I. Summary
Objective
To determine whether the functional properties of tissue-engineered constructs cultured in a chemically-defined medium supplemented briefly with TGF-β3 can be enhanced with the application of dynamic deformational loading.
Methods
Primary immature bovine cells (2–3 months old) were encapsulated in agarose hydrogel (2%, 30×106 cells/ml) and cultured in chemically-defined medium supplemented for the first 2 weeks with transforming growth factor beta 3 (TGF-β3) (10 ug/mL). Physiologic deformational loading (1Hz, 3hrs/day, 10% unconfined deformation initially and tapering to 2% peak-to-peak deformation by day 42) was applied either concurrent with or after the period of TGF-β3 supplementation. Mechanical and biochemical properties were evaluated up to day 56.
Results
Dynamic deformational loading applied concurrently with TGF-β3 supplementation yielded significantly lower (−90%) overall mechanical properties when compared to free-swelling controls. In contrast, the same loading protocol applied after the discontinuation of the growth factor resulted in significantly increased (+10%) overall mechanical properties relative to free-swelling controls. Equilibrium modulus values reach 1,306±79 kPa and glycosaminoglycan levels reach 8.7±1.6 %ww during this 8 week period and are similar to host cartilage properties (994±280kPa, 6.3±0.9 %w.w.).
Conclusions
An optimal strategy for the functional tissue engineering of articular cartilage, particularly to accelerate construct development, may incorporate sequential application of different growth factors and applied deformational loading.
I. Introduction
The application of dynamic compressive loading (DL) within appropriate ranges of magnitude and frequency can be a beneficial tool for the functional tissue engineering of articular cartilage. It has been shown to increase synthesis of cartilage extracellular matrix components such as proteoglycans, collagens and other matrix elements using a variety of loading apparatuses and culture systems when compared to control constructs maintained in free-swelling (FS) culture [1–6]. Although significant efforts have gone into optimizing loading parameters to maximize tissue development in culture, the effect of dynamic loading is strongly influenced by other factors in the tissue-engineering system such as the choice of scaffold, the formulation of feed media, and cellular factors such as species, age, and seeding density [7–10]. Therefore it appears that a universally efficacious culturing protocol that involves physical loading may be elusive, and such protocols must be optimized for a given set of specific experimental conditions adopted. Once established, these parameters do not need to be changed unless new experimental conditions deviate greatly from the conditions on which the loading parameters were originally based.
In our previous studies we optimized a loading protocol for juvenile bovine chondrocytes seeded in agarose hydrogels and cultured in media containing 20% fetal bovine serum. Constructs cultured using this protocol developed Young’s Modulus (EY) values over twice that of FS controls, however the goal of reaching native cartilage values remained elusive [11]. In addition, fetal bovine serum is not a well-characterized culture supplement that can possess batch-to-batch compositional variations [12, 13]. This raises quality control concerns for the clinical application of tissue engineered articular cartilage.
Recent work, using the temporal supplementation of transforming growth factor β3 (TGF-β3) in free-swelling, serum-free cultures of chondrocyte-laden agarose hydrogel constructs has shown great promise. In those studies, a 2-week exposure to TGF-β3 followed by six additional weeks of culture in its absence resulted in the development of constructs possessing cartilage-like compressive mechanical properties (EY>800 kPa) [14]. These values are significantly higher than modulus values obtained for engineered cartilage using any other culture system over the same culture duration; the only comparable outcome previously required over 7 months of continuous cultivation to develop similar properties [15]. Currently, however, there is no data showing how chondrocyte-seeded constructs will respond to the application of dynamic loading under these media conditions - a tissue-engineering strategy that has been shown in other media formulations to significantly increase the mechanical properties of developing tissue over free-swelling culture alone [16]. It is the aim of the present study to extend our earlier work on the mechanical preconditioning of engineered cartilage constructs to include transient supplementation with TGF-β3 in a clinically-relevant, chemically-defined, serum-free media formulation.
II. Materials and Methods
A. Experimental Design
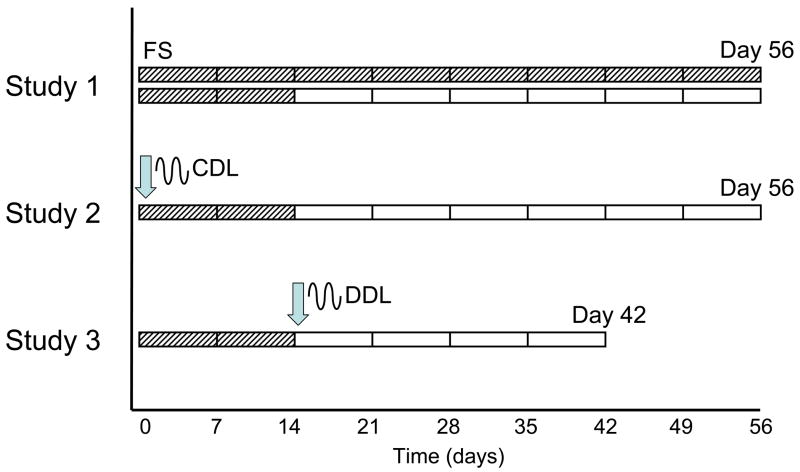
Three consecutive studies are reported here. Study 1 examined the effect of temporal supplementation of TGF- β3 to the basal media; Study 2 examined the effect of dynamic deformational loading applied concurrently with TGF- β3 supplementation; and Study 3 examined the effect of dynamic deformational loading applied non-concurrently with TGF- β3 supplementation (when initiated only after TGF-β3 supplementation was discontinued). Each study was performed independently, using individual cell isolations pooled from different animals. To ensure consistency, Study 3 was repeated twice and results have been pooled.
The timeline of the studies are detailed in Figure 1. There are two variables in the experiments: 1) the day in which TGF- β3 supplementation is discontinued, and 2) the day in which dynamic deformational loading is initiated to the constructs.
Figure 1.
Schematic of TGF-β3 time-course and loading time-course for each study. Loading was initiated at days indicated by arrow; TGF- β3 was supplemented during periods indicated by hatch marks.
Specifically, in Study 1 (n=4 per group), TGF- β3 was supplemented to the media either for the first 14 days only (discontinuous) or it was supplemented throughout the duration of the study (continuous). There was no loading introduced to these developing constructs at any time. Based on the results of Study 1, a protocol of discontinuous TGF-β3 supplementation was adopted for both Study 2 and Study 3.
In Study 2 (n=5 per group), dynamic deformational loading was initiated on day 0 and was continued throughout the culture period. In Study 3 (n=8 per group), dynamic deformational loading was initiated on day 14 (delayed until the day TGF- β3 was discontinued). In all studies dynamic deformational loading is abbreviated CDL when initiated at day 0, and DDL when delayed until after the discontinuation of TGF- β3. A follow up study (n=5 per group) was also performed with loading initiated on day 0 on the basal media without TGF-β3.
Cell Isolation
Articular cartilage was harvested from bovine carpo-metacarpal (CMC) joints of freshly slaughtered 4–6 month old calves. Three to five joints were used for each study and cells were pooled from all joints. Cartilage was rinsed in high-glucose Dulbecco’s Modified Essential Medium (hgDMEM) supplemented with 5% fetal bovine serum (FBS), amino acids (0.5X minimal essential amino acids, 1X non-essential amino acids), buffering agents (10 mM HEPES, 10 mM sodium bicarbonate, 10 mM TES, 10 mM BES), and antibiotics (100 U/ml penicillin, 100 μg/ml streptomycin). The cartilage chunks were then combined and digested in DMEM with 390 U/ml collagenase type VI (Sigma Chemicals, St. Louis, MO) for 11 hours at 37°C with stirring. The resulting cell suspension was then filtered through a 70 μm pore size mesh and sedimented in a bench top centrifuge for 10 minutes at 1000 × g. Viable cells were counted using a hemacytometer and trypan blue. One volume of chondrocyte suspension (at 60 × 106 cells/ml) was then mixed with an equal volume of 4% low-melt agarose (Type VII, Sigma) at 37°C to yield a final cell concentration of 30 × 106 cells/mL in 2% agarose. The chondrocyte/agarose mixture was cast into slabs and cored using a sterile disposable punch (Miltex, York, PA) to final dimensions of 0.3cm diameter and 0.23 cm thickness (0.016 cm3). Constructs were maintained in culture in a chemically defined serum-free growth medium for 42 or up to 56 days depending on the study (Figure 1). Growth medium consisted of hgDMEM supplemented with 1X PSF, 0.1 μM dexamethasone, 50 μg/mL ascorbate 2-phosphate, 40 μg/mL L-proline, 100 μg/mL sodium pyruvate, and 1X ITS+ (Becton Dickinson, Franklin Lakes, NJ). Growth medium was changed every three days and maintained at a cell/media volume ratio of less than 1 million cells/ml media. In some experiments growth medium was further supplemented with 10 ng/mL TGF-β3 (R&D Systems, Minneapolis, MN) for either the first 14 days of culture or the entire culture period as described in Figure 1.
B. Loading Protocol
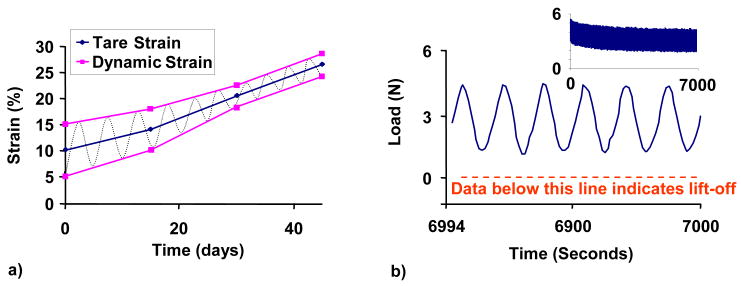
The prescribed loading protocol consisted of a nominal 5% dynamic strain (10% peak-to-peak deformation) above a 10% tare strain in unconfined compression with impermeable platens loading at 1 Hz frequency, for 3 hrs/day, 5 days/week (as had been previously found to be optimal for media formulations containing FBS [17]). The duty cycle consisted of 3 hrs of continuous loading followed by 21 hrs of rest. Deformational loading was carried out at 37°C and 5% CO2 in a humidified incubator. FS controls were positioned adjacent to the loading device. The load and displacement profiles delivered by the bioreactor were analyzed in a small sample of specimens at the completion of all the experiments. In practice, the applied sinusoidal displacement had a consistent frequency of 1.05 Hz, with a total harmonic distortion of 6.03±.95%. Due to inherent compliance of the loading bioreactors, the applied strain amplitude decreased over the culture period, as tissue elaboration produced specimens with increasing stiffness; the dynamic strain amplitude started at 5% and tapered to 2% by day 42 in culture (4% peak-to-peak). This compliance, coupled with the increasing tare strain resulting from growing construct thickness, had the beneficial effect of compensating passively for the increasing construct stiffness to prevent any loading platen lift-off through the entire culture period (Figure 2).
Figure 2.
a) Loading profile adjusted for system compliance delivered by bioreactor over time in culture. Blue line shows increasing tare strain as a result of increasing tissue thickness with time. Purple line shows decreasing applied dynamic strain as a result of tissue stiffening over time. b) Representative load vs. time curve of tissue-engineered constructs on day 42. A load of zero would have indicated platen lift-off. Inset represents full curve.
C. Material Testing
Cylindrical constructs were tested in unconfined compression using a custom computer-controlled testing system [18]. Initially, a series of stress-relaxation tests were conducted for each sample to 5%, 10%, 15%, and 20% strain and the Young’s modulus (EY) of the construct was calculated from the equilibrium stress at each strain value and from the initial cross-sectional area. Since the resulting EY was found to remain invariant across the strain amplitudes tested (not shown), the remaining samples were tested at 10% strain only and at a strain rate of 0.05% strain/sec after an initial 0.02 N tare load. The unconfined dynamic modulus was also measured, after reaching stress-relaxation equilibrium to 10% strain, by superimposing 2% strain at 1 Hz. Tests of static and dynamic compressive properties were selected since the normal physiological loading mode of cartilage is compressive. More specifically, the most functionally relevant mechanical property is the dynamic modulus in compression, since joint loading is typically intermittent [19].
D. Biochemical Content
The biochemical content of each sample was assessed by first measuring sample wet weight, lyophilizing for 72 hours, and then measuring the sample dry weight. Gross water content was determined from the difference. Once dry, the samples were digested in proteinase-K overnight at 56°C, as described previously [17]. Aliquots of digest were analyzed for glycosaminoglycan (GAG) content using the 1,9-dimethylmethylene blue dye-binding assay [20]. A further aliquot was acid hydrolyzed in 12 N HCl at 110°C for 16 hours, dried over NaOH, and resuspended in assay buffer[17]. Ortho-hydroxyproline (OHP) content was then determined via a colorimetric assay by reaction with chloramine T and dimethylaminobenzaldehyde [21], scaled for microplates. OHP content was converted to total collagen content using the conversion of 1:7.64 ratio of OHP:Collagen [22]. DNA content was determined using PicoGreen (Molecular Probes, Carlsbad, CA) assay following the manufacturer’s standard protocols. Each biochemical constituent (GAG and collagen) was normalized to tissue wet weight.
E. Histological Analysis
Samples were fixed in acid formalin ethanol [23], paraffin embedded, sectioned (8 μm thick), and stained with either Safranin O (1% in dH2O, pH 6.7) to view proteoglycan distribution, Picrosirius Red to view collagen distribution, or hematoxylin and eosin to view cell and tissue morphology. Samples were also stained for Type II collagen as follows [24]: sections were digested in 0.5 mg/ml of testicular hyaluronidase, swollen in 0.5 M of acetic acid, blocked in 10% normal goat serum (NGS) and labeled with 10% NGS containing monoclonal primary antibody for types I, and II collagens (Developmental Studies Hybridoma Bank, Iowa City, IA). Non-immune controls were incubated in 10% NGS alone. Alexa 488-conjugated goat anti-mouse secondary antibody labeling (Molecular Probes, Eugene, OR) and propidium iodide nuclear counterstaining (Molecular Probes) were performed to visualize the ECM and cells, respectively. After staining, the slides were coverslipped and sections were analyzed using an inverted microscope with an Olympus Fluoview confocal system (New York/New Jersey Scientific, Middlebush, NJ) with dual wavelengths excitation at 488 and 568 nm (20× to 100×-oil objective lens).
F. Statistics
Statistics were performed with the Statistica (Statsoft,Tulsa, OK) software package. Each data point represents the mean and standard deviation of four or five samples. Groups were examined for significant differences by two-way analysis of variance (ANOVA), with EY, G*, GAG, DNA, or OHP as the dependent variable, and time in culture and loading condition as the independent variables. Tukey’s Honest Significant Difference Test (HSD) post-hoc tests were carried out with a statistical significance set at α =0.05.
III. Results
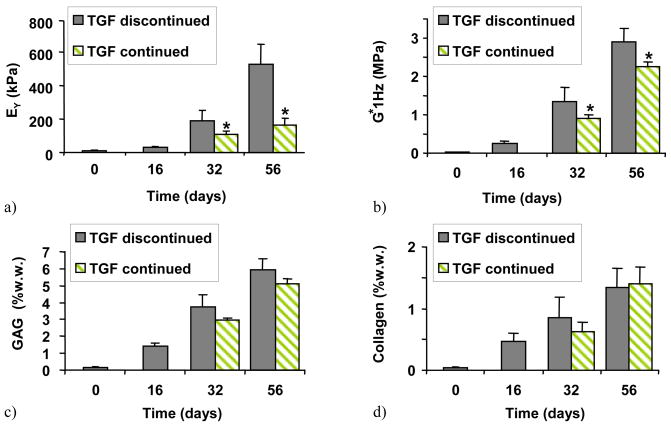
Constructs developed significantly different mechanical properties and biochemical composition depending on culture condition and time. In Study 1, performed in free-swelling cultures, constructs that were transiently exposed to TGF-β3 elaborated significantly stiffer tissue (EY=528±122 kPa, G*=2.9±0.3 MPa) than constructs that were exposed to TGF-β3 continuously (EY=165±42 kPa, G*=2.2±0.1 MPa) (Figure 3a,b, day 56). However, no differences were observed in GAG (TGF discontinued=6.0±0.6% w.w, TGF continued=5.1±0.3% w.w) or collagen (TGF discontinued=1.3±0.3% w.w, TGF continued=1.4±0.3% w.w) content between these groups (Figures 3c,d).
Figure 3.
(Study 1) The effect of temporal application of TGF-β3 to a chemically defined medium: a) EY, b) G* at 1HZ, c) GAG, and d) collagen. *=p<0.005 for TGF continued vs. TGF discontinued (n=4).
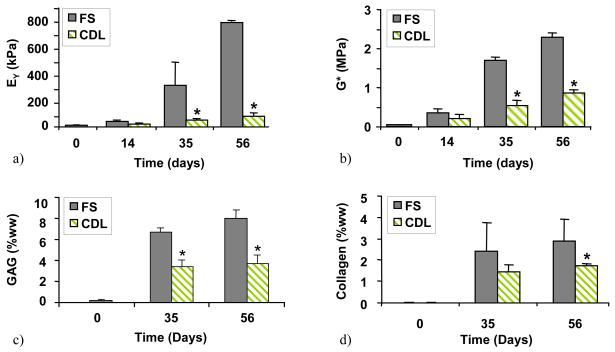
The results of Study 2 demonstrate that the effectiveness of dynamic deformational loading in the continuous presence of TGF-β3 (Figure 4): when loading was applied to constructs in basal media with TGF-β3, the CDL group achieved significantly lower mechanical properties (EY=78±22 kPa, G*=0.88±0.08 MPa) compared to the FS control (E Y=780±8 kPa, G*=2.3±0.1 MPa) (Figure 4a,b, day 56). The GAG content and collagen content also showed significantly lower values in CDL versus FS (GAG: CDL=3.7±0.8% w.w., FS=8.0±0.8% w.w.; collagen: CDL=1.75±0.1%w.w., FS=3.16±1.0%w.w.; Figures 4c,d, day 56).
Figure 4.
(Study 2) The effect of dynamic deformational loading applied concurrently with exposure to TGF-β3: a) EY, b) G* at 1HZ, c) GAG, and d) collagen. *=p<0.005 for FS vs. CDL (n=5).
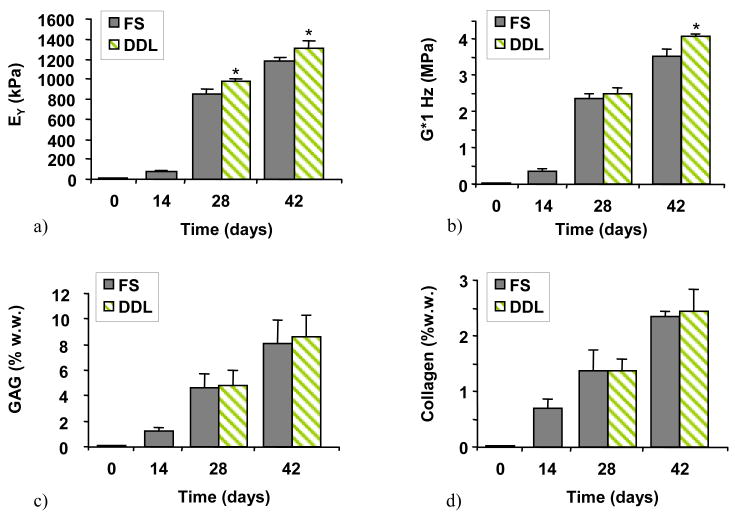
The results of Study 3 show that when loading was applied after the discontinuation of TGF-β3, the DDL group achieved mechanical properties (EY=1,306±79 kPa, G*=4.1±0.1 MPa) significantly higher than FS (EY=1,178±40 kPa, G*=3.5±0.2 MPa) (Figures 5a,b, day 42). However, no differences were observed in GAG (DDL=8.6±1.7% w.w, FS=8.1±1.8% w.w) or collagen (DDL=2.4±0.4% w.w, FS=2.3±0.1% w.w) content (Figures 5,c,d).
Figure 5.
(Study 3) The effect of dynamic deformational loading initiated after the discontinuation of TGF-β3: a) EY, b) G* at 1HZ, c) GAG, and d) collagen. *=p<0.005 for FS vs. DDL (n=8).
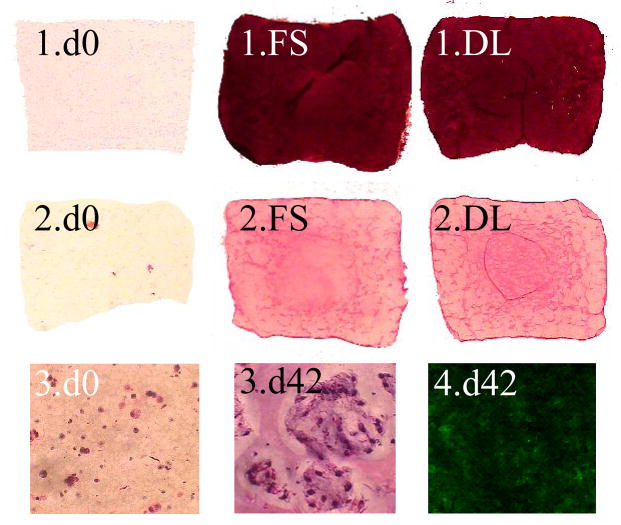
Histological analysis confirmed abundant deposition of GAG throughout the constructs and a uniform distribution of type II collagen (Figure 6) with little or no staining for type I collagen (not shown). Staining indicated that cells multiplied in localized pockets throughout the constructs (Figure 5). Cell proliferated with time, increasing on average 3 fold from day 0 concentrations, but did not differ significantly between any groups reported here.
Figure 6.
(1) Safranin O staining for GAG, (2) Picrosirius Red staining for collagen, (3) hematoxylin and eosin staining for visualization of local multiplication of cell nuclei (Mag. 40x), and (4) Immunohistochemical staining for type II collagen. All slides taken from study 3 on either day 0 or day 42 with either free-swelling (FS) or dynamically-loaded (DL) groups.
For comparison, the mechanical and biochemical properties of juvenile CMC articular cartilage were also measured (n=5) and were found to be EY = 994±280kPa, G*(at 1 Hz) = 13±2.5MPa, GAG = 6.3±0.9 (%w.w.), 24±3.5 (%d.w.), Collagen = 16±0.5 (%w.w.), 66±5.5 (%d.w.). While EY for DDL and FS for Study 3 equaled or exceeded that of native cartilage by day 28 (Figure 5a), G* was at most 32% that of native values at day 42 (Figure 5b). Similarly GAG values equaled or exceeded those of native cartilage in DDL and FS groups (Figure 5c), but collagen content was only 15% that of native tissue (Figure 5d).
IV. Discussion
In this investigation we adopted a protocol of transient supplementation of serum-free media with TGF-β3 and applied a regimen of dynamic deformational loading whose timing was adjusted towards achieving the most robust mechanical properties. Studies by our group [25] and by others [26] have previously demonstrated that mechanical stimulus (physiologic deformational loading) can act synergistically with chemical stimuli (growth factors) to amplify the benefits conferred by either stimulus alone. Furthermore, it has been previously shown that the timing of the application of the growth factor can be critical; free-swelling cultures supplemented transiently with TGF-β3 consistently yielded cartilage-like tissue with higher mechanical properties than those derived from cultures with continuous (or no) growth factor supplementation [14]. Similarly, work has been done demonstrating the utility of sequential growth factor protocols (e.g., TGF-β1/FGF-2 followed by IGF-1) [26]
The findings of this study indicate that coordination of the timing (introduction and duration) of the application of an appropriate chemical stimulus as well as the timing of the introduction of mechanical stimuli represents a strategy to optimize engineered tissue growth (i.e. a sequential loading protocol). In Study 1, we have confirmed the earlier results of Byers et al. [14] who found that discontinuation of TGF-β3 supplementation after two weeks in culture yields much better material properties than under continuous supplementation (Figure 3). In Study 2, we have found that dynamic loading initiated at the same time as TGF-β3 supplementation yields significantly poorer properties than the free-swelling control group, after discontinuation of supplementation (Figure 4). However, the application of deformational loading initiated after culturing with growth factor TGF-β3 for 2 weeks (Study 3) yields significantly stiffer chondrocyte-seeded agarose constructs than free-swelling controls. Using this sequential loading protocol, engineered constructs continued to display the dramatic improvement in properties associated with the removal of the growth factor (Studies 1 and 2) while benefiting from the deformational loading protocol. These constructs achieved the most favorable values for tissue-engineered cartilage constructs reported in the literature to date for the culture period prescribed. Young’s modulus and GAG levels achieved values similar to those of native cartilage after as little as 28 days in culture (Figure 5a). Dynamic modulus values, which are more representative of the functional tissue properties, however, remain at 32% of those manifested by native cartilage, after 42 days in culture (Figure 5b). As has been shown both theoretically [27–29], and in vivo [30], dynamic modulus values are largely influenced by collagen content and organization as well as construct permeability whereas the equilibrium modulus is influenced to a greater degree by GAG content.
Related to this observation, collagen levels for constructs in all the studies presented here remained relatively low (Figures 3d, 4d, 5d) and were not different from levels achieved previously with optimal conditions using serum-supplemented media [25]. This suggests that application of dynamic loading as well as the temporary supplementation of TGF-β3 has a much greater effect on GAG production compared to collagen production. In fact, the increase in the equilibrium compressive modulus over time of developing constructs can be attributed almost entirely to the increase in GAG levels.
While the average content of GAG and collagen were not statistically different between DDL versus FS constructs in Study 3, the compressive moduli were significantly stiffer (~15%) for DDL constructs (Figures 5a,b). We have previously reported that loaded and free-swelling constructs possess differences in levels of other extracellular matrix molecules (such as cartilage oligomeric matrix protein-COMP [31], type IX collagen [24]) and structural organization [17] that may contribute to the disparate material properties observed, and ultimately influence chondrocyte mechanotransduction and the level of cartilage repair after implantation.
The delayed applied loading protocol found to be efficacious in the current studies is in direct contrast to that which we have previously reported to be optimal for constructs cultured with serum supplemented media, where the highest mechanical properties (E Y=185 kPa on day 56 [32]) were obtained when dynamic deformational loading was applied at the earliest possible time (i.e., day 0). One way to explain these results may be to consider the much greater contribution of the growth factor to the observed tissue growth relative to that induced by the application of dynamic loading. The mechanisms behind the drastic increases in growth associated with transient supplementation of TGF-β3 is not yet well understood, however these results suggest that the TGF-β3 preconditions cells toward high anabolic activity that is manifest once the growth factor is removed (Study 1). Continuous loading with or without growth factors in the presence of FBS does not have this same suppressive result [25] and may be due to the presence of other growth factors such as IGF-I or other proteins that can regulate TGF-β growth factors in serum [33, 34].
The mechanism behind the detrimental effect of applied dynamic loading in the presence of TGF-β3 (Study 2) is a new finding that warrants further discussion. One clue may lie in the concentration of the growth factor within the construct. Theoretical models performed by our laboratory for molecules of similar size to TGF-β3 (~25 kDa, R&D Systems) indicate that the concentration of the molecule within the tissue construct under dynamic loading conditions can be elevated ~2–3 fold compared to free diffusion conditions [35]. The presence of TGF-β3 binding proteins in the elaborated matrix [36, 37], such as reported for insulin-like growth factor I (IGF-I) in native cartilage [38, 39], can also result in a greater concentration of growth factor retained in the construct compared to the culture media. Therefore, dynamic loading in combination with binding protein and proteoglycan interactions may increase the concentration of TGF-β3 localized in the construct into the range where the growth factor can begin to elicit a negative response. This threshold concentration where catabolic effects have been observed has been reported to occur at culture media concentrations of approximately 20–50 ng/mL [40, 41]. To test this hypothesized mechanism, a study of the dose response to TGF-β3 with and without dynamic loading is planned for future research. This hypothesis would be supported if doses of TGF-β3 lower than used here were to combine with dynamic loading to yield better properties than free-swelling controls; and if doses of TGF-β3 above a certain threshold were to produce poorer properties than lower doses, under free-swelling conditions.
The results of this study address a number of important issues related to functional tissue engineering of articular cartilage. The most positive outcome is the finding that temporary supplementation of TGF-β3 followed by dynamic loading can produce an equilibrium modulus and GAG content which match those of native tissue over a culture period of 4 to 6 weeks only; the dynamic modulus and collagen content remain lower than in native tissue, but are as good as, or better than reported in previous studies. However, there are a number of practical issues that remain to be addressed. First, the modest improvement observed in the mechanical properties with dynamic loading in Study 3 (~11% for EY and ~17% for G*) suggests that free-swelling culture may be a less costly alternative, precluding the need to load constructs on a daily basis. While this may be true for the culture conditions employed in this study, our previous studies demonstrate that dynamic loading can be far more beneficial than free swelling under other culture conditions[11, 16, 25, 32]. Since the production of higher levels of collagen remains a challenge, it may well be that the elusive culture conditions which can promote rapid protein synthesis might also benefit significantly from dynamic loading, possibly by increasing the expression of cell receptors to growth factors and signaling proteins.
Second, it may be argued than any beneficial outcomes observed with immature chondrocytes are of limited value for current clinical strategies, which rely on mature autologous cells. Indeed, although immature bovine cells respond favorably to supplementation of TGF-β3, preliminary work from our lab (not shown) suggests that, in fact, mature primary chondrocytes do not respond as robustly. This is likely due to known decreases in the expression of TGF-β receptor and signaling proteins that occurs with age [42, 43], and additional strategies are thus required to supplement the successful techniques achieved in this study when using mature cells. It may also be noted that the strategies employed in this study might be successful on alternative sources of immature cells, such as embryonic stem cells.
It appears that, as in bone [44], the structure and function of cartilage reflects the physical demands to which it is subjected. Cartilage from weight-bearing and non-weight bearing regions (the source for autologous grafts) have been reported to be distinct in structural organization as well as cells, with chondrocytes from loaded regions exhibiting greater expression of intermediate filaments than their counterparts in less loading regions [45]. This disparity in chondrocyte populations appears to be an adaptation to their physical environment. Our contention is that chondrocytes subjected to loading during pre-culture (i.e., preconditioning) may better acclimate to the physiologic loading environment that they are exposed to post-implantation. This concept of cell memory has been described in the bone remodeling literature, where it has been suggested that acquired long-term memory of a mechanical loading environment may influence the responsiveness of bone tissue to external stimuli (e.g.[46]). Similarly, the presence (or absence) of extracellular matrix molecules such as type II collagen, the presence or absence of focal adhesions, and mechanical and morphological changes to the cell membrane in response to preconditioning with growth factors [47–50] have all been shown to influence the response of chondrocytes to mechanical loading. Only with in vivo studies, or possibly with the proper in vitro model of cartilage repair [51], can the efficacy of applied loading bioreactors on functional cartilage repair be assessed. The findings of the current study suggest that an optimal strategy using well-characterized conditions for the functional tissue engineering of articular cartilage, particularly to accelerate construct development, may incorporate sequential application of different growth factors and applied deformational loading.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Guilak F, Meyer BC, Ratcliffe A, Mow VC. The effects of matrix compression on proteoglycan metabolism in articular cartilage explants. Osteoarthritis Cartilage. 1994;2:91–101. doi: 10.1016/s1063-4584(05)80059-7. [DOI] [PubMed] [Google Scholar]
- 2.Burton-Wurster N, Vernier-Singer M, Farquhar T, Lust G. Effect of compressive loading and unloading on the synthesis of total protein, proteoglycan, and fibronectin by canine cartilage explants. J Orthop Res. 1993;11:717–729. doi: 10.1002/jor.1100110514. [DOI] [PubMed] [Google Scholar]
- 3.O’Hara BP, Urban JP, Maroudas A. Influence of cyclic loading on the nutrition of articular cartilage. Ann Rheum Dis. 1990;49:536–539. doi: 10.1136/ard.49.7.536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buschmann MD, Gluzband YA, Grodzinsky AJ, Hunziker EB. Mechanical compression modulates matrix biosynthesis in chondrocyte/agarose culture. J Cell Sci. 1995;108(Pt 4):1497–1508. doi: 10.1242/jcs.108.4.1497. [DOI] [PubMed] [Google Scholar]
- 5.Lee DA, Noguchi T, Frean SP, Lees P, Bader DL. The influence of mechanical loading on isolated chondrocytes seeded in agarose constructs. Biorheology. 2000;37:149–161. [PubMed] [Google Scholar]
- 6.Kisiday JD, Jin M, DiMicco MA, Kurz B, Grodzinsky AJ. Effects of dynamic compressive loading on chondrocyte biosynthesis in self-assembling peptide scaffolds. J Biomech. 2004;37:595–604. doi: 10.1016/j.jbiomech.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 7.Hung CT, Mauck RL, Wang CC, Lima EG, Ateshian GA. A paradigm for functional tissue engineering of articular cartilage via applied physiologic deformational loading. Ann Biomed Eng. 2004;32:35–49. doi: 10.1023/b:abme.0000007789.99565.42. [DOI] [PubMed] [Google Scholar]
- 8.Guilak F, Butler DL, Goldstein SA. Functional tissue engineering: the role of biomechanics in articular cartilage repair. Clin Orthop Relat Res. 2001:S295–305. [PubMed] [Google Scholar]
- 9.Hutmacher DW. Scaffold design and fabrication technologies for engineering tissues--state of the art and future perspectives. J Biomater Sci Polym Ed. 2001;12:107–124. doi: 10.1163/156856201744489. [DOI] [PubMed] [Google Scholar]
- 10.Rahaman MN, Mao JJ. Stem cell-based composite tissue constructs for regenerative medicine. Biotechnol Bioeng. 2005;91:261–284. doi: 10.1002/bit.20292. [DOI] [PubMed] [Google Scholar]
- 11.Mauck RL, Seyhan SL, Ateshian GA, Hung CT. Influence of seeding density and dynamic deformational loading on the developing structure/function relationships of chondrocyte-seeded agarose hydrogels. Ann Biomed Eng. 2002;30:1046–1056. doi: 10.1114/1.1512676. [DOI] [PubMed] [Google Scholar]
- 12.Honn KV, Singley JA, Chavin W. Fetal bovine serum: a multivariate standard. Proc Soc Exp Biol Med. 1975;149:344–347. doi: 10.3181/00379727-149-38804. [DOI] [PubMed] [Google Scholar]
- 13.Price PJ, Gregory EA. Relationship between in vitro growth promotion and biophysical and biochemical properties of the serum supplement. In Vitro. 1982;18:576–584. doi: 10.1007/BF02810081. [DOI] [PubMed] [Google Scholar]
- 14.Byers B, Mauck R, Chiang I, Tuan R. ORS. Vol. 31. Chicago: 2006. Temporal Exposure of TGF-beta3 Under Serum-Free Conditions Enhances Biomechanical and Biochemical Maturation of Tissue-Engineered Cartilage; p. 0043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Martin I, Obradovic B, Treppo S, Grodzinsky AJ, Langer R, Freed LE, et al. Modulation of the mechanical properties of tissue engineered cartilage. Biorheology. 2000;37:141–147. [PubMed] [Google Scholar]
- 16.Mauck RL, Soltz MA, Wang CC, Wong DD, Chao PH, Valhmu WB, et al. Functional tissue engineering of articular cartilage through dynamic loading of chondrocyte-seeded agarose gels. J Biomech Eng. 2000;122:252–260. doi: 10.1115/1.429656. [DOI] [PubMed] [Google Scholar]
- 17.Kelly TA, Ng KW, Wang CC, Ateshian GA, Hung CT. Spatial and temporal development of chondrocyte-seeded agarose constructs in free-swelling and dynamically loaded cultures. J Biomech. 2005 doi: 10.1016/j.jbiomech.2005.03.031. [DOI] [PubMed] [Google Scholar]
- 18.Soltz MA, Ateshian GA. Experimental verification and theoretical prediction of cartilage interstitial fluid pressurization at an impermeable contact interface in confined compression. J Biomech. 1998;31:927–934. doi: 10.1016/s0021-9290(98)00105-5. [DOI] [PubMed] [Google Scholar]
- 19.Park S, Hung CT, Ateshian GA. Mechanical response of bovine articular cartilage under dynamic unconfined compression loading at physiological stress levels. Osteoarthritis Cartilage. 2004;12:65–73. doi: 10.1016/j.joca.2003.08.005. [DOI] [PubMed] [Google Scholar]
- 20.Farndale RW, Sayers CA, Barrett AJ. A direct spectrophotometric microassay for sulfated glycosaminoglycans in cartilage cultures. Connect Tissue Res. 1982;9:247–248. doi: 10.3109/03008208209160269. [DOI] [PubMed] [Google Scholar]
- 21.Stegemann H, Stalder K. Determination of hydroxyproline. Clin Chim Acta. 1967;18:267–273. doi: 10.1016/0009-8981(67)90167-2. [DOI] [PubMed] [Google Scholar]
- 22.Vunjak-Novakovic G, Martin I, Obradovic B, Treppo S, Grodzinsky AJ, Langer R, et al. Bioreactor cultivation conditions modulate the composition and mechanical properties of tissue-engineered cartilage. J Orthop Res. 1999;17:130–138. doi: 10.1002/jor.1100170119. [DOI] [PubMed] [Google Scholar]
- 23.Lin W, Shuster S, Maibach HI, Stern R. Patterns of hyaluronan staining are modified by fixation techniques. J Histochem Cytochem. 1997;45:1157–1163. doi: 10.1177/002215549704500813. [DOI] [PubMed] [Google Scholar]
- 24.Kelly TA, Wang CC, Mauck RL, Ateshian GA, Hung CT. Role of cell-associated matrix in the development of free-swelling and dynamically loaded chondrocyte-seeded agarose gels. Biorheology. 2004;41:223–237. [PubMed] [Google Scholar]
- 25.Mauck RL, Nicoll SB, Seyhan SL, Ateshian GA, Hung CT. Synergistic action of growth factors and dynamic loading for articular cartilage tissue engineering. Tissue Eng. 2003;9:597–611. doi: 10.1089/107632703768247304. [DOI] [PubMed] [Google Scholar]
- 26.Pei M, Seidel J, Vunjak-Novakovic G, Freed LE. Growth factors for sequential cellular de- and re-differentiation in tissue engineering. Biochem Biophys Res Commun. 2002;294:149–154. doi: 10.1016/S0006-291X(02)00439-4. [DOI] [PubMed] [Google Scholar]
- 27.Huang CY, Mow VC, Ateshian GA. The role of flow-independent viscoelasticity in the biphasic tensile and compressive responses of articular cartilage. J Biomech Eng. 2001;123:410–417. doi: 10.1115/1.1392316. [DOI] [PubMed] [Google Scholar]
- 28.Ateshian GA, Warden WH, Kim JJ, Grelsamer RP, Mow VC. Finite deformation biphasic material properties of bovine articular cartilage from confined compression experiments. J Biomech. 1997;30:1157–1164. doi: 10.1016/s0021-9290(97)85606-0. [DOI] [PubMed] [Google Scholar]
- 29.Mow VC, Kuei SC, Lai WM, Armstrong CG. Biphasic creep and stress relaxation of articular cartilage in compression? Theory and experiments J Biomech Eng. 1980;102:73–84. doi: 10.1115/1.3138202. [DOI] [PubMed] [Google Scholar]
- 30.Vasara AI, Nieminen MT, Jurvelin JS, Peterson L, Lindahl A, Kiviranta I. Indentation stiffness of repair tissue after autologous chondrocyte transplantation. Clin Orthop Relat Res. 2005:233–242. doi: 10.1097/01.blo.0000150567.00022.2e. [DOI] [PubMed] [Google Scholar]
- 31.Mauck RLWC-C, Chen FH, Lu HH, Ateshian GA, Hung CT. Dynamic deformational loading of chondrocyte-seeded agarose hydrogels modulates deposition and structural organization of matrix constituents. Summer Bioengineering Conference; Key Biscane, FL. 2003. [Google Scholar]
- 32.Mauck RL, Wang CC, Oswald ES, Ateshian GA, Hung CT. The role of cell seeding density and nutrient supply for articular cartilage tissue engineering with deformational loading. Osteoarthritis Cartilage. 2003;11:879–890. doi: 10.1016/j.joca.2003.08.006. [DOI] [PubMed] [Google Scholar]
- 33.McQuillan DJ, Handley CJ, Campbell MA, Bolis S, Milway VE, Herington AC. Stimulation of proteoglycan biosynthesis by serum and insulin-like growth factor-I in cultured bovine articular cartilage. Biochem J. 1986;240:423–430. doi: 10.1042/bj2400423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.O’Connor-McCourt MD, Wakefield LM. Latent transforming growth factor-beta in serum. A specific complex with alpha 2-macroglobulin. J Biol Chem. 1987;262:14090–14099. [PubMed] [Google Scholar]
- 35.Mauck RL, Hung CT, Ateshian GA. Modeling of neutral solute transport in a dynamically loaded porous permeable gel: implications for articular cartilage biosynthesis and tissue engineering. J Biomech Eng. 2003;125:602–614. doi: 10.1115/1.1611512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hildebrand A, Romaris M, Rasmussen LM, Heinegard D, Twardzik DR, Border WA, et al. Interaction of the small interstitial proteoglycans biglycan, decorin and fibromodulin with transforming growth factor beta. Biochem J. 1994;302(Pt 2):527–534. doi: 10.1042/bj3020527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pedrozo HA, Schwartz Z, Gomez R, Ornoy A, Xin-Sheng W, Dallas SL, et al. Growth plate chondrocytes store latent transforming growth factor (TGF)-beta 1 in their matrix through latent TGF-beta 1 binding protein-1. J Cell Physiol. 1998;177:343–354. doi: 10.1002/(SICI)1097-4652(199811)177:2<343::AID-JCP16>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 38.Bhakta NR, Garcia AM, Frank EH, Grodzinsky AJ, Morales TI. The insulin-like growth factors (IGFs) I and II bind to articular cartilage via the IGF-binding proteins. J Biol Chem. 2000;275:5860–5866. doi: 10.1074/jbc.275.8.5860. [DOI] [PubMed] [Google Scholar]
- 39.Garcia AM, Szasz N, Trippel SB, Morales TI, Grodzinsky AJ, Frank EH. Transport and binding of insulin-like growth factor I through articular cartilage. Arch Biochem Biophys. 2003;415:69–79. doi: 10.1016/s0003-9861(03)00215-7. [DOI] [PubMed] [Google Scholar]
- 40.Morales TI, Roberts AB. Transforming growth factor beta regulates the metabolism of proteoglycans in bovine cartilage organ cultures. J Biol Chem. 1988;263:12828–12831. [PubMed] [Google Scholar]
- 41.van Osch GJ, van der Veen SW, Buma P, Verwoerd-Verhoef HL. Effect of transforming growth factor-beta on proteoglycan synthesis by chondrocytes in relation to differentiation stage and the presence of pericellular matrix. Matrix Biol. 1998;17:413–424. doi: 10.1016/s0945-053x(98)90101-9. [DOI] [PubMed] [Google Scholar]
- 42.Blaney Davidson EN, Scharstuhl A, Vitters EL, van der Kraan PM, van den Berg WB. Reduced transforming growth factor-beta signaling in cartilage of old mice: role in impaired repair capacity. Arthritis Res Ther. 2005;7:R1338–1347. doi: 10.1186/ar1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gomez-Camarillo MA, Kouri JB. Ontogeny of rat chondrocyte proliferation: studies in embryo, adult and osteoarthritic (OA) cartilage. Cell Res. 2005;15:99–104. doi: 10.1038/sj.cr.7290273. [DOI] [PubMed] [Google Scholar]
- 44.Rice JC, Cowin SC, Bowman JA. On the dependence of the elasticity and strength of cancellous bone on apparent density. J Biomech. 1988;21:155–168. doi: 10.1016/0021-9290(88)90008-5. [DOI] [PubMed] [Google Scholar]
- 45.Eggli PS, Hunziker EB, Schenk RK. Quantitation of structural features characterizing weight- and less-weight-bearing regions in articular cartilage: a stereological analysis of medial femoral condyles in young adult rabbits. Anat Rec. 1988;222:217–227. doi: 10.1002/ar.1092220302. [DOI] [PubMed] [Google Scholar]
- 46.Turner CHRAG, Duncan RL, Burr DB. Do Bone Cells Behave Like a Neuronal Network? Calcified Tissue International. 2002;70:435–442. doi: 10.1007/s00223-001-1024-z. [DOI] [PubMed] [Google Scholar]
- 47.van der Kraan PM, Buma P, van Kuppevelt T, van den Berg WB. Interaction of chondrocytes, extracellular matrix and growth factors: relevance for articular cartilage tissue engineering. Osteoarthritis Cartilage. 2002;10:631–637. doi: 10.1053/joca.2002.0806. [DOI] [PubMed] [Google Scholar]
- 48.Lee JW, Qi WN, Scully SP. The involvement of beta1 integrin in the modulation by collagen of chondrocyte-response to transforming growth factor-beta1. J Orthop Res. 2002;20:66–75. doi: 10.1016/S0736-0266(01)00073-0. [DOI] [PubMed] [Google Scholar]
- 49.Schneiderbauer MM, Dutton CM, Scully SP. Signaling “cross-talk” between TGF-beta1 and ECM signals in chondrocytic cells. Cell Signal. 2004;16:1133–1140. doi: 10.1016/j.cellsig.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 50.Leipzig ND, Eleswarapu SV, Athanasiou KA. The effects of TGF-beta1 and IGF-I on the biomechanics and cytoskeleton of single chondrocytes. Osteoarthritis Cartilage. 2006;14:1227–1236. doi: 10.1016/j.joca.2006.05.013. [DOI] [PubMed] [Google Scholar]
- 51.Hunter CJ, Levenston ME. The influence of repair tissue maturation on the response to oscillatory compression in a cartilage defect repair model. Biorheology. 2002;39:79–88. [PubMed] [Google Scholar]