Abstract
Spontaneous regeneration of vestibular and auditory receptors and their innervating afferents in birds, reptiles, and amphibians are well known. Here, we produced a complete vestibular receptor loss and epithelial denervation using an ototoxic agent (streptomycin), after which we quantitatively characterized the afferent innervation of the horizontal semicircular canals following completed regeneration. We found that calyx, dimorph, and bouton afferents all regenerate in a manner the recapitulates the epithelial topography of normal birds, but over a slow time course. Similar to previous findings in the vestibular otolith maculae, regeneration occurs according to a three-stage temporal sequence. Bouton afferents regenerate during the first month of regeneration, followed by calyceal-bearing afferents in the second and third months. Calyx afferents were the last to regenerate in the final stage of recovery after 3 mo. We also found that regenerated afferents exhibited terminal morphologies that are significantly smaller, less complex, and innervate fewer receptor cells over smaller epithelial areas than those that develop through normative morphogenesis. These structural fiber changes in afferent innervation correlate to alterations in gaze responses during regeneration, although the exact underlying mechanisms responsible for behavioral changes remain unknown. Plasticity in central vestibular neurons processing motion information seem to be required to explain the observed morphologic and response adaptations observed in regenerating vestibular systems.
INTRODUCTION
Vestibular receptor loss through disease or trauma immediately results not only in compromised oculomotor and postural responses, but also in dysfunctions in more complex orientation and navigation behaviors (Carey et al. 2002; Crane and Demer 1998; Dickman and Lim 2004; Haque et al. 2006; Newlands et al. 2001; Stackman and Taube 1997). For example, it has been long known that certain aminoglycoside antibiotics are ototoxic to both vestibular and auditory receptors and produce receptor cell loss and afferent denervation (Berg 1951; Wersäll and Hawkins Jr 1962). Remarkably, in many vertebrates, including amphibians, reptiles, and birds, spontaneous regeneration of hair cells and reinnervation of the receptor epithelium occurs (Corwin and Cotanche 1988; Masetto and Correia 1997a; Weisleder and Rubel 1992; Zakir and Dickman 2006). In addition, hair cell function (Masetto and Correia 1997b), afferent responses to motion (Boyle et al. 2002; Li and Correia 1998), and vestibular-mediated behavioral responses (Carey et al. 1996; Dickman and Lim 2004; Goode et al. 1999; Haque et al. 2008) have all been shown to recover during regeneration.
To understand the regeneration process, it was first necessary to examine the normal topography and innervation patterns of vestibular receptors. As in all amniotes, birds have two hair cell types (Lindeman 1969; Rüsch et al. 1998; Wersäll 1956) and three classes of vestibular afferent innervation (Brichta and Peterson 1994; Fernandez et al. 1988, 1990, 1995; Haque et al. 2006; Lysakowski and Goldberg 1997; Ramón y Cajal 1908; Schessel et al. 1991; Si et al. 2003; Zakir et al. 2003). Bouton afferents exclusively innervate type II hair cells with bouton terminals. Calyx units innervate type I hair cells with a calyceal terminal that encloses one or multiple hair cells. Dimorph afferents contain calyceal and bouton terminals to innervate both type I and type II hair cells. Both hair cell types and afferent innervation classes exhibit regional topography in bird vestibular epithelia (Haque et al. 2006; Si et al. 2003; Zakir et al. 2003). In pigeon horizontal semicircular canals, calyceal-bearing afferents (calyx and dimorph fibers) are centrally located in the crista, whereas bouton afferents populate the receptor periphery (Haque et al. 2006).
In pigeons, we recently established that complete regeneration of vestibular macular receptors and their afferent innervation following a total receptor loss occurs over many months, according to a specific temporal sequence (Zakir and Dickman 2006). Three stages of regenerative recovery were observed, with different receptor cell types and afferent terminal morphologies developing sequentially. Stage 1 regeneration lasted through the first month following the initial lesion (poststreptomycin treatment [PST]) and was characterized by the exclusive low-density development of type II hair cells and bouton afferents (Masetto and Correia 1997a,b; Zakir and Dickman 2006). No calyceal-bearing afferents were present in the maculae. Stage 2 regeneration (between 6 and 12 wk PST), was characterized by hair-cell densities that had increased to 50% that of normal bouton afferents that exhibited increasing terminal fields and the first calyceal terminals being developed. No true calyx afferents innervating only type I hair cells were observed during most of stage 2 regeneration, although a few rudimentary calyx afferents were observed late at 12 wk PST. Stage 3 regeneration (18–24 wk PST) was marked by the completed morphologic recovery at 6 mo PST. Most notable in stage 3 regeneration was the return of the calyx afferents to the central striolar regions of the maculae. Although regenerative formation reproduced much of the normal receptor phenotype, our findings showed that there were significant differences in afferent innervation following complete regeneration (Zakir and Dickman 2006). For example, regenerated otolith afferents were 20–35% reduced in size (smaller and less branched), innervated fewer hair cells, and covered a smaller area of the epithelium (30% reduction) than did afferents innervating the normal bird maculae (Zakir and Dickman 2006).
Since our previous regeneration morphological investigations were restricted to examination of the otolith macular receptors, we wondered whether regeneration of the semicircular canal receptors followed a similar temporal pattern and resulted in significant differences in innervation phenotype. In the investigation reported here, we produced a total receptor loss and denervation of the horizontal semicircular canal crista in pigeons using an applied single dose of intralabyrinthine ototoxic antibiotic. We then examined afferent reinnervation of the crista sensory epithelium through a 6-mo time course. Quantitative comparisons between afferent terminal morphologies in normal birds and regeneration birds were made. Our present findings show that similar to macular afferents, semicircular canal fibers regenerate slowly over months, with final innervation patterns that exhibit significant differences in their terminal morphologies.
METHODS
Animals
Adult pigeons (Columba livia) ranging in age between 1 and 2 yr before introduction into experiments were used. The birds ranged in weight from 400 to 700 g. Two groups of birds were included for study. Regeneration pigeons were given an ototoxic antibiotic treatment (see following text) to produce a complete vestibular receptor lesion, then allowed to recover fully before study. Normal birds were maintained in identical conditions, although they received no ototoxic treatment. All animals were used in accordance with the guidelines established by the National Institutes of Health Guide for the Care and Use of Animals in Research and those approved by the Institutional Animal Studies Committee. Animals were housed and cared for in the Laboratory Animal Facilities under veterinary supervision.
Receptor lesion
To produce a complete lesion of all horizontal semicircular canal vestibular hair cells and denervation of the receptor cristae, a bilateral intralabyrinthine application of streptomycin was administered (Dye et al. 1999;Frank et al. 1999). Each bird was anesthetized using isoflurane gas and an incision was made over the mastoid bone directly posterior to the external ear canal. Next, a rectangular-shaped bone flap of mastoid cortex was resected, exposing the underlying bony labyrinth. A small-diameter (0.35-mm) hole was trephined into the vestibule promontory bone immediately posterior to the oval window, using a diamond bur. A single dose of streptomycin (2 mg in 1 μl saline; frozen pellet) was carefully placed through the bur hole into the perilymphatic space. A muscle plug was then used to seal the opening, being overlaid with gel foam and bone wax. The mastoid bone flap was approximated and the incision was sutured closed. This procedure was then repeated on the contralateral side. Buprenorphine (0.08 mg/kg, administered intramuscularly [im]) was given for analgesia and the animal was returned to its home cage once standing and alert. Each bird was monitored for behavioral symptoms of vestibular lesion and for weight loss. All birds were hand fed twice daily (Kay-tee formula) for about 2 wk postoperatively until spontaneous feeding resumed and weight loss stabilized.
Neural tracer application
All regeneration birds were allowed to recover for 2 wk to 12 mo following streptomycin treatment. Previous studies in our laboratory using identical ototoxic lesion methods have established that regenerative recovery is complete in the otolith maculae by 6 mo PST (Dye et al. 1999; Zakir and Dickman 2006). Normal birds were composed of appropriately age matched controls. For each animal, biotinylated dextran amine (BDA) was injected into the vestibular nuclei and allowed to transport retrograde along horizontal semicircular canal (HCC) afferents for 12 days (Si et al. 2003; Zakir and Dickman 2006; Zakir et al. 2003). The animal was anesthetized with isoflurane gas, intubated, then placed into a stereotaxic device (Kopf Instruments). An incision over the parietal bone was made and a bone flap resected. A glass micropipette filled with BDA (10% in saline, 10,000 MW; Molecular Probes) was lowered into the brain using predetermined coordinates for the vestibular nuclei (Dickman and Fang 1996). The injection site was purposely varied among animals to provide a complete sampling of HCC afferents due to regional differences in their central projections (Dickman and Fang 1996). BDA was iontophoretically passed into the brain with positive current (8 μA; 50% duty cycle at 7 s for 20 min). Following this, the electrode was allowed to remain in position for a few minutes, then a small negative backing current (0.02 μA) was applied and the electrode retracted. The bone flap was approximated and the skin sutured closed. Buprenorphine (0.08 mg/kg, im) was given for analgesia and the animal was returned to its home cage once standing and alert.
Histology
After 12 days of post-BDA injection survival, the birds were reanesthetized with pentobarbital sodium (20 mg/kg, administered intravenously). The bony labyrinths were quickly exposed and 2-mm openings made into the horizontal and posterior canal walls. A bilateral intralabyrinthine perfusion was performed using 3% glutaraldehyde, 2% paraformaldehyde, and 1% acrolein in 0.1 M phosphate buffer (PBS). Following this, a transcardial perfusion was performed using a sodium nitrite (1% in saline) wash, followed by a 2% glutaraldehyde, 1% paraformaldehyde in 0.1 M sodium phosphate buffer (500 ml). Next, the skull was opened and the whole head was placed in the same aldehyde solution overnight, then rinsed and kept in buffer (PBS). The membranous labyrinths were then excised and the horizontal semicircular canal cristae dissected free. The brain was excised, cryoprotected in 30% sucrose solution overnight, then frozen and serially sectioned (50 μm) in the transverse plane. Next, the cristae and brain sections were blocked from endogenous peroxidase reactions by incubation in 1% H2O2 in 90% methanol for 15 min. These tissues were then processed for BDA histochemistry using a modified diaminobenzidine (DAB) procedure (Brandt and Apkarian 1992). Briefly, the tissues were incubated for 16 h in a solution of 10 μg/ml avidin d-HRP (Vector A-2004) and 0.3% Triton-X100 in PBS. The tissue was then rinsed and reacted using the chromogen diaminobenzidine (DAB, 0.5 mg/ml), with 0.005% nickel chloride and cobalt acetate as enhancers. The reaction was initiated with 0.05% H2O2 until a dark product was visualized, then stopped with PBS rinsing. The reacted maculae were first photographed in whole mount using a Nikon dissecting light microscope. Next, the cristae were embedded into plastic (Durcupan) and serially sectioned (10-μm thickness) using a rotary microtome. Brain sections were mounted on glass slides and counterstained (Richardson et al. 1960).
SEM preparation
In several normal and regeneration birds, the horizontal crista on one side was not used for afferent neural tracing, but was instead prepared for examination by scanning electron microscopy (SEM). The cristae were first rinsed in distilled water and dehydrated using a series of graded acetones (10 min each in 70, 90, and 95% acetone), followed by three washings in 100% acetone for 15 min each. The tissue was then incubated in increasing ratios of a mixture of tetramethylsilane (TMS) and acetone (1:1 and 3:1) for 45 min each, followed by two incubations in 100% TMS for 1 h each. Finally, the tissue was dried at 60°C for 30 min, allowing the TMS to sublimate. The dried maculae were mounted on aluminum studs and gold sputter coated. Tissues were imaged using a Hitachi 2600 SEM (20 kV).
Afferent reconstruction
The injection sites for the BDA within the vestibular nuclei were confirmed from the brain sections and only HCCs from animals without labeled somas in the vestibular efferent nuclei were included for further study. Thus terminal morphologies examined were of primary HCC afferents. First, a contour of the epithelial surface was drawn on every other HCC section (100-μm resolution) using video microscopy (Nikon E600, ×60 oil) and a three-dimensional (3D) image analysis reconstruction program (Neurolucida; MicroBrightfield). Next, a 3D map of the epithelial surface was plotted using the traced contours. Then, labeled afferents were initially quantified as to terminal type and measured locations within the crista sections. Afferent fibers that were darkly stained and sufficiently isolated for accurate reconstruction were traced to establish the complete morphological structure of the terminal innervation within the crista epithelium. Afferents with light staining (ghost fibers) or those that severely overlapped other afferents were not traced. For each traced afferent, a number of morphological parameters were quantified.
Axonal diameter: the overall average diameter of the last 5 μm before penetration through the basement membrane.
Terminal fiber length: summation of all branching segments within the neural epithelium.
Terminal branches: number of branch segments per fiber.
Branch order: defined as the number of branching levels past the initial segment, which was assigned a branch order of 1 (see Fig. 4I).
Bouton terminals: number of identified en passant and boutons terminaux per fiber.
Calyx hair cells: total number of type I hair cells contained in calyceal terminals per fiber.
Calyceal volume: measured 3D volume of all contours drawn at 1-μm focal plane intervals through the calyceal terminal.
Innervation area: calculated by first rotating the reconstructed fiber image in 3D virtual space until an apical view was obtained. Next, the perimeter of the image was drawn using a contoured polygon planimeter (Neurolucida) in the plane of the macular surface. Since the crista surface was curved, the rotation angle differed depending on location within the epithelium. However, each area was calculated from a planar view orthogonal to the epithelial surface. The area of the polygon was then used as the innervation area in μm2.
Terminal density: calculated by dividing the total terminals (number of type I hair cells + number of bouton terminals) by the innervation area. The result was multiplied by a factor of 100, to give a product of terminal density/100 μm2 of epithelial surface area.
Statistical analyses
All afferents were first divided into three main groups based on morphological innervation patterns, including calyx, dimorph, and bouton afferent classes. Comparisons of afferents between classes and within classes between normal and regenerated HCC cristae were made using ANOVAs with commercial software (Statistica; Statsoft, Tulsa, OK). All post hoc comparisons were made using the Sheffé follow-up test.
RESULTS
Horizontal semicircular canal hair cell density
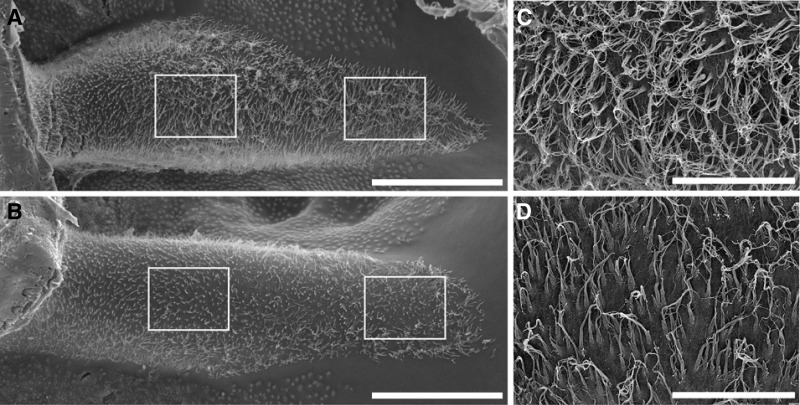
To initially characterize the extent of receptor recovery following complete regeneration, we examined the density of hair cells (HCs) in two areas of the crista epithelium. SEM photomicrographs were obtained from five normal birds (ages between 1.5 and 3 yr) and five regeneration birds (6–12 mo PST). As shown in Fig. 1 A, two 100 × 120-micron counting frames were placed over the apical surface of both the central apex and torus regions (see Fig. 2 for descriptions) of the basal HC crista. These frames did not include hair cells in the planum region (Flock and Orman 1983; Haque et al. 2006; Masetto and Correia 1997a; Fig. 2). All hair cells within the counting frames were quantified by their presence of stereocilia bundles. No attempt was made to broaden the counting frame down the peripheral crista slopes, where difficulties in identification of distinct cell borders arose due to parallax. As shown in Fig. 1, hair cells containing extremely long stereocilia were observed in the torus region, whereas hair cells located in the central apex region had decreased lengths. This was true for both normal (Fig. 1, A and C) and regenerated (Fig. 1, B and D) HC cristae (see Haque et al. 2006 for a more detailed description). In normal birds, the number of hair cells in the apex frame ranged between 208 and 242 receptors, with a mean value of 232 (SD: ±14) hair cells. The mean hair cell density was calculated to be 1.94 (SD: ±0.12) receptors/100 μm2. Although the range of hair cells observed (225–276), mean number of hair cells [245 (SD: ±22) hair cells] and hair cell density [2.04 (SD ±0.18) hair cells/100 μm2] values were slightly higher for the torus region in normal HC cristae, these measures were not significantly different from those of the apex region (ANOVA, P > 0.05). Similar to our previous findings (Dye et al. 1999; Frank et al. 1999; Zakir and Dickman 2006), at 4 days PST the epithelium was completely denuded of hair cells due to streptomycin ototoxicity (data not shown). Complete hair cell loss was verified in three birds by direct histological examination of receptor epithelia (serial cross sections) for one lesioned side and correlated with SEM observations of total stereocilia absence for receptors on the contralateral side. We previously reported a more thorough examination of the complete lesion effects on vestibular receptor epithelia by streptomycin application in pigeons (Dye et al. 1999; Frank et al. 1999; Zakir and Dickman 2006).
FIG. 1.
Scanning electron photomicrographs of horizontal semicircular canal cristae. A: normal hair cell (HC) crista with two 120 × 100-μm counting frames superimposed. B: regeneration HC crista at 9 mo poststreptomycin treatment (PST). C: magnified view of central counting frame in A. D: magnified view of central counting frame in B. Scale bars: A, B = 200 μm; C, D = 50 μm.
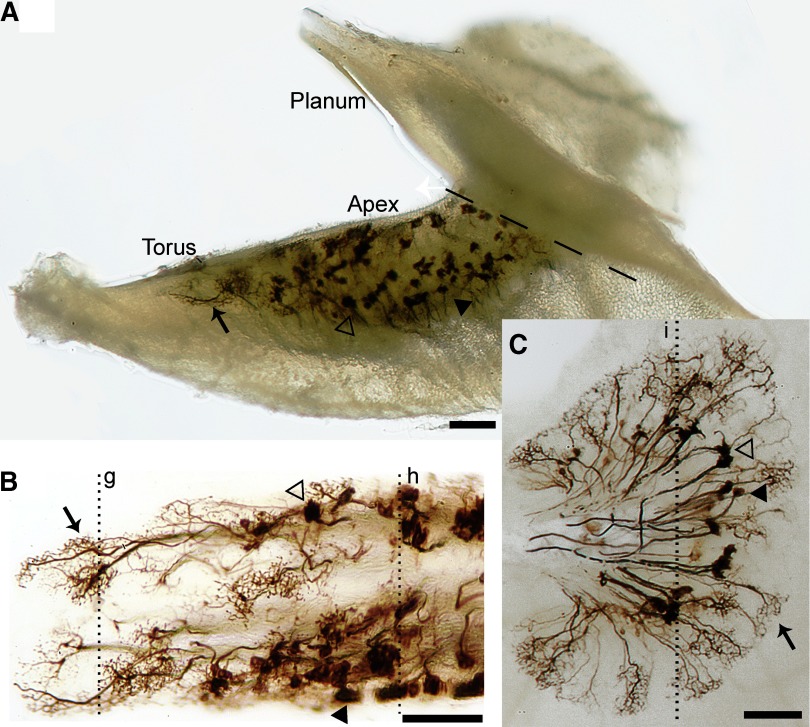
FIG. 2.
Stereo-photomicrophraphs of a single regeneration HC crista. A: HC crista epithelium viewed from the posterolateral surface. Biotinylated dextran amine (BDA)–labeled calyx (black arrowhead), dimorph (open arrowhead), and bouton (black arrows) afferents can be seen throughout the torus (left) and apex regions (middle). The planum (right) contains labeled afferents that are hidden from view. The dashed line indicates the location that a cut was made for better visualization of the apex (B) and planum (C) regions. Dotted lines g–i indicate sections illustrated in Fig. 3. Scale bars: 100 μm.
At 6 mo PST, receptor regeneration was complete. Based on our previous findings for vestibular regeneration, no significant additional hair cell proliferation or afferent innervation occurs with longer recovery periods (Frank et al. 1999; Haque et al. 2006; Zakir and Dickman 2006). As shown in Fig. 1, B and D, following 6 mo of regeneration, the regional differences in hair-cell density, stereocilia formation, and hair-cell polarity were similar to those of normal animals. Although lower cell numbers and densities were sometimes observed in individual bird regenerated HC cristae, the overall mean differences for the five normal and regeneration animals were only marginally different (ANOVA, P = 0.074). The number of regenerated hair cells ranged between 175 and 228 for the apex and between 101 and 308 for the torus, with mean values of 204.8 (±26.5) and 225.2 (±82.5) hair cells, respectively. Accordingly, the cell density was also marginally reduced (ANOVA, P = 0.075) in regeneration birds, with mean values of 1.7 (±0.22) and 1.9 (±0.69) hair cells/100 μm2 for the apex and torus regions, respectively.
Afferent type and regional location
Neural tracer injections into the vestibular nuclei complex produced identifiable retrograde labeled afferents in eight normal and seven regenerated HC cristae at 6 mo PST. As shown in the whole organ view of Fig. 2, the regenerated HC cristae exhibited a regional topography of afferent types. Figure 2A shows a posterolateral view of the crista, with the torus on the left and central apex to the right. Afferents in the planum were hidden from view. Therefore the epithelium was cut through the isthmus of the crista that joins the apex and planum regions (dashed line in Fig. 2A), leaving the central apex and torus regions in one tissue piece (Fig. 2B) and the planum (Fig. 2C) in a second piece laid flat for better visualization. After the whole tissue pieces were photographed, they were sectioned to quantify the 3D morphological structure. Calyceal-bearing afferents were observed only in the apex and central planum regions of the crista. These fibers constituted a mixed population of both calyx (black arrowheads) and dimorph (open arrowheads) afferents, with no clear topographic separation. Bouton afferents (black arrows) were the only fiber type observed in the torus. Bouton fibers were also located in a small portion of the central apex and the peripheral region of the planum. A few scattered bouton fibers were additionally observed in the extreme periphery of the central zone, at the edges of the epithelium. These regional distributions of regenerated calyceal-bearing and bouton-type afferents were largely similar to those observed in normal animals, except that some expansion in the calyceal-bearing fibers was noted (see following text; Haque et al. 2006).
Temporal sequence of regeneration
Recently, we reported that morphological regeneration of the vestibular receptors in the otolith maculae occurs along a three-stage temporal sequence (Zakir and Dickman 2006).
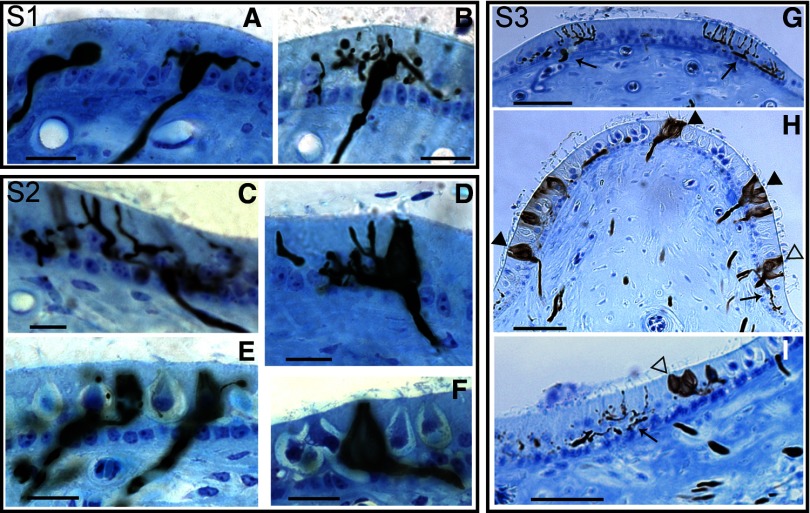
To determine whether the cristae follow a similar recovery time course, we qualitatively examined hair cell and afferent innervation in birds at specific regeneration windows, including 1, 2, 4, 6, 12, and 24 wk PST (26 cristae total). As shown in Fig. 3, three stages of regeneration could be identified. During the first 4 wk (stage 1) of PST regenerative development, BDA-labeled afferents were observed to innervate the entire cristae. However, these fibers contained only bouton and en passant type terminals. No calyceal terminals were ever observed. As shown in Fig. 3A early-regeneration fibers penetrated the basement membrane with growth cones and began to collateralize. For the first 2 wk PST, most fibers observed consisted of a single axonal segment with terminal growth cones. Some fibers (Fig. 3A) initiated simple collateralizations with a few branch segments, each of which terminated in growth cones. Growth cones took various forms, including large canonical or elliptical structures (Fig. 3, A–D), digitiform appendages (Fig. 3, C and D), or rarely, fibril outgrowths (Fink and Morest 1977; Ramón y Cajal 1908; Skaliora et al. 2000; Tosney and Landmesser 1985). Often, fiber segments contained large prominent swellings (Fig. 3, A–E). These appeared to be nodes for budding new branch segments, in that ramifying branches were often observed to emanate from prominences (Fig. 3B). By 4 wk PST (Fig. 3B), a number of fibers had increased arborizations, but many simple fibers were also observed, including single branch fibers just penetrating the basement membrane. A number of developing fibers contained both branches with growth cones and slender branches that terminated in bouton terminals of typical appearance (Fig. 3B). Many of these branches appeared to target and even terminate near developing hair cells.
FIG. 3.
Stages of regeneration. S1: denotes stage 1 regeneration observed between 1 and 4 wk PST. A: 2 wk PST, with growth cone (left) and developing bouton fiber (right). B: more advanced developing bouton fiber at 4 wk PST. S2: denotes stage 2 regeneration, with mostly bouton fibers regenerating at 6 wk PST (C). Calyceal-bearing dimorph afferents first regenerate at 6 wk PST (D). At 12 wk PST, many dimorph afferents are developed, some with only a single calyceal terminal and branch fiber (E). Simple calyx afferents first develop at 12 wk PST (F). S3: denotes completed stage 3 regeneration (24 wk PST). Torus (G), apex (H), and left half of planum (I) regions of the S3 crista are shown. Bouton afferents (black arrow) were the only fiber type observed in the torus region, but were also found in the peripheral apex and planum regions. Calyceal-bearing afferents, including calyx (black arrowhead) and dimorph (open arrowhead) fibers, were located only in the apex and central planum regions. Scale bars: 10 μm (A–F); 25 μm (G–I).
During the second stage of regeneration, extending from 6 to 12 wk PST, a significant change in innervation was observed by the first appearance of calyceal terminals. Although new initial segment fibers could still be observed to enter the epithelium at 6 wk PST, many other existing fibers exhibited increased terminal arborization complexity. As shown for a bouton afferent in the torus region in Fig. 3C, newly developed branches were present, some with growth cones and some with terminal boutons. In addition, regenerating calyceal terminals and identifiable mature type I hair cells were also observed for the first time at 6 wk PST (Fig. 3D). Although often difficult to discriminate type I and type II hair cells during development or regeneration without high-resolution electron microscopy (Rüsch et al. 1988), we conservatively identified mature type I hair cells by morphologic phenotype indicators, including: 1) amphora shape, 2) apical location of nucleus, 3) constricted neck, and 4) calyceal terminal. Many calyceal terminals were incomplete, with partial encapsulations that extended from the basal toward the apical portion of the type I hair cell. Whether partial or complete, all of the observed calyceal terminals were contained on dimorph afferents, since these fibers all exhibited additional branches with either growth cones or terminal boutons. In the latter part of stage 2 regeneration (12 wk PST), the epithelium became more densely populated by afferent fibers. There were fewer entering initial fibers observed and most of these were located in the central regions of the apex and planum regions of the crista. Most of the observed bouton fibers continued to mature, with few growth cones and increased numbers of en passant and boutons terminaux. As shown in Fig. 3E, many simple dimorph fibers were present, most with one or several small branch segments. Other more complex dimorph afferents were also observed. Type I hair cells were increasing in concentration in the central regions of the crista (Fig. 3E). In addition, at 12 wk PST, calyx afferents were observed for the first time, as illustrated in Fig. 3F. Calyx afferents were confined to the central apex region and were not very numerous. Of the observed calyx afferents, all had simple structures, innervating 1 or 2 hair cells and small innervation areas. A count of all BDA-filled fibers in three 12 wk PST HC cristae resulted in the following distribution: 56% bouton, 38% dimorph, and 6% calyx afferents (n = 288).
At 6 mo PST, afferent reinnervation of the epithelium was complete, the terminal point of stage 3 regeneration. Here we observed that bouton, dimorph, and calyx afferents all densely innervated all regions of the receptor epithelium. For all afferents, branch segments ended with either calyceal or bouton terminals and no new growth cones were observed. In addition, no new initial fibers were observed to enter the crista epithelium. Based on these criteria, we concluded that vestibular afferent regeneration was essentially complete, similar to our previous observations for regeneration of the otolith maculae (Zakir and Dickman 2006). Unlike the earlier stages of regeneration in the HC cristae, we quantified afferent innervation at the end of stage 3 regeneration. In Fig. 3, three sections (dotted lines) taken from the HC crista of Fig. 2, including the torus (Fig. 3G), apex (Fig. 3H), and planum (Fig. 3I) regions are illustrated, respectively. Only type II hair cells were located in the torus, whereas in the apex and planum regions a mixture of type II and type I hair cells was observed. In the torus, regenerated bouton fibers quickly ramified into multiple branches and exhibited en passant and bouton terminals (Fig. 3, G–I, black arrows). These fibers were exclusive to the torus and peripheral planum regions. A few boutons were also observed in the extreme peripheral edges of the apex (Fig. 3H). In contrast, calyx (black arrowheads) and dimorph afferents (open arrowheads) were heterogeneously distributed through the apex of the crista, with no clear topographic organization (Fig. 3H). In the planum, a high concentration of dimorph afferents was observed, whereas only a few calyx fibers were noted (Fig. 3I). As shown in the sectioned tissue (Fig. 3, G–I), the HC cristae are not flat. In the torus region, the crista contains a slight central enlargement. However, in the apex, the crista is steeply curved into a U-shaped epithelium with afferents innervating the entire sensory surface. The planum is bisected by the stromal wall of the crista, with only the left half being shown in Fig. 3I. The planum sensory epithelium is flattened, with only a slight inward curvature.
Innervation patterns of the regenerated HC crista
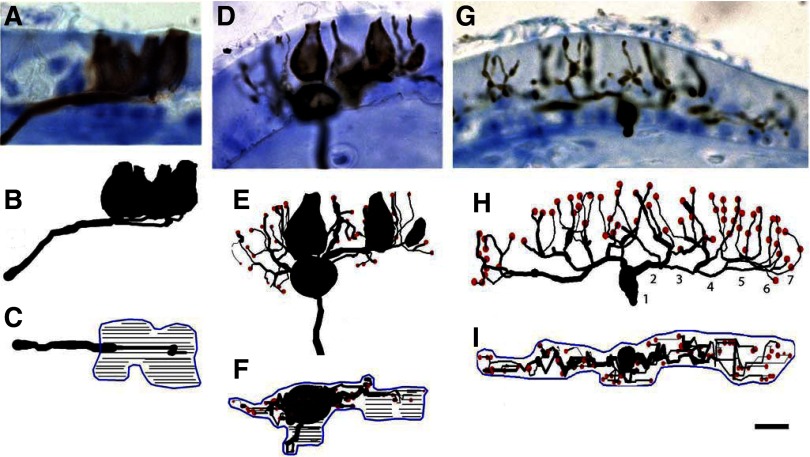
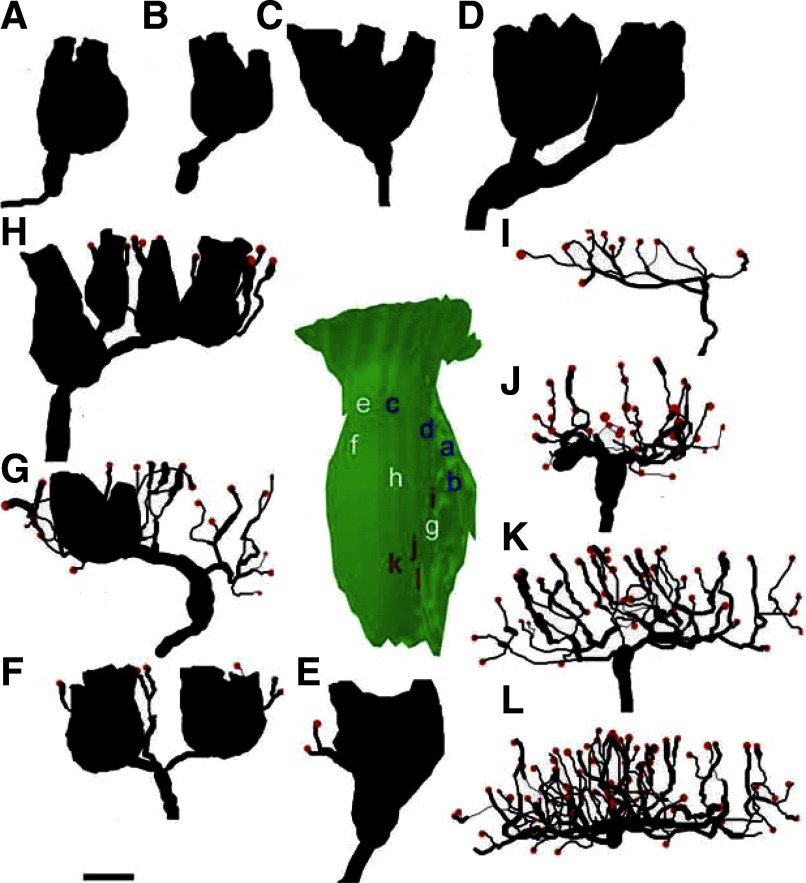
In a recent study from our laboratory, we characterized the regional distribution and terminal morphology of vestibular afferents innervating the horizontal semicircular canal crista in adult normal pigeons (Haque et al. 2006). The data from that study (91 afferents) were combined with new additional normative data (65 afferents) currently obtained for comparisons against the innervation patterns of regenerated fibers. In all, 156 normal and 132 regenerated stage 3 HC afferents were quantified through anatomical reconstructions. Figure 4 shows photomicrographs and anatomical reconstructions of the terminal innervation patterns for representative calyx, dimorph, and bouton regenerated afferents. Each photomicrograph was taken at the point of axonal penetration through the basement membrane. Below the photomicrograph are two reconstruction views. The first reconstruction view is in a transverse plane equal to that of the section and the second is a 90° rotated apical surface view illustrating the terminal field around which the innervation area contour was drawn. For each type of afferent, two general categorizations could be made based on terminal morphology. First, a subjective examination of the apical surface view revealed that each terminal innervation pattern appeared to be arranged in either a “flower” or a “linear” profile. This was similar to pigeon afferents innervating both the semicircular canals (Haque et al. 2006) and otolith maculae (Si et al. 2003; Zakir et al. 2003). Flower profiles (Fig. 4, A–F) consisted of terminals (calyceal and/or boutons) arranged in a rounded or petal formation. Linear profiles (Fig. 4, G–I) consisted of elongated, narrow terminal fields. Second, each afferent type could be classified as being either simple or complex, depending on the number of calyceal terminals, hair cells per calyx, and/or branch order. The locations of all regenerated afferents that were quantified through anatomical reconstructions are shown in Fig. 5.
FIG. 4.
Photomicrographs and anatomical reconstructions of horizontal semicircular canal regenerated afferents. Photomicrographs and anatomical reconstructions are illustrated for 3 BDA-labeled fibers. Calyx (A–C), dimorph (D–F), and bouton (G–I) afferents with transverse section micrographs (A, D, G) and reconstructions for the transverse (B, E, H) and surface (C, F, I) views, respectively. In the surface reconstructions, calyceal contours were drawn at focal plane intervals of 1 μm (thin black lines) and innervation areas were measured using a digital planimeter around the apical surface view of the terminal pattern (blue lines). Terminal boutons are indicated by red circles. In H, numbers indicate branch order of fiber segments. Scale bar: 5 μm.
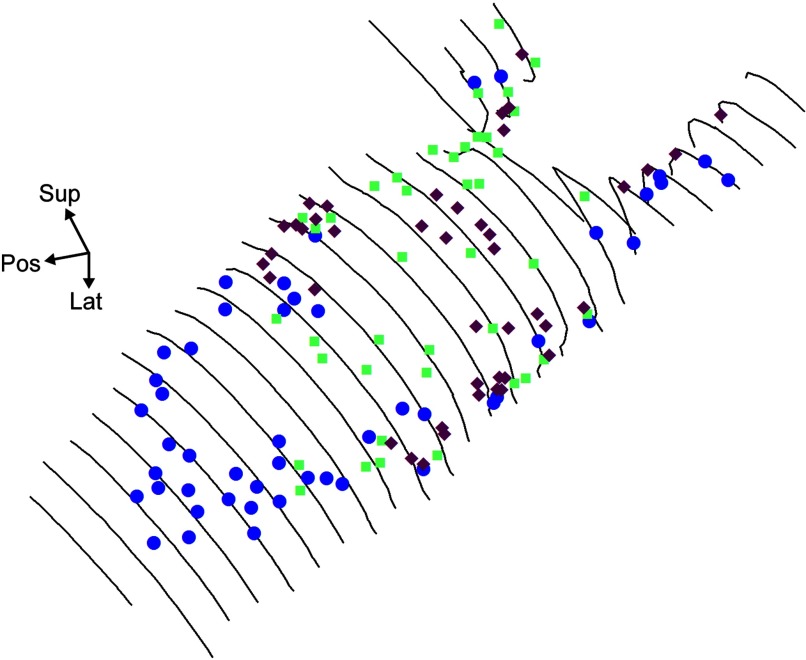
FIG. 5.
Wireframe surface map of the HC crista with locations of reconstructed regeneration afferents. Surface contours are separated by 100-μm sections. Calyx (diamonds) and dimorph (green) afferents were primarily observed in the central regions of the crista. Bouton (blue) afferents were found in the torus and peripheral regions of the crista.
CALYX AFFERENTS.
In all, 43 regenerated calyx units were reconstructed. Calyx afferents were located in the central portions of the apex, isthmus, and planum regions of the HC crista (Fig. 5). Similar to normal birds, calyx units exclusively innervated one or more type I hair cells with calyceal terminals (Fig. 4, A–C). The size, structure, and complexity of the calyx afferent innervation patterns, from the simple to the more complex, are illustrated in Fig. 6. Also shown is the epithelial location for each. Table 1 shows the range and mean values for the regenerated afferent terminal innervation parameters. Generally, regenerated calyx fibers had short, unbranched fiber segments that terminated adjacent to the epithelial penetration. The simplest calyx fiber observed had a single branch fiber and one calyceal terminal that contained one hair cell, although these very simple innervations were extremely rare (2/43 reconstructed). More typical were afferents with a single calyceal terminal innervating two to four hair cells (Fig. 6, A and B). These fibers had smaller calyceal volumes, with small terminal fields and innervation areas. Since the terminal density was defined by the number of hair cells within the terminal area, these values were also characteristically low. The more complex calyx afferents had a single large calyceal terminal that innervated five or six type I hair cells (Fig. 6C). Accordingly, the calyceal volumes, innervation area, and terminal density also increased. Although in our previous study of normal HC crista, complex calyces were defined as contacting at least seven type I hair cells, only one such afferent was observed in regenerated epithelia and it had a single calyceal terminal that innervated nine hair cells. Finally, several regenerated calyx fibers (6/43) exhibited multiple branches and two to three calyceal terminals with two to three hair cells each (Fig. 6D). These units had smaller calyces, but large innervation areas.
FIG. 6.
Anatomical reconstructions for stage 3 regenerated HC crista afferents. Calyx (A–D), dimorph (E–H), and bouton (I–L) afferent reconstructions arranged in increasing order of complexity. For all reconstructions, fiber branch segments and calyceal terminals are in black, bouton terminals in red. Composite surface map (green) of the HC epithelium shows the relative locations of the afferents featured in the reconstructions. Scale bar: 10 μm.
TABLE 1.
Statistical comparisons for regenerated afferents
| Comparison | Mean—Range |
Main Effect Value | Scheffé Post Hoc | ||||
|---|---|---|---|---|---|---|---|
| Calyx | Dimorph | Bouton | Calyx vs. Dimorph | Calyx vs. Bouton | Dimorph vs. Bouton | ||
| Axon diameter | 2.5 (0.9) 0.5–5.0 | 2.5 (0.7) 1.3–4.1 | 2.3 (0.58) 1.3–3.9 | F(2,129) = 1.07 | P = 0.99 | P = 0.45 | P = 0.46 |
| Branches | 1.2 (0.6) 1–3 | 10.6 (6.9) 1–36 | 25.3 (16.9) 9–75 | F(2,129) = 56.3 | P < 0.001 | P < 0.001 | P < 0.001 |
| Branch order | 1.1 (0.4) 1–2 | 4.1 (1,5) 1–8 | 6.1 (2.0) 3–12 | F(2,129) = 125.6 | P < 0.001 | P < 0.001 | P < 0.001 |
| Fiber length | 33.3 (19.9) 4.5–87 | 130.6 (79.7) 28–413 | 258.2 (165.7) 89.0–800.3 | F(2,129) = 47.9 | P < 0.001 | P < 0.001 | P < 0.001 |
| Fiber volume | 241.2 (168.8) 27–677 | 304.4 (232.8) 57–1,136 | 292.7 (238.6) 75.6–1,491.7 | F(2,129) = 1.03 | P = 0.41 | P = 0.53 | P = 0.97 |
| Innervation area | 298.8 (130.5) 63–610 | 377.3 (138.7) 83–731 | 309.6 (196.6) 70.2–755.8 | F(2,129) = 3.0 | P = 0.8 | P = 0.95 | P = 0.14 |
| Type 1 HC | 3.7 (1.5) 1–9 | 3.0 (1.4) 1–7 | — | F(1,82) = 6.0 | P < 0.05* | — | — |
| Calyceal volume | 3,506 (1,406) 1,133–7,702 | 2,514 (1,906) 204–8,360 | — | F(1,82) = 7.4 | P < 0.01* | — | — |
| Boutons | — | 7.0 (5.0) 1–25 | 23.6 (15.4) 5–72 | F(1,87) = 43.7 | — | — | P < 0.001* |
| Terminal density | 1.4 (0.7) | 3.1 (2.6) 0.6–4.8 | 8.3 (3.6) 0.7–16.9 3–21 | F(2,129) = 83.6 | P < 0.05 | P < 0.001 | P < 0.001 |
Values are means, with SD in parentheses.
Denotes one-way ANOVA only.
DIMORPH AFFERENTS.
In all, 41 regenerated dimorph afferents were reconstructed. Similar to normal fibers, each dimorph unit contained both calyceal and bouton terminals (Fig. 4, D–F). The dimorph units were more widely distributed than were calyx afferents, with fibers being observed throughout the planum, isthmus, and apex regions of the crista (Fig. 5). Unlike normal birds, a few regenerated dimorph units were also observed at the edge of the torus and in the peripheral planum regions (Fig. 5). Apparently, some regenerated dimorph units had expanded beyond the range of epithelial locations normally observed (Haque et al. 2006). Similar to normal dimorph afferents, regenerated terminal innervation patterns varied in complexity (Table 1). Simple dimorph patterns included a single calyceal terminal that contained one to three hair cells and very few branch segments with terminal boutons (Fig. 6E). These units accounted for roughly one third of the reconstructed fibers. Similar to the calyx units, dimorph calyceal terminals containing only a single hair cell were rarely observed (3/41). More typical were regenerated dimorphs with one or two calyceal terminals innervating a total of three to four hair cells. These units also contained a number of branch fibers with multiple (3–11) bouton terminals (Fig. 6, F and G). The most complex dimorph afferents (5/41) had innervation patterns that could contain a combination of either several calyceal terminals (3–4) and/or many branch segments and numerous (11–25) bouton terminals (Fig. 6H). Statistical comparisons between the innervation patterns of regenerated dimorph and calyx units showed that there were differences between the two classes of fibers that were characteristic of those of normally developed units (Table 2). Dimorph afferents had significantly longer fibers with more branch segments, complex arborization patterns (branch order), larger terminal fields, and higher terminal densities. In contrast, calyx afferents innervated significantly more hair cells with larger calyceal terminals. These differences in terminal morphological characteristics of regenerated calyx and dimorph afferents are graphically represented in Fig. 7.
TABLE 2.
Statistical comparisons for pooled regenerated versus normal afferents
| Calyx |
Main Effect | Dimorph | Main Effect | Bouton | Main Effect | ||||
|---|---|---|---|---|---|---|---|---|---|
| Normal | Regenerated | Normal | Regenerated | Normal | Regenerated | ||||
| Diameter | 3.2 (0.9) | 2.5 (0.7) | F(1,92) = 17.1; P < 0.001 | 3.3 (0.8) | 2.5 (0.7) | F(1,93) = 21.2; P < 0.001 | 2.6 (1.1) | 2.3 (0.6) | F(1,96) = 2.8; P = 0.1 |
| Branches | 1.2 (0.8) | 1.2 (0.6) | P = 0.71 | 12.4 (8.3) | 10.6 (6.9) | P < 0.26 | 33.8 (13.9) | 25.3 (15.4) | F(1,96) = 7.5; P < 0.01 |
| Branch order | 1.1 (0.5) | 1.1 (0.4) | P = 0.63 | 4.2 (2.0) | 4.1 (1.5) | P < 0.87 | 7.2 (2.3) | 6.1 (2.0) | F(1,96) = 5.3; P < 0.05 |
| Length | 28.5 (15.9) | 33.3 (19.9) | P = 0.20 | 171.9 (105.5) | 130.6 (79.7) | F(1,93) = 4.4; P < 0.05 | 365.4 (134.8) | 258.2 (165.7) | F(1,96) = 12.4; P < 0.001 |
| Fiber volume | 245.1 (164.1) | 241.2 (168.8) | P = 0.84 | 555.9 (468.4) | 304.4 (232.8) | F(1,93) = 9.9; P < 0.005 | 628.8 (481.5) | 292.7 (238.6) | F(1,96) = 18.9; P < 0.001 |
| Area | 384.5 (196.1) | 298.8 (130.5) | F(1,92) = 9.8; P < 0.005 | 510.0 (248.8) | 377.3 (138.7) | F(1,93) = 9.4; P < 0.005 | 429.2 (204.3) | 309.6 (196.6) | F(1,96) = 8.7; P < 0.01 |
| Type 1 HC | 5.1 (2.8) | 3.7 (1.5) | F(1,92) = 7.8; P < 0.01 | 4.9 (3.5) | 3.0 (1.4) | F(1,93) = 10.8; P < 0.005 | — | — | — |
| Calyceal volume | 5,116 (2,751) | 3,506 (1,023) | F(1,92) = 12.1; P < 0.001 | 4,095 (2,237) | 2,524.2 (1,906) | F(1,93) = 13.2; P < 0.001 | — | — | — |
| Boutons | — | — | — | 9.4 (7.2) | 7.0 (5.0) | F(1,93) = 21.2; P = 0.068 | 34.0 (17.1) | 23.2 (15.4) | F(1,96) = 9.7; P < 0.01 |
| Density | 1.3 (2.4) | 1.4 (0.7) | P = 0.40 | 2.9 (1.2) | 3.1 (2.6) | P = 0.62 | 8.3 (2.5) | 8.3 (3.6) | P = 0.97 |
Values are means, with SD in parentheses.
FIG. 7.
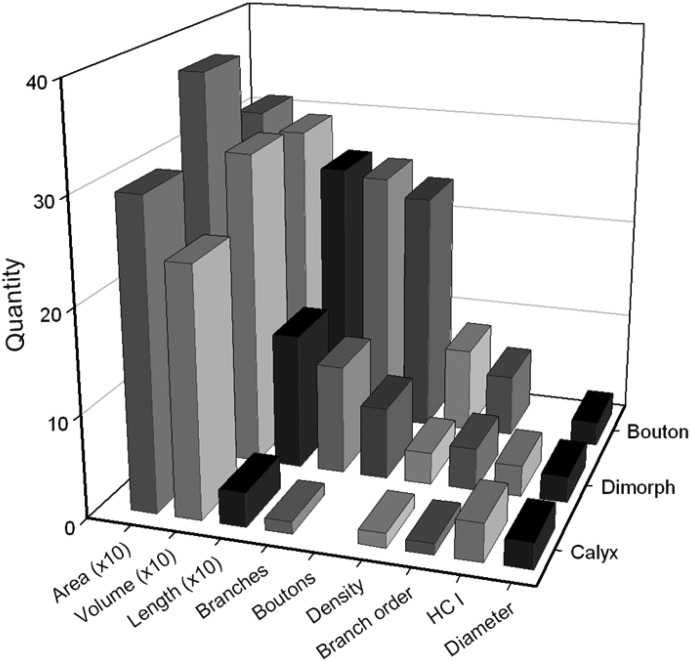
Comparison of afferent innervation morphological properties for stage 3 (6–12 mo PST) calyx, dimorph, and bouton HC fibers. Area, innervation area of receptor epithelium (μm2); Volume, sum of all fiber branch segment volumes (μm3); Length, sum of all branch segments (μm); Branches, number of branch segments/fiber; Bouton, number of bouton terminals/fiber; Branch order, number of branch levels; HC I, number of type hair cells/fiber; Diameter, axonal fiber diameter (μm).
BOUTON AFFERENTS.
In all, 48 bouton afferents were reconstructed. There appeared to be fewer bouton afferents than any other fiber type. These fibers exclusively innervated type II hair cells with bouton terminals over larger terminal fields (Fig. 4, G–I). Most bouton innervations exhibited rounded or flower patterns (Fig. 2B), with fewer units exhibiting terminal fields that were arranged in a linear fashion (Figs. 2, A and B and 4I). Bouton afferents predominantly innervated the torus and peripheral planum regions. Very few bouton afferents were observed in the peripheral margins of the apex. This area was dominated by calyx units (Fig. 5). The innervation patterns of regenerated bouton fibers exhibited differing degrees of complexity (Table 1). Nearly a third of the regenerated fibers were simple bouton units (15/48) that contained a few branch segments with low numbers (5–13) of bouton terminals (Fig. 6I). Consequently, these units covered a small area of the epithelium with low terminal densities. Nearly half of the regenerated bouton fibers (25/48) had innervation patterns with multiple branches (15–30) and moderate numbers (20–40) of bouton terminals (Figs. 4C and 6, J and K). The majority of these fibers had innervation areas of several hundred square microns and moderate terminal densities (3–7 terminals/100 μm2). A small number of bouton fibers (8/48) had the most complex bouton innervation patterns noted, which consisted of numerous branch segments and many (>40) bouton terminals (Fig. 6L). These complex fibers had large innervation areas (>500 μm2) with high terminal densities (>6 terminals/100 μm2).
Comparisons between all three fiber types were performed. As shown in Fig. 7 and statistically verified (Table 1), regenerated bouton afferents were characterized by having the longest fibers with the most branches, the highest branch order, and the densest innervations. Bouton units also had significantly more bouton terminals than those of dimorph units (Table 1), but had similar terminal field sizes. Interestingly, bouton units had longer fibers with more branches than those of dimorph afferents, but similar volumes. This indicates that bouton fibers were generally of smaller diameter than that of dimorphs throughout their length.
Comparison of regenerated and normal HC afferents
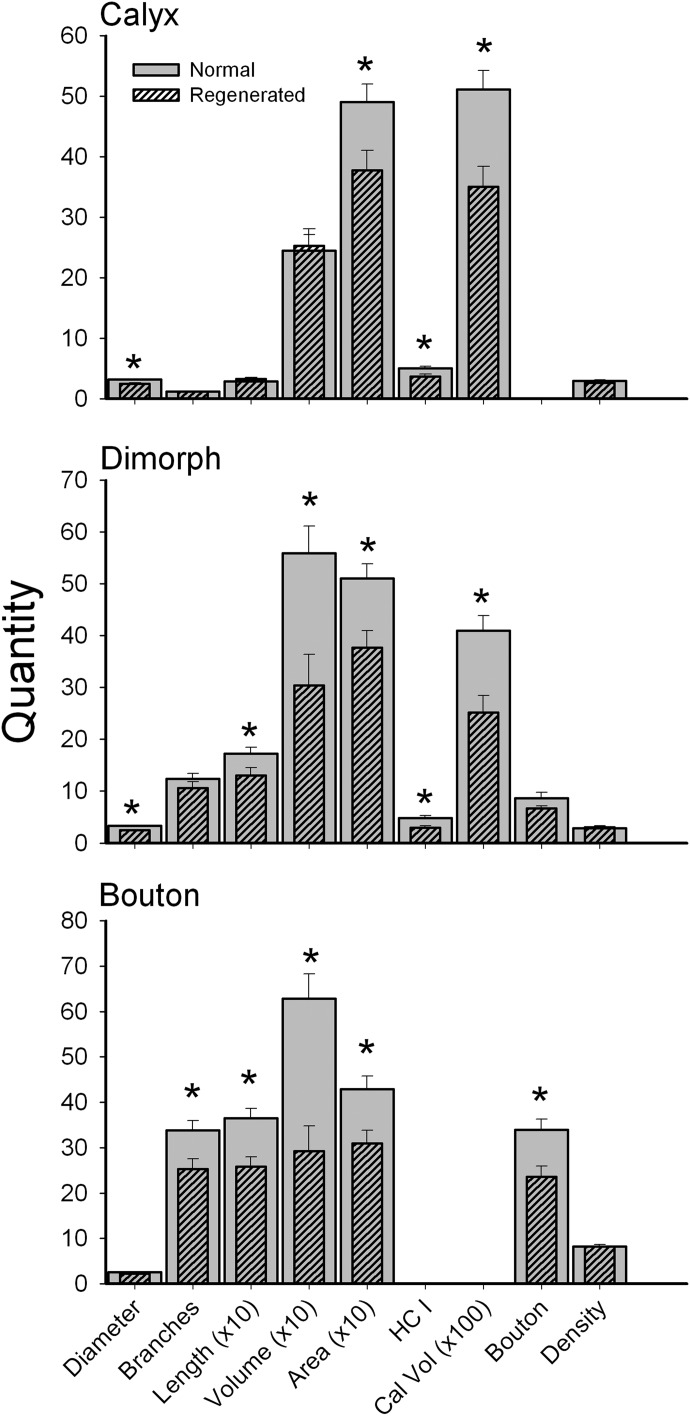
For purposes of quantitative comparisons, the terminal innervation patterns of 51 calyx, 55 dimorph, and 50 bouton afferents were reconstructed from normal pigeons (92 of these normal fibers were obtained from Haque et al. 2006). As noted earlier, much of the topographic organization of afferent type across the epithelium was reproduced in regenerated cristae similar to that observed in normal birds. However, quantitative comparisons of the afferent innervation patterns for individual fibers found significant differences between the terminal fields of regenerated afferents and those developed normally. Figure 8 shows the mean comparisons for the three classes of HC afferents, including calyx, dimorph, and bouton fibers. Regenerated calyx fibers had significantly (asterisks) smaller diameter axons, smaller calyceal terminals, fewer type I hair cells, and smaller innervation areas than those of afferents innervating normally developed cristae (Table 2). Since calyx afferents typically consisted of unbranched short segments, no differences in the arborization, length, or fiber volume were noted. Dimorph afferents were the most numerous fiber type found in the HC crista. As also shown in Fig. 8, regenerated dimorph afferents had significantly smaller diameter axons, shorter length, lower volume, and smaller innervation areas than those of normally developed afferents (Table 2). In addition, similar to calyx afferents, regenerated dimorph fibers exhibited smaller calyceal terminals that contained fewer type I hair cells than normal (Fig. 8). Last, the number of bouton terminals per regenerated dimorph afferent was marginally less than that observed in normal animals (Table 2). Regenerated bouton afferents showed similar significant reductions in number of fiber branches, fiber length, fiber volume, and terminal field innervation area compared with those of normally developed afferents (Table 2). In addition, regenerated bouton fibers contained fewer bouton terminals. Since all three fiber types in regenerated birds exhibited fewer terminal contacts (bouton or type I hair cells) and smaller terminal field size, there was no net change in mean terminal density, compared with fibers in normal birds (Fig. 8, Table 2).
FIG. 8.
Comparison of regeneration and normal afferent innervation morphological properties. Mean values are shown for each parameter for normal (gray) and regeneration (hatched) afferents. Diameter, average fiber diameter (μm); Branches, number of branch segments/fiber; Length, sum of all branch segments (μm); Volume, sum of all fiber branch segment volumes (μm3); Area, innervation area of receptor epithelium (μm2); HC I, number of type hair cells/fiber; Bouton, number of bouton terminals/fiber; Cal Vol, volume of calyceal terminal; Terminals, sum of bouton terminals and calyceal hair cells; Density, terminals/innervation area (#/μm2). For clarity, quantities for fiber length, innervation area, and volume are plotted as a factor of 10 reduction. Error bars: SEs. *P < 0.05 level (ANOVA).
DISCUSSION
Our findings present the first comprehensive examination of afferent innervation of the vestibular semicircular canals during regeneration in any species. We found that regeneration occurred slowly over months, according to a temporal staged sequence, similar to that previously observed for pigeon otolith maculae (Zakir and Dickman 2006). Regeneration of the HC crista was complete after 6 mo of recovery, where our quantitative comparisons between normal and regenerated afferents were performed. We found that much of the normally observed regional topography for hair-cell distribution and afferent type was reproduced during regeneration. However, small increases in the extent of the areas covered by calyceal-bearing dimorph afferents were also noted in regenerated cristae. We also found that following complete regenerative recovery, the terminal morphologies of the calyceal-bearing and bouton afferents were significantly different from the innervation patterns exhibited in normal cristae.
Temporal pattern of regeneration
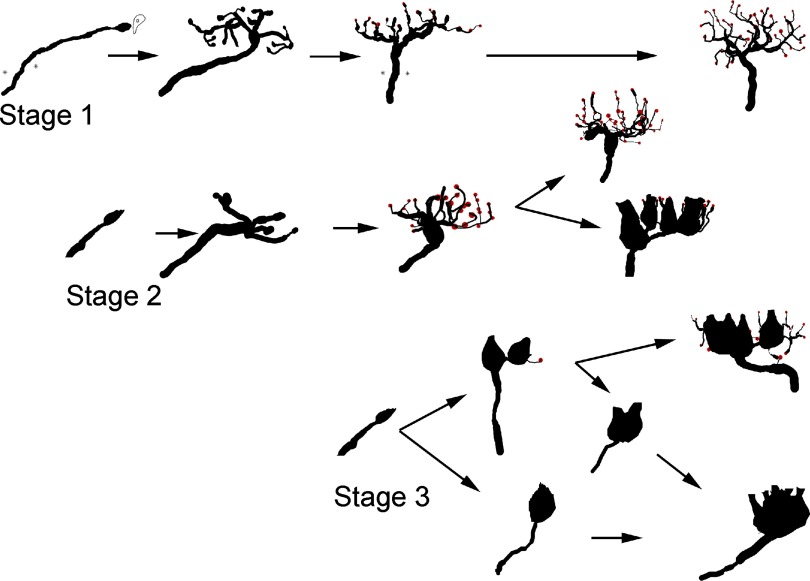
Recently, we reported that morphological regeneration of the vestibular receptors in the otolith maculae occurs along a three-stage temporal sequence (Zakir and Dickman 2006). Our present findings confirm a corresponding pattern of recovery in the HC crista. We previously proposed (Zakir et al. 2006) that vestibular afferents may regenerate through transdifferentiation and our current data support that view, as illustrated in Fig. 9. One possible mechanism that could guide the innervation pattern decision process would be the presence/absence of as yet unknown trophic signaling cues released by developing hair cells. In support of that idea are the following observations. First, during stage 1 regeneration, exclusive formation of bouton afferents and hair cells exhibiting a type II morphologic phenotype were observed. Coincident to the afferent innervation development, we previously found that hair cells regenerated primarily in the peripheral regions of the canal cristae and otolith maculae, with fewer cells present in the central apex or striolar regions (Dye et al. 1999; Haque et al. 2008; Zakir et al. 2006). Still, some afferents and hair cells did occupy these central regions and, when present, were always observed to exhibit a bouton innervation pattern. This was true even though these central regions would later be devoid of bouton fibers when regeneration was complete and would be populated by dimorph and calyx afferents. Either these central regenerated bouton fibers died or they transdifferentiated into dimorph afferents (Fig. 9). Further, bouton fibers exclusively develop in the torus and peripheral regions of the planum. Here only type II hair cells are observed, again consistent with the suggestion that cell type and afferent morphology have common, possibly symbiotic determinants. Second, as presently observed and reported previously for the otolith receptors (Zakir et al. 2006), the distinguishing characteristic of stage 2 regeneration was the appearance of the calyceal terminal and identifiable type I hair cell morphologic phenotype. Yet, throughout most of stage 2 recovery, the regenerated afferents were dimorph fibers, innervating both type II and type I hair cells. In fact, often partial calyces were observed developing on established afferents with several or many branches. This suggests that calyceal terminal formation occurs on existing bouton fibers when type I hair cells begin to develop nearby (Fig. 9). Third, calyx afferents develop last, mostly during stage 3 regeneration. Coincident with calyx afferent formation is an increase in the density of type I hair cells in the central regions of the cristae and maculae. We propose that as type II hair cells diminish, so too do the available cues to maintain bouton terminals. These signaling cascades elicit simple dimorph afferents to shed their small bouton branch segments producing the birth of calyx afferents, largely due to the higher concentration of neighboring type I hair cells (Fig. 9). Simultaneously, trophic cues from the more abundant type I hair cells induce calyceal formation, including larger more complex calyceal structures incorporating several or a number of type I hair cells. In essence, the “decision” to differentiate into either a dimorph or calyx afferent would depend primarily on the concentration of neighboring cell type. Whether afferent transdifferentiation actually occurs is unknown, but the proposed regeneration scheme is consistent with our observations for both cristae and macular recovery.
FIG. 9.
Timeline of proposed transdifferentiation of regenerated vestibular afferents. Stage 1 afferents regenerate as bouton fibers due to the exclusive signaling cues from type II hair cells. During stage 2 regeneration, initial developing fibers and existing fibers develop as bouton afferents. When appropriate cues arise from developing type I hair cells, calyceal terminal formation occurs. High concentrations of type II of hair cells promote bouton afferent phenotype. The presence of mixed type I and type II hair cell signals promotes dimorph development. Stage 3 regeneration occurs primarily in the central regions of the cristae and macular regions. Here, dimorph fibers develop early during regeneration and later lose their bouton terminal branches to become calyx afferents if high concentrations of type I hair cells are present. If mixed type I and type II hair cells occupy the neighboring epithelial space, more complex dimorph afferent phenotypes develop. Newly entering initial fibers could also immediately form as calyx afferents depending on the density of surrounding type I hair cells.
Comparison between regeneration and normal afferent innervation
Our present findings of completed regeneration in the HC crista also observed differences in the terminal afferent innervation patterns. In pigeons, similar to other vertebrates, calyceal-bearing afferents populate the central regions of the cristae, whereas bouton afferents are located in the periphery (Brichta and Peterson 1994; Desai et al. 2005; Haque et al. 2006). This highly conserved regional topography was largely reproduced during regeneration of the HC crista. However, small increases in the range of regenerated dimorph afferents was noted, as was also true for regenerated macular fibers (Zakir et al. 2006). In birds, like mammals, calyceal-bearing afferents comprise the majority of the fibers innervating the crista (Fernandez et al. 1988, 1995; Haque et al. 2006). Together, calyx and dimorph afferents are distributed over a larger region of the epithelial surface than are bouton afferents. In our current study, we compared the relative terminal morphologies between regenerated calyx and dimorph fibers. We found that regenerated calyx afferents had larger calyceal terminals that contained more type I hair cells than did dimorph units, similar to normally developed HC crista fibers. In contrast, regenerated dimorph afferents exhibited larger terminal fields, with more branches, longer segments, and increased innervation areas compared with those of calyx units, again similar to normal afferents. However, when comparisons were made between normal and regenerated calyx and dimorph fibers, significant reductions in size and complexity were observed. Regenerated calyceal-bearing afferents on average had smaller-diameter axons, shorter and less voluminous segments, a 30% reduction in calyceal terminal size, a 30% reduction in the number of hair cells contained, and a 25% reduction in terminal field size (area), compared with those of normal afferents. In the pigeon maculae, regenerated calyceal-bearing afferents were also significantly reduced, but to a lesser degree than HC fibers (Zakir and Dickman 2006). Regenerated bouton fibers exhibited the largest, most complex, terminal innervation patterns compared with those of calyx and dimorph units, similar to the relative differences observed in normal animals. However, regenerated bouton fibers also exhibited significant morphological differences in complexity and size compared with those observed in normative development, with a 30% reduction in length, number of branch segments, number of bouton terminals and innervation area, a 15% reduction in branch order, and a 50% reduction in fiber volume.
Functional considerations of regeneration afferents
In mammals, spontaneous regeneration of vestibular receptors following complete receptor loss does not typically occur, but may vary depending on species (Kawamoto et al. 2009; Meiteles and Raphael 1994). Still, head and eye responses partially recover (Stapely et al. 2006), presumably using extravestibular motion cues to enhance central neural compensation (Newlands and Perachio 1990a,b; Zennou-Azogi et al. 1994) and adaptation (Anastasio 1992; Serafin et al. 1999) processes. Recently, we demonstrated that vestibular-mediated gaze stabilization during rotational motion recovers fully in birds undergoing regeneration, with a time course lasting several months (Haque et al. 2008). Interestingly, gaze stability during high-frequency motion recovered first, at a time when only bouton afferents innervated the vestibular epithelia (stage 1 regeneration). Further, these early-regeneration gaze responses consisted almost entirely of a head response component, with the eye response component contributing very little. As regeneration progressed (late stage 2), gaze stability returned for the mid-frequency rotations, followed lastly by recovery during slow rotational head movements. These later recovery dynamics coincided with the regeneration of calyceal-bearing afferents that innervated the central crista regions (Haque et al. 2008). In mammals, the central regions of the cristae contain irregularly firing high-gain dimorph and low-gain calyx afferents with large dynamic ranges (Baird et al. 1988; Fernandez et al. 1988; Lysakowski et al. 1995). We previously suggested that these central regions appear to provide the signals needed for low-frequency gaze stability because gaze responses to the lowest frequencies of rotation became significant only at the last stage of regeneration. Our current findings of the later development of regenerating calyx and dimorph afferents in the central crista would support that view. In a related manner, we presently found that the regenerated calyceal-bearing HC afferents had terminal morphologies that were significantly reduced in complexity compared with normal fibers. Whether these differences in afferent innervation are related to the change in functional contribution between the head and eye components to gaze stability in regenerated birds is unknown. However, although pigeon gaze stability recovers to normal values, the relative contributions from the head and eye components change during regenerative recovery (Haque et al. 2008). In fact, the eye component during head-free rotations increases, whereas the head component decreases in significance to total gaze stability, in converse to the behavior observed in normal birds (Haque and Dickman 2005; Haque et al. 2008). Our findings suggest that the same calyx and dimorph afferents regenerating to contribute low-frequency dynamic information are also increasing their signal efficacy to eye-movement–related central vestibular neurons in the production of gaze responses. Plasticity in central vestibular neurons processing motion information would appear to be required to explain the observed morphologic and response adaptations observed in regenerating vestibular systems. If true, how central vestibular neurons compensate to the changing vestibular afferent inputs during regeneration remains a topic of substantial interest.
GRANTS
This work was supported in part by Howard Hughes Medical Institute Grant 57003555, the American Otological Society, and the National Institute on Deafness and Other Communication Disorders (NIDCD) Grants F31-DC-006374, DC-003286, DC-007618, and DC-006913. The content is solely the responsibility of the authors and does not necessarily represent the official views of the Howard Hughes Medical Institute, the American Otological Society, the NIDCD, or the National Institutes of Health.
Acknowledgments
We thank D. Huss and I. Acevedo for invaluable contributions.
REFERENCES
- Anastasio 1992.Anastasio TJ Simulating vestibular compensation using recurrent back-propagation. Biol Cybern 66: 389–397, 1992. [DOI] [PubMed] [Google Scholar]
- Angelaki et al. 1992.Angelaki DE, Perachio AA, Mustari MJ, Strunk CL. Role of irregular otolith afferents in the steady-state nystagmus during off-vertical axis rotation. J Neurophysiol 68: 1895–1900, 1992. [DOI] [PubMed] [Google Scholar]
- Baird et al. 1988.Baird RA, Desmadryl G, Fernandez C, Goldberg JM. The vestibular nerve of the chinchilla. II. Relation between afferent response properties and peripheral innervation patterns in the semicircular canals. J Neurophysiol 60: 182–203, 1988. [DOI] [PubMed] [Google Scholar]
- Berg 1951.Berg K The toxic effect of streptomycin on the vestibular and cochlear apparatus; an experimental study on cats. Acta Otolaryngol Suppl 97: 1–77, 1951. [PubMed] [Google Scholar]
- Boyle et al. 2002.Boyle R, Highstein SM, Carey JP, Xu J. Functional recovery of anterior semicircular canal afferents following hair cell regeneration in birds. J Assoc Res Otolaryngol 3: 149–166, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt and Apkarian 1992.Brandt HM, Apkarian AV. Biotin-dextran: a sensitive anterograde tracer for neuroanatomic studies in rat and monkey. J Neurosci Methods 45: 35–40, 1992. [DOI] [PubMed] [Google Scholar]
- Brichta and Peterson 1994.Brichta AM, Peterson EH. Functional architecture of vestibular primary afferents from the posterior semicircular canal of a turtle, Pseudemys (Trachemys) scripta elegans. J Comp Neurol 344: 481–507, 1994. [DOI] [PubMed] [Google Scholar]
- Cajal 1995.Cajal S Histology of the Nervous System of Man and Vertebrates. New York: Oxford Univ. Press, 1995.
- Carey et al. 1996.Carey JP, Fuchs AF, Rubel EW. Hair cell regeneration and recovery of the vestibuloocular reflex in the avian vestibular system. J Neurophysiol 76: 3301–3312, 1996. [DOI] [PubMed] [Google Scholar]
- Carey et al. 2002.Carey JP, Minor LB, Peng GC, Della Santina CC, Cremer PD, Haslwanter T. Changes in the three-dimensional angular vestibulo-ocular reflex following intratympanic gentamicin for Meniere's disease. J Assoc Res Otolaryngol 3: 430–443, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corwin and Cotanche 1988.Corwin JT, Cotanche DA. Regeneration of sensory hair cells after acoustic trauma. Science 240: 1772–1774, 1988. [DOI] [PubMed] [Google Scholar]
- Crane and Demer 1998.Crane BT, Demer JL. Gaze stabilization during dynamic posturography in normal and vestibulopathic humans. Exp Brain Res 122: 235–246, 1998. [DOI] [PubMed] [Google Scholar]
- Desai et al. 2005.Desai SS, Zeh C, Lysakowski A. Comparative morphology of rodent vestibular periphery. I. Saccular and utricular maculae. J Neurophysiol 93: 251–266, 2005. [DOI] [PubMed] [Google Scholar]
- Dickman and Fang 1996.Dickman JD, Fang Q. Differential central projections of vestibular afferents in pigeons. J Comp Neurol 367: 110–131, 1996. [DOI] [PubMed] [Google Scholar]
- Dickman and Lim 2004.Dickman JD, Lim I. Posture, head stability, and orientation recovery during vestibular regeneration in pigeons. J Assoc Res Otolaryngol 5: 323–336, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dye et al. 1999.Dye BJ, Frank TC, Newlands SD, Dickman JD. Distribution and time course of hair cell regeneration in the pigeon utricle. Hear Res 133: 17–26, 1999. [DOI] [PubMed] [Google Scholar]
- Fernandez et al. 1988.Fernandez C, Baird RA, Goldberg JM. The vestibular nerve of the chinchilla. I. Peripheral innervation patterns in the horizontal and superior semicircular canals. J Neurophysiol 60: 167–181, 1988. [DOI] [PubMed] [Google Scholar]
- Fernandez et al. 1990.Fernandez C, Goldberg JM, Baird RA. The vestibular nerve of the chinchilla. III. Peripheral innervation patterns in the utricular macula. J Neurophysiol 63: 767–780, 1990. [DOI] [PubMed] [Google Scholar]
- Fernandez et al. 1995.Fernandez C, Lysakowski A, Goldberg JM. Hair-cell counts and afferent innervation patterns in the cristae ampullares of the squirrel monkey with a comparison to the chinchilla. J Neurophysiol 73: 1253–1269, 1995. [DOI] [PubMed] [Google Scholar]
- Fink and Morest 1977.Fink DJ, Morest DK. Formation of synaptic endings by colossal fibers in the vestibular epithelium of the chick embryo. Neuroscience 2: 229–252, 1977. [DOI] [PubMed] [Google Scholar]
- Flock and Orman 1983.Flock A, Orman S. Micromechanical properties of sensory hairs on receptor cells of the inner ear. Hear Res 11: 249–260, 1983. [DOI] [PubMed] [Google Scholar]
- Frank et al. 1999.Frank TC, Dye BJ, Newlands SD, Dickman JD. Streptomycin ototoxicity and hair cell regeneration in the adult pigeon utricle. Laryngoscope 109: 356–361, 1999. [DOI] [PubMed] [Google Scholar]
- Goode et al. 1999.Goode CT, Carey JP, Fuchs AF, Rubel EW. Recovery of the vestibulocolic reflex after aminoglycoside ototoxicity in domestic chickens. J Neurophysiol 81: 1025–1035, 1999. [DOI] [PubMed] [Google Scholar]
- Haque and Dickman 2005.Haque A, Dickman JD. Vestibular gaze stabilization: different behavioral strategies for arboreal and terrestrial avians. J Neurophysiol 93: 1165–1173, 2005. [DOI] [PubMed] [Google Scholar]
- Haque et al. 2006.Haque A, Huss D, Dickman JD. Afferent innervation patterns of the pigeon horizontal crista ampullaris. J Neurophysiol 96: 3293–3304, 2006. [DOI] [PubMed] [Google Scholar]
- Haque et al. 2008.Haque A, Zakir M, Dickman JD. Recovery of gaze stability during vestibular regeneration. J Neurophysiol 99: 853–865, 2008. [DOI] [PubMed] [Google Scholar]
- Kawamoto and Izumikawa et al. 2009.Kawamoto K, Izumikawa M, Beyer LA, Atkin GM, Raphael Y. Spontaneous hair cell regeneration in the mouse utricle following gentamicin ototoxicity. Hear Res 247: 17–26, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li and Correia 1998.Li W, Correia MJ. Recovery of semicircular canal primary afferent activity in the pigeon after streptomycin ototoxicity. J Neurophysiol 80: 3297–3311, 1998. [DOI] [PubMed] [Google Scholar]
- Lindeman 1969.Lindeman HH Studies on the morphology of the sensory regions of the vestibular apparatus with 45 figures. Ergeb Anat Entwicklungsgesch 42: 1–113, 1969. [PubMed] [Google Scholar]
- Lysakowski and Goldberg 1997.Lysakowski A, Goldberg JM. A regional ultrastructural analysis of the cellular and synaptic architecture in the chinchilla cristae ampullares. J Comp Neurol 389: 419–443, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lysakowski 1995.Lysakowski A, Minor LB, Fernandez C, Goldberg JM. Physiological identification of morphologically distinct afferent classes innervating the cristae ampullares of the squirrel monkey. J Neurophysiol 73: 1270–1281, 1995. [DOI] [PubMed] [Google Scholar]
- Masetto and Correia 1997a.Masetto S, Correia MJ. Electrophysiological properties of vestibular sensory and supporting cells in the labyrinth slice before and during regeneration. J Neurophysiol 78: 1913–1927, 1997a. [DOI] [PubMed] [Google Scholar]
- Masetto and Correia 1997b.Masetto S, Correia MJ. Ionic currents in regenerating avian vestibular hair cells. Int J Dev Neurosci 15: 387–399, 1997b. [DOI] [PubMed] [Google Scholar]
- Meiteles and Rapheal 1994.Meiteles LZ, Rapheal Y. Scar formation in the vestibular sensory epithelium after aminoglycoside toxicity. Hear Res 79: 26–38, 1994. [DOI] [PubMed] [Google Scholar]
- Minor and Goldberg 1991.Minor LB, Goldberg JM. Vestibular-nerve inputs to the vestibulo-ocular reflex: a functional-ablation study in the squirrel monkey. J Neurosci 11: 1636–1648, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newlands 2001.Newlands SD, Hessen SV, Haque A, Angelaki DE. Head unrestrained horizontal gaze shifts after unilateral labyrinthectomy in the rhesus monkey. Exp Brain Res 140: 25–33, 2001. [DOI] [PubMed] [Google Scholar]
- Newlands and Perachio 1990.Newlands SD, Perachio AA. Compensation of horizontal canal related activity in the medial vestibular nucleus following unilateral labyrinth ablation in the decerebrate gerbil. I. Type I neurons. Exp Brain Res 82: 359–372, 1990. [DOI] [PubMed] [Google Scholar]
- Ramón 1908.Ramón y Cajal S Textura del Sistema Nervioso del Hombre y de los Vertebrados (Histology of the Nervous System of Man and Vertebrates), translated by Swanson N, Swanson LW. New York: Oxford Univ. Press, English translation, 1995; original Spanish version, 1908.
- Richardson et al. 1960.Richardson KC, Jarrett L, Finke EH. Embedding in epoxy resins for ultrathin sectioning in electron microscopy. Stain Technol 35: 313–323, 1960. [DOI] [PubMed] [Google Scholar]
- Rüsch et al. 1998.Rüsch A, Lysakowski A, Mattock RA. Postnatal development of type I and type II hair cells in the mouse utricle: acquisition of voltage-gated conductances and differentiated morphology. J Neurosci 18: 7487–7501, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schusses et al. 1991.Schusses DA, Ginsberg R, Highstein SM. Morph physiology of synaptic transmission between type I hair cells and vestibular primary afferents. An intracellular study employing horseradish peroxidase in the lizard, Calottes vesicular. Brain Res 544: 1–16, 1991. [DOI] [PubMed] [Google Scholar]
- Serafin et al. 1999.Serafin M, Ris L, Bernard P, Muhlethaler M, Godaux E, Vidal P-P. Neuronal correlates of vestibulo-ocular reflex adaptation in the alert guinea-pig. Eur J Neurosci 11: 1827–1830, 1999. [DOI] [PubMed] [Google Scholar]
- Si and Zakir 2003.Si X, Zakir MM, Dickman JD. Afferent innervation of the utricular macula in pigeons. J Neurophysiol 89: 1660–1677, 2003. [DOI] [PubMed] [Google Scholar]
- Skaliora et al. 2000.Skaliora I, Adams R, Blakemore C. Morphology and growth patterns of developing thalamocortical axons. J Neurosci 20: 3650–3662, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stackman and Taube 1997.Stackman RW, Taube JS. Firing properties of head direction cells in the rat anterior thalamic nucleus: dependence on vestibular input. J Neurosci 17: 4349–4358, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stapley et al. 2006.Stapley PJ, Ting LH, Kuifu C, Everaert DG, Macpherson JM. Bilateral vestibular loss leads to active destabilization of balance during voluntary head turns in the standing cat. J Neurophysiol 95: 3783–3797, 2006. [DOI] [PubMed] [Google Scholar]
- Tosney and Landmesser 1985.Tosney KW, Landmesser LT. Growth cone morphology and trajectory in the lumbosacral region of the chick embryo. J Neurosci 5: 2345–2358, 1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisleder and Rubel 1992.Weisleder P, Rubel EW. Hair cell regeneration in the avian vestibular epithelium. Exp Neurol 115: 2–6, 1992. [DOI] [PubMed] [Google Scholar]
- Wersäll 1956.Wersäll J Studies on the structure and innervation of the sensory epithelium of the cristae ampulares in the guinea pig; a light and electron microscopic investigation. Acta Otolaryngol Suppl 126: 1–85, 1956. [PubMed] [Google Scholar]
- Wersäll and Hawkings 1962.Wersäll J, Hawkings JE Jr. The vestibular sensory epithelia in the cat labyrinth and their reactions in chronic streptomycin intoxication. Acta Otolaryngol 54: 1–23, 1962. [DOI] [PubMed] [Google Scholar]
- Zakir and Dickman 2006.Zakir M, Dickman JD. Regeneration of vestibular otolith afferents after ototoxic damage. J Neurosci 26: 2881–2893, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakir et al. 2003.Zakir M, Huss D, Dickman JD. Afferent innervation patterns of the saccule in pigeons. J Neurophysiol 89: 534–550, 2003. [DOI] [PubMed] [Google Scholar]
- Zennou-Azogui et al. 1994.Zennou-Azogui Y, Xerri C, Harlay F. Visual sensory substitution in vestibular compensation: neuronal substrates in the alert cat. Exp Brain Res 98: 457–473, 1994. [DOI] [PubMed] [Google Scholar]