Abstract
Human contrast sensitivity in low scotopic conditions is regulated according to the deVries–Rose law. Previous cat behavioral data, as well as cat and mice electrophysiological data, have not confirmed this relationship. To resolve this discrepancy at the behavioral level, we compared sensitivity in dim light for cats and humans in parallel experiments using the same visual stimuli and similar behavioral paradigms. Both species had to detect Gabor functions (SD = 1.5°, spatial frequencies from 0 to 4 cpd, temporal frequency 4 Hz) presented 8° to the right or left of a central fixation point over an 8 log-unit range of adaptation levels spanning scotopic vision and extending well into the mesopic range. Cats had 0.74 log unit greater absolute sensitivity than that of humans for spatial frequencies ≤1/8 cpd. Cats had better contrast sensitivity overall for spatial frequencies <1/2 cpd, whereas humans were more sensitive for spatial frequencies above this. However, most of the cat's sensitivity advantage for low spatial frequencies could be accounted for by the greater light-concentrating abilities of its optics. Contrast sensitivity to 4 cpd was poor or absent in the scotopic range for both species. For both, scotopic increment thresholds were proportional to the square root of retinal illuminance, in accordance with the deVries–Rose law. Overall, cat and human visual systems appear to operate under very similar constraints for rod vision, including the regulation of contrast sensitivity across adaptation levels. A companion paper compares sensitivity of neurons in the lateral geniculate nucleus to these behavioral data.
INTRODUCTION
Sensitivity and acuity are fundamental descriptors of visual systems, with considerable variation across species on the extent to which one or the other is stressed. Here we compare the dim-light vision of two species that occupy different points on the sensitivity–acuity continuum, the cat and human. Although the empirical data for either species alone are of interest, in combination they provide unique opportunities for interspecies comparisons.
For human cone vision, contrast thresholds are fairly constant across adaptation levels, so increment threshold—i.e., the smallest increment of light that is reliably detectable from the background—is a fixed fraction of retinal illuminance, in accordance with Weber's law (Walraven et al. 1990). This results from gain control mechanisms that avoid response saturation and keep visual contrast sensitivity constant over a wide range of background illumination, maintaining perceptual constancy for contrast in the face of changes in illumination (Shapley and Enroth-Cugell 1984). For rod vision, where sensitivity is paramount, this gain control is abandoned and, under appropriate conditions, increment threshold follows the deVries–Rose law: increment threshold is proportional to the square root of the background illumination (Rose 1948). DeVries–Rose behavior is expected when the biological gain is already maximal and quantal fluctuations in the stimulus (i.e., stimulus noise) constitute the limiting factor in sensitivity because the noise caused by quantal fluctuations is proportional to the square root of photon density. In contrast, for cone vision stimulus intensity is high enough so that quantal noise is not a significant factor.
Adherence to the deVries–Rose law is reliably observed in the low scotopic range for sinusoidal gratings, as well as for small, short-duration, stationary spots (for a review, see Shapley and Enroth-Cugell 1984). However, previously published cat behavioral data for scotopic vision do not follow the deVries–Rose law (Supplemental Material, section I). 1 Furthermore, as outlined by Kang and Malpeli (2009), this is also true of previous electrophysiological studies. It is unclear whether these discrepancies result from fundamental differences in the regulation of scotopic sensitivity or whether they are incidental to differences in experimental paradigms. This is a critical issue because access to the physiological circuits underpinning the regulation of scotopic vision is currently available only via animal models.
Here we measured contrast sensitivity for Gabor functions as a function of adaptation level over the scotopic and most of the mesopic range for cats and humans. Gabor functions were presented at the same rod-rich region of the retinas of both species for spatial frequencies that spanned the cat's acuity range at this eccentricity. The data were obtained from both species with the same visual display, viewing distance, retinal location, adaptation period, and method of measuring pupil size. The behavioral paradigms were essentially the same and methods of data analysis identical.
Under these circumstances, most fundamental properties of cat and human vision turn out to be quite similar. For both, scotopic contrast threshold strictly follows the deVries–Rose law and the maximum spatial frequency that can be perceived in the scotopic range is about the same. The cat's absolute threshold is nearly one log unit lower than that of the human's, but when the cat's optical advantages are factored out, the two species are comparable in scotopic sensitivity, suggesting that their intrinsic neural sensitivities are similar at this retinal eccentricity.
In a companion paper (Kang and Malpeli 2009) we show that contrast thresholds of the most sensitive categories of cells in the lateral geniculate complex of the awake cat agree closely with the cat psychophysical data presented here, including adherence to the deVries–Rose law under scotopic conditions.
METHODS
Subjects
Two head-fixed, adult female cats and three humans (the first three authors, ages ranging from 23 to 36 yr) served as subjects. The cats' eye positions were sampled at 500 Hz; eye positions of the humans were not monitored. Two human subjects were myopic and their vision was corrected to normal with eyeglasses; the third required no correction. For the cats, surgical procedures and methods of recording eye movements were identical to those of Cui and Malpeli (2003). Care and use of animals were in accordance with guidelines of the American Physiological Society, Society for Neuroscience, and University of Illinois Institutional Animal Care and Use Committee. Human subjects were treated in accordance with procedures approved by the University of Illinois Institutional Review Board; all were familiar with the experimental procedures and goals of the study and signed statements to this effect.
Visual stimuli
Visual stimuli were binocularly viewed. Small red laser spots displayed on a rear-projection screen (DA-Plex DA100; Da-Lite) served as central fixation points for both cats and humans and as saccade targets during cat training sessions. Gray-scale Gabor functions used as visual stimuli for contrast threshold estimation were generated using 256 gray levels and imaged on the screen by an LCD projector (Hopper XG20; Philips; 1,024 × 768-pixel resolution; 60-Hz refresh rate). The screen was located 67 cm from the eyes and subtended 60° horizontally and 48° vertically. The cats' heads were rigidly immobilized via a surgically implanted skull fixture; human subjects positioned their heads against a chin/forehead rest.
A cassette of neutral density filters (Schott Glass Technologies) placed in front of the projector lens allowed the display intensity to be adjusted in roughly 0.3 log-unit steps without vignetting of the image. At maximum brightness, the display screen had a mean (unattenuated) luminance of 62.87 cd/m2.
Gabor functions had a fixed-width Gaussian of 1.5° SD, truncated at the intensity step nearest the average display luminance, and spatial frequencies of 0, 1/8, 1/4, 1/2, 1, 2, or 4 cpd (cycles/degree). Gratings drifted upwardly at a fixed temporal frequency of 4 Hz, chosen because this is within the temporal frequency range (2–6 Hz) where cat behavioral contrast sensitivity is highest for low spatial frequencies (1/4 to 1/2 cpd; Blake and Camisa 1977). Also, 4 Hz is close to the temporal frequency at which both X and Y cells in the cat dorsal lateral geniculate nucleus are most sensitive at their optimal spatial frequency (Troy 1983). Contrast was defined as [(maximum luminance − minimum luminance)/(maximum luminance + minimum luminance)] × 100%, following the Rayleigh–Michelson contrast.
To avoid temporal transients, onsets of Gabor functions followed a temporal Gaussian envelope with a 125-ms SD, reaching the intended contrast in 500 ms. For the humans, Gabor functions disappeared gradually over 500 ms, following the reverse temporal profile of their onset (see Behavioral paradigms). The mean luminance of the Gabor functions was, within limits imposed by the gray-scale resolution, the same as the background luminance of the display.
Display luminance was carefully calibrated and contrasts of Gabor functions were empirically measured (Supplemental Material, sections A and B). All measurements of display luminance and retinal illuminance are reported in human photopic units.
Tapetal reflection
To take optical differences between species into account, it was necessary to estimate the efficacy of the cat tapetum in scotopic conditions. In brief, the spectral characteristics of the projector were measured (Supplemental Material, Fig. S1) and the amount of light transmitted through the retina was calculated from an estimate of retinal absorption. Finally, the reflective properties of the cat tapetum (Weale 1953) were used to estimate the additional light available to the retina due to the tapetum. This analysis suggests that the tapetum increased effective retinal illuminance for our light source by about 29%, or 0.11 log units (Supplemental Material, section C).
Estimation of pupil size
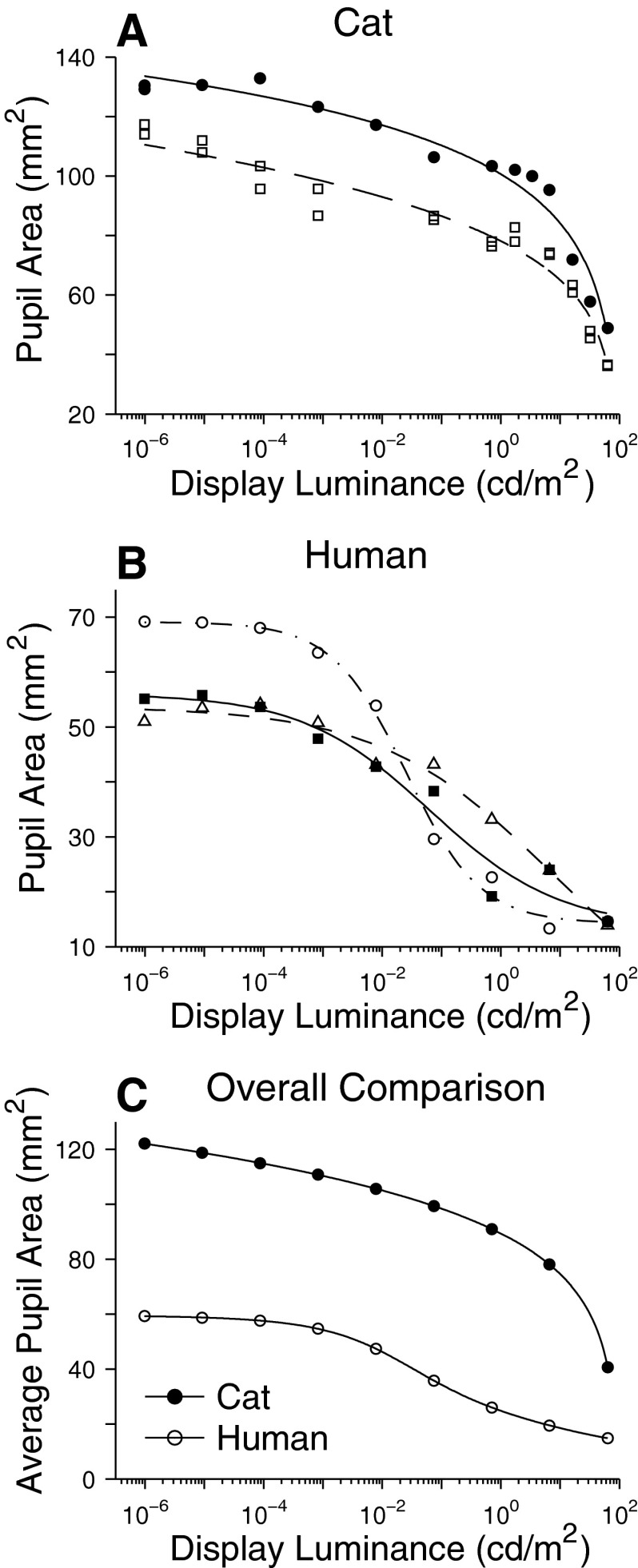
To convert display luminance to retinal illuminance (trolands; td = pupil area in mm2 × luminance in cd/m2), digital photographs of the eyes of all subjects were taken at adaptation levels spanning those used for contrast threshold estimations. For each subject, pupil area averaged for both eyes was fitted with an empirical curve (a log function for the cat and a sigmoid for the human) as a function of adaptation level (Fig. 1) and these functions were used to determine pupil area at each adaptation level for each subject.
FIG. 1.
Estimation of pupil area for individual cats (A) and humans (B), along with a comparison of the average pupil areas of the 2 species (C).
For all adaptation levels used for cats but the highest (i.e., from 10−6.0 to 101.5 cd/m2) and for the full range of adaptation levels for humans (10−6.0 to 101.8 cd/m2), the relationships between display luminance (DL, cd/m2) and retinal illuminance (RI, td) derived from the average pupil area shown in Fig. 1C were closely fit by the following functions: log RI = 0.9964 log DL + 1.9137 for the cats; log RI = 0.9191 log DL + 1.3974 for the humans. We use these equations here and in the companion paper (Kang and Malpeli 2009) only to convert literature estimates of the cone threshold from retinal illuminance to display luminance. When data on individual subjects are presented (e.g., Figs. 6–8), the functions fitted for individual pupil sizes shown in Fig. 1, A and B are used.
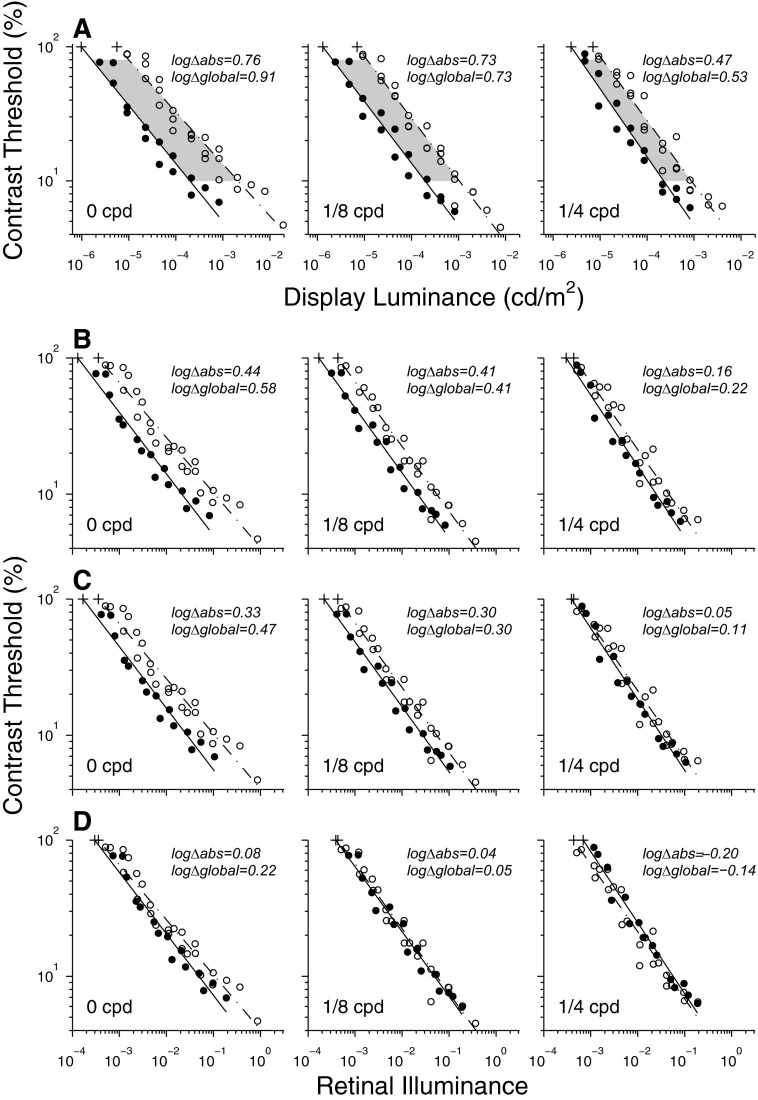
FIG. 6.
Contribution of optical factors to the cat's scotopic contrast sensitivity advantage over humans at low spatial frequency. A: contrast thresholds for the lowest 3 spatial frequencies tested. The cat's absolute luminance advantage (i.e., the horizontal distance between the “+” signs) is denoted by logΔabs in each panel. The cat's global luminance advantage (i.e., the average horizontal distance between regression lines for contrast thresholds from 10 to 80%, indicated by shading) is denoted by logΔglobal. B: comparative contrast sensitivity after factoring out the effect of the cat's larger pupil size at each luminance level. C: comparative contrast sensitivity after factoring out the effects of the cat's larger pupil and its reflective tapetum. D: comparative contrast sensitivity after factoring out the effects of the cat's larger pupil, tapetum, and shorter focal length.
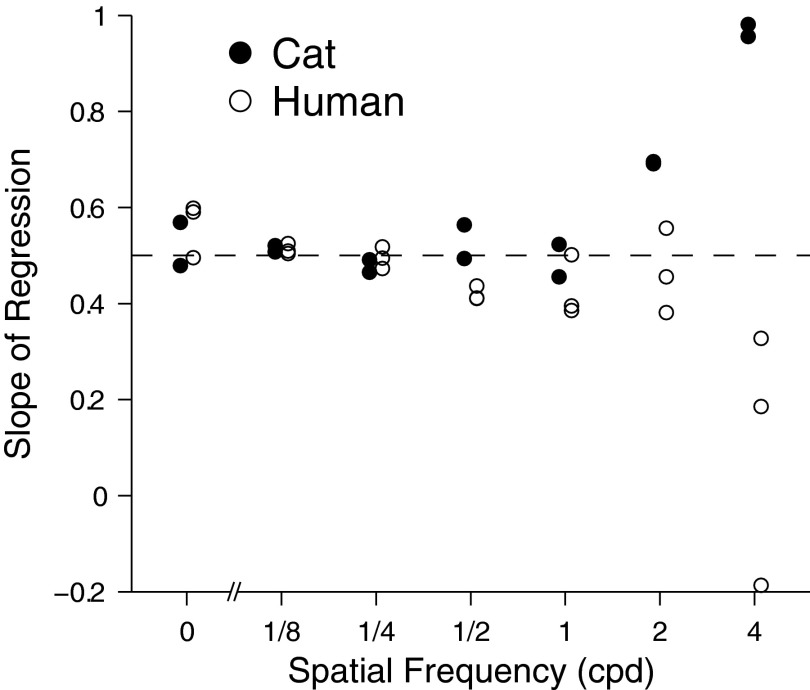
FIG. 8.
Slopes of increment-threshold functions shown in Fig. 7, as a function of spatial frequency. Horizontal dashed line marks a slope of 0.5. For spatial frequency ≤1 cpd (for which vision is primarily scotopic), the mean slope is 0.50 for cats, 0.48 for humans, and 0.49 for both (whether averaged across individuals or species). Symbols are slightly shifted horizontally for clarity.
Behavioral paradigms
The task for both species was fundamentally similar: a Gabor function appeared to one side of a central fixation point and the subject had to indicate whether it was to the right or left, with an eye movement toward the target for the cats and a button press for the humans.
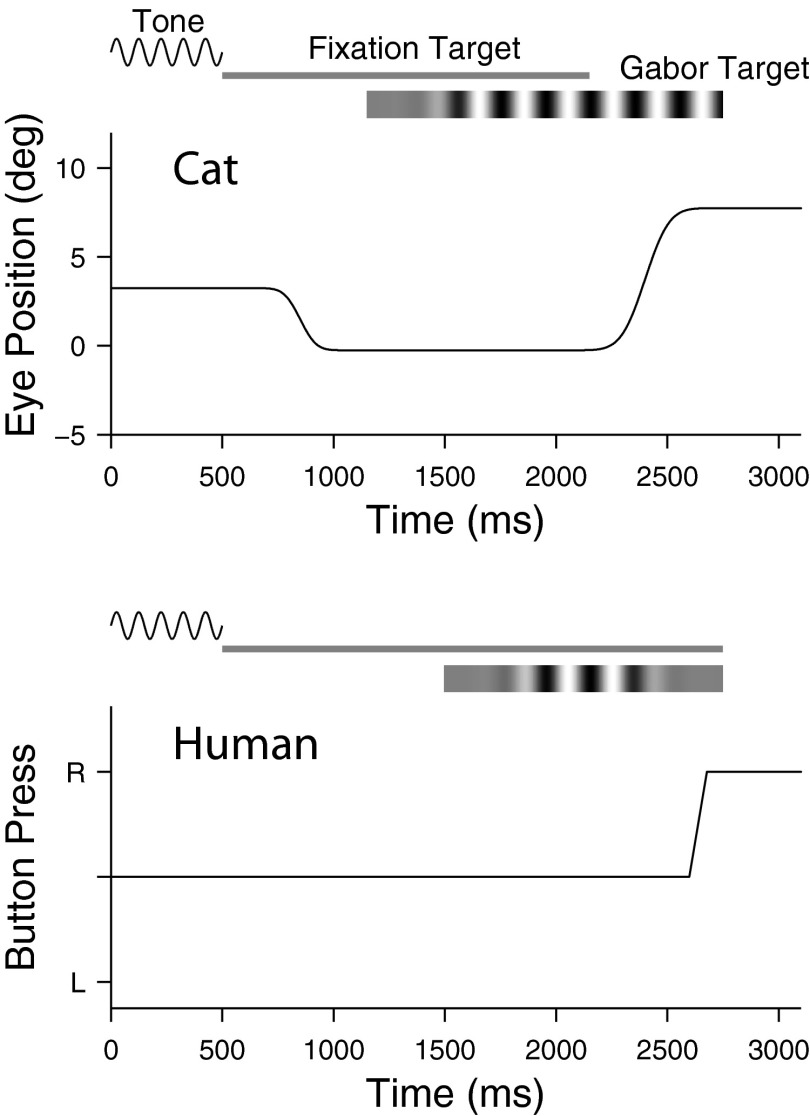
The task used to measure the cats' contrast threshold is schematically illustrated in Fig. 2, top (see Supplemental Material, section D for training procedures). At trial start, a 500-ms warning tone was followed by presentation of a laser spot at the center of the screen. If the cat acquired the central fixation spot within 1,500–3,000 ms and maintained fixation within a square window (±2–2.5°) for an additional 200–1,100 ms, a Gabor function appeared pseudorandomly 8° to the right or left with a 500-ms gradual onset; otherwise, the trial was aborted. With this fixation criterion, the retinal eccentricity of the center of the Gabor functions could vary from 5.5 to 10.5°, but in practice the cat's fixations were quite stable and well clustered around the fixation target, deviating <1.0° for >76% of the trials (Supplemental Material, section E). To reduce the frequency of premature choices, the central fixation spot remained on for 1 s after Gabor images began to appear. Gabor functions were not presented on one side more than twice in a row to avoid behavioral biases. Although in principle the cats could have modified their behavioral strategy to take advantage of these contingencies, in practice they did not (Supplemental Material, section F). A choice was recorded if an eye movement was made beyond the central fixation window within 6 s of Gabor onset. Specifically, for a correct choice the horizontal component of eye position had to cross the lateral edge of the central fixation window toward the Gabor function and remain beyond the edge for ≥200 ms, after which the trial terminated with a reward. If the horizontal component of eye position crossed the opposite edge of the fixation window, the trial was terminated after 500 ms and counted as a miss. Failure to respond within 6 s or an eye movement that crossed the upper or lower edge of the fixation window terminated the trial without reward; these trials were excluded from data analysis.
FIG. 2.
Behavioral paradigms. Progression of a trial is schematically illustrated for cats (top) and humans (bottom). Cats responded with an eye movement toward the stimulus; humans, by pressing a button with the hand corresponding to target location (R or L). For cats the stimulus was extinguished by the response, whereas for humans stimulus duration was fixed. See methods for details.
A schematic trial progression for the human is illustrated in the bottom panel of Fig. 2. After a 500-ms tone, a laser spot, which the subject was instructed to fixate, appeared at the center of the screen. One second later, a Gabor function was presented for 1,250 ms (including the 500-ms gradual onset and offset) 8° right or left of the spot. The central laser spot remained on to aid stable fixation and was extinguished at the same time as the Gabor function. The subjects had to make a decision about target location within 4 s of the onset of the Gabor function. Instead of responding with eye movements, they held a response button in each hand and pressed the one corresponding to the perceived (or guessed) location of the Gabor function. Unlike the cat sessions, the left–right location of the Gabor function was completely randomized across trials. Both humans and cats were dark-adapted for 30 min before a session began.
For both species, the progression of trials for a given combination of spatial frequency and adaptation level followed a modified staircase method (Cornsweet 1962) in which contrast of the Gabor function in the following trial was raised one step for each miss or reduced a step for two consecutive hits (Fig. 3, A and C). This hit/miss ratio stabilizes performance at a threshold of 71% correct (Levitt 1971), which efficiently produces a high density of trials around the 75% threshold level for a two-alternative forced-choice task. Data collection began with Gabor functions at the highest available contrast. Contrast initially changed in large steps to quickly approach threshold (six levels, equally spaced over the available range of contrast). After the subject's performance reached a rough threshold (typically after three or five reversals), the contrast step size was reduced and the number of contrast levels increased to 20. Additional trials (roughly 60 to 70 for humans and 100 to 200 for cats) were given until stable behavior near threshold was obtained. For cats, occasional catch trials (5% of the total) were inserted to estimate the guessing rate.
FIG. 3.
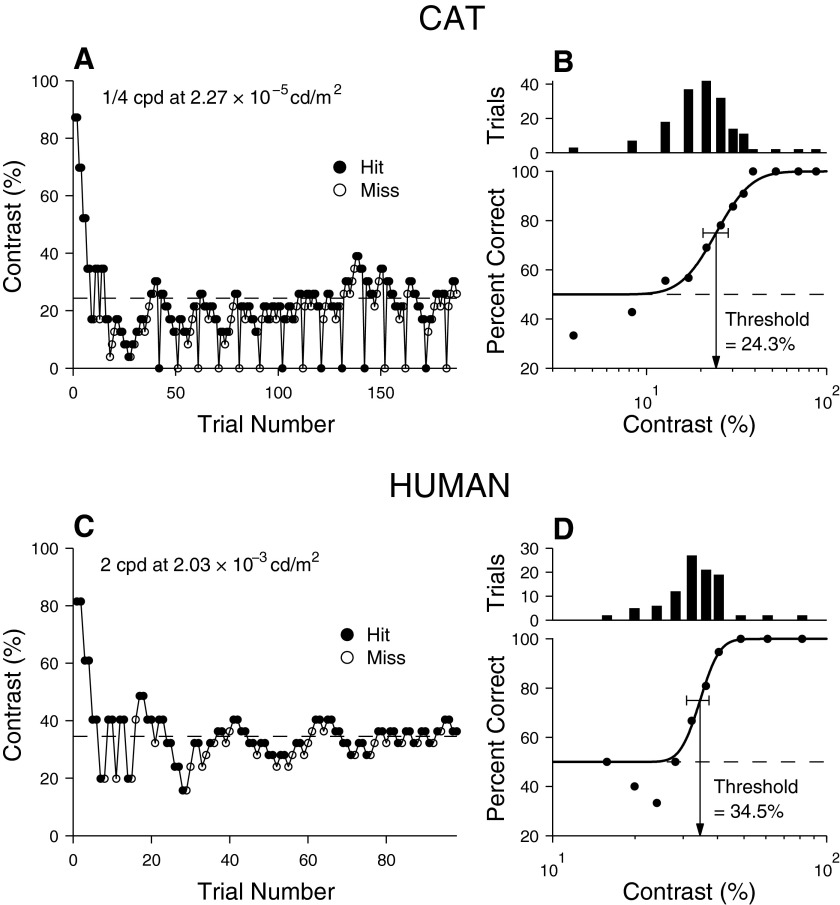
Staircase procedure and derivation of psychometric functions. Examples of behavioral sessions, in which a modified staircase method was used to determine contrast threshold, are shown for cats (A) and humans (C). Data points on the abscissa for cats are catch trials. Performance (percentage correct) is plotted as a function of stimulus contrast for cats (B) and humans (D), along with fitted psychometric functions (sigmoidal curves) and frequency histograms showing the number of trials presented for each stimulus contrast. The horizontal segments bracketing psychometric functions at the threshold criterion of 75% correct (corresponding to contrasts of 24.3% for the cat and 34.5% for the human, as indicated by downward arrows) indicate 95% confidence intervals determined by bootstrap simulations (7.8 and 6.4% for cat and human, respectively). These thresholds are also shown in A and C (horizontal dashed lines) to illustrate how the subjects' performance stabilized as trials progressed. For both cats and humans, at contrasts well below threshold, there is a tendency for performance to appear worse than chance. This was due to the early part of the staircase procedure, where the contrast was driven well below threshold by large downward contrast steps before small steps allow performance to stabilize around threshold.
Data analysis
All analyses were done with Matlab (Version 7.0, The MathWorks). Unless otherwise noted, statistical significance was determined with permutation tests. Contrast thresholds were determined from psychometric functions fitted to the percentage correct response as a function of stimulus contrast on a logarithmic scale (Fig. 3, B and D). Cumulative Gaussians with low asymptote set at 50% were used to fit psychometric functions and two free parameters determining the position and slope of the functions were estimated using a maximum-likelihood method (Wichmann and Hill 2001). Threshold was defined as the stimulus contrast at which performance was 50% correct on the underlying cumulative Gaussian curve, which corresponds to 75% correct in the psychometric function. A 95% confidence interval for estimated thresholds was determined from a parametric bootstrap simulation (Efron and Tibshirani 1993), as implemented by Wichmann and Hill (2001), and goodness-of-fit for psychometric functions was assessed by the Pearson chi-square (χ2) test. Statistical comparisons of the goodness-of-fit of psychometric functions and the confidence intervals of estimated thresholds show that the cat and human data were comparable in quality (Supplemental Material, section G).
These fitting procedures can estimate contrast thresholds above (below) the maximum (minimum) stimulus contrast used. However, we rejected these from analysis except for a few cases where the data were well behaved and the estimated threshold was close to (≤1.4% above) the maximum contrast.
To make the cat data equivalent to those obtained with the humans' two-alternative forced-choice task, trials were excluded if the cats failed to make an eye movement beyond the central window. Only 1.2% of the trials were excluded for this reason because once the cats were familiar with the behavioral paradigm they developed guessing strategies, responding in 90.8% of the catch trials. The catch trials were originally intended to estimate guessing rate for use as the low asymptotes of the psychometric functions. However, because the cats made a choice on a high proportion of trials, the no-choice trials were discarded and 50% was used as the low asymptote. For both species, trials were excluded from analysis when the decision was made within 250 ms of stimulus onset because such decisions were likely premature guesses given the gradual onset of the stimuli.
RESULTS
Contrast thresholds of cats and humans
Typical examples of data obtained with the staircase procedure are illustrated in Fig. 3. Stimulus contrast usually approached and stabilized near threshold in 20 to 50 trials for both species. The average number of trials for a given combination of spatial frequency and adaptation level was 189 for cats (excluding catch trials) and 104 for humans. As mentioned in methods, the staircase procedure efficiently clustered a large number of trials around threshold, giving more weight to data points near the most critical part of the psychometric functions (see the histograms in the top panels of Fig. 3, B and D).
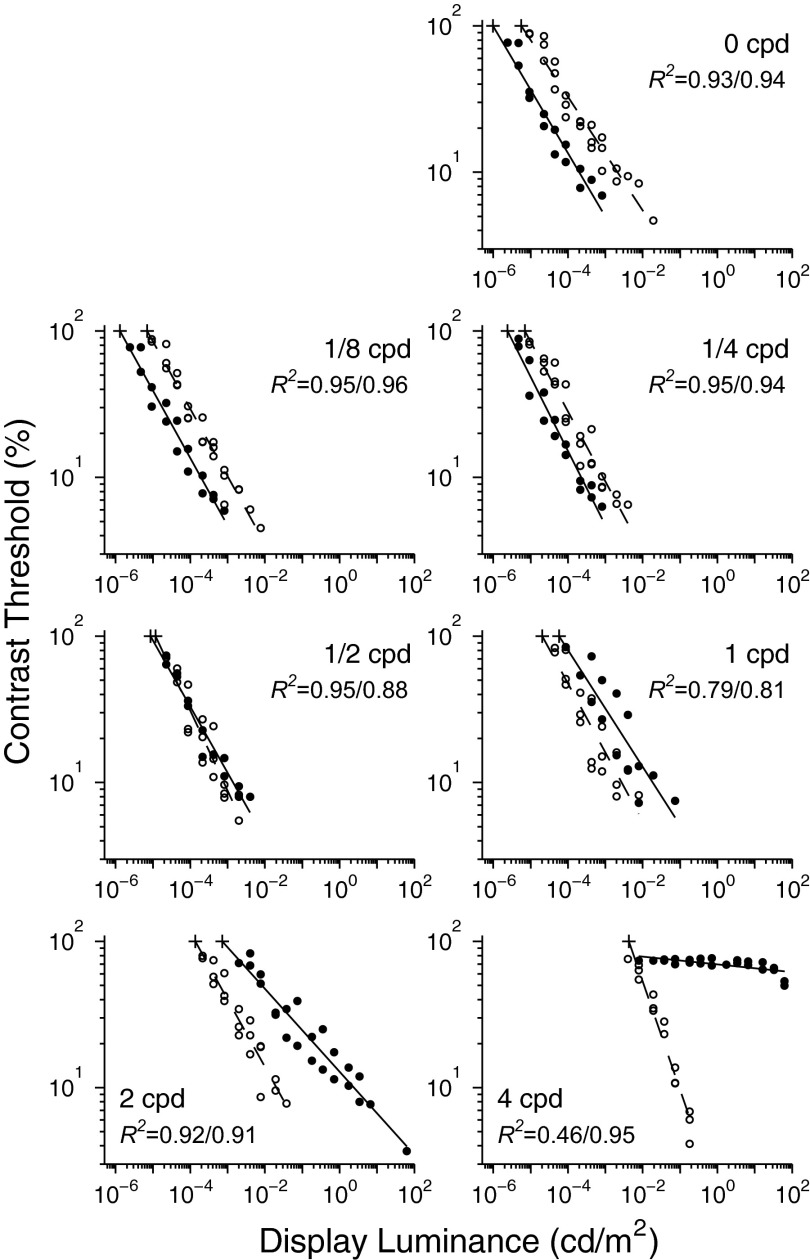
A total of 121 and 144 contrast thresholds were estimated for cats and humans, respectively, from data obtained in about 150 h of testing for each cat and 50 h for each human. Generally, contrast thresholds decreased linearly with display luminance on a log-log scale, although the regression for cats at 4 cpd is virtually flat (Fig. 4). Cats were more sensitive for low spatial frequencies and humans for high, with the crossover point at about 1/2 cpd. Both species were less sensitive for high spatial frequencies (i.e., the curves shift to the right as spatial frequency increases). For most spatial frequencies, linear fits were excellent, explaining >90% of the variance (see R2 values in each panel of Fig. 4). The relatively lower R2 values at 1 cpd are the result of systematic differences in sensitivity between individual subjects: at 1 cpd R2 for individual cats and humans ranged from 0.940 to 0.995. The greatest divergence from the general pattern was for cats at 4 cpd, where contrast threshold was essentially constant at about 73% from adaptation levels of 6.6 to 0.008 cd/m2, below which the animals abruptly failed to perform above chance level. For these data, a linear regression produced a relatively poor fit, primarily because threshold decreased abruptly with display luminance ≳10 cd/m2. We suspect this sudden increase in sensitivity marks the upper end of the mesopic range (see also Kang and Malpeli 2009).
FIG. 4.
Contrast threshold as a function of display luminance, plotted separately for each spatial frequency for cats (filled circles) and humans (open circles). Lines are linear regression lines. The 2 R2 values in each panel are for cats and humans, respectively. Absolute luminance thresholds (marked with pluses) are display luminances extrapolated from the regression lines to 100% contrast threshold.
One way of comparing cat and human sensitivity is to estimate absolute luminance thresholds for each spatial frequency, i.e., the adaptation level at which a subject could detect a 100% contrast stimulus half the time (75% correct response equates to 50% detection in this task). These were defined from the linear fits for all spatial frequencies in both species (see Fig. 4, pluses at the top of each regression), with the exception of 4 cpd in cats. For low spatial frequencies, the cats had much lower absolute thresholds: at 0 cpd, 9.72 × 10−7 for cats and 5.59 × 10−6 cd/m2 for humans; at 1/8 cpd, 1.30 × 10−6 for cats and 6.94 × 10−6 cd/m2 for humans. For the lowest two spatial frequencies, the cats could detect Gabor functions of 100% contrast at adaptation levels dimmer, on average, by 0.74 log units than those detectable by humans. Note that absolute luminance thresholds defined for Gabor functions are not equivalent to absolute rod threshold. All human subjects reported that they could perceive the background illumination of the display at an adaptation level at least one log unit below the lowest absolute luminance threshold.
For the highest spatial frequency used, 4 cpd, humans and cats had similar absolute luminance thresholds. The human absolute threshold, determined from the regression analysis, was −2.37 log cd/m2. It is inappropriate to estimate the absolute luminance thresholds of the cats for 4 cpd from the regression analysis because the contrast threshold was virtually constant over a wide range of display luminance and then behavior abruptly dropped to chance at the low end of this range (Fig. 4, last panel). Instead, the cat's absolute luminance threshold was taken as the display luminance one step below the dimmest adaptation level at which a threshold <100% could be determined, −2.40 log cd/m2.
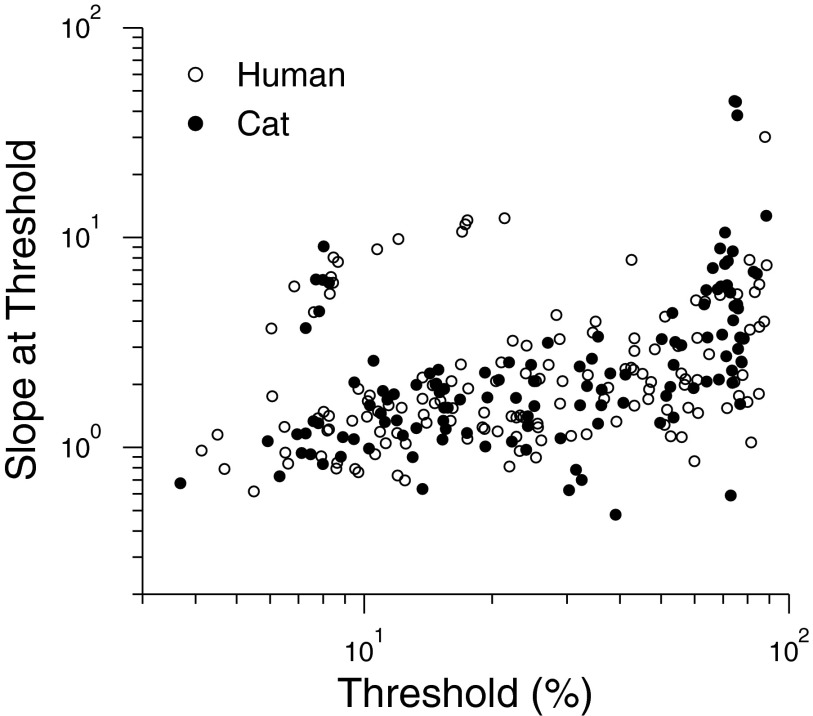
We also examined the slopes of the psychometric functions at the 75% correct performance level and these followed the same pattern for cats and humans, increasing with contrast threshold (Fig. 5). This suggests that the two species have equal discriminability for contrast variations around threshold.
FIG. 5.
Slope of psychometric function as a function of contrast threshold. Each point is the slope of a single psychometric function for a given spatial frequency and adaptation level. Neither slopes nor intercepts of linear regressions fitted to the cat and human data differed significantly (P > 0.05 for t-test applied to both slope and intercept; Zar 1999).
The human and cat paradigms differed mainly in the control of stimulus duration: for humans, nominally set at 1,250 ms; for cats, determined by reaction times. To the extent that threshold decreased with longer presentations, systematic differences in stimulus duration could bias sensitivity in favor of one species and this could confound the comparison of scotopic sensitivity. An analysis of critical duration (i.e., the exposure time beyond which sensitivity no longer improves) suggests that this was not a factor. Savage (1996) examined human critical duration for Gabor functions in the scotopic range. From his data, we estimate critical duration to vary from about 125 ms at the brightest adaptation level to about 300 ms at the dimmest. The cat's critical duration is unknown, but if we assume it is similar to the human's, stimulus duration as estimated from reaction times exceeded critical duration at all adaptation levels for the bulk of trials. As an empirical control, we also reanalyzed the cat contrast-threshold data after eliminating trials with short reaction times to see how much this would lower threshold. This analysis suggests that differences in stimulus presentation times between cats and humans do not significantly influence the comparisons of their contrast sensitivities (Supplemental Material, section H).
Optical versus neural factors
Here we compare contrast thresholds of cats and humans after sequentially factoring out three major optical factors: pupil size, focal length, and tapetum. This analysis is restricted to the lowest three spatial frequencies, 0, 1/8, and 1/4 cpd, where the cat has a sensitivity advantage and contrast sensitivity is probably not limited by the spatial resolution of either species' optics (see discussion).
Figure 6A compares contrast thresholds for each of the three lowest spatial frequencies, replicating a subset of the panels in Fig. 4. The data for the two species fall along lines that are almost, but not quite, parallel. We sought to extract for each panel the horizontal displacement of the data to quantify the cat's advantage in luminance for detecting stimuli. For this, first we calculated the difference in absolute luminance thresholds (i.e., horizontal distance between “+” signs in each panel), which we refer to as the absolute luminance advantage (denoted as logΔabs in each panel). We also calculated the average horizontal distance between the regression lines across contrasts from 10 to 80% (shaded region in Fig. 6A), which is effectively the same as the difference in luminance threshold at the contrast midway between 10 and 80% (i.e., 28.3% on log scale). We will refer to this as the global luminance advantage (denoted as logΔglobal in each panel). The global luminance advantage of the cat was 0.91, 0.73, and 0.53 log units for 0, 1/8, and1/4 cpd, respectively.
We begin the compensation for optical differences between cats and humans by factoring out pupil size, converting display luminance to retinal illuminance (i.e., trolands: display luminance multiplied by pupil area) separately for each adaptation level and subject. This reduces the cat's global luminance advantage—for example, from 0.73 to 0.41 log units for 1/8 cpd (Fig. 6B, middle). Next we factor out the contribution of the tapetum. Tapetal reflection increases the light absorbed by photoreceptors by about 29% (see methods and Fig. S3, Supplemental Material, section C), which improves the cat's sensitivity by 0.11 log units, shifting the cat's regression lines in Fig. 6B rightward (toward higher adaptation levels) by 0.11 log units. This reduces the cat's global luminance advantage to 0.30 log units for 1/8 cpd (Fig. 6C). Finally we factor out focal length. The intensity of light incident on a unit retinal area is inversely proportional to the square of the focal length of the eye. Taking 12.50 mm as the posterior nodal distance for the cat's eye (Vakkur and Bishop 1963) and 16.68 mm for the human eye (LeGrand 1946; as described in Wyszecki and Stiles 1982), the cat's focal length is shorter by a factor of about 0.75 than that of the human's, which accounts for about 0.25 log units of the cat's sensitivity advantage. When this is taken into account by shifting the cat regression lines rightward by an additional 0.25 log units, both species have roughly the same sensitivity (Fig. 6D). Thus it appears that the superior scotopic contrast sensitivity of the cat can be accounted for mostly by differences in optical elements and that the intrinsic neural sensitivities of the two species are similar at 8° retinal eccentricity. However, the match is not perfect: although the regressions are virtually superimposed for 1/8 cpd, the global luminance advantage of the cat is 0.22 log units at 0 cpd, whereas at 1/4 cpd the absolute and global luminance advantages of the human are 0.20 and 0.14 log units, respectively.
Increment threshold as a function of mean retinal illuminance in scotopic conditions
For sinusoidal gratings or Gabor functions, retinal increment threshold is contrast threshold multiplied by mean retinal illuminance, expressed in trolands. When plotted for each spatial frequency as functions of retinal illuminance on a log-log scale, increment thresholds were mostly well fit by linear regressions (Fig. 7). Overall, the cat data are somewhat tighter: R2 values of linear regressions averaged across individual subjects and spatial frequencies were 0.98 for cats and 0.92 for humans.
FIG. 7.
Increment threshold as a function of retinal illuminance for individual cat (filled circles) and human (open circles) subjects for each spatial frequency tested. The fitted lines are linear regressions. For clarity, the scales for human and cat data are offset horizontally and vertically. In addition, with the exception of the last panel, data for individual subjects are offset vertically (raised in 0.5 log unit steps; the subjects with the lowest increment thresholds are plotted veridically).
For humans, increment thresholds were mostly proportional to the square root of retinal illuminance (i.e., the regression slopes are close to 0.5), in accordance with the deVries–Rose law (Figs. 7 and 8, open circles). The exception is 4 cpd, where the slopes were well below 0.5 (Fig. 8, open circles). The considerable variation in slope for humans at 4 cpd in Fig. 8 is misleading. When the increment-threshold data are plotted against retinal illuminance and viewed on the same scale, it can be seen that the three subjects' increment-threshold functions are not actually disparate, but fall on roughly the same curve (Fig. 7, last panel).
For cats, increment thresholds followed the deVries–Rose law for spatial frequencies ≤1 cpd (Figs. 7 and 8, filled circles). The mean slope of the linear regressions increased to 0.70 at 2 cpd and 0.97 at 4 cpd. A slope of 1 is in keeping with Weber's law, which predicts that increment thresholds are proportional to retinal illuminance. Similar shifts from the deVries–Rose law to Weber's law with increasing illuminance have been thoroughly documented for the human (see Walraven et al. 1990 for a summary). Although the data in Fig. 8 are presented as functions of spatial frequency, the cat's transition from deVries–Rose to Weber behavior could as readily be explained by retinal luminance, since the cats detected only 4 cpd stimuli at illuminances that are probably at and above cone threshold.
DISCUSSION
Sensitivity and acuity of cats and humans in dim-light conditions
When tested with Gabor functions, the similarities between cat and human scotopic vision are more striking than the differences. Our results are consistent with the view that overall sensitivity is limited mainly by up-front elements, i.e., optics and receptors, and that neural circuits involved in postreceptor processing are fundamentally alike in the two species.
For the lowest spatial frequencies, cats see at adaptation levels considerably dimmer than humans for all contrasts. However, the cat's dim-light advantage arises mainly from superior light-concentrating capabilities of its eye. One might think this counterintuitive because, for the relevant eccentricity, the cat has many more rods. We estimated the rod density of the cat retina at 8° eccentricity to be nearly 445,000/mm2 from Fig. 13B of Steinberg et al. (1973). The measurements by Østerberg (1935) as plotted in Fig. 13B of Steinberg et al. (1973) and those of Curcio et al. (1990) yield estimates of roughly 120,000/mm2 for the human rod density at this eccentricity. Thus the cat's rod density is about 3.7-fold that of the human's at 8° eccentricity. However, photon capture depends on photopigment density, not on rod density. For both species, outer segments are closely packed in paracentral retina (Curcio et al. 1990; Hendrickson and Drucker 1992; Steinberg et al. 1973) and the bulk are from rods. Thus to the extent that cat and human rod outer segments are of similar length (an issue unaddressed by the literature), they have roughly the same total volume. In short, there is at present no evidence to suggest dramatic differences in rhodopsin density beyond the most central visual fields. Although there are other factors that potentially influence sensitivity, such as synaptic noise, this analysis suggests that at the limits of useful light levels, efficiency of photon capture is the dominant factor—i.e., once photons are captured, subsequent stages of retinal processing are comparably efficient in cats and humans.
The horizontal shifts between contrast-threshold functions in Fig. 4 illustrate relative performance in terms of the lowest luminance for which a given stimulus is perceived. By this criterion, the cat's scotopic vision is superior for spatial frequencies <1/2 cpd and the human is better at spatial frequencies above this. In the scotopic range, cat and human functions are fairly parallel, so the relative advantages are functions of spatial frequency, not stimulus contrast.
Another similarity between cats and humans is that both failed to resolve 4 cpd at the same display luminance: −2.40 and −2.37 log cd/m2 for cats and humans, respectively. The average absolute thresholds in terms of retinal illuminance were −0.37 log td for cat and −0.67 log td for human subjects, values not inconsistent with estimates of the upper limit of the scotopic range of the two species. Estimates of the cat cone threshold vary widely. From among the published studies in which sufficient information was provided to determine cone threshold in equivalent units (log photopic td), we extracted the following values: −0.5 (Barlow and Levick 1968), −0.35 (Enroth-Cugell et al. 1977), and 1.0 (Daw and Pearlman 1969). Estimates of the human cone threshold are in better agreement, converging at about −0.6 log td for foveal vision (for a summary, see Makous 2004). Thus it appears that the acuity limit of rod vision for both species in these conditions is roughly 4 cpd. Of course, we cannot necessarily generalize this conclusion to other conditions, such as temporal frequencies other than 4 Hz or other retinal eccentricities. Nor do we know whether this similarity is the result of some fundamental property common to both species (such as similar Nyquist limits for rod pathways) or the interplay of a variety of factors that incidentally converged at the conditions of our study.
Although the major differences in sensitivity between cats and humans at low spatial frequencies can be accounted for by the cat's brighter optics, our data suggest other optical and/or neural contributions. After accounting for retinal illuminance, modest differences in contrast sensitivity remain at 0 and 1/4 cpd (Fig. 6D). For 0 cpd, cats show an advantage of 0.22 log units (averaged across contrasts) that decreases with adaptation level and virtually disappears at absolute luminance threshold, which might result from central signal averaging (Kang and Malpeli 2009). For 1/4 cpd, the human is slightly more sensitive by a fixed amount across adaptation levels. This might reflect a slightly higher resolution of the human at this spatial frequency, whether optical or neural. For spatial frequencies >1/4 cpd, optical degradation of contrast becomes much more of a limitation for the cat than for the human (e.g., compare Robson and Enroth-Cugell 1978 with Campbell and Gubisch 1966).
deVries–Rose law and scotopic sensitivity
Our human subjects followed the deVries–Rose law throughout the scotopic range for parafoveal Gabor functions. Retinal illuminances high enough to have produced Weber scaling were not explored. The human data contain one anomaly: at 4 cpd, increment-threshold slopes dropped to <0.5 at the highest luminance levels tested, in the upper mesopic range. A possible explanation is a reduction in contrast sensitivity at these illuminances as a result of rod–cone cancellation, a threshold elevation at critical temporal frequencies caused by phase lags in rod signals (Stockman and Sharpe 2006).
For cats, increment thresholds <2 cpd (scotopic conditions) followed the deVries–Rose law, for 4 cpd (mesopic) increment thresholds followed Weber's law, and for 2 cpd (upper scotopic and lower mesopic) they were intermediate between deVries–Rose and Weber behavior. At any display luminance, retinal illuminance was higher for the cat, which is probably why Weber scaling was observed for the cat only. The human and cat increment-threshold functions have very similar slopes over the scotopic range, suggesting that retinal sensitivity is regulated in the same way in both species in dim-light conditions and that physiological data obtained from cats are directly relevant to humans, at least for rod vision.
Previous psychophysical studies
In general, estimates of human contrast threshold for shared adaptation levels and spatial frequencies are in reasonable agreement with one another and with the present study (e.g., Daitch and Green 1969; Hess et al. 1987; Koenderink et al. 1978; Rovamo et al. 1994; Smith Jr 1973; van Meeteren and Vos 1972; van Nes and Bouman 1967). Estimates of spatial acuity of the cat's central vision range from 3.5 to 9 cpd (Blake et al. 1974; Jacobson et al. 1976; Harris 1978), with acuity decreasing with eccentricity (Blake and Bellhorn 1978; Pasternak and Horn 1991). The only prior study on cat contrast sensitivity spanning most of the scotopic range is that of Pasternak and Merigan (1981). Their results are qualitatively similar to ours, but they reported somewhat lower acuity limits (<3 cpd for free viewing) and their data translate into increment-threshold slopes much above those consistent with deVries–Rose scaling (Supplemental Material, section I). There were numerous differences in the way the two studies were conducted that might contribute to these discrepancies. For example, the highest stimulus contrast they used was below the contrast threshold for 4 cpd estimated in our study, so their cats could not have demonstrated sensitivity to this spatial frequency in any event. On the other hand, their data are well behaved and do not show evidence of truncation of the sort expected by using insufficiently high contrasts. Another possibility is the nature of the behavioral tasks: the nose-press task they used may have been more demanding than the eye-movement task of the current study, resulting in different levels of performance. 2 deVries–Rose behavior may also be dependent on details of the stimulus paradigm. Pasternak and Merigan (1981) used large extended stationary gratings with free viewing and it is not clear whether these conditions would have been conducive to deVries–Rose behavior (see Shapley and Enroth-Cugell 1984 for a review of much of the relevant literature). Finally, their adaptation levels did not extend as low as ours and measurements were made at larger steps (2 log units) of adaptation level (Supplemental Material, Fig. S5), so deVries–Rose behavior might have been obscured by a steeper increase in increment threshold at mesopic and photopic levels. Whatever the reasons for these different outcomes, we are satisfied that the current study adequately describes the cat's behavioral sensitivity under the conditions used because, as demonstrated in the companion paper (Kang and Malpeli 2009), neural sensitivity measures obtained from awake cats using the same display apparatus closely parallel our behavioral data.
GRANTS
This work was supported by U.S. Department of Health and Human Services Public Health Grant PHS 5 P41-RR-003155 and National Eye Institute Grant EY-02695.
Supplementary Material
Acknowledgments
We thank W. Busen for programming and reading of the manuscript, E. Dzhafarov for advice on statistical approaches, personnel at the Laboratory for Fluorescence Dynamics at the University of Illinois for measuring the spectral characteristics of our display projector, and the Beckman Imaging Technology Group for use of the digital camera to measure image contrast.
Present addresses: I. Kang, Department of Neurobiology, Harvard Medical School, 220 Longwood Ave., Boston, MA 02115; R. Reem, Department of Ophthalmology and Visual Sciences, Case Western Reserve University, 2085 Adelbert Rd., Rm 303, Cleveland, OH 44106; A. Kaczmarowski, Medical College of Wisconsin, 8701 Watertown Plank Road, Milwaukee, WI 53226.
Footnotes
The online version of this article contains supplemental data.
Pasternak and Horn (1991) reported monocular acuity <4 cpd over a range of retinal eccentricities for cats tested in brighter conditions as well (high mesopic). The same comments apply: the highest contrast used was below contrast threshold for 4 cpd and the task was even more demanding than that used by Pasternak and Merigan (1981).
REFERENCES
- Barlow and Levick 1968.Barlow HB, Levick WR. The Purkinje shift in the cat retina. J Physiol 198: 2P–3P, 1968. [Google Scholar]
- Blake and Bellhorn 1978.Blake R, Bellhorn RW. Visual acuity in cats with central retinal lesions. Vision Res 18: 15–18, 1978. [DOI] [PubMed] [Google Scholar]
- Blake and Camisa 1977.Blake R, Camisa JM. Temporal aspects of spatial vision in the cat. Exp Brain Res 28: 325–333, 1977. [DOI] [PubMed] [Google Scholar]
- Blake et al. 1974.Blake R, Cool SJ, Crawford LJ. Visual resolution in the cat. Vision Res 14: 1211–1217, 1974. [DOI] [PubMed] [Google Scholar]
- Campbell and Gubisch 1966.Campbell FW, Gubisch RW. Optical quality of the human eye. J Physiol 186: 558–578, 1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornsweet 1962.Cornsweet TN The staircase method in psychophysics. Am J Psychol 75: 485–491, 1962. [PubMed] [Google Scholar]
- Cui and Malpeli 2003.Cui H, Malpeli JG. Activity in the parabigeminal nucleus during eye movements directed at moving and stationary targets. J Neurophysiol 89: 3128–3142, 2003. [DOI] [PubMed] [Google Scholar]
- Curcio et al. 1990.Curcio CA, Sloan KR, Kalina RE, Hendrickson AE. Human photoreceptor topography. J Comp Neurol 292: 497–523, 1990. [DOI] [PubMed] [Google Scholar]
- Daitch and Green 1969.Daitch JM, Green DG. Contrast sensitivity of the human peripheral retina. Vision Res 9: 947–952, 1969. [DOI] [PubMed] [Google Scholar]
- Daw and Pearlman 1969.Daw NW, Pearlman AL. Cat colour vision: one cone process or several? J Physiol 201: 745–764, 1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Efron and Tibshirani 1993.Efron B, Tibshirani RJ. An Introduction to the Bootstrap. New York: Chapman & Hall, 1993.
- Enroth-Cugell et al. 1977.Enroth-Cugell C, Hertz BG, Lennie P. Cone signals in the cat's retina. J Physiol 269: 273–296, 1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris 1978.Harris LR Contrast sensitivity and acuity of a conscious cat measured by the occipital evoked potential. Vision Res 18: 175–178, 1978. [DOI] [PubMed] [Google Scholar]
- Hendrickson and Drucker 1992.Hendrickson A, Drucker D. The development of parafoveal and mid-peripheral human retina. Behav Brain Res 49: 21–31, 1992. [DOI] [PubMed] [Google Scholar]
- Hess et al. 1987.Hess RF, Nordby K, Pointer JS. Regional variation of contrast sensitivity across the retina of the achromat: sensitivity of human rod vision. J Physiol 388: 101–119, 1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson et al. 1976.Jacobson SG, Franklin KBJ, McDonald WI. Visual acuity of the cat. Vision Res 16: 1141–1143, 1976. [DOI] [PubMed] [Google Scholar]
- Kang and Malpeli 1978.Kang I, Malpeli JG. Dim-light sensitivity of cells in the awake cat's lateral geniculate and medial interlaminar nuclei: a correlation with behavior. J Neurophysiol 102: 841–852, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenderink et al. 1978.Koenderink JJ, Bouman MA, Bueno de Mesquita AE, Slappendel S. Perimetry of contrast detection thresholds of moving spatial sine wave patterns. IV. The influence of the mean retinal illuminance. J Opt Soc Am 68: 860–865, 1978. [DOI] [PubMed] [Google Scholar]
- LeGrand 1946.LeGrand Y Optique Physiologique. La dioptrique de l'oeil et sa correction. Paris: Éditions de la Revue d'Optique, 1946, vol. I.
- Levitt 1971.Levitt H Transformed up-down methods in psychoacoustics. J Acoust Soc Am 49: 467–477, 1971. [PubMed] [Google Scholar]
- Makous 2004.Makous W Scotopic vision. In: The Visual Neurosciences, edited by Chalupa LM, Werner JS. Cambridge, MA: The MIT Press, 2004, vol. 1, p. 838–850.
- Østerberg 1935.Østerberg GA Topography of the layer of rods and cones in the human retina. Acta Ophthalmol 13, Suppl. 6: 1–97, 1935.
- Pasternak and Horn 1991.Pasternak T, Horn K. Spatial vision of the cat: variation with eccentricity. Vis Neurosci 6: 151–158, 1991. [DOI] [PubMed] [Google Scholar]
- Pasternak and Merigan 1981.Pasternak T, Merigan WH. The luminance dependence of spatial vision in the cat. Vision Res 21: 1333–1339, 1981. [DOI] [PubMed] [Google Scholar]
- Robson and Enroth-Cugell 1978.Robson JG, Enroth-Cugell C. Light distribution in the cat's retinal image. Vision Res 18: 159–173, 1978. [DOI] [PubMed] [Google Scholar]
- Rose 1948.Rose A Sensitivity performance of the human eye on an absolute scale. J Opt Soc Am 38: 196–208, 1948. [DOI] [PubMed] [Google Scholar]
- Rovamo et al. 1994.Rovamo J, Mustonen J, Näsänen R. Modeling contrast sensitivity as a function of retinal illuminance and grating area. Vision Res 34: 1301–1314, 1994. [DOI] [PubMed] [Google Scholar]
- Savage 1996.Savage GL Temporal summation for grating patches detected at low light levels. Optom Vis Sci 73: 404–412, 1996. [DOI] [PubMed] [Google Scholar]
- Shapley and Enroth-Cugell 1984.Shapley R, Enroth-Cugell C. Visual adaptation and retinal gain controls. In: Progress in Retinal Research, edited by Osborne N, Chader G. London: Pergamon Press, 1984, vol. 3, p. 263–346.
- Smith 1973.Smith RA Luminance-dependent changes in mesopic visual contrast sensitivity. J Physiol 230: 115–135, 1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg et al. 1973.Steinberg RH, Reid M, Lacy PL. The distribution of rods and cones in the retina of the cat (Felis domesticus). J Comp Neurol 148: 229–248, 1973. [DOI] [PubMed] [Google Scholar]
- Stockman and Sharpe 2006.Stockman A, Sharpe LT. Into the twilight zone: the complexities of mesopic vision and luminous efficiency. Ophthalmic Physiol Opt 26: 225–239, 2006. [DOI] [PubMed] [Google Scholar]
- Troy 1983.Troy JB Spatio-temporal interaction in neurones of the cat's dorsal lateral geniculate nucleus. J Physiol 344: 419–432, 1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vakkur and Bishop 1963.Vakkur GJ, Bishop PO. The schematic eye in the cat. Vision Res 3: 357–381, 1963. [DOI] [PubMed] [Google Scholar]
- van Meeteren and Vos 1972.van Meeteren A, Vos JJ. Resolution and contrast sensitivity at low luminances. Vision Res 12: 825–833, 1972. [DOI] [PubMed] [Google Scholar]
- van Nes and Bouman 1967.van Nes FL, Bouman MA. Spatial modulation transfer in the human eye. J Opt Soc Am 57: 401–406, 1967. [DOI] [PubMed] [Google Scholar]
- Walraven et al. 1990.Walraven J, Enroth-Cugell C, Schnapf JL, MacLeod DIA, Hood DC. The control of visual sensitivity. In: Visual Perception: The Neurophysiological Foundations, edited by Spillman L, Werner JS. San Diego, CA: Academic Press, 1990, p. 53–101.
- Weale 1953.Weale RA The spectral reflectivity of the cat's tapetum measured in situ. J Physiol 119: 30–42, 1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wichmann and Hill 2001.Wichmann FA, Hill NJ. The psychometric function: I. Fitting, sampling, and goodness of fit. Percept Psychophys 63: 1293–1313, 2001. [DOI] [PubMed] [Google Scholar]
- Wyszecki and Stiles 1982.Wyszecki G, Stiles WS. Color Science: Concepts and Methods, Quantitative Data and Formulae (2nd ed.). New York: Wiley, 1982.
- Zar 1999.Zar JH Biostatistical Analysis (4th ed.). Upper Saddle River, NJ: Prentice Hall, 1999.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.