Abstract
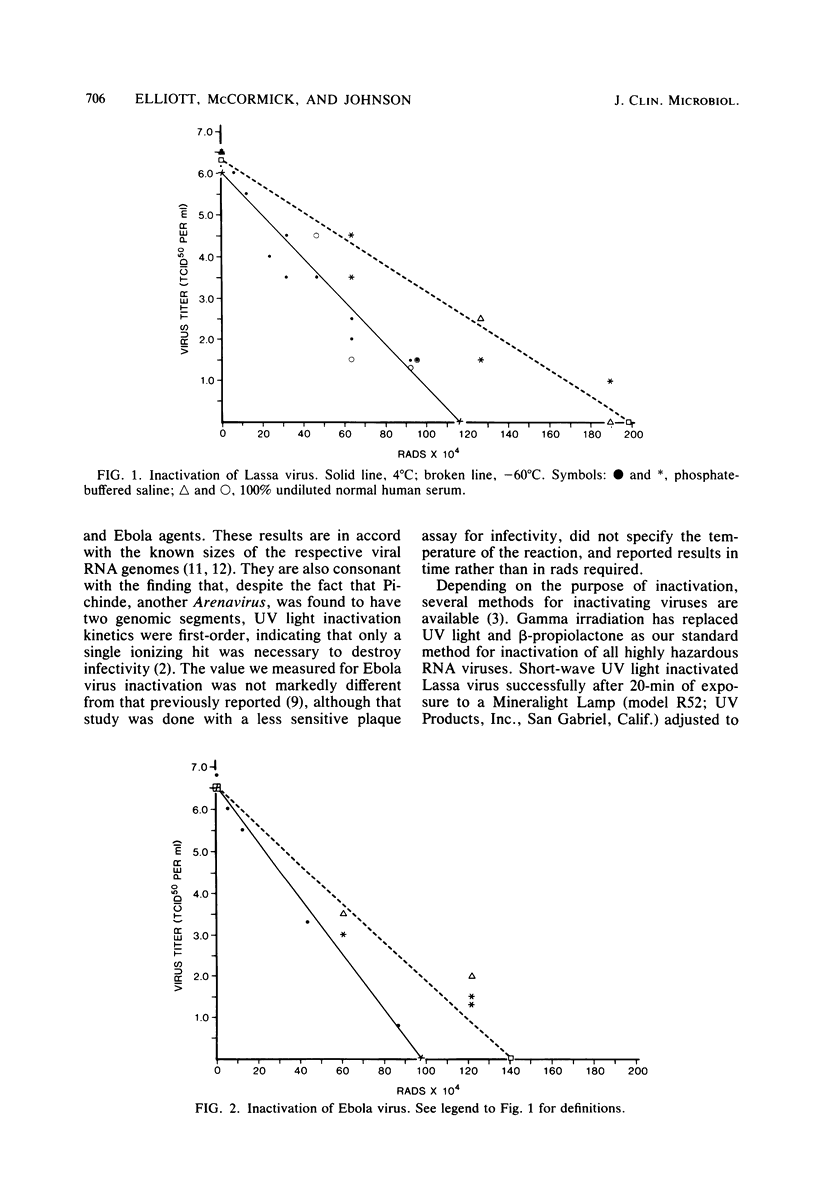
Because of the cumbersome conditions experienced in a maximum containment laboratory, methods for inactivating highly pathogenic viruses were investigated. The infectivity of Lassa, Marburg, and Ebola viruses was inactivated without altering the immunological activity after radiation with Co60 gamma rays. At 4 degrees C, Lassa virus was the most difficult to inactivate with a rate of 5.3 X 10(-6) log 50% tissue culture infective dose per rad of CO60 radiation, as compared with 6.8 X 10(-6) log 50% tissue culture infective dose per rad for Ebola virus and 8.4 X 10(-6) log 50% tissue culture infective dose per rad for Marburg virus. Experimental inactivation curves, as well as curves giving the total radiation needed to inactivate a given concentration of any of the three viruses, are presented. We found this method of inactivation to be superior to UV light or beta-propiolactone inactivation and now routinely use it for preparation of material for protein-chemistry studies or for preparation of immunological reagents.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Buckley S. M., Casals J. Lassa fever, a new virus disease of man from West Africa. 3. Isolation and characterization of the virus. Am J Trop Med Hyg. 1970 Jul;19(4):680–691. doi: 10.4269/ajtmh.1970.19.680. [DOI] [PubMed] [Google Scholar]
- Carter M. F., Murphy F. A., Brunschwig J. P., Noonan C., Rawls W. E. Effects of actinomycin D and ultraviolet and ionizing radiation on Pichinde virus. J Virol. 1973 Jul;12(1):33–38. doi: 10.1128/jvi.12.1.33-38.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldwasser R. A., Elliott L. H., Johnson K. M. Preparation and use of erythrocyte-globulin conjugates to Lassa virus in reversed passive hemagglutination and inhibition. J Clin Microbiol. 1980 Jun;11(6):593–599. doi: 10.1128/jcm.11.6.593-599.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson C. V., Riggs J. L., Lennette E. H. Photochemical inactivation of DNA and RNA viruses by psoralen derivatives. J Gen Virol. 1978 Aug;40(2):345–358. doi: 10.1099/0022-1317-40-2-345. [DOI] [PubMed] [Google Scholar]
- Johnson K. M., Elliott L. H., Heymann D. L. Preparation of polyvalent viral immunofluorescent intracellular antigens and use in human serosurveys. J Clin Microbiol. 1981 Nov;14(5):527–529. doi: 10.1128/jcm.14.5.527-529.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson K. M., Lange J. V., Webb P. A., Murphy F. A. Isolation and partial characterisation of a new virus causing acute haemorrhagic fever in Zaire. Lancet. 1977 Mar 12;1(8011):569–571. doi: 10.1016/s0140-6736(77)92000-1. [DOI] [PubMed] [Google Scholar]
- Lupton H. W. Inactivation of Ebola virus with 60Co irradiation. J Infect Dis. 1981 Feb;143(2):291–291. doi: 10.1093/infdis/143.2.291. [DOI] [PubMed] [Google Scholar]
- Murphy F. A. Arenavirus taxonomy: a review. Bull World Health Organ. 1975;52(4-6):389–391. [PMC free article] [PubMed] [Google Scholar]
- Pfau C. J., Bergold G. H., Casals J., Johnson K. M., Murphy F. A., Pedersen I. R., Rawls W. E., Rowe W. P., Webb P. A., Weissenbacher M. C. Arenaviruses. Intervirology. 1974;4(4):207–214. doi: 10.1159/000149964. [DOI] [PubMed] [Google Scholar]
- Regnery R. L., Johnson K. M., Kiley M. P. Virion nucleic acid of Ebola virus. J Virol. 1980 Nov;36(2):465–469. doi: 10.1128/jvi.36.2.465-469.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegert R., Shu H. L., Slenczka W., Peters D., Müller G. Zur Atiologie einer umbekannten, von Affen ausgegangenen menschlichen Infektionskrankheit. Dtsch Med Wochenschr. 1967 Dec 22;92(51):2341–2343. doi: 10.1055/s-0028-1106144. [DOI] [PubMed] [Google Scholar]
- Wulff H., Lange J. V. Indirect immunofluorescence for the diagnosis of Lassa fever infection. Bull World Health Organ. 1975;52(4-6):429–436. [PMC free article] [PubMed] [Google Scholar]


