Abstract
Within the second synaptic layer of the retina, bipolar cell (BC) output to ganglion cells is regulated by inhibitory input to BC axon terminals. GABAA receptors (GABAARs) mediate rapid synaptic currents in BC terminals, whereas GABAC receptors (GABACRs) mediate slow evoked currents and a tonic current, which is strongly regulated by GAT-1 GABA transporters. We have used voltage-clamp recordings from BC terminals in goldfish retinal slices to determine the source of GABA for activation of these currents. Inhibition of vesicular release with concanamycin A or tetanus toxin significantly inhibited GABAAR inhibitory postsynaptic currents and glutamate-evoked GABAAR and GABACR currents but did not reduce the tonic GABACR current, which was also not dependent on extracellular Ca2+. The tonic current was strongly potentiated by inhibition of GABA transaminase, under both normal and Ca2+-free conditions, and was activated by exogenous taurine; however inhibition of taurine transport had little effect. The tonic current was unaffected by GAT-2/3 inhibition and was potentiated by GAT-1 inhibition even in the absence of vesicular release, indicating that it is unlikely to be evoked by reversal of GABA transporters or by ambient GABA. In addition, GABA release does not appear to occur via hemichannels or P2X7 receptors. BC terminals therefore exhibit two forms of GABACR-mediated inhibition, activated by vesicular and by nonvesicular GABA release, which are likely to have distinct functions in visual signal processing. The tonic GABACR current in BC terminals exhibits similar properties to tonic GABAAR and glutamate receptor currents in the brain.
INTRODUCTION
The retina performs significant processing of visual signals by means of lateral inhibitory connections within its two synaptic layers. In the inner plexiform layer (IPL), amacrine cells modulate ganglion cell activity both directly and by reducing bipolar cell (BC) glutamate release (Lukasiewicz and Werblin 1994; Zhang and Slaughter 1995). Amacrine cells form numerous conventional and reciprocal GABAergic synapses with BC axon terminals (Marc and Liu 2000), which express two subtypes of ionotropic GABA receptor: GABAA and GABAC receptors (GABAARs and GABACRs).
GABAAR and GABACR currents evoked by GABA or by light differ significantly in their kinetics: GABAAR currents are rapid and transient, whereas GABACR currents are slower and more sustained (Eggers and Lukasiewicz 2006a,b; Hull et al. 2006; Lukasiewicz and Shields 1998; Qian and Dowling 1995; Zhang and Slaughter 1995). GABACRs also have a higher GABA affinity and lower single-channel conductance than GABAARs (Feigenspan and Bormann 1994a; Palmer 2006; Qian and Dowling 1995). The receptor subtypes are therefore likely to have distinct roles in retinal processing and indeed have recently been shown to differentially regulate BC output (Eggers and Lukasiewicz 2006a,b). GABACRs appear to be particularly important for limiting postsynaptic N-methyl-d-aspartate (NMDA) receptor activation and generating transient ganglion cell light responses (Dong and Werblin 1998; Matsui et al. 2001; Sagdullaev et al. 2006; Zhang et al. 1997). Consistent with GABAARs and GABACRs subserving distinct functions in BC terminals, there is evidence that the receptor pathways are independently activated and regulated. For example, GABAARs and GABACRs do not appear to be co-localized either anatomically (Koulen et al. 1998) or functionally (Palmer 2006), and GABACRs but not GABAARs are strongly regulated by GAT-1 GABA transporters (Hull et al. 2006; Ichinose and Lukasiewicz 2002; Palmer 2006).
In addition to mediating slow evoked currents in BC terminals, GABACRs generate a spontaneous tonic current that regulates the ability of the terminals to fire Ca2+-dependent action potentials (Hull et al. 2006). Tonic GABA currents have also been described in brain regions such as the hippocampus, cerebellum, and thalamus, where they are mediated by extrasynaptic GABAARs that, like GABACRs, have a high GABA affinity and slow rate of desensitization and are strongly regulated by GABA uptake (Glykys and Mody 2007a). In some cases, tonic GABAAR currents are not dependent on GABA released from synaptic vesicles (Rossi et al. 2003; Wu et al. 2003, 2006). To determine the source of GABA for activation of spontaneous and evoked GABAR currents in BC terminals, we have made voltage-clamp recordings directly from BC terminals in goldfish retinal slices. Our findings reveal novel functional properties of GABACR-mediated inhibition in the IPL.
METHODS
Goldfish (Carassius auratus) were dark-adapted for 1 h and killed by decapitation followed immediately by destruction of the brain and spinal cord under Schedule 1 of the UK Animals (Scientific Procedures) Act 1986. The eyeballs were removed and retinae dissected out and treated for 25 min with hyaluronidase to remove vitreous humor. Each retina was quartered, placed ganglion cell layer down on filter paper and kept at 4°C in medium comprising (in mM): 127 NaCl, 2.5 KCl, 1.0 MgCl2, 0.5 CaCl2, 5 HEPES, and 12 glucose, adjusted to pH 7.45 with NaOH.
Slices were cut at 250-μm intervals, transferred to the recording chamber, and perfused (1 ml/min) with medium comprising (in mM): 108 NaCl, 2.5 KCl, 1.0 MgCl2, 2.5 CaCl2, 24 NaHCO3, and 12 glucose, gassed with 95% O2-5% CO2, pH 7.4. For nominally Ca2+-free solution, CaCl2 was replaced by MgCl2 (2.5 mM) and EGTA (2.0 mM) was added. For incubation in concanamycin A and tetanus toxin, and for the interleaved control experiments, slices were immersed in HEPES-buffered solution (as in the preceding text but with 2.5 mM CaCl2) and not perfused to prevent wash-out of the toxins except in experiments requiring the subsequent application of drugs. Slices treated with concanamycin A/tetanus toxin and interleaved control slices were sequentially prepared from retinal quarters from the same fish. Slice preparation and recordings were performed at room temperature in daylight conditions. Drugs were bath-applied via the extracellular solution and locally-applied via pressure application from a low-resistance glass micropipette, positioned 20–50 μm from the recorded terminal, using a Picospritzer II (Intracell, Royston, UK). Drugs and salts were obtained from Tocris (Bristol, UK), Sigma-Aldrich (Gillingham, UK), Fisher Scientific (Loughborough, UK), and Toronto Research Chemicals.
Whole cell voltage-clamp recordings were obtained from large Mb-type BC terminals as described previously (Palmer et al. 2003). Most recordings were made from axon-severed terminals (determined by their capacitive current) (see Palmer et al. 2003) to eliminate currents arising from somatodendritic receptors and reduce the leak current. However, no differences in GABAR currents were observed between isolated terminals and the terminals of intact BCs. Patch pipettes (5–8 MΩ) were pulled from borosilicate glass and filled with solution comprising (in mM) 115 CsCl, 25 HEPES, 10 TEA-Cl, 3 Mg-ATP, 0.5 Na-GTP, 0.5 EGTA, pH 7.2. CsCl-based intracellular solution was used to increase the driving force through GABARs at a holding potential of −60 mV. Membrane current (IM) was recorded via an EPC-10 patch-clamp amplifier controlled by Patchmaster software (HEKA, Lambrecht/Pfalz, Germany). Series resistance (RS) was monitored and recordings were not used for analysis if IM changes were accompanied by changes in RS.
Off-line analysis was performed using IgorPro software (WaveMetrics, Lake Oswego, OR). Inhibitory postsynaptic currents (IPSCs) were identified and analyzed using macros kindly provided by Dr H. Taschenberger. The tonic current was measured as the average IM during recording periods lacking IPSCs. Current variance was measured following subtraction of a straight-line fit of 3-s current traces selected to be free from IPSCs. Pooled data are expressed as means ± SE; statistical significance was assessed using Student's t-test with P < 0.05 considered significant.
RESULTS
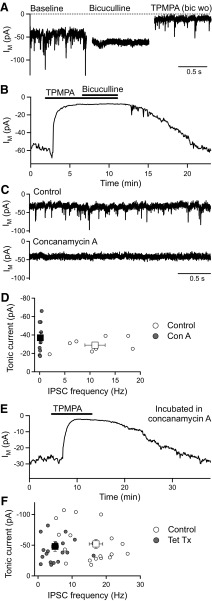
To investigate the source of GABA that activates spontaneous and evoked GABAR currents in BC terminals, we tested the effect of inhibiting vesicular GABA release. Spontaneous GABAAR and GABACR currents, recorded using CsCl-based intracellular solution at −60 mV, are readily distinguished: GABAARs mediate fast, transient IPSCs that are sensitive to bicuculline, whereas GABACRs mediate a tonic current that is sensitive to (1,2,5,6-tetrahydropyridin-4-yl)methylphosphinic acid (TPMPA) (Palmer 2006) (Fig. 1 A). In BC terminals, GABAARs do not contribute to the tonic current (Hull et al. 2006). Thus TPMPA (50–100 μΜ) reduced the tonic current from −57 ± 5 to −13 ± 2 pA (n = 3, P < 0.05), but subsequent application of bicuculline (50 μΜ) caused no further reduction (−17 ± 4 pA, n = 3; Fig. 1B).
FIG. 1.
The tonic GABACR current in bipolar cell (BC) terminals is activated by GABA released via a nonvesicular mechanism. A: spontaneous GABAR-mediated membrane current (IM) recorded from BC terminals with CsCl-based intracellular solution at −60 mV. The GABAAR antagonist bicuculline (50 μM) inhibits the fast inhibitory postsynaptic currents (IPSCs), whereas the GABACR antagonist TPMPA (150 μM) inhibits the tonic current component (bic wo = bicuculline washed-out). B: example plot of IM against time showing that TPMPA (50 μM) inhibits the tonic current but subsequent application of bicuculline (50 μM) has no further effect. C: example IM recordings from BC terminals in control slices and slices incubated in concanamycin A (3.5 μΜ), an inhibitor of vesicular release. Concanamycin A eliminated the fast IPSCs but did not reduce the tonic current. D: tonic current vs. IPSC frequency for individual BC terminal recordings in control slices and slices incubated in concanamycin A (3.5 μΜ; circles), and mean data for these recordings (squares). E: application of TPMPA (150 μM) to a slice incubated in concanamycin A (3.5 μΜ) caused a reversible reduction in the tonic current. F: tonic current vs. IPSC frequency for individual BC terminal recordings in control slices and slices incubated in tetanus toxin (1–4 μg/ml; circles), and mean data for these recordings (squares). Tetanus toxin significantly reduced the IPSC frequency but did not inhibit the tonic current. In this and subsequent figures, error bars represent SE.
Spontaneous GABAR currents were recorded from BC terminals in retinal slices incubated in concanamycin A (3.5 μΜ for ∼50 min), an inhibitor of the proton-translocating ATPase that powers GABA uptake into synaptic vesicles (Drose and Altendorf 1997; Zhou et al. 2000), and compared with GABAR currents in interleaved control slices (incubated in the same concentration of the solvent DMSO). BC terminals in concanamycin A-treated slices exhibited a greatly reduced frequency of spontaneous IPSCs (control: 11 ± 2 Hz, n = 8; concanamycin A: 0.1 ± 0.1 Hz, n = 9, P < 0.01) but no reduction in the amplitude of the tonic current (control: −29 ± 3 pA, n = 8; concanamycin A: −37 ± 6 pA, n = 9; Fig. 1, C and D). Application of TPMPA (100–150 μΜ) to concanamycin A-treated slices reduced the tonic current in a reversible manner (from −36 ± 9 to −11 ± 3 pA, n = 5, P < 0.05; Fig. 1E), confirming that it was mediated by GABACRs.
Vesicular exocytosis was also inhibited by incubating slices in tetanus toxin (1–4 μg/ml for ∼50 min), which acts by cleaving the synaptic vesicle protein synaptobrevin (Grumelli et al. 2005). GABAR currents in tetanus toxin-treated slices were compared with GABAR currents in interleaved control slices. On average, spontaneous IPSC frequency was significantly reduced in tetanus toxin-treated slices (control: 17 ± 2 Hz, n = 20; tetanus toxin: 5 ± 1 Hz, n = 19, P < 0.01; Fig. 1F). Tetanus toxin was less effective at blocking vesicular release than concanamycin A, and the extent of the inhibition was variable between recordings. However, the amplitude of the tonic current was not reduced by tetanus toxin (control: −52 ± 7 pA, n = 20; tetanus toxin: −48 ± 8 pA, n = 19), and there was no correlation between the size of the tonic current and IPSC frequency (Fig. 1F). Application of TPMPA (100 μM) to tetanus toxin-treated BC terminals reduced the tonic current from −60 ± 6 to −17 ± 2 pA (n = 6, P < 0.01). These results strongly suggest that the tonic GABACR current is not activated by vesicular GABA release.
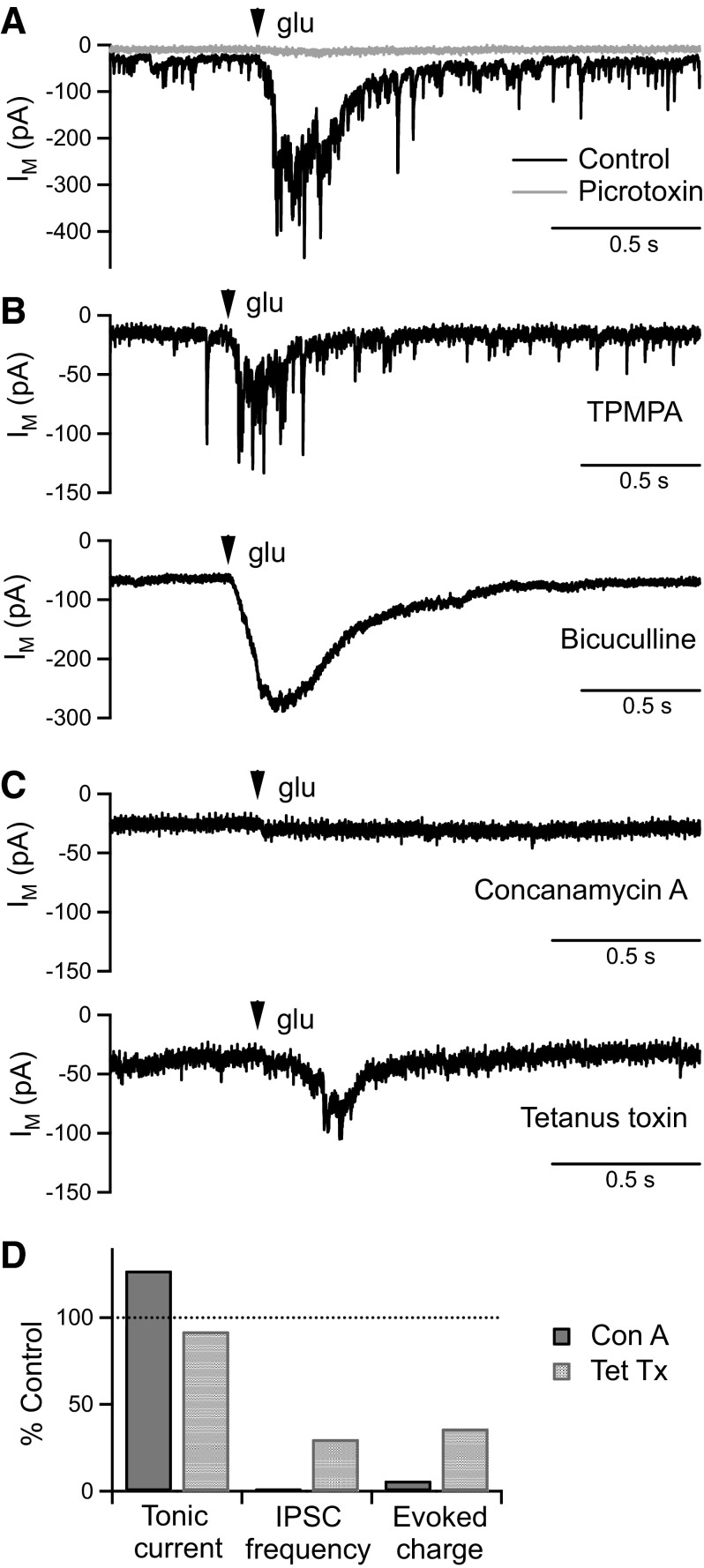
In addition to mediating a tonic current in BC terminals, GABACRs mediate more transient currents that can be evoked by activation of reciprocal amacrine cell synapses (Chavez et al. 2006; Vigh et al. 2005). To determine the source of GABA for evoked GABACR currents, we activated reciprocal synapses by local pressure-application of glutamate. Glutamate application (100 μM, 10 ms, 10 psi) evoked a large inward current that was almost completely inhibited by the GABAAR and GABACR antagonist picrotoxin (50 μM; reduced from −56 ± 17 to −3 ± 1 pC, n = 5, P < 0.05; Fig. 2 A). A small inward current remained that was not observed in the presence of the glutamate transporter inhibitor dl-threo-β-benzyloxyaspartic acid (TBOA) (50 μM) (Palmer et al. 2003). However, as TBOA significantly increased both the tonic current and the size of evoked GABA responses (data not shown) and as these glutamate application parameters evoked only small glutamate transporter currents, TBOA was not used in subsequent experiments.
FIG. 2.
Glutamate-evoked GABAR currents are dependent on vesicular GABA release. A: example BC terminal current responses to focal application of glutamate (glu; 100 μM, 10 ms, 10 psi) in control conditions and following perfusion of the GABAAR and GABACR antagonist picrotoxin (50 μM). B: example glu-evoked responses mediated by GABAARs (in 150 μM TPMPA), and GABACRs (in 50 μM bicuculline). C: example glu-evoked responses from slices incubated in concanamycin A (3.5 μΜ) and tetanus toxin (4 μg/ml). The glu-evoked response was virtually abolished by concanamycin A and reduced by tetanus toxin. D: the degree of inhibition of the glu-evoked response by concanamycin A and tetanus toxin was similar to the degree of inhibition of spontaneous IPSCs [tonic current and IPSC frequency data from Fig. 1; glu-evoked response charge data: concanamycin A (n = 9) compared with interleaved controls (n = 8), tetanus toxin (n = 12) compared with interleaved controls (n = 12)].
To determine the contribution of GABAARs and GABACRs to the glutamate-evoked response, recordings were made in the presence of TPMPA (100–150 μΜ) or bicuculline (50 μΜ). Glutamate-evoked responses were observed under both conditions, but their kinetics differed. Evoked GABAAR responses contained many fast IPSC-like events, whereas GABACR responses exhibited a much smoother time course (Fig. 2B). Although we were specifically interested in the release mechanism of GABA mediating the GABACR component, mixed GABAAR and GABACR responses were recorded to avoid the potentiating effects of bicuculline on GABACR currents that result from amacrine cell disinhibition (Palmer 2006; Zhang et al. 1997).
Glutamate-evoked GABA responses were compared between slices incubated in concanamycin A or tetanus toxin and interleaved control slices. Concanamycin A (3.5 μΜ) virtually abolished the glutamate-evoked response (control: −124 ± 58 pC, n = 8; concanamycin A: −7 ± 2 pC, n = 9; P < 0.05; Fig. 2, C and D). Tetanus toxin (4 μg/ml) also significantly inhibited the glutamate-evoked response (control: −137 ± 29 pC, n = 12; tetanus toxin: −49 ± 15 pC, n = 12; P < 0.05; Fig. 2, C and D), although the size of the response was variable between recordings. On average, the amount of inhibition of the glutamate-evoked GABA response by tetanus toxin or concanamycin A was very similar to the amount of inhibition of spontaneous IPSCs (Fig. 2D). These results are consistent with glutamate-evoked GABA release occurring via a conventional vesicular mechanism and do not support the existence of an evoked GABACR component of nonvesicular origin. Tonic and evoked GABACR currents in BC terminals therefore appear to be activated via distinct mechanisms.
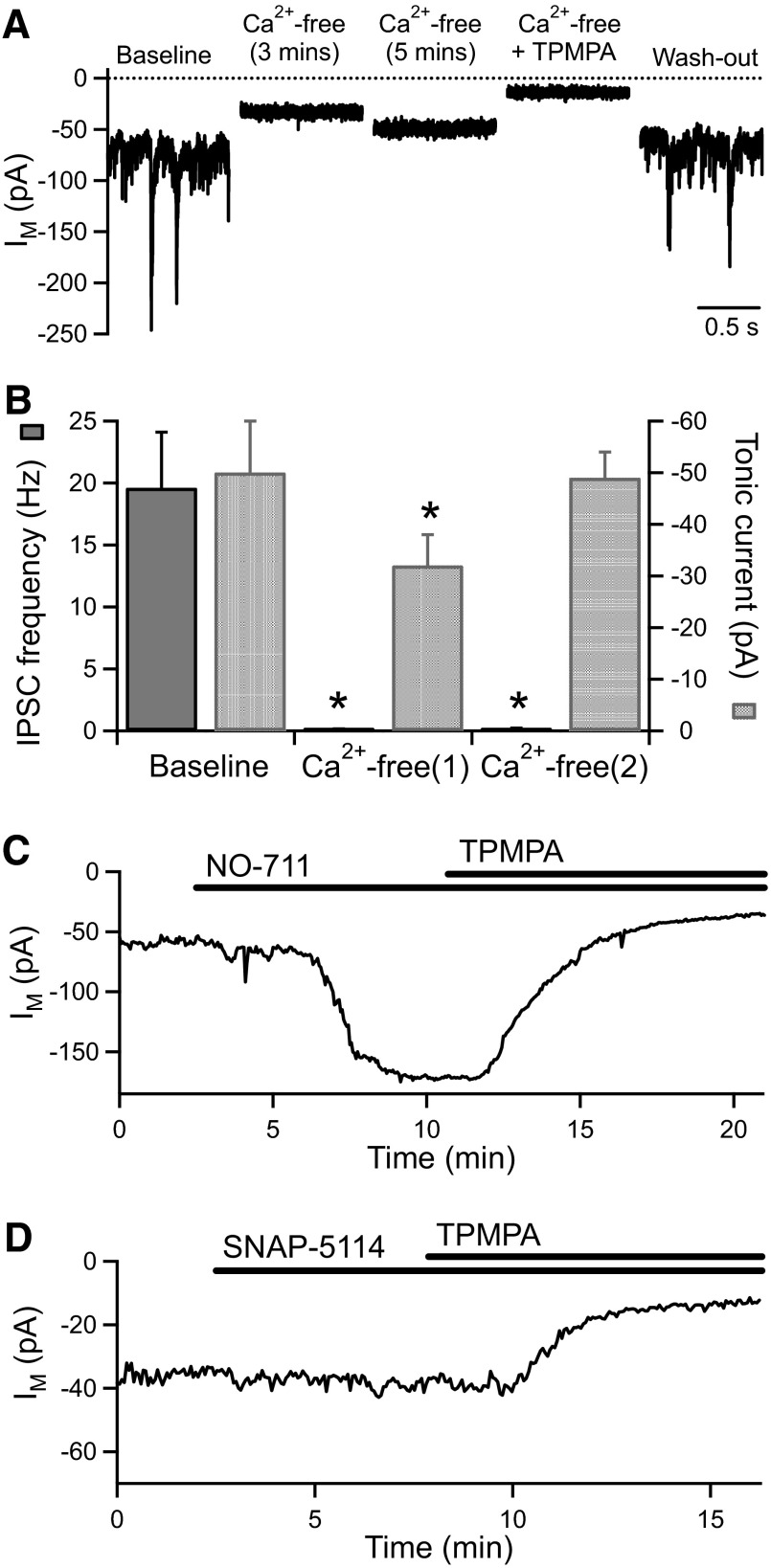
To further investigate the source of GABA for activation of tonic GABACR currents, we examined whether the tonic current is Ca2+ dependent. Application of nominally Ca2+-free extracellular solution (containing 3.5 mM Mg2+) virtually abolished GABAAR-mediated IPSCs (frequency reduced from 20 ± 5 to 0.1 ± 0.03 Hz, n = 5, P < 0.05) and initially caused a small but significant reduction in the tonic current (from −50 ± 10 to −32 ± 6 pA, n = 5, P < 0.05; Fig. 3, A and B). However, in the continued absence of Ca2+, while the IPSCs remained inhibited (0.2 ± 0.06 Hz, n = 5), the tonic current returned to baseline levels (−49 ± 5 pA, n = 5; Fig. 3, A and B). TPMPA (100 μM) inhibited the tonic current in Ca2+-free solution (reduced to −12 ± 2 pA, n = 6, P < 0.05; Fig. 3A). The tonic GABACR current in BC terminals is therefore not dependent on extracellular Ca2+ although it is transiently reduced when Ca2+ is removed.
FIG. 3.
The tonic GABACR current is Ca2+ independent and is not activated by GABA transporter reversal. A: example traces from an experiment showing the effect of perfusion of Ca2+-free extracellular solution (containing 3.5 mM Mg2+) and the sensitivity of the tonic current in Ca2+-free solution to TPMPA. B: mean changes in IPSC frequency and tonic current for BC terminals exposed to Ca2+-free solution (n = 5). The data for Ca2+-free(1) and Ca2+-free(2) were collected at the peak of the inhibitory effect and after the tonic current had stabilized, respectively. In this and subsequent figures, *, P < 0.05 compared with baseline. C: application of the GAT-1 antagonist NO-711 (3 μM) causes a large increase in the tonic current that is fully reversed by addition of TPMPA (100 μM). D: an example experiment showing that application of the GAT-2/3 antagonist SNAP-5114 (50 μM) has no effect on the tonic current.
Nonvesicular, Ca2+-independent GABA release can occur via reversal of the GABA transporter GAT-1 under normal, nonpathological conditions (Wu et al. 2007). GAT-1 is localized to amacrine cell processes surrounding BC terminals (S. M. Jones, D. N. Furness, and M. J. Palmer, unpublished observations); however, inhibition of GAT-1 with the nontransportable antagonist NO-711 significantly enhances rather than reduces the tonic GABACR current (Hull et al. 2006) (Fig. 3C). To investigate a possible role for GAT-2 or GAT-3 in GABA release, we applied 1-[2-[tris(4-methoxyphenyl)methoxy]ethyl]-(s)-3-piperidinecarboxylic acid (SNAP)-5114 (50 μM), but this had no significant effect on the amplitude of the tonic current (baseline: −50 ± 7 pA; SNAP-5114: −65 ± 13 pA, n = 6; Fig. 3D). GAT-2 and GAT-3 therefore do not appear to be involved in regulating the tonic GABACR current, either via GABA uptake or release.
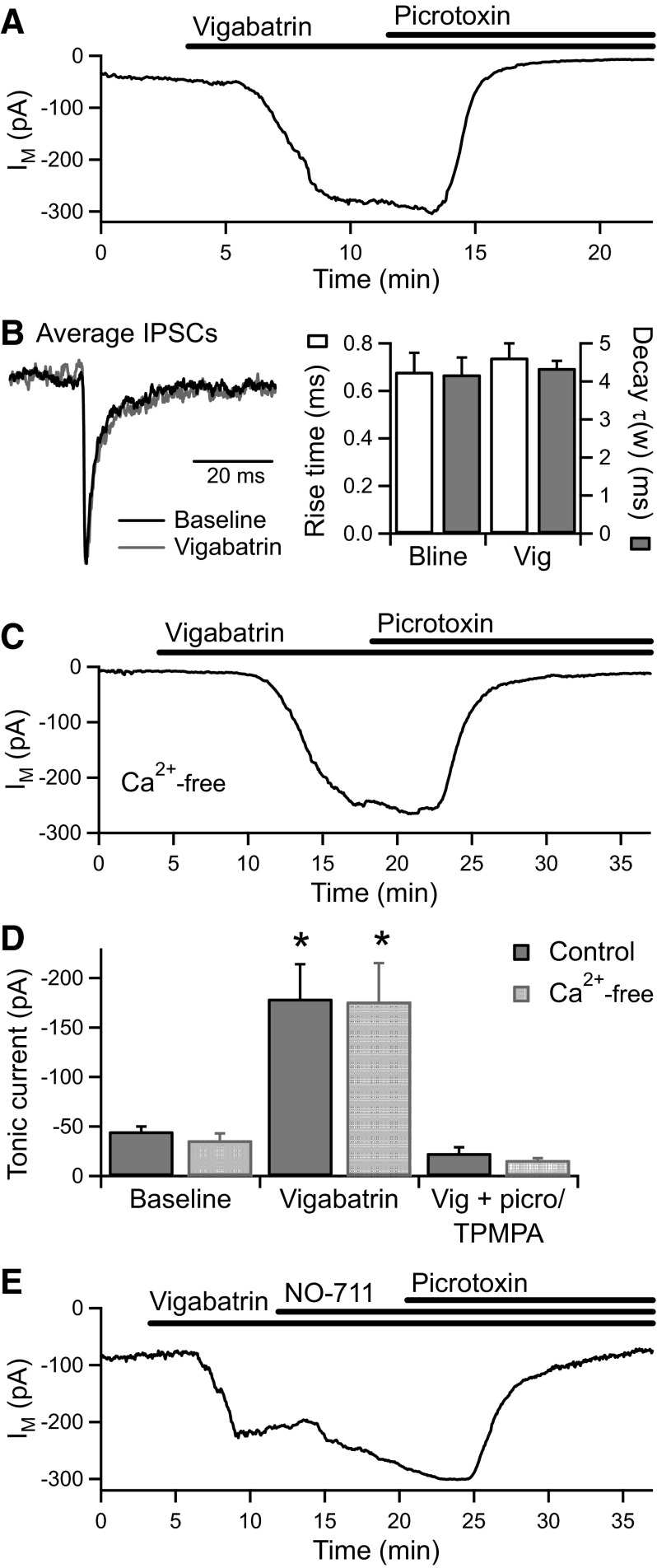
To confirm that the tonic GABACR current is activated by GABA rather than another amino-acid such as taurine, we examined the effect of increasing the intracellular GABA concentration by inhibiting GABA transaminase with vigabatrin (γ-vinyl GABA). Vigabatrin (500 μM) caused a significant increase in the tonic current (from −45 ± 5 to −179 ± 35 pA, n = 5, P < 0.05) that was fully reversed by subsequent application of picrotoxin (100 μM) or TPMPA (100 μM; −23 ± 6 pA, n = 4, P < 0.05; Fig. 4, A and D). Vigabatrin potentiated the tonic current without affecting the kinetics of GABAAR-mediated IPSCs (n = 5; Fig. 4B), a similar effect to that of NO-711 (Palmer 2006). Furthermore, in the presence of Ca2+-free solution to inhibit vesicular release, vigabatrin (500 μM) caused a similarly large increase in the tonic current (from −36 ± 7 to −176 ± 39 pA, n = 5, P < 0.05) that was again fully reversed by picrotoxin (100 μM; −16 ± 2 pA, n = 4, P < 0.05; Fig. 4, C and D). In cultured hippocampal neurons, vigabatrin increases the tonic GABAAR current by inducing reversal of GABA transporters (Wu et al. 2003). We therefore investigated the effect of inhibiting GAT-1 following potentiation of the tonic current by vigabatrin (500 μM). NO-711 (3 μM) caused a further potentiation of the tonic current (from −165 ± 28 to −245 ± 32 pA, n = 5, P < 0.05) that was returned to baseline by picrotoxin (200 μM; −52 ± 8 pA, n = 5, P < 0.05; Fig. 4E). Therefore even under conditions of increased intracellular GABA, GABA release does not occur via reversal of GAT-1.
FIG. 4.
Inhibition of GABA transaminase strongly potentiates the tonic GABACR current. A: an example experiment showing that under normal conditions, application of the GABA transaminase inhibitor vigabatrin (500 μM) causes a large increase in the tonic current that is fully reversed by the addition of picrotoxin (100 μM). B, left: peak-scaled average IPSCs from the recording in A, generated from spontaneous events occurring before and during vigabatrin application. Right: vigabatrin did not affect the kinetics of the IPSCs (IPSC decay expressed as the weighted time constant of a biexponential fit; n = 5). C: an example experiment showing that vigabatrin (500 μM) also caused a large increase in the tonic current in the presence of Ca2+-free extracellular solution, which was reversed by picrotoxin (100 μM). D: average data for vigabatrin application in normal (n = 5) and Ca2+-free (n = 5) conditions. E: an example experiment showing that application of NO-711 (3 μM) in the presence of vigabatrin (500 μM) further potentiates the tonic current, which is subsequently inhibited by picrotoxin (200 μM).
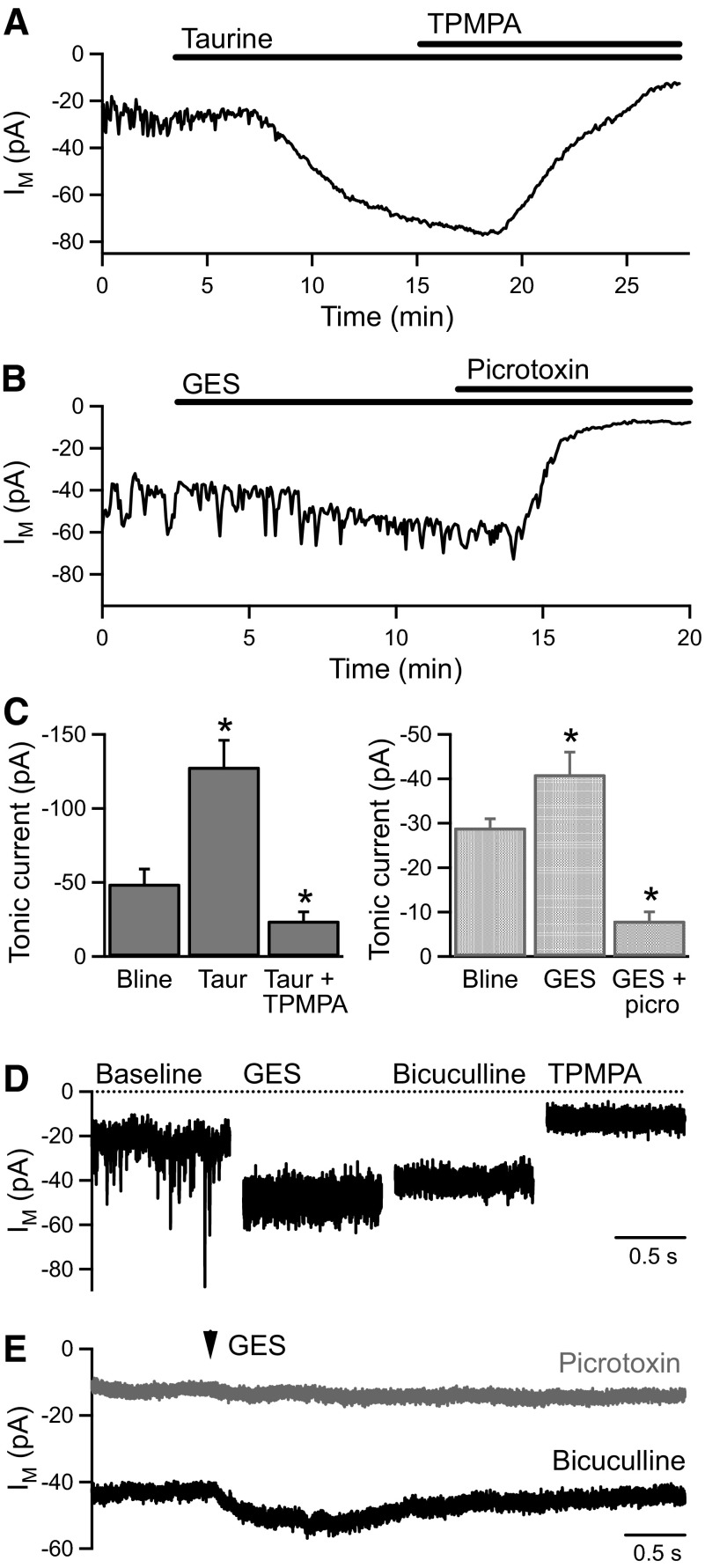
The tonic current in BC terminals is therefore highly sensitive to both the intra- and extracellular GABA concentration. However, as taurine is known to act as an agonist at GABACRs (Ochoa-de la Paz et al. 2008; Pan et al. 2005) and to be present in the retina (Lombardini 1991), we investigated the effect of taurine application on the tonic current. Taurine (1 mM) evoked an increase in the tonic current (from −49 ± 10 to −128 ± 18 pA, n = 5, P < 0.01) that was fully reversed by addition of TPMPA (100 μM; −24 ± 6 pA, n = 3, P < 0.05; Fig. 5, A and C). To determine the potential contribution of endogenous taurine to the tonic current, we applied guanidinoethylsulphonate (GES) to inhibit taurine uptake. GES (100 μM) evoked a very small increase in the tonic current (from −29 ± 2 to −41 ± 5 pA, n = 5, P < 0.05; Fig. 5, B and C). A higher concentration of GES (1 mM) evoked a slightly larger increase in the tonic current (from −35 ± 6 to −68 ± 9 pA, n = 14, P < 0.01) and also caused a significant reduction in the frequency of IPSCs (from 12.3 ± 2.3 to 1.4 ± 0.5 Hz, n = 14, P < 0.01) and a prominent increase in the current variance (Fig. 5D). The large current variance in GES was reduced by application of bicuculline (50 μM; from 21.8 ± 3.6 to 9.6 ± 1.8 pA2, n = 7, P < 0.01; Fig. 5D), consistent with GES acting as a high-affinity, low-efficacy agonist at GABAARs (Mellor et al. 2000). The increase in the tonic current evoked by GES (1 mM) was not significantly inhibited by bicuculline but was reduced by picrotoxin (100 μM) or TPMPA (100–500 μM; from −75 ± 12 to −18 ± 4 pA, n = 11, P < 0.01; Fig. 5D). It was noted that an unusually high concentration of TPMPA was required to maximally inhibit the tonic current in the presence of GES, suggesting that GES may also be acting as a high-affinity, low-efficacy agonist at GABACRs. To investigate this possibility, GES was applied locally to recorded BC terminals by pressure ejection in the presence of bicuculline (50 μM in the bath and ejection pipette). GES application (1 mM, 0.1–1.0 s) evoked a small inward current (−12.8 ± 3.1 pA) that was inhibited by picrotoxin (100 μM; −1.0 ± 0.3 pA, n = 3; Fig. 5E). This rapid, localized effect is consistent with a direct action of GES on GABACRs in BC terminals, which is likely to account for the increase in the tonic current evoked by bath application of GES. The limited effect of GES (100 μM) indicates that endogenous taurine is unlikely to be a significant activator of the tonic current. In addition, it is inconsistent with the tonic current being activated via reversal of taurine transporters, which can also transport GABA (Tomi et al. 2008).
FIG. 5.
The tonic GABACR current is activated by exogenous taurine. A: application of taurine (1 mM) evokes an increase in the tonic current that is reversed by addition of TPMPA (100 μM). B: application of guanidinoethylsulphonate (GES, 100 μM) evokes a very small increase in the tonic current, which is inhibited by picrotoxin (100 μM). C: mean data for taurine (1 mM, n = 5) and GES (100 μM; n = 5) application. D: example traces from an experiment showing the effect of GES (1 mM) application. The large current variance in GES was inhibited by bicuculline (50 μM), and the tonic current was inhibited by TPMPA (100 μM). E: local pressure application of GES (1 mM, 1.0 s, 10 psi) in the presence of bicuculline (50 μM) evoked a small inward current (average of 10 GES applications at 30-s intervals). Both the tonic curent and the GES-evoked current were inhibited by picrotoxin (100 μM; average of 7 GES applications at 30-s intervals).
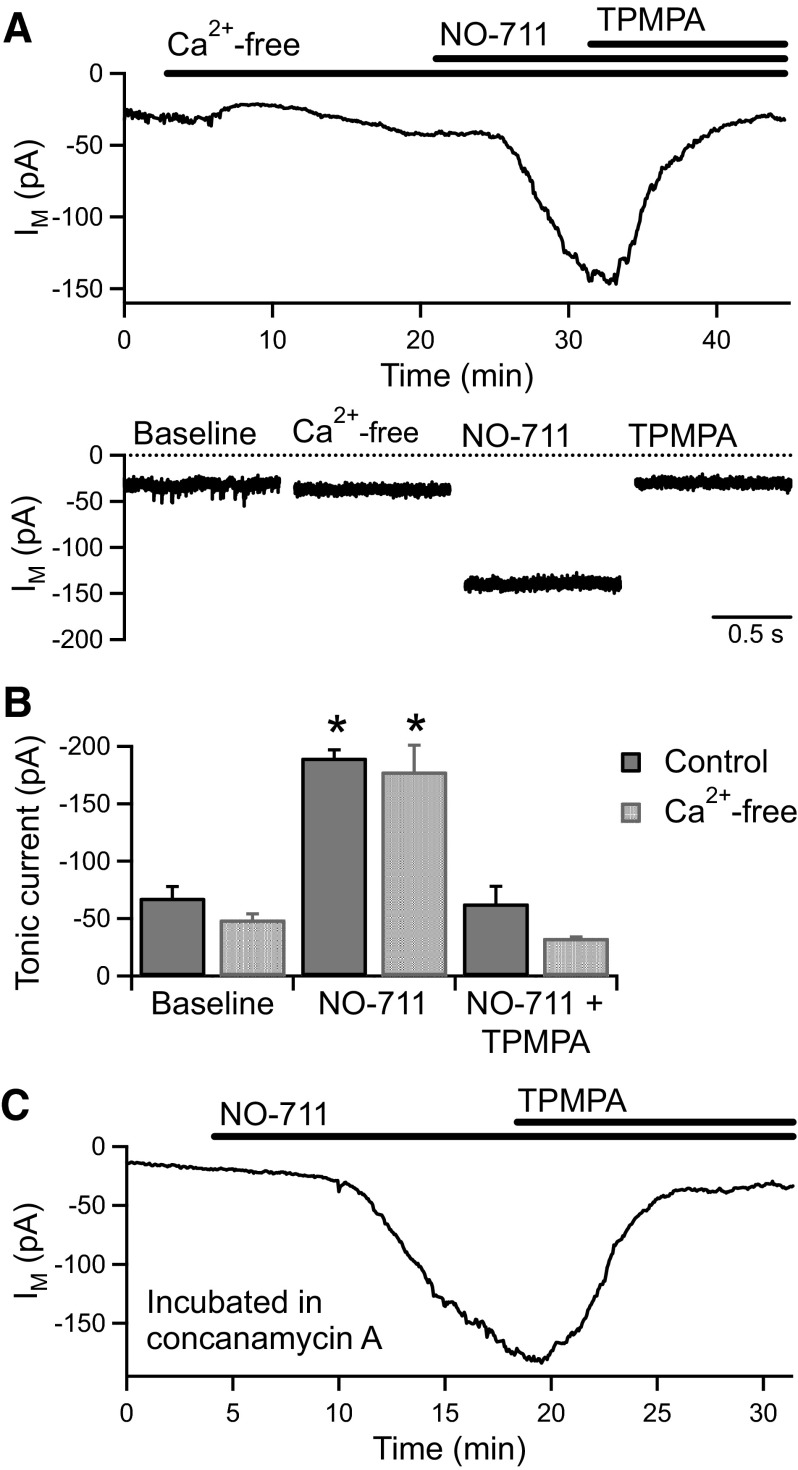
It has been proposed that high-affinity extrasynaptic GABA receptors may be activated by ambient GABA as GABA transporters cannot lower the extracellular GABA concentration below a value determined by their ionic stoichiometry (Cavelier et al. 2005; Richerson and Wu 2003). If the GABACR tonic current in BC terminals is activated by ambient GABA, we reasoned that inhibition of GAT-1 in the absence of vesicular release would be unlikely to produce the large increase in the tonic current that occurs under normal conditions (Fig. 3C). We therefore tested the effect of applying NO-711 (3 μM) following removal of extracellular Ca2+. NO-711 caused a significant increase in the tonic current (from −49 ± 5 to −178 ± 23 pA, n = 5, P < 0.01) that was fully reversed by the addition of TPMPA (100 μM; −33 ± 1 pA, n = 3; Fig. 6, A and B). The magnitude of the tonic current increase evoked by NO-711 in Ca2+-free solution (−129 ± 26 pA) was very similar to that evoked in interleaved experiments in normal Ca2+ solution (−122 ± 7 pA, n = 4; Fig. 6B). In addition, in two BC terminals in which vesicular release had been blocked by concanamycin A (4.6 μM; IPSC frequency <0.2 Hz), the tonic current was strongly potentiated by NO-711 (Fig. 6C). These results are inconsistent with activation of the tonic GABACR current by ambient GABA and instead indicate an active, nonvesicular mechanism for GABA release.
FIG. 6.
The tonic GABACR current is unlikely to be activated by ambient GABA. A: IM vs.time (top) and example current traces (bottom) from an experiment showing the potentiating effect of NO-711 (3 μM) in the presence of Ca2+-free extracellular solution. The increase in the tonic current was reversed by TPMPA (100 μM). B: The increase in the tonic current evoked by NO-711 (3 μM) was similar in control (n = 4) and Ca2+-free (n = 5) conditions. C: Application of NO-711 (3 μM) also caused a large increase in the tonic current in BC terminals incubated in concanamycin A (4.6 μΜ), which was reversed by addition of TPMPA (100 μM).
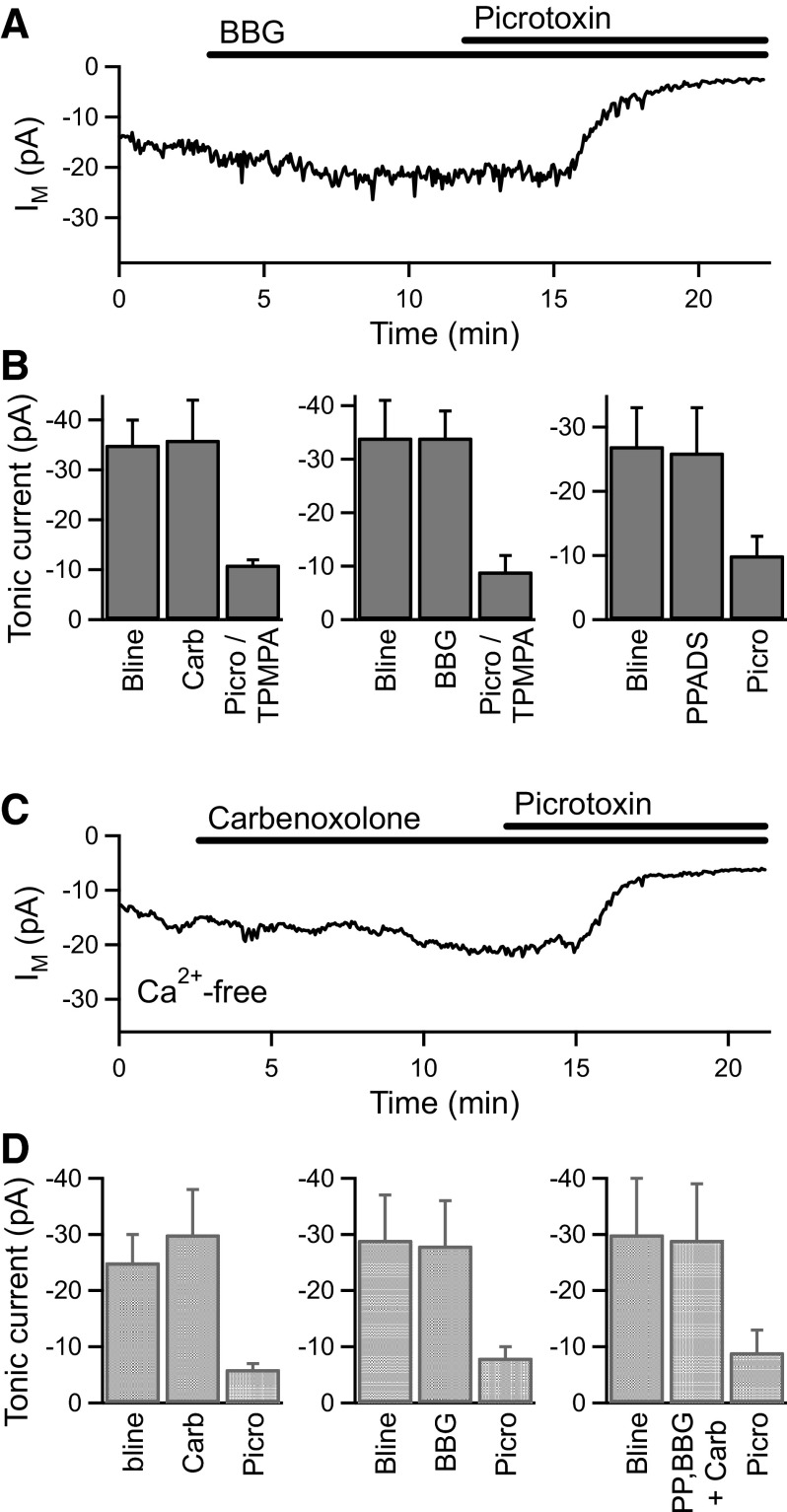
We therefore investigated two potential nonvesicular, Ca2+-independent mechanisms that have been shown to mediate glutamate release from astrocytes: hemichannels (uncoupled gap junctions) (Ye et al. 2003) and large-pore P2X7 receptors (Duan et al. 2003). Under normal conditions, application of the hemichannel inhibitor carbenoxolone (100–200 μM; n = 4), the nonselective P2 receptor antagonist pyridoxalphosphate-6-azophenyl-2′,4′-disulphonic acid (PPADS, 50–100 μM; n = 3), or the P2X7 receptor antagonist Brilliant Blue G (BBG, 1 μM; n = 4) had no significant effect on the amplitude of the tonic current in BC terminals (Fig. 7, A and B). Subsequent application of TPMPA (50–100 μM) or picrotoxin (50–100 μM) confirmed that tonic GABACR current was present in these recordings (Fig. 7, A and B). The hemichannel and P2X7 receptor antagonists were also tested in Ca2+-free extracellular solution (with 3.5 mM Mg2+) to inhibit vesicular release. Application of carbenoxolone (100–200 μM; n = 4) or BBG (1 μM; n = 4) alone, or in combination with PPADS (50–100 μM; n = 4), had no effect on the tonic current in Ca2+-free conditions (Fig. 7, C and D). GABA release therefore appears to occur via a nonvesicular, Ca2+-independent but as yet unidentified mechanism to activate the tonic GABACR current in BC terminals.
FIG. 7.
GABA is not released via hemichannels or P2X7 receptors. A: an example experiment showing the lack of effect of the P2X7 receptor antagonist Brilliant Blue G (BBG, 1 μM) on the tonic current, which was subsequently reduced by picrotoxin (100 μM). B: mean data for the application of carbenoxolone (Carb, 100–200 μM, n = 4), BBG (1 μM, n = 4), and PPADS (50–100 μM, n = 3) under normal conditions. C: an example experiment showing the lack of effect of carbenoxolone (100 μM) in the presence of Ca2+-free extracellular solution, with subsequent inhibition of the tonic current by picrotoxin (100 μM). D: mean data for the application of carbenoxolone (100 μM, n = 4), BBG (1 μM, n = 4), and carbenoxolone plus BBG and PPADS (PP, 50–100 μM, n = 4) under Ca2+-free conditions.
DISCUSSION
To determine the source of GABA that activates the tonic GABACR current in goldfish BC terminals, we investigated the effect of inhibiting vesicular release. As expected, this strongly reduced the frequency of spontaneous GABAAR IPSCs but, somewhat surprisingly, did not inhibit the tonic GABACR current. By contrast, glutamate-evoked GABAR currents, comprising both GABAAR and GABACR components, were dependent on the vesicular release of GABA. These results strongly suggest that evoked and tonic GABACR currents in BC terminals are activated by GABA originating from vesicular and nonvesicular sources, respectively. BC terminals also exhibit GABACR-mediated miniature IPSCs (Palmer 2006), which were not apparent following inhibition of vesicular release. However, as it is difficult to reliably discriminate these small, slow events from the tonic current noise, it was not possible to quantify this effect. The partial, transient reduction in the tonic GABACR current on exposure to Ca2+-free solution may reflect a contribution of vesicular GABA release to the tonic current that, when removed, is compensated for by upregulation of the nonvesicular mechanism.
By comparison, tonic GABAAR currents in the brain are, in some cases, dependent on vesicular GABA release but in other cases are activated by GABA from nonvesicular sources including ambient GABA, GABA transporter reversal, and unidentified nonvesicular mechanisms (Brickley et al. 1996; Bright et al. 2007; Glykys and Mody 2007b; Rossi et al. 2003; Wall and Usowicz 1997; Wu et al. 2006, 2007). Furthermore, tonic NMDA receptor (NMDAR) currents in hippocampal neurons are activated by glutamate released via a Ca2+-independent, nonvesicular mechanism (Cavelier and Attwell 2005; Jabaudon et al. 1999; Le Meur et al. 2007). The activation of tonic currents by nonvesicular neurotransmitter release may therefore be a general feature of CNS function.
It is likely that evoked and tonic GABACR currents in BC terminals are mediated by distinct populations of receptors localized to synaptic and extrasynaptic sites, respectively. GABACRs have been immunolocalized within synapses in BC terminals (Fletcher et al. 1998; Koulen et al. 1998) but are also present throughout the extrasynaptic membrane (Jones, Furness and Palmer, unpublished observations). Tonic GABAAR and NMDAR currents are believed to be mediated by peri- or extrasynaptic receptors that have a distinct subunit composition from the synaptic receptors that mediate phasic currents (Farrant and Nusser 2005; Glykys and Mody 2007a; Le Meur et al. 2007). Similarly, synaptic and extrasynaptic GABACRs in BC terminals may differ in their ρ subunit composition. For example, perch ρ1 homomers have a higher GABA sensitivity, slower deactivation kinetics, and lower single-channel conductance than receptors containing ρ2 (Pan et al. 2006; Qian et al. 1998; Zhu et al. 2007), properties that would make them particularly suitable for mediating the tonic GABACR current.
A high concentration of exogenous taurine was capable of activating the tonic GABACR current in BC terminals, similar to the activation by taurine of a tonic GABAAR current in the thalamus (Jia et al. 2008). However, it is likely that GABA is the major activator of the spontaneous tonic current in BC terminals due to the large potentiation of the tonic current that results from inhibiting either the uptake or breakdown of GABA and the very minor effect of inhibiting taurine uptake. The observed action of the taurine transporter inhibitor GES as a GABAAR and GABACR agonist means that it should be used with caution in experiments where GABAR activation could cause confounding results (Mellor et al. 2000). Taurine has been shown to biphasically modulate the GABA responses of human ρ1 receptors expressed in Xenopus oocytes, with inhibition at taurine concentrations exceeding 0.3 mM (Ochoa-de la Paz et al. 2008). Our observed increase in the tonic current evoked by 1 mM taurine may reflect differences in the sensitivity to taurine and/or GABA of heterologously expressed ρ1 receptors and native GABACRs.
GABA release via either GABA or taurine transporter reversal does not appear to be involved in generating the tonic GABACR current in BC terminals. Even under conditions of increased intracellular GABA, GAT-1 continues to operate in the forward direction to lower the extracellular GABA concentration. Ambient extracellular GABA has been proposed to activate extrasynaptic receptors mediating tonic currents (Cavelier et al. 2005; Richerson and Wu 2003), but we found that inhibition of GAT-1 in the absence of vesicular GABA release produced an increase in the tonic current of similar magnitude to control conditions. These results are most consistent with the tonic GABACR current being activated by GABA that is continuously released via a Ca2+-independent, nonvesicular mechanism and removed via the activity of GAT-1.
Nonvesicular glutamate release occurring via P2X7 receptors can evoke a tonic NMDAR current in hippocampal pyramidal neurons (Fellin et al. 2006). However, inhibition of P2X7 receptors had no effect on the tonic GABACR current in BC terminals. Similarly, inhibition of hemichannels was without effect. The mechanism of GABA release, as well as its cellular source, therefore remain to be ascertained. Nonvesicular GABA release may occur from amacrine cells and/or from retinal astrocytes or Müller cells. Müller cells are known to release neurotransmitters such as ATP and possibly glutamate, leading to changes in ganglion cell activity (Newman 2004; Newman and Zahs 1998). Glial cells are also likely to be the source of nonvesicular glutamate release in the hippocampus because tonic NMDAR currents are potentiated by inhibition of the glial enzyme glutamine synthetase (Cavelier and Attwell 2005; Jabaudon et al. 1999; Le Meur et al. 2007).
In addition to determining the source and mechanism of release for GABA, it will be important to establish how the tonic GABACR current is regulated. For example, it may be regulated by changes in the rate of GABA release, the rate of GABA uptake by GAT-1, and/or by changes in the number or properties of BC terminal GABACRs. When expressed in cell lines, GABACR subunits are internalized in response to activation of protein kinase C (PKC) (Filippova et al. 1999; Kusama et al. 2000), which is concentrated in the axon terminals of certain types of BC (Greferath et al. 1990; Suzuki and Kaneko 1990). Activation of PKC or metabotropic receptors linked to the phospholipase C pathway causes a reduction in GABACR currents in rat BCs (Feigenspan and Bormann 1994b). Similarly, the activity of GAT-1 can be modulated on a time scale of minutes by phosphorylation-induced insertion or removal of transporters from the cell membrane (Beckman et al. 1999; Deken et al. 2003; Whitworth and Quick 2001).
Tonic GABAAR currents in the brain regulate a variety of neuronal processes, including synaptic integration and network excitability, via their effects on membrane conductance (Farrant and Nusser 2005). In BC terminals, the tonic GABACR current regulates the ability of the terminals to fire Ca2+-dependent action potentials (Hull et al. 2006) and is likely to operate on a slow time scale to regulate BC output by setting the responsiveness of the terminal to somatic depolarization and synaptic feedback mechanisms. By contrast, the relatively rapid and transient inhibition resulting from synaptically evoked GABACR activation may be important for dynamically limiting BC output during light responses (Lukasiewicz and Werblin 1994; Zhang and Slaughter 1995).
In conclusion, we have found that different forms of GABACR-mediated inhibition in BC terminals are evoked by vesicular and by nonvesicular GABA release. These two forms of inhibition are likely to be independently activated and regulated and to have distinct functions, further increasing the diversity of mechanisms that regulate BC terminal output. The specific roles and relative importance of these mechanisms in retinal processing within the IPL remain to be determined.
GRANTS
This work was funded by a Medical Research Council (MRC) Career Development Award, a MRC New Investigator Research Grant, a MRC Studentship, and a Keele University Dandelion Award.
Acknowledgments
We thank Dr. Dave Furness for his assistance with related projects.
REFERENCES
- Beckman et al. 1999.Beckman ML, Bernstein EM, Quick MW. Multiple G protein-coupled receptors initiate protein kinase C redistribution of GABA transporters in hippocampal neurons. J Neurosci 19: RC9, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brickley et al. 1996.Brickley SG, Cull-Candy SG, Farrant M. Development of a tonic form of synaptic inhibition in rat cerebellar granule cells resulting from persistent activation of GABAA receptors. J Physiol 497: 753–759, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bright et al. 2007.Bright DP, Aller MI, Brickley SG. Synaptic release generates a tonic GABAA receptor-mediated conductance that modulates burst precision in thalamic relay neurons. J Neurosci 27: 2560–2569, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavelier and Attwell 2005.Cavelier P, Attwell D. Tonic release of glutamate by a DIDS-sensitive mechanism in rat hippocampal slices. J Physiol 564: 397–410, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavelier et al. 2005.Cavelier P, Hamann M, Rossi D, Mobbs P, Attwell D. Tonic excitation and inhibition of neurons: ambient transmitter sources and computational consequences. Prog Biophys Mol Biol 87: 3–16, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavez et al. 2006.Chavez AE, Singer JH, Diamond JS. Fast neurotransmitter release triggered by Ca2+ influx through AMPA-type glutamate receptors. Nature 443: 705–708, 2006. [DOI] [PubMed] [Google Scholar]
- Deken et al. 2003.Deken SL, Wang D, Quick MW. Plasma membrane GABA transporters reside on distinct vesicles and undergo rapid regulated recycling. J Neurosci 23: 1563–1568, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong and Werblin 1998.Dong CJ, Werblin FS. Temporal contrast enhancement via GABAC feedback at bipolar terminals in the tiger salamander retina. J Neurophysiol 79: 2171–2180, 1998. [DOI] [PubMed] [Google Scholar]
- Drose and Altendorf 1997.Drose S, Altendorf K. Bafilomycins and concanamycins as inhibitors of V-ATPases and P-ATPases. J Exp Biol 200: 1–8, 1997. [DOI] [PubMed] [Google Scholar]
- Duan et al. 2003.Duan S, Anderson CM, Keung EC, Chen Y, Chen Y, Swanson RA. P2X7 receptor-mediated release of excitatory amino acids from astrocytes. J Neurosci 23: 1320–1328, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggers and Lukasiewicz 2006a.Eggers ED, Lukasiewicz PD. GABAA, GABAC and glycine receptor-mediated inhibition differentially affects light-evoked signalling from mouse retinal rod bipolar cells. J Physiol 572: 215–225, 2006a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggers and Lukasiewicz 2006b.Eggers ED, Lukasiewicz PD. Receptor and transmitter release properties set the time course of retinal inhibition. J Neurosci 26: 9413–9425, 2006b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrant and Nusser 2005.Farrant M, Nusser Z. Variations on an inhibitory theme: phasic and tonic activation of GABAA receptors. Nat Rev Neurosci 6: 215–229, 2005. [DOI] [PubMed] [Google Scholar]
- Feigenspan and Bormann 1994a.Feigenspan A, Bormann J. Differential pharmacology of GABAA and GABAC receptors on rat retinal bipolar cells. Eur J Pharmacol 288: 97–104, 1994a. [DOI] [PubMed] [Google Scholar]
- Feigenspan and Bormann 1994b.Feigenspan A, Bormann J. Modulation of GABAC receptors in rat retinal bipolar cells by protein kinase C. J Physiol 481: 325–330, 1994b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fellin et al. 2006.Fellin T, Pozzan T, Carmignoto G. Purinergic receptors mediate two distinct glutamate release pathways in hippocampal astrocytes. J Biol Chem 281: 4274–4284, 2006. [DOI] [PubMed] [Google Scholar]
- Filippova et al. 1999.Filippova N, Dudley R, Weiss DS. Evidence for phosphorylation-dependent internalization of recombinant human rho1 GABAC receptors. J Physiol 518: 385–399, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher et al. 1998.Fletcher EL, Koulen P, Wassle H. GABAA and GABAC receptors on mammalian rod bipolar cells. J Comp Neurol 396: 351–365, 1998. [DOI] [PubMed] [Google Scholar]
- Glykys and Mody 2007a.Glykys J, Mody I. Activation of GABAA receptors: views from outside the synaptic cleft. Neuron 56: 763–770, 2007a. [DOI] [PubMed] [Google Scholar]
- Glykys and Mody 2007b.Glykys J, Mody I. The main source of ambient GABA responsible for tonic inhibition in the mouse hippocampus. J Physiol 582: 1163–1178, 2007b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greferath et al. 1990.Greferath U, Grunert U, Wassle H. Rod bipolar cells in the mammalian retina show protein kinase C-like immunoreactivity. J Comp Neurol 301: 433–442, 1990. [DOI] [PubMed] [Google Scholar]
- Grumelli et al. 2005.Grumelli C, Verderio C, Pozzi D, Rossetto O, Montecucco C, Matteoli M. Internalization and mechanism of action of clostridial toxins in neurons. Neurotoxicology 26: 761–767, 2005. [DOI] [PubMed] [Google Scholar]
- Hull and von Gersdorff 2006.Hull C, Li GL, von Gersdorff H. GABA transporters regulate a standing GABAC receptor-mediated current at a retinal presynaptic terminal. J Neurosci 26: 6979–6984, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichinose and Lukasiewicz 2002.Ichinose T, Lukasiewicz PD. GABA transporters regulate inhibition in the retina by limiting GABAC receptor activation. J Neurosci 22: 3285–3292, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jabaudon et al. 1999.Jabaudon D, Shimamoto K, Yasuda-Kamatani Y, Scanziani M, Gahwiler BH, Gerber U. Inhibition of uptake unmasks rapid extracellular turnover of glutamate of nonvesicular origin. Proc Natl Acad Sci USA 96: 8733–8738, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia et al. 2008.Jia F, Yue M, Chandra D, Keramidas A, Goldstein PA, Homanics GE, Harrison NL. Taurine is a potent activator of extrasynaptic GABA(A) receptors in the thalamus. J Neurosci 28: 106–115, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koulen et al. 1998.Koulen P, Brandstatter JH, Enz R, Bormann J, Wassle H. Synaptic clustering of GABAC receptor rho-subunits in the rat retina. Eur J Neurosci 10: 115–127, 1998. [DOI] [PubMed] [Google Scholar]
- Kusama et al. 2000.Kusama T, Hatama K, Saito K, Kizawa Y, Murakami H. Activation of protein kinase C induces internalization of the GABAC receptors expressed in Xenopus oocytes. Jpn J Physiol 50: 429–435, 2000. [DOI] [PubMed] [Google Scholar]
- Le Meur et al. 2007.Le Meur K, Galante M, Angulo MC, Audinat E. Tonic activation of NMDA receptors by ambient glutamate of non-synaptic origin in the rat hippocampus. J Physiol 580: 373–383, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombardini 1991.Lombardini JB Taurine: retinal function. Brain Res Brain Res Rev 16: 151–169, 1991. [DOI] [PubMed] [Google Scholar]
- Lukasiewicz and Shields 1998.Lukasiewicz PD, Shields CR. Different combinations of GABAA and GABAC receptors confer distinct temporal properties to retinal synaptic responses. J Neurophysiol 79: 3157–3167, 1998. [DOI] [PubMed] [Google Scholar]
- Lukasiewicz and Werblin 1994.Lukasiewicz PD, Werblin FS. A novel GABA receptor modulates synaptic transmission from bipolar to ganglion and amacrine cells in the tiger salamander retina. J Neurosci 14: 1213–1223, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marc and Liu 2000.Marc RE, Liu W. Fundamental GABAergic amacrine cell circuitries in the retina: nested feedback, concatenated inhibition, and axosomatic synapses. J Comp Neurol 425: 560–582, 2000. [DOI] [PubMed] [Google Scholar]
- Matsui et al. 2001.Matsui K, Hasegawa J, Tachibana M. Modulation of excitatory synaptic transmission by GABAC receptor-mediated feedback in the mouse inner retina. J Neurophysiol 86: 2285–2298, 2001. [DOI] [PubMed] [Google Scholar]
- Mellor et al. 2000.Mellor JR, Gunthorpe MJ, Randall AD. The taurine uptake inhibitor guanidinoethyl sulphonate is an agonist at gamma-aminobutyric acid(A) receptors in cultured murine cerebellar granule cells. Neurosci Lett 286: 25–28, 2000. [DOI] [PubMed] [Google Scholar]
- Newman 2004.Newman EA Glial modulation of synaptic transmission in the retina. Glia 47: 268–274, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman and Zahs 1998.Newman EA, Zahs KR. Modulation of neuronal activity by glial cells in the retina. J Neurosci 18: 4022–4028, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochoa-de la Paz et al. 2008.Ochoa-de la Paz LD, Martinez-Davila IA, Miledi R, Martinez-Torres A. Modulation of human GABArho1 receptors by taurine. Neurosci Res 61: 302–308, 2008. [DOI] [PubMed] [Google Scholar]
- Palmer 2006.Palmer MJ Functional segregation of synaptic GABAA and GABAC receptors in goldfish bipolar cell terminals. J Physiol 577: 45–53, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer et al. 2003.Palmer MJ, Taschenberger H, Hull C, Tremere L, von Gersdorff H. Synaptic activation of presynaptic glutamate transporter currents in nerve terminals. J Neurosci 23: 4831–4841, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan et al. 2005.Pan Y, Khalili P, Ripps H, Qian H. Pharmacology of GABAC receptors: responses to agonists and antagonists distinguish A- and B-subtypes of homomeric rho receptors expressed in Xenopus oocytes. Neurosci Lett 376: 60–65, 2005. [DOI] [PubMed] [Google Scholar]
- Pan et al. 2006.Pan Y, Ripps H, Qian H. Random assembly of GABA rho1 and rho2 subunits in the formation of heteromeric GABAC receptors. Cell Mol Neurobiol 26: 289–305, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian and Dowling 1995.Qian H, Dowling JE. GABAA and GABAC receptors on hybrid bass retinal bipolar cells. J Neurophysiol 74: 1920–1928, 1995. [DOI] [PubMed] [Google Scholar]
- Qian et al. 1998.Qian H, Dowling JE, Ripps H. Molecular and pharmacological properties of GABA-rho subunits from white perch retina. J Neurobiol 37: 305–320, 1998. [DOI] [PubMed] [Google Scholar]
- Richerson and Wu 2003.Richerson GB, Wu Y. Dynamic equilibrium of neurotransmitter transporters: not just for reuptake anymore. J Neurophysiol 90: 1363–1374, 2003. [DOI] [PubMed] [Google Scholar]
- Rossi et al. 2003.Rossi DJ, Hamann M, Attwell D. Multiple modes of GABAergic inhibition of rat cerebellar granule cells. J Physiol 548: 97–110, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagdullaev et al. 2006.Sagdullaev BT, McCall MA, Lukasiewicz PD. Presynaptic inhibition modulates spillover, creating distinct dynamic response ranges of sensory output. Neuron 50: 923–935, 2006. [DOI] [PubMed] [Google Scholar]
- Suzuki and Kaneko 1990.Suzuki S, Kaneko A. Identification of bipolar cell subtypes by protein kinase C-like immunoreactivity in the goldfish retina. Vis Neurosci 5: 223–230, 1990. [DOI] [PubMed] [Google Scholar]
- Tomi et al. 2008.Tomi M, Tajima A, Tachikawa M, Hosoya K. Function of taurine transporter (Slc6a6/TauT) as a GABA transporting protein and its relevance to GABA transport in rat retinal capillary endothelial cells. Biochim Biophys Acta 1778: 2138–2142, 2008. [DOI] [PubMed] [Google Scholar]
- Vigh et al. 2005.Vigh J, Li GL, Hull C, von Gersdorff H. Long-term plasticity mediated by mGluR1 at a retinal reciprocal synapse. Neuron 46: 469–482, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wall and Usowicz 1997.Wall MJ, Usowicz MM. Development of action potential-dependent and independent spontaneous GABAA receptor-mediated currents in granule cells of postnatal rat cerebellum. Eur J Neurosci 9: 533–548, 1997. [DOI] [PubMed] [Google Scholar]
- Whitworth and Quick 2001.Whitworth TL, Quick MW. Substrate-induced regulation of gamma-aminobutyric acid transporter trafficking requires tyrosine phosphorylation. J Biol Chem 276: 42932–42937, 2001. [DOI] [PubMed] [Google Scholar]
- Wu et al. 2007.Wu Y, Wang W, ez-Sampedro A, Richerson GB. Nonvesicular inhibitory neurotransmission via reversal of the GABA transporter GAT-1. Neuron 56: 851–865, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu et al. 2003.Wu Y, Wang W, Richerson GB. Vigabatrin induces tonic inhibition via GABA transporter reversal without increasing vesicular GABA release. J Neurophysiol 89: 2021–2034, 2003. [DOI] [PubMed] [Google Scholar]
- Wu et al. 2006.Wu Y, Wang W, Richerson GB. The transmembrane sodium gradient influences ambient GABA concentration by altering the equilibrium of GABA transporters. J Neurophysiol 96: 2425–2436, 2006. [DOI] [PubMed] [Google Scholar]
- Ye et al. 2003.Ye ZC, Wyeth MS, Baltan-Tekkok S, Ransom BR. Functional hemichannels in astrocytes: a novel mechanism of glutamate release. J Neurosci 23: 3588–3596, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang and Slaughter 1997.Zhang J, Jung CS, Slaughter MM. Serial inhibitory synapses in retina. Vis Neurosci 14: 553–563, 1997. [DOI] [PubMed] [Google Scholar]
- Zhang et al. 1995.Zhang J, Slaughter MM. Preferential suppression of the ON pathway by GABAC receptors in the amphibian retina. J Neurophysiol 74: 1583–1592, 1995. [DOI] [PubMed] [Google Scholar]
- Zhou et al. 2000.Zhou Q, Petersen CC, Nicoll RA. Effects of reduced vesicular filling on synaptic transmission in rat hippocampal neurones. J Physiol 525: 195–206, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu et al. 2007.Zhu Y, Ripps H, Qian H. A single amino acid in the second transmembrane domain of GABA rho receptors regulates channel conductance. Neurosci Lett 418: 205–209, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]