Kidney failure is a known complication of hematologic malignancy. Common causes include direct parenchymal infiltration by leukemic cells, intrarenal leukostasis, tumor lysis syndrome, Fanconi syndrome, cryoglobulinemia, paraprotein deposition disease, obstruction, and chemotherapy-induced tubular or vascular toxicity. 1 Although these causes of kidney injury are well characterized, other less common causes of malignancy-associated kidney disease exist. These may be poorly recognized because they have been less frequently reported. We present a patient with chronic myelomonocytic leukemia (CMML) who developed an unusual kidney complication of his malignancy. The cause of his decreased kidney function was diagnosed only after performing kidney biopsy.
CASE REPORT
Clinical History
A 76-year-old man with a chronic idiopathic peripheral neuropathy, hypertension, dyslipidemia, and CMML-associated thrombocytopenia was referred to us for evaluation of serum creatinine level of 1.7 mg/dL (150.28 mmol/L). His medications included gabapentin, metoprolol, and atorvastatin. Social and family history was unremarkable. On examination, blood pressure was 142/73 mm Hg. Fundoscopic and cardiovascular examination findings were within normal limits and chest was clear to auscultation. There was moderate splenomegaly, but no lymphadenopathy. Peripheral edema was absent. He had reduced sensation below the knees. Urinalysis was notable for 2+ protein, rare white and red blood cells, and occasional coarse granular casts. Initial laboratory investigations showed the following values: white blood cells, 72,800/µL; hemoglobin, 10.5 g/dL; platelets, 80,000/µL; serum potassium, 3.4 mEq/L (3.4 mmol/L); blood urea nitrogen, 34 mg/dL (12.1 mmol/L); serum creatinine, 1.7 mg/dL (150.28 µmol/L); serum calcium, 8.4 mg/dL (2.1 mmol/L); serum globulin, 5.9 g/dL; urine protein-creatinine ratio, 0.54 g/g; and urine albumin-creatinine ratio, 0.17 g/g. Serological test results for antineutrophil cytoplasmic antibody, anti–glomerular basement membrane antibody, and cryoglobulins were negative. Immunofixation for monoclonal gammopathy also was negative.
A presumptive diagnosis of hypertensive nephropathy and/or chronic thrombotic microangiopathy was made. Kidney biopsy initially was deferred because of the profound long-term thrombocytopenia and expected slow progression of kidney disease. During the next 6 months, serum creatinine level increased to 3.1 mg/dL (274.04 mmol/L), urine protein-creatinine ratio increased to 1.6 g/g, and numerous coarse granular casts appeared in the urine. Repeated laboratory tests showed improvement in thrombocytopenia to platelets of 183,000/µL. Because of the unclear cause of his accelerated decrease in kidney function and now improved platelet count, a kidney biopsy was performed.
Kidney Biopsy
Light Microscopy
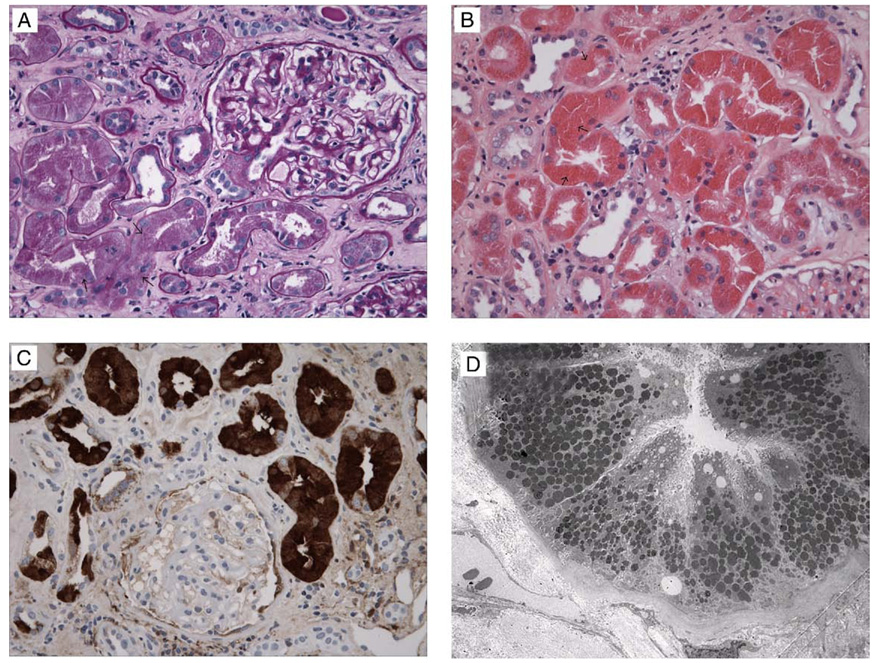
Kidney tissue was prepared by using standard procedures. One of 8 glomeruli present in the sample was obsolescent. The remaining glomeruli showed normal cellularity with mild diffuse mesangial expansion and sclerosis. On periodic acid–Schiff and Jones’ silver stain, glomerular basement membrane thickness was normal. Tubules showed degenerative changes with loss of brush borders in proximal segments and prominent eosinophilic protein-reabsorption granules (Fig 1A and B). Approximately 50% of the sample showed tubular atrophy and interstitial fibrosis by means of trichrome stain. Scattered CD3-positive T cells and CD20-positive B cells were present in the interstitium. Direct infiltration by CD34-positive CMML cells was not present. By means of light microscopy, the presence of coarse protein reabsorption granules in the proximal tubules raised the suspicion of lysozyme-induced kidney injury, especially with the background of CMML and increased serum creatinine level and proteinuria.
Figure 1.
Kidney biopsy findings. (A) Kidney cortical tissue shows an intact glomerulus, proximal tubules with degenerative changes of the cells, and an increase in number of protein reabsorption granules (arrows), interstitial fibrosis, and mild infiltration of the interstitium by mononuclear inflammatory cells (periodic acid–Schiff). (B) Hematoxylin and eosin stain highlights the eosinophilic characteristics of the protein reabsorption granules (arrows) in proximal tubules. (C) Immunohistochemistry shows strong positive staining of granules in the proximal tubule (immunoperoxidase for lysozyme). (D) Electron microscopy shows an increase in number and size of lysosomes in proximal tubules.
Immunofluorescence Microscopy
The specimen was incubated with antibodies specific for the heavy chains of immunoglobulin G (IgG), IgA, IgM, κ and λ light chains, C3, C1q, albumin, and fibrin-related antigens. Diffuse granular deposition of IgM (2+/4+), IgA (trace/4+), C3+, and C4+ was present in mesangial areas, but there was no significant deposition of κ and λ light chain, IgG, or complement components. In addition, scattered interstitial fibrin deposition and occasional distal tubular casts reactive for polyclonal IgA were present.
Immunohistochemical Study
Kidney tubules showed prominent reabsorption granules with dull reactivity for albumin, IgG, and C3 by using immunofluorescence microscopy and strong positive staining for lysozyme by using immunoperoxidase (Fig 1C).
Electron Microscopy
Glomeruli were unremarkable. However, proximal tubular cytoplasm was filled with large and prominent lysosomes (Fig 1D). Epithelial cells also showed degenerative changes.
Summary
The acute and chronic tubular injury was associated with marked proximal tubular reabsorption of lysozymes secondary to CMML.
Diagnosis
Lysozyme-induced kidney injury.
Clinical Follow-up
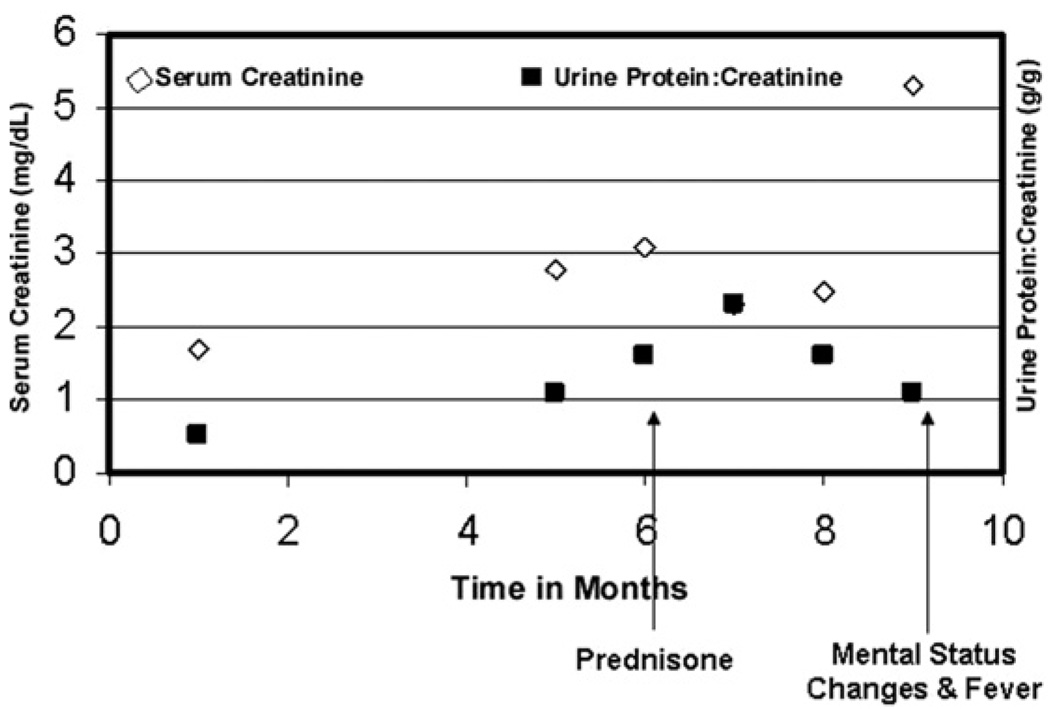
After the kidney biopsy, prednisone, 60 mg/d, was prescribed to treat the underlying CMML. Serum lysozyme levels were increased (>20 µg/mL; normal range, 4 to 10.4 µg/mL), supporting the evidence of increased lysozyme production. Serum creatinine level improved to 2.3 mg/dL (203 µmol/L); however, pronounced leukocytosis, possibly caused by prednisone-induced leukocyte demargination, ensued after several weeks of therapy. Leukocytosis improved with a decrease in prednisone dosage to 20 mg/d (white blood cell count decreased from 204,000/ to 58,000/µL). Kidney function and urine protein excretion were stable. Unfortunately, 3 months after the kidney biopsy, the patient presented with acute-on-chronic kidney failure, confusion, severe leukocytosis, and disseminated intravascular coagulation. After a brief trial of dialysis therapy, comfort care measures were instituted according to the patient’s family, and the patient died. Figure 2 shows the trend of serum creatinine levels and proteinuria during the course of management.
Figure 2.
Line graph shows serum creatinine and urine protein-creatinine ratio values over time. 1 on x-axis represents time of presentation to the kidney clinic.
Autopsy showed marked enlargement of the liver, spleen, and kidneys. The right kidney weighed 205 g, and the left weighed 210 g (normal, ~150 g). Clusters of atypical mononuclear cells consistent with infiltration by CMML were present throughout the kidney parenchyma, papillae, and peripapillary fatty tissue. Dural and leptomeningeal infiltration by atypical mononuclear cells was also noted.
DISCUSSION
Leukemia can adversely impact on the kidneys in several ways. In most cases, leukemia-associated decreased kidney function is caused by parenchymal infiltration of leukemia cells, tumor lysis, thrombotic microangiopathy, radiation injury, or toxicity from chemotherapy.1,2 Other causes of decreased kidney function need to be excluded, including prerenal conditions (ie, volume depletion, heart failure, and medications affecting the renin-angiotensin-aldosterone system) and postrenal causes (ie, obstruction of ureters from lymphadenopathy; Box 1). CMML, a hybrid disorder characterized by overlap of myelodysplastic and myeloproliferative disorders,3 affects predominantly elderly patients.4 The disorder affects kidneys through infiltration of monocytes in glomeruli,1,5 amyloid deposition, 6 production of tumor necrosis factor α,7 and, rarely, increased production and excretion of lysozyme. The case presented here is an example of the latter entity, in which overproduction of lysozyme, an enzyme stored in myelomonocytic cells, is released into the circulation, filtered by glomeruli, and taken up by tubular cells. Tubule cells presumably undergo toxic injury leading to atrophy, interstitial fibrosis, and progressive kidney disease. This mechanism of kidney injury has been reported only rarely in the modern literature.8–12
Box 1.
Cause of Acute Kidney Injury in Leukemia
| Prekidney causes | Intravascular volume depletion: hemorrhage, gastrointestinal losses, “third-space loss” |
| Decrease in cardiac output: chemotherapy induced |
|
| Renal vasoconstriction: hypercalcemia, liver disease, sepsis, renin-angiotensin- aldosterone blockers, nonsteroidal anti-inflammatory agents |
|
| Intrinsic kidney causes |
Diseases of glomeruli: glomerulonephritis or vasculitis, hyperviscosity syndrome, thrombotic microangiopathy from chemotherapy |
| Diseases of tubulointerstitium: parenchymal infiltration by leukemia cells, lysozyme, allergic, infectious |
|
| Diseases of tubules: acute tubular necrosis from chemotherapy, radiocontrast agents, light chain, myeloma, uric acid, tumor lysis |
|
| Postkidney causes |
Obstruction of ureters from lymphadenopathy, tumor masses, nephrolithiasis |
Osserman and Lawlor7 investigated the cause of the high incidence of decreased kidney function in patients with CMML. They observed that patients with CMML excreted large quantities of lysozyme in urine, and nephrotic-range proteinuria was common, with lysozyme accounting for as much as 40%, whereas albumin constituted less than 25% of total urine protein excretion.7
Lysozyme is a small cationic protein with a molecular weight of 15,000 Da that is produced primarily by monocytes and macrophages. It also is known as muramidase, an enzyme with a remarkable capacity to lyse cell walls of certain bacteria (notably Micrococcus lysodeikticus). This activity led to its discovery by Fleming13 in 1922. The mechanism by which lysozyme induces kidney failure is incompletely understood. Filtered lysozyme appears to be a direct tubular toxin,7,8 and animal studies suggest that endocytic reabsorption of such low-molecular-weight cationic proteins as lysozyme by the proximal tubules exacerbates superimposed ischemic tubular damage.1
The cationic charge of the protein may also have a role in kidney injury. Filtered lysozyme is reabsorbed in the proximal convoluted tubule, and its concentration in the cortex may be 10 to 25 times greater than in the medulla or serum.14 The high level of tubular lysozyme may induce significant kaliuresis with severe hypokalemia. 15,16 Alternatively, lysozyme may induce direct tubular injury and kidney failure.7,8 It is worth mentioning that kidney injury from other cationic macromolecules has been reported in the literature. Cationic light chains have been shown to cause tubular and glomerular injury independent of cast formation17 in patients with multiple myeloma and B-cell lymphoproliferative disorders. Experimental evidence also suggests direct tubular injury by meglumine, a small cationic molecule commonly used in intravenous contrast agents.18 Protamine sulfate, another small cationic molecule, has been shown to facilitate albumin filtration through the glomerular barrier by neutralizing other polyanions.19 Our patient was not exposed to these agents and had no evidence of light chain overproduction.
Genetic variants of lysozyme that cause reduced kidney function have also been identified. At least 3 mutations in the lysozyme gene have been described. Phe57Ile, a mutant form of lysozyme,20 has been associated with kidney amyloidosis in several members of 1 Italian-Canadian family.21 Because our patient did not have a family history of hematologic malignancy, genetic analysis was not carried out.
Lysozyme-induced kidney disease is characterized by proteinuria and progressive decrease in kidney function. Because lysozyme migrates in the γ region in protein electrophoresis,22 increased lysozyme levels may lead to increased serum and urine total protein and γ-globulin levels in the absence of monoclonal immunoglobulin, as occurred in our patient. Additionally, patients with mono- and myelomonocytic leukemia may have hypergammaglobulinemia because of increased production of immunoglobulins, in contrast to the hypogammaglobulinemia frequently observed in patients with other leukemias. In urine, nonalbumin polyclonal protein excretion within the γ-globulin fraction typically predominates, but monoclonal paraproteins occasionally are present and may contribute to kidney damage14 if a patient has concomitant plasma cell dyscrasia. Urine electrophoresis in such patients may fail to show the presence of lysozyme because it is masked by a monoclonal protein spike in the same region. However, immunofixation electrophoresis shows distinct bands of these proteins.23 Thus, in appropriate settings, performing immunofixation electrophoresis would help clinch the diagnosis. We measured serum lysozyme, but were not able to measure urine lysozyme. We therefore cannot estimate the ratio of urinary lysozyme to urinary albumin or immunoglobulin. Harrison et al24 performed extensive analysis of urine and serum lysozyme in the context of various disease states. Their data suggests that lysozyme excretion may be highly variable in patients with this disorder. This emphasizes the importance of measuring both urine and serum lysozyme when this entity is suspected.
Other causes of kidney failure should be excluded in patients with CMML before considering a diagnosis of lysozyme-induced kidney failure because these patients are susceptible to typical causes of neoplasia-associated kidney failure. Additionally, urinary and serum lysozyme levels may be increased in patients with many other disease states because of concomitant increases in monocyte/macrophage activity (Box 2). For example, stimulation of monocytes/macrophages results in increased production of angiotensin-converting enzyme.25 This finding has been exploited in clinical practice to diagnose and monitor disease activity in patients with certain disease states, such as sarcoidosis, in which serum lysozyme was shown to be a more sensitive marker for diagnosis of sarcoidosis than angiotensin-converting enzyme.26 Thus, increased lysozyme concentration alone is not diagnostic except in the right clinical situation.15 In our patient, serum lysozyme was measured after the biopsy because the diagnosis of lysozyme-induced kidney injury was not contemplated before the kidney biopsy. The patient had extremely high serum lysozyme levels, which confirmed the biopsy findings.
Box 2.
Disease States Associated With Increased Lysozyme Levels
| Malignancy |
| Leukemia: particularly CMML, CMoL |
| Multiple myeloma |
| Hodgkin disease |
| Carcinoma: particularly stomach |
| Hemodynamic compromise |
| Acute tubular necrosis |
| Perforated peptic ulcer |
| Severe congestive heart failure |
| Myocardial infarction |
| Severe pneumonia |
| Miscellaneous |
| Cadmium poisoning |
| Sarcoidosis |
| Typhoid fever |
| Renal tubular acidosis |
| Amyloidosis |
Note: Increased lysozyme levels: urine lysozyme greater than 1.9 µg/mL, serum lysozyme greater than 14 µg/mL.
Abbreviations: CMML, chronic myelomonocytic leukemia; CMoL, chronic monocytic leukemia.
Treatment for patients with CMML is largely supportive. Treatment may be offered when symptoms, organ involvement, or markedly altered blood counts develop. In the case of kidney disease caused by direct infiltration of leukemia cells or excess lysozyme production, treatment of the underlying leukemia may improve kidney function.27 Newer drugs, namely the DNA-methylation inhibitors azacitidine and decitabine, are approved by the US Food and Drug Administration and may have clinical activity in CMML,27,28 although the effect on kidney function has not been specifically reported.
The present case is an excellent biopsy teaching case because it highlights a forgotten cause of kidney injury in patients with CMML. Lysozyme-induced kidney injury should be in the differential diagnosis of reduced kidney function in this patient population. This entity has been underdiagnosed and underrecognized. First, contemporary oncologists have been aggressive in treating the leukemias, thereby minimizing the number of cases with uncontrolled production of lysozyme. Second, the population affected by chronic leukemia is elderly with premorbid conditions (diabetes and hypertension) that place them at risk of proteinuria and renal insufficiency. Patients with this disorder thus may not be referred by oncologists or primary care physicians. Finally, the nephrologist may be unaware of this entity and thus never pursue the diagnosis. Although this disease is uncommon, in the appropriate clinical setting, knowing that profound lysozymuria can cause proteinuria may obviate the need for additional invasive investigations. In situations in which concomitant glomerular pathological states are suspected, urinary albumin excretion also should be measured. The kidney biopsy specimen has a characteristic appearance on light and electron microscopy, and the diagnosis is confirmed by immunohistochemistry for lysozyme.
ACKNOWLEDGEMENTS
Support: This work was supported by National Institutes of Health Grant T32-DK07527-23 to Dr Patel.
Footnotes
Financial Disclosure: None.
REFERENCES
- 1.Perazella MA, Eisen RN, Frederick WG, Brown E. Renal failure and severe hypokalemia associated with acute myelomonocytic leukemia. Am J Kidney Dis. 1993;22:462–467. doi: 10.1016/s0272-6386(12)70154-3. [DOI] [PubMed] [Google Scholar]
- 2.Humphreys BD, Sharman JP, Henderson JM, et al. Gemcitabine-associated thrombotic microangiopathy. Cancer. 2004;100:2664–2670. doi: 10.1002/cncr.20290. [DOI] [PubMed] [Google Scholar]
- 3.Onida F, Kantarjian HM, Smith TL, et al. Prognostic factors and scoring systems in chronic myelomonocytic leukemia: A retrospective analysis of 213 patients. Blood. 2002;99:840–849. doi: 10.1182/blood.v99.3.840. [DOI] [PubMed] [Google Scholar]
- 4.Stark AN, Thorogood J, Head C, Roberts BE, Scott CS. Prognostic factors and survival in chronic myelomonocytic leukaemia (CMML) Br J Cancer. 1987;56:59–63. doi: 10.1038/bjc.1987.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Robinson GT, Sundaram KR, Dilly SA, Bevan DH, Andrews PA. Renal failure in a patient with chronic myelomonocytic leukaemia. Nephrol Dial Transplant. 1997;12:1500–1502. doi: 10.1093/ndt/12.7.1500. [DOI] [PubMed] [Google Scholar]
- 6.Morschhauser F, Wattel E, Pagniez D, et al. Glomerular injury in chronic myelomonocytic leukemia. Leuk Lymphoma. 1995;18:479–483. doi: 10.3109/10428199509059648. [DOI] [PubMed] [Google Scholar]
- 7.Osserman EF, Lawlor DP. Serum and urinary lysozyme (muramidase) in monocytic and monomyelocytic leukemia. J Exp Med. 1966;124:921–952. doi: 10.1084/jem.124.5.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Saitoh T, Murakami H, Uchiumi H, et al. Myelodysplastic syndromes with nephrotic syndrome. Am J Hematol. 1999;60:200–204. doi: 10.1002/(sici)1096-8652(199903)60:3<200::aid-ajh6>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 9.Mok CC, Tam SC, Kwong YL. Pseudonephrotic syndrome caused by lysozymuria. Ann Intern Med. 1994;121:818. doi: 10.7326/0003-4819-121-10-199411150-00020. [DOI] [PubMed] [Google Scholar]
- 10.Thomson M, de Arriba G, Ordi J, Oliva H, Hernando L. Acute myelogenous leukemia treated with daunomycin associated with nephrotic syndrome. Nephron. 1989;51:261–264. doi: 10.1159/000185296. [DOI] [PubMed] [Google Scholar]
- 11.Morino N, Nojima Y, Mimura T, et al. Nephrotic syndrome developed in a patient with acute promyelocytic leukemia treated with daunomycin. Nephron. 1995;70:374–375. doi: 10.1159/000188623. [DOI] [PubMed] [Google Scholar]
- 12.Aguado MJ, Garcia de Bustos J, Ojeda E, Quevedo E, Foncillas MA, Hernandez Navarro F. Proteinuria caused by lysozymuria mimics nephrotic syndrome. Nephron. 2000;86:183. doi: 10.1159/000045738. [DOI] [PubMed] [Google Scholar]
- 13.Fleming A. On a remarkable bacteriolytic element found in tissues and secretions. Proc R Soc London. 1922;93:306–317. [Google Scholar]
- 14.Sussman M, Asscher AW, Jenkins JA. The intrarenal distribution of lysozyme (muramidase) Invest Urol. 1968;6:148–152. [PubMed] [Google Scholar]
- 15.Muggia FM, Heinemann HO, Farhangi M, Osserman EF. Lysozymuria and renal tubular dysfunction in monocytic and myelomonocytic leukemia. Am J Med. 1969;47:351–366. doi: 10.1016/0002-9343(69)90219-8. [DOI] [PubMed] [Google Scholar]
- 16.Rosenthal DS, Maglio R, Moloney WC. Muramidasuria and hyperkaluria in the chloroleukemic rat. Proc Soc Exp Biol Med. 1972;141:499–500. doi: 10.3181/00379727-141-36807. [DOI] [PubMed] [Google Scholar]
- 17.Picken MM, Shen S. Immunoglobulin light chains and the kidney: An overview. Ultrastruct Pathol. 1994;18:105–112. doi: 10.3109/01913129409016279. [DOI] [PubMed] [Google Scholar]
- 18.Humes HD, Hunt DA, White MD. Direct toxic effect of the radiocontrast agent diatrizoate on renal proximal tubule cells. Am J Physiol. 1987;252:F246–F255. doi: 10.1152/ajprenal.1987.252.2.F246. [DOI] [PubMed] [Google Scholar]
- 19.Bernard A, Amor AO, Viau C, Lauwerys R. The renal uptake of proteins: A nonselective process in conscious rats. Kidney Int. 1988;34:175–185. doi: 10.1038/ki.1988.163. [DOI] [PubMed] [Google Scholar]
- 20. [Accessed August 27];Online Medelian Inheritance in Man for Lysozyme. 2008 Available at http://www.ncbi.nlm.nih.gov/entrez/dispomim.cgi?id=153450.
- 21.Yazaki M, Farrell SA, Benson MD. Anovel lysozyme mutation Phe57Ile associated with hereditary renal amyloidosis. Kidney Int. 2003;63:1652–1657. doi: 10.1046/j.1523-1755.2003.00904.x. [DOI] [PubMed] [Google Scholar]
- 22.Pruzanski W, Platts ME. Serum and urinary proteins, lysozyme (muramidase), and renal dysfunction in monoand myelomonocytic leukemia. J Clin Invest. 1970;49:1694–1708. doi: 10.1172/JCI106387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Levinson SS, Elin RJ, Yam L. Light chain proteinuria and lysozymuria in a patient with acute monocytic leukemia. Clin Chem. 2002;48:1131–1132. [PubMed] [Google Scholar]
- 24.Harrison JF, Parker RW, De Silva KL. Lysozymuria and acute disorders of renal function. J Clin Pathol. 1973;26:278–284. doi: 10.1136/jcp.26.4.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Studdy PR, Lapworth R, Bird R. Angiotensin-converting enzyme and its clinical significance—A review. J Clin Pathol. 1983;36:938–947. doi: 10.1136/jcp.36.8.938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tomita H, Sato S, Matsuda R, et al. Serum lysozyme levels and clinical features of sarcoidosis. Lung. 1999;177:161–167. doi: 10.1007/pl00007637. [DOI] [PubMed] [Google Scholar]
- 27.Aribi A, Borthakur G, Ravandi F, et al. Activity of decitabine, a hypomethylating agent, in chronic myelomonocytic leukemia. Cancer. 2007;109:713–717. doi: 10.1002/cncr.22457. [DOI] [PubMed] [Google Scholar]
- 28.Kantarjian H, Oki Y, Garcia-Manero G, et al. Results of a randomized study of 3 schedules of low-dose decitabine in higher-risk myelodysplastic syndrome and chronic myelomonocytic leukemia. Blood. 2007;109:52–57. doi: 10.1182/blood-2006-05-021162. [DOI] [PubMed] [Google Scholar]