Abstract
Objective
Increasing patient knowledge about the value of colorectal cancer (CRC) screening is a goal of most public health education efforts. We hypothesized that a cohort of women at average risk for CRC, but non-compliant with standard screening recommendations, would demonstrate low levels of CRC related knowledge and underestimate their personal risk for developing CRC.
Methods
Baseline survey results are reported from a prospective trial designed to improve CRC screening participation in women at average risk, but non-complaint with screening recommendations. Women scheduled for a routine gynecological office visit were identified and contacted by telephone approximately 4 weeks before their index appointment. All participants completed a 75 item baseline survey that included items assessing demographic information, CRC knowledge, risk perception and intention to participate in screening.
Results
Women (n=318) enrolled from June, 2006-May, 2007 are included. Participants demonstrated high levels of CRC and CRC screening related knowledge answering an average of 8.6/10 items correctly (SD 1.30). The majority of these non-compliant women (1) estimated their CRC risk incorrectly (60%) and (2) had no intention of CRC screening participation in the future (65%). Multivariate models found no consistent relationships between knowledge, risk perception, and screening intent.
Conclusions
Greater “knowledge” alone is an inadequate stimulus to CRC screening adherence. Future interventions will require a greater understanding of the interplay between CRC-related knowledge, beliefs, risk perception, and other affective responses.
Colorectal cancer (CRC) is the third most common form of cancer in the United States among men and women. Approximately 154,000 new CRC cases will be diagnosed this year as well as 52,000 deaths. (1) These statistics are particularly disheartening considering that CRC incidence and mortality can largely be reduced through routine CRC screening. (2, 3)
Effective CRC-screening techniques are widely available including fecal occult blood testing (FOBT), barium enema (BE), flexible sigmoidoscopy (FS) and colonoscopy. In addition, new tests like virtual colonoscopy are on the horizon. Many authorities have published screening guidelines; however there is no consensus supporting a particular method of CRC screening. Instead, healthcare providers have been urged to concentrate on increasing screening rates for their patients through periodic use of any of the recommended techniques. (4)
Unfortunately, participation rates for people at average risk, approximately 90% of the general population, continue to lag behind those of other recommended preventive health services. (5) Although slowly rising, only about 50% of the population receives any CRC screening test within the recommended time frames.(6) It is unlikely that the goals of Healthy People 2010, a nationwide health promotion and disease prevention initiative, will be met unless there is a substantial increase in screening uptake. (7)
Many barriers to CRC screening have been described. Provider (e.g., absence of appropriate physician recommendation) and healthcare system (e.g., lack of access to screening or a primary care provider) factors have a negative effect on screening uptake. (8) Patient-based factors also play a significant role in screening non-adherence. To conquer patient-related barriers, much effort has focused on cognitive factors, particularly overcoming a lack of knowledge about CRC or CRC screening. (8,9,10,11) Overall, the general population tends to be poorly informed about its CRC risk (11, 12), as well as routine screening recommendations. (10) Both patients and providers perceive a lack of patient awareness and knowledge as a major barrier to screening. (8) We have previously demonstrated that screening adherence is greater among average risk women who understand that screening can reduce their CRC risk. (13)
Most public health authorities have primarily emphasized enhancing knowledge about CRC when designing educational outreach programs. (14) However, receiving information about CRC risk factors and screening recommendations does not necessarily translate directly into more informed decision making or action. For example, an educational intervention about CRC risk factors did not result in more accurate identification by participants of their own lifestyle and occupational risk factors. (15) Non-cognitive factors must also play an important role. For example, believing that one is at above average risk is positively associated with CRC screening. (13) Prior research has identified the negative impact of emotional factors, including anxiety and fear on screening adherence. (13, 16)
We have initiated a randomized, prospective study comparing several interventions intended to increase CRC screening among non-compliant average risk women making routine visits to their gynecologists. The gynecological study platform is particularly relevant to CRC cancer prevention since 55% of all women, and 45% of women ages 50-65 years, consider a gynecologist as their primary care physician. (17) For this study, baseline data is collected that characterizes general CRC-related knowledge, personal risk perceptions, affective responses to screening, and screening intentions. All participants have health insurance, make regular visits to primary care physicians, and have access to other common screening like mammography. Because access to care and insurance coverage is not a major barrier for these women, this study population allows for a greater focus on patient-based factors.
We hypothesized that at baseline these non-compliant women would demonstrate low levels of general knowledge about CRC and CRC prevention, underestimate their personal risk, and show higher levels of screening procedure-related concerns. In addition, we hypothesized that higher CRC knowledge would be positively associated with intention to participate n screening and to accuracy of cancer risk perception. Here, we report baseline data findings on the first 318 women enrolled in the randomized trial (June, 2006-May, 2007).
Methods
This study was reviewed and approved by the institutional review boards of Fox Chase Cancer Center, the Geisinger Health System and Emory University.
Potential participants were identified through the linked patient scheduling, billing and electronic medical record systems at Geisinger and Emory. These databases were first searched for any woman scheduled for a routine gynecology office visit 4 to 6 weeks from the query date. Electronic screens, reflecting the inclusion/exclusion criteria (see below) were then applied to most efficiently identify potentially eligible participants. Women were eligible for participation if they were: 1) 50 years and older; 2) at average risk for CRC defined as no personal history of colorectal polyps or cancer, inflammatory bowel disease, or a family history of familial adenomatous polyposis, hereditary non-polyposis colorectal cancer or CRC in more than one first-degree relative; 3) non-compliant with standard CRC screening recommendations at the time of index gynecology appointment, defined as no completion of three card FOBT in the last 12 months, FS in the past 5 years, BE in the past 5 years, or routine colonoscopy in the past 10 years; and 4) able to communicate/consent in English. As one of the study intervention arms required internet access, a final eligibility criterion was internet access at home or work.
Women identified in this manner were then contacted by telephone. After consent was obtained, they were asked to complete a 75-item survey. In addition to demographic information and medical history (including personal cancer history, compliance with mammography and PAP smear recommendations), respondents were queried about their basic knowledge regarding CRC, CRC screening and their beliefs about their risk for developing CRC in relation to other average risk women and men using scales adapted from previous work (13, 18). Intention to participate in CRC screening, or motivational readiness, was measured using a 5-point scale based on the Transtheoretical Model of Change(19-22), frequently referred to as the “Stages of Change” Model. Specifically, items span from 1 = I am up to date with colorectal cancer screening; 2 = I am planning to undergo screening for colorectal cancer in the next 30 days; 3 = I am planning to undergo screening for colorectal cancer in the next 6 months; 4 = I am thinking about undergoing screening for colorectal cancer, but I'm not really sure and have made no specific plan; to 5 = I am not thinking about undergoing screening for colorectal cancer. Additional survey items included standardized measures of depression, CRC risk-related distress and dispositional attentional style (CES-D (23), RIES (24) and MBSS Short Form (25), respectively). Each of these scales has demonstrated internal consistency and reliability. (23)
Analysis
To investigate the intention to obtain CRC screening, we created a binary variable measuring whether a woman had no specific plans about CRC screening (scale values 4 and 5; variable=1) or had plans to become up-to-date with screening withinthe next six months (scale values 2 and 3; variable=0). The intention to screen variable measures whether a woman is in a precontemplative/contemplative state in which she doesn't view the target behavior as in need of change, and is clearly not ready to even consider making changes or is weighing the pros and cons, but still not at the point where active behavior change efforts will begin vs. the preparation state in which she is beginning to consider behavior change regarding CRC screening. We used a multiple logistic regression to explore the multivariable associations with intention to be screened.
To investigate the multivariable associations with risk perceptions, we used multiple linear regressions of the two risk perception variables describing how women assess their own CRC risk relative to other average-risk women and relative to average-risk men. The variables were measured as five-level ordinal variables, with levels ranging from much lower than average risk to much higher than average risk. Since the measures were not truly continuous, we confirmed the results using cumulative logistic models assuming proportional odds. We chose to report the findings from the multiple linear regressions since the parameters in such models are more readily interpretable than those in cumulative logit models. To account for the non-normality of the risk perception variables, we used robust standard errors with the linear models.
We included age in the model using a restricted cubic spline with three knots. (26) Restricted cubic splines provide a flexible means of accounting for potentially non-linear effects in models.
To account for missing data, we used the multiple imputation method of Raghunathan and colleagues with 10 imputed datasets. (27) Multiple imputation corrects for potential bias that might result from not accounting for missing data in analyses and inflates standard errors to account for the uncertainty in the imputation process. The only variable with missing data in more than 10% of cases was annual income as approximately 30% of participants declined to answer this item. We imputed missing demographic and knowledge items used in multivariable analyses; we did not impute the intention or risk perception response variables. For imputed data, table statistics represent the average of the statistics from the 10 imputed datasets. In sensitivity analyses, we examined models in which we did not impute knowledge items, but presumed that a non-response to the knowledge item indicated an incorrect response.
Results
Demographic, Healthcare Utilization and Psychosocial Characteristics
Forty nine percent (N=318) of eligible women successfully contacted by telephone enrolled. Table 1 displays demographic characteristics and selected health care utilization patterns of the sample. Although information could not be collected about women refusing participation without their consent, study participants parallel the overall patient populations seen in the Geisinger Health System, the site from which the majority of participants were drawn. Participants were predominantly Caucasian (96.2%), married (74.2%), and employed (64.5%) with an average age of 56.7 (SD=7.1) years. Overall, the sample was relatively well-educated with over 65% reporting some college attendance. Less than 5% reported not completing high school; because of the small size of this group, these women were included with those who completed high school in analyses. The median household income was reported in the $45,001-$60,000 range. Health care utilization in the past year for prevention activities was high for the study population. Seventy percent or more of women reported having a physical examination, mammogram and clinical breast examination in that period, while nearly 60% had a Pap smear. Based on CES and RIES results, overall the study population displayed sub-clinical levels of depression and anxiety. The MBSS score is comparable to other average risk populations. (28)
Table 1.
Demographics, healthcare utilization and psychosocial characteristics of sample
| Variable | Statistic |
|---|---|
| Sample Size | 318 |
| Average age (standard deviation-SD) | 56.7 (7.1) |
| Caucasian | 96.2% |
| Household income | |
| <$30,001 | 22.7% |
| $30,001-$60,000 | 33.8% |
| >$60,000 | 43.5% |
| Employment Status | |
| Employed | 64.5% |
| Disabled | 5.0% |
| Retired | 18.9% |
| Other | 11.6% |
| Married or Domestic Partner | 74.2% |
| Education | |
| High school degree or less | 34.6% |
| Some college or vocational education | 30.2% |
| College graduate | 35.2% |
| Healthcare utilization in past year | |
| Physical Examination | 100% |
| Clinical Breast Examination | 70% |
| Mammogram | 74% |
| Pap Smear | 59% |
| MBSS Score | 4.3 (1.8) |
| CESD | 8.4 (3.4) |
| RIES - Avoidance | 4.6 (6.6) |
| RIES - Intrusion | 2.1 (4.4) |
CRC Knowledge, CRC Screening Intentions, and CRC Risk Perceptions
Table 2 presents knowledge, screening intentions, and risk perception responses. Overall, the majority of participants demonstrated a high level of basic knowledge about CRC and CRC screening, with participants answering an average of 8.6 (SD 1.30) out of a possible 10 items correctly. For example, greater than 98% of the women correctly answered questions about the clinical benefit of early detection and treatment, the presence of blood in the stool as a potential indicator of CRC, the utility of CRC screening in asymptomatic persons, and regular CRC screening as a recommendation for everyone 50 years or older. However, variability did occur on several items. The questions most frequently answered incorrectly pertained to gender differences in incidence of CRC, CRC as a preventable disease, and any association of pain with screening. Specifically, 40.4% erroneously stated that men get CRC more frequently then women, nearly 25% did not endorse that CRC is a preventable disease, and 15% incorrectly endorsed that screening would be very painful.
Table 2.
General knowledge and risk perception characteristics of the sample
| Knowledge Items | Percentage Correct |
|---|---|
| Most people who get colon cancer do very well if the cancer is found and treated at an early stage. | 99.0% |
| Blood in your bowel movement means you have colon cancer for sure. | 98.7% |
| You need to be screened for colon cancer even if your bowel habits are normal. | 98.4% |
| Everyone 50 years or older should be screened regularly for colon cancer. | 98.1% |
| You would always have pain if you had colon cancer. | 94.3% |
| Your chances of getting colon cancer are greater if you have a family member who had cancer of the bowel. | 92.0% |
| Colon cancer is always a deadly disease. | 89.3% |
| Screening for colon cancer would be very painful. | 85.0% |
| Colon cancer is a preventable disease. | 77.3% |
| Men get colon cancer more often than women. | 59.6% |
| Average number of knowledge items answered correctly. | 8.6 (SD 1.3) |
|
| |
| Screening Intentions | |
|---|---|
| No plans to obtain CRC screening within 6 months. | 65.7% |
| Plans to obtain CRC screening within 6 months. | 34.3% |
|
| |
| Risk Perceptions | |
|---|---|
| Compared to women your age and with your family history, what are your chances of developing colorectal cancer at this point in your life? | |
| Lower than average | 52.1% |
| Average | 39.9% |
| Higher than average | 8.0% |
| Compared to men your age and with your family history, what are your chances of developing colorectal cancer at this point in your life? | |
| Lower than average | 52.9% |
| Average | 40.1% |
| Higher than average | 7.0% |
Almost two-thirds (65.7%) stated they had no need and/or plans (i.e., precontemplative/contemplative state) to undergo CRC screening, while 34.3% reported plans (i.e., preparation state) to be screened within a 6-month period. In comparison to women of the same age and with a similar family history, greater than 60% incorrectly estimated their relative risk for CRC. Specifically, compared to other women, 52.1% underestimated and 8% overestimated their risk. Similar results occurred when asked to compare to their risk to men of the same age and family background; nearly 60% inaccurately perceived their CRC risk, with 52.9% underestimating and 7% overestimating their personal risk.
There was no association with screening intention or risk perception in multivariable models (p>0.20 in all cases) when we considered the knowledge variable as a summary measure (number of correct answers from 10 questions). We then examined the association of individual knowledge items with screening intention and risk perceptions among those variables that had both true and false response proportions of 5% or greater (Table 3). Women who erroneously thought that CRC screening would be very painful have lower intentions of attending CRC screening within the next 6 months (OR= 2.77, p=.02; [95% CI: 1.18, 6.50] after controlling for other variables in the model. Table 3 also displays the relationship between other study factors and screening intention. In the model, increased age was associated with having lower intentions of being screened within the next six months (p<0.03 for the joint Wald test of both parameters (see Figure 1). Non-Caucasians had a greater intention of adhering to CRC screening within the next six months (OR=0.23, p=0.03, [95% CI 0.06-0.86]).
Table 3.
Regression Estimates
| Screening Intention Model n=315 | Risk Compared to Average Women n=313 | Risk Compared to Average Man n=299 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Variable | Odds Ratio | P-value | 95% CI 95% CI | Coefficient | P-value | 95% CI 95% CI | Coefficient | P-value | 95% CI 95% CI |
| Age | 1.12 | 0.03 * | 1.00,1.24 | 0.01 | 0.88* | -0.03,0.05 | 0.02 | 0.10 * | -0.02,0.06 |
| Age Spline term 1 | 0.80 | 0.51,1.25 | -0.05 | -0.25,0.15 | 0.00 | -0.18,0.19 | |||
| Non-Caucasian | 0.23 | 0.03 | 0.06,0.86 | 0.11 | 0.76 | -0.59,0.80 | 0.27 | 0.40 | -0.37,0.91 |
| Income | 0.97 | 0.77 | 0.77,1.21 | -0.01 | 0.84 | -0.11,0.09 | -0.01 | 0.76 | -0.10,0.07 |
| Not employed/disabled/retired | Reference | Reference | Reference | ||||||
| Employed | 1.43 | 0.39 | 0.64,3.19 | -0.37 | 0.02 | -0.68, -0.06 | -0.29 | 0.05 | -0.59,0.00 |
| Retired | 0.79 | 0.65 | 0.27,2.25 | -0.20 | 0.30 | -0.59,0.18 | -0.25 | 0.19 | -0.61,0.12 |
| Married/Partnered | 0.84 | 0.63 | 0.42,1.69 | -0.07 | 0.62 | -0.36,0.21 | -0.09 | 0.54 | -0.36,0.19 |
| High School or Less | Reference | Reference | Reference | ||||||
| Some College | 1.12 | 0.74 | 0.58,2.18 | -0.26 | 0.07 | -0.54,0.02 | -0.22 | 0.11 | -0.50,0.05 |
| College Graduate | 0.63 | 0.19 | 0.31,1.26 | -0.27 | 0.09 | -0.59,0.04 | -0.19 | 0.21 | -0.48,0.10 |
| Monitor Score | 1.02 | 0.81 | 0.88,1.19 | -0.04 | 0.26 | -0.11,0.03 | -0.04 | 0.21 | -0.11,0.02 |
| Answered the following incorrectly: | |||||||||
| Men get colon cancer more often than women. | 0.93 | 0.81 | 0.54,1.61 | -0.02 | 0.85 | -0.26,0.22 | -0.21 | 0.06 | -0.43,0.01 |
| Colon cancer is a preventable disease. | 0.71 | 0.30 | 0.37,1.35 | -0.21 | 0.13 | -0.49,0.07 | -0.25 | 0.07 | -0.51,0.02 |
| Screening for colon cancer would be very painful. | 2.77 | 0.02 | 1.18,6.50 | 0.02 | 0.92 | -0.30,0.33 | -0.05 | 0.76 | -0.34,0.25 |
| You would always have pain if you had colon cancer. | 0.39 | 0.14 | 0.11,1.38 | -0.30 | 0.29 | -0.86,0.25 | -0.18 | 0.46 | -0.65,0.29 |
| Your chances of getting colon cancer are greater if you have a family member who had cancer of the bowel. | 0.77 | 0.59 | 0.29,2.02 | 0.03 | 0.87 | -0.36,0.43 | 0.19 | 0.30 | -0.17,0.56 |
| Colon cancer is always a deadly disease. | 1.61 | 0.35 | 0.59,4.36 | 0.19 | 0.32 | -0.18,0.56 | 0.03 | 0.86 | -0.31,0.37 |
| Intercept | 1.98 | 0.43 | 0.36,10.86 | 3.19 | 0.00 | 2.48,3.90 | 3.00 | 0.00 | 2.33,3.66 |
The p-value of the age coefficients represents the joint hypothesis test that both age coefficient terms are zero
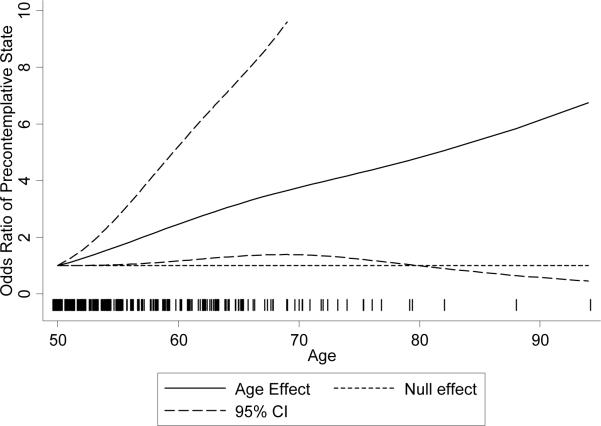
Figure 1.
Odds ratio for the age effect in the intention to be screened analysis. The odds ratio describes the effect relative to the baseline odds at age 50. Older women have greater odds of being in the precontemplative state, and the association is statistically significant since the pointwise 95% confidence intervals generally do not cover one except where data is sparse. The vertical lines at the bottom of the figure, sometimes described as the rug, represent the distribution of the observed data.
Multiple linear regression analyses of CRC risk perceptions were used to explore associations with CRC knowledge. The model of a woman's perception of her personal risk relative to other women did not reveal any significant relationships with the knowledge items. However, women who were employed rated their risk as lower on average compared to the ratings of women who were not employed, retired, or disabled, after controlling for other covariates in the model (β=-.37, p=.02; [95% CI: -.68, -.06]).
The model of a woman's perception of her personal risk relative to men similarly indicated that employed women rated their risk relative to men as lower on average compared to ratings of the reference group (β=-.29, p=.05; [95% CI: -.59, .00]). Women who believe that CRC is more likely to occur in men and those who believe that CRC is preventable tend to rate their risk relative to men as lower than women who believed otherwise, but the effect is only marginally significant (β=-.21, p<.06; [95% CI: -.43, .01], and β=-.25, p<.07; [95% CI: -.51, .02], respectively). The magnitude of effects in the risk perception models was generally modest, with the coefficients suggesting that having the relevant characteristics shifted the average risk response lower by approximately one-fifth to one-third of a point on a 5 point scale.
In sensitivity analyses, we used cumulative logit models assuming proportional odds instead of multiple linear regressions for the risk perception regressions. In general, the inferences did not change, although the education coefficients became slightly more statistically significant in the cumulative logit model of women's own risk perception relative to women their own age (p=0.04 for the some college parameter and p=0.08 for the college graduate parameter).
Discussion
Improving CRC screening rates remains an important public health goal. Although the number of states where ≥60% of the population have been screened has nearly doubled from 2002 to 2004 (29), in many states more than half of age-eligible residents are not screened. In this report, we describe the characteristics of a population of non-compliant women at average risk for CRC. Since it is estimated that 50%-60% of CRC deaths might be prevented through routine screening (4) it is imperative to understand more fully the barriers to screening and to develop more focused and salient interventions to increase participation.
An often cited barrier to greater uptake of CRC screening is limited knowledge on the part of the general public about basic CRC facts and the benefits of existing prevention methods. Indeed, education about CRC risk factors and screening recommendations is the primary focus of most public health efforts designed to increase screening. (7), (14) Therefore, we hypothesized that the non-compliant women in our study would display only limited knowledge levels in these areas. Our results revealed the opposite: study participants demonstrated uniformly high levels of knowledge regarding CRC-related basic facts and prevention. Further, despite greater knowledge, most study participants had no intention to participate in screening in the future. These findings are troubling since many of the often cited provider or system-based barriers to screening, for example access to physicians or healthcare services, or socio-economic status as reflected by income or insurance status were not relevant for this population.
For this study, we chose to use widely employed, true/false knowledge items adapted from theory- and evidence-based measures with demonstrated discriminant validity. (13) (18) We focused on basic clinical facts about CRC (e.g., “Blood in a bowel movement always means you have CRC”) and the utility of screening/early detection (e.g., “Most people who get colon cancer do very well if the cancer if found and treated at an early stage”). No emphasis was placed on the more fine-grained aspects of screening such as the biological mechanisms that underlie why screening helps prevent CRC (i.e., the detection and removal of precancerous polyps) or the specifics of each screening mechanism (i.e., the appropriate intervals between normal FOBT or colonoscopy examinations).
However, allowing for the absence of a standardized “knowledge” measure, women in this study demonstrated greater apparent levels of basic knowledge about CRC than in previous work. There were some important exceptions. Nearly 25% of women in our study continue to display a lack of specific knowledge with respect to CRC's preventability. Future work should explore the degree to which the general population understands the critical role that routine CRC screening plays in prevention as well as the idea that CRC is preventable. This is not a simple message since prevention through the removal of polyps only occurs in endoscopic procedures either as a first line screening modality or as diagnostic follow-up for FOBT screening. As overall general knowledge has increased, new messages addressing these more complex issues in CRC screening are needed.
A substantial minority of our participants thought that CRC was a “man's” disease with women less often affected, consistent with earlier observations. (30) (31) Health communication efforts must continue to target women about how CRC is an “equal opportunity” cancer with respect to gender, an area that straddles both knowledge and risk perception.
With regard to other barriers, a subset of women expressed a belief that screening would be very painful; a belief that was associated with less intention to comply with recommendations. This finding is similar to barriers reported in other studies.(13)(14) Although we did not explore if this concern was specifically linked to endoscopy based recommendations, these findings are particularly relevant as colonoscopy is increasingly used to satisfy screening requirements. (5) Relying on vicarious modeling approaches of women who have had successful screening experiences may help to mitigate these beliefs. (32) Communicating the mounting data that screening, including endoscopy based methods, is generally not as objectionable as anticipated may be a useful component of future health messages. (33)
To improve screening interventions will require a greater understanding of the interplay between CRC-related knowledge, beliefs, personal risk perceptions, and affective responses. Findings like ours demonstrate that greater “knowledge” alone is an inadequate stimulus to screening adherence. Women in this study also underestimated their personal CRC risk, perceiving it as lower than average and perhaps as a result had little intention to participate in screening. In other cancer contexts, perceived vulnerability for disease has been found to play a key role in the adoption of screening recommendations. (34) (35) A heightened perception of individual risk has been associated with CRC screening participation. (13) The present finding that over 50% of the non-compliant women studied perceived their risk to be less than comparable men or women suggests an important target for subsequent efforts to improve screening uptake. For these women, generic educational efforts do not seem to raise the personal threat to a level that motivates preventive action. Further studies are needed to provide a deeper understanding of this lower risk perception in order to guide the development of more potent interventions to raise susceptibility and potentially increase screening behavior.
To more effectively design intervention programs will require a better understanding of what average risk and higher or lower risk means to patients, including the fact that average or even lower than average risk does not negate the utility of prevention maneuvers. Interestingly, women in this study who were employed tended to perceive less personal CRC risk. The basis for this relationship is unclear. Health-care insurance is often linked with employment; therefore women with insurance would have one less barrier to screening. Perhaps these women find CRC less threatening since access to care is available if needed. Older women also expressed less intent to be screened. Most studies suggest that older persons are more likely to have participated in CRC screening. (5) However, our study population included only women who were not compliant with CRC screening, suggesting that advancing age may be another barrier to screening in a non-compliant population.
Risk perception remains a key element as individuals need to be made sufficiently aware of susceptibility to motivate adaptive action, but should not become so anxious that avoidant responses are activated. (36) (37) Recent data suggests that he manner in which risk is described may influence behavior. (38) (39) In addition, there is a growing literature regarding the impact of tailored risk information. (15, 40)
There are several limitations to the present study. In general, the study cohort was employed and well-educated. All participants had access to routine, preventive health-care. Participants were regular users of other preventive services like routine physical examinations and mammography. It is therefore reasonable to infer that lack of interest or participation in CRC screening was not due to access alone or bias against preventive health services in general. However, our results cannot necessarily be extrapolated to populations without regular health-care access. In addition, there is some evidence that men and women utilize CRC screening services in different ways. (41) Our study cannot address the issue of whether this is because men have greater knowledge about CRC risk or perceive cancer risk differently than women.
Our study population was identified from women scheduled to see their gynecologists for a routine office appointment. Gynecologists, although an important source of primary care for many women, may think other physicians will take responsibility for CRC screening discussions, or may promote CRC screening mechanisms (for example office-based FOBT rather than take home FOBT) not supported by expert guidelines. In a recent study, 26% of primary care physicians (and a larger percentage of Ob/Gyns) utilized a single in-office FOBT as their preferred method for CRC screening. (42) Some patients may mistakenly believe that this single FOBT satisfies CRC screening requirements. This issue requires greater study, demonstrating the need to pursue physician-directed, as well as patient-based, efforts to improve CRC screening rates.
Conclusion
In this population of average risk, non-compliant women, barriers regarding access and insurance were almost eliminated but still almost two-thirds were not considering CRC screening. Basic knowledge about CRC screening was generally high suggesting that education alone is an inadequate method to improve screening utilization. Inaccurate risk perceptions about CRC and the belief that screening might be painful were among the key factors that contributed to reduce the intention to participate in CRC screening. While continuing to strive for public education, future efforts to improve CRC screening will need to address how women process CRC risk information and screening recommendations cognitively and affectively in order to attain broader uptake of CRC screening.
Acknowledgments
Supported in part by NIH Grant R01CA102695 (PI: David Weinberg)
References
- 1.Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ. Cancer statistics, 2007. CA Cancer J Clin. 2007;57(1):43–66. doi: 10.3322/canjclin.57.1.43. [DOI] [PubMed] [Google Scholar]
- 2.Weinberg DS. In the clinic. Colorectal cancer screening. Ann Intern Med. 2008;148(3):ITC2-1–ITC2-16. doi: 10.7326/0003-4819-148-3-200802050-01002. [DOI] [PubMed] [Google Scholar]
- 3.Sarfaty M, Wender R. How to increase colorectal cancer screening rates in practice. CA Cancer J Clin. 2007;57(6):354–66. doi: 10.3322/CA.57.6.354. [DOI] [PubMed] [Google Scholar]
- 4.Pignone M, Rich M, Teutsch SM, Berg AO, Lohr KN. Screening for colorectal cancer in adults at average risk: a summary of the evidence for the U.S. Preventive Services Task Force. Ann Intern Med. 2002;137(2):132–41. doi: 10.7326/0003-4819-137-2-200207160-00015. [DOI] [PubMed] [Google Scholar]
- 5.Society AC. Cancer Prevention and Detection: Facts and Figures. American Cancer Society; Atlanta: 2007. [Google Scholar]
- 6.Meissner HI, Breen N, Klabunde CN, Vernon SW. Patterns of colorectal cancer screening uptake among men and women in the United States. Cancer Epidemiol Biomarkers Prev. 2006;15(2):389–94. doi: 10.1158/1055-9965.EPI-05-0678. [DOI] [PubMed] [Google Scholar]
- 7.Healthy People 2010 Mid-Course Review: Cancer. International Medical Publishing; McLean: [Google Scholar]
- 8.Klabunde CN, Vernon SW, Nadel MR, Breen N, Seeff LC, Brown ML. Barriers to colorectal cancer screening: a comparison of reports from primary care physicians and average-risk adults. Med Care. 2005;43(9):939–44. doi: 10.1097/01.mlr.0000173599.67470.ba. [DOI] [PubMed] [Google Scholar]
- 9.Straus WL, Mansley EC, Gold KF, Wang Q, Reddy P, Pashos CL. Colorectal cancer screening attitudes and practices in the general population: a risk-adjusted survey. J Public Health Manag Pract. 2005;11(3):244–51. doi: 10.1097/00124784-200505000-00010. [DOI] [PubMed] [Google Scholar]
- 10.Ford JS, Coups EJ, Hay JL. Knowledge of colon cancer screening in a national probability sample in the United States. J Health Commun. 2006;11(Suppl 1):19–35. doi: 10.1080/10810730600637533. [DOI] [PubMed] [Google Scholar]
- 11.Beeker C, Kraft JM, Southwell BG, Jorgensen CM. Colorectal cancer screening in older men and women: qualitative research findings and implications for intervention. J Community Health. 2000;25(3):263–78. doi: 10.1023/a:1005104406934. [DOI] [PubMed] [Google Scholar]
- 12.Wilcox S, Stefanick M. Knowledge and perceived risk of major diseases in middle-aged and older women. Health Psychology. 1999;18:346–53. doi: 10.1037//0278-6133.18.4.346. [DOI] [PubMed] [Google Scholar]
- 13.Weinberg DS, Turner BJ, Wang H, Myers RE, Miller S. A survey of women regarding factors affecting colorectal cancer screening compliance. Prev Med. 2004;38(6):669–75. doi: 10.1016/j.ypmed.2004.02.015. [DOI] [PubMed] [Google Scholar]
- 14.Seeff LC, Nadel MR, Klabunde CN, et al. Patterns and predictors of colorectal cancer test use in the adult U.S. population. Cancer. 2004;100(10):2093–103. doi: 10.1002/cncr.20276. [DOI] [PubMed] [Google Scholar]
- 15.Lipkus IM, Skinner CS, Green LS, Dement J, Samsa GP, Ransohoff D. Modifying attributions of colorectal cancer risk. Cancer Epidemiol Biomarkers Prev. 2004;13(4):560–6. [PubMed] [Google Scholar]
- 16.Harewood GC, Wiersema MJ, Melton LJ., 3rd. A prospective, controlled assessment of factors influencing acceptance of screening colonoscopy. Am J Gastroenterol. 2002;97(12):3186–94. doi: 10.1111/j.1572-0241.2002.07129.x. [DOI] [PubMed] [Google Scholar]
- 17.Horton JA, Cruess DF, Pearse WH. Primary and preventive care services provided by obstetrician-gynecologists. Obstet Gynecol. 1993;82(5):723–6. [PubMed] [Google Scholar]
- 18.Weinrich SP, Weinrich MC, Boyd MD, Johnson E, Frank-Stromborg M. Knowledge of colorectal cancer among older persons. Cancer Nurs. 1992;15(5):322–30. [PubMed] [Google Scholar]
- 19.Prochaska J, DiClemente C. Toward a comprehensive model of change. In: Miller W, Heather N, editors. Treating addictive behaviors: Process of change. Plenum Press; New York: 1986. pp. 3–27. [Google Scholar]
- 20.Prochaska JO, DiClemente CC, Norcross JC. In search of how people change. Applications to addictive behaviors. Am Psychol. 1992;47(9):1102–14. doi: 10.1037//0003-066x.47.9.1102. [DOI] [PubMed] [Google Scholar]
- 21.Prochaska JO, Redding CA, Harlow LL, Rossi JS, Velicer WF. The transtheoretical model of change and HIV prevention: a review. Health Educ Q. 1994;21(4):471–86. doi: 10.1177/109019819402100410. [DOI] [PubMed] [Google Scholar]
- 22.Rakowski W. The potential variances of tailoring in health behavior interventions. Ann Behav Med. 1999;21(4):284–9. doi: 10.1007/BF02895959. [DOI] [PubMed] [Google Scholar]
- 23.Radloff L. The CES-D scale: A self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1:385–401. [Google Scholar]
- 24.Horowitz M, Wilner N, Alvarez W. Impact of Event Scale: a measure of subjective stress. Psychosom Med. 1979;41(3):209–18. doi: 10.1097/00006842-197905000-00004. [DOI] [PubMed] [Google Scholar]
- 25.Miller SM. Monitoring versus blunting styles of coping with cancer influence the information patients want and need about their disease. Implications for cancer screening and management. Cancer. 1995;76(2):167–77. doi: 10.1002/1097-0142(19950715)76:2<167::aid-cncr2820760203>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 26.Harrell F. Regression Modeling Strategies. Springer; New York: 2001. [Google Scholar]
- 27.Raghunathan T. A multivariate technique for multiply imputing missing values using a sequence of regression models. Survey Methodology. 2001;(27):85–95. [Google Scholar]
- 28.Steptoe A. An abbreviated version of the Miller Behavioral Style Scale. Br J Clin Psychol. 1989;28(Pt 2):183–4. doi: 10.1111/j.2044-8260.1989.tb00830.x. [DOI] [PubMed] [Google Scholar]
- 29.Hughes E, McCracken M, Roberts H, et al. Surveillance for certain health behaviors among states and selected local areas--behavioral risk factor surveillance system, United States, 2004. MMWR Surveill Summ. 2006;55(7):1–124. [PubMed] [Google Scholar]
- 30.Donovan JM, Syngal S. Colorectal cancer in women: an underappreciated but preventable risk. J Womens Health. 1998;7(1):45–8. doi: 10.1089/jwh.1998.7.45. [DOI] [PubMed] [Google Scholar]
- 31.Stockwell DH, Woo P, Jacobson BC, et al. Determinants of colorectal cancer screening in women undergoing mammography. Am J Gastroenterol. 2003;98(8):1875–80. doi: 10.1111/j.1572-0241.2003.07577.x. [DOI] [PubMed] [Google Scholar]
- 32.Turner BJ, Weiner M, Berry SD, Lillie K, Fosnocht K, Hollenbeak CS. Overcoming poor attendance to first scheduled colonoscopy: a randomized trial of peer coach or brochure support. J Gen Intern Med. 2008;23(1):58–63. doi: 10.1007/s11606-007-0445-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ko CW, Riffle S, Shapiro JA, et al. Incidence of minor complications and time lost from normal activities after screening or surveillance colonoscopy. Gastrointest Endosc. 2007;65(4):648–56. doi: 10.1016/j.gie.2006.06.020. [DOI] [PubMed] [Google Scholar]
- 34.van Dijk S, Otten W, van Asperen CJ, et al. Feeling at risk: how women interpret their familial breast cancer risk. Am J Med Genet A. 2004;131(1):42–9. doi: 10.1002/ajmg.a.30322. [DOI] [PubMed] [Google Scholar]
- 35.Bloom JR, Stewart SL, Oakley-Girvans I, Banks PJ, Chang S. Family history, perceived risk, and prostate cancer screening among African American men. Cancer Epidemiol Biomarkers Prev. 2006;15(11):2167–73. doi: 10.1158/1055-9965.EPI-05-0738. [DOI] [PubMed] [Google Scholar]
- 36.Thompson RS, Michnich ME, Gray J, Friedlander L, Gilson B. Maximizing compliance with hemoccult screening for colon cancer in clinical practice. Med Care. 1986;24(10):904–14. doi: 10.1097/00005650-198610000-00004. [DOI] [PubMed] [Google Scholar]
- 37.Miller C, Kruus L. Tuning in and tuning out: Confronting the effects of confrontation. In: Krohne H, editor. Attention and Avoidance. Hogrefe & Huber; Seattle: 1993. [Google Scholar]
- 38.Halvorsen PA, Selmer R, Kristiansen IS. Different ways to describe the benefits of risk-reducing treatments: a randomized trial. Ann Intern Med. 2007;146(12):848–56. doi: 10.7326/0003-4819-146-12-200706190-00006. [DOI] [PubMed] [Google Scholar]
- 39.Woloshin S, Schwartz LM, Welch HG. The effectiveness of a primer to help people understand risk: two randomized trials in distinct populations. Ann Intern Med. 2007;146(4):256–65. doi: 10.7326/0003-4819-146-4-200702200-00004. [DOI] [PubMed] [Google Scholar]
- 40.Lipkus IM, Rimer BK, Halabi S, Strigo TS. Can tailored interventions increase mammography use among HMO women? Am J Prev Med. 2000;18(1):1–10. doi: 10.1016/s0749-3797(99)00106-3. [DOI] [PubMed] [Google Scholar]
- 41.McQueen A, Vernon SW, Meissner HI, Klabunde CN, Rakowski W. Are there gender differences in colorectal cancer test use prevalence and correlates? Cancer Epidemiol Biomarkers Prev. 2006;15(4):782–91. doi: 10.1158/1055-9965.EPI-05-0629. [DOI] [PubMed] [Google Scholar]
- 42.Nadel MR, Shapiro JA, Klabunde CN, et al. A national survey of primary care physicians' methods for screening for fecal occult blood. Ann Intern Med. 2005;142(2):86–94. doi: 10.7326/0003-4819-142-2-200501180-00007. [DOI] [PubMed] [Google Scholar]