Abstract
In the normal guinea pig, contralateral sound inhibits more than a third of ventral cochlear nucleus (VCN) neurons but excites <4% of these neurons. However, unilateral conductive hearing loss (CHL) and cochlear ablation (CA) result in a major enhancement of contralateral excitation. The response properties of the contralateral excitation produced by CHL and CA are similar, suggesting similar pathways are involved for both types of hearing loss. Here we used the neurotoxin melittin to test the hypothesis that this “compensatory” contralateral excitation is mediated either by direct glutamatergic CN-commissural projections or by cholinergic neurons of the olivocochlear bundle (OCB) that send collaterals to the VCN. Unit responses were recorded from the left VCN of anesthetized, unilaterally deafened guinea pigs (CHL via ossicular disruption, or CA via mechanical destruction). Neural responses were obtained with 16-channel electrodes to enable simultaneous data collection from a large number of single- and multiunits in response to ipsi- and contralateral tone burst and noise stimuli. Lesions of each pathway had differential effects on the contralateral excitation. We conclude that contralateral excitation has a fast and a slow component. The fast excitation is likely mediated by glutamatergic neurons located in medial regions of VCN that send their commissural axons to the other CN via the dorsal/intermediate acoustic striae. The slow component is likely mediated by the OCB collateral projections to the CN. Commissural neurons that leave the CN via the trapezoid body are an additional source of fast, contralateral excitation.
INTRODUCTION
The cochlear nucleus (CN) is the first site in the central auditory system where convergence of binaural information occurs. Interaction between the two cochlear nuclei can take place by way of the CN-commissural pathway (Cant and Gaston 1982; Schofield and Cant 1996; Shore et al. 1992) or by descending inputs from the superior olivary complex (SOC) and inferior colliculus (IC) (Shore et al. 1991; Spangler et al. 1987). In the normal hearing adult guinea pig, contralateral sound stimulation inhibits the activity of ∼30% of ventral CN (VCN) neurons (Ingham et al. 2006; Shore et al. 2003) and produces only occasional excitatory responses (Shore et al. 2003). The inhibition is thought to be mediated by glycinergic neurons of the CN-commissural pathway that travel via the acoustic striae (Alibardi 1998, 2000; Babalian et al. 2002; Needham and Paolini 2003, 2006, 2007; Schofield and Cant 1996; Shore et al. 1992, 2003; Wenthold et al. 1987). In addition, it may be mediated indirectly via glycinergic neurons in the medial nucleus of the trapezoid body (MNTB) (Bledsoe et al. 1990; Godfrey et al. 1988; Moore and Caspary 1983; Wenthold et al. 1987) that project to the VCN (Schofield 1994).
In contrast to normal animals, guinea pigs that have been ipsilaterally deafened display a short onset (within 2 h) and lasting increase in the proportion of VCN neurons with excitatory responses to contralateral sound (≤40%) with no persistent changes in the percentage of neurons inhibited by contralateral sound. This enhancement of contralateral excitation occurs after both conductive hearing loss (CHL) (Sumner et al. 2005) and cochlear ablation (CA) (Shore et al. 2006). The excitatory responses have latencies of ≥6 ms; this is consistent with activation by a path involving more than one synapse or a slow pathway and inconsistent with acoustic cross-talk. The latencies and rate-level functions (RLFs) of contralateral excitation are similar to those seen occasionally in normal hearing animals. This, plus the fast onset of the increase in contralateral excitation, suggest an upregulation of existing excitatory pathways rather than a disinhibition of silent synapses (e.g., Calford 2002) because unilateral deafness produces no lasting change in the proportion of neurons that are inhibited by contralateral sound (Sumner et al. 2005).
The contralateral excitatory responses may be mediated by cholinergic neurons in the ventral nucleus of the trapezoid body (VNTB) that send excitatory projections to the VCN via collaterals of the olivocochlear bundle (OCB) (Brown et al. 1988; Fujino and Oertel 2001; Godfrey et al. 1987b; Mulders et al. 2003; Winter et al. 1989) or directly via the trapezoid body (Sherriff and Henderson 1994). In support of this notion, we have shown that melittin lesions of the VNTB on both sides of the brain eliminated the compensatory excitation induced by CA, while lesions of the VNTB contralateral to the cochlear damage only partially eliminated the excitation (Shore et al. 2006). Tucci and colleagues (2006) reported that CHL produces increases in the contralateral round window noise and compound action potential amplitudes in the ear contralateral to the CHL, suggesting also that alterations in OCB pathways play at least a partial role in compensatory excitation. Additional support for cholinergic OCB pathways mediating the excitation is provided by reports of upregulation of choline acetyltransferase and acetylcholine receptor binding (Jin and Godfrey 2006; Jin et al. 2005) and GAP43 associated with OCB inputs (Illing and Horvath 1995; Illing et al. 1997) in the ipsilateral VCN following CA.
Another potential source of contralateral excitation is the CN-commissural pathway. Although this mostly glycinergic pathway likely mediates the inhibitory contralateral responses (Needham and Paolini 2003; Shore et al. 2003), a few of the neurons constituting the CN-commissural pathway have anatomical characteristics indicative of excitatory neurotransmission (Alibardi 1998, 2000; Shore et al. 1992; Zhou et al. 2007). They also terminate in granule cell regions (Alibardi 2004; Shore and Moore 1998), increasing the likelihood of slow temporal integration, which, even in principal cells in the CN, can last 10 ms (Palmer and Winter 1996). Thus this monosynaptic pathway could be involved even though the excitatory responses have latencies of ≥6 ms. Support for the contralateral excitation being mediated by monosynaptic glutamatergic projections is also provided by recent findings from our laboratory that the terminals of CN-commissural projections co-label with the vesicular glutamate transporter 2, (VGLUT2) (Zhou et al. 2007a,b). The cell bodies of these neurons are located in the medial shell areas of VCN, and the fibers exit the CN via both the dorsal/intermediate (D/IAS) acoustic striae and the trapezoid body (TB) (Zhou et al. 2007a,b).
Thus there are at least three potential sources for the contralateral excitation. In the present study, we tested the hypotheses that “compensatory” (post unilateral hearing loss) contralateral excitation is mediated by direct glutamatergic CN-commissural projections via the D/IAS; direct glutamatergic CN-commissural projections via the TB; or cholinergic neurons of the OCB that send excitatory collaterals to the VCN. Figure 1 is a schematic of the brain stem pathways connecting the two cochlear nuclei and the placement of lesions that tested these hypotheses. As depicted in Fig. 1, lesions (L) of the D/IAS (L1, Fig. 1) and TB (L2, Fig. 1) as they exit the contralateral CN identify whether contralateral excitation is mediated by the D/IAS or the TB. Lesions of the OCB near the floor of the fourth ventricle (L3, Fig. 1) elucidate the role played by the crossed and uncrossed OCB (COCB and UCOCB, respectively). Recent studies by Smith et al. (2005) indicate that many of the fibers of the D/IAS cross the brain ventral to the OCB, allowing us to make discrete lesions of the OCB.
FIG. 1.
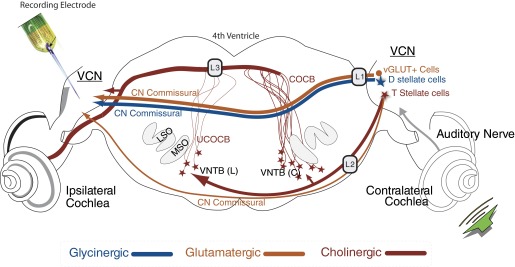
Schematic of the pathways to the ventral cochlear nucleus (VCN) of the guinea pig that originate in the contralateral ear. Illustrated are: 1) the direct inhibitory glycinergic pathway via the dorsal/intermediate acoustic striae (D/IAS); 2) direct glutamatergic CN commissural projections via the D/IAS; 3) direct glutamatergic CN commissural projections via the trapezoid body (TB); or 4) cholinergic neurons of the olivocochlear bundle (OCB) that send excitatory collaterals to the VCN. Lesions of the D/IAS (L1) and TB (L2) as they exit the contralateral CN identify whether contralateral excitation is mediated by the D/IAS or the TB. L1 lesions would also disrupt glycinergic inhibition. Lesions of the OCB near the floor of the 4th ventricle (L3) elucidate the role played by the crossed and uncrossed OCB (COCB and UCOCB, respectively) (modified from de Venecia et al. 2005).
METHODS
The methods used here are as previously reported for deafening (Shore et al. 2006; Sumner et al. 2005), lesioning brain stem structures (Bledsoe et al. 1990; Gardi and Bledsoe 1981; Le Prell et al. 2003), and recording from VCN neurons (Shore et al. 2003, 2008; Sumner et al. 2005). Experiments were performed on 10 healthy female adult pigmented guinea pigs (Elm Hill Breeding Labs, Chelmsford, MA) with normal Preyer's reflexes, weighing 250–400 g. Of these, two were unilaterally impaired by a CHL and eight were unilaterally deafened by a mechanical CA. The database of the CHL animals also included three animals previously reported as zero survival day animals in Sumner et al. (2005).
All procedures were approved by the University Committee on Use and Care of Animals (UCUCA) at the University of Michigan. These procedures were performed in accordance with the National Institutes of Health guidelines for the care and use of laboratory animals (National Institutes of Health publication No. 80-23), guidelines provided by the UCUCA, and Policies on the Use of Animals and Humans in Neuroscience Research approved by the Society for Neuroscience.
General experimental design
Single- and multiunit neural responses were measured in the VCN in animals treated with an ossicular disarticulation (CHL) or mechanical CA to produce a sensorineural hearing loss. Ossicular disruptions and CAs were always performed on the left ear. Unit recordings were always made in the left VCN, ipsilateral to the hearing loss. Responses to contralateral acoustic stimulation were obtained beginning immediately after the deafening procedures, and for ≤4 h post deafening.
Surgical preparation
Animals were anesthetized with ketamine (40 mg/kg) and xylazine (10 mg/kg) and held in a stereotaxic device (Kopf) with hollow ear bars for sound delivery. Rectal temperature was monitored and maintained at 38 ± 0.5°C with a thermostatically controlled heating pad. Supplemental anesthesia (0.25–0.5 times initial dose) was given approximately hourly, after performing a digital pinch test to elicit paw withdrawal. The bone overlying the cerebellum and posterior occipital cortex was removed, and a small amount of cerebellum was aspirated to reveal the surface of the dorsal CN (DCN) and, more rostrally, the VCN. A recording electrode mounted to the stereotaxic device was visually inserted directly into the VCN. The electrodes were pretreated with fluorescent compounds (see histology, in the following text) to enable postmortem reconstruction of electrode tracks.
Deafening procedures
The compensatory contralateral excitatory responses that occur following CHL and CA have similar response properties (Shore et al. 2006; Sumner et al. 2005). In the present study, we quantified features of the excitatory responses produced by both CHL and CA to confirm this observation. For ossicular disruption, the tympanic membrane (TM) was visualized through the ear canal using a microscope. Jeweler's forceps were used to puncture the TM, and the malleus was grasped and rotated to dislocate it from the incus. For CA, the animal was placed in the stereotaxic frame and a metal rod was inserted three to four times through the ear bar to damage the TM and cochlea. Visual inspection of the middle ear at the end of the experiment confirmed that the cochlea was damaged. CA allowed us to examine the changes in response properties of the same neurons before and after the deafening. This was not possible in CHL animals because the procedure had to be performed with the animals out of the stereotaxic frame. Thus the lesion studies were performed only on animals deafened by CA.
Lesion techniques
Once contralateral excitation was established after CA, lesions were produced in the D/IAS, the TB, or the OCB. The techniques used were similar to those we have described previously (Bledsoe et al. 1990; Gardi and Bledsoe 1981; Le Prell et al. 2003). A pulled glass pipette (tip diameter, ∼20–50 μm) was affixed to a 1.0-μl glass microsyringe (Model No. 700; Hamilton, Reno, NV). The syringe and tip were filled with melittin (Sigma Chemical, St. Louis, MO). Melittin is a cytolytic peptide that does not diffuse great distances in tissue. In addition, it produces very discrete lesions with sharp boundaries due the steep relationship between melittin concentration and cytolysis (Killion and Dunn 1986). Prior to use, melittin was dissolved in normal Ringer solution [containing (in mM) 145 NaCl, 2.7 KCl, 2.0 MgSO4, 1.2 CaCl2, and 5.0 HEPES; pH 7.40; osmolality = 280–285 mosM] for a final concentration of 10 mM melittin in solution (pH 7.0 ± 0.5; osmolality = 419 mosM). The solution was aliquotted for storage at 0°C and warmed to room temperature prior to filling the microsyringe/pipette assembly. Fluoro-Gold or -Ruby was added to the melittin solution to aid in histological reconstruction of the lesions. Once filled, the microsyringe was affixed to a micromanipulator and positioned in the appropriate brain stem structure using stereotaxic coordinates and, in most cases, sound-evoked near-field potentials recorded from the pipette (Gardi and Bledsoe 1981). After melittin was expelled (0.2–0.5 μl), the pipette was left in place for 1 min. Contralateral excitatory responses were recorded at least twice before and for ≥3 h after the lesion at 15- to 20-min intervals.
Data acquisition
All recordings were made in a sound-attenuating single-walled booth. Single-shank, 16-channel Michigan electrodes were used to record unit activity thus enabling us to record from many units simultaneously. In all animals, the electrode was inclined to an angle of 35–45° from vertical and positioned at a point on the VCN surface 0.5–0.75 mm medial to the parafloccular recess. The electrode was advanced 2.0–3.0 mm below the surface of the VCN in a ventro-rostral direction. Recording sites spanned 1.5 mm (100-μm spacing of recording sites) from the tip of the probe. Thus we were able to sample from much of the depth of the VCN without moving the probe. In a few CA animals, the electrode had to be repositioned until robust sustained responses to ipsilateral acoustic stimulation were obtained prior to the CA. In these cases, data were accepted only when no more than two to three penetrations had to be made.
The 16-channel electrode was connected by a 16-channel preamplifier and digitizer to a Tucker-Davis Technologies (TDT) data-acquisition system that provided gain (1,000×), filtering (bandwidth, 300–7.5 kHz) and A/D conversion. A/D conversion was performed by simultaneously sampling 12-bit converters at 25 kHz/channel. Signals were then routed to multiple digital signal processors for computer-controlled spike waveform capture. A spike detection threshold was set independently for each recording channel to 4 SD above the mean background noise voltage. Time stamps and associated waveforms were collected at each threshold crossing.
Off-line sorting
In ∼20% of the recordings, neural waveforms had threshold values ≥4 SD above the root mean square (RMS) noise floor. These units were sorted using the Plexon off-line sorter program (Plexon, Dallas, TX) using cluster analysis of principal component amplitudes. It was possible to sort some of the waveforms into more than one single unit per channel using statistical criteria (P < 0.05) provided by the program, thus increasing our yield of individually isolated units. Units separated using automatic cluster analysis methods were manually verified in terms of their amplitude consistency over trials and interspike intervals. Only single-unit data were used to obtain measures of response latency and to classify unit types according to their temporal response patterns in poststimulus time histograms (PSTHs).
Acoustic stimuli
Acoustic stimuli were 100-ms broadband noise (BBN) or tone bursts (1.5-ms rise/fall times) presented at different levels to assess neural thresholds, latencies, and RLFs. Stimuli were delivered to the ears via Beyer dynamic earphones coupled to the hollow ear bars using TDT system III hardware for D/A conversion and analog attenuation. Digital signals were generated and delivered to the TDT hardware by a Pentium PC using a custom MATLAB software package. Stimuli were generated using a sampling rate of 50 kHz at 16-bit resolution. Tones were calibrated using a 1/4-in microphone coupled to the ear with a 0.5-ml tube. The microphone output was measured using custom MATLAB software. Noise was calibrated with the 1/4-in microphone and coupler attached to a sound level meter set to measure the bandwidth of interest (200 Hz to 20 kHz for BBN). Equalization to correct for the system response was performed in the digital frequency domain. The stimulus variable sequences in pseudorandom order were generated from within MATLAB. The maximum output of the system was 80 dB SPL. Tone and BBN bursts were used to assess the effects of ipsi- and contralateral sound on the activity of VCN neurons. In CA animals, responses were recorded to ipsi- and contralateral tones and BBN prior to ipsilateral deafening. Prior to deafening, a few contralateral sounds produced excitatory responses. The longer latencies of these responses (>6 ms) served to rule out cross-talk. Also, if there was no response to an ipsilateral stimulus at 80 dB SPL after deafening, then any contralateral response at that level could not be attributed to cross-talk. After CHL and CA, ipsilateral BBN thresholds were used to assess the extent of deafness. Prior to and after brain stem lesions RLFs to contralateral BBN were obtained. The 100-ms BBN (100 Hz to 22 kHz) was presented to the contralateral ear and the level of the noise varied in 10-dB increments from silence to 80 dB SPL.
Data analysis
The data analysis was performed using a custom toolbox in MATLAB both during and after the experiments. This system generated PSTHs, RLFs, and thresholds. A response threshold was taken to be the (linearly interpolated) sound level at which the difference in the mean spike rate between the driven response and the spontaneous activity satisfied a Student's t-test for statistical significance at a level of P < 0.01. This algorithm gave reliable thresholds that agreed closely with visual inspection of PSTHs and RLFs. Threshold was verified visually by comparing the response to the next higher level, at which a strong response around the same latency was observed. Latency was the point in time at which the firing rate was 2 SD above the average firing rate preceding stimulation.
Histology
BRAIN HISTOLOGY.
The location of the recording electrode in the CN and the lesions in the brain stem were verified post mortem. To mark electrode tracks, the recording electrodes were dipped in 10% 1,1-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate (di-I, Molecular Probes) or Fluoro-Gold (2%), before being inserted into the brain. In addition to 10 mM melittin, the solution in the lesion pipette contained either Fluoro-Gold (2%) or Fluoro-Ruby (2%) to aid in histological reconstructions of the lesions. At the end of each experiment, the animal was perfused transcardially with saline followed by 4% paraformaldehyde. The brain was removed from the skull and immersed in 20% sucrose solution (Shore and Moore 1998; Shore et al. 1992). The following day, the brain was cryosectioned at 40–60 μm, placed on slides, and examined under epifluorescence for evidence of recording and lesion locations.
COCHLEAR HAIR CELL ASSESSMENTS.
In the animals treated with disarticulation to produce a CHL, a lack of cochlear damage was confirmed by demonstrating a normal number of surviving hair cells in cytocochleograms. The animals were perfused transcardially as described in the preceding text. The temporal bones were removed, the round and oval windows were exposed, and the cochleae were fixed by intrascalar infusion of 4% paraformaldehyde in phosphate-buffered saline through the round window. Following fixation, cochleae were micro-dissected and stained with 1% rhodamine phalloidin. Surface preparations of the cochlear spiral were prepared, and individual turns of the organ of Corti were mounted on glass slides. Surface preparation assessment was performed under epifluorescent illumination on a Leitz photomicroscope. Surviving hair cells were counted in 0.19-mm reticules and plotted as cytocochleograms (percentage of inner and outer hair cell loss, relative to normal hearing ears) as previously described (Shore et al. 2008).
Controls and correcting for acoustic cross-talk
A potential concern when conducting these experiments is cross-talk: signals can stimulate the opposite ear directly without any conduction by a neural pathway. This can occur by bone conduction, air conduction, or vibration of the supporting apparatus (Gibson 1982; see also Ozdamar and Stein 1981). We assessed cross-talk in our setup previously when we performed several control experiments in normal hearing animals (Sumner et al. 2005). Those data revealed that cross-talk excitation could occur beginning at 70 dB SPL in normal hearing animals. In other studies in which cross-talk was examined after inducing hearing loss akin to that induced in this study, cross-talk was not observed until tone levels exceeded 85 dB SPL (Le Prell et al. 2004). Although we accepted excitatory responses only in cases where cross-talk could be completely ruled out as described previously (Sumner et al. 2005), it should be noted that with severe hearing losses in the ipsilateral ear, any cross-talk to contralateral stimulation should be well above the dynamic range of the acoustic stimuli used in the present study.
RESULTS
Units were included in this study if histological reconstructions revealed that the recording electrode was located in the VCN and the lesions were confined to the target structure with no obvious effects on adjacent structures. Results are based on responses from 160 units that were located in the anterior VCN (AVCN). Approximately 80% of these units were multiunit recordings and the other 20% were sortable into single units. Both types of unit recordings were used in the population data. Broadband noise (BBN) was used as the primary acoustic stimulus for contralateral excitation to maximize the number of neural responses recorded.
Responses of VCN units to contralateral sound are excitatory after ossicular disruption or cochlear damage
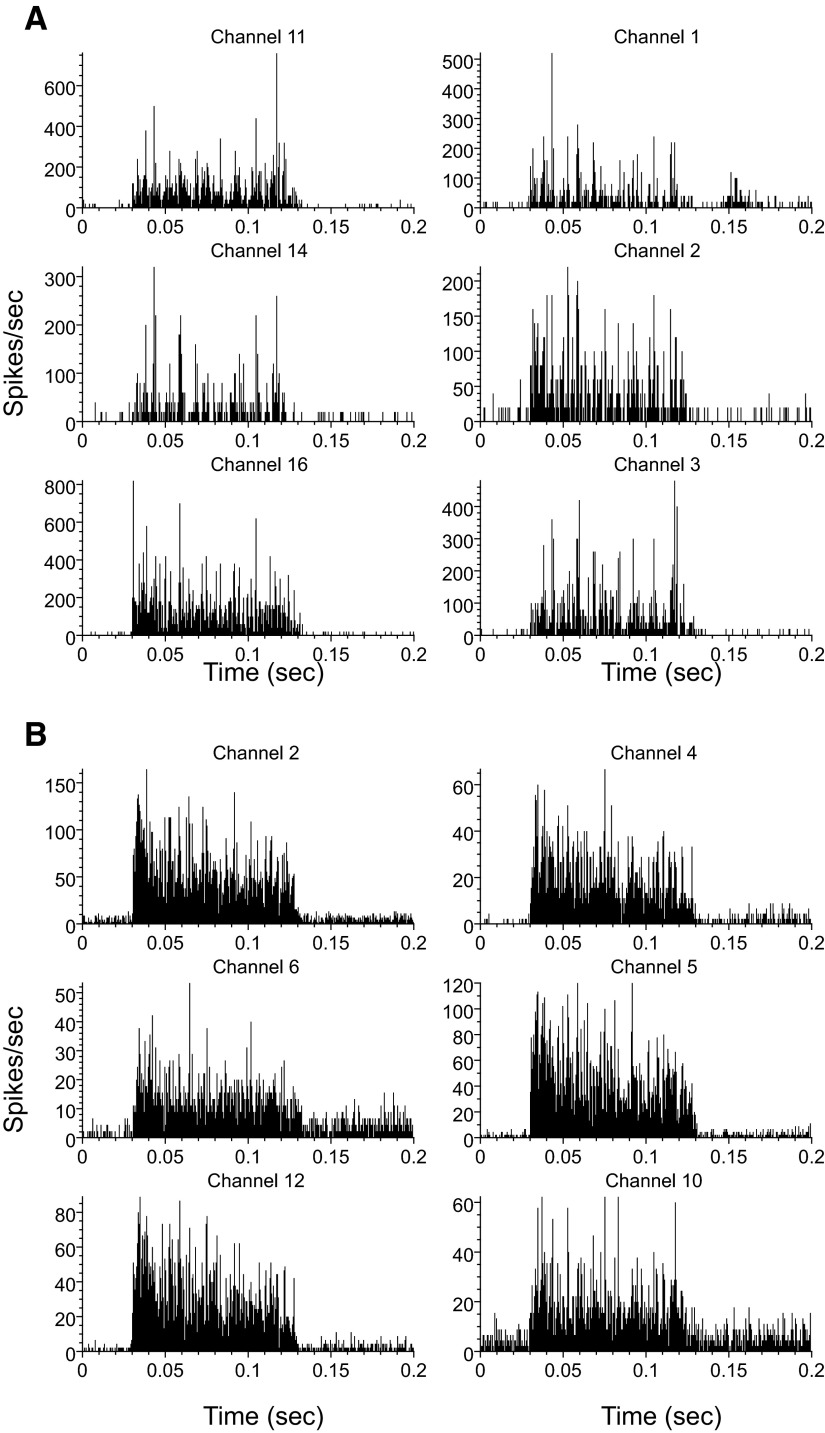
In animals with unilateral CHL and CA, the ipsilateral excitatory thresholds to BBN were high, normally >60 dB SPL. In many cases, for CA, no ipsilateral responses were measurable at the highest levels of BBN generated by our system (80 dB SPL). The contralateral excitatory response thresholds in unilateral deafened animals, by contrast, were usually much lower than the ipsilateral excitatory thresholds. The excitatory responses to contralateral sound were also much more common than in normal animals. In normal animals, only 4% are excited while it can be ≤40% for CHL and >60% for CA. Example PSTHs of contralateral excitatory responses in CHL and CA animals are shown in Fig. 2 (A and B, respectively). The PSTHs look very similar to VCN responses commonly recorded in response to ipsilateral BBN but were more robust following CA than CHL. None of these units responded to an 80-dB SPL ipsilateral noise.
FIG. 2.
Example poststimulus time histograms (PSTHs, 100 repeats, 1-ms bin width) of the excitatory responses in a conductive hearing loss (CHL, A) and a cochlear ablation (CA, B) animal. Contralateral sound was broadband noise (BBN, 100-ms duration, 1.5 ms rise-fall time) at 80 db SPL. Responses are from multiunit clusters. The stimulus start time was 25 ms.
There were no systematic differences in the attributes of contralateral excitatory responses induced by CHL and CA. Because the CHL was performed before placing the animals in the stereotaxic, it was not possible to compare precisely the onset of the excitation with both types of deafening. Excitatory responses were observed in the CHL animals when the neural recordings commenced (∼1.5 h post disruption). In the CA animals, there was a tendency for the contralateral excitation to become more robust 1–2 h after the CA. Thus the time course of the increased excitability is fairly rapid and probably similar with the two types of deafening.
CHL causes an increase in spontaneous activity with mean values almost doubling, from 28.3 spike/s in normal hearing animals to 50 spike/s after CHL (Sumner et al. 2005). In four of the CA animals in this study, we were able to record responses for ≥1.5 h prior to producing lesions. At 61% of the recording sites the spontaneous rates increased during this time by a mean value of 49%. Considering that the effects reported by Sumner et al. (2005) took several hours, the increase in spontaneous activity 1.5 h after CA suggests a similar shift in the distribution of spontaneous rates occurs after CA. In cats, Koerber et al. (1966) reported there was virtually no spontaneous activity in the VCN after complete CAs. However, they found considerable spontaneous activity after incomplete ablations. All the ablations in the guinea pigs in this study were partial. Thus our findings are consistent with those of Koerber et al. (1966).
The latencies of the excitatory responses to BBN stimulation of the contralateral ear were also similar for CHL and CA. The values were determined from the first peak of the excitatory response, which was determined manually on each PSTH. At 80 dB SPL, the latencies for both types of deafness were always >6 ms. Typically, at 40–80 dB SPL they ranged between 6 and 10 ms, but occasionally latencies as long as 15–50 ms were seen in both CHL and CA animals.
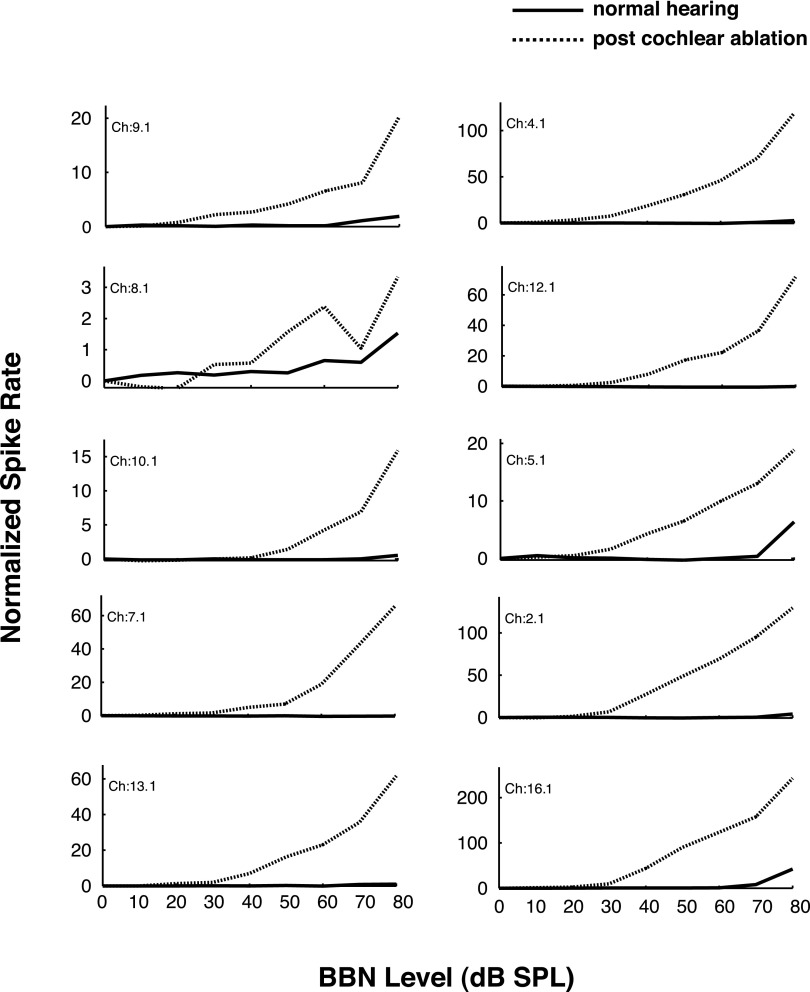
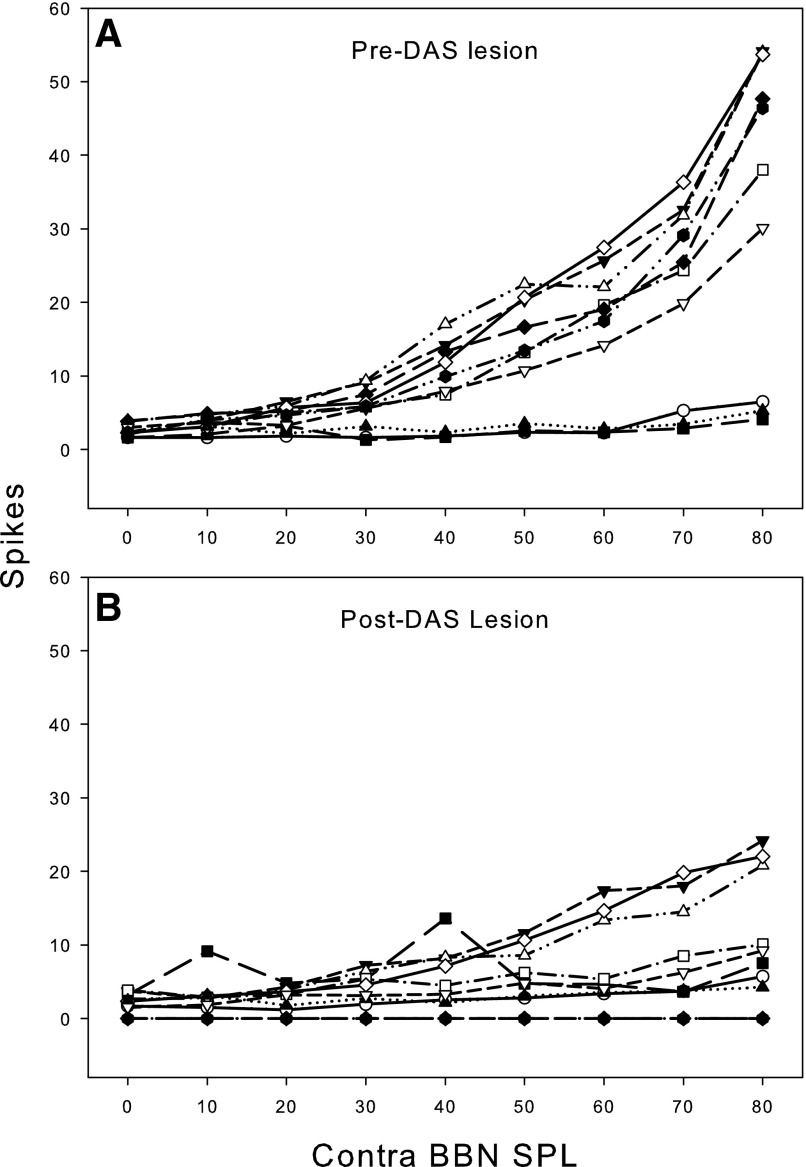
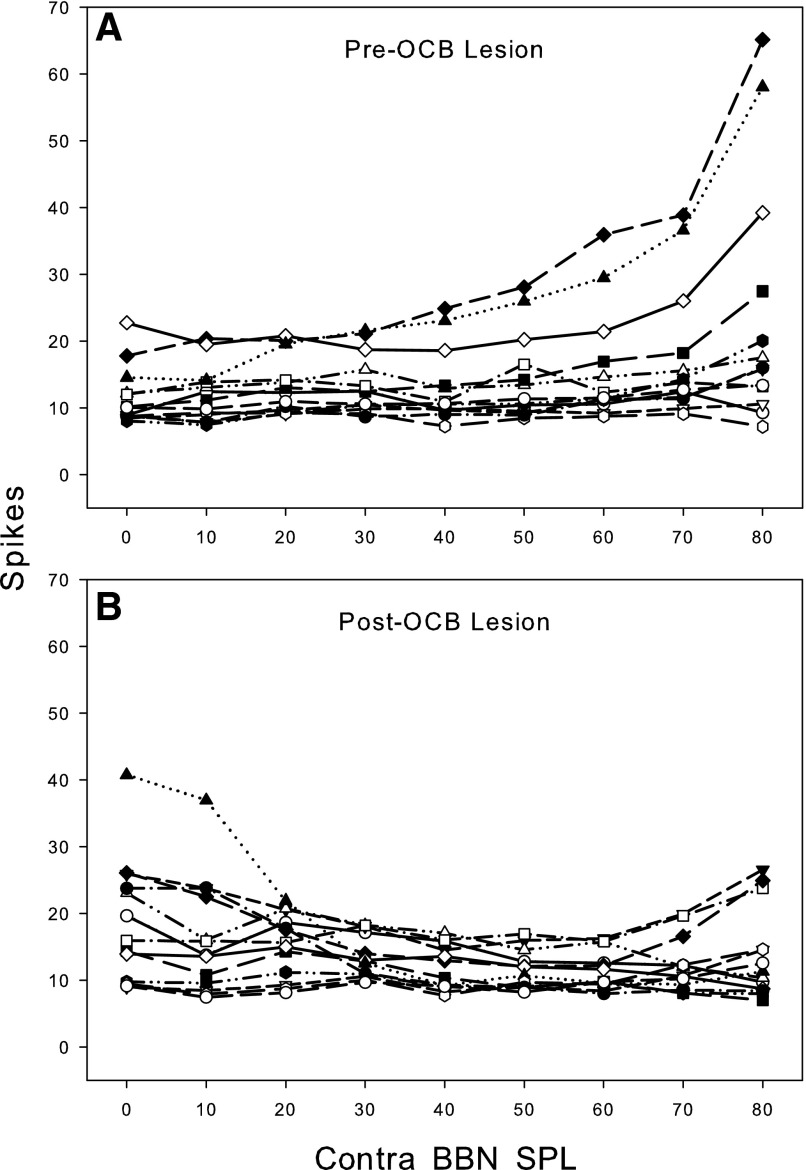
In response to BBN, the thresholds for contralateral excitation were similar in CHL and CA animals, typically ranging from 20 to 40 dB SPL in both groups of animals. The RLFs were also similar. Figures 3 and 4 show single-unit RLFs for the excitatory responses to contralateral BBN stimulation for CA and CHL animals, respectively. Only those responses that could not be attributed to cross-talk are included. Excitatory responses in both CHL and CA animals exhibit monotonically increasing functions that are quantitatively very similar. For the CHL animals, cytocochleograms revealed no loss of inner or outer hair cells, confirming that these were CHLs and not sensorineural. Similarly, examination of the middle and inner ears in the CA animal revealed a near complete destruction of the cochlea confirming a sensorineural hearing loss.
FIG. 3.
Rate-level functions for 10 sorted single units in the left VCN of 1 animal. Responses to contralateral (right ear) sound stimulation are shown before and after left cochlear ablation. Stimulus: 100-ms duration BBN. Spike rate is normalized to spontaneous rate. Only 1 unit (Ch 8.1) showed noticeable excitation to contralateral sound before the ablation, but all units were excited post cochlear ablation.
FIG. 4.
Rate-level functions for 10 sorted single units in the left VCN of 1 animal. Responses to ipsilateral (left ear) and contralateral (right ear) sound stimulation are shown after ossicular disruption of the left middle ear. Stimulus: 100-ms duration BBN. Spike rate is normalized to spontaneous rate. As for the cochlear ablation in Fig. 3, most units were excited by contralateral sound following the ossicular disruption.
Lesion experiments
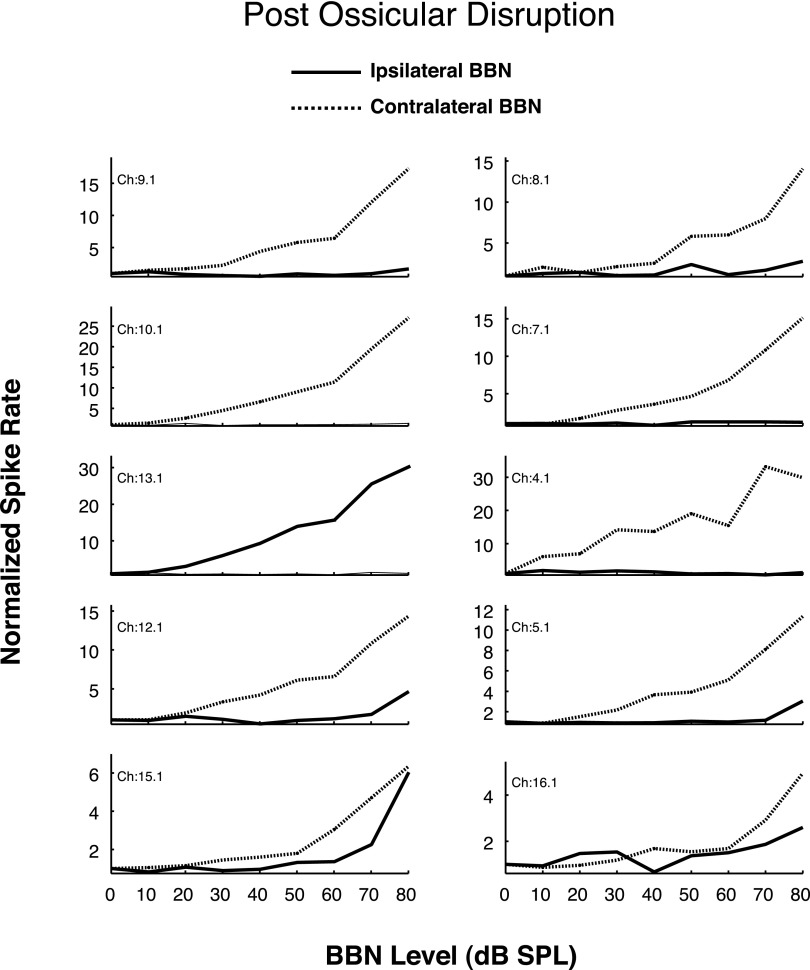
The lesion experiments tested the hypotheses that the contralateral excitation is mediated by direct glutamatergic CN-commissural projections via the D/IAS; direct glutamatergic CN-commissural projections via the TB; or cholinergic neurons of the OCB that send excitatory collaterals to the VCN. For the electrophysiological results to be included in the database, the lesions had to be histologically confirmed as confined to their respective targets. Thus we report here results from five animals with D/IAS lesions (L1, Fig. 1), two animals with TB lesions (L2, Fig. 1), and three animals with OCB lesions (L3, Fig. 1). Results reveal that lesions of the D/IAS, TB, and OCB all reduce the contralateral excitation but have different effects on the numbers of neurons affected and the magnitude of the reductions. Post lesion PSTHs reveal that the onset and steady-state portions of the excitation to the BBN stimulus are also differentially affected. These changes were not observed in animals in which the target structure was missed as revealed by the Fluoro-Gold or -Ruby tracers being found ventral, dorsal, caudal, or rostral to the target.
Commissural pathway lesions of the dorsal/intermediate acoustic striae (D/IAS, L1) reduce contralateral excitation
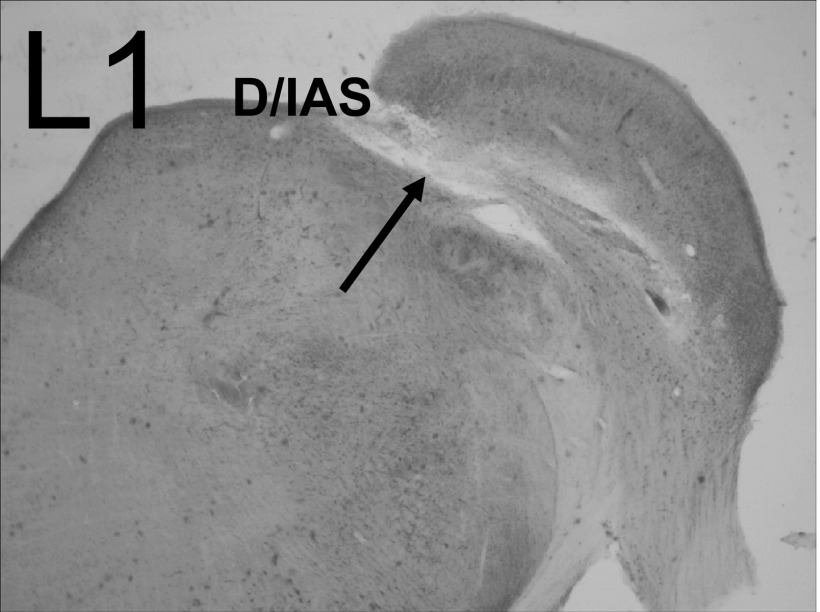
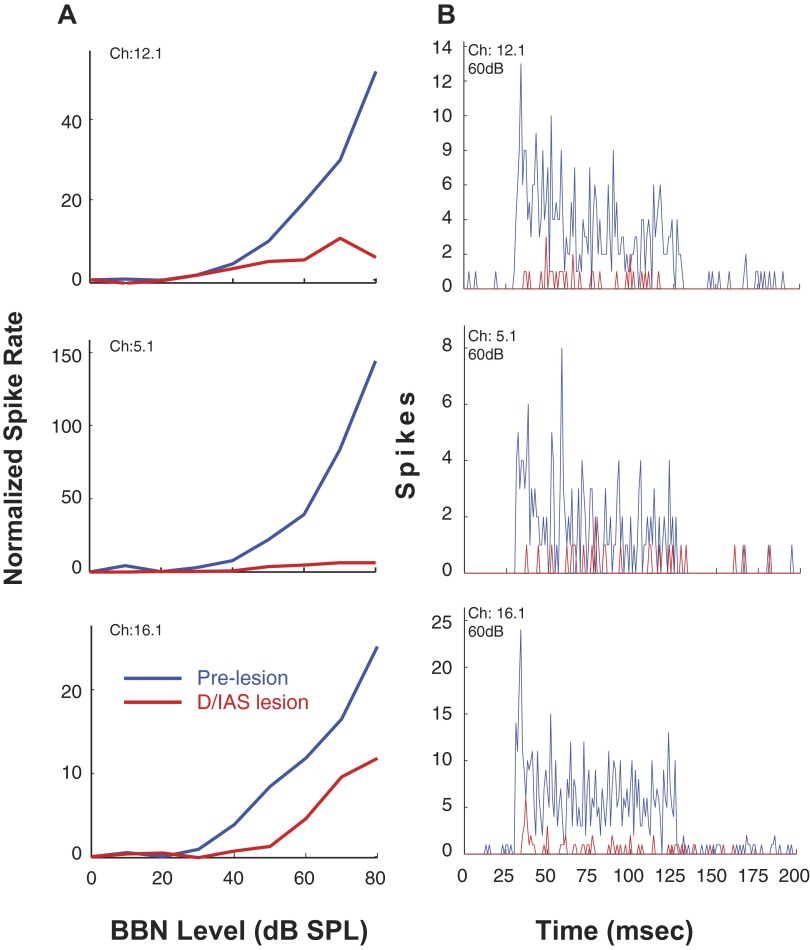
As depicted in Fig. 1, lesions of the D/IAS (L1) as they exit the contralateral CN would be expected to disturb direct excitatory, glutamatergic CN-commissural projections in addition to inhibitory glycinergic CN-commissural projections. An example of this type of lesion is shown in the photomicrograph in Fig. 5 (arrow). The melittin injection produced a lesion with a rostral-caudal extent of 560 μm (∼37% of the D/IAS extent) that damaged only the D/IAS. In this animal, we were able to sort single units from multiunit activity at three of the 16 recording sites. Results for these single units are depicted in Fig. 6, A and B. Figure 6A shows excitatory RLFs to contralateral BBN before and 30 min after the lesion. At 80 dB SPL, the excitation of the units on channels 12.1, 5.1, and 16.1 is reduced 88, 95, and 53%, respectively. In Fig. 6B, the postlesion PSTHs for these three units at 60 dB SPL reveal that the lesion greatly reduced the excitation across the entire time course of the contralateral BBN stimulus. The onset excitation is virtually eliminated for the responses on channels 12.1 and 5.1 and the steady-state portions of the excitation are greatly reduced for all three. Of the 80 single- and multiunits recorded in five animals with confirmed lesions confined to the D/IAS, the contralateral excitation was greatly reduced in a similar manner as depicted in Fig. 6, A and B at 80% of the sites. Figure 7 depicts multiunit RLFs from one of the five animals. The D/IAS lesion produced a widespread reduction in contralateral excitation that was similar to the reduction seen for the sorted single units in Fig. 6A.
FIG. 5.
Photomicrograph of a lesion in the D/IAS (L1). An injection of 0.2 μl melittin into the region of the D/IAS, produced a lesion with a rostral-caudal extent of 560 μm that damaged only the D/IAS (arrow). Electrophysiological results from this animal are depicted in Fig. 6, A and B.
FIG. 6.
A: rate-level functions for 3 sorted single units from 1 animal to contralateral sound after cochlear ablation. Responses are shown before and after a melittin lesion in the D/IAS (L1). Spike rate is normalized to spontaneous rate. B: PSTHs of single-unit responses to contralateral sound after cochlear ablation (bin width, 1 ms). Responses shown are pre- and post-lesion (as indicated). Melittin lesion is in the D/IAS (L1). Photomicrographs of the lesion in this animal are shown in Fig. 5. Stimulus: 100-ms duration BBN.
FIG. 7.
Rate-level functions for 11 multiunit recording sites from 1 animal to contralateral sound after cochlear ablation; from a different animal than the 1 shown in Fig. 6, A and B. Responses are shown before (A) and after (B) a melittin lesion in the D/IAS (L1). Stimulus: 100-ms duration BBN.
TB lesions reduce contralateral excitation
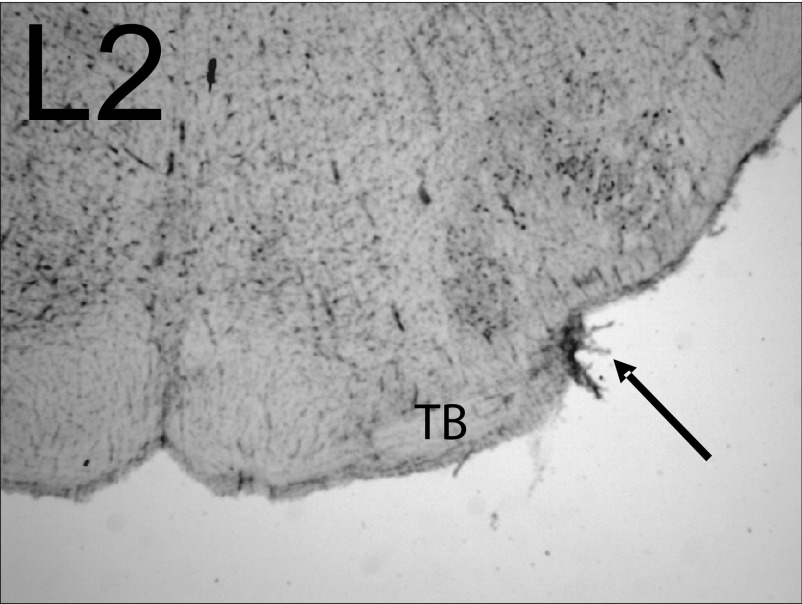
As depicted in Fig. 1, lesions of the TB (L2) as it exits the contralateral CN would be expected to disrupt indirect excitatory projections to CN via collaterals of OCB neurons whose cell bodies are in the VNTB as well as direct glutamatergic ventral commissural projections. An example of this type of lesion is shown in the photomicrograph in Fig. 8 (arrow). The melittin injection produced a small lateral lesion with a rostral-caudal extent of 350 μm that began in the most caudal regions of the SOC and damaged only the TB (∼27% of the TB extent).
FIG. 8.
Photomicrograph of a lesion in the trapezoid body (TB; L2). Following an injection of 0.3 μl melittin into the ventral region of the superior olivary complex (SOC), a small lateral lesion of the TB is observed with a rostral-caudal extent of 350 μm. The lesion began in the most caudal regions of the SOC, and damaged only the trapezoid body (TB, arrow). Electrophysiological results from this animal are depicted in Fig. 9, A and B.
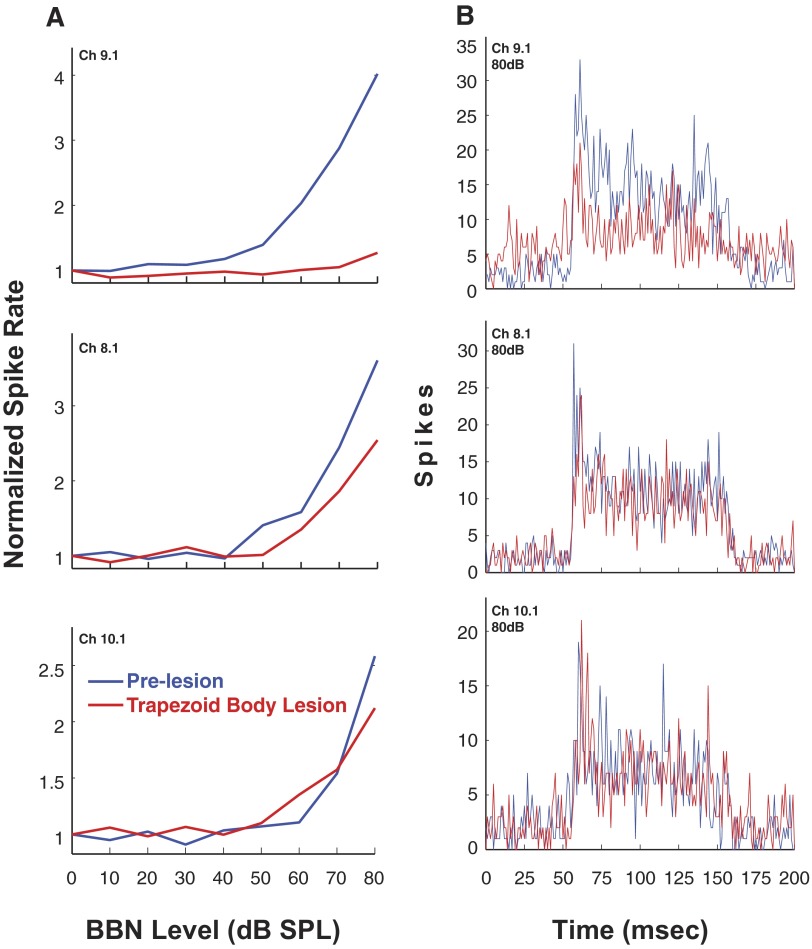
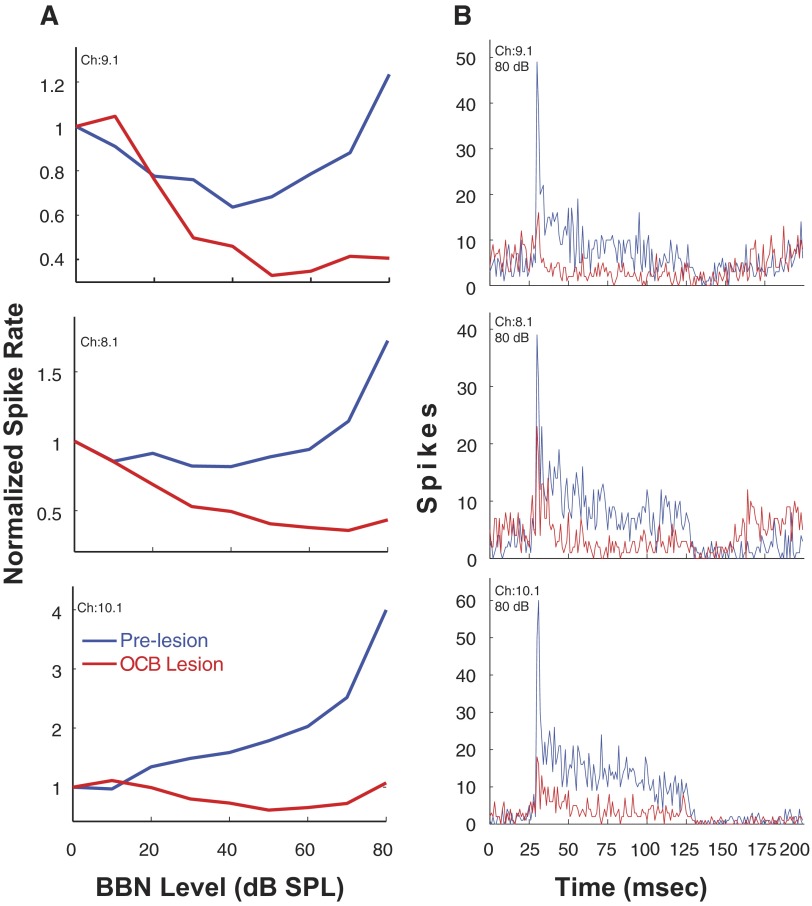
In this animal, the results for three single units sorted from multiunit activity at 3 of the 16 recording sites are depicted in Fig. 9, A and B. Figure 9A shows excitatory RLFs to contralateral BBN before and 30 min after the lesion. At 80 dB SPL, the excitation is reduced 87% for the unit on channel 9.1 but only 38% for the unit on channel 8.1. For channel 10.1, there is very little, if any, effect of the lesion on the RLF except perhaps at 80 dB where the excitation is reduced 26%. In Fig. 9B, the postlesion PSTHs for these three units at 80 dB SPL revealed that the lesion reduced both onset and steady-state portions of the excitation across the entire time course of the contralateral BBN stimulus. A smaller number of VCN neurons were affected by TB lesions, compared with D/IAS lesions, and, the effects were of lower magnitude in terms of spike rate. Specifically, although TB lesions did reduce contralateral excitation, the reductions were only observed at ∼10% of the recording sites (32 single- and multiunits recorded in 2 animals). Figure 10 depicts multiunit RLFs from one of the two animals. The TB lesion produced a moderate reduction in contralateral excitation that was similar to the reduction seen for the sorted single units in Fig. 9A. The less robust reduction in contralateral excitation following TB lesions may have been due to the small size of the lesions produced when injecting melittin near the ventral edge of the brain stem to avoid other auditory structures in the SOC, or it may reflect a less prominent input from the contralateral ear than the D/IAS. It may also be due to the caudal location of the lesions in the TB combined with the rostral location of the neural recordings in the VCN.
FIG. 9.
A: rate-level functions for 3 sorted single units from 1 animal to contralateral sound after cochlear ablation. Responses are shown before and after a melittin lesion in the TB (L2.) Spike rate is normalized to spontaneous rate. B: PSTHs of single-unit responses to contralateral sound after cochlear ablation (bin width, 1 ms). Responses shown are pre- and postlesion (as indicated). Melittin lesion is in the TB (L2). Photomicrograph of the lesion in this animal is shown in Fig. 8. Stimulus: 100-ms duration BBN.
FIG. 10.
Rate-level functions for 13 multiunit recording sites from 1 animal to contralateral sound after cochlear ablation. From a different animal than the 1 shown in Fig. 9, A and B. Responses are shown before (A) and after (B) a melittin lesion in the trapezoid body (L2). Stimulus: 100-ms duration BBN.
OCB lesions reduce contralateral excitation
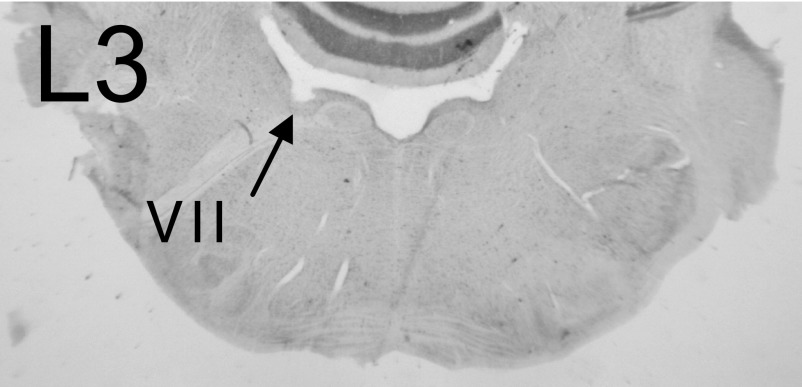
As depicted in Fig. 1, lesions of the OCB (L3) near the floor of the fourth ventricle ipsilateral to the left VCN would be expected to disrupt both the COCB and UCOCB and compromise cholinergic neurons of the OCB that send excitatory collaterals to the VCN. An example of this type of lesion is shown in the photomicrograph in Fig. 11 (arrow). The melittin injection produced a lesion with a rostral-caudal extent of 720 μm (∼100% of the OCB extent) that appeared to damage only the OCB.
FIG. 11.
Photomicrograph of a lesion of the olivocochlear bundle (OCB; L3). An injection of 0.3 μl melittin produced a lesion with a rostral-caudal extent of 720 μm that appeared to damage only the OCB (see arrow). The cochlear nucleus on the left is folded over the section. Electrophysiological results from this animal are depicted in Fig. 12, A and B.
In this animal, the results for three single units sorted from multiunit activity at 3 of the 16 recording sites are depicted in Fig. 12, A and B. Figure 12A shows excitatory RLFs to contralateral BBN before and 30 min after the lesion. At 80 dB SPL, the excitation of the units on channels 9.1, 8.1, and 10.1 is reduced 88, 85, and 82%, respectively. In Fig. 12B, the postlesion PSTHs for these three units at 80 dB SPL revealed that the lesion reduced both onset and steady-state portions of the excitation across the entire time course of the contralateral BBN stimulus as occurred with D/IAS lesions (Fig. 6B). Both steady-state and onset excitation appear less affected by the OCB lesion than by the D/IAS lesion (Fig. 6B) due in part to higher firing rate of the units depicted in Fig. 12B. The steady-state effects were comparable to the reduction that occurred after D/IAS lesions with an almost complete elimination of contralateral steady-state excitation. However, the onset excitation, albeit reduced by the OCB lesions, is still present, whereas it was eliminated by the D/IAS lesion for two of the three units depicted in Fig. 6B. Of the 48 single- and multiunits recorded in three animals with confirmed lesions confined to the OCB, the contralateral excitation was greatly reduced (i.e., in a manner similar to that depicted in Fig. 12, A and B at 65% of the recording sites. Figure 13 depicts multiunit RLFs from one of the three animals. The OCB lesion produced extensive reductions in contralateral excitation that was similar to the reduction seen for the sorted single units in Fig. 12A. It is worth noting that for the animals with an OCB lesion, there is a decrease in firing rate for some units with increasing noise burst intensity. This is seen most strikingly in Fig. 12A and further supports our conclusion that the OCB lesions resulted in an almost complete elimination of contralateral steady-state excitation.
FIG. 12.
A: rate-level functions for 3 sorted single units from 1 animal to contralateral sound after cochlear ablation. Responses are shown before and after a melittin lesion of the OCB (L3). Spike rate is normalized to spontaneous rate. B: PSTHs of single-unit responses to contralateral sound after cochlear ablation (bin width, 1 ms). Responses shown are pre- and postlesion (as indicated). Melittin lesion is in the OCB (L3). Photomicrograph of the lesion in this animal is shown in Fig. 11. Stimulus: 100-ms duration BBN.
FIG. 13.
Rate-level functions for 14 multiunit recording sites from 1 animal to contralateral sound after cochlear ablation. From a different animal than the 1 shown in Fig. 12, A and B. Responses are shown before (A) and after (B) a melittin lesion in the OCB (L3). Stimulus: 100-ms duration BBN.
DISCUSSION
In this study, we have confirmed our initial report (Shore et al. 2006) that unilateral cochlear damage results in excitation of VCN neurons ipsilateral to the damaged ear by contralateral sound stimulation. We have confirmed that the effects of CHL (see also Sumner et al. 2005) and CA are similar in that they both produce contralateral excitation of neurons in the VCN that would normally be inhibited by contralateral sound (Ingham et al. 2006; Shore et al. 2003). The attributes of the compensatory excitation that occurs following both CHL and CA are, in general, similar: the latencies and RLFs exhibit no systematic differences. The onset of the compensatory excitation is fairly rapid, occurring within hours, with both types of deafness. This suggests that the pathways involved and underlying mechanisms are the same with the two types of impairment.
Finally, we have extended this work in new directions and provide evidence that at least three different pathways are involved in this compensatory excitation. Lesions of the D/IAS, TB, and OCB have differential effects on the post CA contralateral excitation, suggesting that each pathway contributes to the contralateral excitation in a different manner with contrasting effects on onset versus steady-state responses.
Compensatory contralateral excitation
The increase in contralateral excitation is a consequence of synaptic activation. Because the animals had severe hearing loss in the ipsilateral ear, thresholds to contralateral sound were well below those that could be activated by acoustic cross-over or bone conduction (Sumner et al. 2005). Furthermore, the response latencies were longer than those for ipsilateral activation of the same neurons. In many cases, especially after CA, there was no response to ipsilaterally presented sound. The short onset (1–2 h) of the changes suggests an increase in the effectiveness of existing synapses. This hypothesis is supported by the occasional observation of contralateral excitatory responses in normal hearing animals (Shore et al. 2003; Sumner et al. 2005). Sumner et al. (2005) reported there was no lasting change in the proportion of neurons inhibited by contralateral sound after CHL. Thus it is likely that the excitation after CA reflects upregulation of existing excitatory pathways connecting the two cochlear nuclei rather than a disinhibition of normally “silent” synapses (Calford 2002).
Excitatory contralateral pathways and mechanisms
Connections between the cochlear nuclei were originally suggested by the responses of DCN neurons to acoustic stimulation of the contralateral ear (Klinke et al. 1969; Mast 1973; Young and Brownell 1976). The latencies of these inhibitory responses ranged widely, some in the range of hundreds of milliseconds, whereas others were compatible with only one synaptic delay (Hochfeld 1973; Pfalz 1962; Pirsig and Pfalz 1967; Pirsig et al. 1968). Subsequent anatomical studies have demonstrated the existence of a CN-commissural connection that is comprised primarily of large type II glycinergic multipolar cells but also small multipolar cells and possibly even bushy cells that are most likely excitatory and glutamatergic (Alibardi 2000; Cant and Gaston 1982; Schofield and Cant 1996; Shore and Moore 1998; Shore et al. 1992; Wenthold et al. 1987; Zhou et al. 2007). The latency range for contralateral excitation of VCN neurons is much smaller than in DCN (Ingham et al. 2006), reflecting the direct CN projections primarily to the VCN.
Only a few physiological studies have focused on the VCN as a binaural nucleus. Electrical stimulation of contralateral VIIIth nerve in an in vitro preparation (Babalian et al. 1999, 2002) produced inhibitory postsynaptic potentials (IPSPs) in ≤70% of principal cells in the VCN. These effects were blocked by strychnine (Babalian et al. 2002), supporting the anatomical evidence of a primarily glycinergic CN-commissural projection. Most latencies were suggestive of mono-, di- and trisynaptic connections. All principal cell types were affected. In vivo, the effect of contralateral sound stimulation on the firing rates of neurons in the VCN (Shore et al. 2003) demonstrated that in normal-hearing animals, a small percentage (<4%) of neurons showed excitation by contralateral stimulation, which could not be attributed to acoustic crossover. This contralateral excitation is consistent with findings that the CN-commissural pathway is additionally composed of neurons that may be excitatory, such as multipolar type I and perhaps globular bushy cells, as mentioned in the preceding text (Alibardi 1998; Schofield and Cant 1996; Shore et al. 1992; Spangler et al. 1987; Zhou et al. 2007). The principal projections of multipolar type I neurons are excitatory to the contralateral IC (Adams 1979b). Globular bushy cells send an excitatory connection to the MNTB (Smith et al. 1991). Because these cells may also contribute minor components to the CN-commissural projection, they may be partially responsible for excitatory responses to contralateral sound stimulation in the VCN, and in neurons of the deep DCN (Adams 1979a,b; Mast 1973).
Contralateral sound stimulation has been shown to suppress VIIIth nerve activity (Brown 1989; Gummer et al. 1988; Liberman and Brown 1986). These suppressive effects, present in almost all units, have latencies close to 200 ms, durations >200 ms, and are maximal if the contralateral tone is at BF and are thus unlikely mediators of the effects described here. However, collaterals of the OCB send a projection to the CN that has been postulated to be excitatory (Benson and Brown 1990; Brown 1993; Brown and Benson 1992; Brown et al. 1991). Electrical stimulation of OCB axons in silence results in predominantly excitation of CN neurons (Mulders et al. 2003). It is therefore possible that this OCB collateral pathway could be responsible for some of the contralateral excitation described in this study.
The results of the lesion studies presented here support the notion that at least three of these pathways are involved! First, lesions of the D/IAS as they exit the contralateral CN reduced the contralateral excitation by ∼80%. The postlesion PSTHs revealed that the reduction in excitation was across the entire time course of the sound stimulus (i.e., onset and steady state are affected), suggesting that this pathway is fast acting and likely to be mediated by glutamate. This is consistent with our recent findings (Zhou et al. 2007b) (see preceding text) that commissural neurons that exit the CN via the D/IAS co-label with VGLUT2. Other studies have shown increased glutamatergic release in the CN after CHL and cochlear damage as well as decreased uptake (Muly et al. 2004; Potashner et al. 1997). This, together with an upregulation of glutamate receptors (Rubio 2006; Suneja et al. 2000), would render any remaining glutamatergic inputs more efficient. In the DCN, this upregulation has been observed as early as 4 h after CA (Rubio 2006).
Caution needs to be exercised in interpreting the results of the D/IAS lesion too narrowly as simply interrupting commissural pathways. A number of other pathways may be involved. For example, one alternative explanation for this lesion could be that the D/IAS inputs to the IC are interrupted by the lesion, which interrupts descending input to the olivocochlear neurons, which prevents their excitation of CN neurons.
Second, lesions of the contralateral TB reduced onset and steady-state contralateral excitation by ∼10%. The small effects seen may relate to the small amount of damage from the lesions. However, the changes are consistent with mediation by an indirect pathway through the VNTB via the OCB neurons. Another possible indirect pathway is the cholinergic projection from VNTB to the CN (Godfrey et al. 1987b; Sherriff and Henderson 1994; Yao and Godfrey 1998). It could also indicate mediation via a direct, ventral commissural pathway via the TB, consistent with the results of Doucet and Lenihan (2006, 2007) demonstrating a variety of small neurons projecting directly to the CN via the TB, likely to be the VGLUT-positive cells demonstrated by Zhou et al. (2007).
Third, lesions that targeted the OCB resulted in an almost complete elimination of contralateral steady-state excitation. However, the onset excitation was less affected than that produced by a lesion of the DAS. This suggests that the OCB collaterals are primarily responsible for a slower delayed-onset excitation elicited by contralateral sounds. Other studies provide evidence that indeed the cholinergic fibers of the OCB that innervate the CN en route to the cochlea are upregulated after unilateral cochlear damage (Jin et al. 2005; Kraus and Illing 2005), providing further support for the VNTB as one source of the contralateral excitation following unilateral deafness. An increase in the sensitivity of cholinergic receptors has also been observed after deafness in slice preparations of DCN (Chang et al. 2002).
Additional mechanisms
The action of olivocochlear efferents to the nondeafened (right) cochlea might also be altered after CHL or CA of the left ear, producing an increased excitation of VIIIth nerve fibers in the ear contralateral to the damaged ear (Benson and Brown 1990, 1996; Brown and Benson 1992; Brown et al. 1988, 1991; Godfrey et al. 1987a; Mulders et al. 2002; Robertson and Winter 1988). Under normal circumstances, activity of PVCN neurons activates SOC neurons on both sides of the brain, including OCB neurons that project to the cochlea on the other side and suppress AN activity (Cant and Casseday 1986; de Venecia et al. 2005; Schofield 1995; Smith et al. 1993). After CHL and CA, spontaneous AN activity decreases (Cook et al. 2002; Kiang et al. 1965). Thus the driving force to OCB neurons might decrease, altering the OCB modulation of the opposite cochlea. Thus the normally suppressive action (Liberman and Brown 1986) of the OCB will be diminished, resulting in a subsequent increase in VIIIth nerve activity and possible increase in excitation of CN neurons. A contributing factor may be retrograde effects on the activity of olivocochlear neurons after destruction of their terminals in the cochlea by CA (Jin et al. 2005). This would not occur with CHL, however.
Another possibility for the contralateral excitation observed in this study is that the inhibitory action of ipsilateral CN projections to the contralateral (right) CN might be reduced after CHL or CA of the left ear, releasing the right CN from inhibition resulting in an unmasking of reciprocal excitation. This, coupled with the increased VIIIth nerve activity discussed above may result in increased excitation not only in the VCN ipsilateral to the CHL or CA but also in other ipsilateral and contralateral parts of the auditory neuraxis (e.g., IC), which needs to be studied as it may be involved in the generation of tinnitus.
Conclusions
The properties of contralateral excitatory responses produced by CHL and CA are very similar, suggesting similar pathways are involved in the expression of the contralateral excitation induced by unilateral conductive and sensorineural hearing loss. The results of the lesion studies indicate that each pathway makes different contributions to the contralateral excitation and has differential effects on onset versus steady-state responses, leading to the conclusion that contralateral excitation has a fast and a slow component. The fast excitation is likely mediated by glutamatergic neurons located in medial regions of VCN that send their commissural axons to the other CN either via the D/IAS or the TB. The slow component is most likely mediated by cholinergic neurons of the olivocochlear pathway the interneurons of which are located in periolivary regions and send collateral projections to the CN.
The increase in contralateral excitation associated with unilateral hearing impairment is an intriguing phenomenon with implications for binaural processing. With the demonstration of functional connections between the cochlear nuclei (Davis 2005; Ingham et al. 2006; Needham and Paolini 2003; Shore et al. 2003), it is clear that the CN plays a role in binaural processing and is the first auditory structure in which binaural processing is altered after damage to the auditory periphery. The increase in excitability may partially compensate for the discrepancy in input between the ears after unilateral deafness. In the gerbil, 2-deoxyglucose uptake is substantially diminished in the major afferent projection from an ear with a unilateral CHL, particularly during early development (postnatal day 21). In animals with a mature auditory system (postnatal day 42), the left–right discrepancy between sides of the central auditory system is less marked (Tucci et al. 1999), perhaps as a consequence of the compensatory mechanisms reported in this study.
GRANTS
This work was supported by the Royal National Institute for Deaf People (RNID) and National Institute on Deafness and Communication Disorder Grants DC-05415, DC-004825, P01 DC-00078, and P30 DC-05188.
Acknowledgments
We are grateful to Ben Yates and Mark Oldakowski for excellent technical assistance.
Present address of J. Zhou: Dept. of Physical Medicine and Rehabilitation, Albany Medical Center, Albany, NY 12208.
REFERENCES
- Adams 1979a.Adams JC Identification of cochlear nucleus projections by removal of HRP reaction product. Brain Res 177: 165–169, 1979a. [DOI] [PubMed] [Google Scholar]
- Adams 1979b.Adams JC Ascending projections to the inferior colliculus. J Comp Neurol 183: 519–538, 1979b. [DOI] [PubMed] [Google Scholar]
- Alibardi 1998.Alibardi L Ultrastructural and immunocytochemical characterization of commissural neurons in the ventral cochlear nucleus of the rat. Anat Anz 180: 427–438, 1998. [DOI] [PubMed] [Google Scholar]
- Alibardi 2000.Alibardi L Cytology, synaptology and immunocytochemistry of commissural neurons and their putative axonal terminals in the dorsal cochlear nucleus of the rat. Anat Anz 182: 207–220, 2000. [DOI] [PubMed] [Google Scholar]
- Alibardi 2004.Alibardi L Mossy fibers in granule cell areas of the rat dorsal cochlear nucleus from intrinsic and extrinsic origin innervate unipolar brush cell glomeruli. J Submicrosc Cytol Pathol 36: 193–210, 2004. [PubMed] [Google Scholar]
- Babalian et al. 1999.Babalian AL, Ryugo DK, Vischer MW, Rouiller EM. Inhibitory synaptic interactions between cochlear nuclei: evidence from an in vitro whole brain study. Neuroreport 10: 1913–1917, 1999. [DOI] [PubMed] [Google Scholar]
- Babalian et al. 2002.Babalian AL, Jacomme AV, Doucet JR, Ryugo DK, Rouiller EM. Commissural glycinergic inhibition of bushy and stellate cells in the anteroventral cochlear nucleus. Neuroreport 13: 555–558, 2002. [DOI] [PubMed] [Google Scholar]
- Benson and Brown 1990.Benson TE, Brown MC. Synapses formed by olivocochlear axon branches in the mouse cochlear nucleus. J Comp Neurol 295: 52–70, 1990. [DOI] [PubMed] [Google Scholar]
- Benson and Brown 1996.Benson TE, Brown MC. Synaptic input to cochlear nucleus dendrites that receive medial olivocochlear synapses. J Comp Neurol 365: 27–41, 1996. [DOI] [PubMed] [Google Scholar]
- Bledsoe et al. 1990.Bledsoe SC Jr, Snead CR, Helfert RH, Prasad V, Wenthold RJ, Altschuler RA. Immunocytochemical and lesion studies support the hypothesis that the projection from the medial nucleus of the trapezoid body to the lateral superior olive is glycinergic. Brain Res 517: 189–194, 1990. [DOI] [PubMed] [Google Scholar]
- Brown 1989.Brown MC Morphology and response properties of single olivocochlear fibers in the guinea pig. Hear Res 40: 93–109, 1989. [DOI] [PubMed] [Google Scholar]
- Brown 1993.Brown MC Fiber pathways and branching patterns of biocytin-labeled olivocochlear neurons in the mouse brain stem. J Comp Neurol 337: 600–613, 1993. [DOI] [PubMed] [Google Scholar]
- Brown and Benson 1992.Brown MC, Benson TE. Transneuronal labeling of cochlear nucleus neurons by HRP-labeled auditory nerve fibers and olivocochlear branches in mice. J Comp Neurol 321: 645–665, 1992. [DOI] [PubMed] [Google Scholar]
- Brown et al. 2003.Brown MC, de Venecia RK, Guinan JJ, Jr. Responses of medial olivocochlear neurons. Specifying the central pathways of the medial olivocochlear reflex. Exp Brain Res 153: 491–498, 2003. [DOI] [PubMed] [Google Scholar]
- Brown et al. 1988.Brown MC, Liberman MC, Benson TE, Ryugo DK. Brain stem branches from olivocochlear axons in cats and rodents. J Comp Neurol 278: 591–603, 1988. [DOI] [PubMed] [Google Scholar]
- Brown et al. 1991.Brown MC, Pierce S, Berglund AM. Cochlear-nucleus branches of thick (medial) olivocochlear fibers in the mouse: a cochleotopic projection. J Comp Neurol 303: 300–315, 1991. [DOI] [PubMed] [Google Scholar]
- Calford 2002.Calford MB Dynamic representational plasticity in sensory cortex. Neuroscience 111: 709–738, 2002. [DOI] [PubMed] [Google Scholar]
- Cant and Cassedy 1986.Cant NB, Cassedy JH. Projections from the anteroventral cochlear nucleus to the lateral and medial superior olivary nuclei. J Comp Neurol 247: 457–476, 1986. [DOI] [PubMed] [Google Scholar]
- Cant and Gaston 1982.Cant NB, Gaston KC. Pathways connecting the right and left cochlear nuclei. J Comp Neurol 212: 313–326, 1982. [DOI] [PubMed] [Google Scholar]
- Chang et al. 2002.Chang H, Chen K, Kaltenbach JA, Zhang J, Godfrey DA. Effects of acoustic trauma on dorsal cochlear nucleus neuron activity in slices. Hear Res 164: 59–68, 2002. [DOI] [PubMed] [Google Scholar]
- Cook et al. 2002.Cook RD, Hung TY, Miller RL, Smith DW, Tucci DL. Effects of conductive hearing loss on auditory nerve activity in gerbil. Hear Res 164: 127–137, 2002. [DOI] [PubMed] [Google Scholar]
- Davis 2005.Davis KA Contralateral effects and binaural interactions in dorsal cochlear nucleus. J Assoc Res Otolaryngol 6: 280–296, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Venecia et al. 2005.de Venecia RK, Liberman MC, Guinan JJ Jr, Brown MC. Medial olivocochlear reflex interneurons are located in the posteroventral cochlear nucleus: a kainic acid lesion study in guinea pigs. J Comp Neurol 487: 345–360, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doucet and Lenihan 2006.Doucet J, Lenihan N. Multipolar neurons in the ventral cochlear nucleus: somatic innervation of cells that project to the contralateral cochlear nucleus. Abstr Assoc Res Otolaryngol 29: 43, 2006. [Google Scholar]
- Doucet and Lenihan 2007.Doucet J, Lenihan N. The neural source of commissural connections between the cochlear nuclei: distinct components revealed by axonal trajectory. Abstr Assoc Res Otolaryngol 30: 208, 2007. [Google Scholar]
- Ebert and Ostwald 1995.Ebert U, Ostwald J. GABA can improve acoustic contrast in the rat ventral cochlear nucleus. Exp Brain Res 104: 310–322, 1995. [DOI] [PubMed] [Google Scholar]
- Fujino and Oertel 2001.Fujino K, Oertel D. Cholinergic modulation of stellate cells in the mammalian ventral cochlear nucleus. J Neurosci 21: 7372–7383, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardi and Bledsoe 1981.Gardi JN, Bledsoe SC Jr. The use of kainic acid for studying the origins of scalp-recorded auditory brainstem responses in the guinea pig. Neurosci Lett 26: 143–149, 1981. [DOI] [PubMed] [Google Scholar]
- Gibson 1982.Gibson DJ Interaural crosstalk in the cat. Hear Res 7: 325–333, 1982. [DOI] [PubMed] [Google Scholar]
- Godfrey et al. 1987a.Godfrey DA, Park-Hellendall JL, Dunn JD, Ross CD. Effect of olivocochlear bundle transaction on choline acetyltransferase activity in the rat cochlear nucleus. Hear Res 28: 237–252, 1987a. [DOI] [PubMed] [Google Scholar]
- Godfrey et al. 1987b.Godfrey DA, Park-Hellendall JL, Dunn JD, Ross CD. Effects of trapezoid body and superior olive lesions on choline acetyltransferase activity in the rat cochlear nucleus. Hear Res 28: 253–270, 1987b. [DOI] [PubMed] [Google Scholar]
- Godfrey et al. 1988.Godfrey DA, Parli JA, J.D. Dunn JD, Ross CD. Neurotransmitter microchemistry of the cochlear nucleus and superior olivary complex. In: Auditory Pathway, edited by Syka J, Masterton RB. New York: Plenum, 1988, p. 107–121.
- Gummer et al. 1988.Gummer M, Yates GK, Johnstone BM. Modulation transfer function of efferent neurons in the guinea pig cochlea. Hear Res 36: 41–51, 1988. [DOI] [PubMed] [Google Scholar]
- Hochfeld 1973.Hochfeld P Binaural Interactions in the Cat's Cochlear Nucleus (Master's thesis). Cambridge, MA: Massachusetts Institute of Technology, 1973.
- Illing and Horvath 1995.Illing RB, Horvath M. Re-emergence of GAP-43 in cochlear nucleus and superior olive following cochlear ablation in the rat. Neurosci Lett 194: 9–12, 1995. [DOI] [PubMed] [Google Scholar]
- Illing et al. 1997.Illing RB, Horvath M, Laszig R. Plasticity of the auditory brainstem: effects of cochlear ablation on GAP-43. J Comp Neurol 382: 116–138, 1997. [DOI] [PubMed] [Google Scholar]
- Illing et al. 2005.Illing RB, Kraus KS, Meidinger MA. Reconnecting neuronal networks in the auditory brain stem following unilateral deafening. Hear Res 206: 185–199, 2005. [DOI] [PubMed] [Google Scholar]
- Ingham et al. 2006.Ingham NJ, Bleeck S, Winter IM. Contralateral inhibitory and excitatory frequency response maps in the mammalian cochlear nucleus. Eur J Neurosci 24: 2515–2529, 2006. [DOI] [PubMed] [Google Scholar]
- Jin and Godfrey 2006.Jin YM, Godfrey DA. Effects of cochlear ablation on muscarinic acetylcholine receptor binding in the rat cochlear nucleus. J Neurosci Res 83: 157–166, 2006. [DOI] [PubMed] [Google Scholar]
- Jin et al. 2005.Jin YM, Godfrey DA, Sun Y. Effects of cochlear ablation on choline acetyltransferase activity in the rat cochlear nucleus. J Neurosci Res 81: 91–101, 2005. [DOI] [PubMed] [Google Scholar]
- Kiang et al. 1965.Kiang NY, Watanabe T, Thomas EC, Clark LF. Discharge Patterns of Single Fibers in the Cat's Auditory Nerve. Cambridge, MA: MIT Press, 1965.
- Killion and Dunn 1986.Killion JJ, Dunn JD. Differential cytolysis of murine spleen, bone-marrow and leukemia cells by melittin reveals differences in membrane topography. Biochem Biophys Res Commun 139: 222–227, 1986. [DOI] [PubMed] [Google Scholar]
- Klinke et al. 1969.Klinke R, Boerger G, Gruber J. Studies of the functional significance of efferent innvervation in the auditory system: afferent neuronal activity as influenced by contralaterally-applied sound. Pfluegers Ges Physiol 306: 165–175, 1969. [DOI] [PubMed] [Google Scholar]
- Koerber et al. 1966.Koerber KC, Pfeiffer RR, Warr WB, Kiang NYS. Spontaneous spike discharges from single units in the cochlear nucleus after destruction of the cochlea. Exp Neurol 16: 119–130, 1966. [DOI] [PubMed] [Google Scholar]
- Kraus and Illing 2005.Kraus KS, Illing RB. Cell death or survival: molecular and connectional conditions for olivocochlear neurons after axotomy. Neuroscience 134: 467–481, 2005. [DOI] [PubMed] [Google Scholar]
- Le Prell et al. 2003.Le Prell CG, Shore SE, Hughes LF, Bledsoe SC Jr. Disruption of lateral efferent pathways: functional changes in auditory evoked responses. J Assoc Res Otolaryngol 4: 276–290, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Prell et al. 2004.Le Prell CG, Yagi M, Kawamoto K, Beyer LA, Atkin G, Raphael Y, Dolan DF, Bledsoe SC Jr, Moody DB. Chronic excitotoxicity in the guinea pig cochlea induces temporary functional deficits without disrupting otoacoustic emissions. J Acoust Soc Am 116: 1044–1056, 2004. [DOI] [PubMed] [Google Scholar]
- Liberman and Brown 1986.Liberman MC, Brown MC. Physiology and anatomy of single olivocochlear neurons in the cat. Hear Res 24: 17–36, 1986. [DOI] [PubMed] [Google Scholar]
- Mast 1973.Mast TE Dorsal cochlear nucleus of the chinchilla: excitation by contralateral sound. Brain Res 62: 61–70, 1973. [DOI] [PubMed] [Google Scholar]
- Moore and Caspary 1983.Moore MJ, Caspary DM. Strychnine blocks binaural inhibition in lateral superior olivary neurons. J Neurosci 3: 237–242, 1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mossop et al. 2000.Mossop JE, Wilson MJ, Caspary DM, Moore DR. Down-regulation of inhibition following unilateral deafening. Hear Res 147: 183–187, 2000. [DOI] [PubMed] [Google Scholar]
- Mulders et al. 2003.Mulders WH, Paolini AG, Needham K, Robertson D. Olivocochlear collaterals evoke excitatory effects in onset neurons of the rat cochlear nucleus. Hear Res 176: 113–121, 2003. [DOI] [PubMed] [Google Scholar]
- Mulders et al. 2002.Mulders WH, Winters IM, Robertson D. Dual action of olivocochlear collaterals in the guinea pig cochlear nucleus. Hear Res 174: 264–280, 2002. [DOI] [PubMed] [Google Scholar]
- Muly et al. 2004.Muly SM, Gross JS, Potashner SJ. Noise trauma alters D-[3H]aspartate release and AMPA binding in chinchilla cochlear nucleus. J Neurosci Res 75: 585–596, 2004. [DOI] [PubMed] [Google Scholar]
- Needham and Paolini 2003.Needham K, Paolini AG. Fast inhibition underlies the transmission of auditory information between cochlear nuclei. J Neurosci 23: 6357–6361, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Needham and Paolini 2006.Needham K, Paolini AG. Neural timing, inhibition and the nature of stellate cell interaction in the ventral cochlear nucleus. Hear Res 216: 31–42, 2006. [DOI] [PubMed] [Google Scholar]
- Needham and Paolini 2007.Needham K, Paolini AG. The commissural pathway and cochlear nucleus bushy neurons: an in vivo intracellular investigation. Brain Res 1134: 113–121, 2007. [DOI] [PubMed] [Google Scholar]
- Ostapoff et al. 1990.Ostapoff EM, Morest DK, Potashner SJ. Uptake and retrograde transport of [3H]GABA from the cochlear nucleus to the superior olive in the guinea pig. J Chem Neuroanat 3: 285–295, 1990. [PubMed] [Google Scholar]
- Ozdamar and Stein 1981.Ozdamar O, Stein L. Auditory brain stem response (ABR) in unilateral hearing loss. Laryngoscope 91: 565–574, 1981. [DOI] [PubMed] [Google Scholar]
- Palmer et al. 2003.Palmer AR, Wallace MN, Arnott RH, Shackleton TM. Morphology of physiologically characterized ventral cochlear nucleus stellate cells. Exp Brain Res 153: 418–426, 2003. [DOI] [PubMed] [Google Scholar]
- Palmer and Winter 1996.Palmer AR, Winter IM. The temporal window of two-tone facilitation in onset units of the ventral cochlear nucleus. Audiol Neurootol 1: 12–30, 1996. [DOI] [PubMed] [Google Scholar]
- Pfalz 1962.Pfalz R Centrifugal inhibition of afferent secondary neurons in the cochlear nucleus by sound. J Acoust Soc Am 34: 1472–1477, 1962. [Google Scholar]
- Pirsig and Pfalz 1967.Pirsig W, Pfalz R. Neurons in the ventral cochlear nucleus, homolaterally stimulated by electrical stimulation on the base of the cochlear coil: centrifugal inhibition by contralateral sound application (guinea pigs). Arch Klin Exp Ohren Nasen Kehlkopfheilkd 189: 135–157, 1967. [PubMed] [Google Scholar]
- Pirsig et al. 1968.Pirsig W, Pfalz R, Sadanaga M. Postsynaptic auditory crossed efferent inhibition in the ventral cochlear nucleus and the blocking of it by strychnine nitrate (guinea pig). Kumamoto Med J 21: 75–82, 1968. [PubMed] [Google Scholar]
- Potashner et al. 1997.Potashner SJ, Suneja SK, Benson CG. Regulation of D-aspartate release and uptake in adult brain stem auditory nuclei after unilateral middle ear ossicle removal and cochlear ablation. Exp Neurol 148: 222–235, 1997. [DOI] [PubMed] [Google Scholar]
- Puel et al. 1995.Puel JL, Saffiedine S, Gervais d'Aldin C, Eybalin M, Pujol R. Synaptic regeneration and functional recovery after excitotoxic injury in the guinea pig cochlea. C R Acad Sci 318: 67–75, 1995. [PubMed] [Google Scholar]
- Robertson and Winter 1988.Robertson D, Winter IM. Cochlear nucleus inputs to olivocochlear neurons revealed by combined anterograde and retrograde labeling in the guinea pig. Brain Res 462: 47–55, 1988. [DOI] [PubMed] [Google Scholar]
- Rubio 2006.Rubio ME Redistribution of synaptic AMPA receptors at glutamatergic synapses in the dorsal cochlear nucleus as an early response to cochlear ablation in rats. Hear Res 216- 217: 154–167, 2006. [DOI] [PubMed] [Google Scholar]
- Saint Marie 1996.Saint Marie RL Glutamatergic connections of the auditory midbrain: selective uptake and axonal transport of D-[3H]aspartate. J Comp Neurol 373: 255–270, 1996. [DOI] [PubMed] [Google Scholar]
- Schofield 1994.Schofield BR Projections to the cochlear nuclei from principal cells in the medial nucleus of the trapezoid body in guinea pigs. J Comp Neurol 344: 83–100, 1994. [DOI] [PubMed] [Google Scholar]
- Schofield 1995.Schofield BR Projections from the cochlear nucleus to the superior paraolivary nucleus in guinea pigs. J Comp Neurol 360: 135–149, 1995. [DOI] [PubMed] [Google Scholar]
- Schofield 2001.Schofield BR Origins of projections from the inferior colliculus to the cochlear nucleus in guinea pigs. J Comp Neurol 429: 206–220, 2001. [DOI] [PubMed] [Google Scholar]
- Schofield and Cant 1992.Schofield BR, Cant NB. Organization of the superior olivary complex in the guinea pig. II. Patterns of projection from the periolivary nuclei to the inferior colliculus. J Comp Neurol 317: 438–455, 1992. [DOI] [PubMed] [Google Scholar]
- Schofield and Cant 1996.Schofield BR, Cant NB. Origins and targets of commissural connections between the cochlear nuclei in guinea pigs. J Comp Neurol 375: 128–146, 1996. [DOI] [PubMed] [Google Scholar]
- Sherriff and Henderson 1994.Sherriff FE, Henderson Z. Cholinergic neurons in the ventral trapezoid nucleus project to the cochlear nuclei in the rat. Neuroscience 58: 627–633, 1994. [DOI] [PubMed] [Google Scholar]
- Shore et al. 1991.Shore S, Helfert R, Bledsoe SJ, Altschuler R, Godfrey D. Descending projections to the guinea pig cochlear nucleus. Hear Res 52: 255–268, 1991. [DOI] [PubMed] [Google Scholar]
- Shore et al. 1992.Shore SE, Godfrey DA, Helfert RH, Altschuler RA, Bledsoe SC Jr. Connections between the cochlear nuclei in guinea pig. Hear Res 62: 16–26, 1992. [DOI] [PubMed] [Google Scholar]
- Shore et al. 2008.Shore SE, Koehler S, Oldakowski M, Hughes LF, Syed S. Dorsal cochlear nucleus responses to somatosensory stimulation are enhanced after noise-induced hearing loss. Eur J Neurosci 27: 155–168, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shore et al. 2006.Shore S, LePrell C, Koehler S. Contralateral excitation of cochlear nucleus neurons following cochlear damage is mediated by peri-olivary nuclei. Abstr Assoc Res Otolaryngol 29: 44, 2006. [Google Scholar]
- Shore and Moore 1998.Shore SE, Moore JK. Input to the cochlear granule cell domain: systems identified in the guinea pig by axonal transport. Hear Res 116: 33–42, 1998. [DOI] [PubMed] [Google Scholar]
- Shore et al. 2003.Shore SE, Sumner CJ, Bledsoe SC, Lu J. Effects of contralateral sound stimulation on unit activity of ventral cochlear nucleus neurons. Exp Brain Res 153: 427–435, 2003. [DOI] [PubMed] [Google Scholar]
- Sie and Rubel 1992.Sie KC, Rubel EW. Rapid changes in protein synthesis and cell size in the cochlear nucleus following eighth nerve activity blockade or cochlea ablation. J Comp Neurol 320: 501–508, 1992. [DOI] [PubMed] [Google Scholar]
- Smith et al. 1991.Smith PF, Joris PX, Carney LH, Yin TCT. Projections of physiologically characterized globular bushy cell axons from the cochlear nucleus of the cat. J Comp Neurol 304: 387–407, 1991. [DOI] [PubMed] [Google Scholar]
- Smith et al. 1993.Smith PH, Joris PX, Yin TC. Projections of physiologically characterized spherical bushy cell axons from the cochlear nucleus of the cat: evidence for delay lines to the medial superior olive. J Comp Neurol 331: 245–260, 1993. [DOI] [PubMed] [Google Scholar]
- Smith et al. 2005.Smith PH, Massie A, Joris PX. Acoustic stria: anatomy of physiologically characterized cells and axonal projection patterns. J Comp Neurol 482: 349–371, 2005. [DOI] [PubMed] [Google Scholar]
- Synder and Sinex 2002.Synder RL, Sinex DG. Immediate changes in tuning of inferior colliculus neurons following acute lesions of cat spiral ganglion. J Neurophysiol 87: 434–452, 2002. [DOI] [PubMed] [Google Scholar]
- Spangler et al. 1987.Spangler K, Cant N, Henkel C, Farley G, Warr W. Descending projections from the superior olivary complex to the cochlear nucleus of the cat. J Comp Neurol 259: 452–465, 1987. [DOI] [PubMed] [Google Scholar]
- Sumner et al. 2005.Sumner CJ, Tucci DL, Shore SE. Responses of ventral cochlear nucleus neurons to contralateral sound after conductive hearing loss. J Neurophysiol 94: 4234–4243, 2005. [DOI] [PubMed] [Google Scholar]
- Suneja et al. 2000.Suneja SK, Potashner SJ, Benson CG. AMPA receptor binding in adult guinea pig brain stem auditory nuclei after unilateral cochlear ablation. Exp Neurol 165: 355–369, 2000. [DOI] [PubMed] [Google Scholar]
- Tucci et al. 1999.Tucci DL, Cant NB, Durham D. Conductive hearing loss results in a decrease in central auditory system activity in the young gerbil. Laryngoscope 109: 1359–1371, 1999. [DOI] [PubMed] [Google Scholar]
- Tucci et al. 2006.Tucci DL, Halsey K, Dolan D, Shore S. Contralateral excitation of cochlear nucleus neurons following conductive impairment may involve olivocochlear pathways. Abstr Assoc Res Otolaryngol 29: 44, 2006. [Google Scholar]
- Warren and Liberman 1989a.Warren EH 3rd, Liberman MC. Effects of contralateral sound on auditory-nerve responses. I. Contributions of cochlear efferents. Hear Res 37: 89–104, 1989a. [DOI] [PubMed] [Google Scholar]
- Warren and Liberman 1989b.Warren EH 3rd, Liberman MC. Effects of contralateral sound on auditory-nerve responses. II. Dependence on stimulus variables. Hear Res 37: 105–121, 1989b. [DOI] [PubMed] [Google Scholar]
- Wenthold et al. 1987.Wenthold RJ, Huie D, Altschuler RA, Reeks KA. Glycine immunoreactivity localized in the cochlear nucleus and superior olivary complex. Neuroscience 22: 897–912, 1987. [DOI] [PubMed] [Google Scholar]
- Wickesberg 1996.Wickesberg RE Rapid inhibition in the cochlear nuclear complex of the chinchilla. J Acoust Soc Am 100: 1691–1702, 1996. [DOI] [PubMed] [Google Scholar]
- Winter et al. 1989.Winter IM, Robertson D, Cole KS. Descending projections from auditory brain stem nuclei to the cochlea and cochlea nucleus of the guinea pig. J Comp Neurol 280: 143–157, 1989. [DOI] [PubMed] [Google Scholar]
- Winter and Palmer 1995.Winter IM, Palmer AR. Level dependence of cochlear nucleus onset unit responses and facilitation by second tones or broadband noise. J Neurophysiol 73: 141–159, 1995. [DOI] [PubMed] [Google Scholar]
- Yao and Godfrey 1998.Yao W, Godfrey DA. Immunohistochemical evaluation of cholinergic neurons in the rat superior olivary complex. Microsc Res Tech 41: 270–283, 1998. [DOI] [PubMed] [Google Scholar]
- Young and Brownell 1976.Young ED, Brownell WE. Responses to tones and noise of single cells in dorsal cochlear nucleus on unanesthetized cats. J Neurophysiol 39: 282–300, 1976. [DOI] [PubMed] [Google Scholar]
- Zhou et al. 2007.Zhou J, Cui Y, Shore S. Cochlear nucleus commissural neurons co-label with vesicular glutamate transporter 2. Abstr Assoc Res Otolaryngol 30: 126, 2007a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou et al. 2007.Zhou J, Nannapaneni N, Shore S. Vesicular glutamate transporters 1 and 2 are differentially associated with auditory nerve and spinal trigeminal inputs to the cochlear nucleus. J Comp Neurol 500: 777–787, 2007b. [DOI] [PubMed] [Google Scholar]