Abstract
Background
PI3K/AKTsignaling pathway plays an important role in cell growth, proliferation, and tumorgenesis of various malignancies. This signaling pathway has been shown to be frequently altered in several human cancers including ovarian cancers. However the role of this oncogenic signaling pathway has not been explored in the Middle Eastern epithelial ovarian cancer (EOC). Therefore, we investigated PI3K/AKT genetic alterations such as PIK3CA amplification, PIK3CA mutation, PTEN protein loss and their relationships with various clinicopathological characteristics in 156 EOCs.
Results
Fluorescence in situ hybridization (FISH) technique and DNA sequencing were used to analyze PIK3CA amplification and mutation respectively. Expression of PIK3CA protein expression (p110 α), PTEN, p-AKT and Ki-67 was analyzed by immunohistochemistry. PIK3CA amplification was seen in 54 of 152 (35.5%) EOC cases analyzed; PIK3CA gene mutations in 6/153 EOC (3.9%); KRAS mutations in 3/154 EOC (1.9%), BRAF mutations in 3/156 EOC (1.9%), p53 mutation in 50/154 EOC (32.5%), and loss of PTEN protein expression in 33/144 EOC (22.9%). p110 α overexpression was associated with increased phosphorylation of AKT-Ser 473 and with the proliferation marker Ki-67.
Conclusion
Our data showed mutual exclusivity between the molecular event of PIK3CA amplification and mutations in PIK3CA, KRAS, BRAF genes, which suggests that each of these alterations may individually be sufficient to drive ovarian tumor pathogenesis independently. High prevalence of genetic alterations in PI3K/AKT pathway in a Middle Eastern ovarian carcinoma provides genetic evidence supporting the notion that dysregulated PI3K/AKT pathways play an important role in the pathogenesis of ovarian cancers.
Background
Ovarian carcinoma is the most lethal gynecological malignancy because most tumours are detected in advanced stages [1,2]. It is the fifth leading cause of cancer-related death among U.S. women. In Middle Eastern ovarian cancer, a similar pattern of incidence has been seen [3]. Epithelial cancer of the ovary derives from malignant transformation of the epithelium of the ovarian surface, which is contiguous with the peritoneal mesothelium [4,5]. Epithelial ovarian neoplasms are subclassified histologically into serous, mucinous, endometrioid, clear cell, transitional (Brenner), squamous cell, and undifferentiated subtypes. Serous carcinomas (SC) are the most common histology, accounting for about two thirds of ovarian carcinomas [6]. A better understanding of the factors and mechanisms determining the aggressive behavior of some EOC is critical in developing new treatment.
Increased mitogenic signaling through receptors has proven to play a major role in ovarian cancer. One of the major downstream mediators of signaling initiated by these receptors is the phosphatidylinositol 3-kinase (PI3K)/AKT pathway. PI3K/AKT is activated in multiple cancers leading to oncogenic transformation [7,8]. Activation may result from activating mutation of the catalytic subunit of p110α of PIK gene (PIK3CA) or amplification of PIK3CA or as a result of inactivating mutation in the tumor suppressor gene, phosphatase and tensin homolog (PTEN). Activation of this pathway will cause AKT to translocate to the plasma membrane, where it is activated by phosphorylation allowing it to mediate many of the biological consequences of PI3K activation. Several studies have showed PIK3CA gene amplification in ovarian cancers [9-13]. Mutations in the PIK3CA have also been identified in ovarian cancers and though relatively common in endometrioid ovarian carcinomas (EC), are uncommon in serous carcinomas (SC) [11].
Rat sarcoma viral oncogene (RAS) proteins are located on the inner surface of the plasma membrane and are attached to the membrane by a farnesyl residue. RAS proteins transmit extracellular signals that promote the growth, proliferation, differentiation and survival of cells. The major downstream target of RAS-GTP is mitogen-activated protein kinases (MAPKs), but it is also known to activate other targets like PI3K [14,15]. The MAPK pathway is hyperactivated in 30% of human cancer [14]. RAS mutation can promote ovarian tumorigenesis through MAPK pathway or through its interaction with PI3K/AKT pathway [14,15]. In ovarian cancer, KRAS mutation has been identified in 35% low-grade serous tumors and 33% of borderline tumors, whereas, BRAF mutations occur in 30% of low-grade serous carcinomas and 28% of borderline tumors [16,17]. KRAS and BRAF mutations are less common in high grade ovarian cancers [16,17].
Several pervious studies have shown the oncogenic role of PI3K/AKT pathway in EOC. Elucidation of the characteristics of ovarian cancer with PI3K alterations seems to be important for the execution of personalized medicine in future. However, all the studies on PI3K alteration reported until now dealt with Caucasian ovarian cancers. It seems interesting to compare the frequency of PI3K alterations between two ethnicities, Middle Eastern and Caucasian EOC, and explore the contribution of PI3K alterations to pathogenesis of Middle Eastern EOC. Therefore, in the present study, we have analyzed the prevalence of genetic alterations of PI3K in Middle Eastern EOC and their interrelationships with other genetic alterations like p53, KRAS and BRAF. Furthermore, correlation of the PI3K alterations with various clinicopathological characteristics was investigated in a large cohort of Middle Eastern EOCs.
Methods
Patient selection and tissue microarray construction
156 patients with ovarian carcinoma diagnosed between 1991–2007 were selected from the files of the King Faisal Specialist Hospital and Research Centre. All samples were analyzed in a tissue microarray (TMA) format. TMA construction was performed as described earlier [18,19]. Two cores of ovarian carcinoma were arrayed from each case. The Institutional Review Board of the King Faisal Specialist Hospital & Research Centre approved the study.
The patients were diagnosed histologically and received follow-up care in the Departments of Obstetrics and Gynecology and Oncology at King Faisal Specialist Hospital and Research Centre. The histological subtype of each ovarian tumor sample was determined by pathologist (PB) according to accepted criteria [20]. Department of Obstetrics and Gynecology, King Faisal Specialist Hospital and Research Center provided long-term follow-up data for these patients. The primary pathological diagnosis was serous 125 patients (80.1%), endometrioid in 22 (14.1%), clear cell in 4 (2.6%) and undifferentiated/mixed Epithelial in 5 (3.2%). The ages of the patients ranged from 19–86 years, with a median age of 56 years. The majority of patients underwent primary surgical staging or cytoreduction. In some patients who were not fit for primary surgery, primary neoadjuvant chemotherapy was followed by interval debulking surgery. The distribution by FIGO stage at diagnosis was: stage I-II in 8 patients (5.1%), stage III-IV in 137 (87.8%), and unknown in 11(6.1%). The median follow-up time was 14.9 months (range, 2–130 months). Progression free survival was computed from date of surgery for patients who underwent primary cytoreduction and from date of diagnosis by biopsy or cytology in those who underwent primary neoadjuvant chemotherapy. Since the majority of patients are lost to follow-up as their disease reaches its terminal stages, it was impossible to determine overall survival in this specific patient population.
DNA extraction and purification
Genomic DNAs were extracted from paraffin embedded neoplastic primary tissues using Gentra Kit (Minneapolis, MN, USA) following a slight modification to the manufacturer's recommendation.
Mutation analysis of PIK3CA, p53, KRAS, and BRAF genes
Step-down cycling condition was used for BRAF T1799A transversion mutation in exon 15 of the BRAF gene [21]. After a 10-min denaturing at 95°C, the PCR was run with each temperature for 1 min at five step-down steps, for two cycles each. The denaturing temperature was 95°C, and extension temperature was 72°C for each step, with the annealing temperature of 66, 64, 62, 60 and 58°C from the first to the last step. The PCR was finally run at 95, 58, and 72°C each for 1 min for 35 cycles, followed by an elongation at 72°C for 5 min. PCR was performed in a total volume of 25 μL using 50 ng of genomic DNA, 2.5 μL 10 × Taq buffer, 1.5 μL MgCl2 (25 mM), 0.05 μL dNTP (10 mM), 0.2 μL Taq polymerase (1 U/μL) (all reagents were from Qiagen Inc), 1 μL of each primer (2.5 μM), and water. As majority of KRAS mutations were found in exon 1 of KRAS gene, we focused our mutation analysis on KRAS.[21] The PCR mixture contained the same components as in PCR reaction for the BRAF gene. The PCR condition was as follows: after a 10 min denaturation at 95°C, 30 s of annealing at 53°C, and 1 min of extension at 72°C, with an extension of 72°C for 7 min at the last step [21]. The efficiency and quality of the amplification PCR were confirmed by running the PCR products on a 2% agarose gel. The PCR products were subsequently subjected to direct sequencing PCR with BigDye terminator V 3.0 cycle sequencing reagents (Applied Biosystems, Foster City, CA). The samples were finally analyzed on an ABI PRISM 3100 xl Genetic Analyzer (Applied Biosystems, Foster City, CA). Sequencing of PIK3CA exons 9, 20 was done by PCR amplification and direct sequencing of both strands for all CRC cases and their matched normal samples as previously described [22,23].
For p53 mutational analysis, exons 5–8 of the P53 gene were amplified separately as previously [24]. The following primers were used: exon-5-forward: 5'GACTTTCAACTC-TGTCTC3', reverse: 5'CTGGGGACCCCTGGGCAAC3'; exon-6-forward: 5'GAGAC-GACAGGGCTGGTT3', reverse: 5'CCACTGAC-AACCACCCTT3'; exon-7-forward 5'CCAAGGCGCACTGGCCTC3', reverse: 5'GCGGCAAGCAGAGGCTGG3'; and exon-8-forward: 'CCTTACTG-CCTCTTGCTT3', reverse 5'TGAATCTGAGGCATAA-CTGC3'. The samples were finally analyzed on an ABI PRISM 3100 xl Genetic Analyzer (Applied Biosystems, Foster City, CA). Mutational analysis was done using DNA SEQMAN software (DNASTAR Inc., Madison, WI).
Immunohistochemistry (IHC)
TMA slides were processed and stained manually. The streptavidin-biotin peroxidase technique with diaminobenzidine as chromogen was applied. For antigen retrieval, Dako Target Retrieval Solution pH 9.0 (Catalogue number S2368) was used, and the slides were boiled in a pressure cooker (Pascal Pressure Cooker, Dako Cytomation, Model: S2800, USA). Primary antibodies used, their dilutions, and incidences are listed in Table 1. Endogenous peroxidase activity was quenched using 3% hydrogen peroxidase. Endogenous biotin was blocked and all slides were counterstained with hematoxylin, dehydrated, cleared, and cover slipped with premount. Only fresh cut slides were stained simultaneously to minimize the influence of slide ageing and maximize repeatability and reproducibility of the experiment.
Table 1.
Antibodies used for tissue microarray Immunohistochemical analysis
| Antibody (Subcellular Localization) | $Positive cases (%) | Clone | Company | Source | Dilution Overnight in incubation | Retrieval | Detection System |
| PTEN@ | 111/144 77.1% |
6H2-1 | Cascade Bioscience | Mouse monoclonal | 1:100 | pH 9, Pressure Cooker | EnVision+ |
| p-AKT (Cytoplasmic & Nuclear) |
75/144 52.1 |
Ser473 | Cell Signaling | Mouse Mono-clonal |
Predilute | pH 9, microwave | Survival Marker; Signal Stain IHC detection Kit |
| PIK3CA-110 | 78/140 55.7% |
1G12E9 | Everest Biotech | Goat Polyclonal | 1:400 | pH 6, Pressure Cooker | EnVision+ |
| P53 | 68/138 49.3% |
DO-7 | D9K0 | Mouse monoclonal | 1:50 | pH 9, microwave | Ventana Bechmark |
@Normal expression of PTEN was seen in 77.1% cases and PTEN inactivation (loss/reduced expression) was seen in 33/144(22.9%) of EOC cases.
$ Non representative spots for the various antibodies ranged from18 cases for p53 to 12 spots for PTEN IHC
p-AKT scoring was done as described earlier [21,25]. For purposes of statistical analysis, all cases staining at level 0 or 1 were grouped as p-AKT negative and all cases staining at level 2 and level 3 were grouped as p-AKT positive. For PTEN scoring cases staining at level 2 or 3 were considered as normal expression and cases staining at level 0 or 1 were considered to have PTEN inactivation. Only fresh cut slides were stained simultaneously to minimize the influence of slide ageing and maximize repeatability and reproducibility of the experiment. Two types of negative controls were used for p-AKT. One was the negative control in the kit in which the primary antibody was omitted. A preabsorption experiment using p-AKT Ser 473 blocking peptide (Cell Signaling Technology, Beverly, MA, Product No 1140) was used as the second negative control. PIK3CA protein (p110 α expression) was categorized by doing an H score as described earlier [26]. Each TMA spot was assigned an intensity score from 0–3(I0, I1–3) and proportion of the tumor staining for that intensity was recorded as 5% increments from a range of 0–100(P0, P1–3). A final H score (range 0–300) was obtained by adding the sum of scores obtained for each intensity and proportion of area stained (H score = I1XP1+I2XP2+I3XP3). Ovarian tumors were grouped into 2 groups using X-tile bioinformatics software: low p110 α expression (H score = 100) and the other group showed high p110 α expression (H score >100) [27].
Flourescent in situ hybridization (FISH) methodology
FISH on tissue micorarray was performed as previously described [21]. Briefly the search for FISH probe was done by browsing Ensemble Genome Browser http://www.ensembl.org/ for bacterial artificial chromosome (BAC) corresponding to PIK3CA gene. BAC RP11-245 C23 was purchased from Childrens Hospital Oakland Reseach Institute (Oakland, California), was cultured and DNA isolated. BAC DNA probe was labeled with digoxigenin using the DIG-nick translating kit from Roche. FISH was performed with a digoxigenin-labeled BAC DNA probe, containing the PIK3CA gene and a Spectrum Orange-labeled chromosome 3 centromeric probe (CEP3) as a reference (purchased from Vysis). TMA sections were treated according to the Paraffin Pretreatment Reagent Kit protocol (Vysis, IL, USA) before hybridization. For the ovarian cancer TMA study, hybridization and post-hybridization washes were according to the Vysis LSI procedure. Probe visualization using fluorescent isothiocyanate (FITC)-conjugated sheep anti-digoxigenin (Roche Diagnostics, Indianapolis, IN, USA) was as described (Wagner et al). Slides were then counterstained with 125 ng/ml 4',6-diamino-2-phenylindole in an antifade solution and screened with a Olympus BX51 fluorescent microscope. Tissue samples were classified with a PIK3CA/centromere 3 ratio of 1.0 as normal, between 1.0 and 2.0 as having PIK3CA gains. A PIK3CA/centromere 3 ratio of more than 2.0 was considered as amplified.
Quantitative real-time PCR
Ovarian tumors with increased copy number by FISH of PIK3CA gene were selected for validation by quantitative real time PCR. DNA content was normalized to that of long interspersed elements (LINE1), a repetitive element for which copy number per haploid genome are similar both in normal DNA sample and neoplastic cells. Primers were designed by Primer express 3.0 software (Applied Biosystems Foster City CA) hybridize to sequences of genomic DNA for PIK3CA and LINE 1. Primers to genomic sequences were (PIK3CA forward, 5'-TATGGTTGTC-TGTCAATCGGTGA-3'; reverse,5'-GCCTTTGCAGTGAATT-TGCAT-3') and (LINE1 forward, 5'-CCGCTCAACTACATGGAAACTG-3' reverse, 5'-GCGTCCCAGAGATTCTGGTATG-3'). Conditions for all PCRs were optimized in gradient cycler (MJ Research, MA, United States) with regard to Taq DNA polymerase, forward and reverse primers, MgCl2 concentrations, dNTP concentrations and various annealing temperatures (55–65°C). Specificity of the PCR product was confirmed by agarose gel electrophoresis. Optimized results were transferred on the following Light Cycler PCR protocol.
All reactions were performed in glass capillaries (Roche, Mannheim, Germany) with a final reaction volume of 10 μl of 1× LightCycler-FastStart DNA Master SYBR Green I reaction mixture (Roche, Mannheim, Germany) containing FastStart Taq, reaction buffer, and deoxynucleoside triphosphate, 1 mM MgCl2, and final concentrations of 0.5 μM for each primer. MgCl2 concentrations were optimized for each target gene (varied from 2–4 mM). Thermocycling and detection were performed on the LightCycler (Roche Diagnostics, Mannheim, Germany). An initial preheating step of 10 min at 95°C was used to activate the DNA polymerase, then, a touch-down procedure, consisting of 10 s at 95°C, annealing for 5 s at temperatures decreasing from 63 to 59°C, and ending with an extension step at 72°C for 10 s. A total of 45 cycles were performed, followed by melting curve program (60–95°C with a heating rate of 0.1°C per second and a continuous fluorescence measurement), and finally a cooling step to 40°C.
Pfaffle method for relative quantification was used to calculate fold of changes for normal and ovarian cancer samples [28]. The relative copy number ratio of a target gene is calculated based on efficiency (E) and crossing point (CP) deviation of samples (normal) versus (ovarian tumor), and expressed in comparison to a reference gene (LINE1). For a normal cell the copy number of a gene per haploid genome should be one.
Statistics
All Statistical analysis will be performed using the Statview JMP software (version 7.0). Fisher's exact chi-square (χ2) test was used to assess associations between categorical variables. Kaplan-Meier survival analyses were carried out for progression free survival, using the log-rank test for differences between groups. Results were considered statistically significant when p from a two-tailed test was < 0.05.
Results
PIK3CA mutations, PIK3CA amplification and PIK3CA 110 alpha subunit protein expression (p110 α expression) in EOC
PIK3CA mutation was seen in 6 of 153 (3.9%, Fig. 1) EOC's analyzed and FISH analysis revealed the presence of PIK3CA amplification in 54 of 152 (35.5%, Fig. 2) EOC cases analyzed. PIK3CA amplification results were further validated by real-time PCR. No significant associations were observed with PIK3CA mutations or PIK3CA amplifications and clinicopathological parameters. Immunohistochemical analysis showed overexpression of p110 α expression in 78 of 140 (55.7%, Fig. 3) EOC cases analyzed and its association with various clinicopathological parameters was analyzed (Table 2). p110 α expression overexpression did not coorelate with age, tumor stage, FIGO grade and progreesion free survival. PIK3CA 110 overexpression was associated with overexpression of p-AKT-Ser 473 (p = 0.0260) and a trend was noted with the proliferation marker Ki-67 (p = 0.0639). Of the 6 EOCS with PIK3CA mutation, 5 of them showed overexpression of PIK3CA 110 protein. No association was seen with PIK3CA amplification and overexpression of p110 α expression (p = 0.2320).
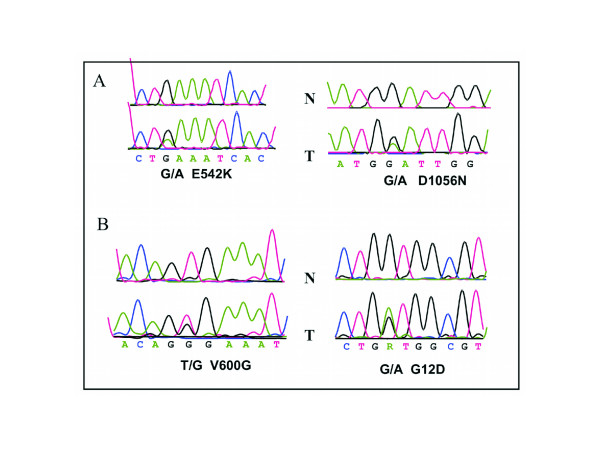
Figure 1.
Examples of PIK3CA, KRAS, and BRAF mutations in EOC cases. A. Typical sequencing traces of PIK3CA mutations are shown for exon 9 (right) and 20 (left) with cancer mutant sequence (bottom) and normal wild-type sequence (top). B. Sequencing traces of BRAF (right) and KRAS (left) mutations with cancer mutant sequence (bottom) and normal wild-type sequence (top). Arrows denote position of the missense mutations with amino acid changes noted.
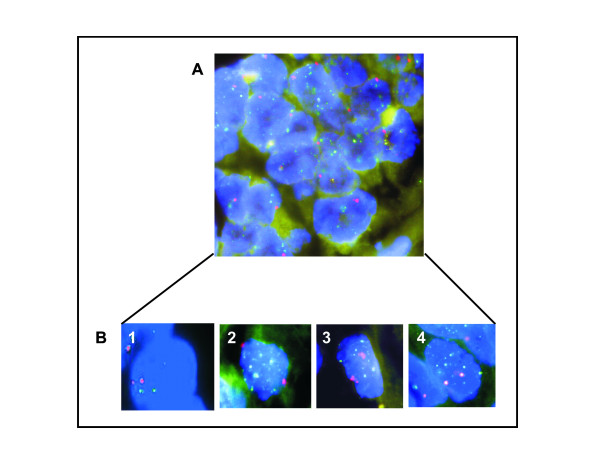
Figure 2.
Determination of PIK3CA gene copy number by FISH on ovarian tissue (A). B. Sample 1 is normal cell selected according to FISH analysis. Samples 2–4 are PIK3CA amplified ovarian tumor cells selected according to FISH. FISH images show cell nuclei (blue) from selected cases, hybridized with probes directed against PIK3CA gene (green RP11-245 C23) and centromere 3 (red). (1). Normal cell (blue) shows 2 centromeric signals (red) and 2 (green) PIK3CA signals. (2–4). Representative cells show amplification two (red) centromeric signals and green PIK3CA amplified Signals.
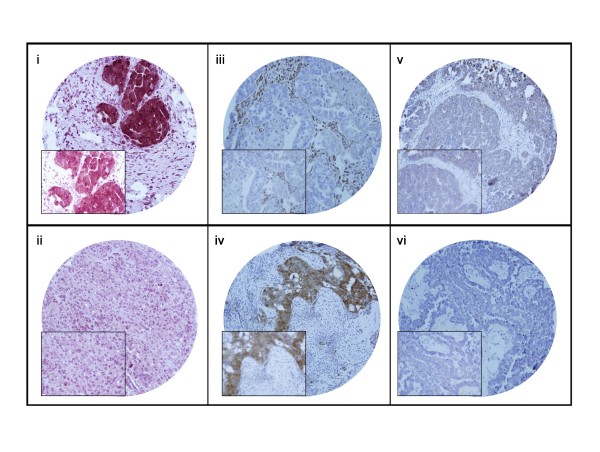
Figure 3.
Immunohistochemical analysis of p-AKT, PI3K-110 alpha subunit protein expression and PTEN in epithelial ovarian carcinoma (EOC). (i) p-AKT over expression was observed along with (iii) low PTEN expressionand (v) over expression of PI3K-110 alpha in EOC TMA specimen and, (ii) Low expression for p-AKT was seen along with (iv) high PTEN expression and (vi) reduced expression for PI3K-110 alpha in EOC TMA specimen. 20 × magnifications with the inset showing a 100 × magnified view of the same.
Table 2.
Correlation between PIK3CA-110-Alpha status and clinicopathological features in Epithelial Ovarian Carcinoma
| High p110 α expression | Low p110 α expression | P value | |||||
| N | % | N | % | N | % | ||
| Total Number of Cases | 140 | 78 | 55.7 | 62 | 44.3 | ||
| Age | |||||||
| < = 50 years | 54 | 38.6 | 31 | 57.4 | 23 | 42.6 | 0.7491 |
| > 50 years | 86 | 61.4 | 47 | 54.7 | 39 | 45.3 | |
| Tumour Stage | |||||||
| Stage I-II | 8 | 6.1 | 4 | 50.0 | 4 | 50.0 | 0.7525 |
| Stage III-IV | 122 | 93.9 | 68 | 55.7 | 54 | 44.3 | |
| Histopathology | |||||||
| Clear cell | 4 | 2.9 | 3 | 75.0 | 1 | 25.0 | 0.8585 |
| Endometriod | 19 | 13.6 | 11 | 57.9 | 8 | 42.1 | |
| Serous | 113 | 80.7 | 62 | 54.9 | 51 | 45.1 | |
| Undifferentiated | 4 | 2.9 | 2 | 50.0 | 2 | 50.0 | |
| FIGO Grade | |||||||
| Well differentiated | 27 | 19.3 | 12 | 44.4 | 15 | 55.6 | 0.4114 |
| Moderately Diff | 73 | 52.1 | 42 | 57.5 | 31 | 42.5 | |
| Poorly Diff | 40 | 28.6 | 24 | 60.0 | 16 | 40.0 | |
| PAKT (Ser473) | |||||||
| High (2–3) | 69 | 51.5 | 46 | 66.7 | 23 | 33.3 | 0.0260 |
| Low (0–1) | 65 | 48.5 | 31 | 47.7 | 34 | 52.3 | |
| PTEN | |||||||
| Low (0–1) | 31 | 23.3 | 17 | 54.8 | 14 | 45.2 | 0.7675 |
| High (2–3) | 102 | 76.7 | 59 | 57.8 | 43 | 42.2 | |
| Ki-67 | |||||||
| Above 50 | 51 | 37.0 | 34 | 66.7 | 17 | 33.3 | 0.0639 |
| Below = 50 | 87 | 63.0 | 44 | 50.6 | 43 | 49.4 | |
| P53 | |||||||
| Negative | 65 | 50.0 | 37 | 56.9 | 28 | 43.1 | 0.5923 |
| Positive | 65 | 50.0 | 40 | 61.5 | 25 | 38.5 | |
| KRAS Mutation | |||||||
| Present | 2 | 1.4 | 0 | 0.0 | 2 | 100.0 | 0.0720 |
| Absent | 136 | 98.6 | 76 | 55.9 | 60 | 44.1 | |
| BRAF Mutation | |||||||
| Present | 3 | 2.1 | 1 | 33.3 | 2 | 66.7 | 0.4302 |
| Absent | 137 | 97.9 | 77 | 56.2 | 60 | 43.8 | |
| PIK3CA Mutation | |||||||
| Present | 6 | 4.4 | 5 | 83.3 | 1 | 16.7 | 0.1298 |
| Absent | 131 | 95.6 | 70 | 53.4 | 61 | 46.6 | |
| P53 Mutation | |||||||
| Present | 47 | 34.1 | 25 | 53.2 | 22 | 46.8 | 0.7497 |
| Absent | 91 | 65.9 | 51 | 56.0 | 40 | 44.0 | |
| PIK3CA FISH | |||||||
| Amplified | 49 | 35.5 | 24 | 49.0 | 25 | 51.0 | 0.2320 |
| Non-Amplified | 89 | 64.5 | 53 | 59.6 | 36 | 40.4 | |
| PFS-Median (months) | 16.2 | 17.1 | 0.6578 | ||||
KRAS, BRAF, and TP53 mutation analysis
We found that six EOCs contained PIK3CA mutation, three EOC contained activating KRAS mutations and three EOCs had BRAF mutations at codon 599 (Fig. 1). The presence of KRAS and BRAF mutations was mutually exclusive. EOCs with PI3K mutation did not display BRAF or KRAS mutations. Thus PIK3CA mutations were also mutually exclusive with KRAS and BRAF mutation.
TP53 mutation analysis revealed a mutation incidence rate of 32.5% (50 of 154). No significant associations were found between TP53 mutations and clinicopathological data like FIGO staging, tumor grading, tumor type, patient age and progression free survival. Also TP53 mutations were not associated with mutations in PIK3CA, BRAF, and KRAS genes or with activation of AKT, loss of PTEN expression and PIK3CA amplification.
Using the mouse monoclonal anti p53 antibody (Table 1), p53 overexpression by immunohistochemistry was seen in 68/138 (49.3%) EOCs analyzed. p53 overexpression was significantly associated with p53 mutations (P = 0.0002) and a trend of increased expression noted in poorly differentiated EOCs (P = 0.0961).
AKT activation in EOC and its correlation with PIK3CA mutations, PIK3CA amplification, KRAS, BRAF mutations
Because it is suggested that PIK3CA mutations activate AKT function through its phosphorylation [29], we investigated the relationship between PIK3CA mutation, PIk3CA amplification and activation of p-AKT. The correlation between the activation of AKT with clinicopathological parameters and mutational status of K-KRAS and BRAF genes and TP53 mutation was also explored. AKT activation was evaluated by assessing phosphorylation of AKT by immunohistochemistry at Ser473 and an EOC case was considered to show AKT activation when it was scored as 2+ or 3+ for p-AKT Ser 473. Activation of AKT was observed in 52.1% (75 of 144) of EOC cases.
Though p-AKT Ser 473 overexpression correlated significantly with proliferation marker, Ki-67 expression (p = 0.0262), no association was seen with clinicopathological parameters including progression free survival. Also there was a lack of association between EOCs that harbored KRAS mutations or BRAF mutations and AKT activation.
PTEN inactivation
All the EOC cases with lost or reduced PTEN expression were considered as EOCs with PTEN inactivation. PTEN inactivation was seen in 33 of 144 (22.9%) EOC's analyzed and was associated with histology subtype of clear cell and undifferentiated carcinomas (p = 0.0491); overexpression of p53 protein (p = .0413) and a trend was seen with older age (p = 0.0936). No significant associations were noted with clinicopathological features and the various mutations analyzed.
Mutual exclusivity among the genetic alterations in the PI3K/Akt and MAPK pathways in Middle Eastern EOC cases
Because PIK3CA copy gain is the most common genetic alteration in the PI3K/Akt pathway in EOCs in this Middle Eastern cohort, we analyzed its relationship with each of the gene mutations in the PI3K/Akt pathway. As shown in Table 3, PIK3CA gene copy gain was uncommonly overlapped with gene mutations in EOC; mutations were mostly seen in the group of EOC without PIK3CA copy gain. The mutual exclusivity between PIK3CA copy gain and any of the PIK3CA, KRAS, and BRAF mutations was not statistically significant, probably due to the small number of each of these mutations. There was not a single overlap among these alterations. Additionally, the majority of the amplified PIK3CA cases harbored normal PTEN protein expression. However, overlap between the PTEN protein loss and PIK3A amplification was seen in a number of cases of EOC (5.7% [4/140]; data not shown).
Table 3.
Summary of individual cases of EOC with genetic alterations in PIK3CA, BRAF and KRAS genes
| KRAS Mutation | PIK3CA Mutation | BRAF Mutation | ||||||||
| Case number | Exon/codon | Nucleotide exchange | Amino acid | PIK3CA amplify | Exon/codon | Nucleotide exchange | Amino acid | Exon/codon | Nucleotide exchange | Amino acid |
| 1 | Normal | 20/1047 | CAT>CGT | His>Arg | ||||||
| 2 | Normal | 20/1056 | GAT>AAT | Asp>Asn | ||||||
| 3 | Normal | 20/1115 | TCT>TTT | Ser>Phe | ||||||
| 4 | Normal | 9/542 | GAA>AAA | Glu>Lys | ||||||
| 5 | Normal | 9/545 | GAG>AAG | Glu>Lys | ||||||
| 6 | Normal | 9/530 | CAG>CGG | Gln>Arg | ||||||
| 7 | 1/12 | GGT>GTT | GLy>Val | Normal | ||||||
| 8 | 1/13 | GGC>GAC | GLy>Asp | Normal | ||||||
| 9 | 1/12 | GGT>GAT | GLy>Asp | Normal | ||||||
| 10 | Normal | 15/594 | GGT>GAT | Asp>Gly | ||||||
| 11 | Normal | 15/600 | GTG>GGG | Val>Gly | ||||||
| 12 | Normal | 15/600 | GTG>GAG | Val>Glu | ||||||
| 13 | Amplified | |||||||||
| 14 | Amplified | |||||||||
| 15 | Amplified | |||||||||
Discussion
Similarities in the prevalence of PI3K alterations in Western and Asian ovarian cancer [29-32], have raised an interest to study these alterations in other ethnic groups. There is a mixed report on the incidence of PIK3CA mutation in ovarian cancer. Reported incidence of PIK3CA mutation in epithelial ovarian cancer varies from 3.6% [30] to 12% [31]. Our study showed a lower incidence of PIK3CA mutations (3.9%), indicating that the PIK3CA gene mutation is not a common mechanism in the activation of PIK3CA in Middle Eastern ovarian tumors. Earlier studies have reported PIK3CA gene amplification to be in the range of 13 to 24.5% [10-13,33]. Only one study done on a limited number of ovarian samples (n = 12) has reported a higher incidence of PIK3CA amplification (40%) [9]. On the other hand, our study demonstrated PIK3CA amplification in ovarian tumors, with a relatively high frequency (35.5%, Fig. 2), which suggests the major role of PIK3CA amplification in the activation of these ovarian cancers. Interestingly, there was an almost perfect reciprocal association of the presence of gene amplification and a somatic PIK3CA mutations suggesting that these mutations mainly occur in tumors without amplification. In agreement with an earlier report by Woenckhaus J et al., 2007 we also did not find any association between PIK3CA amplification and p110 α protein expression [13]. This lack of association could be explained by transcriptional or post transcriptional mechanisms which finely tune the expression level of p110 α expression. However, we found p110 α expression to be correlated with p-AKT (P = 0.0260, Table 2) and a trend was noted with the proliferation marker Ki-67 (P = 0.0639).
Next, PTEN protein expression was investigated by immunohistochemistry and 33 of 144 (22.9%, Fig. 3) EOCs were showing PTEN inactivation, which was in concordance with early report [34]. Correlation analysis between PIK3CA amplification and PTEN protein loss by IHC revealed that the majority of PIK3CA amplifications were found in cases with high PTEN protein expression. This data suggests that a single oncogenic alteration along this pathway is sufficient to drive ovarian cell transformation. As a read out of PI3K functional activation, we tested AKT phosphorylation (activation) in EOC. Our findings show that AKT is activated in a large proportion of EOCs (52.1%), which reflect the activity of that pathway in our EOC. However, no significant association was found between AKT and PIK3CA mutation/amplification. Currently, multiple pathways have been implicated as having role in AKT activation such as MAP kinase [35]. Furthermore, in agreement with an earlier report [36], we have found significant correlation between fatty acid synthase (FAS) protein expression and AKT activation in Middle Eastern EOC (data not shown). Therefore, FAS overexpression might be one of the signaling pathways to activate AKT by a mechanism independent of the PI3K pathway.
We were also interested in studying the relationship between PI3K/AKT pathway and p53 (tumor suppressor gene) since interactions between the p53 and PI3K/AKT pathways play a significant role in the determination of cell death/survival. Interrelation between these pathways occurs through the transcriptional regulation of PTEN and PIK3CA by p53, which is required for p53-mediated apoptosis [37-39]. P53 gene mutation is among the frequent genetic alteration in ovarian carcinomas and its incidence in Middle Eastern EOCs is 32%, which is relatively lower to what has been reported in the west (50 to 80%) [40-42]. Recently, Astanehe et al., 2008 have suggested that p53 inactivation results in increases of PIK3CA transcripts beyond those that result from amplification of PIK3CA alone [37]. Thus, the combined effects of PIK3CA amplification and p53-mediated regulation of PIK3CA and PTEN expression should contribute greatly to the increased signaling through PI3K pathway in Middle Eastern EOC.
We also analyzed MAPK signaling pathway by investigating the mutational status of key genes of that pathway, BRAF and KRAS. Presence of KRAS and BRAF mutations was significantly more common in serous borderline ovarian tumors as compared to serous carcinomas (p = 0.0221; KRAS mutations) and (p < 0.0001; BRAF mutations); data not shown). This is in concordance with earlier reports [16,17]. The mutual exclusivity of BRAF and KRAS mutations (Table 3) in our ovarian carcinoma is consistent with previous findings in ovarian carcinoma, melanoma, and colorectal carcinoma [43-45]. This also supports the view that BRAF and KRAS mutations have equivalent effects on tumorigenesis [44-46]. Although the possibility that other downstream targets of BRAF are mutated in conventional high-grade ovarian carcinomas cannot be completely ruled out, it would appear that the development of high grade serous carcinomas involves a pathway distinct from the MAP kinase signaling pathway.
In examining the relationship between PI3K/AKT and MAPK pathways, we found that PIK3CA amplification was the single most common genetic alteration in our Middle Eastern EOC cases and was mutually exclusive with gene mutations in both pathways (PIK3CA, KRAS, and BRAF). This represents strong genetic evidence that PIK3CA amplification possesses similar oncogenic function as the classical gene mutations in these pathways in ovarian cancer pathogenesis. The collective prevalence of genetic alterations (PIK3CA amplification, mutation, PTEN protein loss) in the PI3K/Akt pathway was particularly high in Middle Eastern EOC (60%), suggesting a significant role of the PI3K/Akt pathway in the pathogenesis of EOC.
Conclusion
Our results showed that though mutation of PIK3CA is not a common event in the EOCs, its amplification is very common and may be a novel mechanism in activating the PI3K/AKT pathway in some EOCs. High prevalence of PI3K/AKT genetic alterations along with a low incidence of KRAS and BRAF mutations in Middle Eastern ovarian carcinomas, mutual exclusivity among genetic alterations in PI3K/AKT and MAPK pathways, support the notion that dysregulated PI3K/AKT pathways play an important role in the pathogenesis of ovarian cancers regardless of ethnic background. Understanding the alternative molecular pathways that result in epithelial ovarian cancers will allow specific biological targeting for therapy and prevention of ovarian cancer.
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
JA carried out all the mutational analysis, helped in the project planning, as well as interpretation of data and contributed to the draft of the manuscript. PB contributed to the performance of immunohistochemistry, statstical analysis, and writing of the manuscript. WH carried out most of the PCR optimization and sequencing. ZJ conducted FISH analysis and real-time PCR validation. MS conducted some of the mutation analysis. AM helped in collecting the samples and provided us with the clinical data. SU participated in experimental design and writing of the manuscript. KSA conceived of the study, coordinated the study, and contributed to the draft of the manuscript.
Acknowledgments
Acknowledgements
We thank Sriraman Devarajan for data abstraction and statistical analysis. The skillful technical assistance of Azadali Moorji, Hassan Al-Dossari, Valerie Atizado and Valorie Balde is greatly appreciated.
Contributor Information
Jehad Abubaker, Email: jabubakr@kfshrc.edu.sa.
Prashant Bavi, Email: PBavi@kfshrc.edu.sa.
Wael Al-Haqawi, Email: whaqawi@kfshrc.edu.sa.
Zeenath Jehan, Email: jzeenath@yahoo.com.
Adnan Munkarah, Email: AMunkarah@Kfshrc.edu.sa.
Shahab Uddin, Email: Shahab@kfshrc.edu.sa.
Khawla S Al-Kuraya, Email: kkuraya@kfshrc.edu.sa.
References
- Fishman DA, Bozorgi K. The scientific basis of early detection of epithelial ovarian cancer: the national ovarian cancer early detection program (NOCEDP) In: Stack MS, Fishman DA, editor. Cancer Treatment and Research: Ovarian Cancer. Boston: Kluwer Academic Publishers; 2001. pp. 3–28. [DOI] [PubMed] [Google Scholar]
- Fader AN, Rose PG. Role of surgery in ovarian carcinoma. J Clin Oncol. 2007;25:2873–2883. doi: 10.1200/JCO.2007.11.0932. [DOI] [PubMed] [Google Scholar]
- Khoja TA. The Five-Year Cancer Incidence, 1998–2002 Report. Gulf centre for cancer registration. 2006. pp. 41–43.
- Cannistra SA. Medical progress: Cancer of the ovary. New England Journal of Medicine. 2004;351:2519–2529. doi: 10.1056/NEJMra041842. [DOI] [PubMed] [Google Scholar]
- Jemal A, Tiwari RC, Murray T, Ghafoor A, Samuels A, Ward E, Feuer EJ, Thun MJ. American Cancer Society. Cancer statistics. CA Cancer J Clin. 2004;54:8–29. doi: 10.3322/canjclin.54.1.8. [DOI] [PubMed] [Google Scholar]
- Barda G, Menczer J, Chetrit A, Lubin F, Beck D, Piura B, Glezerman M, Modan B, Sadetzki S. Comparison between primary peritoneal and epithelial ovarian carcinoma: a population-based study. Am J Obstet Gynecol. 2004;190:1039–45. doi: 10.1016/j.ajog.2003.09.073. [DOI] [PubMed] [Google Scholar]
- Cantley LC. The phosphoinositide 3-kinase pathway. Science. 2002;296:1655–1657. doi: 10.1126/science.296.5573.1655. [DOI] [PubMed] [Google Scholar]
- Vivanco I, Sawyers CL. The phosphatidylinositol 3-kinase AKT pathway in human cancer. Nat Rev Cancer. 2002;2:489–501. doi: 10.1038/nrc839. [DOI] [PubMed] [Google Scholar]
- Shayesteh L, Lu Y, Kuo WL, Baldocchi R, Godfrey T, Collins C, Pinkel D, Powell B, Mills GB, Gray JW. PIK3CA is implicated as an oncogene in ovarian cancer. Nat Genet. 1999;21:99–102. doi: 10.1038/5042. [DOI] [PubMed] [Google Scholar]
- Willner J, Wurz K, Allison KH, Galic V, Garcia RL, Goff BA, Swisher EM. Alternate molecular genetic pathways in ovarian carcinomas of common histological types. Hum Pathol. 2007;38:607–13. doi: 10.1016/j.humpath.2006.10.007. [DOI] [PubMed] [Google Scholar]
- Campbell IG, Russell SE, Choong DY, Montgomery KG, Ciavarella ML, Hooi CS, Cristiano BE, Pearson RB, Phillips WA. Mutation of the PIK3CA gene in ovarian and breast cancer. Cancer Res. 2004;64:7678–81. doi: 10.1158/0008-5472.CAN-04-2933. [DOI] [PubMed] [Google Scholar]
- Zhang L, Huang J, Yang N, Greshock J, Liang S, Hasegawa K, Giannakakis A, Poulos N, O'Brien-Jenkins A, Katsaros D. Integrative genomic analysis of phosphatidylinositol 3'-kinase family identifies PIK3R3 as a potential therapeutic target in epithelial ovarian cancer. Clin Cancer Res. 2007;13:5314–21. doi: 10.1158/1078-0432.CCR-06-2660. [DOI] [PubMed] [Google Scholar]
- Woenckhaus J, Steger K, Sturm K, Münstedt K, Franke FE, Fenic I. Prognostic value of PIK3CA and phosphorylated AKT expression in ovarian cancer. Virchows Arch. 2007;450:387–95. doi: 10.1007/s00428-006-0358-3. [DOI] [PubMed] [Google Scholar]
- Kodaki T, Woscholski R, Hallberg B, Rodriguez-Viciana P, Downward J, Parker PJ. Phosphatidylinositol-3-OH kinase as a direct target of KRAS. Nature. 1994;370:527–32. doi: 10.1038/370527a0. [DOI] [PubMed] [Google Scholar]
- Xing M. BRAF mutation in thyroid cancer. Endocr Relat Cancer. 2005;12:245–62. doi: 10.1677/erc.1.0978. [DOI] [PubMed] [Google Scholar]
- Singer G, Oldt R, 3rd, Cohen Y, Wang BG, Sidransky D, Kurman RJ, Shihe M. Mutations in BRAF and KRAS characterize the development of low-grade ovarian serous carcinoma. JNCI. 2003;95:484–486. doi: 10.1093/jnci/95.6.484. [DOI] [PubMed] [Google Scholar]
- Sieben NL, Macropoulos P, Roemen GM, Kolkman-Uljee SM, Jan Fleuren G, Houmadi R, Diss T, Warren B, Al Adnani M, De Goeij AP, Krausz T, Flanagan AM. In ovarian neoplasms, BRAF, but not KRAS, mutations are restricted to low-grade serous tumours. J Pathol. 2004;202:336–340. doi: 10.1002/path.1521. [DOI] [PubMed] [Google Scholar]
- Bavi P, Jehan Z, Atizado V, Al-Dossari H, Al-Dayel F, Tulbah A, Amr SS, Sheikh SS, Ezzat A, El-Solh H, Uddin S, Al-Kuraya K. Prevalence of fragile histidine triad expression in tumors from saudi arabia: a tissue microarray analysis. Cancer Epidemiol Biomarkers Prev. 2006;15:1708–1718. doi: 10.1158/1055-9965.EPI-05-0972. [DOI] [PubMed] [Google Scholar]
- Kononen J, Bubendorf L, Kallioniemi A, Bärlund M, Schraml P, Leighton S, Torhorst J, Mihatsch MJ, Sauter G, Kallioniemi OP. Tissue microarrays for high-throughput molecular profiling of tumor specimens. Nat Med. 1998;4:844–847. doi: 10.1038/nm0798-844. [DOI] [PubMed] [Google Scholar]
- Russel P. Surface epithelial-stromal tumors of the ovary. In: Kurman RJ, editor. Blaustein's Pathology of the Female Genital Tract. New York: Springer-Verlag; 1995. pp. 705–782. [Google Scholar]
- Abubaker J, Jehan Z, Bavi P, Sultana M, Al-Harbi S, Ibrahim M, Al-Nuaim A, Ahmed M, Amin T, Al-Fehaily M, Al-Sanea O, Al-Dayel F, et al. Clinicopathological analysis of papillary thyroid cancer with PIK3CA alterations in a Middle Eastern population. J Clin Endocrinol Metab. 2008;93:611–8. doi: 10.1210/jc.2007-1717. [DOI] [PubMed] [Google Scholar]
- Abubaker J, Bavi PP, Al-Harbi S, Siraj AK, Al-Dayel F, Uddin S, Al-Kuraya K. PIK3CA mutations are mutually exclusive with PTEN loss in diffuse large B-cell lymphoma. Leukemia. 2007;21:2368–70. doi: 10.1038/sj.leu.2404873. [DOI] [PubMed] [Google Scholar]
- Saal LH, Holm K, Maurer M, Memeo L, Su T, Wang X, Yu JS, Malmström PO, Mansukhani M, Enoksson J, Hibshoosh H, Borg A, et al. PIK3CA mutations correlate with hormone receptors, node metastasis, and ERBB2, and are mutually exclusive with PTEN loss in human breast carcinoma. Cancer Res. 2005;65:2554–2559. doi: 10.1158/0008-5472-CAN-04-3913. [DOI] [PubMed] [Google Scholar]
- Bavi PP, Abubaker JA, Jehan ZD, Al-Jomah NA, Siraj AK, Al-Harbi SR, Atizado VL, Abduljabbar AS, Alhomoud SJ, Ashari LH, Al-Dayel FH, Uddin S, et al. Colorectal carcinomas from Middle East. Molecular and tissue microarray analysis of genomic instability pathways. Saudi Med J. 2008;29:75–80. [PubMed] [Google Scholar]
- Uddin S, Hussain AR, Siraj AK, Manogaran PS, Al-Jomah NA, Moorji A, Atizado V, Al-Dayel F, Belgaumi A, El-Solh H, et al. Role of phosphatidylinositol 3'-kinase/AKT pathway in diffuse large B-cell lymphoma survival. Blood. 2006;108:4178–86. doi: 10.1182/blood-2006-04-016907. [DOI] [PubMed] [Google Scholar]
- Uddin S, Siraj AK, Al-KRASheed M, Ahmed M, Bu R, Myers JN, Al-Nuaim A, Al-Sobhi S, Al-Dayel F, Bavi P, Hussain AR, Al-Kuraya KS. Fatty acid synthase and AKT pathway signaling in a subset of papillary thyroid cancers. J Clin Endocrinol Metab. 2008;93:4088–4097. doi: 10.1210/jc.2008-0503. [DOI] [PubMed] [Google Scholar]
- Camp RL, Dolled-Filhart M, Rimm DL. X-tile: a new bio-informatics tool for biomarker assessment and outcome-based cut-point optimization. Clin Cancer Res. 2004;10:7252–7259. doi: 10.1158/1078-0432.CCR-04-0713. [DOI] [PubMed] [Google Scholar]
- Pfaffl MW, Horgan GW, Dempfle L. Relative expression software tool (REST(C)) for group-wise comparison and statistical analysis of relative expression results in real-time PCR. Nucl Acids Res. 2002;30:e36. doi: 10.1093/nar/30.9.e36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuels Y, Diaz LA, Jr, Schmidt-Kittler O, Cummins JM, Delong L, Cheong I, Rago C, Huso DL, Lengauer C, Kinzler KW, Vogelstein B, Velculescu VE. Mutant PIK3CA promotes cell growth and invasion of human cancer cells. Cancer Cell. 2005;7:561–73. doi: 10.1016/j.ccr.2005.05.014. [DOI] [PubMed] [Google Scholar]
- Wang Y, Helland A, Holm R, Kristensen GB, Børresen-Dale AL. PIK3CA mutations in advanced ovarian carcinomas. Hum Mutat. 2005;25:322. doi: 10.1002/humu.9316. [DOI] [PubMed] [Google Scholar]
- Levine DA, Bogomolniy F, Yee CJ, Lash A, Barakat RR, Borgen PI, Boyd J. requent mutation of the PIK3CA gene in ovarian and breast cancers. Clin Cancer Res. 2005;11:F2875–8. doi: 10.1158/1078-0432.CCR-04-2142. [DOI] [PubMed] [Google Scholar]
- Qiao YH, Cheng J, Guo RX. Expression of phosphorylated protein kinase B and PTEN protein in ovarian epithelial cancer. Zhonghua Fu Chan Ke Za Zhi. 2007;42:325–9. [PubMed] [Google Scholar]
- Nakayama K, Nakayama N, Kurman RJ, Cope L, Pohl G, Samuels Y, Velculescu VE, Wang TL, Shih IeM. Sequence mutations and amplification of PIK3CA and AKT2 genes in purified ovarian serous neoplasms. Cancer Biol Ther. 2006;5:779–85. doi: 10.4161/cbt.5.7.2751. [DOI] [PubMed] [Google Scholar]
- Kurose K, Zhou XP, Araki T, Cannistra SA, Maher ER, Eng C. Frequent loss of PTEN expression is linked to elevated phosphorylated Akt levels, but not associated with p27 and cyclin D1 expression, in primary epithelial ovarian carcinomas. Am J Pathol. 2001;158:2097–106. doi: 10.1016/S0002-9440(10)64681-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cully M, You H, Levine AJ, Mak TW. Beyond PTEN mutations: the PI3K pathway as an integrator of multiple inputs during tumorigenesis. Nat Rev Cancer. 2006;6:184–92. doi: 10.1038/nrc1819. [DOI] [PubMed] [Google Scholar]
- Wang HQ, Altomare DA, Skele KL, Poulikakos PI, Kuhajda FP, Di Cristofano A, Testa JR. Positive feedback regulation between AKT activation and fatty acid synthase expression in ovarian carcinoma cells. Oncogene. 2005;24:3574–82. doi: 10.1038/sj.onc.1208463. [DOI] [PubMed] [Google Scholar]
- Astanehe A, Arenillas D, Wasserman WW, Leung PC, Dunn SE, Davies BR, Mills GB, Auersperg N. Mechanisms underlying p53 regulation of PIK3CA transcription in ovarian surface epithelium and in ovarian cancer. J Cell Sci. 2008;121:664–74. doi: 10.1242/jcs.013029. [DOI] [PubMed] [Google Scholar]
- Maruyama N, Miyoshi Y, Taguchi T, Tamaki Y, Monden M, Noguchi S. Clinicopathologic analysis of breast cancers with PIK3CA mutations in Japanese women. Clin Cancer Res. 2007;13:408–414. doi: 10.1158/1078-0432.CCR-06-0267. [DOI] [PubMed] [Google Scholar]
- Singh B, Reddy PG, Goberdhan A, Walsh C, Dao S, Ngai I, Chou TC, O-Charoenrat P, Levine AJ, Rao PH, Stoffel A. p53 regulates cell survival by inhibiting PIK3CA in squamous cell carcinomas. Genes Dev. 2002;16:984–993. doi: 10.1101/gad.973602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan WY, Cheung KK, Schorge JO, Huang LW, Welch WR, Bell DA, Berkowitz RS, Mok SC. Bcl-2 and p53 protein expression, apoptosis, and p53 mutation in human epithelial ovarian cancers. Am J Pathol. 2000;156:409–417. doi: 10.1016/S0002-9440(10)64744-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohler MF, Marks JR, Wiseman RW, Jacobs IJ, Davidoff AM, Clarke-Pearson DL, Soper JT, Bast RC, Jr, Berchuck A. Spectrum of mutation and frequency of allelic deletion of the p53 gene in ovarian cancer. J Natl Cancer Inst. 1993;85:1513–1519. doi: 10.1093/jnci/85.18.1513. [DOI] [PubMed] [Google Scholar]
- Wen WH, Reles A, Runnebaum IB, Sullivan-Halley J, Bernstein L, Jones LA, Felix JC, Kreienberg R, el-Naggar A, Press MF. p53 mutations and expression in ovarian cancers: correlation with overall survival. Int J Gynecol Pathol. 1999;18:29–41. doi: 10.1097/00004347-199901000-00005. [DOI] [PubMed] [Google Scholar]
- Singer G, Oldt R, 3rd, Cohen Y, Wang BG, Sidransky D, Kurman RJ, Shih IeM. Mutations in BRAF and KRAS characterize the development of low-grade ovarian serous carcinoma. J Natl Cancer Inst. 2003;95:484–6. doi: 10.1093/jnci/95.6.484. [DOI] [PubMed] [Google Scholar]
- Davies H, Bignell GR, Cox C, Stephens P, Edkins S, Clegg S, Teague J, Woffendin H, Garnett MJ, Bottomley W, Davis N, Dicks E, et al. Mutations of the BRAF gene in human cancer. Nature. 2002;417:949–54. doi: 10.1038/nature00766. [DOI] [PubMed] [Google Scholar]
- Kim JS, Lee C, Foxworth A, Waldman T. B-Raf is dispensable for KRAS-mediated oncogenesis in human cancer cells. Cancer Res. 2004;64:1932–7. doi: 10.1158/0008-5472.CAN-03-3862. [DOI] [PubMed] [Google Scholar]
- Rajagopalan H, Bardelli A, Lengauer C, Kinzler KW, Vogelstein B, Velculescu VE. Tumorigenesis: RAF/KRAS oncogenes and mismatch-repair status. Nature. 2002;418:934. doi: 10.1038/418934a. [DOI] [PubMed] [Google Scholar]