Abstract
Whereas the entorhinal cortex (EC) receives profuse cholinergic innervations from the basal forebrain and activation of cholinergic receptors has been shown to modulate the activities of the principal neurons and promote the intrinsic oscillations in the EC, the effects of cholinergic receptor activation on GABAergic transmission in this brain region have not been determined. We examined the effects of muscarinic receptor activation on GABAA receptor-mediated synaptic transmission in the superficial layers of the EC. Application of muscarine dose-dependently increased the frequency and amplitude of spontaneous inhibitory postsynaptic currents (IPSCs) recorded from the principal neurons in layer II/III via activation of M3 muscarinic receptors. Muscarine slightly reduced the frequency but had no effects on the amplitude of miniature IPSCs recorded in the presence of tetrodotoxin. Muscarine reduced the amplitude of IPSCs evoked by extracellular field stimulation and by depolarization of GABAergic interneurons in synaptically connected interneuron and pyramidal neuron pairs. Application of muscarine generated membrane depolarization and increased action potential firing frequency but reduced the amplitude of action potentials in GABAergic interneurons. Muscarine-induced depolarization of GABAergic interneurons was mediated by inhibition of background K+ channels and independent of phospholipase C, intracellular Ca2+ release, and protein kinase C. Our results demonstrate that activation of muscarinic receptors exerts diverse effects on GABAergic transmission in the EC.
INTRODUCTION
The entorhinal cortex (EC) mediates the majority of connections between the hippocampus and other cortical areas (Witter et al. 1989, 2000a) and thus is regarded as the gateway to the hippocampus. Sensory inputs from olfactory structures, parasubiculum, perirhinal cortex, claustrum, and amygdala converge onto the superficial layers (layers II/III) of the EC (Burwell 2000) that give rise to dense projections to the hippocampus; the axons of the stellate neurons in layer II of the EC form the perforant path that innervates the dentate gyrus and CA3 (Steward and Scoville 1976), whereas those of the pyramidal neurons in layer III form the temporoammonic pathway that synapses onto the distal dendrites of pyramidal neurons in CA1 and the subiculum (Steward and Scoville 1976; Witter et al. 2000a,b). Moreover, neurons in the deep layers of the EC (layers V/VI) relay a large portion of hippocampal output projections back to the superficial layers of the EC (Dolorfo and Amaral 1998a,b; Kohler 1986; van der Linden and Lopes da Silva 1998) and to other cortical areas (Witter et al. 1989). The EC is part of a network that aids in the consolidation and recall of memories (Dolcos et al. 2005; Haist et al. 2001; Squire et al. 2004; Steffenach et al. 2005). Neuronal pathology and atrophy of the EC are potential contributors to Alzheimer's disease (Hyman et al. 1984; Kotzbauer et al. 2001) and schizophrenia (Arnold et al. 1991; Falkai et al. 1988; Joyal et al. 2002; Prasad et al. 2004). Furthermore, the EC participates in the induction and maintenance of temporal lobe epilepsy (Avoli et al. 2002; Spencer and Spencer 1994).
The cholinergic system plays a fundamental role in cortical function with respect to attention, learning, and memory (Hasselmo 2006). Cholinergic innervation of the cerebral cortex originates mainly from the basal forebrain (Amaral and Kurz 1985; Rye et al. 1984; Shute and Lewis 1967); the EC receives a profuse cholinergic innervation from the nucleus basalis of Meynert in the basal forebrain, the septum, and the nucleus pallidus, which terminates primarily in layers II and V (Alonso and Kohler 1984; Gaykema et al. 1990; Lewis and Shute 1967; Lysakowski et al. 1989; Mellgren and Srebro 1973; Milner et al. 1983), two layers that gate hippocampal input and output, respectively. Accordingly, activation of cholinergic receptors especially the muscarinic receptors promotes intrinsic oscillation (Dickson and Alonso 1997; Golebiewski et al. 1994; Klink and Alonso 1997b; Konopacki and Golebiewski 1992) and inhibits synaptic transmission (Hamam et al. 2007; Richter et al. 1999). Furthermore, muscarinic receptor activation depolarizes layer II EC neurons (Klink and Alonso 1997a) and generates graded persistent activity (Egorov et al. 2002) by both activating a nonspecific cationic conductance and inhibiting a K+ conductance (Shalinsky et al. 2002). However, the effects of cholinergic receptors on GABAergic inhibitory transmission in the EC have not been determined, although the principal neurons in the EC receive extensive inhibitory innervations from local GABAergic interneurons. Here we examined the roles of muscarinic receptors in GABAergic transmission onto the principal neurons in the superficial layers of the EC, and our results demonstrate that activation of muscarinic receptors exerts diverse effects on GABAergic transmission.
METHODS
Slice preparation
Horizontal brain slices (400 μm) including the EC, subiculum, and hippocampus were cut using a vibrating blade microtome (VT1000S; Leica, Wetzlar, Germany) from 13- to 20-day-old Sprague Dawley rats as described previously (Deng and Lei 2006–2008; Lei et al. 2007). After being deeply anesthetized with isoflurane, rats were decapitated, and their brains were dissected out in ice-cold saline solution that contained (in mM) 130 NaCl, 24 NaHCO3, 3.5 KCl, 1.25 NaH2PO4, 0.5 CaCl2, 5.0 MgCl2, and 10 glucose, saturated with 95% O2-5% CO2 (pH 7.4). Slices were initially incubated in the preceding solution at 35°C for 40 min for recovery and then kept at room temperature (∼24°C) until use. All animal procedures conformed to the guidelines approved by the University of North Dakota Animal Care and Use Committee.
Recordings of spontaneous, miniature, and evoked IPSCs from the principal neurons in the superficial layers of the EC
Whole cell patch-clamp recordings using a Multiclamp 700B amplifier (Molecular Devices, Sunnyvale, CA) in voltage-clamp mode were made from the principal neurons in layer II/III of the EC visually identified with infrared video microscopy (BX51WI; Olympus, Tokyo, Japan) and differential interference contrast optics (Deng et al. 2006, 2007; Lei et al. 2007; Xiao et al. 2009). The recording electrodes were filled with the following solution (in mM): 100 cesium gluconate, 0.6 EGTA, 5 MgCl2, 8 NaCl, 2 ATP2Na, 0.3 GTPNa, 40 HEPES, and 1 QX-314 (pH 7.3). The extracellular solution contained (in mM) 130 NaCl, 24 NaHCO3, 3.5 KCl, 1.25 NaH2PO4, 1.5, MgCl2, 2.5 CaCl2, and 10 glucose, saturated with 95% O2-5% CO2 (pH 7.4). To record GABAA receptor-mediated spontaneous inhibitory postsynaptic currents (sIPSCs), the external solution was supplemented with dl-2-amino-5-phosphonovaleric acid (d-APV; 100 μM) and 6,7-dinitroquinoxaline-2,3(1H, 4H)-dione (DNQX; 10 μM) to block N-methyl-d-aspartate (NMDA) and AMPA receptor-mediated responses, respectively. sIPSCs were recorded at a holding potential of +30 mV (Deng and Lei 2006, 2008; Deng et al. 2006). Under these conditions, the recorded inhibitory currents were completely blocked by bicuculline methobromide (10 μM), confirming that they were mediated by GABAA receptors. Miniature IPSCs (mIPSCs) were recorded by including TTX (1 μM) in the preceding external solution to block action potential (AP)-dependent responses. Evoked IPSCs (eIPSCs) were recorded from stellate and pyramidal neurons in the EC using the same internal and external solution at a holding potential of +30 mV by placing a stimulation electrode 100 μm from the recorded cell in layer III. Synaptic responses were evoked at 0.2 Hz by low-intensity stimulation (80–100 μs duration; 10–40 μA intensity) via a constant-current isolation unit (A360; World Precision Instrument, Sarasota, FL) connected to a patch electrode filled with oxygenated extracellular solution. Series resistance was rigorously monitored by the delivery of a −5-mV voltage step after each evoked current. Experiments were discontinued if the series resistance changed by >10%. Data were filtered at 2 kHz, digitized at 10 kHz, and acquired on-line using pCLAMP 9 (Clampex) software (Molecular Devices). The recorded sIPSCs and mIPSCs were subsequently analyzed by Mini Analysis 6.0.1 (Synaptosoft, Decatur, GA). Each detected event was inspected visually to exclude obvious artifacts before analysis. The threshold for detection was set to three times the SD of the noise as recorded in an event-free stretch of data (Clements and Bekkers 1997). Mean amplitude, frequency, cumulative amplitude, and frequency histograms were calculated by this program. To avoid potential desensitization induced by repeated bath applications of muscarine, one slice was limited to only one application of muscarine.
Recordings from layer III GABAergic interneurons
AP firing was recorded from interneurons in layer III of the EC with the intracellular solution containing (in mM) 130 K+-gluconate, 0.5 EGTA, 2 MgCl2, 5 NaCl, 2 ATP2Na, 0.4 GTPNa, and 10 HEPES (pH 7.4). Usually for most of the cells a positive current injection was needed to bring the membrane potential to approximately −50 mV to induce AP firing. Muscarine was applied after the AP firing had been stable for 5∼10 min. The frequency of the APs was calculated by Mini Analysis 6.0.1.
Holding currents (HCs) at −60 mV were recorded in the extracellular solution supplemented with TTX (1 μM) to block potential indirect effects of muscarine on synaptic transmission. The intracellular solution was the K+-containing solution described in the preceding text. HCs were recorded every 3 s and then averaged per minute. We subtracted the average of the HCs recorded for the last minute prior to the application of muscarine from those recorded at different time points to zero the basal level of the HCs for better comparison. For the experiment involving N-methyl-d-glucamine (NMDG), the extracellular NaCl concentration was replaced by the same concentration of NMDG and HCl was used to adjust pH to 7.4.
Voltage-current curves were constructed from interneurons in layer III of the EC. K+-gluconate-containing internal solution was used and the external solution contained (in μM) 1 TTX, 100 CdCl2, 10 DNQX, 50 dl-APV, and 10 bicuculline. Voltage-current relationship was obtained by using a ramp protocol from −120 to −60 mV at a rate of 0.1 mV/ms. We compared the voltage-current curves recorded prior to and during the application of muscarine for 5–10 min.
Dual electrodes recordings from synaptically connected interneuron and pyramidal neuron pairs in the EC
The electrode sealed to interneurons contained the above K+-gluconate intracellular solution, and the electrode sealed to pyramidal neurons was filled with the preceding Cs+-containing intracellular solution except that Cs+-gluconate was replaced with the same concentration of CsCl. Interneuron was held in current-clamp mode and stimulated at a frequency of 0.3 Hz by brief current pulses (duration: 10 ms, amplitude: 0.2–0.25 nA) to initiate APs. Pyramidal neuron was held in voltage-clamp mode (holding potential: −60 mV). The recorded currents were completely blocked by application of bicuculline (10 μM), indicating that they were mediated by GABAA receptors.
Data analysis
Data are presented as the means ± SE. Concentration-response curve of muscarine was fit by Hill equation: I = Imax × {1/[1 + (EC50/[ligand])n]}, where Imax is the maximum response, EC50 is the concentration of ligand producing a half-maximal response, and n is the Hill coefficient. Student's paired or unpaired t-test or ANOVA was used for statistical analysis as appropriate; P values are reported throughout the text and significance was set as P < 0.05. For sIPSC or mIPSC cumulative probability plots, events recorded 5 min prior to and 5 min after reaching the maximal effect of muscarine were selected. Same bin size (25 ms for frequency and 2 pA for amplitude) was used to analyze data from control and muscarine treatment. Kolmogorov-Smirnoff test was used to assess the significance of the cumulative probability plots. N, number in the text, represents the numbers of cells examined.
Chemicals
Pirenzepine, AF-DX 116, PD 102807, SKF96365, tertiapin, linopirdine, and U73122 were purchased from TOCRIS (Ellisville, MO). Ro318220, GDP-β-S, and GTP-γ-S were from BIOMOL (Plymouth Meeting, PA). 1-O-octadecyl-2-O-methyl-rac-glycero-3-phosphorylcholine (edelfosine) was purchased from Calbiochem (Darmstadt, Germany). Muscarine, atropine, McN-A-343, 1,1-dimethyl-4-diphenylacetoxypiperidinium iodide (4-DAMP), bupivacaine, and other chemicals were products of Sigma-Aldrich (St. Louis, MO).
RESULTS
Muscarine increases the frequency and amplitude of sIPSCs recorded from the principal neurons in the EC
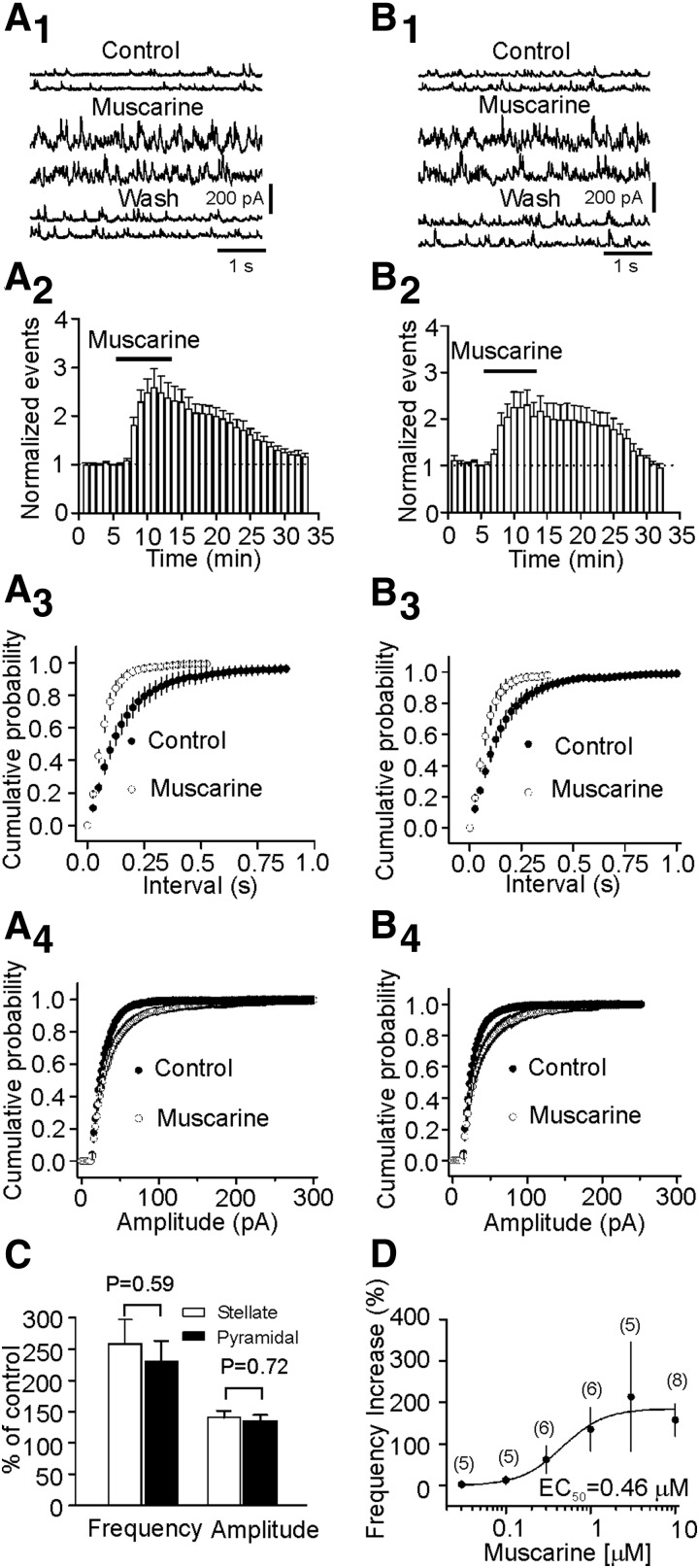
Stellate and pyramidal neurons are the two types of principal cells in the superficial layers of the EC. Here we identified these two types of neurons by their morphology and location because previous studies demonstrate that these neurons can be reliably differentiated by these two criteria (Deng and Lei 2007; Lei et al. 2007). Stellate neurons are usually located in layer II or the border of layer II and III, and they have larger and polygonal soma with variable number of main dendrites radiating out from the cell body but are devoid of a clearly dominant dendrite. Pyramidal neurons have a pyramidal or elongated soma with dendrites orientated in a bidirectional way; an apical dendrite running to the surface of the cortex and basal dendrites extending toward the deeper layers. We recorded sIPSCs from both stellate and pyramidal neurons in layer II/III of the EC. Bath application of muscarine (10 μM) significantly increased the frequency (258 ± 39% of control, P = 0.005, Fig. 1A, 1–3) and amplitude (141 ± 11% of control, P = 0.009, Fig. 1A4) of sIPSCs in eight of eight stellate neurons examined. Similarly, application of muscarine (10 μM) significantly increased the frequency (230 ± 34% of control, P = 0.008, Fig. 1B, 1–3) and amplitude (136 ± 9% of control, P = 0.009, Fig. 1B4) of sIPSCs in seven of seven pyramidal neurons examined. Because there were indistinguishable differences for muscarine-induced increases in sIPSC frequency (P = 0.59, unpaired t-test) and amplitude (P = 0.72, unpaired t-test) recorded from stellate neurons and pyramidal neurons (Fig. 1C), suggesting that the muscarinic effects are not cell-specific, we performed the rest of the experiments on both stellate and pyramidal neurons. The EC50 value was measured to be 0.46 μM (Fig. 1D).
FIG. 1.
Muscarine increases the frequency and amplitude of spontaneous inhibitory postsynaptifc currents (sIPSCs) recorded from stellate (A, 1–4) and pyramidal (B, 1–4) neurons. A1: sIPSCs recorded from a stellate neuron before, during, and after the application of muscarine (10 μM). A2: pooled time course of sIPSC frequency from 8 stellate neurons. A3: cumulative probability of sIPSC frequency prior to and during the application of muscarine (n = 8). Note that muscarine increased sIPSC frequency (reduction in the intervals of events). A4: cumulative probability of sIPSC amplitude before and during the application of muscarine (n = 8). Note that muscarine increased sIPSC amplitude. B, 1–4: data were from 7 pyramidal neurons. The figures are arranged in the same way. C: there were no significant differences for muscarine-induced increases in sIPSC frequency (P = 0.59) and amplitude (P = 0.72) between stellate (n = 8) and pyramidal (n = 7) neurons. D: concentration-response curve by plotting the percentage of increase in frequency versus the concentrations of muscarine. Numbers in the parenthesis are number of cells recoded.
Involvement of M3 receptors
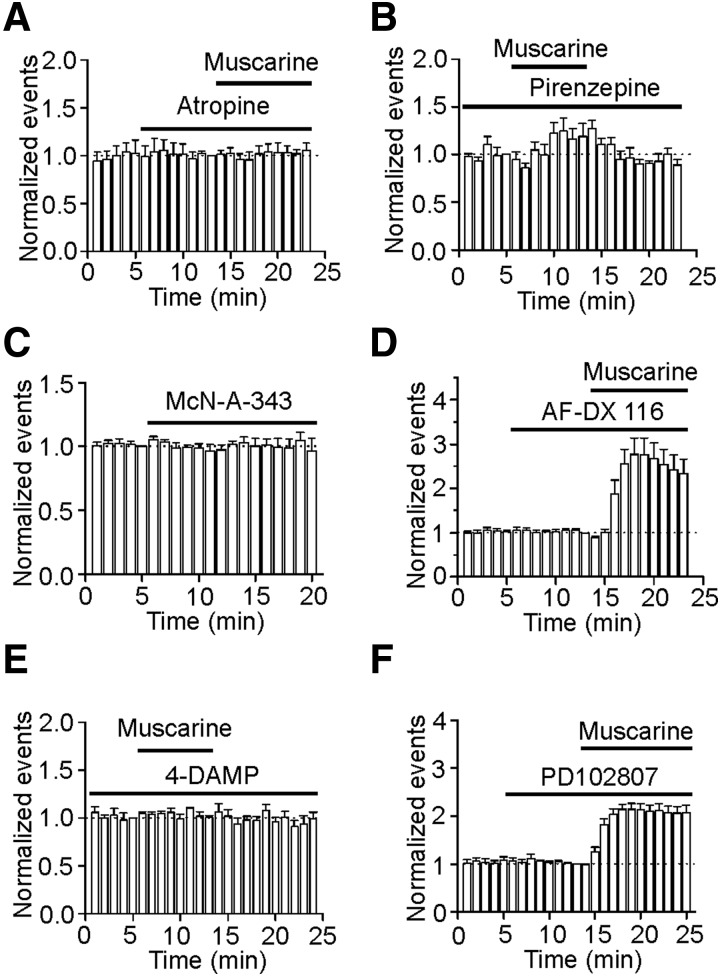
We next examined whether the muscarinic effects on sIPSCs were mediated by activation of muscarinic receptors. Application of atropine (5 μM), a broad-spectrum muscarinic receptor antagonist, did not significantly alter sIPSC frequency (109 ± 9% of control, n = 6, P = 0.38, Fig. 2A) and amplitude (99 ± 4% of control, n = 6, P = 0.89) by itself and completely blocked muscarine-induced increases in sIPSC frequency (98 ± 5% of control, n = 6, P = 0.68, A) and amplitude (99 ± 3% of control, n = 6, P = 0.8), suggesting that muscarine increases sIPSCs via activation of muscarinic receptors. We further probed the roles of muscarinic receptor subtypes in muscarine-induced increases in sIPSCs. Muscarinic receptors comprise the M1-like (M1, M3, M5) and M2-like (M2, M4) receptors (Hammer et al. 1980; Lucas-Meunier et al. 2003). The M1-like receptors are coupled to Gαq/11 and stimulate phospholipase C (PLC) resulting in an increase in intracellular Ca2+ release and activation of protein kinase C (PKC), whereas the M2-like receptors are coupled to Gαi proteins leading to an inhibition of protein kinase A and a reduction in cyclic AMP (Caulfield and Birdsall 1998; Lucas-Meunier et al. 2003). Pirenzepine was used to differentiate these two families of muscarinic receptors because this compound selectively blocks M1-like receptors (Caulfield and Birdsall 1998; Lucas-Meunier et al. 2003). Pretreatment of slices with and continuous bath application of pirenzepine (300 nM) blocked muscarine-induced increases in sIPSC frequency (124 ± 14% of control, n = 5, P = 0.16, Fig. 2B) and amplitude (97 ± 4% of control, n = 5, P = 0.47), suggesting that the effects of muscarine are mediated via activation of M1-like receptors. We then used the available antagonists or agonists for the subtypes of muscarinic receptors to identify the roles of the M1-like (M1, M3, M5) family in muscarine-mediated increases in sIPSCs. Application of McN-A-343 (100 μM), a selective M1 receptor agonist (Guo and Chiappinelli 2001), did not significantly change sIPSC frequency (96 ± 10% of control, n = 6, P = 0.72, Fig. 2C) and amplitude (105 ± 4% of control, n = 6, P = 0.25), suggesting that the effects of muscarine were not mediated by activation of M1 receptors. Application of AF-DX116 (10 μM), a selective M2 receptor antagonist (Lucas-Meunier et al. 2003), did not significantly change sIPSC frequency (92 ± 7% of control, n = 6, P = 0.3, Fig. 2D) and amplitude (99 ± 4% of control, n = 6, P = 0.81) by itself and failed to block muscarine-induced increases in sIPSC frequency (277 ± 37% of control, n = 6, P = 0.005, Fig. 2D) and amplitude (134 ± 4% of control, n = 6, P < 0.001) excluding the involvement of M2 receptors. Pretreatment of slices and continuous bath application of 4-DAMP (200 nM), a selective M3 receptor antagonist (Guo and Chiappinelli 2000), completely blocked muscarine-induced increases in sIPSC frequency (107 ± 8% of control, n = 5, P = 0.47, Fig. 2E) and amplitude (98 ± 3% of control, n = 5, P = 0.71). Bath application of 4-DAMP (2 μM) without preincubation failed to change significantly sIPSC frequency (112 ± 12% of control, n = 6, P = 0.38) and amplitude (105 ± 4% of control, n = 6, P = 0.27), and also completely blocked muscarine-induced increases in sIPSC frequency (102 ± 2% of control, n = 6, P = 0.46) and amplitude (97 ± 4% of control, n = 6, P = 0.48, data not shown), suggesting that the effects of muscarine on sIPSCs are mediated via activation of M3 receptors. Furthermore, application of PD102807 (10 μM), a selective M4 receptor antagonist (Lawrence et al. 2006), did not change sIPSC frequency (94 ± 7% of control, n = 6, P = 0.44, Fig. 2F) and amplitude (111 ± 12% of control, n = 6, P = 0.4) by itself and failed to block muscarine-induced increases in sIPSC frequency (207 ± 15% of control, n = 6, P = 0.001, Fig. 2F) and amplitude (154 ± 12% of control, n = 6, P = 0.005), suggesting that M4 receptors are unlikely to be involved. Together, these results indicate that muscarine increases sIPSC frequency and amplitude via activation of M3 receptors.
FIG. 2.
Muscarine-mediated increases in sIPSC frequency and amplitude are mediated via activation of M3 receptors. A: application of atropine (5 μM) completely blocked muscarine-induced increases in sIPSC frequency (n = 6). B: pretreatment of slices with and continuous bath application of pirenzepine (300 nM) blocked muscarine-induced increases in sIPSC frequency (n = 5). C: application of McN-A-343 (100 μM), a M1 receptor agonist, failed to change sIPSC frequency (n = 6). D: application of AF-DX116 (10 μM), a M2 receptor antagonist, failed to block muscarine-induced increases in sIPSC frequency (n = 6). E: pretreatment of slices with and continuous bath application of 1,1-dimethyl-4-diphenylacetoxypiperidinium iodide (4-DAMP, 200 nM), a selective M3 receptor antagonist, blocked muscarine-induced increases in sIPSC frequency (n = 5). F: application of PD102807 (10 μM), a M4 receptor antagonist, did not block muscarine-induced increases in sIPSC frequency (n = 6).
Muscarine slightly depresses mIPSC frequency and markedly inhibits the amplitudes of eIPSCs
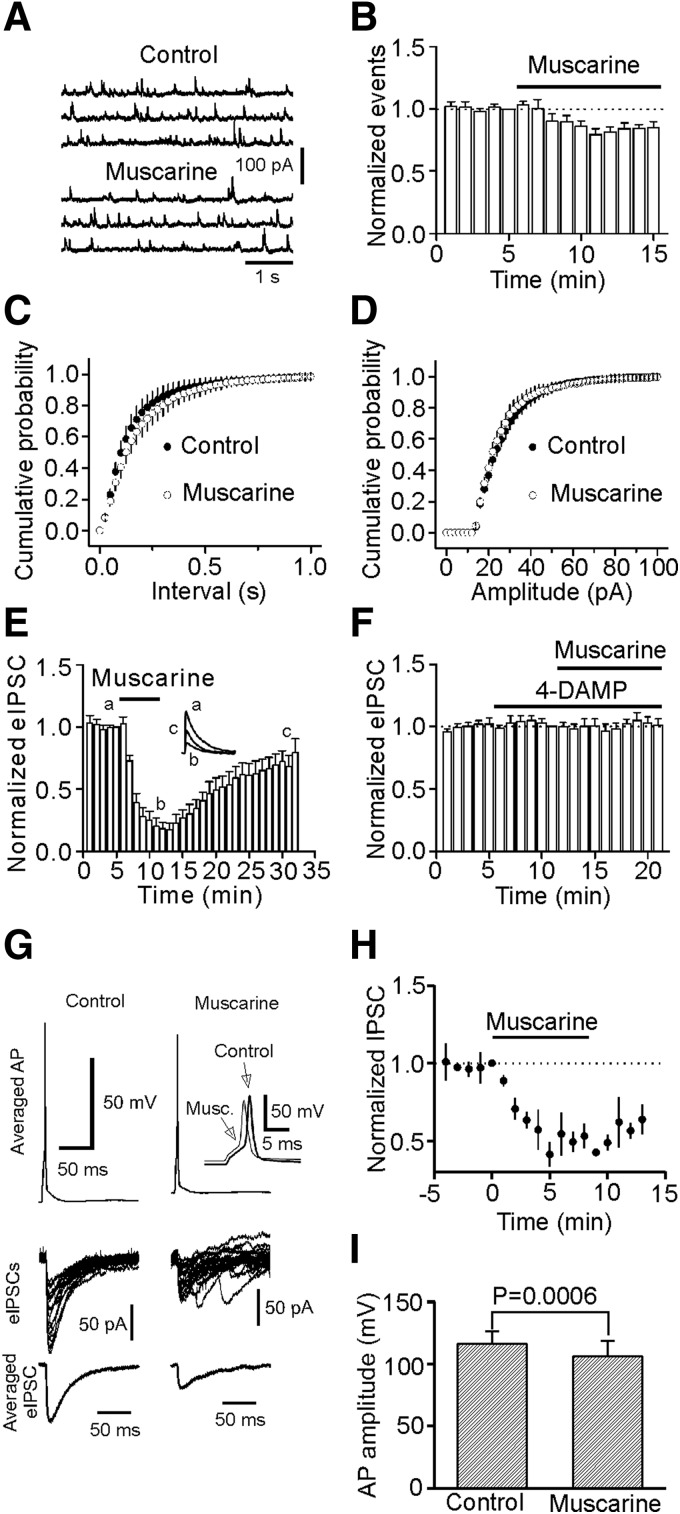
We next examined the effects of muscarine on mIPSCs recorded in the presence of TTX (1 μM). Application of muscarine (10 μM) did not increase but slightly reduced mIPSC frequency (85 ± 5% of control, n = 7, P = 0.02, Fig. 3, A–C). The amplitude of mIPSCs was not significantly changed after application of muscarine (95 ± 3% of control, n = 7, P = 0.1, Fig. 3D), suggesting that muscarine has no effects on postsynaptic GABAA receptors. We then examined the effects of muscarine on eIPSCs by placing a stimulation electrode in layer III (∼100 μm from the recorded cell) to stimulate GABAergic inputs. Application of muscarine (10 μM) did not increase but instead remarkably reduced the amplitude of eIPSCs (17 ± 5% of control, n = 5, P < 0.001, Fig. 3E), consistent with previous results (Salgado et al. 2007). Muscarine-induced depression of eIPSC amplitude was blocked by co-application of 4-DAMP (2 μM, 101 ± 5% of control, n = 6, P = 0.81, Fig. 3F), suggesting that the effect of muscarine on eIPSCs is mediated via activation of M3 receptors. This phenomenon (increases in sIPSCs but reduction in eIPSCs) has also been observed for norepinephrine in the hippocampus (Madison and Nicoll 1988), nicotine in striatum (Liu et al. 2007), and 5-HT in the EC (Deng and Lei 2008).
FIG. 3.
Muscarine slightly depresses miniature IPSC (mIPSC) frequency and markedly inhibits the amplitudes of evoked IPSCs (eIPSCs) by extracellular stimulation and depolarization of GABAergic interneurons in synaptically connected interneuron and pyramidal neuron pairs. A: mIPSCs recorded from a neuron before and during the application of muscarine (10 μM) in the presence of TTX (1 μM). B: pooled time course of mIPSC frequency (n = 7). Note that muscarine slightly inhibited mIPSC frequency. C: cumulative probability of mIPSC frequency before and during the application of muscarine (n = 7). D: cumulative probability of mIPSC amplitude before and during the application of muscarine (n = 7). E: application of muscarine (10 μM) decreased eIPSC amplitude (n = 5). Inset: the current averaged from 10 traces before, during, and after application of muscarine. Note that muscarine reversibly depressed eIPSC amplitude. F: application of 4-DAMP (2 μM) blocked muscarine-induced depression of eIPSC amplitude (n = 6). G: application of muscarine (10 μM) reduced the amplitudes of AP recorded from interneurons and eIPSCs recorded from pyramidal neurons in interneuron-pyramidal neuron pairs. Top: averaged trace from 20 individual action potentials (APs) prior to (left) and during (right) the application of muscarine (10 μM) recorded from an interneuron. Inset: the enlarged overlay of the 2 APs. Note that application of muscarine induced depolarization and slightly reduced the amplitude of AP. Middle panel: Superimposed IPSCs evoked by individual APs before (left) and during (right) the application of muscarine. Note that application of muscarine reduced the amplitude of the IPSCs and increased the frequency of spontaneous IPSCs. Bottom: averaged IPSC before (left) and during (right) the application of muscarine. H: summarized time course of muscarine-mediated inhibition of IPSC amplitudes from 4 interneuron-pyramidal neuron pairs. I: summarized AP amplitude recorded from interneurons before and during the application of muscarine (n = 4 pairs).
We then used dual electrodes and recorded simultaneously APs from an interneuron and eIPSCs from a pyramidal neuron in synaptically connected interneuron and pyramidal neuron pairs in layer III of the EC. The electrode sealed to interneurons contained K+-gluconate, whereas that sealed to pyramidal neurons contained CsCl. Pyramidal neurons were held at - 60 mV. Interneurons were differentiated from pyramidal neurons in layer III by their smaller sizes and fast firing spikes and pronounced afterhyperpolarization. As demonstrated previously (Deng and Lei 2008), there are two types of interneurons in layer III of the EC. Type I interneurons showed little voltage sag in response to hyperpolarizing current injection with no rebound burst firing, whereas type II interneurons displayed prominent voltage sag in response to hyperpolarizing current injection with rebound burst firing (Deng and Lei 2008). Under these conditions, bath application of muscarine (10 μM) significantly reduced the amplitudes of eIPSCs (49 ± 6% of control, n = 4 pairs, P = 0.004, Fig. 3, G and H). Examination of the APs simultaneously recorded from interneurons showed that muscarine significantly depolarized the resting membrane potentials (RMPs; control: −64.8 ± 1.3 mV, muscarine: −61.2 ± 1.5 mV, n = 4, P = 0.02, paired t-test, Fig. 3G) and reduced the amplitudes of APs (control: 116.3 ± 10.3 mV, muscarine: 106.4 ± 12.4 mV, n = 4, P = 0.0006, paired t-test, Fig. 3, G and I). Together, these results suggest that a reduction of presynaptic AP amplitudes may contribute to muscarine-mediated depression of eIPSCs in the EC.
Muscarine increases the excitability of GABAergic interneurons in the EC
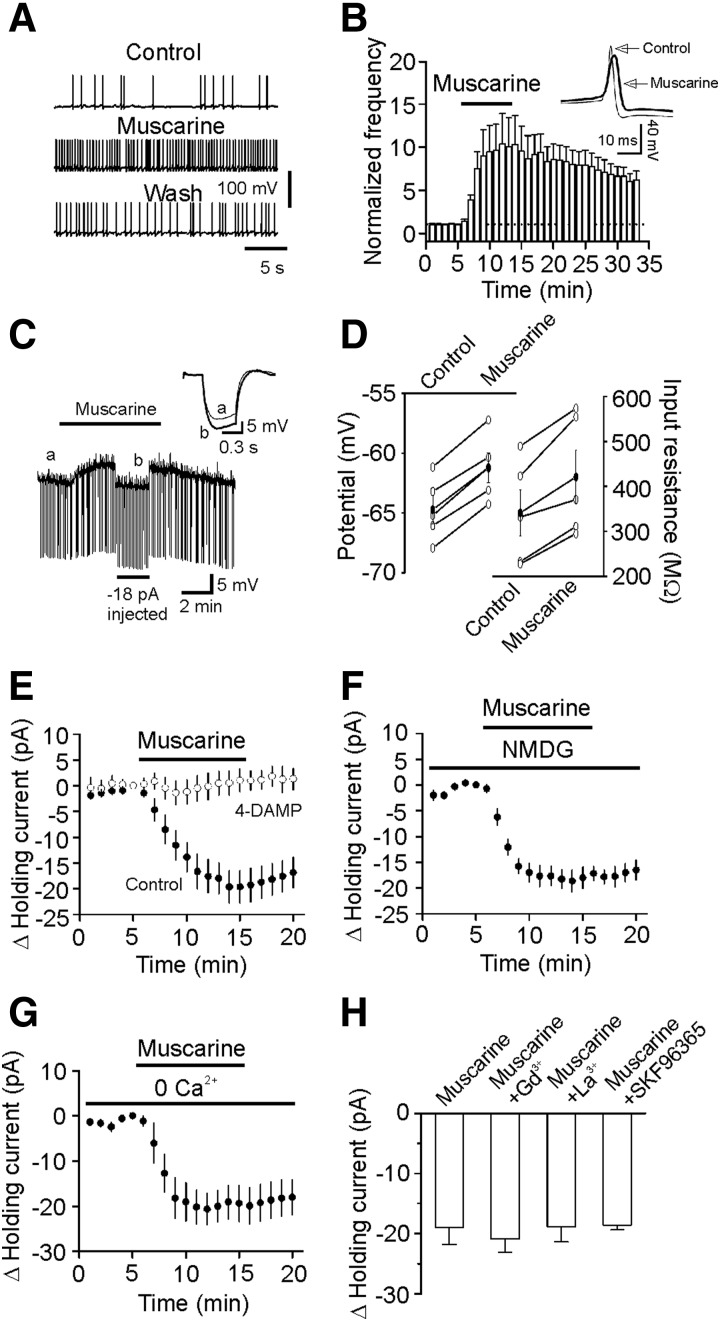
The preceding results suggest that muscarine exerts distinct effects on sIPSCs, mIPSCs, and eIPSCs. Both sIPSCs and eIPSCs are AP-dependent, whereas mIPSCs are not. We next determined the effects of muscarine on the firing frequency of APs recorded from identified GABAergic interneurons in layer III of the EC. Layer III interneurons did not show spontaneous firing. We therefore persistently injected minimal positive current to induce firing. Under these circumstances, the firing frequency (103 ± 15% of control, n = 5, P = 0.83) and amplitude (98 ± 2% of control, n = 5, P = 0.36, data not shown) of the APs were not significantly changed after continuous recordings for 35 min. However, bath application of muscarine (10 μM) significantly increased the frequency (1042 ± 349% of control, n = 7, P = 0.04, Fig. 4, A and B) but decreased the amplitudes (91 ± 2% of control, n = 7, P = 0.02,). Among the seven interneurons recorded, there were four type I and three type II interneurons. Because each interneuron responded to muscarine, we pooled the data obtained from the types I and II interneurons for the rest of the experiments.
FIG. 4.
Muscarine depolarizes interneurons and increases neuronal input resistance in layer III of the entorhinal cortex (EC). A: APs recorded from an interneuron prior to, during, and after the application of muscarine (10 μM). B: pooled time course of AP firing frequency before, during, and after application of muscarine (10 μM, n = 7). Inset: the averaged APs before and during the application of muscarine. Note that AP amplitude was reduced in the presence of muscarine. C: application of muscarine (10 μM) generated membrane depolarization and increased input resistance in a layer III interneuron of the EC. RMP was recorded in current-clamp mode and a hyperpolarizing current (−50 pA, 500 ms) was injected every 5 s to measure the input resistance. To exclude the influence of muscarine-induced membrane depolarization on input resistance, a constant negative current (−18 pA, indicated by the horizontal bar) was injected briefly to bring the membrane potential back to the initial level. Inset: the voltage traces taken before (thin) and during (thick) the application of muscarine. D: summarized data for muscarine-induced changes in resting membrane potentials (RMPs, left) and input resistance (right) in layer III interneurons (n = 5). E: application of muscarine (10 μM) induced an inward Holding current (HC) in interneurons (n = 9) and muscarine-induced increases in inward HCs were blocked by 1,1-dimethyl-4-diphenylacetoxypiperidinium iodide (4-DAMP), demonstrating the involvement of M3 receptors. F: replacement of extracellular NaCl with the same concentration of N-methyl-d-glucamine (NMDG)-Cl failed to change muscarine-induced increases in inward HCs (n = 8). G: omission of extracellular Ca2+ did not alter muscarine-induced increases in inward HCs (n = 6). H: muscarine-induced increases in inward HCs were not significantly altered in the presence of Gd3+ (10 μM, n = 5), La3+ (10 μM, n = 6), and SKF96365 (50 μM, n = 6).
We then recorded muscarine-induced changes in RMPs in current-clamp mode from layer III interneurons in the extracellular solution containing TTX (0.5 μM) to block potential indirect effects from synaptic transmission. A negative current (−50 pA for 500 ms) was injected every 5 s to assess the changes of input resistance induced by muscarine (Fig. 4C). Under these circumstances, application of muscarine (10 μM) generated membrane depolarization (control: −65.1 ± 1.5 mV, muscarine: −61.6 ± 1.0 mV, n = 5, P = 0.008, Fig. 4, C and D) and increased the input resistance (control: 341 ± 52 MΩ, muscarine: 421 ± 60 MΩ, n = 5, P = 0.006, Fig. 4, C and D), suggesting that muscarine reduces membrane conductance.
We also examined muscarine-induced depolarization by recording the HCs at −60 mV in voltage-clamp mode in the extracellular solution containing TTX (1 μM). Application of muscarine (10 μM) induced an inward HC (−18.9 ± 2.9 pA, n = 9, P < 0.001, Fig. 4E). Muscarine-induced increases in inward HCs were blocked when 4-DAMP (2 μM) was applied (-1.4 ± 2.3 pA, n = 6, P = 0.57, Fig. 4E), indicating that muscarine depolarizes entorhinal interneurons via activation of M3 receptors.
Muscarine induces membrane depolarization by inhibiting background K+ channels in interneurons
We then tested whether muscarine depolarizes GABAergic interneurons by opening a cationic conductance. If this is the case, the extracellular Na+ and Ca2+ should be the major cations to mediate membrane depolarization. We initially replaced the extracellular NaCl with the same concentration of NMDG-Cl. In this condition, application of muscarine (10 μM) still induced a comparable inward HC (control: −18.9 ± 2.9 pA, n = 9; NMDG: −18.0 ± 2.1 pA, n = 8, P = 0.81, unpaired t-test, Fig. 4F). Furthermore, omission of extracellular Ca2+ failed to change significantly muscarine-induced increases in inward HCs (control: −18.9 ± 2.9 pA, n = 9; 0 Ca2+: −19.4 ± 4.0 pA, n = 6, P = 0.92, unpaired t-test, Fig. 4G). Finally, muscarine-induced increases in inward HCs were insignificantly changed (versus muscarine alone, Fig. 4H) when the extracellular solution contained other cation channel blockers: Gd3+ (10 μM, −20.8 ± 2.3 pA, n = 5, P = 0.66), La3+ (10 μM, −18.8 ± 2.5 pA, n = 6, P = 0.98), and SKF96365 (50 μM, −18.6 ± 0.8 pA, n = 6, P = 0.93). These results together suggest that muscarine-induced depolarization is not mediated via activation of a cationic conductance.
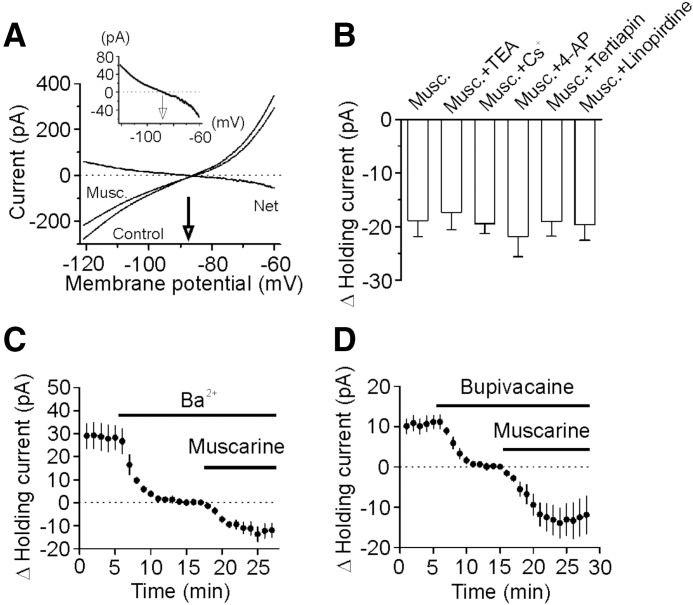
We then tested the hypothesis that muscarine inhibits a resting K+ conductance to generate membrane depolarization. If so, the muscarine-induced currents should have a reversal potential close to the K+ reversal potential. We used a ramp protocol (from −120 to −60 mV, at a speed of 0.1 mV/ms) to construct the voltage-current curve before and during the application of muscarine (10 μM). The extracellular solution was supplemented with (in μM) 1 TTX, 100 Cd2+, 100 Ni2+, 10 DNQX, 50 dl-APV, and 10 bicuculline to block synaptic current and other voltage-gated ion channels. Under these conditions, muscarine induced a current which had a reversal potential (−88.5 ± 2.9 mV, n = 5) close to the calculated K+ reversal potential (−92.2 mV) in our recording conditions (Fig. 5A), suggesting that muscarine induces membrane depolarization by inhibiting resting membrane K+ channels. This result was also consistent with the previous data showing that muscarine increased the input resistance (Fig. 4, C and D).
FIG. 5.
Muscarine generates membrane depolarization in entorhinal interneurons via inhibition of background K+ channels. A: voltage-current relationship recorded by a ramp protocol (from −120 to −60 mV, at a speed of 0.1 mV/ms) before and during the application of muscarine (10 μM) when extracellular K+ concentration was 3.5 mM. Traces in the figure were averaged traces from 5 cells. Muscarine-induced net current had a reversal potential of −88.5 ± 2.9 mV (n = 5), close to the calculated K+ reversal potential (−92.2 mV). Inset: the enlarged net current induced by muscarine. B: muscarine-induced increases in HCs were insensitive to TEA (n = 9), Cs+ (n = 9), 4-AP (n = 6), tertiapin (n = 7), and linopirdine (n = 6). C: bath application of Ba2+ (3 mM) induced an inward HC but did not significantly change muscarine-induced increases in inward HCs (n = 9). D: bath application of bupivacaine (200 μM) induced an inward HC but failed to change significantly muscarine-induced increases in inward HCs (n = 10).
We next tried to characterize the properties of the involved K+ channels. Muscarine-induced increase in inward HCs recorded from interneurons in the EC was not significantly changed (vs. muscarine alone, Fig. 5B) by application of TEA (10 mM, −17.4 ± 3.2 pA, n = 9, P = 0.73), Cs+ (3 mM, −19.5 ± 1.9 pA, n = 9, P = 0.89), or 4-aminopyridine (4-AP, 2 mM, −21.8 ± 3.8 pA, n = 6, P = 0.55), suggesting that muscarine-mediated inhibition of K+ channels is insensitive to the classic K+ channel blockers. Moreover, application of tertiapin (50 nM), an inward rectifier K+ channel inhibitor, failed to change significantly muscarine-induced increases in inward HCs (−19.0 ± 2.8 pA, n = 7, P = 0.98 versus muscarine alone, Fig. 5B) suggesting that muscarine-induced membrane depolarization is unlikely to be mediated by the inward rectifier K+ channels although this type of K+ channels is involved in the modulation of RMPs. The muscarinic effect was unlikely to be mediated by M-type K+ channels because application of linopirdine (10 μM), a selective M-channel blocker, failed to change muscarine-induced increases in inward HCs significantly (−19.7 ± 2.9 pA, n = 6, P = 0.86 vs. muscarine alone, Fig. 5B) and the voltage-threshold for M-channel activation is positive to −60 mV rendering this type of channels less likely to be involved in controlling RMPs.
Because the two pore-domain K+ channels (K2P) are involved in controlling RMPs and they are insensitive to the classic K+ channel blockers (TEA, 4-AP, Cs+), we next examined the roles of K2P in muscarine-induced membrane depolarization in GABAergic interneurons. The family of K2P channels includes TWIK, THIK, TREK, TASK, TALK, and TRESK (Bayliss et al. 2003; Lesage 2003), some of which are sensitive to Ba2+ and bupivacaine. We therefore tested the roles of Ba2+ and bupivacaine in muscarine-induced membrane depolarization. Application of Ba2+ (3 mM) alone induced an inward HC (−28.2 ± 5.2 pA, n = 9, P < 0.001, Fig. 5C) by itself, suggesting that Ba2+-sensitive K+ channels have a significant role in controlling RMPs. However, muscarine-induced increases in inward HCs were not significantly changed in the presence of Ba2+ (−13.7 ± 3.3 pA, n = 9, P = 0.25 vs. muscarine alone, unpaired t-test, Fig. 5C). Likewise, bath application of bupivacaine (200 μM) induced an inward HC (−11.1 ± 1.7 pA, n = 10, P < 0.001, Fig. 5D) and failed to alter muscarine-induced increases in inward HCs (−14.0 ± 3.7 pA, n = 10, P = 0.32 vs. muscarine alone, unpaired t-test, Fig. 5D).
Signal transduction mechanism
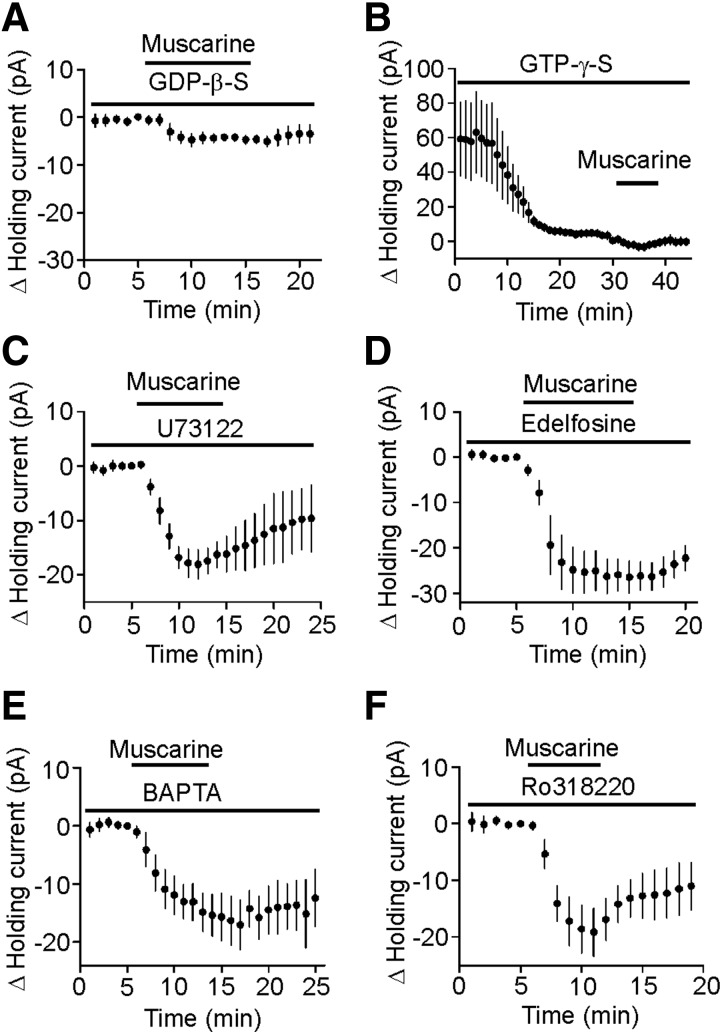
Our results demonstrated that muscarine modulates GABAergic transmission by activating M3 receptors on GABAergic interneurons. Activation of M3 receptors is coupled to Gαq/11 resulting in activation of PLC that further generates two intracellular messengers, IP3 to increase intracellular Ca2+ release and diacylglycerol to activate PKC. We therefore examined the role of this pathway in muscarine-induced depolarization in interneurons. We first tested whether G-proteins were involved in muscarine-induced increases in inward HCs. We replaced GTP in the intracellular solution with GDP-β-S (4 mM), a G protein inactivator, and recorded HCs from interneurons in layer III. Application of muscarine (10 μM) in the presence of GDP-β-S significantly reduced muscarine-induced inward HCs (−4.7 ± 0.9 pA, n = 8, P < 0.001 vs. muscarine alone, unpaired t-test, Fig. 6A). Furthermore, intracellular application of GTP-γ-S (0.5 mM), a nonhydrolysable GTP analogue, induced an inward HC by itself (−59.0 ± 21.3 pA, n = 6, P = 0.04, Fig. 6B) and significantly reduced muscarine-induced increases in inward HCs (−3.7 ± 0.9 pA, n = 6, P = 0.001 vs. muscarine alone, unpaired t-test, Fig. 6B). Together these results suggest that G-proteins are involved in muscarine-induced increases in inward HCs.
FIG. 6.
Muscarine-induced depolarization of entorhinal interneurons is G protein dependent but independent of PLC, intracellular Ca2+ release, and PKC activity. A: intracellular application of GDP-β-S (4 mM) via the recording pipettes significantly reduced muscarine-induced increases in inward HCs (n = 8). B: intracellular application of GTP-γ-S (0.5 mM) induced an inward HC by itself and significantly reduced muscarine-induced increases in inward HCs (n = 6). C: pretreatment of slices with and co-application of U73122 (20 μM) for >2 h failed to block muscarine-induced enhancement of inward HC (n = 5). D: pretreatment of slices with and co-application of edelfosine (20 μM) failed to alter muscarine-induced enhancement of inward HCs (n = 8). E: intracellular dialysis of bis-(o-aminophenoxy)-N,N,N′,N′-tetraacetic acid (20 mM) failed to change significantly muscarine-induced increases in inward HCs (n = 5). F: pretreatment of slices with and continuous bath application of Ro318220 (1 μM) did not significantly change muscarine-induced increases in inward HCs (n = 5).
Because activation of M3 receptors increases PLC activity, we next tested whether PLC was required for muscarine-induced membrane depolarization. Slices were pretreated for >2 h with U73122 (20 μM), a PLC inhibitor, and the bath was continuously perfused with the same concentration of U73122. However, application of U73122 failed to change significantly muscarine-induced increases in inward HCs (−19.3 ± 2.7 pA, n = 5, P = 0.94 vs. muscarine alone, unpaired t-test, Fig. 6C). Similar treatment of slices with edelfosine (20 μM), another PLC inhibitor, did not alter muscarine-induced increases in HCs (−25.4 ± 4.6 pA, n = 8, P = 0.24 versus muscarine alone, unpaired t-test, Fig. 6D). We also examined the requirement of intracellular Ca2+ release and PKC in muscarine-mediated depolarization of interneurons. Intracellular dialysis of BAPTA (20 mM) via the recording pipettes failed to change significantly muscarine-induced increases in inward HCs (−17.0 ± 4.3 pA, n = 5, P = 0.72 vs. muscarine alone, Fig. 6E). Pretreatment of slices with and continuous bath application of Ro318220 (1 μM), a selective PKC inhibitor, did not significantly alter muscarine-induced increases in inward HCs (−19.1 ± 4.2 pA, n = 5, P = 0.96 vs. muscarine alone, Fig. 6F). These results suggest that muscarine-mediated depolarization in entorhinal interneurons is independent of intracellular Ca2+ release and PKC activity.
DISCUSSION
Whereas the effects of muscarine on principal neurons and neural network activity in the EC are under extensive investigations, its potential actions on GABAergic transmission remain unexplored. Our results demonstrate for the first time that activation of muscarinic receptors modulates GABAergic transmission in the EC in distinct modes; activation of M3 muscarinic receptors increases sIPSC frequency and amplitude but inhibits the amplitude of eIPSCs and the frequency of mIPSCs with no effects on the amplitude of mIPSCs. Muscarine increases the firing frequency but reduces the amplitude of APs. Muscarine depolarizes GABAergic interneurons by inhibiting background K+ channels that are insensitive to the classic K+ channel blockers (TEA, Cs+, 4-AP, tertiapin), Ba2+, and bupivacaine. Muscarine-induced modulation of GABAergic transmission requires the function of G proteins but is independent of PLC, intracellular Ca2+ release and PKC activity.
We have shown that bath application of muscarine profoundly increases sIPSC frequency and amplitude but inhibits the amplitudes of IPSCs evoked by extracellular stimulation or depolarization of GABAergic interneurons in synaptically connected interneuron and pyramidal neuron pairs. Muscarine slightly inhibits the frequency of mIPSCs recorded in the presence of TTX with no effects on mIPSC amplitude. The result that muscarine does not modulate mIPSC amplitude indicates that activation of muscarinic receptors does not modulate postsynaptic GABAA receptors. Muscarine has been shown to reduce GABA release in rat auditory cortex (Salgado et al. 2007), lateral amygdala, nucleus accumbens, and striatum (Sugita et al. 1991). However, these studies were conducted by recording eIPSCs. By contrast, muscarine has also been reported to increase GABA release when sIPSCs were recorded (Guo and Chiappinelli 2000; Yigit et al. 2003). Consistent with our results, Guo and Chiappinelli (2001) observed an increase in sIPSC frequency but a reduction in eIPSC amplitude in chick lateral spiriform nucleus neurons. Our results can clearly explain the discrepancy of these results because muscarine indeed remarkably increases sIPSC frequency and amplitude but reduces eIPSC amplitude. The results that muscarine increases sIPSC frequency and amplitude suggest that muscarine increases GABA release. However, muscarine also profoundly reduces eIPSC amplitude and slightly attenuates mIPSC frequency. These two pieces of evidence suggest that muscarine decreases GABA release as well. Because sIPSCs, eIPSCs, and mIPSCs represent three distinct modes of GABAergic transmission, these results suggest that muscarine could either increase or decrease GABAergic transmission possibly depending on the status of neuronal network activities. Because the present study was conducted on juvenile (13–20 days old) rats, we cannot exclude the possibility that the observed diverse effects reflect the function of muscarinic receptors on GABAergic transmission in a particular period of development.
What are the mechanisms underlying muscarine-mediated diverse effects on GABAergic transmission? Muscarine-mediated modest inhibition of mIPSC frequency could be due to a direct interaction with the release machineries because mIPSCs are usually considered to be AP independent. Muscarine depolarizes GABAergic interneurons to enhance AP firing frequency. Muscarine-induced increases in AP firing frequency of GABAergic interneurons could explain its effect on sIPSC frequency. Muscarine-mediated increases in sIPSC amplitude could be due to the synchronization of increased sIPSC events because if sIPSC frequency is increased, more than one event could be summated and the amplitude of the integrated sIPSCs would be larger. Another possible mechanism whereby muscarine increases sIPSC amplitude is that GABA concentration in the synaptic cleft is elevated by muscarine-induced enhancement of AP firing of interneurons. Elevation of GABA concentration in the synaptic cleft likely results in increased sIPSC amplitude. However, the precise mechanisms whereby muscarine reduces eIPSC amplitude are unknown. Because we have observed a small but significant reduction in AP amplitude recorded from GABAergic interneurons, one explanation is that small AP amplitude could result in attenuated eIPSC amplitude. Alternatively, muscarine-induced increases in sIPSC frequency could deplete or at least reduce the readily releasable pool of GABA at the GABAergic synapses if sIPSCs and eIPSCs share the same pool of GABA, which leads to insufficient GABA release when the terminals or the soma of interenurons are stimulated exogenously. Analogously, norepinephrine has been shown to increase sIPSC frequency and amplitude but exerts heterogeneous effects on eIPSCs (Braga et al. 2004; Hirono and Obata 2006; Lei et al. 2007; Madison and Nicoll 1988). 5-HT also increases sIPSC frequency and amplitude but attenuates eIPSC amplitude in the EC (Deng and Lei 2008).
Muscarine has been reported to depolarize the principal neurons in layer II of the EC via a combination of both activation of a nonselective cationic conductance and inhibition of a K+ channel (Shalinsky et al. 2002). However, our results indicate that it is unlikely that muscarine increases GABA release, demonstrated by increased sIPSC frequency and amplitude, via activation of cation channels although Ca2+ influx via either cation channels per se or subsequent activation of voltage-gated Ca2+ channels due to the membrane depolarization induced by cation channel openings could facilitate GABA release. First, if the effect of muscarine on GABA release is mediated via activation of cation channels, application of muscarine should increase mIPSCs recorded in the presence of TTX because under this circumstance Ca2+ influx via the activated cation channels or voltage-gated Ca2+ channels should increase GABA release. We clearly showed that muscarine slightly depressed mIPSC frequency with no effects on mIPSC amplitude. Second, replacement of extracellular Na+ with NMDG or omission of extracellular Ca2+ failed to change muscarine-induced increases in inward HCs recorded from GABAergic interneurons. Third, application of cation channel blockers (Gd3+, La3+, SKF96365) did not change muscarine-induced increases in inward HCs recorded from interneurons. Fourth, muscarine increased neuronal input resistance indicating that muscarine reduces membrane conductance. If muscarine-induced depolarization of interneurons is mediated by opening cationic channels, muscarine should reduce neuronal input resistance. Finally, the reversal potential of muscarine-induced net currents is close to K+ reversal potential suggesting that the targets of muscarine are K+ not cation channels in entorhinal interneurons.
Muscarine has been reported to interact with several K+ channels including M-type (Delmas and Brown 2005), inward rectifier (Bajic et al. 2002), TASK (Boyd et al. 2000; Meuth et al. 2003, 2006), and TREK-2 (Kang et al. 2006) K+ channels. Our results suggest that M-type K+ channels are not involved in muscarine-mediated increases in GABA release in the EC. First, M-type K+ channels are voltage dependent and the voltage range of activation is positive to −60 mV, whereas muscarine-induced net currents in entorhinal interneurons could be recorded at voltages negative to −60 mV (Fig. 5A). Second, application of the selective M-type K+ channel blocker, linopirdine, failed to alter muscarine-induced increases in inward HCs. Neither do our results support a role for inward rectifier K+ channels. First, the inward rectifier K+ channels are sensitive to TEA and Ba2+, whereas muscarine-induced increases in inward HCs are not sensitive to TEA and Ba2+. Second, application of tertiapin, a selective inward rectifier K+ channel inhibitor, failed to change muscarine-induced increases in inward HCs. K2P channels are generally insensitive to the classic K+ channel blockers, and they are involved in controlling RMPs; our results suggest that muscarine inhibits K2P channels to depolarize interneurons. K2P channels can be divided into six subfamilies: TWIK, THIK, TREK, TASK, TALK, and TRESK (Bayliss et al. 2003; Lesage 2003). Whereas muscarinic receptor activation has been shown to inhibit TASK (Boyd et al. 2000; Meuth et al. 2003, 2006) and TREK-2 (Kang et al. 2006) channels, our results do not support the involvement of TASK and TREK-2 channels because these channels are sensitive to extracellular Ba2+(Han et al. 2002; Kim et al. 2000), but our results demonstrate that muscarine-induced depolarization is insensitive to Ba2+. In addition to TASK and TREK-2 channels, other K2P channels including TREK-1 (Fink et al. 1996), TWIK-1 (Lesage et al. 1996) and TRESK (Kang et al. 2004; Sano et al. 2003) are also sensitive to Ba2+. Because our results demonstrated that muscarine-induced depolarization is Ba2+ insensitive, we conclude that muscarine depolarizes entorhinal interneurons by inhibiting K2P channels other than TASK, TREK-1, TREK-2, TWIK-1, and TRESK.
We have demonstrated that muscarine-mediated depolarization of GABAergic interneurons in the EC is mediated via activation of M3 receptors. M3 receptors are coupled to Gαq/11 resulting in activation of PLC. We have shown that the muscarine-induced membrane depolarization in interneurons is dependent on G proteins but independent of PLC, intracellular Ca2+ release, and PKC. Our results support an action mode in which activation of muscarinic receptors depolarizes entorhinal interneurons via intracellular signals other than PLC or a direct interaction of G proteins with K2P channels. Consistent with our results, Gq-mediated inhibition of K2P channels is mediated via either intracellular signaling molecules (Chemin et al. 2003; Kang et al. 2006; Mathie 2007; Veale et al. 2007) or direct G protein coupling (Chen et al. 2006; Deng et al. 2006) depending on the involved types of K2P channels. Specifically, in line with our results, application of U73122, a PLC inhibitor, failed to block muscarine-induced depolarization in neonatal rat cerebellar granule cells (Boyd et al. 2000).
GABAergic interneurons synchronize neural network activities and serve as the precision clockwork for gamma and theta oscillations. Neural oscillatory events are thought to be crucially involved in different sleep states, in various cognitive processes including selective attention, and in the encoding and binding of information for associating features into unified perceived objects at the cortical level (Freund 2003). Furthermore, oscillation and synchronization of neural activity is also important for epileptogenesis (Tolner et al. 2005). Muscarinic receptor activation in the EC promotes intrinsic oscillations both in vivo (Alonso and Garcia-Austt 1987a,b; Dickson et al. 1995; Mitchell and Ranck 1980) and in vitro (Klink and Alonso 1997a,b). Muscarinic receptor-induced synchronization of neural activity in the EC requires concurrent glutamatergic and GABAergic synaptic inputs (Dickson and Alonso 1997). However, the roles of muscarinic receptor activation on GABAergic function in the EC have not been explored previously. Our results clearly filled this gap and suggest that muscarinic modulation of GABAergic transmission in the EC may contribute to the synchronous events induced by muscarinic receptor activation. Moreover, synchronized synaptic potentials observed in epileptic human mesial temporal lobe tissue were also found to be dependent on fast GABAergic transmission (Schwartzkroin and Haglund 1986) suggesting that the epileptogenic synaptic events are probably the compound action of both glutamatergic-mediated excitatory postsynaptic potentials and GABAergic-mediated inhibitory postsynaptic potentials (Schwartzkroin and Knowles 1984). Similar data have been reported during perfusion of hippocampal slices with 4-AP (Rutecki et al. 1987), and simultaneous firing of interneurons and principal cells has been described in the high potassium model of epilepsy in the hippocampus in vitro (McBain 1994), as well as during paroxysmal events in vivo (Bragin et al. 1995; Bragin et al. 1997; Steriade and Contreras 1995; Steriade et al. 1994). Our results therefore provide a cellular and molecular mechanism that could explain the roles of muscarinic receptors in epilepsy as well.
GRANTS
This work was supported by National Institutes of Health Grants R01MH-082881 and 5P20RR-017699 to S. Lei.
REFERENCES
- Alonso and Garcia-Austt 1987a.Alonso A, Garcia-Austt E. Neuronal sources of theta rhythm in the entorhinal cortex of the rat. I. Laminar distribution of theta field potentials. Exp Brain Res 67: 493–501, 1987a. [DOI] [PubMed] [Google Scholar]
- Alonso and Garcia-Austt 1987b.Alonso A, Garcia-Austt E. Neuronal sources of theta rhythm in the entorhinal cortex of the rat. II. Phase relations between unit discharges and theta field potentials. Exp Brain Res 67: 502–509, 1987b. [DOI] [PubMed] [Google Scholar]
- Alonso and Kohler 1984.Alonso A, Kohler C. A study of the reciprocal connections between the septum and the entorhinal area using anterograde and retrograde axonal transport methods in the rat brain. J Comp Neurol 225: 327–343, 1984. [DOI] [PubMed] [Google Scholar]
- Amaral and Kurz 1985.Amaral DG, Kurz J. An analysis of the origins of the cholinergic and noncholinergic septal projections to the hippocampal formation of the rat. J Comp Neurol 240: 37–59, 1985. [DOI] [PubMed] [Google Scholar]
- Arnold et al. 1991.Arnold SE, Hyman BT, Van Hoesen GW, Damasio AR. Some cytoarchitectural abnormalities of the entorhinal cortex in schizophrenia. Arch Gen Psychiatry 48: 625–632, 1991. [DOI] [PubMed] [Google Scholar]
- Avoli et al. 2002.Avoli M, D'Antuono M, Louvel J, Kohling R, Biagini G, Pumain R, D'Arcangelo G, Tancredi V. Network and pharmacological mechanisms leading to epileptiform synchronization in the limbic system in vitro. Prog Neurobiol 68: 167–207, 2002. [DOI] [PubMed] [Google Scholar]
- Bajic et al. 2002.Bajic D, Koike M, Albsoul-Younes AM, Nakajima S, Nakajima Y. Two different inward rectifier K+ channels are effectors for transmitter-induced slow excitation in brain neurons. Proc Natl Acad Sci USA 99: 14494–14499, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayliss et al. 2003.Bayliss DA, Sirois JE, Talley EM. The TASK family: two-pore domain background K+ channels. Mol Interv 3: 205–219, 2003. [DOI] [PubMed] [Google Scholar]
- Boyd et al. 2000.Boyd DF, Millar JA, Watkins CS, Mathie A. The role of Ca2+ stores in the muscarinic inhibition of the K+ current IK(SO) in neonatal rat cerebellar granule cells. J Physiol 529: 321–331, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braga et al. 2004.Braga MF, Aroniadou-Anderjaska V, Manion ST, Hough CJ, Li H. Stress impairs alpha(1A) adrenoceptor-mediated noradrenergic facilitation of GABAergic transmission in the basolateral amygdala. Neuropsychopharmacology 29: 45–58, 2004. [DOI] [PubMed] [Google Scholar]
- Bragin et al. 1995.Bragin A, Jando G, Nadasdy Z, van Landeghem M, Buzsaki G. Dentate EEG spikes and associated interneuronal population bursts in the hippocampal hilar region of the rat. J Neurophysiol 73: 1691–1705, 1995. [DOI] [PubMed] [Google Scholar]
- Bragin et al. 1997.Bragin A, Penttonen M, Buzsaki G. Termination of epileptic afterdischarge in the hippocampus. J Neurosci 17: 2567–2579, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burwell 2000.Burwell RD The parahippocampal region: corticocortical connectivity. Ann NY Acad Sci 911: 25–42, 2000. [DOI] [PubMed] [Google Scholar]
- Caulfield and Birdsall 1998.Caulfield MP, Birdsall NJ. International Union of Pharmacology. XVII. Classification of muscarinic acetylcholine receptors. Pharmacol Rev 50: 279–290, 1998. [PubMed] [Google Scholar]
- Chemin et al. 2003.Chemin J, Girard C, Duprat F, Lesage F, Romey G, Lazdunski M. Mechanisms underlying excitatory effects of group I metabotropic glutamate receptors via inhibition of 2P domain K+ channels. EMBO J 22: 5403–5411, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen et al. 2006.Chen X, Talley EM, Patel N, Gomis A, McIntire WE, Dong B, Viana F, Garrison JC, Bayliss DA. Inhibition of a background potassium channel by Gq protein alpha-subunits. Proc Natl Acad Sci USA 103: 3422–3427, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clements and Bekkers 1997.Clements JD, Bekkers JM. Detection of spontaneous synaptic events with an optimally scaled template. Biophys J 73: 220–229, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delmas and Brown 2005.Delmas P, Brown DA. Pathways modulating neural KCNQ/M (Kv7) potassium channels. Nat Rev Neurosci 6: 850–862, 2005. [DOI] [PubMed] [Google Scholar]
- Deng and Lei 2006.Deng PY, Lei S. Bidirectional modulation of GABAergic transmission by cholecystokinin in hippocampal dentate gyrus granule cells of juvenile rats. J Physiol 572: 425–442, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng and Lei 2007.Deng PY, Lei S. Long-term depression in identified stellate neurons of juvenile rat entorhinal cortex. J Neurophysiol 97: 727–737, 2007. [DOI] [PubMed] [Google Scholar]
- Deng and Lei 2008.Deng PY, Lei S. Serotonin increases GABA release in rat entorhinal cortex by inhibiting interneuron TASK-3 K+ channels. Mol Cell Neurosci 39: 273–284, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng et al. 2006.Deng PY, Porter JE, Shin HS, Lei S. Thyrotropin-releasing hormone increases GABA release in rat hippocampus. J Physiol 577: 497–511, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng et al. 2007.Deng PY, Poudel SK, Rojanathammanee L, Porter JE, Lei S. Serotonin inhibits neuronal excitability by activating two-pore domain k+ channels in the entorhinal cortex. Mol Pharmacol 72: 208–218, 2007. [DOI] [PubMed] [Google Scholar]
- Dickson and Alonso 1997.Dickson CT, Alonso A. Muscarinic induction of synchronous population activity in the entorhinal cortex. J Neurosci 17: 6729–6744, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson et al. 1995.Dickson CT, Kirk IJ, Oddie SD, Bland BH. Classification of theta-related cells in the entorhinal cortex: cell discharges are controlled by the ascending brainstem synchronizing pathway in parallel with hippocampal theta-related cells. Hippocampus 5: 306–319, 1995. [DOI] [PubMed] [Google Scholar]
- Dolcos et al. 2005.Dolcos F, LaBar KS, Cabeza R. Remembering one year later: role of the amygdala and the medial temporal lobe memory system in retrieving emotional memories. Proc Natl Acad Sci USA 102: 2626–2631, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolorfo and Amaral 1998a.Dolorfo CL, Amaral DG. Entorhinal cortex of the rat: organization of intrinsic connections. J Comp Neurol 398: 49–82, 1998a. [DOI] [PubMed] [Google Scholar]
- Dolorfo and Amaral 1998b.Dolorfo CL, Amaral DG. Entorhinal cortex of the rat: topographic organization of the cells of origin of the perforant path projection to the dentate gyrus. J Comp Neurol 398: 25–48, 1998b. [PubMed] [Google Scholar]
- Egorov et al. 2002.Egorov AV, Hamam BN, Fransen E, Hasselmo ME, Alonso AA. Graded persistent activity in entorhinal cortex neurons. Nature 420: 173–178, 2002. [DOI] [PubMed] [Google Scholar]
- Falkai et al. 1988.Falkai P, Bogerts B, Rozumek M. Limbic pathology in schizophrenia: the entorhinal region–a morphometric study. Biol Psychiatry 24: 515–521, 1988. [DOI] [PubMed] [Google Scholar]
- Fink et al. 1996.Fink M, Duprat F, Lesage F, Reyes R, Romey G, Heurteaux C, Lazdunski M. Cloning, functional expression and brain localization of a novel unconventional outward rectifier K+ channel. EMBO J 15: 6854–6862, 1996. [PMC free article] [PubMed] [Google Scholar]
- Freund 2003.Freund TF Interneuron Diversity series: rhythm and mood in perisomatic inhibition. Trends Neurosci 26: 489–495, 2003. [DOI] [PubMed] [Google Scholar]
- Gaykema et al. 1990.Gaykema RP, Luiten PG, Nyakas C, Traber J. Cortical projection patterns of the medial septum-diagonal band complex. J Comp Neurol 293: 103–124, 1990. [DOI] [PubMed] [Google Scholar]
- Golebiewski et al. 1994.Golebiewski H, Eckersdorf B, Blaszczyk M, Grabowski R, Konopacki J. Muscarinic (M1) mediation of carbachol-induced theta in the cat entorhinal cortex in vitro. Neuroreport 5: 1989–1992, 1994. [DOI] [PubMed] [Google Scholar]
- Guo and Chiappinelli 2001.Guo J, Chiappinelli VA. Distinct muscarinic receptors enhance spontaneous GABA release and inhibit electrically evoked GABAergic synaptic transmission in the chick lateral spiriform nucleus. Neuroscience 104: 1057–1066, 2001. [DOI] [PubMed] [Google Scholar]
- Guo and Chiappinelli 2000.Guo JZ, Chiappinelli VA. Muscarinic receptors mediate enhancement of spontaneous GABA release in the chick brain. Neuroscience 95: 273–282, 2000. [DOI] [PubMed] [Google Scholar]
- Haist et al. 2001.Haist F, Bowden Gore J, Mao H. Consolidation of human memory over decades revealed by functional magnetic resonance imaging. Nat Neurosci 4: 1139–1145, 2001. [DOI] [PubMed] [Google Scholar]
- Hamam et al. 2007.Hamam BN, Sinai M, Poirier G, Chapman CA. Cholinergic suppression of excitatory synaptic responses in layer II of the medial entorhinal cortex. Hippocampus 17: 103–113, 2007. [DOI] [PubMed] [Google Scholar]
- Hammer et al. 1980.Hammer R, Berrie CP, Birdsall NJ, Burgen AS, Hulme EC. Pirenzepine distinguishes between different subclasses of muscarinic receptors. Nature 283: 90–92, 1980. [DOI] [PubMed] [Google Scholar]
- Han et al. 2002.Han J, Truell J, Gnatenco C, Kim D. Characterization of four types of background potassium channels in rat cerebellar granule neurons. J Physiol 542: 431–444, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasselmo 2006.Hasselmo ME The role of acetylcholine in learning and memory. Curr Opin Neurobiol 16: 710–715, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirono and Obata 2006.Hirono M, Obata K. Alpha-adrenoceptive dual modulation of inhibitory GABAergic inputs to Purkinje cells in the mouse cerebellum. J Neurophysiol 95: 700–708, 2006. [DOI] [PubMed] [Google Scholar]
- Hyman et al. 1984.Hyman BT, Van Hoesen GW, Damasio AR, Barnes CL. Alzheimer's disease: cell-specific pathology isolates the hippocampal formation. Science 225: 1168–1170, 1984. [DOI] [PubMed] [Google Scholar]
- Joyal et al. 2002.Joyal CC, Laakso MP, Tiihonen J, Syvalahti E, Vilkman H, Laakso A, Alakare B, Rakkolainen V, Salokangas RK, Hietala J. A volumetric MRI study of the entorhinal cortex in first episode neuroleptic-naive schizophrenia. Biol Psychiatry 51: 1005–1007, 2002. [DOI] [PubMed] [Google Scholar]
- Kang et al. 2006.Kang D, Han J, Kim D. Mechanism of inhibition of TREK-2 (K2P10.1) by the Gq-coupled M3 muscarinic receptor. Am J Physiol Cell Physiol 291: C649–656, 2006. [DOI] [PubMed] [Google Scholar]
- Kang et al. 2004.Kang D, Mariash E, Kim D. Functional expression of TRESK-2, a new member of the tandem-pore K+ channel family. J Biol Chem 279: 28063–28070, 2004. [DOI] [PubMed] [Google Scholar]
- Kim et al. 2000.Kim Y, Bang H, Kim D. TASK-3, a new member of the tandem pore K(+) channel family. J Biol Chem 275: 9340–9347, 2000. [DOI] [PubMed] [Google Scholar]
- Klink and Alonso 1997a.Klink R, Alonso A. Ionic mechanisms of muscarinic depolarization in entorhinal cortex layer II neurons. J Neurophysiol 77: 1829–1843, 1997a. [DOI] [PubMed] [Google Scholar]
- Klink and Alonso 1997b.Klink R, Alonso A. Muscarinic modulation of the oscillatory and repetitive firing properties of entorhinal cortex layer II neurons. J Neurophysiol 77: 1813–1828, 1997b. [DOI] [PubMed] [Google Scholar]
- Kohler 1986.Kohler C Intrinsic connections of the retrohippocampal region in the rat brain. II. The medial entorhinal area. J Comp Neurol 246: 149–169, 1986. [DOI] [PubMed] [Google Scholar]
- Konopacki and Golebiewski 1992.Konopacki J, Golebiewski H. Theta rhythms in the rat medial entorhinal cortex in vitro: evidence for involvement of muscarinic receptors. Neurosci Lett 141: 93–96, 1992. [DOI] [PubMed] [Google Scholar]
- Kotzbauer et al. 2001.Kotzbauer PT, Trojanowsk JQ, Lee VM. Lewy body pathology in Alzheimer's disease. J Mol Neurosci 17: 225–232, 2001. [DOI] [PubMed] [Google Scholar]
- Lawrence et al. 2006.Lawrence JJ, Statland JM, Grinspan ZM, McBain CJ. Cell type-specific dependence of muscarinic signalling in mouse hippocampal stratum oriens interneurons. J Physiol 570: 595–610, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei et al. 2007.Lei S, Deng PY, Porter JE, Shin HS. Adrenergic facilitation of GABAergic transmission in rat entorhinal cortex. J Neurophysiol 98: 2868–2877, 2007. [DOI] [PubMed] [Google Scholar]
- Lesage 2003.Lesage F Pharmacology of neuronal background potassium channels. Neuropharmacology 44: 1–7, 2003. [DOI] [PubMed] [Google Scholar]
- Lesage et al. 1996.Lesage F, Guillemare E, Fink M, Duprat F, Lazdunski M, Romey G, Barhanin J. TWIK-1, a ubiquitous human weakly inward rectifying K+ channel with a novel structure. EMBO J 15: 1004–1011, 1996. [PMC free article] [PubMed] [Google Scholar]
- Lewis and Shute 1967.Lewis PR, Shute CC. The cholinergic limbic system: projections to hippocampal formation, medial cortex, nuclei of the ascending cholinergic reticular system, and the subfornical organ and supra-optic crest. Brain 90: 521–540, 1967. [DOI] [PubMed] [Google Scholar]
- Liu et al. 2007.Liu Z, Otsu Y, Vasuta C, Nawa H, Murphy TH. Action-potential-independent GABAergic tone mediated by nicotinic stimulation of immature striatal miniature synaptic transmission. J Neurophysiol 98: 581–593, 2007. [DOI] [PubMed] [Google Scholar]
- Lucas-Meunier et al. 2003.Lucas-Meunier E, Fossier P, Baux G, Amar M. Cholinergic modulation of the cortical neuronal network. Pfluegers 446: 17–29, 2003. [DOI] [PubMed] [Google Scholar]
- Lysakowski et al. 1989.Lysakowski A, Wainer BH, Bruce G, Hersh LB. An atlas of the regional and laminar distribution of choline acetyltransferase immunoreactivity in rat cerebral cortex. Neuroscience 28: 291–336, 1989. [DOI] [PubMed] [Google Scholar]
- Madison and Nicoll 1988.Madison DV, Nicoll RA. Norepinephrine decreases synaptic inhibition in the rat hippocampus. Brain Res 442: 131–138, 1988. [DOI] [PubMed] [Google Scholar]
- Mathie 2007.Mathie A Neuronal two-pore-domain potassium channels and their regulation by G protein-coupled receptors. J Physiol 578: 377–385, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBain 1994.McBain CJ Hippocampal inhibitory neuron activity in the elevated potassium model of epilepsy. J Neurophysiol 72: 2853–2863, 1994. [DOI] [PubMed] [Google Scholar]
- Mellgren and Srebro 1973.Mellgren SI, Srebro B. Changes in acetylcholinesterase and distribution of degenerating fibres in the hippocampal region after septal lesions in the rat. Brain Res 52: 19–36, 1973. [DOI] [PubMed] [Google Scholar]
- Meuth et al. 2006.Meuth SG, Aller MI, Munsch T, Schuhmacher T, Seidenbecher T, Meuth P, Kleinschnitz C, Pape HC, Wiendl H, Wisden W, Budde T. The contribution of TWIK-related acid-sensitive K+-containing channels to the function of dorsal lateral geniculate thalamocortical relay neurons. Mol Pharmacol 69: 1468–1476, 2006. [DOI] [PubMed] [Google Scholar]
- Meuth et al. 2003.Meuth SG, Budde T, Kanyshkova T, Broicher T, Munsch T, Pape HC. Contribution of TWIK-related acid-sensitive K+ channel 1 (TASK1) and TASK3 channels to the control of activity modes in thalamocortical neurons. J Neurosci 23: 6460–6469, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milner et al. 1983.Milner TA, Loy R, Amaral DG. An anatomical study of the development of the septo-hippocampal projection in the rat. Brain Res 284: 343–371, 1983. [DOI] [PubMed] [Google Scholar]
- Mitchell and Ranck 1980.Mitchell SJ, Ranck JB, Jr. Generation of theta rhythm in medial entorhinal cortex of freely moving rats. Brain Res 189: 49–66, 1980. [DOI] [PubMed] [Google Scholar]
- Prasad et al. 2004.Prasad KM, Patel AR, Muddasani S, Sweeney J, Keshavan MS. The entorhinal cortex in first-episode psychotic disorders: a structural magnetic resonance imaging study. Am J Psychiatry 161: 1612–1619, 2004. [DOI] [PubMed] [Google Scholar]
- Richter et al. 1999.Richter M, Schilling T, Muller W. Muscarinic control of intracortical connections to layer II in rat entorhinal cortex slice. Neurosci Lett 273: 200–202, 1999. [DOI] [PubMed] [Google Scholar]
- Rutecki et al. 1987.Rutecki PA, Lebeda FJ, Johnston D. 4-Aminopyridine produces epileptiform activity in hippocampus and enhances synaptic excitation and inhibition. J Neurophysiol 57: 1911–1924, 1987. [DOI] [PubMed] [Google Scholar]
- Rye et al. 1984.Rye DB, Wainer BH, Mesulam MM, Mufson EJ, Saper CB. Cortical projections arising from the basal forebrain: a study of cholinergic and noncholinergic components employing combined retrograde tracing and immunohistochemical localization of choline acetyltransferase. Neuroscience 13: 627–643, 1984. [DOI] [PubMed] [Google Scholar]
- Salgado et al. 2007.Salgado H, Bellay T, Nichols JA, Bose M, Martinolich L, Perrotti L, Atzori M. Muscarinic M2 and M1 receptors reduce GABA release by Ca2+ channel modulation through activation of PI3K/Ca2+-independent and PLC/Ca2+-dependent PKC. J Neurophysiol 98: 952–965, 2007. [DOI] [PubMed] [Google Scholar]
- Sano et al. 2003.Sano Y, Inamura K, Miyake A, Mochizuki S, Kitada C, Yokoi H, Nozawa K, Okada H, Matsushime H, Furuichi K. A novel two-pore domain K+ channel, TRESK, is localized in the spinal cord. J Biol Chem 278: 27406–27412, 2003. [DOI] [PubMed] [Google Scholar]
- Schwartzkroin and Haglund 1986.Schwartzkroin PA, Haglund MM. Spontaneous rhythmic synchronous activity in epileptic human and normal monkey temporal lobe. Epilepsia 27: 523–533, 1986. [DOI] [PubMed] [Google Scholar]
- Schwartzkroin and Knowles 1984.Schwartzkroin PA, Knowles WD. Intracellular study of human epileptic cortex: in vitro maintenance of epileptiform activity? Science 223: 709–712, 1984. [DOI] [PubMed] [Google Scholar]
- Shalinsky et al. 2002.Shalinsky MH, Magistretti J, Ma L, Alonso AA. Muscarinic activation of a cation current and associated current noise in entorhinal-cortex layer-II neurons. J Neurophysiol 88: 1197–1211, 2002. [DOI] [PubMed] [Google Scholar]
- Shute and Lewis 1967.Shute CC, Lewis PR. The ascending cholinergic reticular system: neocortical, olfactory and subcortical projections. Brain 90: 497–520, 1967. [DOI] [PubMed] [Google Scholar]
- Spencer and Spencer 1994.Spencer SS, Spencer DD. Entorhinal-hippocampal interactions in medial temporal lobe epilepsy. Epilepsia 35: 721–727, 1994. [DOI] [PubMed] [Google Scholar]
- Squire et al. 2004.Squire LR, Stark CE, Clark RE. The medial temporal lobe. Annu Rev Neurosci 27: 279–306, 2004. [DOI] [PubMed] [Google Scholar]
- Steffenach et al. 2005.Steffenach HA, Witter M, Moser MB, Moser EI. Spatial memory in the rat requires the dorsolateral band of the entorhinal cortex. Neuron 45: 301–313, 2005. [DOI] [PubMed] [Google Scholar]
- Steriade and Contreras 1995.Steriade M, Contreras D. Relations between cortical and thalamic cellular events during transition from sleep patterns to paroxysmal activity. J Neurosci 15: 623–642, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steriade et al. 1994.Steriade M, Contreras D, Amzica F. Synchronized sleep oscillations and their paroxysmal developments. Trends Neurosci 17: 199–208, 1994. [DOI] [PubMed] [Google Scholar]
- Steward and Scoville 1976.Steward O, Scoville SA. Cells of origin of entorhinal cortical afferents to the hippocampus and fascia dentata of the rat. J Comp Neurol 169: 347–370, 1976. [DOI] [PubMed] [Google Scholar]
- Sugita et al. 1991.Sugita S, Uchimura N, Jiang ZG, North RA. Distinct muscarinic receptors inhibit release of gamma-aminobutyric acid and excitatory amino acids in mammalian brain. Proc Natl Acad Sci USA 88: 2608–2611, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolner et al. 2005.Tolner EA, Kloosterman F, van Vliet EA, Witter MP, Silva FH, Gorter JA. Presubiculum stimulation in vivo evokes distinct oscillations in superficial and deep entorhinal cortex layers in chronic epileptic rats. J Neurosci 25: 8755–8765, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Linden and Lopes da Silva 1998.van der Linden S, Lopes da Silva FH. Comparison of the electrophysiology and morphology of layers III and II neurons of the rat medial entorhinal cortex in vitro. Eur J Neurosci 10: 1479–1489, 1998. [DOI] [PubMed] [Google Scholar]
- Veale et al. 2007.Veale EL, Kennard LE, Sutton GL, MacKenzie G, Sandu C, Mathie A. G(alpha)q-mediated regulation of TASK3 two-pore domain potassium channels: the role of protein kinase C. Mol Pharmacol 71: 1666–1675, 2007. [DOI] [PubMed] [Google Scholar]
- Witter et al. 1989.Witter MP, Groenewegen HJ, Lopes da Silva FH, Lohman AH. Functional organization of the extrinsic and intrinsic circuitry of the parahippocampal region. Prog Neurobiol 33: 161–253, 1989. [DOI] [PubMed] [Google Scholar]
- Witter et al. 2000a.Witter MP, Naber PA, van Haeften T, Machielsen WC, Rombouts SA, Barkhof F, Scheltens P, Lopes da Silva FH. Cortico-hippocampal communication by way of parallel parahippocampal-subicular pathways. Hippocampus 10: 398–410, 2000a. [DOI] [PubMed] [Google Scholar]
- Witter et al. 2000b.Witter MP, Wouterlood FG, Naber PA, Van Haeften T. Anatomical organization of the parahippocampal-hippocampal network. Ann NY Acad Sci 911: 1–24, 2000b. [DOI] [PubMed] [Google Scholar]
- Xiao et al. 2009.Xiao Z, Deng PY, Rojanathammanee L, Yang C, Grisanti L, Permpoonputtana K, Weinshenker D, Doze VA, Porter JE, Lei S. Noradrenergic depression of neuronal excitability in the entorhinal cortex via activation of TREK-2 K+ channels. J Biol Chem 284: 10980–10991, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yigit et al. 2003.Yigit M, Keipert C, Backus KH. Muscarinic acetylcholine receptors potentiate the GABAergic transmission in the developing rat inferior colliculus. Neuropharmacology 45: 504–513, 2003. [DOI] [PubMed] [Google Scholar]