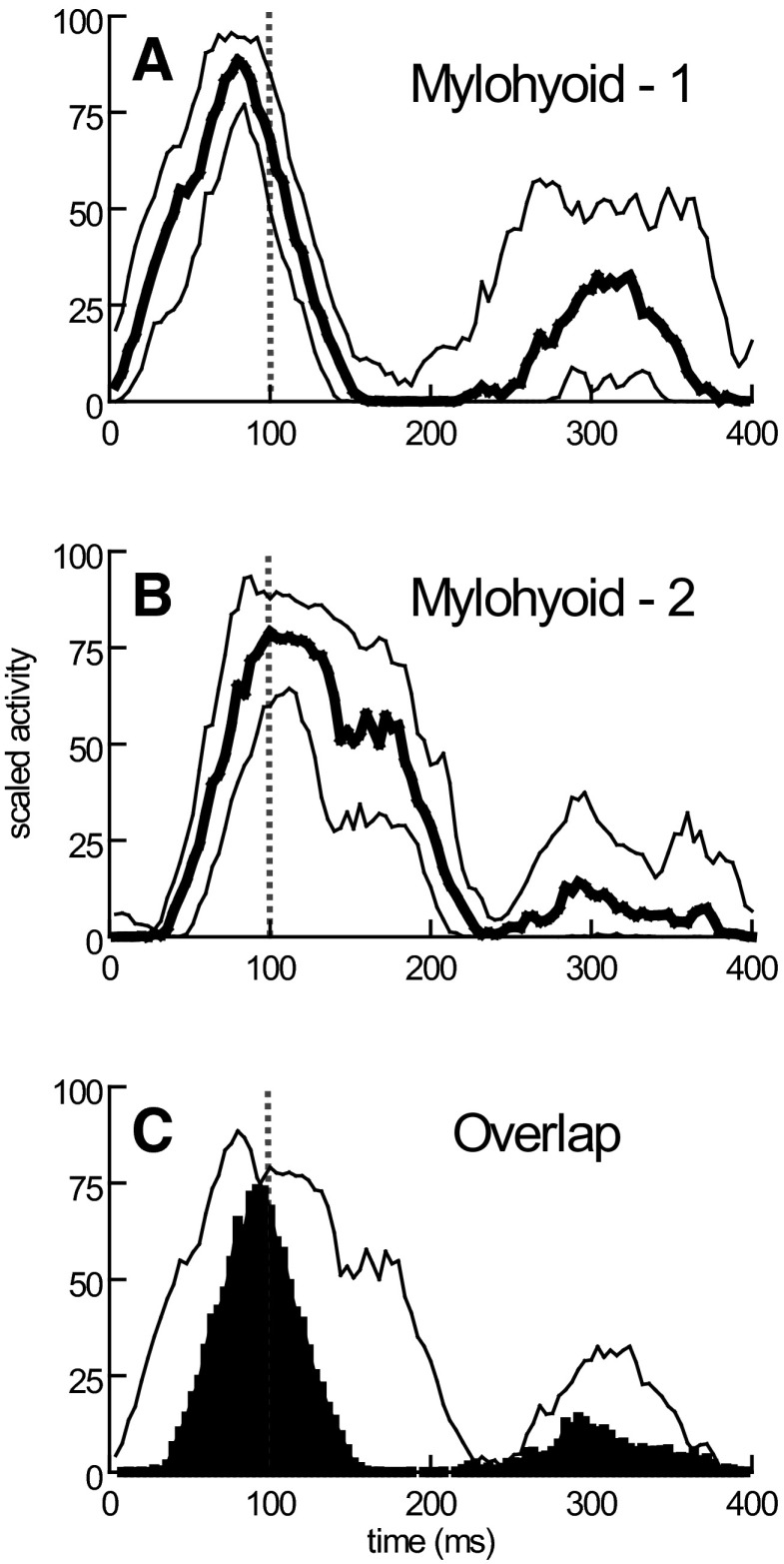
Duplicate electrodes in stylohyoid, thyrohyoid, omohyoid, and cricothyroid recorded relatively similar signals (Fig. 3, Table 1). However, duplicate electrodes in other muscles showed clear differences in the timing of EMG activity at different electrode sites; this was particularly evident in the mylohyoid (Fig. 4; Table 1) and, to a lesser extent, in the digastric.
FIG. 4.
Mylohyoid EMG activity recorded by duplicate EMG patch electrodes located at 2 different sites (A and B) on the muscle. The median and quartile profiles of activity indicate both large measures of temporal variability at each site and differences between the timing of signals detected at the 2 sites. The 2 median profiles from A and B are shown in C with their temporal overlap shown in black. The common (black) area is expressed as a percentage of the total area of the 2 median profiles to obtain a measure of the similarity of timing of EMG signals at 2 sites in the same muscle.
Because of temporal variability in the recorded signals, individual swallow records were of limited use. Instead, for each specified muscle, subsequent analyses used the ensemble median profiles across sites, swallows, and animals.
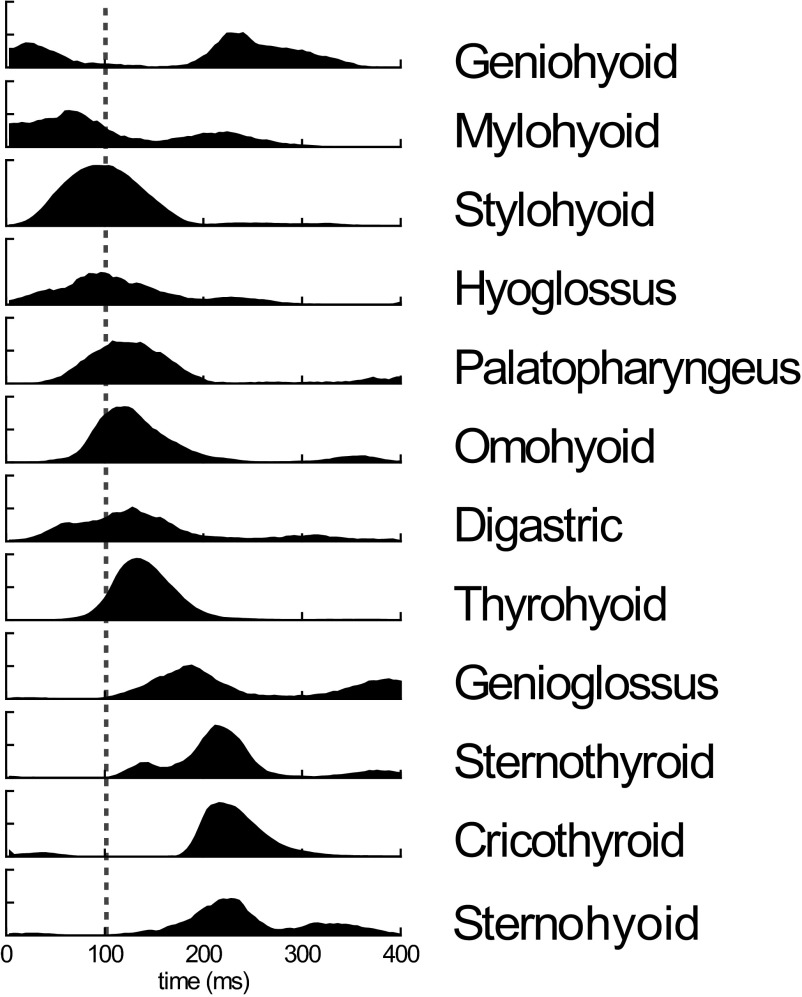
Ensemble median profiles of activity
The ensemble median profiles of the different muscles indicated a sequential, but temporally staggered activation (Fig. 5). Within the 400-ms analysis period, the principal peaks of activity occurred in the following order: geniohyoid, mylohyoid, stylohyoid, hyoglossus, palatopharyngeus, omohyoid, digastric, thyrohyoid, genioglossus, and sternothyroid (Fig. 5). However, synchronous activity in sternothyroid, cricothyroid, and sternohyoid muscles occurred just before the time of the second peak of activity in geniohyoid (Fig. 5).
FIG. 5.
The median profiles of activity during swallowing, across all muscles/animals yielding clean records. ···, the timing of the onset of epiglottal flexion. The successive delays of the profiles of EMG activity were subsequently quantified by cross-correlation, using hyoglossus as the standard.
While the principal bursts of activities of the different muscles were generally staggered in time, some muscles showed two distinct bursts within the period analyzed. In particular, geniohyoid, mylohyoid, and genioglossus, exhibited two well-separated bursts of activity (Fig. 5). The peak activity in geniohyoid occurred ∼140 ms after epiglottal flexion but an earlier (by ∼220 ms) and lower-amplitude peak preceded both the time of epiglottal flexion and the peak of EMG activity in the mylohyoid. The mylohyoid, however, had an extended period of activity. The main genioglossus activity occurred ∼80 ms after epiglottal flexion with a secondary peak ∼200 ms later.
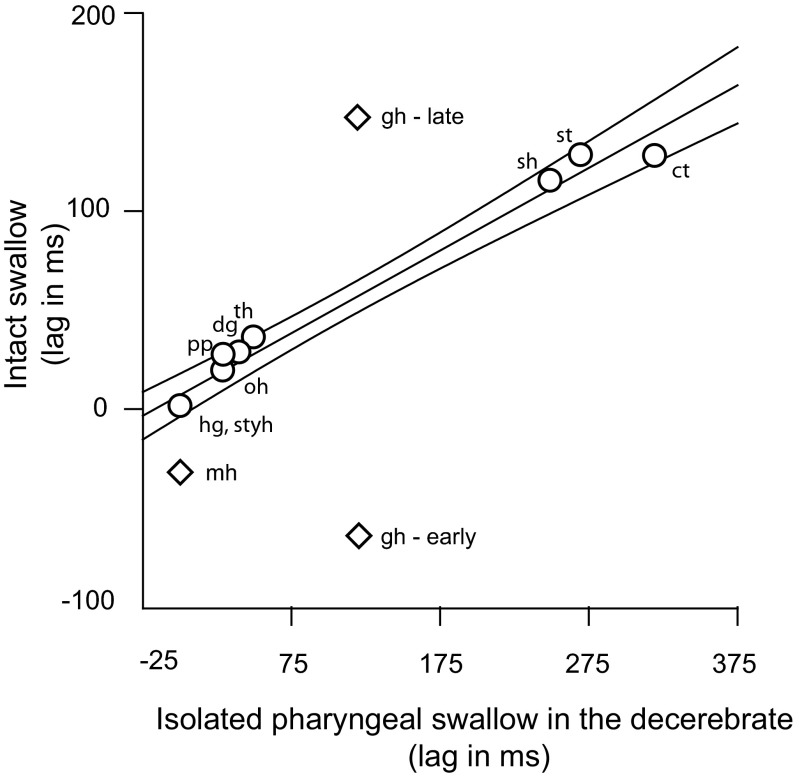
Comparison between conscious and decerebrate data
The relative timings of the EMG activities (Fig. 6) in the intact swallow and in those previously described for the isolated pharyngeal swallow of the decerebrate (Thexton et al. 2007) were largely linear with a narrow 95% confidence interval (CI based on the timings of the muscles represented with circles). The order of firing among the earliest activated muscles (stylohyoid, hyoglossus, palatopharyngeus, omohyoid, digastric, and thyrohyoid) was virtually the same in both animal models, and the order among the later activated group (sternothyroid, sternohyoid and cricothyroid) was fully preserved. The significant difference between the two models was in the timing of geniohyoid and mylohyoid activity. Neither of the two bursts of geniohyoid (diamonds, Fig. 6) fell inside the 95% CI of the line describing the time lags among the other muscles. Mylohyoid activity, which occurred simultaneously with hyoglossus in the decerebrate isolated pharyngeal swallow, occurred earlier in the intact animal.
FIG. 6.
Plot of the relative timings of muscle activities in the swallow of an intact conscious animal and the previously reported activity in the isolated pharyngeal swallow of the decerebrate. ○, muscles used in calculating the regression line and the indicated 95% confidence interval (CI). Only 1 value of geniohyoid timing existed in the isolated, decerebrate swallow, but in the data from the intact swallow, there was an early and late burst. Both bursts, and the mylohyoid burst lie outside the CI for the line.
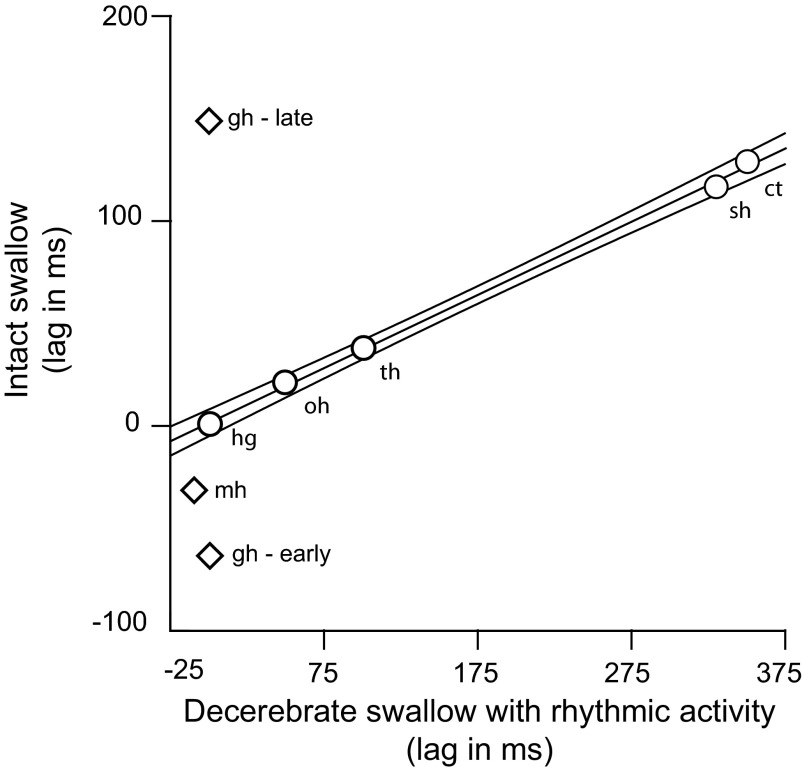
The relationship between the timings of EMG activity, in intact animals and in decerebrate animals with rhythmic oral activity (Fig. 7), was similar the relationship in Fig. 6 between the intact animals and the arrhythmic decerebrates. However, the tight linear relationship in Fig. 7 was based on a smaller set of muscles i.e., hyoglossus, omohyoid, thyrohyoid, sternothyroid, and cricothyroid. Again both the early and late bursts of geniohyoid activity and the mylohyoid activity (diamonds, Fig. 7) fell outside the 95% confidence intervals.
FIG. 7.
Plot of the relative timings of muscle activities in the swallow of an intact conscious animal and the previously reported activity in the swallow of the decerebrate exhibiting rhythmic oral activity. Muscles represented by circles were used in calculating the regression line and the indicated 95% confidence interval. Only 1 value of geniohyoid timing existed in the decerebrate swallow with rhythmic activity, but in the data from the intact swallow, there was an early and late burst. Both bursts and the mylohyoid burst lie outside the CI for the line.
DISCUSSION
Variation in EMG signals
The onset of epiglottal flexion has previously been found to be a reliable time registration point for the pharyngeal swallow (Thexton et al. 2007), the flexion being caused by the sphincteric action of the palatopharyngeus (Crompton et al. 2008). However, the within-muscle variation in the timing of EMG activity differed among the muscles in this study. This is evidenced by the separation between the median and quartile profiles of the burst and suggests differences in the control of differing groups of motor units within the muscle (German et al. 2008).
In the stylohyoid, thyrohyoid, omohyoid, and cricothyroid muscles, there were only small differences between the median and the quartile profiles of EMG activity (Fig. 3). This indicated that the EMG activity was consistently timed relative to epiglottal flexion and that there was minimal variation in the form of the signal. In addition, the large overlap of EMG profiles from multiple electrodes located at different sites within these muscles (Table 1) indicated that the majority of the motor units within these muscles were functioning in a similar way during successive swallows.
In contrast, other muscles had high levels of within-muscle temporal variability of the EMG signals (Table 1) as well as variable overlap of the median profile of signals recorded simultaneously from duplicate electrodes (Fig. 4). In the case of the digastric, the large signal variations between electrode sites suggested that the different motor units might subserve different functions within the swallow cycle. It is known that, in nonswallowing cycles, a strong primary activation of the digastric muscle produces jaw opening but that additional periods of EMG activity can occur at quite separate times during the jaw cycle (Gorniak and Gans 1980; Schwartz et al. 1989; Thexton and McGarrick 1994). The reasons for these additional periods of activity are not fully established.
The EMG signals obtained from palatopharyngeus and sternohyoid were frequently so extremely variable (IQR%>200) that the data for these muscles had to be excluded from analysis of many feeding sequences. Because these signals were recorded simultaneously with the minimally variable signals from muscles such as stylohyoid and thyrohyoid, the high variability of the EMG signals of palatopharyngeus and sternohyoid could not have been caused by errors in the timing of epiglottal flexion. The implication was that the signals recorded in palatopharyngeus and sternohyoid were particularly poorly time locked to epiglottal flexion. The sphincteric action of the palatopharyngeus is the prime factor that initiates and then maintains epiglottal flexion for ∼190 ms during the swallow (Crompton et al. 2008). The variable timing of the firing of motor units in this muscle may consequently be due to the fact that while some motor units must have been synchronized specifically to the onset of epiglottal flexion, others must have been recruited later to maintain the sphincteric action of this muscle.
Sternohyoid differs from palatopharyngeus in that it has multiple functions beyond swallowing. The large temporal variability in sternohyoid activity could be due to these multiple functions that include not only swallowing, sucking, drinking, and licking (Lang et al. 2002; Thexton et al. 1998) but also retching (Lang et al. 2002), vocalization (Hong et al. 1997), cervical flexion (O'Leary et al. 2007), and respiration (Van de Graaff 1984; van Lunteren et al. 1987). The variability in sternohyoid EMG signals may, therefore have been due to recording from a mix of different motor units that were variably recruited for some of these other functions in addition to swallowing.
Swallow EMG profiles in the intact conscious and the decerebrate
The general pattern of activation of muscles in the pharyngeal swallow of the intact animal was that activity occurred firstly in the muscles anterior to the hyoid, then those superior to it, and finally the muscles posterior/inferior to the hyoid. Thus the muscles of the oral floor (geniohyoid, mylohyoid) that moved the hyoid upward and/or forward fired first, followed by muscle activities that moved the hyoid upward/backward and retracted the tongue (stylohyoid, hyoglossus). This was followed by activation of a muscle retracting the hyoid (omohyoid) and another potentially opening the jaw (digastric). The thyrohyoid, the most superior of the infrahyoid muscles, fired next, finally followed by subhyoid and subthyroid muscles i.e., sternothyroid, cricothyroid, and sternohyoid.
This pattern of activation mirrors the pattern for the isolated pharyngeal swallow of the decerebrate. The isolated, reflexive decerebrate swallow is itself a simple temporal sequence of activities and specifically excludes any rhythmic components (Thexton et al. 2007). The strong correlation between these two temporal patterns of pharyngeal swallow (Fig. 6) indicated that much of the sequence from the reflexive swallow was inserted unchanged into the rhythmic activity of the normal animal, supporting our first hypothesis.
One qualitative difference between the EMG activity in the reflexive swallow and in the swallow of the intact animal was the presence of two distinct periods of activation in the geniohyoid and genioglossus muscles. In both these muscles, the interval between the peaks of activity was of the order of 220–260 ms, which approximates to the duration of suckling cycles (200–250 ms) previously described in the intact conscious animal (German et al. 1997; Thexton et al. 1998). Similarly, two peaks of geniohyoid activity are found in the swallows of cerebrally intact primates (Doty et al. 1967).
These two bursts of activity could represent an aspect of the ongoing rhythmic activity into which the pharyngeal swallow was inserted. The signal processing analysis used in the present study retrieved EMG activity that was time locked to epiglottal flexion. If signals reflecting rhythmic activity were retrieved, they also must have been time locked to the epiglottal flexion or vice versa. This means that the insertion of the pharyngeal swallow occurred only at a specific time point within a suckling cycle when it was converted into a suck/swallow cycle.
Earlier videoradiographic studies of suckling also show that the pharyngeal swallows occur at a particular time in oral cycles during the early jaw opening phase (Thexton and Crompton 1998; Thexton et al. 1998). The insertion of the linear sequence of EMG activities specifically into early jaw opening is similar to the phase-sensitive insertion of a flexor reflex into the locomotor cycles of intact and spinalized cats, where the flexor reflex functions as a stumbling corrective reaction (Forssberg 1979). The inserted flexor reflex consists of differing latency excitations or inhibitions of a series of muscles, which is also a characteristic of the pharyngeal swallow (Jean 2001). In both cases, the modified cycle is part of a normal behavioral repertoire.
Much of the pattern of EMG activity, recorded when the swallow is elicited during rhythmic oral activity in a decerebrate is also highly correlated with the EMG pattern of the swallow in the arrhythmic decerebrate (Thexton et al. 2008). The results presented here showed that components of the linear sequence of muscle activations of the isolated pharyngeal swallow were also incorporated into the rhythmic activity of the conscious, neurally intact animal in exactly the same way. The linear EMG sequence of the pharyngeal swallow consequently appears to be broadly constant across all three models studied (Thexton et al. 2007, 2008) and to be capable of incorporation into cycles of rhythmic oral activity whether or not the cerebral hemispheres are present.
Outliers in the swallowing EMG pattern of different models
The timing of mylohyoid and geniohyoid activities differed across the three models (Thexton et al. 2007, 2008) (see also Figs. 6 and 7). In contrast to the relatively constant timings of the core components of the pharyngeal swallow in all three models, the differences in the timings of mylohyoid and geniohyoid activity suggested that those muscles were not subject to the same neural drives as the other muscles. In both decerebrate models, the geniohyoid EMG activity consists of a single burst of activity. However, in the decerebrate model exhibiting rhythmic oral activity, the burst of geniohyoid activity peaks before that in the thyrohyoid while, in the arrhythmic model, the burst peaks after that in the thyrohyoid (Thexton et al. 2007, 2008). In both cases, the timings are derived from cross-correlations and refer only to activity that is time locked to epiglottal flexion.
Current data on hyoid movement in the conscious swallows of a variety of mammals suggest (Thexton et al. 2008) that the two bursts of geniohyoid activity correspond to the forward movement of the hyoid early in the swallow and to jaw opening with additional forward movement of the hyoid later in the swallow. Although the single bursts of geniohyoid activity in decerebrates occur “earlier” or “later” in the swallow, depending on the presence of RTJM, the latency differences between the two are not regularly of sufficient magnitude to correspond directly to the two well separated bursts of geniohyoid activity in the conscious swallow. However, cortical areas generating oro-motor behavior may have the ability to produce facilitation and entrainment of those bursts to the suckling rhythm.
Relative to the other muscles of the pharyngeal swallow, differences in the timing of geniohyoid activity between the two decerebrate models (Thexton et al. 2007, 2008) could only have been due to bulbar mechanisms. However, the timing differences that exist between the rhythmic decerebrate model and the intact animal model, where geniohyoid activity occurred even earlier, are likely to have an additional neurological basis. The facilitatory influences that descend from cerebral structures to act on brain stem mechanisms (Amarasena et al. 2003; Sumi 1969) represent a source of influence on the activities of the muscles of interest. Such cerebral influences are clearly significant because both of the decerebrate models exhibit only one burst of geniohyoid activity, although with different latencies, whereas the intact animal exhibits two bursts.
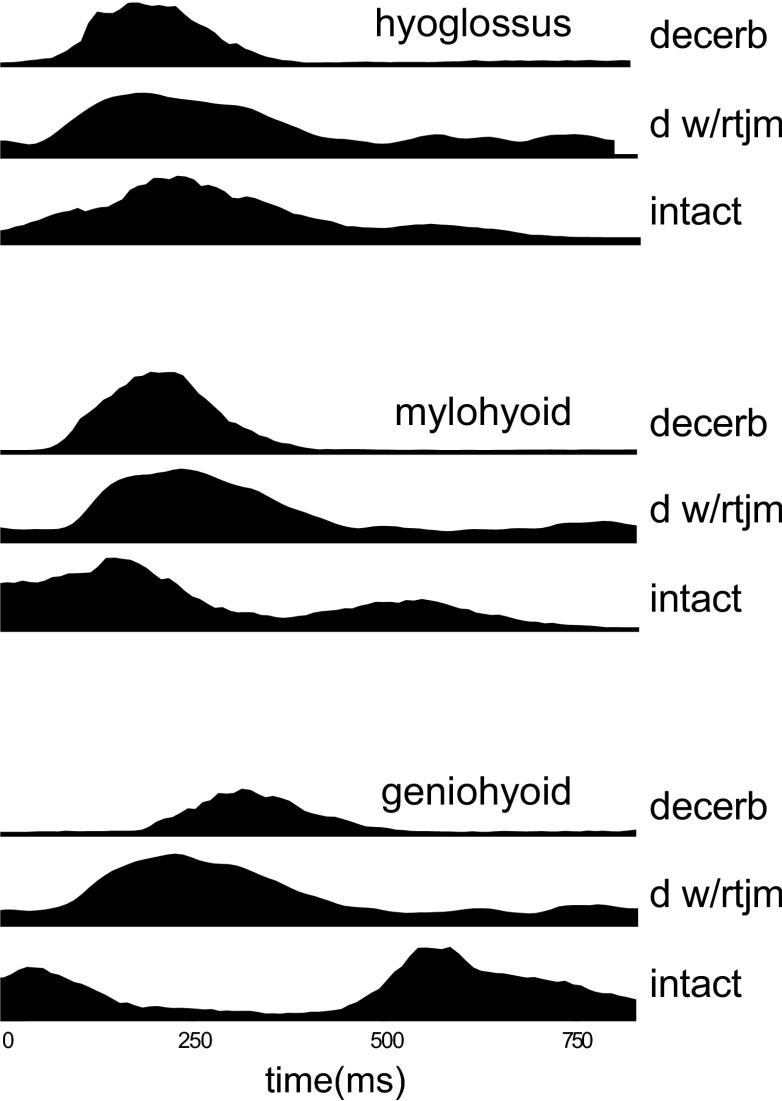
The relationship between the EMG activities in three key muscles, hyoglossus, mylohyoid, and geniohyoid, in the three different animal models (Thexton et al. 2007, 2008; present results) shows the significance of cerebral functioning to their activity (Fig. 8). While the profiles of mylohyoid activity indicate a slightly earlier response occurring in the presence of cerebral hemispheres, the timing and characteristics of the geniohyoid activity were substantially altered by the presence of cerebral function.
FIG. 8.
The relationship between the EMG activities in three key muscles for arrhythmic decerebrates (dec), decerebrates exhibiting rhythmic tongue and jaw movement (dec+rtjm) and conscious intact suckling pigs (intact). The data from each of the 3 sources has been aligned to the profiles of hyoglossus activity using the cross-correlations between the median profiles of hyoglossus EMG activity to mylohyoid and geniohyoid EMG activity. The time scale is the relative 100 units used for each swallow because of the differences in swallow durations among the 3 models. The median profiles of mylohyoid EMG activity suggest a slightly earlier response in the presence of cerebral hemispheres while the median profiles of geniohyoid EMG activity indicate a substantially changed pattern of response.
It is known that neural activity in the lower precentral cerebral cortex generates swallowing (Miller and Bowman 1977; Sumi 1969), possibly by lowering the threshold for the reflex swallow (Ertekin and Aydogdu 2003; Sumi 1969). These “swallowing” sites, however, overlap with cortical sites from which rhythmic oral movements can be elicited by the same stimuli (Iriki et al. 1988; Sumi 1969). When the descending pathways from these cortical “rhythmic oromotor” sites are activated, they similarly reduce the threshold for the generation of rhythmic oral activity by peripheral stimuli (Bremer 1923; Lund and Dellow 1971). The overlap of the two types of cortical site and the close functional association of the two activities suggest that natural activation of such areas of the cortex during suckling might explain the appearance of rhythmicity in the geniohyoid activity of the pharyngeal swallow when that was incorporated into conscious sucking.
Conclusion
The findings of this study are consistent with the incorporation into conscious suck-swallow cycles of most of the EMG activities of the isolated pharyngeal swallow with little change in their relative timings. Most of the components of the pharyngeal swallow recorded in the rhythmically active decerebrate animal also had the same temporal relationship as in the swallow of the conscious intact animal. The exception, to this insertion of a temporally linear series of EMG bursts, was a small change in the timing of mylohyoid activity and a large change in the timing of geniohyoid activity.
Because the linear components of the isolated pharyngeal swallow can be inserted into rhythmic oral activity when the cerebral hemispheres are absent, it appears likely that their insertion into the conscious suck-swallow cycle would also be independent of cerebral activity.
On the other hand, when a pharyngeal swallow was incorporated into the rhythmic activity of an intact animal, the double bursting and the substantially different timing of geniohyoid activity appeared to be directly related to the presence of cerebral hemispheres.
GRANTS
This work was funded by National Institute on Deafness and Other Communication Disorder Grant DC-03604.
Acknowledgments
We thank L. Kelchner, C. Musinsky, and C. Sullivan for assistance with data collection. N. Konow provided valuable insight into the results. C. Musinsky drew Fig. 1.
REFERENCES
- Amarasena et al. 2003.Amarasena J, Ootaki S, Yamamura K, Yamada Y. Effect of cortical masticatory area stimulation on swallowing in anesthetized rabbits. Brain Res 965: 222–238, 2003. [DOI] [PubMed] [Google Scholar]
- Basmajian and Stecko 1962.Basmajian J, Stecko G. A new bipolar electrode for electromyography. J Appl Physiol 17: 849, 1962. [Google Scholar]
- Bremer 1923.Bremer F Physiologie nerveuse de la mastication chez le chat et le lapin. Arch Int Physiol 21: 309–352, 1923. [Google Scholar]
- Buchthal and Schmalbruch 1980.Buchthal F, Schmalbruch H. Motor unit of mammalian muscle. Physiol Rev 60: 901–942, 1980. [DOI] [PubMed] [Google Scholar]
- Crompton et al. 2008.Crompton AW, German RZ, Thexton AJ. Development of the movement of the epiglottis in infant and juvenile pigs. Zoology 111: 339–349, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doty and Bosma 1967.Doty RW, Bosma JF. Effect of medullary lesions on coordination of deglutition. Exp Neurol 17: 91–106, 1967. [DOI] [PubMed] [Google Scholar]
- Ertas et al. 1995.Ertas M, Stalberg E, Falck B. Can the size principle be detected in conventional EMG recordings? Muscle Nerve 18: 43–59, 1995. [DOI] [PubMed] [Google Scholar]
- Ertekin and Aydogdu 2003.Ertekin C, Aydogdu I. Neurophysiology of swallowing. Clin Neurophysiol 114: 2226–2244, 2003. [DOI] [PubMed] [Google Scholar]
- Forssberg 1979.Forssberg H Stumbling corrective reaction: a phase-dependent compensatory reaction during locomotion. J Neurophysiol 42: 936–953, 1979. [DOI] [PubMed] [Google Scholar]
- German et al. 1997.German RZ, Crompton AW, Hertweck DW, Thexton AJ. Determinants of rhythm and rate in suckling. J Exp Zool 278: 1–8, 1997. [DOI] [PubMed] [Google Scholar]
- German et al. 1992.German RZ, Crompton AW, Levitch LC, Thexton AJ. The mechanism of suckling in two species of infant mammal: miniature pigs and long-tailed macaques. J Exp Zool 261: 322–330, 1992. [DOI] [PubMed] [Google Scholar]
- German et al. 2008.German RZ, Crompton AW, Thexton AJ. Variation in EMG activity: a hierarchical approach. Integr Comp Bio 283–293, 2008. [DOI] [PMC free article] [PubMed]
- Gorniak and Gans 1980.Gorniak GC, Gans C. Quantitative assay of electromyograms during mastication in domestic cats (Felis catus). J Morphol 163: 253–281, 1980. [DOI] [PubMed] [Google Scholar]
- Hiiemae and Crompton 1985.Hiiemae KM, Crompton AW. Mastication, food transport and swallowing. In: Functional Vertebrate Morphology, edited by Hildebrand M, Bramble D, Liem K, Wake D. Cambridge, MA: Harvard University Press, 1985, p. 262–290.
- Hong et al. 1997.Hong KH, Ye M, Kim YM, Kevorkian KF, Berke GS. The role of strap muscles in phonation—in vivo canine laryngeal model. J Voice 11: 23–32, 1997. [DOI] [PubMed] [Google Scholar]
- Iriki et al. 1988.Iriki A, Nozaki S, Nakamura Y. Feeding behavior in mammals: corticobulbar projection is reorganized during conversion from sucking to chewing. Brain Res Dev Brain Res 44: 189–196, 1988. [DOI] [PubMed] [Google Scholar]
- Jean 2001.Jean A Brain stem control of swallowing: neuronal network and cellular mechanisms. Physiol Rev 81: 929–969, 2001. [DOI] [PubMed] [Google Scholar]
- Khalili et al. 2005.Khalili K, Razavi S, Karimzadgan D. High resolution measurements using a low resolution system. Measurement Sci Rev 5: 56–59, 2005. [Google Scholar]
- Laitman and Crelin 1980.Laitman JT, Crelin ES. Tantalum markers as an aid in identifying the upper respiratory structures. Lab Anim Sci 30: 245–248, 1980. [PubMed] [Google Scholar]
- Laitman et al. 1977.Laitman JT, Crelin ES, Conlogue GJ. The function of the epiglottis in monkey and man. Yale J Biol Med 50: 43–48, 1977. [PMC free article] [PubMed] [Google Scholar]
- Lang et al. 2002.Lang IM, Dana N, Medda BK, Shaker R. Mechanisms of airway protection during retching, vomiting and swallowing. Am J Physiol Gastrointest Liver Physiol 283: G529–G536, 2002. [DOI] [PubMed] [Google Scholar]
- Larson and Herring 1996.Larson JE, Herring SW. Movement of the epiglottis in mammals. Am J Phys Anthropol 100: 71–82, 1996. [DOI] [PubMed] [Google Scholar]
- Loeb and Gans 1986.Loeb G, Gans, C. Electromyography for Experimentalists. Chicago: University of Chicago Press, 1986, p. 119–120.
- Loeb et al. 1987.Loeb GE, Yee WJ, Pratt CA, Chanaud CM, Richmond FJ. Cross-correlation of EMG reveals widespread synchronization of motor units during some slow movements in intact cats. J Neurosci Methods 21: 239–249, 1987. [DOI] [PubMed] [Google Scholar]
- Lund and Dellow 1971.Lund JP, Dellow PG. The influence of interactive stimuli on rhythmical masticatory movements in rabbits. Arch Oral Biol 16: 215–233, 1971. [DOI] [PubMed] [Google Scholar]
- Miller and Bowman 1977.Miller A, Bowman J. Precentral cortical modulation of mastication and swallowing. J Dent Res 56: 1154, 1977. [DOI] [PubMed] [Google Scholar]
- Miller 2002.Miller AJ Oral and pharyngeal reflexes in the mammalian nervous system: their diverse range in complexity and the pivotal role of the tongue. Crit Rev Oral Biol Med 13: 409–425, 2002. [DOI] [PubMed] [Google Scholar]
- O'Leary et al. 2007.O'Leary S, Falla D, Jull G, Vicenzino B. Muscle specificity in tests of cervical flexor muscle performance. J Electromyogr Kinesiol 17: 35–40, 2007. [DOI] [PubMed] [Google Scholar]
- Sack 1982.Sack WO Pig Anatomy and Atlas. Ithaca, NY: 1982.
- Schwartz et al. 1989.Schwartz G, Enomoto S, Valiquette C, Lund JP. Mastication in the rabbit: a description of movement and muscle activity. J Neurophysiol 62: 273–287, 1989. [DOI] [PubMed] [Google Scholar]
- Spiro et al. 1994.Spiro J, Rendell JK, Gay T. Activation and coordination patterns of the suprahyoid muscles during swallowing. Laryngoscope 104: 1376–1382, 1994. [DOI] [PubMed] [Google Scholar]
- Sumi 1969.Sumi T Some properties of cortically evoked swallowing and chewing in rabbits. Brain Res 15: 107–120, 1969. [DOI] [PubMed] [Google Scholar]
- Thexton 1996.Thexton AJ A randomisation method for discriminating between signal and noise recordings of rhythmic electromyographic activity. J Neurosci Methods 66: 93–98, 1996. [DOI] [PubMed] [Google Scholar]
- Thexton and Crompton 1998.Thexton AJ, Crompton AW. Control of swallowing. In: Frontiers of Oral Biology, edited by LInden R. Basle: Karger, 1998, p. 168–222.
- Thexton et al. 2007.Thexton AJ, Crompton AW, German RZ. Electromyographic activity during the reflex pharyngeal swallow in the pig: Doty and Bosma (1956) revisited. J Appl Physiol 102: 587–600, 2007. [DOI] [PubMed] [Google Scholar]
- Thexton et al. 2008.Thexton AJ, Crompton AW, Owerkowicz T, German RZ. Impact of rhythmic oral activity on the timing of muscle activation in the swallow of the decerebrate pig. J Neurophysiol 101: 1386–1393, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thexton et al. 1998.Thexton AJ, Crompton AW, German RZ. Transition from suckling to drinking at weaning: a kinematic and electromyographic study in miniature pigs. J Exp Zool 280: 327–343, 1998. [DOI] [PubMed] [Google Scholar]
- Thexton et al. 2004.Thexton AJ, Crompton AW, Owerkowicz T, German RZ. Correlation between intraoral pressures and tongue movements in the suckling pig. Arch Oral Biol 49: 567–575, 2004. [DOI] [PubMed] [Google Scholar]
- Thexton and McGarrick 1994.Thexton AJ, McGarrick JD. The electromyographic activities of jaw and hyoid musculature in different. Arch Oral Biol 39: 599–612, 1994. [DOI] [PubMed] [Google Scholar]
- Uchida et al. 1994.Uchida K, Yamada Y, Sato T. The coordination of rhythmical drinking behavior with swallowing in rabbits. Physiol Behav 55: 795–801, 1994. [DOI] [PubMed] [Google Scholar]
- Van de Graaff et al. 1984.Van de Graaff WB, Gottfried, S.B, Mitra, J, van Lunteren, E, Cherniack, N.S. Strohl, K.P. Respiratory function of hyoid muscle and hyoid arch. J Appl Physiol 57: 1984. [DOI] [PubMed]
- van Lunteren et al. 1987.van Lunteren E, Haxhiu MA, Cherniack NS. Relation between upper airway volume and hyoid muscle length. J Appl Physiol 63: 1443–1449, 1987. [DOI] [PubMed] [Google Scholar]