Abstract
Experience-dependent plasticity in the central sensory systems depends on activation of both the sensory and neuromodulatory systems. Sensitization or nonspecific augmentation of central auditory neurons elicited by pseudo-conditioning with unpaired conditioning tonal (CS) and unconditioned electric leg (US) stimuli is quite different from tone-specific plasticity, called best frequency (BF) shifts, of the neurons elicited by auditory fear conditioning with paired CS and US. Therefore the neural circuits eliciting the nonspecific augmentation must be different from that eliciting the BF shifts. We first examined plastic changes in the response properties of collicular neurons of the big brown bat elicited by pseudo-conditioning and found that it elicited prominent nonspecific augmentation—an auditory response increase, a frequency-tuning broadening, and a threshold decreas—and that, in addition, it elicited a small short-lasting BF shift only when the CS frequency was 5 kHz lower than the BF of a recorded neuron. We examined the role of acetylcholine and the auditory and somatosensory cortices in these collicular changes. The development of the nonspecific augmentation was affected little by a muscarinic acetylcholine receptor antagonist applied to the inferior colliculus and by a GABAA receptor agonist applied to the auditory or somatosensory cortex. However, these drugs abolished the small short-lasting BF shift as they abolished the large long-lasting cortical and short-lasting collicular BF shifts elicited by the conditioning. These results indicate that, different from the BF shift, the nonspecific augmentation of the inferior colliculus depends on neither the cholinergic neuromodulator nor the auditory and somatosensory cortices.
INTRODUCTION
Auditory fear conditioning elicits tone-specific plasticity, i.e., best frequency (BF) shifts, in the central auditory system (Gao and Suga 1998; Ji et al. 2001; Weinberger 1998). In our conditioning experiments (Gao and Suga 1998; Ji and Suga 2003; Ji et al. 2001), a conditioning tonal stimulus (CS) is followed by an unconditioned electric leg-stimulus (US) with a fixed time delay, and this CS-US pair is repeatedly delivered to an animal with a fixed time interval. Pseudo-conditioning elicits cortical nonspecific plasticity, i.e., sensitization (Bakin et al. 1992) or nonspecific augmentation, in the central auditory system (Ji and Suga 2008). In pseudo-conditioning, the CS and US are unpaired by delivering them pseudorandomly. The CS and US, respectively, activate the auditory and somatosensory systems regardless of whether they are paired or unpaired, but they elicit totally different plastic changes depending on whether they are paired or unpaired. Does this difference in plasticity simply depend on whether the pseudo-randomized CS and/or US elicit greater arousal than the unrandomized ones? Are the neuromodulators involved in eliciting the nonspecific augmentation different from those involved in eliciting the BF shifts? How is the neural circuit for the nonspecific augmentation different from that for the BF shift?
The neural circuit involved in eliciting the BF shifts has been extensively studied (for review, see Suga and Ma 2003), whereas that involved in eliciting nonspecific augmentation has been studied only a little, perhaps, because of the reasonable speculation that the strong arousal evoked by randomized CS and/or US elicits the nonspecific augmentation (Bakin et al. 1992). In the initial steps of our research on the neural circuit for the nonspecific augmentation, we studied the differences in response properties between the nonspecific augmentation and the BF shifts of neurons in the primary auditory cortex, AI (Ji and Suga 2008), and also in the central nucleus of the inferior colliculus, ICc (this study). These studies examined the following aspects related to the neural circuit for the nonspecific augmentation: 1) whether the pseudo-conditioning evoked the small short-term collicular BF shift in addition to the nonspecific collicular augmentation, 2) whether bilateral inactivation of the somatosensory cortex (SI) with an agonist of GABAA receptors, muscimol, abolished the collicular BF shift and/or nonspecific augmentation, 3) whether inactivation of AI abolished the collicular BF shift and/or nonspecific augmentation elicited by pseudo-conditioning, and 4) whether an antagonist of muscarinic ACh receptors, atropine, applied to the ICc abolished the collicular BF shift and/or nonspecific augmentation. Here, we first report the collicular tone-specific (BF shift) and nonspecific (augmentation) plasticities of the bat's ICc elicited by the pseudo-conditioning. We report that muscimol applied to SI or AI and atropine applied to the ICc abolished the collicular BF shift but not the nonspecific plasticity. These data indicate that, unlike the collicular BF shift, the collicular nonspecific augmentation depends on neither the corticofugal system nor the cholinergic system.
METHODS
Experimental subjects
Thirty adult big brown bats (Eptesicus fuscus, 18–24 g body weight) were used for the experiments. All experimental procedures were approved by the Animal Studies Committee of Washington University in St. Louis and were the same as those previously described (Gao and Suga 2000; Ji et al. 2001).
Surgical and recording procedures
Under neuroleptanalgesia (Innovar 4.08 mg/kg of body weight, 0.4 ml Fentanyl citrate in 20 mg/ml Droperidol), a 1.5-cm-long metal post was glued to the dorsal surface of the bat's skull. Experiments began 3–4 days after the surgery. An awake bat was placed in a polyethylene-foam body-mold suspended at the center of a soundproof room maintained at 31°C. The bat's head was immobilized by fixing the metal post glued on the skull onto a metal rod with set screws to ensure a uniform and stable position of the head, which directly faced a loudspeaker located 80 cm away. A tungsten-wire microelectrode with a tip diameter of ∼7 μm was inserted dorsoventrally into the ICc, and a single-unit recording was made at a depth between 50 and 1,000 μm. Only one neuron was studied in a 1-day experiment. The BF shift and nonspecific augmentation of cortical auditory neurons elicited by the pseudo-conditioning recover within 3.5 h, so the same animal could be used for the experiments every day. However, our 1-day experiment lasted ≤8 h, so it might be traumatic to the animal if we did so. Accordingly, we gave the animal a 3-day rest between experiments. Local anesthetic (5% lidocaine, E. Fougera and Co.) and antibiotic (0.2% nitrofurazone, RXV Products) ointments were applied to the surgical wound. The recording session lasted ∼7 h. Water was provided with a dropper, and lidocaine was reapplied every 2 h. The bat was neither anesthetized nor tranquilized during the experiments. If the bat continued to show signs of discomfort, recordings were terminated, and the bat was returned to its cage.
Acoustic stimulation
Acoustic stimuli were 20-ms tone bursts, including a 0.5-ms rise-decay time, delivered to the bat at a rate of 4/s from a leaf tweeter with real-time processors 2.1 (Tucker-Davis Technologies, Alachua, FL). The frequency and amplitude of the tone bursts were manually varied or computer-controlled to measure the BF and the minimum threshold of a given neuron. Frequency-tuning curves and iso-spike count contour lines were obtained from the response of a single neuron to a frequency-amplitude scan that was repeated 10 times. The scan consisted of 21 250-ms time blocks for a frequency scan and 13 250-ms time blocks for an amplitude scan. Therefore each 250-ms time block of the 273 time blocks represented a specific combination of a frequency and an amplitude. The frequency and amplitude of the tone burst were randomly varied by 1.0-kHz steps over 20 kHz and 5 dB steps over 60 dB, respectively. The receptive field of a neuron is the excitatory response area (i.e., the area inside of a frequency-tuning curve) derived from the response to the frequency-amplitude scan. To measure a frequency-response curve and a BF shift, the amplitude of the tone burst was set at 10 dB above the minimum threshold of a given neuron. The frequency of the tone burst was randomly varied in 0.5- or 1.0-kHz steps. This frequency scan consisted of 21 250-ms time blocks and was repeated 50 times. These frequency-amplitude and frequency scans were delivered by the stimulus control and recording software (Brainware version 8.0). The amplitude of the tone bursts delivered from the leaf tweeter was calibrated with a microphone (Brüel and Kjær Instruments, Naerum, Denmark). It was flat within ±5 dB from 10.0 to 70.0 kHz. The amplitudes of the tone bursts were expressed in decibels in sound pressure level (dB SPL) referred to 20 μPa r.m.s.
Pseudo-conditioning
To explore the neural circuit for the nonspecific augmentation, it is more analytical to randomize the CS or US and not both. Therefore in our experiments (Ji and Suga 2008; this paper), the CS was delivered at a fixed time interval the same as that in our conditioning experiments (Gao and Suga 1998, 2000; Ji et al. 2001), because the CS (10-ms, 50-dB SPL tone bursts) was hardly aversive to an animal and seemed to play a minor role in eliciting the nonspecific augmentation. On the other hand, the US was pseudorandomized (Ji and Suga 2008), because the US (50-ms, 0.4-mA electric pulse) was aversive to the animal and seemed to play the major role in eliciting it.
In the big brown bat, auditory fear conditioning elicits the largest BF shifts of the cortical (Gao and Suga 2000) and collicular (Gao and Suga 1998, 2000) neurons with a BF 6.0–7.0 kHz higher than the frequency of the CS, whereas it does not elicit the BF shifts of the neurons with a BF >10 kHz lower or >15 kHz higher than the CS frequency. Furthermore, it has been known that a 30-min-long repetitive tone burst stimulation evokes the small BF shift of a neuron with a BF ∼5 kHz higher than the tone burst frequency (Gao and Suga 1998; Ma and Suga 2001, 2003). Therefore to show tone-specific and nonspecific plasticity evoked by pseudo-conditioning, the frequency of the CS was adjusted to be either the same as, 10 kH higher, 5.0 kHz lower, or 15 kHz lower than the BF of a given neuron. We particularly examined whether the pseudo-conditioning with the CS at 5.0-kHz lower than the BF of a given neuron elicited a BF shift in addition to the nonspecific augmentation. In the pseudo-conditioning, the CS was unpaired with the US, so this unpaired CS is hereafter abbreviated as the CSu.
The CS in our auditory fear conditioning was a train of tone bursts that were 50 dB SPL, 10-ms-long and 33/s over 1.0 s to mimic the biosonar pulses emitted by the bat at the middle of the approach phase of echolocation, and this CS was delivered every 30 s over 30 min (Gao and Suga 1998, 2000; Ji and Suga 2003; Ji et al. 2001). We kept all the parameters characterizing the CSu for pseudo-conditioning exactly the same as those of the CS for the auditory fear conditioning, and this CSu was also delivered every 30 s for 30 min. The unpaired US was a 50-ms, 0.1- to 0.4-mA monophasic electric pulse as in our auditory fear conditioning. However, the the unpaired US was randomly delivered to the bat in a time interval between 5 and 25 s after the CSu, to avoid being delivered in a period between 5.0 s before and 5.0 s after the CSu. The mean interval of the CSu and unpaired US was 14.8 s. There were 60 CSu and 60 unpaired US in total per training session.
The stimulus designed to elicit behavioral changes is different between sensitization and pseudo-conditioning, although the behavioral changes evoked by them might be almost the same (Erickson and Walters 1988). Therefore as before (Ji and Suga 2008), we call the changes in neural responses evoked by pseudo-conditioning “nonspecific augmentation” instead of sensitization. The ascending reticular activating system evokes “general augmentation” for cortical arousal, so we avoid using this term.
Drug applications
In the big brown bat, the drug applied to the dorsal surface of the IC was 0.2 μl of 0.4 M atropine (Sigma Chemical, St. Louis, MO) dissolved in a 0.9% saline solution. Auditory responses can be recorded within the 5.7-mm2 cortical area (Dear et al. 1993; Shen et al. 1997). The approximate center of AI is dorsoventrally crossed by a 30-kHz iso-BF line. The approximate mid-point of this iso-BF line was first electrophysiologically located by recording auditory responses at five to six cortical loci. A hole, ∼1.0 mm in diameter, was made there for an ipsilateral application of 0.2 μl of 8.8 M muscimol to AI. SI was localized by recording neural responses to touch stimuli (Gao and Suga 1998, 2000; Krubitzer and Calford 1992). Muscimol, 0.2 μl of 8.8 mM, was bilaterally applied to the SI surface in the same way as did Gao and Suga (1998, 2000). The drug applications were made 2–5 min before the pseudo-conditioning with a 1.0-μl Hamilton syringe. The dura mater was not removed but punctured by the penetration of the recording electrode, so that the drug applied to the AI, SI, or IC was diffused through these puncture holes.
Cortical inactivation by a cryoloop (Lomber et al. 1999) may be more appropriate for inactivation, but the auditory cortex, including AI and non-AI, of the big brown bat is ∼5.7 mm2 (Dear et al. 1993; Shen et al. 1997). The cryoloop is thus too large to selectively inactivate AI of the big brown bat. In our experiments, muscimol was applied to the surface of the dura matter through holes made by penetrations of a recording wire electrode into AI, allowing muscimol to diffuse into AI. The cortical inactivation by this method is highly selective and not traumatic to the bat, because, under cortical muscimol inactivation, the mustached bat, which is similar to the big brown bat in size, can perform a discrimination task in the frequency domain, but not in the time domain or vice versa, depending on which subdivision of the auditory cortex is inactivated (Riquimaroux et al. 1991).
The pseudo-conditioning induces nonassociative learning and nonspecific augmentation that are frequency independent. That is, they are equally induced at different frequencies (Ji and Suga 2008), as reported by Bakin et al. (1992). Therefore in our these studies, drug effects on the collicular plasticity were studied at the CSu frequency 5.0 kHz lower than the BF of a given collicular neuron instead of at different CSu frequencies, because the pseudo-conditioning with the CSu 5.0 kHz lower than the BF of the neuron evoked both the small BF shift and the nonspecific augmentation of the neuron in AI (Ji and Suga 2008) and the ICc (Figs. 1B and 2B). The CSu frequency was adjusted to be 5.0 kHz lower than the BF of a given neuron.
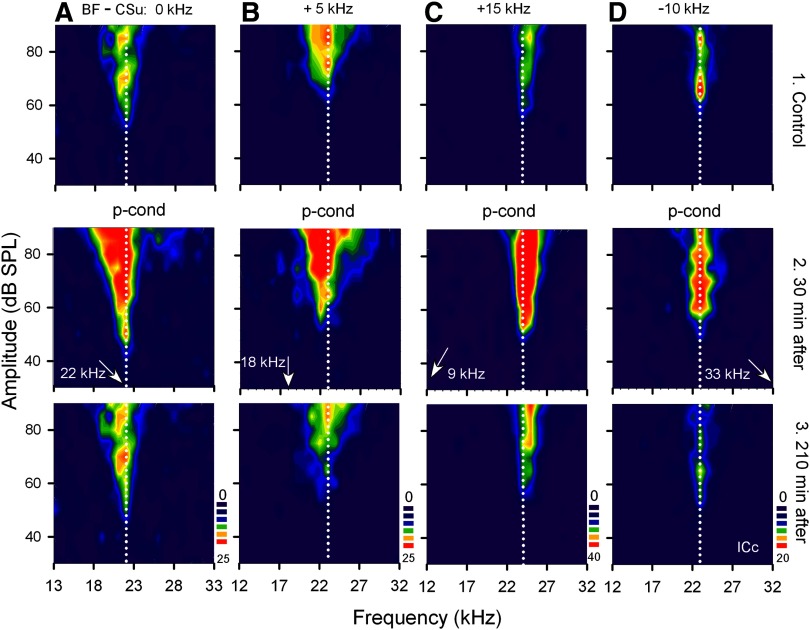
FIG. 1.
Changes in the receptive fields of 4 different collicular neurons elicited by pseudo-conditioning (p-cond). The best frequencies (BFs) of the 4 single neurons (A–D) were either 22.0, 23.0, 24.0, or 23.0 kHz (vertical dotted lines). The arrows along the frequency axis indicate the frequencies of the unpaired conditioning stimulus tones (CSu), which were either 0 (A), 5 (B), 10 (D), or 15 (C) kHz different from the BFs of the recorded neurons. In each column, 1–3 show the receptive fields obtained before (control), 30 and 210 min after the onset of p-cond, respectively. The scale bars from dark blue to dark red show low to high spike counts per 10 stimuli. Note a small BF shift toward 18 kHz (i.e., tone-specific change) in B2. Note the nonspecific augmentation: the augmentation of the responses, broadening of the receptive field and decrease in threshold and increase in background discharges.
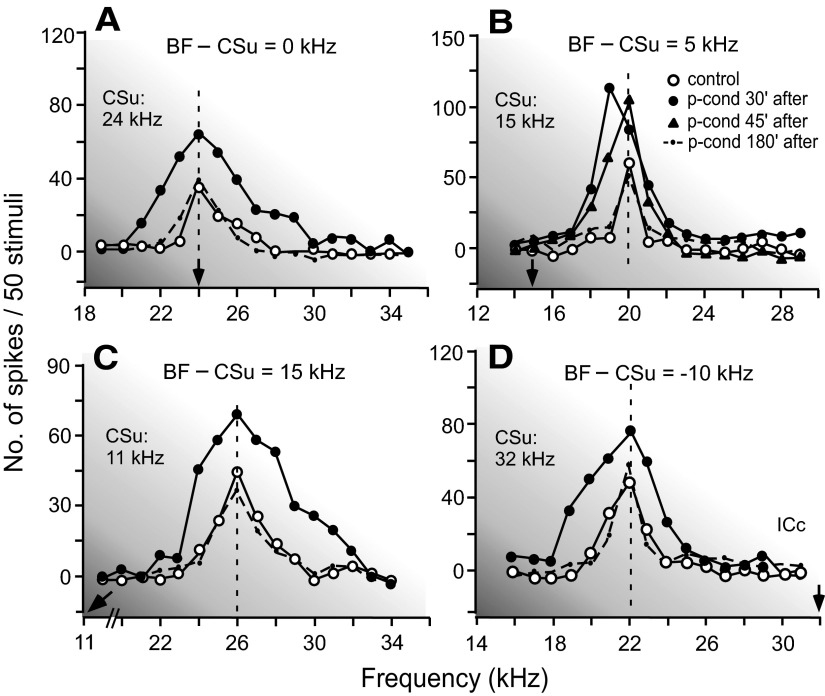
FIG. 2.
Changes in the frequency-response curves of 4 different collicular neurons showing nonspecific augmentation (A–D) and a BF shift (B) elicited by p-cond. The BFs of the neurons in A–D were 24.0, 20.0, 26.0, and 22.0 kHz, respectively (open circles on the vertical dashed lines). All the curves were obtained with tone bursts at 10 dB above the minimum threshold of a given neuron. The arrows along the frequency axis indicate the frequencies of CSus. The difference between the BF and CSu frequency is shown at the top of each graph. The key of the curves is shown in the inset. Note the overall augmentation of the responses 30 min after the onset of p-cond and, in addition, the 1.0-kHz BF shift in B.
Data acquisition
A tungsten-wire electrode (∼7 μm diam at the tip) inserted into the ICc commonly recorded action potentials originating from a few neurons. We used the BrainWare data acquisition software (Tucker-Davis Technologies) for sorting and selecting the action potentials of a single collicular neuron. At the beginning of the data acquisition, the waveform of an action potential was stored and displayed on the monitor screen. This action potential (i.e., template) was compared with other action potentials obtained during data acquisition. An array of poststimulus time histograms displaying the auditory responses to the frequency scan repeated 50 times or the frequency-amplitude scan repeated 10 times was acquired every 15 or 30 min after the onset of the pseudo-conditioning, as long as action potentials visually matched the template. The response latency of a neuron was determined to be the time between the onset of a tone burst at the bat's ears and the onset of the response of the neuron to it.
Off-line data processing
To obtain the frequency-response curve of a neuron with the frequency scan, the magnitude of the auditory response of the neuron was expressed by the number of spikes per 50 identical stimuli as a function of the frequency of the tone burst. The BF of the neuron was defined as the frequency at which the frequency-response curve peaked. To study the development of the nonspecific augmentation of auditory responses, the magnitude of the response at the BF was plotted as a function of time. Because an identical frequency scan was delivered 50 times, there were 50 samples of BFs that could be used to compute a mean and SE of the BFs and to perform statistical analysis. To determine whether there were differences in response magnitude between a BF and the adjacent frequencies, or between the BFs obtained before and after the pseudo-conditioning or a drug application, a two-tailed unpaired t-test was used for a significant difference of P < 0.01 or P < 0.05.
For processing the data obtained with the frequency-amplitude scan, the magnitude of the response of a neuron was expressed by the number of spikes per 10 identical stimuli and was plotted in the frequency-amplitude coordinates, and iso-spike count contour lines were drawn by using Sigma plot 8.0 software. The recovery of an auditory response was defined as the changed response at the BF recovered to that of the control response within ±10%.
RESULTS
BFs of collicular neurons and the frequencies of CSu
The ICc of the big brown bat represents 10∼80 kHz sounds along its dorso-ventral axis (Casseday and Covey 1992; Ferragamo et al. 1998), so the BFs of ICc neurons recorded in dorsoventral electrode penetrations systematically increased, as previously reported. Without a drug application, we examined the effects of pseudo-conditioning on 34 collicular neurons with BFs ranging between 20 and 34 kHz [22.3 ± 0.6 (SE) kHz]. Their BFs were either the same as (10 neurons), 5.0 kHz higher (12 neurons), 15.0 kHz higher (6 neurons), or 10.0 kHz lower (6 neurons) than a CSu frequency (Table 1, group 1).
TABLE 1.
Plastic changes in ICc and Al after p-cond with or without drugs
| Amount of Changes at 60 min After p-cond |
||||||
|---|---|---|---|---|---|---|
| Groups | Recording Site | Response Increase at MT + 10 dB, % | BW Increase at MT + 10 dB, kHz | Threshold Decrease at BF, dB | Background Discharge Increase, % | BF shift at CSu + 5 kHz |
| 1. P-cond with saline | ICc | 45.8 ± 6.8 (34)† | 2.0 ± 0.1† | 5.1 ± 0.3† | 20 ± 2.9* | −1.0 ± 0.1 (8/12)* |
| Al | 56.7 ± 3.4 (23)** | 3.8 ± 0.4** | 6.1 ± 0.8 | 14 ± 4.1 | −1.0 ± 0.1 (5/10)* | |
| 2. P-cond with Atr to ICc | ICc | 38.0 ± 9.5 (19)§ | 1.7 ± 0.1‡ | 0.0 ± 0.5 | 12 ± 2.6 | 0 (19/19) |
| Atr to Al | Al | 48.6 ± 5.1 (12)‡ | 3.2 ± 0.3 | 5.4 ± 0.7 | 10 ± 9.6 | 0 (14/14) |
| 3. P-cond with Mus to Al | ICc | 36.3 ± 4.0 (17)§ | 1.9 ± 0.2 | 5.2 ± 0.5* | 21 ± 2.8 | 0 (17/17) |
| 4. p-cond with Mus to Sl | ICc | 42.3 ± 5.7 (15) | 2.1 ± 0.1‡ | 5.0 ± 0.5* | 13 ± 3.3 | 0 (15/15) |
| Al | 58.2 ± 4.6 (16)** | 3.7 ± 0.2 | 5.0 ± 1.0 | 16 ± 9.8 | 0 (16/16) | |
| 5. Groups 1+4 | ICc | 44.7 ± 6.4 (49) | 2.1 ± 0.1 | 5.1 ± 0.3 | 17 ± 3.0 | |
| Al | 57.3 ± 3.8 (39)** | 3.8 ± 0.3** | 5.6 ± 0.6 | 15 ± 6.1 | ||
Values are means ± SE (n).
P < 0.05,
P < 0.01, t-test; the effects of p-cond are compared with pre-p-cond, i.e., control.
P < 0.05,
P < 0.01, t-test; the drug effects are compared with p-cond with saline.
P < 0.01, t-test; the effects of p-cond are compared between ICc and Al. ICc, central nucleus of the inferior colliculos; AI, primary auditory cortex; p-cond, pseudo-conditioning; MT, minimum threshold; BF, best frequency; CSu, unpaired conditioning tonal stimulus; Atr, atropine; Mus, Muscimol; SI, Somatosensory Cortex. The AI data were obtained by Ji and Suga (2008).
The effects of drugs were studied on the changes elicited by the pseudo-conditioning in 51 collicular neurons: 17 and 15 neurons for the effect of muscimol applied to AI and SI, respectively, and 19 neurons for the effect of atropine applied to the ICc (Table 1, groups 2–4). The BFs of these collicular neurons ranged between 12.5 and 31 kHz (22.8 ± 0.5 kHz). For the studies of the drug effects on both nonspecific and tone-specific collicular changes elicited by the pseudo-conditioning, the CSu frequencies were always 5.0 kHz lower than the BFs of the neurons studied.
Nonspecific and tone-specific plasticity of collicular neurons elicited by pseudo-conditioning
For the pseudo- conditioning, all 34 collicular neurons studied increased their responses to tone bursts, broadened their frequency-tuning curves, lowered their thresholds and/or increased their background discharges. All these changes were nonspecific to the CSu frequencies. In addition to the above changes, the pseudo-conditioning elicited a small short-lasting BF shift when the CSu was 5.0 kHz lower than the BF of a recorded collicular neuron (Table 1, group 1). These collicular changes are shown in Figs. 1–4.
In Fig. 1, the top row shows the receptive fields of four different collicular neurons with the color-coded iso-spike count contours. The outer edge of the faint blue zone represents the excitatory frequency-tuning curve of a given neuron. When the pseudo-conditioning was delivered to the bat with the CSu at the frequency being the same as (Fig. 1A), 5.0 kHz lower (Fig. 1B), 15.0 kHz lower (Fig. 1C), or 10.0 kHz higher (Fig. 1D) than the BF of a given collicular neuron, the responses of all these four neurons were augmented, as shown by the large red areas (Fig. 1, middle row). This augmentation gradually reduced and disappeared within 210 min after the onset of the pseudo-conditioning (Fig. 1, bottom row). For example, the neuron in Fig. 1A (top) was tuned to 22.0 kHz. Its maximum response was 20 spikes/10 stimuli, which was evoked by a tone burst at 22.0 kHz and 85 and 75 dB SPL. After the pseudo-conditioning with the CSu at 22.0 kHz, its responses to tone bursts were augmented so that 25 spikes/10 stimuli occurred at many different frequency-amplitude combinations within the large red area. The neuron in Fig. 1C was tuned to 24.0 kHz. Its responses to tone bursts were also augmented by the pseudo-conditioning with the CSu at 9.0 kHz. The neuron in Fig. 1D was tuned to 23.0 kHz. Its responses to tone bursts were augmented by the pseudo-conditioning with the CSu at 33.0 kHz.
The lowest and highest BFs of the collicular neurons studied were 20.0 and 34.0 kHz, respectively. The responses of the 20.0-kHz tuned neuron to tone bursts were augmented by the pseudo-conditioning with the CSu at 30.0 kHz and those of the 34.0-kHz tuned neuron were augmented by the pseudo-conditioning with the CSu at 19.0 kHz. Therefore the augmentation of the collicular neurons was nonspecific to the CSu frequencies. On the average, the nonspecific augmentation of the response to the tone burst at the BF and 10 dB above the minimum threshold was 45.8 ± 6.8% (n = 34). In Fig. 1, it is clear that the nonspecific augmentation of the response was accompanied with a broadening of the frequency-tuning curve, a decrease in minimum threshold and an increase in background discharges. The amounts of all these changes were calculated and listed in the first row of Table 1. All these changes disappeared (i.e., the neurons recovered) 3.0 ± 0.3 h after the onset of the pseudo-conditioning.
The sharpness of a frequency-tuning curve is usually expressed by a bandwidth or quality factor (Q-value) at a given dB above the minimum threshold of a neuron, so that we calculated changes in bandwidth and Q-value of a tuning curve elicited by the pseudo-conditioning at 10 and 40 dB above the minimum threshold of a given collicular neuron. Here, the minimum thresholds were those measured before and after the pseudo-conditioning. The percent increase in 10 dB bandwidth and the percent decrease in Q-10 dB value (mean and SE) were 28.4 ± 4.5 (n = 18; t-test, P = 0.0026) and 20.4 ± 2.5% (n = 18; P = 0.0039), respectively. The percent increase in 40 dB bandwidth and the percent decrease in Q-40 dB value were 65.3 ± 4.8 (n = 14; P = 0.0024) and 38.8 ± 1.8% (n = 14; P = 0.0018), respectively. Therefore the frequency-tuning curves of the collicular neurons were significantly broadened by pseudo-conditioning.
The pseudo-conditioning elicited a small short-lasting BF shift in addition to the nonspecific augmentation when the CSu was 5.0 kHz lower than the BF of a given neuron. For example, Fig. 1B (middle) shows the BF shift in addition to the nonspecific augmentation of the neuron tuned to 23.0 kHz. The BF shift toward the CSu at 18.0 kHz was small, 1.0 kHz, and disappeared within 45 min after the onset of the pseudo-conditioning. This small short-lasting BF shift may be behaviorally insignificant, but it is important in understanding the neural circuit evoking it (see discussion).
The nonspecific augmentation and tone-specific BF shift are further shown with the frequency-response curves of four other collicular neurons obtained at 10 dB above their minimum thresholds. Their BFs were either the same as (A), 5.0 kHz higher (B), 15.0 kHz higher (C), or 10.0 kHz lower (D) than the CSu frequencies (Fig. 2, ○). The responses of all four neurons to tone bursts were augmented by the pseudo-conditioning regardless of whether the BFs of the neurons were the same as or different from the CSu frequencies (Fig. 2, •). The amount of augmentation was 62% in A, 40% in B, 57% in C, and 51% in D when measured at their BFs 30 min after the onset of the pseudo-conditioning.
In Fig. 2B, the BF of the collicular neuron was 5.0 kHz higher than the CSu frequency. It showed a 1.0-kHz BF shift 30 min after the onset of pseudo-conditioning (•). This BF shift disappeared at ∼45 min after the onset of the pseudo-conditioning (▴), whereas the nonspecific augmentation took ∼180 min to disappear (dashed line). The BF shift elicited by the pseudo-conditioning was small (1.0 ± 0.1 kHz, n = 8; Table 1, 1st row) and short-lasting. These BF shifts occurred toward the CSu frequencies and were statistically significant in 8 of the 12 neurons studied (P < 0.01).
Shortening of the response latencies of collicular neurons elicited by pseudo-conditioning
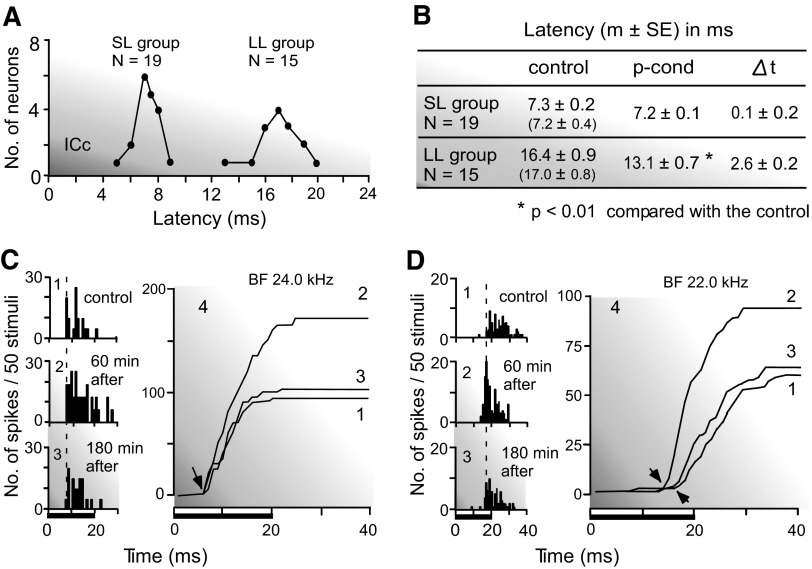
It is a common phenomenon that the stronger the response, the shorter the response latency is. Therefore the response latency of a collicular neuron was measured before and after the nonspecific augmentation. It was defined as the time interval between the onset of a tone burst at the BF and 10 dB above the minimum threshold of a given neuron and the deflection point of a poststimulus time cumulative histogram displaying the response of the neuron to the tone burst. The response latencies of 34 neurons studied showed a bimodal distribution (Fig. 3A). The mean and SE of the latencies in the control condition were 7.3 ± 0.2 ms (n = 19) for the short-latency group and 16.4 ± 0.9 ms (n = 15) for the long-latency group. The long-latency responses appeared before the offset of the 20-ms-long tone burst, so that they were on-responses. The nonspecific augmentation had little effect on the response latencies of the short-latency neurons (Δt = 0.1 ± 0.2 ms), but it significantly shortened those of the long-latency neurons (Δt = 2.6 ± 0.2 ms). The poststimulus time and poststimulus time cumulative histograms in Fig. 3, C and D, display the responses of single neurons to 50 identical tone bursts at their BFs and 10 dB above their minimum thresholds. The response latency of the neuron in Fig. 3C was short, 7.0 ms in the control condition. It did not shorten despite a 56% increase in the response magnitude elicited by the pseudo-conditioning. On the other hand, the response latency of the neuron in Fig. 3D was long: 16.5 ms in the control condition. Its response magnitude increased by 40% for the pseudo-conditioning, and its latency shortened from 16.5 to 14.0 ms. The response latencies reverted back to the control values ∼180 min after the onset of the pseudo-conditioning (Fig. 3B, table).
FIG. 3.
Shortening of the response latency accompanied with the augmentation of a response elicited by p-cond. A: the bimodal distribution of the latencies of collicular neurons: short (SL)- and long (LL)-latency groups. B: the means and SE of the response latencies of neurons in these 2 groups measured before and after the p-cond. The latencies in the recovered condition are shown in the parentheses. Δt: change in latency. C and D: peristimulus time (PST; 1–3) and PST cumulative (4) histograms show the responses of 2 neurons (C and D) to tone bursts delivered 50 times. The tone burst was 24.0 kHz, 35 dB SPL (C) or 22.0 kHz, 40 dB SPL (D). Its duration was 20 ms (horizontal bars at bottom). The minimum threshold of the neuron was 25 (C) or 30 (D) dB SPL. 1–3, control, 60 and 180 min after the onset of the p-cond. The arrows in C indicate no latency shift, but those in D indicate the shift.
The short- and long-latency groups were not different from each other in BF (21.5 ± 0.45 vs. 23.3 ± 1.2 kHz), nonspecific augmentation measured at their BFs (55.7 ± 7.6 vs. 43.9 ± 6.4%), and bandwidth at 10 dB above a minimum threshold (1.8 ± 0.23 vs. 2.1 ± 0.27 kHz; P > 0.1).
Time course of the collicular nonspecific augmentation and small BF shift elicited by pseudo-conditioning
The collicular nonspecific augmentation gradually developed to a peak over 30 min after the onset of the pseudo-conditioning. It slowly decreased and disappeared ∼180 min after that on the average (Fig. 4A, curve 1). The array of the poststimulus time histograms in Fig. 4B1 shows the time course of such a nonspecific augmentation of a collicular neuron tuned to 24.0 kHz. The small BF shift elicited by the pseudo-conditioning could be detected 30 min after the onset of pseudo-conditioning, but it was hardly detected 45 min after. On the average, it disappeared 40 ± 10 min after the pseudo-conditioning. Therefore we did not obtain enough data points for plotting the time course of the small BF shift. This short-lasting BF shift is shown in Figs. 2B, 6A, 7A, and 8A. It was noticed that the time course of the large collicular BF shift elicited by fear conditioning (Gao and Suga 1998; Ji et al. 2001) was similar to that of the nonspecific augmentation shown by curve 1 of Fig. 4.
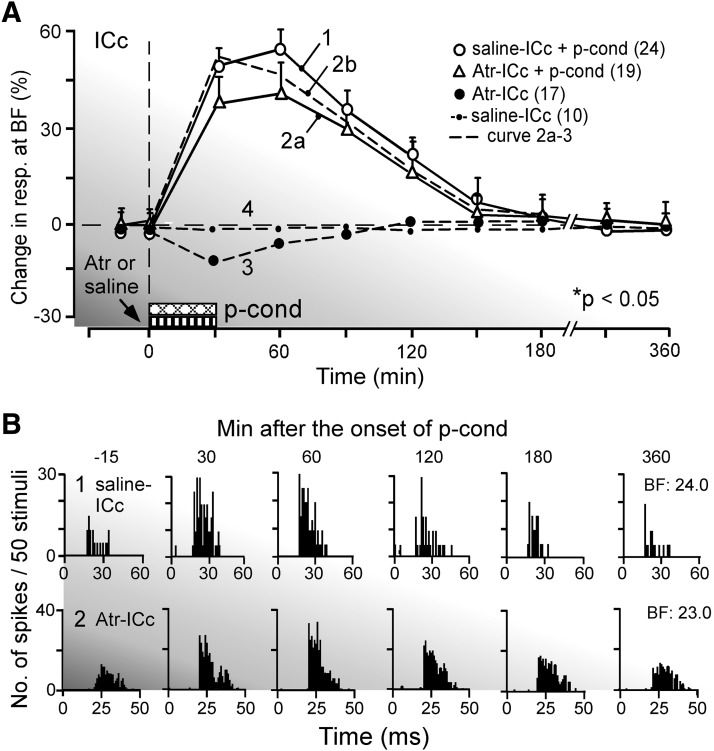
FIG. 4.
The time course of nonspecific augmentation elicited by p-cond. A: the change in response magnitude after the onset of p-cond. 1: the p-cond delivered immediately after a saline application to the central nucleus of the inferior colliculus (ICc). 2a: the p-cond delivered immediately after an atropine application to the ICc. 3: an atropine application to the ICc without the p-cond. 4: a saline application to the ICc without the p-cond. The change is expressed in the percentage of the response obtained before the p-cond. Each data point represents a mean and SE of the data obtained from several single neurons indicated by the numbers in the parentheses. 2b: curve 2a –curve 3. Curve 2a was different from curve 1 (P < 0.05), whereas curve 2b was not different from curve 1. B: the arrays of PST histograms in 1 and 2, respectively, show the responses at the BFs of 2 collicular neurons to tone bursts obtained −15 (control), 30, 60, 120, 180, and 360 min after the p-cond with a saline (1) or atropine (2) application to the ICc. The tone burst was either 24.0 (B1) or 23.0 (B2) kHz and was repeated 50 times to obtain each histogram.
Effects of muscarinic acetycholine receptor-antagonist applied to the IC on the collicular BF shift and nonspecific augmentation elicited by pseudo-conditioning
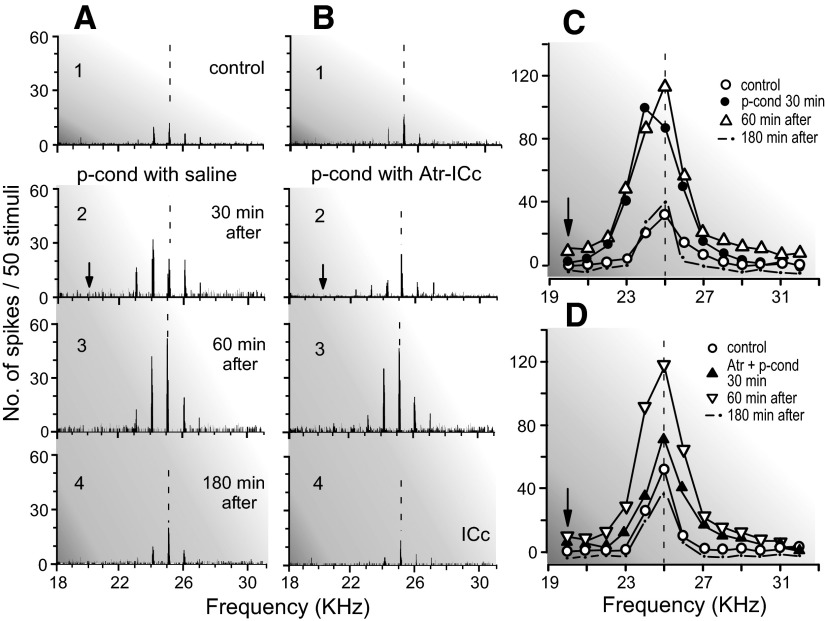
Because the collicular BF shift evoked by auditory fear conditioning is abolished by atropine applied to the ICc (Ji et al. 2001), atropine was also applied to the ICc to test whether the small collicular BF shift elicited by the pseudo-conditioning was abolished. None of the 19 neurons studied with atropine showed BF shifts but showed nonspecific augmentation for the pseudo-conditioning. It has been shown that atropine applied to the ICc, without the pseudo-conditioning, reduces collicular auditory responses by 14 ± 7.0% at 30 min after its application and that the reduction gradually disappears within 90 min after that (Fig. 4A, curve 3; Ji et al. 2001). Therefore it was expected that atropine applied to the ICc before the pseudo-conditioning would reduce the pseudo-conditioning–dependent augmentation of the auditory responses of collicular neurons. In fact, atropine reduced the augmentation of the auditory response by 15 ± 9.2% on average (Fig. 4A, curve 2a). This reduction in the augmentation was the same as that in the response elicited by atropine (curve 3). When the direct atropine effect (curve 3) was subtracted from curve 2a, the time course of the augmentation caused by the expression of the pseudo-conditioning under atropine is obtained (curve 2b, dashed line). Curve 2b indicates that the development of the nonspecific augmentation was little affected by atropine. A saline solution applied to the ICc had no effect at all on the auditory responses of collicular neurons (Fig. 4A, curve 4). The array of the poststimulus time histograms in Fig. 4B2 shows the change evoked by atropine and the pseudo-conditioning in the response of a collicular neuron tuned to 23.0 kHz. These histograms show a slightly reduced augmentation, as represented by curve 2a in Fig. 4A. Table 1 indicates that the augmentation elicited by the pseudo-conditioning alone was 45.8 ± 6.8% (group 1), but the augmentation elicited by that with atropine was 38.0 ± 9.5% (group 2). The difference between these two was insignificant. When the direct atropine effect on the auditory response (−14.0 ± 7.0%; Ji et al. 2001) was subtracted from 38.0 ± 9.5%, the sum was 52.0 ± 8.3%, which was also insignificantly different from 45.8 ± 6.8%.
The collicular neuron in Fig. 5A, for example, was sharply tuned to 22.0 kHz (Fig. 5A1). Its response to a 22.0-kHz tone burst was augmented by 40% at its BF and 10 dB above its minimum threshold; its minimum threshold was lowered from 50 dB SPL to 40 dB SPL by the pseudo-conditioning (Fig. 5A3), but the neuron showed no BF shift toward the CSu frequency: 17.0 kHz. The effects of atropine on the BF shift and nonspecific augmentation are further shown in Fig. 6 with the array of poststimulus time histograms displaying the responses and the frequency-response curves of a collicular neuron tuned to 25.0 kHz. Because a collicular neuron did not necessarily show a BF shift for the pseudo-conditioning (Table 1, group 1), the BF shift of the neuron was first confirmed (Fig. 6A), and then the pseudo-conditioning accompanied with an atropine application to the ICc was delivered to the animal (Fig. 6B). As shown in Fig. 6A, the pseudo-conditioning with a saline application evoked both the BF shift and nonspecific augmentation of the neuron 30 min after the onset of its delivery (Fig. 6A2). It also augmented background discharges of the neuron. The BF shift disappeared 60 min after that, but the augmentation remained (Fig. 6A3). After the recovery of the neuron from the effect of the pseudo-conditioning, the second pseudo-conditioning accompanied with atropine was delivered to the animal, and the same neuron as that in Fig. 6A was restudied. It did not show the BF shift. However, the augmentation of its response to tone bursts and background discharges occurred 30 min after the onset of the pseudo-conditioning (Fig. 6B, 2 and 3). These data obtained with atropine indicate that the neuromodulator for the nonspecific augmentation is different from that for the BF shift.
FIG. 5.
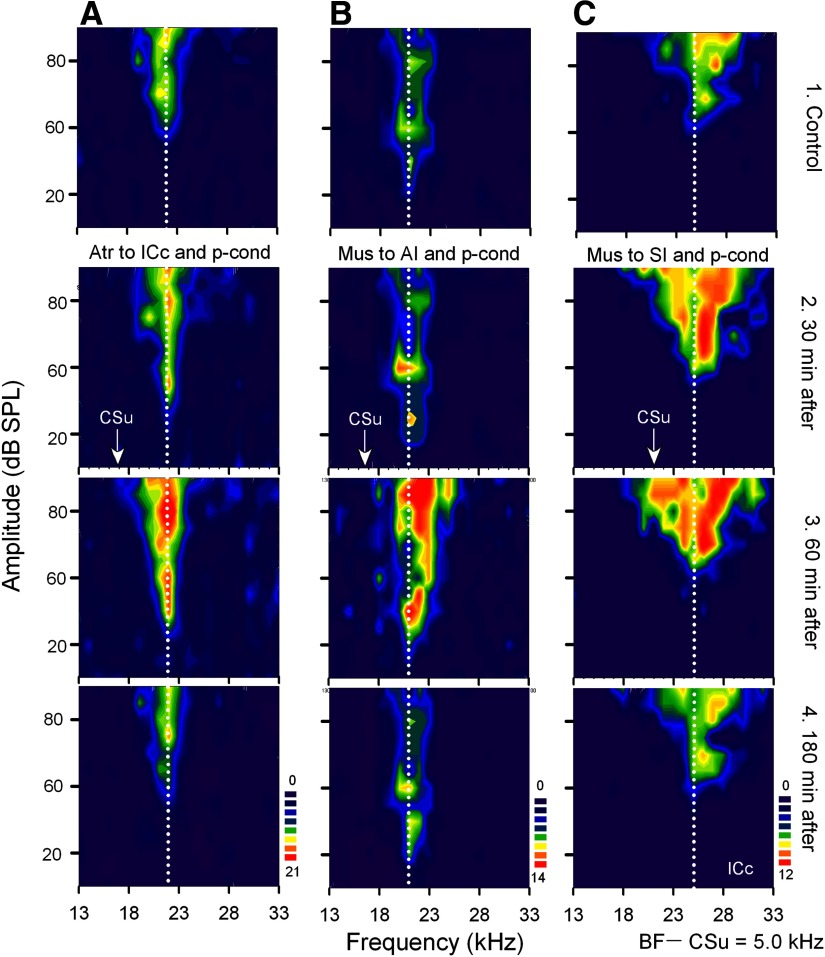
Effects of atropine applied to the ICc (A) and of muscimol applied to the primary auditory cortex (AI) (B) or somatosensory cortex (SI) (C) on the nonspecific augmentation and BF shift of collicular neurons elicited by p-cond. Atropine (Atr) and muscimol (Mus) were homolaterally applied to the ICc or AI, respectively, whereas muscimol was bilaterally applied to SI. The BFs of the 3 different collicular neurons (A–C) were 22.0, 21.0, and 25.0 kHz (vertical dotted lines). The arrows along the frequency axes indicate the frequencies of the CSu, which were 5.0 kHz lower than the BFs of the recorded neurons. In each column, 1–4 show the receptive fields obtained before (1), 30 (2), 60 (3), and 180 min after (4) the onset of the p-cond. See Fig. 1 legend for the key.
FIG. 6.
Effects of atropine (Atr) applied to the ICc on the nonspecific augmentation and BF shift of a single collicular neuron elicited by p-cond. The BF of the neuron (25.0 kHz) was 5.0 kHz higher than the CSu frequency (arrows). The arrays of the PST histograms in A and B show the responses of the same single neuron recorded before (1, control), 30 (2), 60 (3), and 180 (4) min after the p-cond without (A) or with (B) an atropine application to the ICc, respectively. The frequency-response curves in C and D originated from A1-4 and B1-4, respectively. The vertical dashed lines indicate the BF of the neuron in the control condition. Note that atropine applied to the ICc blocked the BF shift but not the nonspecific augmentation.
Effects of an inactivation of AI or SI on the collicular BF shift and nonspecific augmentation elicited by pseudo-conditioning
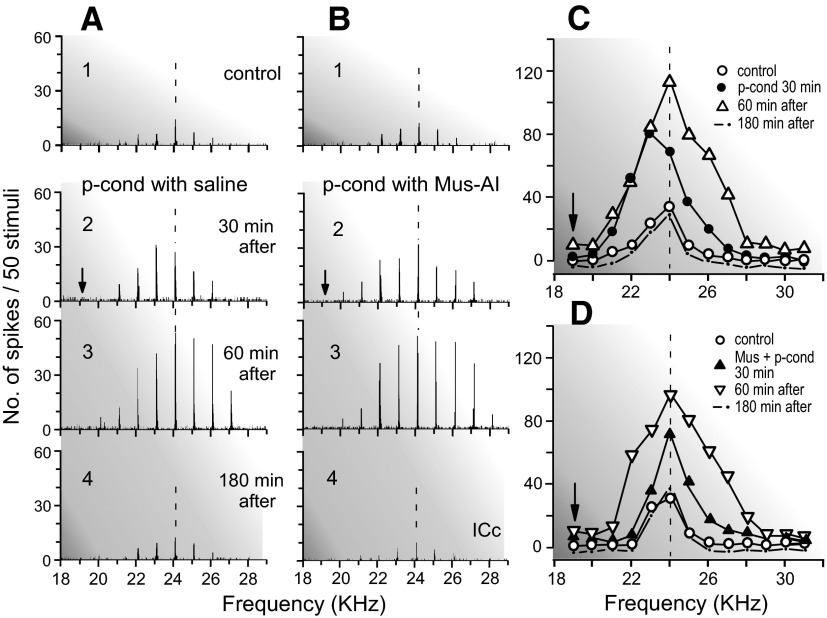
Ipsilateral inactivation of AI by muscimol abolishes the collicular BF shifts elicited by auditory fear conditioning without affecting their auditory responses (Gao and Suga 1998, 2000). Therefore the effect of an ipsilateral muscimol application to AI was studied on the BF shift and nonspecific augmentation of 17 collicular neurons elicited by the pseudo-conditioning. None of these 17 neurons showed BF shifts but delayed nonspecific augmentation (Table 1, group 3). There was no sign that their auditory responses were directly affected by muscimol applied to AI.
Figure 5B shows the receptive field of a collicular neuron tuned to 21.0 kHz (Fig. 5B1). When muscimol was ipsilaterally applied to AI before the pseudo-conditioning, the neuron did not show a BF shift, but the nonspecific augmentation, which, 30 min after the onset of the pseudo-conditioning, was smaller than that elicited by the pseudo-conditioning without muscimol. However, it was prominent ∼60 min after the onset of the pseudo-conditioning (Fig. 5B3) and disappeared ∼180 min after that (Fig. 5B4). The effects of muscimol applied to AI on the BF shift and nonspecific augmentation are further shown with the array of poststimulus time histograms and the frequency-response curves of a neuron tuned to 24.0 kHz (Fig. 7). First, the pseudo-conditioning was delivered to the animal with an application of a saline solution to AI (Fig. 7A). Both the BF shift and nonspecific augmentation were clear 30 min after the pseudo-conditioning (Fig. 7A2). The BF shift disappeared, but the augmentation was further developed 60 min after that (Fig. 7A3). About 30 min after the recovery of the neuron from the changes elicited by the pseudo-conditioning, the second pseudo-conditioning was delivered to the animal together with a muscimol application to AI, and the same collicular neuron was restudied (Fig. 7B). The neuron did not show a BF shift but nonspecific augmentation 30 min after the onset of the second pseudo-conditioning (Fig. 7B2). The augmentation further developed between 30 and 60 min after that (Fig. 7B3).
FIG. 7.
Effects of muscimol (Mus) ipsilaterally applied to AI on the nonspecific augmentation and BF shift of a single collicular neuron elicited by p-cond. The BF of the neuron (24.0 kHz) was 5.0 kHz higher than the CSu frequency (arrows). A and B show the arrays of the PST histograms displaying the responses of the neuron recorded before (1), 30 (2), 60 (3), and 180 (4) min after the onset of the p-cond without (A) and with (B) muscimol application to AI, respectively. C and D show the frequency-response curves originated from A1–4 and B1–4, respectively. Note that muscimol applied to AI blocked the BF shift but not the nonspecific augmentation.
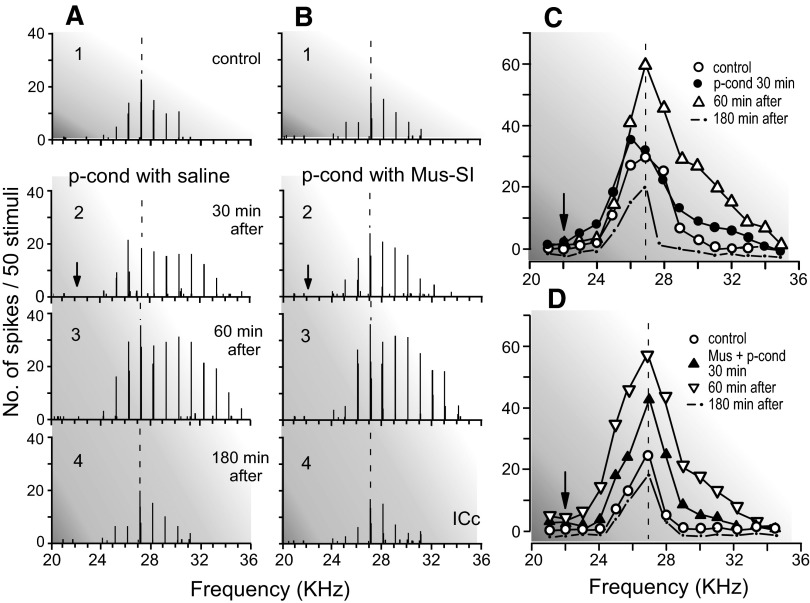
The effects of muscimol bilaterally applied to SI were studied on the BF shifts and nonspecific augmentation of 15 collicular neurons elicited by the pseudo-conditioning. With the muscimol application, none of these 15 neurons showed any BF shifts but showed the nonspecific augmentation (Table 1, group 4). Different from muscimol applied to AI, the nonspecific augmentation was prominent 30 min after the onset of the pseudo-conditioning. There was no sign that these collicular neurons were directly influenced by muscimol applied to SI, because their auditory responses to tone bursts were not reduced at all.
Figure 5C shows the receptive field of a collicular neuron tuned to 25.0 kHz (Fig. 5C1). When muscimol was bilaterally applied to SI before the pseudo-conditioning, the neuron showed no BF shift, but showed the nonspecific augmentation (Fig. 5C2). The nonspecific augmentation was strong ∼60 min after the onset of the pseudo-conditioning (Fig. 5C3). It disappeared ∼180 min after that (Fig. 5C4). The effects of muscimol bilaterally applied to SI on the BF shift and nonspecific augmentation are further shown with the poststimulus time histograms and frequency-response curves of a collicular neuron tuned to 27.0 kHz (Fig. 8). The pseudo-conditioning reduced its response at 27.0 kHz and increased its response at 26.0 kHz, so the BF of the neuron shifted toward the CSu frequency 30 min after the onset of the pseudo-conditioning. In addition to the BF shift, the nonspecific augmentation was also clear (Fig. 8A2). The pseudo-conditioning and SI inactivation with muscimol, however, did not evoke the BF shift of the neuron (Fig. 8B2). The nonspecific augmentation 60 min after the onset of the pseudo-conditioning with the SI inactivation (Fig. 8B3) was the same as that elicited by the pseudo-conditioning alone (Fig. 8A3). The data obtained with muscimol indicate that neither AI nor SI is involved in evoking the collicular nonspecific augmentation.
FIG. 8.
Effects of muscimol (Mus) bilaterally applied to SI on the nonspecific augmentation and BF shift of a single collicular neuron elicited by p-cond. The BF of the neuron (27.0 kHz) was 5.0 kHz higher than the CSu frequency (arrows). A and B show the arrays of the PST histograms displaying the responses of the same single neuron recorded before (1), 30 (2), 60 (3), and 180 (4) min after the onset of the p-cond without (A) and with (B) a muscimol application to SI, respectively. C and D show the frequency-response curves originated from A1–4 and B1–4, respectively. Note that muscimol applied to SI blocked the BF shift but not the nonspecific augmentation.
DISCUSSION
Collicular BF shifts elicited by pseudo-conditioning
In our current experiments, the unpaired US was not delivered to the animal in the time period between 5 s before and after the CSu to avoid CSu -unpaired US association. However, one may argue that weak CSu -unpaired US association might occur and cause the small collicular BF shift, although the pseudo-conditioning was nonassociative training. This simple and straightforward speculation may not necessarily be correct, because it has been repeatedly shown that the small short-term BF shift is evoked without the CS-US association, as listed below. 1) A “long” repetitive tone burst stimulation lasting 30 min evokes a small short-term collicular BF shift (Gao and Suga 1998; Ma and Suga 2001, 2003; Yan and Suga 1998). 2) A “short” repetitive tone burst stimulation lasting 1.0 s delivered every 30 s over 30 min (i.e., CS) evokes the subthreshold (for the measurement of a BF shift, the frequency of a tone burst is varied by 0.5-kHz steps; therefore the BF shift <0.5 kHz is statistically insignificant and is defined as a subthreshold BF shift) collicular BF shift (Gao and Suga 1998), which is augmented by ACh released by the nucleus basalis (NB) into the cortex (Ji et al. 2001). 3) Acoustic stimulation paired with electric stimulation of the NB evokes the collicular BF shift (Ma and Suga 2003; Zhang et al. 2005). 4) Electric stimulation of AI evokes the collicular BF shift, which is augmented by electric stimulation of SI; this augmentation is blocked by a lesion of the NB (Ma and Suga 2001, 2003). 5) Electric stimulation of the ventral division of the medial geniculate body (MGBv; Wu and Yan 2007) or the ICc (Zhang and Suga 2005) evokes the collicular BF shift. 6) An increase in the cortical ACh level is essential for augmenting the subthreshold cortical BF shift elicited by CS alone. Sensory stimuli excite a corresponding sensory cortex and, without CS-US association, increases the cortical ACh level through the prefrontal cortex and NB (Golmayo et al. 2003; Rasmusson et al. 2007; Zaborszky et al. 1999).
Interpretation of the effect of atropine applied to the IC on the collicular BF shift and nonspecific augmentation
Atropine applied to the ICc reduces the collicular response to a tone burst by 14% (Ji et al. 2001). In the normal condition, therefore, the response is slightly augmented by ACh spontaneously released into the ICc by the cholinergic brain stem nucleus (Motts and Schofield 2006). Atropine applied to AI abolishes the conditioning-elicited cortical BF shift and reduces the conditioning-elicited collicular BF shift by ∼30%. Atropine applied to the ICc blocks the conditioning-elicited collicular BF shift and reduces the conditioning-elicited cortical BF shift by ∼25% (Ji et al. 2001). Focal electric stimulation of AI evokes the collicular BF shift (Ma and Suga 2001, 2003; Yan and Suga 1998; Zhang et al. 1997). These experiments indicate that the ICc can produce the BF shift with the corticofugal signals and ACh. In our current experiments, atropine applied to the ICc blocked the pseudo-conditioning-elicited collicular BF shift. However, it did not block the nonspecific augmentation. The pseudo-conditioning-elicited collicular nonspecific augmentation apparently depends on neither the corticofugal system nor ACh. Atropine applied to AI blocks the pseudoconditioning-elicited cortical BF shift, but it does not block the pseudoconditioning-elicited cortical nonspecific augmentation (Ji and Suga 2008; Table 1, line 4). Therefore the cortical nonspecific augmentation also does not depend on ACh. Noncholinergic neuromodulators presumably play an important role in evoking the collicular and cortical nonspecific augmentation.
Interpretation of the effect of muscimol applied to AI on the collicular BF shift and nonspecific augmentation
As described above, the collicular BF shift is evoked by focal electric stimulation of AI through the corticofugal system (Ma and Suga 2001, 2003; Yan and Suga 1998; Zhang et al. 1997). Inactivation of AI with muscimol abolishes the collicular BF shift elicited by the conditioning without affecting the collicular frequency tuning (Gao and Suga 1998, 2000). It also abolishes the small collicular BF shift elicited by the pseudo-conditioning without abolishing the collicular nonspecific augmentation. These findings indicate that muscimol applied to AI does not diffuse into the ICc.
The corticofugal modulation in the big brown bat (for review, see Suga et al. 2000) and rodents (Yan et al. 2005) consists of facilitation focused on BF-matched subcortical neurons and widespread inhibition of BF-unmatched subcortical neurons at their BFs. Such corticofugal modulation seems to be unsuitable for evoking the subcortical nonspecific augmentation dependent on the pseudo-conditioning. In fact, muscimol applied to AI did not block the collicular nonspecific augmentation. Therefore the collicular nonspecific augmentation was not evoked by the corticofugal system. Muscimol applied to AI temporarily reduced the collicular responses to tone bursts by 38 ± 8.1% (Gao and Suga 1998) and delayed the augmentation. This reduction can be explained by blocking the on-going corticofugal facilitation in the normal condition as dscribed above.
Interpretation of the effect of muscimol applied to SI on the collicular BF shift and nonspecific augmentation
Bilateral inactivation of SI by muscimol blocks the development of the cortical and collicular BF shifts elicited by auditory fear conditioning without affecting their auditory responses and frequency tunings (Gao and Suga 2000). Electric stimulation of SI after, not before, electric stimulation of AI augments the small BF shift evoked by the AI stimulation (Ma and Suga 2001, 2003). Furthermore, Ji and Suga (2008) and our current experiments showed that the pseudo-conditioning elicited not only the nonspecific augmentation but also the small short-lasting cortical and collicular BF shifts and that bilateral SI inactivation with muscimol abolishes the development of these BF shifts without affecting the auditory responses and nonspecific augmentation of these auditory neurons. These inactivation and activation experiments clearly indicate that muscimol applied to SI does not diffuse into either AI or ICc, that SI plays an important role in evoking the conditioning-elicited and pseudo-conditioning–elicited cortical and collicular BF shifts, and that the interpretation—SI does not play a role in eliciting the conditioning-dependent cortical BF shift (Weinberger 2004, 2007)—is not supported.
Comparison between the collicular and cortical nonspecific augmentation
Because muscimol applied to SI had no direct effect on the auditory system, nonspecific augmentation elicited by the pseudo-conditioning with SI inactivation was the same as that elicited by the pseudo-conditioning alone, so the data shown in groups 1 and 4 of Table 1 are added together for a statistical analysis of the difference in nonspecific augmentation between AI and the ICc (group 5). The increase in response and frequency-tuning width both are significantly larger for AI than for the ICc. This may be partly attributed to the nonspecific augmentation evoked by the medial division of the medial geniculate body (MGBm) and the posterior intralaminar nucleus (PIN) projecting to the auditory cortex, i.e., by the nonlemniscal pathway. Namely, we may speculate that the nonspecific augmentation of AI neurons consists of the lemniscal (44.7% × 100%/57.3% = 78.0%) and nonlemniscal (12.6% × 100%/57.3% = 22.0%) origins, and that the increase in the frequency-tuning width of AI neurons also consists of the lemniscal (2.1% × 100%/3.8% = 55.2%) and nonlemniscal (1.7% × 100%/3.8% = 44.7%) origins.
Our current data and the Gao-Suga and Suga models
The Gao-Suga model (Gao and Suga 1998; for review, see Suga and Ma 2003) states that the ventral division of the medial geniculate body (MGBv), AI, and corticofugal feedback are involved in evoking the subthreshold or small cortical and collicular BF shifts, that these BF shifts are augmented by the cholinergic neuromodulator, and that SI and AI both play a role in indirectly activating this cholinergic neuromodulator. Our current and previous (Ji and Suga 2008) data clearly indicate that both SI and the cholinergic neuromodulator are involved in evoking the BF shifts regardless of whether they are elicited by the pseudo-conditioning or conditioning. The Suga model (Suga 2008) states that the MGBm and PIN are involved in evoking a small cortical nonspecific augmentation; that this augmentation is further augmented by noncholinergic neuromodulators; and that SI is not involved in evoking the nonspecific augmentation. This model did not hypothesize anything about the collicular nonspecific augmentation. Our current data clearly indicate that the pseudo-conditioning elicits the collicular nonspecific augmentation in addition to the collicular BF shift and that SI and a cholinergic neuromodulator are not involved in evoking this nonspecific augmentation. This collicular nonspecific augmentation would be transferred to AI through the MGBv. However, the cortical nonspecific augmentation does not simply depend on the collicular one, because the former is 22.0% larger than the latter. The additional augmentation is presumably evoked in AI by the MGBm and PIN as hypothesized by Suga (2008). The Suga model should be incorporated with the neural circuit for the collicular nonspecific augmentation, which remains to be further explored.
Our current data indicate that the nonspecific augmentation does not depend on ACh. There are a number of noncholinergic neuromodulators. It remains to be identified which neuromodulators play an important role in evoking the nonspecific augmentation: dopamine, noradrenaline, histamine, etc.
Our previous experiment (Ji and Suga 2008) indicates that the pseudo-randomized US plays an essential role in eliciting the nonspecific augmentation of cortical auditory neurons, and the CS or CSu, not pseudo-randomized, plays a role in eliciting a small short-lasting cortical BF shift. Bakin et al. (1992) trained a guinea pig with pseudo-randomized visual and electric foot stimuli. Its cortical auditory neurons were sensitized to auditory stimuli. This finding also indicates that the pseudo-randomized US plays an essential role in eliciting the nonspecific augmentation. If the CSu is simultaneously delivered with the randomized US keeping their onsets in register, they may elicit nonspecific augmentation, because randomized electric leg-shock alone elicits it (Ji and Suga 2008).
The unpaired US is an aversive stimulus. The neural substrate for evoking defensive behaviors, such as escape, jumping, crouching, startle, freezing, and vocal responses, has been called the “brain aversion” system. This system consists of the medial hypothalamus, amygdala, and dorsal periaqueductal gray. In addition, it may include the inferior and superior colliculi (Brandao et al. 2003 for review). The ICc becomes active after an electrical or chemical stimulus of the dorsal periaqueductal gray (Brandao et al. 2008) or exposure of animals to aversive environmental stimuli (Silveira et al. 1993, 1995, 2001). The collicular activity is modulated by dopamine when animals show defensive behaviors (Cuadra et al. 2000). Electrical and chemical stimulation of the ICc elicits the defensive behaviors, which are the same as those elicited by the stimulation of the dorsal periaqueductal gray (for review, see Brandao et al. 2008), and these behaviors are accompanied by changes in the collicular auditory-evoked potentials (Brandao et al. 2001). Therefore the collicular nonspecific augmentation evoked by the pseudo-conditioning might be mediated by the brain aversion system and dopamine.
GRANTS
This work was supported by National Institute on Deafness and Other Communication Disorders Grant DC-000175.
Acknowledgments
We thank S. E. Miller for editing this manuscript.
REFERENCES
- Bakin et al. 1992.Bakin JS, Lepan B, Weinberger NM. Sensitization induced receptive field plasticity in the auditory cortex is independent of CS-modality. Brain Res 577: 226–235, 1992. [DOI] [PubMed] [Google Scholar]
- Brandao et al. 2001.Brandao ML, Coimbra NC, Osaki MY. Changes in the auditory-evoked potentials induced by fear-evoking stimulations. Physiol Behav 72: 365–372, 2001. [DOI] [PubMed] [Google Scholar]
- Brandao et al. 2003.Brandao ML, Troncoso AC, De Souza Silva MA, Huston JP. The relevance of neuronal substrates of defense in the midbrain tectum to anxiety and stress: empirical and conceptual considerations. Eur J Pharmacol 463: 225–233, 2003. [DOI] [PubMed] [Google Scholar]
- Brandao et al. 2008.Brandao ML, Zanoveli JM, Ruiz-Martinez RC, Oliveira LC, Landeira-Fernandez J. Different patterns of freezing behavior organized in the periaqueductal gray of rats: association with different types of anxiety. Behav Brain Res 188: 1–13, 2008. [DOI] [PubMed] [Google Scholar]
- Casseday and Covey 1992.Casseday JH, Covey E. Frequency tuning properties of neurons in the inferior colliculus of an FM bat. J Comp Neurol 319: 34–50, 1992. [DOI] [PubMed] [Google Scholar]
- Cuadra et al. 2000.Cuadra G, Zurita A, Macedo CE, Molina VA, Brandao ML. Electrical stimulation of the midbrain tectum enhances dopamine release in the frontal cortex. Brain Res Bull 52: 413–418, 2000. [DOI] [PubMed] [Google Scholar]
- Dear et al. 1993.Dear SP, Fritz J, Haresign T, Ferragamo M, Simmons JA. Tonotopic and functional organization in the auditory cortex of the big brown bat, Eptesicus fuscus. J Neurophysiol 70: 1988–2009, 1993. [DOI] [PubMed] [Google Scholar]
- Erickson and Walters 1988.Erickson MT, Walters ET. Differential expression of pseudoconditioning and sensitization by siphon responses in Aplysia: novel response selection after training. J Neurosci 8: 3000–3010, 1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferragamo et al. 1998.Ferragamo MJ, Haresign T, Simmons JA. Frequency tuning, latencies, and responses to frequency-modulated sweeps in the inferior colliculus of the echolocating bat, Eptesicus fuscus. J Comp Physiol [A] 182: 65–79, 1998. [DOI] [PubMed] [Google Scholar]
- Gao and Suga 1998.Gao E, Suga N. Experience-dependent corticofugal adjustment of midbrain frequency map in bat auditory system. Proc Natl Acad Sci USA 95: 12663–12670, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao and Suga 2000.Gao E, Suga N. Experience-dependent plasticity in the auditory cortex and the inferior colliculus of bats: role of the corticofugal system. Proc Natl Acad Sci USA 97: 8081–8086, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golmayo et al. 2003.Golmayo L, Nunez A, Zaborszky L. Electrophysiological evidence for the existence of a posterior cortical-prefrontal-basal forebrain circuitry in modulating sensory responses in visual and somatosensory rat cortical areas. Neuroscience 119: 597–609, 2003. [DOI] [PubMed] [Google Scholar]
- Ji et al. 2001.Ji W, Gao E, Suga N. Effect of acetylcholine and atropine on plasticity of central auditory neurons caused by conditioning in bats. J Neurophysiol 86: 211–225, 2001. [DOI] [PubMed] [Google Scholar]
- Ji and Suga 2003.Ji W, Suga N. Development of reorganization of the auditory cortex caused by fear conditioning: effect of atropine. J Neurophysiol 90: 1904–1909, 2003. [DOI] [PubMed] [Google Scholar]
- Ji and Suga 2008.Ji W, Suga N. Tone-specific and nonspecific plasticity of the auditory cortex elicited by pseudoconditioning: role of acetylcholine receptors and the somatosensory cortex. J Neurophysiol 100: 1384–1396, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krubitzer and Calford 1992.Krubitzer LA, Calford MB. Five topographically organized fields in the somatosensory cortex of the flying fox: microelectrode maps, myeloarchitecture, and cortical modules. J Comp Neurol 317: 1–30, 1992. [DOI] [PubMed] [Google Scholar]
- Lomber et al. 1999.Lomber SG, Payne BR, Horel JA. The cryoloop: an adaptable reversible cooling deactivation method for behavioral or electrophysiological assessment of neural function. J Neurosci Methods 86: 179–194, 1999. [DOI] [PubMed] [Google Scholar]
- Ma and Suga 2001.Ma X, Suga N. Plasticity of bat's central auditory system evoked by focal electric stimulation of auditory and/or somatosensory cortices. J Neurophysiol 85: 1078–1087, 2001. [DOI] [PubMed] [Google Scholar]
- Ma and Suga 2003.Ma X, Suga N. Augmentation of plasticity of the central auditory system by the basal forebrain and/or somatosensory cortex. J Neurophysiol 89: 90–103, 2003. [DOI] [PubMed] [Google Scholar]
- Motts et al. 2006.Motts SD, Slusarczyk AS, Sowick CS, Schofield BR. Distribution of cholinergic cells in guinea pig brainstem. Neuroscience 154: 186–195, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmusson et al. 2007.Rasmusson DD, Smith SA, Semba K. Inactivation of prefrontal cortex abolishes cortical acetylcholine release evoked by sensory or sensory pathway stimulation in the rat. Neuroscience 149: 232–241, 2007. [DOI] [PubMed] [Google Scholar]
- Riquimaroux et al. 1991.Riquimaroux H, Gaioni SJ, Suga N. Cortical computational maps control auditory perception. Science 251: 565–568, 1991. [DOI] [PubMed] [Google Scholar]
- Shen et al. 1997.Shen JX, Chen QC, Jen PH. Binaural and frequency representation in the primary auditory cortex of the big brown bat, Eptesicus fuscus. J Comp Physiol [A] 181: 591–597, 1997. [DOI] [PubMed] [Google Scholar]
- Silveira et al. 1995.Silveira MC, Sandner G, Di Scala G, Graeff FG. c-fos immunoreactivity in the brain following electrical or chemical stimulation of the medial hypothalamus of freely moving rats. Brain Res 674: 265–274, 1995. [DOI] [PubMed] [Google Scholar]
- Silveira et al. 1993.Silveira MC, Sandner G, Graeff FG. Induction of Fos immunoreactivity in the brain by exposure to the elevated plus-maze. Behav Brain Res 56: 115–118, 1993. [DOI] [PubMed] [Google Scholar]
- Silveira et al. 2001.Silveira MC, Zangrossi H, de Barros Viana M, Silveira R, Graeff FG. Differential expression of Fos protein in the rat brain induced by performance of avoidance or escape in the elevated T-maze. Behav Brain Res 126: 13–21, 2001. [DOI] [PubMed] [Google Scholar]
- Suga 1995.Suga N Sharpening of frequency tuning by inhibition in the central auditory system: tribute to Yasuji Katsuki. Neurosci Res 21: 287–299, 1995. [DOI] [PubMed] [Google Scholar]
- Suga 2008.Suga N Role of corticofugal feedback in hearing. J Comp Physiol A Neuroethol Sens Neural Behav Physiol 194: 169–183, 2008. [DOI] [PubMed] [Google Scholar]
- Suga et al. 2000.Suga N, Gao E, Zhang Y, Ma X, Olsen JF. The corticofugal system for hearing: recent progress. Proc Natl Acad Sci USA 97: 11807–11814, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suga and Ma 2003.Suga N, Ma X. Multiparametric corticofugal modulation and plasticity in the auditory system. Nat Rev Neurosci 4: 783–794, 2003. [DOI] [PubMed] [Google Scholar]
- Suga and Manabe 1982.Suga N, Manabe T. Neural basis of amplitude-spectrum representation in auditory cortex of the mustached bat. J Neurophysiol 47: 225–255, 1982. [DOI] [PubMed] [Google Scholar]
- Suga and Tsuzuki 1985.Suga N, Tsuzuki K. Inhibition and level-tolerant frequency tuning in the auditory cortex of the mustached bat. J Neurophysiol 53: 1109–1145, 1985. [DOI] [PubMed] [Google Scholar]
- Suga et al. 2002.Suga N, Xiao Z, Ma X, Ji W. Plasticity and corticofugal modulation for hearing in adult animals. Neuron 36: 9–18, 2002. [DOI] [PubMed] [Google Scholar]
- Weinberger 2007.Weinberger NM Associative representational plasticity in the auditory cortex: a synthesis of two disciplines. Learn Mem 14: 1–16, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberger 2004.Weinberger NM Specific long-term memory traces in primary auditory cortex. Nat Rev Neurosci 5: 279–290, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberger 1998.Weinberger NM Physiological memory in primary auditory cortex: characteristics and mechanisms. Neurobiol Learn Mem 70: 226–251, 1998. [DOI] [PubMed] [Google Scholar]
- Wu and Yan 2007.Wu Y, Yan J. Modulation of the receptive fields of midbrain neurons elicited by thalamic electrical stimulation through corticofugal feedback. J Neurosci 27: 10651–10658, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan et al. 2005.Yan J, Zhang Y, Ehret G. Corticofugal shaping of frequency tuning curves in the central nucleus of the inferior colliculus of mice. J Neurophysiol 93: 71–83, 2005. [DOI] [PubMed] [Google Scholar]
- Yan and Suga 1998.Yan W, Suga N. Corticofugal modulation of the midbrain frequency map in the bat auditory system. Nat Neurosci 1: 54–58, 1998. [DOI] [PubMed] [Google Scholar]
- Zaborszky et al. 1999.Zaborszky L, Pang K, Somogyi J, Nadasdy Z, Kallo I. The basal forebrain corticopetal system revisited. Ann NY Acad Sci 877: 339–367, 1999. [DOI] [PubMed] [Google Scholar]
- Zhang et al. 2005.Zhang Y, Hakes JJ, Bonfield SP, Yan J. Corticofugal feedback for auditory midbrain plasticity elicited by tones and electrical stimulation of basal forebrain in mice. Eur J Neurosci 22: 871–879, 2005. [DOI] [PubMed] [Google Scholar]
- Zhang and Suga 2005.Zhang Y, Suga N. Corticofugal feedback for collicular plasticity evoked by electric stimulation of the inferior colliculus. J Neurophysiol 94: 2676–2682, 2005. [DOI] [PubMed] [Google Scholar]
- Zhang et al. 1997.Zhang Y, Suga N, Yan J. Corticofugal modulation of frequency processing in bat auditory system. Nature 387: 900–903, 1997. [DOI] [PubMed] [Google Scholar]