Abstract
Slow finger movements in man are not smooth, but are characterized by 8- to 12-Hz discontinuities in finger acceleration thought to have a central source. We trained two macaque monkeys to track a moving target by performing index finger flexion/extension movements and recorded local field potentials (LFPs) and spike activity from the primary motor cortex (M1); some cells were identified as pyramidal tract neurons by antidromic activation or as corticomotoneuronal cells by spike-triggered averaging. There was significant coherence between finger acceleration in the approximately 10-Hz range and both LFPs and spikes. LFP–acceleration coherence was similar for flexion and extension movements (0.094 at 9.8 Hz and 0.11 at 6.8 Hz, respectively), but substantially smaller during steady holding (0.0067 at 9.35 Hz). The coherence phase showed a significant linear relationship with frequency over the 6- to 13-Hz range, as expected for a constant conduction delay, but the slope indicated that LFP lagged acceleration by 18 ± 14 or 36 ± 8 ms for flexion and extension movements, respectively. Directed coherence analysis supported the conclusion that the dominant interaction was in the acceleration to LFP (i.e., sensory) direction. The phase relationships between finger acceleration and both LFPs and spikes shifted by about π radians in flexion compared with extension trials. However, for a given trial type the phase relationship with acceleration was similar for cells that increased their firing during flexion or during extension trials. We conclude that movement discontinuities during slow finger movements arise from a reciprocally coupled network, which includes M1 and the periphery.
INTRODUCTION
Slow voluntary finger movements are characterized by approximately 8- to 12-Hz discontinuities in finger acceleration (Vallbo and Wessberg 1993). These discontinuities are unlikely to be a purely mechanical phenomenon because their frequency remains constant even when moving against different constant torque loads (Vallbo and Wessberg 1993). Extensor and flexor muscles modulate their activity in a pulsatile fashion, locked to the discontinuities in acceleration (Vallbo and Wessberg 1993), and single motor units exhibit a synchronized 8- to 12-Hz modulation (Kakuda et al. 1999), implying a neural basis for the discontinuities. Spinal stretch reflexes have been suggested to contribute to physiological tremor in this frequency range (Elble and Koller 1990; Lippold 1970; Vallbo and Wessberg 1993), but detailed analysis of the amplitude and phase of single muscle spindle afferent firing relative to the discontinuities seen in slow finger movements rules out oscillations in the stretch reflex arc as a major source (Wessberg and Vallbo 1995, 1996).
By a process of elimination, the most likely cause of these discontinuities is thus a neural oscillator within the CNS. In agreement with this, similar discontinuities can be seen during smooth-pursuit eye movements (McAuley et al. 1999a). The discontinuities synchronize between eye and hand during eye–hand tracking tasks (McAuley et al. 1999a) and in some circumstances between the two hands (Evans and Baker 2003; McAuley et al. 1999a). Only a central oscillator could explain the coupling between such anatomically distant structures.
An obvious candidate for this central oscillator is the primary motor cortex (M1), since in primates it has monosynaptic connections to motoneurons via the corticospinal tract (Porter and Lemon 1993). Oscillations generated in M1 could thus directly influence motor outflow. However, several studies in humans using noninvasive recording methods have failed to find cortical activity coherent with peripheral discontinuities (Marsden et al. 2001; Williams et al. 2005). Raethjen et al. (2002) used electrocorticogram recordings of M1 activity and found coherence with physiological tremor. Using a novel analytical method (dynamic imaging of coherent sources, or DICS; see Gross et al. 2001) applied to magnetoencephalographic (MEG) recordings, one study reported close to 7-Hz corticomuscular coherence between right extensor digitorum and the sensorimotor cortex (Gross et al. 2002). Other regions also appeared to be involved; the authors concluded that a complex circuit involving the primary motor, premotor and somatosensory cortices, thalamus, and cerebellum were responsible for generating discontinuities around 10 Hz.
Thus far, this system has not been investigated using invasive recordings of neural activity in an animal model, which would allow for better spatial resolution. To provide more detailed information on the role of M1, we recorded local field potential (LFP) and single-unit activity directly from M1 in monkeys trained to produce slow ramp movements with the index finger. Motor cortical cells were synchronized with the peripheral discontinuities. However, from the temporal relationship between cells and the peripheral acceleration it appeared unlikely that this activity was generated purely in M1 and then simply passed to the periphery over fast motor pathways. Rather, M1 is likely to form part of an interconnected network (including sensory feedback from the periphery), which generates these oscillations as an emergent phenomenon.
METHODS
Behavioral task
Two female Macaca mulatta monkeys (weight ∼8 and 5.2 kg) were trained to perform a finger flexion/extension task for food reward. The index finger of one hand was inserted into a narrow tube, which restricted movement to the metacarpophalangeal (MCP) joint. Each tube was custom made to fit the monkey's index finger well, minimizing movement around the more distal finger joints. The tube was attached to a lever that rotated coaxially with the MCP joint; a motor exerted torque in a direction to oppose flexion. Lever angular displacement was sensed by an optical encoder and fed back to the animal via a cursor on a computer screen. A displacement of 0° indicated the neutral position, where the finger was in the same plane as the palm. Positive angles denoted finger flexion. During each trial the palm and digits 1, 3, 4, and 5 lay horizontally against a flat surface and the elbow and upper arm were held in a sleeve. The contralateral arm was unrestrained throughout the task.
A trial commenced when a rectangular target appeared at either 12 or 24° displacement. The monkey moved the cursor into this target and held for 1 s. The target then moved over a linear ramp lasting 1 s, ending at a displacement of 24 or 12°. The trial ended after a further 1-s hold period. Maintenance of the cursor within the target (allowed error ±1.4°) for the entire 3-s-long sequence led to a food reward. We refer to “flexion” or “extension” trials, denoting 12 → 24° and 24 → 12° movement sequences, respectively. An accelerometer (Isotron 25B, Endevco, San Juan Capistrano, CA) attached to the lever measured physiological tremor during the hold phases and movement discontinuities during the target ramp (band-pass, 1–100 Hz).
Surgical preparation
Following training, the animal was implanted under general anesthesia (3.0–5.0% sevoflurane inhalation, intravenous infusion of 0.025 mg·kg−1·h−1 alfentinil) and aseptic conditions with electromyogram (EMG) patch electrodes over 9–10 forearm and hand muscles (flexor digitorum superficialis [FDS], flexor digitorum profundus [FDP], flexor carpi ulnaris [FCU], flexor carpi radialis [FCR], abductor pollicis brevis [AbPB], extensor carpi ulnaris [ECU], extensor digitorum communis [EDC], abductor pollicis longus [AbPL], first dorsal interosseous [1DI], extensor carpi radialis [ECR]). After an appropriate recovery period, a further surgery implanted a stainless steel headpiece for head fixation and a recording chamber over the primary motor cortex (M1) contralateral to the trained arm. Two insulated tungsten stimulating electrodes (LF501G, Microprobe, Potomac, MD) were chronically implanted into the pyramidal tract (PT) for antidromic identification of pyramidal tract neurons (PTNs; see Baker et al. 1999; Lemon 1984). All procedures were carried out under the authority of licenses issued by the UK Home Office under the Animals (Scientific Procedures) Act (1986) and were approved by the local ethical review panel of Newcastle University.
Recordings
An Eckhorn microdrive (Thomas Recording, Giessen, Germany) was used to make up to ten simultaneous microelectrode penetrations into M1 during daily recording sessions. Electrodes were platinum insulated with quartz glass and had a shaft diameter of 80 μm and impedance of 1–2 MΩ (Thomas Recording). Cells that responded at constant latency to PT stimulation through the chronically implanted electrodes (maximum stimulus intensity: 400-μA, 0.2-ms pulse, 1 Hz) and that passed a collision test were identified as PTNs. Cells that could not be so activated were classified as unidentified neurons (UIDs). Single-unit activity (band-pass, 300 Hz to 10 kHz) and LFPs (band-pass, 1–100 Hz) were then recorded while the animal performed the task, together with acceleration, lever displacement, and EMG activity (band-pass, 30 Hz to 2 kHz).
Off-line, spike waveforms were discriminated into the occurrence times of well-isolated single units using custom-written cluster-cutting software (Getspike, SN Baker). Only single units with a consistent spike waveform and no interspike intervals <1 ms were used in subsequent analysis.
Analysis
Analysis was carried out in the MATLAB environment (The MathsWorks, Natick, MA). Power and coherence spectra were calculated using a sampling rate of 500 Hz for all signals, which was the rate used to digitize LFP and finger acceleration. Spike trains were converted to a waveform sampled at 500 Hz by counting the number of spikes in 2-ms bins (Baker et al. 2003). EMGs, which were originally sampled at 5 kHz, were full-wave rectified, digitally low-pass filtered (250 Hz), and then downsampled to 500 Hz (“resample” function in MATLAB). Analysis used nonoverlapping 512 sample-point (1.024 s) sections, providing a frequency resolution of 0.98 Hz. Coherence and its significance limits were calculated using formulae given in full in Baker et al. (2006) (in these formulae, X denoted the acceleration and Y either the single-unit spikes or LFP). Multiple coherence estimates were combined by calculating average coherence, with significance limits found as described in Evans and Baker (2003).
Coherence phase was calculated for bins with coherence significantly different from zero as the argument of the cross-spectrum and 95% confidence limits were calculated as follows (Rosenberg et al. 1989)
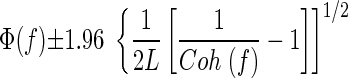 |
(1) |
where Coh (f) is the coherence at the frequency of interest and L is the number of disjoint sections used in the calculation (see Baker et al. 2006). Multiple estimates of the phase spectra were combined by taking the circular mean of the phases at each frequency, for spectra that had coherence significantly different from zero at that frequency. The 95% confidence limits for the average phase were calculated as
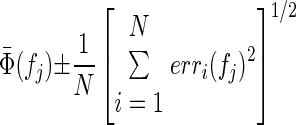 |
(2) |
where N is the number of phase spectra averaged and erri is the 95% confidence limit for phase spectrum i at frequency fj. Delays were estimated from the slope of a straight line fitted to the phase spectra by linear regression (“regress” function in MATLAB); 95% confidence limits on the estimate of slope generated by the regression algorithm provided confidence limits on the delays.
To obtain further insight into the direction of coupling between cortical recordings and acceleration we calculated directed coherence, following methods outlined in detail in Baker et al. (2006). For this analysis, both LFP and acceleration signals were downsampled to 100 Hz; the frequency resolution of directed coherence spectra was 2.94 Hz. An autoregressive (AR) model was fitted to data from each flexion period. The averaged AR models were then used to calculate the directed coherence. The directed coherence was normalized according to
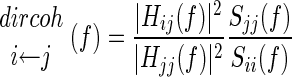 |
(3) |
where Sii and Sjj represent the power spectra and Hij and Hjj represent the transfer functions giving the influence of signal j on i and j on j, respectively (see Baker et al. 2006).
Significance limits were estimated by numerical Monte Carlo simulation. Two signals were generated as independent Gaussian random vectors with the same number of trials as the original data and the directed coherence was calculated. This was repeated 50 times with different random numbers. Directed coherence estimates at all frequencies and for all simulations were rank ordered and the 95th percentile was used as an approximate P < 0.05 significance level.
Power spectra were normalized as described in Witham and Baker (2007). The time dependence of power spectra was assessed using the wavelet method described in detail in Baker and Baker (2003).
All measures were first computed using data from each animal separately; in most cases results were very similar and data were therefore combined between animals for presentation in this study.
Corticomotoneuronal cells
The discharges of all antidromically identified PTNs were used to compile spike-triggered averages (STAs) of rectified EMG from all available muscle recordings, in a window of ±100 ms around the triggering spike. The baseline was estimated by convolving the STA with a Gaussian kernel (width parameter, 50 ms). Where an average revealed a postspike facilitation (PSF) that rose above a baseline region plus 2SDs, the peak width at half-maximum (PWHM) of the PSF was measured. PSFs with PWHM <7 ms were assumed to result from monosynaptic connections between the triggering cell and motoneurons innervating the recorded muscle (Baker and Lemon 1998). Such PTNs were thus further classified as corticomotoneuronal (CM) cells.
RESULTS
Discontinuities in slow finger movements in monkey
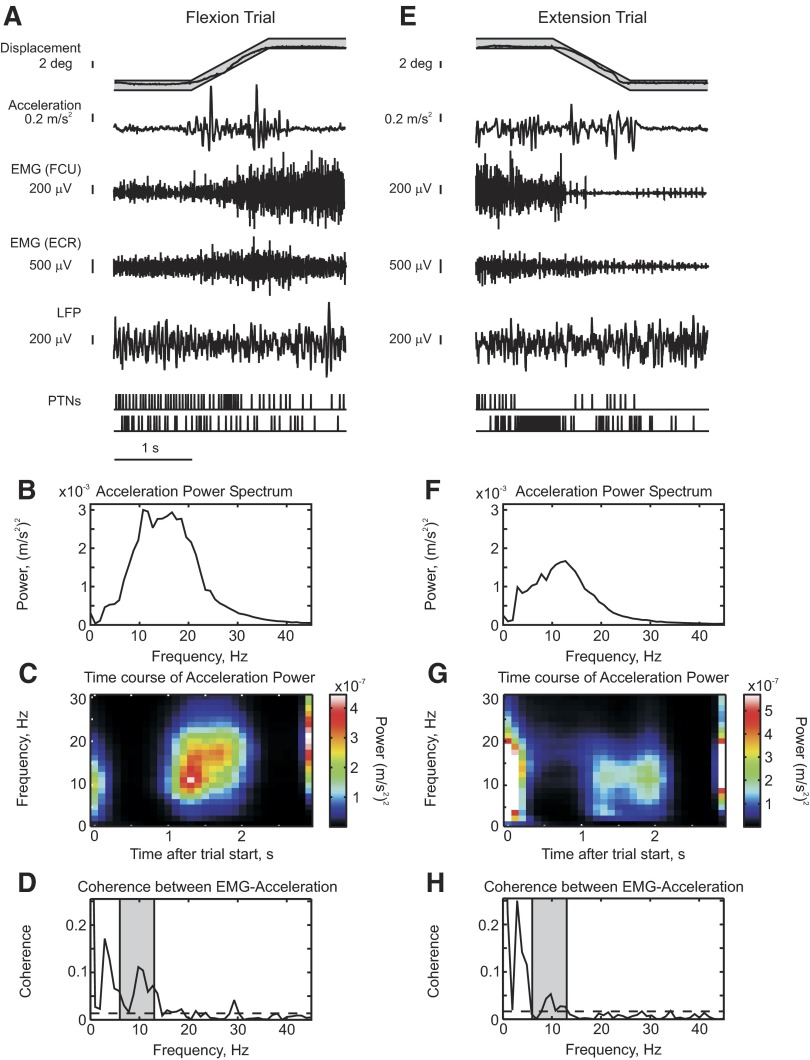
Figure 1 presents data on the phenomenon of discontinuities during slow finger movements in monkey, which hitherto have been investigated only in man. Figure 1A shows raw data from a single flexion trial of the task; Fig. 1E shows raw data from a single extension trial in the same session. After training, monkeys were able to complete this demanding tracking task, performing around 340 (range, 167–618) successful trials within one recording session. However, the task remained challenging, as shown by the fact that on about 60% of trials the finger displacement strayed outside the allowed target error window, resulting in termination of the trial. Only trials that were successfully completed are used in the analysis presented herein. During the ramp tracking phase of the task there were clear oscillations in the finger acceleration. The EMG from a finger flexor muscle modulated with task performance, as did two simultaneously recorded M1 PTNs.
FIG. 1.
Raw data showing discontinuities during slow finger movements in monkey. A: examples of raw data during a single trial of the behavioral task. Actual finger displacement (black) is shown against the allowed target window (gray). EMG is shown from FCU and ECR muscles. B: acceleration power during flexion ramp phase of the task. C: time course of acceleration power during performance of flexion task. D: coherence between EMG from FCU muscle and acceleration. B–D were calculated using all available successful flexion trials of the task from one day's recording session. E–H are the same as A–D but for extension ramps. H: coherence between EMG from ECR muscle and acceleration. All data from monkey D. EMG, electromyogram; ECR, extensor carpi radialis; FCU, flexor carpi ulnaris.
Figure 1B shows the power spectrum of the finger acceleration, calculated using data from the ramp phase of all successful flexion trials from one recording session. There was a broad spectral peak from 5 to 30 Hz. Unlike similar recordings in man, there was no well-defined peak at 8–12 Hz. There was also a similar peak in the acceleration during the extension trial (Fig. 1F). In Fig. 1, C and G the acceleration power was computed as a function of time during the trial. This makes it clear that the increase in oscillations was confined to the ramp phases of the task. Power at a wider range of frequencies was seen at the beginning and end of the trial, corresponding to the initial movement of the lever into the target zone and the release of the lever at trial completion.
The increase in approximately 10-Hz oscillations could be due to mechanical resonant properties of the finger. However, there was significant coherence between the acceleration and EMG from a flexor muscle (FCU) at about 10 Hz (Fig. 1D) during the flexion ramp and between the acceleration and an extensor EMG (ECR) at about 10 Hz (Fig. 1H) during the extension ramp. The oscillations close to 10 Hz produced during the ramp were thus not due to the mechanical properties of the arm but had a neural component.
There was also a significant peak in the EMG–acceleration coherence at about 3 Hz during the ramp period (Fig. 1, D and H). This coherence peak occurs as a consequence of visual feedback and has been well documented by previous studies in man (McAuley et al. 1999a,b; Vallbo and Wessberg 1993).
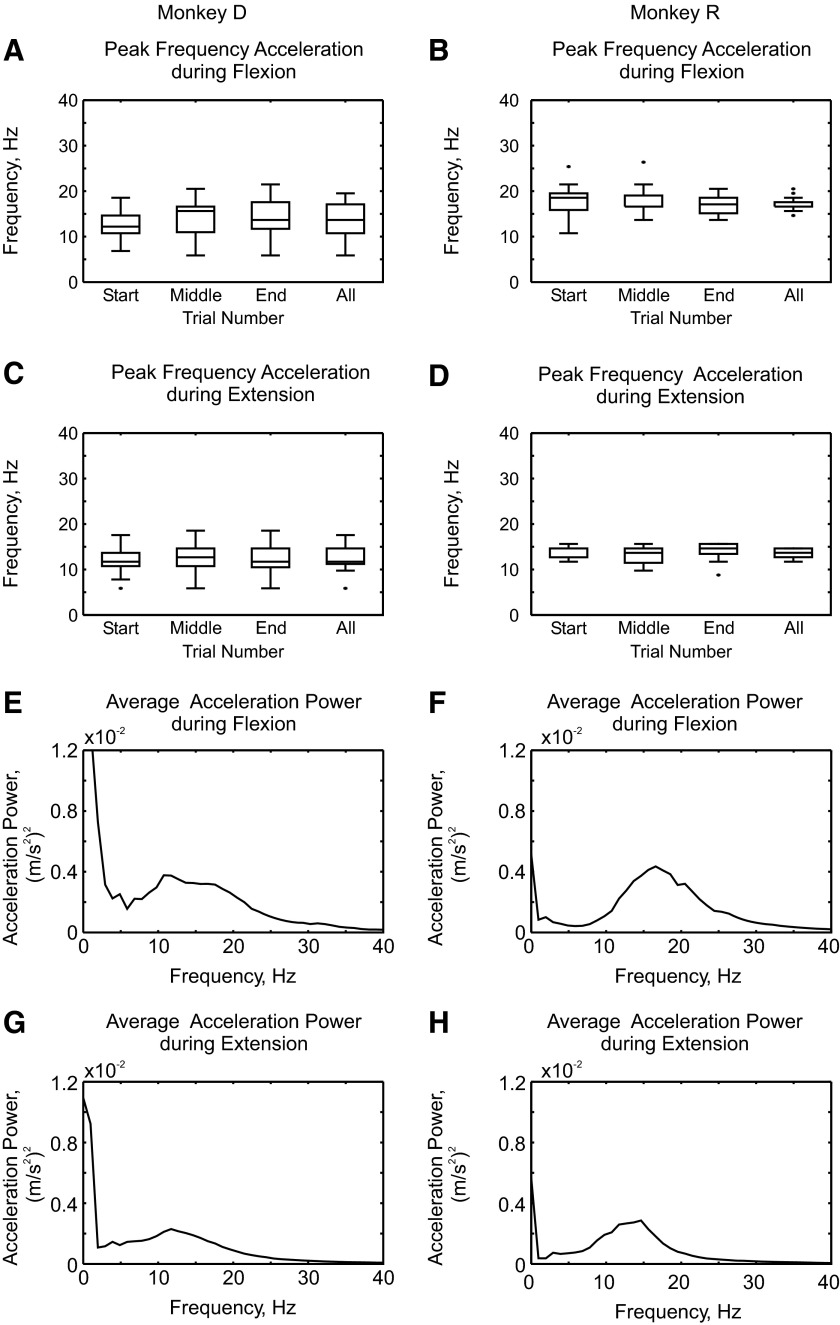
Figure 1 shows that, under these experimental conditions, monkeys have discontinuities during slow finger movement that occur over a broad frequency range. Further analysis of the variation in the frequency of this phenomenon is provided by Fig. 2, which shows box plots of the peak frequency of the acceleration power. These are presented separately for each monkey and for different parts of the experimental session (10 trials from the start, middle, or end of the session, or using all available trials). There was no consistent change in the frequency peak throughout sessions (Fig. 2, A–D), suggesting that there were no changes associated with fatigue. The acceleration peak frequency calculated using all trials was higher for monkey R than that for monkey D during both flexion (Fig. 2, A and B; mean frequency, 17 ± 1.3 vs. 14 ± 3.6 Hz) and extension (Fig. 2, C and D; mean frequency, 13.6 ± 1.1 vs. 12 ± 3.1 Hz). Figure 2, E–H shows the average acceleration power spectra. In all cases, there was a broad peak from about 5 to 30 Hz; this was at slightly higher frequencies in monkey R.
FIG. 2.
Acceleration power during each session. A–D: box plots of the peak frequency of the acceleration power spectrum for each monkey during flexion (A and B) and during extension (C and D). Bars show peak frequency calculated using 10 trials from the start, middle, and end of each session, as well as frequency estimated from all available trials. Outliers are shown with a dot. E–H: average power spectra from each monkey during flexion (E and F) and during extension (G and H). A, C, E, and G show data from monkey D; B, D, F, and H from monkey R.
The acceleration power spectrum for the discontinuities in humans is also quite wide and includes frequencies outside the 8- to 12-Hz range (Wessberg and Kakuda 2001). A 6- to 13-Hz frequency range has been suggested as more reasonable to encompass physiological tremor (Halliday and Redfearn 1956; Raethjen et al. 2002). This range also appeared to include the significant peak in EMG–acceleration coherence in the present study (Fig. 1, D and H, gray shading) and we therefore also adopted it as our frequency range of interest.
Coherence between M1 LFP and finger acceleration
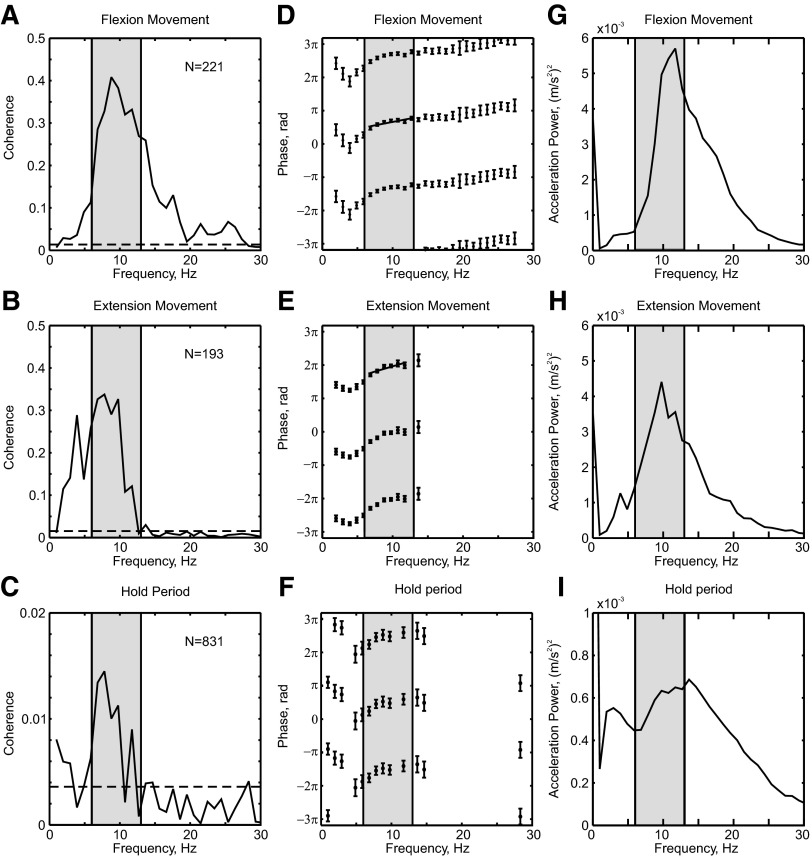
Previous studies in humans have disagreed on whether M1 activity is coherent with finger discontinuities during slow movements. In our data using finer-scale recordings of LFP from M1, we routinely found coherence between the cortex and periphery. This is illustrated for a single recording session in Fig. 3. Regardless of whether we analyzed the ramp phase of flexion (Fig. 3A) or extension trials (Fig. 3B), coherence was above significance in the 6- to 13-Hz region (gray shading). The shape of the coherence peak was similar to that of the acceleration power spectrum (Fig. 3, G and H). The coherence magnitude was high compared with our previous work on corticomuscular coherence at about 20 Hz, where peaks often do not rise above coherence levels of 0.1 (Baker et al. 1997; Kilner et al. 2000; Riddle and Baker 2005). This probably reflects the better signal-to-noise ratios in acceleration recordings compared with EMG; we verified that EMG–LFP coherence at these frequencies was smaller and closer to the levels we previously reported in the beta band (data not illustrated).
FIG. 3.
Local field potential (LFP)–acceleration coherence, coherence phase, and acceleration power for an example recording session. Example of coherence and coherence phase between primary motor cortex (M1) LFP from a single recording site and finger acceleration, during (A) flexion ramps, (B) extension ramps, and (C) hold periods. Horizontal dashed lines show significance limits (P < 0.05), below which coherence is not significantly different from zero. D–F: coherence phase corresponding to coherence shown in A–C; phase is presented only for bins with significant coherence. Solid lines mark linear regression fits with slopes significantly different from zero. G–I: power spectra of acceleration. Throughout, gray-shaded regions mark the 6- to 13-Hz range. All data are from monkey D.
It was also possible to see significant coherence between LFP and acceleration during the hold phase of the task (Fig. 3C), corresponding to physiological tremor (Fig. 3I). However, in this case the magnitude was substantially smaller (peak of 0.014 at 7.8 Hz, compared with 0.41 and 0.34 for flexion and extension ramps, respectively).
Our results confirm that M1 activity is coherent with the discontinuities. Further insight can be obtained by examining the coherence phase. If the peripheral discontinuities resulted from purely efferent propagation of M1 oscillations to the muscles, there would be a constant time delay between the two signals. Coherence phase would then show a linear phase–frequency relationship (Rosenberg et al. 1989). Figure 3, D–F shows the coherence phase corresponding to the coherence spectra in Fig. 3, A–C. For both extension and flexion movements, there was an approximately linear phase–frequency relationship (Fig. 3, D and E), which gave a linear regression slope significantly different from zero. The delays calculated from the slopes were 20.4 ± 12.1 and 31.1 ± 21.8 ms for flexion and extension movements, respectively; however, the slopes were positive. With the conventions we used to compute the coherence, this indicates that the LFP lagged the acceleration. Far from providing evidence that M1 causes the discontinuities, the data are more consistent with the idea that M1 cells respond to afferent input from the oscillating digit.
Phase values for coherence calculated during the hold period of the task appeared similar to those for the ramp; however, the smaller magnitude of coherence resulted in more variable phase estimates and the linear trend was not significantly different from zero.
Figure 3, G–I shows the acceleration power spectra for flexion, extension, and hold, respectively. There are broad peaks between 5 and 30 Hz in all cases, whereas the LFP–acceleration coherence was concentrated over a narrower range.
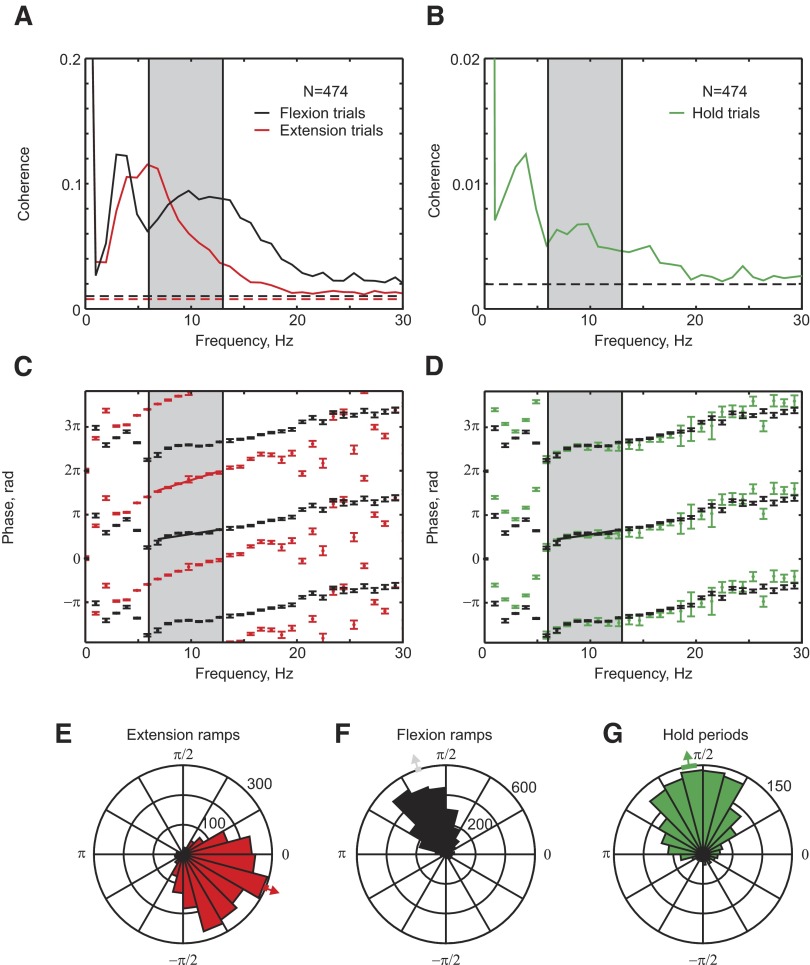
Single-session coherence spectra were comparable for both monkeys; we thus produced a population result by averaging the spectra across all available recording sites from the two animals (shown in Fig. 4). Coherence amplitude was similar in size for flexion and extension ramps, although the peak was broader for flexion (Fig. 4A). As with the single session previously illustrated, the average coherence during the task hold period was substantially smaller than that during flexion and extension ramps (Fig. 4B; note the ordinate scale is different from that in Fig. 4A).
FIG. 4.
Average coherence and phase between LFP and acceleration. A: average coherence between LFP and acceleration during flexion ramp (black) and extension ramp (red) phases. B: average coherence between LFP and acceleration during hold period (green). Averages have been compiled over all available recording sites, in both monkeys. C and D: circular mean coherence phase corresponding to averaged coherences shown in A and B. Black points in D relate to flexion trials (same data as black points in C) for comparison. E–G: phase distribution of coherence between single LFP sites and acceleration for bins in the 6- to 13-Hz range, during (E) extension ramp, (F) flexion ramp, (G) hold period.
Figure 4C shows the average coherence phase spectra for flexion and extension ramp periods. There was a significant linear regression of phase on frequency over the 6- to 13-Hz region, with slopes implying that LFP lagged acceleration by 18 ± 14 and 36 ± 8 ms for flexion and extension movements, respectively. In addition, there was a phase difference between the two types of trials, which ranged from 3.4 to 4.2 radians over the 6- to 13-Hz range (mean, 3.8 radians). The coherence phase measured during the task hold period (green, Fig. 4D) was similar to that seen during flexion trials (overlaid in black for comparison, Fig. 4D).
Figure 4, E–G examines the coherence phase in more detail as a rose plot. This shows the distribution of phase estimates. Each significant LFP-coherence bin in the 6- to 13-Hz range from every single recording session contributed one count to these circular histograms. Phase estimates were tightly clustered, with means and 95% confidence limits of 1.77 ± 0.031, 5.50 ± 0.036, and 1.66 ± 0.071 radians, respectively, for flexion, extension, and hold trials. The mean phase was significantly different between extension and flexion trials and between extension and hold phases (χ12 test, with P < 0.05; see Fisher 1993). The acceleration–LFP coherence phase thus differed by around π radians (i.e., an antiphase relationship) between the ramp phase of the flexion and extension trials.
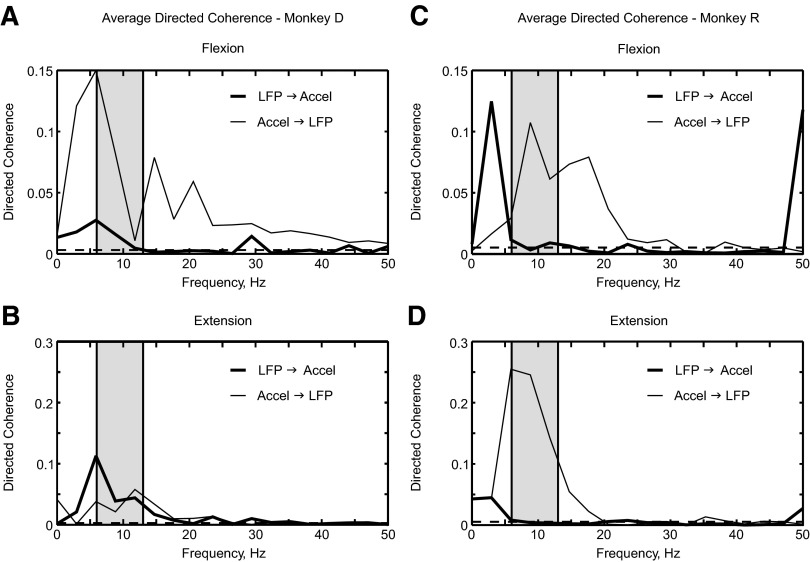
The coherence phase–frequency relationships suggested that the cortical LFP lagged the acceleration. However, it can be difficult to interpret such phases in a system that potentially has bidirectional coupling. Baker et al. (2006) used a form of Granger causality analysis (directed coherence) to determine the direction of influences between signals. We applied a similar method to the acceleration and LFP recorded in the present experiments, with the results shown in Fig. 5. Unlike the coherence analysis, there were differences in the results from the two monkeys that we studied and accordingly we did not combine data across animals. In both monkeys, directed coherence in the acceleration → LFP direction was large and significant in the 6- to 13-Hz band (Fig. 5, A and C, thin line) during flexion ramps. For monkey D, directed coherence in the LFP → acceleration direction was also significant, although smaller (Fig. 5A, thick line). For monkey R, directed coherence in the descending (LFP → acceleration) direction hovered close to the significance limit (Fig. 5C, thick line). Similar results were obtained for the extension ramp in monkey R (Fig. 5D); in monkey D, directed coherence appeared comparable in the two directions (Fig. 5B).
FIG. 5.
Directed coherence for flexion and extension ramps between LFP and acceleration. Results are shown separately for monkeys D and R (A and B vs. C and D), and for causal influences in an ascending (thin line) and descending (thick line) direction. A and C show results from flexion ramp and B and D extension ramp phases of the task.
Directed coherence analysis therefore supports the conclusions from examination of the coherence phase—that sensory feedback of peripheral discontinuities onto the LFP was the dominant effect. By contrast, under these experimental conditions descending influences (LFP → acceleration) were present but weaker.
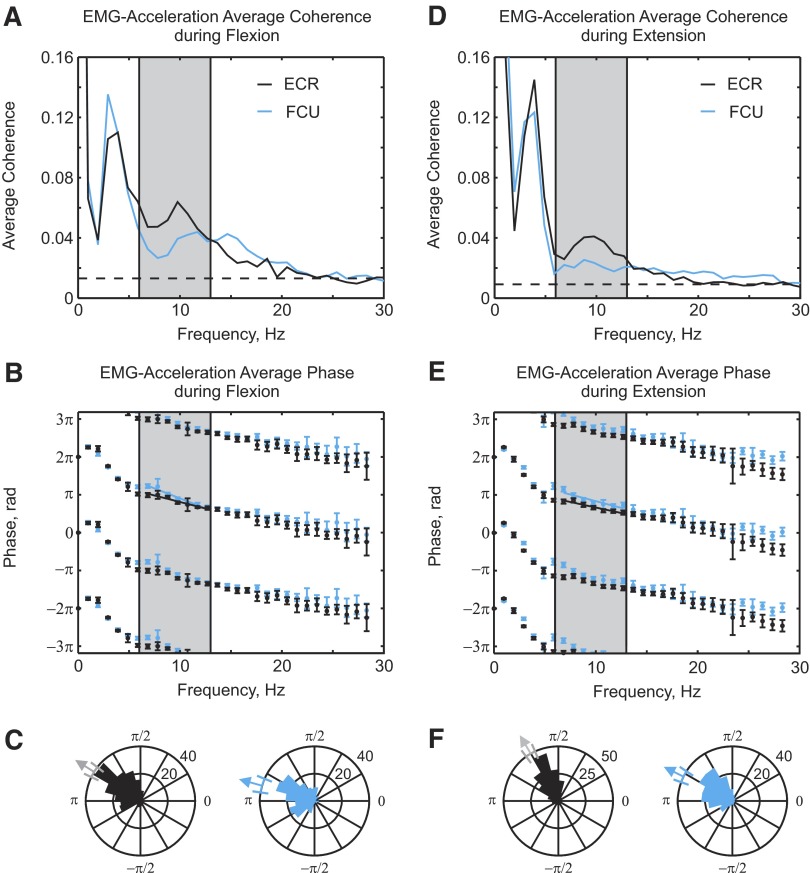
EMG–acceleration coherence during flexion and extension
In Fig. 4 there was a nearly π phase difference between LFP–acceleration coherence during flexion and extension trials. This could be caused by a shift in the phase relationship between muscle activity and acceleration between the two task types. Figure 6 shows the coherence between EMG and acceleration during flexion (Fig. 6A) and extension (Fig. 6B) for two different muscles (ECR and FCU). In all cases the EMG–acceleration coherence had a significant peak between 6 and 13 Hz. Figure 6, B and E shows the coherence phase–frequency relationship. The phase spectra from the two muscles were almost overlapping during both flexion and extension. This can be more easily seen from the circular histograms of the phase (Fig. 6, C and F). The phase relationship of flexor and extensor muscles to acceleration was similar with means and 95% confidence limits (for ECR and FCU, respectively) of 2.58 ± 0.11, 2.86 ± 0.13 radians during flexion and 2.10 ± 0.095, 2.68 ± 0.11 radians during extension. There was a significant difference in mean phase between flexion and extension movements for both muscles (difference of 0.48 radian for ECR, 0.18 radian for FCU), but this was small compared with the close to π radians phase shift seen for the LFP–acceleration coherence (Fig. 4, E and F).
FIG. 6.
EMG–acceleration coherence and phase. Average coherence between EMG and acceleration during flexion (A) and extension (D) for muscles ECR (black) and FCU (blue). Corresponding coherence phase spectra are shown in B and E. C and F: phase distribution of coherence between EMG and acceleration for bins in the 6- to 13-Hz range for both muscles during flexion (C) and extension (F).
Cell response profiles during task performance
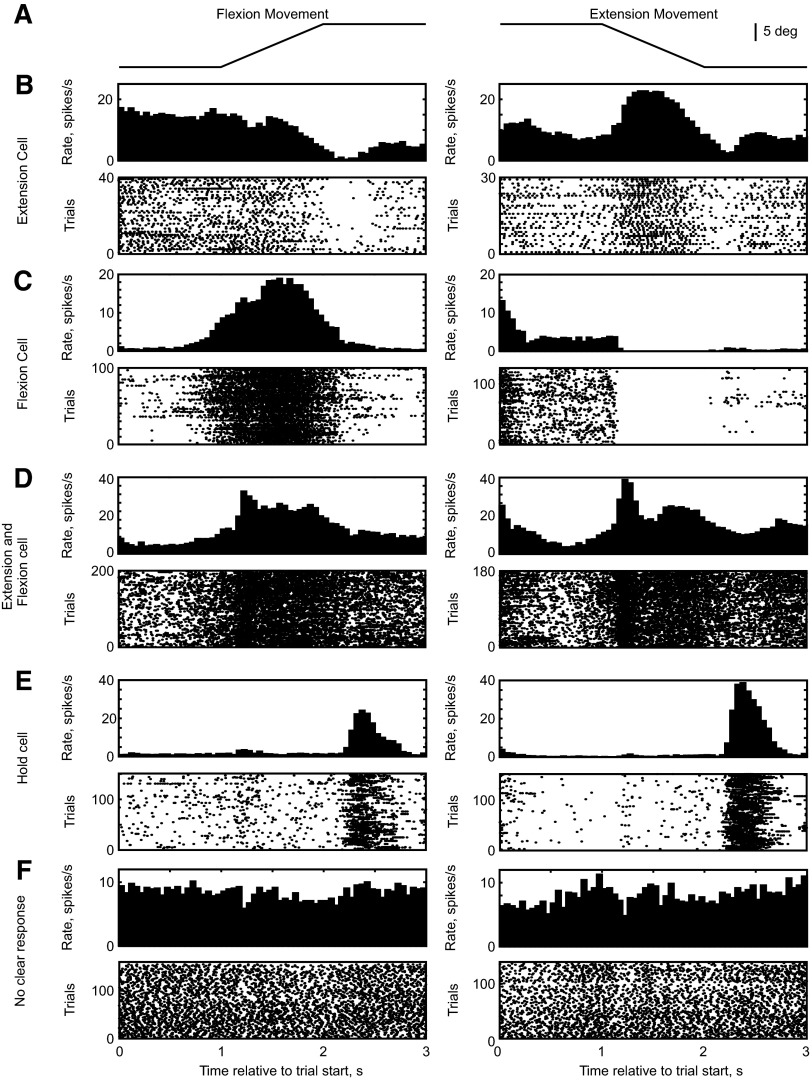
LFP reflects the averaged, synchronous synaptic inputs to many cells surrounding the electrode tip. However, cell spiking in M1 shows highly heterogeneous response profiles during performance of a behavioral task. In the finger ramp tracking task examined here, some cells fired strongly during extension and others during flexion movements. We were interested to examine the coherence of different functional classes of M1 cells with the peripheral discontinuities, to determine whether the phase of coherence would be different for flexion and extension cells. This first required a means of subclassifying the different firing profiles.
Figure 7 shows poststimulus time histogram (PSTH) and raster displays of the task-related firing of five example cells recorded from M1. The heterogeneous nature of the response profiles is evident: there were cells that responded best to extension (Fig. 7B), flexion (Fig. 7C), or to both directions of the ramp movement (Fig. 7D). Some cells fired strongly during the hold period (Fig. 7E); others exhibited no obvious task-related firing (Fig. 7F). It is not possible fully to represent this great diversity of response patterns in any straightforward way. We thus chose simply to measure the average firing rate during the ramp phase (Fig. 8A, gray bars) and to compare this with the average during the first hold phase (Fig. 8A, black bars). The difference between these average firing rates produced a measure we refer to as w. Using all successfully performed flexion trials, if the spike counts from single trials in the ramp and hold phase increased significantly (paired t-test, two-tailed, P < 0.05), the cell was classified as a flexion cell. Cells producing suppression effects were not classified. A similar analysis was applied to the extension trials; a cell could thus be classified as unmodulated (neither flexion nor extension), flexion, extension, or both.
FIG. 7.
Poststimulus time histogram (PSTH) and raster displays of example cells recorded from M1. A: schematic representation of the time course of the finger displacement during flexion and extension trials. B–F: PSTH and raster plots of different cells during flexion trials (left column) and extension trials (right column).
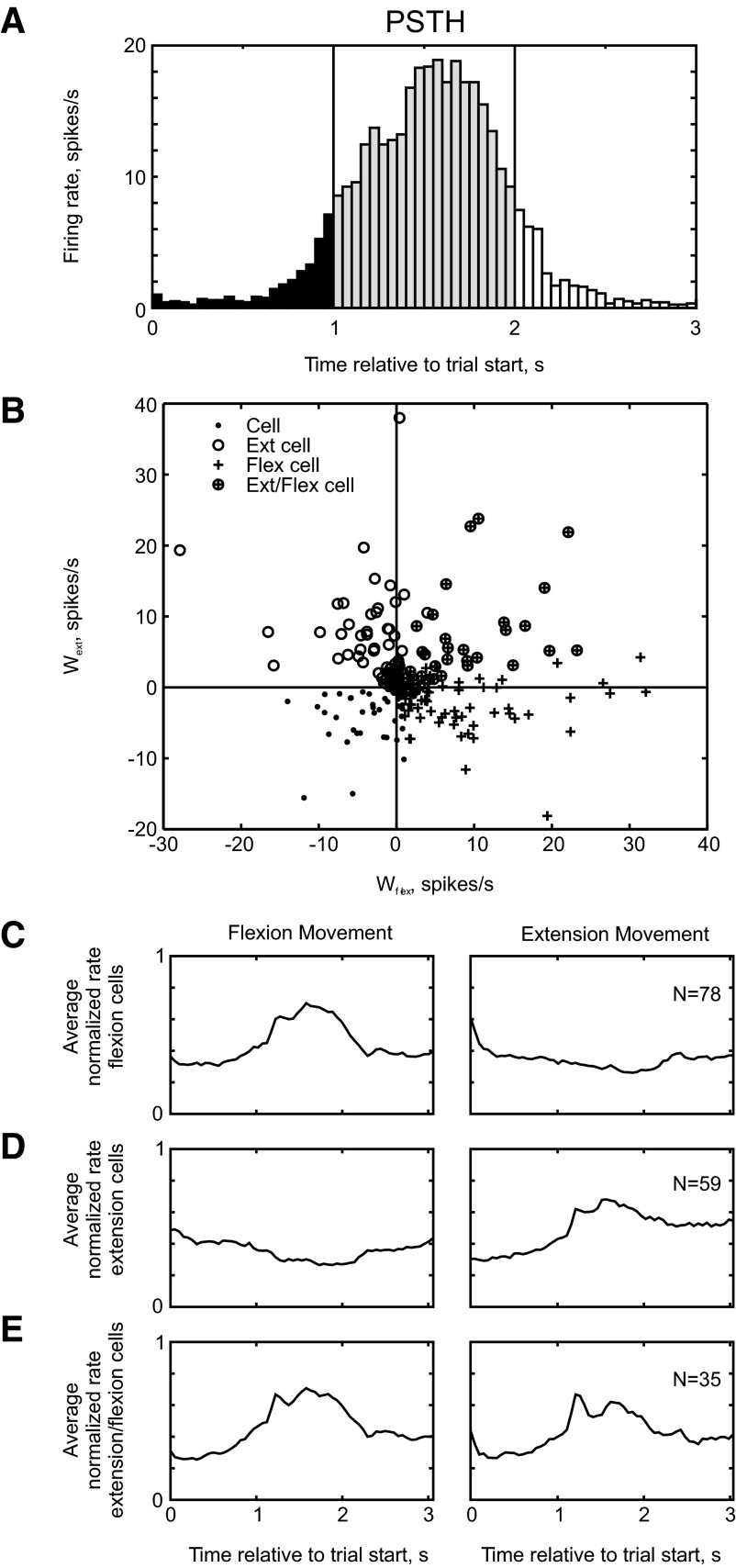
FIG. 8.
Classification of cells as flexion, extension, or flexion/extension. A: example PSTH of a cell recorded from M1. Comparison of the mean rate during the ramp phase (gray shading) vs. the initial hold phase (black shading) indicated whether the cell was significantly modulated. B: scatterplot of wext vs. wflex. Points are marked according to significant flexion cells (cross), significant extension cells (open circle), significant extension and flexion cells (open circle with cross), and cells with no significant facilitation (filled circle). C–E: averaged PSTH during flexion and extension trials (left and right columns, respectively), for cells categorized as flexion only (C), extension only (D), and flexion/extension (E). Each constituent PSTH was first normalized by its maximum rate before averaging.
Figure 8B plots wext against wflex for each of the 241 cells recorded in this study (124 from monkey D, 117 from monkey R). Significant flexion cells are denoted by a cross (78 cells) and significant extension cells by a circle (59 cells). Cells that responded significantly to both movements are marked by a coincident cross and circle (35 cells). Figure 8, C–E presents the mean PSTHs calculated for cells with only a significant flexion modulation (Fig. 8C), only a significant extension modulation (Fig. 8D), and both a flexion and an extension modulation (Fig. 8E). These averaged PSTHs confirm that we have successfully selected functionally distinct subtypes.
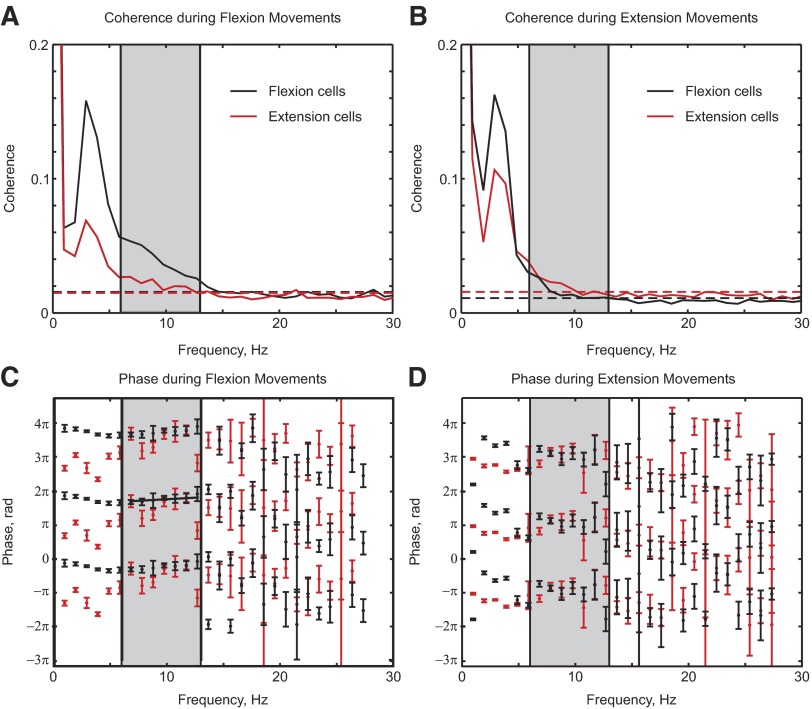
Coherence between cell spikes and acceleration
Figure 9, A and B shows the average coherence between cells and finger acceleration, plotted separately for flexion and extension cells (black and red lines) and flexion and extension trials (Fig. 9, A and B). In all cases, there was significant coherence in the 6- to 13-Hz range (gray shading). Coherence was larger for flexion-related cells than that for extension-related cells during flexion movements (mean over shaded range 0.039 vs. 0.021, P < 0.05, t-test). It was conversely larger for extension-related cells than for flexion-related cells during extension movements (mean over shaded range 0.020 vs. 0.016, P < 0.05, t-test). This result could come about from the different amounts of data available for each cell class, which would slightly change the bias of the coherence estimates (this also produces the different significant limits visible in Fig. 9B; Benignus 1969), although this is a small effect. It is known that coherence between cell spiking and LFP is increased at high firing rates (Baker et al. 2003; Witham et al. 2007); it is most likely that the coherence differences seen in Fig. 9, A and B result from the rate differences imposed by the subclassification of the cells.
FIG. 9.
Average coherence between cells and acceleration. A and B: average coherence between cell spiking and acceleration, for flexion cells (black) and extension cells (red). Results are plotted for flexion ramp phase (A) and extension ramp phase (B). Averages were compiled over all available cells from both monkeys. C and D: circular mean coherence phase corresponding to A and B.
Figure 9, C and D shows the phase spectra corresponding to the coherence illustrated in Fig. 9, A and B. For a given trial type, phase was similar for flexion and extension cells, although there was a phase difference between flexion and extension trials, as seen for LFP–acceleration coherence. Flexion cells during the flexion task showed a positive phase slope, indicating the cells lagged the acceleration by 9.1 ± 6.7 ms. No linear relationship between phase and frequency was apparent for any of the other average phase spectra shown (P > 0.05, linear regression on 6- to 13-Hz region). This may result from the greater uncertainty of the phase estimates for cell–acceleration coherence compared with those for LFP–acceleration coherence, caused by the lower coherence magnitude or from an interplay of both ascending and descending interactions between M1 and the periphery, as suggested by the directed coherence analysis of Fig. 5.
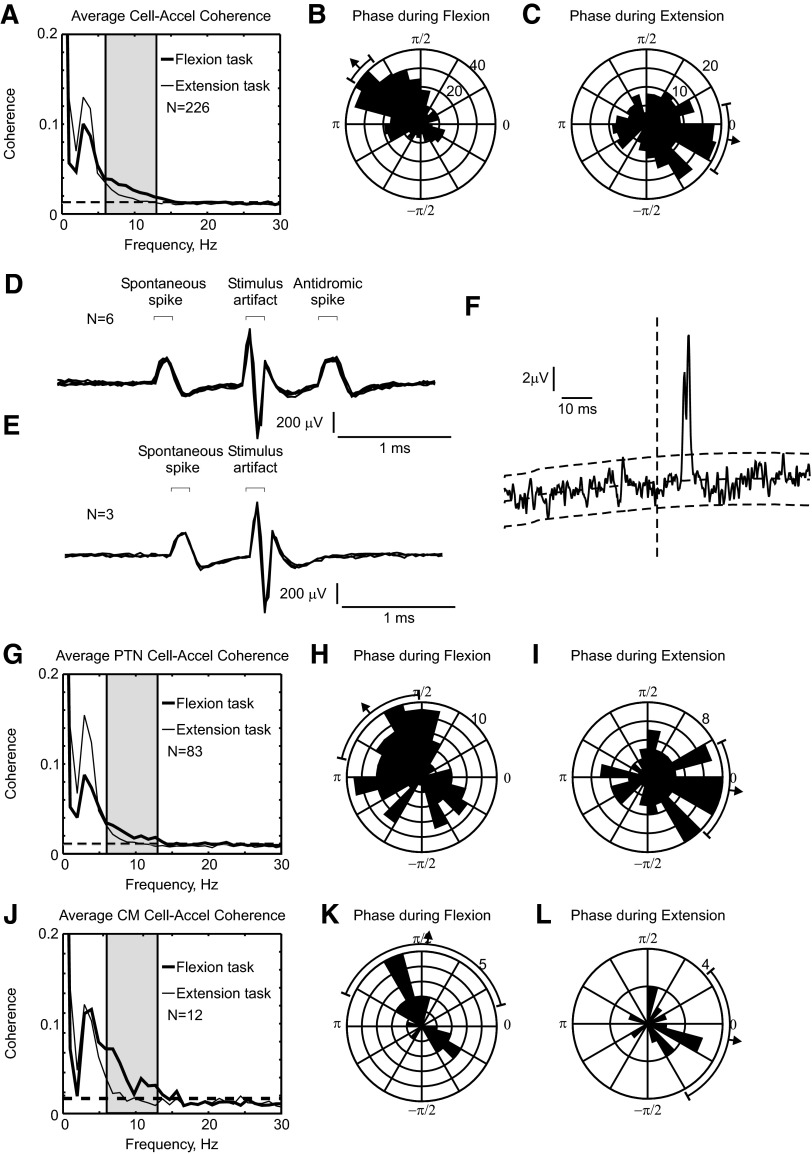
Since flexion and extension cells have a similar coherence phase during a given task, it is valid to analyze the entire population of recorded cells together. Figure 10A shows the average cell–acceleration coherence; significant population coherence is seen across the 6- to 13-Hz range. In Fig. 10, B and C the coherence phase for individual cells and frequency bins (across the 6- to 13-Hz range) is presented as a circular distribution histogram (similar to the data of Fig. 4, E and F for LFP–acceleration coherence). The mean phase for flexion trials was 2.4 ± 0.18 radians (circular mean ±95% confidence limits); for extension trials it was 6.1 ± 0.43 radians. These were significantly different from each other (χ12 test, with P < 0.05; Fisher 1993); the difference was close to π radians.
FIG. 10.
Average coherence between all cells, pyramidal tract neurons (PTNs), corticomotoneuronal (CM) cells, and acceleration. A: averaged coherence between spikes from all available cells and finger acceleration, calculated separately for flexion and extension trials (thick vs. thin lines). B and C: corresponding coherence phase distribution for flexion (B) and extension trials (C) for the 6- to 13-Hz range. D: antidromic response of cell to PT stimulation (500 μA). Stimulus was delivered 0.8 ms after a spontaneous spike. E: shortening the trigger interval to 0.7 ms resulted in collision of the antidromic spike, identifying the cell as a PTN. F: spike-triggered average of rectified EMG from the 1DI muscle, triggered by the spikes of cell shown in D and E. Dotted lines show baseline estimate, produced by convolving the average with a Gaussian kernel (width parameter 50 ms), and the 95% confidence limits on the baseline. n = 142413 trigger spikes. G–I: like A–C, but only using identified PTNs. J–L: like A–C, but only using identified CM cells. Bars outside circular histograms show mean phase and 95% confidence limits on this mean.
M1 contains many of the cells of origin of the corticospinal tract, which in primates is the major pathway for communication of descending commands from brain to spinal cord. Some of the neurons that we recorded were antidromically identified as pyramidal tract neurons (PTNs); an example of an antidromic response (and its collision by a spontaneous spike) is illustrated in Fig. 10, D and E. Figure 10, G–I presents an analysis of cell–acceleration coherence compiled selectively for these identified output cells. The results were similar to those for the whole cell population, although more scatter is evident in the circular histograms due to the smaller sample size. The mean phase for flexion trials was 2.3 ± 0.66 radians and for extension trials, 6.1 ± 0.60 radians. These were significantly different from each other (χ12 test, with P < 0.05) and, again, the difference was close to π radians.
Of the identified PTNs, 12 cells were further identified as corticomotoneuronal cells by spike-triggered averaging of rectified EMG (see Fig. 10F for an example postspike facilitation). All 12 of these cells were recorded for a minimum of 10,000 spikes. Figure 10, J–L presents the coherence analysis compiled for only these cells, which we know make monosynaptic connections to motoneurons innervating hand and arm muscles. The results were broadly very similar to those for the whole cell population in Fig. 10, A–C, although it is possible that the phase for flexion trials showed a bimodal distribution.
DISCUSSION
Discontinuities during finger tracking movements in monkey
This study marks the first attempt to use invasive electrophysiological recordings in an animal model of slow finger movement discontinuities; such recordings can provide improved spatial resolution (even to the single-unit level) over those typically available in humans. We have shown that it is possible to train monkeys to produce well-controlled ramp displacements of the finger that are associated with discontinuities in acceleration. The power spectrum of the acceleration during the ramp period had a broader peak than has been previously reported in human studies (Fig. 1B; Vallbo and Wessberg 1993). This may reflect an interspecies difference, for example in the mechanical properties of the hand, which is smaller in monkeys than in humans. Additionally, the experimental arrangement used with the monkeys was necessarily more constrained than in human work. The animals placed the index finger in a tube attached to a lever and torque motor; this introduced extra inertia and a springlike resisting load. By contrast, most human studies have used methods in which the finger is unloaded (Marsden et al. 2001; McAuley et al. 1999a; Vallbo and Wessberg 1993; Wessberg and Kakuda 2001). The different mechanical arrangements may have produced the different acceleration power spectra seen. However, the coherence spectrum between EMG and acceleration showed a peak around 10 Hz (Fig. 1D), similar to previous reports in humans. This suggests that the underlying neural generators of the discontinuities are the same in the two species, even if the peripheral manifestation is altered by nonneural factors.
Role of M1 in discontinuities
Using direct intracortical recordings, we found that M1 activity was coherent with peripheral oscillations around 10 Hz. This agrees with previous work (Gross et al. 2002; Marsden et al. 2001; Raethjen et al. 2002). However, examination of the LFP–acceleration coherence phase showed a linear relationship, with acceleration leading LFP by around 18 ms. Directed coherence analysis resulted in the same conclusion, although this conflicts with previous reports (Gross et al. 2002; Raethjen et al. 2002), which suggested that M1 recordings led activity measured from the periphery.
Gross et al. (2002) used whole head MEG and calculated a “directional index”(Rosenblum and Pikovsky 2001), which indicated an influence from M1 on finger muscle EMG, rather than vice versa. Raethjen et al. (2002) performed an analysis similar to that of ours on electrocoricogram recordings, using linear fits to coherence phase spectra to estimate the direction and delay associated with coupling. In six patients, only two had spectra from which delays could be measured and these indicated that M1 led acceleration. Both of these studies used signals with lower spatial resolution than LFP recorded from penetrating microelectrodes as used here, which can lead to important differences (Mehring et al. 2004). The additional spatial averaging may result in cancellation of signals that change over a fine scale. In addition, both MEG and electrocorticogram are surface recordings, whereas our electrodes were probably located mainly within layer V of the cortex, as judged by the proximity of identified PTNs. These differences in the recorded signals may be responsible for the differences in the dominant direction of coupling that were identified.
It would be surprising if sensory feedback of the discontinuities from the periphery did not affect M1 activity to some extent. It is known that cells in M1—including identified PTNs and CM cells—receive powerful input from the periphery. This comes both via S1 and also directly from the thalamus (Cheney and Fetz 1984; Lemon and Porter 1976; Rosen and Asanuma 1972). Muscle spindle afferent discharges are modulated by the movement discontinuities (Tracey et al. 1980). Measurement of coherence between M1 and the periphery therefore presumably reflects the relative balance between descending and ascending pathways (Wessberg and Vallbo 1995). Even for a CM cell, it is certainly possible that the consequences on cell firing of sensory feedback from movement could be greater than the descending influence of that cell's spiking on motor output.
The discrepancy in the direction of M1–periphery interaction between our data in monkey and that previously obtained in humans may be due to details of the experimental configuration that differently emphasized ascending or descending pathways. As already mentioned, the mechanical configuration was quite different, with the finger connected to a torque motor in our studies, but free in air in previous work. This may have increased the importance of sensory feedback. Additionally, changes in the relative efficacy of ascending or descending connections evoked by different task configurations may play a role. The corticospinal connections from M1 to motoneurons are direct and might be only slightly amenable to modulation of their efficacy (Jackson et al. 2006; but see Davidson et al. 2007). By contrast, peripheral input passes via cuneate nucleus and thalamus and is subject to a highly developed system of descending control and gating (Aguilar et al. 2003; Chapman et al. 1988; Rapisarda et al. 1992; Tsumoto et al. 1975). In our task, monkeys were required constantly to keep finger displacement in a narrow target zone to achieve a successful trial. Human studies by contrast usually impose only weak accuracy constraints. This may have led to an increased emphasis on sensory feedback in our monkey data.
Differences between flexion and extension movements
An important finding of the present study was that the phase relationship between M1 LFP and acceleration alters by nearly π radians for flexion compared with extension ramp movements. One possible explanation for this might be that different muscle groups contribute during the different directions of ramp movement. If the dominant activation were of flexors during flexion ramps, and extensors during extension ramps, then the averaged muscle activity might reverse its phase relationship with the digit acceleration between the two movements. If M1 activity controls the muscles, a phase reversal would also be seen between LFP and acceleration.
This explanation is unlikely to be correct. In this task there was often cocontraction of flexor and extensor muscles to produce the finely controlled finger movements that were required (Fig. 1, A and E). Oscillations in both flexors and extensors had the same relationship to discontinuities in acceleration (Fig. 6) and this did not change during flexion and extension movements. By contrast, the coherence phase between M1 cell spiking and acceleration differed between flexion and extension movements. Cells that fire preferentially during flexion movements presumably mainly contribute to activation of flexors. If the motor output of these cells was the most important influence on their coherence with acceleration, we would expect that the phase relationship of spiking from these cells should not change between flexion and extension movements. In fact, similar changes with task type were seen as for LFP–acceleration coherence (Figs. 9, C and D and 10, B and C).
As discussed earlier, the slope of the coherence phase–frequency relationship suggests that LFP lags acceleration and that sensory feedback from the periphery is more important than descending control of movement in mediating the coherence between M1 and finger discontinuities. It is possible that the dominant muscles providing spindle feedback could change from flexion to extension movements, reflecting a shift in fusimotor drive. If flexor muscle spindles provide the majority of the sensory feedback to M1 during flexor movements, but extensor spindles during extensor movements, this would lead to the observed phase shift not only in LFP, but also in cell activity. Equally, there could be differences in cutaneous feedback between the extension and flexion tasks. The differences between flexion and extension trials may thus provide further indirect evidence for a predominantly sensory contribution to the observed coupling between cortex and the periphery. A further possibility arises from the nature of the behavioral task: in both task variants the manipulandum exerted an extension torque on the finger. Flexion movements were thus active flexions, whereas extension movements were performed by a controlled release of the manipulandum lever. It is conceivable that this asymmetry of task configuration could have led to the differences seen in the phases.
Conclusions
Early work on physiological tremor sought to distinguish between the hypotheses that tremor was produced by resonance in the monosynaptic stretch reflex feedback arc or that it was caused by the output of central neural oscillators (for review see Elble and Koller 1990). Wessberg and Vallbo (1996) likewise investigated whether the monosynaptic stretch reflex alone could account for the nearly 10-Hz discontinuities seen during slow finger movements; they concluded that it could not.
Our results indicate that M1 activity is coherent with peripheral oscillations during slow finger movements. The oscillatory activity in M1 included identified output neurons (Fig. 10, G and J) and directed coherence analysis showed significant interactions in the M1 to acceleration direction. Motor cortex is thus clearly part of the circuit that generates this distinctive peripheral oscillatory phenomenon. However, multiple lines of evidence converge to suggest that there are also strong interactions in the ascending (sensory) direction, by which peripheral oscillations influence M1 activity. The situation thus seems to represent a synthesis of earlier, apparently conflicting ideas: peripheral oscillations are partially generated by a central oscillator that involves M1, but this oscillator receives strong feedback from the periphery. It is likely that other motor structures are also involved, as suggested by Gross et al. (2002), leading to a complex interacting system in which it makes little sense to think of any one center as being the “origin” of the activity. Some of these systems may actually act to reduce oscillations, as suggested by Hore and Flament (1986) for the cerebellum and by Williams and Baker (2009b) for Renshaw cells in the spinal cord.
Beta-band oscillations in the motor system during steady contraction have been well studied; we have recently accumulated evidence that this system also has reciprocal coupling between M1 and the periphery (Baker 2007; Baker et al. 2006; Riddle and Baker 2005; Williams and Baker 2009a; Witham et al. 2007). Such reciprocal coupling is probably unavoidable, given the known anatomy and the strong dependence of movement on proprioceptive reafference. For the beta-band activity, we have speculated that this feedback loop may have a functional role (Baker 2007); whether this is the case for the lower-frequency oscillations studied herein is at present unknown.
GRANTS
This work was supported by The Wellcome Trust and the Medical Research Council (UK).
Acknowledgments
The authors thank T. Jackson for assistance with animal training.
REFERENCES
- Aguilar et al. 2003.Aguilar J, Rivadulla C, Soto C, Canedo A. New corticocuneate cellular mechanisms underlying the modulation of cutaneous ascending transmission in anesthetized cats. J Neurophysiol 89: 3328–3339, 2003. [DOI] [PubMed] [Google Scholar]
- Baker and Baker 2003.Baker MR, Baker SN. The effect of diazepam on motor cortical oscillations and corticomuscular coherence studied in man. J Physiol 546: 931–942, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker 2007.Baker SN Oscillatory interactions between sensorimotor cortex and the periphery. Curr Opin Neurobiol 17: 649–655, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker et al. 2006.Baker SN, Chiu M, Fetz EE. Afferent encoding of central oscillations in the monkey arm. J Neurophysiol 95: 3904–3910, 2006. [DOI] [PubMed] [Google Scholar]
- Baker and Lemon 1998.Baker SN, Lemon RN. A computer simulation study of the production of post-spike facilitation in spike triggered averages of rectified EMG. J Neurophysiol 80: 1391–1406, 1998. [DOI] [PubMed] [Google Scholar]
- Baker et al. 1997.Baker SN, Olivier E, Lemon RN. Task dependent coherent oscillations recorded in monkey motor cortex and hand muscle EMG. J Physiol 501: 225–241, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker et al. 1999.Baker SN, Philbin N, Spinks R, Pinches EM, Wolpert DM, MacManus DG, Pauluis Q, Lemon RN. Multiple single unit recording in the cortex of monkeys using independently moveable microelectrodes. J Neurosci Methods 94: 5–17, 1999. [DOI] [PubMed] [Google Scholar]
- Baker et al. 2003.Baker SN, Pinches EM, Lemon RN. Synchronization in monkey motor cortex during a precision grip task. II. Effect of oscillatory activity on corticospinal output. J Neurophysiol 89: 1941–1953, 2003. [DOI] [PubMed] [Google Scholar]
- Benignus 1969.Benignus VA Estimation of the coherence spectrum and its confidence interval using the fast Fourier transform. IEEE Trans Acoust 17: 145–150, 1969. [Google Scholar]
- Chapman et al. 1988.Chapman CE, Jiang W, Lamarre Y. Modulation of lemniscal input during conditioned arm movements in the monkey. Exp Brain Res 72: 316–334, 1988. [DOI] [PubMed] [Google Scholar]
- Cheney and Fetz 1984.Cheney PD, Fetz EE. Corticomotoneuronal cells contribute to long-latency stretch reflexes in the rhesus monkey. J Physiol 349: 249–272, 1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson et al. 2007.Davidson AG, Chan V, O'Dell R, Schieber MH. Rapid changes in throughput from single motor cortex neurons to muscle activity. Science 318: 1934–1937, 2007. [DOI] [PubMed] [Google Scholar]
- Elble and Koller 1990.Elble RJ, Koller WC. Tremor. Baltimore, MD: Johns Hopkins University Press, 1990.
- Evans and Baker 2003.Evans CMB, Baker SN. Task dependent inter-manual coupling of 10-Hz discontinuities during slow finger movements. Eur J Neurosci 18: 453–456, 2003. [DOI] [PubMed] [Google Scholar]
- Fisher 1993.Fisher N Statistical Analysis of Circular Data. Cambridge, UK: Cambridge Univ. Press, 1993.
- Gross et al. 2001.Gross J, Kujala J, Hamalainen M, Timmermann L, Schnitzler A, Salmelin R. Dynamic imaging of coherent sources: studying neural interactions in the human brain. Proc Natl Acad Sci USA 98: 694–699, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross et al. 2002.Gross J, Timmermann L, Kujala J, Dirks M, Schmitz F, Salmelin R, Schnitzler A. The neural basis of intermittent motor control in humans. Proc Natl Acad Sci USA 99: 2299–2302, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliday and Redfearn 1956.Halliday AM, Redfearn JW. An analysis of the frequencies of finger tremor in healthy subjects. J Physiol 134: 600–611, 1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hore and Flament 1986.Hore J, Flament D. Evidence that a disordered servo-like mechanism contributes to tremor in movements during cerebellar dysfunction. J Neurophysiol 56: 123–136, 1986. [DOI] [PubMed] [Google Scholar]
- Jackson et al. 2006.Jackson A, Baker SN, Fetz EE. Tests for presynaptic modulation of corticospinal terminals from peripheral afferents and pyramidal tract in the macaque. J Physiol 573: 107–120, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakuda et al. 1999.Kakuda N, Nagaoka M, Wessberg J. Common modulation of motor unit pairs during slow wrist movement in man. J Physiol 520: 929–940, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilner et al. 2000.Kilner JM, Baker SN, Salenius S, Hari R, Lemon RN. Human cortical muscle coherence is directly related to specific motor parameters. J Neurosci 20: 8838–8845, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemon 1984.Lemon RN Methods for Neuronal Recording in Conscious Animals. London: Wiley, 1984.
- Lemon and Porter 1976.Lemon RN, Porter R. Afferent input to movement-related precentral neurones in conscious monkeys. Proc R Soc Lond B Biol Sci 194: 313–339, 1976. [DOI] [PubMed] [Google Scholar]
- Lippold 1970.Lippold OC Oscillation in the stretch reflex arc and the origin of the rhythmical, 8–12 C-S component of physiological tremor. J Physiol 206: 359–382, 1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsden et al. 2001.Marsden JF, Brown P, Salenius S. Involvement of the sensorimotor cortex in physiological force and action tremor. Neuroreport 12: 1937–1941, 2001. [DOI] [PubMed] [Google Scholar]
- McAuley et al. 1999a.McAuley JH, Farmer SF, Rothwell JC, Marsden CD. Common 3 and 10 Hz oscillations modulate human eye and finger movements while they simultaneously track a visual target. J Physiol 515: 905–917, 1999a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAuley et al. 1999b.McAuley JH, Rothwell JC, Marsden CD. Human anticipatory eye movements may reflect rhythmic central nervous activity. J Neurosci 94: 339–350, 1999b. [DOI] [PubMed] [Google Scholar]
- Mehring et al. 2004.Mehring C, Nawrot MP, de Oliveira SC, Vaadia E, Schulze-Bonhage A, Aertsen A, Ball T. Comparing information about arm movement direction in single channels of local and epicortical field potentials from monkey and human motor cortex. J Physiol (Paris) 98: 498–06, 2004. [DOI] [PubMed] [Google Scholar]
- Porter and Lemon 1993.Porter R, Lemon RN. Corticospinal Function and Voluntary Movement. Oxford, UK: Oxford Univ. Press, 1993, p. 428.
- Raethjen et al. 2002.Raethjen J, Lindemann M, Dumpelmann M, Wenzelburger R, Stolze H, Pfister G, Elger CE, Timmer J, Deuschl G. Corticomuscular coherence in the 6–15 Hz band: is the cortex involved in the generation of physiologic tremor? Exp Brain Res 142: 32–40, 2002. [DOI] [PubMed] [Google Scholar]
- Rapisarda et al. 1992.Rapisarda C, Palmeri A, Sapienza S. Cortical modulation of thalamo-cortical neurons relaying exteroceptive information: a microstimulation study in the guinea pig. Exp Brain Res 88: 140–150, 1992. [DOI] [PubMed] [Google Scholar]
- Riddle and Baker 2005.Riddle CN, Baker SN. Manipulation of peripheral neural feedback loops alters human corticomuscular coherence. J Physiol 566: 625–639, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen and Asanuma 1972.Rosen I, Asanuma H. Peripheral afferent inputs to the forelimb area of the monkey motor cortex: input–output relations. Exp Brain Res 14: 257–273, 1972. [DOI] [PubMed] [Google Scholar]
- Rosenberg et al. 1989.Rosenberg JR, Amjad AM, Breeze P, Brillinger DR, Halliday DM. The Fourier approach to the identification of functional coupling between neuronal spike trains. Prog Biophys Mol Biol 53: 1–31, 1989. [DOI] [PubMed] [Google Scholar]
- Rosenblum and Pikovsky 2001.Rosenblum MG, Pikovsky AS. Detecting direction of coupling in interacting oscillators. Phys Rev E 64: 045202, 2001. [DOI] [PubMed] [Google Scholar]
- Tracey et al. 1980.Tracey DJ, Asanuma C, Jones EG, Porter R. Thalamic relay to motor cortex: afferent pathways from brain stem, cerebellum, and spinal cord in monkeys. J Neurophysiol 44: 532–554, 1980. [DOI] [PubMed] [Google Scholar]
- Tsumoto et al. 1975.Tsumoto T, Nakumura S, Iwama K. Pyramidal tract control over cutaneous and kinaesthetic sensory transmission in the cat thalamus. Exp Brain Res 22: 281–294, 1975. [DOI] [PubMed] [Google Scholar]
- Vallbo and Wessberg 1993.Vallbo AB, Wessberg J. Organization of motor output in slow finger movements in man. J Physiol 469: 673–691, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessberg and Kakuda 2001.Wessberg J, Kakuda N. Single motor unit activity in relation to pulsatile motor output in human finger movements. J Physiol 517: 273–285, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessberg and Vallbo 1995.Wessberg J, Vallbo AB. Coding of pulsatile motor output by human muscle afferents during slow finger movements. J Physiol 485: 271–282, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessberg and Vallbo 1996.Wessberg J, Vallbo AB. Pulsatile motor output in human finger movements is not dependent on the stretch reflex. J Physiol 493: 895–908, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams and Baker 2009a.Williams ER, Baker SN. Circuits generating corticomuscular coherence investigated using a biophysically based computational model. I. Descending systems. J Neurophysiol 101: 31–41, 2009a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams and Baker 2009b.Williams ER, Baker SN. Renshaw cell recurrent inhibition improves physiological tremor by reducing corticomuscular coupling at 10 Hz. J Neurosci 29: 6616–6624, 2009b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams et al. 2005.Williams ER, Soteropoulos D, Baker MR, Baker SN. Motor cortical activity is synchronous with 10 Hz discontinuities during slow finger movements in monkey. Proc Physiol Soc UCL Meeting: C17, 2005.
- Witham and Baker 2007.Witham CL, Baker SN. Network oscillations and intrinsic spiking rhythmicity do not covary in monkey sensorimotor areas. J Physiol 580 801–814, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witham et al. 2007.Witham CL, Wang M, Baker SN. Cells in somatosensory areas show synchrony with beta oscillations in monkey motor cortex. Eur J Neurosci 26: 2677–2686, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]