Abstract
Human ability to switch from one cognitive task to another involves both endogenous preparation without an external stimulus and exogenous adjustment in response to the external stimulus. In an event-related functional MRI study, participants performed pairs of two tasks that are either the same (task repetition) or different (task switch) from each other. On half of the trials, foreknowledge about task repetition or task switch was available. On the other half, it was not. Endogenous preparation seems to involve lateral prefrontal cortex (BA 46/45) and posterior parietal cortex (BA 40). During preparation, higher activation increases in inferior lateral prefrontal cortex and superior posterior parietal cortex were associated with foreknowledge than with no foreknowledge. Exogenous adjustment seems to involve superior prefrontal cortex (BA 8) and posterior parietal cortex (BA 39/40) in general. During a task switch with no foreknowledge, activations in these areas were relatively higher than during a task repetition with no foreknowledge. These results suggest that endogenous preparation and exogenous adjustment for a task switch may be independent processes involving different brain areas.
Goal-directed human cognitive processes can be characterized as the deliberate application of intention to achieve the currently relevant goal. In doing so, cognitive control is critical, especially when cognitive resources are limited, and executive decisions are required regarding when and how to initiate a certain task or to switch from one task to another. Recently, the task-switching paradigm has appealed to cognitive psychologists as a tool to study the role of executive control mechanisms (1–4). In this paradigm, participants rapidly repeat the same task or alternate between different tasks. A consistent finding is that latencies are longer to perform a switched task than to perform a repeated task, and this deficit is called the switch cost.
The switch cost is remarkably robust even in a situation in which a participant can supposedly prepare for a task switch, such as when the identity of the upcoming task is already known and two tasks are apart from each other with a sufficiently long time interval (2, 3). With foreknowledge about the upcoming task, the switch cost was indeed reduced as the time interval between two tasks increased (2, 3). The reduction of the switch cost indicates that participants could prepare themselves for the upcoming task before the stimulus for the task was presented, the process we call the endogenous preparation. The extent or the likelihood of endogenous preparation should increase as a function of time, which facilitates task-switching performance and reduces the switch cost. However, the cost may not be completely eliminated even with a very long interval (1–3), implying that endogenous preparation may not be sufficient but switching to the new task can only be complete with the presentation of the external stimulus. We call this process the exogenous adjustment for a new task, in the sense that the process is completed on the basis of the external stimulus.
We recently have constructed an ACT-R model of both the endogenous preparation and the exogenous adjustment (M.-H.S. and J.R.A., unpublished work). ACT-R (6) is a general theory of cognition in which one can develop computer simulation models for various cognitive tasks. With foreknowledge that the upcoming task will be repeated, the model just maintains its task preparation from the first task, whereas the model engages in an effort to prepare for the upcoming trial with foreknowledge of a switch trial. This preparation involves active retrieval of task relevant knowledge. Therefore, the model predicts that there will be special preparatory activity just in the foreknowledge switch condition before the upcoming task is presented. When there is no foreknowledge of the upcoming task, the model predicts that participants will have to expend less effort when repeating the previous task because the relevant knowledge is primed by the first task. In contrast, in the switch condition, unprimed knowledge has to be retrieved. Therefore, the model predicts greater effort in the switch condition with no foreknowledge during a task switch.
The purpose of the current study is to identify brain regions that may be responsible for the above two mechanisms of task-switching—endogenous preparation and exogenous adjustment. Studies using functional neuroimaging techniques have shown that the superior posterior parietal cortex (BA 39/40) as well as dorsolateral prefrontal cortex (BA 9/46) may be related to cognitive control. Dorsolateral prefrontal cortex activates more when sequences of items have to be held in working memory (7), when dual task is performed rather than single task (8), when the relevant task dimension switches (9), or when the to-be-switched task dimension is cognitively more demanding (10). It also has been hypothesized that subareas of prefrontal cortex seem to be associated with even further specialized functions, such as processing predictable or unpredictable sequences of tasks (11). Whereas dorsolateral prefrontal cortex activation is related to relative difficulty of task demands, superior posterior parietal cortex seems to be associated with allocating attention in general (12), not necessarily reflecting the task difficulty. In a spatial cueing paradigm, superior posterior parietal cortex activates more during cueing period, reflecting that attention is allocated to a cued target location to prepare for an upcoming target that may appear at the location (10). The ACT-R model mentioned earlier assumes that endogenous preparation involves retrieval and maintenance of information on the basis of foreknowledge. Together, superior posterior parietal cortex and dorsolateral prefrontal cortex may be involved in endogenous preparation for a task before an actual stimulus is provided.
The residual switch cost mentioned earlier suggests that, even when a new task can be prepared endogenously, there may be exogenous adjustment after the relevant stimulus actually is presented. Our ACT-R model suggests that the residual switch cost is caused by extra retrieval of the task-relevant knowledge on arrival of an exogenous stimulus. Although the task domain is different, spatial attention studies have shown that inferior posterior parietal cortex (BA 39) may be associated with redirecting attention after an actual stimulus is presented to a certain target location. For example, the ability to detect a target at an unattended spatial location is seriously compromised after damages in inferior posterior parietal cortex (13). Moreover, inferior posterior parietal cortex activates more after a target stimulus is actually presented and even more when the target appears at an unattended location (12). Based on these results, we expect that inferior posterior parietal cortex may be involved in the processes specific to performing a task switch compared with a task repetition.
Hence, the predictions of the ACT-R theory are that there will be special activation in dorsolateral prefrontal cortex and superior posterior parietal cortex during the preparatory period, especially in the switch condition with foreknowledge. Also, there should be greater activation in inferior posterior parietal cortex during a task switch than during a task repetition with no foreknowledge.
Methods
Behavioral Protocol.
To identify the regions associated with endogenous preparation and stimulus-dependent exogenous adjustment, we conducted an event-related functional MRI study. Twelve healthy right-handed participants (three female/nine male, aged 18–36 years) completed the study during a functional MRI scanning session, in which they performed a pair of tasks on every trial. The tasks involved simple classification of letters and digits, and a response was made by pressing hand-held response buttons (see Fig. 1 for details). The tasks within each pair were either the same (task repetition) or different (task switch). On half of the blocks, participants knew that they would perform task repetition or task switch, foreknowledge condition, and did not know on the other half, no-foreknowledge condition (see Table 1).
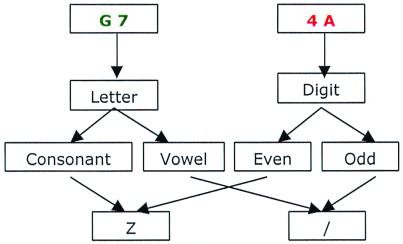
Figure 1.
Task description. For each task, a stimulus consisted of one letter and one digit. The task to be performed was indicated by the color of the stimulus. For example, if the color is green, the task is a letter task, which is to decide whether the presented letter is a consonant or a vowel. If the color is red, the task is a digit task, which is to decide whether the presented digit is even or odd. The response was made by pressing one of two buttons (indicated as Z and /) of a hand-held response glove by using right index and middle fingers. Consonant and even responses were assigned to one button and vowel and odd responses to another. The response to button mapping was balanced across participants.
Table 1.
An illustration of the foreknowledge manipulation in experiments 1 and 2
| Condition | Type of block | Task 1 | Task 2 |
|---|---|---|---|
| Foreknowledge | Repetition block | Letter | Letter |
| condition | Digit | Digit | |
| Switch block | Digit | Letter | |
| Letter | Digit | ||
| No foreknowledge | Mixed block | Letter | Letter |
| condition | Letter | Digit | |
| Digit | Letter | ||
| Digit | Digit |
Functional MRI Procedures.
The experiment was administered in 16 scanning blocks consisting of four foreknowledge-repetition, four foreknowledge-switch, and eight no-foreknowledge blocks. Each block included eight trials. Foreknowledge and no-foreknowledge blocks were alternated, so that a foreknowledge-repetition block and foreknowledge-switch were always separated by a no-foreknowledge block. This design helped prevent confounding effects such as order of presentation or scanner drift. Participants received standard instructions to respond both quickly and accurately. The mel software package was used to present stimuli and to collect behavioral performance (14). Participants provided written informed consent in accordance with the guidelines at the University of Pittsburgh.
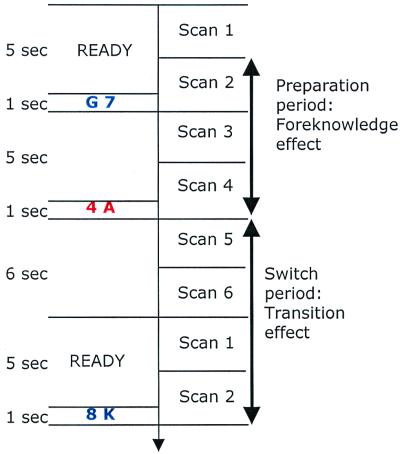
Twenty 3.8-mm oblique-axial slices covering most of the forebrain were acquired every 3 sec. A 1.5-Tesla General Electric scanner with a standard head coil acquired all images. Three T1-weighted scouts (in the axial, coronal, and sagital planes) were used to localize the AC/PC line. T2*-weighted two-shot spiral scans (3.8 mm3 voxels, repetition time = 1,500 ms, echo time = 34 ms, flip = 50°) allowed full image acquisition every 3 sec. Scanning was synchronized with each trial with six 3-sec scans per trial (see Fig. 2).
Figure 2.
Each trial lasted for 18 sec, and the tasks within a trial were separated by 5-sec interstimulus interval. Shown is a sequence of events in a trial. Every trial began with a “ready” signal (5 sec), followed by task 1 stimulus (1 sec), interstimulus interval (5 sec), task 2 stimulus (1 sec), blank screen (6 sec), then the next trial began with a new “ready” signal (see Fig. 1). The 16 blocks (eight trials each), were of three types: foreknowledge-repetition (task 1, not predictable; task 2, same as task 1, i.e., colors of stimuli were repeated between task 1 and task 2); foreknowledge-switch (task 1, not predictable; task 2 different from task 1, i.e., color of task 2 always different from that of task 1 stimulus), and no foreknowledge (task repetition and task switch randomly mixed). The foreknowledge and no-foreknowledge blocks alternated. Before each block began, participants were instructed on the type of the block, so that in the foreknowledge block information about task 2 became available as soon as task 1 stimulus was presented.
Images were realigned by using 12-parameters air (15) and then cross-registered to a common reference brain by minimizing signal intensity differences, after which functional images were set to a standard mean intensity, smoothed (8-mm full width half maximum three-dimensional Gaussian kernel) and pooled across subjects to improve signal-to-noise ratio. Spatial F-maps were generated by using ANOVA models that are described later with subjects as a random factor. Within specified anatomical areas, regions were identified by thresholding spatial F-maps with the requirement of six contiguous pixels, P < = 0.005 to control for type I error (16). Time series of individual activations were displayed as the average change from the anchor (see functional MRI results section for details) of all pixels in the regions identified in the group analysis. These data were pooled for planned-contrasts and evaluated for correlations with behavioral data.
Results
Behavioral Performances.
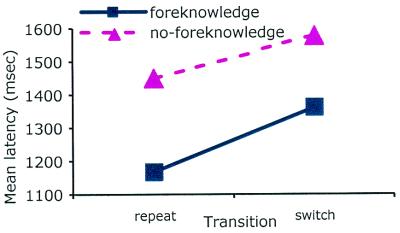
A trial was counted as correct when responses for both task 1 and task 2 were correct. Overall accuracy was 0.95, and no main effects or interaction was significant, P > 0.2. For latency, we analyzed only task 2 reaction times from correct trials. Fig. 3 shows reaction time data. Reaction times were faster with foreknowledge (1,263 ms) than with no foreknowledge (1,513 ms), F(1,11) = 15.89, P < 0.01, mean square error = 47273. Also, reaction times were faster with task repetition (1308 ms) than with task switch (1,468 ms), F(1,11) = 47.68, P < 0.0001, mean square error = 6458. There was no interaction between foreknowledge and task transition, P > 0.1. The current result is consistent with the ACT-R model described earlier with the foreknowledge effect reflecting endogenous preparation and the switch cost reflecting exogenous adjustment.
Figure 3.
Mean task 2 reaction time.
Functional MRI Results.
Endogenous preparation.
In our paradigm, the endogenous preparation should be reflected in the foreknowledge effect, and the exogenous adjustment should be reflected in the transition effect. Regions involved in the endogenous preparation were identified by using foreknowledge × scan interaction with scans 2–4, because this was the preparation period during which foreknowledge could have its effect. We expected that the hemodynamic function caused by preparation would increase from scan 2 and peak at scan 4. The ANOVA model used to select regions of interest (ROIs) of foreknowledge effect was 2 (foreknowledge and no foreknowledge) × 3 (scans 2–4). The interaction was examined in each voxel, and the selected regions met the criteria of minimum six contiguous voxels with significant interaction at P < = 0.005.
Having identified the ROIs, we performed another ANOVA to examine whether the percent increase activation of scans 3 and 4 was above or below the anchor (scan 2) and whether these percent changes are different depending on foreknowledge conditions and task transitions. The ANOVA model was 3 (scan) × 2 (foreknowledge and no foreknowledge) × 2 (repetition and switch). Our expectation was that because task 1 was performed at the end of scan 2 (see Fig. 2), scan 2 formed an anchor against which the subsequent effects of preparing for task 2 could be measured. The blood oxygenation level-dependent response during scans 3 and 4 would reflect endogenous preparatory processes uncontaminated by task 2 that occurred only at the end of scan 4.
Table 2 shows the ROIs identified and average F values of foreknowledge × scan interactions of these regions. The coordinates of the voxel with the highest F value in the ROI were given in terms of Talairach and Tournoux (17) coordinates. During the preparation period (scans 2–4), left superior posterior parietal cortex (BA 40), right lateral prefrontal cortex (BA 46/45), right temporal areas (BA 22) showed greater increase in the foreknowledge condition than with no foreknowledge. Activation in the motor cortex (BA 1), motor/parietal (BA 2), and thalamus increased more with no foreknowledge than with foreknowledge. Activation in the caudate nucleus decreased more with foreknowledge than with no foreknowledge. It appears to be that these regions are localized in one hemisphere, primarily because of the very strict criterion (P < = 0.005) in selecting the ROIs analysis. With lower criterion (P < = 0.05), many regions we mentioned earlier showed bilateral activations.
Table 2.
Foreknowledge effects
| Regions | Size | Maximum F (average F) | Coordinates*
|
||
|---|---|---|---|---|---|
| x | y | z | |||
| Left motor/parietal (BA 2) | 12 | 8.91 (7.82) | 54 | −32 | 49 |
| Left superior parietal (BA 40) | 34 | 28.36 (12.97) | 36 | −51 | 41 |
| Left motor (BA 1) | 18 | 11.50 (9.25) | 40 | −16 | 40 |
| Caudate nucleus | 12 | 11.34 (8.37) | −8 | 3 | 16 |
| Right thalamus | 7 | 11.33 (8.96) | −23 | −4 | 11 |
| Right lateral prefrontal cortex (BA 46/45) | 17 | 9.55 (8.18) | −53 | 27 | 6 |
| Right temporal (BA 22) | 10 | 8.95 (7.66) | −53 | −3 | 4 |
Regions that revealed significant foreknowledge (foreknowledge and no foreknowledge) × scan (scans 2, 3, and 4) interaction with criteria of P <= 0.005, six contiguous voxels.
Coordinates of voxel with the maximum F.
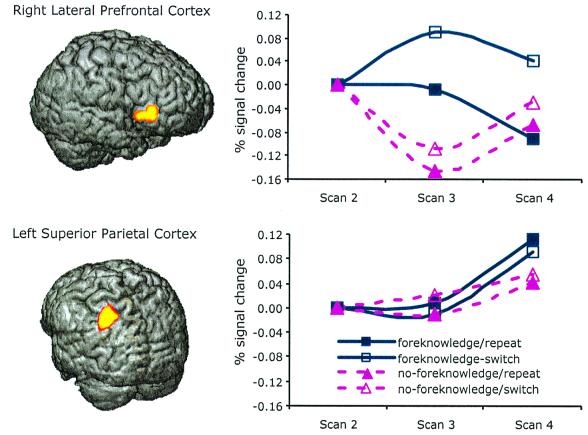
Fig. 4 shows percent activation increases in right lateral prefrontal cortex (BA 46/45) and left superior posterior parietal cortex (BA 40) as a function of scan in each condition. In these two areas, activation increase compared with the anchor was in general greater with foreknowledge than with no foreknowledge, reflecting endogenous preparation. With respect to the right lateral prefrontal cortex, we conducted a specific contrast to test if the rise was greater in the foreknowledge switch condition as hypothesized by the ACT-R model. This contrast compared average of the foreknowledge switch condition during scans 3 and 4 with the average of the foreknowledge repetition condition. This contrast is significant, t(11) = 1.80, P < 0.05, one-tailed test . We also tested whether a greater rise in activation in the foreknowledge switch condition predicted faster reaction time. We used a median-split analysis, in which trials from the same condition were ranked within a block in the order of the average activation of scans 3 and 4 and were classified as either lower half or higher half. Median-split analysis showed that the trials associated with a higher activation in lateral prefrontal cortex during preparation of switch with foreknowledge were faster than the trials associated with relatively low activation in this area, t(11) = 2.48, P < 0.05, one-tailed test. This was not true with foreknowledge-repeat but note that there was virtually no activation change in this condition. This trend was also not significant with respect to the superior posterior parietal cortex region.
Figure 4.
Right lateral prefrontal cortex (BA 46/45) and left superior posterior parietal cortex (BA 40) identified by foreknowledge × scan ANOVA with six contiguous voxels, P < = 0.005, and the time series of activation change against scan 2 of the current trial.
Exogenous adjustment.
Regions involved in the exogenous adjustment were identified by using transition × scan interaction with scans 5 and 6 of the current trial and scans 1 and 2 of the next trial. In our paradigm, scan 4 was the time when the task 2 stimulus was presented, indicating either a task switch or a task repetition. Therefore, we expected that the hemodynamic function caused by a task switch would begin to rise at scan 5 and go back to baseline by scan 2 of the next trial. Because this analysis involves consecutive two trials, the last trial of each block had to be discarded. The ANOVA model used to select ROIs of transition effect was 2 (repeat and switch) × 4 (scans 5, 6, 1, and 2). Selection criterion was the same as that used for the endogenous preparation. We analyzed the transition effect separately with foreknowledge and no foreknowledge, because, with foreknowledge, differences caused by repetition versus switch may have been processed already during the endogenous preparation. In contrast, without foreknowledge, the difference between repetition and switch can be reflected only after the task 2 information is available. For exogenous preparation, scan 2 of the next trial would serve as an anchor because this is the point farthest from performing task 2, so that hemodynamic function caused by performing task 2 will be stabilized as possible. For the identified ROIs, we tested whether the percent changes of scans 5, 6, and 1 were above or below the anchor (scan 2) and whether these changes were different depending on conditions. The ANOVA model was 4 (scans) × 2 (repetition and switch). We expected that the blood oxygenation level-dependent response during scans 5, 6, and 1, especially with no foreknowledge, would reflect exogenous adjustment processes uncontaminated by task 2 preparation.
Table 3 shows the ROIs identified and average F values of transition × scan interactions of these regions. With no foreknowledge, activation was relatively higher with a task switch than with a task repetition in superior prefrontal cortex (BA 8), left posterior parietal cortex (BA 39/40), posterior cingulate cortex (BA 31), and occipital cortex (BA 19). With foreknowledge, activation was higher with a task repetition than with a task switch in the posterior cingulate cortex (BA 31) and right occipital cortex (BA 19).
Table 3.
Transition effects
| Regions | Size | Maximum F (average F) | Coordinates*
|
||
|---|---|---|---|---|---|
| x | y | z | |||
| With foreknowledge | |||||
| Posterior cingulate (BA 31) | 18 | 6.29 (9.27) | −6 | −22 | 37 |
| Right occipital (BA 19) | 11 | 6.17 (7.81) | −18 | −61 | 38 |
| With no foreknowledge | |||||
| Right superior prefrontal cortex (BA 8) | 13 | 5.78 (6.34) | −26 | 23 | 43 |
| Left posterior parietal cortex (BA 39/40) | 43 | 6.00 (8.17) | 37 | −72 | 30 |
| Posterior cingulate (BA 31) | 36 | 6.48 (10.47) | −14 | −52 | 23 |
| Right occipital (BA 19) | 21 | 7.01 (10.58) | −18 | −69 | 16 |
Regions that revealed significant transition (repetition and switch) × scan (scans 5, 6, 1, and 2) interaction with criteria of P <= 0.005, six contiguous voxels.
Coordinates of voxel with the maximum F.
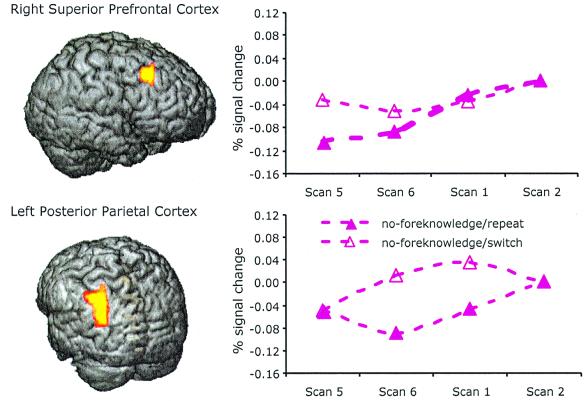
Fig. 5 shows scan by scan activation changes in right superior prefrontal cortex and left posterior parietal cortex as a function of scan and task transition in the no-foreknowledge condition. In these two regions, the percent activation change against the anchor (scan 2) with a task switch was generally higher than the activation change with a task repetition during the exogenous adjustment period. In an analysis not reported here, we examined the activation levels in the scans that preceded scan 5. This analysis showed that in both superior prefrontal and left posterior parietal cortices, activation decreases from scan 2 to scan 4, and this pattern was not different regardless of a task repetition or a task switch. In the case of the right superior prefrontal cortex, there was a marked rise from scan 4 to scan 5. As can be seen in Fig. 5, in the case of the left posterior parietal cortex the functions do not diverge until scan 6. One may note that the activation level at scan 5 is generally lower compared with the anchor (scan 2). We suspect that this uprising activation in the end of a trial may reflect the arousal level for the upcoming trial. We tested whether the relatively high level of activation in the no-foreknowledge switch condition predicted reaction time in this condition. Median-split analysis was done by taking the average of scans 5 and 6. This analysis showed that the trials associated with a higher activation in superior prefrontal cortex during performance of no-foreknowledge switch were slower than the trials associated with relatively low activation in this area, t(11) = 2.44, P < 0.05, one-tailed test. This was not true with no-foreknowledge repeat. Also, this trend was not true in posterior parietal cortex.
Figure 5.
Right superior prefrontal cortex (BA 8) and left posterior parietal cortex (BA 39/40) identified by transition × scan ANOVA with six contiguous voxels in the no-foreknowledge condition, P < = 0.005, and the time series of activation change against scan 2 of the following trial.
Discussion
The ACT-R model described earlier assumes knowledge retrieval as the mechanism for both endogenous preparation and exogenous adjustment. During preparation, the task-relevant knowledge is retrieved on the basis of foreknowledge. Similarly, during exogenous adjustment, the task-relevant knowledge is retrieved on the basis of the external stimulus. During the preparation period, maintenance or rehearsal of the retrieved information is highly important, because the retrieved information cannot be yet applied before the task 2 stimulus is presented. In contrast, during the exogenous adjustment, the retrieved information can be immediately applied to perform the task, lowering the maintenance load. Whereas the maintenance load is lower during exogenous adjustment than preparation, selection load is higher because attention has to be focused on the right aspect of the stimulus so that the appropriate information can be retrieved. Supporting this idea, different areas of prefrontal cortex were identified for preparation (BA 46/45) and exogenous adjustment (BA 8). The inferior part of dorsolateral prefrontal cortex (BA 46/45) has been regarded as responsible for maintaining information in working memory (18). However, it is relatively unknown how the superior prefrontal cortex would be involved with working memory functions such as retrieving task relevant information. One possibility is that premotor areas are involved in selection of one of several (in this case two) alternative stimulus-response mapping rules. This hypothesis is on the basis of the observation that the higher activation in the area was associated with slower reaction times. This suggests that activity in this region reflected a reaction time cost associated with conflict between competing stimulus response mappings.
The close connection between different areas of prefrontal cortex and different processes involved in task switching also has been established by correlation between activation change and behavioral performance. In the case of inferior lateral prefrontal cortex (BA 46/45), higher activation during the preparation period was associated with faster reaction times for task 2 in the foreknowledge switch condition. In contrast, higher activation in superior prefrontal cortex (BA 8) during the switch period was associated with slower performance for task 2. Note that the activation in inferior lateral prefrontal cortex (BA 46/45) during the preparation period is pretask activation, which reflects the level of preparation for the upcoming task, and the activation in superior prefrontal cortex (BA 8) during the switch period is on-task activation, which reflects the current effort put into the performance of the task. The different pattern of behavioral correlation also may suggest the preparation-adjustment distinction. That is, the more prepared a person is for a task as reflected in higher activation in inferior lateral prefrontal cortex (BA 46/45), the faster the performance. However, during performance of switch, participants have to select task-relevant information against task-irrelevant information. The slower reaction times may reflect less efficient selection and therefore more effort in the selection process as reflected in higher activation during the switch period in superior prefrontal cortex (BA 8).
The idea that dorsolateral prefrontal cortex (BA 46) may be involved in active maintenance of information that is newly loaded into working memory is also consistent with the studies using Wisconsin Card Sorting Test (WCST) or Stroop task. When performing WCST, frontal lobe patients show greater difficulty than a normal population in shifting the relevant dimension when the previously relevant dimension stays as distractor dimension (19). Also with WCST, the activity in this area seems to increase with an increase in the number of dimensions shifted (9). Consistent with this, MacDonald et al. (10) showed that, when performing a Stroop task, BA 46 activated more when the to-be-attended dimension was a difficult one (i.e., color naming) than when it was an easy one (i.e., word naming). Perhaps, BA 46 may be involved in actively maintaining the new task-relevant information as well as discarding the previous task configuration, so that performance would not be disturbed by the irrelevant task dimension. One has to be careful, however, to accept our interpretation of results, because the lateral prefrontal cortex areas we identified are not exactly the same as the areas found in other studies for similar functions. For example, the BA 46/45 in the current study is somewhat inferior to the areas normally attributed to maintaining items in working memory (18).
In inferior lateral prefrontal cortex, activation increase with switch foreknowledge was greater than activation increase with repetition foreknowledge. However, in superior posterior parietal cortex, there was no difference between repetition and switch during the preparation period, but only a difference between foreknowledge and no foreknowledge. This difference in superior posterior parietal cortex and lateral prefrontal cortex during preparation may indicate that different kinds of endogenous preparation might be possible. One, involving the lateral prefrontal cortex, is preparation that is specific to the task that would be performed in task 2. Another, involving the superior posterior parietal cortex, is more task-general metastrategy. Note that in the foreknowledge condition, participants could have two kinds of benefit compared with the no-foreknowledge condition. One is advance preparation for task 2 on the basis of foreknowledge, which we argue to be the source of the foreknowledge benefit. The other is that they could deal with only one type of task transition in a particular block with foreknowledge, as opposed to two transitions in a block with no foreknowledge. To prepare for a repetition, participants need to simply maintain the current task. In contrast, to prepare for a task switch, participants need to discard the current one and adopt a new task. It was a well-established fact that people adopt different strategies depending on whether the experimental conditions are blocked or random (5). People can adopt an optimal strategy for each of experimental conditions, when they are blocked. However, when the conditions are randomly mixed in a block, people adopt rather a general strategy that works for all of the conditions even though it is not optimal for any of the conditions. Therefore, with a blocked task transition as in the foreknowledge condition, participants could apply the same strategy across different trials within a block, which could not be possible with no foreknowledge. Because the foreknowledge conditions allows one strategy in one block, a participant may have been more efficient in managing attention allocation so that performance would become more efficient. Perhaps, the superior posterior parietal cortex is involved in this attention management process.
The different patterns identified within posterior parietal cortex in relation to task preparation and task performance is somewhat consistent with findings from spatial attention literature (12). Different regions of the right posterior parietal cortex have been known to be responsible for spatial or visual attention. When a specific spatial location is cued, superior posterior parietal cortex is activated more than when there are no such cues. However, when a target is actually presented to the validly or invalidly cued location, superior posterior parietal cortex does not reveal any differences. Rather, inferior posterior parietal cortex shows different activation depending on whether the spatial attention has to retreat from one location and to be redirected to another location. These results indicate that voluntary goal-directed attentional shift is different from involuntary stimulus-dependent attentional shift. In the current study, left superior posterior parietal cortex was highly activated with foreknowledge, indicating that this area may be associated with endogenous reconfiguration of a task set. However, both superior and inferior posterior parietal cortex areas were activated more with a task switch than with a task repetition with no foreknowledge, indicating that the broader area of posterior parietal cortex may be involved in exogenous adjustment. Although more research needs to be done to establish a close connection, it seems that posterior parietal cortex areas found in our study may be left hemisphere homologues of the voluntary/involuntary spatial attention. That is, superior posterior parietal cortex may be responsible for endogenous goal-directed preparation for a task set, and other areas including inferior posterior parietal cortex may be responsible for stimulus-driven completion of adopting a task set.
In conclusion, the current study showed that endogenous preparation and exogenous adjustment of task switching are indeed separate processes, serving as sources of switch cost, by identifying different brain regions that seem to be responsible for these processes. The results with lateral prefrontal cortex are consistent with the maintenance hypothesis for BA 46 in the current literature. Although it is a tentative hypothesis that our task can be viewed as conceptual homologue of spatial cueing task, the results with posterior parietal cortex are also consistent with endogenous orientation and exogenous reorienting in spatial attention.
Acknowledgments
We thank Julie Fiez for helpful comments on earlier drafts. This work was supported by National Science Foundation Grant SBR-9873465 (to J.R.A.), National Institutes of Health Grant MH59883 (to C.S.C.), and a National Alliance for Research on Schizophrenia and Depression Young Investigator Award (to C.S.C.).
Abbreviation
- ROI
region of interest
Footnotes
This contribution is part of the special series of Inaugural Articles by members of the National Academy of Sciences elected on April 27, 1999.
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.240460497.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.240460497
References
- 1.Allport D A, Styles E A, Hsieh S. In: Attention and Performance XV. Umilta C, Moscovitch M, editors. Cambridge, MA: MIT Press; 1994. pp. 421–452. [Google Scholar]
- 2.Meiran N. J Exp Psychol Learn Mem Cognit. 1996;22:1423–1442. [Google Scholar]
- 3.Rogers R D, Monsell S. J Exp Psychol Gen. 1995;124:207–231. [Google Scholar]
- 4.Sohn, M.-H. & Carlson, R. A. (2000) J. Exp. Psychol. Learn. Mem. Cognit., in press. [DOI] [PubMed]
- 5.Strayer D L, Kramer A F. J Exp Psychol Learn Mem Cognit. 1994;20:318–342. [Google Scholar]
- 6.Anderson J R, Lebiere C. The Atomic Component of Thought. Mahwah, NJ: Erlbaum; 1998. [Google Scholar]
- 7.Cohen J D, Perlstein W M, Braver T S, Nystrom L E. Nature (London) 1997;386:604–608. doi: 10.1038/386604a0. [DOI] [PubMed] [Google Scholar]
- 8.Courtney S M, Petit L, Maisog J M, Ungerleider L G, Haxby J V. Science. 1998;279:1347–1351. doi: 10.1126/science.279.5355.1347. [DOI] [PubMed] [Google Scholar]
- 9.Konish S, Nakajima K, Uchida I, Kaneyama M, Nakahara K, Sekihara K, Miyahsita Y. Nat Neurosci. 1998;1:80–84. doi: 10.1038/283. [DOI] [PubMed] [Google Scholar]
- 10.MacDonald A W, III, Cohen J D, Stenger V A, Carter C S. Science. 2000;288:1835–1838. doi: 10.1126/science.288.5472.1835. [DOI] [PubMed] [Google Scholar]
- 11.Koechlin E, Corrado G, Pietrini P, Grafman J. Proc Natl Acad Sci USA. 2000;97:7651–7656. doi: 10.1073/pnas.130177397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Corbetta M, Kincade J M, Ollinger J M, McAvoy M P, Shulman G L. Nat Neurosci. 2000;3:292–297. doi: 10.1038/73009. [DOI] [PubMed] [Google Scholar]
- 13.Morrow L A, Ratcliff G. Psychobiology. 1988;16:261–269. [Google Scholar]
- 14.Micro Experimental Lab. Psychology Software Tools. PA: Pittsburgh; 2000. [Google Scholar]
- 15.Woods R P, Grafton S T, Holmes C J, Cherry S R, Mazziotta J C. J Comput Assist Tomogr. 1998;22:139–152. doi: 10.1097/00004728-199801000-00027. [DOI] [PubMed] [Google Scholar]
- 16.Forman S D, Cohen J D, Fitzgerald M, Eddy W F, Mintun M A, Noll D C. Magn Reson Imaging Method. 1995;33:636–647. doi: 10.1002/mrm.1910330508. [DOI] [PubMed] [Google Scholar]
- 17.Talairach J, Tournoux P. Coplanar Stereotaxic Atlas of the Human Brain. New York: Thieme; 1988. [Google Scholar]
- 18.Goldman-Rakic P S. Annu Rev Neurosci. 1988;11:137–156. doi: 10.1146/annurev.ne.11.030188.001033. [DOI] [PubMed] [Google Scholar]
- 19.Owen A A, Roberts A C, Hodges J R, Summers B A, Polkey C E, Robbins T W. Brain. 1993;116:1159–1175. doi: 10.1093/brain/116.5.1159. [DOI] [PubMed] [Google Scholar]