Abstract
Background
Several studies have documented an inverse association between adherence to the Mediterranean diet and risk of coronary heart disease (CHD), but little data are available on the relationship between Mediterranean diet and risk of stroke.
Methods and results
74,886 women aged 38-63 in the Nurses' Health Study, a cohort study of women nurses, without a history of cardiovascular disease and diabetes were followed from 1984 to 2004. We computed an alternate Mediterranean diet score (aMed) from self-reported dietary data collected through validated food frequency questionnaires administered 6 times between 1984 and 2002. Relative risks for incident CHD, stroke, and combined fatal cardiovascular disease (CVD) were estimated using Cox proportional hazards models adjusted for cardiovascular risk factors. During 20 years of follow-up, 2391 incident cases of CHD, 1763 incident cases of stroke, and 1077 CVD deaths (fatal CHD and strokes combined) were ascertained. Women in the top aMed quintile were at lower risk for both CHD and stroke compared with the bottom quintile (RR=0.71 (95%CI=0.62-0.82; p trend< 0.0001) for CHD; RR=0.87 (95% CI=0.73-1.02; p trend=0.03) for stroke). CVD mortality was significantly lower among women in the top quintile of the aMed (RR=0.61, 95% CI=0.49-0.76, p trend<0.0001).
Conclusion
A greater adherence to the Mediterranean diet, as reflected by a higher aMed score, was associated with a lower risk of incident CHD and stroke in women.
Keywords: stroke, coronary disease, diet, nutrition
The traditional Mediterranean diet is characterized by a high intake of monounsaturated fat, plant proteins, whole grains, fish, moderate intake of alcohol, and low consumption of red meat, refined grains, and sweets1. An intervention trial has recently shown that the Mediterranean diet is more effective in promoting weight loss and lowering total: HDL cholesterol ratio in obese individuals than a low fat diet2. Previously, the Lyon Heart Study showed that the Mediterranean diet was more effective than a low fat diet in secondary prevention of cardiac events3, 4. Since then, the Mediterranean diet pattern has been shown in several prospective studies from around the world to be inversely associated with total and cardiovascular (CVD) mortality5, 6. However, data are limited for the relationship with non-fatal cardiovascular events. To our knowledge, no studies have specifically focused on incidence of stroke or stroke mortality. In addition, in investigating the association between diet and disease with a slow progression such as CHD and stroke, the availability of multiple dietary measurements over time provides a better estimate of overall diet over the follow-up period.
We have previously constructed a Mediterranean diet adherence score for the Nurses' Health Study7, based on a prior scoring system developed for Greek populations8. This Alternate Mediterranean Diet Score (aMed) focuses on higher consumption of plant foods including plant proteins, monounsaturated fat, fish, and lower consumption of animal products and saturated fat. In this analysis, we used data from multiple dietary assessments to prospectively examine the association between the aMed and risk of incident CHD and stroke, as well as CVD mortality in women. We also combined nonfatal and fatal CHD and stroke incidence to assess the association of aMed with major CVD events.
Methods
Study Population
The Nurses' Health Study (NHS) is a cohort study of 121,700 female nurses aged 30-55 years living in 11 U.S. states at the time of inception (1976). The first questionnaire regarding medical, lifestyle, and other health-related information was sent at the time9. Since then, questionnaires have been sent biennially to update this information. Follow-up was complete for more than 95% of the potential person time up to 2004. In 1980, the participants completed a 61-item food frequency questionnaire (FFQ). In 1984, the FFQ was expanded to 116 items. Similar FFQs were sent in 1986, 1990, 1994, 1998, and 2002.
For this analysis, we included women who completed the 1984 FFQ with fewer than 70 missing items and total energy intake (as calculated from the FFQ) between 500 and 3500 kcal/day10. At baseline, we excluded those with a history of CHD, stroke, or diabetes because diagnoses of these conditions may lead to changes in diet. Thus, 76,522 women with follow-up from 1984 through 2004 were included in the analyses. This study was approved by the Institutional Review Board of the Brigham and Women's Hospital, Boston, MA.
Dietary Assessment
Self-reported FFQs were designed to assess average food intake over the preceding year. A standard portion size and nine possible frequency of consumption responses, ranging from “never, or less than once per month” to “six or more times per day” were given for each food. Total energy and nutrient intake was calculated by summing up energy or nutrients from all foods. Previous validation studies in this cohort revealed good correlations between nutrients assessed by the FFQ and multiple weeks of food records completed over the preceding year10. For example, correlation coefficients between 1986 FFQ and 4 weeks of diet records obtained in 1986 were 0.68 for saturated fat and 0.78 for crude fiber. The mean correlation coefficient between frequencies of intake of 55 foods assessed by two FFQ 12 months apart was 0.5710, 11.
The aMed score was adapted from the Mediterranean diet scale by Trichopoulou et al8. Our components include vegetables (excluding potatoes), fruits, nuts, whole grains, legumes, fish, monounsaturated-to-saturated fat ratio, red and processed meats, and alcohol. Participants with intake above the median intake received 1 point for these categories; otherwise they received 0 points. Red and processed meat consumption below the median received 1 point. We assigned 1 point for alcohol intake between 5-15 g/d. This represents approximately one 12-oz can of regular beer, 5 oz of wine, or 1.5 oz of liquor. The possible score range for aMed was 0–9, with a higher score representing closer resemblance to the Mediterranean diet. Table 1 shows the intake of aMed components during the follow-up periods. Consumption of each food group was stable across time except for a trend toward a decrease in alcohol and red/processed meat intake.
Table 1.
Participant characteristics and median (inter-quartile range) intake* of aMed components for the years which FFQ was administered
| 1984 | 1986 | 1990 | 1994 | 1998 | 2002 | |
|---|---|---|---|---|---|---|
| Mean aMed score | 3.9 | 4.0 | 4.0 | 4.5 | 4.4 | 4.3 |
| Alcohol (g/d) | 2.0 (0-9.5) | 1.8 (0–7.8) | 1.1 (0–6.0) | 1.1 (0–6.1) | 1.0 (0–6.3) | 1.2 (0–7.8) |
| Red/processed meat | 0.9 (0.6–1.3) | 0.8 (0.5–1.1) | 0.6 (0.4–1.0) | 0.6 (0.4–0.9) | 0.6 (0.3–0.9) | 0.5 (0.2–0.9) |
| Fish | 0.2 (0.1–0.4) | 0.3 (0.1–0.4) | 0.3 (0.1–0.5) | 0.2 (0.1–0.4) | 0.2 (0.1–0.3) | 0.2 (0.1–0.3) |
| Whole grains | 0.6 (0.2–1.1) | 1.1 (0.6–1.8) | 1.1 (0.5–1.8) | 1.0 (0.5–1.7) | 1.0 (0.5–1.6) | 1.0 (0.4–1.7) |
| Legumes | 0.3 (0.2–0.6) | 0.3 (0.2–0.6) | 0.3 (0.2–0.6) | 0.3 (0.2–0.6) | 0.3 (0.2–0.5) | 0.3 (0.1–0.5) |
| Nuts | 0.1 (0–0.4) | 0.2 (0.1–0.4) | 0.1 (0–0.4) | 0.1 (0–0.4) | 0.1 (0–0.4) | 0.2 (0.1–0.6) |
| Fruits | 1.9 (1.2–2.8) | 2.3 (1.4–3.3) | 2.1 (1.3–3.1) | 2.2 (1.4–3.1) | 2.2 (1.4–3.2) | 1.9 (1.1–2.9) |
| Vegetables**** | 2.6 (1.8–3.8) | 3.1 (2.1–4.4) | 2.7 (1.8–3.8) | 2.9 (2.0–4.1) | 2.7 (1.8–3.9) | 2.5 (1.5–3.7) |
| Monounsaturated to saturated fat ratio | 1.0 (0.9–1.1) | 1.0 (0.9–1.1) | 1.1 (1.0–1.2) | 1.1 (1.0–1.3) | 1.1 (1.0–1.3) | 1.1 (1.0–1.3) |
servings per day except otherwise stated
assessed as hours of total physical activity only
Median
Potatoes and French fries are not included
Endpoint Ascertainment
For these analyses, we ascertained incident cases of CHD (non-fatal myocardial infarct (MI) or fatal CHD) and stroke that occurred after the return of the 1984 questionnaire but before June 1, 2004. We requested permission to review medical records from women who reported having a nonfatal MI or stroke on each biennial questionnaire. Physicians unaware of the self-reported risk factor status reviewed the records. For each case, the year and month of diagnosis was recorded as the diagnosis date. For MI, we noted whether it was fatal or nonfatal. MI was classified as confirmed if the criteria of the World Health Organization were met, specifically, symptoms and either echocardiogram changes or elevated cardiac enzyme levels12. If medical records were not available, the case was considered probable. We included confirmed and probable cases for the analyses. Fatal CHD events were confirmed by hospital records or by an autopsy or by CHD listed as the cause of death on the death certificate, if it was listed as an underlying and the most plausible cause of death, and if evidence of previous CHD was available.
Strokes were confirmed by medical record review using National Survey of Stroke criteria13, which require a constellation of neurologic deficits, sudden or rapid in onset, and duration of at least 24 hours or until death. We classified strokes as ischemic (embolic or thrombotic) or hemorrhagic (subarachnoid or intracerebral) or undetermined type according to medical record evidence as well as computed tomography, magnetic resonance imaging, or autopsy findings. Deaths were identified from state vital statistics records and the National Death Index or reported by the families and the postal system. Strokes for which medical records were not available were considered probable. In our cohort, approximately 17% of all strokes and 24% of myocardial infarctions were classified as probable. Confirmed and probable cases were combined in our analyses.
Assessment of covariates
Body mass index (BMI) was calculated from weight reported on each biennial questionnaire and height reported in 1976. In each biennial questionnaire, we also assessed smoking status (including number of cigarettes), frequency and number of aspirin tablets used, multivitamin intake, menopausal status and use of postmenopausal hormone. Leisure-time physical activity was measured biennially beginning in 1986 with a validated questionnaire asking about average time spent on 10 common activities. The information was then summed and calculated as Metabolic Equivalent Hours (METs)14.
Statistical Analysis
We used Cox proportional hazard modeling to assess the association between the aMed score and risk of CHD and stroke, including separate models for fatal and nonfatal CHD and ischemic and hemorrhagic stroke, as the etiology of subtypes may differ. We combined all CHD and stroke cases as total major CVDs, and fatal CHD and fatal stroke as fatal major CVDs. For individuals with confirmed diagnoses of stroke and CHD on the same year and month, we included both endpoints in the CHD or stroke analyses. However, these individuals only contributed to one endpoint in the total major CVD analysis.
To reduce random within-person variation and to best represent long-term dietary intake, we calculated cumulative averages of the aMed score from our repeated FFQs15. For example, aMed score in 1984 was used to predict CHD and stroke occurrence from 1984 to 1986, and the average score from 1984 and 1986 was used to predict CHD and stroke risk from 1986 to 1990, and so forth. An overall risk ratio for the entire follow-up period is then computed using Cox Proportional Hazard Model. We adjusted for the following potential confounders, which were updated at each 2-year cycle: age (continuous), smoking (never, past, current with cigarette use of 1-14/day, 15-24/day, 25+/day, missing), BMI (<22, 22.1-23.0, 23.1-24.9, 25.0-29.9, 30+), menopausal status and postmenopausal hormone use (pre-menopausal, never, past, current hormone use), energy intake (quintiles), multivitamin intake (yes/no), alcohol intake (0g/day, up to 5g/day, 5-15g/day, >15g/day), family history (yes/no), physical activity (quintiles), and aspirin use (<1/wk, 1-2/wk, 3-6/wk, 7-14/wk, 15+/wk). Statistical analysis was conducted with SAS version 9.
In secondary analyses, we additionally adjusted for use of cholesterol-lowering and antihypertensive medications, history of hypertension, hypercholesterolemia, and diabetes diagnosed during follow-up. We also stratified by major risk factors at baseline to evaluate potential interactions between these factors and the aMed in relation to CHD and stroke risk. In addition, we assessed the association between changes in aMed score between 1984 and 1990 and risk of CHD and stroke from 1990-2004.
The authors had full access to the data and take responsibility for its integrity. All authors have read and agree to the manuscript as written.
Results
During up to 20 years of follow-up, we ascertained 2391 cases of CHD, of which 1597 were non-fatal and 794 were fatal. We also ascertained 1763 cases of stroke, of which 959 cases were ischemic and 329 cases were hemorrhagic, and 475 cases could not be clearly classified. Of all strokes, 1480 cases were non-fatal and 283 cases were fatal. At baseline, women with higher aMed score tended to exercise more and were less likely to be smokers (table 2). They also consumed more calories and fiber, but less saturated fat.
Table 2.
Age standardized baseline (1984) characteristics according to quintiles of 1984 aMed scores.
| aMed | |||||
|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | Q5 | |
| Participant characteristics | |||||
| BMI | 24.9 | 24.9 | 24.9 | 24.9 | 24.6 |
| Current smokers % | 30 | 26 | 23 | 20 | 16 |
| Leisure time physical activity MET/wk | 11 | 12 | 14 | 16 | 19 |
| History of hypertension % | 7 | 8 | 7 | 8 | 8 |
| History of hypercholesterolemia % | 2 | 3 | 3 | 3 | 4 |
| Family history of CHD | 19 | 19 | 18 | 19 | 20 |
| Dietary intake* | |||||
| Energy (kcal) | 1546 | 1644 | 1738 | 1849 | 1986 |
| Glycemic load | 98 | 99 | 99 | 99 | 102 |
| Carbohydrates (g) | 179 | 183 | 185 | 187 | 194 |
| Protein (g) | 68 | 70 | 72 | 73 | 75 |
| Monounsaturated fat (g) | 24 | 23 | 23 | 22 | 21 |
| Saturated fat (g) | 25 | 23 | 22 | 21 | 19 |
| Trans fat (g) | 4 | 4 | 3 | 3 | 3 |
| Long chain omega-3 fatty acids (g) | 0.13 | 0.17 | 0.21 | 0.24 | 0.30 |
| Dietary fiber (g) | 13 | 15 | 16 | 18 | 20 |
| Components of aMed score** | |||||
| Alcohol (g) | 6.8 | 6.9 | 7.1 | 7.0 | 7.2 |
| Monounsaturated to saturated fat ratio | 0.97 | 1.02 | 1.03 | 1.04 | 1.08 |
| Fish | 0.2 | 0.2 | 0.3 | 0.4 | 0.5 |
| Red/processed meat | 1.0 | 1.0 | 0.9 | 0.9 | 0.8 |
| Whole grains | 0.4 | 0.7 | 0.9 | 1.1 | 1.6 |
| Legumes | 0.2 | 0.3 | 0.4 | 0.5 | 0.6 |
| Fruit | 1.3 | 1.7 | 2.1 | 2.6 | 3.2 |
| Vegetables | 1.8 | 2.4 | 3.0 | 3.6 | 4.4 |
| Nuts | 0.1 | 0.3 | 0.3 | 0.4 | 0.5 |
energy adjusted except for energy.
servings per day unless otherwise stated.
After adjusting for potential confounders, we observed a significant inverse association between aMed and risk of CHD. Women in the top quintile of aMed score had a relative risk of 0.71 (95% CI=0.62-0.82, p trend<0.0001) compared with the bottom quintile (table 3). The association appears somewhat stronger for fatal CHD, with RR=0.58 (95% CI=0.45-0.75, p trend<0.0001) comparing the extreme quintiles.
Table 3.
Relative risks of CHD by quintiles of aMED score.
| Average mean score (range) | Q1 1.8 (0-2.5) |
Q2 3.1 (2.5-3.4) |
Q3 4.0 (3.5-4.4) |
Q4 4.9 (4.5-5.4) |
Q5 6.3 (5.5-9.0) |
P trend |
|---|---|---|---|---|---|---|
| TOTAL CHD | ||||||
| Cases | 528 | 518 | 466 | 474 | 405 | |
| Person years | 271,209 | 285,181 | 276,345 | 274,812 | 293,382 | |
| Age & energy adjusted | 1 | 0.81 (0.72-0.92) | 0.71 (0.63-0.81) | 0.66 (0.58-0.75) | 0.50 (0.43-0.57) | <0.0001 |
| Multivariate adjusted* | 1 | 0.92 (0.82-1.04) | 0.87 (0.77-0.99) | 0.87 (0.76-0.99) | 0.71 (0.62-0.82) | <0.0001 |
| NON-FATAL CHD | ||||||
| Cases | 335 | 333 | 317 | 318 | 294 | |
| Age & energy adjusted | 1 | 0.83 (0.71-0.97) | 0.78 (0.67-0.91) | 0.72 (0.61-0.84) | 0.59 (0.50-0.70) | <0.0001 |
| Multivariate adjusted * | 1 | 0.91 (0.78-1.07) | 0.90 (0.77-1.06) | 0.88 (0.75-1.04) | 0.78 (0.66-0.93) | 0.0008 |
| FATAL CHD | ||||||
| Cases | 193 | 185 | 149 | 156 | 111 | |
| Age & energy adjusted | 1 | 0.78 (0.64-0.96) | 0.60 (0.48-0.75) | 0.57 (0.46-0.71) | 0.35 (0.27-0.45) | <0.0001 |
| Multivariate adjusted * | 1 | 0.94 (0.77-1.15) | 0.81 (0.65-1.00) | 0.85 (0.68-1.07) | 0.58 (0.45-0.75) | <0.0001 |
adjusted for age (continuous), smoking (never, past, current with cigarette use of 1-14/day, 15-24/day, 25+/day, missing), BMI (<22, 22.1-23.0, 23.1-24.9, 25.0-29.9, 30+), menopausal status and postmenopausal hormone use (pre-menopausal, never, past, current hormone use), energy intake (quintiles), multivitamin intake (yes/no), alcohol intake (0g/day, up to 5g/day, 5-15g/day, >15g/day), family history (yes/no), physical activity (quintiles), and aspirin use (<1/wk, 1-2/wk, 3-6/wk, 7-14/wk, 15+/wk).
For stroke, a significant inverse association was also observed when comparing the top to bottom quintiles, with RR=0.87 (95% CI=0.73-1.02, p trend=0.03) (table 4). A similar magnitude of risk reduction was noted for ischemic and hemorrhagic strokes although statistical significance was not reached, likely due to the reduced power in subtype analysis. The association with aMed appeared to be stronger for fatal strokes (RR for extreme quintiles=0.69, 95% CI=0.44-1.07, p trend=0.10) and non-fatal strokes (RR=0.90, 95% CI=0.75-1.08, p trend=0.12). Results remained essentially unchanged after additional adjustment for use of cholesterol-lowering and antihypertensive medications, history of hypercholesterolemia, hypertension, and diabetes that occurred during follow-up for both CHD and stroke.
Table 4.
Relative risks of stroke by quintiles of aMED.
| Q1 | Q2 | Q3 | Q4 | Q5 | P trend | |
|---|---|---|---|---|---|---|
| TOTAL Stroke | ||||||
| Cases | 341 | 380 | 370 | 341 | 331 | |
| Person years | 271,209 | 285,181 | 276,345 | 274,812 | 293,382 | |
| Age & energy adjusted | 1 | 0.91 (0.79-1.06) | 0.88 (0.76-1.03) | 0.75 (0.64-0.88) | 0.65 (0.55-0.77) | <0.0001 |
| Multivariate adjusted * | 1 | 1.00 (0.86-1.16) | 1.03 (0.89-1.20) | 0.92 (0.79-1.08) | 0.87 (0.73-1.02) | 0.03 |
| Ischemic† | ||||||
| Cases | 163 | 210 | 209 | 188 | 189 | |
| Age & energy adjusted | 1 | 1.04 (0.85-1.28) | 1.01 (0.82-1.25) | 0.84 (0.67-1.04) | 0.74 (0.59-0.92) | 0.0004 |
| Multivariate adjusted * | 1 | 1.12 (0.91-1.40) | 1.13 (0.91-1.40) | 0.98 (0.79-1.23) | 0.94 (0.74-1.18) | 0.24 |
| Hemorrhagic† | ||||||
| Cases | 71 | 74 | 71 | 56 | 57 | |
| Age & energy adjusted | 1 | 0.89 (0.64-1.24) | 0.87 (0.62-1.22) | 0.64 (0.45-0.92) | 0.60 (0.41-0.87) | 0.002 |
| Multivariate adjusted * | 1 | 0.99 (0.71-1.37) | 1.01 (0.72-1.42) | 0.77 (0.55-1.15) | 0.79 (0.54-1.16) | 0.17 |
| Non-fatal | ||||||
| Cases | 283 | 317 | 305 | 282 | 293 | |
| Age & energy adjusted | 1 | 0.92 (0.78-1.08) | 0.88 (0.74-1.03) | 0.75 (0.63-0.89) | 0.69 (0.58-0.82) | <0.0001 |
| Multivariate adjusted * | 1 | 1.00 (0.85-1.17) | 1.00 (0.85-1.19) | 0.90 (0.75-1.07) | 0.90 (0.75-1.08) | 0.12 |
| Fatal | ||||||
| Cases | 58 | 63 | 65 | 59 | 38 | |
| Age & energy adjusted | 1 | 0.88 (0.62-1.27) | 0.91 (0.64-1.31) | 0.77 (0.53-1.12) | 0.46 (0.30-0.71) | 0.0001 |
| Multivariate adjusted * | 1 | 1.04 (0.72-1.49) | 1.17 (0.81-1.68) | 1.07 (0.73-1.58) | 0.69 (0.44-1.07) | 0.10 |
adjusted for the same variables as in table 2.
Strokes that could not be clearly classified as ischemic or hemorrhagic were included in the analysis of total stroke but not in ischemic or hemorrhagic strokes.
In stratified analyses, we observed consistent results according to all covariates except for BMI (tables 5, 6). The inverse association between aMed and CHD was stronger among women with BMI<25 (RR=0.57, 95% CI=0.46-0.71, p trend<0.0001) than those with BMI>=25 (RR=0.84, 95% CI=0.69-1.02, p trend=0.08), p interaction=0.005. However, such an interaction was not found for stroke.
Table 5.
Multivariate RR (95% CI) of total CHD by quintiles of aMED stratified by selected cardiovascular risk factors.
| Q1 | Q2 | Q3 | Q4 | Q5 | P trend | P interaction | |
|---|---|---|---|---|---|---|---|
| BMI<25 (case=1054) | 1 | 0.89 (0.73-1.06) | 0.73 (0.60-0.89) | 0.74 (0.61-0.90) | 0.57 (0.46-0.71) | <0.0001 | |
| BMI>=25 (case=1338) | 1 | 0.96 (0.81-1.14) | 0.99 (0.84-1.18) | 0.99 (0.83-1.19) | 0.84 (0.69-1.02) | 0.08 | 0.005 |
| Physical activity>median (case=880) | 1 | 0.97 (0.77-1.23) | 0.86 (0.67-1.09) | 0.92 (0.73-1.16) | 0.77 (0.60-0.98) | 0.006 | |
| Physical activity=<median (case=1511) | 1 | 0.90 (0.78-1.05) | 0.88 (0.76-1.03) | 0.83 (0.70-0.97) | 0.66 (0.55-0.80) | <0.0001 | 0.56 |
| Non smokers (case=1921) | 1 | 0.88 (0.76-1.01) | 0.81 (0.70-0.94) | 0.80 (0.69-0.93) | 0.66 (0.56-0.77) | <0.0001 | |
| Current smokers (case=470) | 1 | 0.99 (0.76-1.28) | 0.97 (0.73-1.29) | 1.05 (0.78-1.42) | 0.81 (0.56-1.17) | 0.37 | 0.16 |
| No history of HTN (case=2011) | 1 | 0.91 (0.80-1.04) | 0.84 (0.73-0.96) | 0.85 (0.74-0.98) | 0.70 (0.60-0.81) | <0.0001 | |
| History of HTN (case=380) | 1 | 0.91 (0.64-1.29) | 0.95 (0.66-1.35) | 0.95 (0.66-1.35) | 0.83 (0.57-1.22) | 0.63 | 0.14 |
| No history of hypercholesterolemia (case=2235) | 1 | 0.95 (0.84-1.08) | 0.87 (0.76-0.99) | 0.87 (0.76-1.00) | 0.70 (0.60-0.81) | <0.0001 | |
| History of hypercholesterolemia (case=156) | 1 | 0.41 (0.21-0.80) | 0.67 (0.37-1.21) | 0.52 (0.28-1.97) | 0.58 (0.31-1.06) | 0.33 | 0.60 |
| Family history of CHD (case=1695) | 1 | 0.92 (0.80-1.07) | 0.89 (0.77-1.04) | 0.86 (0.73-1.00) | 0.71 (0.60-0.84) | <0.0001 | |
| No family history of CHD (case=696) | 1 | 0.98 (0.77-1.24) | 0.84 (0.65-1.07) | 0.94 (0.73-1.20) | 0.76 (0.58-0.99) | 0.03 | 0.89 |
adjusted for the same variables as in table 2, except for the variable of stratification.
Table 6.
Multivariate RR of total stroke by quintiles of aMED stratified by selected cardiovascular risk factors.
| Q1 | Q2 | Q3 | Q4 | Q5 | P trend | P interaction | |
|---|---|---|---|---|---|---|---|
| BMI<25 (case=838) | 1 | 1.06 (0.84-1.33) | 1.18 (0.94-1.48) | 0.97 (0.76-1.23) | 0.99 (0.77-1.26) | 0.48 | |
| BMI>=25 (case=925) | 1 | 0.94 (0.77-1.15) | 0.91 (0.74-1.12) | 0.87 (0.70-1.87) | 0.75 (0.60-0.95) | 0.02 | 0.12 |
| Physical activity>median (case=660) | 1 | 0.96 (0.72-1.27) | 1.14 (0.87-1.49) | 0.82 (0.61-1.08) | 0.80 (0.60-1.03) | 0.06 | |
| Physical activity=<median (case=1103) | 1 | 0.98 (0.82-1.17) | 0.92 (0.76-1.11) | 0.95 (0.78-1.15) | 0.86 (0.70-1.06) | 0.06 | 0.44 |
| Non smokers (case=1503) | 1 | 0.95 (0.80-1.12) | 0.98 (0.83-1.16) | 0.88 (0.74-1.04) | 0.85 (0.71-1.02) | 0.04 | |
| Current smokers (case=260) | 1 | 1.10 (0.78-1.57) | 1.09 (0.74-1.59) | 1.06 (0.70-1.61) | 0.63 (0.36-1.11) | 0.17 | 0.96 |
| No history of HTN (case=1509) | 1 | 0.98 (0.84-1.15) | 1.03 (0.87-1.21) | 0.92 (0.77-1.09) | 0.86 (0.72-1.03) | 0.05 | |
| History of HTN (case=254) | 1 | 1.16 (0.77-1.74) | 0.98 (0.64-1.50) | 0.95 (0.61-1.48) | 0.79 (0.50-1.26) | 0.21 | 0.55 |
| No history of hypercholesterolemia (case=1672) | 1 | 1.00 (0.86-1.16) | 1.03 (0.88-1.20) | 0.94 (0.80-1.11) | 0.86 (0.73-1.02) | 0.04 | |
| History of hypercholesterolemia (case=91) | 1 | 0.99 (0.43-2.24) | 0.73 (0.31-1.70) | 0.66 (0.27-1.62) | 0.71 (0.30-1.68) | 0.31 | 0.63 |
| No family history of CHD (case=1356) | 1 | 1.04 (0.88-1.23) | 1.06 (0.89-1.26) | 0.93 (0.78-1.12) | 0.87 (0.20-1.05) | 0.05 | |
| Family history of CHD (case=407) | 1 | 0.91 (0.66-1.25) | 0.97 (0.70-1.34) | 0.94 (0.67-1.31) | 0.86 (0.61-1.21) | 0.44 | 0.70 |
*adjusted for the same variables as in table 2, except for the variable of stratification.
Women who remained in the highest quintiles (4th or 5th, 294 CHD cases) between 1984 and 1990 had an RR of 0.72 (95% CI=0.61-0.84, p<0.0001) for developing CHD during follow-up from 1990-2004 when compared with those who remained in the lowest quintiles (1st or 2nd, 410 cases). Risk for CHD for women who changed from low score to high (126 cases), or high to low (118 cases) was not significantly different from those who remained low.
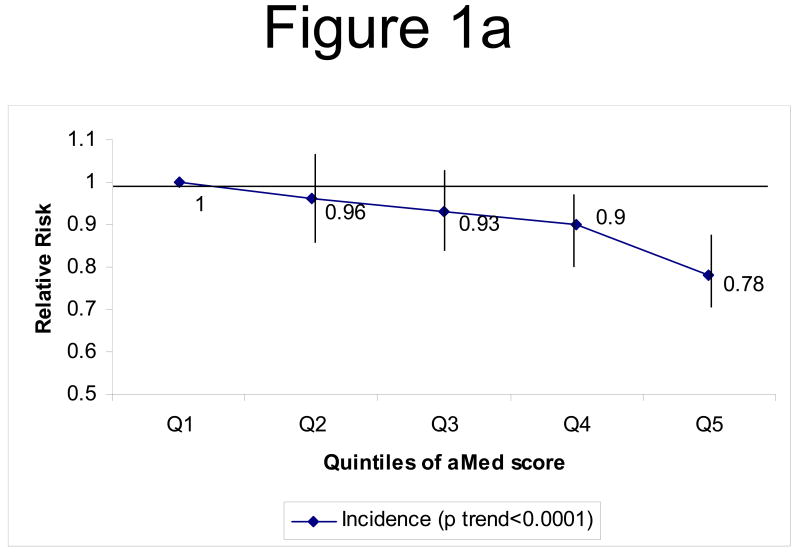
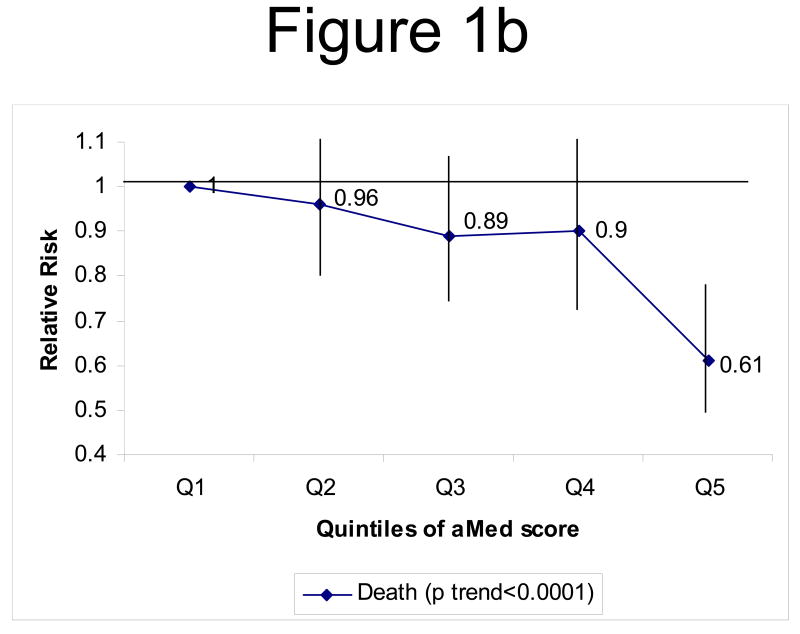
When we evaluated total CVD (combined CHD and stroke incidence), we noted a 22% risk reduction comparing extreme quintiles of aMed score (p trend<0.0001) after multivariate adjustment (figure 1a). Fatal CVD (fatal CHD and fatal stroke combined) risk was also lower among women in the top quintile of the aMed score (RR=0.61, 95% CI=0.49-0.76, p trend<0.0001), compared to those in the lowest quintile (figure 1b).
Figure 1.
Figure 1a: Multivariate* RR of CVD (combined CHD and stroke) by quintiles of aMed. ◆ Incidence of CVD (p trend<0.0001)
Figure 1b: Multivariate* RR of fatal CVD (combined CHD and stroke mortality) by quintiles of aMed. ◆ Incidence of fatal CVD (p trend<0.0001)
*adjusted for the same variables as in table 2
Discussion
In this large cohort of women with 20 years of follow-up, greater adherence to a Mediterranean dietary pattern, as measured by a higher aMed score, was significantly associated with lower risk of incident CHD and stroke. We also observed lower CVD mortality with higher aMed score.
Previous studies generally support an inverse association between adherence to the Mediterranean dietary pattern and risk of CHD. Among middle-aged American, a high aMed score (>=6) was associated with a 22% reduction in cardiovascular mortality in men, and a 19% reduction in mortality in women, when compared to those with a low score (<=3)6. Elderly Europeans with no history of cardiovascular disease who adhered to the Mediterranean diet more closely had a lower risk of CHD mortality8. However, we are unaware of any prior reports that specifically examined the association between Mediterranean diet and stroke incidence.
A Mediterranean dietary pattern shares components with several other healthy eating patterns that have previously been shown to reduce cardiovascular disease risk. The Alternate Healthy Eating Index emphasizing plant foods and unsaturated oils was shown to reduce CVD mortality in both men and women16. In addition, the Dietary Approaches to Stop Hypertension (DASH) diet pattern which also emphasizes high intake of plant foods, low intake of animal protein, and low intake of sweets17 was associated with lower risk of CHD and stroke in this cohort18.
The Mediterranean diet has been linked to beneficial effects on inflammatory markers, lipids, and blood pressure. In cross-sectional analyses, adherence to the Mediterranean diet as measured by various indices was associated with lower levels of C-reactive protein (CRP)19 and Interleukin-6 (IL-6)20, and markers of endothelial functions19, 21. A two year randomized trial with a Mediterranean-style diet was effective in reducing CRP and IL-6 in individuals with metabolic syndrome22. A higher Mediterranean diet score was associated with more favorable levels of adiponectin23, an adipo-cytokine linked to CHD risk24. In a Greek population without hypertension, a higher Mediterranean diet score was associated with lower systolic and diastolic blood pressure25. In another three month randomized trial, traditional Mediterranean diet was more effective in reducing oxidized low-density lipoprotein levels26 and blood pressure, than a low fat diet27. In a 2-year randomized trial, the Mediterranean diet has resulted in significant weight loss, and more favorable total:high density liproprotein cholesterol ratio, and was more effective than a low fat diet2.
The association between Mediterranean dietary pattern appeared even stronger for fatal than nonfatal CHD. Fatal CHD events are generally characterized by either more severe disease or arrhythmia. This may reflect that higher fish intake, an important component of the aMed, has been strongly associated with lower risk of CHD deaths28, 29. A stronger association with fatal CVD events is consistent with the idea that the Mediterranean dietary pattern is not only beneficial for prevention of nonfatal CVD events, but can also improve survival among patients with existing CVD3, 4.
The aMed is constructed based on the literature and our a priori hypotheses. A score always involves some level of arbitrary decision in the type and number of foods to be included, and assignment of points to different levels of intake. Although our score is largely similar to those reported in the literature in the choice of food groups, there are some differences. The score used by Pitsavos et al30 and the one developed by Trichopoulou et al8 awarded points for potato intake, but ours did not. The Trichopoulou score included a dairy component but not the aMed. Also, several dietary factors that have been demonstrated to be important for CHD risk, such as trans fat31, n-6 polyunsaturated fatty acids32, and glycemic load33, 34 were not included in any of the Mediterranean diet scores. However, the omitted dietary components are likely to be associated with foods that are included in the score, and thus the score does account for them to a certain extent. For example, in our cohort, women with high aMed score appeared to have lower trans fat intake.
Another difference between scoring systems is the assignment of points. The score used by Pitsavos classified adherence by assigning 0 to 5 points for each food component30, while the scoring by Trichopoulou8 and aMed used dichotomous point. However, results from different studies generally showed more favorable health outcomes in individuals with a higher Mediterranean diet score, regardless of the scoring criteria. The aMed score assignment depended upon intake relative to the level in the population. Therefore, when the scoring algorithm is applied to different populations, individuals from these populations may have the same score but actual intake of each component could vary substantially.
The major sources of monounsaturated fat differ between the U.S. and other countries, especially Mediterranean countries, as well as by time period. In the first half of the follow-up period, the major sources (over 30%) of monounsaturated fat in our cohort were beef and other meats. In the second half of follow-up, the contribution of beef and other meats dropped to about 18%. At the same time, olive oil consumption increased considerably, but it still contributed to only about 10% of all monounsaturated fat intake. In contrast, in traditional Mediterranean diets of the 1960s, olive oil, along with other plant foods, is the primary source of monounsaturated fat intake1.
The long follow-up period in this study allowed us to assess long-term associations between the aMed score and CVD. The prospective assessment of diet and lifestyle information in this analysis reduces the probability of recall bias. A high rate of follow-up reduced potential selection bias due to systematic loss to follow-up. We used repeated measurements of diet to obtain a better assessment of long-term overall diet and to reduce measurement error, and CVD ascertainment was not influenced by risk factor or dietary intake of the cases. Confounding is always a concern in observational studies, and some level of residual confounding is unavoidable. Given our detailed and updated adjustment for potential confounders, it is unlikely that this would account for the observed results. As this analysis is conducted in women, and that it is the first report on the Mediterranean diet on stroke, our results need to be replicated in other populations, especially men.
In conclusion, a greater adherence to the Mediterranean diet, as reflected by a higher aMed score, was associated with a lower risk of incident CHD and stroke in women.
Acknowledgments
Funding source: NIH grants CA87969, HL60712, HL34594, HL88521
Footnotes
Clinical Perspective: This study examined the association between the Alternate Mediterranean Diet score (aMed) and risk of CHD and stroke in the Nurses' Health Study. A higher score represents higher intake of vegetables (excluding potatoes), fruits, nuts, whole grains, legumes, fish, monounsaturated-to-saturated fat ratio; lower intake of red and processed meats; and alcohol intake between 5-15 g/day. Although there have been a number of studies on various scores that measures adherence to the Mediterranean diet, most of these focused on total or CHD mortality, and none have examined stroke as a separate outcome. This study differs by examining incidence of CHD and stroke, which includes both fatal and non-fatal events. After adjusting for known risk factors for CVD and energy intake, women at the highest 20% of the aMed score had a lower risk for both CHD and stroke. Therefore, greater adherence to the Mediterranean diet, as reflected by a higher aMed score, was associated with a lower risk of incident CHD and stroke in women.
Disclosures: There is no conflict of interest from any of the authors.
References
- 1.Willett WC, Sacks F, Trichopoulou A, Drescher G, Ferro-Luzzi A, Helsing E, Trichopoulos D. Mediterranean diet pyramid: a cultural model for healthy eating. American Journal of Clinical Nutrition. 1995;61:1402s–1406s. doi: 10.1093/ajcn/61.6.1402S. [DOI] [PubMed] [Google Scholar]
- 2.Shai I, Schwarzfuchs D, Henkin Y, Shahar DR, Witkow S, Greenberg I, Golan R, Fraser D, Bolotin A, Vardi H, Tangi-Rozental O, Zuk-Ramot R, Sarusi B, Brickner D, Schwartz Z, Sheiner E, Marko R, Katorza E, Thiery J, Fiedler GM, Bluher M, Stumvoll M, Stampfer MJ Group DIRCTD. Weight Loss with a Low-Carbohydrate, Mediterranean, or Low-Fat Diet. New England Journal of Medicine. 2008;359:229–241. doi: 10.1056/NEJMoa0708681. [DOI] [PubMed] [Google Scholar]
- 3.de Lorgeril M, Renaud S, Mamelle N, Salen P, Martin JL, Monjaud I, Guidollet J, Touboul P, Delaye J. Mediterranean alpha-linolenic acid-rich diet in secondary prevention of coronary heart disease. Lancet. 1994;343:1454–1459. doi: 10.1016/s0140-6736(94)92580-1. [DOI] [PubMed] [Google Scholar]
- 4.De Lorgeril M, Salen P, Martin JL, Monjaud I, Delaye J, Mamelle N. Mediterranean diet, traditional risk factors and the rate of cardiovascular complications after myocardial infarction. Final report of the Lyon Diet Heart Study. Circulation. 1999;99:779–785. doi: 10.1161/01.cir.99.6.779. [DOI] [PubMed] [Google Scholar]
- 5.Knoops KT, de Groot LC, Kromhout D, Perrin AE, Moreiras-Varela O, Menotti A, van Staveren WA. Mediterranean diet, lifestyle factors, and 10-year mortality in elderly European men and women: the HALE project. Journal of the American Medical Association. 2004;292:1433–1439. doi: 10.1001/jama.292.12.1433. [DOI] [PubMed] [Google Scholar]
- 6.Mitrou PN, Kipnis V, Thiebaut Ac, Reedy J, Subar AF, Wirfalt E, Flood A, Mouw T, Hollenbeck AR, Letizmann M, Schatzkin A. Mediterranean diet ary pattern and prediction of all-cause mortality in a U.S. population: results from the NIH-AARP Diet and Health Study. Archives of Internal Medicine. 2007;167:2461–2468. doi: 10.1001/archinte.167.22.2461. [DOI] [PubMed] [Google Scholar]
- 7.Fung TT, McCullough ML, Newby PK, Manson JE, Meigs JB, Rifai N, Willett WC, Hu FB. Diet-quality scores and plasma concentrations of markers of inflammation and endothelial dysfunction. American Journal of Clinical Nutrition. 2005;82:163–173. doi: 10.1093/ajcn.82.1.163. [DOI] [PubMed] [Google Scholar]
- 8.Trichopoulou A, Costacou T, Bamia C, Trichopoulous D. Adherence to a Mediterranean diet and survival in a Greek population. New England Journal of Medicine. 2003;348:2599–2608. doi: 10.1056/NEJMoa025039. [DOI] [PubMed] [Google Scholar]
- 9.Colditz GA, Martin P, Stampfer MJ, Willett WC, Sampson L, Rosner BA, Hennekens CH, Speizer FE. Validation of questionaire information on risk factors and disease outcomes in a prospetive cohort of women. American Journal of Epidemiology. 1986;123:894–900. doi: 10.1093/oxfordjournals.aje.a114319. [DOI] [PubMed] [Google Scholar]
- 10.Willett WC. Nutritional Epidemiology. New York: Oxford Univeristy Press; 1998. [Google Scholar]
- 11.Salvini SD, Hunter DJ, Sampson L, Stampfer MJ, Colditz GA, Rosner BA, Willett WC. Food-based validation of a dietary questionnaire: the effects of week-to-week variation in food consumption. International Journal of Epidemiology. 1989;18:858–867. doi: 10.1093/ije/18.4.858. [DOI] [PubMed] [Google Scholar]
- 12.Rose GA, Blackburn H, Gillum R, Prineas R. Cardiovascular Survey Methods. Geneva, Switzerland: World Health Organization; 1982. [Google Scholar]
- 13.Walker AE, Robins M, Weinfeld FD. The National Survey of Stroke. Clinical findings. Stroke. 1981;12(2 pt 2 suppl 1):I13–44. [PubMed] [Google Scholar]
- 14.Ainsworth BE, Haskell WL, Whitt MC, Irwin ML, Swartz AM, Strath SJ, O'Brien WL, Bassett DRJ, Schmitz KH, Emplaincourt PO, Jacobs DRJ, Leon AS. Compendium of physical activities: an update of activity codes and MET intensities. Medicine and Science in Sports and Exercise. 2000;32(9 Suppl):S498–504. doi: 10.1097/00005768-200009001-00009. [DOI] [PubMed] [Google Scholar]
- 15.Hu FB, Stampfer MJ, Rimm E, Ascherio A, Rosner BA, Spiegelman D, Willett WC. Dietary fat and coronary heart disease: a comparison of approaches for adjusting for total energy intake and modeling repeated dietary measurements. American Journal of Epidemiology. 1999;149:531–540. doi: 10.1093/oxfordjournals.aje.a009849. [DOI] [PubMed] [Google Scholar]
- 16.McCullough ML, Feskanich D, Stampfer MJ, Giovannucci EL, Rimm EB, Hu FB, Spiegelman D, Colditz GA, Hunter DJ, Willett WC. Diet quality and major chronic disease risk in men and women: moving toward improved dietary guidance. American Journal of Clinical Nutrition. 2002;(76):1261–1271. doi: 10.1093/ajcn/76.6.1261. [DOI] [PubMed] [Google Scholar]
- 17.Appel LJ, Moore TJ, Obarzanek E, Vollmer WM, Svetkey LP, Sacks FM, Bray GA, Vogt TM, Culter JA, Windhauser MM, Lin PH, Karanja N. A clinical trial of the effects of dietary patterns on blood pressure. DASH Collaborative Research Group. New England Journal of Medicine. 1997;336:1117–1124. doi: 10.1056/NEJM199704173361601. [DOI] [PubMed] [Google Scholar]
- 18.Fung TT, Chiuve SE, McCullough ML, Rexrode KM, Logroscino G, Hu FB. Adherence to a DASH-style diet and risk of coronary heart disease and stroke in women. Archives of Internal Medicine. 2008;168:713–720. doi: 10.1001/archinte.168.7.713. [DOI] [PubMed] [Google Scholar]
- 19.Fung TT, McCullough ML, Newby PK, Manson JE, Meigs JB, Rifai N, Willett WC, Hu FB. Diet-quality scores and plasma concentrations of markers of inflammation and endothelial dysfunction. American Journal of Clinical Nutrition. 2005;82:163–173. doi: 10.1093/ajcn.82.1.163. [DOI] [PubMed] [Google Scholar]
- 20.Dai J, Miller AH, Bremner JD, Goldberg J, Jones L, Shallenberger L, Buckham R, Murrah NV, Veledar E, Wilson PW, Vaccarino V. Adherence to the Mediterranean diet is inversely associated with circulating interleukin-6 among middle-aged men. A twin study. Circulation. 2007 Dec 17; doi: 10.1161/CIRCULATIONAHA.107.710699. Epub. epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Serrano-Martinez M, Palacios M, Martinez-Losa E, Lezaun R, Maravi C, Prado M, Martinez JA, Martinez-Gonzalez MA. A Mediterranean dietary style influences TNF-alpha and VCAM-1 coronary blood levels in unstable angina patients. European Journal of Nutrition. 2005;44:348–354. doi: 10.1007/s00394-004-0532-9. [DOI] [PubMed] [Google Scholar]
- 22.Esposito K, Marfella R, Ciotola M, Di Palo C, Giugliano F, Giugliano G, D'Armiento M, D'Andrea F, Guigliano D. Effect of a mediterranean-style diet on endothelial dysfunction and markers of vascular inflammation in the metabolic syndrome: a randomized trial. Journal of the American Medical Association. 2004;292:1440–1446. doi: 10.1001/jama.292.12.1440. [DOI] [PubMed] [Google Scholar]
- 23.Mantzoros CS, Williams CJ, Manson JE, Meigs JB, Hu FB. Adherence to the Mediterranean dietary pattern is positively associated with plasma adiponectin concentrations in diabetic women. American Journal of Clinical Nutrition. 2006;84:328–335. doi: 10.1093/ajcn/84.1.328. [DOI] [PubMed] [Google Scholar]
- 24.Pischon T, Girman CJ, Hotamisligil GS, Rifai N, Hu FB, Rimm E. Plasma adiponectin levels and risk of myocardial infarction in men. Journal of the American Medical Association. 2004;291:1730–1737. doi: 10.1001/jama.291.14.1730. [DOI] [PubMed] [Google Scholar]
- 25.Psaltopoulou T, Naska A, Orfanos P, Trichopoulos D, Mountokalakis T, Trichopoulou A. Olive oil, the Mediterranean diet, and arterial blood pressure: the Greek European Prospective Investigation into Cancer and Nutrition (EPIC) study. American Journal of Clinical Nutrition. 2004;80:1012–1018. doi: 10.1093/ajcn/80.4.1012. [DOI] [PubMed] [Google Scholar]
- 26.Fito M, Guzens M, Corella D, Saez G, Estruch R, de la Torre R, Frances F, Cabezas C, Lopez-Sabater Mdel C, Marrugat J, Garcia-Arellano A, Aros F, Ruiz-Gutierrez V, Ros E, Salas-Salvado J, Fiol M, Sola R, Covas MI Investigators tPS. Effect of a traditional Mediterranean diet on lipoprotein oxidation: a randomized controlled trial. Archives of Internal Medicine. 2007;167:1195–1203. doi: 10.1001/archinte.167.11.1195. [DOI] [PubMed] [Google Scholar]
- 27.Estruch R, Martinez-Gonzalez MA, Corella D, Salas-Salvado J, Ruiz-Gutierrez V, Covas MI, Fiol M, Gomez-Gracia E, Lopez-Sabater MC, Vinyoles E, Aros F, Conde M, Lahoz C, Lapetra J, Saez G, Ros E, Investigators tPS Effects of a Mediterranean diet on cardiovascular risk factors: a randomized trial. Annals of Internal Medicine. 2006;145:1–11. doi: 10.7326/0003-4819-145-1-200607040-00004. [DOI] [PubMed] [Google Scholar]
- 28.He K, Song Y, Daviglus ML, Liu K, van Horn L, Dyer AR, Greenland P. Accumulated evidence on fish consumption and coronary heart disease mortality: a meta-analysis of cohort studies. Circulation. 2004;109:2705–2711. doi: 10.1161/01.CIR.0000132503.19410.6B. [DOI] [PubMed] [Google Scholar]
- 29.Mozaffarian D, Rimm E. Fish intake, contaminants, and human health: evaluating the risks and the benefits. Journal of the American Medical Association. 2006;296:1885–1899. doi: 10.1001/jama.296.15.1885. [DOI] [PubMed] [Google Scholar]
- 30.Pitsavos C, Panagiotakos DB, Tzima N, Chrysohoou c, Economou M, Zampelas A, Stefanadis C. Adherence to the Mediterranean diet is associated with total antioxidant capacity in healthy adults: the ATTICA study. American Journal of Clinical Nutrition. 2005;82:694–699. doi: 10.1093/ajcn.82.3.694. [DOI] [PubMed] [Google Scholar]
- 31.Oh K, Hu FB, Manson JE, Stampfer MJ, Willett WC. Dietary fat intake and risk of coronary heart disease in women: 20 years of follow-up of the nurses' health study. American Journal of Epidemiology. 2005;161:672–679. doi: 10.1093/aje/kwi085. [DOI] [PubMed] [Google Scholar]
- 32.Kuller LH. Nutrition, lipids, and cardiovascular disease. Nutrition Review. 2006;64:S15–26. doi: 10.1111/j.1753-4887.2006.tb00230.x. [DOI] [PubMed] [Google Scholar]
- 33.Levitan EB, Mittleman MA, Hakansson N, Wolk A. Dietary glycemic index, dietary glycemic load, and cardiovascular disease in middle-aged and older Swedish men. American Journal of Epidemiology. 2007;85:1521–1526. doi: 10.1093/ajcn/85.6.1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu S, Willett WC. Dietary glycemic load and atherothrombotic risk. Currently Atherosclerosis Report. 2002;4:454–461. doi: 10.1007/s11883-002-0050-2. [DOI] [PubMed] [Google Scholar]