Abstract
Modulation of dendrodendritic synapses by the noradrenergic system in the accessory olfactory bulb (AOB) plays a key role in the formation of memory in olfactory-mediated behaviors. We have recently shown that noradrenaline (NA) inhibits mitral cells by increasing γ-aminobutyric acid inhibitory input onto mitral cells in the AOB, suggesting an excitatory action of NA on granule cells (GCs). Here, we show that NA (10 μM) elicits a long-lasting depolarization of GCs. This effect is mediated by activation of α1-adrenergic receptors as the depolarization is mimicked by phenylephrine (PE, 30 μM) and completely blocked by the α1-adrenergic receptor antagonist prazosin (300 nM). In addition to this depolarization, application of NA induced the appearance of a slow afterdepolarization (sADP) following a stimulus-elicited train of action potentials. Similarly, the group I metabotropic glutamate receptor (mGluR1) agonist DHPG (10–30 μM) also produced a depolarization of GCs and the appearance of a stimulus-induced sADP. The ionic and voltage dependence and sensitivity to blockers of the sADP suggest that it is mediated by the nonselective cationic conductance ICAN. Thus the excitatory action resulting from the activation of these receptors could be mediated by a common transduction target. Surprisingly, the excitatory effect of PE on GCs was completely blocked by the mGluR1 antagonist LY367385 (100 μM). Conversely, the effect of DHPG was not antagonized by the α1-adrenergic receptor antagonist prazosin (300 nM). These results suggest that most of the noradrenergic effect on GCs in the AOB is mediated by potentiation of a basal activity of mGluR1s.
INTRODUCTION
In the olfactory bulb, the most abundant neuronal type—the granule cells (GCs)—forms ubiquitous dendrodendritic synapses with projection neurons and mitral and tufted cells (MTCs) (Shepherd and Greer 1998). Lateral and recurrent inhibition at dendrodendritic synapses between GCs and MTCs are thought to be the most relevant physiological mechanisms contributing to olfactory processing in the bulb. In the accessory olfactory bulb (AOB), a region of the bulb involved in the processing of olfactory information relevant to social and reproductive behaviors, modulation of dendrodendritic synapses is thought to play an important role in learning processes associated with olfactory-mediated behaviors. Dendrodendritic synapses have been extensively studied in the main olfactory bulb (MOB) and are thought to function in a similar fashion in the AOB. Briefly, they consist of excitatory glutamatergic input from MTCs to GCs, inducing the release of γ-aminobutyric acid (GABA) from the dendrites of GCs and, in turn, inhibiting MTCs (Schoppa and Urban 2003). However, it has recently been shown that the release of GABA at dendrodendritic synapses in the AOB can occur by activation of group I metabotropic glutamate receptors (mGluR1s) and that the activation of these receptors occurs under physiological conditions, suggesting that the reciprocal synapses between GCs and MTCs in the AOB may differ functionally from those in the MOB (Castro et al. 2007; Heinbockel et al. 2007a,b).
Information processing in the AOB is highly influenced by modulatory afferent systems. Among them, the noradrenergic system plays an important role in promoting synaptic changes at dendrodendritic synapses, which are thought to underlie the ability to learn the stud's odor signals during mating in mice. The recognition of the stud by female mice after mating is critical because the presence of a strange male can induce pregnancy block, also known as the “Bruce effect” (Brennan 2004; Bruce 1959; Kaba and Nakanishi 1995). The AOB receives an extensive noradrenergic projection (McLean et al. 1989; Shipley et al. 1985) and, during mating, noradrenaline (NA) levels in the AOB of females are increased (Brennan et al. 1995; Rosser and Keverne 1985). It has been postulated that NA produces disinhibition of MTCs by reducing the release of GABA from GCs at dendrodendritic synapses in females during mating (Brennan 2004). Disinhibition of MTCs leads to an increased release of glutamate from MTCs, which then induces the long-term changes underlying the formation of memory to the male. Interestingly, activation of group II mGluRs in the AOB can also promote disinhibition of MTCs and activation of these receptors can induce the formation of memory to the male in the absence of mating (Hayashi et al. 1993; Kaba et al. 1994). Thus the activation of adrenergic receptors and mGluRs has been postulated to promote the synaptic changes underlying the formation of memory in the AOB in females during mating in mice (Brennan 2004).
We have recently shown that NA inhibits MTCs in the AOB by increasing GABA inhibitory activity (Araneda and Firestein 2006). This finding was surprising, given that several studies have indicated that the overall effect of noradrenergic modulation in the olfactory bulb is excitatory (Ciombor et al. 1999; Hayar et al. 2001; Jahr and Nicoll 1982; Mouly et al. 1995; Trombley and Shepherd 1992; but see McLennan 1971; Okutani et al. 1998; Perez et al. 1987; Salmoiraghi et al. 1964). Moreover, despite the proposed models on the mechanisms underlying the changes induced by NA in the AOB during mating, only a few studies have addressed the cellular actions of this neuromodulatory transmitter in AOB neurons (Brennan et al. 1995; Kaba and Huang 2005; Kaba and Keverne 1988). The increase in GABA inhibitory activity in MTCs could result from a direct excitatory action of NA on GCs (Araneda and Firestein 2006). To address this possibility we characterized the cellular actions of NA in GCs, using whole cell recordings. In agreement with our previous work, we describe an excitatory action of NA on GCs; NA depolarized GCs and induced the appearance of a slow afterdepolarization (sADP) following a stimulus-elicited train of action potentials. These excitatory effects are mediated by activation of α1-adrenergic receptors. Similarly, activation of mGluR1s also depolarizes GCs and then induces the appearance of the sADP. Furthermore, we present evidence for an unexpected interaction between the excitation of GCs produced by activation of adrenergic receptors and the activity of mGluR1s.
METHODS
Slice preparation
Experiments were performed in AOB slices obtained from 3- to 6-wk-old C57/BL6 mice; both male and female mice were used in our studies because we did not find any gender differences in the effects of NA. Animals were deeply anesthetized with isoflurane and decapitated. Brain slices were prepared in a modified artificial cerebral spinal fluid (ACSF) of the following composition (in mM): 222 sucrose, 27 NaHCO3, 1.25 NaH2PO4, 3 KCl, 1 CaCl2, and 3 MgCl2. The whole brain was quickly removed and placed in oxygenated ice-cold sucrose ACSF. A block of tissue, containing part of the frontal lobes and the olfactory bulbs, was glued with cyanoacrylate to a microslicer stage and bathed in chilled sucrose ACSF. Sagittal sections (250–300 μm) of the olfactory bulb, containing the AOB, were sliced using a vibrating microslicer (Leica, Redding, CA). The slices were then transferred to an incubation chamber containing normal ACSF (see following text) and allowed to recuperate first at 34°C for 30 min and then at room temperature for another hour. In all experiments, unless otherwise indicated, the extracellular solution is ACSF of the following composition (in mM): 125 NaCl, 25 NaHCO3, 1.25 NaH2PO4, 3 KCl, 2 CaCl2, 1 MgCl2, 3 myo-inositol, 0.3 ascorbic acid, 2 Na-pyruvate, and 15 glucose, continuously oxygenated (95% O2-5% CO2) to give pH 7.4 and osmolarity of about 305 mOsm.
Slices were placed in a submerged recording chamber mounted on the stage of an Olympus BX51, fixed-stage, upright microscope, fitted with differential infrared interference contrast optics. Slices were observed with a ×40 water-immersion objective and visualized using a CoolSNAP EZ camera (Photometrix, Tucson, AZ). Granule cells were recognized by their morphology and position in the slices; in the AOB the lateral olfactory tract separates the granule cell layer from the MTCs and glomerular layers (Meisami and Bhatnagar 1998; Takami and Graziadei 1991). Most experiments, unless indicated, were carried out in the current-clamp mode using standard patch pipettes (3- to 7-MΩ resistance) pulled on a horizontal puller (Sutter, Novato, CA). All experiments were performed at room temperature.
Data acquisition and analysis
Membrane potential was recorded using a dual EPC10 amplifier (HEKA, Union City, NY). Data analysis was performed using macros written for the IGOR Pro software (WaveMetrics, Lake Oswego, OR) and the Mini60 software (Synaptosoft, Fort Lee, NJ). To elicit the slow afterdepolarization (sADP), GCs were stimulated every 30 s with a depolarizing current stimulus (500 ms) of variable intensity adjusted to elicit 3–12 action potentials. The afterhyperpolarization (AHP) was measured as the most negative value of membrane potential following the depolarizing stimulus and it usually occurred within 100 ms after the end of the pulse. The sADP was measured as the most positive value of membrane potential after the end of the pulse and it generally occurred within a few seconds of the end of pulse. The value of membrane potential prestimulus (baseline) was subtracted from each of these values and thus the reported values of AHP and sADP correspond to the ΔV (in mV). The duration of the sADP was quantified by fitting a single exponential, from the peak of the response to the end of the stimulus protocol (30 s), expressed as the τ of decay. The size and duration of the sADP in the presence of agonists were variable from cell to cell and differences in the membrane potential from which it was elicited also contributed to this variability. In general, we found a small positive correlation between the size of the sADP and the voltage prestimulus; larger sADPs were reliably observed at more depolarized potentials (data not shown). Nevertheless, the size and kinetics (time to peak and decay) of the sADP reported here correspond to averages of the largest sADP obtained in different cells in the presence of agonist (or agonist in the presence of antagonist). To obtain the reversal potential of the current underlying the ADP, we used a “hybrid” current/voltage-clamp protocol. In these experiments the amplifier was switched from voltage- to current-clamp 200 ms before a depolarizing current pulse was applied (500 ms, 20–45 pA) and back to voltage-clamp 50 ms after the end of the stimulus. In voltage-clamp cells were held at −60 mV and a dual ramp was conducted from −100 to −30 mV and back to −100 mV (70 mV/s). Control ramps were subtracted from the ramps obtained in the presence of phenylephrine (PE) once the depolarizing pulse elicited the sADP in current-clamp. The reversal potential was obtained from the current–voltage relation from the ramp in the negative direction by extrapolating, to the voltage axis, a polynomial fitting of the curves. The kinetic analysis of the GABA inhibitory postsynaptic currents (IPSCs) shown in Fig. 6B was performed in segments of 2 min for control and in the presence of LY367385 (LY) and 1.5–2 min after PE alone or in the presence of LY. Statistical differences were assessed by the paired t-test and the Kolmogorov–Smirnov test for the distributions shown in Fig. 7. Values reported correspond to results from at least three different trials and error bars indicate the SE.
Solutions and pharmacological agents
In current-clamp experiments the internal solution had the following composition (in mM): 120 K-gluconate, 10 Na-gluconate, 4 NaCl, 10 HEPES-K, 10 Na phosphocreatine, 2 Na-ATP, 4 Mg-ATP, and 0.3 GTP (adjusted to pH 7.3 with KOH). For the experiments with BAPTA, the internal solution had the following composition (in mM): 80 K-gluconate, 25 K-BAPTA, 7.5 CaCl2, 2 MgCl2, 5 NaCl, 10 HEPES-K, 2 Na-ATP, 4 Mg-ATP, and 0.3 GTP (adjusted to pH 7.3 with KOH). To record GABA IPSCs in MTCs, the internal solution had the following composition (in mM): 125 Cs-gluconate, 4 NaCl, 2 MgCl2, 2 CaCl2, 10 EGTA, 10 HEPES, 2 Na-ATP, 4 Mg-ATP, and 0.3 GTP (adjusted to pH 7.3 with CsOH). The osmolarity of the internal solutions was adjusted to 290–305 mOsm. For the experiments in Fig. 4A the extracellular Na concentration in the ACSF was reduced. This modified ACSF (low-Na) had the same composition as that of the normal ACSF (see preceding text) except for the following (in mM): 10 NaCl, 100 N-methyl-d-glucamine, and 10 glucose (the pH of this solution was adjusted to pH 7.4 with HCl). The following drugs were bath applied: noradrenaline (NA), (R)-(−)-1-(3-hydroxyphenyl)-2-methylaminoethanol hydrochloride (phenylephrine, PE), (RS)-1-aminoindan-1,5-dicarboxylic acid (AIDA), (RS)-3,5-dihydroxyphenylglycine (DHPG), 1-(4-amino-6,7-dimethoxy-2-quinazolinyl)-4-(2-furanylcarbonyl) piperazine hydrochloride (prazosin), (S)-(+)-α-amino-4-carboxy-2-methylbenzeneacetic acid (LY367385, LY), d-2-amino-5-phosphonopentanoic acid (APV), 2,3-dihydroxy-6-nitro-7-sulfamoyl-benzo[f]quinoxaline-2,3-dione (NBQX), bicuculline (Bic), tetrodotoxin (TTX), 1-[β-[3-(4-methoxyphenyl)propoxy]-4-methoxyphenethyl]-1H-imidazole hydrochloride (SKF96365, SKF), and flufenamic acid (FFA).
The volume of the chamber and the speed of perfusion allowed for full exchange of the solution in <1 min. Antagonists were applied for ≥10 min. All drugs were purchased from Tocris Cookson (Bristol, UK) except for NA and bicuculline, which were purchased from Sigma (St. Louis, MO).
RESULTS
Noradrenaline depolarizes granule cells
Most GCs had hyperpolarized membrane potential and did not fire spontaneously (−68 ± 1.1, n = 47). As shown in Fig. 1A (top trace), application of NA (10 μM) depolarized GCs, with some of these cells reaching threshold, followed by firing of action potentials (9.7 ± 0.9 mV; n = 13). When we recorded the response of two consecutive applications of NA at different potentials in the same cells the depolarization was larger at more depolarized potentials. Thus in the range of −80 to −70 mV the average was 6.0 ± 2.3 mV, whereas at depolarized potentials it was 12.5 ± 2.5 mV (−68 to −59 mV, P < 0.03, n = 4). The NA-induced depolarization had a slow onset (127 ± 37 s to peak, n = 5) and lasted several minutes (>10 min), particularly when it was accompanied by cell firing. The time course of response of the GCs to NA closely resembles that of the increase in GABA IPSCs elicited by NA in MTCs, lasting several minutes (Araneda and Firestein 2006).
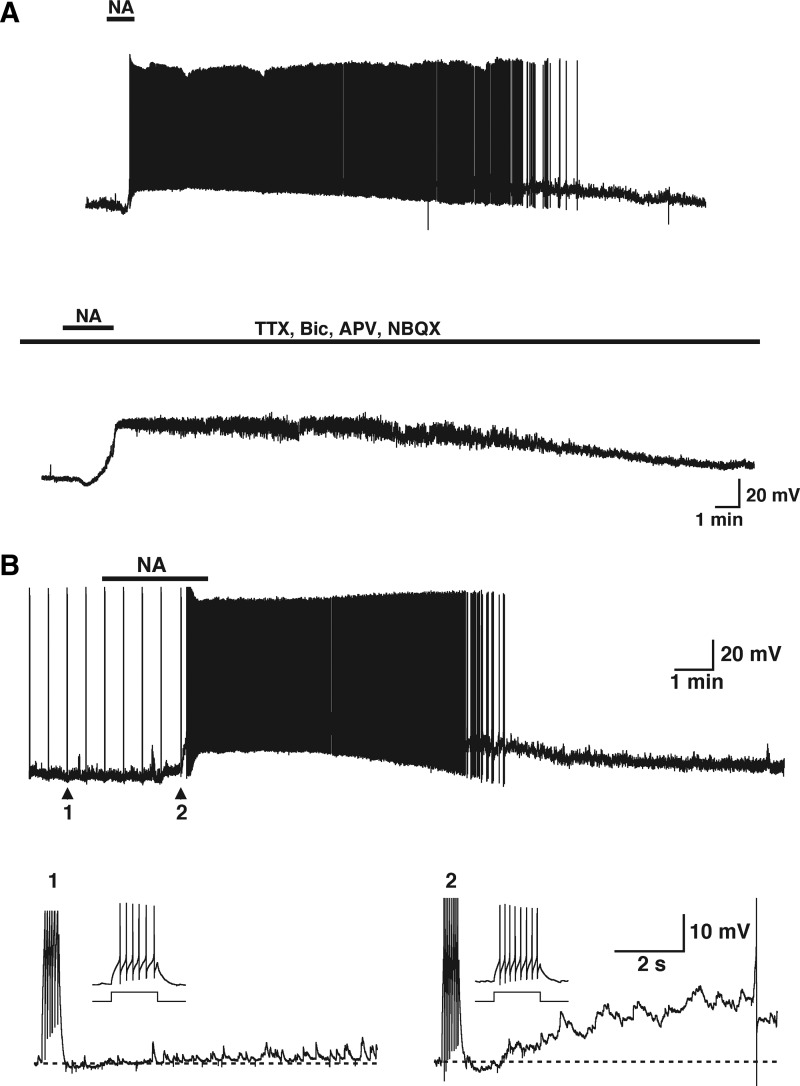
FIG. 1.
Noradrenaline (NA) directly excites granule cells (GCs). A, top trace: bath application of NA (10 μM, 2 min, top bar) produced a membrane potential depolarization and action potential firing in this GC. The responses evoked by NA had a slow onset (>40 s) and lasted several minutes (>10 min), after which the membrane potential returned to resting levels. Bottom trace: in the same cell, application of blockers of fast synaptic transmission [synaptic blockers; d-2-amino-5-phosphonopentanoic acid (APV), 2,3-dihydroxy-6-nitro-7-sulfamoyl-benzo[f]quinoxaline-2,3-dione (NBQX), bicuculline (Bic)] and tetrodotoxin (TTX) to block voltage-gated Na channels, reduced the spontaneous synaptic activity but NA still induced a robust depolarization of GCs (resting membrane potential is −60 mV). B: in addition to membrane potential depolarization, NA (10 μM, 160 s, top bar) induced the appearance of a slow afterdepolarization (sADP) following a stimulus-induced train of action potentials. In this and the next figures GCs are stimulated every 30 s and the stimulus-elicited action potentials appear as upward lines due to compression in the time axis. Bottom traces: under control conditions (arrow 1 in top trace) the depolarizing current stimulus (30 pA, 500 ms) elicited 6 action potentials in this cell (inset). At the end of the stimulus the membrane potential was slightly hyperpolarized and then returned to resting levels. In the presence of NA (arrow 2, top trace) the same stimulus induced 8 action potentials (inset) and was followed by a small hyperpolarization; however, during the next seconds an sADP developed that greatly enhanced the excitation of the cell (see text for values and number of GCs tested). The dotted lines, in this and the following figures, indicate the resting membrane potential before the stimulus, −72 mV (1) and −65 mV (2).
The depolarization of GCs could be mediated by an indirect action of NA, such as a stimulatory action on afferent excitatory terminals from other brain regions. For example, the olfactory bulb receives an extensive excitatory projection from the olfactory cortex and olfactory nucleus (Shepherd and Greer 1998). To test this possibility we recorded the actions of NA (10 μM) in the presence of blockers of excitatory transmission, the N-methyl-d-aspartate (NMDA) receptor antagonist APV (100 μM) and the α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor antagonist NBQX (10 μM). In addition, we included the GABAA receptor antagonist bicuculline (20 μM) to block inhibitory transmission and TTX (1 μM) to block voltage-gated Na+ channels. In the presence of fast synaptic transmission blockers NA still produced a large, long-lasting depolarization with a time course similar to that observed under control conditions (8.5 ± 4.8 mV, Fig. 1A, bottom trace, n = 4), suggesting a direct action of NA on GCs.
Noradrenaline induces the appearance of a slow afterdepolarization
In addition to the depolarization of GCs, NA (10 μM, Fig. 1B, top trace) induced the appearance of a slow afterdepolarization (sADP) following a stimulus-induced train of action potentials (Fig. 1B, bottom traces). Under resting conditions a depolarizing current stimulus (20–100 pA, 500 ms) elicited several nonaccommodating spikes (Fig. 1B, trace 1, 10.2 ± 0.5 Hz, n = 46) that were followed by a small afterhyperpolarization at the end of the pulse (AHP, −2.2 ± 0.2 mV, P < 0.0001). Following this AHP, the membrane potential was slightly depolarized from baseline (0.2 ± 0.1 mV, P < 0.03). Application of NA resulted in an increase in the number of action potentials elicited by the stimulus (17.7 ± 1.1 Hz, n = 27), consistent with a depolarization of the cell. As in the control condition, a small AHP followed these action potentials (−1.3 ± 0.4 mV, n = 25); however, over the next several seconds, a large, long-lasting (>15 s) sADP developed (8.0 ± 0.8 mV, n = 27). In most cells the sADP further depolarized GCs to threshold, resulting in the firing of action potentials, which greatly exacerbated the underlying depolarization induced by NA (Fig. 1B; 13.7 ± 1.3 mV, n = 29). The sADP peaked within a few seconds (2.8 ± 0.3 s, n = 19; see Fig. 5B) and its duration was variable as the result of differences in membrane potential from which it was elicited (Fig. 5B, τ = 17.6 ± 3.7 s, n = 14; see methods) during the depolarization produced by NA; however, the sADP could still be elicited independent of this depolarization (see following text).
Effect of NA is mediated by activation of α1-adrenergic receptors
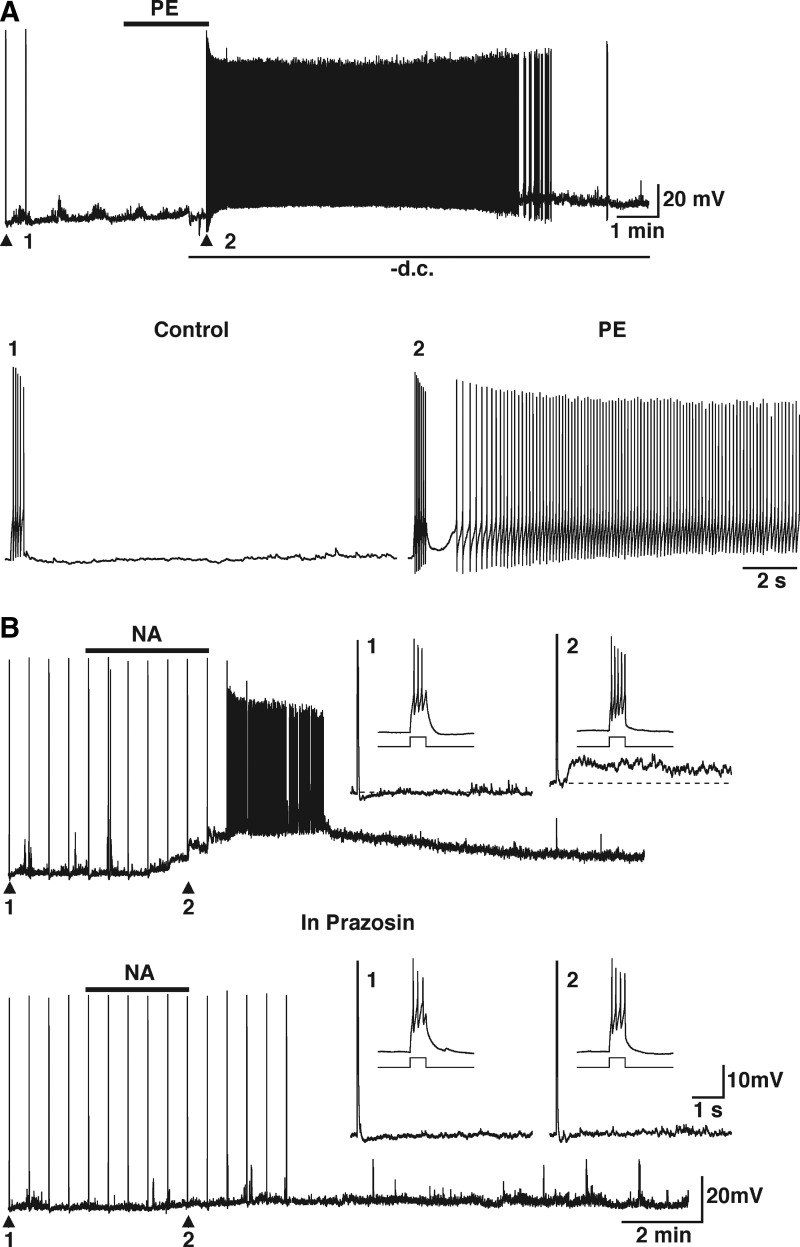
We have previously shown that the inhibitory effect of NA on MTCs is due to the activation of α1-adrenergic receptors, which increases the GABA inhibitory input onto MTCs (Araneda and Firestein 2006). In agreement with our previous findings, application of the α1-adrenergic receptor agonist phenylephrine (PE, 30 μM) mimicked the effect of NA on GCs. PE induced a long-lasting depolarization with a time course similar to that of the depolarization of GCs induced by NA (Fig. 2A, top trace, 15.4 ± 1.8, n = 21). This depolarization did not desensitize when the two PE (30 μM) applications were ≥25 min apart (first application, 15.6 ± 4.1 mV; second application, 14.7 ± 3.7 mV, n = 3; data not shown). In addition, PE also induced the sADP, following a stimulus-induced train of action potentials that had characteristics similar to those of the sADP induced by NA (Fig. 2A, bottom traces; 8.6 ± 1.1 mV, n = 18; time to peak, 3.1 ± 0.7 s, n = 11; τ = 23.3 ± 8.1 s; n = 4; see also Fig. 3B). As shown in Fig. 2A, the sADP could still be induced by the depolarizing stimulus when the membrane depolarization produced by PE was counteracted by manually injecting negative current into the cell to keep the membrane potential as close as possible to the resting value (i.e., before PE). Under these conditions the stimulus-elicited action potentials were still followed by the sADP, indicating that the slow depolarization of the cells is not a requirement for appearance of the sADP. To further characterize the pharmacology of this response we used the selective α1-adrenergic receptor antagonist prazosin. In the presence of prazosin (300 nM) both the depolarization (11.7 ± 1.9 mV) and the stimulus-induced sADP (7.0 ± 0.8 mV) produced by NA (10 μM, n = 3) were completely blocked (Fig. 2B, bottom traces; depolarization, 0.0 ± 1.0 mV; sADP, 0.0 ± 0.0 mV). Taken together, these results indicate that both the depolarization and sADP produced by NA in GCs are mediated by activation of α1-adrenergic receptors.
FIG. 2.
The depolarization and sADP elicited by NA is due to activation of α1-adrenergic receptors. A: the selective α1-adrenergic receptor agonist phenylephrine (PE, 30 μM, 2 min, top bar) depolarized this GC and induced the appearance of an sADP following a stimulus-induced train of action potentials. During the application of PE the membrane potential was manually maintained at the resting value by passing negative current, thus counteracting the PE-induced depolarization. Nevertheless, a depolarizing stimulus (20 pA, 500 ms) still induced the sADP, which resulted in firing of the cell (bottom traces in expanded timescale; 1 and 2, control and in PE, respectively; membrane potential is −54 mV). B: the depolarization and ADP induced by NA are completely abolished by the selective α1-adrenergic receptor antagonist prazosin. NA (10 μM, 150 s, top bar) depolarized this GC and induced the appearance of the sADP after a stimulus-induced train of action potentials (30 pA, 250 ms; insets 1 and 2: dotted line indicates the membrane potential and −63 and −55 mV, respectively). Bottom traces: in the same cell and in the presence of prazosin (300 nM) both the ADP (insets 1 and 2) and the depolarization induced by NA (10 μM, 150 s, top bar) are completely abolished.
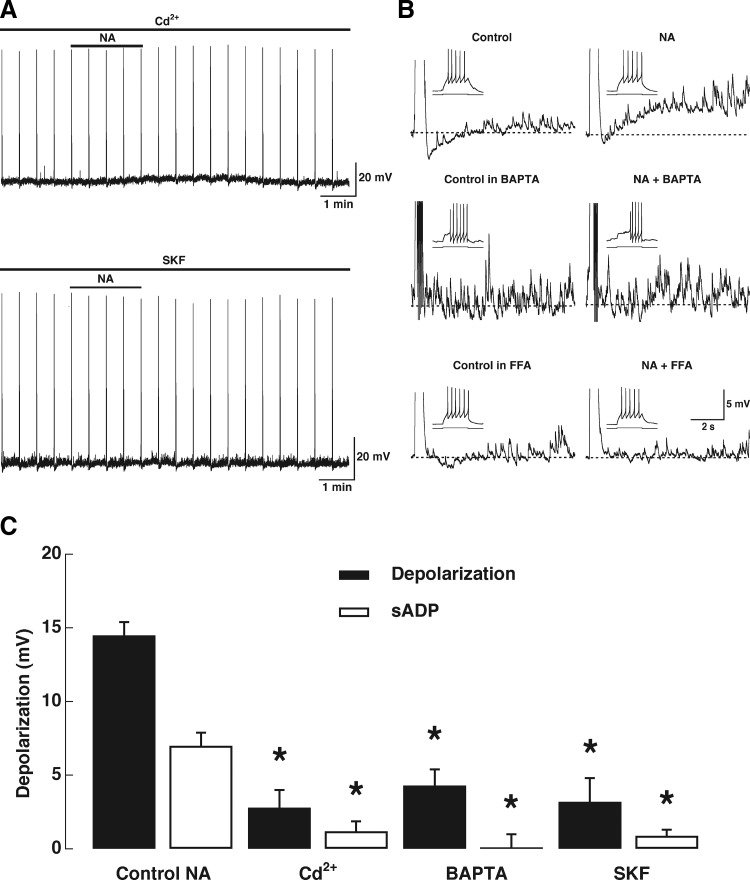
FIG. 3.
Pharmacology and Ca2+ dependence of the NA-induced afterdepolarization. A: the depolarization and ADP induced by NA (10 μM, top bar; stimulus 47 pA, 500 ms) are completely abolished in the presence of the voltage-gated Ca2+ channel blocker cadmium (Cd2+, 200 μM, top left trace). In another cell the receptor-operated Ca2+ channel blocker SKF96365 (SKF, 30 μM, bottom left trace) also completely blocked the depolarization and ADP induced by NA (10 μM, top bar; stimulus 45 pA, 500 ms). B, top right traces: control and NA-induced sADP. Middle traces: in another GC recorded with the Ca2+ chelator BAPTA, the sADP induced by NA is completely abolished. Bottom traces: the response to NA is also completely abolished in the presence of the nonselective cation channel blocker flufenamic acid (FFA, 30 μM). C: graph bar summarizing the effects of different blockers on the depolarization and sADP elicited by NA (10 μM); both the depolarization and sADP are reduced by these blockers; the asterisks indicate a significance of ≥P < 0.01 (see text).
Properties of the afterdepolarization in granule cells
The sADP induced by α1-adrenergic receptor activation in GCs has the characteristics of the ADP-induced NA and other neurotransmitters, in various brain regions, including the olfactory bulb (Araneda and Andrade 1991; Constanti et al. 1993; Egorov et al. 2006; Haj-Dahmane and Andrade 1999; Hall and Delaney 2002; Pressler et al. 2007). This ADP is thought to result from the activation of a Ca2+-dependent nonselective cationic current (ICAN). To address this possibility, we first recorded the responses to NA (10 μM) in the presence of the voltage-gated Ca2+ channel blocker Cd2+. Application of Cd2+ (200 μM) almost completely abolished both the depolarization induced by NA (Fig. 3A, control NA, 14.5 ± 0.9 mV; NA in Cd2+, 2.8 ± 1.2 mV, P < 0.01, n = 6) and the sADP (control NA, 5.8 ± 1.0 mV; 1.2 ± 0.7 mV, P < 0.005). Similar results were obtained when we tested the response to NA in the presence of both Cd2+ and Ni2+ (data not shown). To further characterize the Ca2+ dependence of the sADP, a group of GCs were recorded with an intracellular solution containing the Ca2+ chelator BAPTA. In all these cells the NA (10 μM) induced sADP was completely abolished (Fig. 3B, middle traces, 0.1 ± 0.9 mV, n = 5), whereas the depolarization was reduced by about 75% (4.3 ± 1.1 mV). These results indicate that the response to NA depends on Ca2+ entry.
In addition, the sADP was markedly abolished by the putative blocker of the transient-receptor potential canonical (TRPC) channel, SKF96365 (SKF, 30 μM; Fig. 3A, bottom trace) (Kim et al. 2003). As with the experiments using Ca2+ channel blockers the sADP induced by NA (10 μM) was abolished by SKF (Fig. 3C, 0.9 ± 0.4 mV, n = 5), as well as the depolarization (3.2 ± 1.6 mV). We also tested the ability of FFA, a partially selective inhibitor of ICAN (Partridge and Valenzuela 2000), to block the sADP induced by NA (10 μM). In general, the effect of FFA was difficult to reverse and its application resulted in recordings that were unstable. Nevertheless, when we tested different concentrations of FFA in different GCs (n = 6) we found that the size of the sADP was greatly reduced. Thus in two cells each, tested with FFA at 200, 100, and 30 μM, the average size of the ADP after NA was 0.9, 0.5, and 1.8 mV, respectively (see Fig. 3B, bottom traces). To further explore the possibility that ICAN underlies the sADP, we tested the effect of PE under conditions where the external concentration of Na ions was reduced, thus decreasing the driving force for Na. Under control conditions PE (30 μM), in the presence of TTX (0.5 μM), produced a robust stimulus-elicited sADP; however, reducing the Na concentration to 10 mM substantially reduced the sADP (Fig. 4A, top and bottom traces, n = 4). Lowering the Na in the external solution decreased the depolarization induced by PE (Fig. 4A, bottom graph; control PE, 18.3 ± 0.7 mV; PE in low Na, 7.3 ± 0.5 mV, P < 0.002, n = 4) and the sADP (control PE, 4.0 ± 0.4 mV; PE in low Na, 0.8 ± 0.1 mV, P < 0.002). It should be noted that the AHP was not affected by the Na substitution, indicating that the ionic substitution did not affect the equilibrium potential in the time course of these experiments (control, −2.0 ± 0.0 mV; low Na, −2.0 ± 0.25 mV, n = 4). Similarly, we estimated the reversal potential of the current underlying the sADP by conducting a “hybrid” current/voltage-clamp protocol (see methods and Fig. 4B). The inward current induced by PE (30 μM) showed a marked decrease toward more negative potentials (Fig. 4B, −3.2 ± 2.2 pA at −100 mV; −15.2 ± 2.9 pA at −30 mV, P < 0.01, n = 4), but failed to reverse at the value of EK (−95 mV, under our recordings conditions), suggesting that the PE-induced depolarization is not due to the closure of a K conductance. At more positive potentials, the estimated value for the reversal potential of the current is −20.6 ± 2.2 mV. However, it should be noted that the Ca2+ dependence of this current precludes an accurate measurement of the reversal potential because in this range (i.e., >40 mV) Ca2+ currents are also active. Nevertheless, this value of reversal potential for the current underlying the ADP is in agreement with that obtained for the muscarinic-induced ADP in granule cells in the MOB using a more direct method: flash photolysis of Ca2+ (Pressler et al. 2007). Together these results strongly suggest that both the depolarization and the sADP occur via activation of ICAN, although the contribution of other currents is also possible.
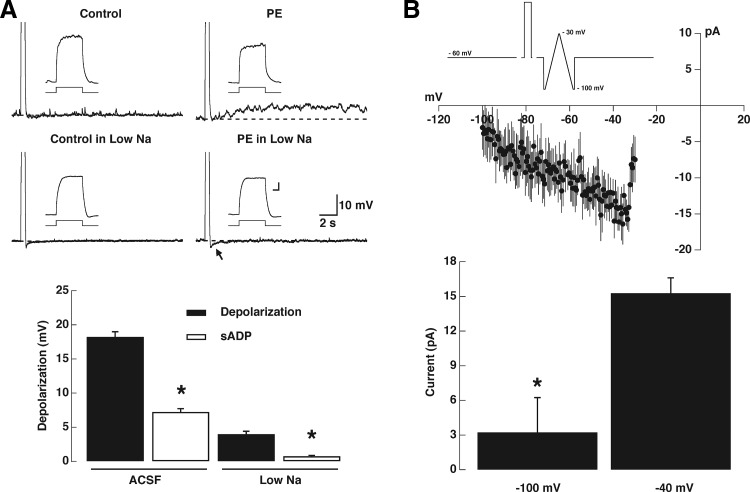
FIG. 4.
The sADP is voltage sensitive and Na dependent. A: the sADP induced by PE (30 μM, top left traces; stimulus 50 pA, 500 ms; 6 mV in this cell) is abolished when the extracellular Na concentration is reduced to 10 mM (bottom left traces; stimulus 50 pA, 500 ms). The calibration bar for the inset is 100 ms and 10 mV and the dotted line indicates the membrane potential before the depolarizing stimulus, control, and during PE (Control: −62 and −51 mV; low Na, −60 and −54 mV, respectively). The arrow indicates the AHP following the stimulus, which is not reduced in the low-Na solution. Left: graph bar summarizing the effects of reducing the extracellular Na concentration on the depolarization and sADP elicited by PE (30 μM); both the depolarization and sADP are reduced by reducing the Na; the asterisk indicates a significance of ≥P < 0.02 (see text). B, top: average current from subtracted current–voltage relationships obtained from a ramp under control conditions and in the presence of PE (30 μM; see methods). The PE-induced current was significantly larger at −40 than that at −100 mV (P < 0.01).
Activation of metabotropic glutamate receptors also activates sADP
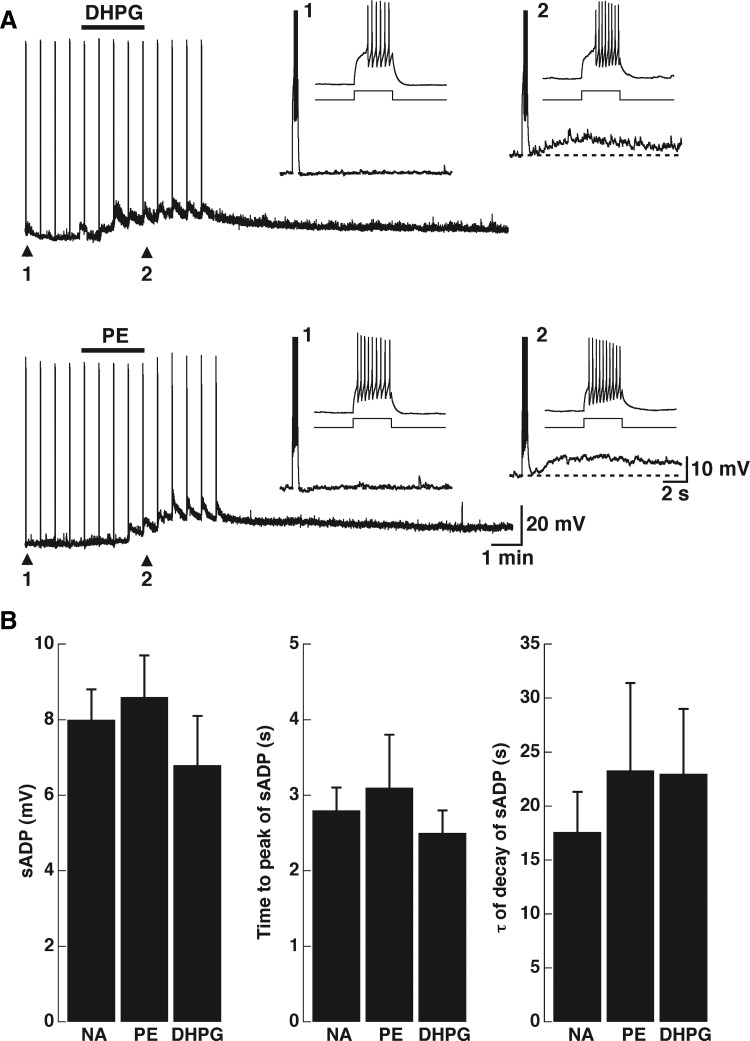
The olfactory bulb contains abundant expression of mGluRs; both group I and group II mGluRs are highly expressed in the olfactory bulb (for a review see Ennis et al. 2007). Recent studies have indicated that activation of group I mGluRs excites GCs in both the MOB and AOB (Castro et al. 2007; Heinbockel et al. 2007a,b). Furthermore, in the AOB, activation of mGluR1s has been shown to have a physiological role at dendrodendritic synapses between MTCs and GCs, promoting the release of GABA at these synapses (Castro et al. 2007). Thus we reasoned that activation of mGluR1 could also induce the appearance of sADP. As shown in Fig. 5A, and in agreement with previous work (Castro et al. 2007), the group I agonist DHPG (30 μM) produced a depolarization in GCs (14.3 ± 1.4 mV, n = 12). Importantly, application of DHPG also resulted in the appearance of a stimulus-induced sADP (6.8 ± 1.3 mV, n = 11). In the same cells, the depolarization produced by PE had a time course similar to that of DHPG (30 μM; Fig. 6A, bottom trace). Moreover, the size and kinetics parameters of the sADP induced by NA, PE, and DHPG were not different (Fig. 5B, time to peak, 2.5 ± 0.3 s; τ = 23 ± 6 s; n = 10). These results suggest that activation of mGluR1 and α1-adrenergic receptors leads to the activation of ICAN. The depolarization produced by DHPG (30 μM) was abolished by LY (100 μM), which at this concentration selectively blocks mGluR1s (see Fig. 6B; control DHPG, 14.7 ± 2.6 mV; DHPG in LY, 2.0 ± 0.6 mV, P < 0.03, n = 3). Likewise, the sADP induced by DHPG was also reduced in the presence of LY (DHPG, 5.8 ± 1.3 mV; DHPG in LY, 0.3 ± 0.3 mV, P < 0.03). Last, as with the effect of NA, both the depolarization and the sADP induced by DHPG were not reduced in the presence of APV and NBQX (data not shown).
FIG. 5.
Metabotropic glutamate receptor (mGluR) activation depolarizes and elicits the appearance of the sADP in GCs. A, top traces: the group I mGluR agonist (RS)-3,5-dihydroxyphenylglycine (DHPG, 10 μM, top bar) depolarized and induced the appearance of the slow ADP (inset: 1, control; 2 in DHPG). In the same cell the depolarization and stimulus-induced sADP elicited by PE (30 μM, top bar) has a time course similar to that elicited by DHPG (inset: 1, control; 2 in PE). The dotted lines indicate the membrane potential before the stimulus (top traces: −66 and −60 mV, 1 and 2, respectively; bottom traces: −62 and −57 mV, 1 and 2, respectively). B: graph bars showing the size (left), the time to peak (middle), and the duration (right) of the sADP elicited by the different agonists. These parameters were not significantly different for the sADP elicited by NA (10 μM), PE (30 μM), and DHPG (30 μM).
FIG. 6.
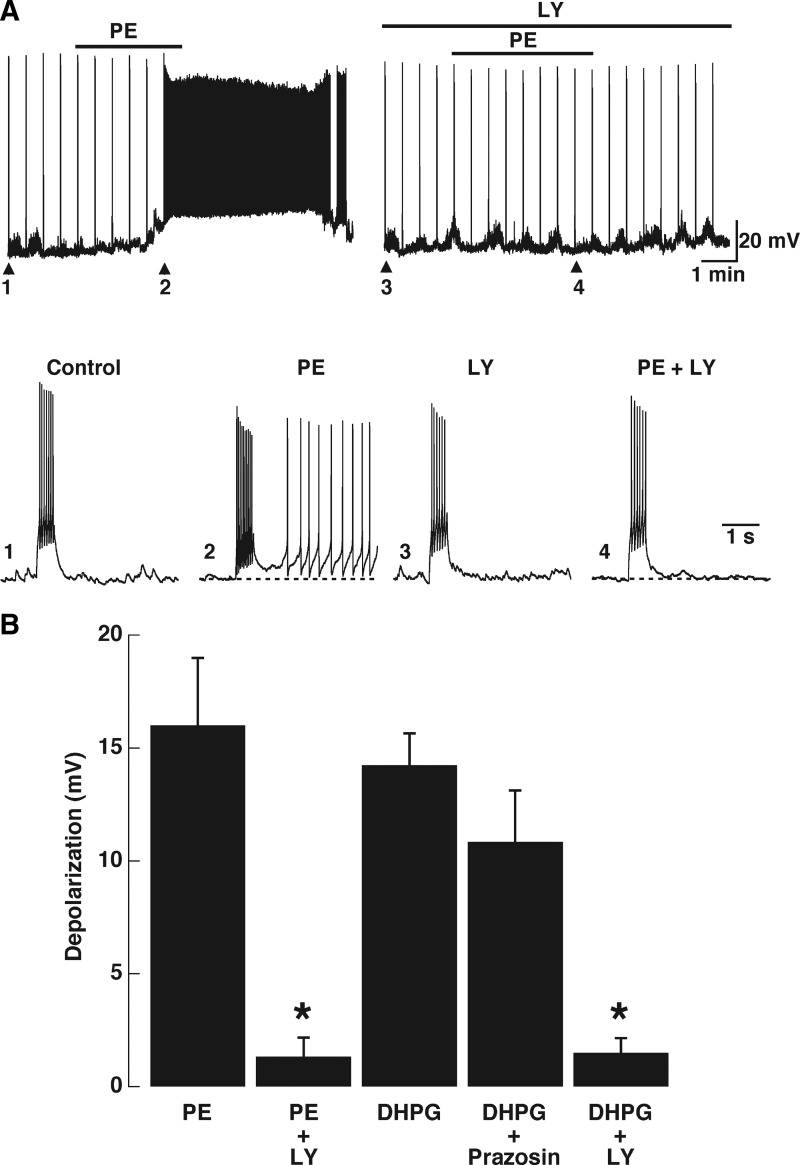
A group I mGluR antagonist abolished the excitatory response of PE in GCs. A: under control conditions PE (30 μM, top bar) depolarized and induced the sADP. In the presence of LY367385 (LY, 100 μM, upper top bar) the effect of PE was completely abolished. Bottom traces: expanded stimulus-induced responses, indicated by arrow and numbers in the top traces. In this cell the PE-induced sADP reached threshold and the cell fired (2); in the presence of LY, PE failed to induce the appearance of the sADP (4). The responses to the depolarizing stimulus in control and LY were not different (compare 1 and 3; in both A and B the stimulus is 50 pA, 500 ms). B: summary of the effect of blockers, LY effectively reduced the depolarizing response of PE (*P < 0.001). Conversely the depolarization induced by DHPG was not antagonized by the selective α1-adrenergic receptor antagonist prazosin (300 nM).
Effect of PE is reduced in the presence of an mGluR1 antagonist
Surprisingly, we found that LY, at concentrations that selectively block mGluR1s (Castro et al. 2007), abolished the excitatory responses to PE in GCs. Under control conditions PE (30 μM; Fig. 6A, top bar) depolarized and induced the sADP. In the presence of LY (100 μM) the depolarization induced by PE was completely abolished (Fig. 6B; control, 16.00 ± 2.99 mV; PE in LY, 1.33 ± 0.84 mV, P < 0.001, n = 7). The responses to a depolarizing stimulus in control and LY were not different; however, in the presence of LY the PE-induced sADP was also abolished (Fig. 6A; PE sADP, 7.33 ± 1.58 mV; PE in LY sADP, 0.33 ± 0.21 mV, P < 0.005). However, the depolarization and sADP produced by DHPG (30 μM) were not antagonized by the selective α1-adrenergic receptor antagonist prazosin (300 nM). We also tested a lower concentration of LY (30 μM; data not shown); at this lower concentration of blocker the depolarization induced by PE (30 μM) was reduced by 31% (control, 13.9 ± 3.1 mV; in LY, 9.6 ± 2.9 mV; P < 0.02, n = 4), whereas the depolarization induced by DHGP (30 μM) was reduced by 49% (control, 15.8 ± 2.4 mV; in LY, 8.1 ± 1.1 mV; P < 0.02; n = 5). These results suggest that the depolarizing effect and the sADP produced by activation of α1-adrenergic receptors are mediated largely, if not completely, by mGluR1 activity.
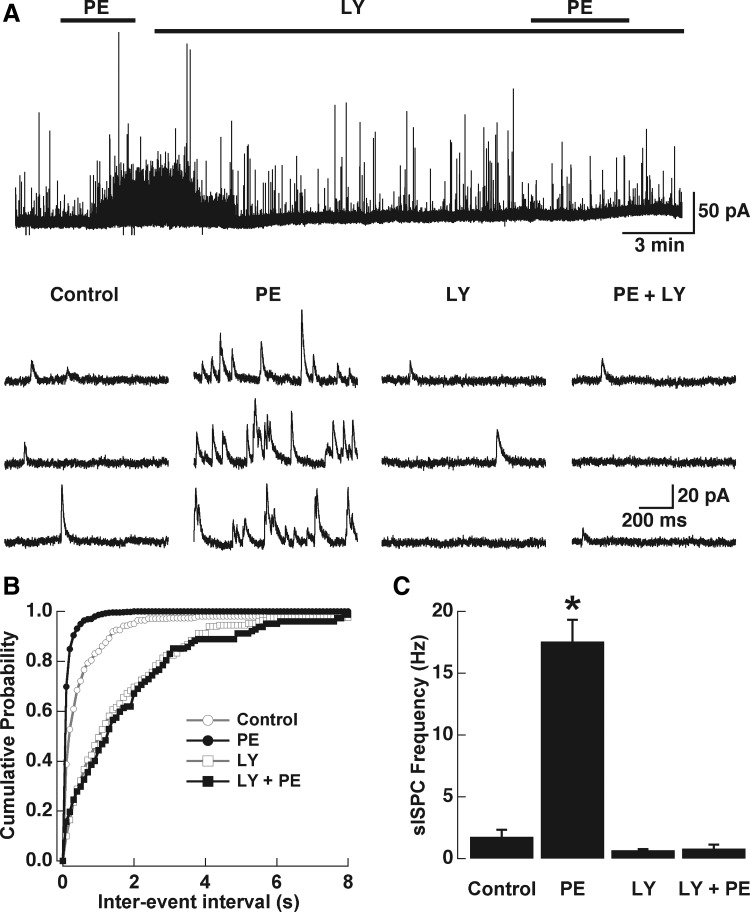
We have previously shown that NA, acting on α1-adrenergic receptors, greatly enhances both the frequency of miniature (m)IPSCs in MTCs and the evoked release of GABA from GCs (Araneda and Firestein 2006). Thus to further characterize the potential interaction of the excitatory effect produced by α1-adrenergic receptor activation and activation of mGluR1s, we recorded GABA IPSCs in MTCs in the presence of TTX (0.5–1 μM). In agreement with our results in GCs and our previous work, PE (30 μM) substantially increased the frequency of GABA IPSCs (Fig. 7A; control, 1.76 ± 0.55 Hz; PE, 17.58 ± 1.75 Hz, P < 0.004, n = 4), whereas the mean amplitude of the GABA IPSCs was not changed by PE (control, 24.12 ± 5.08 pA; PE, 19.66 ± 1.42 pA, P > 0.5, n = 4; data not shown). Application of the mGluR1 antagonist LY (100 μM) quickly reversed the increase in mean frequency of GABA IPSCs produced by PE, which we have previously shown lasts for several minutes (>10 min). Importantly, a second application of PE, in the presence of LY, completely failed to increase the mean frequency of GABA IPSCs, indicating that LY effectively blocked the effect of PE (control in LY, 0.66 ± 0.1 Hz; PE in LY, 0.8 ± 0.32 Hz, n = 4). It was previously shown that LY alone could reduce the basal mean frequency of GABA IPSCs in MTCs, suggesting that under physiological conditions mGluRs participate in the release of GABA from GCs (Castro et al. 2007). Under our recording conditions, LY produced a reduction in the mean frequency of IPSCs, although it did not reach significance (1.76 ± 0.55 vs. 0.66 ± 0.1 Hz, P > 0.07, n = 4). To examine the possibility that the blocking effect LY has on the PE-induced increase in the frequency of IPSCs could be limited to this particular antagonist, we also tested the effect of the selective group I antagonist AIDA. In the presence of AIDA (100 μM) the increase in IPSCs produced by PE (30 μM, n = 4) was also abolished, suggesting that this is not an effect restricted to LY (control, 0.92 ± 0.25 Hz; PE, 7.95 ± 1.83 Hz, P < 0.03; control AIDA, 1.90 ± 0.61 Hz; PE in AIDA, 3.17 ± 1.32 Hz, P > 0.1). Last, in agreement with the observed excitatory effect of DHGP on GCs, application of DHPG (30 μM) increased the mean frequency of GABA IPSCs in MTCs (control, 2.88 ± 1.25 Hz; DHPG, 8.15 ± 1.97 Hz, P < 0.03, n = 7). As with PE, the mean amplitude of the IPSCs was not affected by DHPG (control, 15.85 ± 1.97 pA; DHPG, 14.62 ± 1.46 pA, P > 0.5; data not shown), suggesting a presynaptic effect (i.e., on GCs). As with the excitatory response in GCs, DHPG still increased the mean frequency of IPSCs in the presence of the selective α1-adrenergic receptor antagonist prazosin (300 nM) (control in prazosin, 0.84 ± 0.27; DHPG and prazosin, 5.21 ± 1.77, P < 0.04, n = 7).
FIG. 7.
The increase in the frequency of γ-aminobutyric acid (GABA) inhibitory postsynaptic currents (IPSCs) induced by PE in mitral cells is reduced by a mGluR1 antagonist. A: PE (30 μM, top bar) greatly increased the frequency of GABA IPSCs in mitral and tufted cells (MTCs) recorded in the presence of tetrodotoxin (TTX, 1 μM). Application of the mGluR1 antagonist LY (100 μM, long top bar) quickly reversed the PE-induced increase in frequency of IPSCs and a 2nd application of PE in the presence of LY completely failed to produce and increase in IPSCs. B: selected traces for mIPSCs for the same cell shown in A. The frequency but not the amplitude was increased in the presence of PE (see text). B: average cumulative interinterval distributions for the IPSCs (n = 4); control, gray line, circles; in the presence of PE, dark line, filled circles; in LY, gray line, empty squares; PE in the presence of LY, dark line, filled squares. Left: bar graph showing the effects of PE and LY on the mean frequency of IPSCs. PE significantly increased the frequency of IPSCs by about 10-fold (*P < 0.004). LY produced a small reduction in the mean frequency of IPSCs albeit not significantly (N.S., control vs. LY, P > 0.07). In the presence of LY PE failed to increase the frequency of IPSCs (see text for details). The holding potential in these experiments was 0 mV.
DISCUSSION
The present study shows that NA, acting through α1-adrenergic receptors, excites GCs in the AOB. In addition, NA induced the appearance of the sADP following a stimulus-induced train of action potentials. The Ca2+ dependence, ionic sensitivity, and pharmacology suggest that the sADP is mediated by activation of ICAN. Activation of mGluR1s also depolarized GCs and induced the sADP, suggesting that the excitatory action resulting from the activation of these receptors could be mediated by a common transduction target. Surprisingly, an antagonist of mGluR1s almost completely abolished the excitation of GCs produced by α1-adrenergic receptor activation, whereas the mGluR1-mediated excitation was not sensitive to a selective α1-adrenergic receptor antagonist. These results indicate that the actions of NA in the AOB may be more complex than previously appreciated.
The olfactory bulb receives a prominent noradrenergic input from the locus coeruleus (Shipley et al. 1985) and noradrenergic modulation of olfactory bulb neurons is thought to play an important role in olfactory processing (Brennan 2004; Doucette et al. 2007; Gire and Schoppa 2008). We have recently shown that NA inhibits MTCs in the AOB by increasing the frequency of GABA IPSCs, suggesting that noradrenergic system activity promotes GABA release from GCs (Araneda and Firestein 2006). In agreement with those findings, we show here that activation of α1-adrenergic receptors significantly increased the excitability of GCs. NA depolarized GCs and induced the appearance of the sADP following a stimulus-induced train of action potentials (see following text); these excitatory effects contributed to produce a long-lasting depolarization of GCs (<15 min). The time course and pharmacology of this excitation closely resemble the time course and the pharmacology of the increase in frequency of GABA IPSCs produced by NA in MTCs (Araneda and Firestein 2006). Furthermore, our results indicate that this excitatory action is likely due to a direct effect of NA on GCs because blockers of fast synaptic transmission and TTX did not affect the excitatory response of NA in GCs. The distribution of noradrenergic fibers and α1-adrenergic receptor expression pattern in the AOB further support this conclusion. In the AOB the majority of the noradrenergic fibers are found in the internal plexiform layer, which contains the GC dendrites and the GC layer. Also, our results are in agreement with in situ hybridization experiments that show high expression levels of α1-adrenergic receptors, particularly the α1A subtype, in the AOB (Day et al. 1997; Domyancic and Morilak 1997; McCune et al. 1993; Pieribone et al. 1994). Virtually no noradrenergic innervation in the glomerular layer is observed (McLean et al. 1989). Moreover, our results are in agreement with the reported effects of NA at other synapses in the CNS where NA has been shown to exert presynaptic enhancement of inhibitory transmission (Braga et al. 2004).
The olfactory bulb contains abundant expression of mGluRs—both group I and group II mGluRs are highly expressed in the olfactory bulb (Ennis et al. 2007). In the MOB, group I mGluR can enhance synaptic inhibition of MTCs in the MOB (Heinbockel et al. 2007a,b). In the AOB activation of the group II receptor, mGluR2, is thought to play an important role in the formation of memory during mating in mice (Hayashi et al. 1993; Kaba et al. 1994). Furthermore, it has been shown that under physiological conditions mGluR1s participate in the release of GABA at dendrodendritic synapses in the AOB (Castro et al. 2007). Most of the mGluR1 protein, assessed by immunoreactivity, is found in the internal plexiform layer of the AOB where MTCs are found, suggesting that the increase in GABA release is mediated by mGluR1s at dendrodendritic synapses (Sahara et al. 2001). We found that activation of mGluR1s produced a response strikingly similar to that observed with α1-adrenergic receptor activation. The group I agonist DHPG depolarizes GCs and induces sADP and these effects were blocked by LY at a concentration that selectively blocks mGluR1s. The Ca2+ dependence, voltage sensitivity, sensitivity to blockers, and external Na of the sADP induced by activation of mGluR1s and α1-adrenergic receptors suggest that this depolarization is mediated by activation of ICAN. Activation of these receptors in other brain regions, including the olfactory cortex, has also been shown to induce the appearance of an ADP (Araneda and Andrade 1991; Greene et al. 1994; Kim et al. 2003; Libri et al. 1997). Interestingly, this current has recently been described in a subset of inhibitory neurons in the main olfactory bulb—the Blanes cells—where it is thought to play a role in the initiation of persistent firing, although in these cells ICAN can be elicited in the absence of neuromodulators (Pressler and Strowbridge 2006). On the other hand, activation of type M1 muscarinic receptors also induces the appearance of an ADP following stimulus-elicited action potentials in GCs of the MOB as well as in other brain regions. This ADP is also thought to result from the activation of ICAN (Constanti et al. 1993; Haj-Dahmane and Andrade 1999; Pressler et al. 2007). Taken together these results suggest that in GCs second-messenger pathways activated by different receptors can converge onto a common final target—the activation of ICAN—that in turn depolarizes GCs.
Surprisingly, in addition to the blocking effect on mGluR1-mediated excitation of GCs, LY dramatically reduced the excitatory response to PE. In agreement with this blocking action, we found that the increase in mean frequency of GABA IPSCs on MTCs produced by PE was also blocked by LY. It is worth noting that the response to PE was shortened when LY was included in the perfusion shortly after the onset of the PE response. Although we cannot completely rule out a nonspecific effect of LY, the blockade by this group I mGluR antagonist of the α1-adrenergic response is not exclusive because AIDA, a general group I mGluR antagonist, also blocked the response to PE, both in GCs and in MTCs. In addition, LY (100 μM) does not block the excitatory α1-adrenergic response in neurons of the dorsal raphe nucleus (S. Haj-Dahmane, personal communication). Taken together, these unexpected results suggest that activation of α1-adrenergic receptors occurs upstream from the activation of mGluR1s and perhaps acts to potentiate a tonic glutamatergic metabotropic activity on GCs, which in turn induces the release of GABA at dendrodendritic synapses. This proposed scheme is in agreement with our findings that the responses to DHPG, both in GCs and MTCs, were insensitive to the α1-adrenergic receptor antagonist prazosin. Moreover, this possibility is further supported by a recent report that showed endogenous release of glutamate does in fact activate mGluR1s and can participate in recurrent inhibition in the AOB under physiological conditions (Castro et al. 2007). The same study showed that LY significantly reduced the mean basal frequency of GABA IPSCs recorded in MTCs, suggesting that there is a tonic activity of these receptors. We also observed a reduction in the basal mean frequency of GABA IPSCs in MTCs in the presence of LY, although this value did not reach significance within the sample of GCs tested in these experiments.
What is the possible source of this potentiation? Both α1-adrenergic receptors and mGluR1s, coupling through Gq/11, activate phospholipase C and induce changes in intracellular Ca2+ (Conn and Pin 1997; Zhong and Minneman 1999). Additionally, activation of α1-adrenergic receptors by NA has been shown to increase intracellular Ca2+ in cultured olfactory bulb interneurons (Tani et al. 1992). One possibility is that activation of α1-adrenergic receptors induces a Ca2+ increase in GCs, which in turn potentiates a tonic mGluR1 response. It has been shown that synaptic activation of GCs can induce a long-lasting depolarization (LLD), which is paralleled by a long-lasting increase in intracellular Ca2+ that could induce GABA release at dendrodendritic synapses. Interestingly, this Ca2+ signal is also dependent on ICAN, raising the possibility that mGluR1 activation could be responsible, in part, for the activation of these LLDs (Egger 2008). In those experiments the induction of the LLD is also dependent on NMDA receptor activation, although the effects of α1-adrenergic receptors and mGluR1s (depolarization and sADP), reported here, were not reduced by blockers of ionotropic glutamate receptors (APV and NBQX), indicating that they may activate a different mechanism involved in GABA release. It is also possible that a second-messenger pathway activated by α1-adrenergic receptors could exert a modulatory action of mGluR1, which results in enhancement of their activity. Finally, we cannot exclude the possibility that α1-adrenergic receptor activation could decrease the uptake of glutamate released by MTCs. However, in this case the increase in glutamate concentration at dendrodendritic synapses would have to selectively increase mGluR1 activity but not the activity of ionotropic glutamate receptors (i.e., NMDA and/or AMPA) because both the increase in GABA IPSCs frequency in MTCs and the excitatory action in GCs produced by NA are not affected by the presence of blockers of synaptic transmission (i.e., APV and NBQX). Furthermore, we find that activation of mGluR1s and α1-adrenergic receptors increases the mean frequency of GABA IPSCs, without any significant change in their amplitude. Also, the insensitivity to blockers of synaptic transmission by the excitatory responses produced by activation of these receptors in GCs strongly suggests that these receptors are located in the GCs. However, it remains to be elucidated whether these receptors are located in the same or different parts of these neurons. Further experiments are needed to distinguish between these possibilities.
The relationship between the basal activity of mGluR1s and the enhancement of this inhibitory action by the noradrenergic system could have an important physiological role in dendrodendritic function. Eliciting the sADP greatly enhanced the excitability of GCs and, in most cases, the sADP was large enough to reach threshold and induce a long-lasting firing of GCs. In general, GCs exhibit a hyperpolarized membrane potential; thus we predict that the level of excitatory input to GCs will be an important determinant of the extent that noradrenergic activation could enhance the release of GABA at dendrodendritic synapses. These excitatory inputs onto GCs are mainly through dendrodendritic synapses and from afferent fibers from the olfactory cortex. Basal dendrites and the soma of GCs receive synapses from centrifugal fibers, as well as axon collaterals from MCs. It has been suggested that this segregated pattern of connectivity is likely to have an important physiological role in GC functioning (Balu et al. 2007; Whitman and Greer 2007). Excitation driven by either of these inputs (or both) could be enhanced by tonic activity of the noradrenergic system. It has been suggested that persistent spiking in the olfactory bulb GCs can be induced by a pattern of synaptic input similar to that observed during sniffing and this pattern could lead to correlated spiking in MTCs (Inoue and Strowbridge 2008). In these studies the maintenance of this persistent spiking depends on muscarinic receptor activation and we propose a similar role for the persistent spiking induced by the noradrenergic system and mGluR1s in the AOB. The persistent spiking induced by these systems could act in concert throughout the learning of chemosignals from the male during mating in mice. Thus it appears that a more complex mechanism than a simple excitation of MTCs by the noradrenergic system during mating is needed to explain its role in the Bruce effect (Brennan 2004). Finally, our results also indicate that studies addressing the physiological role of the noradrenergic system in the olfactory bulb and other brain regions should consider the potential contribution of mGluR activation.
GRANTS
This work was partially supported by a summer research fellowship from the Marine Biological Laboratory to R. C. Araneda. R. S. Smith is a Howard Hughes Medical Institute Undergraduate Research Fellow.
Acknowledgments
We thank members of the Araneda lab for helpful comments on the manuscript.
REFERENCES
- Araneda and Andrade 1991.Araneda R, Andrade R. 5-Hydroxytryptamine 2 and 5-hydroxytryptamine 1A receptors mediate opposing responses on membrane excitability in rat association cortex. Neuroscience 40: 399–412, 1991. [DOI] [PubMed] [Google Scholar]
- Araneda and Firestein 2006.Araneda RC, Firestein S. Adrenergic enhancement of inhibitory transmission in the accessory olfactory bulb. J Neurosci 26: 3292–3298, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balu et al. 2007.Balu R, Pressler RT, Strowbridge BW. Multiple modes of synaptic excitation of olfactory bulb granule cells. J Neurosci 27: 5621–5632, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braga et al. 2004.Braga MF, Aroniadou-Anderjaska V, Manion ST, Hough CJ, Li H. Stress impairs alpha(1A) adrenoceptor-mediated noradrenergic facilitation of GABAergic transmission in the basolateral amygdala. Neuropsychopharmacology 29: 45–58, 2004. [DOI] [PubMed] [Google Scholar]
- Brennan 2004.Brennan PA The nose knows who's who: chemosensory individuality and mate recognition in mice. Horm Behav 46: 231–240, 2004. [DOI] [PubMed] [Google Scholar]
- Brennan et al. 1995.Brennan PA, Kendrick KM, Keverne EB. Neurotransmitter release in the accessory olfactory bulb during and after the formation of an olfactory memory in mice. Neuroscience 69: 1075–1086, 1995. [DOI] [PubMed] [Google Scholar]
- Bruce 1959.Bruce HM An exteroceptive block to pregnancy in the mouse (Letter). Nature 184: 105, 1959. [DOI] [PubMed] [Google Scholar]
- Castro et al. 2007.Castro JB, Hovis KR, Urban NN. Recurrent dendrodendritic inhibition of accessory olfactory bulb mitral cells requires activation of group I metabotropic glutamate receptors. J Neurosci 27: 5664–5671, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciombor et al. 1999.Ciombor KJ, Ennis M, Shipley MT. Norepinephrine increases rat mitral cell excitatory responses to weak olfactory nerve input via alpha-1 receptors in vitro. Neuroscience 90: 595–606, 1999. [DOI] [PubMed] [Google Scholar]
- Conn and Pin 1997.Conn PJ, Pin JP. Pharmacology and functions of metabotropic glutamate receptors. Annu Rev Pharmacol Toxicol 37: 205–237, 1997. [DOI] [PubMed] [Google Scholar]
- Constanti et al. 1993.Constanti A, Bagetta G, Libri V. Persistent muscarinic excitation in guinea-pig olfactory cortex neurons: involvement of a slow post-stimulus afterdepolarizing current. Neuroscience 56: 887–904, 1993. [DOI] [PubMed] [Google Scholar]
- Day et al. 1997.Day HE, Campeau S, Watson SJ Jr, Akil H. Distribution of alpha 1a-, alpha 1b- and alpha 1d-adrenergic receptor mRNA in the rat brain and spinal cord. J Chem Neuroanat 13: 115–139, 1997. [DOI] [PubMed] [Google Scholar]
- Domyancic and Morilak 1997.Domyancic AV, Morilak DA. Distribution of alpha1A adrenergic receptor mRNA in the rat brain visualized by in situ hybridization. J Comp Neurol 386: 358–378, 1997. [DOI] [PubMed] [Google Scholar]
- Doucette et al. 2007.Doucette W, Milder J, Restrepo D. Adrenergic modulation of olfactory bulb circuitry affects odor discrimination. Learn Mem 14: 539–547, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egger 2008.Egger V Synaptic sodium spikes trigger long-lasting depolarizations and slow calcium entry in rat olfactory bulb granule cells. Eur J Neurosci 27: 2066–2075, 2008. [DOI] [PubMed] [Google Scholar]
- Egorov et al. 2006.Egorov AV, Unsicker K, von Bohlen, Halbach O. Muscarinic control of graded persistent activity in lateral amygdala neurons. Eur J Neurosci 24: 3183–3194, 2006. [DOI] [PubMed] [Google Scholar]
- Ennis et al. 2007.Ennis M, Hamilton KA, Hayar A. Neurochemistry of the Main Olfactory System. New York: Springer, 2007.
- Gire and Schoppa 2008.Gire DH, Schoppa NE. Long-term enhancement of synchronized oscillations by adrenergic receptor activation in the olfactory bulb. J Neurophysiol 99: 2021–2025, 2008. [DOI] [PubMed] [Google Scholar]
- Greene et al. 1994.Greene CC, Schwindt PC, Crill WE. Properties and ionic mechanisms of a metabotropic glutamate receptor-mediated slow afterdepolarization in neocortical neurons. J Neurophysiol 72: 693–704, 1994. [DOI] [PubMed] [Google Scholar]
- Haj-Dahmane and Andrade 1999.Haj-Dahmane S, Andrade R. Muscarinic receptors regulate two different calcium-dependent non-selective cation currents in rat prefrontal cortex. Eur J Neurosci 11: 1973–1980, 1999. [DOI] [PubMed] [Google Scholar]
- Hall and Delaney 2002.Hall BJ, Delaney KR. Contribution of a calcium-activated non-specific conductance to NMDA receptor-mediated synaptic potentials in granule cells of the frog olfactory bulb. J Physiol 543: 819–834, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayar et al. 2001.Hayar A, Heyward PM, Heinbockel T, Shipley MT, Ennis M. Direct excitation of mitral cells via activation of alpha1-noradrenergic receptors in rat olfactory bulb slices. J Neurophysiol 86: 2173–2182, 2001. [DOI] [PubMed] [Google Scholar]
- Hayashi et al. 1993.Hayashi Y, Momiyama A, Takahashi T, Ohishi H, Ogawa-Meguro R, Shigemoto R, Mizuno N, Nakanishi S. Role of a metabotropic glutamate receptor in synaptic modulation in the accessory olfactory bulb. Nature 366: 687–690, 1993. [DOI] [PubMed] [Google Scholar]
- Heinbockel et al. 2007a.Heinbockel T, Hamilton KA, Ennis M. Group I metabotropic glutamate receptors are differentially expressed by two populations of olfactory bulb granule cells. J Neurophysiol 97: 3136–3141, 2007a. [DOI] [PubMed] [Google Scholar]
- Heinbockel et al. 2007b.Heinbockel T, Laaris N, Ennis M. Metabotropic glutamate receptors in the main olfactory bulb drive granule cell-mediated inhibition. J Neurophysiol 97: 858–870, 2007b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue and Strowbridge 2008.Inoue T, Strowbridge BW. Transient activity induces a long-lasting increase in the excitability of olfactory bulb interneurons. J Neurophysiol 99: 187–199, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahr and Nicoll 1982.Jahr CE, Nicoll RA. Noradrenergic modulation of dendrodendritic inhibition in the olfactory bulb. Nature 297: 227–229, 1982. [DOI] [PubMed] [Google Scholar]
- Kaba et al. 1994.Kaba H, Hayashi Y, Higuchi T, Nakanishi S. Induction of an olfactory memory by the activation of a metabotropic glutamate receptor. Science 265: 262–264, 1994. [DOI] [PubMed] [Google Scholar]
- Kaba and Huang 2005.Kaba H, Huang GZ. Long-term potentiation in the accessory olfactory bulb: a mechanism for olfactory learning. Chem Senses 30, Suppl. 1: i150–i151, 2005. [DOI] [PubMed] [Google Scholar]
- Kaba and Keverne 1988.Kaba H, Keverne EB. The effect of microinfusions of drugs into the accessory olfactory bulb on the olfactory block to pregnancy. Neuroscience 25: 1007–1011, 1988. [DOI] [PubMed] [Google Scholar]
- Kaba and Nakanishi 1995.Kaba H, Nakanishi S. Synaptic mechanisms of olfactory recognition memory. Rev Neurosci 6: 125–141, 1995. [DOI] [PubMed] [Google Scholar]
- Kim et al. 2003.Kim SJ, Kim YS, Yuan JP, Petralia RS, Worley PF, Linden DJ. Activation of the TRPC1 cation channel by metabotropic glutamate receptor mGluR1. Nature 426: 285–291, 2003. [DOI] [PubMed] [Google Scholar]
- Libri et al. 1997.Libri V, Constanti A, Zibetti M, Postlethwaite M. Metabotropic glutamate receptor subtypes mediating slow inward tail current (IADP) induction and inhibition of synaptic transmission in olfactory cortical neurones. Br J Pharmacol 120: 1083–1095, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCune et al. 1993.McCune SK, Voigt MM, Hill JM. Expression of multiple alpha adrenergic receptor subtype messenger RNAs in the adult rat brain. Neuroscience 57: 143–151, 1993. [DOI] [PubMed] [Google Scholar]
- McLean et al. 1989.McLean JH, Shipley MT, Nickell WT, Aston-Jones G, Reyher CK. Chemoanatomical organization of the noradrenergic input from locus coeruleus to the olfactory bulb of the adult rat. J Comp Neurol 285: 339–349, 1989. [DOI] [PubMed] [Google Scholar]
- McLennan 1971.McLennan H The pharmacology of inhibition of mitral cells in the olfactory bulb. Brain Res 29: 177–184, 1971. [DOI] [PubMed] [Google Scholar]
- Meisami and Bhatnagar 1998.Meisami E, Bhatnagar KP. Structure and diversity in mammalian accessory olfactory bulb. Microsc Res Tech 43: 476–499, 1998. [DOI] [PubMed] [Google Scholar]
- Mouly et al. 1995.Mouly AM, Elaagouby A, Ravel N. A study of the effects of noradrenaline in the rat olfactory bulb using evoked field potential response. Brain Res 681: 47–57, 1995. [DOI] [PubMed] [Google Scholar]
- Okutani et al. 1998.Okutani F, Kaba H, Takahashi S, Seto K. The biphasic effects of locus coeruleus noradrenergic activation on dendrodendritic inhibition in the rat olfactory bulb. Brain Res 783: 272–279, 1998. [DOI] [PubMed] [Google Scholar]
- Partridge and Valenzuela 2000.Partridge LD, Valenzuela CF. Block of hippocampal CAN channels by flufenamate. Brain Res 867: 143–148, 2000. [DOI] [PubMed] [Google Scholar]
- Perez et al. 1987.Perez H, Hernandez A, Almli CR. Locus coeruleus stimulation modulates olfactory bulb evoked potentials. Brain Res Bull 18: 767–770, 1987. [DOI] [PubMed] [Google Scholar]
- Pieribone et al. 1994.Pieribone VA, Nicholas AP, Dagerlind A, Hokfelt T. Distribution of alpha 1 adrenoceptors in rat brain revealed by in situ hybridization experiments utilizing subtype-specific probes. J Neurosci 14: 4252–4268, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pressler et al. 2007.Pressler RT, Inoue T, Strowbridge BW. Muscarinic receptor activation modulates granule cell excitability and potentiates inhibition onto mitral cells in the rat olfactory bulb. J Neurosci 27: 10969–10981, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pressler and Strowbridge 2006.Pressler RT, Strowbridge BW. Blanes cells mediate persistent feedforward inhibition onto granule cells in the olfactory bulb. Neuron 49: 889–904, 2006. [DOI] [PubMed] [Google Scholar]
- Rosser and Keverne 1985.Rosser AE, Keverne EB. The importance of central noradrenergic neurones in the formation of an olfactory memory in the prevention of pregnancy block. Neuroscience 15: 1141–1147, 1985. [DOI] [PubMed] [Google Scholar]
- Sahara et al. 2001.Sahara Y, Kubota T, Ichikawa M. Cellular localization of metabotropic glutamate receptors mGluR1, 2/3, 5 and 7 in the main and accessory olfactory bulb of the rat. Neurosci Lett 312: 59–62, 2001. [DOI] [PubMed] [Google Scholar]
- Salmoiraghi et al. 1964.Salmoiraghi GC, Bloom FE, Costa E. Adrenergic mechanisms in rabbit olfactory bulb. Am J Physiol 207: 1417–1424, 1964. [DOI] [PubMed] [Google Scholar]
- Schoppa and Urban 2003.Schoppa NE, Urban NN. Dendritic processing within olfactory bulb circuits. Trends Neurosci 26: 501–506, 2003. [DOI] [PubMed] [Google Scholar]
- Shepherd and Greer 1998.Shepherd GM, Greer CA. Olfactory bulb. In: The Synaptic Organization of the Brain (4th ed.), edited by Shepherd GM. Oxford, UK: Oxford Univ. Press, 1998, p. 159–204.
- Shipley et al. 1985.Shipley MT, Halloran FJ, de la Torre J. Surprisingly rich projection from locus coeruleus to the olfactory bulb in the rat. Brain Res 329: 294–299, 1985. [DOI] [PubMed] [Google Scholar]
- Takami and Graziadei 1991.Takami S, Graziadei PP. Light microscopic Golgi study of mitral/tufted cells in the accessory olfactory bulb of the adult rat. J Comp Neurol 311: 65–83, 1991. [DOI] [PubMed] [Google Scholar]
- Tani et al. 1992.Tani A, Yoshihara Y, Mori K. Increase in cytoplasmic free Ca2+ elicited by noradrenalin and serotonin in cultured local interneurons of mouse olfactory bulb. Neuroscience 49: 193–199, 1992. [DOI] [PubMed] [Google Scholar]
- Trombley and Shepherd 1992.Trombley PQ, Shepherd GM. Noradrenergic inhibition of synaptic transmission between mitral and granule cells in mammalian olfactory bulb cultures. J Neurosci 12: 3985–3991, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitman and Greer 2007.Whitman MC, Greer CA. Adult-generated neurons exhibit diverse developmental fates. Dev Neurobiol 67: 1079–1093, 2007. [DOI] [PubMed] [Google Scholar]
- Zhong and Minneman 1999.Zhong H, Minneman KP. Alpha1-adrenoceptor subtypes. Eur J Pharmacol 375: 261–276, 1999. [DOI] [PubMed] [Google Scholar]