Abstract
The excitability of the human primary motor cortex (M1) as tested with transcranial magnetic stimulation (TMS) depends on its previous history of neural activity. Homeostatic plasticity might be one important physiological mechanism for the regulation of corticospinal excitability and synaptic plasticity. Although homeostatic plasticity has been demonstrated locally within M1, it is not known whether priming M1 could result in similar homeostatic effects in the homologous M1 of the opposite hemisphere. Here, we sought to determine whether down-regulating excitability (priming) in the right (R) M1 with 1-Hz repetitive transcranial magnetic stimulation (rTMS) changes the excitability-enhancing effect of intermittent theta burst stimulation (iTBS) applied over the homologous left (L) M1. Subjects were randomly allocated to one of four experimental groups in a sham-controlled parallel design with real or sham R M1 1-Hz TMS stimulation always preceding L M1 iTBS or sham by about 10 min. The primary outcome measure was corticospinal excitability in the L M1, as measured by recruitment curves (RCs). Secondary outcome measures included pinch force, simple reaction time, and tapping speed assessed in the right hand. The main finding of this study was that preconditioning R M1 with 1-Hz rTMS significantly decreased the excitability-enhancing effects of subsequent L M1 iTBS on RCs. Application of 1-Hz rTMS over R M1 alone and iTBS over L M1 alone resulted in increased RC in L M1 relative to sham interventions. The present findings are consistent with the hypothesis that homeostatic mechanisms operating across hemispheric boundaries contribute to regulate motor cortical function in the primary motor cortex.
INTRODUCTION
Application of noninvasive brain stimulation techniques, such as repetitive transcranial magnetic stimulation (rTMS), theta burst stimulation (TBS), and transcranial DC stimulation (tDCS) modulate cortical excitability in the human motor cortex (M1) (Fitzgerald et al. 2006; Huang et al. 2005; Nitsche and Paulus 2000; Reis et al. 2008). Accordingly, noninvasive brain stimulation can contribute to the understanding of mechanisms of rehabilitative processes and might have a therapeutic potential in the treatment of a variety of neurological disorders (Fregni and Pascual-Leone 2007; Hummel and Cohen 2006). rTMS can induce long-lasting bidirectional changes in cortical excitability within the stimulated cortical area, depending on the stimulation protocol (Chen et al. 1997; Di Lazzaro et al. 2002; Muellbacher et al. 2000; Peinemann et al. 2000; for review see Fitzgerald et al. 2006). Although direct evidence on mechanisms mediating the rTMS-induced aftereffects is relatively scanty, it has been proposed that changes in synaptic efficacy including long-term potentiation (LTP)–like and long-term depression (LTD)–like mechanisms might play a contributory role (Cooke and Bliss 2006; Huang et al. 2008; Iyer et al. 2003; Siebner and Rothwell 2003; Siebner et al. 2004).
Previous studies provided evidence that the response to noninvasive brain stimulation protocols critically depends on the previous history of neural activity (Iyer et al. 2003; Ziemann et al. 2004). These findings have been interpreted within the framework of homeostatic plasticity as proposed in the Bienenstock–Cooper–Munro (BCM) theory (Bienenstock et al. 1982). Fundamental to the BCM theory is a time-variable induction threshold for synaptic plasticity. For example, prolonged low levels of postsynaptic activity decrease the induction threshold, thereby increasing the probability for LTP. Conversely, a history of enhanced postsynaptic activity will increase the threshold for LTP and therefore increase the likelihood for LTD induction. The concept of homeostatic plasticity in human subjects has so far been demonstrated only when priming the target area (Lang et al. 2004; Muller et al. 2007; Siebner et al. 2004) or priming an interconnected brain area within the same hemisphere such as the dorsal premotor cortex (Hartwigsen et al. 2008). In the present study we asked whether priming the motor cortex on the opposite hemisphere could result in similar homeostatic effects. As a priming stimulation we used a suprathreshold low-frequency (1-Hz) rTMS that is known not only to decrease cortical excitability within the stimulated cortical area but also to increase excitability in the homologous M1 (Schambra et al. 2003). It has been proposed that this phenomenon relies on interhemispheric disinhibition between both M1 (for review see Hummel and Cohen 2006). Interhemispheric disinhibition is also a promising method for boosting the beneficial effects of training-based interventions in neurorehabilitation, since the concept of interhemispheric disinhibition induced by rTMS has been proposed in stroke patients to facilitate motor function (Duque et al. 2005; Mansur et al. 2005; Murase et al. 2004; Takeuchi et al. 2005) or speech production in aphasia (Naeser et al. 2005b).
We hypothesized that the excitability-enhancing effect of intermittent TBS (iTBS) over L M1 would be diminished by preconditioning the contralateral M1 with suprathreshold 1-Hz rTMS. If so, we reasoned, these data could provide evidence for the operation of homeostatic mechanisms across both primary motor cortices in normal volunteers.
METHODS
Experimental procedures
Subjects
We studied 34 healthy subjects between 22 and 32 yr of age (16/34 females). They gave written informed consent to participate in the experiment according to the declaration of Helsinki and the NINDS Institutional Review Board approved the study. Prior to participation, all healthy volunteers underwent a comprehensive neurological examination. They were not taking any medication. Subjects that did not meet the protocol criteria and/or had contraindications for the TMS measurements were excluded from participation. According to the Oldfield questionnaire for the assessment of handedness (Oldfield 1971), all subjects were right-handed.
Experimental design
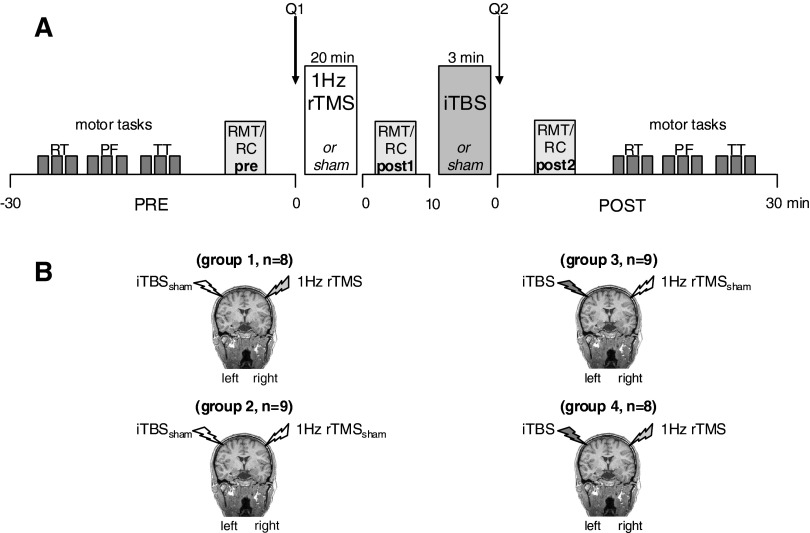
Subjects were randomly allocated to one of four experimental groups in a sham-controlled, single-blinded parallel study design (Fig. 1). We conservatively chose a factorial design to avoid within-subject possible carryover effects across session, acknowledging that it may have resulted in underestimating the efficacy of interventions and biasing our study against our hypothesis. The only difference between each group was the type of the rTMS protocol (real/sham) applied to the left (L) and right (R) M1. R M1 stimulation using suprathreshold 1-Hz rTMS or 1-Hz rTMSsham always preceded L M1 stimulation (iTBS or iTBSsham) by about 10 min. Group 1 received 1-Hz rTMS followed by iTBSsham (1-Hz rTMS + iTBSsham, n = 8), group 2 received 1-Hz rTMSsham followed by iTBSsham (1-Hz rTMSsham + iTBSsham, n = 9), group 3 received 1-Hz rTMSsham followed by iTBS (1-Hz rTMSsham + iTBS, n = 9), and group 4 received 1-Hz rTMS followed by iTBS (1-Hz rTMS + iTBS, n = 8).
FIG. 1.
A: experimental setup. Subjects were randomly allocated to one of 4 experimental groups in a parallel design. Each subject participated in only one single session, receiving only one combined intervention. In each session we tested recruitment curves (RCs, primary endpoint measure) and motor thresholds (RMTs) at baseline, immediately after each 1-Hz transcranial magnetic stimulation (TMS) or sham (post 1) and after intermittent theta burst stimulation (iTBS) or sham (post 2). Subjects rated their level of attention, their perception of fatigue, and their discomfort using a visual analog scale before (Q1) and after (Q2) the application of 2 subsequent stimulation protocols. Reaction times (RTs), pinch force (PF), and index finger tapping (TT) were evaluated at pre and post 2. B: the 4 interventions were: 1-Hz repetitive (r)TMS over right primary motor cortex (R M1) followed by sham iTBS over left (L) M1 (group 1), sham 1-Hz rTMS over R M1 followed by sham iTBS over L M1 (group 2), sham 1-Hz rTMS over R M1 followed by iTBS over L M1 (group 3), and 1-Hz rTMS over R M1 followed by iTBS over L M1 (group 4).
During the experiment, subjects were seated in an armchair with both arms relaxed and were instructed to keep their eyes open. Surface electromyogram (EMG) was recorded bilaterally using surface Ag/AgCl electrodes positioned on the skin overlying the first dorsal interosseous (FDI) hand muscle in a bipolar montage. The signal was amplified using a Counterpoint EMG device (Dantec Electronics, Skovlunde, Denmark) with band-pass filtering between 50 and 2,000 Hz. The signal was digitized at a frequency of 5,000 Hz and fed off-line to a data acquisition system built within LabVIEW (LabVIEW version 7.1, National Instruments, Austin, TX) for further analysis. The absence of voluntary contraction was monitored on-line by visual inspection of the EMG signal and off-line by inspection of each individual trace. Trials with background EMG were excluded from the analysis (a single subject showed occasional background activity in a few trials that were excluded from the analysis).
Corticomotor excitability was studied using TMS stimuli delivered from a Magstim 200 (Magstim, Whitland, South West Wales, UK) through a figure-of-eight 70-mm coil. Initially, the position of the coil was identified over the motor cortex with the handle of the coil pointing posterolaterally with a 45° angle to the sagittal plane, to elicit the largest and most consistent motor-evoked potential (MEP) amplitude in the right FDI hand muscle—this position was marked on the scalp (i.e., the motor hotspot; Rossini et al. 1994). The motor hotspot of the FDI muscle representation was identified as the scalp position at which single TMS pulses at slightly suprathreshold intensity induced the most consistent MEP amplitudes in the relaxed muscle. TMS measurements, including resting motor threshold (RMT) and recruitment curves (RCs) were performed before (pre) after the first (post 1) and second (post 2) rTMS interventions (Fig. 1A). For the application of 1-Hz rTMS and iTBS, we used a Magstim Super Rapid stimulator that produced a biphasic waveform connected with a figure-of-eight coil (internal wing diameter, 7 cm; peak magnetic field strength, 2.2 Tesla; peak electric field strength, 660 V/m).
Each session started with determination of baseline measurements in the right hand (reaction time task [RT], index-finger tapping task [TT], pinch force task [PF]) as well as RMT and RC measurements of L M1 (pre). Subsequently, either 1-Hz rTMS or 1-Hz rTMSsham was applied over the R M1 followed by measurement of RMT and RC (post 1, Fig. 1A). Immediately afterward, iTBS or iTBSsham was applied over L M1. RMT and RC measurements in L M1 and determination of motor performance were repeated immediately after the end of the second intervention (post 2, Fig. 1A).
The 1-Hz rTMS was applied at suprathreshold intensity (115% RMT) for 20 min and consisted of 1,200 consecutive pulses. For iTBS, the stimulation intensity was set to 80% of active motor threshold, defined as the minimal TMS stimulus intensity required to elicit contralateral FDI MEPs of >200 μV in 5 of 10 trials when subjects exerted background facilitation (20% of maximum force). The stimulation pattern of iTBS consisted of bursts containing three pulses at 50 Hz repeated at 200-ms intervals (i.e., at 5 Hz) for 2 s. These 2-s blocks were repeated once every 10 s for a total of 20 times. Thus the complete iTBS block lasted 192 s (including gaps) and contained a total of 600 stimuli (Huang et al. 2005). Sham stimulation was delivered through a specially designed sham figure-of-eight magnetic coil (Magstim), with an internal wing diameter of 7 cm that provides slight sensory stimulation on the head and discharge noise similar to the usual coil without stimulating cortical tissue.
RMT over L and R M1 was defined as the lowest intensity capable of evoking 5 of 10 MEPs with amplitudes of ≥50 μV in the relaxed FDI muscle of the right/left hand (Rossini et al. 1994). For recruitment curves, TMS pulses were delivered at 0.1 Hz to the L (target) M1 position optimal for activation of the FDI muscle, a rate that at rest does not affect cortical excitability (Chen et al. 1997). Mean MEP amplitudes obtained in response to 10 consecutive TMS stimuli were recorded at four systematically increased stimulus intensities (100, 110, 130, and 150% of RMT). We also evaluated pinch force in the right hand according to a protocol that exhibits good validity and test–retest reliability (Mathiowetz et al. 1984, 1985) using a pinch gauge. Pinch force measurements of five consecutive trials separated by 30 s were taken for each time point and averaged. Each block containing five maximal pinch force measurements was repeated three times. Between each block there was a rest period of 1 min to avoid fatigue. Trials in which pinch force was produced during the rest period were excluded from analysis. For the index finger tapping task, subjects were instructed to press a key on a keyboard with the index finger of the right hand as many times as possible during an epoch of 30 s. For the simple reaction time task (RT) subjects were instructed to perform 20 index finger abduction motions with their right hand as fast as possible in response to an auditory cue. RT was defined as the time between the auditory cue and the onset of the EMG response (at the time where it first exceeded 100-μV peak-to-peak amplitude).
Questionnaires
Using a visual analogue scale (VAS), subjects rated their level of attention (range: 1–10; 1 = not attentive, 10 = very attentive), their perception of fatigue (range 1–10; 1 = strong fatigue, 10 = no fatigue), and their discomfort (range 1–10; 1 = no discomfort, 10 = strong discomfort) before (pre) and after (post 2) the application of the two rTMS interventions (Fig. 1A).
Statistical analysis
Data were analyzed using the SPSS software package for Windows version 12.0.1. We used repeated-measures ANOVA (ANOVARM), post hoc analysis (Bonferroni-corrected), and Students' paired t-test to compare motor performance before and after the application of both interventions within each group. To test the effects of 1-Hz rTMS or sham followed by iTBS or sham, ANOVARMs, if necessary with Huynh–Feld sphericity corrections, were performed with the independent factors GROUP (four groups 1–4), TIME (pre, post 1, post 2), and INTENSITY (100, 110, 130, and 150% of RMT). All figures represent group data. Error bars refer to SE. Differences were considered significant at P ≤ 0.05.
RESULTS
Attention, fatigue, and discomfort
There was no difference in attention [ANOVARM with factor GROUP × TIME: F(3,30) = 0.428; P = 0.735], fatigue [ANOVARM with factor GROUP × TIME: F(3,30) = 1.576; P = 0.216], or discomfort [ANOVARM with factor GROUP × TIME: F(3,30) = 0.916; P = 0.445] between or within groups along the experiment (see Table 1).
TABLE 1.
Changes in attention, fatigue, and discomfort for all groups tested before (pre) and after (post) application of the interventions
| Group | Attention |
Fatigue | Discomfort | |||
|---|---|---|---|---|---|---|
| Pre | Post | Pre | Post | Pre | Post | |
| 1-Hz rTMS + iTBSsham | 7.50 ± 0.46 | 8.12 ± 0.35 | 4.50 ± 0.80 | 4.12 ± 0.79 | 1.75 ± 0.31 | 1.12 ± 0.12 |
| 1-Hz rTMSsham + iTBSsham | 8.22 ± 0.40 | 8.55 ± 0.41 | 4.44 ± 0.67 | 4.67 ± 0.87 | 1.66 ± 0.44 | 2.00 ± 0.55 |
| 1-Hz rTMSsham + iTBS | 7.44 ± 0.50 | 7.44 ± 0.62 | 4.55 ± 0.90 | 3.77 ± 0.85 | 1.55 ± 0.24 | 1.77 ± 0.32 |
| 1-Hz rTMS + iTBS | 8.50 ± 0.46 | 9.00 ± 0.36 | 4.37 ± 0.84 | 3.00 ± 0.73 | 1.75 ± 0.41 | 1.00 ± 0.79 |
Values are means ± SE. Ranges are as follows: attention (1–10), where 1 = not attentive and 10 = very attentive; fatigue (1–10), where 1 = strong fatigue and 10 = no fatigue; and discomfort (1–10), where 1 = no discomfort and 10 = strong discomfort. Note that there was no significant change within or across groups.
Resting motor thresholds
RMTs of the L M1 were comparable across the four groups, as estimated by univariate ANOVA with factor GROUP [F(3,30) = 1.384; P = 0.267] and did not change during the experiment [ANOVARM with factor GROUP × TIME: F(3,30) = 1.212; P = 0.322; Table 2].
TABLE 2.
Resting motor thresholds (RMTs) for groups 1–4 before (pre) and after (post 1) 1-Hz rTMS/1-Hz rTMSsham as well as after (post 2) iTBS/iTBSsham
| RMT | Group 1: 1-Hz rTMS + iTBSsham | Group 2: 1-Hz rTMSsham + iTBSsham | Group 3: 1-Hz rTMSsham + iTBS | Group 4: 1-Hz rTMS + iTBS |
|---|---|---|---|---|
| pre | 43.11 ± 2.16 | 42.66 ± 2.05 | 47.11 ± 2.82 | 48.50 ± 3.72 |
| post 1 | 42.71 ± 1.93 | 42.44 ± 2.17 | 46.44 ± 2.66 | 48.87 ± 3.76 |
| post 2 | 40.85 ± 2.05 | 41.89 ± 2.34 | 46.78 ± 2.34 | 47.75 ± 3.84 |
Values are means ± SE. Note that there was no significant change in RMT within or between each group.
Recruitment curves
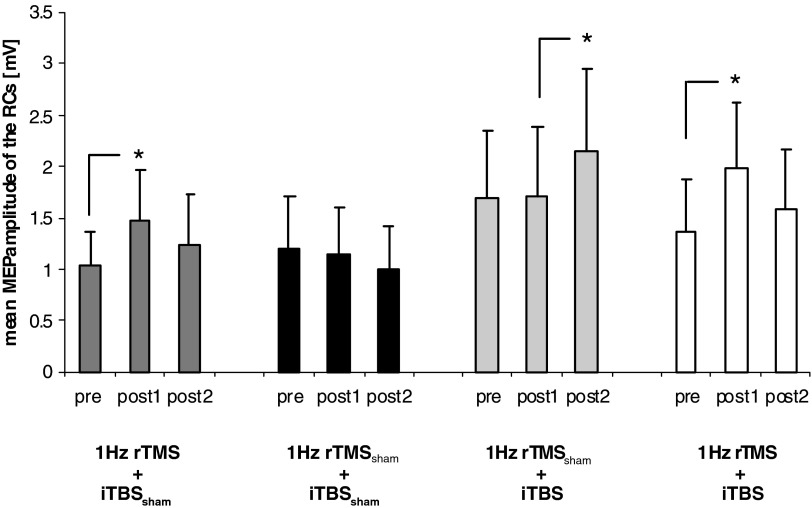
ANOVARM showed a triple interaction INTENSITY × TIME × GROUP [F(18,180) = 1.671; P = 0.05] on RCs, suggesting a difference in MEP amplitudes over time in at least one of the experimental groups at one or more of the stimulus intensities tested. Importantly, there was a significant GROUP × TIME interaction [ANOVARM: F(6,60) = 4.651; P = 0.001; see also Fig. 2], indicating a clear difference in RC over time in at least one of the experimental groups. Further analysis revealed a significant effect of TIME on RC for subjects that received 1-Hz rTMS + iTBS [ANOVARM: F(2,14) = 4.929; P = 0.024], 1-Hz rTMSsham + iTBS [ANOVARM: F(2,16) = 4.670; P = 0.025], and 1-Hz rTMS + iTBSsham [ANOVARM: F(2,14) = 5.742; P = 0.015]. However, no such changes could be observed for sham stimulation [1-Hz rTMSsham + iTBSsham, ANOVARM: F(2,16) = 2.497; P = 0.114; Fig. 2]. These results indicate that in all groups, except for the sham condition, RC after L M1 stimulation showed a significant amplitude change in at least one of the time points (pre, post 1, or post 2) measured.
FIG. 2.
Mean motor-evoked potential (MEP) amplitudes of the recruitment curves (RCs) for the 4 different groups (1-Hz rTMS + iTBSsham, 1-Hz rTMSsham + iTBSsham, 1-Hz rTMSsham + iTBS, and 1-Hz rTMS + iTBS) tested at 3 different time points (pre, post 1, post 2). Significant differences in mean MEP amplitudes are marked with an asterisk (P ≤ 0.05). For details see text.
Group 1: 1-Hz rTMS + iTBSsham (Fig. 1B).
A 1-Hz rTMS of the R M1 resulted in larger RC of L M1 at post 1 compared with pre [ANOVARM with factor TIME: F(1,7) = 12.518; P = 0.009], whereas the interaction of TIME × INTENSITY [F(3,21) = 2.615; P = 0.078] was not significant (Table 2). After iTBSsham (post 2), RC returned to baseline levels (pre), indicating the reversibility of 1-Hz rTMS- induced effects [ANOVARM with factor SESSION: F(1,7) = 1.843; P = 0.217] on corticospinal excitability after about 15 min (see Fig. 4 and Table 3).
TABLE 3.
Absolute changes (mV) in recruitment curves (RCs) for all groups tested at three different time points (pre, post 1, post 2)
| Change in MEP size (mV) | 100% RMT | 110% RMT | 130% RMT | 150% RMT |
|---|---|---|---|---|
| post 1 vs. pre | ||||
| 1-Hz rTMS + iTBSsham | 0.16 ± 0.06 | 0.31 ± 0.13 | 0.39 ± 0.16 | 0.91 ± 0.37 |
| 1-Hz rTMSsham + iTBSsham | −0.06 ± 0.06 | 0.09 ± 0.03 | −0.06 ± 0.20 | 0.02 ± 0.26 |
| 1-Hz rTMSsham + iTBS | −0.05 ± 0.05 | −0.08 ± 0.13 | 0.14 ± 0.14 | 0.04 ± 0.19 |
| 1-Hz rTMS + iTBS | 0.18 ± 0.09 | 0.51 ± 0.32 | 0.85 ± 0.29 | 0.83 ± 0.22 |
| post 2 vs. post 1 | ||||
| 1-Hz rTMS + iTBSsham | −0.18 ± 0.05 | −0.35 ± 0.09 | −0.08 ± 0.19 | −0.33 ± 0.34 |
| 1-Hz rTMSsham + iTBSsham | −0.02 ± 0.02 | 0.03 ± 0.10 | −0.25 ± 0.18 | −0.38 ± 0.17 |
| 1-Hz rTMSsham + iTBS | 0.12 ± 0.06 | 0.53 ± 0.18 | 0.87 ± 0.37 | 0.37 ± 0.35 |
| 1-Hz rTMS + iTBS | −0.17 ± 0.06 | −0.34 ± 0.32 | −0.78 ± 0.22 | −0.27 ± 0.40 |
| post 2 vs. pre | ||||
| 1-Hz rTMS + iTBSsham | −0.01 ± 0.04 | −0.04 ± 0.12 | 0.31 ± 0.25 | 0.58 ± 0.35 |
| 1-Hz rTMSsham + iTBSsham | −0.08 ± 0.06 | −0.07 ± 0.09 | −0.30 ± 0.24 | −0.36 ± 0.25 |
| 1-Hz rTMSsham + iTBS | 0.08 ± 0.07 | 0.45 ± 0.14 | 0.95 ± 0.44 | 0.42 ± 0.33 |
| 1-Hz rTMS + iTBS | 0.01 ± 0.04 | 0.18 ± 0.24 | 0.07 ± 0.28 | 0.57 ± 0.25 |
Values are means ± SE, as a group average. For details see text.
Group 2: 1-Hz rTMSsham + iTBSsham (Fig. 1B).
There was no significant effect of TIME [ANOVARM: F(2,16) = 2.497; P = 0.114] or the interaction of TIME × INTENSITY [ANOVARM: F(6,48) = 0.894; P = 0.507] on RC, indicating that two subsequent sham stimulation protocols (sham 1-Hz rTMS over R M1 followed by sham iTBS over L M1) did not influence RC on the L M1 (Table 3).
Group 3: 1-Hz rTMSsham + iTBS (Fig. 1B).
iTBS of the L M1 (post 2) resulted in larger RC of L M1 compared with post 1 [ANOVARM with factor TIME: F(2,16) = 4.670; P = 0.025], whereas the interaction of TIME × INTENSITY was not significant [ANOVARM: F(6,48) = 2.007; P = 0.083; see Fig. 3 and Table 3].
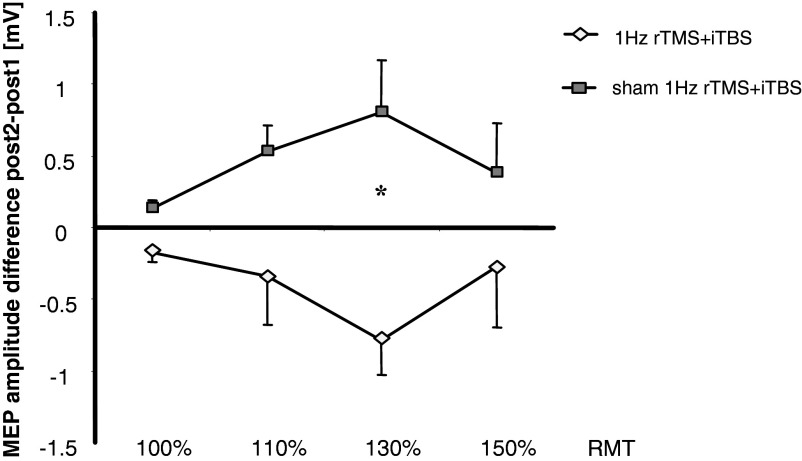
FIG. 3.
Change in MEP amplitudes evoked by stimulation of L M1 at different stimulus intensities when iTBS was applied following sham (dark symbols) and real (light symbols) 1-Hz rTMS (post 2, Fig. 1A) relative to the post 1 measurement (immediately after either sham or 1-Hz rTMS over R M1). Asterisk indicates the significant changes in L M1 MEP amplitudes at 130% RMT intensity (increase when preconditioning stimulation of R M1 was sham and decrease when it was real 1-Hz rTMS). Note that preconditioning R M1 with 1-Hz TMS (1-Hz rTMS + iTBS) significantly decreased the excitability-enhancing effects of L M1 iTBS on RCs at 130% RMT.
Group 4: 1-Hz rTMS + iTBS (Fig. 1B).
Similar to group 1, 1-Hz rTMS of the R M1 resulted in larger RC after stimulation of L M1 at post 1 compared with pre [ANOVARM with factor TIME: F(1,7) = 11.974; P = 0.01], whereas the interaction of TIME × INTENSITY [F(3,21) = 2.286; P = 0.141] was not significant. Subsequent iTBS over L M1 resulted in no significant additive change on RCs [ANOVARM with factor TIME (post 1 vs. post 2): F(1,7) = 3.088; P = 0.122]. Therefore 1-Hz rTMS over R M1 diminished the excitability-enhancing effect of subsequent iTBS over L M1 as seen in group 3 [univariate ANOVA with factors INTENSITY and GROUP: F(7,60) = 3.340; P = 0.005].
Post hoc analyses revealed significant differences in post 2 MEP amplitudes at 130% RMT in 1-Hz rTMSsham + iTBS and in 1-Hz rTMS + iTBS groups relative to post 1 measurements (P = 0.006, Bonferroni-corrected), but not at 100, 110, or 150% RMT (Figs. 3 and 4).
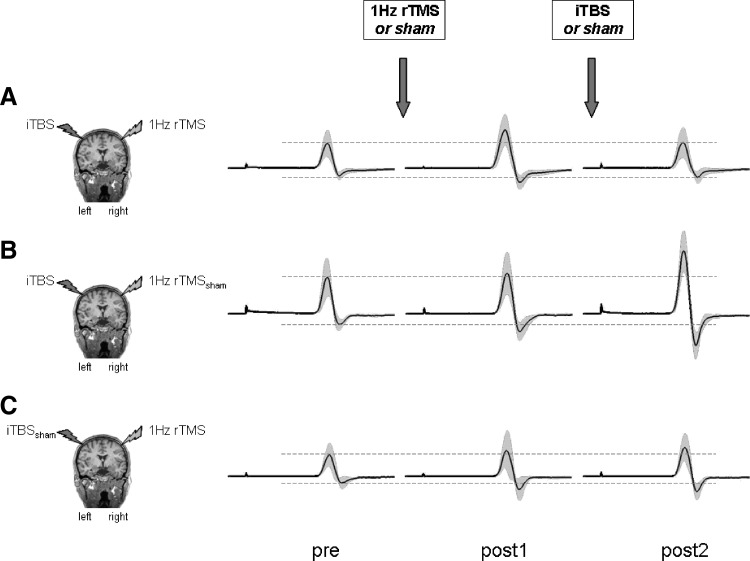
FIG. 4.
Change in MEP amplitudes over L M1 at 130% RMT at different time points (pre, post 1, and post 2) in 3 representative subjects receiving either (A) 1-Hz rTMS + iTBS, (B) 1-Hz rTMSsham + iTBS, or (C) 1-Hz rTMS + iTBSsham. Displayed are the mean MEP amplitudes of 10 MEPs at 130% RMT for each time point tested (solid lines) and SDs (gray area). Dashed lines indicate the mean MEP amplitudes (pre) before the application of the 2 brain-stimulation protocols, indicated by the arrows. Note that the overt excitability-enhancing effect of iTBS over L M1 (see subject depicted in B at post 2) was decreased by preconditioning R M1 with 1-Hz rTMS (see subject depicted in A at post 2). The subject depicted in C received only 1-Hz rTMS, leading to a mild increase in RC in L M1 at post 1.
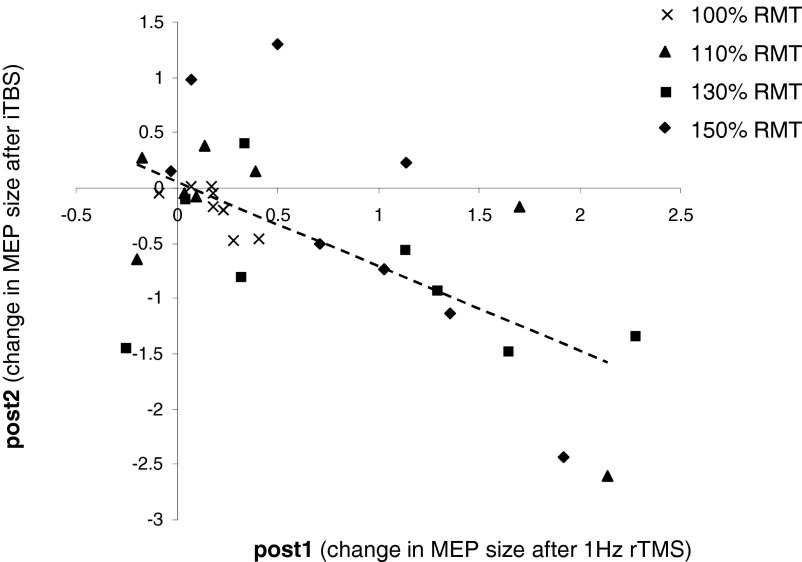
Within the 1-Hz rTMS + iTBS group, there was an inverse correlation between absolute changes in MEP amplitude for all subjects and the four stimulus intensities tested (100, 110, 130, and 150%) immediately after 1-Hz rTMS to R M1 (post 1) and the subsequent aftereffects of iTBS to L M1 (post 2) (r = −0.634, P < 0.001, separate correlations: 100% RMT: r = −0.753, P = 0.031; 110% RMT: r = −0.706, P = 0.05; 130% RMT: r = −0.426, P = 0.293; 150% RMT: r = −0.819, P = 0.013; see Fig. 5). Subjects that experienced the most prominent facilitation of L M1 excitability by 1-Hz rTMS of the R M1 (x-axis) were those with more pronounced decrease in excitability at post 2, following iTBS to the L M1.
FIG. 5.
Individual MEP amplitude changes for all stimulus intensities tested (100, 110, 130, and 150%) in the 1-Hz rTMS + iTBS group. The abscissa shows post 1–pre change in MEP amplitudes after preconditioning R M1 with 1-Hz rTMS. The ordinate shows post 2–post 1 change in MEP amplitudes after subsequent iTBS over L M1. Note that subjects that experienced the most prominent MEP amplitude facilitation by 1-Hz rTMS of the R M1 (x-axis) are those who subsequently had larger MEP amplitude decreases after iTBS of the L M1 at post 2 (y-axis). The regression line refers to the whole data set.
Behavioral measurements
Neither PF, TT, nor RT changed in any of the four groups (see Table 4) nor predicted the individual changes in RCs (P > 0.05).
TABLE 4.
Summary of reaction time (RT), pinch force (PF), and tapping speed (TT) performance of the right hand before (pre) and after (post) application of the two interventions of each group
| Motor Tasks | Group 1: 1-Hz rTMS + iTBSsham | Group 2: 1-Hz rTMSsham + iTBSsham | Group 3: 1-Hz rTMSsham + iTBS | Group 4: 1-Hz rTMS + iTBS |
|---|---|---|---|---|
| RT, ms | ||||
| pre | 150.52 ± 12.36 | 155.46 ± 12.49 | 169.09 ± 9.93 | 152.24 ± 15.74 |
| post | 146.01 ± 7.03 | 154.22 ± 10.73 | 159.23 ± 10.61 | 154.24 ± 16.60 |
| PF, N | ||||
| pre | 14.32 ± 1.86 | 16.33 ± 1.56 | 17.56 ± 1.53 | 19.91 ± 2.93 |
| post | 14.58 ± 1.92 | 16.51 ± 1.43 | 17.60 ± 1.30 | 21.05 ± 2.86 |
| TT, key strokes/30 s | ||||
| pre | 174.41 ± 9.88 | 172.03 ± 8.32 | 174.31 ± 5.94 | 184.79 ± 8.16 |
| post | 169.00 ± 8.10 | 170.92 ± 8.15 | 171.51 ± 6.78 | 180.79 ± 10.47 |
Values are means ± SE, as an average of the three blocks tested for each task. Note that there was no significant change in motor tasks within or between each group.
DISCUSSION
The present results show that the excitability-enhancing effect of iTBS over L M1 was reversed by preconditioning the R M1 with suprathreshold 1-Hz rTMS. Notably, healthy subjects that experienced the most prominent facilitation of L M1 excitability by 1-Hz rTMS of the R M1 were those with more excitability drop at post 2, following iTBS to the L M1.
Decreasing excitability in one motor cortex by 1-Hz rTMS results in well-described increments in MEP amplitudes in the opposite M1 (Kobayashi et al. 2004; Plewnia et al. 2003; Schambra et al. 2003). These findings provided a rationale for the development of interventional strategies in neurorehabilitation focused on down-regulating activity in the healthy hemisphere of patients with stroke to facilitate, perhaps disinhibiting, the lesioned hemisphere (Boggio et al. 2007; Hummel and Cohen 2006; Naeser et al. 2005a). Although to date proof-of-principle studies documented these effects, the physiological mechanisms underlying interhemispheric inhibitory interactions between the primary motor cortices are still not well understood (Perez and Cohen 2008, 2009). The purpose of this investigation was to evaluate the ability of an M1 disinhibited by 1-Hz rTMS of the opposite M1 to respond to an excitability-enhancing iTBS protocol (Huang et al. 2005) in normal volunteers. First, we found that excitability of the L M1 was facilitated by 1-Hz rTMS to the R M1, consistent with previous results (Plewnia et al. 2003; Schambra et al. 2003). Importantly, the effects of iTBS on motor cortical excitability were opposite when applied over L M1 devoid of any other influence (facilitatory in group 3) and over a disinhibited L M1 (inhibitory in group 4). One possible way to interpret these findings is in the framework of what has been referred to as homeostatic plasticity (Rutherford et al. 1998; Turrigiano et al. 1998).
In humans, previous work demonstrated excitability changes consistent with homeostatic plasticity within specific cortical areas of one hemisphere (Iyer et al. 2003; Lang et al. 2004; Muller et al. 2007; Siebner et al. 2004) including M1 (Gentner et al. 2008; Iyer et al. 2003; Muller et al. 2007; Siebner et al. 2004), primary somatosensory cortex (Bliem et al. 2008), and the visual cortex (Silvanto and Muggleton 2008). It has been proposed that abnormalities in patterns of homeostatic plasticity in M1 may contribute to clinical disorders such as writer's cramp (Quartarone et al. 2003) or migraine with visual aura (Antal et al. 2008). Practice of rapid thumb abduction movements prevents a subsequent excitability-enhancing paired-associative stimulation (PAS) protocol from inducing LTP-like plasticity, whereas it enhanced PAS-induced LTD-like plasticity (Ziemann et al. 2004). Similarly, voluntary motor activation prior to theta burst stimulation leads to rapid polarity-reversing effects (Gentner et al. 2008). Overall, these findings clearly supported that the effects of different stimulation protocols over a particular cortical area depend on the previous history of activity in that area. The timing of changes in activity of the stimulated area seems to be important as well. One recent study provided evidence that the induction of homeostatic plasticity in human subjects critically depends on the timing and type of the preconditioning protocol. It was found that preconditioning M1 by using either excitability-enhancing (anodal) or inhibiting (cathodal) tDCS did not influence PAS homeostatically. On the other hand, if the two stimulation protocols were applied simultaneously, cathodal tDCS resulted in a prolonged facilitation by PAS, whereas anodal tDCS turned it into inhibition (Nitsche et al. 2007). Our results now—demonstrating that preconditioning L M1 by 1-Hz rTMS of R M1 can reverse the response of the L M1 to an excitability-enhancing iTBS protocol—suggest that homeostatic mechanisms might also contribute to regulate plasticity across human motor cortices. One factor to take in consideration is that duration of the aftereffects may vary across individuals and experiments.
The influence of these homeostatic mechanisms on motor behavior in health and disease seems to be more complex. In our experiments, we found no effects on performance of RT, pinch force, or finger tapping. Pointing to the still unclear link between motor cortical excitability and motor performance, a previous study showed that reducing or enhancing motor cortex excitability before performing a serial reaction time task did not affect procedural learning according to homeostatic rules (Kuo et al. 2008). On the other hand, in the somatosensory domain, consecutive application of PAS targeting primary somatosensory cortex (S1) followed by high-frequency peripheral nerve stimulation revealed changes in excitability and tactile spatial discrimination behavior that closely followed the rules of homeostatic plasticity (Bliem et al. 2008). It is possible then that either homeostatic plasticity rules operate in a task-specific manner or that the behavioral measurements used in our investigation were not sensitive enough. Additional investigations would be important to address the influence of principles of (interhemispheric) homeostatic plasticity in neurorehabilitation following brain lesions.
In summary, we showed that the excitability-enhancing effect of iTBS over L M1 was reversed by preconditioning R M1 with suprathreshold R M1 1-Hz rTMS. These results are consistent with homeostatic rules and might be relevant to the design of interventions geared to purposefully modulate motor cortical excitability and cortical plasticity. The present findings suggest that interhemispheric disinhibition can revert the effects of excitability-enhancing protocols over the primary motor cortex in a homeostatically effective manner in normal volunteers. However, principles of homeostatic plasticity may operate to a different extent according to disease, task, and/or specific learning protocols used.
GRANTS
This research was supported by Deutsche Forschungsgemeinschaft Grant Ra 13911-1 to P. Ragert and by the Intramural Research Program of the National Institute of Neurological Disorders and Stroke (NINDS). Y. Vandermeeren and M. A. Dimyan were supported by a NINDS Competitive Fellowship Grant. Y. Vandermeeren was supported by a postdoctoral grant from the Research Council of the University of Louvain (Belgium).
REFERENCES
- Antal et al. 2008.Antal A, Lang N, Boros K, Nitsche M, Siebner HR, Paulus W. Homeostatic metaplasticity of the motor cortex is altered during headache-free intervals in migraine with aura. Cereb Cortex 18: 2701–2705, 2008. [DOI] [PubMed] [Google Scholar]
- Bienenstock et al. 1982.Bienenstock EL, Cooper LN, Munro PW. Theory for the development of neuron selectivity: orientation specificity and binocular interaction in visual cortex. J Neurosci 2: 32–48, 1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bliem et al. 2008.Bliem B, Muller-Dahlhaus JF, Dinse HR, Ziemann U. Homeostatic metaplasticity in the human somatosensory cortex. J Cogn Neurosci 20: 1517–1528, 2008. [DOI] [PubMed] [Google Scholar]
- Boggio et al. 2007.Boggio PS, Nunes A, Rigonatti SP, Nitsche MA, Pascual-Leone A, Fregni F. Repeated sessions of noninvasive brain DC stimulation is associated with motor function improvement in stroke patients. Restor Neurol Neurosci 25: 123–129, 2007. [PubMed] [Google Scholar]
- Chen et al. 1997.Chen R, Classen J, Gerloff C, Celnik P, Wassermann EM, Hallett M, Cohen LG. Depression of motor cortex excitability by low-frequency transcranial magnetic stimulation. Neurology 48: 1398–1403, 1997. [DOI] [PubMed] [Google Scholar]
- Cooke and Bliss 2006.Cooke SF, Bliss TV. Plasticity in the human central nervous system. Brain 129: 1659–1673, 2006. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro et al. 2002.Di Lazzaro V, Oliviero A, Mazzone P, Pilato F, Saturno E, Dileone M, Insola A, Tonali PA, Rothwell JC. Short-term reduction of intracortical inhibition in the human motor cortex induced by repetitive transcranial magnetic stimulation. Exp Brain Res 147: 108–113, 2002. [DOI] [PubMed] [Google Scholar]
- Duque et al. 2005.Duque J, Hummel F, Celnik P, Murase N, Mazzocchio R, Cohen LG. Transcallosal inhibition in chronic subcortical stroke. Neuroimage 28: 940–946, 2005. [DOI] [PubMed] [Google Scholar]
- Fitzgerald et al. 2006.Fitzgerald PB, Fountain S, Daskalakis ZJ. A comprehensive review of the effects of rTMS on motor cortical excitability and inhibition. Clin Neurophysiol 117: 2584–2596, 2006. [DOI] [PubMed] [Google Scholar]
- Fregni and Pascual-Leone 2007.Fregni F, Pascual-Leone A. Technology insight: noninvasive brain stimulation in neurology: perspectives on the therapeutic potential of rTMS and tDCS. Nat Clin Pract Neurol 3: 383–393, 2007. [DOI] [PubMed] [Google Scholar]
- Gentner et al. 2008.Gentner R, Wankerl K, Reinsberger C, Zeller D, Classen J. Depression of human corticospinal excitability induced by magnetic theta-burst stimulation: evidence of rapid polarity-reversing metaplasticity. Cereb Cortex 18: 2046–2053, 2008. [DOI] [PubMed] [Google Scholar]
- Hartwigsen et al. 2008.Hartwigsen G, Bergmann TO, Woerbel S, Granert O, Siebner HR. Conditioning left dorsal premotor cortex with low-frequency rTMS can sensitize the supramarginal gyrus to the disruptive effect of high-frequency online rTMS. Brain Stimulation 1: 279–280, 2008. [Google Scholar]
- Huang et al. 2005.Huang YZ, Edwards MJ, Rounis E, Bhatia KP, Rothwell JC. Theta burst stimulation of the human motor cortex. Neuron 45: 201–206, 2005. [DOI] [PubMed] [Google Scholar]
- Huang et al. 2008.Huang YZ, Rothwell JC, Edwards MJ, Chen RS. Effect of physiological activity on an NMDA-dependent form of cortical plasticity in human. Cereb Cortex 18: 563–570, 2008. [DOI] [PubMed] [Google Scholar]
- Hummel and Cohen 2006.Hummel FC, Cohen LG. Non-invasive brain stimulation: a new strategy to improve neurorehabilitation after stroke? Lancet Neurol 5: 708–712, 2006. [DOI] [PubMed] [Google Scholar]
- Iyer et al. 2003.Iyer MB, Schleper N, Wassermann EM. Priming stimulation enhances the depressant effect of low-frequency repetitive transcranial magnetic stimulation. J Neurosci 23: 10867–10872, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi et al. 2004.Kobayashi M, Hutchinson S, Theoret H, Schlaug G, Pascual-Leone A. Repetitive TMS of the motor cortex improves ipsilateral sequential simple finger movements. Neurology 62: 91–98, 2004. [DOI] [PubMed] [Google Scholar]
- Kuo et al. 2008.Kuo MF, Unger M, Liebetanz D, Lang N, Tergau F, Paulus W, Nitsche MA. Limited impact of homeostatic plasticity on motor learning in humans. Neuropsychologia 46: 2122–2128, 2008. [DOI] [PubMed] [Google Scholar]
- Lang et al. 2004.Lang N, Siebner HR, Ernst D, Nitsche MA, Paulus W, Lemon RN, Rothwell JC. Preconditioning with transcranial direct current stimulation sensitizes the motor cortex to rapid-rate transcranial magnetic stimulation and controls the direction of after-effects. Biol Psychiatry 56: 634–639, 2004. [DOI] [PubMed] [Google Scholar]
- Mansur et al. 2005.Mansur CG, Fregni F, Boggio PS, Riberto M, Gallucci-Neto J, Santos CM, Wagner T, Rigonatti SP, Marcolin MA, Pascual-Leone A. A sham stimulation-controlled trial of rTMS of the unaffected hemisphere in stroke patients. Neurology 64: 1802–1804, 2005. [DOI] [PubMed] [Google Scholar]
- Mathiowetz et al. 1985.Mathiowetz V, Kashman N, Volland G, Weber K, Dowe M, Rogers S. Grip and pinch strength: normative data for adults. Arch Phys Med Rehabil 66: 69–74, 1985. [PubMed] [Google Scholar]
- Mathiowetz et al. 1984.Mathiowetz V, Weber K, Volland G, Kashman N. Reliability and validity of grip and pinch strength evaluations. J Hand Surg (Am) 9: 222–226, 1984. [DOI] [PubMed] [Google Scholar]
- Muellbacher et al. 2000.Muellbacher W, Ziemann U, Boroojerdi B, Hallett M. Effects of low-frequency transcranial magnetic stimulation on motor excitability and basic motor behavior. Clin Neurophysiol 111: 1002–1007, 2000. [DOI] [PubMed] [Google Scholar]
- Muller et al. 2007.Muller JF, Orekhov Y, Liu Y, Ziemann U. Homeostatic plasticity in human motor cortex demonstrated by two consecutive sessions of paired associative stimulation. Eur J Neurosci 25: 3461–3468, 2007. [DOI] [PubMed] [Google Scholar]
- Murase et al. 2004.Murase N, Duque J, Mazzocchio R, Cohen LG. Influence of interhemispheric interactions on motor function in chronic stroke. Ann Neurol 55: 400–409, 2004. [DOI] [PubMed] [Google Scholar]
- Naeser et al. 2005a.Naeser MA, Martin PI, Nicholas M, Baker EH, Seekins H, Helm- Estabrooks N, Cayer-Meade C, Kobayashi M, Theoret H, Fregni F, Tormos JM, Kurland J, Doron KW, Pascual-Leone A. Improved naming after TMS treatments in a chronic, global aphasia patient: case report. Neurocase 11: 182–193, 2005a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naeser et al. 2005b.Naeser MA, Martin PI, Nicholas M, Baker EH, Seekins H, Kobayashi M, Theoret H, Fregni F, Maria-Tormos J, Kurland J, Doron KW, Pascual-Leone A. Improved picture naming in chronic aphasia after TMS to part of right Broca's area: an open-protocol study. Brain Lang 93: 95–105, 2005b. [DOI] [PubMed] [Google Scholar]
- Nitsche and Paulus 2000.Nitsche MA, Paulus W. Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. J Physiol 527: 633–639, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitsche et al. 2007.Nitsche MA, Roth A, Kuo MF, Fischer AK, Liebetanz D, Lang N, Tergau F, Paulus W. Timing-dependent modulation of associative plasticity by general network excitability in the human motor cortex. J Neurosci 27: 3807–3812, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldfield 1971.Oldfield RC The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia 9: 97–113, 1971. [DOI] [PubMed] [Google Scholar]
- Peinemann et al. 2000.Peinemann A, Lehner C, Mentschel C, Munchau A, Conrad B, Siebner HR. Subthreshold 5-Hz repetitive transcranial magnetic stimulation of the human primary motor cortex reduces intracortical paired-pulse inhibition. Neurosci Lett 296: 21–24, 2000. [DOI] [PubMed] [Google Scholar]
- Perez and Cohen 2008.Perez MA, Cohen LG. Mechanisms underlying functional changes in the primary motor cortex ipsilateral to an active hand. J Neurosci 28: 5631–5640, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez and Cohen 2009.Perez MA, Cohen LG. Interhemispheric inhibition between primary motor cortices: what have we learned? J Physiol 587: 725–726, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plewnia et al. 2003.Plewnia C, Lotze M, Gerloff C. Disinhibition of the contralateral motor cortex by low-frequency rTMS. Neuroreport 14: 609–612, 2003. [DOI] [PubMed] [Google Scholar]
- Quartarone et al. 2003.Quartarone A, Bagnato S, Rizzo V, Siebner HR, Dattola V, Scalfari A, Morgante F, Battaglia F, Romano M, Girlanda P. Abnormal associative plasticity of the human motor cortex in writer's cramp. Brain 126: 2586–2596, 2003. [DOI] [PubMed] [Google Scholar]
- Reis et al. 2008.Reis J, Swayne OB, Vandermeeren Y, Camus M, Dimyan MA, Harris-Love M, Perez MA, Ragert P, Rothwell JC, Cohen LG. Contribution of transcranial magnetic stimulation to the understanding of cortical mechanisms involved in motor control. J Physiol 586: 325–351, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossini et al. 1994.Rossini PM, Barker AT, Berardelli A, Caramia MD, Caruso G, Cracco RQ, et al. Non-invasive electrical and magnetic stimulation of the brain, spinal cord and roots: basic principles and procedures for routine clinical application. Report of an IFCN committee. Electroencephalogr Clin Neurophysiol 91: 79–92, 1994. [DOI] [PubMed] [Google Scholar]
- Rutherford et al. 1998.Rutherford LC, Nelson SB, Turrigiano GG. BDNF has opposite effects on the quantal amplitude of pyramidal neuron and interneuron excitatory synapses. Neuron 21: 521–530, 1998. [DOI] [PubMed] [Google Scholar]
- Schambra et al. 2003.Schambra HM, Sawaki L, Cohen LG. Modulation of excitability of human motor cortex (M1) by 1 Hz transcranial magnetic stimulation of the contralateral M1. Clin Neurophysiol 114: 130–133, 2003. [DOI] [PubMed] [Google Scholar]
- Siebner et al. 2004.Siebner HR, Lang N, Rizzo V, Nitsche MA, Paulus W, Lemon RN, Rothwell JC. Preconditioning of low-frequency repetitive transcranial magnetic stimulation with transcranial direct current stimulation: evidence for homeostatic plasticity in the human motor cortex. J Neurosci 24: 3379–3385, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siebner and Rothwell 2003.Siebner HR, Rothwell J. Transcranial magnetic stimulation: new insights into representational cortical plasticity. Exp Brain Res 148: 1–16, 2003. [DOI] [PubMed] [Google Scholar]
- Silvanto and Muggleton 2008.Silvanto J, Muggleton NG. Testing the validity of the TMS state-dependency approach: targeting functionally distinct motion-selective neural populations in visual areas V1/V2 and V5/MT+. Neuroimage 40: 1841–1848, 2008. [DOI] [PubMed] [Google Scholar]
- Takeuchi et al. 2005.Takeuchi N, Chuma T, Matsuo Y, Watanabe I, Ikoma K. Repetitive transcranial magnetic stimulation of contralesional primary motor cortex improves hand function after stroke. Stroke 36: 2681–2686, 2005. [DOI] [PubMed] [Google Scholar]
- Turrigiano et al. 1998.Turrigiano GG, Leslie KR, Desai NS, Rutherford LC, Nelson SB. Activity-dependent scaling of quantal amplitude in neocortical neurons. Nature 391: 892–896, 1998. [DOI] [PubMed] [Google Scholar]
- Ziemann et al. 2004.Ziemann U, Iliac TV, Pauli C, Meintzschel F, Ruge D. Learning modifies subsequent induction of long-term potentiation-like and long-term depression-like plasticity in human motor cortex. J Neurosci 24: 1666–1672, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]