Abstract
Hypophonia is an early symptom in Parkinson's disease (PD) that involves an increase in laryngeal muscle activity, interfering with voice production. Our aim was to use an animal model to better understand the role of different dopamine receptor subtypes in the control of laryngeal neurophysiology. First, we evaluated the combined effects of SCH23390—a D1 receptor antagonist with a D2 receptor antagonist (eticlopride) on laryngeal neurophysiology, and then tested the separate effects of selective receptor antagonists. Thyroarytenoid (TA) and gastrocnemius (GN) muscle activity was measured at rest and while stimulating the internal branch of superior laryngeal nerve to elicit the laryngeal adductor response (LAR) in alpha-chloralose–anesthetized rats. Paired stimuli at different interstimulus intervals between 250 and 5,000 ms measured central conditioning of the LAR. Changes in resting muscle activity, response latency, amplitude, and LAR conditioning after each drug were compared with the saline control. SCH23390 alone increased the resting TA muscle activity (P < 0.05). With the combined SCH23390 + eticlopride or SCH23390 alone, response latency decreased (P < 0.01), amplitude increased (P < 0.01), and the test LAR was reduced at 2,000-ms ISI (P < 0.01). No LAR changes occurred when eticlopride was administered alone at a low dose and only a tendency to suppress responses was found at a high dose. No changes in GN muscle activity occurred in any of the groups. The results suggest that a loss of stimulation of D1 receptors plays a significant role in laryngeal pathophysiology in PD.
INTRODUCTION
In Parkinson's disease (PD), laryngeal motor control abnormalities frequently occur early in the disorder, affecting voice and speech production (Logemann et al. 1978). When laryngeal muscle control was examined prior to treatment early in the disease, increased muscle activity was associated with vocal fold bowing and greater impairment in voice onset and offset control for speech (Gallena et al. 2001). Similar increases in background muscle activity were found in labial muscles that interfered with speech production in untreated patients with PD (Leanderson et al. 1971). In both studies, the abnormally high levels of muscle activity were reduced and speech production improved when the patients were administered a therapeutic dose of levodopa (Gallena et al. 2001; Leanderson et al. 1971).
As the disease progresses, however, levodopa becomes less effective for reducing some symptoms such as speech impairment, abnormal posture, gait, and balance (Rascol et al. 2003). Further, the effects of deep brain stimulation on speech and voice are varied, compared with benefits on limb control (Dromey et al. 2000; Rascol et al. 2003; Rousseaux et al. 2004). These observations led to the suggestion that the disease mechanisms underlying laryngeal and speech symptoms may differ from those mediating the effects on other motor symptoms (Dromey et al. 2000) and that speech symptoms are less benefited by levodopa than are other motor symptoms (Plowman-Prine et al. 2009). On the other hand, a careful examination of different speech attributes found that some speech symptoms relate to motor symptoms whereas others do not in persons with PD (Goberman 2005).
A number of motor control characteristics might provide explanations for the possible differences in response to treatment between limb and speech and voice deficits in PD. Speech is a fine motor control task, more like handwriting than walking, in that it requires precision and skill. However, fine motor control tasks are not necessarily less sensitive to levodopa because handwriting appears to be highly responsive to dopamine enhancement in PD (Visser et al. 2006). As PD progresses, different effects may alter midline brain stem motor control affecting laryngeal control in PD relative to other brain regions. Speech may show limited benefit from dopamine enhancement similar to other midline functions such as gait, posture, and postural stability (Visser et al. 2006). Midline brain stem motor control regions may be affected earlier by the disease process than other brain regions in PD. Some have proposed a caudal to rostral spread of the disease, moving from involvement of the dorsal motor nucleus of the vagus in the brain stem upward through the medulla, the pontine tegmentum in the midbrain, and later reaching the cerebral cortex (Braak et al. 2003). Others have not found support for this in that the substantia nigra was involved in 100% of cases and only half of the cases fit the pattern of caudal to rostral spread (Kalaitzakis et al. 2008).
The effect of dopamine deficiency on laryngeal neurophysiology is of importance for attempting to understand the mechanisms involved in the voice abnormalities in PD and whether these mechanisms are different from those mediating limb motor control abnormalities. Few animal studies have addressed laryngeal pathophysiology in PD partly because the mammalian phonatory system differs considerably from the human speech system (Jurgens 2002). Mammals express innately programmed vocalizations during isolation, pain, and reproductive/sexual functions. These vocalizations are controlled by central pattern generators in the periaqueductal gray and the parvocellular pontine reticular formation with inputs to laryngeal and respiratory motoneuron pools in the brain stem (Jurgens 2009). Speech in humans, on the other hand, is cortically driven and inputs to the motoneurons in the brain stem descend via rapidly conducting corticobulbar pathways (Jurgens 2002, 2009). Because of the neural control differences between human and animal vocalizations, we chose to assess the effects of dopamine system dysfunction at the final common pathway for both animal vocalizations and voice and speech in humans: that is, the brain stem systems involved in laryngeal muscle control.
Laryngeal sensory stimulation can elicit laryngeal muscle responses, referred to as the laryngeal adductor response (LAR), via a well-defined pathway in the brain stem in the cat (Ambalavanar et al. 2004). Further, LAR response conditioning using paired sensory stimuli can quantify central excitation and inhibition in the brain stem as has been done in humans (Ludlow et al. 1995). In the present study, we used LAR conditioning in the rat to investigate changes in central excitation and inhibition in the LAR brain stem system with dopamine receptor antagonism. Because the LAR is very similar to the blink reflex (Bhabu et al. 2003), it was predicted that loss of dopamine receptor stimulation might have effects on the LAR similar to those seen with the blink reflex. Studies of blink reflex conditioning in persons with PD have shown a marked loss of inhibition of blink reflex conditioning in humans with PD (Lozza et al. 1997). Based on the previous findings on the blink reflex in PD, we hypothesized that with dopamine depletion the laryngeal adductor response would be hyperexcitable during response conditioning.
Because levodopa is nonselective in its action, previous studies of the neurophysiological effects of levodopa on laryngeal and labial muscles in persons with early PD (Gallena et al. 2001; Leanderson et al. 1971) did not address the different dopaminergic receptor subtypes. There are two classes of dopamine receptors: D1-like receptors and D2-like receptors (Memo 1990; Seeman et al. 2000). If selective receptor blockers have specific actions within the basal ganglia that alter laryngeal neurophysiology differently, more targeted approaches for addressing patients' deficits in this system might be indicated. The processes initiated by stimulation of D1 and D2 receptor subtypes may interact in a synergistic way to alter basal ganglia output (Pollack 2004; Walters et al. 1987; Weick and Walters 1987) and may modulate laryngeal neurophysiology differently.
Decreased blink rate is exhibited in PD (Agostino et al. 2008) and both D1 and D2 dopamine receptor agonists increase blink rate in primates (Elsworth et al. 1991), although these two receptor subtypes may interact. For example, D2 receptor activation could inhibit D1-mediated increases in eye blinking (Jutkiewicz and Bergman 2004). Spontaneous blinking, however, differs from blink reflex excitability. Although the blink rate is reduced in PD patients (Agostino et al. 2008), these patients have hyperexcitable blink reflex conditioning (Lozza et al. 1997). We hypothesized that D1 and D2 dopamine receptor blocking may have different effects on laryngeal muscle activity and LAR conditioning. Such information may increase our understanding of laryngeal neurophysiological deficits in PD and the clinical effects of levodopa on voice and speech function.
Based on the abnormalities in laryngeal muscle control that occur in untreated PD patients, we hypothesized that some selective dopamine receptor antagonists would have an excitatory effect on resting laryngeal muscle activity that would differ from the effects on limb muscles and that different subtypes of dopamine receptor antagonists would have different effects on laryngeal muscle activity. Further, based on previous findings of blink reflex conditioning abnormalities in PD, we hypothesized that R2 initial conditioning responses would show enhanced excitability of the LAR to sensory input with the administration of the dopamine receptor antagonists and that R2 test responses would show reduced suppression of the LAR during conditioning following the administration of selective dopamine receptor antagonists.
To examine this in the present study, we measured laryngeal resting muscle activity and the LAR evoked by stimulation of the internal branch of superior laryngeal nerve (iSLN) in the rat. To study the effects of dopamine depletion on laryngeal muscle activity and sensorimotor modulation, we used a combination of SCH23390, a selective D1 receptor antagonist, and eticlopride, a selective D2 receptor antagonist. Furthermore, we administered SCH23390 and eticlopride separately to examine the effects of modulating each of these selective dopamine receptor subtypes independently on laryngeal neurophysiology. Here we used selective dopamine receptor antagonists to study the effects of dopamine depletion on laryngeal neurophysiology. Others have proposed using unilateral injection of 6-hydroxydopamine (6-OHDA) or haloperidol to study the effects of dopaminergic depletion on ultrasonic vocalizations in the rat (Ciucci et al. 2007). We chose not to use this model because the laryngeal system is a bilateral midline system and is less likely to show deficits following a unilateral 6-OHDA injection than limb control. For example, vocalization calls were not reduced in number or duration despite severe deficits on contralateral limb movement following unilateral 6-OHDA injections in rats (Ciucci et al. 2009). Further, although levodopa and haloperidol are used clinically to augment or depress the dopamine systems in PD and schizophrenia, respectively, neither has selective actions and could affect other neurotransmitters besides dopamine. Because changes in limb muscle activity have been induced by dopamine antagonists in rats (Hemsley and Crocker 2001; Hemsley et al. 2002), we measured activity in the gastrocnemius muscle—a limb muscle—to simultaneously compare the effects of dopamine on both limb and laryngeal muscles.
METHODS
Animals and experimental design
Thirty-seven adult male Sprague–Dawley rats weighing between 250 and 400 g (Harlan, Indianapolis, IN) were maintained on a 12-h light/dark cycle and given unrestricted access to food and water. All procedures were carried out in accordance with National Institute of Health guidelines on the care and use of laboratory animals and the study protocol was approved by the National Institute of Neurological Disorders and Stroke Animal Care and Use Committee. All the drugs were dissolved in 0.25-ml volumes of normal saline for intravenous (iv) injection in all groups. The rats were divided into three groups: group 1 (0.5 mg/kg SCH23390 + 0.5 mg/kg eticlopride, n = 11), group 2 (0.5 mg/kg SCH23390, n = 15), and group 3 (0.5 mg/kg eticlopride, n = 11). Ten animals had recordings made from both the right and left thyroarytenoid (TA) muscles in each group; however, because the gastrocnemius (GN) was not available in all of these animals, additional animals were added to each group to gather comparable data on the GN muscle to have similar group sizes for the laryngeal and limb muscles. For the SCH23390 + eticlopride, 7 animals had both the TA and GN muscles recorded, 3 had just TA recordings, and an additional animal had only the GN recorded, bringing the total with TA recordings to 10 and the total with GN recordings to 8 animals. For the SCH23390 alone, 5 animals had both TA and GN recordings, 5 had only TA recordings, and an additional 5 animals had only the GN recorded, bringing the total number with TA recordings to 10 and the total with GN to 10 animals. For eticlopride alone, 8 has TA and GN recordings, 2 had only TA recordings, and an additional animal had GN alone, bringing the total with TA recordings to 10 and the total number with GN recordings to 9 animals.
Surgery and electrical stimulation
Each animal was anesthetized with a 3–4% mixture of isoflurane (Sigma, St. Louis, MO) and 100% oxygen, in an induction chamber, then moved to the surgical table and a 16-gauge endotracheal cannula was inserted via the oral cavity for maintenance on 3% isoflurane. After percutaneous exposure of the trachea, a 14-gauge curved tracheostomy cannula was inserted at the fifth tracheal ring and connected to the anesthesia machine (MDS Matrx, Orchard Park, NY) with a pressure-controlled ventilator (Kent Scientific, Torrington, CT). The oral cannula was then removed and the animal was maintained on the ventilator with 3% isoflurane between 40 and 60 breaths/min, adjusted by weight, with a 2-ml tidal volume. An ocular lubricant was placed on the cornea and lidocaine gel into the ear canals before ear bars were placed and the head was fixed onto a stereotactic frame (Stoelting, Indianapolis, IN) in the supine position. Cardiac and respiratory rates were monitored continuously by EKG and endotracheal CO2. Heart rate, respiratory rate, oxygen saturation, and CO2 levels were recorded every 15 min. The rectal temperature was maintained at 37 ± 0.5°C with a circulating water heating blanket (Gaymar Industries, Orchard Park, NY) to prevent hypothermia. A tail vein iv provided saline at a rate of 3 ml/kg/h. Because the laryngeal response is suppressed by isoflurane, following surgery isoflurane was gradually reduced while a 15 mg/ml alpha-chloralose solution was administered by iv drip between 18 and 36 μl/min, at 340 to 360 mg/kg for 4 h.
Superior laryngeal nerve stimulation and muscle recordings
The right/left superior laryngeal nerve (SLN) was exposed and positioned over a hooked bipolar platinum stimulating electrode (FHC, Bowdoinham, ME), with 1.75-mm-coated diameter and 0.8-cm spacing between the two poles, and connected to a stimulus isolator (A365, WPI). The nerve stimulation sites were immersed in warm mineral oil.
For electromyographic (EMG) recordings, two Teflon-coated stainless steel wires (0.011-mm-coated diameter) with 1-mm bared tips contained in a 27-gauge needle were inserted through the cricothyroid space into the TA muscle on each side. The same type of electrode was inserted into the GN muscle of each hind leg. All four muscles, the left and right TA and GN muscles, were recorded throughout the study on a computer (AD Instruments).
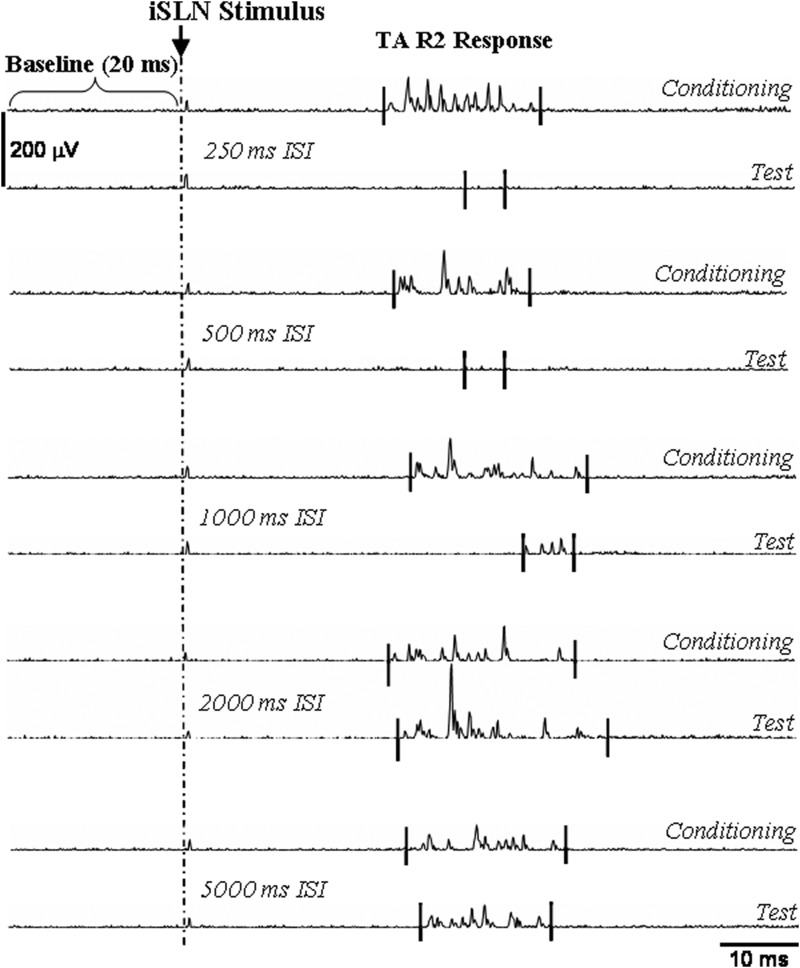
After sectioning the external branch of the SLN to prevent cricothyroid muscle contraction from interfering with TA muscle recordings animals were stabilized for 20 min. Electrical stimulation of the iSLN on one side began at 10 μA with a pulse width of 20 ms and was increased until the threshold level for eliciting a reliable laryngeal adductor R2 response was determined (Fig. 1). The LAR responses in the rat consisted of an ipsilateral short-latency R1 (7.35 ± 0.26 ms) and a bilateral long-latency R2 response (22.20 ± 2.08 ms) of the TA muscles. In this study, R1 responses occurred in only three animals and were irregular. Therefore only the consistent R2 responses were recorded and analyzed. The stimulus intensity was then set at threefold the stimulation threshold for eliciting R2 responses and the same level was used throughout the experiment. Between five and six pairs of conditioning stimuli each followed by a test stimulus were administered at each interstimulus interval (ISI) of 250, 500, 1,000, 2,000, and 5,000 ms. The R2 response to the first stimulus (the conditioning stimulus in a pair) was the conditioning response and the response to the second stimulus (the test stimulus) was the test response (Fig. 1). A pilot study confirmed that complete suppression of test R2 responses occurred at intervals of ≤500 ms, whereas test response amplitudes increased at 2,000-ms ISI. At least 40 s occurred between stimulus pairs to avoid habituation. The stimulation rate was programmed using Master-8 (AWPI, Jerusalem, Israel). The EMG signals were amplified (Grass Telefactor, Model RPS312RM, Grass Technologies, West Warwick, RI), filtered between 30 Hz and 10 kHz, monitored on-line with a digital oscilloscope (Tektronix TDS 420, Beaverton, OR), and digitized at 20K Hz with Chart 5 for Windows (AD Instruments) for off-line analysis. Means of the conditioning and test responses were computed for each set at each ISI using Matlab-customized software before statistical analysis.
FIG. 1.
Conditioning and test responses recorded in the thyroarytenoid (TA) muscle. Examples are shown of conditioning and test R2 responses at each interstimulus interval (250-, 500-, 1,000-, 2,000-, 5,000-ms ISI). Test responses were suppressed at 250-, 500-, and 1,000-ms ISIs and increased at 2,000-ms ISI relative to conditioning responses. The baseline (the resting muscle activity before the internal branch of superior laryngeal nerve [iSLN] stimulus) was measured for 20 ms before stimulation onset.
Drug administration
The first part of the study measured changes with the administration of saline in resting muscle activity and the response latency and amplitude to the conditioning stimuli and the response characteristics after the test stimuli as the control condition. Initially, the laryngeal responses were recorded in each animal before saline infusion and included five to six trials at each ISI of 250, 500, 1,000, 2,000, and 5,000 ms. Animals were then maintained under quiet conditions for ≤30 min. After a 0.25-ml bolus saline solution was administered by iv, another set of laryngeal responses was recorded beginning 5 min later (postsaline). Following the second stimulation set, the animals were maintained under quiet conditions for another 30 min and then a third set of laryngeal responses (predrug) was recorded at 30 min post saline infusion. After 30 min of further stabilization, a 0.25-ml volume bolus containing either the mixture of SCH23390 (0.5 mg/kg) + eticlopride (0.5 mg/kg) (Sigma), SCH23390 (0.5 mg/kg), or eticlopride (0.5 mg/kg) was administered by iv and a fourth set of laryngeal responses was recorded 5 min later (5 min postdrug). Finally, 1 h following drug administration, a fifth set of laryngeal responses was recorded (1 h postdrug).
At the end of each experiment, the recording wires were cut from the outside (leaving the tips of the wires inside the laryngeal muscles), animals were administered an overdose of propofol (10 mg/ml, iv), and the larynx was dissected to confirm the position of the recording wires in the TA muscles. Only data collected from animals with confirmed electrode placement in the TA muscles were analyzed.
Data analysis
EMG signals were digitized at 20K samples/s with antialiasing filtering at 10K Hz, stored on a computer, rectified, and visually analyzed off-line using an interactive software program written in Matlab R2006a, allowing the operator to mark the onset and offset of each laryngeal R2 response. After marking, the program computed the response latency, duration, and the integrated area under the curve from the rectified TA EMG response. For each response, the mean baseline activity during a 20-ms interval before the first stimulus onset was computed and multiplied by the duration of the following response before being subtracted from the total area under the curve of the response to correct for any changes that may have occurred due to changes in muscle activity. The corrected integrated amplitude was computed as follows
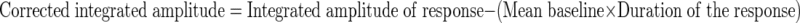 |
All responses were reviewed and confirmed by two members of the research team blinded to animal and conditions. In instances where no response was observed, an interval of 5 ms was marked as the response. When the baseline activity × response duration was then subtracted, the response area under the curve was close to zero. For each condition, the following measures were made: the mean level of baseline activity, the latency of a response to the stimulus, and the total area under the curve of a response in microvolt-milliseconds after subtracting the corresponding baseline activity. The conditioning R2 responses for an animal were averaged over 25–30 trials (5–6 per interval for each of the ISIs) to compute mean ± SD of R2 responses on the ipsilateral and contralateral sides to iSLN stimulation. The responses from both sides were averaged for analysis after finding no differences between the R2 responses in the ipsilateral and the contralateral sides.
To compare the percentage change of prepost saline with the percentage change of pre to post drug for the resting muscle activity, response latency, and response amplitude of the conditioning R2 responses, the percentage change between pre and post measures after saline and after drug were computed as
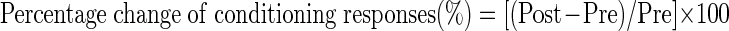 |
To measure the percentage change in a test response from a conditioning response at each ISI, we computed
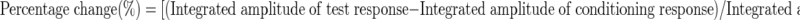 |
The mean percentage change (computed over five to six trials) between pre and post saline and between pre and post drug was then computed for each ISI for each animal in each condition.
Statistical analyses
One-way repeated-measures ANOVAs were used to compare different conditions within the same group of animals including:
the change in resting activity in the TA muscle post saline with the change post SCH23390 + eticlopride and similarly the change post SCH23390 with post saline and the change post eticlopride alone with post saline (P < 0.05).
the change in LAR conditioning response latency and amplitude post saline with the change post SCH23390 + eticlopride and similarly the change post SCH23390 with post saline and the change post eticlopride alone with post saline (using a Bonferroni-corrected P ≤ 0.025).
the test response percentage change from the conditioning LAR response amplitude before and after drug while testing for interaction effects with ISIs for SCH23390 + eticlopride, SCH23390 alone, and eticlopride alone. If before versus after drug effects or interactions with ISI were significant (P ≤ 0.05), post hoc Wilcoxon signed-rank tests were used to compare before versus after drug effects at each ISI.
Kruskal–Wallis comparisons were conducted between changes in resting activity in the TA with the change in resting activity of the GN post saline with post SCH23390 + eticlopride and, similarly, the change in the two muscles post saline were compared with post SCH23390 alone and with post eticlopride alone (P ≤ 0.05).
One-way ANOVAs between animal groups were used to compare the effects of the two dopamine receptor antagonists:
on changes in resting muscle activity post saline with change post each antagonist.
on changes in latency and amplitude of LAR responses to conditioning stimuli post saline with change post each antagonist (P < 0.025).
One-way ANOVAs between groups were used to determine whether eticlopride modified the effect of SCH23390 on muscle activity, response latency, and integrated conditioning response amplitude between SCH23390 + eticlopride and SCH23390 alone using a Bonferroni-corrected P value of 0.0167 for statistical significance.
Finally, to examine whether any changes in test responses with antagonists were secondary to increases in the amplitude of the conditioning response with antagonists, we computed r values between the percentage increase in amplitude in the conditioning response post antagonist in relation to the change in percentage conditioning on the test response at ISIs, in which there were significant changes in conditioning effects with the antagonist (P ≤ 0.05).
RESULTS
Effects of dopamine receptor antagonists on resting TA muscle activity
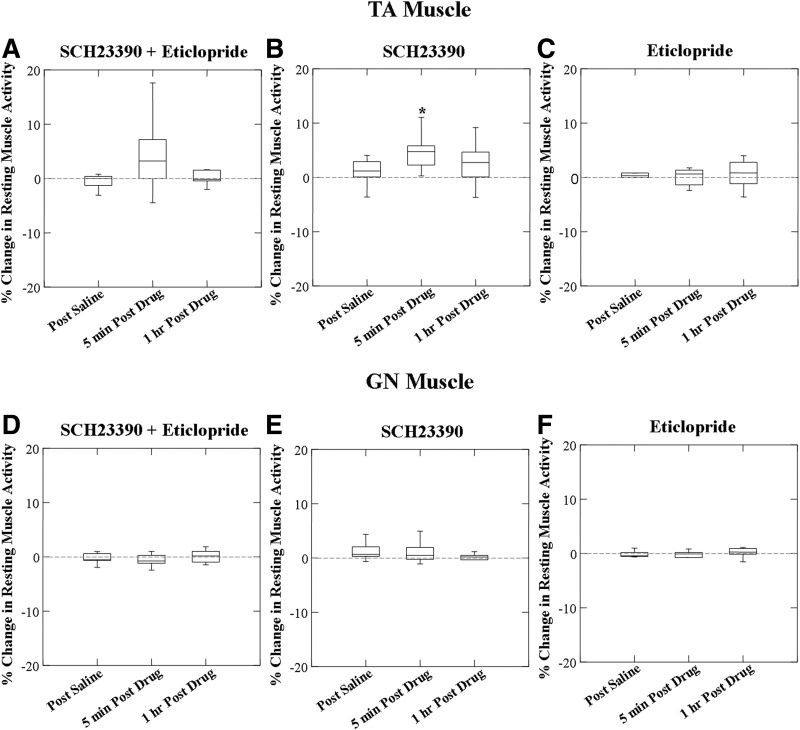
No significant change in the resting TA muscle activity occurred 5 min (F = 2.222; P = 0.174) and 1 h (F = 0.145; P = 0.714) post SCH23390 + eticlopride injection (Fig. 2A). When the effects of SCH23390 alone were compared with saline 5 min post drug, there was a significant increase in muscle activity (F = 5.986; P = 0.037), which did not persist at 1 h post drug (F = 1.725; P = 0.225) (Fig. 2B). No effects on resting TA muscle activity were found in eticlopride alone (F = 0.089; P = 0.773; Fig. 2C). Further, no differences were found between the effects of SCH23390 + eticlopride and SCH23390 alone on resting TA muscle activity (F = 0.132; P = 0.721).
FIG. 2.
Box plots of percentage change from resting activity of TA and gastrocnemius (GN) muscles in response to SCH23390 + eticlopride, SCH23390, and eticlopride. No statistically significant increase of resting TA muscle activity was found in SCH23390 + eticlopride (A, n = 10) or eticlopride (C, n = 10) alone. Resting TA muscle activity increased 5 min postadministration of SCH23390 alone (B) (*P = 0.037, n = 10). Neither SCH23390 + eticlopride (D, n = 8), SCH23390 (E, n = 10), nor eticlopride (F, n = 9) had effects on the resting GN muscle activity.
Comparison of the effects of dopamine receptor antagonists on resting TA and GN muscle activity
No significant changes in GN resting muscle activity were found following SCH23390 + eticlopride (F = 0.117; n = 8, P = 0.743, Fig. 2D). No significant changes in GN muscle activity were found following either SCH23390 (F = 0.535; n = 10, P = 0.483, Fig. 2E) or eticlopride alone (F = 0.110; n = 9, P = 0.750, Fig. 2F).
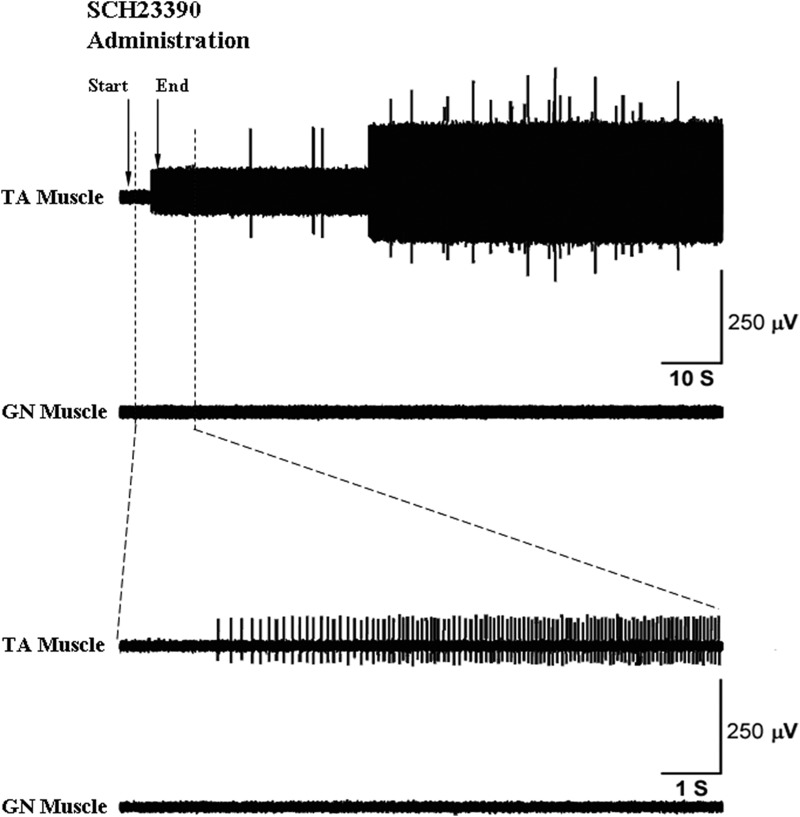
Comparisons between the effects of drug on TA versus GN were between groups because some of the animals were different in the muscles recorded. Kruskal–Wallis group comparisons were used because of group differences in the SDs. For SCH23390 + eticlopride there were no differences between effects in muscle activity between the TA and GN (Mann–Whitney U = 24; P = 0.155). For SCH23390 alone no significant difference was found between muscles (Mann–Whitney U = 21; P = 0.028) and for eticlopride alone there was no difference in change in activity between the TA and GN muscles (Mann–Whitney U = 33; P = 0.327). A typical recording of increased resting muscle activity in TA (increased motor unit firing) but not in GN muscles following SCH23390 (0.5 mg/kg, iv) infusion alone is shown in Fig. 3.
FIG. 3.
Effects of SCH23390 on the resting TA and GN muscle activity. A representative recording of electromyographic (EMG) activity shows increased resting TA muscle activity (top trace) but no change in resting GN muscle activity (2nd trace) post SCH23390 administration. In the top trace, the arrows mark the time points for the start and end of the intravenous injection, which took <5 s. Resting TA muscle activity increased before the end of the injection. The bottom 2 traces, with an expanded view of the first 10 s following injection, show increased motor unit firing in the TA post SCH23390 administration, with no resting activity change in the GN muscle.
Comparison of the effects of D1 and D2 antagonists on resting muscle activity
A significant difference was found between animals receiving SCH23900 and those receiving eticlopride (F = 4.545; P = 0.047), with the mean percentage difference following SCH23390 = 5.242 ± 4.704 (mean ± SD) and the mean percentage difference following eticlopride = 0.968 ± 4.249.
Effects of dopamine receptor antagonists on the conditioning laryngeal R2 responses
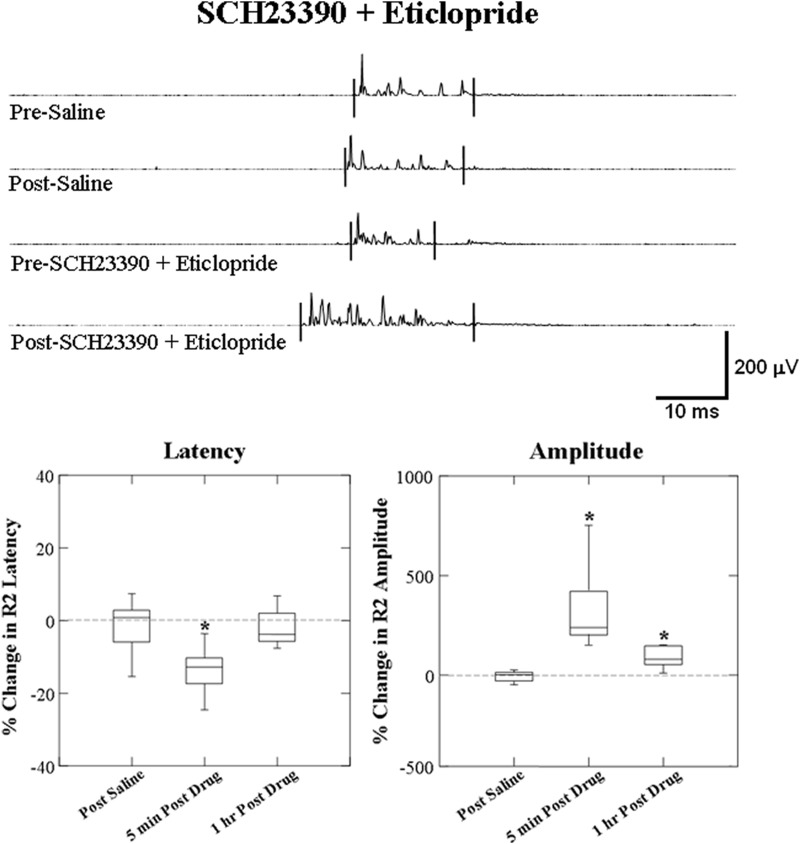
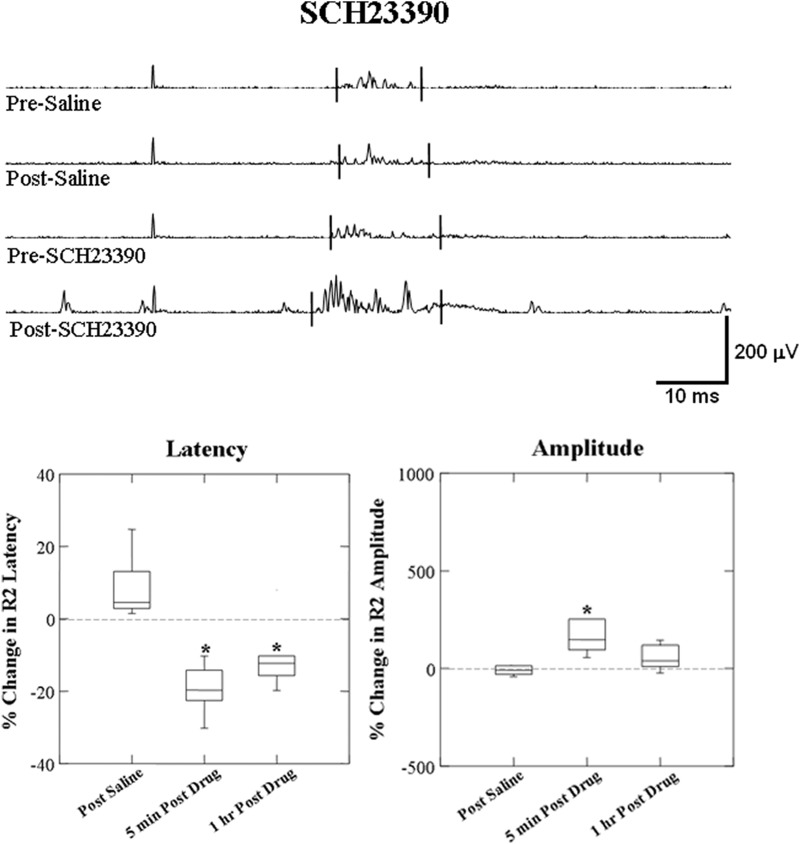
For SCH23390 + eticlopride administration, conditioning R2 responses were reduced in latency (F = 11.167; P = 0.01) from 23.18 ± 1.41 to 19.64 ± 1.00 ms and increased in amplitude (F = 66.362; P < 0.0001) at 5 min post. At 1 h post SCH23390 + eticlopride administration, the amplitude remained increased (F = 15.708; P = 0.004) but the response latency returned to predrug level at 22.57 ± 1.69 ms (F = 0.249; P = 0.631, Fig. 4).
FIG. 4.
Box plots of percentage change from effects of SCH23390 + eticlopride on the latency and amplitude of the laryngeal conditioning R2 response. The top traces show representative EMG recording of conditioning R2 responses changes induced by SCH23390 + eticlopride compared with the saline. Latency of conditioning R2 response decreased and amplitude increased post SCH23390 + eticlopride administration. Percentage changes of the response latency decreased and amplitude increased at 5 min after administration and the latency returned to predrug level and amplitude remained increasing 1 h after SCH23390 + eticlopride administration compared with the saline control (*P ≤ 0.01, n = 10).
For SCH23390 administration alone, a similar trend to SCH23390 + eticlopride for a reduced latency from 24.23 ± 1.17 to 19.33 ± 0.93 ms (F = 62.792; P < 0.001) and increased amplitude (F = 12.187; P = 0.007, Fig. 5) 5 min post administration. At 1 h post SCH23390 administration, the latency continued to be decreased around 21.34 ± 1.20 ms (F = 20.031; P = 0.002), whereas the amplitude still showed a trend toward increasing (F = 5.529; P = 0.047).
FIG. 5.
Box plots of percentage change from effects of SCH23390 alone on the latency and amplitude of laryngeal conditioning R2 responses. The top traces show representative EMG recording of conditioning R2 responses changes induced by SCH23390 alone compared with the saline. Latency of conditioning R2 response decreased and amplitude increased after SCH23390 administration. Percentage changes of the response latency decreased at 5 min post SCH23390 administration and the latency maintained decreasing 1 h post SCH23390 compared with the saline control (*P ≤ 0.01, n = 10).
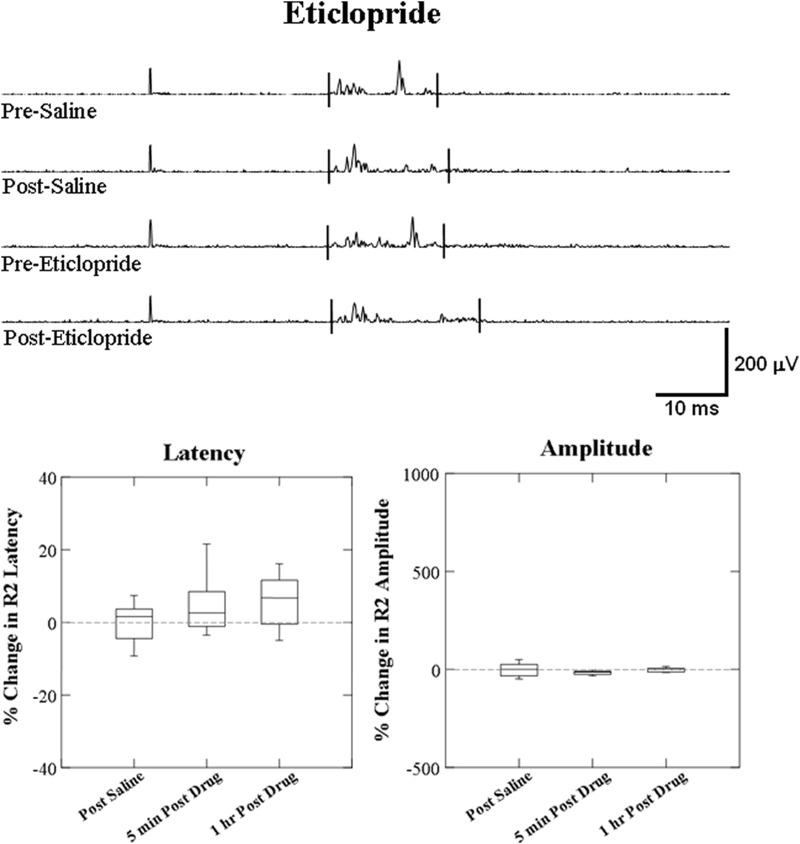
No changes were found at 5 min or 1 h post eticlopride administration in both the latency (F = 2.643, P = 0.138; F = 2.744, P = 0.132) and the amplitude (F = 2.388, P = 0.157; F = 0.234, P = 0.640) (Fig. 6).
FIG. 6.
Box plots of percentage change from effects of eticlopride (0.5 mg/kg) alone on the latency and amplitude of the laryngeal conditioning R2 response. The top traces show representative EMG recording of conditioning R2 responses changes induced by eticlopride alone compared with the saline. No effects of eticlopride (0.5 mg/kg) were found on the latency and amplitude of conditioning R2 response at 5 min and 1 h after eticlopride administration compared with the saline control (n = 10).
No differences were found between the combined SCH23390 + eticlopride compared with SCH23390 alone on latency (F = 3.004, P = 0.100) and integrated amplitude (F = 0.846, P = 0.370) of the conditioning R2 responses 5 min after administration.
Effects of dopamine antagonists on the conditioning changes in laryngeal R2 responses
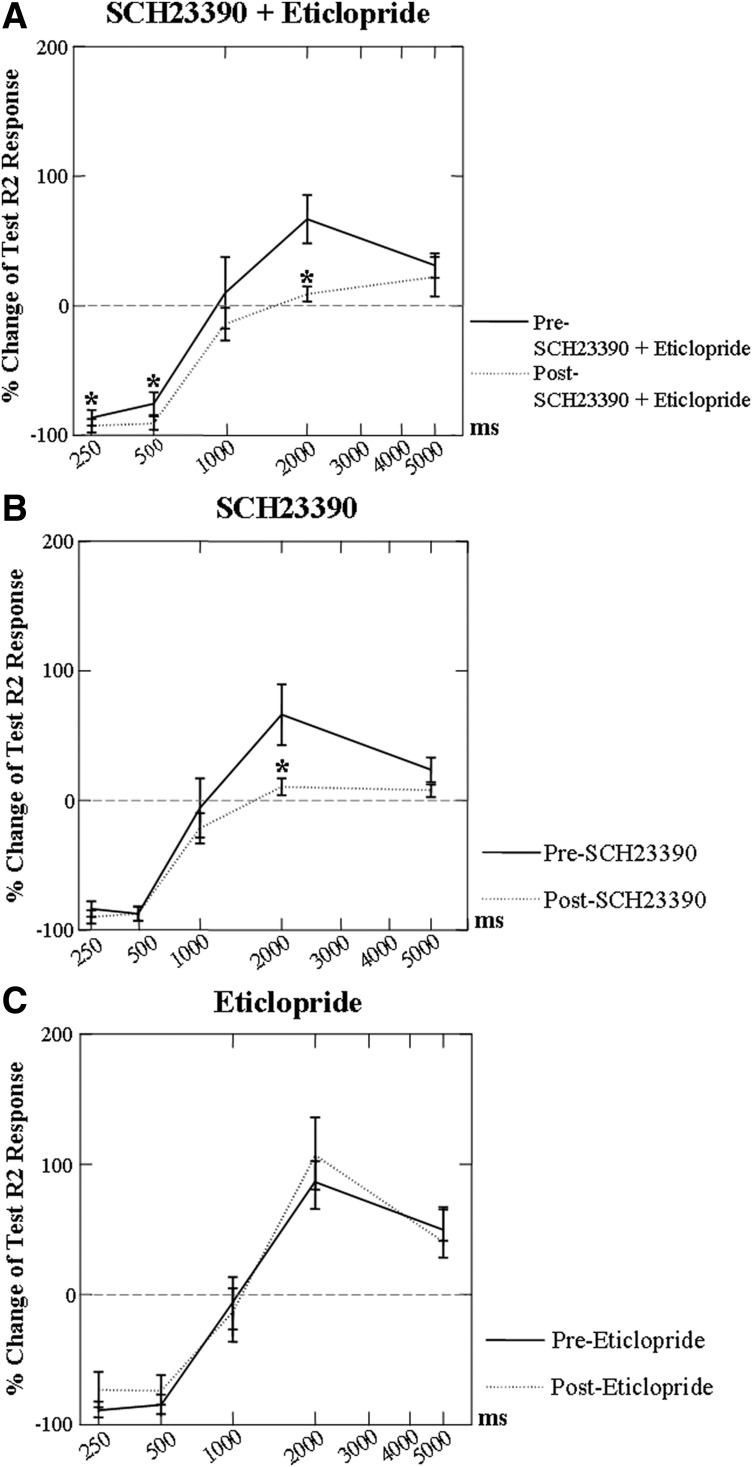
The combination of SCH23390 + eticlopride had a significant effect (F = 6.778; P = 0.029) on conditioning changes in laryngeal R2 test responses. Post hoc Wilcoxon signed-rank testing indicated a significant increase in conditioning suppression at 250-ms ISI (Z = −2.497; P = 0.013) and 500-ms ISI (Z = −2.293; P = 0.022) and reduction in conditioning facilitation at 2,000-ms ISI (Z = −2.599; P = 0.009, Fig. 7A). The percentage change in test R2 responses between the pre drug and 5 min post drug at each of the ISIs also showed a significant change in conditioning effects with SCH23390 (F = 10.602; P = 0.01) and a significant interaction with ISI (F = 4.068; P = 0.008). Post hoc testing found a decrease in conditioning facilitation at 2,000-ms ISI (Z = −2.395; P = 0.017, Fig. 7B). No effects of eticlopride were found on conditioning (F = 0.114; P = 0.743, Fig. 7C).
FIG. 7.
Percentage change of test R2 responses induced by SCH23390 + eticlopride, SCH23390, and eticlopride. Conditioning facilitation was decreased by either the combination of SCH23390 + eticlopride (A, *P < 0.05, n = 10) or the SCH23390 alone (B, *P < 0.05, n = 10) at 2,000-ms ISI. Conditioning suppression was increased by the SCH23390 + eticlopride at 250- and 500-ms ISIs (B). No effects on conditioning changes were found in eticlopride administration group (C) at any ISIs (n = 10).
We tested whether the conditioning changes in test responses could be related to increases in the conditioning responses with the antagonist. For SCH23390 + eticlopride, no relationship with change in amplitude of the conditioning R2 response was found at 250-ms ISI (r = 0.225; P = 0.532), at 500-ms ISI (r = 0.452; P = 0.190), or at 2,000-ms ISI (r = 0.052; P = 0.887). This suggested that the significant increase in conditioning suppression at 250- and 500-ms ISI and the significant reduction in facilitation at 2,000-ms ISI were not related to the increase in the conditioning response amplitude post SCH23390 + eticlopride.
For SCH23390 alone, a significant relationship was found between the conditioning R2 response amplitude and the reduced facilitation of the test response at 2,000-ms ISI (r = −0.688; P = 0.028). This suggested that the greatest reduction in facilitation at 2,000-ms ISI occurred when there was a greater percentage increase in muscle activity in response to the conditioning stimulus post SCH23390.
The lack of effects of eticlopride (0.5 mg/kg) on the LAR was further examined by increasing the dosage from 0.5 to 1 mg/kg in four animals. Because only four animals were studied, no statistical analyses were conducted.
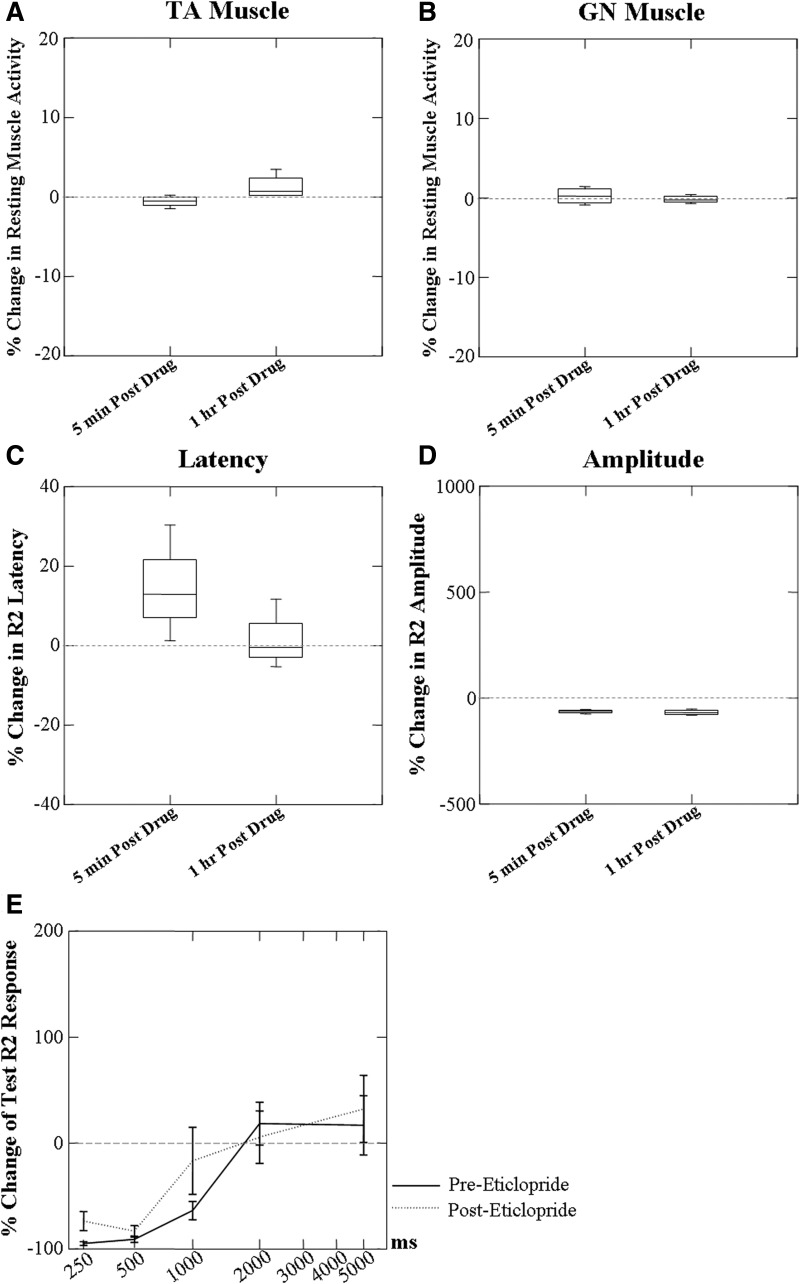
Effects of high-dose eticlopride on the resting muscle activity of TA and GN
Resting muscle activity was not changed in either the TA or the GN muscles with the higher dosage of eticlopride (Fig. 8, A and B). Similar results were found at 5 min and 1 h post the high-dose eticlopride.
FIG. 8.
Effects of high-dose eticlopride (1 mg/kg) on baseline activity of TA and GN muscles, the latency, amplitude of the laryngeal conditioning R2 response, and conditioning changes in the test R2 response. No effects of high-dose eticlopride were found on the resting muscle activity of TA (A) and GN (B) muscles. Conditioning R2 response latency (C) showed a tendency to increase and amplitude (D) decrease 5 min post administration. The amplitude tended to remain decreased and the latency remained somewhat increased 1 h later. No effects of high-dose eticlopride were found on the conditioning changes in the test R2 response at each ISI (E) (n = 4).
Effects of high-dose eticlopride on the conditioning laryngeal R2 responses
Conditioning R2 responses 5 min post 1 mg/kg eticlopride administration showed a tendency to increase in latency (Fig. 8C) and decrease in amplitude (Fig. 8D). At 1 h post eticlopride administration the amplitude tended to remain decreased and the response latency remained somewhat increased.
Effects of high-dose eticlopride on the laryngeal R2 test responses
No marked changes were seen in the percentage change in the test responses post high-dose eticlopride (Fig. 8E).
DISCUSSION
The data demonstrate that D1 and D2 dopamine receptor antagonists have different effects on the regulation of the laryngeal neurophysiology and sensorimotor responses in rats.
Changes in resting muscle activity with dopamine receptor blockade
Comparisons of changes in resting muscle activity with the administration of D1 and D2 receptor antagonists showed increases in laryngeal muscle activity only with administration of the D1 receptor antagonist. Further, the effects of the selective D1 and D2 receptor antagonists showed different effects on laryngeal muscle activity and neither altered limb muscle activity. These results may indicate that increases in laryngeal muscle activity found in persons with PD may have been due to the loss of dopamine binding at D1 receptors (Gallena et al. 2001). Other studies have shown dopamine D1 and D2 receptors in the ventral striatum and D1 receptors in the substantia nigra (SN) regulated limb muscle activity in awake rats (Hemsley and Crocker 2001). A nonselective dopamine receptor antagonist that blocks both D1 and D2 receptors administered either centrally or systemically can increase the limb muscle activity in awake rats (Double and Crocker 1995; Hemsley and Crocker 1998). However, in this study only the D1 antagonist alone was effective, whereas the D2 antagonist and the combined D1 and D2 antagonists did not change the laryngeal muscle activity. The results, however, did not suggest that these dopamine receptor subtypes may modulate muscle activity differently because there was no difference between the effects of the two antagonists combined versus the D1 antagonist alone.
On the other hand, the effects of the D1 receptor subtype on the laryngeal and limb muscles differed, although we cannot discount the potential role of alpha-chloralose anesthesia used in this study, whereas the studies in the limb muscles were performed in awake animals (Hemsley and Crocker 2001). Alpha-chloralose anesthesia induces a chemical restraint without altering autonomic reflex activity or myocardial function. The laryngeal muscles are part of the respiratory system and are active at rest (Bartlett Jr et al. 1973; Kuna et al. 1988, 1994), whereas the GN is quiet in a supine animal under anesthesia. The D1 receptor antagonist increased the muscle activity in the laryngeal but not the limb muscles. Differences in the resting levels of motor neuron firing in the two muscle systems under alpha-chloralose anesthesia might explain these results. We maintained our animals at level 3 anesthesia in this study. At this stage, breathing becomes shallow and the animal no longer responds to foot pinch, although respiratory-related laryngeal muscle activity is present (McKelvey and Hollingshead 2000; Whelan and Flecknell 1992). We used tracheal ventilation to modulate the respiratory rate and pressure close to the natural rhythm and maintained normal respiratory activity in the laryngeal muscles. If ventilation was stopped, the animal's respiratory rhythm reappeared. However, limb muscle activity was absent throughout the experiment. This may account for the lack of modulation of the GN muscles by dopamine receptor antagonists under anesthesia. Whether dopamine receptor antagonists differentially modulate the spinal and brain stem respiratory systems or exert different effects on laryngeal and limb muscles under the alpha-chloralose anesthesia is not clear.
Dopamine receptor blockade and laryngeal sensorimotor responses
We postulated that D1 and D2 receptor antagonists would enhance the excitability of laryngeal R2 muscle responses to conditioning sensory stimuli. Both the combined D1 and D2 receptor antagonists and the D1 receptor antagonist alone increased the amplitude and decreased the latency of laryngeal R2 responses to conditioning stimuli, thus supporting our hypothesis. However, neither the low nor the high dosage of the D2 receptor antagonist had significant effects on laryngeal R2 responses and no differences were found between the combined D1 and D2 receptor antagonists and the D1 receptor antagonist alone. Therefore the results only support an active effect of the D1 receptor blocker on the laryngeal adductor response.
D1 and D2 receptors are the most heavily expressed in the striatal part of the basal ganglia and mediate the responses of striatal neurons to dopaminergic input from the midbrain (Bouthenet et al. 1991; Civelli et al. 1991; Van Tol et al. 1991). Although striato-GPi/SN neurons express D1 and D2 receptors the majority mainly express D1, whereas the vast majority of striato-GPe neurons express D2 receptors (Deng et al. 2006). Dopamine is thought to modulate locomotion through activation of different dopamine receptors in the direct and/or indirect pathways. A recent study supports the notion that dopamine receptors are segregated on striatal projection neurons (Gantois et al. 2007). Some neurons in the dopamine pathways coexpress D1 and D2 receptors, which may have opposing, synergistic, or independent effects. Here, we showed only the excitatory effects of D1 receptor antagonism on the laryngeal responses and no interaction between the two antagonists. These data strongly suggested that only the D1 receptors have effects on modulating laryngeal response control in the brain stem.
Previously, D1 and D2 receptor modulation altered the blink reflex differently across studies. In one study both D1 and D2 agonists enhanced blink rate independently in primates (Elsworth et al. 1991), whereas in another study a D2 agonist attenuated blinking and a D1 agonist induced increased in eye blinking (Jutkiewicz and Bergman 2004). The present results indicate that D1 receptor activation may normally suppress laryngeal sensorimotor responses. This may suggest that D1 receptor activation may have a somewhat different effect on the LAR than that on the blink rate.
However, drug effects may differ between the blink rate and the blink reflex. A study of the effect of haloperidol-induced dopamine antagonism on the blink reflex in healthy volunteers found no effect on R2 latencies or amplitude but that the amplitude of the R1 blink response was enhanced with dopamine antagonism (Raffaele et al. 1988). Because of the many differences between these two studies (only R2 was elicited for the LAR in the anesthetized rat) it is difficult to know whether the effects of D1 antagonism on the LAR and the blink reflex are similar. Both the LAR and the blink are defensive cranial reflexes with similar short- (R1) and long-latency (R2) components. These results suggest that different mechanisms may modulate the LAR and blink reflexes with dopamine antagonism. However, these differences could also be due to possible interactions with the alpha-chloralose anesthesia used in this study.
Dopamine receptors and conditioned laryngeal responses
We found that conditioning suppression increased at 250 and 500 ms when D1 and D2 receptor antagonists were combined. Neither of these changes could be explained by increases in amplitude of the conditioning R2 response. On the other hand, facilitation was reduced at 2,000-ms ISI with the combined D1 and D2 receptor antagonists. This also occurred with the D1 receptor antagonist alone, which was related to the percentage increase in the conditioning response amplitude with D1 receptor antagonism. Here the method of computation may have contributed to the finding of decreased facilitation at 2,000-ms ISI with D1 receptor antagonism alone.
We can thus conclude that the increased conditioned suppression of the test LAR responses with D1 + D2 receptor antagonists in the brain demonstrate increased suppression of laryngeal responses to sensory stimuli with repeated stimulation. These effects of dopamine neurotransmission antagonism may underlie the reduction in upper airway responses such as cough in PD. In the late stages of PD, some patients have reduced cough reflex sensitivity (Ebihara et al. 2003).
The increased suppression with conditioning of the LAR in the rat with D1 + D2 antagonism at ISIs <1,000 ms also differs from facilitation of the conditioned blink reflex found contralateral to a complete 6-OHDA lesion in the rat (Basso et al. 1993). Similarly, in PD patients, less suppression of test R2 responses was seen in the R2 blink reflex with conditioning (Kimura 1973) at ISIs between 50 and 600 ms (Lozza et al. 1997). Methods for studies of the blink and this study of the LAR were not comparable; a unilateral 6-OHDA lesion likely has effects different from those of the systemic selective receptor antagonists used here. Further, the effects of reduced dopamine neurotransmission on conditioning effects on the LAR have not been studied in patients with PD as was done with the blink reflex (Kimura 1973; Lozza et al. 1997). As proposed earlier, the LAR and the blink may have different mechanisms involved; a comparison of the two in the same animal model or in PD patients is needed to determine whether the physiological effects of reductions in dopamine neurotransmission on the blink and the LAR differ.
We cannot generalize our findings in a rat model of selective receptor antagonists to voice disorders in PD patients. However, abnormally high levels of muscle activity in the laryngeal and labial muscles have been observed in untreated patients with PD (Gallena et al. 2001; Leanderson et al. 1971; Zarzur et al. 2007). In PD patients treated with clinically effective levels of levodopa, the firing rate of the TA motor units and labial muscle activity are both decreased (Gallena et al. 2001; Leanderson et al. 1971; Luschei et al. 1999). The reduction in TA activity in PD patients treated to effect with levodopa later in the disease course may also reflect sensory gating abnormalities occurring in the more severe form (Jobst et al. 1997; Schneider et al. 1986). Levodopa is the most commonly used medication for PD, although others have proposed that it may not be as effective for speech disorders as for limb control in PD patients (Wolfe et al. 1975). Similarly, no significant benefits of apomorphine on voice and speech articulation were found in comparison with placebo control (Kompoliti et al. 2000). Both levodopa and apomorphine are nonselective dopamine agonists in the brain with a higher affinity for the D2 receptor in the brain (Jenner 2002). No studies have explored the effects of selective D1 or D2 agonists on laryngeal physiology in PD patients. The depletion of dopamine in PD patients that affects the laryngeal sensorimotor modulation may be via different dopamine receptors and/or different neural pathways from limb motor control (Jurgens and Ehrenreich 2007). In addition, D1-like receptors are further divided into D1A, D1B, and D5 and D2-like receptors are divided into D2S and D2L, D2, D3, and D4 receptor subtypes (Memo 1990; Seeman et al. 2000). Our study found a higher level of resting laryngeal muscle activity, increased LAR R2 responses, and decreased facilitation in a D1 dopamine receptor antagonist-induced rat model. This may suggest that loss of D1 receptors may play an important role in laryngeal abnormalities in PD. However, further studies are needed to explore the effects of the specific subtypes of dopamine receptors on laryngeal physiology to determine the mechanisms of dopamine neurotransmission on the cranial musculature involved in laryngeal functions in dopamine-depleted animal models. Studies in animals may provide the bases for exploring the potential of future selective dopamine enhancement therapies for voice deficits in PD in humans.
Conclusions
We conclude that reduced neurotransmission via the D1 receptor subtypes serves to increase resting muscle activity similar to that seen in early untreated PD. Further, laryngeal adductor responses to afferent input have a shorter latency, increased amplitudes, and reduced excitation of test responses after reductions in D1 neurotransmission. D1 dopamine receptors may modulate the control of laryngeal responses to sensory inputs in the brain stem and may affect laryngeal neurophysiology differently from limb movements. Further studies are needed to address these issues in humans.
GRANTS
This work was supported by the Intramural Program of the National Institute of Neurological Disorders and Stroke, National Institutes of Health.
Acknowledgments
We thank Dr. Kristina Simonyan for comments on an earlier version of the manuscript.
REFERENCES
- Agostino et al. 2008.Agostino R, Bologna M, Dinapoli L, Gregori B, Fabbrini G, Accornero N, Berardelli A. Voluntary, spontaneous, and reflex blinking in Parkinson's disease. Mov Disord 23: 669–675, 2008. [DOI] [PubMed] [Google Scholar]
- Ambalavanar et al. 2004.Ambalavanar R, Tanaka Y, Selbie WS, Ludlow CL. Neuronal activation in the medulla oblongata during selective elicitation of the laryngeal adductor response. J Neurophysiol 92: 2920–2932, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartlett et al. 1973.Bartlett D, Remmers JE, Gautier H. Laryngeal regulation of respiratory airflow. Respir Physiol 18: 194–204, 1973. [DOI] [PubMed] [Google Scholar]
- Basso et al. 1993.Basso MA, Strecker RE, Evinger C. Midbrain 6-hydroxydopamine lesions modulate blink reflex excitability. Exp Brain Res 94: 88–96, 1993. [DOI] [PubMed] [Google Scholar]
- Bhabu et al. 2003.Bhabu P, Poletto C, Mann E, Bielamowicz S, Ludlow CL. Thyroarytenoid muscle responses to air pressure stimulation of the laryngeal mucosa in humans. Ann Otol Rhinol Laryngol 112: 834–840, 2003. [DOI] [PubMed] [Google Scholar]
- Bouthenet et al. 1991.Bouthenet ML, Souil E, Martres MP, Sokoloff P, Giros B, Schwartz JC. Localization of dopamine D3 receptor mRNA in the rat brain using in situ hybridization histochemistry: comparison with dopamine D2 receptor mRNA. Brain Res 564: 203–219, 1991. [DOI] [PubMed] [Google Scholar]
- Braak et al. 2003.Braak H, Del Tredici K, Rub U, de Vos RA, Jansen Steur EN, Braak E. Staging of brain pathology related to sporadic Parkinson's disease. Neurobiol Aging 24: 197–211, 2003. [DOI] [PubMed] [Google Scholar]
- Ciucci et al. 2009.Ciucci MR, Ahrens AM, Ma ST, Kane JR, Windham EB, Woodlee MT, Schallert T. Reduction of dopamine synaptic activity: degradation of 50-kHz ultrasonic vocalization in rats. Behav Neurosci 123: 328–336, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciucci et al. 2007.Ciucci MR, Ma ST, Fox C, Kane JR, Ramig LO, Schallert T. Qualitative changes in ultrasonic vocalization in rats after unilateral dopamine depletion or haloperidol: a preliminary study. Behav Brain Res 182: 284–289, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Civelli et al. 1991.Civelli O, Bunzow JR, Grandy DK, Zhou QY, Van Tol HH. Molecular biology of the dopamine receptors. Eur J Pharmacol 207: 277–286, 1991. [DOI] [PubMed] [Google Scholar]
- Deng et al. 2006.Deng YP, Lei WL, Reiner A. Differential perikaryal localization in rats of D1 and D2 dopamine receptors on striatal projection neuron types identified by retrograde labeling. J Chem Neuroanat 32: 101–116, 2006. [DOI] [PubMed] [Google Scholar]
- Double and Crocker 1995.Double KL, Crocker AD. Dopamine receptors in the substantia nigra are involved in the regulation of muscle tone. Proc Natl Acad Sci USA 92: 1669–1673, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dromey et al. 2000.Dromey C, Kumar R, Lang AE, Lozano AM. An investigation of the effects of subthalamic nucleus stimulation on acoustic measures of voice. Mov Disord 15: 1132–1138, 2000. [DOI] [PubMed] [Google Scholar]
- Ebihara et al. 2003.Ebihara S, Saito H, Kanda A, Nakajoh M, Takahashi H, Arai H, Sasaki H. Impaired efficacy of cough in patients with Parkinson disease. Chest 124: 1009–1015, 2003. [DOI] [PubMed] [Google Scholar]
- Elsworth et al. 1991.Elsworth JD, Lawrence MS, Roth RH, Taylor JR, Mailman RB, Nichols DE, Lewis MH, Redmond DE Jr. D1 and D2 dopamine receptors independently regulate spontaneous blink rate in the vervet monkey. J Pharmacol Exp Ther 259: 595–600, 1991. [PubMed] [Google Scholar]
- Gallena et al. 2001.Gallena S, Smith PJ, Zeffiro T, Ludlow CL. Effects of levodopa on laryngeal muscle activity for voice onset and offset in Parkinson disease. J Speech Lang Hear Res 44: 1284–1299, 2001. [DOI] [PubMed] [Google Scholar]
- Gantois et al. 2007.Gantois I, Fang K, Jiang L, Babovic D, Lawrence AJ, Ferreri V, Teper Y, Jupp B, Ziebell J, Morganti-Kossmann CM, O'Brien TJ, Nally R, Schutz G, Waddington J, Egan GF, Drago J. Ablation of D1 dopamine receptor-expressing cells generates mice with seizures, dystonia, hyperactivity, and impaired oral behavior. Proc Natl Acad Sci USA 104: 4182–4187, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goberman 2005.Goberman AM Correlation between acoustic speech characteristics and non-speech motor performance in Parkinson disease. Med Sci Monit 11: CR109–CR116, 2005. [PubMed] [Google Scholar]
- Hemsley and Crocker 1998.Hemsley KM, Crocker AD. The effects of an irreversible dopamine receptor antagonist, N-ethoxycarbonyl-2-ethoxy-1,2-dihydroquinoline (EEDQ), on the regulation of muscle tone in the rat: the role of the substantia nigra. Neurosci Lett 251: 77–80, 1998. [DOI] [PubMed] [Google Scholar]
- Hemsley and Crocker 2001.Hemsley KM, Crocker AD. Changes in muscle tone are regulated by D1 and D2 dopamine receptors in the ventral striatum and D1 receptors in the substantia nigra. Neuropsychopharmacology 25: 514–526, 2001. [DOI] [PubMed] [Google Scholar]
- Hemsley et al. 2002.Hemsley KM, Farrall EJ, Crocker AD. Dopamine receptors in the subthalamic nucleus are involved in the regulation of muscle tone in the rat. Neurosci Lett 317: 123–126, 2002. [DOI] [PubMed] [Google Scholar]
- Jenner 2002.Jenner P Pharmacology of dopamine agonists in the treatment of Parkinson's disease. Neurology 58: S1–S8, 2002. [DOI] [PubMed] [Google Scholar]
- Jobst et al. 1997.Jobst EE, Melnick ME, Byl NN, Dowling GA, Aminoff MJ. Sensory perception in Parkinson disease. Arch Neurol 54: 450–454, 1997. [DOI] [PubMed] [Google Scholar]
- Jurgens 2002.Jurgens U A study of the central control of vocalization using the squirrel monkey. Med Eng Phys 24: 473–477, 2002. [DOI] [PubMed] [Google Scholar]
- Jurgens 2009.Jurgens U The neural control of vocalization in mammals: a review. J Voice 23: 1–10, 2009. [DOI] [PubMed] [Google Scholar]
- Jurgens and Ehrenreich 2007.Jurgens U, Ehrenreich L. The descending motorcortical pathway to the laryngeal motoneurons in the squirrel monkey. Brain Res 1148: 90–95, 2007. [DOI] [PubMed] [Google Scholar]
- Jutkiewicz and Bergman 2004.Jutkiewicz EM, Bergman J. Effects of dopamine D1 ligands on eye blinking in monkeys: efficacy, antagonism, and D1/D2 interactions. J Pharmacol Exp Ther 311: 1008–1015, 2004. [DOI] [PubMed] [Google Scholar]
- Kalaitzakis et al. 2008.Kalaitzakis ME, Graeber MB, Gentleman SM, Pearce RK. The dorsal motor nucleus of the vagus is not an obligatory trigger site of Parkinson's disease: a critical analysis of alpha-synuclein staging. Neuropathol Appl Neurobiol 34: 284–295, 2008. [DOI] [PubMed] [Google Scholar]
- Kimura 1973.Kimura J Disorder of interneurons in parkinsonism. The orbicularis oculi reflex to paired stimuli. Brain 96: 87–96, 1973. [DOI] [PubMed] [Google Scholar]
- Kompoliti et al. 2000.Kompoliti K, Wang QE, Goetz CG, Leurgans S, Raman R. Effects of central dopaminergic stimulation by apomorphine on speech in Parkinson's disease. Neurology 54: 458–462, 2000. [DOI] [PubMed] [Google Scholar]
- Kuna et al. 1988.Kuna ST, Insalaco G, Woodson GE. Thyroarytenoid muscle activity during wakefulness and sleep in normal adults. J Appl Physiol 65: 1332–1339, 1988. [DOI] [PubMed] [Google Scholar]
- Kuna et al. 1994.Kuna ST, Smickley JS, Vanoye CR, McMillan TH. Cricothyroid muscle activity during sleep in normal adult humans. J Appl Physiol 76: 2326–2332, 1994. [DOI] [PubMed] [Google Scholar]
- Leanderson et al. 1971.Leanderson R, Meyerson BA, Persson A. Effect of L-dopa on speech in Parkinsonism. An EMG study of labial articulatory function. J Neurol Neurosurg Psychiatry 34: 679–681, 1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logemann et al. 1978.Logemann JA, Fisher HB, Boshes B, Blonsky ER. Frequency and cooccurrence of vocal tract dysfunctions in the speech of a large sample of Parkinson patients. J Speech Hear Disord 43: 47–57, 1978. [DOI] [PubMed] [Google Scholar]
- Lozza et al. 1997.Lozza A, Pepin JL, Rapisarda G, Moglia A, Delwaide PJ. Functional changes of brainstem reflexes in Parkinson's disease. Conditioning of the blink reflex R2 component by paired and index finger stimulation. J Neural Transm 104: 679–687, 1997. [DOI] [PubMed] [Google Scholar]
- Ludlow et al. 1995.Ludlow CL, Schulz GM, Yamashita T, Deleyiannis FW. Abnormalities in long latency responses to superior laryngeal nerve stimulation in adductor spasmodic dysphonia. Ann Otol Rhinol Laryngol 104: 928–935, 1995. [DOI] [PubMed] [Google Scholar]
- Luschei et al. 1999.Luschei ES, Ramig LO, Baker KL, Smith ME. Discharge characteristics of laryngeal single motor units during phonation in young and older adults and in persons with Parkinson disease. J Neurophysiol 81: 2131–2139, 1999. [DOI] [PubMed] [Google Scholar]
- McKelvey and Hollingshead 2000.McKelvey D, Hollingshead KW. Small Animal Anesthesia and Analgesia (2nd ed.). St. Louis, MO: Mosby, 2000, p. 49–54.
- Memo 1990.Memo M Pharmacological and molecular basis for dopamine D-2 receptor diversity. Mol Neurobiol 4: 181–196, 1990. [DOI] [PubMed] [Google Scholar]
- Plowman-Prine et al. 2009.Plowman-Prine EK, Okun MS, Sapienza CM, Shrivastav R, Fernandez HH, Foote KD, Ellis C, Rodriguez AD, Burkhead LM, Rosenbek JC. Perceptual characteristics of parkinsonian speech: a comparison of the pharmacological effects of levodopa across speech and non-speech motor systems. NeuroRehabilitation 24: 131–144, 2009. [DOI] [PubMed] [Google Scholar]
- Pollack 2004.Pollack A Coactivation of D1 and D2 dopamine receptors: in marriage, a case of his, hers, and theirs. Sci STKE 2004: pe50, 2004. [DOI] [PubMed]
- Raffaele et al. 1988.Raffaele R, Emery P, Palmeri A, Ricca G, Perciavalle V. Influences of dopaminergic systems on the blink reflex. Ital J Neurol Sci 9: 351–354, 1988. [DOI] [PubMed] [Google Scholar]
- Rascol et al. 2003.Rascol O, Payoux P, Ory F, Ferreira JJ, Brefel-Courbon C, Montastruc JL. Limitations of current Parkinson's disease therapy. Ann Neurol 53, Suppl. 3: S3–S15, 2003. [DOI] [PubMed] [Google Scholar]
- Rousseaux et al. 2004.Rousseaux M, Krystkowiak P, Kozlowski O, Ozsancak C, Blond S, Destee A. Effects of subthalamic nucleus stimulation on parkinsonian dysarthria and speech intelligibility. J Neurol 251: 327–334, 2004. [DOI] [PubMed] [Google Scholar]
- Schneider et al. 1986.Schneider JS, Diamond SG, Markham CH. Deficits in orofacial sensorimotor function in Parkinson's disease. Ann Neurol 19: 275–282, 1986. [DOI] [PubMed] [Google Scholar]
- Seeman et al. 2000.Seeman P, Nam D, Ulpian C, Liu IS, Tallerico T. New dopamine receptor, D2(Longer), with unique TG splice site, in human brain. Brain Res Mol Brain Res 76: 132–141, 2000. [DOI] [PubMed] [Google Scholar]
- Van Tol et al. 1991.Van Tol HH, Bunzow JR, Guan HC, Sunahara RK, Seeman P, Niznik HB, Civelli O. Cloning of the gene for a human dopamine D4 receptor with high affinity for the antipsychotic clozapine. Nature 350: 610–614, 1991. [DOI] [PubMed] [Google Scholar]
- Visser et al. 2006.Visser M, Marinus J, Stiggelbout AM, van Hilten JJ. Responsiveness of impairments and disabilities in Parkinson's disease. Parkinsonism Relat Disord 12: 314–318, 2006. [DOI] [PubMed] [Google Scholar]
- Walters et al. 1987.Walters JR, Bergstrom DA, Carlson JH, Chase TN, Braun AR. D1 dopamine receptor activation required for postsynaptic expression of D2 agonist effects. Science 236: 719–722, 1987. [DOI] [PubMed] [Google Scholar]
- Weick and Walters 1987.Weick BG, Walters JR. Effects of D1 and D2 dopamine receptor stimulation on the activity of substantia nigra pars reticulata neurons in 6-hydroxydopamine lesioned rats: D1/D2 coactivation induces potentiated responses. Brain Res 405: 234–246, 1987. [DOI] [PubMed] [Google Scholar]
- Whelan and Flecknell 1992.Whelan G, Flecknell PA. The assessment of depth of anaesthesia in animals and man. Lab Anim 26: 153–162, 1992. [DOI] [PubMed] [Google Scholar]
- Wolfe et al. 1975.Wolfe VI, Garvin JS, Bacon M, Waldrop W. Speech changes in Parkinson's disease during treatment with L-dopa. J Commun Disord 8: 271–279, 1975. [DOI] [PubMed] [Google Scholar]
- Zarzur et al. 2007.Zarzur AP, Duprat AC, Shinzato G, Eckley CA. Laryngeal electromyography in adults with Parkinson's disease and voice complaints. Laryngoscope 117: 831–834, 2007. [DOI] [PubMed] [Google Scholar]