Abstract
We describe experiments on behaving rats with electrodes implanted on the cornea, in the optic chiasm, and on the visual cortex; in addition, two red light-emitting diodes (LED) are permanently attached to the skull over the left eye. Recordings timelocked to the LED flashes reveal both the local events at each electrode site and the orderly transfer of visual information from retina to cortex. The major finding is that every stimulus, regardless of its luminance, duration, or the state of retinal light adaptation, elicits an optic nerve volley with a latency of about 10 ms and a duration of about 300 ms. This phenomenon has not been reported previously, so far as we are aware. We conclude that the retina, which originates from the forebrain of the developing embryo, behaves like a typical brain structure: it translates, within a few hundred milliseconds, the chemical information in each pattern of bleached photoreceptors into a corresponding pattern of ganglion cell neuronal information that leaves via the optic nerve. The attributes of each rat ganglion cell appear to include whether the retinal neuropile calls on it to leave after a stimulus and, if so when, within a 300-ms poststimulus epoch. The resulting retinal analysis of the scene, on arrival at the cortical level, is presumed to participate importantly in the creation of visual perceptual experiences.
Keywords: retinal ganglion cells, optic chiasm, visual perception
Visual stimuli normally activate the entire retina, as when one drives an automobile or reads text like this. In such situations, saccadic eye movements jerk the eyeballs from one place to the next, coming to rest several times a second with the scene in focus on the retinal surface. To gain further insights into how information is transferred from retina to brain in such situations, we have been studying behaving rats with light-emitting diodes (LED) attached to the skull and electrodes implanted at the anatomical beginning, middle, and end of the visual system. Flashes of light from the LED, which appear to activate the entire retina (1), are varied in duration to simulate natural exposures such as a lightning flash, or the fixation at the end of a saccade, or an attempt to stare at an object without moving the eyes. We present here four simple experiments in which changes in stimulus parameters are correlated with the responses produced throughout the system.
Readers will find the data presented here to be only distantly related to the mainstream of current electrophysiological research on animal visual systems. We are not interested in the activities of individual cells in the retinal, thalamic, or cortical neuropiles. We describe instead the activities recordable by large electrodes at three major stations of the system. We emphasize here the retinal neuronal output. We report that ganglion cell axons invariably leave the eyeball in a rigidly predetermined order during about 300 ms, even when the stimuli are delivered at a rate of three Hz. We interpret the data to mean that retinas normally create and deliver to the central nervous system several elaborately processed neuronal versions of the scene every second.
Methods and Analysis
What follows summarizes a description of the methods already published (1) and adds a few new details.
Electrodes.
Fig. 1A diagrams a typical mammalian visual system and points to the three sites at which electrodes were implanted in Wistar rats under halothane anesthesia. The electroretinogram (ERG) electrode was a cluster of seven fine stainless steel wires contacting the corneal surface immediately beneath the upper eyelid; no pathology was found in histological sections of two such corneas implanted several weeks earlier. The optic chiasm electrode was one of a pair of insulated tungsten wires, electrolytically sharpened, with 10- to 15-μm tips spaced about 1 mm apart; they were aimed stereotaxically at (bregma) caudal −1.1 and lateral 0.6 (2). Cortical potentials (VEP, or evoked visual cortex potentials) were recorded through an extradural stainless steel screw turned into the skull. The indifferent electrode in all recordings was a 4 × 8-mm flat piece of stainless steel insulated on one side and slipped under the skin over the left masseter muscle. Electrode and stimulus wires were soldered to separate plugs fixed to the skull with dental cement.
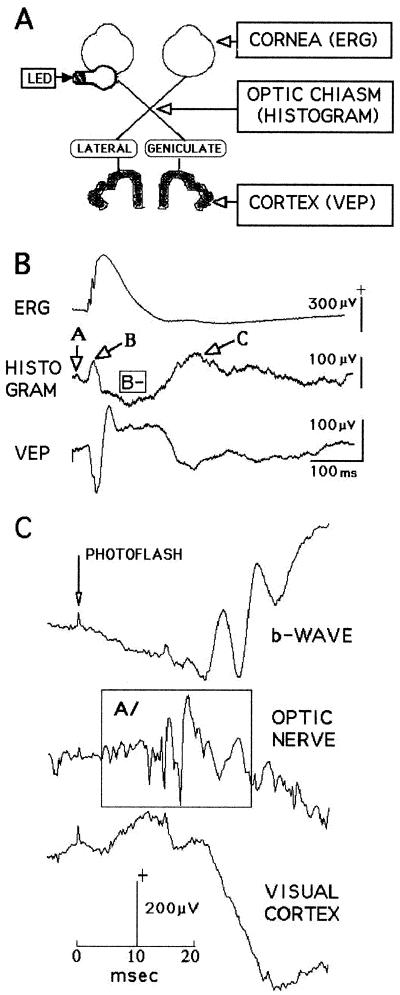
Figure 1.
Background information essential for understanding the experiments. (A) Diagram of the rat visual system showing where the recording electrodes were implanted. (B) Typical electrophysiological events recorded at the electrode sites after flashes delivered to the eye. At the chiasm electrode, three positive deflections, A/B/C/, and a major negative deflection, B−, appear (rat J14). (C) The earliest chiasm event, A/, has a latency of about 10 ms and precedes the appearance of the retinal b-wave (rat NO). Further details in the text.
Stimuli.
Visual stimuli were generated by a photoflash or by light emitting diodes (LED) permanently fixed to the skull over the left eye. Two red LEDs were usually implanted. The StimulusLED was a 5 × 8-mm cylinder illuminated by current pulses adjusted for duration and amplitude by a square wave generator. The AdaptLED was a low emission LED 3 × 4 mm long illuminated continuously at one of six different levels to produce stable light adapted states in complete ambient darkness. The photoflash stimulator was a conventional camera photoflash device having an output of approximately 17,000 lux.
Calibrations and Controls.
Both LEDs were calibrated by using a Lunasix 3 luxmeter in the dark experimental room where the meter read 0.17 lux (its lowest mark). Nine StimulusLED luminance levels between 8 and 5,500 lux could be selected by adjusting nine series resistors in the StimulusLED circuit. The AdaptLED output was similarly adjusted by six series resistors. StimulusLED durations between 10 μs and 5 s were controlled by a pulse generator.
Limitations of the Calibrations.
The current-to-lux functions of the LEDs in a given lot from the same manufacturer were found to differ by as much as 20%. Furthermore, the implanted LEDs radiate photons in all directions (the rat's entire head glows red), which means unquantifiable numbers reach unknowable retinal regions by unknown paths. The LED flash illuminated both retinas about equally in control experiments, and through skull and sclera rather than pupil (1). Responses change for several days after implantation, then stabilize. In a “good” rat, approximately the same number of photons must reach the retinas day after day because the corneal, chiasm, and cortical responses can show little change when tested almost daily for weeks.
Response Acquisition, Storage, and Analysis.
The data and conclusions published here are based on experiments involving more than 100 animals since 1994. Rats were tested during implantation and thereafter for up to 4 mo. During testing, the rat was in a box with glass walls (25 × 25 × 40 cms tall) inside a large, light-tight and sound-deadened Faraday cage, usually between 8 a.m. and 6 p.m., when they tend to sleep. Sleep state was estimated by on-line examination of the electroencephalogram, often confirmed by later examination of the printed record. Except as noted, the LED was manually triggered only when the rat was in slow wave sleep (SWS) according to Gottesmann's criteria (3). Averages consist of 25 or more (often 100) individual responses acquired at intervals of at least 3 s. Data collection during SWS immediately stopped when the rat wakened or moved into rapid eye movement (REM) sleep, and resumed when the SWS state returned; such pauses could last many minutes.
Conventional amplifiers (bandpass 0.1–10 KHz) delivered signals to the A/D converters of a Cambridge Electronic Devices signal analysis system (Cambridge, U.K.; either CED 1401 or CED 1401micro) and were averaged by using either its SIGAVG 6.0 program or SIGNAL 1.82. Samples 400–1,400 ms in duration were converted at 2.2, 3.0, or 4.0 kHz, depending on the number of recording channels in use. Responses included at least 10 pre-trigger points and were usually averaged and stored as ASCII text files, but single traces were often stored for later statistical manipulations. Calculations used original scripts written in MATLAB for the WINDOWS 5.1 software environment (MathWorks, Natick, MA), and statistical calculations used the STATISTICA 5.1 (StatSoft, Tulsa, OK) program package.
Results
Orientation.
Fig. 1A diagrams a typical mammalian visual system and shows the three regions where electrodes were implanted in our rats. The corneal electrode records the ERG. Three processes take place in the retina as the ERG is being recorded: (i) light bleaches the photoreceptors, (ii) the optic nerve activity is created, and (iii) the retina returns to its resting state ready for the next stimulus. The optic chiasm electrode monitors the optic nerve activity as it moves toward the lateral geniculate nuclei (LGN) where synapses will relay it to the cortex. Finally, the cortical electrode indexes the arrival of the visual information and its subsequent distribution throughout the nearby cortical structures. The light bulb cartoon represents the LEDs attached with dental cement to the skull above the left eye.
Fig. 1B illustrates typical averaged responses recorded simultaneously at the three electrode sites from a rat in SWS. The stimuli were 1-ms LED flashes (n = 50), and the recordings are all monopolar, positive up, against the common indifferent plate electrode. The ERG in the top line shows the retinal b-wave with its three small deflections (the oscillating potentials). The middle line displays the averaged optic nerve traffic that has left the retina; it is a time-varying voltage representing the ion currents leaving and entering about 100,000 axons in the process of moving the information extracted from the bleached photoreceptors toward the cortex. Its pattern varies with electrode location, but its three regions, here labeled A/B/C/, are often identified. We also use the term histogram¶ for this A/B/C/ pattern. Finally, the VEP usually approximates, as in this case, a phase-inverted version of the histogram.
Fig. 1C enlarges the first 40 ms of three responses like those shown in Fig. 1B. This rat was awake, the stimulus was a single photoflash, and the nerve electrode was located behind the eyeball in the retrobulbar space. The earliest optic nerve response (A/, enclosed in the box) is a short burst with a latency of about 10 ms; in a rabbit study the first axons to leave the retina did so within 4.5 ms (4). At the moment A/ begins, the corneal response is in transition between the classical ERG a-wave (negative-going) and the cornea-positive b-wave.
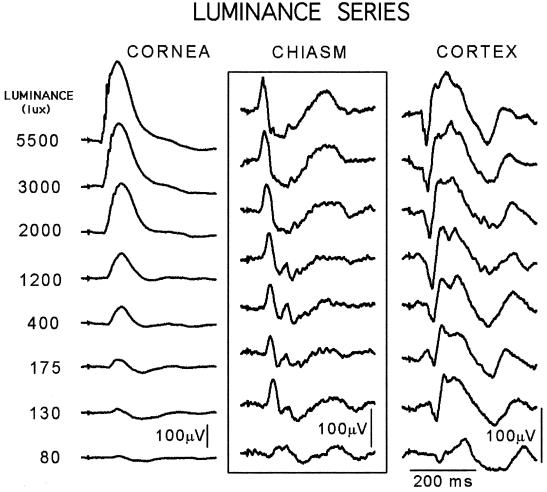
Stimulus Luminance.
Fig. 2 shows responses to 1-ms LED flashes presented at luminances that increase stepwise almost 3 log units, from near-threshold at the bottom of the column to 5,500 lux at the top. These flashes evoked the ERG (corneal) traces shown in the left column, where the b-wave amplitude is seen to increase regularly from a 20-μV almost-purely negative event near threshold to a 350-μV positive deflection after 5,500 lux flashes. Robson and Frishman recorded similar large and systematic luminance-dependent changes in b-wave amplitude and polarity from the cat retina (5).
Figure 2.
Activity evoked at the three levels of a dark-adapted visual system by 1-ms LED flashes (n = 50) graded in luminance. At the retinal level (Left), the near-threshold flashes (bottom trace) evoked a barely visible b-wave response that increased systematically as the luminance rose through about three log units. The other output of the retina, the optic nerve activity recorded at the chiasm level (Center), behaves in an entirely different manner: its near-threshold triphasic waveshape is apparent in every suprathreshold response. The cortical activity (Right) closely resembles a mirror image of the corresponding chiasm response, as already noted in Fig. 1 (Rat J23).
By contrast, all of the A/B/C/ histograms (Fig. 2 Center) resemble each other; even the near-threshold response retains the approximately triphasic waveshape and 300- to 400-ms duration that characterizes them all. Although similar in amplitude, waveshape, and duration, these histograms are not identical; the measurable differences, which presumably carry the luminance information, include small amplitude and latency changes near threshold and a series of approximately 15-Hz wavelets in the B-minus response region (defined in Fig. 1B). The ganglion cell output to every stimulus we have ever presented is a similar ca. 300-ms arrangement of tens of thousands of axon discharges. Note that the two retinal outputs, b-wave and histogram, do not covary in latency, duration, or amplitude, which indicates that their retinal antecedents differ. This difference and other b-wave phenomena are subjects deserving study.
The cortical responses (Fig. 2 Right) do not resemble the corresponding ERGs, but, if each one were to be inverted, it would resemble, more or less, the pattern of the corresponding histogram. Meeren et al. recently described just such an approximate mirror-image relationship between rat LGN and cortical responses (6). We will present our data on this relationship in another place, along with a discussion of the fact that our inverted cortical responses resemble the retinal ganglion cell outputs.
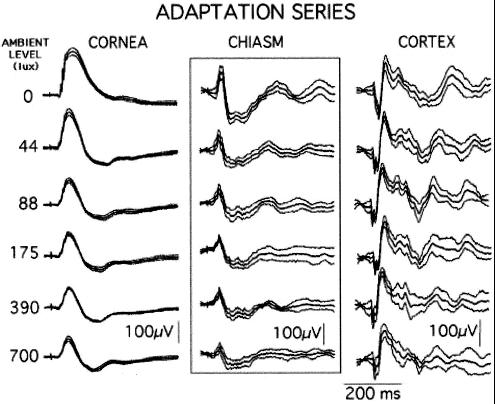
Light Adaptation.
If a rat has two LEDs attached to its head, one of them can be continuously illuminated to light-adapt the retinas while the other is flashed intermittently to produce transient responses. Fig. 3 shows five such light-adapted responses along with the dark-adapted one. In the Fig. 3 Left, the ERG amplitudes decline steeply as the ambient level rises, and their durations progressively narrow; these events mark systematic changes in the b-wave generating mechanism. By contrast, and as in Fig. 2, the histograms in Fig. 3 Center are all similar triphasic events in which small stimulus-dependent changes can be identified. Finally, if the cortical responses were inverted electronically, they would resemble the corresponding histogram waveshapes rather than the corneal ones, as is usually the case.
Figure 3.
Responses at the six levels to LED flashes (1 ms, n = 25) delivered to the dark- and light-adapted retina. As the ambient luminance rises, the b-wave declines in amplitude and changes its waveshape; the chiasm and corresponding cortical waveshapes decline somewhat in amplitude but retain the typical triphasic waveshape throughout. Each trace here and in Fig. 4 shows the mean ± SD (Rat J30).
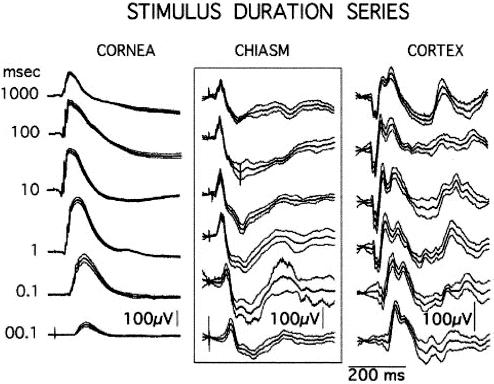
Stimulus Duration.
Fig. 4 shows the responses made by a dark-adapted rat when stimulated by LED flashes that varied in duration over five log units (10 μs to 1,000 ms). These major changes in stimulus parameter are accompanied by relatively minor changes in the chiasm and cortical responses. Once again, the rat displays our three main findings: first, all stimuli produce similar histograms; second, the other retinal output, the b-wave, is highly variable; and third, each cortical response resembles a mirror image of the corresponding chiasm response.
Figure 4.
Cornea, chiasm, and cortical responses (n = 25) to LED flashes varied in duration over five log units, 10 μs to 1,000 ms. The b-wave undergoes large changes whereas the chiasm and cortical responses vary relatively little in either waveshape or amplitude (Rat J30).
Stimulus Repetition Rate.
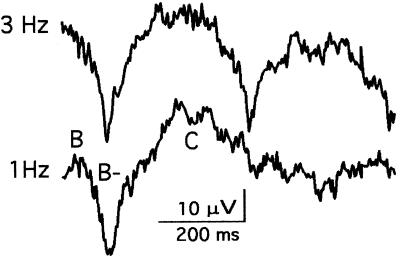
Fig. 5 shows chiasm responses to LED flashes continuously delivered at rates of 3 and 1 Hz. It demonstrates that the rat retina can create three separate, normal histograms every second, which is of interest because three Hz is also the rate at which human reading saccades move the eye from one fixation to the next.
Figure 5.
Chiasm responses to 1-ms LED flashes continuously delivered at rates of 1 and 3 Hz. Each trace represents the average of about 50 samples, 750 ms in duration timelocked to a flash. At the repetition rate of 1 Hz, the histogram has a duration of about 350 ms and displays typical B/, B−, and C/ deflections. The 3-Hz responses resemble the 1-Hz versions, which shows that this retina continuously created separate, complete histograms of three photoreceptor bleach patterns every second (Rat J37).
Discussion
The A/B/C/ Histogram.
We are unaware of any other claim that the major product of a mammalian retina is a timelocked series of ganglion cell axons lasting a few hundred milliseconds; however, Gaarder reached a similar conclusion in a theoretical treatise (7). We list here our evidence supporting the claim. First, a histogram like those in Figs. 1–5 has been produced by every adequate stimulus ever delivered to our rats, asleep or awake, and regardless of its luminance, duration, repetition rate (to 3 Hz), or the ambient illumination at the time. More than 2,000 individual responses make up the averages shown in Figs. 1–5, and tens of thousands of them have been included in the hundreds of similar averaged histograms recorded from more than 30 rats in our optic chiasm electrode series.
Our data and conclusions exist only because problems of stimulus control effectively disappear when the rat carries its own LED stimulators. The AdaptLED attached to the skull unerringly bleaches both retinas to fixed levels in an otherwise light-free environment, and a button press delivers a StimulusLED flash of known duration and relative luminance at any time of the day or night. However, it has been suggested that rat histograms are somehow artifacts of the method. This possibility appears to be ruled out by the fact that the ERG and VEP responses simultaneously recorded with every histogram resemble those recorded after stimuli delivered through the pupil. Thus, our rat b-waves look like cat b-waves evoked by stimuli delivered through the pupil, as already noted (5), and they resemble human ones as well. Similarly, the VEPs look like the ones Meeren et al. collected from rats after stimuli delivered through the pupil (6). When both ERG and VEP are normal in our rats, it is unlikely that the optic chiasm activity recorded at the same time is not.
Third, we have been told that it is “impossible” for the retina to organize hundreds of thousands of ganglion cells (over a million in man) into the histograms we describe. However, it should not come as a surprise that a structure that arises embryologically from the forebrain primordium can precisely organize its output, brain-like, into the compact neuronal event we invariably record.
Our evidence clearly supports the conclusion that activated ganglion cell axons leave the eyeball in a specific order determined in the neuropile. Each individual histogram is the neuronal version of the chemical information in a particular photoreceptor bleach pattern; it is delivered via the LGN to the cortex where further processing takes place. Evidently, rat visual perceptual experiences are the joint product of a retinal analysis and a cortical analysis, and, unless the second follows the first, there will be no perception.
However, the retina is a complex structure, and many laboratory experiments show that our obligatory A/B/C/ histogram is not its only neuronal product. An interesting empirical question is whether a retina that produces histograms to full-field stimuli is producing some other kind of ganglion cell activity at the same time. Another answerable empirical question is whether other mammalian retinas behave like rat retinas. Extrapolating from the existing information, we propose that the following series of events is initiated whenever an eye such as ours comes to rest after a saccade. The retinal surface is exposed to light energy for the duration of the fixation (about 200 ms). From the moment of fixation onward, the neuropile performs its three tasks. First, it converts every detail the bleach pattern contains—its onset, contrasts, changes because of movement, and colors—into exact neuronal equivalents. Next, it outputs this analysis sequentially into the optic nerve in the form of the A/B/C/ histogram. Third, and probably concurrently with whatever else is going on, it restores the photoreceptor surface to the biological equivalent of unexposed photographic film ready for the next fixation to bleach. Every ca. 300-ms histogram created in this way is a unique, quantitative statement of where the retinal surface was bleached, when, for how long, and by what photon wavelengths. Finally, Fig. 5 shows that the rat retina, and presumably ours as well, easily completes three of these cycles every second.
History.
Modern retinologists are mostly interested in the b-wave output of the retina; in fact, the structure is sometimes poisoned to prevent ganglion cell responses (5). However, the A/B/C/ histogram is so readily recorded from rats that it must have been seen and not recognized in many optic nerve and tract records. The published literature we have examined shows parts but not the whole of it. For instance, the A/B/ pair but not C/ was recorded many years ago from rabbit optic nerve (4), and (probably) from man and monkey as well (8). Shaw shows B/, possibly A/, and not C/ in cat chiasm recordings (9). B/C/ appears alone several times in the classic Doty and Kimura cat study (10). An early example from the huge literature on single ganglion cell responses is the Steinberg cat units that seem to belong in either the B/ or C/ category (ref. 11, Fig. 4, and ref. 6). In all of the above experiments, the stimulus was a full field flash, which may be essential for the demonstration of ganglion cell histograms. However, Levick and Zacks (12) did deliver flashes to the receptive field center of cat ganglion cells and found, as in our Figs. 2 and 4, that large changes in stimulus luminance and duration produced small response changes. Unfortunately they did not also record the entire ganglion cell output so it cannot be known whether their stimuli produced histograms in addition.
The Order in Which Axons Leave the Retina.
The axons of 20 or so different ganglion cell types make up an optic nerve such as that of the rat. Each chiasm trace represents activity in a fraction of these axons, and the fractions sampled by different electrodes will never be identical. Most of our electrodes end up recording B/C/ and little or no A/, but one electrode site produced A/ and C/ but no B/, and another only C/. Our terms “triphasic A/B/C/” and the equivalent “histogram” designate what is not always displayed in a particular record, namely, the temporal distribution of the entire complement of activated ganglion cell axons.
Are the axon types segregated in the A/B/C/ subdivisions? Do the ones relaying contrast and movement information leave the retina at the same or different times? Is the magno/parvocellular dichotomy already evident in the distribution? Microelectrode measurements could presumably answer such questions, but a few inferences and correlations based on known facts may be useful.
A/, which is infrequently recorded, probably originates in axons that are both large and rare. The obvious candidate is the large alpha cell axon that makes up 3–4% of the (cat) total (13). Alpha cell dendritic fields are large, cover the entire retinal surface, and overlap, which suggests that a small illuminance change anywhere will activate them. A/ may mark for the brain the upcoming arrival of the rest of the histogram and prompt or initiate the cortical extraction and analyzing processes.
B/ and the rising limb of the b-wave are coincident in time, which suggests that the retinal processes these two deflections index may be linked. Unfortunately for that hypothesis, Fig. 2 demonstrates that, whereas every stimulus creates B/, not all stimuli create b-waves. B/ probably represents a powerful synchronized ganglion cell discharge carrying the essential information about every scene. The relatively small standard deviations (SD) in the A/B/ regions of Figs. 3 and 4 suggest that axons belonging to the magnocellular system may produce these early and similar responses to stimuli of every type, intensity, and duration.
B− follows B/ (see Fig. 1); it is a prolonged negative wave of variable duration. A labile positive deflection on its descending limb, 2B/, is often absent during sleep. The entire B− area may contain oscillations (15–100 Hz) of unknown origin (and often reported, e.g., refs. 10 and 11). B− may represent the progress of the powerful B/ volley as it moves past the chiasm electrode. SD in the B− response region are generally larger than those found earlier.
C/ is the most variable deflection, as the SD in Figs. 3 and 4 demonstrate. It may have three peaks (at about 5 Hz) and presumably conveys detailed information about the scene. For instance, movement on the retinal surface (“smear”) during a 200-ms fixation could be sensed by late-firing C/ fibers that compare the time between beginning and end of the smear.
Triggers.
When similar histograms are produced by both 1-s and 10-μs flashes (see Fig. 4), it is clear the trigger for them both is related to stimulus onset. When similar histograms emerge from both light- and dark-adapted retinas (see Fig. 3), triggers seem to be related to changes in the retinal bleach pattern. And when near-threshold stimuli evoke fully formed histograms (see Fig. 2), small and large bleach pattern changes appear to be equally effective.
Further clues to histogram triggers come from the mostly forgotten human stabilized image experiments, where the effect of eye movement is eliminated and the image remains in the same place on the retinal surface (14–16). The ensuing temporary blindness immediately disappears if the image is moved across the retinal surface, or if the ambient level is changed. Ditchburn gives 2.0 arc min as the smallest image displacement that always destabilizes a stabilized image, and about 2.0 arc min of the amplitude of a 10-Hz sinusoid as the minimum luminance change (17). We propose here that these small local changes in the distribution of light energy on the human retinal surface successfully restore vision because each one triggers an optic nerve histogram that reaches the cortex via the LGN.
Ditchburn and others have measured how far and how frequently human eyes move in typical involuntary saccades; how far eyes move often exceeds his 2.0 arc min minima during normal viewing behavior (18). Thus, local changes in retinal illumination capable of triggering histograms take place almost continuously because of saccades, blinks, head movements, or changes in the scene. We propose that any event capable of initiating a histogram will do so provided it follows a similar event no sooner than about 200 ms (i.e., a temporal interval that order of magnitude is required to create and dispatch each histogram).
Integrating New and Old Facts.
During normal viewing, according to our claim, the retina generates an endless series of discrete ganglion cell volleys triggered by events that alter the distribution of light on the retinal surface. However, and despite almost 50 years of microelectrode research, the conventional receptive field/center-surround view of retinal function has never to our knowledge developed data that call for or support this conclusion. Hence, two apparently conflicting data sets coexist, each supported by strong experimental evidence. It is interesting to speculate why the conflicting data sets coexist and to consider what might bring them together.
The conventional microelectrode view is a spatial concept; in the typical experiment an isolated retinal territory is illuminated, perhaps for seconds, often with the goal of uncovering synaptic links within and between the layers of intrinsic neurons that eventually converge on the ganglion cell final common paths. The rat histogram, by contrast, is a temporal concept; whatever happens on the retinal surface and in its depths ends up as a time series of ganglion cell discharges. Thus, the spatial and temporal data sets represent two different views of the same thing, each of them incomplete.
What brings them together is the fact that it takes time to move the information contained in a given photoreceptor bleach pattern from the rod and cone pedicles through the intrinsic neuronal maze to end up as information distributed throughout a population of active axons that leaves the eyeball. That time interval is given by the rat A/B/C/ histogram. All of the remarkable retinal activities uncovered by microelectrode physiologists since Kuffler's original description take place in about a third of a second if the entire retina is exposed to the stimulus. When it is not, the retina can fail to generate histograms, and then the central processing of activated ganglion cells is handled in a different way.
The Perceptual Experience.
For a century or so, it has been known that the perceptual experience called reading begins when eyes move across a page such as this one and pause in fixations about three times each second (19, 20). Although the evidence is circumstantial, it must be true that human retinas can and do create several similar, complete ganglion cell volleys every second, each one a unique neuronal replica of all of the information on a segment of a printed page. Furthermore, stabilized image experiments show that normal visual experience is impossible if the retina is prevented from generating these intermittent ganglion cell episodes. Given these facts, the rat ca. 300-ms histograms experimentally validated here could be modeling the retinal contribution every vertebrate brain requires if it is to create visual perceptions.
A recent publication correlating monkey saccades with cortical unit activities shares our view of the relation between saccades and the visual perceptual experience but not our concept of the important contribution retinas make to the process (21). The abstract in ref. 21 states: “To understand how microsaccades sustain perception, we studied their relation to firing of cells in primary visual cortex.” Our study, by contrast, began at the retinal level and uncovered the A/B/C/ histograms, one of which almost surely leaves each retina and reaches the cortex every time a monkey's eyeballs come to rest at the end of a saccade.
We conclude that the normal neuronal input to rat, human, and monkey visual cortex is a precisely ordered time series that originated in the retina. For the cortical analysis to begin, the major analysis already performed at the retinal level must first arrive and be in the A/B/C/ format. In our view, the improved understanding of how a vertebrate brain creates a visual perceptual experience awaits more data about those histograms the cortex gets from the retina, and what it does with them.
Acknowledgments
We thank T. H. Bullock, R. W. Doty, S. A. Hillyard, H. Karten, C. J. Karwoski, D. Purves, and R. Rodieck for comments, criticism, and advice. This work was supported in part by Hungarian Science Foundation Grant OTKA 628.
Abbreviations
- ERG
electroretinogram
- VEP
evoked visual cortex potential
- LED
light-emitting diode
- SWS
slow wave sleep
- LGN
lateral geniculate nuclei
Footnotes
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.240448697.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.240448697
Webster's Third New International Dictionary defines histogram as follows: “A graphical representation of a frequency distribution by means of rectangles whose widths represent the class intervals and whose heights represent the class magnitudes.” Microelectrode physiologists use the term to cover single unit interval and latency distributions whereas others plot phase histograms, two dimensional histograms, etc. We use the term here for the poststimulus time distribution, at the computer sampling rate, of the ion current amplitude that axons produce as they sweep past a fixed electrode.
References
- 1.Galambos, R., Juhász, G., Kékesi, A. K., Nyitrai, G. & Szilágyi, N. (1994) Proc. Natl. Acad. Sci. USA91(11): 5153–5157. [DOI] [PMC free article] [PubMed]
- 2.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 3rd Ed. San Diego: Academic; 1997. [DOI] [PubMed] [Google Scholar]
- 3.Gottesmann C. Neurosci Biobehav Rev. 1992;16:31–38. doi: 10.1016/s0149-7634(05)80048-x. [DOI] [PubMed] [Google Scholar]
- 4.Yonemura D, Tsuchida Y. Jpn J Physiol. 1968;18:703–722. doi: 10.2170/jjphysiol.18.703. [DOI] [PubMed] [Google Scholar]
- 5.Robson J G, Frishman L J. Vis Neurosci. 1995;12:837–850. doi: 10.1017/s0952523800009408. [DOI] [PubMed] [Google Scholar]
- 6.Meeren H K, Van Luijtelaar E L, Coenen A M. Electroencephalogr Clin Neurophysiol. 1998;108:306–319. doi: 10.1016/s0168-5597(97)00101-9. [DOI] [PubMed] [Google Scholar]
- 7.Gaarder K R. Eye Movement, Vision and Behavior. Washington, DC: Hemisphere Pub. Corp. (Wiley); 1975. p. 156. [Google Scholar]
- 8.Møller A R, Burgess J E, Sekhar L N. Electroencephalogr Clin Neurophysiol. 1987;67:549–555. doi: 10.1016/0013-4694(87)90057-5. [DOI] [PubMed] [Google Scholar]
- 9.Shaw N A. Physiol Behav. 1998;63:615–620. doi: 10.1016/s0031-9384(97)00509-x. [DOI] [PubMed] [Google Scholar]
- 10.Doty R W, Kimura D S. J Physiol. 1963;168:205–218. doi: 10.1113/jphysiol.1963.sp007187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Steinberg R H. J Neurophysiol. 1966;29:139–156. doi: 10.1152/jn.1966.29.2.139. [DOI] [PubMed] [Google Scholar]
- 12.Levick W R, Zacks J L. J Physiol. 1970;206:677–700. doi: 10.1113/jphysiol.1970.sp009037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wässle H, Peichl L, Boycott B B. Proc R Soc London Ser B. 1981;212:157–175. doi: 10.1098/rspb.1981.0032. [DOI] [PubMed] [Google Scholar]
- 14.Ditchburn R W, Ginsborg B L. Nature. 1952;170:36. doi: 10.1038/170036a0. [DOI] [PubMed] [Google Scholar]
- 15.Riggs L A, Ratliff F, Cornsweet J C, Cornsweet T N. J Opt Soc Am. 1953;43:495–501. doi: 10.1364/josa.43.000495. [DOI] [PubMed] [Google Scholar]
- 16.Yarbus A L. Eye Movements and Vision. New York: Plenum; 1967. p. 222. (English translation). [Google Scholar]
- 17.Ditchburn R W. J Opt Soc Am. 1987;4:405–406. doi: 10.1364/josaa.4.000405. [DOI] [PubMed] [Google Scholar]
- 18.Ditchburn R W. Eye-Movements and Visual Perception. Oxford: Clarendon; 1973. p. 421. [Google Scholar]
- 19.Kolers P A. In: Eye Movements and Psychological Processes. Monty R A, Senders J W, editors. Hillsdale, NJ: Erlbaum; 1976. pp. 373–395. [Google Scholar]
- 20.Rodieck R W. The First Steps in Seeing. Sunderland, MA: Sinauer; 1998. p. 562. [Google Scholar]
- 21.Martinez-Conde S, Macknik S L, Hubel D H. Nat Neurosci. 2000;3:251–258. doi: 10.1038/72961. [DOI] [PubMed] [Google Scholar]