Abstract
An interplay of mechanical and chemical factors is integral to cartilage maintenance and/or degeneration. Interleukin-1 (IL-1) is a pro-inflammatory cytokine that is present at elevated concentrations in osteoarthritic joints and initiates the rapid degradation of cartilage when cultured in vitro. Several short-term studies have suggested that applied dynamic deformational loading may have a protective effect against the catabolic actions of IL-1. In the current study we examine whether the long-term (42 days) application of dynamic deformational loading on chondrocyte-seeded agarose constructs can mitigate these catabolic effects. Three studies were carried out using two IL-1 isoforms (IL-1α and IL-1β) in chemically defined medium supplemented with a broad range of cytokine concentrations and durations. Physiologic loading was unable to counteract the long-term catabolic effects of IL-1 under any of the conditions tested, and in some cases led to further degeneration over unloaded controls.
Keywords: Cartilage Tissue Engineering, Inflammatory Cytokines, Interlukin-1, Physical stimulus
Introduction
Articular cartilage is a specialized connective tissue that bears load and reduces friction across moving joints. It is composed of an extracellular matrix that contains no nerves or blood vessels and relatively few cells. Articular cartilage does not heal well, but instead often degenerates further, leading to pain and loss of function (Hangody and Modis 2006). Tissue-engineering offers great hope for expanding the range of treatment options by generating healthy replacement cartilage from a combination of isolated, living cells embedded in a scaffold carrier (Cima et al. 1991; Capito and Spector 2003; Hung et al. 2004; Giannoudis and Pountos 2005; Giannoni and Cancedda 2006; Habibovic et al. 2006; Raghunath et al. 2007; Schulz and Bader 2007).
In order to function within a defect site an engineered implant must have both the mechanical competency and the chemical fortitude to survive and flourish within an environment that is likely to contain potent catabolic mediators stemming from chronic inflammation (van den Berg and Bresnihan 1999; Schiff 2000; Lotz 2001; Smeets et al. 2003). Interleukin-1 (IL-1) is a pro-inflammatory cytokine that has been shown to be elevated in osteoarthritis (Towle et al. 1997) and leads to cartilage degradation in in vitro tests (Ratcliffe et al. 1986; Morales and Hascall 1989; Temple et al. 2006). The catabolic effects of IL-1 may be especially pronounced in underdeveloped engineered cartilage (Xu et al. 1996; Cook et al. 2000; Rotter et al. 2005; Lima et al. 2008) whose chondrocytes are not yet fully embedded in a dense chondroprotective cartilaginous extracellular matrix (Li et al. 2003).
As the interplay of mechanical and chemical factors is integral to cartilage maintenance and/or degeneration, it motivates researchers to examine the combined effects of chemical and mechanical stimuli (Mauck et al. 2003). The chondrocyte-seeded agarose system has clear basic science and tissue-engineering applications in which both chemical and mechanical stimuli can be carefully controlled. Several short-term studies using an agarose culture model have suggested that applied loading may have a protective effect against the catabolic actions of IL-1 (Gassner et al. 1999; Honda et al. 2000; Xu et al. 2000; Agarwal et al. 2001; Chowdhury et al. 2001)
Chowdury et al. have shown that dynamic loading counteracts IL-1-induced increase of nitric oxide (NO) and PGE2 in chondrocyte-seeded agarose constructs (Chowdhury et al. 2001; Chowdhury et al. 2003). Mio and co-workers have reported that RNA expression of anabolic factors (aggrecan and type II collagen) in chondrocyte-seeded agarose constructs increases with application of dynamic loading for 24 hours
Long-term culture of chondrocytes in agarose results in the formation of a functional matrix (Buschmann et al. 1992; Buschmann et al. 1995) and applied deformational loading can enhance development of tissue properties (Mauck et al. 2000; Mauck et al. 2003; Lima et al. 2007). The culture system preserves the chondrocyte phenotype by maintaining a physiologic three-dimensional environment and produces extracellular matrix components with a proteoglycan composition and corresponding Young’s modulus similar to that of native cartilage (Mouw et al. 2005)
In the current in vitro study we examine the effects of IL-1 on the mechanical and biochemical properties of engineered tissue and explore whether the long-term application of physiological levels of dynamic deformational loading on chondrocyte-seeded agarose constructs can mitigate these effects.
Materials and Methods
A. Experimental Design
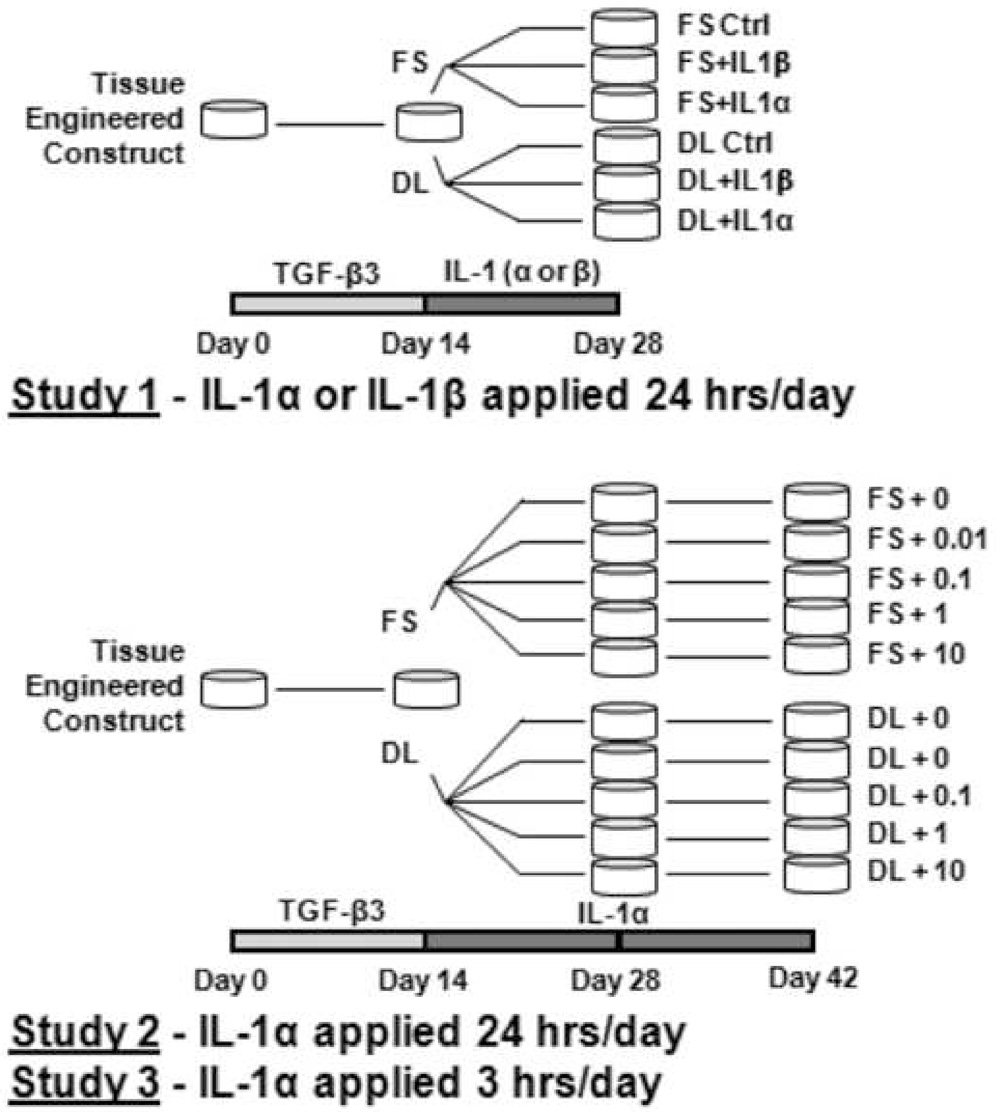
Three studies were carried out in this set of experiments. In Study 1 (n=5–7/group) we establish a broad range of response by culturing constructs for 28 days with or without dynamic deformational loading in a chemically-defined medium with one of two IL-1 isoforms (IL-1α and IL-1β, 10 ng/mL). Based on these results, we selected the most potent isoform (IL-1α) for the remaining two studies. In Study 2 (n=6–9/group) we examine the dependence on cytokine concentration by testing a logarithmic range from 0.01 to 10 ng/mL over 42 days in culture. In Study 3 (n=8/group) we repeat the logarithmic range of concentrations, however we restrict IL-1 exposure to periods of deformational loading only. Thus in Study 3 IL-1 is present for 3 hours/day (for both loaded and unloaded groups) while in Studies 1 and 2 IL-1 is present 24 hours/day. The timelines of the studies are detailed in Figure 1. Each study was performed independently, using individual cell isolations pooled from different animals, and repeated twice. Values reported were averaged across repeat studies
Figure 1.
Study 1: (Testing IL-1 isoform Dependence) Tissue engineered constructs were cultured for 14 days in a chemically-defined medium (CD) supplemented with TGF-β3. For the subsequent 14 days constructs were exposed to either IL-1α or IL-1β and either dynamic loaded 3 hrs/day (DL) or maintained in free-swelling (FS) without TGF-β3. Study 2: (Testing IL-1 concentration): Constructs were cultured for 14 days in CD medium with TGF-β3 and for the next 28 days were exposed to IL-1α in a logarithmic range of concentrations (0, 0.01, 0.1, 1, or 10 ng/mL) with or without loading. Study 3: (Testing IL-1 exposure time): Constructs were cultured as in Study 2 with the exception that IL-1α was added only during loading periods (3hrs/day).
B. Cell Isolation
Articular cartilage was harvested from bovine carpo-metacarpal (CMC) joints of freshly slaughtered 1–3 month old calves. The cartilage tissue was digested in high-glucose Dulbecco's Modified Eagle's Medium (hgDMEM, 7.5 ml/g) with collagenase type IV (390 activity units/ml, Sigma Chemicals, St. Louis, MO) for 11 hours at 37°C with stirring. The resulting cell suspension was filtered, combined, and cast into slabs with a final cell concentration of 30 × 106 in 2% agarose (Type VII, Sigma). The slabs were cored to final construct dimensions (Ø0.4cm × 0.23 cm) and maintained in culture in one of two medium formulations (described below) for up to 42 days depending on the study (Figure 1).
C. Growth medium
Chemically-defined (CD) medium consisted of hgDMEM supplemented with lx PSF, 0.1 µM dexamethasone, 50 µg/mL ascorbate 2-phosphate, 40 µg/mL L-proline, 100 µg/mL sodium pyruvate, and 1X ITS+ premix (insulin, human transferrin, and selenous acid, Becton Dickinson, Franklin Lakes, NJ). CD medium was further supplemented with 10 ng/mL of TGF-β3 (R&D Systems, Minneapolis, MN) for the first 14 days of culture. All culture media was changed every other day. This protocol has been shown to promote significant matrix elaboration that results in engineered tissue with native equilibrium modulus and proteoglycan content (Lima et al. 2007) . The final mechanical and biochemical properties attained within the culture period can vary depending on the cell isolation, as is typical of the native tissue.
D. Interleukin Supplementation
In Study 1, cell-seeded agarose constructs were cultured for 14 days in CD medium without IL-1. For the subsequent 14 days constructs were exposed to either IL-1α or IL-1β at 10 ng/mL and either dynamic loaded 3 hrs/day (DL) or maintained in free-swelling (FS). Mechanical testing and biochemical analysis was carried out as described below on day 0, day 14, and day 28.
In Studies 2 and 3, constructs were cultured for 14 days in CD medium without IL-1 as above. In Study 2, for the subsequent 28 days the culture medium was supplemented with to 0.01, 0.1, 1, or 10 ng/mL IL-1α and constructs were loaded or remained in free swelling. In Study 3, for the subsequent 28 days the constructs were exposed to 0.1, 1, or 10 ng/mL IL-1α during loading times only (both FS and DL constructs were transferred to Petri dishes with new IL-1α supplemented medium). For concentrations and time-courses see Figure 1.
E. Loading Protocol
Dynamic sinusoidal strain was applied at 1 Hz, with a nominal amplitude of 5% (10% peak-to-peak deformation) above a 10% tare strain, in unconfined compression with impermeable platens. Loading was applied continuously for 3 hrs/day, 5 days/week, beginning on day 14 (previously found to be optimal CD medium formulations (Lima et al. 2007)). Built-in compliance in the loading devices compensated for increasing stiffness in developing constructs, altering the load and displacement profiles and circumventing platen lift-off through the entire culture period as described previously (Lima et al. 2007).
F. Material Testing
Cylindrical constructs were tested in unconfined compression using a custom computer-controlled testing system (Soltz and Ateshian 1998). Samples were loaded to 10% strain at a strain rate of 0.05% strain/sec, after an initial 0.02 N tare load. After achieving stress-relaxation equilibrium, the unconfined compression dynamic modulus G* was measured by superimposing 2% peak to peak sinusoidal strain at 1 Hz.
G. Biochemical Content
The biochemical content of each sample was assessed according to wet weight. Samples were digested in proteinase-K overnight at 56°C and analyzed for either glycosaminoglycan (GAG) content using the 1,9-dimethylmethylene blue dye-binding assay (Farndale et al. 1982) or ortho-hydroxyproline (OHP) content via a colorimetric assay by reaction with chloramine T and dimethylaminobenzaldehyde (Stegemann and Stalder 1967), as described previously (Kelly et al. 2005). Cell viability (not shown) was measured via Live/Dead assay (Molecular Probes).
H. Histological Analysis
Samples were fixed in acid formalin ethanol (Lin et al. 1997), paraffin embedded, sectioned (8 µm thick), and stained with Safranin 0 (1% in dH2O, pH 6.7) to view proteoglycan distribution. To ensure consistency, all time points and experimental groups were stained at the same time in a single batch, with native explant groups serving as controls. Distribution of intensity across a section was quantified using ImageJ (NIH). Grey scale intensity was normalized to mean intensity at center of histological section for the ordinate and percentage across the cross section for the abscissa and averaged for all samples in each group.
I. Statistics
Statistics were performed with the Statistica (Statsoft, Tulsa, OK) software package. Groups were examined for significant differences by two-way analysis of variance (α = 0.05) using Tukey’s Honest Significant Difference Test (HSD) with EY, G*, GAG, or OHP as the dependent variable, and time in culture and loading condition as the independent variables.
Results
Study 1 (Testing Medium and IL-1 isoform dependency)
All experimental groups cultured in CD medium without the addition of IL-1 grew in culture, developing significant differences in EY, G*, and GAG by day 28 when compared to day 14 (Figure 2A, 2B, 2C).
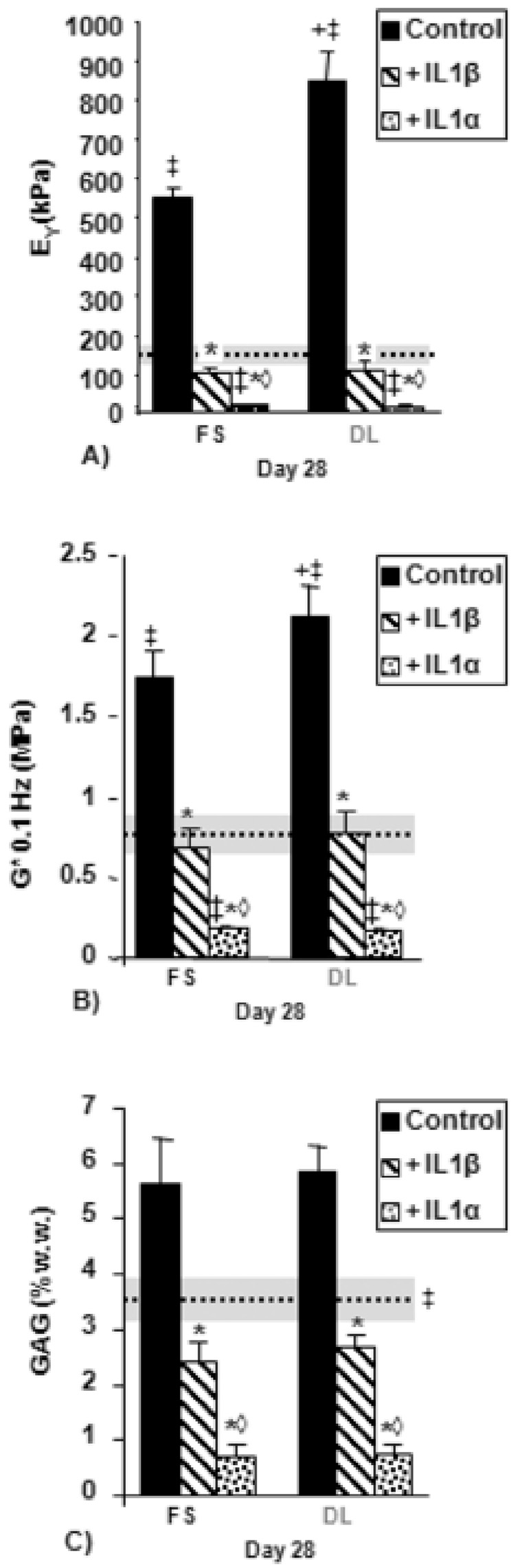
Figure 2.
A) Equilibrium Young’s modulus, B) Dynamic modulus at 0.1 Hz, and C) GAG (% wet weight) for tissue-engineered constructs (TE) cultured first in free-swelling (FS) culture (14 days) and then an additional 14 days in either FS or dynamic loading (DL) culture in chemically-defined (CD) medium with IL-1α or IL-1β. Dotted line indicates day 14 time point. Horizontal shaded bar indicates standard deviation. ‡p<0.05 vs. day 14, *p<0.01 vs. FS or DL control, +p<0.03 for DL Control vs. FS control, ◊p < 0.01 vs. IL-1β.
The application of dynamic loading resulted in significantly increased mechanical properties (Figures 2A and 2B) by day 28 compared to FS controls, but with no significant differences in GAG (Figures 2C) or collagen (not shown).
The presence of interleukin resulted in lower EY, G*, and GAG when compared to IL-1 free controls, regardless of the isoform used (Figures 2A and 2B). The application of dynamic loading did not mitigate this effect; both the DL+IL-1α and the DL+IL-1β groups developed no significant mechanical or biochemical differences compared to the FS+IL-1α or the FS+IL-1β groups.
Between the two isoforms, IL-1α was the more potent; both FS+IL-1α and DL+IL-1α were significantly lower in EY, G*, and GAG compared to FS+IL-1β and DL+IL-1β, but there were no significant differences in collagen values between the two groups (not 4 shown).
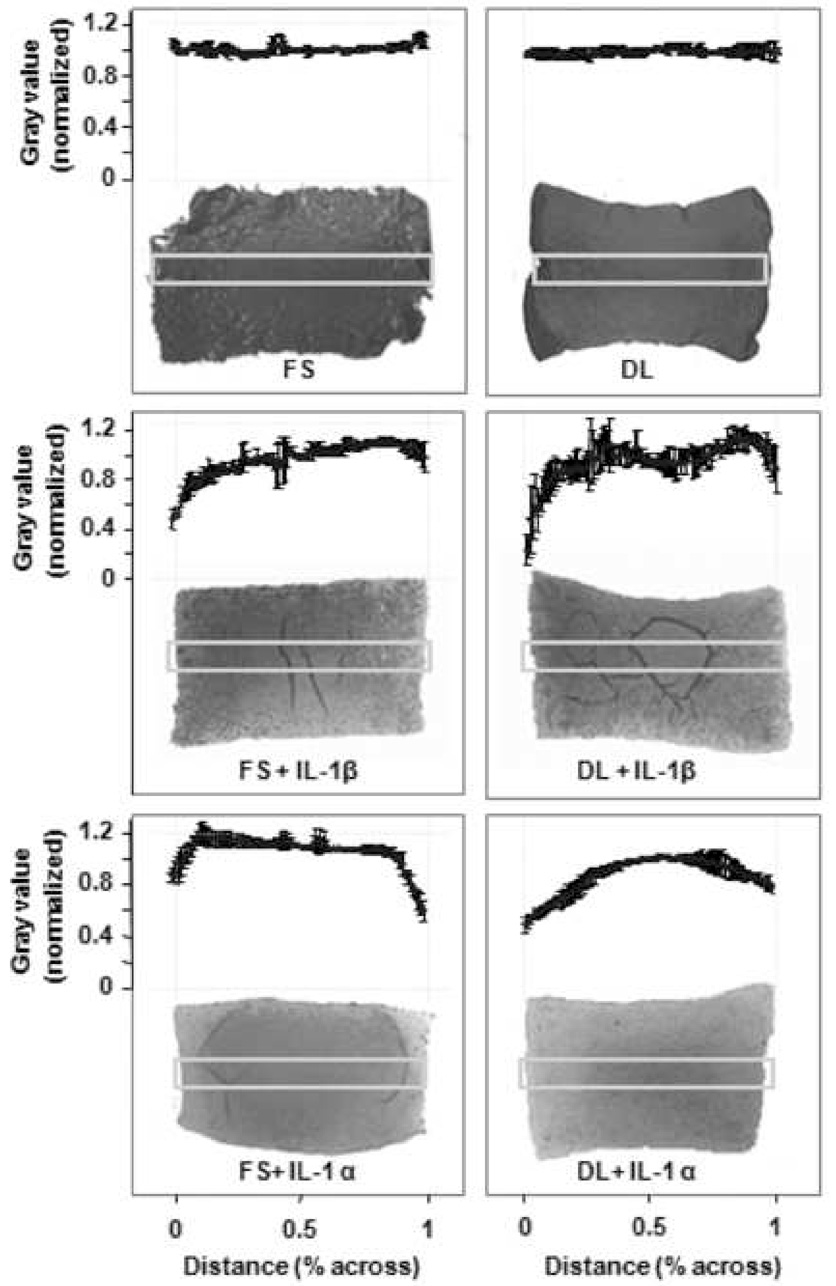
Safranin-O staining indicated clear differences between the control groups and the cytokine-treated groups: whereas both FS and DL control groups had a largely uniform distribution of staining across their cross-sections, constructs that had been treated with IL-1α or IL-1β had an uneven distribution of staining, with the majority of GAG loss occurring at the periphery (Figure 3).
Figure 3.
Safranin O staining for GAG comparing free-swelling (FS) and dynamically-loaded (DL) constructs with IL-1α, IL-1β, or as untreated control (Study 1, day 35, chemically-defined medium). Graph indicates grey scale intensity normalized to mean intensity at center of histological section for the ordinate and percentage across the cross section for the abscissa (each data point represents 1% across). Boxes indicate region used for quantification. Graphs represent the average of four samples within each group.
Study 2 (Testing the effect of IL-1α concentration)
All experimental groups cultured in CD medium without the addition of IL-1 grew in culture, developing significant differences in EY, G*, and GAG at each time point tested (Figure 4A, 4B, 4C).
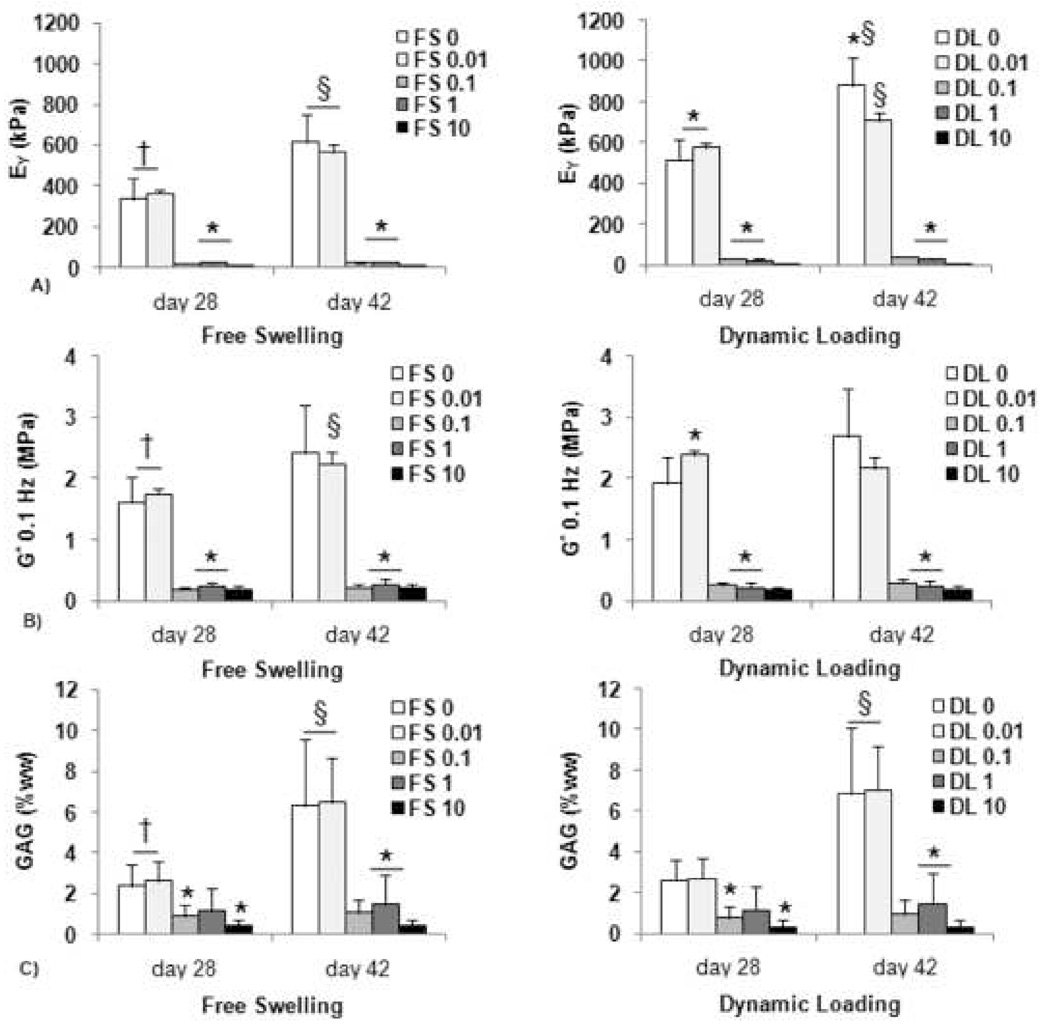
Figure 4.
A) Equilibrium Young’s modulus, B) Dynamic modulus at 0.1 Hz, and C) GAG (% wet weight) for constructs cultured first for 14 days in free-swelling (FS) culture and then an additional 14 days in either FS or dynamic loading (DL) culture in chemically-defined medium. DL groups were additionally exposed to IL-1α in a logarithmic range of concentrations (0, 0.01, 0.1, 1, or 10 ng/mL). *p<0.01 for vs. FS 0 and FS 0.01 on same day. †p<0.05 for FS on day 28 vs. DL on day 42. §p<0.05 for day 42 groups vs. same groups on day 28.
The application of dynamic loading resulted in significantly increased mechanical properties (Figures 4A and 4B) compared to FS controls by day 28. There continued to be a significant increase in EY for the DL control by day 42, but with no further significant changes in G*, GAG (Figures 4B, 4C). Collagen values were unaffected by supplementation with IL-1α (not shown).
Exposure to the cytokine seemed to have an all-or nothing effect. At concentrations above 0.01 ng/mL constructs did not develop mechanical or biochemical properties above their day 14 values, with no significant differences between the treated groups. This result was true regardless of whether constructs were maintained in DL or FS culture. Exposure at the lowest dosage tested (0.01 ng/mL) did not inhibit development, with no significant differences from untreated controls on day 28 or day 42 (Figure 4). There were no detrimental effects on cell viability (not shown) with any of the concentrations tested.
Study 3 (IL-I added only during loading)
Similarly to Study 2, all experimental groups grew and developed significant higher EY, 12 G*, and GAG at each time point tested during the 42 day culture period and the application of dynamic loading significantly increased EY and G* compared to FS controls by day 42 (not shown). The 3 hour daily exposure to IL-1α at 0.1 and 1 ng/mL, however, did not result in significant mechanical or biochemical differences from controls regardless of the dosage. At the highest concentration (10 ng/mL) IL-1α did result in significantly lower GAG and this was not counteracted by the application of dynamic loading.
Discussion
The findings of the current set of studies suggest that physiologic loading is unable to counteract the long-term catabolic effects of IL-1 in chondrocyte-seeded agarose constructs. Under the conditions of this study, interleukin-induced tissue degradation was apparent from the decreased material and biochemical properties (but with no loss of cell-viability) after tissue treatment irrespective of the application of loading. Conversely, the stimulatory effects of mechanical loading, in the absence of IL-1, was noted by the significantly increased stiffness of dynamically-loaded constructs compared to free-swelling constructs.
It was our goal to establish a controlled, long-term in vitro model that approximates both the chemical and mechanical environment within the joint. In Study 1 we attempted to establish a broad foundation for this model by examining the effect of dynamic deformational loading on constructs cultured in a chemically defined medium formulation and exposed to one of two cytokine conditions.
We adopted a dynamic deformational loading tissue culture protocol that we have demonstrated to foster development of functional tissue engineered cartilage (Lima et al. 2007). This protocol is based on the sequential application of growth factor (TGF-β3) followed by physiologic deformational loading (i.e. loading is introduced starting at day 14 in culture when TGF-β3 is discontinued). As such, we chose to introduce interleukin treatment coincident with applied loading, either during the 3 hour loading period (Study 3) or continuously in culture (Study 1, 2).
Similarly, both IL-1α and IL-1β iso-forms have been widely used as inducers of inflammation and degradation in experimental models of osteoarthritis (Smith et al. 1991; Pattoli et al. 2005). We found that IL-1α induced a stronger response than IL-1β in our bovine chondrocytes-seeded agarose constructs; however the application of dynamic deformational loading did not ameliorate these catabolic effects over the long-term with either iso-form.
Interestingly, the decrease in GAG content seemed greatest at the periphery of the dynamically loaded and interleukin (DL+IL-1α) group, indicating that dynamic loading may not only fail to protect against degenerative effects, but increase the rate of matrix loss. There are a number of experimental parameters to consider that together will modulate the degree of cellular exposure to the inflammatory cytokine. These include the concentration of IL-1, the day in culture that IL-1 was first introduced, the total number of days of IL-1 treatment, and the amount of time per day of IL-1 treatment. The application of dynamic deformational loading may be yet another way to modulate the degree of cellular exposure to the cytokine.
Theoretical analysis of dynamic deformational loading of neutrally charged gels (such as agarose) predict that loading can lead to enhanced transport of solutes such as growth factors and cytokines (Mauck et al. 2003; Mauck et al. 2003; Chahine et al. 2005). The transient concentration of these solutes within the gel during loading can even be enhanced to values many times above the surrounding bathing solution. Thus, although the application of dynamic deformational loading has clearly been shown to reduce the catabolic response of chondrocytes in the short term –reducing or abolishing IL-1β induced release of NO and PGE2 (Chowdhury et al. 2001; Chowdhury et al. 2006), we speculated that long-term development may be inhibited by higher concentrations of the cytokine within the gel.
We indirectly examined this possibility in Studies 2 and 3 by modulating the dosage and exposure time of IL-1α. We anticipated that the application of dynamic deformational loading at lower concentrations would result in increased degradation of the dynamically loaded group over the free swelling group, as these DL constructs would be exposed to transitory spikes in the concentration of IL-1 above that of free-swelling. This was not the case. In Study 2, modulating the concentration of IL-1 did not result in a gradient of mechanical and biochemical properties, but instead had an equal degradative effect at 0.1, 1, and 10 ng/mL and no effect at a 0.01 ng/mL concentration. Thus, exposure to the cytokine seemed to have an all-or nothing effect once the cytokine dosage was above an activation threshold.
We were interested to see if there was evidence in the literature of an activation threshold beyond which further increases in IL-1 concentration would not lead to further degradative response. In a study by Kuroki et al (Kuroki et al. 2005), canine chondrocytes were cultured in agarose for 9 days in a serum-free medium with a gradient of IL-1α and IL-1β concentrations (20, 50, 100 ng/mL). They found all concentrations were equally inhibitory as measured by RNA expression. Chowdhury et al also examined short-term IL-1β response in both bovine (Chowdhury et al. 2001) and human (Chowdhury et al. 2006) chondrocytes in agarose with a range (0.1–100 ng/mL) of concentrations. They found that nitrite and PGE2 production by bovine chondrocytes increased in a step-like manner with increasing IL-1β concentrations, but that the human response increased rapidly at 0.1 ng/mL with no further significant increases at higher concentrations of cytokine. Thus although the evidence in the literature is somewhat mixed, the results of the current investigation lead us to believe that transient increases in IL-1α concentration due to loading are not in themselves responsible for further cell-mediated catabolic degradation.
The transient concentration of cytokine may also be critical in interpreting the results of Study 3 where IL-1α was included for 3 hours/day. In this study there was little or no effect of the cytokine regardless of the bathing concentration. Since the solutes take time to diffuse through the scaffold, it is likely that the actual cellular exposure to IL-1α was at a significantly lower concentration than the bathing medium due to the relatively short treatment duration (3 hours). This limited concentration and transient exposure to the cytokine could account for the lack of cytokine-induced tissue degradation. In an arthritic patient exposure to pro-inflammatory IL-1α within the joint is likely to be of a near constant duration (as in Study 2) rather than transitory in nature (as in Study 3) and at average concentration of 0.17 ng/mL (Marks and Donaldson 2005; Pattoli et al. 2005)
It is important to note that results of the current experiments differ from those in the literature that have reported protective effects of applied loading on IL-1 treated chondrocyte constructs (Chowdhury et al. 2001). In these earlier studies, experiments were performed on agarose encapsulated chondrocytes before significant matrix was elaborated and for short culture durations, whereas our study examined cultures grown as long as 42 days with concomitant matrix elaboration and a much longer duration of interleukin exposure. Differences in matrix composition between engineered constructs can lead to disparities in the manner by which IL-1, physical stimuli, or the combination of both is presented to chondrocytes. At early time points in culture, chondrocyte deformation is similar to that which is applied to the agarose matrix, but in as little as six days cells can elaborate a pericellular matrix that is stiffer than the surrounding hydrogel matrix, shielding the chondrocytes from full deformation (Lee and Bader 1995). At later culture times, coalescence of elaborated matrix re-establishes loading-induced cell deformation in the culture system (Guilak et al. 1995; Chahine et al. 2007). The results of the current study suggest the application of dynamic deformational loading is insufficient to protect against degradation over the long term.
A greater understanding of the potential interplay between mechanical stimuli and cytokines may help to elucidate mechanisms that underlie cartilage degeneration in OA, including the effects of load, proinflammatory cytokines, and their combination on both intact articular cartilage and on developing engineered tissue. The findings of this study reject the hypothesis that physiologic loading counteracts the catabolic effects of IL-1 in chondrocyte-seeded agarose constructs.
Acknowledgments
The research presented in this manuscript was funded in part by the National Institute for Health grants numbered: AR46568 and AR53530
Footnotes
Conflict of Interest Statement
There were no financial and personal relationships that could have inappropriately influenced the work presented herein.
References
- Agarwal S, Long P, Gassner R, Piesco NP, Buckley MJ. Cyclic tensile strain suppresses catabolic effects of interleukin-1beta in fibrochondrocytes from the temporomandibular joint. Arthritis Rheum. 2001;443:608–617. doi: 10.1002/1529-0131(200103)44:3<608::AID-ANR109>3.0.CO;2-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buschmann MD, Gluzband YA, Grodzinsky AJ, Hunziker EB. Mechanical compression modulates matrix biosynthesis in chondrocyte/agarose culture. J Cell Sci. 1995;108(Pt 4):1497–1508. doi: 10.1242/jcs.108.4.1497. [DOI] [PubMed] [Google Scholar]
- Buschmann MD, Gluzband YA, Grodzinsky AJ, Kimura JH, Hunziker EB. Chondrocytes in agarose culture synthesize a mechanically functional extracellular matrix. J Orthop Res. 1992;106:745–758. doi: 10.1002/jor.1100100602. [DOI] [PubMed] [Google Scholar]
- Capito RM, Spector M. Scaffold-based articular cartilage repair. IEEE Eng Med Biol Mag. 2003;225:42–50. doi: 10.1109/memb.2003.1256271. [DOI] [PubMed] [Google Scholar]
- Chahine NO, Hung CT, Ateshian GA. In-situ measurements of chondrocyte deformation under transient loading. Eur Cell Mater. 2007;13:100–111. doi: 10.22203/ecm.v013a11. discussion 111. [DOI] [PubMed] [Google Scholar]
- Chahine NO, Lima EG, Wei V, Hung CT, Ateshian GA. Dynamic deformational loading significantly enhances the transport of dextran molecules into agarose hydrogels. Washington, D.C.: ORS; 2005. [Google Scholar]
- Chowdhury TT, Bader DL, Lee DA. Dynamic compression inhibits the synthesis of nitric oxide and pge(2) by il-1beta-stimulated chondrocytes cultured in agarose constructs. Biochem Biophys Res Commun. 2001;2855:1168–1174. doi: 10.1006/bbrc.2001.5311. [DOI] [PubMed] [Google Scholar]
- Chowdhury TT, Bader DL, Lee DA. Dynamic compression counteracts il-1 beta-induced release of nitric oxide and pge2 by superficial zone chondrocytes cultured in agarose constructs. Osteoarthritis Cartilage. 2003;119:688–696. doi: 10.1016/s1063-4584(03)00149-3. [DOI] [PubMed] [Google Scholar]
- Chowdhury TT, Bader DL, Lee DA. Dynamic compression counteracts il-1 beta induced inos and cox-2 activity by human chondrocytes cultured in agarose constructs. Biorheology. 2006;433–434:413–429. [PubMed] [Google Scholar]
- Cima LG, Vacanti JP, Vacanti C, Ingber D, Mooney D, Langer R. Tissue engineering by cell transplantation using degradable polymer substrates. J Biomech Eng. 1991;1132:143–151. doi: 10.1115/1.2891228. [DOI] [PubMed] [Google Scholar]
- Cook JL, Anderson CC, Kreeger JM, Tomlinson JL. Effects of human recombinant interleukin-1beta on canine articular chondrocytes in three-dimensional culture. Am J Vet Res. 2000;617:766–770. doi: 10.2460/ajvr.2000.61.766. [DOI] [PubMed] [Google Scholar]
- Farndale RW, Sayers CA, Barrett AJ. A direct spectrophotometric microassay for sulfated glycosaminoglycans in cartilage cultures. Connect Tissue Res. 1982;94:247–248. doi: 10.3109/03008208209160269. [DOI] [PubMed] [Google Scholar]
- Gassner R, Buckley MJ, Georgescu H, Studer R, Stefanovich-Racic M, Piesco NP, Evans CH, Agarwal S. Cyclic tensile stress exerts anti anflammatory actions on chondrocytes by inhibiting inducible nitric oxide synthase. J Immunol. 1999;1634:2187–2192. [PMC free article] [PubMed] [Google Scholar]
- Giannoni P, Cancedda R. Articular chondrocyte culturing for cell-based cartilage repair: Needs and perspectives. Cells Tissues Organs. 2006;1841:1–15. doi: 10.1159/000096946. [DOI] [PubMed] [Google Scholar]
- Giannoudis PV, Pountos I. Tissue regeneration. The past, the present and the future. Injury. 2005;36 Suppl 4:S2–S5. doi: 10.1016/j.injury.2005.10.006. [DOI] [PubMed] [Google Scholar]
- Guilak F, Ratcliffe A, Mow VC. Chondrocyte deformation and local tissue strain in articular cartilage: A confocal microscopy study. J Orthop Res. 1995;133:410–421. doi: 10.1002/jor.1100130315. [DOI] [PubMed] [Google Scholar]
- Habibovic P, Woodfield T, de Groot K, van Blitterswijk C. Predictive value of in vitro and in vivo assays in bone and cartilage repair--what do they really tell us about the clinical performance? Adv Exp Med Biol. 2006;585:327–360. doi: 10.1007/978-0-387-34133-0_22. [DOI] [PubMed] [Google Scholar]
- Hangody L, Modis L. surgical treatment options for weight bearing articular surface defect. Orv Hetil. 2006;14746:2203–2212. [PubMed] [Google Scholar]
- Honda K, Ohno S, Tanimoto K, Ijuin C, Tanaka N, Doi T, Kato Y, Tanne K. The effects of high magnitude cyclic tensile load on cartilage matrix metabolism in cultured chondrocytes. Eur J Cell Biol. 2000;799:601–609. doi: 10.1078/0171-9335-00089. [DOI] [PubMed] [Google Scholar]
- Hung CT, Mauck RL, Wang CC, Lima EG, Ateshian GA. A paradigm for functional tissue engineering of articular cartilage via applied physiologic deformational loading. Ann Biomed Eng. 2004;321:35–49. doi: 10.1023/b:abme.0000007789.99565.42. [DOI] [PubMed] [Google Scholar]
- Kelly TA, Ng KW, Wang CC, Ateshian GA, Hung CT. Spatial and temporal development of chondrocyte-seeded agarose constructs in free-swelling and dynamically loaded cultures. J Biomech. 2005 doi: 10.1016/j.jbiomech.2005.03.031. [DOI] [PubMed] [Google Scholar]
- Kuroki K, Stoker AM, Cook JL. Effects of proinflammatory cytokines on canine articular chondrocytes in a three-dimensional culture. Am J Vet Res. 2005;667:1187–1196. doi: 10.2460/ajvr.2005.66.1187. [DOI] [PubMed] [Google Scholar]
- Lee DA, Bader DL. The development and characterization of an in vitro system to study strain-induced cell deformation in isolated chondrocytes. In Vitro Cell Dev Biol Anim. 1995;3111:828–835. doi: 10.1007/BF02634565. [DOI] [PubMed] [Google Scholar]
- Li KW, Wang AS, Sah RL. Microenvironment regulation of extracellular signal-regulated kinase activity in chondrocytes: Effects of culture configuration, interleukin-1, and compressive stress. Arthritis Rheum. 2003;483:689–699. doi: 10.1002/art.10849. [DOI] [PubMed] [Google Scholar]
- Lima EG, Bian L, Ng KW, Mauck RL, Byers BA, Tuan RS, Ateshian GA, Hung CT. The beneficial effect of delayed compressive loading on tissue-engineered cartilage constructs cultured with tgf-beta3. Osteoarthritis Cartilage. 2007;159:1025–1033. doi: 10.1016/j.joca.2007.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lima EG, Tan AR, Tai T, Bian L, Stoker AM, Ateshian GA, Cook JL, Hung CT. Differences in interleukin-1 response between engineered and native cartilage. Tissue Eng. 2008 doi: 10.1089/ten.tea.2007.0347. [DOI] [PubMed] [Google Scholar]
- Lin W, Shuster S, Maibach HI, Stern R. Patterns of hyaluronan staining are modified by fixation techniques. J Histochem Cytochem. 1997;458:1157–1163. doi: 10.1177/002215549704500813. [DOI] [PubMed] [Google Scholar]
- Lotz M. Cytokines in cartilage injury and repair. Clin Orthop Relat Res. 2001;391 Suppl:S108–S115. doi: 10.1097/00003086-200110001-00011. [DOI] [PubMed] [Google Scholar]
- Marks PH, Donaldson ML. Inflammatory cytokine profiles associated with chondral damage in the anterior cruciate ligament-deficient knee. Arthroscopy. 2005;2111:1342–1347. doi: 10.1016/j.arthro.2005.08.034. [DOI] [PubMed] [Google Scholar]
- Mauck RL, Hung CT, Ateshian GA. Modeling of neutral solute transport in a dynamically loaded porous permeable gel: Implications for articular cartilage biosynthesis and tissue engineering. J Biomech Eng. 2003;1255:602–614. doi: 10.1115/1.1611512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauck RL, Nicoll SB, Seyhan SL, Ateshian GA, Hung CT. Synergistic action of growth factors and dynamic loading for articular cartilage tissue engineering. Tissue Eng. 2003;94:597–611. doi: 10.1089/107632703768247304. [DOI] [PubMed] [Google Scholar]
- Mauck RL, Soltz MA, Wang CC, Wong DD, Chao PH, Valhmu WB, Hung CT, Ateshian GA. Functional tissue engineering of articular cartilage through dynamic loading of chondrocyte-seeded agarose gels. J Biomech Eng. 2000;1223:252–260. doi: 10.1115/1.429656. [DOI] [PubMed] [Google Scholar]
- Morales TI, Hascall VC. Effects of interleukin-1 and lipopolysaccharides on protein and carbohydrate metabolism in bovine articular cartilage organ cultures. Connect Tissue Res. 1989;192–194:255–275. doi: 10.3109/03008208909043900. [DOI] [PubMed] [Google Scholar]
- Mouw JK, Case ND, Guldberg RE, Plaas AH, Levenston ME. Variations in matrix composition and gag fine structure among scaffolds for cartilage tissue engineering. Osteoarthritis Cartilage. 2005;139:828–836. doi: 10.1016/j.joca.2005.04.020. [DOI] [PubMed] [Google Scholar]
- Pattoli MA, MacMaster JF, Gregor KR, Burke JR. Collagen and aggrecan degradation is blocked in interleukin-1-treated cartilage explants by an inhibitor of ikappab kinase through suppression of metalloproteinase expression. J Pharmacol Exp Ther. 2005;3151:382–388. doi: 10.1124/jpet.105.087569. [DOI] [PubMed] [Google Scholar]
- Raghunath J, Rollo J, Sales KM, Butler PE, Seifalian AM. Biomaterials and scaffold design: Key to tissue-engineering cartilage. Biotechnol Appl Biochem. 2007;46(Pt 2):73–84. doi: 10.1042/BA20060134. [DOI] [PubMed] [Google Scholar]
- Ratcliffe A, Tyler JA, Hardingham TE. Articular cartilage cultured with interleukin 1. Increased release of link protein, hyaluronate-binding region and other proteoglycan fragments. Biochem J. 1986;2382:571–580. doi: 10.1042/bj2380571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotter N, Ung F, Roy AK, Vacanti M, Eavey RD, Vacanti CA, Bonassar LJ. Role for interleukin lalpha in the inhibition of chondrogenesis in autologous implants using polyglycolic acid-polylactic acid scaffolds. Tissue Eng. 2005;111–112:192–200. doi: 10.1089/ten.2005.11.192. [DOI] [PubMed] [Google Scholar]
- Schiff MH. Role of interleukin 1 and interleukin 1 receptor antagonist in the mediation of rheumatoid arthritis. Ann Rheum Dis. 2000;59 Suppl 1:i103–i108. doi: 10.1136/ard.59.suppl_1.i103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz RM, Bader A. Cartilage tissue engineering and bioreactor systems for the cultivation and stimulation of chondrocytes. Eur Biophys J. 2007;364–365:539–568. doi: 10.1007/s00249-007-0139-1. [DOI] [PubMed] [Google Scholar]
- Smeets TJ, Barg EC, Kraan MC, Smith MD, Breedveld FC, Tak PP. Analysis of the cell infiltrate and expression of proinflammatory cytokines and matrix metalloproteinases in arthroscopic synovial biopsies: Comparison with synovial samples from patients with end stage, destructive rheumatoid arthritis. Ann Rheum Dis. 2003;627:635–638. doi: 10.1136/ard.62.7.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith RJ, Chin JE, Sam LM, Justen JM. Biologic effects of an interleukin-1 receptor antagonist protein on interleukin-1-stimulated cartilage erosion and chondrocyte responsiveness. Arthritis Rheum. 1991;341:78–83. doi: 10.1002/art.1780340112. [DOI] [PubMed] [Google Scholar]
- Soltz MA, Ateshian GA. Experimental verification and theoretical prediction of cartilage interstitial fluid pressurization at an impermeable contact interface in confined compression. J Biomech. 1998;3110:927–934. doi: 10.1016/s0021-9290(98)00105-5. [DOI] [PubMed] [Google Scholar]
- Stegemann H, Stalder K. Determination of hydroxyproline. Clin Chim Acta. 1967;182:267–273. doi: 10.1016/0009-8981(67)90167-2. [DOI] [PubMed] [Google Scholar]
- Temple MM, Xue Y, Chen MQ, Sah RL. Interleukin- 1alpha induction of tensile weakening associated with collagen degradation in bovine articular cartilage. Arthritis Rheum. 2006;5410:3267–3276. doi: 10.1002/art.22145. [DOI] [PubMed] [Google Scholar]
- Towle CA, Hung HH, Bonassar LJ, Treadwell BV, Mangham DC. Detection of interleukin-1 in the cartilage of patients with osteoarthritis: A possible autocrine/paracrine role in pathogenesis. Osteoarthritis Cartilage. 1997;55:293–300. doi: 10.1016/s1063-4584(97)80008-8. [DOI] [PubMed] [Google Scholar]
- van den Berg WB, Bresnihan B. Pathogenesis of joint damage in rheumatoid arthritis: Evidence of a dominant role for interleukin-i. Baillieres Best Pract Res Clin Rheumatol. 1999;134:577–597. doi: 10.1053/berh.1999.0047. [DOI] [PubMed] [Google Scholar]
- Xu C, Oyajobi BO, Frazer A, Kozaci LD, Russell RG, Hollander AP. Effects of growth factors and interleukin-1alpha on proteoglycan and type ii collagen turnover in bovine nasal and articular chondrocyte pellet cultures. Endocrinology. 1996;1378:3557–3565. doi: 10.1210/endo.137.8.8754787. [DOI] [PubMed] [Google Scholar]
- Xu Z, Buckley MJ, Evans CH, Agarwal S. Cyclic tensile strain acts as an antagonist of il-1 beta actions in chondrocytes. J Immunol. 2000;1651:453–460. doi: 10.4049/jimmunol.165.1.453. [DOI] [PMC free article] [PubMed] [Google Scholar]