Abstract
Dynamic regulation of the expression of surface AMPA receptors (AMPARs) is a key mechanism to modulate synaptic strength and efficacy in the CNS and also to regulate auditory sensitivity. Here we address the role of surface AMPAR expression in excitotoxicity by blocking clathrin-mediated AMPAR endocytosis in auditory neurons. We used a membrane-permeable, dynamin-derived, myristoylated peptide (myr-Dyn) to inhibit surface AMPAR endocytosis induced by glutamate receptor agonists in culture and by noise exposure in vivo. Myr-Dyn infused into the mouse cochlea induced excitotoxic responses to acoustic stimuli that were normally not excitotoxic. These included vacuolization in the nerve terminals and spiral ganglion as well as irreversible auditory brain stem response threshold shifts. In cultured spiral ganglion neuronal cells, blockade of the reduction of surface AMPARs exacerbated neuronal death by incubation with N-methyl-d-aspartate and AMPA. This excitotoxic neuronal death could be prevented by calpeptin, a calpain-specific inhibitor. These results suggest that the reduction of surface AMPAR by endocytosis during excitatory stimulation plays an important role in limiting the excitotoxic damage to the neuron.
INTRODUCTION
Dynamic changes in the expression of surface AMPA receptors (AMPARs) have been shown to regulate synaptic strength and efficacy and are thought to underlie mechanisms of long-term potentiation and depression in hippocampal neurons (Barry and Ziff 2002; Bredt and Nicoll 2003; Collingridge et al. 2004; Derkach et al. 2007; Malinow and Malenka 2002; Man et al. 2000; Newpher and Ehlers 2008; Sheng and Lee 2001; Song and Huganir 2002). In auditory neurons, we have shown that surface AMPAR expression is reversibly decreased both in response to acoustic overstimulation in vivo and with application of glutamate receptor agonists in culture (Chen et al. 2007). The reversible reduction of surface AMPARs following acoustic stimulation correlated with a decrease in the acoustic sensitivity of auditory neural responses.
Auditory neurons display excitotoxic responses to acoustic overstimulation characterized by cellular swelling (vacuolization) in the spiral ganglion and vacuolization of dendritic endings near the inner hair cells (Pujol and Puel 1999; Wang et al. 2002). This process also can be seen in response to exogenous glutamate receptor agonists such as AMPA and kainate and is blocked by AMPAR antagonists (Khan et al. 2000; Le Prell et al. 2004; Ruel et al. 2000; Sun et al. 2001). It also can be exacerbated by drugs that block desensitization of AMPARs (Ruel et al. 1999). Thus the excitotoxic effect in auditory neurons is thought to be mediated via activation of AMPARs. Recent evidence suggests that the excitotoxic response to acoustic overstimulation is followed by denervation of the inner hair cells and neuronal cell death over a period of days to months (Kujawa and Liberman 2006).
It is reasonable to hypothesize that the reduction in surface AMPA receptors with acoustic overstimulation serves not only to regulate auditory sensitivity but may also ameliorate potential excitotoxic effects of loud sounds. In the present study, we examined this hypothesis by blocking the removal of surface AMPARs during a normally nonexcitotoxic acoustic exposure and observing signs of neuronal excitotoxicity. We blocked the sound-induced removal of surface AMPARs in vivo by infusing the mouse cochlea with a membrane-permeable, dynamin-derived, myristoylated peptide (myr-Dyn), which inhibits clathrin-mediated AMPAR endocytosis (Marks and McMahon 1998; Nong et al. 2003). We specifically looked at the GluR2 subunit of the AMPAR. Myr-Dyn-infused ears demonstrated smaller reductions in surface GluR2 receptors and increased excitotoxic-like neuronal vacuolization. Noise-induced threshold shifts were exacerbated in myr-Dyn-infused ears, and, unlike noise-only ears, auditory sensitivity did not recover during a 6-h postexposure period of monitoring. We also showed that normally nonexcitotoxic concentrations of glutamate receptor agonists could induce excitotoxic responses in the presence of myr-Dyn in auditory neuronal culture. The excitotoxic response in auditory neurons was prevented by calpeptin, a specific calpain inhibitor. Therefore we suggest that the decreased expression of surface AMPARs we observe in response to acoustic stimulation may serve to protect auditory neurons from the excitotoxic effects of acoustically mediated AMPAR activation.
METHODS
Cell culture
Auditory spiral ganglion neurons were cultured as described previously (Chen et al. 2007). The bulla was retrieved from postnatal day 3–5 CBA/CaJ mice and placed in ice-cold Hank's balanced salt solution (HBSS, Gibco). The modiolus was isolated from the surrounding tissue, cut into approximately three pieces, and transferred to an enzymatic solution containing collagenase type IV (0.5 mg/ml) and trypsin (2.5 mg/ml) at 37°C for 25 min. The enzymatic digestion was terminated by replacing the supernatant with culture medium (see following text). The tissue was dissociated by gentle mechanical trituration with a pipette. The cell suspension was enriched for spiral ganglion cells by taking advantage of the observation that nonneuronal cells tend to stick to plastic petri dishes while neuronal cells do not. The suspension was subjected to three sequential platings onto dry 35-ml dishes. After each plating, the suspension was poured off into another plate. The final plating was transferred into poly-l-ornithine-coated eight-well-chamber coverglass (Nalge Nunc International, Naperville, IL) at a density of two cochleae per eight-well plate. Cultures were maintained in a humidified 5% CO2 incubator at 37°C for 15–18 h. Culture medium contained Dulbecco's modified eagle's medium (DMEM) and F-12 (1:1 vol:vol), 10% FBS, 5% horse serum, NT-3 (20 ng/ml), BDNF (5 ng/ml), 2% B-27 supplement, penicillin (100 U/ml), and streptomycin (100 μg/ml).
Immunostaining of surface AMPA receptors
Immediately prior to treatment, the cultures were gently washed three times with artificial perilymph (AP), which was composed of (in mM) 120 NaCl, 3.5 KCl, 1.5 CaCl2, 5.5 glucose, and 20 HEPES; titrated with NaOH to pH 7.5; total Na+ = 130 mM. The cultures were treated with 20 μM myr-Dyn for 10 min followed by myr-Dyn with 20 μM N-methyl-d-aspartate (NMDA) or with 20 μM AMPA for another 10 min at 37°C. As controls, the cells were treated with either 20 μM NMDA, 20 μM AMPA, 20 μM myr-Dyn, or AP alone for 10 min. The cells were then washed three times with AP and fixed immediately with 4% formaldehyde/4% sucrose for 20 min and then blocked with 10% horse serum without permeabilization at room temperature.
Surface GluR2 was labeled by incubating for 2 h with mouse anti-GluR2 antibody recognizing the extracellular epitopes of GluR2 (1:200, Chemicon). Goat anti-mouse biotin (1:500, Jackson ImmunoResearch, West Grove, PA) was incubated with the culture for 1 h to amplify the GluR2 signal, and the biotin-bound GluR2 was saturated with fluorescein-conjugated streptavidin (1:500, Jackson ImmunoResearch) for 1 h. The cells were then permeabilized with 0.15% Triton X-100 in 10% horse serum for 1 h and incubated with rabbit anti-neurofilament M antibody for 2 h. The neuronal marker was saturated with Cy3-conjugated goat anti-rabbit secondary antibody (1:500, Jackson ImmunoResearch) for 1 h.
Fluorescence was visualized using a Zeiss Axiovert 200 inverted microscope and images were acquired with a 16-bit, cooled CCD camera (Princeton Instruments MicroMAX) driven by a LabView software package (National Instruments, Austin, TX). The exposure time was 0.3 s for Cy3 and 1 s for fluorescein.
For quantification of GluR2 receptors, wells were examined for clusters of neurons, based on neurofilament (Cy3) label. Filter sets were then switched and images taken of GluR2 (FITC) label. Five images, randomly taken based on the presence of neurons, were obtained from each well. The quantification of GluR2 labeling was performed using National Institutes of Health ImageJ software (http://rsb.info.nih.gov/ij/) on the 16-bit images. Background values within the image were thresholded out, and GluR2 intensity per neuron was calculated as the mean pixel intensity multiplied by total pixel number and divided by the number of neurons.
Cellular viability assay and neuronal staining
To quantify excitotoxic cell death in auditory neuronal cultures, dead cells were labeled with ethidium homodimer-1 (EthD-1, Live/Dead Viability/Cytotoxicity kit; Invitrogen, Carlsbad, CA), and neurons were identified by labeling with rabbit anti-neurofilament M. The cultures were pretreated with 20 μM myr-Dyn for 10 min followed by myr-Dyn with 20 μM NMDA or with 20 μM AMPA or myr-Dyn alone for another 10 min at 37°C. In some experiments, calpeptin (50 μM) was added to myr-Dyn with NMDA or AMPA. Treatment with AP, 20 μM NMDA, 20 μM AMPA, or 50 μM calpeptin alone served as control. The cells were returned to the incubator with culture medium for 6 h and then incubated with 4 μM EthD-1 for 20 min at room temperature. Thereafter the culture was fixed with 4% formaldehyde/4% sucrose for 20 min at room temperature. After permeabilization with 0.15% Triton X-100 in 10% horse serum for 1 h, the cells were incubated with rabbit anti-neurofilament M antibody overnight. The neuronal marker was saturated with FITC-conjugated goat anti-rabbit secondary antibody (1:100, Jackson ImmunoResearch) for 1 h. Images from six to seven random regions were taken from each well based on the presence of neurons. Neuronal death was quantified as the number of EthD-1-positive neurons divided by total neurons. Incubation of the cells with 300 μM AMPA or 300 μM NMDA with or without 50 μM calpeptin for 24 h served as positive control and the staining was performed as in the preceding text.
Auditory function measurements
CBA/CaJ mice (6–8 wk) were randomly assigned to one of the following groups: myr-Dyn infusion only; noise exposure only; noise exposure with artificial perilymph infusion; and noise exposure with myr-Dyn infusion. In the myr-Dyn-only group, auditory function was tested immediately prior to the infusion and at regular intervals for 7.5 h during the infusion. For the noise-exposure-only group and the noise-with-AP-infusion group, auditory function was tested immediately prior to noise exposure and at regular intervals for 60–80 min postnoise exposure [data from these 2 groups have been previously published in a different format (Chen et al. 2007)]. For the noise exposure with infusion of myr-Dyn group, auditory function was tested immediately prior to noise exposure and at regular intervals for ∼6 h posttreatment.
The mice were anesthetized (ketamine, 100 mg/kg ip; xylazine, 10 mg/kg ip; boosters of 1/3 to 1/2 the original dose as needed) and kept in a warmed sound-attenuating chamber throughout the experiment. Auditory brain stem responses (ABRs) and distortion product otoacoustic emissions (DPOAEs) were recorded at the right ear using our standard methods (Chen et al. 2006). Briefly, ABRs were measured under computer control in response to tone pips (8, 20, and 45.2 kHz; 5-ms duration; 0.5-ms rise/fall; cos2 shaping; 30/s) with level adjusted in 5-dB steps over the range required to capture threshold. Responses were detected with subcutaneous stainless electrodes placed at the vertex and ventrolateral to the ipsilateral ear with a ground near the tail. The response was amplified (10,000 times), filtered (0.1–3 kHz band-pass), and averaged (across 512 sweeps at each frequency-level combination; artifact reject = 15 μV peak to peak). Threshold was defined as the lowest stimulus level at which response peaks were clearly and reproducibly present. These visual detection threshold judgments were confirmed following termination of the experiment by off-line display and analysis of the stored waveforms.
The 2f1-f2 DPOAEs were recorded as response amplitude versus primary level functions (L1 = 10–75 dB SPL; L2 = L1-10; primaries incremented together in 5-dB steps) for f2 = 8, 20, 45.2 kHz (f2/f1 = 1.2). Ear-canal sound pressure was amplified, digitally sampled, averaged (20 discrete spectra at each frequency-level combination) and fast Fourier transforms were computed from the averaged pressures. DPOAE level at 2f1-f2 and surrounding noise floor values (±50 Hz of 2f1-f2) were extracted and stored. Iso-response contours (L2 levels required to generate a DPOAE amplitude criterion of 0 dB SPL) were constructed from amplitude versus sound level data.
Noise exposure
The sound overexposure was a broadband noise (1–40 kHz), generated by a custom-built noise generator, filtered (Rockland Brickwall), amplified (Crown d-75), and presented, using a sound-delivery tweeter (Radio Shack 40-1377), directly to the right ear of the animal at 93 dB SPL for 10 min.
Intra-cochlear drug delivery
To test the role of removal of surface AMPAR in noise-induced neurotoxicity and hearing loss, we infused myr-Dyn into the mouse cochlea using a delivery method described previously (Chen et al. 2006). Briefly, animals were anesthetized as in the preceding text, and the surgical procedure was performed in the warmed chamber. A ventral skin incision was made longitudinally to expose the digastric muscle, which was cut to expose the underlying tympanic bulla and stapedial artery. The stapedial artery was cauterized at the entrance to the bulla and the surrounding fat tissue was removed to expose the inferior-medial aspect of the bulla. A cochleostomy was drilled ∼300 μm beneath the stapedial artery stump. The tip of a 10-cm length of fused silica tubing (144 μm OD, 75 μm ID; World Precision Instruments) was then inserted into the cochleostomy. Dental cement was applied to seal the hole and to secure the tubing to the bulla.
An infusion/withdrawal syringe pump (Harvard Apparatus PHD 2000) was operated at an infusion flow rate of 1 μl/h. A three-way miniature manifold (Warner Instruments, now Harvard Apparatus MM-2) was placed in between the silica tubing and the pump to allow for changes of infusion solutions. Another three-way valve was placed between the manifold and the pump and an open/shut valve on another end of the drain line. When the drain open/shut valve was closed, the flow from the pump was directed into the cochlea. To change the injection solution, the drain valve was opened, and the new solution was loaded through the three-way valve into the line between the pump and manifold.
ABRs and DPOAEs were measured before and regularly during the infusion. The initial infusion was of AP (1 μl/h for 30 min), followed by myr-Dyn (200 μM for 90 min). Then the mouse was exposed to the broad-band noise as described in the preceding text for 10 min and ABRs were tested regularly within 6 h following noise exposure until the animal was killed for histological examination. In the control group, myr-Dyn (200 μM) was infused, and ABRs and DPOAEs were monitored for 7.5 h.
Histological preparation
Anesthetized mice were perfused intracardially with 2.5% glutaraldehyde and 1.5% paraformaldehyde in a 0.1 M phosphate buffer. The petrous bone was extracted, and the round and oval windows opened to allow intralabyrinthine perfusion of fixative. The cochlea was kept in the same fixative at 4°C overnight and then osmicated (1% OsO4 in dH2O) for 60 min and decalcified (0.5 M EDTA with 1% glutaraldehyde) overnight. After decalcification, cochleae were dehydrated in ethanol and propylene oxide and then embedded in Araldite resins and sectioned in a horizontal plane parallel to the spiral axis of the upper turn at 20 μm with a carbide steel knife. Sections were mounted in Permount on microscope slides and coverslipped.
The cochlear spiral was reconstructed in 3D using Neurolucida (MicroBrightField, Colchester, VT) software and tracking the heads of pillar cells as the reference point for cochlear lengths (Wang et al. 2002). Distance from base of the cochlea was computed for each section through the cochlear duct using custom software. The cochlear location was converted into characteristic frequency based on the place-frequency map of the mouse cochlea (Muller et al. 2005).
Spiral ganglion neurons at locations corresponding to frequencies of 6, 13, 27, 30, 33, and 60 kHz were visualized. The number of neurons with associated cellular swelling and total neurons were counted with ×100 oil-immersion objectives at all focal depths through the cross section at chosen locations.
Vacuolization around the ganglion cell was identified as a distinctive clear area surrounding the spiral ganglion cells. These distinctive profiles have been examined in detail by electron microscopy (Wang et al. 2002) and have been shown to be associated with separation of the myelin lamina of the myelinating satellite cells with large fluid spaces. All ganglion cells in the mid-modiolar sections were counted, and all ganglion cells with associated cell swelling were counted.
All animal procedures were approved by the Animal Care and Use Committee of the Massachusetts Eye and Ear Infirmary.
RESULTS
If, as we hypothesize, one of the functions of removal of surface GluR2 receptor is to prevent excitotoxic damage in the auditory neurons, then blockade of GluR2 removal should produce excitotoxic responses to even relatively low levels of acoustic stimulation. To test this hypothesis, we infused the mouse cochlea with myr-Dyn to inhibit the removal of surface AMPAR and then exposed the animal to a broadband noise for 10 min. We chose a noise exposure that does not generate excitotoxicity but that we have previously shown to induce removal of surface GluR2 receptors, and we chose a concentration of myr-Dyn that we have previously demonstrated to block the removal of surface receptors in vivo (Chen et al. 2007).
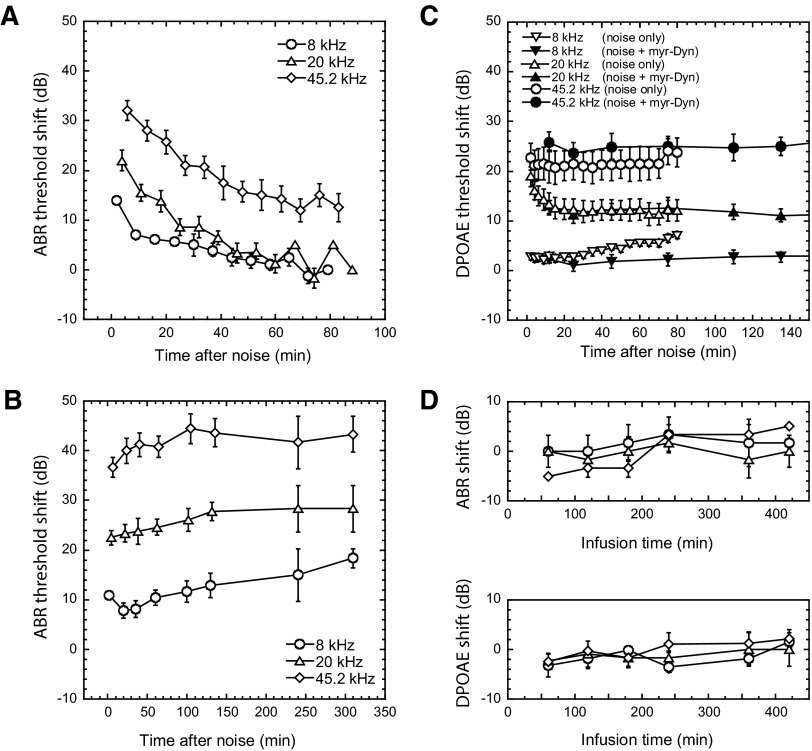
The 10-min noise exposure alone produced a reversible loss in auditory sensitivity in the mouse (Fig. 1 A). Neural-based ABR thresholds were elevated by ∼15 dB at 8 kHz, ∼20 dB at 20 kHz, and ∼30 dB at 45.2 kHz, when measured 2–6 min after noise and returned to near baseline levels within 60–80 min. When we infused the mouse cochlea with myr-Dyn before, during, and after noise exposure, ABR thresholds did not recover; instead, they were ≤15–40 dB poorer, depending on frequency, than those recorded in noise-only ears at the 80-min postexposure time point (Fig. 1B), and they did not recover over the 6 h period over which we monitored. Infusion of myr-Dyn (200 μM for 7.5 h, Fig. 1D) alone did not significantly elevate ABR thresholds (the ∼5 dB maximum shifts across all frequencies were similar to changes we have recorded during extended AP infusion only). Thus an acoustic stimulus that normally induced removal of surface GluR2 receptor to induce a temporary threshold shift, became, in the presence of myr-Dyn, a potentially excitotoxic stimulus.
FIG. 1.
Blocking the removal of cochlear surface AMPA recpetors (AMPARs) with a membrane-permeable, dynamin-derived, myristoylated peptide (myr-Dyn) increased and prolonged auditory brain stem response (ABR) threshold shifts. A: ABR threshold shifts in control animals infused with an artificial perilymph solution and exposed to noise. Ten mice were exposed to a 10-min broad-band noise (1–40 kHz, 93 dB sound pressure level), and ABR thresholds were measured ≤80 min after the noise. There were initial ABR threshold shifts of ∼15 dB at 8 kHz, ∼20 dB at 20 kHz, and ∼30 dB at 45.2 kHz, and the ABR thresholds were nearly recovered at the end of measurements. Means ± SE are plotted. We have previously published, in a different format, the data presented in A (Chen et al. 2007). B: ABR threshold shifts with myr-Dyn infused before, during, and after the same noise exposure. When the animals were exposed to the same noise in the presence of myr-Dyn, ABR thresholds did not recover over the ∼6 h-period monitored after noise. myr-Dyn (200 μM) was infused 90 min before noise exposure and infusion with myr-Dyn continued throughout the experiment. Twelve mice were followed for ≤2.5 h after noise exposure, and 3 of them were followed up to 6 h after noise exposure. C: Myr-Dyn (200 μM) did not alter the noise-induced shifts in distortion product otoacoustic emissions (DPOAEs), suggesting that effects on ABRs were secondary to processes occurring beyond the level of the OHCs. Ten mice were exposed to the same stimuli as in A and B. D: myr Dyn alone (200 μM, 7.5 h, 4 mice examined) in the absence of noise exposure elevated ABR and DPOAE thresholds only slightly (∼5 dB), suggesting its effects on the outer hair cells were minimal.
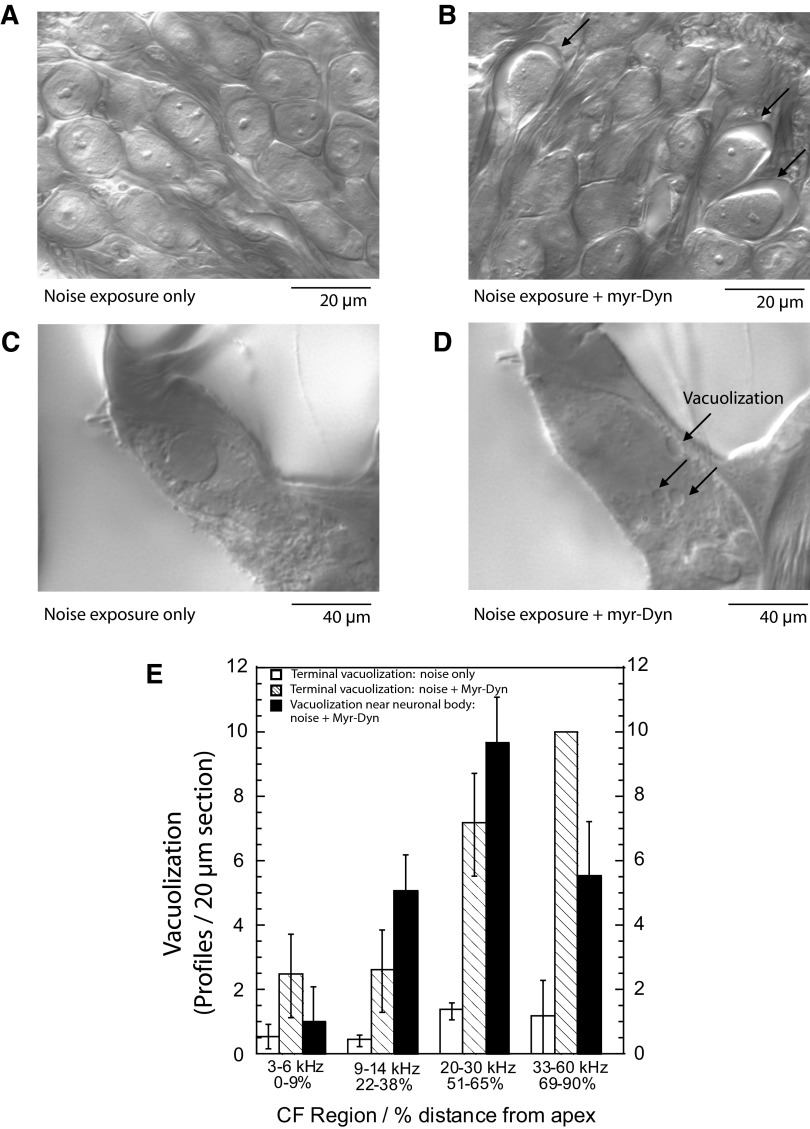
A robust and quantifiable consequence of exposure to intense acoustic stimuli or to glutamate receptor agonists is the appearance of swelling around neurons in the spiral ganglion (Fig. 2 B), which, in transmission electron microscopy, appears as a clear space between the neuron and the Schwann cell (Sun et al. 2001; Wang et al. 2002). Another observation is the appearance of vacuolization at the nerve terminal near the inner hair cell (Pujol and Puel 1999; Wang et al. 2002). Both phenomena are easily observed and quantified with Nomarski optics (Fig. 2, B and D). Because nerve terminal vacuolization can be seen as an artifact of imperfect fixation via arterial perfusion, we only analyzed animals that, after fixation, had a white liver and a hard brain, which are indicants of thorough fixation. To assess excitotoxicity, we quantified the percentage of neurons exhibiting these phenomena 6 h after exposure to the 10-min noise stimulus described in the preceding text. Control animals, exposed either to myr-Dyn alone, or noise alone, showed no ganglion-cell-associated vacuolization (Fig. 2A) and minimal vacuolization in the terminals (Fig. 2C). However, significant numbers of ganglion-cell-associated vacuolizations were identified in ears exposed to noise during myr-Dyn infusion (Fig. 2, B and C). Vacuolization was greatest in the 30 kHz region (11% of cells showed vacuolization) and decreased apically and basally. Similarly, vacuolization in the nerve terminals mirrored the findings for ganglion cell-associated vacuolization.
FIG. 2.
Myr-Dyn infusion before, during, and after noise exposure increased vacuolization associated with auditory neurons. A: noise exposure alone (1–40 kHz, 93 dB sound pressure level) did not generate vacuolization associated with spiral ganglion cell bodies 6 h after exposure. The image is of ganglion cells in the 30 kHz region. B: vacuolization associated with the ganglion cells (arrows) was seen 6 h after the same noise exposure with myr-Dyn infusion. Again, the image is from the 30-kHz region. Three cochleae from each group were examined with similar findings. In the 3 cochlea examined, we observed 118 ganglion-cell-associated vacuolization profiles in 1,763 neurons examined. In controls, we saw no vacuolizations in a similar number of ganglion cells examined. C: noise exposure alone did not induce vacuolization of the auditory nerve terminals at the inner hair cells. D: the same noise exposure with myr-Dyn induced vacuolization in auditory nerve terminals. The degree of vacuolization in afferent terminals and near ganglion cells was quantified for different cochlear regions. E: data were from collected from 12 animals—ganglion-cell-associated vacuolization was quantified in 3 control animals (noise only) and 3 experimental animals (noise + perfusion with myr-Dyn). Vacuolization in the nerve terminals were counted in 3 control animals (noise only) and 3 experimental animals (noise + myr-Dyn). Plotted are means ± SE.
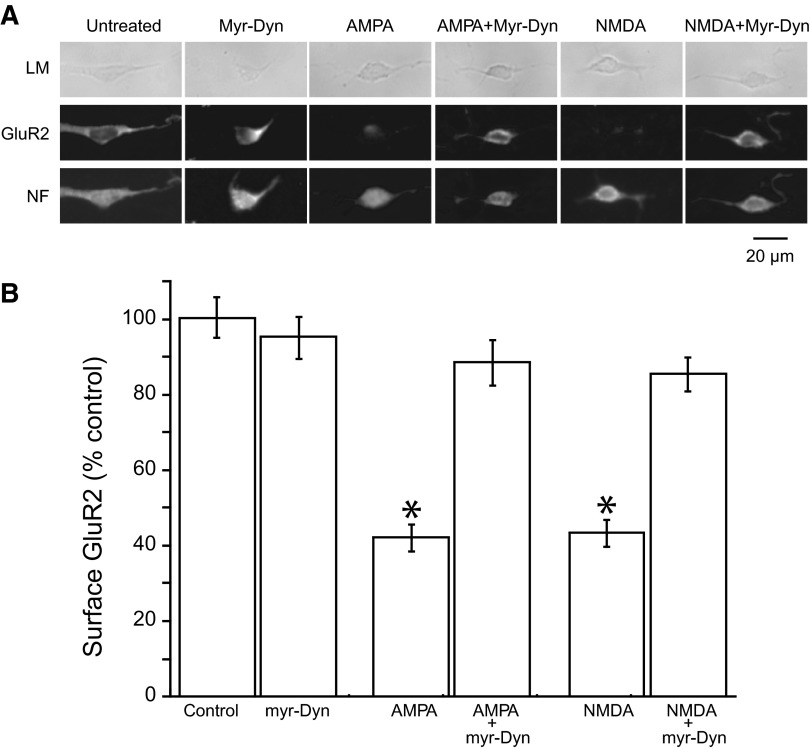
To examine some of the mechanisms of excitotoxicity in auditory neurons, we turned to cultured auditory neurons, where we have previously demonstrated that activation of AMPA or NMDA receptors can induce removal of surface AMPA receptor (Chen et al. 2007). We quantified surface and total AMPA receptors by measuring GluR2 labeling before (surface) and after (total) permeabilizing the cell membrane. Fixation alone slightly increased permeability, as evidenced by an 11% increase in label for neurofilament (an intracellular protein). Consistent with our previous study, a 10-min incubation with NMDA (20 μM) or AMPA (20 μM) produced a ∼55–60% decrease of surface GluR2 labeling. While myr-Dyn (20 μM, 10 min) alone did not significantly alter the presence of surface GluR2, myr-Dyn completely blocked NMDA- or AMPA- induced removal of surface GluR2 receptors from these cultured auditory neurons (Fig. 3, A and B).
FIG. 3.
Removal of surface AMPARs can be blocked by myr-Dyn in cultured auditory neurons. A: representative images of cultured auditory neurons with labeling of surface GluR2 and neurofilament (NF), and images from differential interference contrast light microscopy (LM). Auditory neurons in culture were identified by their appearance in DIC microscopy and by staining with an antibody against neurofilament M, a neuronal marker. Surface GluR2 was labeled by an antibody to an extracellular epitope on the receptor in the absence of permeabilization of membrane. B: myr-Dyn blocked the reduction of surface AMPARs induced by AMPA or N-methyl-d-aspartate (NMDA). Incubation of the cultured neurons with AMPA (20 μM) or NMDA (20 μM) alone for 10 min decreased surface AMPARs to ∼40–45%. Pretreatment of the culture with myr-Dyn (20 μM) for 10 min and during AMPA or NMDA incubation nearly completely blocked the removal of surface AMPARs. Each value represents the analysis of 5 images of neuronal clusters from each of three different cultures. The number of neurons in each image ranged from 3 to 40. Plotted are means ± SE. *, P < 0.0001, compared with control surface GluR2 without treatment as analyzed with a nonpaired Student's t-test.
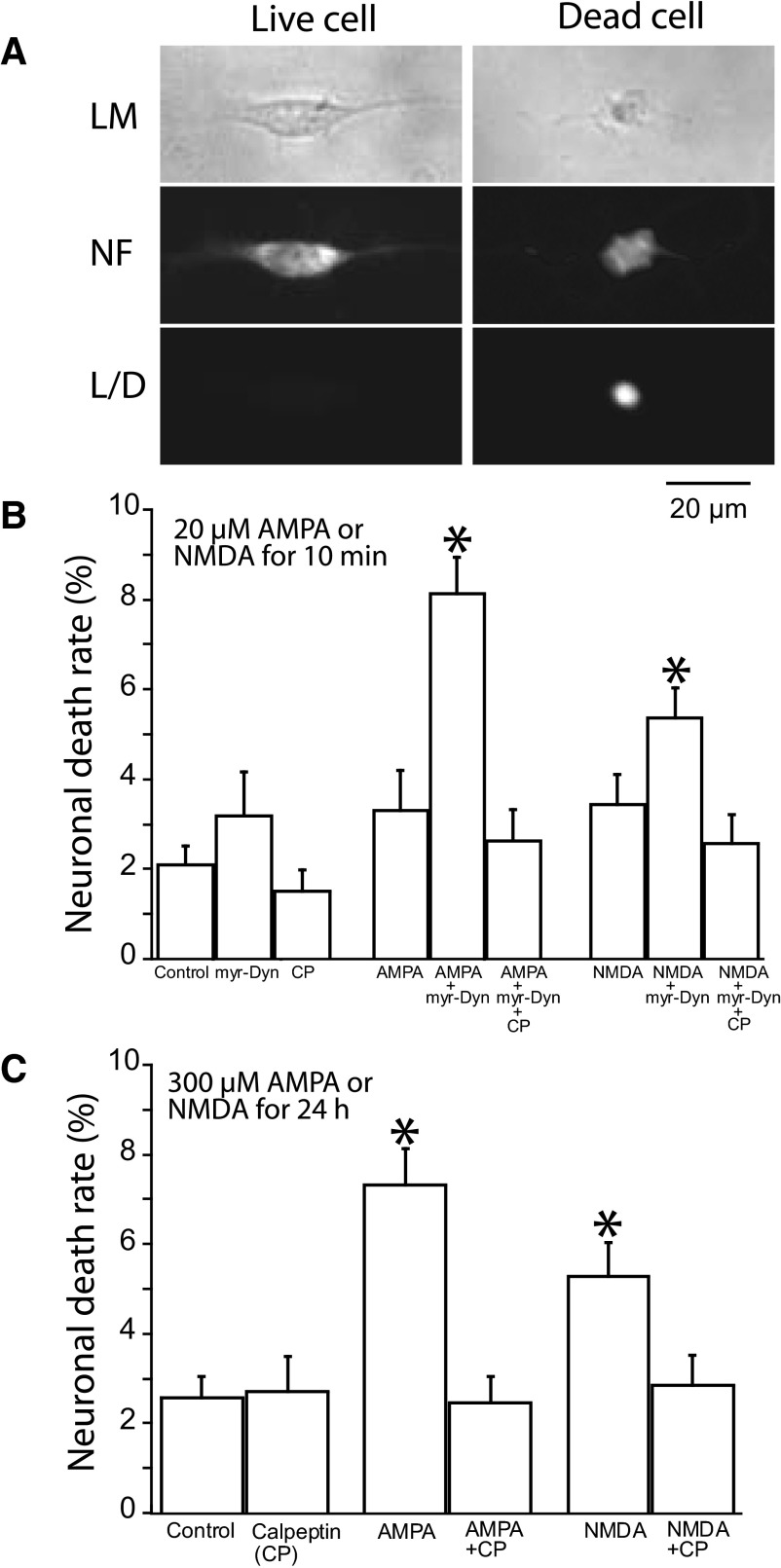
We also assessed the ability of myr-Dyn to enhance the excitotoxic effects of glutamate agonists with a viability assay using EthD-1 to label the nuclei of dead cells. Because calpain activation plays an important role in excitotoxic neuronal death, we determined if calpeptin, a calpain-specific inhibitor, could prevent neuronal death. Cultured auditory neurons were fixed and permeabilized to label neurons with anti-neurofilament M antibody. Live neurons were identified by positive neurofilament labeling with negative EthD-1 staining, whereas dead neurons were both neurofilament- and EthD-1 positive (Fig. 4 A). We observed a relatively low neuronal death rate of ∼2–3% in the control culture group 6 h after the treatment with artificial perilymph (AP; Fig. 4B). This rate was not significantly altered by a 20-min application of myr-Dyn (20 μM) or by calpeptin (50 μM) alone (P > 0.05).
FIG. 4.
Blockade of the removal of surface AMPARs with myr-Dyn increased excitotoxic ganglion cell death by AMPA or NMDA in culture. A: representative images of live and dead neurons. The dead cell has a shrunken or disrupted shape in light microscopy (LM). Label with neurofilament (NF) was used to distinguish neurons from other cells in both live and dead cells. The dead cells were EthD-1 positive, whereas the live cell were EthD-1 negative (L/D). B: blockade of surface AMPA receptor removal with myr-Dyn increased cell death following treatment with AMPA or NMDA. We chose doses of AMPA and NMDA (20 μM, 10 min) that we have previously demonstrated to produce reversible decreases in surface GluR2 (Chen et al. 2007). Treatment with 10 min of myr-Dyn (20 μM), AMPA (20 μM), NMDA (20 μM), or calpeptin (CP) alone, or no treatment resulted in ∼2–3% neuronal death 6 h later. Pretreatment of the culture with myr-Dyn (20 μM) for 10 min and during AMPA or NMDA incubation produced a significantly higher rate of neuronal death 6 h later, with 8.1% in the “AMPA with myr-Dyn” group and 5.3% in the “NMDA with myr-Dyn” group. The increase in neuronal death induced by myr-Dyn was prevented by coincubation with calpeptin (50 μM). C: in the absence of myr-Dyn, induction of ganglion cell death required very long exposure (24 h) at high concentrations (300 μM) of AMPA or NMDA. Treatment of the cultured ganglion cells for 24 h with AMPA (300 μM) alone or with NMDA (300 μM) alone resulted in neuronal cell death rates of 7.3 and 5.3% respectively. The significantly higher death rates with this treatment were nearly blocked by coincubation with CP (50 μM). Each group represents the analysis of 6–7 images of neuronal clusters from each of 5 different cultures. The number of neurons in each image ranged from 3 to 40. Plotted are means ± SE. *, P < 0.0001, compared with control group without treatment as analyzed with a nonpaired Student's t-test. There was ∼3% neuronal cell death with treatment of the cells with CP (50 μM for 24 h) alone or without treatment.
When neurons were treated for 10 min with myr-Dyn followed by a 10-min incubation of myr-Dyn with AMPA or myr-Dyn with NMDA, the neuronal death rates were increased to 8.1 and 5.3%, respectively (Fig. 4B). Both of these increases could be prevented by the addition of 50 μM calpeptin. Thus normally nonlethal AMPA or NMDA concentrations became excitotoxic to auditory neurons when myr-Dyn blocked the removal of AMPARs on the cell surface. It was possible to induce neuronal death without myr-Dyn, but to do so required very high concentrations of glutamate receptor agonists (300 μM) with incubation periods of 24 h. Under those conditions, AMPA induced death in 7.3% and NMDA in 5.3% of the neurons. Both could be prevented by 50 μM calpeptin (Fig. 4C).
DISCUSSION
We suggest that one role of regulated surface AMPAR expression is to limit excitotoxicity that might be induced during acoustic overstimulation. Intense acoustic stimulation can produce histological signs thought to be associated with excitotoxicity, which include vacuolization in the auditory nerve terminals and in the satellite cells near the ganglion cell bodies (Sun et al. 2001; Wang et al. 2002). The noise exposure we employed normally induces removal of surface AMPARs and does not induce signs of excitotoxicity. But when the removal of surface AMPARs was blocked with myr-Dyn, these same stimuli generated excitotoxic responses. In neuronal cultures from the CNS, excitotoxicity can be observed as a calpain-mediated cell death (Bano et al. 2005; Lankiewicz et al. 2000). We showed that a brief exposure to glutamate receptor agonists at concentrations that induce surface AMPAR removal without generating cell death can induce cell death when removal of surface AMPARs is blocked by myr-Dyn.
The myristoylation of dynamin inhibitory peptide enables permeation of the peptide into the cell. Dynamin inhibitory peptide acts to block dynamin-dependent endocytosis by abolishing the recruitment of dynamin to clathrin-coated pits by amphiphysin (Grabs et al. 1997), a process involved in the endocytosis of AMPARs (Man et al. 2000). We cannot say what other cochlear processes are dynamin dependent and thus potentially blocked by myr-Dyn. We do show that fundamental indicators of cochlear function, such as the ABR and DPOAEs are unaffected by myr-Dyn, suggesting limited roles for dynamin-dependent processes that are accessible to the infused drug. We have previously shown that infusion of myr-Dyn into the mouse cochlea can block the decrease of cochlear surface AMPARs induced by sound stimulation (Chen et al. 2007). In the present study, we demonstrated in cultured auditory neurons that myr-Dyn could inhibit the removal of surface AMPARs stimulated by agonists such as AMPA or NMDA.
Excitotoxicity following glutamate receptor activation is thought to result from excessive influx of extracellular ions, mainly Na+ and Ca2+, either directly via NMDA and AMPA receptors, or indirectly through voltage-gated Ca2+ channels or through Na+ loading and subsequent reversal of Ca2+-Na+ exchange (Bano et al. 2005; Budd and Nicholls 1996; Choi 1988; Fryer et al. 1999; Lipton and Rosenberg 1994; Olney 1969; Rothman et al. 1987). A reduction in the number of AMPARs present on the synaptic surface should reduce the amount of ion current entering the auditory neuron for a given amount of transmitter released. We have suggested that regulation of GluR2 in auditory nerve fibers is important in regulating synaptic strength and dynamic range at this synapse. The current findings suggest that a consequence of this regulation is to reduce exposure of the neuron to damaging synaptic current that might induce excitotoxicity.
ABR threshold shifts observed after exposure to myr-Dyn plus noise are not likely due to an effect on the hair cells. First, DPOAEs, which are sensitive to outer hair cell dysfunction, were not different in the presence and absence of myr-Dyn following noise exposure or for myr-Dyn alone. Second, while we did not specifically count hair cells, we did examine histological sections and saw no evidence of hair cell damage, or even stereociliary damage, in regions with vacuolization of nerve terminals.
If, as we have suggested (Chen et al. 2007), the reversible threshold shift following moderate noise exposure is due to removal and return of surface AMPARs, and if, as we have demonstrated, myr-Dyn blocks the removal of surface AMPARs, then we might expect myr-Dyn to prevent the noise induced removal and return of surface AMPARs and eliminate the threshold shift following moderate noise exposure. Instead myr-Dyn had the opposite effect: moderate noise exposure with myr-Dyn induced threshold shifts in the neurally based ABRs that increased over the next 6 h instead of recovering. Our interpretation is that the threshold elevations in the presence of myr-Dyn are a consequence of excitotoxic insult resulting from the inability of the neurons to reduce surface AMPARs in face of prolonged neurotransmitter release. This interpretation is supported by the observation of anatomical signs of excitotoxicity (cell swelling around the spiral ganglion) and by the absence of an action of myr-Dyn on the outer hair cell-based DPOAEs. The time course of the delayed threshold elevation with noise in myr-Dyn is consistent with the finding that the delayed neuronal degeneration occurs over a period of hours due to excess entry of Ca2+ after excitotoxic stimuli (Budd and Nicholls 1996; Lipton and Rosenberg 1994; Rothman et al. 1987).
Upregulation of the calpain pathway has been strongly implicated in the pathophysiology of excitotoxic neuronal death through its action to cleave cellular proteins (Goll et al. 2003; Ray and Banik 2003). We showed that a calpain-specific inhibitor prevented excitotoxic auditory neuronal death in cell culture, which is consistent with previous findings in the neurons from brain, retina, and cochlea (Bartus et al. 1994; Cheng et al. 1999; Das et al. 2006; Kupina et al. 2001; Saatman et al. 1996).
In auditory neurons, cellular vacuolization in the spiral ganglion and vacuolization of auditory nerve terminals following noise exposure is thought to be an excitotoxic effect related to glutamatergic synaptic transmission (Pujol and Puel 1999; Sun et al. 2001; Wang et al. 2002). The afferent terminal vacuolization can be mimicked by cochlear perfusion of glutamate receptor agonists such as AMPA and kainate, and this excitotoxicity can be blocked by antagonists to AMPARs (Ruel et al. 2000).
Noise exposure in the presence of myr-Dyn produced vacuolization around cochlear ganglion cells, with the greatest effects in the 20- to 30-kHz region with less vacuolization apically and basally. This observation is similar to that reported by Wang et al. (2002), who used much more intense acoustic stimulation to induce excitotoxic responses in the auditory nerve. They suggested this was due to more severe damage to hair cells at higher frequencies because of their increased susceptibility, an outcome that could lower the amount of transmitter released at higher frequencies. An alternative explanation, based on Furness and Lawton's (2003) observation of an increased density of glutamate receptors in the middle turn of the cochlea, is that the characteristic frequency regions most susceptible to excitotoxic responses are those with the greatest density of glutamate receptors.
In summary, we have shown that a moderate acoustic exposure, which is normally not excitotoxic, can be made excitotoxic if the auditory neuron is prevented from regulating surface AMPAR removal through application of myr-Dyn. Thus we demonstrate that a functional consequence of regulating synaptic strength by trafficking surface AMPARs is to limit excitotoxicity during acoustic exposure.
GRANTS
The work was supported by National Institutes of Deafness and Other Communication Disorders Grants DC-000767 to W. F. Sewell and DC-008577 to S. G. Kujawa.
Acknowledgments
We thank M. C. Liberman for guidance on analyzing vacuolization in afferent terminals.
REFERENCES
- Bano et al. 2005.Bano D, Young KW, Guerin CJ, Lefeuvre R, Rothwell NJ, Naldini L, Rizzuto R, Carafoli E, Nicotera P. Cleavage of the plasma membrane Na+/Ca2+ exchanger in excitotoxicity. Cell 120: 275–285, 2005. [DOI] [PubMed] [Google Scholar]
- Barry and Ziff 2002.Barry MF, Ziff EB. Receptor trafficking and the plasticity of excitatory synapses. Curr Opin Neurobiol 12: 279–286, 2002. [DOI] [PubMed] [Google Scholar]
- Bartus et al. 1994.Bartus RT, Hayward NJ, Elliott PJ, Sawyer SD, Baker KL, Dean RL, Akiyama A, Straub JA, Harbeson SL, Li Z. Calpain inhibitor AK295 protects neurons from focal brain ischemia. Effects of postocclusion intra-arterial administration. Stroke 25: 2265–2270, 1994. [DOI] [PubMed] [Google Scholar]
- Bredt and Nicoll 2003.Bredt DS, Nicoll RA. AMPA receptor trafficking at excitatory synapses. Neuron 40: 361–379, 2003. [DOI] [PubMed] [Google Scholar]
- Budd and Nicholls 1996.Budd SL, Nicholls DG. Mitochondria, calcium regulation, and acute glutamate excitotoxicity in cultured cerebellar granule cells. J Neurochem 67: 2282–2291, 1996. [DOI] [PubMed] [Google Scholar]
- Chen et al. 2007.Chen Z, Kujawa SG, Sewell WF. Auditory sensitivity regulation via rapid changes in expression of surface AMPA receptors. Nat Neurosci 10: 1238–1240, 2007. [DOI] [PubMed] [Google Scholar]
- Chen et al. 2006.Chen Z, Mikulec AA, McKenna MJ, Sewell WF, Kujawa SG. A method for intracochlear drug delivery in the mouse. J Neurosci Methods 150: 67–73, 2006. [DOI] [PubMed] [Google Scholar]
- Cheng et al. 1999.Cheng AG, Huang T, Stracher A, Kim A, Liu W, Malgrange B, Lefebvre PP, Schulman A, Van de Water TR. Calpain inhibitors protect auditory sensory cells from hypoxia and neurotrophin-withdrawal induced apoptosis. Brain Res 850: 234–243, 1999. [DOI] [PubMed] [Google Scholar]
- Choi 1988.Choi DW Glutamate neurotoxicity and diseases of the nervous system. Neuron 1: 623–634, 1988. [DOI] [PubMed] [Google Scholar]
- Collingridge et al. 2004.Collingridge GL, Isaac JT, Wang YT. Receptor trafficking and synaptic plasticity. Nat Rev 5: 952–962, 2004. [DOI] [PubMed] [Google Scholar]
- Das et al. 2006.Das A, Garner DP, Del Re AM, Woodward JJ, Kumar DM, Agarwal N, Banik NL, Ray SK. Calpeptin provides functional neuroprotection to rat retinal ganglion cells following Ca2+ influx. Brain Res 1084: 146–157, 2006. [DOI] [PubMed] [Google Scholar]
- Derkach et al. 2007.Derkach VA, Oh MC, Guire ES, Soderling TR. Regulatory mechanisms of AMPA receptors in synaptic plasticity. Nat Rev 8: 101–113, 2007. [DOI] [PubMed] [Google Scholar]
- Fryer et al. 1999.Fryer HJ, Knox RJ, Strittmatter SM, Kalb RG. Excitotoxic death of a subset of embryonic rat motor neurons in vitro. J Neurochem 72: 500–513, 1999. [DOI] [PubMed] [Google Scholar]
- Furness and Lawton 2003.Furness DN, Lawton DM. Comparative distribution of glutamate transporters and receptors in relation to afferent innervation density in the mammalian cochlea. J Neurosci 23: 11296–11304, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goll et al. 2003.Goll DE, Thompson VF, Li H, Wei W, Cong J. The calpain system. Physiol Rev 83: 731–801, 2003. [DOI] [PubMed] [Google Scholar]
- Grabs et al. 1997.Grabs D, Slepnev VI, Songyang Z, David C, Lynch M, Cantley LC, De Camilli P. The SH3 domain of amphiphysin binds the proline-rich domain of dynamin at a single site that defines a new SH3 binding consensus sequence. J Biol Chem 272: 13419–13425, 1997. [DOI] [PubMed] [Google Scholar]
- Khan et al. 2000.Khan MJ, Seidman MD, Quirk WS, Shivapuja BG. Effects of kynurenic acid as a glutamate receptor antagonist in the guinea pig. Eur Arch Otorhinolaryngol 257: 177–181, 2000. [DOI] [PubMed] [Google Scholar]
- Kujawa and Liberman 2006.Kujawa SG, Liberman MC. Acceleration of age-related hearing loss by early noise exposure: evidence of a misspent youth. J Neurosci 26: 2115–2123, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupina et al. 2001.Kupina NC, Nath R, Bernath EE, Inoue J, Mitsuyoshi A, Yuen PW, Wang KK, Hall ED. The novel calpain inhibitor SJA6017 improves functional outcome after delayed administration in a mouse model of diffuse brain injury. J Neurotrauma 18: 1229–1240, 2001. [DOI] [PubMed] [Google Scholar]
- Le Prell et al. 2004.Le Prell CG, Yagi M, Kawamoto K, Beyer LA, Atkin G, Raphael Y, Dolan DF, Bledsoe SC Jr, Moody DB. Chronic excitotoxicity in the guinea pig cochlea induces temporary functional deficits without disrupting otoacoustic emissions. J Acoust Soc Am 116: 1044–1056, 2004. [DOI] [PubMed] [Google Scholar]
- Lipton and Rosenberg 1994.Lipton SA, Rosenberg PA. Excitatory amino acids as a final common pathway for neurologic disorders. N Engl J Med 330: 613–622, 1994. [DOI] [PubMed] [Google Scholar]
- Lankiewicz et al. 2000.Lankiewicz S, Marc Luetjens C, Truc Bui N, Krohn AJ, Poppe M, Cole GM, Saido TC, Prehn JH. Activation of calpain I converts excitotoxic neuron death into a caspase-independent cell death. J Biol Chem 275: 17064–17071, 2000. [DOI] [PubMed] [Google Scholar]
- Malinow and Malenka 2002.Malinow R, Malenka RC. AMPA receptor trafficking and synaptic plasticity. Annu Rev Neurosci 25: 103–126, 2002. [DOI] [PubMed] [Google Scholar]
- Man et al. 2000.Man HY, Lin JW, Ju WH, Ahmadian G, Liu L, Becker LE, Sheng M, Wang YT. Regulation of AMPA receptor-mediated synaptic transmission by clathrin-dependent receptor internalization. Neuron 25: 649–662, 2000. [DOI] [PubMed] [Google Scholar]
- Marks and McMahon 1998.Marks B, McMahon HT. Calcium triggers calcineurin-dependent synaptic vesicle recycling in mammalian nerve terminals. Curr Biol 8: 740–749, 1998. [DOI] [PubMed] [Google Scholar]
- Muller et al. 2005.Muller M, von Hunerbein K, Hoidis S, Smolders JW. A physiological place-frequency map of the cochlea in the CBA/J mouse. Hear Res 202: 63–73, 2005. [DOI] [PubMed] [Google Scholar]
- Newpher and Ehlers 2008.Newpher TM, Ehlers MD. Glutamate receptor dynamics in dendritic microdomains. Neuron 58: 472–497, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nong et al. 2003.Nong Y, Huang YQ, Ju W, Kalia LV, Ahmadian G, Wang YT, Salter MW. Glycine binding primes NMDA receptor internalization. Nature 422: 302–307, 2003. [DOI] [PubMed] [Google Scholar]
- Olney 1969.Olney JW Brain lesions, obesity, and other disturbances in mice treated with monosodium glutamate. Science 164: 719–721, 1969. [DOI] [PubMed] [Google Scholar]
- Pujol and Puel 1999.Pujol R, Puel JL. Excitotoxicity, synaptic repair, and functional recovery in the mammalian cochlea: a review of recent findings. Ann NY Acad Sci 884: 249–254, 1999. [DOI] [PubMed] [Google Scholar]
- Ray and Banik 2003.Ray SK, Banik NL. Calpain and its involvement in the pathophysiology of CNS injuries and diseases: therapeutic potential of calpain inhibitors for prevention of neurodegeneration. Curr Drug Targets CNS Neurol Disord 2: 173–189, 2003. [DOI] [PubMed] [Google Scholar]
- Rothman et al. 1987.Rothman SM, Thurston JH, Hauhart RE. Delayed neurotoxicity of excitatory amino acids in vitro. Neuroscience 22: 471–480, 1987. [DOI] [PubMed] [Google Scholar]
- Ruel et al. 2000.Ruel J, Bobbin RP, Vidal D, Pujol R, Puel JL. The selective AMPA receptor antagonist GYKI 53784 blocks action potential generation and excitotoxicity in the guinea pig cochlea. Neuropharmacology 39: 1959–1973, 2000. [DOI] [PubMed] [Google Scholar]
- Ruel et al. 1999.Ruel J, Chen C, Pujol R, Bobbin RP, Puel JL. AMPA-preferring glutamate receptors in cochlear physiology of adult guinea pig. J Physiol 518: 667–680, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saatman et al. 1996.Saatman KE, Murai H, Bartus RT, Smith DH, Hayward NJ, Perri BR, McIntosh TK. Calpain inhibitor AK295 attenuates motor and cognitive deficits following experimental brain injury in the rat. Proc Natl Acad Sci USA 93: 3428–3433, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng and Lee 2001.Sheng M, Lee SH. AMPA receptor trafficking and the control of synaptic transmission. Cell 105: 825–828, 2001. [DOI] [PubMed] [Google Scholar]
- Song and Huganir 2002.Song I, Huganir RL. Regulation of AMPA receptors during synaptic plasticity. Trends Neurosci 25: 578–588, 2002. [DOI] [PubMed] [Google Scholar]
- Sun et al. 2001.Sun H, Hashino E, Ding DL, Salvi RJ. Reversible and irreversible damage to cochlear afferent neurons by kainic acid excitotoxicity. J Comp Neurol 430: 172–181, 2001. [DOI] [PubMed] [Google Scholar]
- Wang et al. 2002.Wang Y, Hirose K, Liberman MC. Dynamics of noise-induced cellular injury and repair in the mouse cochlea. J Assoc Res Otolaryngol 3: 248–268, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]